
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 25 October 2021
Sec. Inflammation Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.721769
This article is part of the Research Topic Inflammatory immune disease: Molecular Mechanisms, Translational Approaches and Therapeutics View all 97 articles
Coronavirus disease (COVID-19) patients with cardiovascular and metabolic disorders have been found to have a high risk of developing severe conditions with high mortality, further affecting the prognosis of COVID-19. However, the effect of hypertension and angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blocker (ARB) agents on the clinical characteristics and inflammatory immune responses in COVID-19 patients is still undefined. In this study, 90 COVID-19 patients were divided into hypertension and nonhypertension groups. The hypertension group was divided into well-controlled and poorly controlled subgroups based on blood pressure levels; moreover, hypertensive patients were also divided into ACEI/ARB and non-ACEI/ARB subgroups according to the administration of ACEI/ARB antihypertensive agents. The clinical characteristics of and inflammatory immune biomarker levels in the different groups of COVID-19 patients were compared, and the association between the combined effect of hypertension with ACEI/ARB antihypertensive agents and the severity of COVID-19 was examined. The results showed that the levels of aminotransferase (AST) and hs-cTnI were higher in the hypertension group compared with the nonhypertension group. The long-term use of ACEI/ARB agents in patients had statistically significantly lower AST, low-density lipoprotein cholesterol (LDL-C), and oxygen uptake and lower white cell count, neutrophil count, and levels of CD4, CD8, CRP, and PCT but without statistical significance. In addition, compared with COVID-19 patients without hypertension, hypertensive patients without the use of ACEI/ARB had a higher risk of developing severity of COVID-19 (for poorly controlled patients: OR = 3.97, 95% CI = 1.03–15.30; for well-controlled patients: OR = 6.48, 95% CI = 1.77–23.81). Hypertension could cause organ damage in COVID-19 patients, but the long-term use of ACEI/ARB agents may be beneficial to alleviate this injury.
Coronavirus disease (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has developed into a high-risk pandemic that has affected the global population. By July 18, 2021, more than 190,000,000 COVID-19 patients have been confirmed worldwide (WHO, 2021). COVID-19 patients with cardiovascular conditions, especially hypertension, have been reported to be at a high risk of developing severe conditions and mortality, in turn affecting the prognosis of COVID-19 (Chen et al., 2020; Zhou et al., 2020; Li et al., 2020; Leung, 2020). Our previous study also provided evidence to confirm this (Xie et al., 2020). Among the well-known pathophysiological elements leading to essential hypertension, the renin–angiotensin–aldosterone system (RAAS) has a vital role (Lu et al., 2015). Many studies have also demonstrated that angiotensin (Ang) II and other components of the RAAS, such as Ang-(1–7) and aldosterone, contribute to the inflammatory response, which suggests that the activation or blockage of the RAAS is related to the inflammatory response in patients with COVID-19 (the RAAS system is presented in Figure 1) (Di Raimondo et al., 2012; El-Hashim et al., 2012). In addition, the dysregulation of immune response in patients with COVID-19 has been demonstrated (Leisman et al., 2020). Therefore, hypertension may contribute to the progression of COVID-19. Moreover, the angiotensin-converting enzyme 2 receptor (ACE2 receptor) in the RAAS is considered to play an important role. As a monocarboxypeptidase, it converts the vasoconstrictor Ang II to Ang-(1–7) and exerts a variety of organ-protective properties (Danser et al., 2020; Serfozo et al., 2020). However, the ACE2 receptor has also been reported to be a functional receptor for coronavirus-induced infection through binding of the spike protein of SARS-CoV-2 and ACE2 (Bosso et al., 2020). Current research has shown that an increase in the ACE2 receptor aids COVID-19 infection (Esler and Esler, 2020). In addition, ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are widely used as antihypertensive drugs, which can enhance ACE2 expression (Gottlieb et al., 1993; Bauer et al., 1995). However, there is insufficient clinical evidence regarding the negative effects of the long-term use of these drugs in COVID-19 patients. Therefore, arbitrarily adjusting the use of antihypertensive drugs is not recommended (Esler and Esler, 2020)
In this study, we described the clinical characteristics, inflammatory markers, and outcomes in COVID-19 patients with hypertension. We also preliminarily explored the effect of ACEI/ARB agents on the clinical characteristics of COVID-19. This study provided additional evidence for the beneficial effects of ACEI/ARB agents on COVID-19 in patients with hypertension.
This study enrolled 90 patients with confirmed COVID-19 infection from the Z6 and Z11 infectious departments of the Union Hospital Tongji Medical College of Huazhong University of Science and Technology. Hospital admission varied from February 15, 2020, to February 28, 2020, and information on all cases was recorded on March 14, 2020.COVID-19 was diagnosed based on the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia released by the National Health Commission of the PRC (Wei, 2020). Blood pressure was monitored and measured during hospitalization, with the patient seated. Hypertension was diagnosed either on the basis of blood pressure measurements (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg) at least twice during the first and second days after hospital admission or on the basis of whether the patient was undergoing hypotensive therapy. Of the enrolled patients, 31 (34.4%) had a confirmed diagnosis of hypertension. According to the current guidelines (Williams et al., 2018), well-controlled hypertension was defined as a blood pressure < 140/90 mmHg during the first and second days after hospital admission in COVID-19 patients with hypertension. Therefore, of the 31 COVID-19 patients with hypertension, 17 were classified as having well-controlled hypertension and 14 were classified as having poorly controlled hypertension. In addition, according to the antihypertensive history before hospital admission, we further divided the hypertension cases into ACEI/ARB (n = 6) and non-ACEI/ARB (n = 25) groups.
To diagnose COVID-19, throat swab samples obtained from all patients were subjected to real-time reverse transcriptase–polymerase chain reaction at least twice according to the protocol described previously (Huang et al., 2020). The test was performed by the Union Hospital Tongji Medical College of Huazhong University of Science and Technology.
The clinical classification of the severity of COVID-19 was based on the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia released by the National Health Commission of the PRC (Huang et al., 2020). Mild COVID-19 was defined as patients with mild symptoms and no imaging abnormalities. Moderate COVID-19 was defined as patients with respiratory infection symptoms, such as fever, cough, and pneumonia manifestation on imaging. Severe COVID-19 was defined as patients with a respiratory rate of ≥30 breaths/min, a resting fingertip oxygen saturation of ≤ 93%, or an oxygen partial pressure (PaO2)/fraction of inspired O2 (FiO2) of ≤ 300 mmHg (1 mmHg = 0.133 kPa). Critical COVID-19 was defined as patients with respiratory failure requiring mechanical ventilation, symptoms of shock, or multiple organ dysfunction requiring intensive care. In our study, mild and moderate cases were categorized as the nonsevere group and severe and critical cases were categorized as the severe group.
All data were collected from electronic medical records. Epidemic data included age, sex, signs and symptoms, comorbidities, and medication history. Laboratory findings included routine blood tests, arterial blood gas, cardiac markers, renal and liver function, blood lipid levels, and immune indicators. Computed tomography on admission was performed for radiological assessment. Oxygen inhalation and the use of antibiotics were recorded.
The data analysis was performed using SPSS software version 24.0. Continuous variables with a nonnormal distribution are presented as median (M) and interquartile range (IQR). Discontinuous variables are described as numbers (n) and percentages. Clinical characteristics and inflammatory immune biomarker levels were first compared between COVID-19 patients with different conditions of hypertension. In addition, these comparisons were also performed between COVID-19 patients with different ACEI/ARB use conditions. All comparisons were conducted using nonparametric tests or Fisher’s exact probability tests. All significance levels were further adjusted using the Bonferroni correction for pairwise comparisons among the three groups. Univariate logistic regressions were performed to preliminarily determine the factors associated with the severity of COVID-19. Multivariate logistic regression was conducted to examine the association between the blood pressure control, ACEI/ARB use, and the severity of COVID-19 adjusted according to age and sex. All statistical tests were two-sided, and p-values less than 0.05 indicated statistical significance.
The median age of the 90 patients was 64.00 years (IQR: 55.50–73.00). Of the included cases, 24 (26.7%) were severe cases. There were 31 (34.4%) patients with hypertension, of which 16 (51.7%) were women and 15 (48.4%) were severe cases. The most documented comorbidities in COVID-19 patients with hypertension were diabetes (32.3%), followed by coronary heart disease (29.0%), heart failure (6.5%), respiratory disease (6.5%), cancer (6.5%), and atrial fibrillation (3.2%). Compared with nonhypertensive patients, the prevalence of diabetes (32.3 vs 11.9%, p = 0.025) and coronary heart disease (29.0 vs 3.4%, p = 0.003) was significantly higher. The most common symptoms of the onset were fever (74.4%) and cough (47.8%) in all COVID-19 patients. In the subgroup of patients with hypertension, 71.0% had fever and 48.4% had cough (Table 1).
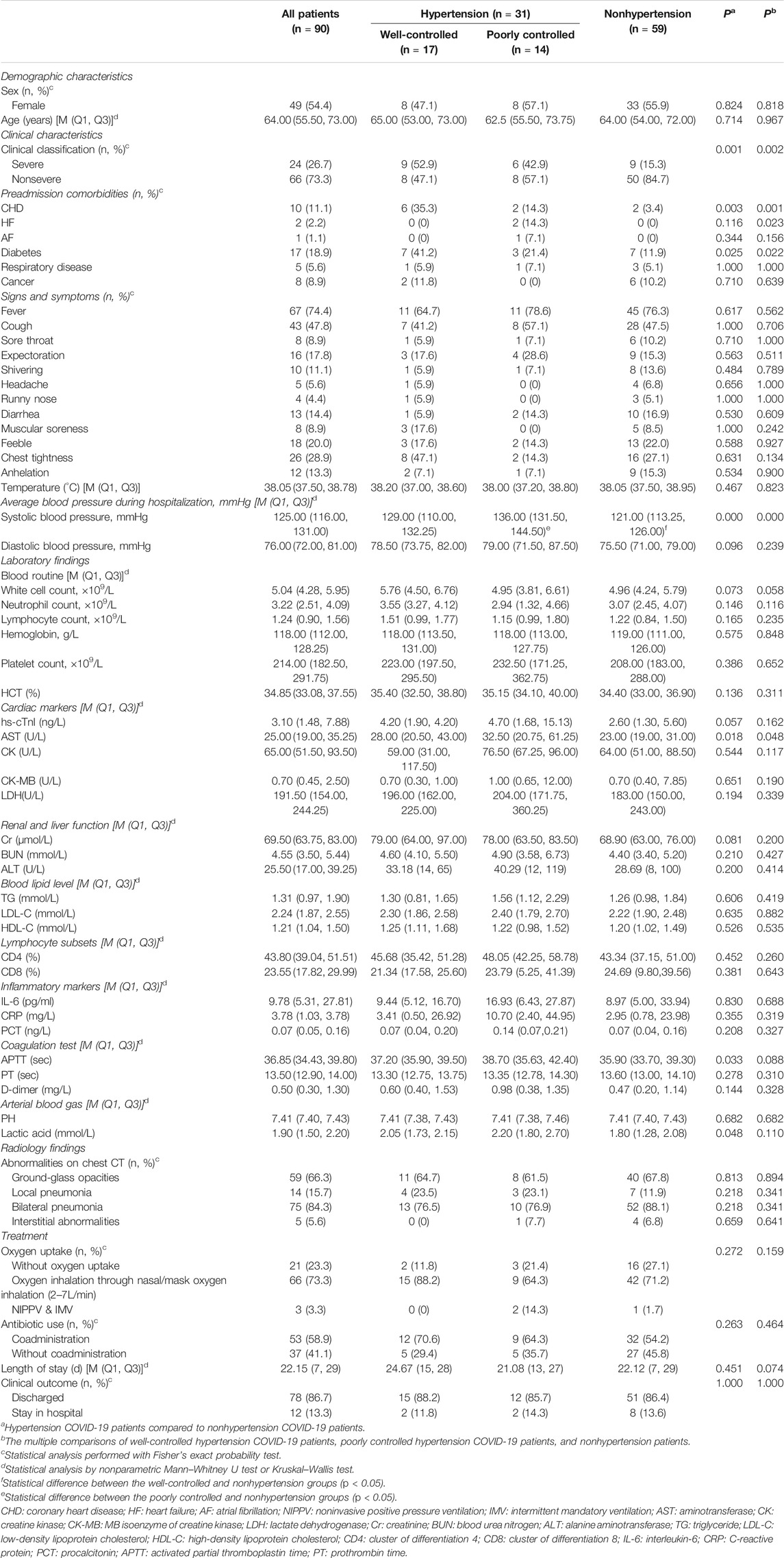
TABLE 1. The comparison of demographic and clinical characteristics, laboratory and radiology findings, clinical measures, clinical outcomes, and severity of COVID-19 among well-controlled, poorly controlled, and nonhypertension groups.
There was no statistical difference in sex ratio and age between the well-controlled, poorly controlled, and nonhypertensive groups. The most documented comorbidity among the three groups was diabetes. The well-controlled hypertension group had a significantly higher prevalence of coronary disease, heart failure, and diabetes mellitus than the other two groups. The comparison between well-controlled hypertension and nonhypertension cases showed a statistically significant difference in the prevalence of coronary heart disease (35.3 vs 3.4%) and diabetes mellitus (41.2 vs 11.9%). There was a statistically significant difference in the prevalence of heart failure between patients with poorly controlled hypertension and those without hypertension (14.3 vs 0%). The ratio of severe COVID-19 in the well-controlled hypertension group was significantly higher than that in the nonhypertension group (Table 1).
There was no significant difference in the constituent sex ratio and average age among the ACEI/ARB, non-ACEI/ARB, and nonhypertensive groups. However, the comorbidities of coronary heart disease (28 vs 3.4%, p = 0.004) were significantly higher in the non-ACEI/ARB group. And heart failure (16.7 vs 0%, p = 0.041) was significantly higher in the ACEI/ARB group. In addition, there was also a statistically higher prevalence of coronary heart disease (35.3 vs 3.4%) and heart failure (16.7 vs 0%) in the ACEI/ARB group than in the nonhypertensive group. In terms of signs and symptoms, there was a statistically significant difference in shivering between the ACEI/ARB and nonhypertensive groups (33.3 vs 0%). The ratio of severe COVID-19 in the non-ACEI/ARB group was significantly higher than that in the nonhypertensive group (Table 2).
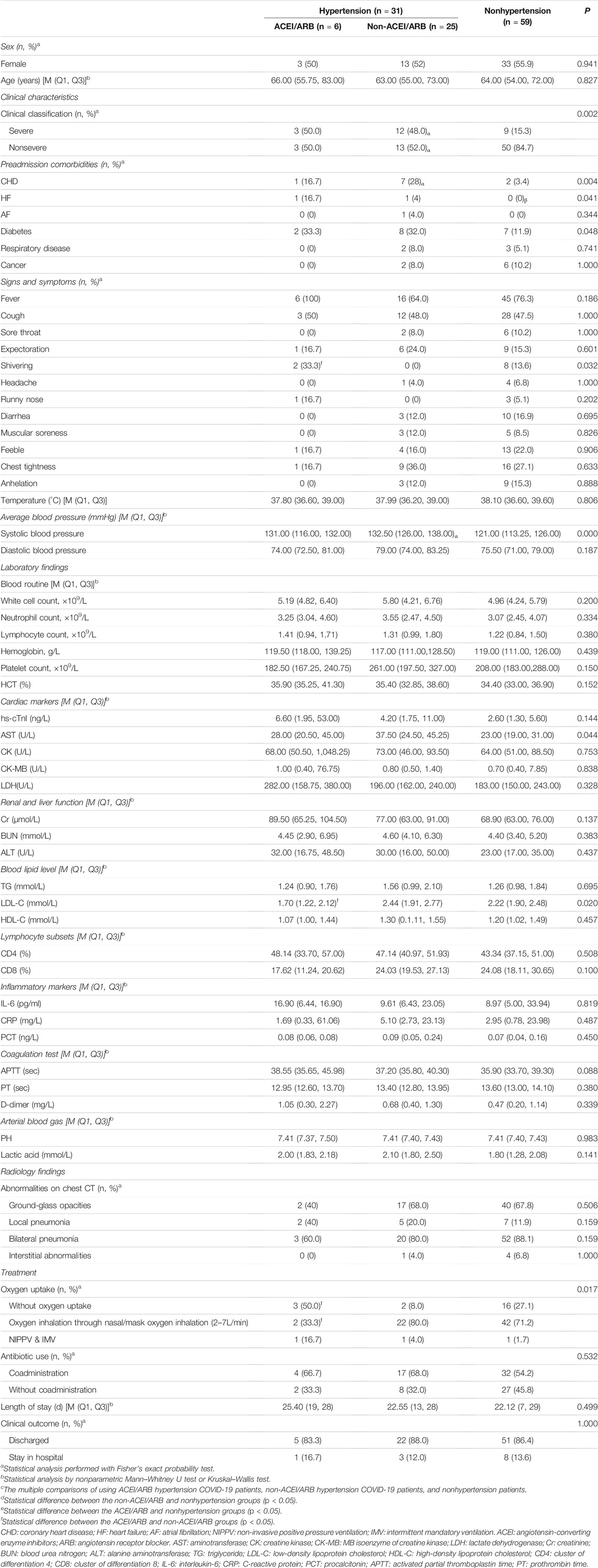
TABLE 2. The comparison of demographic and clinical characteristics, laboratory and radiology findings, clinical measures, clinical outcomes, and severity of COVID-19 among ACEI/ARB, non-ACEI/ARB, and nonhypertension groups.
All patients with hypertension were documented using several kinds of antihypertensive drugs. Among patients using ACEI/ARB agents before hospitalization, 3 (50%) used ACEI agents, whereas the other 3 (50%) used ARB agents. Among those not using ACEI/ARB agents, 14 (56%) patients used calcium channel blockers, 5 (20%) used β-receptor blockers, 1 (4%) used adrenoceptor blocking agents, and 3 (12%) used diuretics. In addition, 6 (24%) patients did not use antihypertensive drugs before hospitalization. Moreover, 30 patients received antihypertensive treatment after admission.
The average blood pressure during hospitalization was recorded for each group. The average blood pressure was found to be statistically different between the hypertension and nonhypertension groups. The average blood pressure during hospitalization showed no statistical difference between well-controlled and poorly controlled groups. Similarly, there was also no statistical difference in the average blood pressure during hospitalization for comparison between ACEI/ARB and non-ACEI/ARB groups (Tables 1 and 2).
The level of aspartate aminotransferase (AST) was significantly higher in the hypertensive group than in the nonhypertensive group (p = 0.018). And there was borderline significance in the comparison of hs-cTnI levels between the hypertension and nonhypertension control groups (p = 0.057). For the coagulation test and arterial blood gas, activated partial thromboplastin time (APTT) and lactic acid levels were substantially lower in the nonhypertension group than in the hypertension group. In comparison among the three subgroups of well-controlled, poorly controlled, and nonhypertensive groups, the results showed a statistically significant difference in AST levels (Table 1).
For cardiac markers and blood lipid levels, AST showed statistically significant differences among the three groups, ACEI/ARB, non-ACEI/ARB, and nonhypertensive groups (28.00U/L vs 37.50U/L vs 23.00U/L, respectively; p = 0.044). The level of LDL-C was significantly higher in the non-ACEI/ARB group than in the ACEI/ARB group (2.44 mmol/L vs 1.70 mmol/L) (Table 2 and Figure 2).
The blood routine, lymphocyte subsets, and inflammatory markers in the three groups of patients were compared. The results of the comparison between the ACEI/ARB, non-ACEI/ARB, and nonhypertensive groups showed no statistical differences in white cell count (5.19/L vs 5.80/L vs 4.96 × 109/L, respectively; p = 0.200), neutrophil count (3.25/L vs 3.55/L vs 3.07 × 109/L, respectively; p = 0.334), and lymphocyte count (1.41/L vs 1.31/L vs 1.22 × 109/L, respectively; p = 0.380) (Table 2 and Figure 2).
Similarly, the comparison for lymphocyte subsets and inflammatory factors between the ACEI/ARB, non-ACEI/ARB, and nonhypertensive groups showed no statistical differences in CD4 (48.14 vs 47.14% vs 43.34%, respectively; p = 0.508), CD8 (17.62 vs 24.03% vs 24.08%, respectively; p = 0.100), IL-6 (16.90 pg/ml vs 9.61 pg/ml vs 8.97 pg/ml, respectively; p = 0.819), C-reactive protein (1.69 mg/L vs 5.10 mg/L vs 2.95 mg/L, respectively; p = 0.487), and procalcitonin (0.08 ng/L vs 0.09 ng/L vs 0.07 ng/L, respectively; p = 0.450) (Table 2 and Figure 2).
Of the 90 patients, 78 were discharged and 12 were transferred to another hospital because of ward closure. The clinical outcomes of COVID-19 patients were as follows: 27 (87.1%) hypertension cases and 51 (86.4%) nonhypertension cases were cured and discharged, and 4 (12.9%) hypertension cases and 8 (13.6%) nonhypertension cases remained in the hospital, including 2 (3.2%) patients requiring intubation and mechanical ventilation. Furthermore, there were no significant differences in oxygen uptake, length of stay, and clinical outcomes among the groups (Table 1).
The comparison of differences in oxygen uptake among the three groups showed statistically significant results (p = 0.017). The nonoxygen uptake cases in the ACEI/ARB group were significantly higher in the pairwise comparison than in the non-ACEI/ARB group (Table 2 and Figure 2).
Risk factors were identified through univariate logistic regression, which included sex, age, uncontrolled hypertension, and antihypertensive medication history. The results revealed that compared with patients without hypertension, poorly controlled (OR = 3.97, 95% CI = 1.03–15.30, p = 0.045) or well-controlled (OR = 6.48, 95% CI = 1.77–23.81, p = 0.005) hypertension patients who did not use ACEI/ARB antihypertensive agents had a higher risk of developing severe COVID-19. A multivariate logistic regression analysis showed similar findings (poorly controlled hypertension patients without using ACEI/ARB: OR = 4.03, 95% CI = 1.01–16.07; well-controlled hypertension patients without using ACEI/ARB: OR = 6.09, 95% CI = 1.77–26.48) (Table 3).
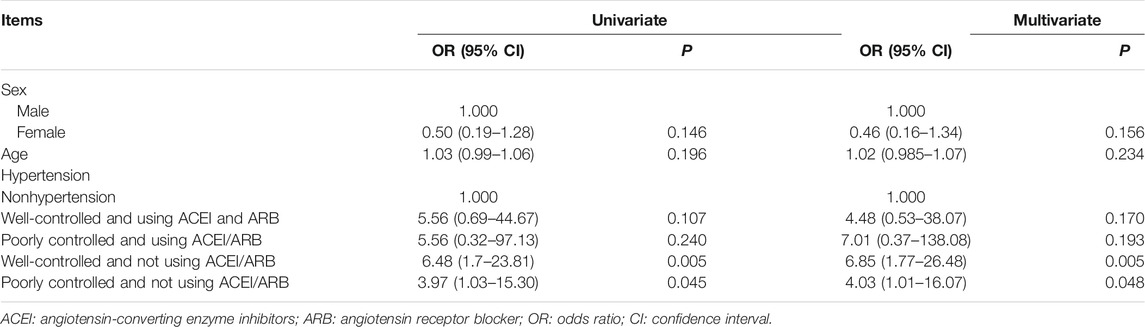
TABLE 3. Univariate logistic regression analysis and multivariate logistic regression analysis of severity of COVID-19-related factors.
This retrospective cohort study initially revealed the clinical characteristics and inflammatory immune responses of COVID-19 patients with and without hypertension. In this study, patients with hypertension were more likely to have increased oxygen demand, myocardial injury, and greater possibilities of developing severe COVID-19, suggesting that hypertension might play an important role in COVID-19. These findings are consistent with the idea that hypertension cases have an increased risk of comorbidity, infection, and multiple organ function damage (Volpe et al., 2015; Qaseem et al., 2017; Guo et al., 2020; Huang et al., 2020). Not only that, in the analysis of different subgroups, the result also showed a difference in the level of AST, LDL-C, and demand for oxygen among subgroups. However, no significant differences in lymphocyte subsets and inflammatory markers were found between the subgroups. These findings are in line with previous studies (Bansal, 2020; Kreutz et al., 2020; Lopes et al., 2021).
Hypertension is an important comorbidity in patients with COVID-19. The immune activation in hypertension patients that was largely augmented under COVID-19 raises a concern about the negative effect of hypertension COVID-19 patients (Trump et al., 2021). In our study, levels of aminotransferase (AST) and hs-cTnI were high in the hypertension group. Although the relationship between myocardial injury and COVID-19 with hypertension is underreported to date, hs-cTnI was to be a gold standard biomarker for diagnosing myocardial injury (Nigam, 2007). So it was theorized that COVID-19 patients with hypertension were more likely to develop myocardial injury. Moreover, people with hypertension also had a higher risk of developing the severity of COVID-19. Considering the impact of hypertension on COVID-19 patients, more attention should be paid to COVID-19 patients with hypertension in clinical practice. In addition, clinicians should pay more attention to the cardiac damage of COVID-19 patients with hypertension and early interventions are warranted.
The RAAS plays a major role in the pathophysiology of hypertension (Lu et al., 2015). SARS-CoV-2 uses ACE2 as a viral entry receptor, (Bosso et al., 2020), although there is no direct evidence for whether the use of RAAS inhibitors is beneficial to the treatment or prognosis of COVID-19. Animal experiments also showed that ACEI/ARBs treatment would increase the ACE2 expression (Trump et al., 2021). It was theorized that these medications could enhance viral binding and cell entry (Esler and Esler, 2020). But in our study, the levels of AST and hs-cTnI were high in the hypertension group, and the long-term use of ACEI/ARB had a statistically significantly lower AST, low-density lipoprotein cholesterol (LDL-C), and oxygen uptake and lower white cell count, neutrophil count, and levels of CD4, CD8, CRP, and PCT but without statistical significance. Contrary to the above theory, the long-term use of ACEI/ARB agents in patients with hypertension might also provide more protection to the lungs and other organs than the nonuse of ACEI/ARB. In addition, the long-term use of ACEI/ARB agents may suppress the inflammatory response in COVID-19 patients. SARS-CoV-2 is driven by ACE2 downregulation, leading to an array of complex and intertwined molecular interactions via at least four axes consisting of the dysregulation of the ACE2/angiotensin II/AT1R axis, attenuation of ACE2/MasR axis, increased activation of ACE2/bradykinin B1R/DABK axis, and activation of the complement cascades, resulting in a tornado of inflammatory cytokine responses (Tisoncik et al., 2012). However, RAAS inhibitors could benefit patients with COVID-19 through their effects on the angiotensin II expression and subsequent increases in Ang-(1–7) and (1–9), which have vasodilatory and anti-inflammatory effects that might attenuate lung injury, myocardial injury, and injury to other organs (Ingelfinger, 2009; Fraga-Silva et al., 2012; Verdecchia et al., 2012; Santos et al., 2013; Arendse et al., 2019). Although the role of RAAS blockers in patients with COVID-19 has not completely been determined, scientific societies have recommended that patients should not discontinue ACEI or ARB therapy during the COVID-19 pandemic (Celutkiene et al., 2020). The results of our study also support this view. Our clinical findings not only provided evidence on the impact of hypertension on COVID-19 but also provided new insights into the benefits of antihypertensive medication on COVID-19.
This study has several limitations. First, it was a retrospective study, and recall bias on testing or diagnosis may occur in some patients. Second, the relatively small number of patients with hypertension who are taking ACEI/ARBs might limit the extension of these results to a broader population. Third, the effect of ACEIs or ARBs on the susceptibility to COVID-19 was not studied because the focus was only on clinical outcomes in patients already been infected. Fourth, the duration and doses of the antihypertensive medication use were not systematically collected.
In conclusion, hypertension, especially poorly controlled hypertension, may play an important role in the severity of COVID-19. COVID-19 patients with hypertension are more likely to develop myocardial injury. The long-term use of ACEI/ARB agents might also provide more protection to the lungs and other organs, as well as suppress the inflammatory response in COVID-19 patients with hypertension. These findings suggest that the use of renin–angiotensin system blockers may be beneficial for COVID-19 patients with hypertension.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s), and minor(s) legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
CW and XF provided overall thinking. SC collected data, CW and LW analyzed data, DX and GQ analyzed results, CW, BW and GQ wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the Cancer Center, Union Hospital, Tongji Medical College, Huazhong Science and Technology University for the clinical testing. We also thank all the patients involved in this study.
Arendse, L. B., Danser, A. H. J., Poglitsch, M., Touyz, R. M., Burnett, J. C., Llorens-Cortes, C., et al. (2019). Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure. Pharmacol. Rev. 71, 539–570. doi:10.1124/pr.118.017129
Bansal, M. (2020). Cardiovascular Disease and COVID-19. Diabetes Metab. Syndr. 14, 247–250. doi:10.1016/j.dsx.2020.03.013
Bauer, I. H., Reams, G. P., Wu, Z., and Lau-Sieckman, A. (1995). Effects of Losartan on the Renin-Angiotensin-Aldosterone axis in Essential Hypertension. J. Hum. Hypertens. 9, 237–243.
Bosso, M., Thanaraj, T. A., Abu-Farha, M., Alanbaei, M., Abubaker, J., and Al-Mulla, F. (2020). The Two Faces of ACE2: The Role of ACE2 Receptor and its Polymorphisms in Hypertension and COVID-19. Mol. Ther. Methods Clin. Dev. 18, 321–327. doi:10.1016/j.omtm.2020.06.017
Celutkiene, J., Pudil, R., López-Fernández, T., Grapsa, J., Nihoyannopoulos, P., Bergler-Klein, J., et al. (2020). Role of Cardiovascular Imaging in Cancer Patients Receiving Cardiotoxic Therapies: a Position Statement on Behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 22, 1504–1524. doi:10.1002/ejhf.195710.1002/ejhf.1949
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 395, 507–513. doi:10.1016/S0140-6736(20)30211-7
Danser, A. H. J., Epstein, M., and Batlle, D. (2020). Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension 75, 1382–1385. doi:10.1161/HYPERTENSIONAHA.120.15082
Di Raimondo, D., Tuttolomondo, A., Buttà, C., Miceli, S., Licata, G., and Pinto, A. (2012). Effects of ACE-Inhibitors and Angiotensin Receptor Blockers on Inflammation. Curr. Pharm. Des. 18, 4385–4413. doi:10.2174/138161212802481282
El-Hashim, A. Z., Renno, W. M., Raghupathy, R., Abduo, H. T., Akhtar, S., and Benter, I. F. (2012). Angiotensin-(1-7) Inhibits Allergic Inflammation, via the MAS1 Receptor, through Suppression of ERK1/2- and NF-κb-Dependent Pathways. Br. J. Pharmacol. 166, 1964–1976. doi:10.1111/j.1476-5381.2012.01905.x
Esler, M., and Esler, D. (2020). Can Angiotensin Receptor-Blocking Drugs Perhaps Be Harmful in the COVID-19 Pandemic? J. Hypertens. 38, 781–782. doi:10.1097/HJH.0000000000002450
Fraga-Silva, R. A., Da Silva, D. G., Montecucco, F., Mach, F., Stergiopulos, N., da Silva, R. F., et al. (2012). The Angiotensin-Converting Enzyme 2/angiotensin-(1-7)/Mas Receptor axis: A Potential Target for Treating Thrombotic Diseases. Thromb. Haemost. 108, 1089–1096. doi:10.1160/TH12-06-0396
Gottlieb, S. S., Dickstein, K., Fleck, E., Kostis, J., Levine, T. B., LeJemtel, T., et al. (1993). Hemodynamic and Neurohormonal Effects of the Angiotensin II Antagonist Losartan in Patients with Congestive Heart Failure. Circulation 88, 1602–1609. doi:10.1161/01.cir.88.4.1602
Guo, T., Fan, Y., Chen, M., Wu, X., Zhang, L., He, T., et al. (2020). Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 5, 811–818. doi:10.1001/jamacardio.2020.1017
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 395, 497–506. doi:10.1016/S0140-6736(20)30183-5
Ingelfinger, J. R. (2009). Angiotensin-converting Enzyme 2: Implications for Blood Pressure and Kidney Disease. Curr. Opin. Nephrol. Hypertens. 18, 79–84. doi:10.1097/MNH.0b013e32831b70ad
Kreutz, R., Algharably, E. A. E.-H., Azizi, M., Dobrowolski, P., Guzik, T., Januszewicz, A., et al. (2020). Hypertension, the Renin-Angiotensin System, and the Risk of Lower Respiratory Tract Infections and Lung Injury: Implications for COVID-19. Cardiovasc. Res. 116, 1688–1699. doi:10.1093/cvr/cvaa097
Leisman, D. E., Deutschman, C. S., and Legrand, M. (2020). Facing COVID-19 in the ICU: Vascular Dysfunction, Thrombosis, and Dysregulated Inflammation. Intensive Care Med. 46, 1105–1108. doi:10.1007/s00134-020-06059-6
Leung, C. (2020). Clinical Features of Deaths in the Novel Coronavirus Epidemic in China. Rev. Med. Virol. 30, e2103. doi:10.1002/rmv.2103
Li, B., Yang, J., Zhao, F., Zhi, L., Wang, X., Liu, L., et al. (2020). Prevalence and Impact of Cardiovascular Metabolic Diseases on COVID-19 in China. Clin. Res. Cardiol. 109, 531–538. doi:10.1007/s00392-020-01626-9
Lopes, R. D., Macedo, A. V. S., de Barros E Silva, P. G. M., Moll-Bernardes, R. J., Dos Santos, T. M., Mazza, L., et al. (2021). Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted with COVID-19: A Randomized Clinical Trial. JAMA 325, 254–264. doi:10.1001/jama.2020.25864
Lu, X., Bi, Y. W., Chen, K. B., and Wang, H. Y. (2015). Protective Effect of Olmesartan against Cardiac Ischemia/reperfusion Injury in Spontaneously Hypertensive Rats. Exp. Ther. Med. 9, 2081–2087. doi:10.3892/etm.2015.2373
Nigam, P. K. (2007). Biochemical Markers of Myocardial Injury. Indian J. Clin. Biochem. 22, 10–17. doi:10.1007/BF02912874
Qaseem, A., Wilt, T. J., Rich, R., Humphrey, L. L., Frost, J., and Forciea, M. A. (2017). Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher versus Lower Blood Pressure Targets: A Clinical Practice Guideline from the American College of Physicians and the American Academy of Family Physicians. Ann. Intern. Med. 166, 430–437. doi:10.7326/M16-1785
Santos, R. A., Ferreira, A. J., Verano-Braga, T., and Bader, M. (2013). Angiotensin-converting Enzyme 2, Angiotensin-(1-7) and Mas: New Players of the Renin-Angiotensin System. J. Endocrinol. 216, R1–R17. doi:10.1530/JOE-12-0341
Serfozo, P., Wysocki, J., Gulua, G., Schulze, A., Ye, M., Liu, P., et al. (2020). Ang II (Angiotensin II) Conversion to Angiotensin-(1-7) in the Circulation Is POP (Prolyloligopeptidase)-Dependent and ACE2 (Angiotensin-Converting Enzyme 2)-Independent. Hypertension 75, 173–182. doi:10.1161/HYPERTENSIONAHA.119.14071
Tisoncik, J. R., Korth, M. J., Simmons, C. P., Farrar, J., Martin, T. R., and Katze, M. G. (2012). Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 76, 16–32. doi:10.1128/MMBR.05015-11
Trump, S., Lukassen, S., Anker, M. S., Chua, R. L., Liebig, J., Thürmann, L., et al. (2021). Hypertension Delays Viral Clearance and Exacerbates Airway Hyperinflammation in Patients with COVID-19. Nat. Biotechnol. 39, 705–716. doi:10.1038/s41587-020-00796-1
Verdecchia, P., Gentile, G., Angeli, F., and Reboldi, G. (2012). Beyond Blood Pressure: Evidence for Cardiovascular, Cerebrovascular, and Renal Protective Effects of Renin-Angiotensin System Blockers. Ther. Adv. Cardiovasc. Dis. 6, 81–91. doi:10.1177/1753944712444866
Volpe, M., Battistoni, A., Savoia, C., and Tocci, G. (2015). Understanding and Treating Hypertension in Diabetic Populations. Cardiovasc. Diagn. Ther. 5, 353–363. doi:10.3978/j.issn.2223-3652.2015.06.02
Wei, P-F. (2020). Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin. Med. J. (Engl) 133, 1087–1095. doi:10.1097/CM9.0000000000000819
WHO, (2021). Weekly Operational Update on COVID Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/publications/m/item/weekly-operational-update-on-covid. (Accessed July, 19-20 2021).
Williams, B., Mancia, G., Spiering, W., Rosei, E. A., Azizi, M., Burnier, M., et al. (2018). 2018 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Hypertension. J. Hypertens. 36 (12), 2284–2309. doi:10.1097/HJH.0000000000001961
Xie, Y., You, Q., Wu, C., Cao, S., Qu, G., Yan, X., et al. (2020). Impact of Cardiovascular Disease on Clinical Characteristics and Outcomes of Coronavirus Disease 2019 (COVID-19). Circ. J. 84, 1277–1283. doi:10.1253/circj.CJ-20-0348
Keywords: inflammatory immune responses, hypertension, RAAS blockers, COVID-19, myocardial injury
Citation: Wu C, Qu G, Wang L, Cao S, Xia D, Wang B, Fan X and Wang C (2021) Clinical Characteristics and Inflammatory Immune Responses in COVID-19 Patients With Hypertension: A Retrospective Study. Front. Pharmacol. 12:721769. doi: 10.3389/fphar.2021.721769
Received: 07 June 2021; Accepted: 18 August 2021;
Published: 25 October 2021.
Edited by:
Jian Gao, Shanghai Children’s Medical Center, ChinaReviewed by:
Zhiyu Ling, Chongqing Medical University, ChinaCopyright © 2021 Wu, Qu, Wang, Cao, Xia, Wang, Fan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changhui Wang, d2FuZ2NoYW5naHVpQGFobXUuZWR1LmNu; Xiaoyun Fan, MTM5NTY5ODg1NTJAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.