
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 05 August 2021
Sec. Neuropharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.718800
 Tzu-Hua Wu1,2†
Tzu-Hua Wu1,2† Chieh-Hsin Lin3,4,5†
Chieh-Hsin Lin3,4,5† Kah Kheng Goh2,6
Kah Kheng Goh2,6 Cynthia Yi-An Chen2,6
Cynthia Yi-An Chen2,6 Chun-Hsin Chen2,6,7
Chun-Hsin Chen2,6,7 Hsien-Yuan Lane4,8
Hsien-Yuan Lane4,8 Mong-Liang Lu2,6,7*
Mong-Liang Lu2,6,7*The aim of the study was to assess the relationship between prolactin levels and sexual dysfunction in patients with schizophrenia who use olanzapine medication. The potential risk factors of hyperprolactinemia and sexual dysfunction were also investigated. Patients with schizophrenia undergoing olanzapine monotherapy were invited to participate in this cross-sectional study. The Arizona Sexual Experiences Scale (ASEX) and the Positive and Negative Syndrome Scale were used to evaluate subjective sexual dysfunction and psychopathology, respectively. Levels of prolactin and metabolic parameters were also measured. In total, 279 participants with schizophrenia were recruited. The overall incidences of hyperprolactinemia, sexual dysfunction, and metabolic syndrome were 51.6, 53.8, and 43.7%, respectively. Higher ASEX scores, higher insulin levels, female sex, and younger age were associated with hyperprolactinemia. Prolactin level was significantly correlated with ASEX score. Elevated prolactin levels, concomitant antidepressant, increased insulin resistance, longer illness duration, and female sex were associated with sexual dysfunction. Female participants recorded higher levels of sexual dysfunction than their male counterparts did, whereas male participants had comparatively lower prolactin levels and lower rates of spousal partnership. Hyperprolactinemia, metabolic syndrome, and sexual dysfunction are prevalent in patients with schizophrenia treated with olanzapine. Clinicians should maintain awareness of these problems and monitor them regularly with their patients.
Schizophrenia is a severe and chronic mental disorder that causes marked functional impairment. Antipsychotic medication is the first-line treatment for patients with schizophrenia. However, antipsychotic drugs may produce unwanted side effects due to their pharmacodynamic characteristics. Antipsychotics often elevate prolactin levels by blocking dopamine D2 receptors in the tuberoinfundibular pathway of the brain (Grigg et al., 2017). As a potent antagonist of dopamine D2 and serotonin 5-HT2 receptors, olanzapine has fewer extrapyramidal side effects and better clinical efficacy compared with first-generation antipsychotics (Callaghan et al., 1999). But several studies reported that olanzapine was associated with elevated prolactin levels to some degree (Barata et al., 2019; Huhn et al., 2019). It is proposed that because 5-HT2 receptor stimulation can induce prolactin release, the 5-HT2 receptor antagonism of olanzapine may partially counteract its prolactin-elevating tendency through its D2 receptor antagonism (Cowen et al., 1990).
Sexual dysfunction is characterized by a significant disturbance in a person’s ability to respond sexually or experience sexual pleasure. Finn et al. (1990) reported that patients with schizophrenia rated antipsychotic-induced sexual dysfunction as more “bothersome” than most psychiatric symptoms of their illness are. The prevalence of sexual dysfunction among people with schizophrenia is higher than that among the rest of the population. Sexual dysfunction has been reported in 30–82% of patients with psychiatric disorders who use antipsychotics, due to hyperprolactinemia as well as α1 and dopamine D2 receptor blockades (De Boer et al., 2015; Dumontaud et al., 2020). A meta-analysis reported that olanzapine users had a higher rate of sexual dysfunction (approximately 40%) as compared with the prolactin-sparing antipsychotics quetiapine, ziprasidone, and aripiprazole (16–27%) (Serretti and Chiesa, 2011). The causal relationship between hyperprolactinemia and sexual dysfunction remains contested. Some studies have suggested a relationship between the disorders (Ahl et al., 2004; Rubio-Abadal et al., 2016), but other studies have discovered no such relationship (Johnsen et al., 2011; Kikuchi et al., 2012). Other factors that have been suggested to be related to sexual dysfunction in patients with schizophrenia include sex, age, disease psychopathology, effects of other medications, substance use, and medical comorbidities (Martin et al., 2018; Dehelean et al., 2020; Dumontaud et al., 2020). Antipsychotic-induced sexual dysfunction can significantly distress patients and subsequently reduce drug compliance (Souaiby et al., 2019); accordingly, evaluating sexual dysfunction and managing it properly are essential.
Metabolic disturbance is a common adverse reaction in patients with schizophrenia under olanzapine treatment (Hirsch et al., 2017; Lu et al., 2018). Several studies reported that metabolic dysfunction is associated with sexual dysfunction in general population (Schulster et al., 2017; Di Francesco et al., 2019). But the relationships between metabolic syndrome and sexual dysfunction in patients with schizophrenia have been poorly explored (Dumontaud et al., 2020).
Our study was aimed to examine the relationships between hyperprolactinemia, metabolic disturbance, and sexual dysfunction among study participants with schizophrenia who received olanzapine medication. Potential links to hyperprolactinemia and sexual dysfunction were also analyzed.
This cross-sectional study was approved by our facility’s institutional review board. After providing a detailed description of the study, we obtained written informed consent from the participants. Patients aged 20–65 years and with schizophrenia as diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition, were included in the study. They were required to have undergone olanzapine monotherapy at the same dose for the last 6 months.
All study participants participated in clinical interviews, underwent anthropometrical parameter assessments, and provided fasting blood samples. A trained research nurse interviewed patients to collect demographic and psychiatric information. Body mass index was calculated as weight in kilograms divided by the square of the height in meters (kg/m2).
The Arizona Sexual Experiences Scale (ASEX) was used to assess sexual dysfunction (Mcgahuey et al., 2000). ASEX is a five-item rating scale that assesses sex drive, arousal, vaginal lubrication/penile erection, ability to reach orgasm, and satisfaction from orgasm. It measures sexual dysfunction on a 6-point Likert scale ranging from hyperfunction (1) to hypofunction (6). A participant with a total ASEX score of ≥19, any one item with a score of ≥5, or any 3 items with a score of ≥4 was deemed to be experiencing sexual dysfunction. We also assessed the participants for psychopathology severity by using the Positive and Negative Syndrome Scale (Kay et al., 1987).
Blood samples were obtained in the morning after overnight fasting. Plasma was stored at −80°C prior to testing. Levels of prolactin were measured using electrochemiluminescence immunoassays. Hyperprolactinemia was considered when the prolactin level exceeded 20 ng/ml in women and 15 ng/ml in men, in accordance with laboratory procedures. Enzymatic colorimetric assays were used to measure fasting serum levels of glucose, high-density lipoprotein cholesterol (HDL-C), and triglycerides. Serum insulin levels were measured using an electrochemiluminescence immunoassay kit. Insulin resistance was calculated using the homeostasis model assessment for insulin resistance (HOMA-IR) as follows (fasting glucose [mmol/L] × fasting insulin [mU/L]/22.5) (Matthews et al., 1985).
In this study, we used the modified Adult Treatment Panel III criteria for Asians to evaluate participants for metabolic syndrome (Tan et al., 2004). A diagnosis of metabolic syndrome required 3 of the following five criteria: (1) abdominal obesity (waist circumference of ≥90 cm in men and ≥80 cm in women), (2) fasting hypertriglyceridemia (≥150 mg/dl); (3) low fasting HDL-C levels (<40 mg/dl in men and <50 mg/dl in women), (4) high blood pressure (≥130/≥85 mm Hg) or current treatment with antihypertensive medication, and (5) high fasting levels of plasma glucose (≥100 mg/dl) or current treatment with antidiabetic medication.
The variables were compared using the Student’s t test for continuous variables and Fisher’s exact test for categorical variables. Nonparametric tests (Mann–Whitney U test) were performed for variables without normal distribution. We used the Pearson correlation method to analyze the correlations between variables and ASEX scores as well as prolactin levels. A multivariate linear regression model with selection of clinically relevant variables related to hyperprolactinemia and sexual dysfunction was utilized. All continuous variables that were entered in the regression were logarithmically transformed to normalize the data, whereas categorical variables were recoded into sets of distinct binary variables. The significance level used was p < 0.05. Analyses were performed using SPSS 19.
In total, 279 participants with schizophrenia, comprising 147 women and 132 men, were recruited for our study. The demographic, clinical, and laboratory data of the study participants with and without hyperprolactinemia are summarized in Table 1. The overall prevalence of hyperprolactinemia was 51.6%. Compared with participants not diagnosed as having hyperprolactinemia, those with hyperprolactinemia were younger, were less likely to have a spouse or partner, and recorded higher ASEX scores, prevalence of sexual dysfunction, insulin levels, and HOMA-IR indexes.
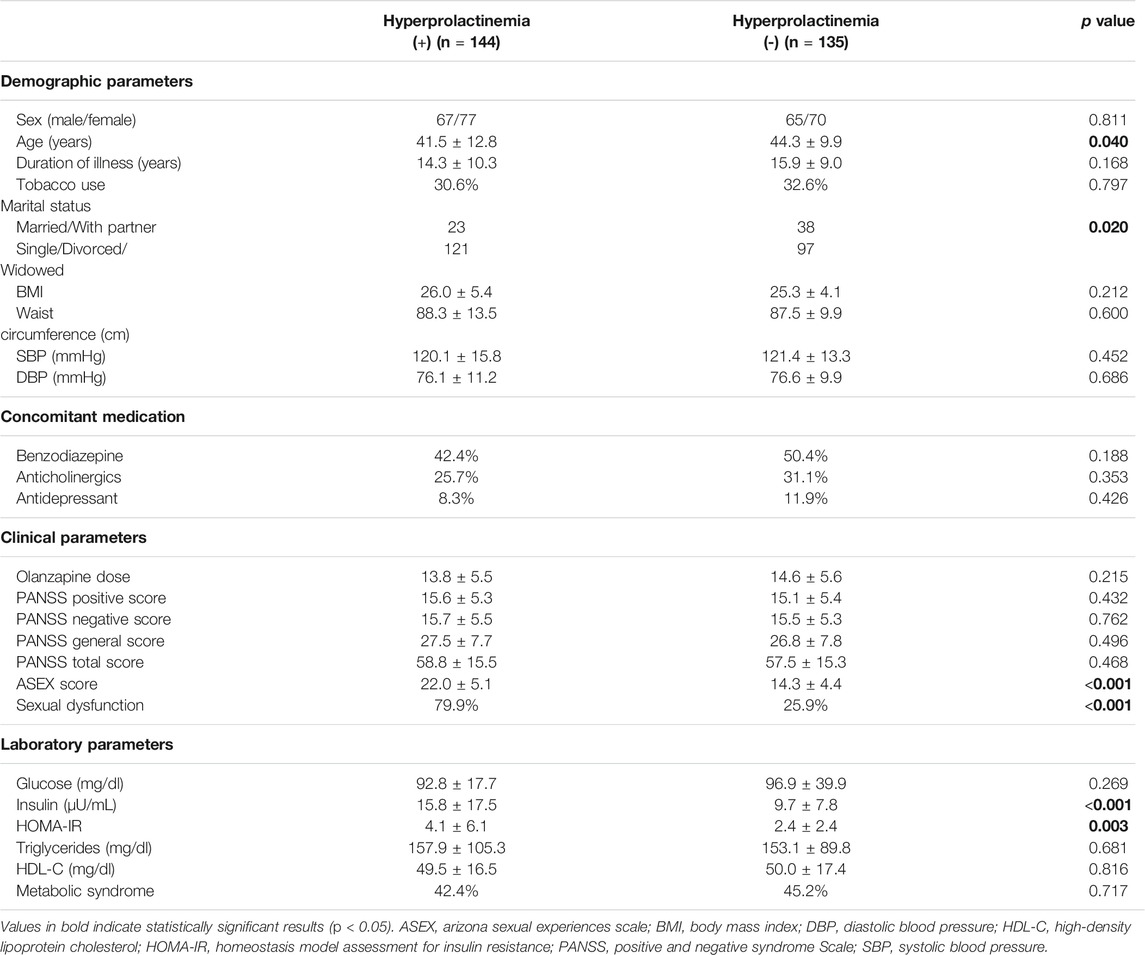
TABLE 1. Demographic, clinical and laboratory characteristics of study subjects by hyperprolactinemia.
A multivariate liner regression model was applied to identify the variables associated with hyperprolactinemia. For all participants with schizophrenia, ASEX score (B = 0.053, p < 0.001), age (B = −0.006, p = 0.004), female sex (B = 0.119, p = 0.015), and insulin level (B = 0.014, p = 0.044) were associated with hyperprolactinemia (Table 2).
Prolactin level was significantly correlated with ASEX score (r = 0.689, p < 0.001) (Figure 1). And prolactin level was also positively correlated with each ASEX item score (all p < 0.001). Other demographic parameters, clinical parameters, or metabolic parameters were not correlated with prolactin level.
The demographic, clinical, and laboratory characteristics of the study participants with and without sexual dysfunction are summarized in Table 3. The overall prevalence of sexual dysfunction was 53.8%. Compared with participants without sexual dysfunction, participants with sexual dysfunction were more likely to be female and advanced in age and more likely to have had concomitant antidepressant, longer durations of illness, and higher prolactin levels. Regarding metabolic parameters, participants with sexual dysfunction had higher glucose levels, insulin levels, and insulin resistance compared with those not experiencing sexual dysfunction. No differences in psychopathology or olanzapine dose between those two groups were observed.
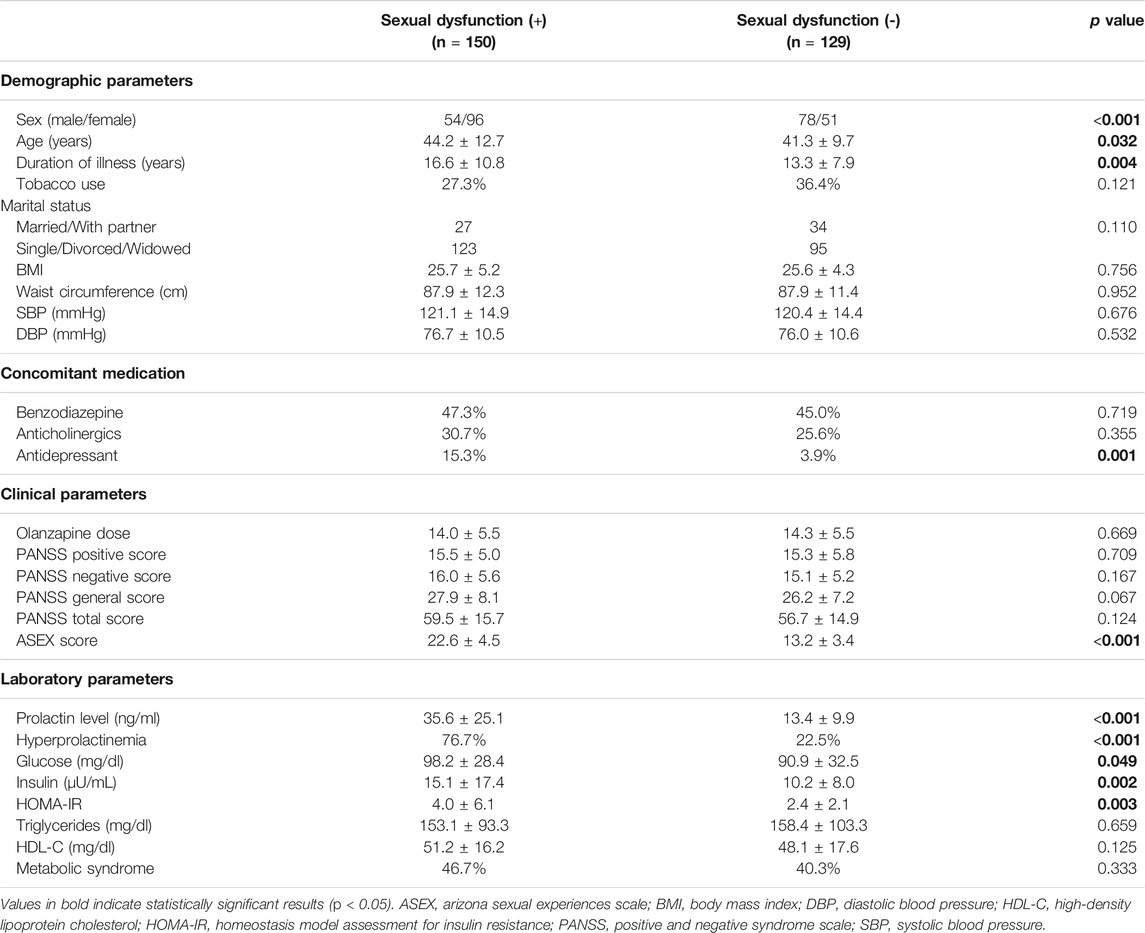
TABLE 3. Demographic, clinical and laboratory characteristics of study subjects by sexual dysfunction.
A multivariate regression model was used to identify the factors associated with sexual dysfunction. For all participants with schizophrenia, prolactin level (B = 0.012, p < 0.001), concomitant antidepressant (B = 0.460, p < 0.001), HOMA-IR (B = 0.018, p = 0.001), duration of illness (B = 0.007, p = 0.008), and female sex (B = 0.0.127, p = 0.013) were associated with sexual dysfunction (Table 4).
With total and individual ASEX item scores as dependent variables, multivariate regression analyses were performed to find the associated factors. Prolactin level was positively associated with total ASEX score and all five item scores (Table 5). Female sex was positively associated with total ASEX score as well as scores of ASEX item 2 (ease of arousal), item 3 (ability to achieve erection/lubrication), and item 4 (ease of reaching orgasm). Concomitant antidepressant was positively associated with total ASEX score as well as scores of item 3 and item 4. Married participants or participants with sex partner were negatively associated with scores of ASEX item 3 and item 4.
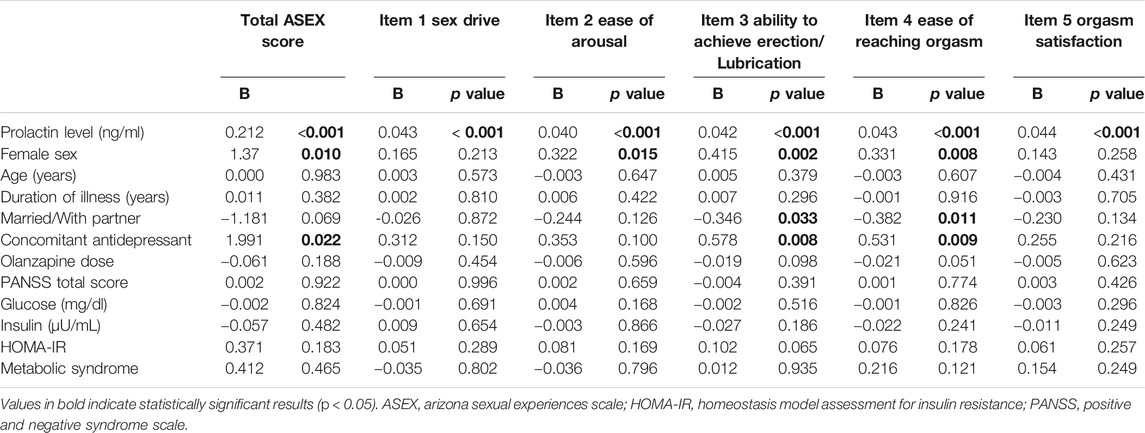
TABLE 5. Multiple linear regression analysis with individual ASEX item scores as dependent variables.
Among all parameters, only prolactin level was correlated with the ASEX score (r = 0.689, p < 0.001).
The demographic, clinical, and laboratory information of the participants by sex are summarized in Supplementary Table S1. Compared with female participants, male participants were more likely to be young and smoke, as well as less likely to have a spouse or partner. Male participants also recorded lower ASEX scores, lower prevalence of sexual dysfunction, lower prolactin levels, lower glucose levels, lower HDL-C levels, larger waist circumferences, and higher blood pressure values. No differences between sexes in the severity of psychopathology or olanzapine dose were observed.
In our study, approximately half of the olanzapine-treated participants with schizophrenia had hyperprolactinemia and sexual dysfunction. Hyperprolactinemia was associated with sexual dysfunction, female sex, higher insulin level, and younger age. Sexual dysfunction was significantly related to higher prolactin levels in olanzapine-treated patients with schizophrenia, with significant differences between the sexes. Concomitant antidepressant, longer durations of illness, and greater insulin resistance were also associated with sexual dysfunction.
Given that prolactin is under negative control by dopamine and positive control by serotonin, olanzapine has been reported to induce moderately elevated prolactin levels in patients with schizophrenia (Peuskens et al., 2014). In our study, the mean prolactin level was 25.3 ± 22.5 ng/ml in all subjects and 51.6% of participants had hyperprolactinemia. Consistent with our result, other study data have revealed prevalence rates of olanzapine-induced hyperprolactinemia ranging between 6 and 60% (Peuskens et al., 2014). However, our results demonstrated that no dose-dependent effect of olanzapine on prolactin levels in patients with schizophrenia exists. Some studies have supported the dose-dependent effect of olanzapine on plasma prolactin levels (Kinon et al., 2008; Citrome et al., 2009; Suzuki et al., 2011), but others have not (Karagianis and Baksh, 2003; Takeuchi et al., 2014).
The prevalence of sexual dysfunction reported by participants in our study was 53.8%. In Taiwan, the prevalence of sexual dysfunction among men and women aged 40–80 years was observed to be 32 and 51%, respectively (Nicolosi et al., 2005). The rates of sexual dysfunction reported by participants with schizophrenia are higher than those of the general population (De Boer et al., 2015; Zhao et al., 2020). Studies using structured interviews or self-report questionnaires have noted the prevalence of antipsychotic-associated sexual side effects in 30–60% of participants (De Boer et al., 2015). Postsynaptic dopamine blockade, prolactin elevation, and α1-receptor antagonism might be the underlying mechanisms in the pathogenesis of antipsychotic-induced sexual dysfunction (De Boer et al., 2015). Our result supports the findings of studies that have indicated that, among second-generation antipsychotics, olanzapine is moderately associated with the increased prevalence of sexual dysfunction (Dumontaud et al., 2020).
Our study supports that the association between higher prolactin levels and sexual dysfunction in patients with schizophrenia (De Hert et al., 2014). Hyperprolactinemia has profound effects on reproductive health and sexual function, including hypogonadism, decreased libido in both sexes, amenorrhea and infertility in women and low sperm count and reduced muscle mass in men. It is proposed that prolactin elevation might reduce sex hormone release via an alteration of the hypothalamic–pituitary–gonadal axis, and consequently lead to sexual dysfunction (Smith et al., 2002).
We found concomitant antidepressant was associated sexual dysfunction. Furthermore, concomitant antidepressant was associated with inability to achieve erection/lubrication and difficulty of reaching orgasm. One possibility is that antidepressant itself induced sexual dysfunction (Clayton et al., 2016). Another possibility is that antidepressant prescription might imply subjects had depressive symptoms. Depressive symptomatology in patients with schizophrenia may contribute to sexual dysfunction (Dumontaud et al., 2020).
We ascertained that patients with longer duration of illness are at greater risk of sexual dysfunction. The relationship between longer illness duration and sexual dysfunction is likely mediated by extended exposure to antipsychotic treatments and the characteristics of the chronic illness itself (Lee et al., 2015). Some studies, however, have reported that longer antipsychotic treatment duration is not associated with sexual dysfunction (Souaiby et al., 2019). Differences between those in the literature may be attributable to the differences in the current and past antipsychotic medications as well as the characteristics of study subjects. But duration of illness was not associated with total ASEX score or individual ASEX item scores in our study. Further studies are warranted to investigate the relationship between sexual dysfunction and duration of illness in patients with schizophrenia.
A difference between the sexes in the prevalence of sexual dysfunction among patients with schizophrenia is inconsistent in previous studies; some authors have reported higher rates of sexual dysfunction in female patients (Bianco et al., 2019; Huang et al., 2019), others have reported the opposite (Cutler, 2003), whereas some have discovered similar rates for both sexes (Fujii et al., 2010). Our study found that female sex was associated with sexual dysfunction in olanzapine-treated patients with schizophrenia. And female sex was positively associated with total ASEX score as well as difficulty of arousal, inability to achieve erection/lubrication, and difficulty of reaching orgasm. Epidemiologic study reported that the prevalence of sexual dysfunction was higher in women (40–45%) than men (20–30%) (Lewis et al., 2004). In the present study, female patients exhibited significantly higher serum levels of prolactin and metabolic parameters than male patients. Those differences between male and female patients may contribute to gender-related differences in sexual dysfunction.
We found that subjects with hyperprolactinemia had higher insulin levels and HOMA-IR indices than those without hyperprolactinemia. Prolactin is not only a lactogenic hormone; it possesses more than 300 physiological effects, including an influence on metabolism (Andersen and Glintborg, 2018). Several large cohort studies among the general population have concluded that low prolactin levels are associated with metabolic disease and represent a risk factor for type 2 diabetes (Macotela et al., 2020). However, other population studies have reported contradictory results (Balbach et al., 2013; Daimon et al., 2017). In patients with schizophrenia spectrum psychosis, a relationship between hyperprolactinemia and insulin resistance was discovered (Petruzzelli et al., 2018). The effects of prolactin on metabolic homeostasis may be different when prolactin levels are within and outside the physiological range (Daimon et al., 2017; Andersen and Glintborg, 2018). Furthermore, Gragnoli et al. hypothesized that the dopamine–prolactin pathway potentially contributes to the comorbidity of schizophrenia and type 2 diabetes (Gragnoli et al., 2016).
Consistent with studies in general population (Schulster et al., 2017; Di Francesco et al., 2019), we found that subjects with sexual dysfunction had higher HOMA-IR indices than those without sexual dysfunction. However, the underlying mechanisms of how insulin resistance results in sexual dysfunction remain unclear. Insulin resistance plays an important role in the pathogenesis of inflammation and endothelial dysfunction which subsequently progress to cardiovascular disease (Muniyappa and Sowers, 2013). In men, insulin resistance impaired vascular nitric oxide production and insulin-induced vasodilation, both of which are likely to cause sexual dysfunction (Schulster et al., 2017; Maiorino et al., 2018). In women, insulin resistance decreased nitric oxide production and vascular vaginal relaxation, both of which might cause sexual dysfunction (Rahmanian et al., 2019). In addition, insulin had an inhibitory effect on the production of sex hormone-binding globulin by the liver (Wallace et al., 2013). Those might explain that insulin resistance plays an important role in the pathogenesis of sexual dysfunction. But HOMA-IR indices were not associated with total ASEX scores or individual ASEX item scores in our study. Further studies are warranted to investigate the relationship between sexual dysfunction and insulin resistance in patients with schizophrenia.
In this study, participants who were married or with sex partner were negatively associated with inability to achieve erection/lubrication and difficulty of reaching orgasm. Consistent with our result, other studies have reported that marital status was positively associated with sexual arousal and sexual satisfaction (Lee et al., 2015; Fanta et al., 2018). The present findings may be attributed to the fact that married subjects are more likely to have the opportunity to engage in sexual relationship and activity. However, marital status was not associated with sexual dysfunction in this study. Further studies with more comprehensive assessment of sexual function to investigate the role of marital status are warranted.
Some studies have reported a relationship between sexual dysfunction and the severity of psychotic symptoms (Kheng Yee et al., 2014; Simiyon et al., 2016), whereas others did not ascertain such an association (Martin et al., 2018). In this study, we did not determine sexual dysfunction to be associated with the severity of psychopathology. Although we did not exclude patients with severe psychopathology, our naturalistic study design may have unintentionally entailed an inclusion bias favoring participants who were stable psychopathologically.
Consistent with other studies (Yasui-Furukori et al., 2010), male participants in our study recorded comparatively lower prolactin levels than female participants did. This might be partly due to higher base levels of prolactin and higher vulnerability to hyperprolactinemia in response to antipsychotic drugs in women (Kinon et al., 2003). The lower prolactin levels in male participants might partially explain their lower ASEX scores and rates of sexual dysfunction. In concordance the research of Häfner (2002), our study also recorded a higher rate of marriage and partnership among female participants compared with the male participants.
Several limitations were present in our study. First, because of the cross-sectional design of this study, potential selection and confounding biases should be assumed and a causal relationship between hyperprolactinemia and sexual dysfunction cannot be concluded. Second, the exact days in the female participants’ menstrual cycles when the blood tests were performed were not recorded in our study. Third, the absence of healthy controls or drug-naïve patients with schizophrenia in our study rendered the differentiation of the effects on disease and medication impossible. Fourth, patients with schizophrenia in this study were clinically stable; hence, the results cannot be generalized to patients in other illness stages.
In conclusion, approximately half of the olanzapine-treated patients with schizophrenia in our study had hyperprolactinemia, metabolic syndrome, and sexual dysfunction. Higher prolactin levels, concomitant antidepressant, greater insulin resistance indices, longer duration of illness, and female sex were associated with sexual dysfunction. Prolactin levels, metabolic parameters, and sexual function in olanzapine-treated patients with schizophrenia should be monitored regularly.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Wan Fang Hospital, Taipei Medical University. The patients/participants provided their written informed consent to participate in this study.
T-HW, C-HL, and H-YL designed and performed the study. KG, CY-AC, and C-HC helped in patient recruitment, monitor of the data quality, and document treatment related outcomes. M-LL performed the statistical analysis and drafted the manuscript. All authors were responsible for critical revision of the manuscript and have accepted the final version.
The study was supported by grants from the Ministry of Science and Technology, Taipei, Taiwan (MOST 109-2314-B-038-083 and MOST110-2314-B-038-072-MY3), and Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan (108TMU-WFH-07 and 110TMU-WFH-04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.718800/full#supplementary-material
Ahl, J., Kinon, B. J., and Liu-Seifert, H. (2004). Sexual Dysfunction Associated with Neuroleptic-Induced Hyperprolactinemia Improves with Reduction in Prolactin Levels. Ann. N. Y Acad. Sci. 1032, 289–290. doi:10.1196/annals.1314.041
Andersen, M., and Glintborg, D. (2018). Metabolic Syndrome in Hyperprolactinemia. Front. Horm. Res. 49, 29–47. doi:10.1159/000486000
Balbach, L., Wallaschofski, H., Volzke, H., Nauck, M., Dorr, M., and Haring, R. (2013). Serum Prolactin Concentrations as Risk Factor of Metabolic Syndrome or Type 2 Diabetes? BMC Endocr. Disord. 13, 12. doi:10.1186/1472-6823-13-12
Barata, P. C., Santos, M. J., Melo, J. C., and Maia, T. (2019). Olanzapine-Induced Hyperprolactinemia: Two Case Reports. Front. Pharmacol. 10, 846. doi:10.3389/fphar.2019.00846
Bianco, C. L., Pratt, S. I., and Ferron, J. C. (2019). Deficits in Sexual Interest Among Adults with Schizophrenia: Another Look at an Old Problem. Ps 70, 1000–1005. doi:10.1176/appi.ps.201800403
Callaghan, J. T., Bergstrom, R. F., Ptak, L. R., and Beasley, C. M. (1999). Olanzapine. Clin. Pharmacokinet. 37, 177–193. doi:10.2165/00003088-199937030-00001
Citrome, L., Stauffer, V. L., Chen, L., Kinon, B. J., Kurtz, D. L., Jacobson, J. G., et al. (2009). Olanzapine Plasma Concentrations after Treatment with 10, 20, and 40 Mg/d in Patients with Schizophrenia. J. Clin. Psychopharmacol. 29, 278–283. doi:10.1097/jcp.0b013e3181a289cb
Clayton, A. H., Alkis, A. R., Parikh, N. B., and Votta, J. G. (2016). Sexual Dysfunction Due to Psychotropic Medications. Psychiatr. Clin. North America 39, 427–463. doi:10.1016/j.psc.2016.04.006
Cowen, P. J., Anderson, I. M., and Gartside, S. E. (1990). Endocrinological Responses to 5-HT. Ann. NY Acad. Sci. 600, 250–257. discussion 257-259. doi:10.1111/j.1749-6632.1990.tb16887.x
Cutler, A. J. (2003). Sexual Dysfunction and Antipsychotic Treatment. Psychoneuroendocrinology 28 (Suppl. 1), 69–82. doi:10.1016/s0306-4530(02)00113-0
Daimon, M., Kamba, A., Murakami, H., Mizushiri, S., Osonoi, S., Yamaichi, M., et al. (2017). Association between Serum Prolactin Levels and Insulin Resistance in Non-diabetic Men. PLoS One 12, e0175204. doi:10.1371/journal.pone.0175204
De Boer, M. K., Castelein, S., Wiersma, D., Schoevers, R. A., and Knegtering, H. (2015). The Facts about Sexual (Dys)function in Schizophrenia: an Overview of Clinically Relevant Findings. Schizophrenia Bull. 41, 674–686. doi:10.1093/schbul/sbv001
De Hert, M., Detraux, J., and Peuskens, J. (2014). Second-generation and Newly Approved Antipsychotics, Serum Prolactin Levels and Sexual Dysfunctions: a Critical Literature Review. Expert Opin. Drug Saf. 13, 605–624. doi:10.1517/14740338.2014.906579
Dehelean, L., Romosan, A. M., Papava, I., Bredicean, C. A., Dumitrascu, V., Ursoniu, S., et al. (2020). Prolactin Response to Antipsychotics: An Inpatient Study. PLoS One 15, e0228648. doi:10.1371/journal.pone.0228648
Di Francesco, S., Caruso, M., Robuffo, I., Militello, A., and Toniato, E. (2019). The Impact of Metabolic Syndrome and its Components on Female Sexual Dysfunction: A Narrative Mini-Review. Curr. Urol. 12, 57–63. doi:10.1159/000489420
Dumontaud, M., Korchia, T., Khouani, J., Lancon, C., Auquier, P., Boyer, L., et al. (2020). Sexual Dysfunctions in Schizophrenia: Beyond Antipsychotics. A Systematic Review. Prog. Neuro-Psychopharmacology Biol. Psychiatry 98, 109804. doi:10.1016/j.pnpbp.2019.109804
Fanta, T., Haile, K., Abebaw, D., Assefa, D., and Hibdye, G. (2018). Assessment of Sexual Dysfunction and Associated Factors Among Patients with Schizophrenia in Ethiopia, 2017. BMC Psychiatry 18, 158. doi:10.1186/s12888-018-1738-3
Finn, S. E., Bailey, J. M., Schultz, R. T., and Faber, R. (1990). Subjective Utility Ratings of Neuroleptics in Treating Schizophrenia. Psychol. Med. 20, 843–848. doi:10.1017/s0033291700036539
Fujii, A., Yasui-Furukori, N., Sugawara, N., Sato, Y., Nakagami, T., Saito, M., et al. (2010). Sexual Dysfunction in Japanese Patients with Schizophrenia Treated with Antipsychotics. Prog. Neuro-Psychopharmacology Biol. Psychiatry 34, 288–293. doi:10.1016/j.pnpbp.2009.11.022
Gragnoli, C., Reeves, G. M., Reazer, J., and Postolache, T. T. (2016). Dopamine-prolactin Pathway Potentially Contributes to the Schizophrenia and Type 2 Diabetes Comorbidity. Transl Psychiatry 6, e785. doi:10.1038/tp.2016.50
Grigg, J., Worsley, R., Thew, C., Gurvich, C., Thomas, N., and Kulkarni, J. (2017). Antipsychotic-induced Hyperprolactinemia: Synthesis of World-wide Guidelines and Integrated Recommendations for Assessment, Management and Future Research. Psychopharmacology 234, 3279–3297. doi:10.1007/s00213-017-4730-6
Häfner, H. (2002). Schizophrenia: Do Men and Women Suffer from the Same Disease? Rev. Psiquiatr. Clín. 29, 267–292. doi:10.1590/s0101-60832002000600002
Hirsch, L., Yang, J., Bresee, L., Jette, N., Patten, S., and Pringsheim, T. (2017). Second-Generation Antipsychotics and Metabolic Side Effects: A Systematic Review of Population-Based Studies. Drug Saf. 40, 771–781. doi:10.1007/s40264-017-0543-0
Huang, Y.-H., Hou, C.-L., Ng, C. H., Chen, X., Wang, Q.-W., Huang, Z.-H., et al. (2019). Sexual Dysfunction in Chinese Rural Patients with Schizophrenia. BMC Psychiatry 19, 218. doi:10.1186/s12888-019-2205-5
Huhn, M., Nikolakopoulou, A., Schneider-Thoma, J., Krause, M., Samara, M., Peter, N., et al. (2019). Comparative Efficacy and Tolerability of 32 Oral Antipsychotics for the Acute Treatment of Adults with Multi-Episode Schizophrenia: a Systematic Review and Network Meta-Analysis. The Lancet 394, 939–951. doi:10.1016/s0140-6736(19)31135-3
Johnsen, E., Kroken, R., Løberg, E. M., Kjelby, E., and Jørgensen, H. A. (2011). Sexual Dysfunction and Hyperprolactinemia in Male Psychotic Inpatients: a Cross-Sectional Study. Adv. Urol. 2011, 686924. doi:10.1155/2011/686924
Karagianis, J. L., and Baksh, A. (2003). High-dose Olanzapine and Prolactin Levels. J. Clin. Psychiatry 64, 1192–1194. doi:10.4088/jcp.v64n1008
Kay, S. R., Fiszbein, A., and Opler, L. A. (1987). The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophrenia Bull. 13, 261–276. doi:10.1093/schbul/13.2.261
Kheng Yee, O., Muhd Ramli, E. R., and Che Ismail, H. (2014). Remitted Male Schizophrenia Patients with Sexual Dysfunction. J. Sex. Med. 11, 956–965. doi:10.1111/jsm.12246
Kikuchi, T., Iwamoto, K., Sasada, K., Aleksic, B., Yoshida, K., and Ozaki, N. (2012). Sexual Dysfunction and Hyperprolactinemia in Japanese Schizophrenic Patients Taking Antipsychotics. Prog. Neuro-Psychopharmacology Biol. Psychiatry 37, 26–32. doi:10.1016/j.pnpbp.2011.11.016
Kinon, B. J., Gilmore, J. A., Liu, H., and Halbreich, U. M. (2003). Hyperprolactinemia in Response to Antipsychotic Drugs: Characterization across Comparative Clinical Trials. Psychoneuroendocrinology 28 (Suppl. 2), 69–82. doi:10.1016/s0306-4530(02)00128-2
Kinon, B. J., Volavka, J., Stauffer, V., Edwards, S. E., Liu-Seifert, H., Chen, L., et al. (2008). Standard and Higher Dose of Olanzapine in Patients with Schizophrenia or Schizoaffective Disorder. J. Clin. Psychopharmacol. 28, 392–400. doi:10.1097/jcp.0b013e31817e63a5
Lee, J.-Y., Kim, S.-W., Lee, Y.-H., Kang, H.-J., Kim, S.-Y., Bae, K.-Y., et al. (2015). Factors Associated with Self-Rated Sexual Function in Korean Patients with Schizophrenia Receiving Risperidone Monotherapy. Hum. Psychopharmacol. Clin. Exp. 30, 416–424. doi:10.1002/hup.2489
Lewis, R. W., Fugl‐Meyer, K. S., Bosch, R., Fugl‐Meyer, A. R., Laumann, E. O., Lizza, E., et al. (2004). Epidemiology/risk Factors of Sexual Dysfunction. J. Sex. Med. 1, 35–39. doi:10.1111/j.1743-6109.2004.10106.x
Lu, M.-L., Chen, C.-H., Kuo, P.-T., Lin, C.-H., and Wu, T.-H. (2018). Application of Plasma Levels of Olanzapine and N -Desmethyl-Olanzapine to Monitor Metabolic Parameters in Patients with Schizophrenia. Schizophrenia Res. 193, 139–145. doi:10.1016/j.schres.2017.07.022
Macotela, Y., Triebel, J., and Clapp, C. (2020). Time for a New Perspective on Prolactin in Metabolism. Trends Endocrinol. Metab. 31, 276–286. doi:10.1016/j.tem.2020.01.004
Maiorino, M. I., Bellastella, G., Giugliano, D., and Esposito, K. (2018). From Inflammation to Sexual Dysfunctions: a Journey through Diabetes, Obesity, and Metabolic Syndrome. J. Endocrinol. Invest. 41, 1249–1258. doi:10.1007/s40618-018-0872-6
Martín, J. C., Acuña, M. J., Labrador, J., Blanco, M., and Casas, C. (2018). Sexual Dysfunction Factors in Patients with Schizophrenia Treated with Second Generation Antipsychotics: Not Only Prolactin. Actas Esp Psiquiatr 46, 217–225.
Matthews, D. R., Hosker, J. P., Rudenski, A. S., Naylor, B. A., Treacher, D. F., and Turner, R. C. (1985). Homeostasis Model Assessment: Insulin Resistance and ?-cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 28, 412–419. doi:10.1007/bf00280883
Mcgahuey, C. A., Gelenberg, A. J., Laukes, C. A., Moreno, F. A., Delgado, P. L., Mcknight, K. M., et al. (2000). The Arizona Sexual Experience Scale (ASEX): Reliability and Validity. J. Sex. Marital Ther. 26, 25–40. doi:10.1080/009262300278623
Muniyappa, R., and Sowers, J. R. (2013). Role of Insulin Resistance in Endothelial Dysfunction. Rev. Endocr. Metab. Disord. 14, 5–12. doi:10.1007/s11154-012-9229-1
Nicolosi, A., Glasser, D. B., Kim, S. C., Marumo, K., Laumann, E. O., and Group, G. I. (2005). Sexual Behaviour and Dysfunction and Help-Seeking Patterns in Adults Aged 40-80 Years in the Urban Population of Asian Countries. BJU Int. 95, 609–614. doi:10.1111/j.1464-410x.2005.05348.x
Petruzzelli, M. G., Margari, M., Peschechera, A., De Giambattista, C., De Giacomo, A., Matera, E., et al. (2018). Hyperprolactinemia and Insulin Resistance in Drug Naive Patients with Early Onset First Episode Psychosis. BMC Psychiatry 18, 246. doi:10.1186/s12888-018-1827-3
Peuskens, J., Pani, L., Detraux, J., and De Hert, M. (2014). The Effects of Novel and Newly Approved Antipsychotics on Serum Prolactin Levels: a Comprehensive Review. CNS Drugs 28, 421–453. doi:10.1007/s40263-014-0157-3
Rahmanian, E., Salari, N., Mohammadi, M., and Jalali, R. (2019). Evaluation of Sexual Dysfunction and Female Sexual Dysfunction Indicators in Women with Type 2 Diabetes: a Systematic Review and Meta-Analysis. Diabetology Metab. Syndr. 11, 73. doi:10.1186/s13098-019-0469-z
Rubio-Abadal, E., Del Cacho, N., Saenz-Navarrete, G., Arranz, B., Cambra, R.-M., Cuadras, D., et al. (2016). How Hyperprolactinemia Affects Sexual Function in Patients under Antipsychotic Treatment. J. Clin. Psychopharmacol. 36, 422–428. doi:10.1097/jcp.0000000000000539
Schulster, M. L., Liang, S. E., and Najari, B. B. (2017). Metabolic Syndrome and Sexual Dysfunction. Curr. Opin. Urol. 27, 435–440. doi:10.1097/mou.0000000000000426
Serretti, A., and Chiesa, A. (2011). A Meta-Analysis of Sexual Dysfunction in Psychiatric Patients Taking Antipsychotics. Int. Clin. Psychopharmacol. 26, 130–140. doi:10.1097/yic.0b013e328341e434
Simiyon, M., Chandra, P. S., and Desai, G. (2016). Sexual Dysfunction Among Women with Schizophrenia-A Cross Sectional Study from India. Asian J. Psychiatry 24, 93–98. doi:10.1016/j.ajp.2016.08.022
Smith, S., Wheeler, M. J., Murray, R., and O’Keane, V. (2002). The Effects of Antipsychotic-Induced Hyperprolactinaemia on the Hypothalamic-Pituitary-Gonadal Axis. J. Clin. Psychopharmacol. 22, 109–114. doi:10.1097/00004714-200204000-00002
Souaiby, L., Kazour, F., Zoghbi, M., Bou Khalil, R., and Richa, S. (2019). Sexual Dysfunction in Patients with Schizophrenia and Schizoaffective Disorder and its Association with Adherence to Antipsychotic Medication. J. Ment. Health, 29 1–8. doi:10.1080/09638237.2019.1581333
Suzuki, Y., Ono, S., Sugai, T., Fukui, N., Watanabe, J., Tsuneyama, N., et al. (2011). Dose-dependent Effects of Olanzapine on QT Intervals and Plasma Prolactin Levels in Japanese Patients with Stable Schizophrenia. Hum. Psychopharmacol. 26, 440–443. doi:10.1002/hup.1218
Takeuchi, H., Suzuki, T., Remington, G., Watanabe, K., Mimura, M., and Uchida, H. (2014). Lack of Effect of Risperidone or Olanzapine Dose Reduction on Metabolic Parameters, Prolactin, and Corrected QT Interval in Stable Patients with Schizophrenia. J. Clin. Psychopharmacol. 34, 517–520. doi:10.1097/jcp.0000000000000142
Tan, C.-E., Ma, S., Wai, D., Chew, S.-K., and Tai, E.-S. (2004). Can We Apply the National Cholesterol Education Program Adult Treatment Panel Definition of the Metabolic Syndrome to Asians?. Diabetes Care 27, 1182–1186. doi:10.2337/diacare.27.5.1182
Wallace, I. R., Mckinley, M. C., Bell, P. M., and Hunter, S. J. (2013). Sex Hormone Binding Globulin and Insulin Resistance. Clin. Endocrinol. 78, 321–329. doi:10.1111/cen.12086
Yasui-Furukori, N., Saito, M., Nakagami, T., Sugawara, N., Sato, Y., Tsuchimine, S., et al. (2010). Gender-specific Prolactin Response to Antipsychotic Treatments with Risperidone and Olanzapine and its Relationship to Drug Concentrations in Patients with Acutely Exacerbated Schizophrenia. Prog. Neuro-Psychopharmacology Biol. Psychiatry 34, 537–540. doi:10.1016/j.pnpbp.2010.02.014
Keywords: olanzapine, metabolic syndrome, prolactin, sexual dysfunction, schizophrenia
Citation: Wu T-H, Lin C-H, Goh KK, Chen CY-A, Chen C-H, Lane H-Y and Lu M-L (2021) The Relationships Between Hyperprolactinemia, Metabolic Disturbance, and Sexual Dysfunction in Patients With Schizophrenia Under Olanzapine Treatment. Front. Pharmacol. 12:718800. doi: 10.3389/fphar.2021.718800
Received: 01 June 2021; Accepted: 26 July 2021;
Published: 05 August 2021.
Edited by:
Philippe De Deurwaerdere, Université de Bordeaux, FranceCopyright © 2021 Wu, Lin, Goh, Chen, Chen, Lane and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mong-Liang Lu, bW9uZ2xpYW5nQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.