- 1Master Program in Clinical Pharmacy, School of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan
- 2Department of Pharmacy, E-Da Hospital, Kaohsiung, Taiwan
- 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, and Department of Occupational Medicine, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University, Kaohsiung, Taiwan
- 4Department of Pharmacy, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 5Department of Pharmacy, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 6Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 7Center for Big Data Research, Kaohsiung Medical University, Kaohsiung, Taiwan
Background: There is limited data on the relative survival rate of first-line therapy of gefitinib, erlotinib (first-generation epidermal growth factor receptor-tyrosine kinase inhibitor [EGFR-TKI]), and afatinib (second-generation EGFR-TKI) in patients with EGFR-mutated advanced lung adenocarcinoma in real-world data, especially in the Asian population. This study aimed to compare the relative survival rate of gefitinib, erlotinib, and afatinib in patients with EGFR-mutated advanced lung adenocarcinoma by real-world data in Taiwan.
Methods: This retrospective cohort population-based study included untreated adult patients diagnosed with advanced lung adenocarcinoma who were identified in the Taiwan National Health Insurance Research Database between 2014 and 2017. The date of EGFR-mutated advanced lung adenocarcinoma diagnosis was referred as index date. This outcome evaluated overall survival (OS) and time to treatment failure (TTF) between gefitinib, erlotinib, and afatinib. Switching EGFR-TKIs or chemotherapy and new development of brain metastases were proxies of TTF. Estimated relative treatment effects on OS and TTF among EGFR-TKIs were adjusted by inverse probability of treatment weighting (IPTW) in Cox proportional hazards model. Propensity score (PS) matched pair analyses were performed as sensitivity analyses.
Results: The study cohort included 3,695 patients initiated with gefitinib, 3,310 with erlotinib, and 3,041 with afatinib. The mean age among the three treatment groups was 70.4 (±11.6), 66.8 (±11.6), and 64.3 (±11.4) years, and the female percentage was 70.4, 58.6, and 57.7%, respectively. Afatinib showed longer median OS than gefitinib (23.9 vs. 21.3 months; adjusted hazard ratio (aHR), 0.87; p < 0.001) and erlotinib (23.9 vs. 21.8 months; aHR, 0.87; p = 0.001). Consistent results were observed with TTF outcomes. For patients with brain metastases at diagnosis, afatinib showed similar OS with erlotinib (p = 0.917) but superior to gefitinib (p = 0.028). PS matching had similar results with IPTW adjustment in the study population.
Conclusion: Afatinib as first-line therapy had better survival outcomes for EGFR-mutated advanced lung adenocarcinoma than gefitinib and erlotinib in the Taiwan population. Both erlotinib and afatinib demonstrated superior treatment effect in patients with initial brain metastases than gefitinib.
Introduction
Lung cancer is the major cause of cancer-related death worldwide (Bray et al., 2018; Ministry of Health and Welfare, 2019). Epidermal growth factor receptor (EGFR) is one of the members of ErbB/HER transmembrane receptor family facilitating cellular regulation and proliferation (Chen et al., 2016). EGFR mutation is correlated to neoplasia, and the incidence in the Asian population is higher than that in the Western population (i.e., 57% in Taiwan, 15% in Europe; 22% in North America) (Midha et al., 2015). Of these, EGFR-tyrosine kinase inhibitor (TKI) was recommended by treatment guidelines as first-line treatment for EGFR-mutated advanced non-small cell lung cancer (Hanna et al., 2017; Clinical Practice Gu, 2018; Wu et al., 2019a).
The superior progression-free survival (PFS) of gefitinib, erlotinib (first-generation EGFR-TKI), and afatinib (second-generation EGFR-TKI) compared to platinum-based doublet therapy was confirmed in clinical trials (Maemondo et al., 2010; Mitsudomi et al., 2010; Zhou et al., 2011; Rosell et al., 2012; Sequist et al., 2013; Wu et al., 2014; Wu et al., 2015). Moreover, several trials had been conducted to investigate head-to-head comparison of EGFR-TKIs. The phase III CTONG 0901 trial that enrolled Chinese patients was designed to compare erlotinib and gefitinib, which indicated no significant difference in PFS (13.0 vs. 10.4 months; hazard ratio [HR], 0.81; 95% confidence interval [CI], 0.62–1.05; p = 0.108) and overall survival (OS) (22.9 vs. 20.1 months; HR, 0.84; 95% CI, 0.63–1.13, p = 0.250) (Yang et al., 2017). Phase IIb Lux-Lung seven trial compared afatinib and gefitinib and revealed longer PFS in the afatinib group (11.0 vs. 10.9 months; p = 0.017) but not in OS (27.9 vs. 24.5 months; p = 0.258). (Paz-Ares et al., 2017).
Nonetheless, the limited sample size of Asian ethnicity in Lux-Lung seven trial may be underpowered to demonstrate the OS discrepancy with gefitinib and afatinib. Second, patients with unstable brain metastases, cardiovascular abnormalities, or gastrointestinal disorders were excluded in trials that were prevalent in clinical practice. Third, erlotinib demonstrated a higher blood-brain barrier penetration rate than gefitinib and afatinib, which may be beneficial to patients with brain metastases. However, there was still lack of population-based evidence compared to erlotinib and afatinib in these patients (Ahluwalia et al., 2018). Lastly, certain nature of difference between trial design and real-world practice should be considered. To complement knowledge of clinical decision-making, real-world data (RWD) could accommodate a more comprehensive population with longer period to address the choice of preliminary EGFR-TKI treatment.
In Taiwan, gefitinib, erlotinib, and afatinib had been reimbursed in first-line treatment for advanced lung adenocarcinoma harboring EGFR mutation in 2011, 2013, and 2014, respectively. A retrospective study using population-based claims data between 2011 and 2015 showed that afatinib had superior OS than gefitinib (adjusted HR [aHR], 0.82; 95% CI, 0.72–0.93; p < 0.001) but not erlotinib (aHR, 0.95; 95% CI, 0.86–1.05, p = 0.159) (Hsieh et al., 2019). The study did not address brain metastases at diagnosis, and the limited observation period of afatinib might lack explanation for long-term survival effect. Furthermore, probable selection bias was presented in the imbalance of baseline characteristics.
This study applied the latest RWD to assess the relative survival rate and time to treatment failure (TTF) of gefitinib, erlotinib, and afatinib in first-line therapy for patients with advanced lung adenocarcinoma with EGFR mutation, and propensity score (PS) method was performed to adjust the estimated HR.
Materials and Methods
Data Source
The retrospective population-based cohort study was executed using the National Health Insurance Research Database (NHIRD), which covered >99% of the Taiwanese population. The NHIRD included the reimbursement records of inpatient and outpatient visit and emergency admission, which provided patient characteristics, disease diagnoses, and medical treatment. The National Health Insurance Administration (NHIA) collated the claims data yearly to ensure the quality for academic research.
Study Design and Patient Cohort
Adult patients diagnosed with lung cancer (ICD-9-CM, 162.x; ICD-10-CM, C33, C34) between 2014 and 2017 were identified, and the first prescription date of gefitinib, erlotinib, or afatinib was defined as the index date (ATC code: L01XE02, L01XE03, L01XE13). In Taiwan, first-line use of the three EGFR-TKIs was reimbursement for advanced (stage IIIb, IIIc or IV) lung adenocarcinoma harboring EGFR mutation, and restricted for monotherapy. A pre-audit approval was warranted to confirm the pathology, cell examination report and EGFR testing result. To refill prescription of EGFR-TKI, the chest radiography or computed tomography image must be checked every 4 weeks to evaluate the treatment response. Patients after 2014 were included since three EGFR-TKIs were reimbursed with the same reimbursement criteria. Wash-out period of 1 year was established to ensure patients were treatment naïve and receive gefitinib, erlotinib or afatinib as first-line treatment. The excluded criteria were applied to confirm the study cohort: 1) patients had any cancer diagnoses or previously received other antineoplastic agents (ATC code, L01.x) prior to 1 year of the index date; 2) patients did not have claims information or NHI eligibility by 1 year of index date; 3) patients had unknown sex; 4) patients had coprescription with other antineoplastic drugs on index date. This study was approved by the Institutional Review Board of Ethical Kaohsiung Medical University, Kaohsiung in Taiwan. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for cohort studies.
Outcomes
The outcome was divided into OS and TTF. The OS would evaluate survival rate from index date to censor or all-cause death, and the follow-up period was defined from index date to the date of death or end date of follow-up (December 31, 2017). The TTF was the time to treatment failure in the follow-up period, which was used as the proxy of PFS. The follow-up period of TTF was determined from the index date to progressive event or censor; progressive events included switching to or adding another antineoplastic therapy or EGFR-TKI, newly diagnosed brain metastases, and death. If patients did not receive any subsequent antineoplastic therapy after the first EGFR-TKI, the end date of first-line EGFR-TKI treatment was considered a progressive event. Subgroup analysis was conducted according to patients’ brain metastases status at diagnosis.
Study Variables
Variables including age, sex, year of diagnosis, brain metastases, and comorbidities were retrieved from the database to depict the patient characteristics. In Taiwan, NHIA implemented ICD-10-CM in the medical expenses reporting system in 2015. Due to the different taxonomies on coding algorithms between original ICD-9-CM and ICD-10-CM, we applied enhanced ICD-9-CM in Supplementary Table S1 in Supplement developed from Quan et al.’s study to improve the comparability between the two coding algorithms (Quan et al., 2005). The diagnosis of brain metastases was according to ICD code (ICD-9-CM, 198.3; ICD-10-CM, C79.31). The Charlson’s Comorbidity Index (CCI) was divided into three groups: 0, 1, and d ≧2. All comorbidities were identified 1 year before the index date and confirmed with at least two outpatient records or one inpatient record.
Statistical Analysis
The descriptive statistics were presented in mean with standard deviation (SD) and median with interquartile range for continuous variables and number and percent for categorical variables. One-way analysis of variance for continuous variables and chi-square test or Fisher’s exact test for categorical variables was used to compare baseline characteristics among three EGFR-TKI arms. Furthermore, standardized mean differences were also used to compare baseline characteristics among the three EGFR-TKI arms before and after PS matching.
The Kaplan-Meier method and log-rank test were applied to estimate the outcome. Cox proportional hazard regression was performed to estimate HR and 95% CI in the univariate and multivariable analyses. Demographic data including age, sex, brain metastases, and CCI score were used for adjustment.
To decrease probable selection bias among EGFR-TKI treatments, the inverse probability of treatment weighting (IPTW) and PS matching were performed for both OS and TTF estimates with variables of age, sex, year of diagnosis, brain metastases, and comorbidities. The weighted approach ensured that patients would not be excluded and mimicked a pseudopopulation to reflect baseline characteristics of the whole population. It remained the representative of the included patients, which was beneficial to generalize the result of the treatment effect (Leslie and Thiebaud, 2007; Brookhart et al., 2013). In PS matching, we initially matched 1:1 with afatinib and gefitinib and then 1:1 with afatinib and erlotinib. Since the two-step PS matching would certainly decrease the sample size, the matched method was conducted for sensitivity analysis.
Two-sided p-value with <0.05 was considered statistically significant. SMD >0.1 was considered as imbalance in the two groups. SAS 4.0 (SAS Institute, Cary, NC, United States) was used to conduct the data analyses.
Results
Patient Characteristics
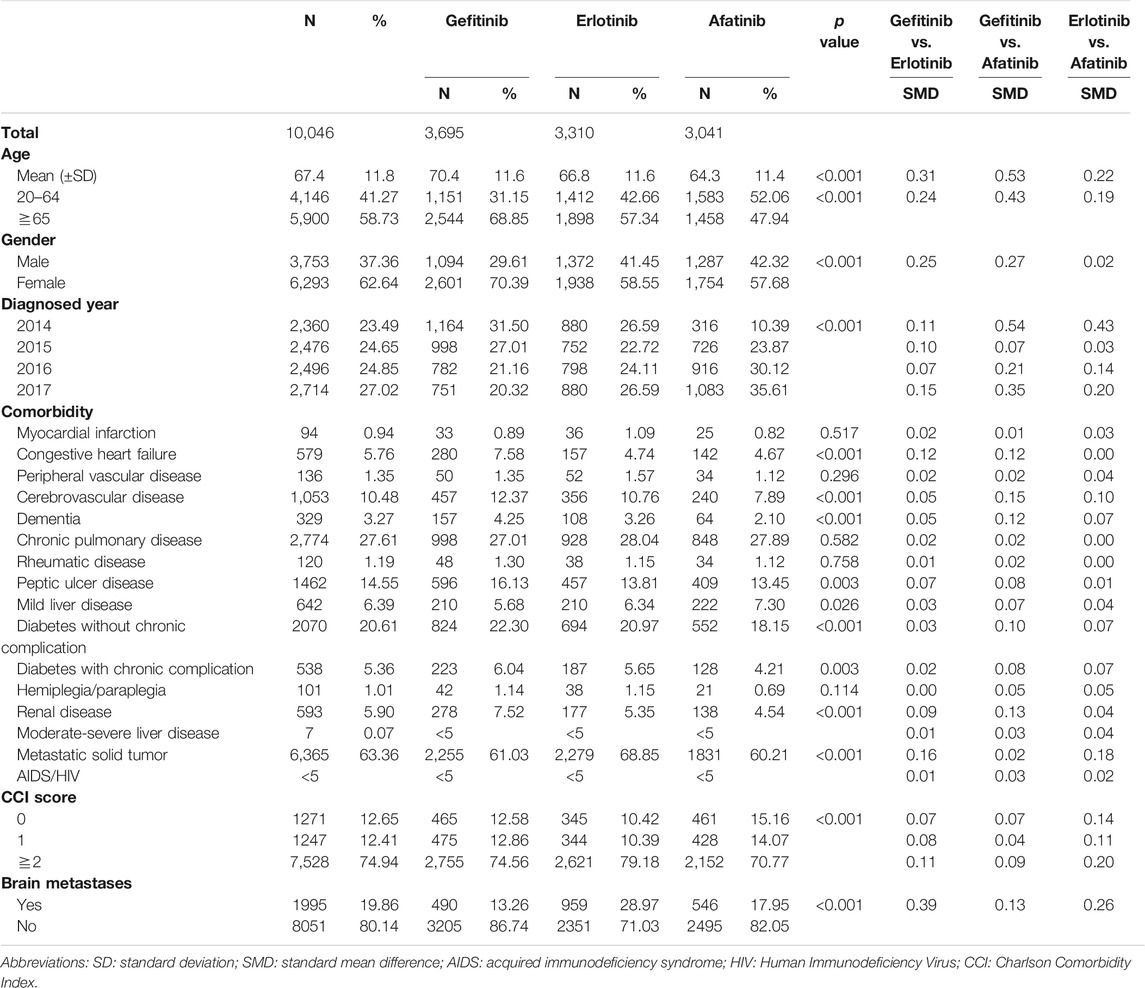
Overall, 93,137 patients with lung cancer were identified from January 1, 2014 and December 31, 2017. After excluding patients aged <20 years, 17,522 patients received either gefitinib, erlotinib, or afatinib following the first lung cancer diagnosis. Next, patients with prior antineoplastic therapy or pre-existing cancer (n = 7,162), lack of prior claims information or NHI eligibility (n = 213), unknown sex (n = 22), and coprescription with other antineoplastic agents (n = 79) were excluded. A total of 10,046 patients with EGFR-mutant advanced lung adenocarcinoma initiated first-line EGFR-TKI therapy with 3,695 patients who received gefitinib, 3,310 who received erlotinib and 3,041 who received afatinib (Supplementary Figure S1 in Supplement).
Table 1 shows the baseline characteristics of the three groups. The SMD was revealed in age, sex, year of diagnosis, brain metastases status, and CCI score. The age of the afatinib group (mean ± SD, 64.3 ± 11.4) was lower than those of the gefitinib (70.4 ± 11.6) and erlotinib groups (66.8 ± 11.6), whereas the percentages of elderly patients were 47.9, 68.9, and 57.3%, respectively. More female patients were prescribed with gefitinib (70.4%) than with erlotinib (58.6%) and afatinib (57.7%). Patients with brain metastases at baseline tended to receive erlotinib as first-line treatment compared to gefitinib and afatinib. The proportions of severe comorbidity (CCI score ≥2) in the gefitinib, erlotinib, and afatinib groups were 74.6, 79.2, and 70.8%, respectively. Patient characteristics after IPTW and PS matching are shown in Supplementary Tables S2A,B in Supplement.
Overall Survival
The median follow-up durations in the gefitinib, erlotinib, and afatinib groups were 16.4, 15.1, and 13.8 months. Kaplan–Meier estimates and log-rank test showed that afatinib was associated with longer OS compared with gefitinib (p < 0.001) and erlotinib (p < 0.001) and the median OS was 25.5, 20.9, and 21.4 months, respectively (Supplementary Figure S2A in Supplement). After IPTW, the median OS of the afatinib group was 23.9 vs. 21.3 months in the gefitinib group (p < 0.001) and 21.8 months in the erlotinib group (p = 0.001) (Supplementary Figure S2B). The 1-year survival rates of gefitinib, erlotinib, and afatinib were 70.0, 71.6, and 74.4%, and the 2-years survival rates were 44.7, 47.6, and 50.3%, respectively.
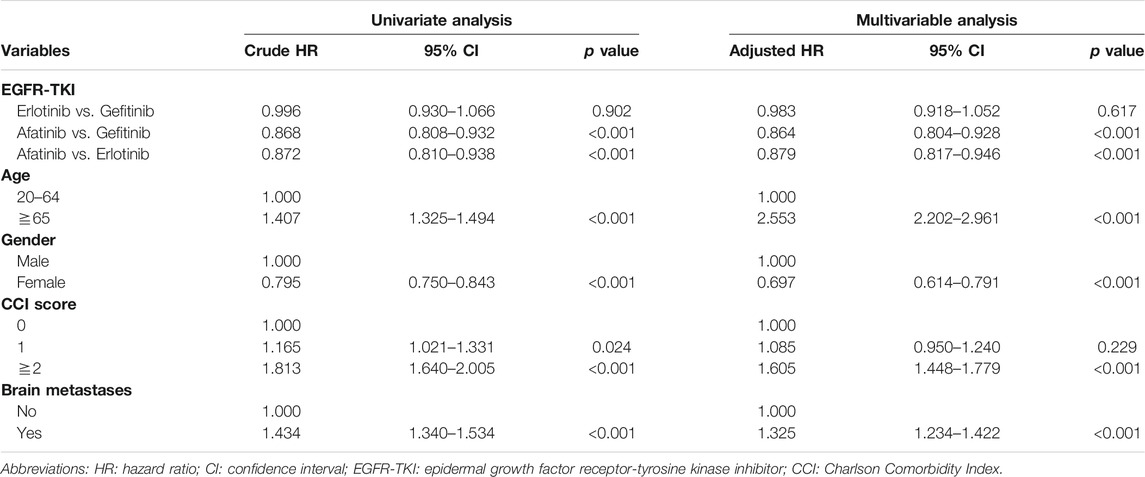
Multivariable analyses demonstrated that afatinib had lower mortality rate than gefitinib (aHR = 0.864; 95% CI, 0.804 to 0.928; p < 0.001) and erlotinib (aHR = 0.879; 95% CI, 0.817 to 0.946; p < 0.001) (Table 2). Baseline factors related to hazard of death included old age, male sex, severe comorbidity (CCI score≧2), and baseline brain metastases.
Time to Treatment Failure
The median follow-up period in the gefitinib, erlotinib, and afatinib groups were 9.5, 9.9, and 10.1 months, respectively. Afatinib showed superior TTF against gefitinib (p < 0.001) and erlotinib (p < 0.001), and the median TTFs were 11.7, 9.7, and 9.7 months, respectively (Supplementary Figure S3A in Supplement). After IPTW, the median TTF of afatinib was 11.5 vs. 9.5 months in the gefitinib group (p < 0.001) and 9.7 months in the erlotinib group (p < 0.001) (Supplementary Figure S3B). The 1-year non-failure rates in the gefitinib, erlotinib, and afatinib were 40.1, 43.2, and 49.6%, and the 2-years estimated rates were 14.3, 16.1, and 21.3%, respectively.
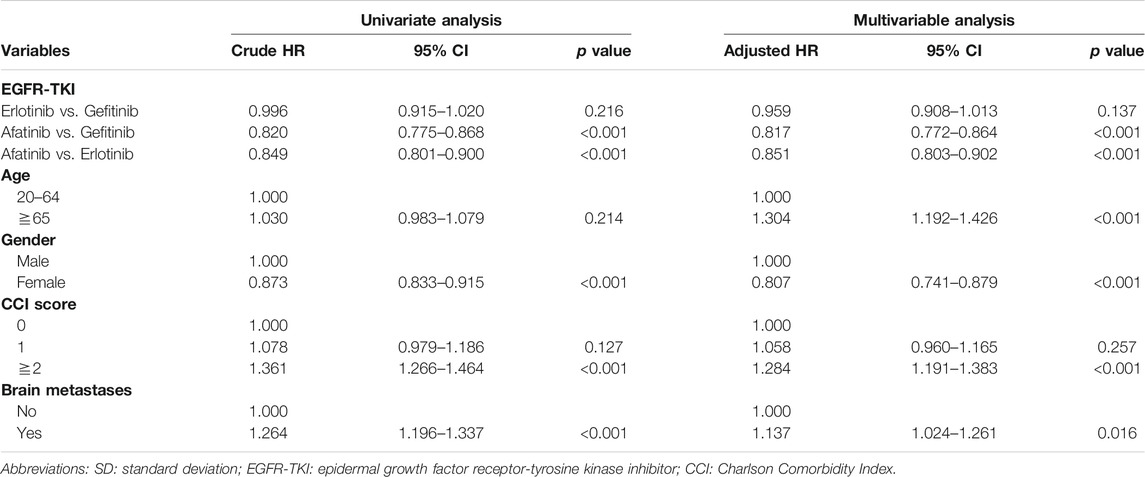
Table 3 shows the multivariable analysis that revealed similar results with OS estimates. Patients in the afatinib group were less likely to have treatment failure with aHR of 0.817 (95% CI, 0.772 to 0.864, p < 0.001) vs. gefitinib and 0.851 (95% CI, 0.803 to 0.902, p < 0.001) vs. erlotinib. The factors associated with treatment failure were consistent with the results of OS.
Subgroup Analysis
The subgroup analysis focused on patients with or without brain metastases at diagnosis. In brain metastases, the crude OS in the gefitinib, erlotinib, and afatinib groups were 15.3, 18.7, and 19.8 months (Supplementary Figure S2C). IPTW estimates were 15.7, 18.7, and 18.3 months, respectively (Supplementary Figure S2D). The crude TTF in the three study groups were 8.5, 9.5, and 11.7 months (Supplementary Figure S3C); IPTW estimates were 8.4, 9.4, and 9.2 months, respectively (Supplementary Figure S3D). OS and TTF were similar between erlotinib and afatinib, and both treatments were superior to the gefitinib group.
In the absence of brain metastases (Supplementary Figures S4, S5 in Supplement), the crude OS in the gefitinib, erlotinib, and afatinib groups were 21.9, 23.0, and 28.4 months; IPTW estimates were 22.6, 23.1, and 25.6 months, respectively. The crude TTF in the three study groups were 10.1, 9.8, and 12.0 months; IPTW estimates were 9.9, 9.9, and 11.9 months, respectively. The afatinib group presented better outcomes compared with groups that received first-generation EGFR-TKI, and there was no statistical difference between the gefitinib and erlotinib groups.
Sensitivity Analysis
PS matching method was conducted for sensitivity analysis. Supplementary Figure S6 in Supplement showed PS matching-adjusted OS was 22.1 months with gefitinib, 22.6 months with erlotinib, and 24.9 months with afatinib. Afatinib had better survival compared with gefitinib (p = 0.007) and erlotinib (p = 0.004). Multivariable analysis favored the afatinib group compared to the gefitinib (aHR = 0.819, 95% CI, 0.735 to 0.912, p < 0.001) and erlotinib groups (aHR = 0.870, 95% CI, 0.781 to 0.968, p = 0.011) (Supplementary Table S3 in Supplement).
The PS matching-adjusted TTF in the three groups were 9.6, 9.9, and 11.7 months, respectively (Supplementary Figure S7 in Supplement). The TTF was significantly longer for patients receiving afatinib (<0.001 vs. those receiving gefitinib and erlotinib). Multivariable analysis revealed that afatinib was associated with aHR of 0.869 (95% CI, 0.764 to 0.988; p = 0.032) vs. gefitinib and 0.892 (95% CI, 0.784 to 1.015; p = 0.083) vs. erlotinib (Supplementary Table S4 in Supplement).
Discussion
This study was conducted with the latest population-based RWD to evaluate relative survival rate and TTF of first-line treatment for EGFR-mutated advanced lung adenocarcinoma in Taiwan. Median OS and TTF were comparable with the results in clinical trials (Park et al., 2016; Yang et al., 2017). The slightly prolonged survival outcomes in the trial setting may result from the inclusion of patients who were younger (CTONG 0901, 58.5 years; Lux-Lung 7, 63 years; RWD, 67 years) and had less baseline brain metastases (CTONG 0901. 18.4%; Lux-Lung 7, 15.7%; RWD, 19.8%) and fewer comorbidities than those of RWD. The present study revealed that patients who preliminarily received afatinib had superior OS and TTF compared with those who received gefitinib and erlotinib, while a similar treatment effect was shown in the first-generation EGFR-TKIs. In OS estimates, Lux-Lung seven trial demonstrated no significant difference between the afatinib and gefitinib groups, but a discrepancy of 3.4 months was observed. The treatment benefit of afatinib in the Asian population was confirmed with sufficient sample size in the real-world situation.
The rigorous reimbursement criteria and regular treatment evaluation ensure consecutive EGFR-TKI treatment toward target patients in Taiwan. Given the real-world setting, the primary choice of EGFR-TKI is subject to the patient characteristics at diagnosis, and the baseline distribution in our study is in concordance with clinical experience. Patients who received afatinib were younger and had relatively mild comorbidity, which was supposed to prevent adverse events. Gefitinib tended to be prescribed for women, and erlotinib was commonly used for patients with initial brain metastases. The prescription patterns were in line with those of previous studies (Park et al., 2016; Hsieh et al., 2019; Kim et al., 2019; Ito et al., 2020). To facilitate the relative effectiveness assessment, two well-established PS methods were performed to adjust the survival. The similar hazard of treatment failure and mortality verified the treatment effect among the three EGFR-TKI treatments.
For patients initially diagnosed with EGFR-mutant advanced lung adenocarcinoma, concurrent brain metastasis was associated with severe morbidity, poor survival outcomes, and quality of life (Economopoulou and Mountzios, 2016). Previous direct comparison of EGFR-TKIs for these patients was investigated in Lux-Lung seven trial, but the sample size between the gefitinib (n = 24) and afatinib (n = 26) groups was limited. In the present study, brain metastasis was prevalent with up to 20% in our cohort, and results exhibited that both erlotinib and afatinib had better OS and TTF compared to gefitinib. As previous research, blood-brain barrier penetration rate of afatinib was lower than erlotinib (Ahluwalia et al., 2018). However, other research showed afatinib permeability rate was enough to inhibit tumor cell and present clinical improvement (Hoffknecht et al., 2015). Moreover, afatinib concentration in CSF was correlated to treatment dosage, so the adverse event tolerability and effective treatment dose should be pondered in clinical practice. Based on the claims data of large sample size, the result provided real-world evidence to address the front-line choice for the subgroup patients.
The third-generation EGFR-TKI osimertinib demonstrated superior treatment benefit compared to gefitinib/erlotinib in the first-line therapy (Soria et al., 2018). However, the drug accessibility was scant because of the high acquisition price and unfavorable cost-effectiveness results worldwide (Aguiar et al., 2018; Ezeife et al., 2018; Wu et al., 2019b; Cai et al., 2019). In Taiwan, osimertinib was covered by the national health insurance program in 2020 and conditionally reimbursed for stage IV non-brain metastatic lung adenocarcinoma as first-line treatment, and patients progressed after first-line gefitinib, erlotinib, or afatinib therapy harboring T790M mutation. Untreated patients who had initial brain metastases could only receive first- or second-generation EGFR-TKI, and the present study supported erlotinib and afatinib to be the best treatment option. Furthermore, a previous study indicated that progressive events in patients receiving first- or second-generation EGFR-TKI mostly originated from T790M mutation (Oxnard et al., 2011; Yu et al., 2013). The secondary EGFR mutation also accounted for most frequent mechanisms of EGFR-TKI resistance in Taiwan (Huang et al., 2018; Lin et al., 2019). Hence, in patients with baseline brain metastases, the front-line treatment option of erlotinib or afatinib would not influence hereafter sequential therapy. Other considerations were treatment-related adverse events. Skin reactions, general disorders, and administration site conditions were commonly associated with EGFR-TKIs in trials and real-world settings; generally, patients are considered to tolerate well every EGFR-TKI (Huang et al., 2020).
Limitation
Several limitations should be noted in the study. First, the nature of non-randomized study design led to selection bias among treatment groups. Otherwise, the limitation of NHIRD leads to lack of smoking status, severity of cancer stage, lifestyle, examination results, performance status, and laboratory data. To our best effort, IPTW and PS matching was applied to adjust well-known prognostic factors in lung cancer treatment, and results in the Cox regression model were robust. Second, TTF used as proxy of PFS could exaggerate the time to progression, although patients with initial response or slow progression may continue EGFR-TKI with local therapy. The TTF endpoint may be more practical to reflect the contribution of EGFR-TKI treatment. Third, the compassionate use of osimertinib was approved in 2016. Approximately 1,000 patients received osimertinib as subsequent therapy, which may compromise relative outcome. Nonetheless, treatment failure rate was higher in first-line gefitinib and erlotinib, which could crossover to use osimertinib, and the results indirectly strengthened treatment benefit of afatinib. Fourth, despite the latest claims data, the follow-up period may not be comprehensive enough to reflect the lifelong duration. Lastly, NHIRD did not provide mutation type information (i.e., L858R or T790M mutation) in our study period. A meta-analysis showed patients with exon 19 deletion had lower risk of progression than L858R mutation (Lee et al., 2015), but only Lux-Lung seven trial conducted the head-to-head comparison of EGFR-TKIs in both mutation types (Paz-Ares et al., 2017). The divergence of OS curves was observed in patients harboring exon 19 mutation, yet the sample size might be underpowered. Future studies with detailed types of mutation and sufficient sample size are warranted to provide a further prospect for direct comparison of EGFR-TKIs.
Conclusion
This RWD with large population suggested that afatinib had longer survival rate and TTF than gefitinib and erlotinib in the Asian population. The treatment preference was shown in gefitinib over female patients; erlotinib over patients with brain metastases; and afatinib over younger patients. In patients with brain metastases, both erlotinib and afatinib contribute to longer OS compared with gefitinib. IPTW and PS matching method enhanced the robustness of estimated OS and progression outcomes. Further research could focus on the sequential therapy in patients with different EGFR mutation types.
Data Availability Statement
The datasets presented in this article are not readily available because legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”. Corresponding author (C-YC) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data are available from the National Health Insurance Research Database (NHIRD) published by the Bureau of National Health Insurance (BNHI) of the Ministry of Health and Welfare. Requests to access the datasets should be directed to amsyOTc1NTI1QGhvdG1haWwuY29t.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The research reported in this publication was supported by grant from Kaohsiung Medical University Research Foundation (KMU-M1100018). The Kaohsiung Medical University Research Foundation was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Disclaimer
The conclusion presented in this study are those of the authors and do not necessarily reflect the views of the BNHI, the Ministry of Health and Welfare.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Center for Medical Informatics and Statistics of Kaohsiung Medical University for providing administrative and funding support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.720687/full#supplementary-material
References
Aguiar, P. N., Haaland, B., Park, W., San Tan, P., del Giglio, A., and de Lima Lopes, G. (2018). Cost-effectiveness of Osimertinib in the First-Line Treatment of Patients WithEGFR-Mutated Advanced Non-small Cell Lung Cancer. JAMA Oncol. 4 (8), 1080–1084. doi:10.1001/jamaoncol.2018.1395
Ahluwalia, M. S., Becker, K., and Levy, B. P. (2018). Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for Central Nervous System Metastases from Non‐Small Cell Lung Cancer. Oncol. 23 (10), 1199–1209. doi:10.1634/theoncologist.2017-0572
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clinicians 68 (6), 394–424. doi:10.3322/caac.21492
Brookhart, M. A., Wyss, R., Layton, J. B., and Stürmer, T. (2013). Propensity Score Methods for Confounding Control in Nonexperimental Research. Circ. Cardiovasc. Qual. Outcomes 6 (5), 604–611. doi:10.1161/circoutcomes.113.000359
Cai, H., Zhang, L., Li, N., Chen, S., Zheng, B., Yang, J., et al. (2019). Cost-effectiveness of Osimertinib as First-Line Treatment and Sequential Therapy for EGFR Mutation-Positive Non-small Cell Lung Cancer in China. Clin. Ther. 41 (2), 280–290. doi:10.1016/j.clinthera.2018.12.007
Chen, J., Zeng, F., Forrester, S. J., Eguchi, S., Zhang, M.-Z., and Harris, R. C. (2016). Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 96, 1025–1069. doi:10.1152/physrev.00030.2015
Economopoulou, P., and Mountzios, G. (2016). Non-small Cell Lung Cancer (NSCLC) and central Nervous System (CNS) Metastases: Role of Tyrosine Kinase Inhibitors (TKIs) and Evidence in Favor or against Their Use with Concurrent Cranial Radiotherapy. Transl. Lung Cancer Res. 5 (6), 588–598. doi:10.21037/tlcr.2016.12.06
Ezeife, D. A., Kirk, V., Chew, D. S., Nixon, N. A., Lee, R., Le, L. W., et al. (2018). Economic Analysis of Osimertinib in Previously Untreated EGFR-Mutant Advanced Non-small Cell Lung Cancer in Canada. Lung Cancer 125, 1–7. doi:10.1016/j.lungcan.2018.08.024
Hanna, N., Johnson, D., Temin, S., Baker, S., Brahmer, J., Ellis, P. M., et al. (2017). Systemic Therapy for Stage IV Non-small-cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. Jco 35 (30), 3484–3515. doi:10.1200/jco.2017.74.6065
Hoffknecht, P., Tufman, A., Wehler, T., Pelzer, T., Wiewrodt, R., Schütz, M., et al. (2015). Efficacy of the Irreversible ErbB Family Blocker Afatinib in Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitor (TKI)-pretreated Non-small-cell Lung Cancer Patients with Brain Metastases or Leptomeningeal Disease. J. Thorac. Oncol. 10 (1), 156–163. doi:10.1097/jto.0000000000000380
Hsieh, Y. Y., Fang, W. T., Lo, Y. W., Chen, Y. H., and Chien, L. N. (2019). Comparing the Effectiveness of Different EGFR-TKIs in Patients with EGFR Mutant Non-small-cell Lung Cancer: A Retrospective Cohort Study in Taiwan. Int. J. Cancer 4, 1107–1116. doi:10.1002/ijc.32841
Huang, J., Meng, L., Yang, B., Sun, S., Luo, Z., and Chen, H. (2020). Safety Profile of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: A Disproportionality Analysis of FDA Adverse Event Reporting System. Scientific Rep. 10 (1), 4803. doi:10.1038/s41598-020-61571-5
Huang, Y.-H., Hsu, K.-H., Tseng, J.-S., Chen, K.-C., Hsu, C.-H., Su, K.-Y., et al. (2018). The Association of Acquired T790M Mutation with Clinical Characteristics after Resistance to First-Line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in Lung Adenocarcinoma. Cancer Res. Treat. 50 (4), 1294–1303. doi:10.4143/crt.2017.512
Ito, K., Murotani, K., Kubo, A., Kunii, E., Taniguchi, H., Shindoh, J., et al. (2020). Propensity Score Analysis of Overall Survival between First‐ and Second‐generation EGFR‐TKIs Using Real‐world Data. Cancer Sci. 111 (10), 3705–3713. doi:10.1111/cas.14560
Kim, Y., Lee, S.-H., Ahn, J. S., Ahn, M.-J., Park, K., and Sun, J.-M. (2019). Efficacy and Safety of Afatinib for EGFR-Mutant Non-small Cell Lung Cancer, Compared with Gefitinib or Erlotinib. Cancer Res. Treat. 51 (2), 502–509. doi:10.4143/crt.2018.117
Lee, C. K., Wu, Y.-L., Ding, P. N., Lord, S. J., Inoue, A., Zhou, C., et al. (2015). Impact of Specific Epidermal Growth Factor Receptor (EGFR) Mutations and Clinical Characteristics on Outcomes after Treatment with EGFR Tyrosine Kinase Inhibitors versus Chemotherapy in EGFR-Mutant Lung Cancer: A Meta-Analysis. Jco 33 (17), 1958–1965. doi:10.1200/jco.2014.58.1736
Leslie, S. R., and Thiebaud, P. (2007). Using propensity scores to adjust for selection bias when assessing the effectiveness of Alcoholics Anonymous in observational studies. Drug Alcohol Depend 2, 56–64. doi:10.1016/j.drugalcdep.2009.03.018
Lin, Y. T., Chen, J. S., Liao, W. Y., Ho, C. C., Hsu, C. L., Yang, C. Y., et al. (2019). Clinical Outcomes and Secondary Epidermal Growth Factor Receptor (EGFR) T790M Mutation Among First‐line Gefitinib, Erlotinib and Afatinib‐treated Non‐small Cell Lung Cancer Patients with Activating EGFR Mutations. Int. J. Cancer 144 (11), 2887–2896. doi:10.1002/ijc.32025
Maemondo, M., Inoue, A., Kobayashi, K., Sugawara, S., Oizumi, S., Isobe, H., et al. (2010). Gefitinib or Chemotherapy for Non-small-cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 362 (25), 2380–2388. doi:10.1056/nejmoa0909530
Midha, A., Dearden, S., and McCormack, R. (2015). EGFR Mutation Incidence in Non-small-cell Lung Cancer of Adenocarcinoma Histology: a Systematic Review and Global Map by Ethnicity (mutMapII). Am. J. Cancer Res. 5 (9), 2892–2911.
Ministry of Health and Welfare (2019). Cause Of Death Statistics. Available at: https://www.mohw.gov.tw/lp-4964-2.html (Accessed October 5, 2020).
Mitsudomi, T., Morita, S., Yatabe, Y., Negoro, S., Okamoto, I., Tsurutani, J., et al. (2010). Gefitinib versus Cisplatin Plus Docetaxel in Patients with Non-small-cell Lung Cancer Harbouring Mutations of the Epidermal Growth Factor Receptor (WJTOG3405): an Open Label, Randomised Phase 3 Trial. Lancet Oncol. 11 (2), 121–128. doi:10.1016/s1470-2045(09)70364-x
NCCN Clinical Practice Guidelines in Oncology: Non-small Cell Lung Cancer, version 3.2018. Available at: https://www.nccn.org/patients/guidelines/cancers.aspx#nsclc (Accessed 19 August 2020).
Oxnard, G. R., Arcila, M. E., Sima, C. S., Riely, G. J., Chmielecki, J., Kris, M. G., et al. (2011). Acquired Resistance to EGFR Tyrosine Kinase Inhibitors in EGFR-Mutant Lung Cancer: Distinct Natural History of Patients with Tumors Harboring the T790M Mutation. Clin. Cancer Res. 17 (6), 1616–1622. doi:10.1158/1078-0432.ccr-10-2692
Park, K., Tan, E.-H., O'Byrne, K., Zhang, L., Boyer, M., Mok, T., et al. (2016). Afatinib versus Gefitinib as First-Line Treatment of Patients with EGFR Mutation-Positive Non-small-cell Lung Cancer (LUX-Lung 7): a Phase 2B, Open-Label, Randomised Controlled Trial. Lancet Oncol. 17 (5), 577–589. doi:10.1016/s1470-2045(16)30033-x
Paz-Ares, L., Tan, E.-H., O’Byrne, K., Zhang, L., Hirsh, V., Boyer, M., et al. (2017). Afatinib versus Gefitinib in Patients with EGFR Mutation-Positive Advanced Non-small-cell Lung Cancer: Overall Survival Data from the Phase IIb LUX-Lung 7 Trial. Ann. Oncol. 28 (2), 270–277. doi:10.1093/annonc/mdw611
Quan, H., Sundararajan, V., Halfon, P., Fong, A., Burnand, B., Luthi, J.-C., et al. (2005). Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 43 (11), 1130–1139. doi:10.1097/01.mlr.0000182534.19832.83
Rosell, R., Carcereny, E., Gervais, R., Vergnenegre, A., Massuti, B., Felip, E., et al. (2012). Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-small-cell Lung Cancer (EURTAC): a Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 13 (3), 239–246. doi:10.1016/S1470-2045(11)70393-X
Sequist, L. V., Yang, J. C.-H., Yamamoto, N., O'Byrne, K., Hirsh, V., Mok, T., et al. (2013). Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. Jco 31 (27), 3327–3334. doi:10.1200/jco.2012.44.2806
Soria, J. C., Ohe, Y., Vansteenkiste, J., Reungwetwattana, T., Chewaskulyong, B., Lee, K. H., et al. (2018). Osimertinib in Untreated EGFR-Mutated Advanced Non-small-cell Lung Cancer. N. Engl. J. Med. 378 (2), 113–125. doi:10.1056/NEJMoa1713137
Wu, B., Gu, X., Zhang, Q., and Xie, F. (2019). Cost‐Effectiveness of Osimertinib in Treating Newly Diagnosed, Advanced EGFR‐Mutation‐Positive Non‐Small Cell Lung Cancer. Oncol. 24 (3), 349–357. doi:10.1634/theoncologist.2018-0150
Wu, Y.-L., Planchard, D., Lu, S., Sun, H., Yamamoto, N., Kim, D.-W., et al. (2019). Pan-Asian Adapted Clinical Practice Guidelines for the Management of Patients with Metastatic Non-small-cell Lung Cancer: a CSCO-ESMO Initiative Endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann. Oncol. 30 (2), 171–210. doi:10.1093/annonc/mdy554
Wu, Y.-L., Zhou, C., Hu, C.-P., Feng, J., Lu, S., Huang, Y., et al. (2014). Afatinib versus Cisplatin Plus Gemcitabine for First-Line Treatment of Asian Patients with Advanced Non-small-cell Lung Cancer Harbouring EGFR Mutations (LUX-Lung 6): an Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 15 (2), 213–222. doi:10.1016/s1470-2045(13)70604-1
Wu, Y.-L., Zhou, C., Liam, C.-K., Wu, G., Liu, X., Zhong, Z., et al. (2015). First-line Erlotinib versus Gemcitabine/cisplatin in Patients with Advanced EGFR Mutation-Positive Non-small-cell Lung Cancer: Analyses from the Phase III, Randomized, Open-Label, ENSURE Study. Ann. Oncol. 26 (9), 1883–1889. doi:10.1093/annonc/mdv270
Yang, J. J., Zhou, Q., Yan, H. H., Zhang, X. C., Chen, H. J., Tu, H. Y., et al. (2017). A Phase III Randomised Controlled Trial of Erlotinib vs Gefitinib in Advanced Non-small Cell Lung Cancer with EGFR Mutations. Br. J. Cancer 116 (5), 568–574. doi:10.1038/bjc.2016.456
Yu, H. A., Arcila, M. E., and Rekhtman, N. (2013). Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. 19, 2240-2247. doi:10.1158/1078-0432.CCR-12-2246
Zhou, C., Wu, Y.-L., Chen, G., Feng, J., Liu, X.-Q., Wang, C., et al. (2011). Erlotinib versus Chemotherapy as First-Line Treatment for Patients with Advanced EGFR Mutation-Positive Non-small-cell Lung Cancer (OPTIMAL, CTONG-0802): a Multicentre, Open-Label, Randomised, Phase 3 Study. Lancet Oncol. 12 (8), 735–742. doi:10.1016/s1470-2045(11)70184-x
Keywords: gefitinib, erlotinib, afatinib, EGFR mutation, real-world effectiveness
Citation: Chen P-Y, Wang C-C, Hsu C-N and Chen C-Y (2021) Association of EGFR Tyrosine Kinase Inhibitor Treatment With Progression-Free Survival Among Taiwanese Patients With Advanced Lung Adenocarcinoma and EGFR Mutation. Front. Pharmacol. 12:720687. doi: 10.3389/fphar.2021.720687
Received: 04 June 2021; Accepted: 28 July 2021;
Published: 09 August 2021.
Edited by:
Emanuel Raschi, University of Bologna, ItalyReviewed by:
Francesco Gelsomino, IRCCS Azienda Ospedaliero-Universitaria di Bologna, ItalyHankil Lee, Ajou University, South Korea
Copyright © 2021 Chen, Wang, Hsu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chung-Yu Chen, amsyOTc1NTI1QGhvdG1haWwuY29t
 Po-Yen Chen1,2
Po-Yen Chen1,2 Chien-Ning Hsu
Chien-Ning Hsu Chung-Yu Chen
Chung-Yu Chen