- 1School of Pharmacy, School of Basic Medicine, Innovative Institute of Chinese Medicine and Pharmacy, School of Acupuncture and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Neijiang Hospital of Traditional Chinese Medicine Affiliated to Chengdu University of Traditional Chinese Medicine, Neijiang, China
- 3Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
The Buxue Tongluo pill (BTP) is a self-made pill with the functions of nourishing blood, promoting blood circulation, dredging collaterals, and relieving pain. It consists of Angelica sinensis (Oliv.) Diels, Pheretima aspergillum (E.Perrier), Panax notoginseng (Burk.) F. H. Chen, Astragalus membranaceus (Fisch.) Bge, and Glycyrrhiza uralensis Fisch. Various clinical practices have confirmed the therapeutic effect of BTP on osteonecrosis of the femoral head (ONFH), but little attention has been paid to the study of its bioactive ingredients and related mechanisms of action. In this study, UPLC/MS-MS combined with GEO data mining was used to construct a bioactive ingredient library of BTP and a differentially expressed gene (DEG) library for ONFH. Subsequently, Cytoscape (3.7.2) software was used to analyze the protein–protein interaction between BTP and DEGs of ONFH to screen the key targets, and functional annotation analysis and pathway enrichment analysis were carried out. Finally, 34 bioactive compounds were screened, which acted on 1,232 targets. A total of 178 DEGs were collected, and 17 key genes were obtained after two screenings. By bioinformatics annotation on these key genes, a total of 354 gene ontology (GO) functional annotation analyses and 42 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were obtained. The present study found that GO and KEGG enrichment were mainly related to apoptosis, suggesting that BTP may exert an anti-ONFH effect by promoting osteoclast apoptosis. Experiments in vitro demonstrated that BTP could increase the mitochondrial membrane potential (MMP) and induce remarkable apoptosis in osteoclasts. Furthermore, we determined the apoptosis marker of cleaved(C)-caspase-3, bcl-2, and bax and found that BTP could upregulate the C-caspase-3 and bax expression in osteoclasts and decrease the expression of bcl-2, p-Akt, and p-PI3K in a dose-dependent manner, indicating that BTP could induce PI3K/Akt-mediated apoptosis in osteoclasts to treat ONFH. This study explored the pharmacodynamic basis and mechanism of BTP against ONFH from the perspective of systemic pharmacology, laying a foundation for further elucidating the therapeutic effects of BTP against ONFH.
Introduction
Osteonecrosis of the femoral head (ONFH) refers to a disease in which the blood supply of the femoral head is damaged or interrupted, leading to the death of bone marrow components and bone cells and the structural change and collapse of the femoral head (Pouya and Kerachian, 2015). It has also been considered a debilitating disease of multifactorial genesis, predominately affecting young people aged 20–40 years with the destruction of hip joints in their third, fourth, or fifth decade of life (Sun and Li, 2013). Currently, approximately 20,000–30,000 patients in the United States are diagnosed with ONFH annually, bringing pain and a substantial economic burden to the patients. Treatment options available for ONFH include pharmaceutical intervention and surgical treatment. Unfortunately, the former often fails to achieve satisfactory results due to the rapid progress of ONFH, and concerns regarding the longevity of the hip replacement have always existed. Thus, ONFH has been widely regarded as a global problem (Ping, 2021). Recently, traditional Chinese medicine (TCM) has been gradually accepted as a complementary and alternative medicine for the treatment of multiple diseases due to low toxicity and good curative effects (Xu et al., 2021; Zhang et al., 2021). A growing amount of TCM compounds and monomers, such as Bushen Huoxue decoction (Sun and Li, 2013), Huangqi Shenggu decoction (Ping, 2021), Taohong Siwu decoction (Zhao, 2020), Gastrodin (Zheng et al., 2014), and luteolin (Yan et al., 2020), have been reported to exert a significant therapeutic effect on ONFH.
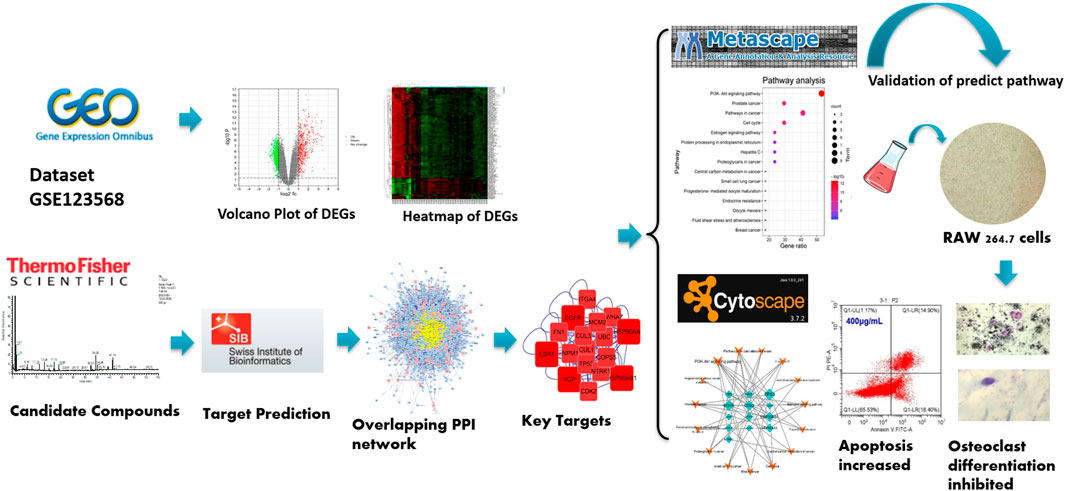
BTP is a TCM formula that has been proven effective in treating ONFH with the functions of “promoting blood supply”. It is composed of Angelica sinensis (Oliv.) Diels, Pheretima aspergillum (E.Perrier), Panax notoginseng (Burk.) F. H. Chen, Astragalus membranaceus (Fisch.) Bge, and Glycyrrhiza uralensis Fisch. Although it is frequently used to treat ONFH in clinical practice, there is no research for the specific mechanism regarding the anti-ONFH effect of BTP. Moreover, the TCM formula is characteristic of multicomponents, multitargets, and multipathways, making it difficult to reveal their mechanisms of action (Xu et al., 2021). Network pharmacology is a technology that can systematically analyze the interaction network of drug components, targets, diseases, and genes, which is consistent with the overall concept of the TCM theoretical system (Hopkins, 2008). At present, increasing studies have confirmed the great potential of network pharmacology in investigating the possible molecular mechanisms of TCM (Shan et al., 2018; Cui et al., 2018; Daina et al., 2019; Wang et al., 2020; Xia et al., 2021). Thus, it is an inevitable trend to apply network pharmacology to investigate the material basis and molecular mechanism of the TCM formula (Qing and Jia, 2021; Qing and Hu, 2021; Qing and Jia, 2019). In the current study, UPLC-MS/MS was used to identify the active ingredients of BTP, and network pharmacology was used to study the molecular mechanisms of BTP against ONFH. Besides, experiments in vitro were performed to provide scientific evidence for the predicted results with the network pharmacology. The general experimental procedures are shown in Figure 1.
Methods
Preparation of the Extracts of BTP
BTP samples were prepared and provided by Neijiang traditional Chinese medicine hospital (Neijiang, China). The powder of BTP (10 g) was ultrasonically dissolved with 300 ml, 75% ethanol, followed by concentrating to 100 ml using a rotary evaporator. Then, the supernatant was filtered by microporous membranes with a pore size of 0.22 μm. Then, the obtained 10 μl filtrates of BTP were analyzed by UPLC-MS/MS.
UPLC-MS/MS Analysis
For qualitative analysis, a Thermo Scientific Q Exactive Orbitrap HRMS (Thermo Fisher Scientific, Massachusetts, United States) was connected to a Thermo Scientific Vanquish UPLC (Thermo Fisher Scientific, Massachusetts, United States). Chromatographic separation was achieved on a Thermo Scientific Accucore C18 (3 × 100 mm, 2.6 μm) in a thermostatically controlled column compartment (30 °C). The aqueous and organic mobile phases used were acetonitrile (A) and 0.1% phosphoric acid aqueous solution (B), respectively. The gradient elution program was set up as follows: 0–20 min, 12–25% A; 20.1–30 min, 25–28% A; 30.1–32 min, 28–40% A; 32.1–40 min, 40–50% A; 40.1–60 min, 50% A; 60.1–65 min, 12% A. The flow rate was set as 0.3 ml/min, and 2 μl of the extraction was injected into the UPLC system. The instrument was operated in the positive ion mode to perform full-scan analysis over an m/z range of 100–1,500. The optimized parameters were set as follows: a sheath gas flow rate of 35 L/min; a spray voltage of 3000 V; a capillary temperature of 320 V; an aux gas flow rate of 10.00 L/min; a max spray current of 100 A; a probe heater temperature of 350 C; and an S-lens RF level of 50.00%.
Establishment of Effective Compounds and the Target Library of BTP
Compounds of BTP were analyzed by UPLC-MS/MS. Subsequently, targets regarding these identified compounds via UPLC-MS/MS were obtained from the online database of SwissTargetPrediction (http://www.swisstargetprediction.ch/) (Daina et al., 2019).
Establishment of the ONFH Target Library
As a common worldwide resource for gene expression studies, the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo) allows open access to high-throughput gene expression and other functional genomics datasets (Clough and Barrett, 2016). The GEO database was searched using “osteonecrosis of femoral head” as the keyword. We aimed to find a comparison of gene expression data sets between femoral head samples from patients with ONFH and normal femoral head samples. In order to ensure the stability and credibility of the analysis results, only data sets with a sample size larger than 10 were selected. Finally, only one suitable data set of ONFH was downloaded from the GEO database, namely, dataset GSE123568. In this dataset, 10 normal femoral head samples and 30 ONFH samples were included.
Acquisition of Differentially Expressed Genes
In order to obtain DEGs, dataset GSE123568 was analyzed with GEO2R, which was the official differential expression analysis tool of the GEO database. In this study, the difference with logFC (fold change) ≥1.3 and p-values <0.01 was statistically significant, and the corresponding genes were identified as DEGs.
Construction of the Protein–Protein Interaction Based on Bisogenet
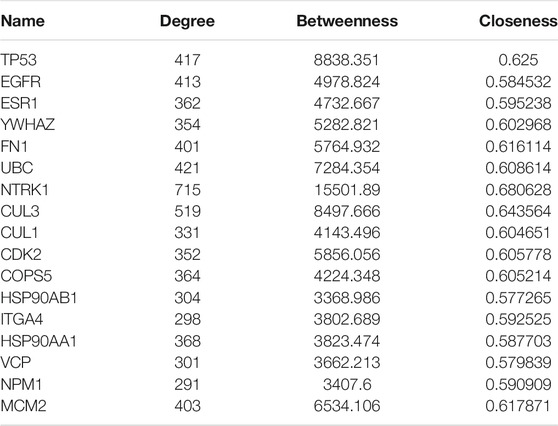
In this study, the protein–protein interaction (PPI) of BTP and DEGs from ONFH were constructed by Cytoscape software (3.7.2). Then, the overlapping PPI network was obtained by using BisoGenet in Cytoscape. The median of three important attribute values, namely, degree, betweenness, and closeness, was employed for screening to obtain the core gene. After two screenings, a PPI network containing 17 nodes and 114 edges was obtained and used for enrichment analysis. The detailed attribute values and screening process of these targets are presented in Table 3 and Figure 3, respectively.
Functional Enrichment and Constructing the Network of BTP-DEGs-Pathway
The Metascape platform (http://metascape.org/gp/index.html) has a comprehensive annotation function and data of gene annotation monthly updated (Zhou et al., 2019). In this study, functional annotation of gene ontology (GO) and KEGG pathways was performed with the Metascape platform. The main biological processes and involved metabolic pathways were analyzed by submitting the core targets of BTP-DEGs from ONFH to Metascape with p < 0.01. Then, the results were saved and visualized by Bioinformatics (http://www.bioinformatics.com.cn/) (Huang, 2009). Afterward, as shown in Figure 4, the network of the BTP-DEGs-Pathway was constructed with Cytoscape (3.7.2).
Experimental Validation
Chemicals and Reagents
Fetal bovine serum (FBS), phosphate-buffered saline (PBS), penicillin–streptomycin, 0.25% trypsin–EDTA (1x), and Dulbecco’s modified eagle’s medium (DMEM) were purchased from GIBCO (Grand Island, NY, United States). A cell counting kit-8 (CCK8) detection kit was purchased from the Beijing 4A Biotech Co., Ltd. (Beijing, China). The assay kits for DCFH-DA, horseradish peroxidase (HPR)-conjugated secondary antibodies, β-actin, BCA protein assay reagents, and primary antibodies for cleaved (C) caspase-3 were purchased from Boster Biol. Tech. (Wuhan, China). Primary antibodies for phosphorylation- (p-) Akt, Akt, PI3K, and P-PI3K were obtained from ImmunoWay Biotechnology Co. (Suzhou, China). Annexin V-FITC/PI apoptosis kits were obtained from US Everbright® Inc (Suzhou, China). Polyvinylidene fluoride (PVDF) membranes were purchased from Sigma-Aldrich (Shanghai, China). 5,5′,6,6′-Tetrachloro-1.1′,3.3′-tetraethyl-imidacarbocyanine iodide (JC-1) was obtained from Jiangsu KeyGen Biotech (Nanjing, China).
Cell Culture
The RAW 264.7 cells were purchased from Wuhan Pu-nuo-sai Life Technology Co., Ltd. (Wuhan, China) and used throughout the study. The RAW264.7 cells were cultured in DMEM containing 10% FBS (v/v) and 1% penicillin–streptomycin (v/v) at 37°C in a humidified atmosphere of 5% CO2.
Cell Activity Detection
The RAW 264.7 cells were incubated in 96-well plates (1×104 cells/well) with different concentrations (0, 5, 10, 20, 40, 60, 80, 100, 150, and 200 μg/ml) of BTP, followed by induction with RANKL (100 ng/ml). After incubation for 24 and 48 h, the supernatant was discarded, and the cells were washed three times with PBS. Then, 10 µl CCK-8 was added to each well and incubated for 0.5 h. The optical density (OD) values were measured at 450 nm by the microtablet reader (Bio-RAD, United States). Each experiment was repeated three times. The concentration- and time-dependent effects of BTP on raw 264.7 cells are presented in Figure 5.
Osteoclastogenesis and TRAP Staining
The RAW 264.7 cells were seeded in 24-well plates (1×105 cells/well) and then pretreated with BTP (0, 50, 75, and 100 μg/ml) for 24 h, followed by induction with RANKL (100 ng/ml) for 7 days. Untreated cells were used as a negative control, while only RANKL-induced cells were used as a positive control group. During the induction period, the medium was changed every other day. After 7 days, the cells were washed with PBS, followed by fixing with 4% paraformaldehyde for 30 min, and then stained to detect TRAP activity. Finally, trap-positive cells were enumerated via a microscope. Mature osteoclasts with more than three nuclei were counted in a random area in each replicate sample.
Bone Resorption Assay
The RAW 264.7 cells were seeded in 96-well plates (1×104 cells/well) containing bovine bone slices and then pretreated with different concentrations of BTP (0, 50, 75, and 100 μg/ml) for 24 h, followed by induction with RANKL (100 ng/ml) for 9 days. Then, bovine bone slices seeded with cells were washed with PBS, fixed in 2.5% glutaraldehyde over 10 min, ultrasonically cleaned with 0.25 mol/l ammonia hydroxide, and stained with 1% toluidine blue over 10 min. After washing with ddH2O, the osteolysis pits were photographed with a light microscope. Bone resorption capacity was defined as the bone resorption relative area (resorption area/total bone area).
Apoptosis Assay Using a Flow Cytometer
Cell apoptosis was detected by flow cytometry (CytoFLEX FCM, Beckman Coulter Inc., Atlanta, Georgia, United States). The RAW 264.7 cells were incubated in 6-well plates with RANKL (100 ng/ml) for 5 days and then treated with BTP (0, 50, 75, and 100 μg/ml) for 24 h. Subsequently, cell apoptosis was detected with the manufacturer’s instructions of the Annexin V/PI apoptosis kit and cell cycle kit; the cells were stained with Annexin V-FITC and PI, respectively, and then detected by flow cytometer analysis. Each experiment was repeated three times.
Mitochondrial Membrane Potential (MMP) Determination
The change of intracellular mitochondrial membrane potential (MMP, ΔΨm) is generally recognized as an important indicator of mitochondrial dysfunction, and JC-1 is commonly considered as an ideal probe to evaluate ΔΨm. In this study, the cells were inoculated in laser confocal dishes (1×104/well), treated with different concentrations of BTP for 24 h, and subsequently induced with RANKL (100 ng/ml) for 5 days. After the induction, the cells were incubated with the JC-1 probe (10 μg/ml) in the dark for 30 min. Confocal laser microscopy was used to observe the green and the red fluorescence (Leica, SP8 SR, Wetzlar, Germany) of cells at 488 and 647 nm, respectively, and the images were recorded.
Immunofluorescence Assay of AKT/P-AKT
The RAW 264.7 cells were seeded in the confocal dish (1×105) and processed as described above. Then, they were washed three times with PBS, fixed with 4% paraformaldehyde for 30 min, and then washed three times with PBS. Afterward, the cells were treated with 0.5% Triton-X-100 for 30 min, followed by blocking with goat serum for 1 h at room temperature, and then, PI3K and P-PI3K antibodies (1:500) were added separately and incubated overnight at 4°C. Soon afterward, antirabbit IgG (H&L) Alexa Fluor® 488 and antimouse IgG (H + L) Alexa Fluor® 647 were added and incubated at room temperature for 1 h in the dark. Then, PBS was used to wash out the unbound secondary antibody, and the nuclei were stained with DAPI. Then, AKT/P-AKT and their fluorescence intensity were observed using a confocal laser microscope (Leica, SP8 SR, Wetzlar, Germany).
Western Blotting Assay
The RAW 264.7 cells were inoculated in 6-well plates (2×106/well) at 37°C in a humidified atmosphere of 5% CO2 and incubated for 24 h. Then, the RAW 264.7 cells were treated with RANKL (100 ng/ml) for 5 days and then treated with BTP (50, 75, and 100 μg/ml) for 24 h. At the end of the treatment, 100 μl of RIPA cell lysates was used to extract the total cell protein. The protein concentrations were determined using BCA protein assay reagents. Then, the total protein was separated by 12% SDS-PAGE and then transferred to the PVDF membrane. After blocking by 5% skimmed milk, the PVDF membrane was incubated overnight with diluted primary antibodies of PI3K, P-PI3K, C-caspase-3, Akt, and p-Akt (dilution 1:1,000) at 4°C. Subsequently, the PVDF membrane was incubated for 1 h with the diluted secondary antibody. Finally, the protein bands were stained with ECL detection kits, and β-actin was used as the internal reference. Image analysis software ImageJ (version 1.51, National Institutes of Health, MD, United States) was utilized for gray analysis.
Statistical Analysis
All the data were presented as the mean ± standard deviations (SDs), statistical comparisons were evaluated using one-way analysis of variance (ANOVA), and significant differences between the mean values were measured using Duncan’s multiple range test. p < 0.01 was deemed statistically significant.
Results
Effective Compounds of BTP
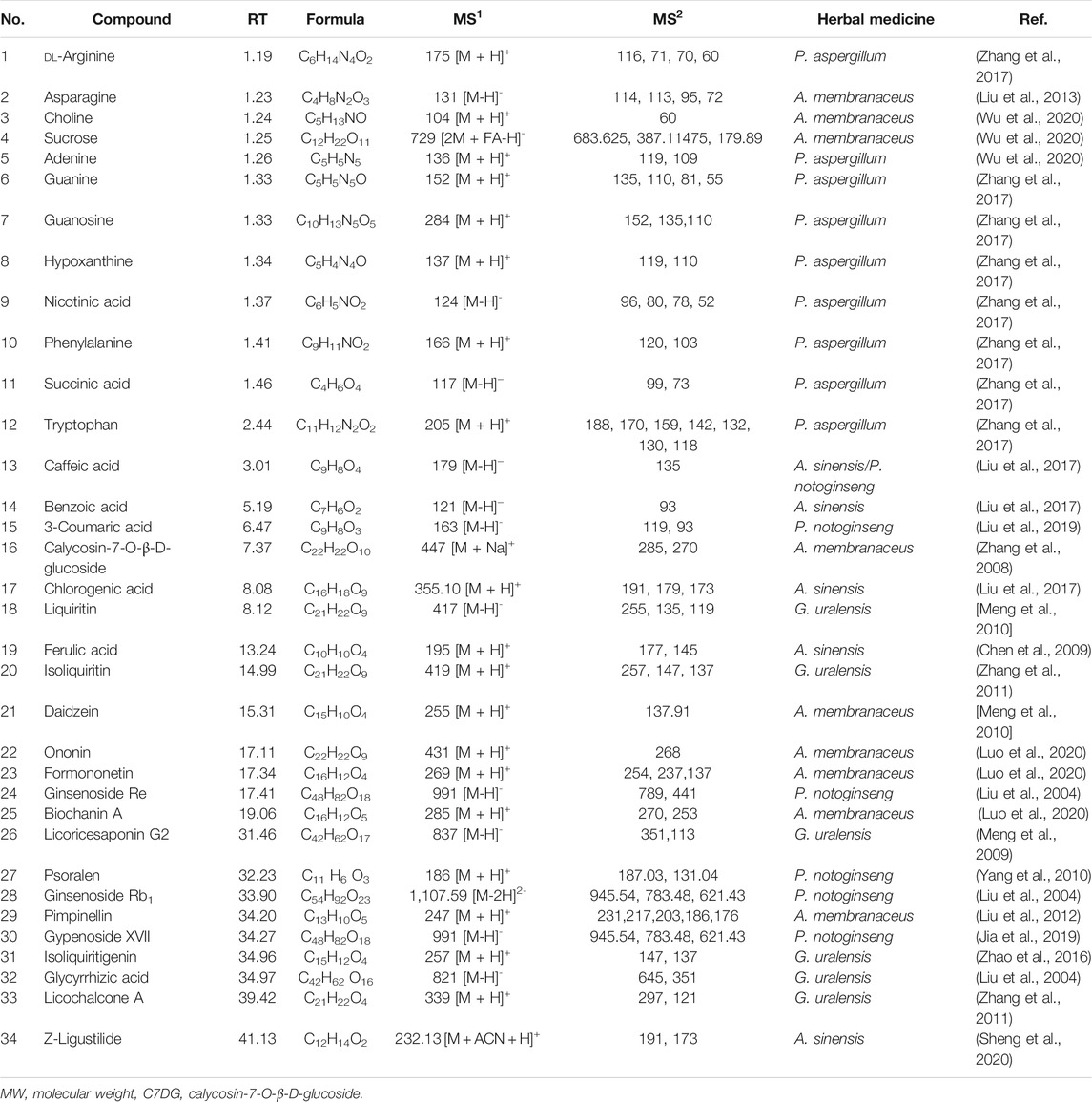
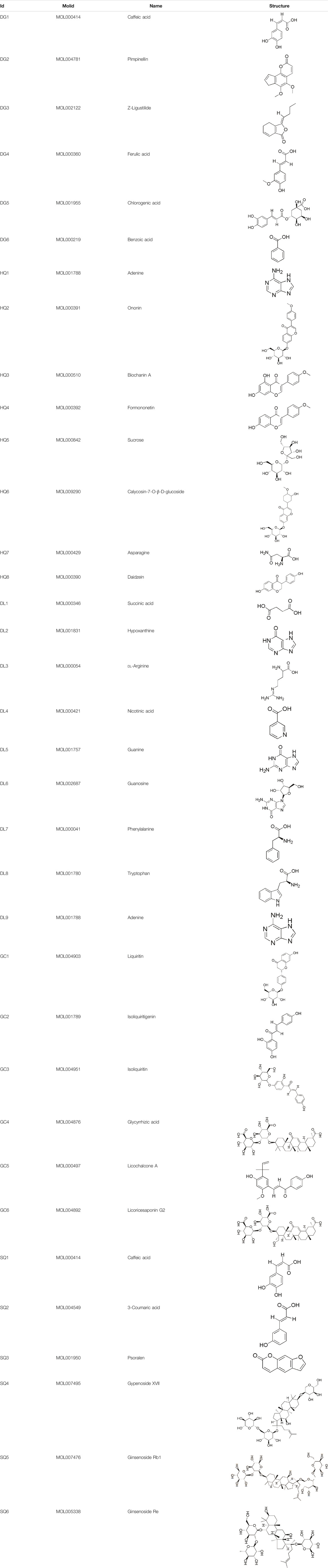
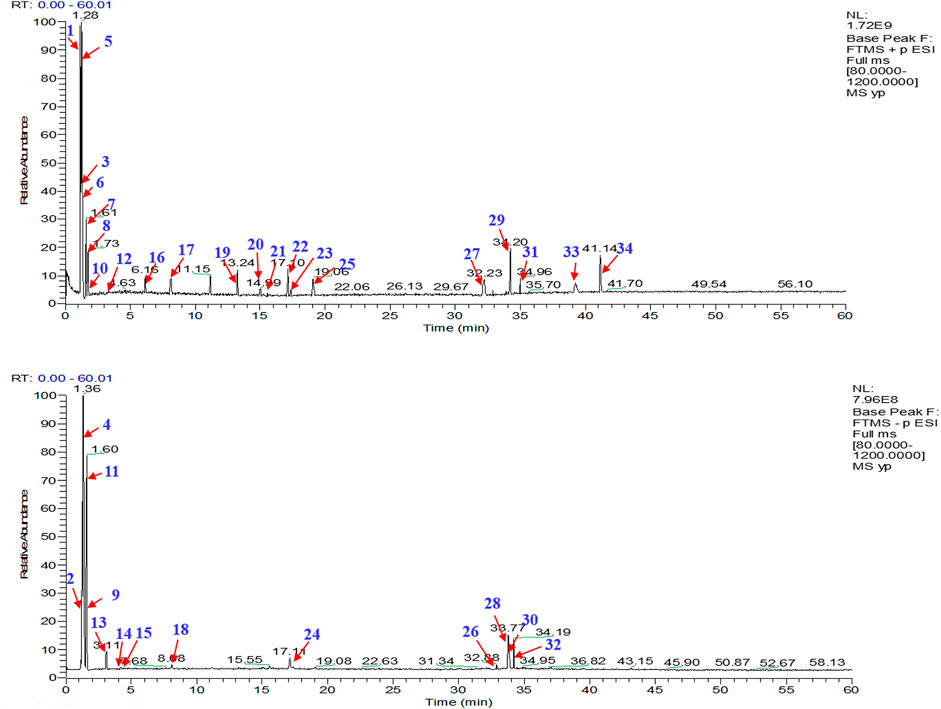
A total of 34 components were identified from BTP, of which six compounds belonged to Angelica sinensis (Oliv.) Diels, seven to Astragalus membranaceus (Fisch.) Bge, six to Glycyrrhiza uralensis Fisch., six to Panax notoginseng (Burk.) F. H. Chen, and nine to Pheretima aspergillum (E.Perrier). Their specific information and total ion current diagram are shown in Table 1, Table 2, and Figure 2, respectively. Moreover, a total of 1,232 related targets were obtained from the online databases.
Establishment of the ONFH Target Library
A total of 178 DEGs were screened out, including 143 upregulated genes and 35 downregulated genes, and were visualized with a volcano plot, as shown in Figure 2. In the volcano plot, the vertical axis represents log2 FC (fold change), and the horizontal axis represents log10 (p-value). The DEGs with log2 FC > 0 were defined as upregulated genes, and the others were downregulated genes. Additionally, the expression situation regarding all the DEGs was visualized with a heatmap, as shown in Figure 3A. In the heatmap, the vertical axis represents DEGs, while the horizontal axis represents the sample. DEGs were effectively divided into normal and ONFH groups. Red indicates the upregulated gene, and green represents the downregulated genes.
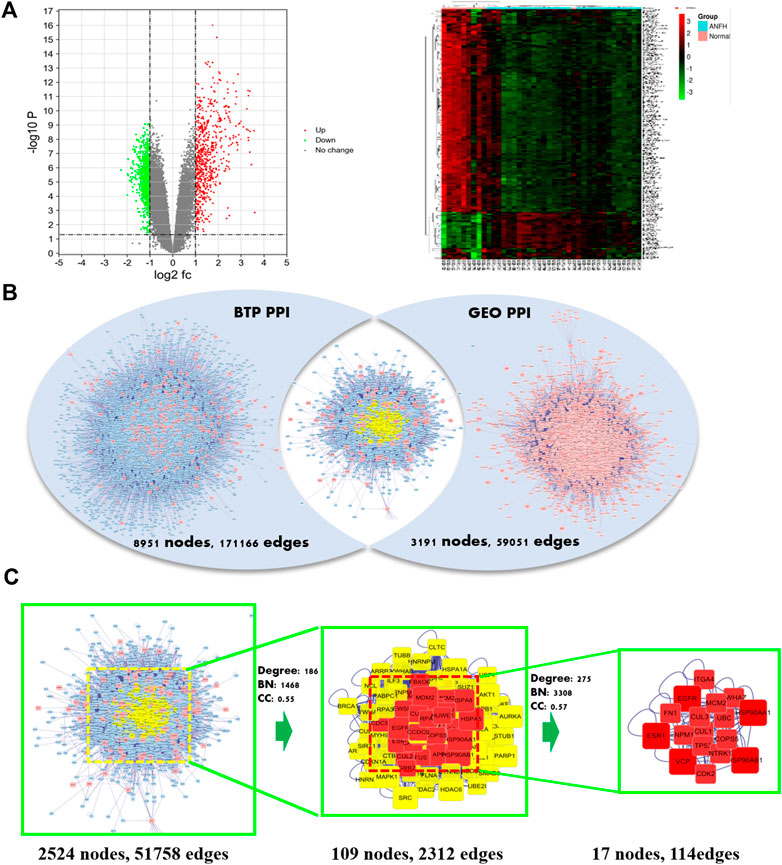
FIGURE 3. Volcano plot (left) and heatmap (right) of differentially expressed genes (DEGs) in microarray data sets of GSE123568 (A). Intersection PPI network of BTP and DEGs. (B) Identification process of core targets of BTP against ONFH. Nodes for genes and edges for interactions between proteins (C).
Acquisition of Core Genes Regarding BTP Against ONFH
Centrality expresses the degree of a node in a certain network in the center of the whole network. The degree is defined as the sum of direct connections between nodes and represents the cohesion of a given node in a certain network. Furthermore, betweenness indicates the number of shortest paths passing through a node. These three factors are the indispensable basis for assessing the importance of a specific target. Through screening, 17 core targets with 114 vital interactions were obtained, and the detailed information and identification process of core targets are shown in Table 3 and Figure 3C, respectively.
Functional Enrichment of Core Targets
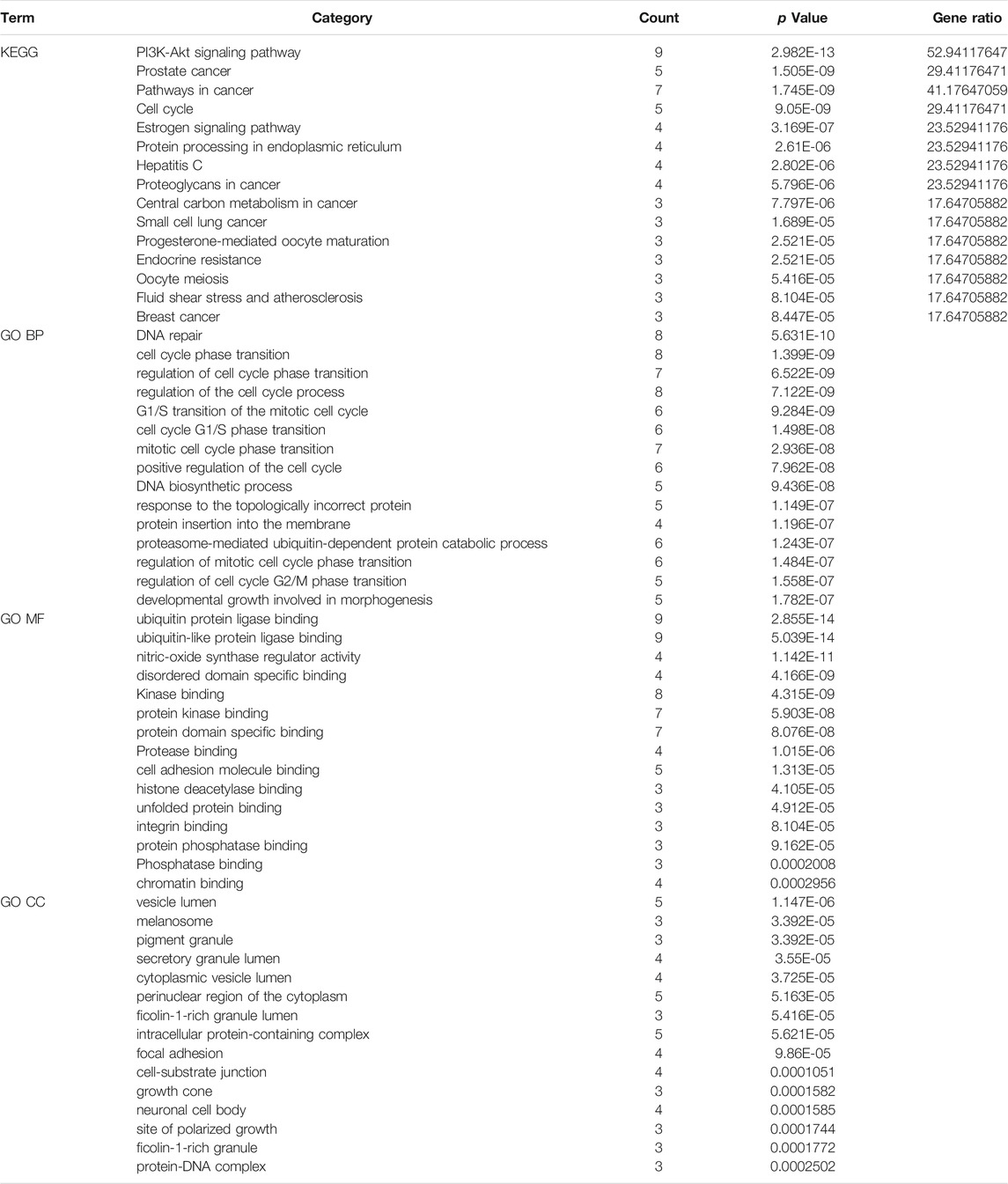
The signal pathway of core targets was analyzed using the Metascape platform, and the results were visualized by bioinformatics. The results showed that the functions of core targets were closely related to the occurrence of ONFH. KEGG and GO enrichment are presented in Table 4 and Figure 4, respectively.
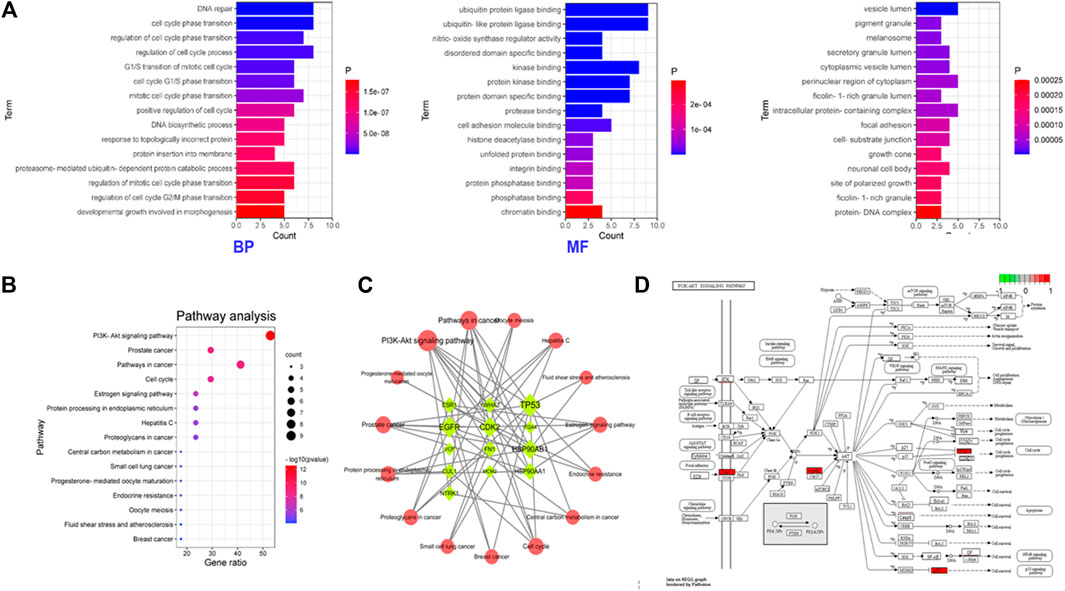
FIGURE 4. Functional enrichment of the core targets of BTP-DEGs. (A) GO enrichment. (B) KEGG enrichment. (C) Network of Key Targets-Pathway. (D) Pathway map of key targets.
Based on the results of KEGG enrichment, as shown in Table 4, multiple pathways jointly contributed to the antiONFH effect of BTP, including the PI3k-Akt signaling pathway, prostate cancer, pathways in cancer, the cell cycle, the estrogen signaling pathway, protein processing in the endoplasmic reticulum, hepatitis c, proteoglycans in cancer, the central carbon metabolism in cancer, small-cell lung cancer, progesterone-mediated oocyte maturation, endocrine resistance, oocyte meiosis, fluid shear stress and atherosclerosis, and breast cancer. Thus, it suggested that the antiONFH effect of BTP might associate with a synergistic effect of multiple pathways.
In conformity with the results of GO enrichment, the biological processes (BPs) in which BTP chiefly participated involved DNA repair, cell cycle phase transition, regulation of cell cycle phase transition, regulation of the cell cycle process, mitotic cell cycle G1/S transition, cell cycle G1/S phase transition, mitotic cell cycle phase transition, positive regulation of the cell cycle, the DNA biosynthetic process, response to topologically incorrect protein, protein insertion into the membrane, the proteasome-mediated ubiquitin-dependent protein catabolic process, regulation of mitotic cell cycle phase transition, regulation of cell cycle G2/M phase transition, and developmental growth involved in morphogenesis.
Such effects were primarily reflected in several aspects, including ubiquitin-protein ligase binding, ubiquitin-like protein ligase binding, nitric-oxide synthase regulator activity, disordered domain specific binding, kinase binding, protein kinase binding, protein domain specific binding, protease binding, cell adhesion molecule binding, histone deacetylase binding, unfolded protein binding, integrin binding, protein phosphatase binding, phosphatase binding, and chromatin binding. The targets of this action were chiefly concentrated in multiple cell sites, including vesicle lumen, melanosomes, pigment granules, secretory granule lumen, cytoplasmic vesicle lumen, the perinuclear region of the cytoplasm, ficolin-1-rich granule lumen, the intracellular protein-containing complex, focal adhesion, the cell–substrate junction, growth cones, neuronal cell bodies, sites of polarized growth, ficolin-1-rich granules, and the protein-DNA complex.
Based on the above bioinformatics analysis, previous reports, and our preliminary experiments, we deduced that the molecular mechanisms of this TCM formula might attribute to PI3K/Akt-related cell apoptosis.
Construction of Key Targets-Pathway Network
The network of key targets-pathway was constructed with Cytoscape (3.7.2), as presented in Figure 3B. V represents the pathway, and the diamond represents the DEGs. The greater the significance, the larger the volume of the graph was. The results showed that the targets of TP53, EGFR, and CDK2 and the pathways of the PI3K-AKT signaling pathway and cell cycle were more critical in the entire network. Therefore, subsequent experiments chose these two pathways for verification.
Cytotoxic Effect of BTP on RAW264.7 Cells
The cytotoxicity of BTP on normal RAW 264.7 cells was assessed by CCK-8 assays. As shown in Figure 5, BTP at concentrations up to 200 mg/ml did not exhibit obvious cytotoxicity on RAW264.7 cells within 24 and 48 h of incubation.
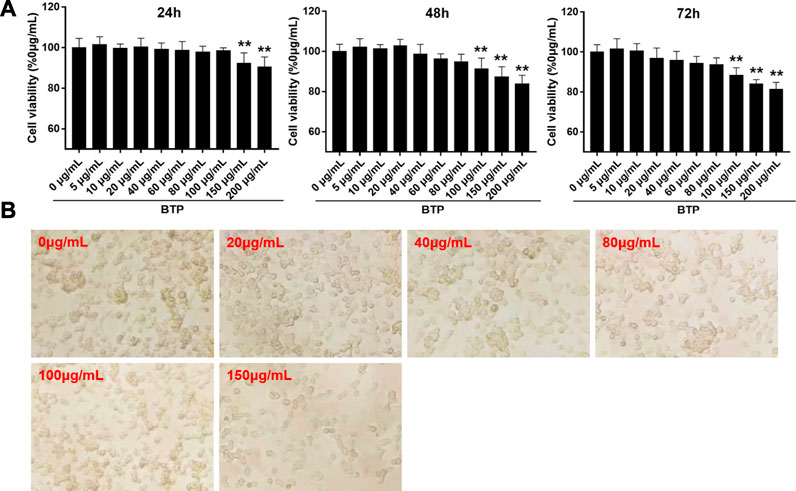
FIGURE 5. Cytotoxic effect of BTP on RAW264.7 cells. (A) Cell viability assays of RAW 264.7 cells treated with series doses of BTP at 24, 48, and 72 h, respectively. (B) Representative figures of RAW264.7 cells treated with series doses of BTP for 24 h.
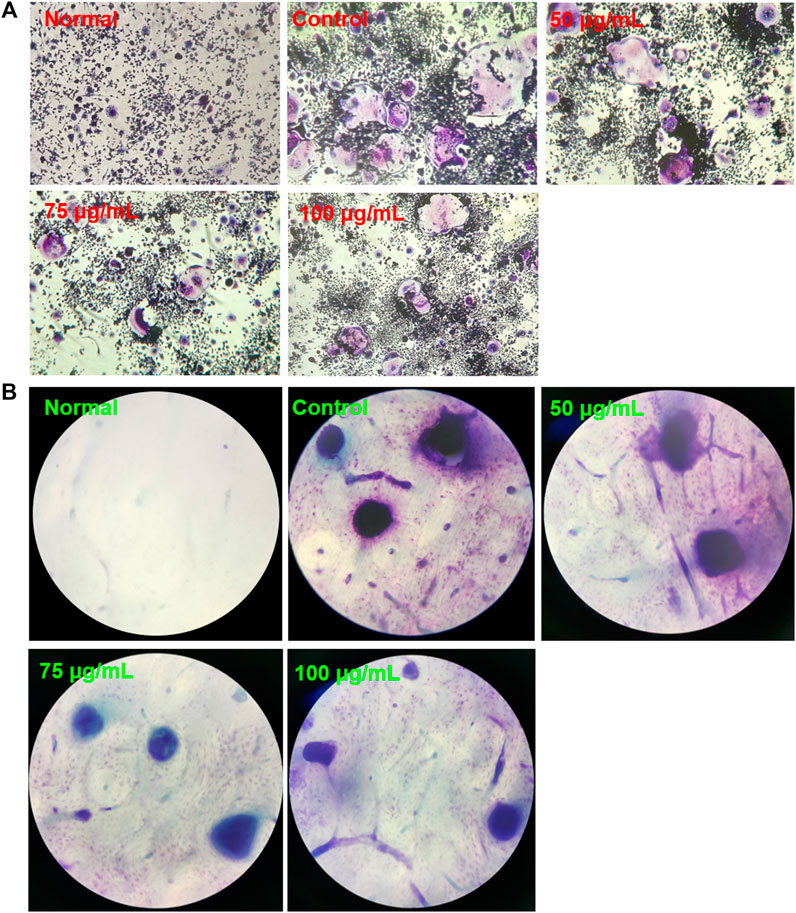
BTP Inhibits Osteoclastogenesis and Bone Resorption in Osteoclasts
At 7 days after inducting RAW 264.7 cells with RANKL (100 ng/ml), TRAP-positive multinucleated cells were observed under a light microscope, as shown in Figure 6. Interestingly, BTP at 20, 40, and 80 μg/ml markedly decreased the number of osteoclasts in a dose-dependent manner (p < 0.05 at 100 μg/ml, p < 0.005 at 200 μg/ml, p < 0.0001 at 400 μg/ml). In addition, the significant inhibitory effect of BTP on bone resorption in vitro has also been confirmed. As shown in Figure 8, compared with the control group, BTP dose-dependently reduced the area of bone resorption.
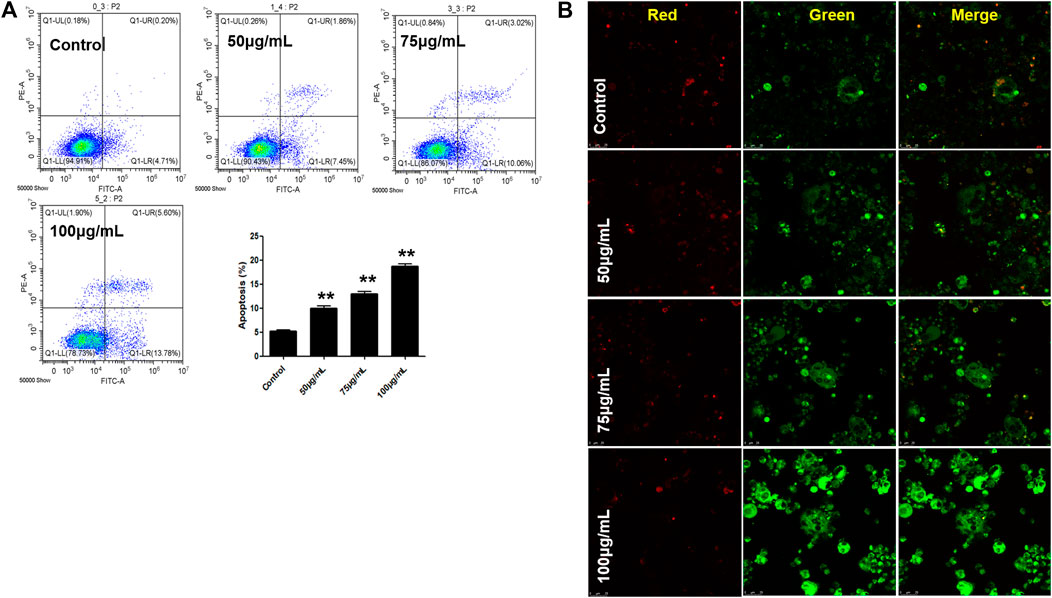
BTP Induces Cell Apoptosis in Osteoclasts
In this study, two comprehensively recognized cell apoptosis detection methods, including AO-EB double staining and FITC-conjugated Annexin V/PI staining, were carried out using a laser confocal microscope and flow cytometry analysis. Therefore, we further explored the apoptosis induced by BTP treatments. As shown in Figure 7A, our present results confirmed our hypothesis. After 5 days of RANKL treatment, the RAW 264.7 cells were induced to osteoclasts. As shown in Figure 7A, BTP at the concentrations of 50, 75, and 100 μg/ml could induce apoptosis in osteoclasts, compared to the control osteoclasts. Moreover, the results of immunofluorescence also presented the same trend. As far as we know, JC-1 aggregates in the mitochondrial matrix to form polymers that emit red fluorescence under normal physiological conditions and green fluorescence when MMP is reduced. Thus, the changes of ΔΨm could be directly reflected by the fluorescence transformation. In Figure 7B, compared with the control group, the green fluorescence of the experimental group was getting stronger, while the red fluorescence was getting weaker, indicating that MMP breakdown happened.
The results suggested that BTP (50, 75, and 100 μg/ml) could induce remarkable apoptosis in osteoclasts (RANKL induced RAW 264.7 cells). Furthermore, the apoptosis markers of C-caspase-3, bcl-2, and bax were determined. As shown in Figure 8, BTP could upregulate the expression of C-caspase three and bax, downregulating bcl-2 expression in osteoclasts in a dose-dependent manner.
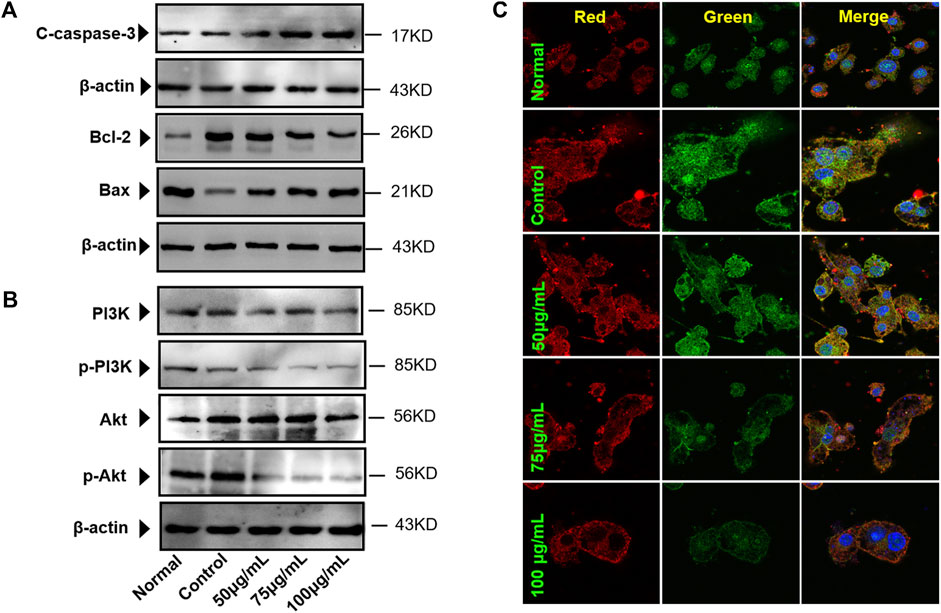
FIGURE 8. BTP regulates apoptotic proteins in osteoclasts. (A) Caspase-3, Bcl-2, and Bax (B) Akt and PI3K in osteoclasts. (C) Akt/p-Akt expressions in cells (× 400), p-Akt (green) and Akt (red).
BTP Regulates the PI3K/AKT Signal Pathway in Osteoclasts
The PI3K/Akt pathway is a classical signaling pathway responsible for regulating cell proliferation, differentiation, apoptosis, and a series of critical physiological activities. Nevertheless, only the phosphorylation of PI3K and Akt mainly participate in these physiological activities. Besides, growing numbers of studies have found that cell apoptosis is closely related to the PI3K/Akt pathway (Xue and Feng, 2018; Zhan and Yan, 2020). Therefore, we evaluated the effect of BTP on the PI3K/Akt pathway in osteoclasts. In the present study, both immunofluorescence and western blot assays were performed to determine the effect of BTP on the PI3K/Akt signaling pathway in osteoclasts. The results revealed that after pretreatment with BTP, the expression of p-Akt and p-PI3K was reduced, while no noticeable change was evident in the expression of AKT and PI3K compared to the control group (Figure 8B). Likewise, as presented in Figure 8C, the result of the immunofluorescence revealed that green fluorescence becomes weaker, indicating that the expression of p-Akt was reduced, and the red fluorescence (the expression of Akt) had no apparent change.
Discussion
It is widely known that TCM has shown broad application prospects in treating various intractable diseases and has become increasingly recognized globally (Zhang et al., 2021; Long et al., 2020). TCM also provides abundant resources for the development of new drugs. However, it remains a challenge to study the mechanisms of action of the TCM formula due to their characteristic of multicomponents, multitargets, and multipathways (Yu and Qi, 2020). Network pharmacology, first put forward by Hopkins in 2007, can analyze the connection of components, targets, and diseases in the network systematically and study the multilayer and multipathway mechanism of action of medications comprehensively, which coincides with the fundamental concept of holism in TCM (Hopkins, 2007; Sheng et al., 2020). Thus, it has become increasingly indispensable for performing qualified basic research of TCM formulas (Zhang et al., 2021). In addition, network pharmacology may realize “drug relocation” and explore new biological activities of drugs, thus expanding the application scope. As a result, it has been widely used in pharmacological research regarding TCM.
BTP has a long history of clinical applications for ONFH treatment and is composed of Angelica sinensis (Oliv.) Diels, Pheretima aspergillum (E.Perrier), Panax notoginseng (Burk.) F. H. Chen, Astragalus membranaceus (Fisch.) Bge, and Glycyrrhiza uralensis Fisch. This TCM formula has the effects of nourishing blood, promoting blood circulation, dredging collaterals, and relieving pain. Although the anti-ONFH effect of BTP has been well documented, its active components and mechanisms of action remain obscure and need further research. In this study, a total of 34 compounds were identified. Among these, six compounds (caffeic acid, pimpinellin, Z-ligustilide, ferulic acid, chlorogenic acid, and benzoic acid) belonged to Angelica sinensis (Oliv.) Diels, seven (ononin, biochanin a, formononetin, sucrose, calycosin-7-O-β-D-glucoside, asparagine, and daidzein) to Astragalus membranaceus (Fisch.)Bge, six (liquiritin, isoliquiritigenin, isoliquiritin, glycyrrhizic acid, licochalcone A, and licoricesaponin g2) to Glycyrrhiza uralensis Fisch., eight (caffeic acid, 3-coumaric acid, psoralen, gypenoside xvii, ginsenoside Rb1, and ginsenoside re) to Panax notoginseng (Burk.), and nine (succinic acid, hypoxanthine, dl-arginine, nicotinic acid, guanine, guanosine, phenylalanine, tryptophan, and inosine) to Pheretima aspergillum (E. Perrier). Formononetin and daidzein have been reported to inhibit RANKL-induced osteoclast differentiation and regulate osteogenic differentiation, indicating that they may become potential drugs for preventing and treating bone destruction in ONFH. In addition, another study has shown that the extract of Panax notoginseng (Burk.) could significantly promote the repair of the femoral head in rabbits with ONFH and improve the bone density and bone microstructure (Gui et al., 2019), which was also supported by clinical research (Jia et al., 2011).
KEGG is one of the most used tools to predict the possible molecular mechanisms of drugs, particularly herbal medicines. In our present study, the KEGG enrichment of the core target genes revealed that these genes were significantly enriched in the PI3K-AKT signaling pathway and cell cycle. Numerous studies have confirmed that cell apoptosis is often accompanied by growth arrest, suggesting that cell cycle arrest correlates with apoptosis closely (Duan et al., 2021; Zhang et al., 2021). When CAF and theobromine, which can shorten the G2/M block time, were added before the exposure, the ratio of the G2/M phase decreased with more noticeable increases in apoptosis. In contrast, when TPA, IBMX, and 3-aminoben extending the G2/M block time were added, the G2/M ratio increased, while the apoptosis ratio decreased (Zhao et al., 1999). Shinomiya et al. used CAF to reduce the G2 blockade of mouse lymphoma cells EL-4 caused by cis-diamminedichloroplatinum and promote the occurrence of apoptosis (Shinomiya et al., 1997). As a result, we designed a series of in vitro experiments to verify our hypothesis. In the present study, CCK-8 results showed that BTP had no toxicity to RAW 264.7 cells under 150 μg/ml for 24 h treatment.
Therefore, we applied the concentration of 50, 75, and 100 μg/ml to the follow-up experiments. TRAP staining results showed that BTP at 75 and 100 μg/ml inhibited the formation of TRAP-positive multinucleated cells. In addition, the area of bone resorption in vitro was also significantly suppressed, illustrating that BTP could inhibit the formation of osteoclasts. In addition, the results of flow cytometry revealed that BTP could induce remarkable apoptosis in osteoclasts (RANKL-induced RAW 264.7 cells) and decrease the mitochondrial membrane potential (MMOP, ΔΨ). Consequently, we determined the apoptosis-related proteins of C-caspase-3, Bax, and Bcl-2, and found that BTP could upregulate the proapoptosis proteins of C-caspase-3 and Bax but downregulate the antiapoptosis protein of Bcl-2, which is consistent with the flow cytometry and MMOP analysis. The PI3K-Akt signaling pathway is closely related to cell apoptosis and has been recognized as a potential target for regulating cell survival and apoptosis (Zhang et al., 2019). Therefore, we speculated that the treatment effects of BTP on ONFH might associate with PI3K/Akt-mediated apoptosis. In our bioinformatics analysis, the results indicated that the effects of BTP might be closely related to the PI3K/Akt signaling pathway, which is also an important upstream signaling for apoptosis. Therefore, we determined the expressions of PI3K/Akt signal-related proteins, including PI3K, phosphorylation (p)-PI3K, Akt, and p-Akt. Our results demonstrated that BTP treatment could downregulate the expressions of p-PI3K and p-Akt, subsequently resulting in cell apoptosis of osteoclasts (RANKL induced RAW 264.7 cells).
Conclusion
In conclusion, our present results suggested that BTP treatment could be beneficial for the osteonecrosis of the femoral head (ONFH), and the possible mechanisms are related to suppressing the formation of osteoclasts and induction of apoptosis in osteoclasts via regulation of the PI3K/Akt signaling pathway (Calder et al., 2004, Dhomen et al., 2012, Huang et al., 2004, Hua, 2017, Kimura et al., 2006, Lanaya et al., 2014, Linder et al., 2018, Lv et al., 2021, Mettey et al., 2003, Okuma et al., 2008, Poeta et al., 2007, Roovers et al., 2007, Ren et al., 2013, Savio et al., 2014, Schneider et al., 2009, Soussi, 2000, Xu et al., 2020, Yu et al., 2020, Zhang et al., 2014).
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: PRIDE (Proteomics Identification Database, https://www.ebi.ac.uk/pride/archive). The Project Name: Analysis of components of Buxuetongluo Pills by LC-MS/MS, Project accession: PXD027269.
Author Contributions
All authors contributed to the article and approved the submitted version. YHC, QR, and XPT conceived and designed this paper. DW, YCL, DT completed the main experiment content. HL, RL processed all the pictures in the article. DW and YCL analyzed the data and wrote the original draft of the paper. YHC reviewed and edited the paper. Additionally, WS helped complete the test of osteogenic differentiation and bone resorption, and SJ helped complete all the confocal imaging.
Funding
This research was supported by the Sichuan Science and Technology Program (Grant No. 2020YFS0525), the International Cooperation and Exchange Project of Sichuan Provincial Science and Technology Department (Grant No. 2017HH0004), the National Natural Science Foundation of China (Grant No. 81603537), the Sichuan Provincial Administration of Traditional Chinese Medicine (Grant No. 2021MS464), and the Youth Scholarship of Chengdu University of Traditional Chinese Medicine (Grant No. QNXZ2019043).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
References
Calder, J. D., Buttery, L., Revell, P. A., Pearse, M., and Polak, J. M. (2004). Apoptosis--a Significant Cause of Bone Cell Death in Osteonecrosis of the Femoral Head. J. Bone Jt. Surg Br 86 (8), 1209–1213. doi:10.1302/0301-620x.86b8.14834
Chen, L., Qi, J., Chang, Y. X., Zhu, D., and Yu, B. (2009). Identification and Determination of the Major Constituents in Traditional Chinese Medicinal Formula Danggui-Shaoyao-San by HPLC-DAD-ESI-MS/MS. J. Pharm. Biomed. Anal. 50 (2), 127–137. doi:10.1016/j.jpba.2009.03.039
Clough, E., and Barrett, T. (2016). The Gene Expression Omnibus Database. Methods Mol. Biol. 1418, 93–110. doi:10.1007/978-1-4939-3578-9_5
Cui, Y., Li, C., Zeng, C., Li, J., Zhu, Z., Chen, W., et al. (2018). Tongmai Yangxin Pills Anti-oxidative Stress Alleviates Cisplatin-Induced Cardiotoxicity: Network Pharmacology Analysis and Experimental Evidence. Biomed. Pharmacother. 108, 1081–1089. doi:10.1016/j.biopha.2018.09.095
Daina, A., Michielin, O., and Zoete, V. (2019). SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 47 (W1), W357–W364. doi:10.1093/nar/gkz382
Dhomen, N. S., Mariadason, J., Tebbutt, N., and Scott, A. M. (2012). Therapeutic Targeting of the Epidermal Growth Factor Receptor in Human Cancer. Crit. Rev. Oncog 17 (1), 31–50. doi:10.1615/critrevoncog.v17.i1.40
Duan, H., Zhang, Q., Liu, J., Li, R., Wang, D., Peng, W., et al. (2021). Suppression of Apoptosis in Vascular Endothelial Cell, the Promising Way for Natural Medicines to Treat Atherosclerosis. Pharmacol. Res. 168, 105599. doi:10.1016/j.phrs.2021.105599
Gui, X., Jiang, Z., Chen, B., Yu, J., and Yang, Y. (2019). Effect of Radix et Rhizoma Notoginseng on the microstructure of femoral head in rabbits with steroid-induced osteonecrosis of femoral head and Its Mechanism. J. New Chin. Med. 51 (12), 9–12.
Hopkins, A. L. (2007). Network Pharmacology. Nat. Biotechnol. 25(10), 1110–1111. doi: doi:10.1038/nbt1007-1110
Hopkins, A. L. (2008). Network Pharmacology: The Next Paradigm in Drug Discovery. Nat. Chem. Biol. 4 (11), 682–690. doi:10.1038/nchembio.118
Hua, Y. (2017). Expression of P53 Gene in the Blood of Patients with Alcohol-Induced Avascular Necrosis of the Femoral Head and its Mechanism on Osteoblast Apoptosis. Anat. Res 39 (02), 136–139.
Huang, da. W., Sherman, B. T., and Lempicki, R. A. (2009). Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 37 (1), 1–13. doi:10.1093/nar/gkn923
Huang, Y., Luo, X., Guo, K., An, H., and Jiang, D. (2004). Effect of Endogenous Glucocorticoid on Expression of P53 and Bcl-2 Proteins in Osteocyte Apoptosis in Avascular Necrosis of the Femoral Head Induced by Alcoholism. Guizhou. Med. J. 12, 1059–1062.
Jia, B., Fan, W., Chen, G., Li, Y., Zhang, G., Yang, Sh., et al. (2019). Development of UPLC-MS Combined with HPLC Technique to Explore the Effects of Exogenous Substances on the Saponin Contents and Growth of Panax Notoginseng Leaves. J. Yunnan Agric. Univ. 34 (01), 116–123. doi:10.12101/j.issn.1004-390X(n).201712011
Jia, F., Sun, R., Li, Y., Liu, B., Zhao, F., and Xiong, Y. (2011). Study on Prevention and Treatment of Femoral Head Necrosis after Femoral Neck Fracture with Sanqi Danshen Tablet. Chin. J. Orthop. Traumatol. 6, 12–14.
Kimura, H., Fujiwara, Y., Sone, T., Kunitoh, H., Tamura, T., Kasahara, K., et al. (2006). EGFR Mutation Status in Tumour-Derived DNA from Pleural Effusion Fluid Is a Practical Basis for Predicting the Response to Gefitinib. Br. J. Cancer 95 (10), 1390–1395. doi:10.1038/sj.bjc.6603428
Lanaya, H., Natarajan, A., Komposch, K., Li, L., Amberg, N., Chen, L., et al. (2014). EGFR Has a Tumour-Promoting Role in Liver Macrophages during Hepatocellular Carcinoma Formation. Nat. Cel Biol. 16 (10), 972–977. doi:10.1038/ncb3031
Linder, M., Hecking, M., Glitzner, E., Zwerina, K., Holcmann, M., Bakiri, L., et al. (2018). EGFR Controls Bone Development by Negatively Regulating mTOR-Signaling during Osteoblast Differentiation. Cell Death Differ 25 (6), 1094–1106. doi:10.1038/s41418-017-0054-7
Liu, J., Wang, X., Cai, S., Katsuko, K., and Tsuneo, N. (2004). Analysis of the Constituents in the Chinese Drug Notoginseng by Liquid Chromatography-Electrospray Mass Spectrometry. Chin. Pharm. J. 13 (4). doi:10.1021/ja961604z
Liu, L., Duan, J., and Tang, Y. (2017). Study on Chemical Component and Synergetic Effect of Concerted Application of Chinese Angelica and Safflower by Using UHPLC-Q-TOF-MS. Chin. J. Tradit. Chin. Med. Pharm. 3, 996–1000.
Liu, M. H., Tong, X., Wang, J. X., Zou, W., Cao, H., and Su, W. W. (2013). Rapid Separation and Identification of Multiple Constituents in Traditional Chinese Medicine Formula Shenqi Fuzheng Injection by Ultra-fast Liquid Chromatography Combined with Quadrupole-Time-Of-Flight Mass Spectrometry. J. Pharm. Biomed. Anal. 74, 141–155. doi:10.1016/j.jpba.2012.10.024
Liu, M., Hui, O., Jia, J., Huang, W., Guo, S., He, M., et al. (2019). Analysis of Anti-complement Activity and Active Ingredients of Gynura Procumbens by UPLC-Q-TOF-MS. Chin. Tradit Pat Med. 41 (11), 2681–2687.
Liu, Z., Jiang, M., Lu, X., Qin, F., Song, Y., Wen, J., et al. (2012). Simultaneous Determination of Pimpinellin, Isopimpinellin and Phellopterin in Rat Plasma by a Validated UPLC-MS/MS and its Application to a Pharmacokinetic Study after Administration of Toddalia Asiatica Extract. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 891-892, 102–108. doi:10.1016/j.jchromb.2012.02.022
Luo, L., Gao, W., Zhang, Y., Liu, C., Wang, G., Wu, H., et al. (2020). Integrated Phytochemical Analysis Based on UPLC-MS and Network Pharmacology Approaches to Explore the Quality Control Markers for the Quality Assessment of Trifolium Pratense L. Molecules 25 (17), 3787. doi:10.3390/molecules25173787
Lv, W., Yu, M., Yang, Q., Kong, P., and Yan, B. (2021). Total Flavonoids of Rhizoma Drynariae Ameliorate Steroid-induced A-vascular N-ecrosis of the F-emoral H-ead via the PI3K/AKT P-athway. Mol. Med. Rep. 23 (5). doi:10.3892/mmr.2021.11984
Meng, X., Li, H., Song, F., Liu, C., Liu, Z., and Liu, S. (2009). Studies on Triterpenoids and Flavones inGlycyrrhiza uralensisFisch. By HPLC-ESI-MSnand FT-ICR-MSn. Chin. J. Chem. 27 (2), 299–305. doi:10.1002/cjoc.200990048
Mettey, Y., Gompel, M., Thomas, V., Garnier, M., Leost, M., Ceballos-Picot, I., et al. (2003). Aloisines, a New Family of CDK/GSK-3 Inhibitors. SAR Study, crystal Structure in Complex with CDK2, Enzyme Selectivity, and Cellular Effects. J. Med. Chem. 46 (2), 222–236. doi:10.1021/jm020319p
Okuma, C., Kaketa, T., Hikita, A., Matsuda, K., Nakamura, M., Nagase, Y., et al. (2008). Potential Involvement of P53 in Ischemia/reperfusion-Induced Osteonecrosis. J. Bone Miner Metab. 26 (6), 576–585. doi:10.1007/s00774-007-0849-6
Ping, C. (2021). Clinical Observation on Non-traumatic Femoral Head Necrosis Treated by Operation Combined with Huangqi Shenggu Decoction. J. Tradit Chin. Med. 37 (03), 427–429.
Poeta, M. L., Manola, J., Goldwasser, M. A., Forastiere, A., Benoit, N., Califano, J. A., et al. (2007). TP53 Mutations and Survival in Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 357 (25), 2552–2561. doi:10.1056/NEJMoa073770
Pouya, F., and Kerachian, M. A. (2015). Avascular Necrosis of the Femoral Head: Are Any Genes Involved?. Arch. Bone Jt. Surg. 3 (3), 149–155. doi:10.22038/abjs.2015.4294
Qing, Z., Xinyue, D., Ruolan, L., Jiayi, S., Jia, L., Wei, P., et al. (2021). Inducing Apoptosis and Suppressing Inflammatory Reactions in Synovial Fibroblasts Are Two Important Ways for Guizhi-Shaoyao-Zhimu Decoction against Rheumatoid Arthritis. J. Inflamm. Res. 14, 217–236. doi:10.2147/jir.s287242
Ren, Y., Zhu, Z., Zhang, W., Zhang, Y., and Zhang, H. (2013). HPLC-MS/MS Determination of Puerarin and Daidzein in Puerarin Root. Phys. Test. Chem Anal B 49 (04), 464–467.
Roovers, R. C., Laeremans, T., Huang, L., De Taeye, S., Verkleij, A. J., Revets, H., et al. (2007). Efficient Inhibition of EGFR Signaling and of Tumour Growth by Antagonistic Anti-EFGR Nanobodies. Cancer Immunol. Immunother. 56 (3), 303–317. doi:10.1007/s00262-006-0180-4
Savio, A. L., da Silva, G. N., de Camargo, E. A., and Salvadori, D. M. (2014). Cell Cycle Kinetics, Apoptosis Rates, DNA Damage and TP53 Gene Expression in Bladder Cancer Cells Treated with Allyl Isothiocyanate (Mustard Essential Oil). Mutat. Res. 762, 40–46. doi:10.1016/j.mrfmmm.2014.02.006
Schneider, M. R., Sibilia, M., and Erben, R. G. (2009). The EGFR Network in Bone Biology and Pathology. Trends Endocrinol. Metab. 20 (10), 517–524. doi:10.1016/j.tem.2009.06.008
Shan, J., Yang, R., Zhang, X., Shen, C., Shen, C., Ji, J., et al. (2018). Network Pharmacological Study of Antitussive and Expectorant Effective of Jiegeng Decotion. Chin. Tradit Herb Drugs 49, 3501–3508.
Sheng, S., Huang, Y., Yang, Z., Li, J., and Xu, F. (2020). Conventional Drugs in New Use: Recognition on Pharmacological Effects of Tanshinone IIA Based on Gene Expression Profile and Connectivity Map. Chin. J. Integr. Med. Cardio-/cerebrovascuiar Dis. 18 (18), 2967–2973.
Shinomiya, N., Takemura, T., Iwamoto, K., and Rokutanda, M. (1997). Caffeine Induces S-phase Apoptosis in Cis-Diamminedichloroplatinum-Treated Cells, whereas Cis-Diamminedichloroplatinum Induces a Block in G2/M. Cytometry 27 (4), 365–373. doi:10.1002/(sici)1097-0320(19970401)27:4<365:aid-cyto8>3.0.co;2-b
Soussi, T. (2000). The P53 Tumor Suppressor Gene: from Molecular Biology to Clinical Investigation. Ann. N. Y Acad. Sci. 910, 121–129. doi:10.1111/j.1749-6632.2000.tb06705.x
Sun, B., and Li, G. (2013). Observation on 38 Cases of Femoral Head Necrosis Treated with Bushen Huoxue Decoction. J. Tradit Chin. Med. 29 (12), 987–988.
Wang, S., Tong, Y., Yang, Y., and Shi, Y. (2020). Mechanism of Wuling Powder in Treatment of Diabetic Kidney Disease Based on Network Pharmacology. World Chin. Med. 15 (22), 3383–3390. doi:10.3969/j.issn.1673-7202.2020.22.004
Wu, H., Zhong, S., Peng, L., Wu, W., Li, Z., and Li, W. (2020). HPLC-ESI-MS/MS Analysis of Active Component Differences in astragalus before and after Honey-Fried. J. Chin. Mass. Spectr. Soc. 41 (06), 117–127. doi:10.7538/zpxb.2019.0121
Xia, W., Hu, S., Wang, M., Xu, F., Han, L., and Peng, D. (2021). Exploration of the Potential Mechanism of the Tao Hong Si Wu Decoction for the Treatment of Postpartum Blood Stasis Based on Network Pharmacology and In Vivo Experimental Verification. J. Ethnopharmacol. 268, 113641. doi:10.1016/j.jep.2020.113641
Xu, H., Chen, S., Zhang, H., Zou, Y., Zhao, J., Yu, J., et al. (2020). Network-based Analysis Reveals Novel Gene Signatures in the Peripheral Blood of Patients with Sporadic Nonsyndromic Thoracic Aortic Aneurysm. J. Cel Physiol 235 (3), 2478–2491. doi:10.1002/jcp.29152
Xu, Q., Guo, Q., Wang, C. X., Zhang, S., Wen, C. B., Sun, T., et al. (2021). Network Differentiation: a Computational Method of Pathogenesis Diagnosis in Traditional Chinese Medicine Based on Systems Science. Artif. Intell. Med. 118():102134, doi:10.1016/j.artmed.2021.102134
Xu, Q., Wang, X., Zhong, N., Wang, Y., Li, Z., and Cai, Y. (2021). A Network Pharmacology and Molecular Docking Study on Treatment Mechanism of Bacterial Dysentery of Huanglian-Huangqin-Huangbo Herb Pair. doi: doi:10.21203/rs.3.rs-147874/v1
Xue, X. H., Feng, Z. H., Li, Z. X., and Pan, X. Y. (2018). Salidroside Inhibits Steroid-Induced Avascular Necrosis of the Femoral Head via the PI3K/Akt Signaling Pathway: In Vitro and In Vivo Studies. Mol. Med. Rep. 17 (3), 3751–3757. doi:10.3892/mmr.2017.8349
Yan, Z., Zhan, J., Qi, W., Lin, J., Huang, Y., Xue, X., et al. (2020). The Protective Effect of Luteolin in Glucocorticoid-Induced Osteonecrosis of the Femoral Head. Front. Pharmacol. 11, 1195. doi:10.3389/fphar.2020.01195
Yang, W., Feng, C., Kong, D., Shi, X., Cui, Y., Liu, M., et al. (2010). Simultaneous and Sensitive Determination of Xanthotoxin, Psoralen, Isoimpinellin and Bergapten in Rat Plasma by Liquid Chromatography-Electrospray Ionization Mass Spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 878 (5-6), 575–582. doi:10.1016/j.jchromb.2009.12.035
Yu, L., Qiyue, Y., Yan, X., Yulu, Z., Jinyan, W., Songyu, L., et al. (2020). Nose to Brain Drug Delivery - a Promising Strategy for Active Components from Herbal Medicine for Treating Cerebral Ischemia Reperfusion. Pharmacol. Res. 159, 104795. doi:10.1016/j.phrs.2020.104795
Zhan, J., Yan, Z., Zhao, M., Qi, W., Lin, J., Lin, Z., et al. (2020). Allicin Inhibits Osteoblast Apoptosis and Steroid-Induced Necrosis of Femoral Head Progression by Activating the PI3K/AKT Pathway. Food Funct. 11, 7830–7841. doi:10.1039/D0FO00837K
Zhang, J.-Z., Gao, W.-Y., Gao, Y., Liu, D., Liu, D.-L., and Huang, L.-Q. (2011). Analysis of Influences of Spaceflight on Chemical Constituents in Licorice by HPLC-ESI-MS/MS. Acta Physiol. Plant 33, 2511–2520. doi:10.1007/s11738-011-0796-7
Zhang, Q., Duan, H. X., Li, R. L., Sun, J. Y., Liu, J., Peng, W., et al. (2021). Inducing Apoptosis and Suppressing Inflammatory Reactions in Synovial Fibroblasts Are Two Important Ways for Guizhi-Shaoyao-Zhimu Decoction against Rheumatoid Arthritis. J. Inflamm. Res. 14, 217–236. doi:10.2147/JIR.S287242
Zhang, Q., Liu, J., Duan, H., Li, R., Peng, W., and Wu, C. (2021). Activation of Nrf2/HO-1 Signaling: An Important Molecular Mechanism of Herbal Medicine in the Treatment of Atherosclerosis via the protection of Vascular Endothelial Cells from Oxidative Stress. J. Adv. Res. doi:10.1016/j.jare.2021.06.023
Zhang, Q., Liu, J., Zhang, M., Wei, S., Li, R., Gao, Y., et al. (2019). Apoptosis Induction of Fibroblast-like Synoviocytes Is an Important Molecular-Mechanism for Herbal Medicine along with its Active Components in Treating Rheumatoid Arthritis. Biomolecules 9, 795. doi:10.3390/biom9120795
Zhang, X., Qi, L. W., Yi, L., Li, P., Wen, X. D., and Yu, Q. T. (2008). Screening and Identification of Potential Bioactive Components in a Combined Prescription of Danggui Buxue Decoction Using Cell Extraction Coupled with High Performance Liquid Chromatography. Biomed. Chromatogr. 22 (2), 157–163. doi:10.1002/bmc.910
Zhang, X., Zhu, J., Liu, F., Li, Y., Chandra, A., Levin, L. S., et al. (2014). Reduced EGFR Signaling Enhances Cartilage Destruction in a Mouse Osteoarthritis Model. Bone. Res. 2, 14015. doi:10.1038/boneres.2014.15
Zhang, Y., Long, Y., Yu, S., Li, D., Yang, M., Guan, Y., et al. (2021). Natural Volatile Oils Derived from Herbal Medicines: A Promising Therapy Way for Treating Depressive Disorder. Pharmacol. Res. 164, 105376. doi:10.1016/j.phrs.2020.105376
Zhang, Y., Wei, W., Huo, J., and Wang, W. (2017). Serum Pharmacochemistry Study of Pheretima Aspergillum by UPLC-Q-TOF-MS. J. Chin. Med. Mat. 40 (004), 848–853.
Zhao, H. (2020). Clinical Observation on 47 Cases of Early Non-traumatic Femoral Head Necrosis Treated by Taohong Siwu Decoction Combined with Acupuncture. Chin. J. Ethn. Ethn. 29 (14), 99–101.
Zhao, Y., Liu, S., Zhang, C., Liu, D., and Zhang, T. (2016). Analysis on chemical constituents from Glycyrrhizae Radix et Rhizoma by HPLC-Q-TOF-M. Chin. Med. Mat 47 (12), 2061–2068. doi:10.7501/j.issn.0253-2670.2016.12.007
Zheng, H., Yang, E., Peng, H., Li, J., Chen, S., Zhou, J., et al. (2014). Gastrodin Prevents Steroid-Induced Osteonecrosis of the Femoral Head in Rats by Anti-apoptosis. Chin. Med. J. (Engl) 127 (22), 3926–3931. doi:10.3760/cma.j.issn.0366-6999.20141371
Keywords: buxue tongluo pill, osteonecrosis of the femoral head, GEO database, apoptosis, PI3K/AKT
Citation: Wang D, Liu Y, Tang D, Wei S, Sun J, Ruan L, He L, Li R, Ren Q, Tian X and Chen Y (2021) Induction of PI3K/Akt-Mediated Apoptosis in Osteoclasts Is a Key Approach for Buxue Tongluo Pills to Treat Osteonecrosis of the Femoral Head. Front. Pharmacol. 12:729909. doi: 10.3389/fphar.2021.729909
Received: 24 June 2021; Accepted: 04 October 2021;
Published: 29 November 2021.
Edited by:
Hong Zhang, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Xiaofei Zhang, Shaanxi University of Chinese Medicine, ChinaLei Chen, Guangdong Pharmaceutical University, China
Copyright © 2021 Wang, Liu, Tang, Wei, Sun, Ruan, He, Li, Ren, Tian and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Ren, NDAxNjI3NDA2QHFxLmNvbQ==; Xiaoping Tian, dGlhbnhpYW9waW5nQGNkdXRjbS5lZHUuY24=; Yunhui Chen, Y2hlbnl1bmh1aUBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Dan Wang1†
Dan Wang1† Ruolan Li
Ruolan Li Yunhui Chen
Yunhui Chen