- 1The Ministry of Education (MOE) Key Laboratory for Standardization of Chinese Medicines, Institute of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Jiangsu Kanion Pharmaceutical Co., Ltd., Lianyungang, China
Diabetes is a common and complex disease that can exacerbate the complications related to cardiovascular disease, and this is especially true for type 2 diabetes mellitus (T2DM). In addition to the standard pharmacological therapies, T2DM has also been treated with nonconventional regimens such as traditional Chinese medicine (TCM), e.g., herbal medicines and TCM prescriptions, although the mechanisms underlying the therapeutic benefits remain unclear. In this regard, many studies have used metabolomics technology to elucidate the basis for the efficacy of TCM for T2DM. Metabolomics has recently attracted much attention with regard to drug discovery and pharmacologically relevant natural products. In this review, we summarize the application of metabolomics to the assessment of TCM efficacy for treating T2DM. Increasing evidence suggests that the metabolic profile of an individual patient may reflect a specific type of T2DM syndrome, which may provide a new perspective for disease diagnosis. In addition, TCM has proved effective for countering the metabolic disorders related to T2DM, and this may constitute the basis for TCM efficacy. Therefore, further determining how TCM contributes to the reversal of metabolic disorders, such as using network pharmacology or by assessing the contribution of host–gut microbiota interactions, will also provide researchers with new potential targets for pharmacologic-based therapies.
Introduction
Diabetes is one of the most prevalent chronic diseases worldwide, affecting 463 million people in 2019. It is expected that this number will rise to 700 million by 2045 (Saeedi et al., 2019). Type 2 diabetes mellitus (T2DM) is the most common type of diabetes and is characterized by insulin resistance. Two troublesome effects of T2DM, cardiovascular disease and metabolic disorders, can cause high morbidity and mortality. T2DM is mainly a consequence of environmental and behavioral factors such as a sedentary lifestyle and/or poor dietary choices, which often cause obesity; however, several genetic susceptibilities contribute to disease onset or exacerbate its severity (Zimmet et al., 2001). These factors lead to persistent hyperglycemia and subsequently to decreased insulin sensitivity, which in turn can lead to a spectrum of metabolic abnormalities (Ferrannini et al., 2013). Moreover, persistent disorders of glucose and lipid metabolism may lead to various complications of the microvasculature and macrovasculature, such as stroke, ischemic heart disease, diabetic nephropathy, cognitive dysfunction, and retinopathy (Brownlee, 2001). These conditions have a severe impact on quality of life. Therefore, new and effective measures are needed to prevent the onset of T2DM and improve the management of patients.
Clinically proven medicines for treating T2DM include thiazolidinediones (Thangavel et al., 2017; Nanjan et al., 2018; Oikonomou et al., 2018) as well as sulfonylureas, which stimulate insulin secretion and increase insulin sensitivity to biguanides (Setter et al., 2003). These drugs activate the genes encoding hepatic adenosine monophosphate-activated protein kinase (AMPK) (Wang Q. et al., 2018; Glosse and Föller, 2018; Różańska and Regulska-Ilow, 2018), phosphatidylinositol 3-kinase, and protein kinase B (Akt) (John et al., 2018; Mabhida et al., 2018; Garcia-Galiano et al., 2019). Stimulation of fatty-acid oxidation in an AMPK- and peroxisome proliferator activated receptor-α-dependent manner inhibits interference with c-Jun amino-terminal kinases and insulin action activated by inflammatory cytokines and free fatty acids (Hirosumi et al., 2002; Ferguson et al., 2013). These approaches and candidate drug targets constitute potential means for reducing blood sugar and the incidence of obesity and diabetes symptoms.
Glucose transporter type 4 also plays an important role in maintaining blood glucose homeostasis, which prevents insulin resistance and facilitates the transfer of glucose from blood to the liver through the phosphatidylinositol 3-kinase and Akt signaling pathways. However, the vasculature can be damaged by dysfunction of several metabolic pathways, including the hexamine pathway, the protein kinase C pathway, the glycosylation end-product pathway, and the classic polyol pathway (Hunt and Wolff, 1991; Sheetz and King, 2002).
In addition to the aforementioned drug classes for treating diabetes (i.e., biguanides, thiazolidinediones, and sulfonylureas), alpha-glucosidase inhibitors are also commonly used (Choi and Kim, 2010; Zhou et al., 2013; Ishii et al., 2018). However, these inhibitors often have considerable negative effects, such as drug resistance, hypoglycemia, edema, and weight gain. Advances in the treatment of diabetes have changed the focus from hyperglycemia to controlling glucose metabolism, enhancing the sensitivity of insulin receptors, inhibiting insulin resistance, regulating the non-enzymatic glycosylation of proteins, and downregulating fatty-acid metabolism, among other treatment modalities (Weyer et al., 2001; de Dios et al., 2007). Although many strategies and drugs have been developed for the prevention and treatment of diabetes, current disease management options fall short of complete containment. Current therapies mainly rely on drugs; however, many recently approved diabetes drugs have serious complications, such as hypoglycemia, liver and kidney function damage, and diarrhea (Bekele, 2019). Conventional therapies treat the symptoms of diabetes but do not mitigate metabolic syndrome, which is the major complication of the disease. Traditional Chinese herbal medicines, however, contain a variety of bioactive ingredients that can provide therapeutic benefit for several conditions. For example, Gynostemma pentaphyllum (Thunb.) Makino (Jiao-Gu-Lan), Coptis chinensis Franch. (Huang-Lian), and Salvia miltiorrhiza Bunge (Dan-Shen) can simultaneously enhance insulin sensitivity, reduce visceral fat, and improve hyperlipidemia (Garcia-Galiano et al., 2019). Chinese herbal medicines can also help treat diabetes complications by reversing abnormalities related to blood viscosity, microcirculation, and oxidative stress. Therefore, there is great potential merit in developing new, safe, and effective natural anti-hyperglycemia agents as alternatives to conventional treatments for diabetes and its complications.
The American Dietary Guidelines and the American Diabetes Association (Evert et al., 2013) recommend that diabetes patients as well as healthy individuals eat less refined grains, red meat or processed meat, and sugary drinks to help prevent the onset of T2DM. In addition, in many countries, T2DM patients often take botanical medicines or alternative medicines to potentiate the therapeutic effects of conventional medicines; among these alternatives, traditional Chinese herbal medicines account for a relatively high proportion. Traditional Chinese medicines (TCMs) and their natural bioactive ingredients have a variety of anti-hyperglycemia effects. For example, by eliminating oxygen free-radicals, they help thwart blood hypercoagulability, inhibit the non-enzymatic glycosylation of proteins, inhibit aldose reductase, modulate the metabolism of fats and proteins, and effectively control or delay the onset of diabetes and its complications (Zhang and Jiang, 2012; Lao et al., 2014; Sun et al., 2016; Sharma et al., 2017; Feng et al., 2018). Although an increasing volume of evidence shows that TCMs have a substantial positive impact on treatment of diabetes, research on TCM efficacy remains incomplete.
For many years, researchers have attempted to understand T2DM to formulate interventions and treatment plans to improve patient health (Floegel et al., 2013; Roberts et al., 2014; Zheng et al., 2016). In this regard, high-throughput metabolomics technology has recently begun providing insight into the pathophysiological pathways underlying T2DM (Rhee et al., 2011; Würtz et al., 2012; Padberg et al., 2014).
Metabolomics is the systematic analysis of metabolites in biological samples (Guasch-Ferré et al., 2016), including low-molecular-weight compounds such as amino acids, organic acids, lipids, nucleotides, and sugars. Metabolomics often utilizes approaches based on nuclear magnetic resonance (NMR) and/or various mass spectrometry (MS) techniques, as these technologies not only identify complex metabolic phenotypes but can also be integrated with other “omics” strategies (i.e., transcriptomics, genomics, and proteomics) and bioinformatics data to elucidate potential biological mechanisms and discover clinically relevant diagnostic and prognostic markers of disease risk.
Metabolomics approaches have also been used to study and understand T2DM. A review of recent research revealed that many studies found correlations between T2DM and metabolomics characteristics (Tai et al., 2010). Metabolomics studies can provide insight into disease mechanisms by monitoring differences in metabolite abundance and/or profiles in patients (Cha, 2008; Chan et al., 2009; Carr et al., 2011). Therefore, metabolomics can be used to describe metabolic abnormalities that occur during diabetes progression. Furthermore, metabolomics also has been used for the discovery of disease-related biomarkers (Madsen et al., 2010; Malik et al., 2010), which are commonly used to assess disease severity and the underlying metabolic pathways (Zhang A. H. et al., 2013). Hence, metabolomics can provide a greater understanding of disease pathology and contribute to the development of new treatments (Cruz et al., 2007; Coen et al., 2008). A comprehensive review of T2DM metabolomics is provided by Sas et al. (2015), Gonzalez-Franquesa et al. (2016), and Guasch-Ferré et al. (2016).
Application of Metabolomics in Studying the Traditional Chinese Medicine Treatment of Type 2 Diabetes Mellitus
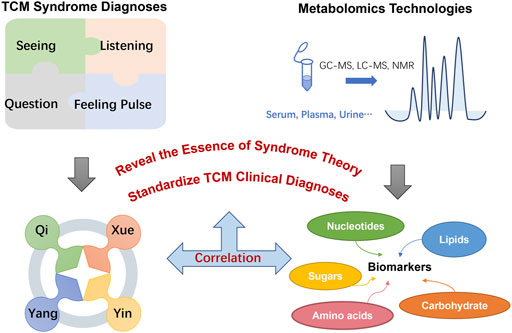
For hundreds of years in China, traditional medicine practices have been used to assess disease through personalized diagnosis, whereas modern medical practice is mainly concerned with treating symptoms. In the classic Chinese publication The Yellow Emperor’s Inner Classic, the symptoms of “drinking more,” “eating more,” “polyuria,” and “weight loss” that are typical of diabetes are classified as xiao ke zheng, for which TCM has long been a treatment (Ning et al., 2009). According to TCM theory, a disease state reflects an imbalance in any or all of four fundamental aspects—Yin (things related to the physical form of an object), Yang (things related to energy quality), Qi (life-force, which animates the forms of the world), and Xue (dense form of body fluids that have been acted upon and excited by Qi) (Wang et al., 2012) – which are in an unbalanced state when people suffer from a disease. Similarly, patients with T2DM could be classified as having deficiency or excess syndromes, which refer to the organs’ insufficiency or excess in Qi, Xue, Yin, and Yang. A disease or syndrome can be diagnosed via comprehensive consideration of symptoms and signs, including tongue appearance and pulse rate, which can also help determine the cause, location, and nature of the disease as well as the patient’s physical condition, disease status, and prognosis. The diagnosis of any particular disease or syndrome is the most notable attribute of TCM, and all diagnosis and treatment methods are derived from this principle.
Metabolomics technology has been applied to help differentiate among T2DM-related syndromes diagnosed based on TCM practices, and thus metabolomics can assist the standardization of TCM clinical diagnoses, inform our understanding of TCM theory, and provide a basis for modernizing TCM practice. Xu et al. (2012) used high-performance liquid chromatography (HPLC) to analyze the profiles of fatty acids in plasma samples from healthy controls and T2DM patients. They measured levels of 12 fatty acids and assessed four lipid parameters: total cholesterol, triglycerides (TG), and high- and low-density lipoproteins. This analysis documented the levels and profiles of common fatty acids in samples from patients with the three TCM syndromes, noting certain significant differences for the group having Qi deficiency vs. the group having both Qi and Yin deficiencies, and found that the unsaturated fatty acids C18:3 and C20:2 in TG and low-density lipoprotein are potential biomarkers and that C20:5, TG, and high-density lipoprotein are candidate biomarkers for Qi deficiency and damp heat. For Qi and Yin deficiency and damp heat, C14:0, C16:0, C18:1, C18:2, and high-density lipoprotein provide the main classification information, each of which is of great relevance to the diagnosis and treatment of T2DM (Xu et al., 2012). Wu et al. used gas chromatography-mass spectrometry (GC/MS) to carry out metabolic profiling of carbohydrates in urine samples from T2DM patients and healthy control subjects (Wu et al., 2012). They also conducted a comparative analysis of 366 subjects using GC/MS combined with kernel-based orthogonal projections to latent structures (K-OPLS) or subwindow permutation analysis (SPA) to 1) compare urinary carbohydrate profiles between T2DM patients and healthy subjects, 2) determine the relationship between urine carbohydrate levels and TCM syndromes in subjects with T2DM, and 3) determine the characteristics and differences in the distribution of TCM syndromes between patients with mild or severe syndromes. They found that, compared with healthy controls, T2DM patients with deficiency or excess syndromes had substantial abnormalities in glucose metabolism. Patients with deficiency syndrome were older than those with excess syndrome, consistent with TCM theory that Qi, Xue, Yin, and Yang are less prevalent in the elderly than the young. Furthermore, two potential biomarkers, xylose and C4 sugar 2, were discovered in the two syndromes using K-OPLS/SPA and Student’s t-test. The analysis revealed elevated levels of xylose and C4 sugar 2 in patients with excess syndrome compared with those with deficiency syndrome.
In summary, syndrome differentiation (Qi, Xue, Yin, and Yang) is the foundation and essence of TCM theory, and the diagnostics mainly depend on overall observation of human symptoms including seeing, listening, questioning, and feeling the pulse rather than “micro” level tests. The metabolic profile is a highly sensitive means of detecting the physiological and pathological changes characteristic of T2DM patients. In addition, a profile can clarify the concept of “syndrome” in the complex physiological system of TCM. Compared with using a single metabolite as a biomarker, using all metabolites to assess human health is more comprehensive and therefore more accurate (Goodacre, 2004; Lu et al., 2009). The research of Wu et al. (2012) demonstrated that the overall application of metabolic profiling in studies of the underlying mechanisms of TCM syndromes is reasonable. Discrimination of different syndromes and the discovery of syndrome-related biomarkers were meaningful for revealing the essence of syndromes. These potential biomarkers reflect dysregulation of metabolism in diabetes patients, which may be helpful for diagnosing diabetes and identifying TCM syndromes, and thus to standardize TCM clinical diagnoses (Figure 1).
Metabolomics Reveals the Mechanisms Underlying the Efficacy of Traditional Chinese Medicine for Treating Type 2 Diabetes Mellitus
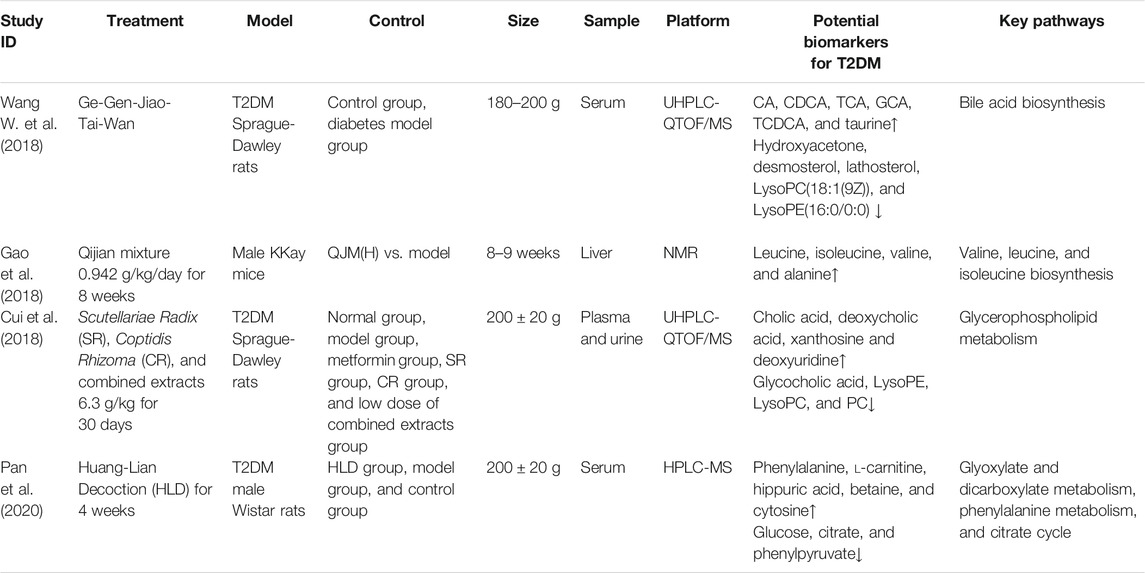
TCM practice has documented success for treatment of T2DM. The guiding principle of TCM is that a variety of traditional Chinese herbal medicines should be used to treat xiao ke zheng, and many classic prescriptions have been passed on through generations of practitioners over thousands of years. The most common Chinese medicine formulations for treating T2DM are Huang-Lian Decoction (HLD) (Pan et al., 2020), Ge-Gen-Jiao-Tai-Wan (GGJTW) (Wang W. et al., 2018), and Qijian mixture (Gao et al., 2018). With continuous practice, experience, and refinement through treatment observations, TCM has formed its own advantages and characteristics relative to modern drugs in terms of regulating glucose and lipid metabolism and countering insulin resistance, having mild yet temporally stable therapeutic effects (Bailey and Day, 1989; Prabhakar and Doble, 2011; Tzeng et al., 2013). For example, Jin-Qi-Jiang-Tang tablets have been proven to improve sugar intake, lipid metabolism, insulin signal transduction, inflammation, and oxidative stress (Liu et al., 2017). Tang-Ning-Tong-Luo formula tablets can reduce liver degeneration, regulate glucose and lipid metabolism, and improve insulin resistance (Cheng et al., 2014). Contemporaneous with treatment, however, patients must adopt healthy dietary habits, emotional and physical exercise habits, and other lifestyle changes to effectively control both blood glucose and blood pressure. To explore the mechanisms underlying these multifaceted interventions, many studies have applied metabolomics methods and produced numerous interesting findings. Table 1 summarizes the various experimental strategies and metabolomics results, including biomarkers and the relevant metabolic pathways.
Two main factors regulate insulin secretion: one is nutrients such as glucose, fatty acids, and amino acids; and the other includes neurotransmitters and hormones. Islet cells maintain a certain steady state of secretion in different physiological states through coordination and integration of these two types of factors. T2DM can cause an imbalance among metabolites, such as lipids, carbohydrates, and amino acids. These imbalances can be detected by metabolomics, toward the goal of identifying biomarkers of early-stage disease.
More than 70 years ago, elevated cellular levels of branched-chain amino acids were found to correlate with insulin resistance and diabetes (Luetscher, 1942; Felig et al., 1969; Felig et al., 1974). Metabolomics studies of 74 obese subjects and 67 lean subjects by Newgard et al. (2009) demonstrated that an overabundance of isoleucine, leucine, and valine correlated with the prevalence of coronary artery disease in a cardiac study cohort and also revealed a strong correlation between glutamate and insulin resistance (Newgard et al., 2009); a lower glutamine:glutamic acid ratio predicted an increased risk of developing diabetes (Cheng et al., 2012). Studies showed that an overabundance of free fatty acids (resulting from phospholipid catabolism) may be the main cause of insulin resistance (Taskinen, 2003; Krauss and Siri, 2004). Also, Liu et al. (2010) conducted metabolomics analyses of fatty acids in serum from healthy subjects, T2DM patients, and patients with postprandial hyperglycemia, revealing that elevated levels of palmitic, stearic, oleic, linolenic, and linolenic acids are key indicators of T2DM (Liu et al., 2010).
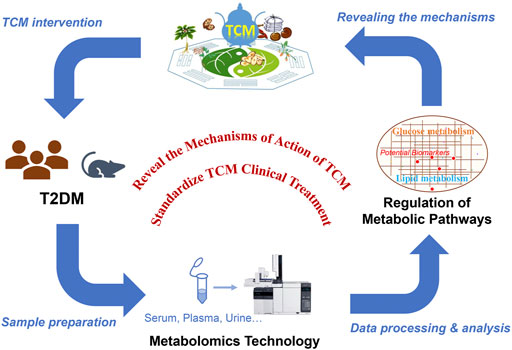
TCM has a long history of treating T2DM, but the underlying mechanism remains unknown. In agreement with the holistic concept of TCM, metabolomics has shown great potential in evaluating efficacy of TCM for treating T2DM. By analyzing endogenous metabolites of small molecules in samples from T2DM patients and examining the metabolic status of the organism, metabolomics can reveal the metabolic changes and the underlying mechanism involved in the pathogenesis of diabetic complications and the efficacy of TCM for treating the disease. Generally, TCM treatment may significantly change some metabolic disorders (e.g., glucose or lipid metabolism) associated with T2DM, promoting metabolic network reorganization through restoring of key metabolites and metabolic pathways, which may be the main mechanism providing the basis of TCM treatment of T2DM. In addition, the discovery of additional diabetes biomarkers would have breakthrough significance for the development of diabetes drugs (Figure 2). Below, we summarize several commonly used herbal medicines for diabetes treatment and the identification of potential T2DM biomarkers.
Georgi root, the dry root of Scutellaria baicalensis Georgi, has several biological activities such as anti-inflammation (Yang et al., 2018), anti-cancer (Cheng et al., 2018), and anti-oxidation (Zhang et al., 2011) and has been used to treat various diseases. The major bioactive components of Georgi root are flavonoids such as baicalin, wogonoside, baicalein, and wogonin. Accumulating research with T2DM patients has shown that baicalin can mitigate insulin resistance (Yang et al., 2012; Song et al., 2013) and suppress gluconeogenesis (Wang et al., 2017). Coptidis Rhizoma, the dried rhizome of Coptis chinensis Franch. (Huang-Lian), contains numerous alkaloids – the major ones being berberine, coptisine, and palmatine—and has been used to treat diarrhea for thousands of years in China. Modern studies have shown that berberine can also substantially lower blood glucose and promote insulin secretion (Zhang et al., 2014; Neag et al., 2018; Xie et al., 2018; Belwal et al., 2020), but the mechanism underlying its role in relieving T2DM symptoms remains unknown. However, potential biomarkers and related pathways have been identified via comparative analysis of plasma and urine metabolomics data for normal and T2DM rats (Cui et al., 2018). Several studies identified eight potential T2DM-related metabolites, including cholic, deoxycholic, and glycocholic acids, the latter of which may inhibit inflammation via TGR5 activation (Agarwal et al., 2018). Cholic and deoxycholic acids block glycocholic acid binding to TGR5. Three other compounds—phosphatidylcholines, lysophosphatidylcholines, and lysophosphatidylethanolamines—are mainly involved in the pathogenesis of inflammation and metabolic diseases such as diabetes. Another compound, xanthosine, promotes the production of uric acid, which is involved in several diabetes complications. The study demonstrated that extracts of Georgi root and Coptidis Rhizoma can have a substantial therapeutic effect for patients with T2DM by modulating the activities of both pro-inflammatory cytokines and enzymes related to glucose metabolism (Cui et al., 2018).
HLD was first described in the classic Chinese medicine volume Treatise on Febrile Diseases (Shang-Han-Lun), mainly with regard to treatment of Yin and Qi deficiencies. Two other ancient books, Physician’s Record (Ming-Yi-Bie-Lu) and Explanation of Materia Medica (Ben-Cao-Jing-Ji-Zhu), also promoted the use of prescriptions containing Coptis chinensis Franch. For more than 1,500 years, these prescriptions were found to benefit patients with T2DM and were first described in Ming-Yi-Bie-Lu during the Wei Jin Dynasty (An and Cui, 2008). HLD is composed of Coptis chinensis Franch. (Huang-Lian); Rhizoma Zingiberis, the fresh rhizome of Zingiber officinale Roscoe (Gan-Jiang); Glycyrrhiza uralensis Fisch. ex DC. (Gan-Cao); Cinnamomum verum J.Presl (Gui-Zhi); Panax ginseng C. A. Mey (Ren-Shen); Pinellia ternata (Thunb.) Makino (Ban-Xia); and Ziziphus jujube Mill. (Da-Zao). Pan et al. (2020) reported that HLD can effectively regulate the levels of numerous compounds in various metabolic pathways that are affected in patients with T2DM (Pan et al., 2020). Using HPLC-MS, they discovered that HLD regulates the production of several compounds – such as cytosine, phenylalanine, glucose, l-carnitine, phenylpyruvate, betaine, citrate, and hippuric acid—that are involved in dicarboxylate and glyoxylate metabolism, phenylalanine metabolism, and the tricarboxylic acid cycle. All these metabolites are related to glucose and lipid metabolism, which are affected by HLD.
GGJTW is composed of Pueraria montana var. lobata (Willd.) Maesen & S.M.Almeida ex Sanjappa & Predeep (Ge-Gen), Coptis chinensis Franch. (Huang-Lian), and Cinnamomum verum J.Presl (Rou-Gui). Jiao-tai-wan, which comprises solely the latter two herbs, was first mentioned as a treatment for insomnia in the classic medical work “Han-Shi-Yi-Tong” of the Ming Dynasty. Puerarin is one of the main bioactive ingredients in Pueraria montana var. lobata (Willd.) Maesen & S.M.Almeida ex Sanjappa & Predeep, and has been used therapeutically for diabetes and its complications (Wu et al., 2013). Puerarin can mitigate insulin resistance (Chen et al., 2018) and protect islet cells (Rojas et al., 2018). GGJTW is used to treat diabetes in China owing to its potent anti-hyperglycemia effect. However, little was known about the underlying metabolic mechanism until 37 potential biomarkers were detected using a metabolomics approach based on ultra-HPLC coupled with quantitative time-of-flight (QTOF) MS. The majority of these biomarkers participate in the biosynthesis of primary bile acids involving increased production of taurine and cholic, chenodeoxycholic, taurocholic, glycocholic, and taurochenodeoxycholic acids. The observed significant changes in the levels of these metabolites demonstrated the anti-hyperglycemia effect of GGJTW on diabetic rats and its potential metabolic mechanism (Wang W. et al., 2018).
The Qijian mixture contains four herbs, each of which is commonly used in TCM to treat diabetes: Astragalus mongholicus Bunge (Huang-Qi); Pueraria montana var. lobata (Willd.) Maesen & S.M.Almeida ex Sanjappa & Predeep (Ge-Gen); Ramulus euonymi, an extract of Euonymus alatus (Thunb.) Sieb. (Gui-Jian-Yu); and Coptis chinensis Franch. (Huang-Lian) (Gao et al., 2018). Astragalus membranaceus was formally described by Carl Linnaeus in his book Plant Species in 1753, and it has a long history in China with wide-ranging clinical applications. Astragalus mongholicus Bunge lowers blood lipids and blood sugar, eliminates edema and oxidative stress (Nozaki et al., 2017), and has a broad range of pharmacological effects on diabetes (Liao et al., 2017). For T2DM in particular, it has a potent therapeutic benefit (He et al., 2018). For example, Gao et al. (2018) used 1H-NMR to assess how the Qijian mixture affects the metabolomic profiles of various liver cell types in samples from T2DM patients and to explore the pharmacodynamics. The major metabolites affected by the Qijian mixture follow: isoleucine, choline, leucine, valine, sn-glycero-3-phosphocholine, citrate, myo-inositol, glycerol, anserine, trimethylamine n-oxide, glutarate, lactate, trimethylamine n-oxide, alanine, glucose, acetate, homoserine, inosine, 3-hydroxybutyrate, glutathione, taurine, niacinamide, xanthine, glycine, and adenine (Table 1). Four metabolites—valine, alanine, isoleucine, and leucine—potentially mediate the anti-hyperglycemia effect. The results demonstrated that the Qijian mixture can regulate amino-acid metabolism by decreasing the levels of these four amino acids. The catabolism of leucine, isoleucine, and valine is linked to insulin sensitivity, i.e., an increase in the levels of these three free-amino-acids in serum promotes insulin sensitivity. The biosynthetic pathways for alanine, aspartate, and glutamate contribute to the pathogenesis of metabolic syndrome in T2DM. Thus, four pathways—branched-chain fatty-acid metabolism and the alanine, aspartate, and glutamate biosynthesis pathways – are closely associated with T2DM, confirming the therapeutic potential of the Qijian mixture for T2DM.
Further Perspectives in Metabolomics Studies of Traditional Chinese Medicine for Treating Type 2 Diabetes Mellitus
Network Pharmacology for Discovery of New Drugs for Treating Type 2 Diabetes Mellitus With Traditional Chinese Medicine
Metabolomics can show the reversal of metabolic disorders caused by TCM treatment, whereas network pharmacology can help to determine how TCM contributes to these changes (Zhang G. B. et al., 2013; Mao et al., 2017). Network pharmacology employs “omics” technologies to detect different molecules (genes, enzymes, and metabolites) and annotate them by comparing with specific databases. Specifically, TCM network pharmacology is used to investigate TCM from a systems perspective and at the molecular level, updating the research paradigm from the current “one target, one drug” mode to a new “network target, multi-components” mode. This method is specialized to prioritize disease-associated genes, to predict the target profiles and pharmacological actions of herbal compounds, to reveal drug–gene–disease co-module associations, to screen synergistic multi-compounds from herbal medicines in a high-throughput manner, and to interpret the combinatorial rules and network regulation effects of herbal medicines (Li and Zhang, 2013). The network-based method has been demonstrated effective to identify the key ingredients in TCM formulations and predict potential molecular targets in T2DM. Below, we summarize some typical examples.
The classic TCM formulation Ge-Gen-Qin-Lian Decoction (GGQLD) has good clinical effects on T2DM which consists of four herbs: Pueraria montana var. lobata (Willd.) Maesen & S.M.Almeida ex Sanjappa & Predeep (Ge-Gen), Scutellaria baicalensis Georgi (Huang-Qin), Coptis chinensis Franch. (Huang-Lian), and Glycyrrhiza glabra L. (Gan-Cao) (Tong et al., 2011; Zhang C. H. et al., 2013). A network pharmacology approach was employed to determine the potential antidiabetic ingredients from the GGQLD formula. Further in vitro antidiabetic trials demonstrated that a predicted antidiabetic ingredient from Ge-Gen, 4-hydroxymephenytoin, can increase the insulin secretion in RIN-5F cells and improve insulin resistance in 3T3-L1 adipocytes (Li et al., 2014), indicating that the network pharmacology strategy is a powerful means for identifying bioactive ingredients and mechanisms of action for TCM herbal formulae.
Compound Lian-Ge granules are composed of Coptis chinensis Franch. (Huang-Lian), Pueraria montana var. lobata (Willd.) Maesen & S.M.Almeida ex Sanjappa & Predeep (Ge-Gen), Salvia miltiorrhiza Bunge (Dan-Shen), and Thlaspi arvense L. (Hai-Zao), resveratrol, and taurine Xue et al. (2019) used network pharmacology methods to predict the 24 bioactive components of compound Lian-Ge granules for lowering blood sugar, including berberine, puerarin, danshinolic acid A, and sinigrin, for which nine targets and 111 metabolic pathways were implicated (Xue et al., 2019). Using network pharmacology methods and technologies, this study predicted the hypoglycemic targets of main active ingredients in Lian-Ge granules and revealed their action modes of in multiple pathways, providing a theoretical basis and a clue for exploration of the hypoglycemic mechanism of compound Lian-Ge granules.
As a traditional Chinese herbal medicine, Astragalus mongholicus Bunge is widely used clinically to treat diabetes. Li et al. (2019) discovered 13 key T2DM targets and found that A. membranaceus can treat T2DM by upregulating the activity of casein kinase, by ensuring the normal regulation of lipid metabolism, and by enhancing insulin resistance, thereby upregulating insulin signaling. Several key targets were randomly selected for quantitative real-time PCR validation, and the results indicated that the analysis of network pharmacology was robust and targets identified via this process were worthy of validation (Li et al., 2019).
In summary, these studies confirmed the feasibility of combining metabolomics and network pharmacology to study the metabolic pathways involved in T2DM that are affected by TCM. In future studies, this combined approach may be a potentially powerful tool for discovery of bioactive components of TCM and elucidation of their action mechanisms for treating T2DM.
Role of Gut Microbiota in Treating Type 2 Diabetes Mellitus
In recent decades, the gut microbiome has emerged as an integral aspect of the efficacy of TCM, and increasing evidence supports a role for the microbiome in the treatment of T2DM. Subjects of T2DM can be distinguished based on a reduced number of gut Clostridiales bacteria (Roseburia species and Faecalibacterium prausnitzii), which produce the short-chain fatty acid butyrate (Qin et al., 2012; Karlsson et al., 2013). Short-chain fatty acids are one of the most important metabolites produced by the gut microbiota, because these metabolites can affect host glucose–insulin homeostasis by modulating the activities of G protein-coupled receptors, promoting the secretion of various hormones, and stimulating the vagus nerve. Finally, another important metabolite of lipopolysaccharide-producing, Gram-negative bacteria has been identified as a trigger of insulin resistance (Cani et al., 2007).
Gegen Qinlian Decoction has long been used to treat common metabolic diseases such as T2DM. Xu et al. found that treatment with both berberine and Gegen Qinlian Decoction significantly altered the overall profile of the gut microbiome and enriched many butyrate-producing bacteria, including Faecalibacterium and Roseburia, thereby reducing intestinal inflammation and lowering blood glucose level (Xu et al., 2020). Sophora flavescens Aiton (Ku-Shen) is a well-known Chinese herbal medicine that has been used to combat T2DM (Jung et al., 2008). It was recently reported that flavonoid compounds in the ethyl-acetate extract of Sophora flavescens Aiton can regulate the metabolism of lipids, carbohydrates, and especially amino acids in patients with T2DM by mediating the host–microbe metabolic axis (Shao et al., 2020). Therefore, it is feasible to identify potential gut microbiota-related targets in T2DM patients for patient-specific treatment with TCM.
Conclusion and Perspective
T2DM is a major global health problem, which is treated as “Xiaoke” in the TCM system, and the related herbal medicines have been used over thousands of years. However, due to its complexity, it is very difficult to decipher the scientific basis and systematic features of TCM for treating T2DM. In the systemic context, metabolomics has a convergence with TCM, therefore it could provide useful tools for uncovering the essence of TCM. Metabolomics can be used to systematically explore the pathophysiology of T2DM and elucidate the overall molecular mechanism underlying the known positive effects of treatment with TCM. Previous studies have shown that metabolomics can help distinguish the various T2DM syndromes. In addition, metabolic disorders in diabetes patients can be mitigated by treatment with TCM, reflecting TCM’s anti-hyperglycemia effect. Research has shown that network pharmacology methods, in combination with experimental pharmacology, can be further used to identify the bioactive ingredients in TCM and their targets, which could inform the development of new therapies for T2DM (Figure 3). The emerging application of metabolomics to elucidate the pathways underlying the efficacy of TCM for T2DM will assist efforts to identify new T2DM biomarkers and develop novel anti-diabetes therapeutics. Moreover, the integration of metabolomics and TCM shows promise in bridging the gap between Chinese and Western medicine and helping to interpret the essence of TCM, and thus perhaps enabling a revolution for future health care.
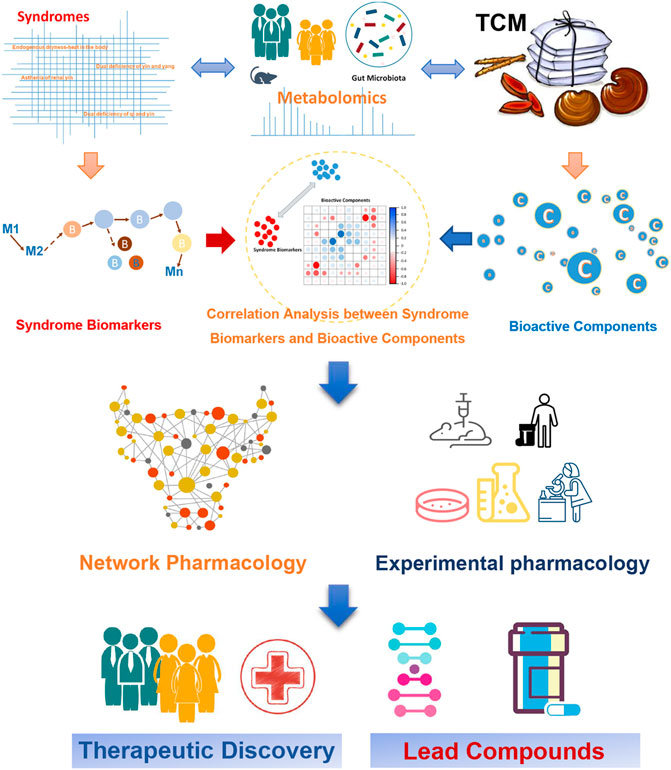
FIGURE 3. Application of metabolomics to assess the efficacy of TCM for treating T2DM, for the purpose of developing new anti-diabetes therapeutics.
It is noteworthy that although a considerable number of potential biomarkers for T2DM have been identified via integration of metabolomic approaches into TCM, there are still some limitations. Due to the lack of a universal analysis platform and a mature and consistent operation plan, most of them have not been analyzed in a standard fashion, and few have been clinically validated. Therefore, it is necessary to establish a comprehensive and complete operation method by expanding the research content of metabolic tissue, improving technical methods, and perfecting the database. Furthermore, it is required to strengthen the combined application of metabolomics technology with other multi-omics, such as genomics and proteomics, to make up for the shortcomings of metabolomics itself, so as to achieve comprehensive and efficient clinical application from research content, and accelerate the standardization and modernization of TCM for treating T2DM.
Author Contributions
YZ, YY, and YX wrote the first draft. LD, ZW, YX, and WX provided critical revisions. All authors approved the final version of the manuscript for submission.
Funding
This work was supported by the National Natural Science Foundation of China Grants (81874335, 32170402, 81920108033, and 81703682), Shanghai Rising-Star Program (18QB1402700, China), and Special Funds for transformation and upgrading of industrial informatization of industry and information technology department of Jiangsu in 2020 (Research on Key technologies of multi-component traditional Chinese medicines).
Conflict of Interest
Authors YY and WX were employed by the company Jiangsu Kanion Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Akt, protein kinase B; AMPK, adenosine monophosphate-activated protein kinase; GC/MS, gas chromatography-mass spectrometry; GGJTW, Ge-Gen-Jiao-Tai-Wan; GGQLD, Ge-Gen-Qin-Lian Decoction; HLD, Huang Lian Decoction; HPLC, high-performance liquid chromatography; K-OPLS, gas chromatography-mass spectrometry; MS, mass spectrometry; NMR, nuclear magnetic resonance; SPA, subwindow permutation analysis; T2DM, type 2 diabetes mellitus; TCM, traditional Chinese medicine; TG, triglycerides.
References
Agarwal, S., Sasane, S., Kumar, J., Deshmukh, P., Bhayani, H., Giri, P., et al. (2018). Evaluation of Novel TGR5 Agonist in Combination With Sitagliptin for Possible Treatment of Type 2 Diabetes. Bioorg. Med. Chem. Lett. 28, 1849–1852. doi:10.1016/j.bmcl.2018.04.011
An, X. P., and Cui, Q. R. (2008). The Reasrch Progress of Rhizoma Coptidis in Treating Diabetes. Gansu. J. TCM. 21, 57–58. doi:10.3969/j.issn.1004-6852.2008.01.039
Bailey, C. J., and Day, C. (1989). Traditional Plant Medicines as Treatments for Diabetes. Diabetes Care 12, 553–564. doi:10.2337/diacare.12.8.553
Bekele, B. B. (2019). The Prevalence of Macro and Microvascular Complications of DM Among Patients in Ethiopia 1990-2017: Systematic Review. Diabetes Metab. Syndr. 13, 672–677. doi:10.1016/j.dsx.2018.11.046
Belwal, T., Bisht, A., Devkota, H. P., Ullah, H., Khan, H., Pandey, A., et al. (2020). Phytopharmacology and Clinical Updates of Berberis Species Against Diabetes and Other Metabolic Diseases. Front. Pharmacol. 11, 41. doi:10.3389/fphar.2020.00041
Brownlee, M. (2001). Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 414, 813–820. doi:10.1038/414813a
Cani, P. D., Amar, J., Iglesias, M. A., Poggi, M., Knauf, C., Bastelica, D., et al. (2007). Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 56, 1761–1772. doi:10.2337/db06-1491
Carr, P. W., Stoll, D. R., and Wang, X. (2011). Perspectives on Recent Advances in the Speed of High-Performance Liquid Chromatography. Anal. Chem. 83, 1890–1900. doi:10.1021/ac102570t
Cha, Y. S. (2008). Effects of L-Carnitine on Obesity, Diabetes, and as an Ergogenic Aid. Asia Pac. J. Clin. Nutr. 17 (Suppl. 1), 306–308. doi:10.6133/apjcn.2008.17.s1.75
Chan, J. C., Malik, V., Jia, W., Kadowaki, T., Yajnik, C. S., Yoon, K. H., et al. (2009). Diabetes in Asia: Epidemiology, Risk Factors, and Pathophysiology. JAMA 301, 2129–2140. doi:10.1001/jama.2009.726
Chen, X., Wang, L., Fan, S., Song, S., Min, H., Wu, Y., et al. (2018). Puerarin Acts on the Skeletal Muscle to Improve Insulin Sensitivity in Diabetic Rats Involving μ-opioid Receptor. Eur. J. Pharmacol. 818, 115–123. doi:10.1016/j.ejphar.2017.10.033
Cheng, S., Rhee, E. P., Larson, M. G., Lewis, G. D., McCabe, E. L., Shen, D., et al. (2012). Metabolite Profiling Identifies Pathways Associated with Metabolic Risk in Humans. Circulation 125, 2222–2231. doi:10.1161/CIRCULATIONAHA.111.067827
Cheng, L., Meng, X. B., Lu, S., Wang, T. T., Liu, Y., Sun, G. B., et al. (2014). Evaluation of Hypoglycemic Efficacy of Tangningtongluo Formula, a Traditional Chinese Miao Medicine, in Two Rodent Animal Models. J. Diabetes Res. 2014, 745419. doi:10.1155/2014/745419
Cheng, C. S., Chen, J., Tan, H. Y., Wang, N., Chen, Z., and Feng, Y. (2018). Scutellaria Baicalensis and Cancer Treatment: Recent Progress and Perspectives in Biomedical and Clinical Studies. Am. J. Chin. Med. 46, 25–54. doi:10.1142/S0192415X18500027
Choi, K., and Kim, Y. B. (2010). Molecular Mechanism of Insulin Resistance in Obesity and Type 2 Diabetes. Korean J. Intern. Med. 25, 119–129. doi:10.3904/kjim.2010.25.2.119
Coen, M., Holmes, E., Lindon, J. C., and Nicholson, J. K. (2008). NMR-based Metabolic Profiling and Metabonomic Approaches to Problems in Molecular Toxicology. Chem. Res. Toxicol. 21, 9–27. doi:10.1021/tx700335d
Cruz, C., Correa-Rotter, R., Sánchez-González, D. J., Hernández-Pando, R., Maldonado, P. D., Martínez-Martínez, C. M., et al. (2007). Renoprotective and Antihypertensive Effects of S-Allylcysteine in 5/6 Nephrectomized Rats. Am. J. Physiol. Ren. Physiol. 293, F1691–F1698. doi:10.1152/ajprenal.00235.2007
Cui, X., Qian, D. W., Jiang, S., Shang, E. X., Zhu, Z. H., and Duan, J. A. (2018). Scutellariae Radix and Coptidis Rhizoma Improve Glucose and Lipid Metabolism in T2DM Rats via Regulation of the Metabolic Profiling and MAPK/PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 19, 3634. doi:10.3390/ijms19113634
de Dios, S. T., Frontanilla, K. V., Nigro, J., Ballinger, M. L., Ivey, M. E., Cawson, E. A., et al. (2007). Regulation of the Atherogenic Properties of Vascular Smooth Muscle Proteoglycans by Oral Anti-hyperglycemic Agents. J. Diabetes Complications 21, 108–117. doi:10.1016/j.jdiacomp.2006.03.003
Evert, A. B., Boucher, J. L., Cypress, M., Dunbar, S. A., Franz, M. J., Mayer-Davis, E. J., et al. (2013). Nutrition Therapy Recommendations for the Management of Adults with Diabetes. Diabetes Care 36, 3821–3842. doi:10.2337/dc13-2042
Felig, P., Marliss, E., and Cahill, G. F. (1969). Plasma Amino Acid Levels and Insulin Secretion in Obesity. N. Engl. J. Med. 281, 811–816. doi:10.1056/NEJM196910092811503
Felig, P., Wahren, J., Hendler, R., and Brundin, T. (1974). Splanchnic Glucose and Amino Acid Metabolism in Obesity. J. Clin. Invest. 53, 582–590. doi:10.1172/JCI107593
Feng, Y., Fang, Y., Wang, Y., and Hao, Y. (2018). Acupoint Therapy on Diabetes Mellitus and its Common Chronic Complications: A Review of its Mechanisms. Biomed. Res. Int. 2018, 3128378. doi:10.1155/2018/3128378
Ferguson, R. D., Gallagher, E. J., Scheinman, E. J., Damouni, R., and LeRoith, D. (2013). The Epidemiology and Molecular Mechanisms Linking Obesity, Diabetes, and Cancer. Vitam. Horm. 93, 51–98. doi:10.1016/B978-0-12-416673-8.00010-1
Ferrannini, E., Natali, A., Camastra, S., Nannipieri, M., Mari, A., Adam, K. P., et al. (2013). Early Metabolic Markers of the Development of Dysglycemia and Type 2 Diabetes and Their Physiological Significance. Diabetes 62, 1730–1737. doi:10.2337/db12-0707
Floegel, A., Stefan, N., Yu, Z., Mühlenbruch, K., Drogan, D., Joost, H. G., et al. (2013). Identification of Serum Metabolites Associated with Risk of Type 2 Diabetes Using a Targeted Metabolomic Approach. Diabetes 62, 639–648. doi:10.2337/db12-0495
Gao, K., Yang, R., Zhang, J., Wang, Z., Jia, C., Zhang, F., et al. (2018). Effects of Qijian Mixture on Type 2 Diabetes Assessed by Metabonomics, Gut Microbiota and Network Pharmacology. Pharmacol. Res. 130, 93–109. doi:10.1016/j.phrs.2018.01.011
Garcia-Galiano, D., Borges, B. C., Allen, S. J., and Elias, C. F. (2019). PI3K Signalling in Leptin Receptor Cells: Role in Growth and Reproduction. J. Neuroendocrinol. 31, e12685. doi:10.1111/jne.12685
Glosse, P., and Föller, M. (2018). AMP-Activated Protein Kinase (AMPK)-Dependent Regulation of Renal Transport. Int. J. Mol. Sci. 19, 3481. doi:10.3390/ijms19113481
Gonzalez-Franquesa, A., Burkart, A. M., Isganaitis, E., and Patti, M. E. (2016). What Have Metabolomics Approaches Taught Us about Type 2 Diabetes? Curr. Diab. Rep. 16, 74. doi:10.1007/s11892-016-0763-1
Goodacre, R. (2004). Metabolic Profiling: Pathways in Discovery. Drug Discov. Today 9, 260–261. doi:10.1016/S1359-6446(04)03027-2
Guasch-Ferré, M., Hruby, A., Toledo, E., Clish, C. B., Martínez-González, M. A., Salas-Salvadó, J., et al. (2016). Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care 39, 833–846. doi:10.2337/dc15-2251
He, K. Q., Li, W. Z., Chai, X. Q., Yin, Y. Y., Jiang, Y., and Li, W. P. (2018). Astragaloside IV Prevents Kidney Injury Caused by Iatrogenic Hyperinsulinemia in a Streptozotocin-induced D-iabetic R-at M-odel. Int. J. Mol. Med. 41, 1078–1088. doi:10.3892/ijmm.2017.3265
Hirosumi, J., Tuncman, G., Chang, L., Görgün, C. Z., Uysal, K. T., Maeda, K., et al. (2002). A central Role for JNK in Obesity and Insulin Resistance. Nature 420, 333–336. doi:10.1038/nature01137
Hunt, J. V., and Wolff, S. P. (1991). Oxidative Glycation and Free Radical Production: A Causal Mechanism of Diabetic Complications. Free Radic. Res. Commun. 12-13 Pt 1, 115–123. doi:10.3109/10715769109145775
Ishii, H., Hayashino, Y., Akai, Y., Yabuta, M., and Tsujii, S. (2018). Dipeptidyl Peptidase-4 Inhibitors as Preferable Oral Hypoglycemic Agents in Terms of Treatment Satisfaction: Results from a Multicenter, 12-week, Open Label, Randomized Controlled Study in Japan (PREFERENCE 4 Study). J. Diabetes Investig. 9, 137–145. doi:10.1111/jdi.12659
John, C. M., Mohamed Yusof, N. I. S., Abdul Aziz, S. H., and Mohd Fauzi, F. (2018). Maternal Cognitive Impairment Associated with Gestational Diabetes Mellitus-A Review of Potential Contributing Mechanisms. Int. J. Mol. Sci. 19, 3894. doi:10.3390/ijms19123894
Jung, H. A., Yoon, N. Y., Kang, S. S., Kim, Y. S., and Choi, J. S. (2008). Inhibitory Activities of Prenylated Flavonoids from Sophora Flavescens against Aldose Reductase and Generation of Advanced Glycation Endproducts. J. Pharm. Pharmacol. 60, 1227–1236. doi:10.1211/jpp.60.9.0016
Karlsson, F. H., Tremaroli, V., Nookaew, I., Bergström, G., Behre, C. J., Fagerberg, B., et al. (2013). Gut Metagenome in European Women with normal, Impaired and Diabetic Glucose Control. Nature 498, 99–103. doi:10.1038/nature12198
Krauss, R. M., and Siri, P. W. (2004). Dyslipidemia in Type 2 Diabetes. Med. Clin. North. Am. 88, 897–x. doi:10.1016/j.mcna.2004.04.004
Lao, Y., Wang, X., Xu, N., Zhang, H., and Xu, H. (2014). Application of Proteomics to Determine the Mechanism of Action of Traditional Chinese Medicine Remedies. J. Ethnopharmacol. 155, 1–8. doi:10.1016/j.jep.2014.05.022
Li, S., and Zhang, B. (2013). Traditional Chinese Medicine Network Pharmacology: Theory, Methodology and Application. Chin. J. Nat. Med. 11, 110–120. doi:10.1016/S1875-5364(13)60037-0
Li, H., Zhao, L., Zhang, B., Jiang, Y., Wang, X., Guo, Y., et al. (2014). A Network Pharmacology Approach to Determine Active Compounds and Action Mechanisms of Ge-Gen-Qin-Lian Decoction for Treatment of Type 2 Diabetes. Evid. Based. Complement. Alternat. Med. 2014, 495840. doi:10.1155/2014/495840
Li, J., Huang, Y., Zhao, S., Guo, Q., Zhou, J., Han, W., et al. (2019). Based on Network Pharmacology to Explore the Molecular Mechanisms of astragalus Membranaceus for Treating T2 Diabetes Mellitus. Ann. Transl. Med. 7, 633. doi:10.21037/atm.2019.10.118
Liao, H., Hu, L., Cheng, X., Wang, X., Li, J., Banbury, L., et al. (2017). Are the Therapeutic Effects of Huangqi (Astragalus Membranaceus) on Diabetic Nephropathy Correlated with its Regulation of Macrophage iNOS Activity? J. Immunol. Res. 2017, 3780572. doi:10.1155/2017/3780572
Liu, L., Li, Y., Guan, C., Li, K., Wang, C., Feng, R., et al. (2010). Free Fatty Acid Metabolic Profile and Biomarkers of Isolated post-challenge Diabetes and Type 2 Diabetes Mellitus Based on GC-MS and Multivariate Statistical Analysis. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 878, 2817–2825. doi:10.1016/j.jchromb.2010.08.035
Liu, Q., Liu, S., Gao, L., Sun, S., Huan, Y., Li, C., et al. (2017). Anti-diabetic Effects and Mechanisms of Action of a Chinese Herbal Medicine Preparation JQ-R In Vitro and in Diabetic KKAy Mice. Acta Pharm. Sin. B 7, 461–469. doi:10.1016/j.apsb.2017.04.010
Lu, D., Wo, X., Wo, L., Li, Y., Tang, L., and Yang, Z. (2009). Effects of Warm and Tonify Kidney-Yang Herbs on Liver Mitochondria Proteome of Kidney-Yang Deficiency Rats. Zhongguo Zhong Yao Za Zhi 34, 1251–1256. doi:10.3321/j.issn:1001-5302.2009.10.017
Luetscher, J. A. (1942). The Metabolism of Amino Acids in Diabetes Mellitus. J. Clin. Invest. 21, 275–279. doi:10.1172/JCI101300
Mabhida, S. E., Dludla, P. V., Johnson, R., Ndlovu, M., Louw, J., Opoku, A. R., et al. (2018). Protective Effect of Triterpenes against Diabetes-Induced β-cell Damage: An Overview of In Vitro and In Vivo Studies. Pharmacol. Res. 137, 179–192. doi:10.1016/j.phrs.2018.10.004
Madsen, R., Lundstedt, T., and Trygg, J. (2010). Chemometrics in Metabolomics-Aa Review in Human Disease Diagnosis. Anal. Chim. Acta 659, 23–33. doi:10.1016/j.aca.2009.11.042
Malik, V. S., Popkin, B. M., Bray, G. A., Després, J. P., and Hu, F. B. (2010). Sugar-sweetened Beverages, Obesity, Type 2 Diabetes Mellitus, and Cardiovascular Disease Risk. Circulation 121, 1356–1364. doi:10.1161/CIRCULATIONAHA.109.876185
Mao, Y., Hao, J., Jin, Z. Q., Niu, Y. Y., Yang, X., Liu, D., et al. (2017). Network Pharmacology-Based and Clinically Relevant Prediction of the Active Ingredients and Potential Targets of Chinese Herbs in Metastatic Breast Cancer Patients. Oncotarget 8, 27007–27021. doi:10.18632/oncotarget.15351
Nanjan, M. J., Mohammed, M., Prashantha Kumar, B. R., and Chandrasekar, M. J. N. (2018). Thiazolidinediones as Antidiabetic Agents: A Critical Review. Bioorg. Chem. 77, 548–567. doi:10.1016/j.bioorg.2018.02.009
Neag, M. A., Mocan, A., Echeverría, J., Pop, R. M., Bocsan, C. I., Crişan, G., et al. (2018). Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 9, 557. doi:10.3389/fphar.2018.00557
Newgard, C. B., An, J., Bain, J. R., Muehlbauer, M. J., Stevens, R. D., Lien, L. F., et al. (2009). A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 9, 311–326. doi:10.1016/j.cmet.2009.02.002
Ning, G., Hong, J., Bi, Y., Gu, W., Zhang, Y., Zhang, Z., et al. (2009). Progress in Diabetes Research in China. J. Diabetes 1, 163–172. doi:10.1111/j.1753-0407.2009.00037.x
Nozaki, T., Minaguchi, J., Takehana, K., and Ueda, H. (2017). Anti-diabetic Activities of Traditional Chinese Herbal Medicine in Streptozotocin-Induced Diabetic Rats. Okajimas Folia Anat. Jpn. 93, 111–118. doi:10.2535/ofaj.93.111
Oikonomou, E., Mourouzis, K., Fountoulakis, P., Papamikroulis, G. A., Siasos, G., Antonopoulos, A., et al. (2018). Interrelationship Between Diabetes Mellitus and Heart Failure: the Role of Peroxisome Proliferator-Activated Receptors in Left Ventricle Performance. Heart Fail. Rev. 23, 389–408. doi:10.1007/s10741-018-9682-3
Padberg, I., Peter, E., González-Maldonado, S., Witt, H., Mueller, M., Weis, T., et al. (2014). A New Metabolomic Signature in Type-2 Diabetes Mellitus and its Pathophysiology. PLoS One 9, e85082. doi:10.1371/journal.pone.0085082
Pan, L., Li, Z., Wang, Y., Zhang, B., Liu, G., and Liu, J. (2020). Network Pharmacology and Metabolomics Study on the Intervention of Traditional Chinese Medicine Huanglian Decoction in Rats with Type 2 Diabetes Mellitus. J. Ethnopharmacol. 258, 112842. doi:10.1016/j.jep.2020.112842
Prabhakar, P. K., and Doble, M. (2011). Mechanism of Action of Natural Products Used in the Treatment of Diabetes Mellitus. Chin. J. Integr. Med. 17, 563–574. doi:10.1007/s11655-011-0810-3
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 490, 55–60. doi:10.1038/nature11450
Rhee, E. P., Cheng, S., Larson, M. G., Walford, G. A., Lewis, G. D., McCabe, E., et al. (2011). Lipid Profiling Identifies a Triacylglycerol Signature of Insulin Resistance and Improves Diabetes Prediction in Humans. J. Clin. Invest. 121, 1402–1411. doi:10.1172/JCI44442
Roberts, L. D., Koulman, A., and Griffin, J. L. (2014). Towards Metabolic Biomarkers of Insulin Resistance and Type 2 Diabetes: Progress from the Metabolome. Lancet. Diabetes Endocrinol. 2, 65–75. doi:10.1016/S2213-8587(13)70143-8
Rojas, J., Bermudez, V., Palmar, J., Martínez, M. S., Olivar, L. C., Nava, M., et al. (2018). Pancreatic Beta Cell Death: Novel Potential Mechanisms in Diabetes Therapy. J. Diabetes Res. 2018, 9601801. doi:10.1155/2018/9601801
Różańska, D., and Regulska-Ilow, B. (2018). The Significance of Anthocyanins in the Prevention and Treatment of Type 2 Diabetes. Adv. Clin. Exp. Med. 27, 135–142. doi:10.17219/acem/64983
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., et al. (2019). Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 157, 107843. doi:10.1016/j.diabres.2019.107843
Sas, K. M., Karnovsky, A., Michailidis, G., and Pennathur, S. (2015). Metabolomics and Diabetes: Analytical and Computational Approaches. Diabetes 64, 718–732. doi:10.2337/db14-0509
Setter, S. M., Iltz, J. L., Thams, J., and Campbell, R. K. (2003). Metformin Hydrochloride in the Treatment of Type 2 Diabetes Mellitus: A Clinical Review with a Focus on Dual Therapy. Clin. Ther. 25, 2991–3026. doi:10.1016/S0149-2918(03)90089-0
Shao, J., Liu, Y., Wang, H., Luo, Y., and Chen, L. (2020). An Integrated Fecal Microbiome and Metabolomics in T2DM Rats Reveal Antidiabetes Effects from Host-Microbial Metabolic Axis of EtOAc Extract from Sophora Flavescens. Oxid. Med. Cel. Longev. 2020, 1805418. doi:10.1155/2020/1805418
Sharma, B. R., Gautam, L. N., Adhikari, D., and Karki, R. (2017). A Comprehensive Review on Chemical Profiling of Nelumbo Nucifera: Potential for Drug Development. Phytother. Res. 31, 3–26. doi:10.1002/ptr.5732
Sheetz, M. J., and King, G. L. (2002). Molecular Understanding of Hyperglycemia's Adverse Effects for Diabetic Complications. JAMA 288, 2579–2588. doi:10.1001/jama.288.20.2579
Song, K. H., Lee, S. H., Kim, B. Y., Park, A. Y., and Kim, J. Y. (2013). Extracts of Scutellaria Baicalensis Reduced Body Weight and Blood Triglyceride in Db/db Mice. Phytother. Res. 27, 244–250. doi:10.1002/ptr.4691
Sun, G. D., Li, C. Y., Cui, W. P., Guo, Q. Y., Dong, C. Q., Zou, H. B., et al. (2016). Review of Herbal Traditional Chinese Medicine for the Treatment of Diabetic Nephropathy. J. Diabetes Res. 2016, 5749857. doi:10.1155/2016/5749857
Tai, E. S., Tan, M. L., Stevens, R. D., Low, Y. L., Muehlbauer, M. J., Goh, D. L., et al. (2010). Insulin Resistance Is Associated with a Metabolic Profile of Altered Protein Metabolism in Chinese and Asian-Indian Men. Diabetologia 53, 757–767. doi:10.1007/s00125-009-1637-8
Taskinen, M. R. (2003). Diabetic Dyslipidaemia: from Basic Research to Clinical Practice. Diabetologia 46, 733–749. doi:10.1007/s00125-003-1111-y
Thangavel, N., Al Bratty, M., Akhtar Javed, S., Ahsan, W., and Alhazmi, H. A. (2017). Targeting Peroxisome Proliferator-Activated Receptors Using Thiazolidinediones: Strategy for Design of Novel Antidiabetic Drugs. Int. J. Med. Chem. 2017, 1069718. doi:10.1155/2017/1069718
Tong, X.-L., Zhao, L.-H., Lian, F.-M., Zhou, Q., Xia, L., Zhang, J.-C., et al. (2011). Clinical Observations on the Dose-Effect Relationship of Gegen Qin Lian Decoction on 54 Out-Patients With Type 2 Diabetes. J. Tradit. Chin. Med. 31, 56–59. doi:10.1016/s0254-6272(11)60013-7
Tzeng, T. F., Liou, S. S., and Liu, I. M. (2013). The Selected Traditional Chinese Medicinal Formulas for Treating Diabetic Nephropathy: Perspective of Modern Science. J. Tradit. Complement. Med. 3, 152–158. doi:10.4103/2225-4110.114893
Wang, L. M., Zhao, X., Wu, X. L., Li, Y., Yi, D. H., Cui, H. T., et al. (2012). Diagnosis Analysis of 4 TCM Patterns in Suboptimal Health Status: A Structural Equation Modelling Approach. Evid. Based. Complement. Alternat. Med. 2012, 970985. doi:10.1155/2012/970985
Wang, T., Jiang, H., Cao, S., Chen, Q., Cui, M., Wang, Z., et al. (2017). Baicalin and its Metabolites Suppresses Gluconeogenesis through Activation of AMPK or AKT in Insulin Resistant HepG-2 Cells. Eur. J. Med. Chem. 141, 92–100. doi:10.1016/j.ejmech.2017.09.049
Wang, Q., Liu, S., Zhai, A., Zhang, B., and Tian, G. (2018). AMPK-Mediated Regulation of Lipid Metabolism by Phosphorylation. Biol. Pharm. Bull. 41, 985–993. doi:10.1248/bpb.b17-00724
Wang, W., Zhao, L., He, Z., Wu, N., Li, Q., Qiu, X., et al. (2018). Metabolomics-Based Evidence of the Hypoglycemic Effect of Ge-Gen-Jiao-Tai-Wan in Type 2 Diabetic Rats via UHPLC-QTOF/MS Analysis. J. Ethnopharmacol. 219, 299–318. doi:10.1016/j.jep.2018.03.026
Weyer, C., Funahashi, T., Tanaka, S., Hotta, K., Matsuzawa, Y., Pratley, R. E., et al. (2001). Hypoadiponectinemia in Obesity and Type 2 Diabetes: Close Association with Insulin Resistance and Hyperinsulinemia. J. Clin. Endocrinol. Metab. 86, 1930–1935. doi:10.1210/jcem.86.5.7463
Wu, T., Yang, M., Wei, H. F., He, S. H., Wang, S. C., and Ji, G. (2012). Application of Metabolomics in Traditional Chinese Medicine Differentiation of Deficiency and Excess Syndromes in Patients with Diabetes Mellitus. Evid. Based. Complement. Alternat. Med. 2012, 968083. doi:10.1155/2012/968083
Wu, K., Liang, T., Duan, X., Xu, L., Zhang, K., and Li, R. (2013). Anti-Diabetic Effects of Puerarin, Isolated from Pueraria lobata (Willd.), on Streptozotocin-Diabetogenic Mice through Promoting Insulin Expression and Ameliorating Metabolic Function. Food Chem. Toxicol. 60, 341–347. doi:10.1016/j.fct.2013.07.077
Würtz, P., Tiainen, M., Mäkinen, V. P., Kangas, A. J., Soininen, P., Saltevo, J., et al. (2012). Circulating Metabolite Predictors of Glycemia in Middle-Aged Men and Women. Diabetes Care 35, 1749–1756. doi:10.2337/dc11-1838
Xie, H., Wang, Q., Zhang, X., Wang, T., Hu, W., Manicum, T., et al. (2018). Possible Therapeutic Potential of Berberine in the Treatment of STZ Plus HFD-Induced Diabetic Osteoporosis. Biomed. Pharmacother. 108, 280–287. doi:10.1016/j.biopha.2018.08.131
Xu, W., Zhang, L., Huang, Y., Yang, Q., Xiao, H., and Zhang, D. (2012). Discrimination of Type 2 Diabetes Mellitus Corresponding to Different Traditional Chinese Medicine Syndromes Based on Plasma Fatty Acid Profiles and Chemometric Methods. J. Ethnopharmacol. 143, 463–468. doi:10.1016/j.jep.2012.06.045
Xu, X., Gao, Z., Yang, F., Yang, Y., Chen, L., Han, L., et al. (2020). Antidiabetic Effects of Gegen Qinlian Decoction via the Gut Microbiota are Attributable to its Key Ingredient Berberine. Genom. Proteom. Bioinform. 18, 721–736. doi:10.1016/j.gpb.2019.09.007
Xue, J., Shi, Y., Li, C., and Song, H. (2019). Network Pharmacology-Based Prediction of the Active Ingredients, Potential Targets, and Signaling Pathways in Compound Lian-Ge Granules for Treatment of Diabetes. J. Cel. Biochem. 120, 6431–6440. doi:10.1002/jcb.27933
Yang, M. D., Chiang, Y. M., Higashiyama, R., Asahina, K., Mann, D. A., Mann, J., et al. (2012). Rosmarinic Acid and Baicalin Epigenetically Derepress Peroxisomal Proliferator-Activated Receptor γ in Hepatic Stellate Cells for Their Antifibrotic Effect. Hepatology 55, 1271–1281. doi:10.1002/hep.24792
Yang, X., Zhang, Q., Gao, Z., Yu, C., and Zhang, L. (2018). Baicalin Alleviates IL-1β-induced Inflammatory Injury via Down-Regulating miR-126 in Chondrocytes. Biomed. Pharmacother. 99, 184–190. doi:10.1016/j.biopha.2018.01.041
Zhang, X. W., Li, W. F., Li, W. W., Ren, K. H., Fan, C. M., Chen, Y. Y., et al. (2011). Protective Effects of the Aqueous Extract of Scutellaria Baicalensis against Acrolein-Induced Oxidative Stress in Cultured Human Umbilical Vein Endothelial Cells. Pharm. Biol. 49, 256–261. doi:10.3109/13880209.2010.501803
Zhang, A. H., Sun, H., Qiu, S., and Wang, X. J. (2013). Recent Highlights of Metabolomics in Chinese Medicine Syndrome Research. Evid. Based. Complement. Alternat. Med. 2013, 402159. doi:10.1155/2013/402159
Zhang, C. H., Xu, G. L., Liu, Y. H., Rao, Y., Yu, R. Y., Zhang, Z. W., et al. (2013). Anti-Diabetic Activities of Gegen Qinlian Decoction in High-Fat Diet Combined with Streptozotocin-Induced Diabetic Rats and in 3T3-L1 Adipocytes. Phytomedicine 20, 221–229. doi:10.1016/j.phymed.2012.11.002
Zhang, G. B., Li, Q. Y., Chen, Q. L., and Su, S. B. (2013). Network Pharmacology: A New Approach for Chinese Herbal Medicine Research. Evid. Based. Complement. Alternat. Med. 2013, 621423. doi:10.1155/2013/621423
Zhang, T. T., and Jiang, J. G. (2012). Active Ingredients of Traditional Chinese Medicine in the Treatment of Diabetes and Diabetic Complications. Expert Opin. Investig. Drugs 21, 1625–1642. doi:10.1517/13543784.2012.713937
Zhang, Z., Zhang, H., Li, B., Meng, X., Wang, J., Zhang, Y., et al. (2014). Berberine Activates Thermogenesis in white and Brown Adipose Tissue. Nat. Commun. 5, 5493. doi:10.1038/ncomms6493
Zheng, Y., Ceglarek, U., Huang, T., Li, L., Rood, J., Ryan, D. H., et al. (2016). Weight-loss Diets and 2-y Changes in Circulating Amino Acids in 2 Randomized Intervention Trials. Am. J. Clin. Nutr. 103, 505–511. doi:10.3945/ajcn.115.117689
Zhou, J., Huang, K., and Lei, X. G. (2013). Selenium and Diabetes-Eevidence from Animal Studies. Free Radic. Biol. Med. 65, 1548–1556. doi:10.1016/j.freeradbiomed.2013.07.012
Keywords: traditional Chinese medicine, metabolomics, type 2 diabetes, metabolic regulation, metabolome
Citation: Zhang Y, Yang Y, Ding L, Wang Z, Xiao Y and Xiao W (2021) Emerging Applications of Metabolomics to Assess the Efficacy of Traditional Chinese Medicines for Treating Type 2 Diabetes Mellitus. Front. Pharmacol. 12:735410. doi: 10.3389/fphar.2021.735410
Received: 02 July 2021; Accepted: 08 September 2021;
Published: 17 September 2021.
Edited by:
XY Zhang, University of Minho, PortugalReviewed by:
You YUN, China Academy of Chinese Medical Sciences, ChinaShao Li, Tsinghua University, China
Copyright © 2021 Zhang, Yang, Ding, Wang, Xiao and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Xiao, eGlhb3lpbmd0Y21Ac2h1dGNtLmVkdS5jbg==; Wei Xiao, eHdfa2FuaW9uQDE2My5jb20=
†These authors have contributed equally to this article
 Yumeng Zhang
Yumeng Zhang Yingbo Yang
Yingbo Yang Lili Ding
Lili Ding Zhengtao Wang
Zhengtao Wang Ying Xiao
Ying Xiao Wei Xiao
Wei Xiao