
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 06 September 2021
Sec. Experimental Pharmacology and Drug Discovery
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.735876
This article is part of the Research Topic Natural Products as Drivers in Drug Development for Neurodegenerative Disorders View all 8 articles
 Yang Yang†
Yang Yang† Lijing Zhang†
Lijing Zhang† Jiaojiao Yu†
Jiaojiao Yu† Zhaobin Ma
Zhaobin Ma Moxiang Li
Moxiang Li Jin Wang
Jin Wang Pengcheng Hu
Pengcheng Hu Jia Zou
Jia Zou Xueying Liu
Xueying Liu Ying Liu
Ying Liu Su An
Su An Cheng Xiang
Cheng Xiang Xiaoxi Guo
Xiaoxi Guo Qian Hao
Qian Hao Tian-Rui Xu*
Tian-Rui Xu*The serotonin receptor 5-HT1B is widely expressed in the central nervous system and has been considered a drug target in a variety of cognitive and psychiatric disorders. The anti-inflammatory effects of 5-HT1B agonists may present a promising approach for Alzheimer’s disease (AD) treatment. Herbal antidepressants used in the treatment of AD have shown functional overlap between the active compounds and 5-HT1B receptor stimulation. Therefore, compounds in these medicinal plants that target and stimulate 5-HT1B deserve careful study. Molecular docking, drug affinity responsive target stability, cellular thermal shift assay, fluorescence resonance energy transfer (FRET), and extracellular regulated protein kinases (ERK) 1/2 phosphorylation tests were used to identify emodin-8-O-β-d-glucopyranoside (EG), a compound from Chinese medicinal plants with cognitive deficit attenuating and antidepressant effects, as an agonist of 5-HT1B. EG selectively targeted 5-HT1B and activated the 5-HT1B-induced signaling pathway. The activated 5-HT1B pathway suppressed tumor necrosis factor (TNF)-α levels, thereby protecting neural cells against beta-amyloid (Aβ)-induced death. Moreover, the agonist activity of EG towards 5-HT1B receptor, in FRET and ERK1/2 phosphorylation, was antagonized by SB 224289, a 5-HT1B antagonist. In addition, EG relieved AD symptoms in transgenic worm models. These results suggested that 5-HT1B receptor activation by EG positively affected Aβ-related inflammatory process regulation and neural death resistance, which were reversed by antagonist SB 224289. The active compounds such as EG might act as potential therapeutic agents through targeting and stimulating 5-HT1B receptor for AD and other serotonin-related disorders. This study describes methods for identification of 5-HT1B agonists from herbal compounds and for evaluating agonists with biological functions, providing preliminary information on medicinal herbal pharmacology.
The serotonergic system is important in regulating crucial processes in the central nervous system (CNS). These effects are mediated by serotonin receptors, such as the serotonin receptor subtype 1B (5-HT1B). These receptors, belonging to the G protein-coupled receptors (GPCRs) superfamily, are abundantly expressed in the CNS and constitute validated as well as putative drug targets in a variety of cognitive and psychiatric disorders, including depression and Alzheimer’s disease (AD), the most common incurable neurodegenerative disease (Monti and Jantos, 2008; Tiger et al., 2018; Gadgaard and Jensen, 2020).
Activation of the serotonergic system blocks the beta-amyloid (Aβ) oligomer-induced inflammatory response in AD (Ledo et al., 2016). Aβ-induced inflammatory response and neuronal death play important roles in AD pathology. Recent studies have identified inflammatory pathways as potential new drug targets for treating AD (Unzeta et al., 2016). Aβ oligomer-mediated activation of pro-inflammatory tumor necrosis factor (TNF)-α signaling in the brain has been reported as a cause of memory loss in mice (Lourenco et al., 2013). Activation of 5-HT1B by its agonist sumatriptan, an anti-migraine agent, significantly diminishes the mRNA levels of TNF-α in rat nerve cells (Khalilzadeh et al., 2018). Moreover, it has been reported that the frontal and temporal cortices of AD patients show a reduction in 5-HT1B/1D receptor levels (Garcia-Alloza et al., 2004). Therefore, the anti-inflammatory effects of 5-HT1B agonists may present a promising approach in AD treatment.
Overall activation of 5-HT1B in the CNS may have antidepressant effects (Sanchez et al., 2015). Several lines of evidence support the therapeutic use of herbal antidepressants in the treatment of AD (Jeon et al., 2019; Chen et al., 2020; Li et al., 2020), wherein various compounds present in the ingredients reduce neuronal inflammation and apoptosis. However, the targets of the herbal antidepressants and the interactions between the active compounds and their targets, which constitute the basic issue of medicinal herbal pharmacology, remain to be elucidated. The functional overlap between herbal antidepressants of traditional Chinese medicine and 5-HT1B stimulation suggests the therapeutic potential of compounds from the ingredients of these medicinal plants, as they can target and stimulate 5-HT1B for AD treatment.
In this study, the active compound emodin-8-O-β-d-glucopyranoside (EG), a component of several Chinese medicinal plants such as Polygonum multiflorum and Rheum officinale that contain herbal antidepressants that could be used in AD treatment (Lin et al., 2015; Jeon et al., 2019; Chen et al., 2020; Li et al., 2020), was identified as a ligand targeting the 5-HT1B receptor. The role of serotonin-system-mediated neural protection was also investigated. The results support the importance of 5-HT1B receptor as a therapeutic target and validate the potential use of 5-HT1B agonists as therapeutic agents for serotonin system-related diseases such as AD.
EG (cas 23,313–21‐5) was obtained from Weikeqi (Sichuan, China), CP‐94253 (4‐{5‐propoxypyrrolo [3,2‐b]pyridin‐3‐yl}‐1.2,3,6‐tetrahydropyridine, catalog # SML0588), DOI (1‐(4‐iodo‐2,5‐dimethoxyphenyl)propan‐2‐amine, catalog #D101), dopamine (4‐(2‐Aminoethyl)benzene‐1,2‐diol, catalog #H8502), and caffeine from Sigma‐Aldrich, ramelteon (N‐[2‐[(8S)‐2.6,7,8‐tetrahydro‐1H‐cyclopenta [e][1]benzoxol‐8‐yl]ethyl]propanamide) from MedChemExpress (catalog # HYA0014), and dihydroergotamine ((2,4, 7R)‐N‐[(1S,2S,4R, 7S)‐7‐benzyl‐2‐hydroxy‐4‐methyl‐5,8‐dioxo‐3‐oxa‐6,9‐diazatricyclo[7.3.0.0 ∧ {2.6}]dodecan‐4‐yl]‐6‐methyl‐6,11‐diazatetracyclo [7.6.1.0∧{2.7}.0 ∧ {12.16}]hexadeca‐1 (16),9,12,14‐tetraene‐4‐carboxamide)and SB 224289 ([4‐[2‐methyl‐4‐(5‐methyl‐1,2,4‐oxadiazol‐3‐yl)phenyl]phenyl]‐(1′‐methylspiro [6,7‐dihydro‐2H‐furo [2,3‐f]indole‐3.4′‐piperidine]‐5‐yl)methanone) from APEx BIO (catalog #B3459 and B6641, respectively). OxB (RSGPPGLQGRAQRLLQASGNHAAGILTM‐NH2), Aβ40 (rat:DAEFGHDSGFEVRHQKLVFFAEDVGSNKGAIIGLMVGGVV; human: DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV) and Aβ42 (rat: DAEFGHDSGFEVRHQKLVFFAEDVGSNKGAIIGLMVGGVVIA; human: DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA) were synthesized by Shangting (Shanghai,China). The Flp‐In™ T‐REx™ 293 cells, Lipofectamine®2000 transfection reagent, and cell culture materials were from Invitrogen (Thermo Fisher Scientific Inc., United States). The anti‐VSV‐G antibody (catalog #V5507) was obtained from Sigma‐Aldrich, and the anti‐ERK1/2‐MAP kinase (catalog # 9102S) and anti‐phospho‐ERK1/2‐MAP kinase (catalog # 9101S) antibodies were from Cell Signaling Technology (Nottingham,United Kingdom).
The VSV-G-mGluR5-5-HT1B, VSV-G-mGluR5-5HT2A, D2, MT2, and OX2 constructs were established by inserting human 5-HT1B cDNA into a pcDNA5/FRT/TO vector (Invitrogen) using the In-Fusion® PCR Cloning System (Clontech, United States) as previously described (Xu et al., 2012). Constructs were introduced into HEK293T cell lines for preliminary transient transfection studies and then into Flp-In™ T-REx™ 293 cells to generate inducible stable cell lines (Ward et al., 2011).
The cells were co-transfected with the pOG44 plasmid and the desired protein cDNA in pcDNA5/FRT/TO in a 9:1 ratio using Lipofectamine®2000 to generate Flp-In™ T-REx™ 293 cells that can inducibly express the indicated constructs (Ward et al., 2011). After 48 h, the medium was supplemented with 200 ng ml−1 hygromycin to select stable transfected cells. The cell pools were tested for inducible expression by adding 100 ng ml−1 doxycycline for 12 h, followed by western blot analysis for phosphorylation of extracellular regulated ERKs corresponding to GPCR activation and VSV-G protein expression.
HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 0.292 g⋅ L−1l-glutamine, whereas PC12 and SH-SY5Y cells were maintained in PRIM 1640 medium and 10% (v/v) fetal bovine serum (FBS) at 37°C in a 5% CO2 humidified atmosphere. The cells were transfected with the indicated constructs using Lipofectamine®2000.
The structure of 5-HT1B, access number 5V54 (Name: Crystal structure of 5-HT1B receptor in complex with methiothepin), was obtained from the PDB database (http://www.rcsb.org/pdb/home/home.do). The 3D structures and properties of CP-94253 and EG were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov). The free molecular docking software AutoDock Tools-1.5.6 was employed, and the Autodock 4 (Department of Molecular Biology of Scripps Research Institute, La Jolla, CA and Department of Cognitive Science of University of California, San Diego, La Jolla, CA) was used for flexible docking, as previously reviewed and discussed (Beuming et al., 2015; Kontoyianni, 2017).
Cells were washed once in cold phosphate-buffered saline (PBS) (120 mM NaCl, 25 mM KCl, 10 mM Na2HPO4, and 3 mM KH2PO4, pH 7.4) and harvested with ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM HEPES, 150 mM NaCl, 1% Triton X-100, and 0.5% sodium deoxycholate, 10 mM NaF, 5 mM EDTA, 10 mM NaH2PO4, and 5% ethylene glycol pH 7.4) supplemented with a protease inhibitor cocktail, as previously described. After heating the samples at 37°C for 5 min, the cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were electrophoretically transferred onto a nitrocellulose membrane, which was then blocked (5% fat-free milk powder in PBS containing 0.1% Tween-20) at room temperature on a rotating shaker for 1 h, washed twice in tris-buffered saline (TBS, pH 7.4) containing 0.1% Tween 20, and incubated with the appropriate primary antibody for 2 h, followed by incubation with a secondary antibody for 1 h at room temperature. Subsequently, the proteins of interest were visualized using the ECL Chemiluminescence System (Santa Cruz Biotechnology) according to the manufacturer’s instructions. Protein levels were quantified by band intensity using the Quantity One 1D Analysis Software.
Intact Flp-In™ T-REx™ 293 cells induced to express mGluR5-VSV-G-5-HT1B were treated with EG, dihydroergotamine, CP-94253, or the vehicle at the indicated concentrations and used for subsequent studies. In the DARTS experiment, cell lysates were subjected to pronase digestion at room temperature for 3 min, then loaded for western blotting to detect the 5-HT1B protein. In the CETSA experiment, the cells were scraped off and lysed via alternate freezing and thawing thrice with liquid nitrogen, then heated to varying temperatures for 5 min. The 5-HT1B protein was detected with western blotting. In both experiments, GAPDH was used as the loading control for quantification.
The 5-HT1B FRET sensor-expressing cells were placed in a microscope chamber containing physiological HEPES-buffered saline solution (130 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, and 10 mM d-glucose, pH 7.4). The cells were then imaged using an inverted Nikon TE2000-E microscope (Nikon Instruments) equipped with a 40× (numerical aperture = 1.3) oil immersion Fluor lens, as previously described (Xu et al., 2012). The monochromator was set at 427 nm/bandwidth (BW) 5 and 504 nm/BW 5 nm to visualize the surface located cyan fluorescent protein (CFP) and FlAsH separately. FRET and donor emission images were recorded simultaneously using a Quadview 2 (QV2) image splitting device (Photometrics, United Kingdom), coupled to a CoolSnap-HQ2 camera connected to the microscope. The FRET and donor signals were detected simultaneously using the following Chroma (Brattleboro, VT) ET dichroic and emitter series mounted in the QV2 cube: ET t505LPXR dichroic, ET535/30 nm, and ET 632/60 nm. Using the streaming capability of the multiple dimensional wavelength acquisition modules of MetaMorph, the ligand-induced changes in intramolecular FRET were recorded directly in the computer’s hard drive at 40 ms intervals during excitation with 427 nm light. The Cool Snap-HQ2 camera was operated in the 14-bit mode, and exposure time, 8 × 8 binning, and camera gain, were kept constant for all streaming experiments. Computerized control of all electronic hardware and camera streaming acquisition was achieved using the MetaMorph software (version 7.7.5 Molecular Devices, Sunnydale, CA). A peristaltic pump, operated at a 5 ml/min flow rate, was used to rapidly add or remove test ligands to or from the imaging chamber. The ratiometric quantification of intramolecular charge changes was then calculated as the average 535 nm emission intensity divided by the average 470 nm emission intensity. The FRET ratiometric was set to 1.0 at the onset of each experiment and plotted over time.
Cells stably expressing inducible GPCRs were placed in 6-well plates and allowed to grow overnight. Expression of the constructs was induced by adding 10 ng ml−1 doxycycline for 12 h and rendered quiescent via serum starvation for 12 h, then stimulated for 5 min by the indicated agonist using FBS as the positive control and deionized water as the negative control. Cells were then placed on ice and harvested with ice-cold RIPA buffer. Phosphorylation of ERK1/2 MAP kinases was detected via western blotting using a phospho-ERK1/2-specific antibody. The nitrocellulose membranes were subsequently stripped of immunoglobulins and re-probed using an anti-ERK1/2 antibody to assess the protein loading equivalence.
Total RNA was isolated from PC12 or worm model cells treated as previously indicated, using TRIzol (Ambion Life Technologies), and the mRNA was reversed-transcribed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, catalog #K1621) according to the manufacturer’s instructions. The primer sequences for quantitative PCR were as follows: TNF-α F (5′-CCCTCACACTCAGATCATCTTCT-3′), TNF-α R (3′-GCTACGACGTGGGCTACAG-5′), β-Actin F (5′-GATTACTGCTCTGGCTCCTAGC-3′), and β-Actin R (3′-GACTCATCGTACTCCTGCTTGC-5′). Previously described primers were used for the serotonin receptor subtypes (Koizumi and Nakajima, 2014) and the worm model (Bellier et al., 2009; Sahu et al., 2012). Three independent experiments were performed on triplicate samples. PCR amplification was performed using the SYBR Green PCR Master Mix Kit (Thermo Fisher). All quantifications were normalized to GAPDH levels.
The PC12 cells were seeded in a 96-well plate at a 5 × 103 density; 12 h after seeding, CP-94253 or EG was added with or without SB 224289. After 24 h of seeding, the amyloid peptides were added, and after 18 h, the cells were subjected to CCK-8 (catalog #C0037, Beyotime, China), MTT (catalog #M1025, Solarbio, Beijing, China), and LDH (catalog # BC0680, Solarbio, Beijing, China) assays following the manufacturers’ instructions.
The C. elegans CL14176 strain was synchronized to the L1 phase and allocated to 160 μL S media with the appropriate treatment drug concentrations. The worms were cultured at 16°C for 24 h and transferred to 25°C, at which point they were activated to express amyloid peptides that would result in paralysis (Dostal and Link, 2010). At 38 h, the worms were counted for the paralysis phenotype. Each group contained approximately 60 worms. The positive control group was set by adding 6.27 mM caffeine.
Variables were compared between non-treated and treated groups using Student’s t-tests. Statistical differences in protein expression, cell viability, and relative mRNA levels between two groups were analyzed using a One-way Analysis of variance (ANOVA). Data were analyzed using GraphPad Prism 6.01 software (GraphPad Software, La Jolla, CA, United States). Data are expressed as mean ± S.D. of values from at least three independent experiments. A value of p < 0.05 was considered statistically significant.
Molecular docking is widely used to identify specific molecular targets, such as GPCRs (Beuming et al., 2015; Kontoyianni, 2017). We used this technique to determine whether EG binds to the human GPCR 5-HT1B. The binding affinity of EG to 5-HT1B was similar to that of the agonist CP-94253, as the binding energies were −8.09 and −8.48 kcal mol−1, respectively. EG (Figures 1D,E) docked into the same 5-HT1B pocket as CP-94253 (Figures 1A,B). In addition, EG (Figure 1F) interacted with 5-HT1B similarly to the agonist CP-94253 (Figure 1C). The specific interaction parameters are shown in Supplementary Figure S1. The results suggested that EG might target 5-HT1B and act similarly to CP-94253 as a ligand of this receptor.
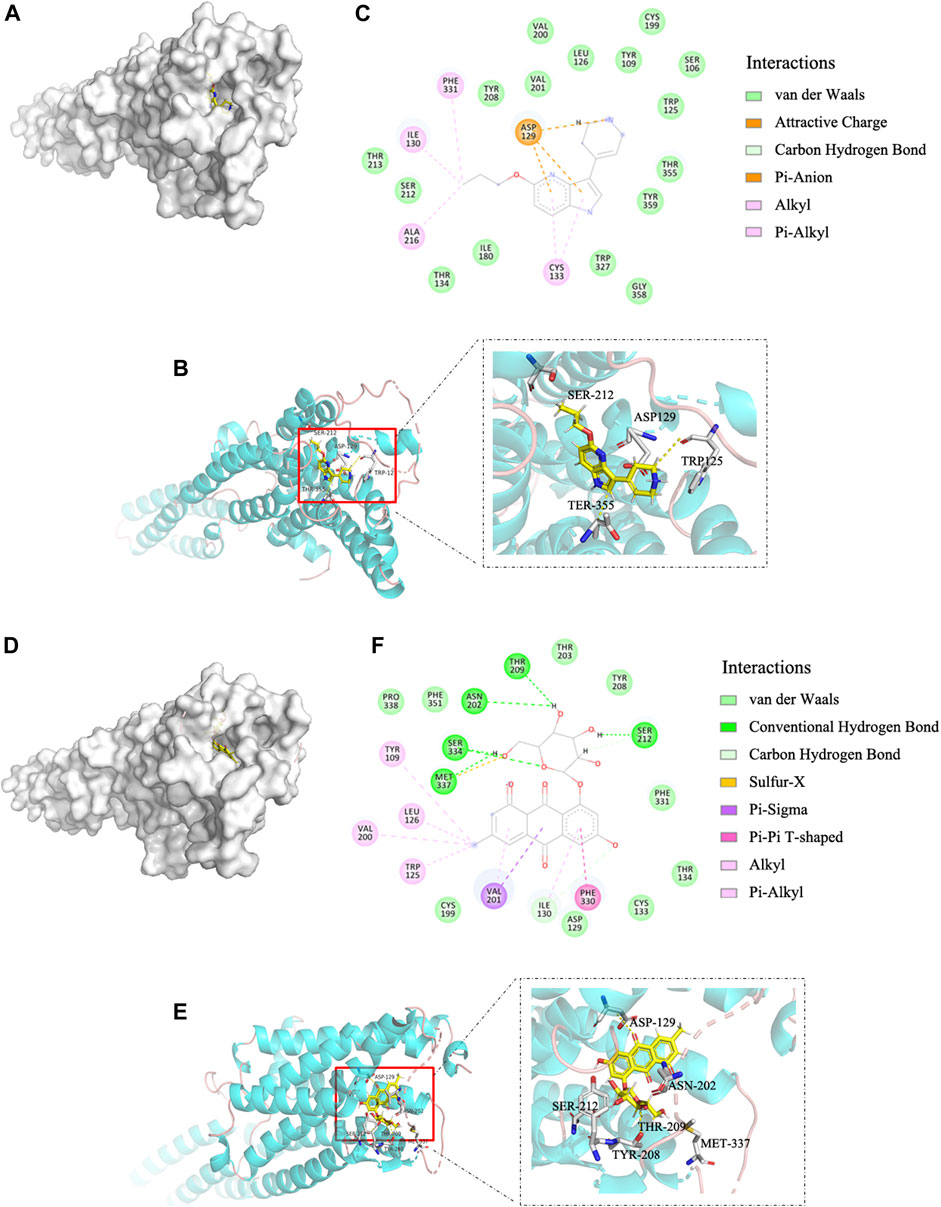
FIGURE 1. EG is a 5-HT1B ligand in molecular docking compared with agonist CP-94253. (A) Electrostatic surface model. (B) Schematic representation of crystal structure. (C) Magnified 2D-view of protein-ligand CP-94253 docking into its 5-HT1B interacting pocket. (D−F) Corresponding representations of EG docking into the same 5-HT1B interacting pocket as CP-94253. EG: emodin-8-O-β-d-glucopyranoside; 5-HT1B: serotonin receptor 1B; CP-94253: 4-{5-propoxypyrrolo [3,2-b]pyridin-3-yl}-1,2,3,6-tetrahydropyridine.
To verify the interaction of EG with the 5-HT1B receptor, we applied DARTS and CETSA, previously reported methods (Chang et al., 2016), for identifying target proteins of natural products without chemical modifications. Unlike that with the single target protein, the conformation and stabilization of the compound-target complex are altered in either DARTS or CETSA, and the interaction between the compound and target protein can be visualized via western blotting (Lomenick et al., 2009; Molina et al., 2013; Chang et al., 2016). In this study, DARTS was performed on the whole-cell lysate of cells stably induced to express 5-HT1B. The proteins were digested with pronase, whereas EG, CP-94253, and dihydroergotamine (a 5-HT1B agonist, FDA-approved for migraine) were used to prevent target depletion. The 5-HT1B protein in the band protected by EG (10−6 M) was enriched approximately 2-fold compared with that in the vehicle, and EG showed a similar protective effect with dihydroergotamine at a low concentration (10−8 M). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was resistant to pronase under this condition and served as a loading indicator (Figures 2A,B). The 5-HT1B protein protected by EG, compared with CP-94253, from digestion is shown in Supplementary Figure S2A. CETSA was performed using intact cells induced to express 5-HT1B. The dihydroergotamine- or vehicle-treated cells were heated to varying temperatures as indicated. The 5-HT1B protein in the soluble fractions was separated from the precipitated destabilized protein and detected with western blotting (Figure 2C). Figure 2D shows ligand-target interaction plotted against temperature to display the shifts. The results revealed a physical interaction between the agonist dihydroergotamine and its target protein 5-HT1B in intact cells. Additionally, EG-induced thermodynamic stabilization of 5-HT1B was observed (Figures 2E,F). The 5-HT1B protein thermodynamically stabilized by EG, compared with CP-94253, is shown in Supplementary Figure S2B.
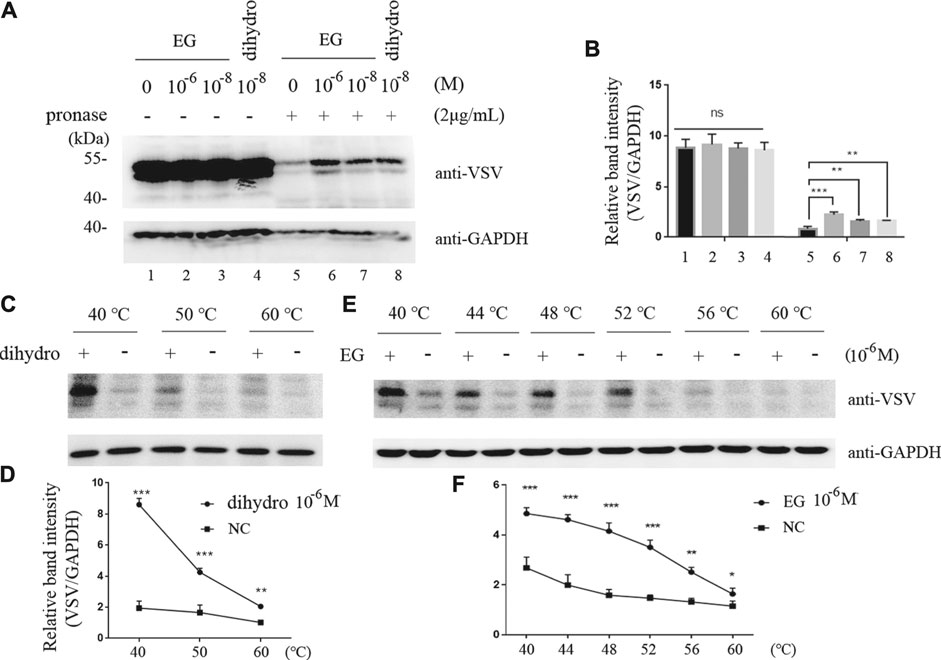
FIGURE 2. EG interaction with 5-HT1B in DARTS and CETSA experiments. (A,B) Intact Flp-In™ T-REx™ 293 cells stably induced to express mGluR5-VSV-G-5-HT1B were treated with EG or dihydro (dihydroergotamine, a 5-HT1B agonist) at indicated concentrations and lysates were subjected to pronase (2 μg/ml) digestion. 5-HT1B protein with EG and dihydro protection in pronase digestion detected by western blotting and enrichment of 5-HT1B protein in pronase-induced depletion quantified by relative band intensity compared with GAPDH. (C,D) Intact Flp-In™ T-REx™ 293 cells of stably expressing mGluR5-VSV-G-5-HT1B treated with dihydro compared to control cells. The harvested cells were lysed three times by alternate freezing and thawing with liquid nitrogen before heating to the indicated temperatures. The 5-HT1B engagement with dihydro was detected by western blotting and quantified by relative band intensity compared with GAPDH. The corresponding 5-HT1B thermodynamic stabilization curves distinguish the treated from non-treated cells. (E,F) Stable cells expressing mGluR5-VSV-G-5-HT1B treated with EG compared to control cells. The cells were alternately frozen and thawed three times followed by heating to the indicated temperatures, and the 5-HT1B engagement with EG was detected by western blotting. The corresponding 5-HT1B thermodynamic stabilization curves distinguished the treated from non-treated cells. The mean values (±S.D.) of three independent experiments are shown. An asterisk indicates statistical significance (*p < 0.05, **p < 0.001, ***p<<0.001).
Intramolecular FRET sensors have been used as real-time optical tools for GPCR ligand binding (Xu et al., 2012; Lohse et al., 2008). We successfully produced effective 5-HT1B sensor constructs using previously described methods (Xu et al., 2012). The full-length human 5-HT1B receptor was added to CFP in-frame with the C-terminal tail (Figures 3A,B), and the 12-amino acid sequence (FLNCCPGCCMEP) containing the fluorescein arsenical hairpin binder via tetra-cysteine (FlAsH) labeling sequence (CCPGCC) was inserted into the third intracellular loop of the receptor. In addition, it contained the VSV-G peptide epitope within the extracellular N-terminal domain (Figure 3B). Functionality of the 5-HT1B sensor has been previously assessed in a study on ERK1/2 MAP kinase phosphorylation (p-ERK) (Xu et al., 2012). The full-length coding sequence of the human 5-HT1B receptor without alterations to the receptor’s intracellular segments was also cloned into the pcDNA5/FRT/TO vector described previously (Xu et al., 2012) and in the Methods section. Then, these constructs were transfected into Flp-In™ T-REx™ 293 cells to generate stable cell lines. In these cells, the doxycycline-induced expression of either the 5-HT1B sensor or the full-length human 5-HT1B was examined using an anti-VSV antibody. The 5-HT1B sensor performed effectively in response to the selective 5-HT1B agonist CP-94253, compared with the full-length human 5-HT1B, at the p-ERK level (Figure 3C). The 5-HT1B sensor was effectively delivered to the cell surface, on which its normal functions are based (Figure 3D). These results showed that the sensor was functionally similar and virtually identical to the wild-type receptor.
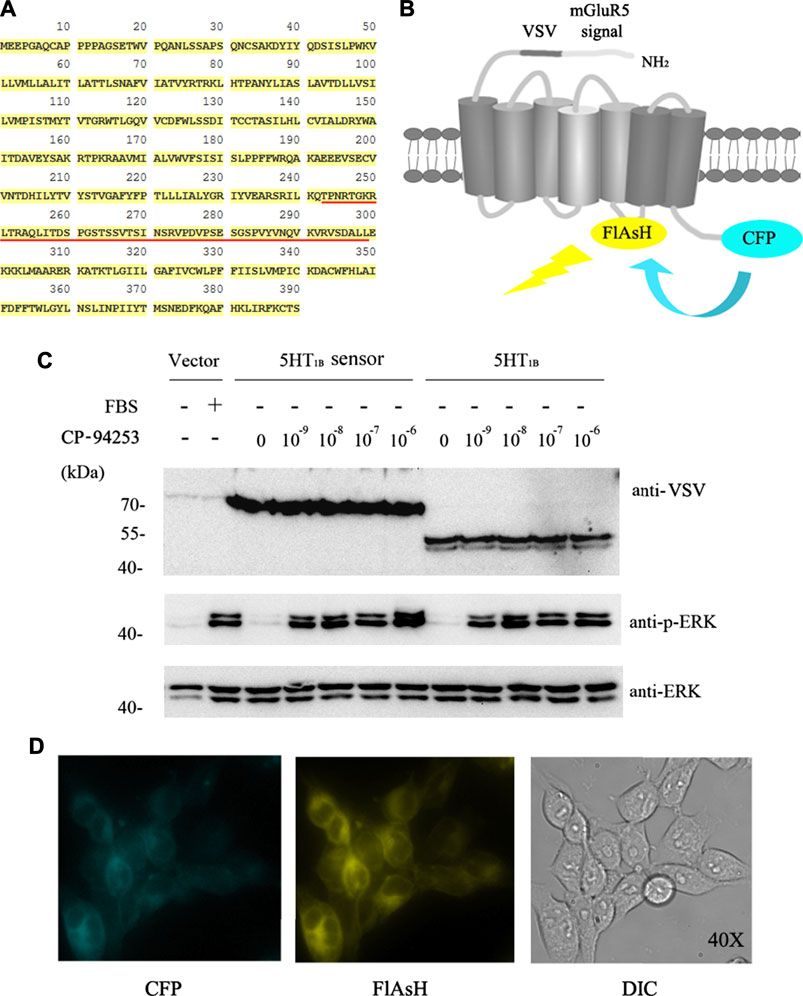
FIGURE 3. 5-HT1B FRET sensor construction. (A) Primary human 5-HT1B-receptor amino acid sequence. FlAsH motif replaces FRET sensor sequence (underlined in red). (B) N terminus modification of human 5-HT1B receptor by adding a leader sequence from mGluR5 followed by the VSV-G peptide sequence, while CFP was added at C terminus. FlAsH motif sequence FLNCCPGCCMEP introduced into third intracellular loop-linking transmembrane domains V and VI. Energy transfer from CFP to FlAsH and subsequent output is illustrated. (C) Stable cell lines harboring inducible 5-HT1B FRET sensor, VSV-G-mGluR5-5-HT1B, and corresponding vector constructs induced by 10 ng ml−1 doxycycline for 12 h, followed by 12 h of serum starvation quiescence, and 5 min stimulation by graded concentrations (10−9 to 10−6 M) of the 5-HT1B selective agonist CP-94253, using FBS as the positive control and deionized water as negative control in vector construct cells. Samples were then subjected to western blotting. The expression of 5-HT1B was determined by its fusion protein tag using VSV antibody. Phosphorylation of ERK 1/2 MAP kinases was detected by a phospho-ERK1/2-specific antibody (anti-p-ERK), and the anti-ERK1/2 antibody was used to assess the protein loading equivalence. (D) 5-HT1B FRET sensor was cloned into the inducible Flp-In™ T-REx™ locus of Flp-In™ T-REx™ 293 cells, and expression was induced by doxycycline. Imaging of CFP (left), labeled FlAsH (middle), and light field (right) demonstrated effective delivery of this sensor to the cell surface. FRET: fluorescence resonance energy transfer; VSV: vesicular stomatitis virus; FlAsH: fluorescein arsenical hairpin binder via tetra-cysteine; ERK: extracellular regulated protein kinases; CFP: cyan fluorescent protein; VSV: vesicular stomatitis virus; EG: emodin-8-O-β-d-glucopyranoside; CP-94253: 4-{5-propoxypyrrolo [3,2-b] pyridin-3-yl}-1.2,3,6-tetrahydropyridine; 5-HT1B: serotonin receptor 1B; FBS: fetal bovine serum; DIC: differential interference contrast; mGluR5: metabotropic glutamate receptor 5.
To study the effects of EG on the 5-HT1B receptor, we imaged the intramolecular rearrangement of the 5-HT1B sensor in response to EG, compared with CP-94253, by measuring emission from CFP and FlAsH. The normalized basal FRET signal in cells induced to express the 5-HT1B sensor decreased immediately upon the addition of CP-94253, then rapidly restored agonist withdrawal in a concentration-dependent manner. In contrast, addition of the 5-HT1B antagonist SB 224289 (10−6 M) did not produce a significant alteration in sensor response beyond the vehicle effect, and, as anticipated, blocked the effect of CP-94253 (Figure 4A). This was similar to the results obtained using EG, although the FRET signal changed in the opposite direction to that of CP-94253 (Figure 4B). Moreover, the variability of FRET changes from EG was approximately 60% of CP-94253 (Figure 4C), illustrating that structure changes in the receptor triggered by binding of CP-94253 and EG were distinct in amplitude and distance, at least between the third intracellular loop and C-terminal, where CFP and FlAsH are located, respectively. 5-HT1B has been previously shown to be internalized from the cell surface via agonist binding in a time-dependent manner (Janoshazi et al., 2007). Our findings revealed that EG induced 5-HT1B internalization, similarly to CP-94253 (Figure 4D). These results demonstrated that EG could be a 5-HT1B agonist.
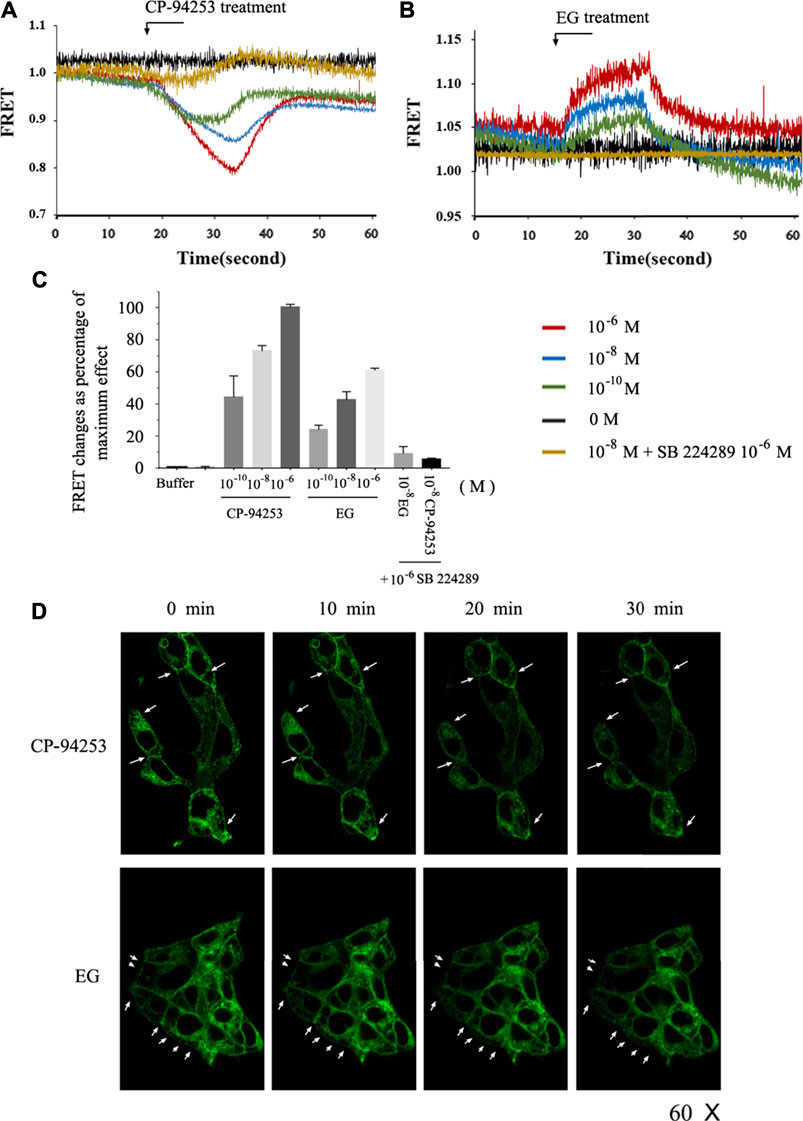
FIGURE 4. Intramolecular FRET sensor imaging of EG as a 5-HT1B agonist. (A,B) Flp-In™ T-REx™ 293 cells induced to express mGluR5-VSV-G-5-HT1B-FlAsH-CFP shown in FRET imaging. CP-94253 (A) or EG (B) at three concentrations (10−10, 10−8 and 10−6 M) added at the indicated times and removed after 15 s. FRET signal was monitored over 60 s. (C) Changes in normalized FRET signals due to ligand addition and effect of 10−6 M CP-94253 defined as 100% and vehicle as 0%. 5-HT1B receptor antagonist SB 224289 did not modulate mGluR5-VSV-G-5-HT1B-FlAsH-CFP FRET signals, but co-addition with CP-94253 or with EG blocked agonists’ effects. Studies were quantified, and data are represented as means ± S.E., n = 6. (D) Flp-In™ T-REx™ 293 cells induced to express mGluR5-VSV-G-5-HT1B-FlAsH-CFP imaged to detect CFP following addition of CP-94253 or EG (10−7 M). CP-94253 or EG-induced internalization, reducing the cell surface mGluR5-VSV-G-5-HT1B-FlAsH-CFP in a time-dependent fashion. Representative examples of n = 3 independent experiments are shown. FRET: fluorescence resonance energy transfer; FlAsH: fluorescein arsenical hairpin binder via tetra-cysteine; CFP: cyan fluorescent protein; VSV: vesicular stomatitis virus; EG: emodin-8-O-β-d-glucopyranoside; CP-94253: 4-{5-propoxypyrrolo [3,2-b]pyridin-3-yl}-1.2,3,6-tetrahydropyridine; SB 224289 [4-[2-methyl-4-(5-methyl-1,2,4-oxadiazol-3-yl)phenyl]phenyl]-(1′-methylspiro [6,7-dihydro-2H-furo [2,3-f]indole-3.4′-piperidine]-5-yl) methanone; 5-HT1B: serotonin receptor 1B; mGluR5: metabotropic glutamate receptor 5; FlAsH: fluorescein arsenical hairpin binder via tetra-cysteine.
To further study the EG target, we used stable cell lines that were induced to overexpress serotonergic-related GPCRs, including the serotonin receptors 1B and 2A (5-HT1B and 5-HT2A), dopamine receptor 2 (D2), melatonin receptor 2 (MT2), and orexin receptor 2 (OX2). In the stable cell lines that over-expressed 5-HT1B, EG promoted the phosphorylation of ERK1/2 MAP kinases in a concentration-dependent manner. This activation through the 5-HT1B receptor-mediated pathway was similar to the 5-HT1B selective agonist, i.e., the positive control CP-94253 (Figure 5A). In addition, the 5-HT1B selective antagonist SB224289 at 10−7 M inhibited the effect of the concentration-dependent promotion of the ERK1/2 MAP kinase phosphorylation by EG and CP-94253 activity (Figure 5B). However, no response to the EG of other 5-HT-related GPCRs activities, such as 5-HT2A, D2, MT2, or OX2, was observed (Figures 5C–F). These results showed that EG exhibited agonistic activity toward 5-HT1B and could selectively activate the 5-HT1B-mediated signaling pathway.
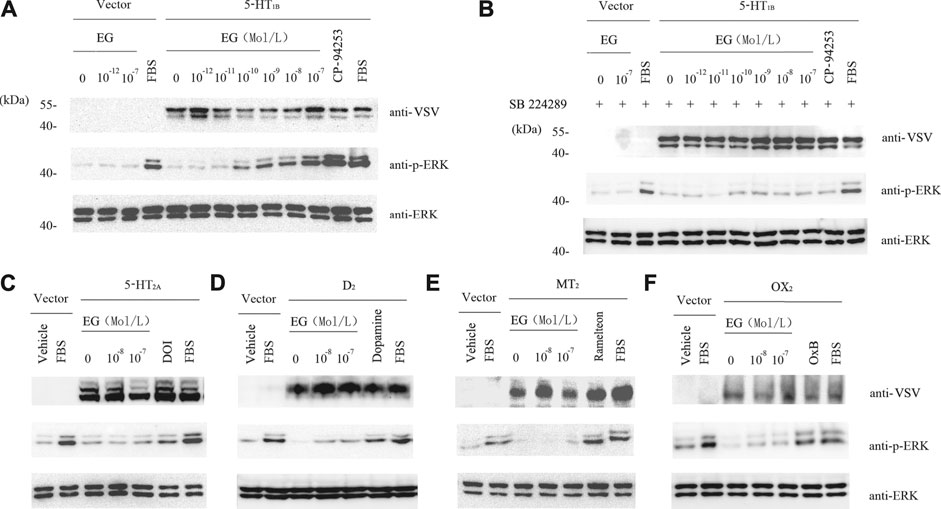
FIGURE 5. EG selectively activates 5-HT1B signaling. (A) Stable cell lines harboring inducible VSV-G-mGluR5-5HT1B and corresponding vector constructs induced by 10 ng ml−1 doxycycline for 12 h, and another 12 h of serum starvation for quiescence, then stimulated for 5 min by graded concentrations of EG (10−12 to 10−7 M in 5-HT1B cells, 10−12 to 10−7 M in vector cells), and the 5-HT1B selective agonist CP-94253 at 10−7 M, using FBS as positive control and deionized water as negative control. Samples were then subjected to western blotting. The expression of 5-HT1B, phosphorylation of ERK1/2 MAP kinases and loading control of ERK1/2 were determined by indicated antibodies described above in legend of Figure 3. (B) Stable cell lines and the inducing and starvation treatments were similar, as described in A above. Cells were pre-incubated with the 5-HT1B selective antagonist SB 224289 at 10−7 M for 20 min, following stimulation and western blotting. (C−F) Stable cell lines harboring inducible VSV-G-mGluR5-5HT2A(C), VSV-G-mGluR5-D2(D), VSV-G-mGluR5-MT2(E), and VSV-G-mGluR5-OX2(F) and the corresponding vector constructs treated similar to A and the effects of EG on the indicated GPCR mediated pathways determined by phosphorylation of ERK1/2 MAP kinases. The induced and quiescent stable cell lines were stimulated for 5 min by graded concentrations of EG (10−8 and 10−7 M), and the selective agonis at 10−7 M, that were DOI, dopamine, ramelteon and OxB, respectively corresponding to 5HT2A, D2, MT2 and OX2 cells, using FBS as positive control and deionized water as negative control. EG: emodin-8-O-β-d-glucopyranoside; FBS: fetal bovine serum; ERK: extracellular regulated protein kinases; CFP: cyan fluorescent protein; 5-HT1B: serotonin receptor 1B; 5-HT2A: serotonin receptor 2A; D2: dopamine receptor 2; MT2: melatonin receptor 2; OX2: orexin receptor 2; VSV: vesicular stomatitis virus; mGluR5: metabotropic glutamate receptor 5; FBS: fetal bovine serum; DOI (1-(4-iodo-2,5-dimethoxyphenyl) propan-2-amine; dopamine: 4-(2-Aminoethyl) benzene-1,2-diol; ramelteon (N-[2-[(8S)-2.6,7,8-tetrahydro-1H-cyclopenta [e] (Gadgaard and Jensen, 2020) benzoxol-8-yl]ethyl]propanamide).
Aβ-induced neuronal toxicity is an important pathology in AD, and there are two principal variants of amyloid peptides in humans, of which Aβ42 is more toxic than Aβ40 (Hamley, 2012). EG and its derivatives exhibit neural protection activity and can be used in AD treatment (Lin et al., 2015). We applied Aβ40 and Aβ42 to PC12 (with rat Aβ) and SH-SY5Y (with human Aβ) cell lines, respectively. The cells were subsequently assessed using the Cell Counting Kit-8 (CCK-8) for viability assay. Cell survival decreased with increased Aβ concentration in both PC12 (Figure 6A) and SH-SY5Y cells (Supplementary Figure S3). No significant differences in viability were observed in the treatments with <5 μM Aβ42 or <10 μM Aβ40. PC12 cell death was observed at 10 μM Aβ42 and reached approximately 18% at 20 μM, whereas cell death in the Aβ40 treatment group began at 20 μM. At 50 μM, 47% of the Aβ42-treated and 52% of the Aβ40-treated cells survived. These results suggest that Aβ peptides dose-dependently induce neuronal toxicity, and support the hypothesis that Aβ42 is more toxic than Aβ40. To determine the effects of EG on neural cells, we pre-treated PC12 cells with or without EG, followed by exposure to the amyloid peptides Aβ40 and Aβ42, respectively. The results showed that EG could alleviate the reduction in Aβ40-and Aβ42-induced cell viability by approximately 8.22 and 8.64%, respectively (Figure 6B).
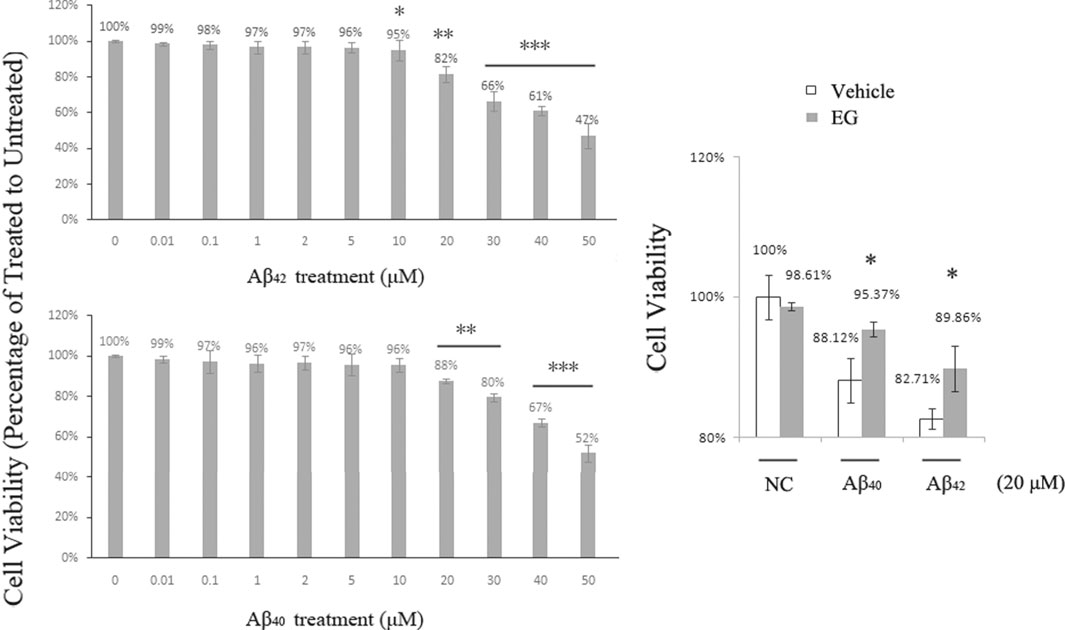
FIGURE 6. Neuronal death induced by Aβ peptides was alleviated by EG. (A) PC12 cells treated with amyloid peptides Aβ42(upper) and Aβ40(lower) for 24 h, at gradient concentrations from 0.01 to 50 μM, followed by Cell Counting Kit-8 (CCK-8) assay for viability. (B) PC12 cells pre-treated with (gray bar) or without (white bar) EG at 10−7 M for 18 h, then further subjected to 20 μM final concentration of amyloid peptides Aβ40 and Aβ42 for 24 h followed by CCK-8 assay for viability. The mean values (±S.D.) of three independent experiments are shown. An asterisk indicates statistical significance (*p < 0.05, **p < 0.001, ***p<<0.001). EG: emodin-8-O-β-d-glucopyranoside; NC: negative control.
As EG could selectively activate 5-HT1B signaling and alleviate Aβ-induced mortality, we further investigated the underlying mechanisms. Activation of 5-HT1B by its agonist reduces the levels of pro-inflammatory TNF-α in rat nerve cells (Khalilzadeh et al., 2018). The inflammatory cascade is one of the most important processes in Aβ-induced toxicity, and the Aβ oligomer-elevated TNF-α signaling is a cause of memory loss in mice (Lourenco et al., 2013); thus, stimulation of 5-HT1B may relieve Aβ-induced TNF-α signaling. The two amyloid peptides Aβ42 and Aβ40 induced the death of PC12 cells to a similar extent (Figure 6A). Therefore, we conducted the following experiments with Aβ42 because it exhibited neuronal toxicity at a lower concentration than Aβ40.
To evaluate TNF-α levels under 5-HT1B-related conditions, we used quantitative RT-PCR to detect TNF-α mRNA levels in 5-HT1B over-expressing stable PC12 cells (Supplementary Figure S4B) with Aβ42 and with or without 5-HT1B activators and blockers (Figure 7A). PC12 cells have endogenous 5-HT1B as well as 5-HT2A/3/6 receptor subtypes (Supplementary Figure S4A). We used 5-HT1B-over-expressing stable PC12 cells to accentuate the effect of 5-HT1B. Consistent with previous studies (Shrewsbury et al., 2008), our results showed that Aβ42 elevated TNF-α expression, which could be reversed by treatment with the 5-HT1B agonist CP-94253 and dihydroergotamine and the novel 5-HT1B activator EG. Moreover, the selective serotonin 5-HT1B antagonist SB 224289 blocked EG, CP-94253, and dihydroergotamine-mediated TNF-α restraint under Aβ42 conditions. The 5-HT1B activators alone could decrease TNF-α levels in the 5-HT1B over-expressed PC12 cells, whereas the antagonist SB 224289 contributed to pro-inflammatory TNF-α signaling and was synergistic with Aβ42 (Figure 7B). These results suggested that EG resorted to the 5-HT1B pathway to reduce Aβ-induced pro-inflammatory TNF-α signaling.

FIGURE 7. Cell death promoting effect of Aβ42 in 5-HT1B over-expressed stable PC12 cells eliminated by TNF-α signaling reduction via 5-HT1B pathway activation. A. TNF-α mRNA level induced by Aβ42 (20 μM).5-HT1B agonists (CP-94253 and dihydroergotamine) and EG added as indicated at 10−7 M. To confirm 5-HT1B mediated pathway role, the 5-HT1B was blocked by its selective antagonist SB 224289 (10−7 M), and the TNF-α mRNA level was measured in the presence of dihydroergotamine, CP-94253 and EG. The mean values (±S.D.) of three independent experiments are shown. Asterisks indicate statistical significance (***p<<0.001). B. Compounds shown in A were separately tested for their effects on inducing TNF-α expression in 5-HT1B over-expressed, stable PC12 cells. C, D, E. 5-HT1B over-expressed stable PC12 cells were pre-treated with the CP-94253, dihydroergotamine, and EG at 10−7 M for 18h, then subjected to Aβ42 (20 μM) or not, for 24 h, followed by CCK-8, MTT Kit viability assay and LDH for cytotoxicity. SB 224289 was applied 20 min before CP-94253, dihydroergotamine, and EG when necessary.
The Aβ-induced inflammatory response and subsequent neuronal death are considered important events in AD pathology (Hamley, 2012; Unzeta et al., 2016). We found that the death of Aβ42-induced 5-HT1B over-expressing stable PC12 cells was significantly reversed by the 5-HT1B agonists and EG, as assessed via the CCK-8 and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays (Figures 7C,D, Supplementary Figure S5A). However, SB 224289 alone did not lead to significant cell death under the indicated conditions (10−7 M, 42 h, Supplementary Figure S6). EG and the 5-HT1B agonists reduced the Aβ42-induced cytotoxicity that could be blocked by SB 224289 (Figure 7E, Supplementary Figure S5B).
To further explore the ability of EG to afford protection against amyloid peptides, we tested transgenic C. elegans for resistance to the induced Aβ expression. The C. elegans CL14176 strain (Link et al., 2003), which could be induced to express Aβ by modulating the temperature and leads to paralysis in 2–3 d, was used. This paralysis phenotype could serve as a measurement of Aβ toxicity to assay the effects of compounds protective against Aβ, using the paralysis delay to indicate the suppression of Aβ toxicity in the worm model (Dostal and Link, 2010). The results showed that EG at different concentrations retarded the paralysis time from 6 to 7 h compared with that for the untreated group (∼52 h). The performance of the 200 μM EG group was superior to that of the positive control caffeine group (Supplementary Table S1, Figure 8A). In this model, the survival rate of the EG group at 200 μM was significantly prolonged (Figure 8B). The expression of immune response-related genes that are analogs of the inflammatory effectors in humans, including the nuclear receptor nhr-57, effectors of hypoxia pathway, pqn-5 (prion-like glutamine (Q)/asparagine (N) -5) gene, part of the unfolded protein response pathway that responded to endoplasmic reticulum stress, the collagen gene col-41, and transthyretin-like protein gene ttr-21, between the untreated and EG groups was observed via quantitative RT-PCR (Bellier et al., 2009; Sahu et al., 2012). The EG could significantly reduce the Aβ-induced expression of the immune response-related genes nhr-57, pqn-5, and col-41. However, it failed to suppress ttr-21 expression (Figure 8C), which suggested that EG protected the worm model from AD in a complex manner rather than arbitrarily suppressing all the immune response pathways.
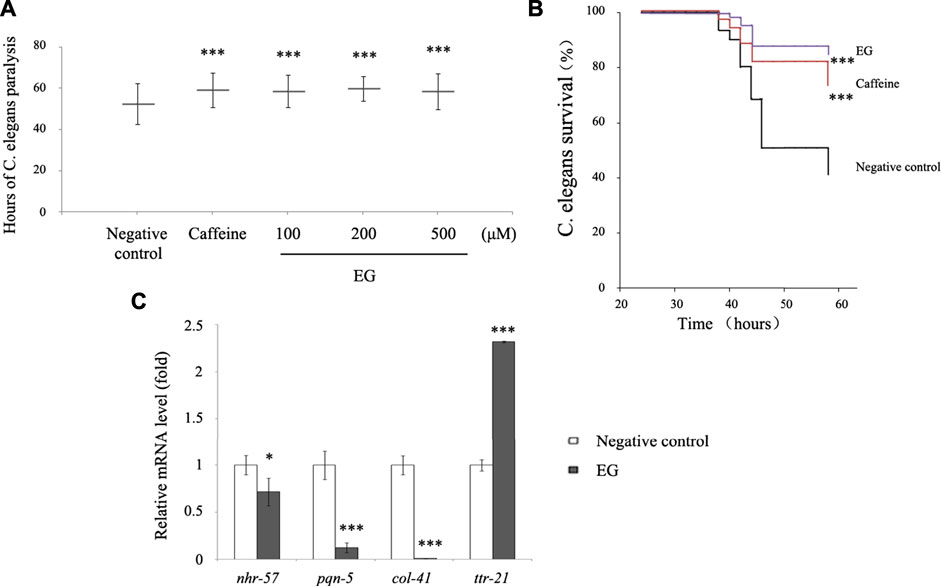
FIGURE 8. EG retarded transgenic C. elegans paralysis in AD model. (A). CL14176 of C. elegans strain synchronized to L1 phase, and cultured at 16°C for 24 h, then transferred to 25°C to express amyloid peptides that would result in paralysis. The negative control was untreated (equal volume of water), the positive was exposed to 6.27 mM caffeine, and the EG was measured. The mean paralysis times and the ranks of the individual paralysis times in each group are shown (see Supplementary Table S1). Asterisks indicate statistical significance (***p<<0.001). (B) Worms were synchronized and cultured at 16°C for 24 h, then transferred to 25°C for activation. The paralysis phenotypes were detected after 38 h, the number of worms not paralyzed was converted to a percentage, and the “non-paralyzed” percentage was plotted against time activation initiation. The negative control was untreated as described above, the positive was exposed to 6.27 mM caffeine, and the test group to 200 μM EG. Asterisks indicate statistical significance (***p<<0.001). (C) Total RNA of the untreated and EG (200 μM) groups was extracted and reverse transcribed to cDNA. The mRNA levels of nhr-57, pqn-5, col-41, and ttr-21 were measured using quantitative RT-PCR. An asterisk indicates statistical significance (*p < 0.05, **p < 0.001, ***p<<0.001). EG: emodin-8-O-β-d-glucopyranoside.
The serotonin receptor 5-HT1B, is an important part of the serotonergic system, which regulates crucial processes of the CNS such as cognition, satiety, anxiety, depression, and sleep. 5-HT1B has been considered a drug target for serotonergic system-related disorders (Gadgaard and Jensen, 2020; Monti and Jantos, 2008; Tiger et al., 2018). We identified 5-HT1B as a target of EG, an active ingredient in several Chinese medicinal plants with antidepressant and neuroprotective effects, by using molecular docking, DARTS, and CETSA. Molecular docking provided a structural basis for EG recognition in 5-HT1B. Additionally, EG docked into the same 5-HT1B pocket as CP-94253, a 5-HT1B agonist (Figure 1). DARTS allows for the determination of the direct binding of natural products and their extracts to their protein targets. As the conformation and stabilization of the compound-target complex, compared with those of the control protein target, are altered by protease-induced digestion, the compound and target protein interactions can be visualized using western blotting (Chang et al., 2016). The CETSA method can detect the physical interaction between a ligand and target protein in intact cells. The thermodynamic stability of target proteins in drug- or vehicle-treated intact cells varies over changing temperatures, and the target proteins can be monitored using western blotting (Chang et al., 2016). The DARTS results showed that EG could protect the 5-HT1B protein from pronase digestion, compared with 5-HT1B agonists dihydroergotamine (Figures 2A,B) and CP-94253 (Supplementary Figure S2A). In addition, EG promoted thermodynamic stabilization of the 5-HT1B protein compared with dihydroergotamine (Figures 2C–F) and CP-94253 (Supplementary Figure S2B). These methods can also be used to identify other 5-HT1B agonists.
We further studied the structural changes in the 5-HT1B receptor after EG binding using FRET. Intramolecular FRET sensors provide real-time optical evidence for GPCR ligand binding and activation kinetics and can be used as screening tools for molecular ligands toward a particular receptor (Lohse et al., 2008; Xu et al., 2012). We produced an effective 5-HT1B sensor (Figure 4) and detected EG as a ligand with 5-HT1B (Figure 5). However, the changes in FRET signals during treatment with CP-94253 (Figure 4A) and EG (Figure 4B) were in opposite directions, demonstrating that binding of CP-94253 closed the distance between the third intracellular loop and C-terminal of the 5-HT1B receptor, whereas EG extended the distance. Additionally, we found that 5-HT1B could be induced to internalization by agonist CP-94253 and EG (Figure 5), suggesting that EG might be an agonist of 5-HT1B. Selective activation of the 5-HT1B-mediated ERK1/2 signaling pathway (Figure 6) by EG established its agonist activity toward 5-HT1B. Together, the intramolecular FRET 5-HT1B sensor and the subsequent tests of 5-HT1B-induced ERK1/2 signaling activation provide a strong evidence that 5-HT1B is a target of and is activated by EG. Further biological functions of EG targeting and 5-HT1B stimulation need to be elucidated.
Overall, activation of central 5-HT1B may have antidepressant (Sanchez et al., 2015), anti-aggression (de Almeida et al., 2001), and antinociceptive effects (Labastida-Ramírez et al., 2020). The therapeutic use of those herbal antidepressants that contain EG has been suggested for the treatment of AD (Jeon et al., 2019; Chen et al., 2020; Li et al., 2020). In this study, the 5-HT1B-mediated ERK1/2 phosphorylation by EG and the antifungal (Qi et al., 2005) as well as acetylcholinesterase I (AChE I)- inhibiting (Lin et al., 2015) activities of EG led us to investigate the EG and 5-HT1B interaction in the context of AD. Activation of ERK1/2 signaling is related to the inhibition of Aβ-induced apoptosis (Galvão et al., 2019). Additionally, a series of interesting studies showed that Aβ might act as an antimicrobial peptide, thereby protecting the CNS from infections in mouse models and innate immunity in worms, whereas dysregulated Aβ leads to AD pathology (Kumar et al., 2016). AChE I has been used as a biomarker in AD diagnosis, and AChE I inhibitors have been clinically used for AD treatment (Lin et al., 2015). Both the classic 5-HT1B agonists (CP-94253 and dihydroergotamine) and EG could singly suppress the expression of pro-inflammatory TNF-α and correspondingly improve the survival rates of 5-HT1B over-expressing stable PC12 cells compared with untreated negative control (Figures 7B,C, Supplementary Figure S5A, B). This result might agree with the finding that activated serotonergic pathways promote proliferation (Chilmonczyk et al., 2017). The data in wild-type PC12 cells pointed toward the same trend, although not as significant as those in the 5-HT1B over-expressing stable PC12 cells. EG alleviated Aβ42-induced cell death and increased cell viability from approximately 82.71–89.86% (Figure 6B), whereas in 5-HT1B over-expressing stable PC12 cells, the increase was from 84.06 to 109.71% (Figure 7C). In addition, PC12 cells possessed some endogenous serotonin receptor subtypes, including 5-HT1B, 5-HT2A, 5-HT3, and 5-HT6 (Supplementary Figure S4A), that could explain why the wild-type PC12 cells also responded to the classic 5-HT1B agonists (data not shown) and EG in reducing TNF-α levels and were prosurvival. Cell viability in the EG treatment (in Aβ42 conditions) was greater than that in the untreated control, indicating the protective effects of EG via the 5-HT1B pathway.
This study provides evidence that 5-HT1B receptor is one of the targets for compounds in Chinese medicinal plant ingredients with cognitive deficit attenuating and antidepressant effects in the treatment of AD. Molecular docking, DARTS, CETSA, FRET, and ERK1/2 phosphorylation tests are all relatively economic, fast, and accurate methods. Further biological functions of the compound targeting 5-HT1B was tested with AD models in cells and worms. However, a variety of herbal compounds are reported to possess cognitive deficit attenuating and antidepressant effects, and the ability of these compounds to target and activate the 5-HT1B still needs to be investigated. Interestingly, caffeine, also a herbal ingredient used as positive control in the worm experiments, was also docked into the 5-HT1B receptor, similar with that of EG, CP94253, and dihydroergotamine (data not shown). These results suggest that caffeine might also be an agonist toward 5-HT1B, and support the 5-HT1B agonists’ potential for treatment of AD.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was funded by The Fund of Yunnan Basic Research Program (202001AS070024 and 202001AT0700) and conducted at the University Based Provincial Key Laboratory of Screening and Utilization of Targeted Drugs of Yunnan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The transgenic C. elegans CL14176 strain was a gift from Ping Yang at the Shanghai Research Center For Model Organisms, Pudong New Area, Shanghai, China.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.735876/full#supplementary-material
CNS, central nervous system; AD, Alzheimer’s disease; EG, emodin-8-O- β-d-glucopyranoside; DARTS, drug affinity responsive target stability; CETSA, cellular thermal shift assays; FRET, fluorescence resonance energy transfer.
Bellier, A., Chen, C. S., Kao, C. Y., Cinar, H. N., and Aroian, R. V. (2009). Hypoxia and the Hypoxic Response Pathway Protect against Pore-Forming Toxins in C. elegans. Plos Pathog. 5 (12), e1000689. doi:10.1371/journal.ppat.1000689
Beuming, T., Lenselink, B., Pala, D., McRobb, F., Repasky, M., and Sherman, W. (2015). Docking and Virtual Screening Strategies for GPCR Drug Discovery. Methods Mol. Biol. 1335, 251–276. doi:10.1007/978-1-4939-2914-6_17
Chang, J., Kim, Y., and Kwon, H. J. (2016). Advances in Identification and Validation of Protein Targets of Natural Products without Chemical Modification. Nat. Prod. Rep. 33 (5), 719–730. doi:10.1039/c5np00107b
Chen, S. Y., Gao, Y., Sun, J. Y., Meng, X. L., Yang, D., Fan, L. H., et al. (2020). Traditional Chinese Medicine: Role in Reducing β-Amyloid, Apoptosis, Autophagy, Neuroinflammation, Oxidative Stress, and Mitochondrial Dysfunction of Alzheimer's Disease. Front. Pharmacol. 11, 497. doi:10.3389/fphar.2020.00497
Chilmonczyk, Z., Bojarski, A. J., Pilc, A., and Sylte, I. (2017). Serotonin Transporter and Receptor Ligands with Antidepressant Activity as Neuroprotective and Proapoptotic Agents. Pharmacol. Rep. 69 (3), 469–478. doi:10.1016/j.pharep.2017.01.011
de Almeida, R. M., Nikulina, E. M., Faccidomo, S., Fish, E. W., and Miczek, K. A. (2001). Zolmitriptan--a 5-HT1B/D Agonist, Alcohol, and Aggression in Mice. Psychopharmacology (Berl) 157 (2), 131–141. doi:10.1007/s002130100778
Dostal, V., and Link, C. D. (2010). Assaying β-amyloid Toxicity Using a Transgenic C. elegans Model. J. Vis. Exp. 44, 1. doi:10.3791/2252
Gadgaard, C., and Jensen, A. A. (2020). Functional Characterization of 5-HT1A and 5-HT1B Serotonin Receptor Signaling through G-Protein-Activated Inwardly Rectifying K+ Channels in a Fluorescence-Based Membrane Potential Assay. Biochem. Pharmacol. 175, 113870. doi:10.1016/j.bcp.2020.113870
Galvão, F., Grokoski, K. C., da Silva, B. B., Lamers, M. L., and Siqueira, I. R. (2019). The Amyloid Precursor Protein (APP) Processing as a Biological Link between Alzheimer's Disease and Cancer. Ageing Res. Rev. 49, 83–91. doi:10.1016/j.arr.2018.11.007
Garcia-Alloza, M., Hirst, W. D., Chen, C. P., Lasheras, B., Francis, P. T., and Ramírez, M. J. (2004). Differential Involvement of 5-HT(1B/1D) and 5-HT6 Receptors in Cognitive and Non-cognitive Symptoms in Alzheimer's Disease. Neuropsychopharmacology 29 (2), 410–416. doi:10.1038/sj.npp.1300330
Hamley, I. W. (2012). The Amyloid Beta Peptide: A Chemist's Perspective. Role in Alzheimer's and Fibrillization. Chem. Rev. 112 (10), 5147–5192. doi:10.1021/cr3000994
Janoshazi, A., Deraet, M., Callebert, J., Setola, V., Guenther, S., Saubamea, B., et al. (2007). Modified Receptor Internalization upon Coexpression of 5-HT1B Receptor and 5-HT2B Receptors. Mol. Pharmacol. 71 (6), 1463–1474. doi:10.1124/mol.106.032656
Jeon, S. G., Song, E. J., Lee, D., Park, J., Nam, Y., Kim, J. I., et al. (2019). Traditional Oriental Medicines and Alzheimer's Disease. Aging Dis. 10 (2), 307–328. doi:10.14336/AD.2018.0328
Khalilzadeh, M., Panahi, G., Rashidian, A., Hadian, M. R., Abdollahi, A., Afshari, K., et al. (2018). The Protective Effects of Sumatriptan on Vincristine - Induced Peripheral Neuropathy in a Rat Model. Neurotoxicology 67, 279–286. doi:10.1016/j.neuro.2018.06.012
Koizumi, K., and Nakajima, H. (2014). Serotonin Induces the Migration of PC12 Cells via the Serotonin Receptor 6/cAMP/ERK Pathway. Biomed. Rep. 2 (1), 29–33. doi:10.3892/br.2013.203
Kontoyianni, M. (2017). Docking and Virtual Screening in Drug Discovery. Methods Mol. Biol. 1647, 255–266. doi:10.1007/978-1-4939-7201-2_18
Kumar, D. K., Choi, S. H., Washicosky, K. J., Eimer, W. A., Tucker, S., Ghofrani, J., et al. (2016). Amyloid-β Peptide Protects against Microbial Infection in Mouse and Worm Models of Alzheimer's Disease. Sci. Transl Med. 8 (340), 340ra72. doi:10.1126/scitranslmed.aaf1059
Labastida-Ramírez, A., Rubio-Beltrán, E., Haanes, K. A., Chan, K. Y., Garrelds, I. M., Johnson, K. W., et al. (2020). Lasmiditan Inhibits Calcitonin Gene-Related Peptide Release in the Rodent Trigeminovascular System. Pain 161 (5), 1092–1099. doi:10.1097/j.pain.0000000000001801
Ledo, J. H., Azevedo, E. P., Beckman, D., Ribeiro, F. C., Santos, L. E., Razolli, D. S., et al. (2016). Cross Talk between Brain Innate Immunity and Serotonin Signaling Underlies Depressive-like Behavior Induced by Alzheimer's Amyloid-β Oligomers in Mice. J. Neurosci. 36 (48), 12106–12116. doi:10.1523/JNEUROSCI.1269-16.2016
Li, J. M., Zhao, Y., Sun, Y., and Kong, L. D. (2020). Potential Effect of Herbal Antidepressants on Cognitive Deficit: Pharmacological Activity and Possible Molecular Mechanism. J. Ethnopharmacol. 257, 112830. doi:10.1016/j.jep.2020.112830
Lin, L., Ni, B., Lin, H., Zhang, M., Li, X., Yin, X., et al. (2015). Traditional Usages, Botany, Phytochemistry, Pharmacology and Toxicology of Polygonum Multiflorum Thunb.: A Review. J. Ethnopharmacol. 159, 158–183. doi:10.1016/j.jep.2014.11.009
Link, C. D., Taft, A., Kapulkin, V., Duke, K., Kim, S., Fei, Q., et al. (2003). Gene Expression Analysis in a Transgenic Caenorhabditis elegans Alzheimer's Disease Model. Neurobiol. Aging 24 (3), 397–413. doi:10.1016/s0197-4580(02)00224-5
Lohse, M. J., Nikolaev, V. O., Hein, P., Hoffmann, C., Vilardaga, J. P., and Bünemann, M. (2008). Optical Techniques to Analyze Real-Time Activation and Signaling of G-Protein-Coupled Receptors. Trends Pharmacol. Sci. 29 (3), 159–165. doi:10.1016/j.tips.2007.12.002
Lomenick, B., Hao, R., Jonai, N., Chin, R. M., Aghajan, M., Warburton, S., et al. (2009). Target Identification Using Drug Affinity Responsive Target Stability (DARTS). Proc. Natl. Acad. Sci. U S A. 106 (51), 21984–21989. doi:10.1073/pnas.0910040106
Lourenco, M. V., Clarke, J. R., Frozza, R. L., Bomfim, T. R., Forny-Germano, L., Batista, A. F., et al. (2013). TNF-α Mediates PKR-dependent Memory Impairment and Brain IRS-1 Inhibition Induced by Alzheimer's β-amyloid Oligomers in Mice and Monkeys. Cell Metab 18 (6), 831–843. doi:10.1016/j.cmet.2013.11.002
Molina, D. M., Jafari, R., Ignatushchenko, M., Seki, T., Larsson, E. A., Dan, C., et al. (2013). Monitoring Drug Target Engagement in Cells and Tissues Using the Cellular Thermal Shift Assay. Science 341 (6141), 84–87. doi:10.1126/science.1233606
Monti, J. M., and Jantos, H. (2008). The Roles of Dopamine and Serotonin, and of Their Receptors, in Regulating Sleep and Waking. Prog. Brain Res. 172, 625–646. doi:10.1016/S0079-6123(08)00929-1
Qi, H. Y., Zhang, C. F., Wang, Z. T., and Zhang, M. (2005). Studies On Constituents and Antifungal Activity of Polygonum Cillinerve Chinese. Pharm. J. 40, 819–822.
Sahu, S. N., Lewis, J., Patel, I., Bozdag, S., Lee, J. H., LeClerc, J. E., et al. (2012). Genomic Analysis of Immune Response against Vibrio cholerae Hemolysin in Caenorhabditis elegans. Plos One 7 (5), e38200. doi:10.1371/journal.pone.0038200
Sanchez, C., Asin, K. E., and Artigas, F. (2015). Vortioxetine, a Novel Antidepressant with Multimodal Activity: Review of Preclinical and Clinical Data. Pharmacol. Ther. 145, 43–57. doi:10.1016/j.pharmthera.2014.07.001
Shrewsbury, S. B., Cook, R. O., Taylor, G., Edwards, C., and Ramadan, N. M. (2008). Safety and Pharmacokinetics of Dihydroergotamine Mesylate Administered via a Novel (Tempo) Inhaler. Headache 48 (3), 355–367. doi:10.1111/j.1526-4610.2007.01006.x
Tiger, M., Varnäs, K., Okubo, Y., and Lundberg, J. (2018). The 5-HT1B Receptor - a Potential Target for Antidepressant Treatment. Psychopharmacology (Berl) 235 (5), 1317–1334. doi:10.1007/s00213-018-4872-1
Unzeta, M., Esteban, G., Bolea, I., Fogel, W. A., Ramsay, R. R., Youdim, M. B., et al. (2016). Multi-Target Directed Donepezil-like Ligands for Alzheimer's Disease. Front. Neurosci. 10, 205. doi:10.3389/fnins.2016.00205
Ward, R. J., Alvarez-Curto, E., and Milligan, G. (2011). Using the Flp-In™ T-Rex™ System to Regulate GPCR Expression. Methods Mol. Biol. 746, 21–37. doi:10.1007/978-1-61779-126-0_2
Keywords: 5-HT1B receptor, molecular docking, drug affinity responsive target stability, fluorescence resonance energy transfer, alzheimer’s disease, emodin-8-O-β-d-glucopyranoside
Citation: Yang Y, Zhang L, Yu J, Ma Z, Li M, Wang J, Hu P, Zou J, Liu X, Liu Y, An S, Xiang C, Guo X, Hao Q and Xu T-R (2021) A Novel 5-HT1B Receptor Agonist of Herbal Compounds and One of the Therapeutic Uses for Alzheimer’s Disease. Front. Pharmacol. 12:735876. doi: 10.3389/fphar.2021.735876
Received: 18 July 2021; Accepted: 23 August 2021;
Published: 06 September 2021.
Edited by:
Rebeca Alvariño, University of Santiago de Compostela, SpainReviewed by:
Eva Alonso, Health Research Institute of Santiago de Compostela (IDIS), SpainCopyright © 2021 Yang, Zhang, Yu, Ma, Li, Wang, Hu, Zou, Liu, Liu, An, Xiang, Guo, Hao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian-Rui Xu, dGlhbnJ1aXh1QGt1c3QuZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.