- 1Department of Gastroenterology, Sir Run Run Shaw Hospital, College of Medicine Zhejiang University, Hangzhou, China
- 2Medical Affairs, Janssen, China
Background and Aims: Ustekinumab (UST) was approved in China for treating moderate-to-severe Crohn’s disease (CD) in 2020. We aimed to identify the reasons and possible contributing factors for UST preference in Chinese patients with CD.
Methods: We conducted a multicenter cross-sectional survey among patients with moderate to severe CD who underwent UST treatment in 27 hospitals. Patients completed a 46-item questionnaire that included information on demographics, clinical characteristics, reasons in favor of UST and shared decision-making perception. Logistic regression analysis was performed to examine the predictive factors of different UST preferences.
Results: Overall, 127 patients (73 males; mean age, 25.9 ± 9.9 years) completed the questionnaire. Most patients (74.8%) had biologic failure. The most common reason for the latest treatment disconnection was unresponsiveness to the previous medications. The major UST information sources were physicians (96.1%). Nearly half of the patients (44.9%) reported shared decision making regarding UST treatment. No difference was found in the decision-making patterns in terms of sex and age. The most influential reason for UST preference was “effectiveness” (77%, 98/127), followed by “safety” (65%, 83/127), “frequency of administration” (39%, 49/127), and “mode of administration” (37%, 47/127). Multivariate logistic regression analysis revealed that a positive self-rated health status was a contributing factor for UST preference with a low frequency of administration.
Conclusion: This is the first multicenter survey of Chinese patients with CD to identify the possible contributing factors for UST preference. Treatment choice should be discussed with patients because individual preferences are determined by diverse factors.
Introduction
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract characterized by a perpetuated mucosal immune response, often requiring life-long medical treatment (Vermeire et al., 2007). Although CD has been considered remarkably rare in Asian countries compared with Western countries, its incidence and prevalence have been increasing recently in China (Qiao and Ran, 2019). The exact etiology of the disease has not been fully clarified; interleukin-23 (IL-23) is regarded as one of the main pathophysiological components involved in the retractable mucosal inflammation of the gut (Petagna et al., 2020).
Therapeutic medications for patients with CD, which have been endorsed by the National Medical Products Administration of China, include glucocorticoids, immunosuppressants, tumor necrosis factor (TNF) antagonists, and integrin inhibitors (Biancone et al., 2003; Colombel et al., 2007; Sandborn et al., 2012). Recently, several anti-IL23 agents, including ustekinumab (UST), guselkumab, tildrakizumab, and risankizumab, have been administered as new therapeutic strategies for achieving sustained clinical remission and mucosal healing in Western populations with CD (Ma et al., 2019; Almradi et al., 2020). However, only UST has been approved in China for intravenous induction therapy of moderate-to-severe CD since 2017 as a fully human monoclonal antibody to IL-12/23p40. Since 2020, UST has been utilized among the Chinese population with CD. UST can be administered by subcutaneous or intravenous injection, the latter of which is the only usage for infliximab (IFX), the most common biological agent for CD treatment in China.
The decision to use UST is based on many factors, including information sources and perceptions about UST. Rigorous patient involvement in decision making plays a vital role in the management of CD because patients, who are actively involved in decision making regarding their treatment, have a greater possibility of acquiring satisfaction and better clinical outcomes (van den Bemt and van Lankveld, 2007; Siegel, 2012). Therefore, it is essential for patients to be actively involved in the decision-making process (Baars et al., 2010). Moreover, a recent study demonstrated that patients with psoriasis living in the Netherlands preferred UST, mainly due to its therapeutic effects (van Muijen et al., 2021). However, no study has been conducted to investigate the reasons and factors associated with UST preference in patients with CD in the Chinese population.
Therefore, we aimed to identify the reasons and possible contributing factors for UST preference in the profiles of Chinese patients. In addition, we also sought to investigate the information sources and decision-making patterns of the Chinese population with CD.
Materials and methods
Study design and population
We conducted a multicenter cross-sectional survey among patients with moderate-to-severe CD who underwent UST treatment. Twenty-seven hospitals in China participated in this study from October 2020 to February 2021, and patients were enrolled consecutively. Male and female patients enrolled in the study had a diagnosis established by a combination of clinical symptoms, endoscopic examination, pathologic examination, and the absence of alternative diagnoses (Lichtenstein et al., 2018). CD activity was classified based on the Crohn’s disease activity index (Conigliaro et al., 2021). The studies involving human participants were reviewed and approved by the China Ethics Committee of Registering Clinical Trials. The patients/participants provided their written informed consent to participate in this study.
Questionnaires
“The Questionnaire in Chinese patients with moderate to severe CD who underwent UST treatment” was done just after the decision of treatment with ustekinumab. It consisted of 46 items, which could be completed by the patients within 10 min. First, the patients were asked 17 questions concerning their demographic information, including the age, body mass index, and the history of alcohol intake. Second, 25 questions about clinical characteristics were asked, including the date of onset, date of diagnosis, history of perianal surgery, and previous medications. Finally, the remaining four questions pertained to the reasons for UST preference, information sources for UST, and shared decision-making perception required answers. The detailed contents of the questionnaire are shown in Supplementary Table S1.
Treatment strategy
Patients received different doses of UST according to their body weight: 1) week 0: UST intravenous injection of 260 mg when weight was ≤55 kg, 390 mg when weight was >55 kg, but ≤85 kg and 520 mg when weight was >85 kg; 2) week 8: UST subcutaneous injection of 90 mg; and 3) every 12 weeks after week 8: subcutaneous or intravenous injection of the same dose of UST, dependent upon the physicians in different centers.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (quartiles, Q1–Q3), and categorical variables are presented as frequencies and percentages. Different sources of information on UST and strategy for UST drug selection were compared between sexes and ages using the Chi-square test. Student’s t-test or the Wilcoxon signed-rank test was used for continuous variables, while the Chi-square test or Fisher’s exact test was used for categorical variables to compare the characteristics between groups (groups with different preferences toward the frequency of UST administration, different preferences toward modes of UST administration, and different preferences toward impacts of UST treatment on daily life). Variables with a p-value ≤0.1 were selected to fit a multivariate logistic model to explore potentially influential factors that led to different reasons for choosing UST. All tests were two sided, and the results were considered statistically significant at a p-value <0.05. All analyses were conducted using SAS 9.4 (SAS Institute, Cary NC, USA), and the graphs were plotted using R software (version 4.1) (R. R Development Core Team, 2021) with the ggplot2 package. (Wickham, 2016)
Results
Baseline characteristics
A total of 127 patients diagnosed with CD were enrolled, and 73 (57.5%) of them were males. The mean age at survey was 31.0 ± 11.3 years, the mean age at diagnosis was 25.9 ± 9.9 years, and the median interval between onset and diagnosis was 9.4 (1.8–48.2) months. Approximately half of the patients (48.8%) had a university education background, and 59 (46.5%) household income per capita of the patients were less than 5,000 yuan/month. Forty (31.5%) and 61 (48.0%) patients had good and fair health statuses, respectively. L1 (ileal) and B2 (stricturing) were the most common location and disease behavior of CD. Majority of the patients (74.8%) had biologic failure (mainly TNF inhibitors); the most common reason for the latest treatment discontinuation was unresponsiveness to the previous medications. Other demographic and clinical characteristics of the participants are presented in Table 1.
As shown in Figure 1, the participants were mostly from the Zhejiang and Hubei Provinces, followed by the Guangdong and Hunan Provinces. Furthermore, there were participants from Fujian, Jiangxi, Anhui, Guizhou, Qinghai, and Hebei Provinces.
Source of information on ustekinumab
As shown in Table 2, the major information source of UST treatment for CD was from physicians (122, 96.1%), followed by family/friends/ associations of patients (21, 16.5%), internet (13, 10.2%), and books or television (5,3.9%). Except for mere sex differences for source from physicians (100% versus 93.2%; p = 0.0497), no significant difference was observed among other information sources. In addition, a similar pattern was found between different age subgroups at diagnosis when comparing the sources of information.
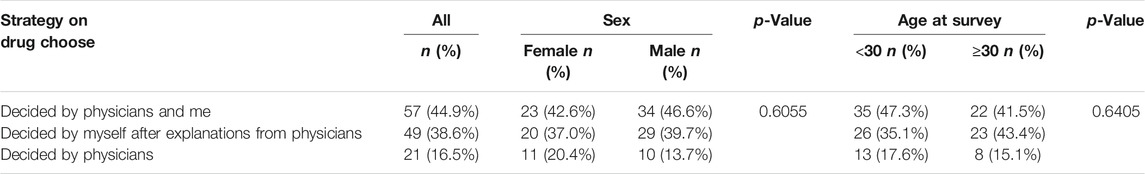
Strategy for ustekinumab drug choice
Table 3 displays the choice strategy for UST among the different sex and age subgroups. Nearly half of the patients (57, 44.9%) reported a shared decision making regarding UST treatment, followed by 49 (38.6%), self-decision with explanations from physicians. No differences were found in the decision-making patterns in terms of sex and age. Considering the pandemic situation (COVID) during the study period, we further analyzed whether there were differences between patients from Hubei Province and other provinces in the aspect of strategy for UST drug choice. As shown in Supplementary Table S2, no significant difference was found in strategies on ustekinumab drug choice between patients from Hubei Province and other provinces.
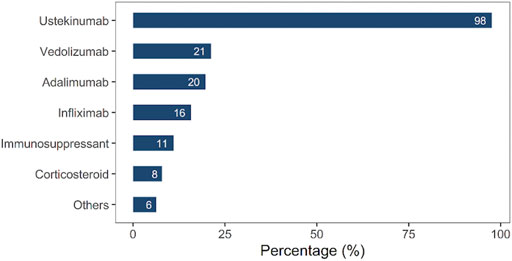
Reasons for ustekinumab preference
As shown in Figure 2, UST (98%) was the major candidate option for CD treatment. Other candidate drugs included vedolizumab (21%), adalimumab (20%), IFX (16%), immunosuppressants (11%), and corticosteroids (8%).
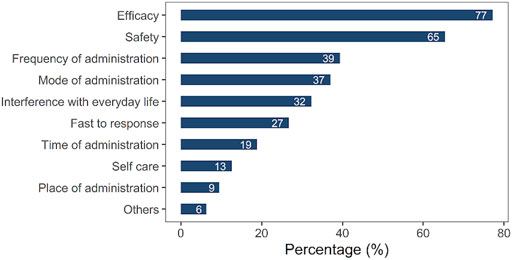
To investigate the various reasons for choosing UST as treatment medication among the Chinese population, we designed multiple choice answers not only in terms of efficacy and safety but also in terms of frequency of administration, mode of administration, interference with everyday life, fast to respond, and time of administration, which had been scarcely detected in previous studies. Efficacy (77%) and safety (65%), frequency of administration (39%), mode of administration (intravenous or subcutaneous) (37%), and decreased interference with everyday life (32%) were the most common options for choosing UST. Unusual reasons included “fast to respond” (27%), “time of administration” (19%), “self-care” (13%), and “place of administration” (9%), as shown in Figure 3. In addition, we also analyzed whether there were differences between patients from Hubei Province and other provinces in reasons for UST preference. As shown in Supplementary Table S3, despite a slightly higher rate of frequency of administration observed in patients from Hubei Province (p = 0.007), no significant difference was found in other reasons for UST preference between patients from Hubei Province and other provinces.
Factors contributing to preference for ustekinumab
Next, we investigated the possible demographic characteristics contributing to the preference for UST with frequency of administration, mode of administration, and interference with everyday life, separately.
In the first subgroup analysis, patients were divided according to different preferences regarding the frequency of UST administration. Table 4 shows that self-rate health was significantly different (p = 0.013) among patients who chose UST for treatment, regardless of the reason for low-frequency administration. Other factors found to be associated with the UST preference, with a low frequency of administration in the univariate analysis, were the age at diagnosis (p = 0.091) and employment status (p = 0.105). In the multivariate analysis, a positive self-rated health status (fair, p = 0.044; good, p = 0.004; and very good, p = 0.007) was a contributing factor for UST preference with a low frequency of administration, as shown in Table 5.

TABLE 4. Characteristics of participants with different preference toward frequency of UST administration.
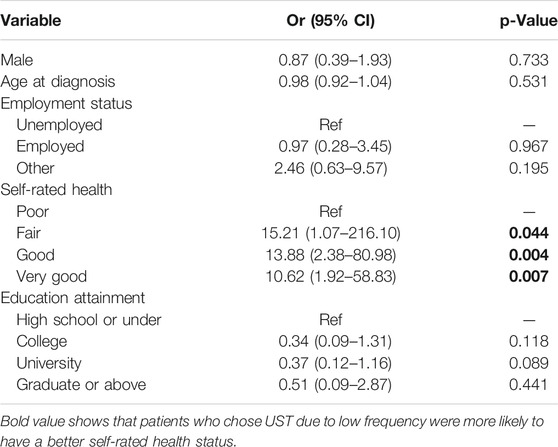
TABLE 5. Multivariate logistic regression analysis of predictive factors for preference toward low frequency of UST administration.
In the second subgroup analysis, patients were divided according to different preferences for the mode of UST administration. History of hormone therapy (p = 0.013), history of biologic agent therapy (p = 0.040), and other reasons for recent drug withdrawal (p = 0.025) showed differences in the univariate analysis. Similar results revealed that self-rate health was a contributing factor for UST preference due to convenient administration both in the univariate analysis (p = 0.010) and multivariate analysis (very good, p = 0.008), as shown in Tables 6 and 7.

TABLE 6. Characteristics of participants with different preference toward mode of UST administration.
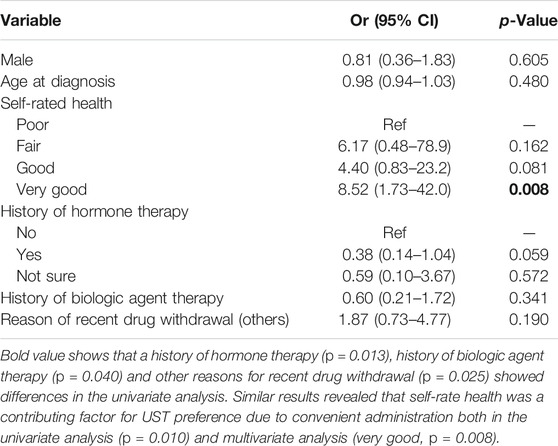
TABLE 7. Multivariate logistic regression analysis of predictive factors for preference toward convenience of UST administration.
When analyzed separately according to different preferences toward the impact of UST administration on everyday life, self-rate health was still significantly different (p = 0.008) between the two groups, regardless of whether they had a low impact on everyday life. Other factors found to be associated with impact preference in the univariate analysis were a history of hormone therapy (p = 0.067) and economic reasons for recent drug withdrawal (p = 0.050) (Table 8). Furthermore, a fitful multivariate logistic model was selected to explore potential factors that were relevant to the impact preference for choosing UST. In line with this, patients with a positive self-rated health status (good, p = 0.020; very good, p = 0.008) were correlated with a higher possibility of UST preference with a low interference in everyday life, as shown in Table 9.
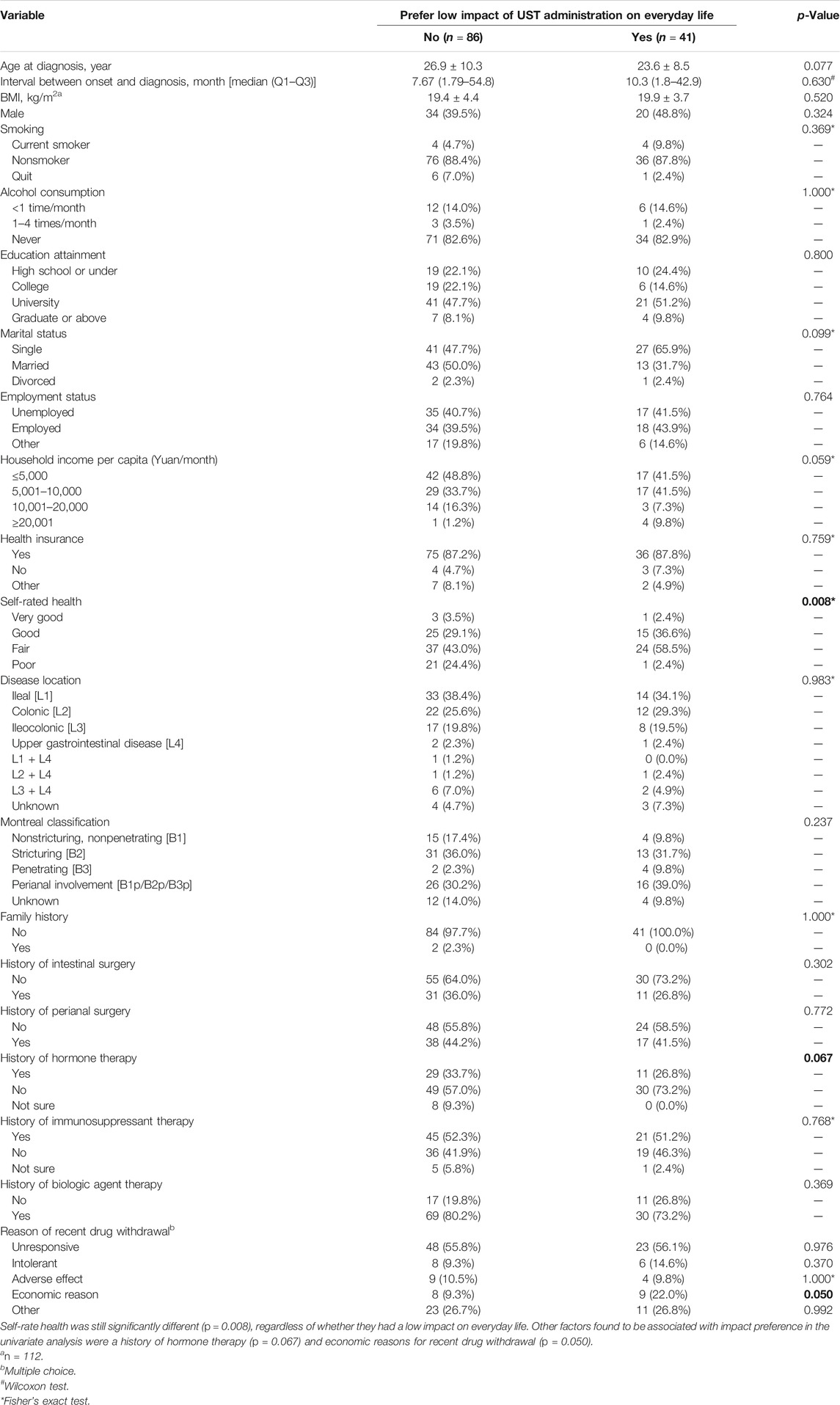
TABLE 8. Characteristics of participants with different preference toward impact of UST administration on everyday life.
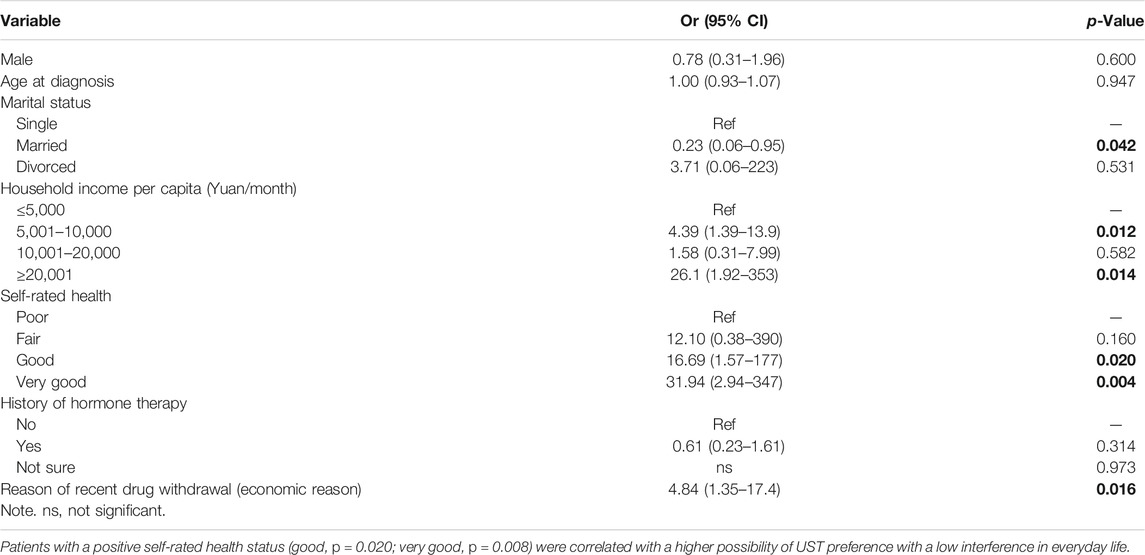
TABLE 9. Multivariate logistic regression analysis of predictive factors for preference toward impact of UST administration on everyday life.
Discussion
The principal aim of this study was to identify the reasons and possible contributing factors for UST preference in the Chinese population with CD. To our knowledge, this is the first and most current multicenter cross-sectional study to investigate the preference for choosing UST and to identify the potential contributing factors for frequency and mode of administration in subgroup analyses among Chinese patients with CD.
This questionnaire-based study demonstrated that effectiveness, safety, frequency of administration, and mode of administration were the primary reasons for choosing UST. Patients who chose UST with a low frequency of administration and a convenient mode of administration were more likely to have a better self-rated health status. In China, most patients with CD chose UST for treatment due to steroid dependence, primary unresponsiveness to IFX, adalimumab, and immunosuppressants, or loss of response in long-term follow-up. IFX is the most common biological agent for the treatment of CD. Despite the high cost of UST, selecting UST also has various advantages compared with IFX, including efficacy and safety. Moreover, UST has a lower frequency of administration (three times in the first 6 months, thereafter once every 12 weeks) than IFX (three times in the first 6 weeks; thereafter, once every 8 weeks), which may be beneficial for patients in terms of saving time. In addition, after the first intravenous treatment of UST at the hospital, patients can subcutaneously inject UST by themselves at home for the second time, which is more convenient for patients who have less time for hospital admittance, such as international students, young people, and businessmen. The results of our study are similar to those of several recent Western studies (Constantinescu et al., 2009; Vavricka et al., 2012). Although the results from those studies were about IFX, all patients were more likely to select a subcutaneous injection strategy.
Patients reported that the major information source for UST information was from physicians, and nearly half of the patients reported shared decision making with respect to UST treatment. In this digital age, patients prefer to educate themselves and research on the benefits and risks of their therapy, and actively participate in the decision-making process of treatment (Guadagnoli and Ward, 1998; Siegel, 2012). Patients are more likely to be involved in the treatment of inflammatory bowel disease because of the uncertainty of the evidence regarding many clinical questions and the heterogeneity of the disease course (Siegel, 2012). Sharing decision making with patients is significant for improving clinical outcomes, resulting from a better adherence to the therapy. We believe that our results will facilitate Chinese patients with CD to make informative decisions.
This study had several strengths. First, patients were from 27 inflammatory bowel disease referral centers in different regions of China and represented a broad and reliable spectrum of the Chinese population. Second, this study was based on a well-designed questionnaire that contained detailed information about demographic and clinical characteristics at diagnosis and preference for UST from the perspectives of the patients. Third, we investigated possible demographic characteristics contributing to preference for UST in terms of frequency of administration, mode of administration, and interference with everyday life separately, which had not been analyzed in previous studies.
However, there are some limitations to the present study. First, generalization and extrapolation of the results in our study are questionable as genotypic and phenotypic differences exist in different regions of Asia. Multicenter studies targeting other Asian populations are needed in further work. Second, the factors for effectiveness and safety in UST preferences were not analyzed. Instead, the current study was the first to focus on the frequency and mode of administration. Finally, noncontinuity and intergenerational effects might have influenced the results owing to a cross-sectional study design. Further prospective studies may be the preferred choice to focus on the following treatment strategies after UST and associated factors.
Conclusion
In conclusion, we found that the effectiveness, safety, and frequency of administration were the three main reasons patients chose UST. Patients who chose UST due to low frequency and administration convenience were more likely to have a better self-rated health status. Treatment choices should be discussed with patients as individual preferences are determined by diverse factors.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the China Ethics Committee of Registering Clinical Trials. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LY, XZ, BS, RL, ZL, JC, and QC contributed to the conception and design of the study. XZ and RL contributed to the acquisition of data. BS performed the statistical analysis. LY, XZ, and LW wrote the first draft of the manuscript. LY and QC critically revised the manuscript. All authors contributed to the article, and read and approved the submitted version.
Conflict of Interest
Authors ZL and JC were employed by the company Janssen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.736149/full#supplementary-material
References
Almradi, A., Hanzel, J., Sedano, R., Parker, C. E., Feagan, B. G., Ma, C., et al. (2020). Clinical Trials of IL-12/IL-23 Inhibitors in Inflammatory Bowel Disease. BioDrugs 34, 713–721. doi:10.1007/s40259-020-00451-w
Baars, J. E., Markus, T., Kuipers, E. J., and van der Woude, C. J. (2010). Patients' Preferences Regarding Shared Decision-Making in the Treatment of Inflammatory Bowel Disease: Results from a Patient-Empowerment Study. Digestion 81, 113–119. doi:10.1159/000253862
Biancone, L., Tosti, C., Fina, D., Fantini, M., De Nigris, F., Geremia, A., et al. (2003). Review Article: Maintenance Treatment of Crohn's Disease. Aliment. Pharmacol. Ther. 17 (Suppl. 2), 31–37. doi:10.1046/j.1365-2036.17.s2.20.x
Colombel, J. F., Sandborn, W. J., Rutgeerts, P., Enns, R., Hanauer, S. B., Panaccione, R., et al. (2007). Adalimumab for Maintenance of Clinical Response and Remission in Patients with Crohn's Disease: the CHARM Trial. Gastroenterology 132, 52–65. doi:10.1053/j.gastro.2006.11.041
Conigliaro, P., Chimenti, M. S., Triggianese, P., D'Antonio, A., Sena, G., Alfieri, N., et al. (2021). Two Years Follow-Up of Golimumab Treatment in Refractory Enteropathic Spondyloarthritis Patients with Crohn Disease: A STROBE-Compliant Study. Medicine (Baltimore) 100, e25122. doi:10.1097/MD.0000000000025122
Constantinescu, F., Goucher, S., Weinstein, A., Smith, W., and Fraenkel, L. (2009). Understanding Why Rheumatoid Arthritis Patient Treatment Preferences Differ by Race. Arthritis Rheum. 61, 413–418. doi:10.1002/art.24338
Guadagnoli, E., and Ward, P. (1998). Patient Participation in Decision-Making. Soc. Sci. Med. 47, 329–339. doi:10.1016/s0277-9536(98)00059-8
Lichtenstein, G. R., Loftus, E. V., Isaacs, K. L., Regueiro, M. D., Gerson, L. B., and Sands, B. E. (2018). ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am. J. Gastroenterol. 113, 481–517. doi:10.1038/ajg.2018.27
Ma, C., Panaccione, R., Khanna, R., Feagan, B. G., and Jairath, V. (2019). IL12/23 or Selective IL23 Inhibition for the Management of Moderate-To-Severe Crohn's Disease? Best Pract. Res. Clin. Gastroenterol. 38-39, 101604. doi:10.1016/j.bpg.2019.02.006
Petagna, L., Antonelli, A., Ganini, C., Bellato, V., Campanelli, M., Divizia, A., et al. (2020). Pathophysiology of Crohn's Disease Inflammation and Recurrence. Biol. Direct 15, 23. doi:10.1186/s13062-020-00280-5
Qiao, Y., and Ran, Z. (2019). Potential Influential Factors on Incidence and Prevalence of Inflammatory Bowel Disease in mainland China. JGH Open 4, 11–15. doi:10.1002/jgh3.12238
R. R Development Core Team, (2021). A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Sandborn, W. J., Gasink, C., Gao, L. L., Blank, M. A., Johanns, J., Guzzo, C., et al. CERTIFI Study Group (2012). Ustekinumab Induction and Maintenance Therapy in Refractory Crohn's Disease. N. Engl. J. Med. 367, 1519–1528. doi:10.1056/NEJMoa1203572
Siegel, C. A. (2012). Shared Decision Making in Inflammatory Bowel Disease: Helping Patients Understand the Tradeoffs between Treatment Options. Gut 61, 459–465. doi:10.1136/gutjnl-2011-300988
van den Bemt, B. J., and van Lankveld, W. G. (2007). How Can We Improve Adherence to Therapy by Patients with Rheumatoid Arthritis? Nat. Clin. Pract. Rheumatol. 3, 681. doi:10.1038/ncprheum0672
van Muijen, M. E., Atalay, S., van Vugt, L. J., Vandermaesen, L. M. D., van den Reek, J. M. P. A., and de Jong, E. M. G. J. (2021). Unmet Personal Patient Needs in Psoriasis Patients with Low Disease Activity on Adalimumab, Etanercept or Ustekinumab. Drugs Real World Outcomes 82, 163–172. doi:10.1007/s40801-021-00227-w
Vavricka, S. R., Bentele, N., Scharl, M., Rogler, G., Zeitz, J., Frei, P., et al. (2012). Systematic Assessment of Factors Influencing Preferences of Crohn's Disease Patients in Selecting an Anti-tumor Necrosis Factor Agent (CHOOSE TNF TRIAL). Inflamm. Bowel Dis. 18, 1523–1530. doi:10.1002/ibd.21888
Vermeire, S., van Assche, G., and Rutgeerts, P. (2007). Review Article: Altering the Natural History of Crohn's Disease-Eevidence for and against Current Therapies. Aliment. Pharmacol. Ther. 25, 3–12. doi:10.1111/j.1365-2036.2006.03134.x
Keywords: Crohn’s disease, ustekinumab (UST), preference, Chinese patients, multicenter cross-sectional study
Citation: Yao L, Zhu X, Shao B, Liu R, Li Z, Wu L, Chen J and Cao Q (2021) Reasons and Factors Contributing to Chinese Patients’ Preference for Ustekinumab in Crohn’s Disease: A Multicenter Cross-Sectional Study. Front. Pharmacol. 12:736149. doi: 10.3389/fphar.2021.736149
Received: 04 July 2021; Accepted: 22 October 2021;
Published: 22 November 2021.
Edited by:
Fernando Gomollón, University of Zaragoza, SpainReviewed by:
Beatriz Sicilia, Burgos University Hospital, SpainSantiago García López, Hospital Universitario Miguel Servet, Spain
Copyright © 2021 Yao, Zhu, Shao, Liu, Li, Wu, Chen and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Cao, Y2FvcUB6anUuZWR1LmNu
 Lingya Yao
Lingya Yao Xiao Zhu
Xiao Zhu Bule Shao1
Bule Shao1 Qian Cao
Qian Cao