- 1Department of Gastroenterology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China
- 2Department of Medical Oncology, Shanghai Medical College, Fudan University Shanghai Cancer Center, Shanghai, China
- 3Department of Internal Medicine, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China
- 4Department of Radiation Oncology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China
- 5School of Medicine, Tsinghua University, Beijing, China
- 6Department of Oncology, Beijing Hospital, National Center of Gerontology, Beijing, China
- 7Department of Pharmacy, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China
- 8Department of Hematology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China
- 9Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Science, Beijing, China
- 10Tsinghua Clinical Research Institute, School of Medicine, Tsinghua University, Beijing, China
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment; however, immune-related adverse events (irAEs) in the gastrointestinal (GI) system commonly occur. In this study, data were obtained from the US Food and Drug Administration adverse event reporting system between July 2014 and December 2020. Colitis, hepatobiliary disorders, and pancreatitis were identified as irAEs in our study. Reporting odds ratio (ROR) with information components (IC) was adopted for disproportionate analysis. A total of 70,330 adverse events were reported during the selected period, 4,075 records of which were associated with ICIs. GI toxicities have been reportedly increased with ICI, with ROR025 of 17.2, 6.7, and 2.3 for colitis, hepatobiliary disorders, and pancreatitis, respectively. The risks of colitis, hepatobiliary disorders, and pancreatitis were higher with anti-CTLA-4 treatment than that with anti-PD-1 (ROR025 2.6, 1.3, and 1.1, respectively) or anti-PD-L1 treatment (ROR025 4.8, 1.3, and 1.3, respectively). Logistic analysis indicated that hepatobiliary disorders and pancreatitis more frequently occurred in female patients (adjusted odds ratio, 1.16 and 1.52; both p < 0.05). Consistently, polytherapy was a strong risk factor for colitis (adjusted odds ratio 2.52, p < 0.001), hepatobiliary disorders (adjusted odds ratio 2.50, p < 0.001), and pancreatitis (adjusted odds ratio 2.29, p < 0.001) according to multivariate logistic analysis. This pharmacovigilance analysis demonstrated an increased risk of all three GI irAEs associated with ICI therapies. The comparative analysis offered supportive insights on selecting GI irAEs for patients treated with ICIs.
Introduction
The increasing clinical use of approved antibodies against programmed cell death protein 1 (PD-1) (pembrolizumab, nivolumab, and cemiplimab), programmed death-ligand 1 (PD-L1) (avelumab, atezolizumab, and durvalumab), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) (ipilimumab) has changed the paradigm of cancer treatment (Ackermann et al., 2020). However, exaggerated immune responses and immune-related toxicities, known as immune-mediated adverse events (irAEs), decreased the patients’ survival. GI toxicities are the second most commonly reported irAEs, such as colitis, hepatotoxicity and biliary abnormality, and pancreatitis (Tan et al., 2020). As the most common type, colitis more commonly occurs after the administration of anti-CTLA-4 antibodies (7–12%) than that of anti-PD-1/PD-L1 antibodies (3%) (Davies and Duffield, 2017). This proportion increased to 12–18% when combining CTLA-4 and PD-1/PD-L1 inhibitors (Motzer et al., 2018). The incidence of immune-associated hepatotoxicity with either ipilimumab or nivolumab was approximately 5–10%, which was lower than that of ipilimumab plus nivolumab treatment (25–30%) (Larkin et al., 2015). Immune-related acute pancreatitis only occurs in <1% of patients (Friedman et al., 2017). Despite the low incidence, it rapidly progresses and is associated with high mortality. Currently, the majority of GI irAEs were detected in clinical trials examining single drugs, making the comparison difficult. Therefore, irAEs related to ICIs should be investigated in the real-world setting. This study characterized the safety profiles of ICIs and performed a disproportionality analysis through data-mining using the US Food and Drug Administration adverse event reporting system (FAERS), a postmarketing safety surveillance database, aimed at providing the new insights into GI toxicities associated with different ICIs or their combination.
Materials and Methods
Data Collection
In this pharmacovigilance study, disproportional analysis was performed in FAERS, which retrospectively collects adverse event (AR) reports submitted by patients, medical professionals, pharmaceutical manufacturers, and others to the FDA to monitor safety risks associated with marketed drugs and biologics. Data of this study were retrieved from the publicly released FAERS (https://www.open.fda.gov/) between July 1, 2014, and December 31, 2020.
Data Processing
Medications used in this study included PD-1 (nivolumab, pembrolizumab, and cemiplimab), PD-L1 (atezolizumab, avelumab, and durvalumab), and CTLA-4 blockade (ipilimumab) alone or in combination. Polytherapy was defined as the combined administration of an anti-CTLA-4 antibody plus an anti-PD-1/PD-L1 antibody. To identify ICI-related records, both brand names and generic names were used. Furthermore, AE reports in FAERS are coded using the preferred term (PT) according to the Medical Dictionary for Regulatory Activities Terminology (MedDRA). Despite the rarity of immune-related pancreatitis (1.9%) (George et al., 2019), immediate and appropriate management was vital to eliminate long-term toxicities. Thus, besides PTs of “colitis” and “hepatobiliary disorders,” PTs involving “pancreatitis” were also obtained from MedDRA version 20.0 (https://www.meddra.org/; details in Supplemental materials) in this study. Gender, age, year and country of reporting, and AE outcomes were also collected and analyzed.
Statistical Analysis
A disproportional analysis is a generally accepted mathematical algorithm used for calculating the association between a specific AE and a drug. We used either proportional reports reporting odds ratio (ROR) or Bayesian confidence propagation neural networks of information components (IC) to calculate disproportionality in this study. The relevant data-mining theory and calculation formulae have been described in detail in previous studies (Sakaeda et al., 2013; Zhai et al., 2019) when the entire database is used as a comparator. ROR was only used when comparing different treatment regimens. tROR025 is defined as significant with a lower 95% confidence interval (CI) boundary of >1 and with ≥3 patients. Conversely, the lower boundary 95%CI of >0 for IC (IC025) was deemed statistically significant. Demographic characteristics (age and gender) and treatment strategy (monotherapy or polytherapy) were used as covariates to predict risk factors for AEs by logistic regression analysis. All analyses were performed with SPSS version 23.0 (IBM Corporation, Armonk, NY, United States).
Results
Descriptive Analysis
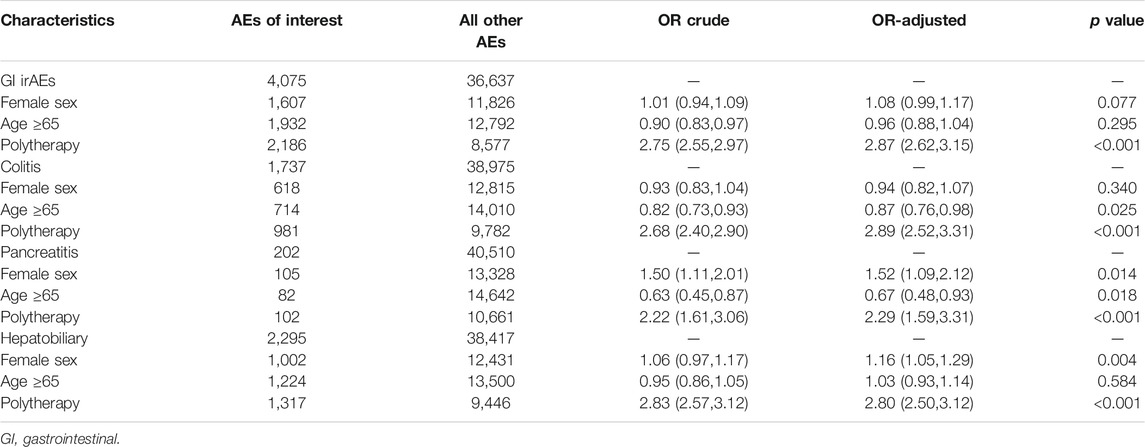
A total of 70,330 reports of gastrointestinal, hepatobiliary, and pancreatic toxicities were identified from FAERS between July 1, 2014, and December 31, 2020. Collectively, 4,075 cases were reported with ICI treatment, including nivolumab (n = 2,267), pembrolizumab (n = 964), cemiplimab (n = 8), atezolizumab (n = 241), avelumab (n = 22), durvalumab (n = 76), and ipilimumab (n = 1,615). The clinical features of events are presented in Table 1. The number of male patients with ICI-related AEs nearly doubled that of female patients (2,093 vs. 1,320 events). AEs were mainly reported from Japan (43.0%), followed by the United States (29.3%) and France (5.4%). Of all the outcomes reported, hospitalization (34.7%) and death (18.5%) were the most common, whereas life-threatening AEs occurred in 173 (4.2%) patients.
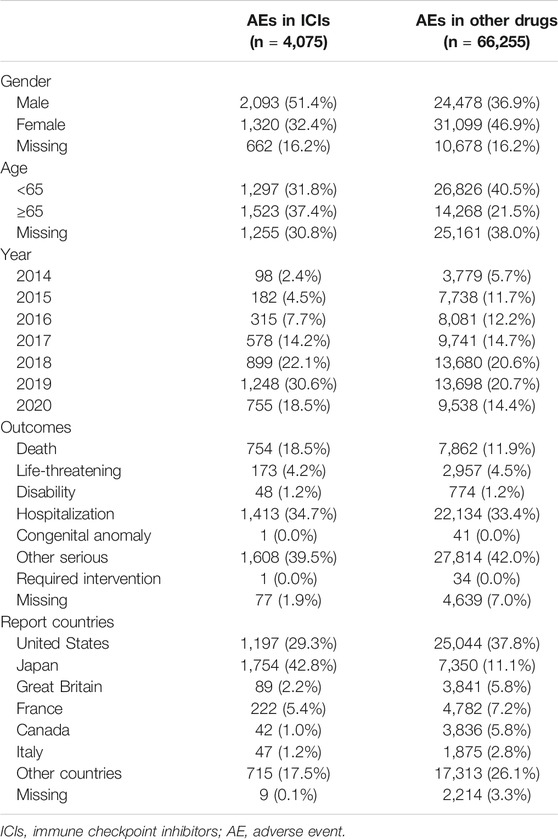
TABLE 1. Demographic and clinical characteristics of patients with ICIs-induced intestinal, hepatobiliary, and pancreatic toxicity.
Associations Between GI Disorders and ICIs
A disproportional analysis was performed to evaluate the associations of the occurrence of colitis, hepatobiliary disorders, or pancreatitis with ICI treatment. Overall, 1,737 colitis events were reported in the ICI group, including 1,229, 78, and 909 from anti-PD-1, anti-PD-L1, and anti-CTLA-4 drugs, respectively (Table 2). An increased risk of colitis was identified with ICI treatment (ROR025 17.2, IC025 3.9). The risk of colitis was higher in patients treated with anti-CTLA-4 antibodies than in those treated with anti-PD-1 (ROR025 2.6) or anti-PD-L1 antibodies (ROR025 4.8). As expected, colitis more frequently occurred in patients receiving anti-PD-1/PD-L1 plus anti-CTLA-4 antibodies (ROR025 1.7, IC025 0.5) than either single agent alone. Similarly, the risk of hepatobiliary disorders was also increased with ICI treatment (ROR025 6.7, IC025 2.6). Similar to colitis, the risk of hepatobiliary disorders in the anti-CTLA-4 antibodies group was speculated to be higher than PD-1/PD-L1 inhibitors (ROR025 1.3 and 1.3, respectively, Table 2). The risk was even higher in the combined anti-PD-1/PD-L1 and anti-CTLA-4 antibody treatment than that in monotherapy (ROR025 1.8). Regarding pancreatitis (Table 2), 202 reports were identified in the ICI group, indicating an increased risk (ROR025 2.3, IC025 1.1). Moreover, anti-PD-1/PD-L1 combined with anti-CTLA-4 inhibitors increased the number of patients treated with monotherapy (ROR025 1.2).
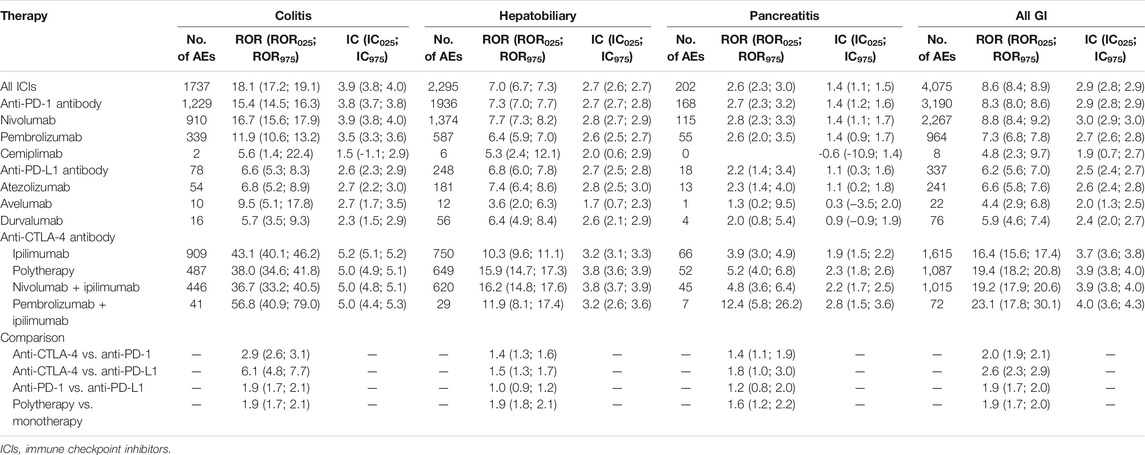
TABLE 2. The associations of induced intestinal, hepatobiliary, and pancreatic toxicity with different ICI regimens.
Risk Factors for GI irAEs
Logistic regression analysis was performed according to GI AE subtypes indicating polytherapy as a strong risk factor for colitis [adjusted odds ratio, 2.89 (95%CI: 2.52, 3.31), p < 0.001], hepatobiliary disorders [adjusted odds ratio, 2.80 (95%CI: 2.50, 3.12), p < 0.001], and pancreatitis [adjusted odds ratio, 2.29 (95%CI: 1.59, 3.31), p < 0.001] (Table 3). Hepatobiliary disorders more frequently occurred in female patients [adjusted odds ratio, 1.16 (95%CI: 1.05, 1.29); p = 0.004]. Meanwhile, patients aged ≥65 years were less likely to develop colitis [adjusted odds ratio, 0.87 (95%CI: 0.76, 0.98); p = 0.025] and pancreatitis [adjusted odds ratio, 0.67 (95%CI: 0.48, 0.93); p = 0.018]. The details of data are shown in Table 3.
Discussion
As an extensive pharmacovigilance analysis on GI irAEs after ICI treatments obtained from the FAERS database, this study demonstrated that increased risk of colitis, hepatobiliary abnormalities, and pancreatitis was associated with ICI monotherapy or combination therapies. Among those administered ICIs, PD-1 plus CTLA-4 polytherapy correlated with increased risk of these three GI irAEs. This comparative analysis provided abundant data on GI profiles of individual ICIs alone or combined, providing supportive safety insights in selecting specific ICI therapies for patients with preexisting GI disorders and identifying posttherapeutic GI irAEs.
Based on a previous meta-analysis, ipilimumab was reportedly correlated with a higher risk of high-grade colitis as compared with anti-PD-1/PD-L1 inhibitors (p = 0.021) (De Velasco et al., 2017). In line with this, the present study also showed that patients receiving ipilimumab were more likely to experience colitis. Moreover, the risk of colitis was also demonstrated to be higher with PD-1 than with PD-L1 inhibitors. As irAE onset has been indicated as a predictor for ICI treatment efficacy (Rogado et al., 2019; Zhou et al., 2020), rechallenging ICI treatment is an option for selected patients after discontinuation due to toxicity or clinical decision (Gobbini et al., 2020). The risk of colitis with different ICI agents may inform tailoring of ICI treatment for patients previously diagnosed with immune-related colitis.
Hepatobiliary disorders commonly occur in patients with cancer. A higher risk of immune-mediated hepatitis secondary to ICIs has been observed (Lin et al., 2020). The risk of increased aspartate aminotransferase level (relative risk 1.80, p = 0.020) has been reportedly associated with ICIs compared with non-ICI treatment (De Velasco et al., 2017). This study confirmed that liver injury may occur with ICI treatment and indicated no difference in hepatic transaminase elevation between CTLA-4 and PD-1/PD-L1 inhibitors.
Gender difference has been observed in the irAE incidence (Valpione et al., 2018; Duma et al., 2019). Recently, a large pharmacovigilance study indicated gender-related differences in endocrine irAEs, with a significantly lower occurrence of thyroid dysfunction in male patients (Morganstein et al., 2017; Zhai et al., 2019). The evidence of GI irAEs is increased further, showing that the occurrence of hepatobiliary disorders more frequently occurs in female patients. This gender difference may be associated with greater antigen-presenting activity, more frequent antibody expression, and higher sex hormone levels in female patients (Shen et al., 2016). Thus, enhanced immunoactivity after the ICI administration may result in increased toxicity in their male counterparts, which could be incorporated into safety evaluation, especially when rechallenging the ICI treatment.
Results in the relationship between age and irAE incidence have been reportedly inconsistent. A previous report demonstrated an increased likelihood (odds ratio, 5.4) of irAE-related hospitalization in patients aged >65 years (Balaji et al., 2019), whereas other reports suggested no increased risk of irAEs or irAE-related hospitalization in older patients administered with PD-1 antibody (Sattar et al., 2019; Ksienski et al., 2020). These inconsistencies could be due to the sample size of the study and the bias of different drugs. Based on the current population-based study, patients aged ≥65 years seem to be less likely to experience colitis and pancreatitis.
An increasing number of published studies have revealed that irAEs due to polytherapy occurred more frequently than those due to monotherapy. Consistent with previous observations, this study presents real-world evidence that combination treatment could result in a considerably higher rate of GI AEs secondary to ICI treatment (Boutros et al., 2016; Khoja et al., 2017). When treating patients with cancer currently treated with or previously exposed to ICIs with GI disorders, general practitioners and GI physicians should consider that this could be some irAE presentation. Medication history and patient demographics and characteristics should be carefully evaluated. Once irAEs are suspected, consultation with medical oncologists is necessary to manage these immune-related GI disorders.
The AE signal mining methods in this study consisted of three main categories: proportional disequilibrium, logistic regression modeling, and association rule mining. Furthermore, ROR and IC in the proportional imbalance algorithm were used for signals in reports obtained from the FAERS database (Dias et al., 2015). The limitations of this study are specific to the use of the FAERS database. Reports are spontaneous; therefore, exposure data may be lacking and cause under- and overreporting. Furthermore, due to the retrospective nature of pharmacovigilance databases, the causality should be cautiously interpreted.
Our real-world pharmacovigilance analysis demonstrated an increased risk of GI AEs due to ICIs. AE patterns greatly vary with different ICI regimens and patient characteristics. With the increasing use of ICIs, studies regarding irAEs and rechallenging ICIs are warranted in the following years to standardize management strategies, minimize irAE-related mortality, and thereby promote survival benefit to patients with cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XB, SJ, YZ and HZ contributed equally to this work. XB and YZ collected and analyzed the data. XB and SJ designed the research study and wrote the paper. HZ updated and re-analyzed the revised data, JJ contributed to the design of the study. YL, GR, YY, KS, and LW revised the draft. All authors have approved the final version of the manuscript.
Funding
The work was supported by the CAMS Innovation Fund for Medical Sciences (2016-I2M-3-001, 2019-I2M-2-007, 2021-1-I2M-001), the National Natural Science Foundation of China (81801632, 81970495), Natural Science Foundation of Beijing Municipality (No. 7202161), and the Fundamental Research Funds for the Center Universities (3332021007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.720776/full#supplementary-material
References
Ackermann, C. J., Adderley, H., Ortega-Franco, A., Khan, A., Reck, M., and Califano, R. (2020). First-line Immune Checkpoint Inhibition for Advanced Non-small-cell Lung Cancer: State of the Art and Future Directions. Drugs 80 (17), 1783–1797. doi:10.1007/s40265-020-01409-6
Balaji, A., Zhang, J., Wills, B., Marrone, K. A., Elmariah, H., Yarchoan, M., et al. (2019). Immune-related Adverse Events Requiring Hospitalization: Spectrum of Toxicity, Treatment, and Outcomes. J. Oncol. Pract. 15 (9), e825–e834. doi:10.1200/JOP.18.00703
Boutros, C., Tarhini, A., Routier, E., Lambotte, O., Ladurie, F. L., Carbonnel, F., et al. (2016). Safety Profiles of Anti-CTLA-4 and Anti-PD-1 Antibodies Alone and in Combination. Nat. Rev. Clin. Oncol. 13 (8), 473–486. doi:10.1038/nrclinonc.2016.58
Davies, M., and Duffield, E. A. (2017). Safety of Checkpoint Inhibitors for Cancer Treatment: Strategies for Patient Monitoring and Management of Immune-Mediated Adverse Events. Immunotargets Ther. 6, 51–71. doi:10.2147/ITT.S141577
De Velasco, G., Je, Y., Bossé, D., Awad, M. M., Ott, P. A., Moreira, R. B., et al. (2017). Comprehensive Meta-Analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 5 (4), 312–318. doi:10.1158/2326-6066.CIR-16-0237
Dias, P., Penedones, A., Alves, C., Ribeiro, C. F., and Marques, F. B. (2015). The Role of Disproportionality Analysis of Pharmacovigilance Databases in Safety Regulatory Actions: A Systematic Review. Curr. Drug Saf. 10 (3), 234–250. doi:10.2174/1574886310666150729112903
Duma, N., Abdel-Ghani, A., Yadav, S., Hoversten, K. P., Reed, C. T., Sitek, A. N., et al. (2019). Sex Differences in Tolerability to Anti-programmed Cell Death Protein 1 Therapy in Patients with Metastatic Melanoma and Non-small Cell Lung Cancer: Are We All Equal? Oncologist 24 (11), e1148–e1155. doi:10.1634/theoncologist.2019-0094
Friedman, C. F., Clark, V., Raikhel, A. V., Barz, T., Shoushtari, A. N., Momtaz, P., et al. (2017). Thinking Critically about Classifying Adverse Events: Incidence of Pancreatitis in Patients Treated with Nivolumab + Ipilimumab. J. Natl. Cancer Inst. 109 (4), djw260. doi:10.1093/jnci/djw260
George, J., Bajaj, D., Sankaramangalam, K., Yoo, J. W., Joshi, N. S., Gettinger, S., et al. (2019). Incidence of Pancreatitis with the Use of Immune Checkpoint Inhibitors (ICI) in Advanced Cancers: A Systematic Review and Meta-Analysis. Pancreatology 19 (4), 587–594. doi:10.1016/j.pan.2019.04.015
Gobbini, E., Toffart, A. C., Pérol, M., Assié, J. B., Duruisseaux, M., Coupez, D., et al. (2020). Immune Checkpoint Inhibitors Rechallenge Efficacy in Non-small-cell Lung Cancer Patients. Clin. Lung Cancer 21 (5), e497–e510. doi:10.1016/j.cllc.2020.04.013
Khoja, L., Day, D., Wei-Wu Chen, T., Siu, L. L., and Hansen, A. R. (2017). Tumour- and Class-specific Patterns of Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Ann. Oncol. 28 (10), 2377–2385. doi:10.1093/annonc/mdx286
Ksienski, D., Wai, E. S., Croteau, N. S., Freeman, A. T., Chan, A., Fiorino, L., et al. (2020). Association of Age with Differences in Immune Related Adverse Events and Survival of Patients with Advanced Nonsmall Cell Lung Cancer Receiving Pembrolizumab or Nivolumab. J. Geriatr. Oncol. 11 (5), 807–813. doi:10.1016/j.jgo.2020.01.006
Larkin, J., Hodi, F. S., and Wolchok, J. D. (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 373 (13), 1270–1271. doi:10.1056/NEJMc1509660
Lin, L. L., Lin, G. F., Yang, F., and Chen, X. Q. (2020). A Systematic Review and Meta-Analysis of Immune-Mediated Liver Dysfunction in Non-small Cell Lung Cancer. Int. Immunopharmacol. 83, 106537. doi:10.1016/j.intimp.2020.106537
Morganstein, D. L., Lai, Z., Spain, L., Diem, S., Levine, D., Mace, C., et al. (2017). Thyroid Abnormalities Following the Use of Cytotoxic T-Lymphocyte Antigen-4 and Programmed Death Receptor Protein-1 Inhibitors in the Treatment of Melanoma. Clin. Endocrinol. (Oxf) 86 (4), 614–620. doi:10.1111/cen.13297
Motzer, R. J., Tannir, N. M., McDermott, D. F., Arén Frontera, O., Melichar, B., Choueiri, T. K., et al. (2018). Nivolumab Plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 378 (14), 1277–1290. doi:10.1056/NEJMoa1712126
Rogado, J., Sánchez-Torres, J. M., Romero-Laorden, N., Ballesteros, A. I., Pacheco-Barcia, V., Ramos-Leví, A., et al. (2019). Immune-related Adverse Events Predict the Therapeutic Efficacy of Anti-PD-1 Antibodies in Cancer Patients. Eur. J. Cancer 109, 21–27. doi:10.1016/j.ejca.2018.10.014
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data Mining of the Public Version of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 10 (7), 796–803. doi:10.7150/ijms.6048
Sattar, J., Kartolo, A., Hopman, W. M., Lakoff, J. M., and Baetz, T. (2019). The Efficacy and Toxicity of Immune Checkpoint Inhibitors in a Real-World Older Patient Population. J. Geriatr. Oncol. 10 (3), 411–414. doi:10.1016/j.jgo.2018.07.015
Shen, Z., Rodriguez-Garcia, M., Patel, M. V., Barr, F. D., and Wira, C. R. (2016). Menopausal Status Influences the Expression of Programmed Death (PD)-1 and its Ligand PD-L1 on Immune Cells from the Human Female Reproductive Tract. Am. J. Reprod. Immunol. 76 (2), 118–125. doi:10.1111/aji.12532
Tan, B., Li, Y., Xu, Y., Chen, M., Wang, M., and Qian, J. (2020). Recognition and Management of the Gastrointestinal and Hepatic Immune-Related Adverse Events. Asia Pac. J. Clin. Oncol. 16 (3), 95–102. doi:10.1111/ajco.13317
Valpione, S., Pasquali, S., Campana, L. G., Piccin, L., Mocellin, S., Pigozzo, J., et al. (2018). Sex and Interleukin-6 Are Prognostic Factors for Autoimmune Toxicity Following Treatment with Anti-CTLA4 Blockade. J. Transl. Med. 16 (1), 94. doi:10.1186/s12967-018-1467-x
Zhai, Y., Ye, X., Hu, F., Xu, J., Guo, X., Zhuang, Y., et al. (2019). Endocrine Toxicity of Immune Checkpoint Inhibitors: A Real-World Study Leveraging Us Food and Drug Administration Adverse Events Reporting System. J. Immunother. Cancer 7 (1), 286. doi:10.1186/s40425-019-0754-2
Keywords: immune checkpoint inhibitors, digestive toxicities, fares, cancer, side effects
Citation: Bai X, Jiang S, Zhou Y, Zhen H, Ji J, Li Y, Ruan G, Yang Y, Shen K, Wang L, Li G and Yang H (2021) Common Immune-Related Adverse Events of Immune Checkpoint Inhibitors in the Gastrointestinal System: A Study Based on the US Food and Drug Administration Adverse Event Reporting System. Front. Pharmacol. 12:720776. doi: 10.3389/fphar.2021.720776
Received: 05 June 2021; Accepted: 26 October 2021;
Published: 29 November 2021.
Edited by:
Halina Was, Military Institute of Medicine, PolandReviewed by:
Kevin Tyan, Harvard Medical School, United StatesMaria-Ioanna Christodoulou, European University Cyprus, Cyprus
Copyright © 2021 Bai, Jiang, Zhou, Zhen, Ji, Li, Ruan, Yang, Shen, Wang, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Yang, WWFuZ2hAcHVtY2guY24=; Guanqiao Li, Z3VhbnFpYW9saUBtYWlsLnRzaW5naHVhLmVkdS5jbg==
†These authors have contributed equally to this work
 Xiaoyin Bai
Xiaoyin Bai Shiyu Jiang2†
Shiyu Jiang2† Yangzhong Zhou
Yangzhong Zhou Hongnan Zhen
Hongnan Zhen Junyi Ji
Junyi Ji Yi Li
Yi Li Yang Yang
Yang Yang Hong Yang
Hong Yang