- 1School of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, China
- 2School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
- 3School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
Guhong injection (GHI) is a drug for ischemic stroke created by combining safflower, a traditional Chinese medicine, and aceglutamide, a Western medicine. In this study, we investigated the curative effect of GHI on cerebral ischemia–reperfusion (I/R) injury via the PKC/HIF-1α pathway in rats. Adult male Sprague Dawley rats were randomly divided into seven groups: sham-operated, middle cerebral artery occlusion (MCAO), GHI, nimodipine injection (NMDP), MCAO + LY317615 (PKC inhibitor), GHI + LY317615, and NMDP + LY317615. After establishing an MCAO rat model, we performed neurological deficit testing, 2,3,5-triphenyltetrazolium chloride staining, hematoxylin and eosin (HE) staining, enzyme-linked immunosorbent assay, Western blotting, and q-PCR to detect the brain damage in rats. Compared with the MCAO group, the GHI and GHI + LY317615 group showed neurological damage amelioration as well as decreases in serum hypoxia-inducible factor-1α (HIF-1α), protein kinase C (PKC), and erythropoietin levels; brain HIF-1α and inducible nitric oxide synthase protein expression; and brain HIF-1α and NOX-4 mRNA expression. These effects were similar to those in the positive control groups NMDP and NMDP + LY317615. Thus, our results confirmed GHI can ameliorate cerebral I/R injury in MCAO rats possibly via the PKC/HIF-1α pathway.
Introduction
Ischemic stroke, a serious cerebrovascular disorder, is a leading cause of death and disability globally (Wang et al., 2013; Yuan et al., 2020). Its pathogenesis includes apoptosis, inflammation, oxidative and nitrative stress, neurotoxicity, and calcium overload (Jin et al., 2017; Sun et al., 2018a; Cheon et al., 2018). Tissue plasminogen activator (tPA) is the only FDA-approved drug for acute stroke treatment; however, only <5% of patients with ischemic stroke receive tPA because it has the potential to cause hemorrhagic transformation (Chalouhi et al., 2013; Bruch et al., 2019; Kuo et al., 2020). Several recent studies have focused on finding effective medicine formulations with significant effects on ischemic stroke.
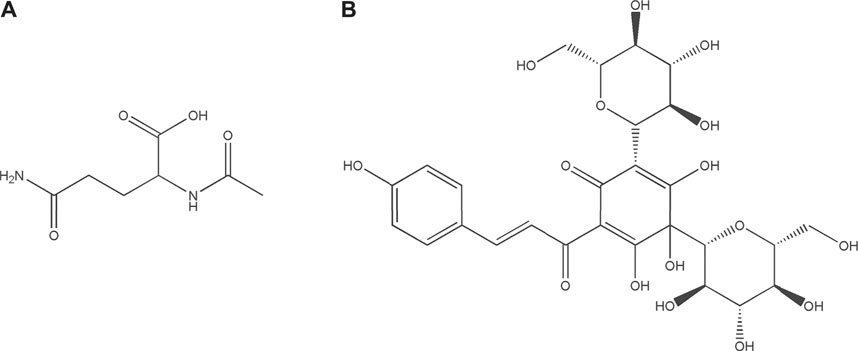
Guhong injection (GHI) is a typical sterile solution created by combining aqueous Carthamus tinctorius Linné [Asteraceae; Carthami Flos] extract (a traditional Chinese medicine) and aceglutamide (a Western medicine) (Wang et al., 2020). In other words, this is a preparation innovation for the sublimation of ethnomedicine or ethnopharmacy. We previously established GHI’s high-performance liquid chromatography fingerprint (He et al., 2015; Ai et al., 2017). GHI has been extensively applied in cerebrovascular disease treatment, mainly because of its anti-inflammatory and antioxidative effects (Ai et al., 2017; Shu et al., 2014; Zhou et al., 2021). Acetylglutamine (Figure 1A) is an acetyl derivative of glutamine—one of the most abundant free amino acids in humans (Xu et al., 2020). Aceglutamide can attenuate cerebral ischemia–reperfusion (I/R) injury (Zhang et al., 2020). Carthamus tinctorius Linné is commonly known as Safflower (honghua in Chinese), which is used in a dried form for treating various cardiovascular and cerebrovascular diseases clinically because its effects include blood stasis removal and meridian dredging (Zhang et al., 2016). Hydroxysafflor yellow A (Figure 1B), a main safflower component, can be used for treating ischemic diseases via platelet aggregation and thrombosis inhibition (Chen et al., 2016; Li et al., 2016). However, the mechanism underlying GHI’s effects on cerebral I/R injury has not been elucidated.
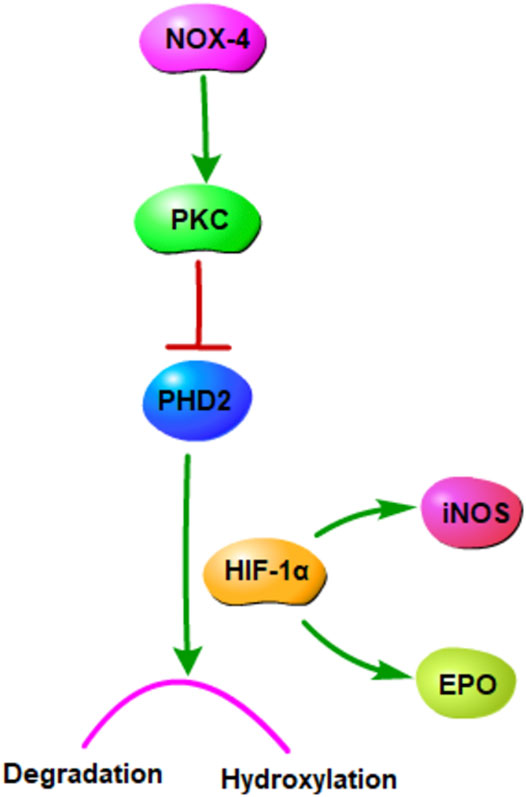
Nicotinamide adenine dinucleotide phosphate oxidase (NOX) 4 is a major reactive oxygen species source, and it plays a key role in cerebral I/R injury treatment (Pushpakumar et al., 2020). It is involved in the oxidative pentose phosphate metabolic pathway and related to myosin light-chain kinase activation (Sun et al., 2018b; Geng et al., 2019). Protein kinase C (PKC), a family of threonine serine kinases involved in many biological processes, is involved in NOX activation (Tang et al., 1994; Gulati and Singh, 2014). Hypoxia-inducible factor-1α (HIF-1α), an critical transcriptional factor regulating hypoxia and ischemia responses (Yu et al., 2020), is activated by PKC and induced hydroxylation and degradation by the enzymes prolyl hydroxylase domain (PHD) (Gibbs et al., 2011). The expression of the HIF-1α downstream gene can produce many products, including erythropoietin (EPO), NOS, EGF, PAI-1, and VEGF (Wang et al., 2019).
In this study, we investigated the pharmacological effects of GHI on cerebral I/R injury in rats and elucidated the underlying mechanism. Our results may lay an experimental foundation for clinical research on GHI in cerebrovascular disease treatment.
Materials and Methods
Reagents and Chemicals
GHI was procured from Tonghua Guhong Pharmaceutical (Jilin, China), nimodipine injection (NMDP) from Bayer Schering AG (Leverkusen, Germany), and 2,3,5-triphenyltetrazolium chloride (TTC) from Hangzhou Dacheng Biotechnology (Hangzhou, China). Moreover, enzyme-linked immunosorbent assay (ELISA) kits for HIF-1α, EPO, and PKC were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). iNOS, PHD2, and HIF-1α antibodies were purchased from abcam Biotechnology Co., Ltd. (Hangzhou, China).
Quality Control of GHI
According to the Chinese national standard [No. WS-10001-(HD-1506)-2004] promulgated by Chinese Pharmacopoeia Commission and the process of GHI: Each 1,000 ml of GHI contains safflower equivalent to 500 g and aceglutamide equivalent to 30 g (Ai et al., 2017). According to the corresponding quality control standard, the content of HYSA should not be lower than 0.15 mg/ml and the content of aceglutamide should be in the range of 27–33 mg/ml when detected by HPLC (Ai et al., 2017; Zhou et al., 2021). In ten batches of GHI, our research group found 21 common peaks (Ai et al., 2017). The contents of seven representative ingredients (aceglutamide, uridine, adenosine, guanosine, syringing, hydroxysafflower yellow A, and anhydrosafflor yellow B) were determined (He et al., 2015). The above studies proved that the preparation process of GHI was stable.
Animals
Adult male Sprague Dawley rats (age = 10–12 weeks, weight = 260–300 g) were purchased from the Animal Center of Zhejiang Chinese Medicine University (laboratory animal certificate: SYXK 2018-0012). The rats were housed at room temperature with humidity 60 ± 5% under a consistent 12-h light–dark cycle with ad libitum access to food and water. All the experiment procedures were approved by the Animal Subjects Review Board of Zhejiang Chinese Medical University.
Middle Cerebral Artery Occlusion Model Establishment
Middle cerebral artery occlusion (MCAO) method was performed according to a previously described method (Yu et al., 2018). In brief, the rats were anesthetized with 2% sodium pentobarbital (30 mg/kg) via intraperitoneal injection, and their common carotid artery (CCA), external carotid artery, and internal carotid artery (ICA) were exposed after the midline neck incision. Next, a 0.28-mm polylysine-coated nylon monofilament was inserted from the CCA into the ICA until the cerebral middle artery was reached (18 ± 2 mm). The nylon monofilament was removed after 1 h of ischemia induction. Effective establishment of MCAO was judged based on the presence of Horner syndrome in the right eye, the contralateral claw of the forelimb, and crawling in a circle to the left.
Animal Grouping and Drug Treatment
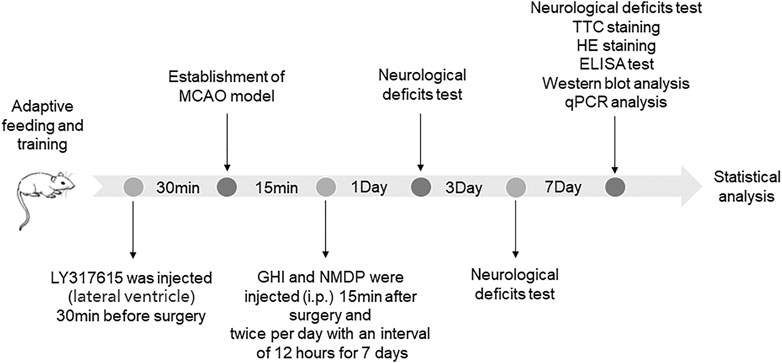
All rats were randomly divided into seven groups (n = 18 per group): sham-operated (Sham), MCAO, GHI, NMDP, MCAO + LY317615, GHI + LY317615, and NMDP + LY317615. The rats were injected with 10 ml/kg twice per day of GHI or NMDP intraperitoneally (Ai et al., 2017; Zhou et al., 2021). The dosage of LY317615 was 10 μl. The Sham and MCAO groups were intraperitoneal injected the same amount of physiological saline. All drugs or saline were administered twice per day with an interval of 12 h for 7 days. The schedule of the experimental procedures is presented in Figure 2.
Behavioral Observations
An examiner blinded to animal treatment scored the neurological deficits 1, 3, and 7 days after MCAO by using the modified version of Garcia sensorimotor (Han et al., 2020) and Longa motor functions and neurologic (Li et al., 2020) scoring systems.
For the Garcia scoring system, spontaneous activity (0–3 points), symmetry of movements (0–3 points), symmetry of forelimbs (0–3 points), climbing wall of wire cage (1–3 points), reaction to touch on either side of trunk (1–3 points), and response to vibrissal touch (1–3 points) were evaluated. The maximum and minimum scores were 18 and 3, respectively.
In the Longa scoring system, the scores were as follows: 0 for no deficits, 1 for contralateral forepaw inflection when tail is lifted, 2 for spontaneous circling to the contralateral side, 3 for hemiplegia to the contralateral side, and 4 for inability to move spontaneously without consciousness.
Infarct Volume Measurement
The infarct volume was determined using 2,3,5-triphenyltetrazolium chloride (TTC) staining. Rat brain samples were collected 7 days after treatment administration and immediately frozen at −20°C for 15 min. The frozen samples were then cut into 2-mm-thick coronal slices and then stained with 2% TTC solution at 37°C for 20 min in the dark. These slices were photographed, and the infarct volume was calculated using ImageJ.
Histopathology Study
The hippocampus samples of brain tissues were collected in a cryotube with 4% paraformaldehyde after reperfusion. The samples were dehydrated and embedded in paraffin, after which coronal sections were prepared. These coronal slices were stained with hematoxylin and eosin (HE) and then used for studying histopathology. Finally, the hippocampal tissues were analyzed by acquiring images on a microscope (Eclipse TI-SR with DS-U3; Nikon).
Serum Biochemistry
Rat blood samples were collected from the heart under deep anesthesia, and the serum samples were prepared by centrifugation at 4,500 rpm for 12 min at 4°C. The supernatant (serum) was than extracted and stored at −20°C before ELISA. Next, by using specific ELISA kits according to the manufacturer's instruction, we determined serum HIF-1α, PKC, and EPO concentrations.
Western Blot Analysis
The protein samples of rat brain were lysed using a lysis buffer, followed by centrifugation at 12,000 rpm for 5 min at 4°C. Then, the samples were electrophoresed via the sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. After we added a blocking solution containing 5% skimmed milk powder with shaking for 2 h, the membrane was incubated in a primary antibody dilution buffer with shaking at 4°C overnight. This was followed by the addition of secondary antibodies and shaking for 1 h. Finally, electrochemiluminescence was observed using a chemi-capture software program.
Quantitative Polymerase Chain Reaction
Total RNA of brain samples were extracted using TRIzol solution, followed by centrifugation at 12,000 rpm and 4°C for 10 min after chloroform addition. Next, we aspirated the supernatant. To this, we added isopropyl alcohol and centrifuged the solution at 12,000 rpm for 15 min at 4°C. The precipitate was washed with absolute ethanol to obtain RNA.
This total RNA was reverse transcribed using the HiFiScript cDNA Synthesis Kit (Kangwei Century Biotechnology). This was followed by the real-time quantitative polymerase chain reaction (q-PCR) with the following cycling parameters: 40 cycles of 95°C for 15 s and 60°C for 60 s. The relative mRNA expression levels were standardized using β-actin (the housekeeping gene). The primer sequences are listed in Table 1.
Statistical Analyses
All statistical analyses were performed on GraphPad Prism (version 8.0; GraphPad Software, San Diego, CA, United States ). Here, one-way analysis of variance was used to compare the differences between multiple groups. All data are presented as means ± standard deviations, with p < 0.05 considered to denote statistically significant.
Results
GHI Alleviated Neurological Deficits
The neurological scores were evaluated 1, 3, and 7 days after MCAO. As shown in Figure 3, the Garcia score for the Sham group was 18, indicating the absence of any neurological deficit. Moreover, compared with the MCAO group, the GHI, NMDP, MCAO + LY317615, GHI + LY317615, and NMDP + LY317615 groups showed significant improvements in neurological deficits on days 1, 3, and 7 (p < 0.05 or p < 0.01).
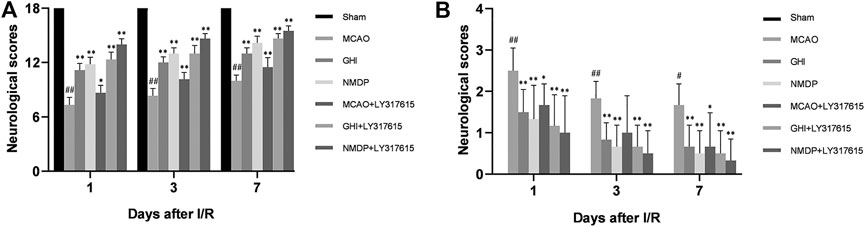
FIGURE 3. The Garcia neurological score (A) and Longa neurological score (B) in rats for each group (
Based on the Longa scores (which were consistent with the Garcia scores), the neurologic deficits were the most serious in the MCAO group. Compared with the MCAO group, the GHI, NMDP, MCAO + LY317615, GHI + LY317615, and NMDP + LY317615 groups showed significant decreased in the Longa scores on days 1, 3, and 7 (P < 0.01), indicating that GHI could alleviate neurological deficits.
GHI Protected Against Increased Brain Infarct Volume
A representative photograph of TTC staining is displayed in Figure 4A, and the infarct volume in each group is presented in Figure 4B. In the Sham group, there was no infarction in rat brain. In the MCAO group, there was obvious infarction compared with the Sham group. Compared with the MCAO group, the infarct volume was significantly lower in the GHI, NMDP, MCAO + LY317615, GHI + LY317615, and NMDP + LY317615 group—indicating that GHI could alleviate the cerebral I/R injury.
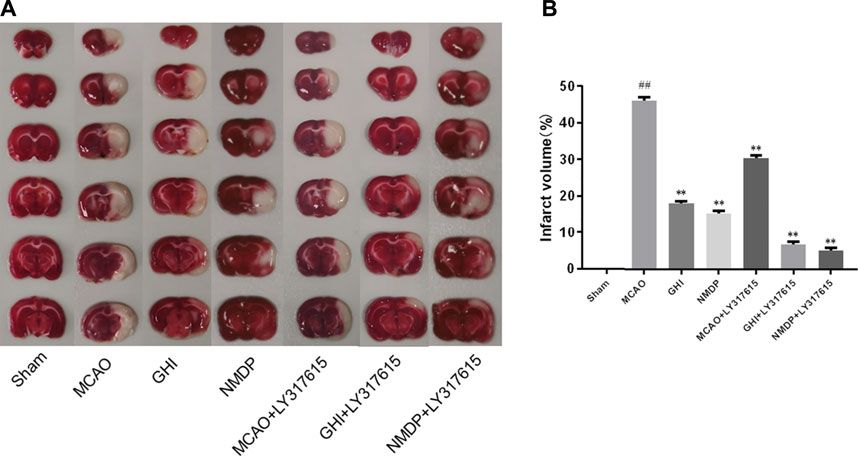
FIGURE 4. TTC staining (A) and infarct volume (B) of each group (
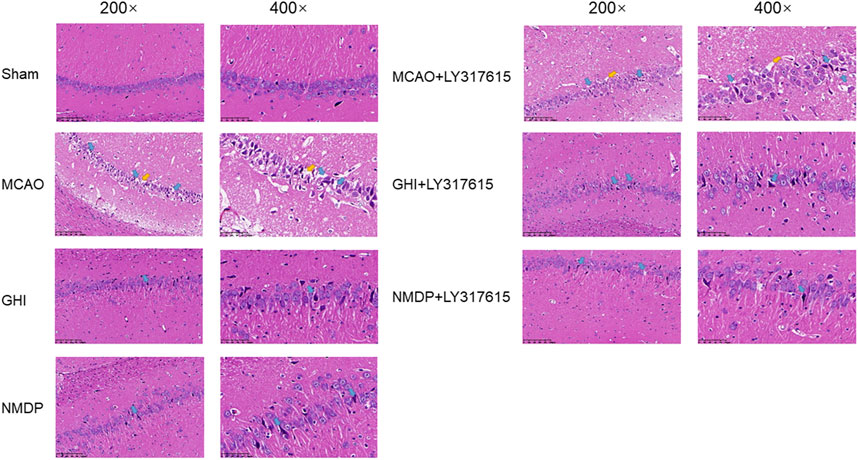
GHI Protected Neurons Against I/R Injury
The morphological changes in the cerebral hippocampus are illustrated in Figure 5. The Sham group showed neurons arranged in an orderly manner without any necrosis. However, the MCAO group had neurons in a disordered arrangement and abnormal cell structures, such as shrunken cytoplasm and deeply stained nuclei. Moreover, the MCAO group had significantly fewer live neurons than did the Sham group. In the GHI, GHI + LY317615, MCAO + LY317615, NMDP, and NMDP + LY317615 groups, the hippocampal neurons were arranged in a more orderly manner and had more lightly stained nuclei than were those in the MCAO group.
GHI Regulated Serum HIF-1α, PKC, and EPO Expression After I/R Injury
Rat serum EPO, HIF-1α, and PKC expression was measured using specific ELISA kits. As shown in Figure 6, compared with the Sham group, the MCAO group significantly higher EPO, HIF-1α, and PKC levels (p < 0.01). In addition, compared with those in the MCAO group, EPO, HIF-1α, and PKC levels in the GHI, GHI + LY317615, MCAO + LY317615, NMDP, and NMDP + LY317615 groups were significant lower. These levels were similar to those in the Sham group.
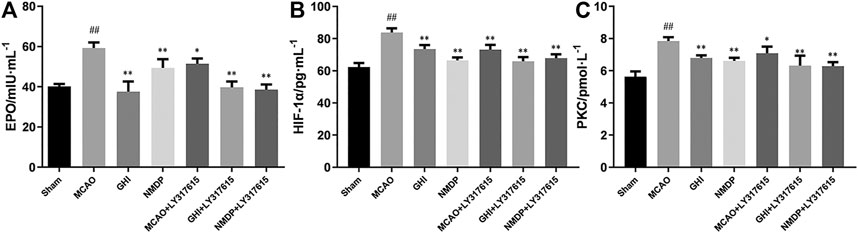
FIGURE 6. The expression of EPO (A), HIF-1α (B) and PKC (C) in rat after I/R for each group (
GHI Inhibited I/R Injury-Induced Activation via the PKC/HIF-1α Pathway
Representative Western blots are shown in Figure 7. Compared with the Sham group, the MCAO group showed significantly higher HIF-1α and iNOS expression and significantly lower PHD2 expression (all p < 0.01). Moreover, compared with the MCAO group, the GHI, NMDP, MCAO + LY317615, GHI + LY317615, and NMDP + LY317615 groups had significantly lower HIF-1 and iNOS expression and significantly higher PHD2 expression (p < 0.05 or p < 0.01). Thus, GHI alleviated cerebral I/R injury possibly via the PKC/HIF-1α pathway.
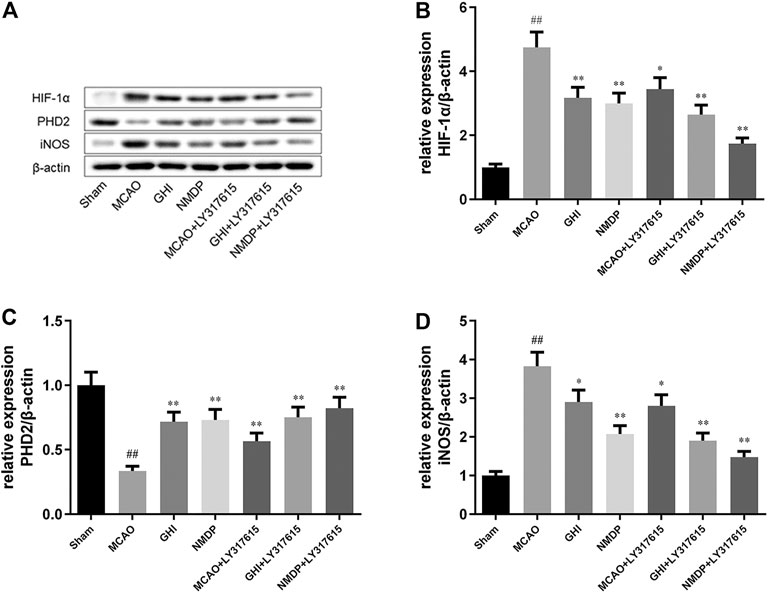
FIGURE 7. Representative photographs of Western blotting (A); the expression of HIF-1α (B), PHD2 (C), iNOS (D) protein in rat after I/R for each group (
GHI Reduced Nox4 and Hif1α mRNA Expression
As shown in Figure 8, compared with the Sham group, the MCAO group had significantly higher Nox4 and Hif1α mRNA expression (p < 0.01). By contrast, compared with the MCAO group, the GHI, NMDP, MCAO + LY317615, GHI + LY317615, and NMDP + LY317615 groups had lower Nox4 and Hif1α mRNA expression (p < 0.05 or p < 0.01).
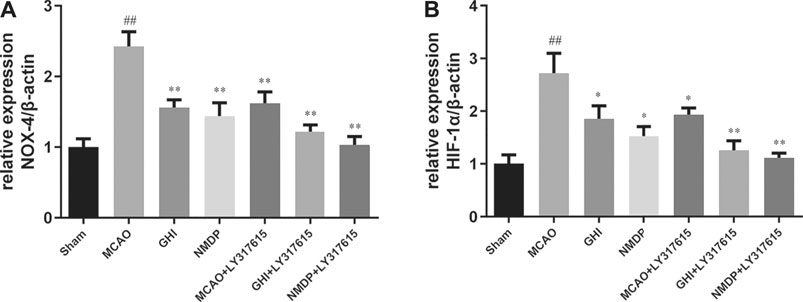
FIGURE 8. The expression of NOX-4 (A) and HIF-1α (B) mRNA in rat after I/R for each group (
Discussion
Nowadays, ethnomedicine or ethnopharmacy is playing a more and more important role in the treatment of tumor, immunity, and inflammation (Michael Heinrich, 2006; Koshak et al., 2018), especially in the treatment of brain diseases.
In the recent years, ischemic stroke has caused high mortality and morbidity worldwide (Zhang et al., 2011). Cerebral ischemia leads to cognitive dysfunction and vascular dementia, which leads to increased economic and mental burden to patients and their families (Uemura et al., 2020). Studies have shown that the mechanisms underlying cerebral ischemia are complex (Liu et al., 2020; Mavroudakis et al., 2020). Moreover, traditional Chinese medicine formulations may be effect in ischemic stroke treatment (Orgah et al., 2019; Shen et al., 2020).
GHI, a formulation made by combining traditional Chinese and Western medicine components, is used for treating cerebral ischemia and coronary microvascular disease (Ai et al., 2017; Jieqin et al., 2020). We have previously studied the pharmacokinetics of the active components of GHI was investigated in rat blood and brain (Yu et al., 2018; Xu et al., 2020). Pharmacological studies have shown that GHI protects against cerebral I/R injury through anti-inflammatory and antithrombotic effects (Shu et al., 2014; Ai et al., 2017). Consistent with these studies, here we found that that GHI exerts a strong amelioration effect on cerebral I/R injury—as indicated by the reduced neurological deficit score, cerebral infarct volume, and neuronal damage. At the same time, other studies also found that GHI can protect hippocampal neurons and endothelial cells from damage by oxygen-glucose deprivation (Dai et al., 2014; Du et al., 2017; Wang et al., 2020). And these studies focus on anti-inflammatory, anti-apoptosis, anti-oxidation and other aspects of experimental research.
In the current study, the rat model of MCAO was established to explore the effects of GHI on cerebral I/R injury and understand their underlying mechanisms. First, the Garcia and Longa scoring systems were used to detected neurological deficit, with the former affording more specific results than the latter. Moreover, the Garcia score included the tests for wire cage wall climbing and tactile reflexes. Based on the results of the neurological deficit scores and TTC staining, the rats in the GHI and GHI + LY317615 groups were found to have less neurological damage and lower infarct volume—indicating that GHI had protective effects on cerebral I/R injury. HE staining showed similar results in that the GHI and GHI + LY317615 groups demonstrated significant improvements in the neuron number and morphology compared with the MCAO group. In general, GHI’s effects were similar to those of the positive control drug, NMDP.
Moreover, hypoxia is a key factor determining cell death or survival in ischemic injury. The mechanism underlying the effects of GHI on cerebral I/R injury was investigated using ELISA, Western blotting, and qPCR. The results revealed that compared with the MCAO group, the GHI and GHI + LY317615 groups had significantly lower serum HIF-1α, PKC, and EPO concentrations and brain Hif1α mRNA and HIF-1α expression. These results indicated that GHI could protect against cerebral I/R injury by inhibiting the PKC/HIF-1α signaling pathway. NOX4 is a major prooxidant enzyme in cerebral ischemia pathogenesis. PKC is a protein family with containing >10 isozymes, and it plays a main role in the NADPH oxidase signaling pathway, MAPK signaling pathway, and NF-κB signaling pathway (Dasu et al., 2007; Tsai et al., 2012). During prolonged periods of cerebral ischemic, PHD2 levels decrease, probably because it is the transcription target of HIF-1α. PKC inhibits PHD2 activation, whereas HIF-1α induces PHD2 hydroxylation and degradation. HIF-1α protects gene transcription by regulating adaptive responses to hypoxia and other stresses. It is crucial in the activation of endogenous substances downstream of cerebral ischemia. Studies have shown that EPO and iNOS are the critical downstream substances that are activated by HIF-1α (Wang et al., 2019). Moreover, EPO is an inducer and transducer of ischemic tolerance that prevents the death of apoptotic cells. In the current study, GHI was found to upregulate brain EPO and iNOS expression in rats with cerebral ischemia injury.
Taken together, the current results demonstrated that GHI has protective effects against cerebral ischemia via the PKC/HIF-1α signal pathway. As a formulation combining traditional Chinese medicine with Western medicine, GHI can have more multitarget pharmacological effects. Therefore, other mechanisms underlying GHI’s protective effects against cerebral ischemia warrant further investigation. Especially in vitro experiments, GHI needs further follow-up verification.
Conclusion
The PKC/HIF-1α pathway in the condition of hypoxia was shown in Figure 9. Our study demonstrated that GHI alleviated cerebral I/R injury in a rodent MCAO model through reducing EPO, HIF-1α, and PKC in plasma, up-regulating PHD2 protein and down-regulating HIF-1α and iNOS protein, reducing NOX4 and HIF-1α mRNA of MCAO rats. These results indicated that PKC/HIF-1α signal pathway may be a potential mechanism which GHI alleviates cerebral I/R injury. We will further verify this mechanism through cell experiments and further study this signal pathway.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by Institutional Review Board of Zhejiang Chinese Medical University.
Author Contributions
Conceptualization, HW and WJ; methodology, YZ; validation, LY, XZ, and YH; formal analysis, LY; investigation, LY and XZ; resources, WJ; writing—original draft preparation, YZ; writing—review and editing, WJ; visualization, WJ; supervision, YH; project administration, HW; funding acquisition, YH and WJ. All authors have read and agreed to the published version of the article.
Funding
This research was funded by National Natural Science Foundation of China, grant number of 81904083, 81873226, 81874366; Zhejiang Basic Public Welfare Research Project, grant number of LGN19C190004, Zhejiang University of Traditional Chinese Medicine School-level Scientific Research Fund Project, grant number of 2019ZG30, and the Key Laboratory of TCM Encephalopathy of Zhejiang Province, grant no. 2020E10012.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.716121/full#supplementary-material
Abbreviations
GHI, Guhong injection; I/R, ischemia-reperfusion; PKC, protein kinase C; HIF-1α, hypoxia-inducible factor-1α; SD, Sprague Dawley; MCAO, middle cerebral artery occlusion; EPO, erythropoietin; iNOS, inducible nitric oxide synthase; NOX-4, nicotinamide adenine dinucleotide phosphate oxidase 4; NMDP, nimodipine injection; HPLC, high performance liquid chromatography; tPA, tissue plasminogen activator; FDA, Food and Drug Administration; PHD, prolyl hydroxylase domain; EGF,epidermal growth factor; PAI-1, plasminogen activator Inhibitor-1; VEGF, vascular endothelial growth factor; TTC, 2,3,5-triphenyltetrazolium chloride; ELISA, enzyme linked immunosorbent assay; CCA, common carotid artery; ECA, the external carotid artery; ICA, internal carotid artery; HE, hematoxylin-eosin; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; PVDF, polyvinylidene difluoride; q-PCR, quantitative polymerase chain reaction.
References
Ai, J., Wan, H., Shu, M., Zhou, H., Zhao, T., Fu, W., et al. (2017). Guhong Injection Protects against Focal Cerebral Ischemia-Reperfusion Injury via Anti-inflammatory Effects in Rats. Arch. Pharm. Res. 40, 610–622. doi:10.1007/s12272-016-0835-4
Bruch, G. E., Fernandes, L. F., Bassi, B. L. T., Alves, M. T. R., Pereira, I. O., Frézard, F., et al. (2019). Liposomes for Drug Delivery in Stroke. Brain Res. Bull. 152, 246–256. doi:10.1016/j.brainresbull.2019.07.015
Chalouhi, N., Dressler, J. A., Kunkel, E. S., Dalyai, R., Jabbour, P., Gonzalez, L. F., et al. (2013). Intravenous Tissue Plasminogen Activator Administration in Community Hospitals Facilitated by Telestroke Service. Neurosurgery 73, 667–672. doi:10.1227/NEU.0000000000000073
Chen, T., Chen, N., Pang, N., Xiao, L., Li, Y., Li, R., et al. (2016). Hydroxysafflor Yellow A Promotes Angiogenesis via the Angiopoietin 1/Tie-2 Signaling Pathway. J. Vasc. Res. 53, 245–254. doi:10.1159/000452408
Cheon, S., Kim, E., Kim, J., and Koo, B. (2018). Cell Type-specific Mechanisms in the Pathogenesis of Ischemic Stroke: The Role of Apoptosis Signal-Regulating Kinase 1. Oxidative Med. Cell. longevity 2018, 2596043. doi:10.1155/2018/2596043
Dai, L., Wan, H., Zhou, H., Yang, J., Zhao, T., Fu, W., et al. (2014). Protective Effect of Guhong Injection on Rat Cerebral Microvascular Endothelial Cells Injured by Oxygen-Glucose Deprivation. Chin. Pharm. J. 49, 1897–1902.
Dasu, M. R., Devaraj, S., and Jialal, I. (2007). High Glucose Induces IL-1beta Expression in Human Monocytes: Mechanistic Insights. Am. J. Physiol. Endocrinol. Metab. 293, E337–E346. doi:10.1152/ajpendo.00718.2006
Du, C., Wan, H., Zhou, H., Zhang, Y., Yang, J., Fan, H., et al. (2017). Protective Effects of Guhong Injection on Neonatal Rat Primary Cultured Hippocampal Neurons Injured by Oxygen-Glucose Deprivation. China J. Traditional Chin. Med. Pharm. 31, 3656–3661.
Geng, J., Zhang, Y., Li, S., Li, S., Wang, J., Wang, H., et al. (2019). Metabolomic Profiling Reveals that Reprogramming of Cerebral Glucose Metabolism Is Involved in Ischemic Preconditioning-Induced Neuroprotection in a Rodent Model of Ischemic Stroke. J. Proteome Res. 18, 57–68. doi:10.1021/acs.jproteome.8b00339
Gibbs, B. F., Yasinska, I. M., Oniku, A. E., and Sumbayev, V. V. (2011). Effects of Stem Cell Factor on Hypoxia-Inducible Factor 1 Alpha Accumulation in Human Acute Myeloid Leukaemia and LAD2 Mast Cells. PloS one 6, e22502. doi:10.1371/journal.pone.0022502
Gulati, P., and Singh, N. (2014). Neuroprotective Mechanism of Ischemic Postconditioning in Mice: a Possible Relationship between Protein Kinase C and Nitric Oxide Pathways. J. Surg. Res. 189, 174–183. doi:10.1016/j.jss.2014.02.019
Han, X., Mao, Z., Wang, S., Xin, Y., Li, P., Maharjan, S., et al. (2020). GYY4137 Protects against MCAO via P38 MAPK Mediated Anti-apoptotic Signaling Pathways in Rats. Brain Res. Bull. 158, 59–65. doi:10.1016/j.brainresbull.2020.02.015
He, Y., Zhou, H., Huang, L., Zhao, T., Fu, W., and Wan, H. (2015). Simultaneous Quantification of Seven Components in Guhong Injection by HPLC-DAD. Chin. J. Pharm. Anal. 35, 954–959. doi:10.16155/j.0254-1793.2015.06.02
Jieqin, Z., Shuling, L., Hairong, C., Xingzhen, D., Yanhong, C., Zilin, J., et al. (2020). Efficacy and Safety of Guhong Injection for Treating Coronary Microvascular Disease: Study Protocol for a Randomized Controlled Trial. Trials 21, 75. doi:10.1186/s13063-019-3990-3
Jin, R., Xiao, A. Y., Chen, R., Granger, D. N., and Li, G. (2017). Inhibition of CD147 (Cluster of Differentiation 147) Ameliorates Acute Ischemic Stroke in Mice by Reducing Thromboinflammation. Stroke 48, 3356–3365. doi:10.1161/STROKEAHA.117.018839
Koshak, A. E., Yousif, N. M., Fiebich, B. L., Koshak, E. A., and Heinrich, M. (2018). Comparative Immunomodulatory Activity of Nigella Sativa L. Preparations on Proinflammatory Mediators: A Focus on Asthma. Front. Pharmacol. 9, 1075. doi:10.3389/fphar.2018.01075
Kuo, P. C., Weng, W. T., Scofield, B. A., Furnas, D., Paraiso, H. C., Intriago, A. J., et al. (2020). Interferon-β Alleviates Delayed tPA-Induced Adverse Effects via Modulation of MMP3/9 Production in Ischemic Stroke. Blood Adv. 4, 4366–4381. doi:10.1182/bloodadvances.2020001443
Li, L., Dong, P., Hou, C., Cao, F., Sun, S., He, F., et al. (2016). Hydroxysafflor Yellow A (HSYA) Attenuates Hypoxic Pulmonary Arterial Remodelling and Reverses Right Ventricular Hypertrophy in Rats. J. Ethnopharmacol 186, 224–233. doi:10.1016/j.jep.2016.04.004
Li, Y., Wang, R., Xue, L., Yang, Y., and Zhi, F. (2020). Astilbin Protects against Cerebral Ischaemia/reperfusion Injury by Inhibiting Cellular Apoptosis and ROS-NLRP3 Inflammasome axis Activation. Int. Immunopharmacol 84, 106571. doi:10.1016/j.intimp.2020.106571
Liu, J., Guo, Z. N., Yan, X. L., Huang, S., Ren, J. X., Luo, Y., et al. (2020). Crosstalk between Autophagy and Ferroptosis and its Putative Role in Ischemic Stroke. Front Cel Neurosci 14, 577403. doi:10.3389/fncel.2020.577403
Mavroudakis, L., Stevens, S., Duncan, K., Stenzel-Poore, M., Laskin, J., and Lanekoff, I. (2020). CpG Preconditioning Reduces Accumulation of Lysophosphatidylcholine in Ischemic Brain Tissue after Middle Cerebral Artery Occlusion. Anal. Bioanal. Chem. doi:10.1007/s00216-020-02987-w
Michael Heinrich, P. B. (2006). Ethnobotany and Ethnopharmacy - Their Role for Anti-cancer Drug Development. Curr. Drug Targets 77 (3), 239–245. doi:10.2174/138945006776054988
Orgah, J. O., Ren, J., Liu, X., Orgah, E. A., Gao, X. M., and Zhu, Y. (2019). Danhong Injection Facilitates Recovery of post-stroke Motion Deficit via Parkin-Enhanced Mitochondrial Function. Restor Neurol. Neurosci. 37, 375–395. doi:10.3233/RNN-180828
Pushpakumar, S., Ren, L., Juin, S. K., Majumder, S., Kulkarni, R., and Sen, U. (2020). Methylation-dependent Antioxidant-Redox Imbalance Regulates Hypertensive Kidney Injury in Aging. Redox Biol. 37, 101754. doi:10.1016/j.redox.2020.101754
Shen, J., Huang, K., Zhu, Y., Xu, K., Zhan, R., and Pan, J. (2020). Buyang Huanwu Decoction Promotes Angiogenesis after Cerebral Ischemia by Inhibiting the Nox4/ROS Pathway. Evid. Based Complement. Alternat Med. 2020, 5264205. doi:10.1155/2020/5264205
Shu, M. C., Wan, H. T., Zhou, H. F., Yang, J. H., Zhao, T., Fu, W., et al. (2014). [Effect and Mechanism of Guhong Injection against Cerebral Ischemia Reperfusion Injury]. Zhongguo Zhong Yao Za Zhi 39, 4829–4833. doi:10.4268/cjcmm20142425
Sun, J. B., Li, Y., Cai, Y. F., Huang, Y., Liu, S., Yeung, P. K., et al. (2018). Scutellarin Protects Oxygen/glucose-Deprived Astrocytes and Reduces Focal Cerebral Ischemic Injury. Neural Regen. Res. 13, 1396–1407. doi:10.4103/1673-5374.235293
Sun, M. S., Jin, H., Sun, X., Huang, S., Zhang, F. L., Guo, Z. N., et al. (2018). Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid Med. Cel Longev 2018, 3804979. doi:10.1155/2018/3804979
Tang, H., Pan, C. S., Mao, X. W., Liu, Y. Y., Yan, L., Zhou, C. M., et al. (1994). Role of NADPH Oxidase in Total Salvianolic Acid Injection Attenuating Ischemia-Reperfusion Impaired Cerebral Microcirculation and Neurons: Implication of AMPK/Akt/PKC. Microcirculation 21 (2014), 615–627. doi:10.1111/micc.12140
Tsai, K. L., Chiu, T. H., Tsai, M. H., Chen, H. Y., and Ou, H. C. (2012). Vinorelbine-induced Oxidative Injury in Human Endothelial Cells Mediated by AMPK/PKC/NADPH/NF-κB Pathways. Cell Biochem Biophys 62, 467–479. doi:10.1007/s12013-011-9333-y
Uemura, M. T., Maki, T., Ihara, M., Lee, V. M. Y., and Trojanowski, J. Q. (2020). Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia. Front. Aging Neurosci. 12, 80. doi:10.3389/fnagi.2020.00080
Wang, H., Niu, F., Fan, W., Shi, J., Zhang, J., and Li, B. (2019). Modulating Effects of Preconditioning Exercise in the Expression of ET-1 and BNP via HIF-1α in Ischemically Injured Brain. Metab. Brain Dis. 34, 1299–1311. doi:10.1007/s11011-019-00450-z
Wang, H., Zhou, H., He, Y., Yu, L., Li, C., Yang, J., et al. (2020). Protective Effect of Naoxintong Capsule Combined with Guhong Injection on Rat Brain Microvascular Endothelial Cells during Cerebral Ischemia-Reperfusion Injury. Chin. J. Integr. Med. doi:10.1007/s11655-020-3215-3
Wang, J. Q., He, J. T., Du, Z. W., Li, Z. S., Liu, Y. F., Mang, J., et al. (2013). Effects of SARA on Oxygen-Glucose Deprivation in PC12 Cell Line. Neurochem. Res. 38, 961–971. doi:10.1007/s11064-013-1004-5
Xu, S., Li, C., Zhou, H., Yu, L., Deng, L., Zhu, J., et al. (2020). A Study on Acetylglutamine Pharmacokinetics in Rat Blood and Brain Based on Liquid Chromatography-Tandem Mass Spectrometry and Microdialysis Technique. Front. Pharmacol. 11, 508. doi:10.3389/fphar.2020.00508
Yu, L., Wan, H. F., Li, C., Yang, J. H., Zhou, H. F., Wan, H. T., et al. (2018). Pharmacokinetics of Active Components from Guhong Injection in Normal and Pathological Rat Models of Cerebral Ischemia: A Comparative Study. Front. Pharmacol. 9, 493. doi:10.3389/fphar.2018.00493
Yu, Z. P., Yu, H. Q., Li, J., Li, C., Hua, X., and Sheng, X. S. (2020). Troxerutin Attenuates Oxygen-glucose D-eprivation and R-eoxygenation-induced O-xidative S-tress and I-nflammation by E-nhancing the PI3K/AKT/HIF-1α S-ignaling P-athway in H9C2 C-ardiomyocytes. Mol. Med. Rep. 22, 1351–1361. doi:10.3892/mmr.2020.11207
Yuan, Y., Ye, Z., Yu, H., Chen, Y., Wang, Y., Zhao, J., et al. (2020). Shrm4 Contributes to Autophagy Inhibition and Neuroprotection Following Ischemic Stroke by Mediating GABA Receptor Activation. FASEB J. 34 (12), 15837–15848. doi:10.1096/fj.202000458rr
Zhang, J., Wang, Y., Wang, G. N., Sun, H., Sun, T., Shi, J. Q., et al. (2011). Clinical Factors in Patients with Ischemic versus Hemorrhagic Stroke in East China. World J. Emerg. Med. 2, 18–23. doi:10.5847/wjem.j.1920-8642.2011.01.003
Zhang, J., Zhou, R., Xiang, C., Fan, F., Gao, J., Zhang, Y., et al. (2020). Enhanced Thioredoxin, Glutathione and Nrf2 Antioxidant Systems by Safflower Extract and Aceglutamide Attenuate Cerebral Ischaemia/reperfusion Injury. J. Cel Mol Med 24, 4967–4980. doi:10.1111/jcmm.15099
Zhang, L. L., Tian, K., Tang, Z. H., Chen, X. J., Bian, Z. X., Wang, Y. T., et al. (2016). Phytochemistry and Pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 44, 197–226. doi:10.1142/S0192415X16500130
Keywords: Guhong injection, cerebral ischemia-reperfusion injury, PKC–protein kinase C, HIF–hypoxia inducible factor, pathway
Citation: Yu L, Zhang Y, Zhao X, Wan H, He Y and Jin W (2021) Guhong Injection Alleviates Cerebral Ischemia–Reperfusion Injury via the PKC/HIF-1α Pathway in Rats. Front. Pharmacol. 12:716121. doi: 10.3389/fphar.2021.716121
Received: 28 May 2021; Accepted: 19 August 2021;
Published: 02 September 2021.
Edited by:
Jiahong Lu, University of Macau, ChinaReviewed by:
Wuwen Feng, Chengdu University of Traditional Chinese Medicine, ChinaShi-Jun Yue, Shaanxi University of Chinese Medicine, China
Copyright © 2021 Yu, Zhang, Zhao, Wan, He and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifeng Jin, amluX3dlaWZlbmdAMTI2LmNvbQ==; Yu He, aGV5dTA5MjNAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Li Yu
Li Yu Yangyang Zhang2†
Yangyang Zhang2† Xixi Zhao
Xixi Zhao Haitong Wan
Haitong Wan Yu He
Yu He Weifeng Jin
Weifeng Jin