- 1The Fifth Department of Oncology, Jinshazhou Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2The Second Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, China
- 3Pediatric Ward, Henan Province Hospital of TCM, Zhengzhou, China
- 4Department of Oncology, Fuda Cancer Hospital Guangzhou, Guangzhou, China
Background: Anti-programmed cell death protein 1 (PD-1) has been successfully used in carcinomas treatment. However, it causes significant adverse effects (AEs), including cutaneous reactions, particularly the life-threatening severe bullous skin reactions (SBSR) and toxic epidermal necrolysis (TEN).
Case summary: Herein, we described for the first time a case report of SBSR induced by anti-PD-1 therapy in a cervical cancer patient. In addition, we revised existing literature on anti-PD-1 induced cutaneous reactions. We reported a cervical cancer patient who was treated with four successive cycles of Sintilimab and Toripalimab injections and developed systemic rashes, bullae, and epidermal desquamation, which worsened and led to infection, eventually causing death after being unresponsive to aggressive treatments.
Conclusion: Anti-PD-1 antibodies commonly cause skin toxicity effects, some of which may be deadly. Therefore, healthcare providers should observe early symptoms and administer proper treatment to prevent aggravation of symptoms.
Introduction
Cervical cancer is the fourth most common cancer in women (Colombo et al., 2012). Its main treatment consists of platinum-based chemotherapy, with limited therapeutic outcomes and severe side effects (Greer et al., 2010; Pfaendler and Tewari, 2016). Since the introduction of programmed death 1 (PD-1) protein monoclonal antibodies, they have shown outstanding clinical efficacy in multiple cancer types, including advanced cervical cancer (Martínez and Del Campo, 2017; Wang and Li, 2019). In this regard, Pembrolizumab was used for advanced cervical cancer in the KEYNOTE-028 clinical study, demonstrating the efficacy of PD-1/PD-L1 inhibitors in advanced cervical cancer (Frenel et al., 2017). PD-1 monoclonal antibodies have been shown to potentiate T lymphocytes cytotoxic activity against tumor cells and control tumor growth (Pedoeem et al., 2014; Tumeh et al., 2014). Most patients tolerated anti-PD-1 therapy, whereas some presented toxic and side effects (Champiat et al., 2016). Major anti-PD-1 associated adverse effects (AEs) included skin toxicity, endocrine reaction, gastrointestinal reaction, hepatitis, and renal dysfunction (Hofmann et al., 2016; Ansell, 2017; Nishijima et al., 2017; O’Kane et al., 2017; Tie et al., 2017; Gubens et al., 2019). The most common AEs involved skin reactions such as lichenoid reaction, eczema, vitiligo, and pruritus (Joseph et al., 2015; Robert et al., 2015a; Weber et al., 2015; Ciccarese et al., 2016; Hwang et al., 2016; Yang et al., 2019). However, the most severe skin reaction observed was toxic epidermal necrolysis (TEN) in three cases of malignant melanoma (Nayar et al., 2016; Vivar et al., 2017; Logan et al., 2020).
Case Presentations
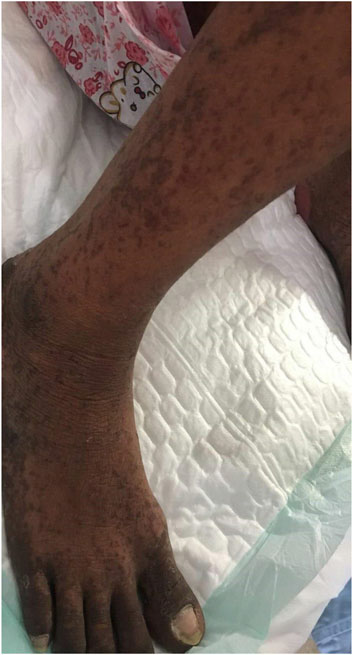
The patient was a 38-year-old Asian female. In June 2019, cervical tumor with invasion of the uterine wall, bladder and rectum walls, and anterior sacral and bilateral inguinal lymphadenopathies was detected by magnetic resonance imaging, which was prescribed because she presented vaginal bleeding. Biopsy pathological results suggested cervical squamous cell carcinoma, FIGO stage IVA. On August 18, 2019, she was intravenously (i. v.) administered 240 mg paclitaxel +90 mg cisplatin chemotherapy, along with 200 mg Sintilimab at 21-days cycle intervals. On September 9, 2019, the patient received a second cycle of the same dose of Sintilimab and chemotherapy. Sintilimab is an innovative monoclonal antibody targeting PD-1, jointly developed by Innovent and Lilly in China, which has been granted marketing approval by the China Food and Drug Administration. The drug was granted orphan drug status by the FDA in April 2020 for the treatment of esophageal cancer. Because of financial issues, Sintilimab was switched to 240 mg Toripalimab in the third cycle on October 1, 2019, for two consecutive weeks per cycle, whereas the chemotherapy regimen remained unaltered. Toripalimab is also an anti-PD-1 monoclonal antibody produced in China. In March 2020, Toripalimab was granted orphan drug status by the US FDA in combination with acytinib for the treatment of mucosal melanoma. A follow-up exam after the third cycle showed progressive disease. In the fourth cycle on October 21, 2019, we modified chemotherapy to 240 mg paclitaxel and 135 mg nedaplatin, combined with 200 mg Sintilimab. Six days after the fourth cycle of treatment, she presented with rashes. Large erythema was observed in many parts of the body, along with some prominent skin areas and pigmentation (Figure 1) and she was given the antihistamine diphenhydramine. The patient further developed shortness of breath and edema of both lower limbs, which was considered a heart failure condition. Cardiotonic, diuretic, and vasodilator agents were then provided. In addition, red blood cell transfusion was given, because her hemoglobin was 61 g/L.
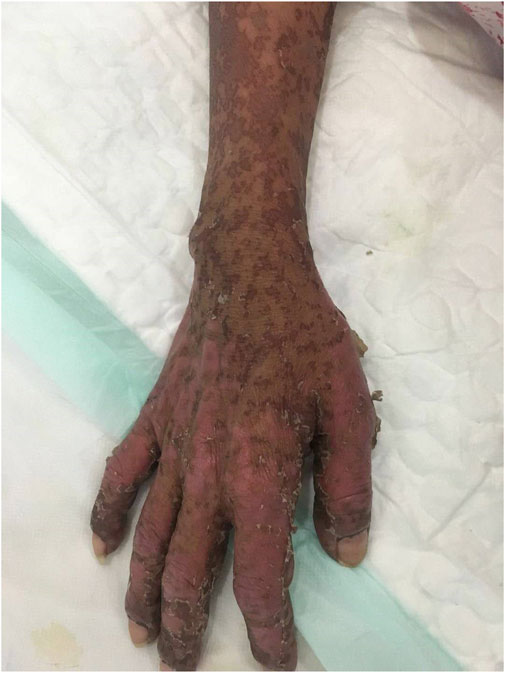
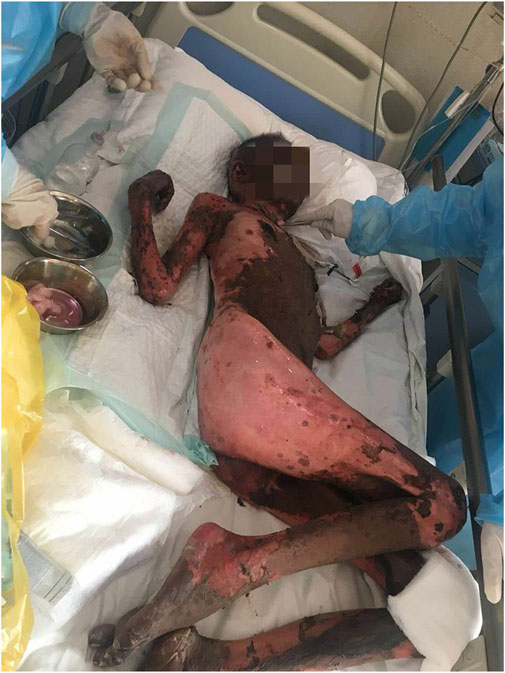
After antihistamine treatment, she presented with worsening symptoms, with increased erythema, local transparent blisters, and tender epidermal loss of less than 10% of the body surface area (BSA). Furthermore, she developed mild swelling of oropharyngeal mucosa with repeated bleeding and scabbing, resulting in mouth opening and swallowing difficulties (Figure 2), but refused to undergo skin biopsy. Stevens-Johnson syndrome (SJS) was diagnosed by a professor of dermatology, and we started i. v. administration of 80 mg/d methylprednisolone, combined with 0.4 g/kg/d immunoglobulins injection every day. We also improved the skin, mouth, and perineal mucous membranes care. Six days after medication, the epidermal peeling aggravated. Clear blisters locally appeared, presenting apparent perineal skin damage, with skin loss over 30% of BSA (Figure 3). The patient also presented fever, with respective C-reactive protein and neutrophil values of 117.33 mg/L and 89.20%. The skin lesion developed to SBSR, accompanied by infection, which was treated with antibiotics. The epidermal exfoliation and infection continued to worsen, and a disturbance of consciousness was observed. The patient died 33 days after the fourth cycle of medication, probably due to sepsis.
Discussion
Cutaneous Adverse Effects of Anti-Programmed Death-1 Therapy
Although docetaxel and paclitaxel share the same pharmaceutical composition, cases of SBSR -causing paclitaxel have not been reported to date (Ohlmann et al., 2007; Kattan et al., 2008; Cohen et al., 2019). The toxicity spectrum of anti-PD-1 is prone to skin-related reactions, excluding the possibility that other drugs used by the patient may cause SBSR, which we believed it was related to anti-PD-1 treatment.
PD-1/PD-L1 pathway inhibits T cell activation, inducing lymphocyte apoptosis, and maintains autoimmune tolerance. In the tumor microenvironment, tumor cells bind to PD-1 on the lymphocyte surface through PD-L1 overexpression to inhibit lymphocytes function, thus escaping the immune surveillance and destruction (Dong and Chen, 2006). PD-1 is an important target in anti-tumor therapy because the aforementioned inhibitory signaling pathways can be blocked, enhancing T cells immune response (Topalian et al., 2012).
In major lung cancer related studies, CheckMate 017 and CheckMate 057 (Borghaei et al., 2015; Brahmer et al., 2015) revealed that anti-PD-1 AEs in lung cancer patients were mainly grade 1–2, in KEYNOTE-010 (Herbst et al., 2016), two cases (less than 1%) were reported as grade 3–4 rash, with a median time of AEs of 5–7 weeks.
In the study of malignant melanoma, CheckMate 066 (Robert et al., 2015a) reported one case of grade 3–4 rash and one case of grade 3–4 pruritus. The incidence of grade 3–4 cutaneous AEs was 0.5%. CheckMate 037 and KEYNOTE-002 did not report grade 3–4 cutaneous AEs (Ribas et al., 2015; Weber et al., 2015). The incidence of Nivolumab and Pembrolizumab associated rashes was 14.3 and 16.7% respectively, and the incidence of rash over grade 3 was 1.2 and 1.9% respectively, according to the meta-analysis of V. R. Belum (Belum et al., 2016). Skin reactions generally occur two to 3 weeks after the administration of the immune inhibitor (Kumar et al., 2017). Studies have shown that progression‐free survival and overall survival in cancer patients with skin reactions are longer than those without skin reactions, after immunotherapy (Sanlorenzo et al., 2015; Hasan Ali et al., 2016; Imafuku et al., 2017; Min Lee et al., 2018; Quach et al., 2019). A similar conclusion was reported in a study of immunotherapy combined with radiotherapy (Haratani et al., 2018). Quach HT (Quach et al., 2019) retrospective analysis of 318 patients treated with anti-PD-1 monoclonal showed that patients who experienced skin toxicity 3 months after the application of the immune inhibitors, had better therapeutic outcomes than those who did not show skin issues, and that a higher response rate was observed in patients with vitiligo and pruritus than patients with only vitiligo.
Clinical manifestations of severe bullous skin reaction caused by PD-1 monoclonal antibody are very similar to those observed in SJS/TEN. However, the former characterizes by the duration of drug application, the involvement of the mucous membrane, and the degree of skin peeling (Lipowicz et al., 2013; Zhao et al., 2018). Research by Robin Reschke et al. (2019) showed that among all the current reports of serious skin reactions caused by checkpoint inhibitor therapy, only a small number are typical SJS/TEN. Therefore, clinicians, especially oncologists, should differentiate them by considering clinical evidence and if necessary, request a dermatologist assistance in diagnosis and treatment.
Case Reports on Toxic Epidermal Necrolysis Induced by Immunotherapy
Although mild dermatotoxicity indicates a better clinical outcome, severe dermatotoxicity may result in treatment interruption (Macdonald et al., 2015), and sometimes it may become a life-threatening condition (Nayar et al., 2016; Vivar et al., 2017; Logan et al., 2020). TEN is the most serious type of drug eruption, characterized by extensive epidermal exfoliation and blistering (Roujeau and Stern, 1994), with a mortality of 25–50% (Mockenhaupt et al., 2008; Kinoshita and Saeki, 2017).
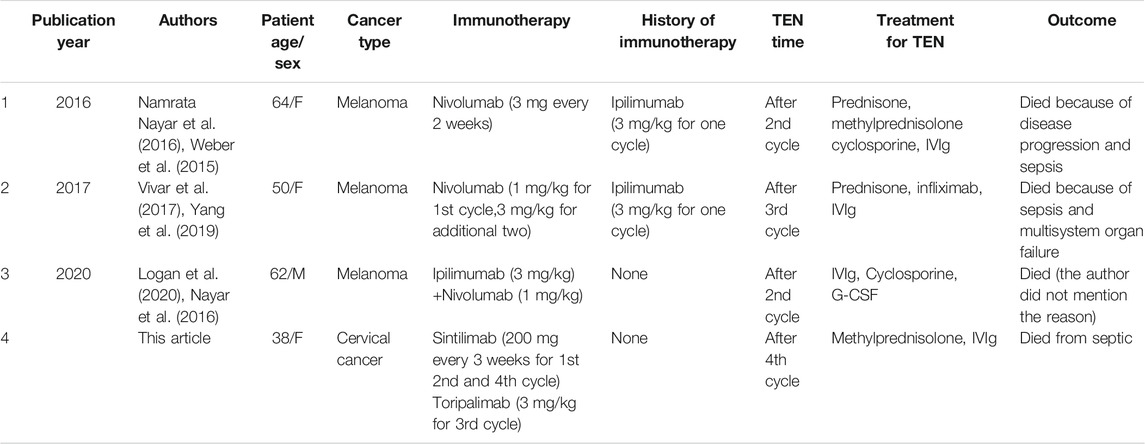
Three cases of TEN induced by anti-PD-1 in melanoma have been reported to date (Nayar et al., 2016; Vivar et al., 2017; Logan et al., 2020), all of which were treated with Nivolumab, and a previous history of Ipilimumab or were treated with these drugs in combination. TEN developed after the second or third cycle of Nivolumab. In all cases, TEN was treated with immunoglobulins (IVIg), two patients used cyclosporines, and other two used glucocorticoids. All patients finally died, including those reported in this article (Table 1).
The incidence of CTLA-4 adverse reactions is as high as 60%, and skin and gastrointestinal tract is the most easily affected organs (Hodi et al., 2010). The incidence of grade 3–4 adverse events was 20% (Schachter et al., 2017). At present, it is believed that the cause of CTLA-4 adverse reactions was the excessive immune response to normal organs after activation of T cells (Blansfield et al., 2005). However, the mechanism that causes TEN is still unclear.
Relationship Between Anti-Programmed Death-1 Monoclonal Antibodies and Cutaneous Reactions
Immune-related AEs may damage any organ in the body (Michot et al., 2016), mostly skin (Minkis et al., 2013; Abdel-Rahman et al., 2015; Naidoo et al., 2015). However, the mechanism of anti-PD-1-induced skin toxicity has not yet been elucidated (Maloney et al., 2020). PD-1/PD-L1 pathway plays an important role in autoimmunity, preventing T cells from responding to autoantigens (Francisco et al., 2010). After anti-PD-1 monoclonal antibody administration, this balance may be broken, causing T cells to attack normal and tumor cells, leading to toxic and side effects (Berman et al., 2015). In the PD-1 knockout mouse model, Okazaki et al. (2003) demonstrated that PD-1 blockade not only affected Treg function, but also participated in the production of autoantibodies. Similar results were observed in melanoma patients treated with Nivolumab (Kanameishi et al., 2016). The three reported cases of TEN induced by anti-PD-1 had a medical history of anti-CTLA-4. Gu et al. (2019) meta-analysis showed that anti-PD-1/PD-L1 combined with anti-CTLA-4 caused a higher incidence of adverse reactions and was prone to treatment discontinuation, as shown in Check-Mate 067 study (Larkin et al., 2015). However, results evidenced higher remission rates when two immune inhibitors were used in combination (Robert et al., 2015b). Choi et al. (2019) reported a case of liver cancer patient who successfully switched to pembrolizumab due to nivolumab allergy. Therefore, different anti-PD-1 monoclonal antibodies share the same mechanism, but may cause different reactions. Our patients presented with TEN after using two different anti-PD-1 monoclonal antibodies. However, interaction between these two drugs may not be discarded.
Treatment of Skin Reactions Caused by Immunotherapy
Early treatment of rash caused by immune agents is particularly important (O’Kane et al., 2017). In general, patients can be topically treated with glucocorticoid ointment and orally with antipruritics (mainly antihistamines) if the patient presents itching. For grade 3–4 toxic side effects caused by anti-PD-1 monoclonal antibodies, glucocorticoids should be orally administered and anti-PD-1 should be discontinued until glucocorticoid dose reduction therapy is completed (Eigentler et al., 2016; Weber et al., 2017). The drug should be immediately discontinued and the patient should be hospitalized if TEN develops. SJS/TEN treatment guidelines from the United Kingdom (Creamer et al., 2016) and the literature review of Friedman et al. (2016) indicate that glucocorticoids, immunoglobulins, and cyclosporine may be used in TEN therapy but such treatment has not been clearly demonstrated over supportive therapy alone. Previous studies (de Sica-Chapman et al., 2010) have shown that G-CSF has immunomodulatory effects and promotes epithelial regeneration. SJS/TEN treatment by plasmapheresis has also been reported (Narita et al., 2011). In this regard, Koštál used plasmapheresis therapy in four patients presenting TEN who did not respond to glucocorticoids and immunoglobulin. Their symptoms improved and necrotic epithelium began to repair (Kostal et al., 2012). Skin care played an important role in the recovery from TEN (Cooper, 2012).
Conclusion
Although most skin reactions caused by anti-PD-1 monoclonal antibodies were mild, they may still cause fatal skin toxicity. Therefore, early recognition, intervention, and treatment are essential to avoid further development of skin reactions. In this regard, early application of glucocorticoids may slow the response.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Jinshazhou Hospital of Guangzhou University of Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The original manuscript was written by XL, L-XQ, and Y-MR, reviewed and edited by CH. All authors read and approved the final manuscript. XL, L-XQ and Y-MR contribute equally to this work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Abbreviations
AEs, adverse effects; BSA, body surface area; PD-1, Anti-programmed cell death protein 1; TEN, toxic epidermal necrolysis.
References
Abdel-Rahman, O., ElHalawani, H., and Fouad, M. (2015). Risk of Cutaneous Toxicities in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors: a Meta-Analysis. Future Oncol. 11 (17), 2471–2484. doi:10.2217/fon.15.118
Ansell, S. M. (2017). Nivolumab in the Treatment of Hodgkin Lymphoma. Clin. Cancer Res. 23 (7), 1623–1626. doi:10.1158/1078-0432.CCR-16-1387
Belum, V. R., Benhuri, B., Postow, M. A., Hellmann, M. D., Lesokhin, A. M., Segal, N. H., et al. (2016). Characterisation and Management of Dermatologic Adverse Events to Agents Targeting the PD-1 Receptor. Oxford, England : 1990. Eur. J. Cancer. 60, 12–25. doi:10.1016/j.ejca.2016.02.010
Berman, D., Korman, A., Peck, R., Feltquate, D., Lonberg, N., and Canetta, R. (2015). The Development of Immunomodulatory Monoclonal Antibodies as a New Therapeutic Modality for Cancer: the Bristol-Myers Squibb Experience. Pharmacol. Ther. 148, 132–153. doi:10.1016/j.pharmthera.2014.11.017
Blansfield, J. A., Beck, K. E., Tran, K., Yang, J. C., Hughes, M. S., Kammula, U. S., et al. (2005). Cytotoxic T-Lymphocyte-Associated Antigen-4 Blockage Can Induce Autoimmune Hypophysitis in Patients with Metastatic Melanoma and Renal Cancer. J. Immunother. 28 (6), 593–598. doi:10.1097/01.cji.0000178913.41256.06
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (17), 1627–1639. doi:10.1056/NEJMoa1507643
Brahmer, J., Reckamp, K. L., Baas, P., Crinò, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (2), 123–135. doi:10.1056/NEJMoa1504627
Champiat, S., Lambotte, O., Barreau, E., Belkhir, R., Berdelou, A., Carbonnel, F., et al. (2016). Management of Immune Checkpoint Blockade Dysimmune Toxicities: a Collaborative Position Paper. Ann. Oncol. 27 (4), 559–574. doi:10.1093/annonc/mdv623
Choi, B., McBride, A., and Scott, A. J. (2019). Treatment with Pembrolizumab after Hypersensitivity Reaction to Nivolumab in a Patient with Hepatocellular Carcinoma. Am. J. Health Syst. Pharm. 76 (21), 1749–1752. doi:10.1093/ajhp/zxz189
Ciccarese, C., Alfieri, S., Santoni, M., Santini, D., Brunelli, M., Bergamini, C., et al. (2016). New Toxicity Profile for Novel Immunotherapy Agents: Focus on Immune-Checkpoint Inhibitors. Expert Opin. Drug Metab. Toxicol. 12 (1), 57–75. doi:10.1517/17425255.2016.1120287
Cohen, E. E. W., Soulières, D., Le Tourneau, C., Dinis, J., Licitra, L., Ahn, M. J., et al. (2019). Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-And-Neck Squamous Cell Carcinoma (KEYNOTE-040): a Randomised, Open-Label, Phase 3 Study. Lancet 393 (10167), 156–167. doi:10.1016/S0140-6736(18)31999-8
Colombo, N., Carinelli, S., Colombo, A., Marini, C., Rollo, D., and Sessa, C. (2012). Cervical Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 23 (Suppl. 7), vii27–32. doi:10.1093/annonc/mds268
Cooper, K. L. (2012). Drug Reaction, Skin Care, Skin Loss. Crit. Care Nurse. 32 (4), 52–59. doi:10.4037/ccn2012340
Creamer, D., Walsh, S. A., Dziewulski, P., Exton, L. S., Lee, H. Y., Dart, J. K. G., et al. (2016). UK Guidelines for the Management of Stevens-Johnson Syndrome/toxic Epidermal Necrolysis in Adults 2016. J. Plast. Reconstr. Aesthet. Surg. 69 (6), e119–e153. doi:10.1016/j.bjps.2016.01.034
de Sica-Chapman, A., Williams, G., Soni, N., and Bunker, C. B. (2010). Granulocyte colony-stimulating Factor in Toxic Epidermal Necrolysis (TEN) and Chelsea & Westminster TEN Management Protocol [corrected]. Br. J. Dermatol. 162 (4), 860–865. doi:10.1111/j.1365-2133.2009.09585.x
Dong, H., and Chen, X. (2006). Immunoregulatory Role of B7-H1 in Chronicity of Inflammatory Responses. Cell Mol Immunol. 3 (3), 179–187.
Eigentler, T. K., Hassel, J. C., Berking, C., Aberle, J., Bachmann, O., Grünwald, V., et al. (2016). Diagnosis, Monitoring and Management of Immune-Related Adverse Drug Reactions of Anti-PD-1 Antibody Therapy. Cancer Treat. Rev. 45, 7–18. doi:10.1016/j.ctrv.2016.02.003
Francisco, L. M., Sage, P. T., and Sharpe, A. H. (2010). The PD-1 Pathway in Tolerance and Autoimmunity. Immunol. Rev. 236, 219–242. doi:10.1111/j.1600-065X.2010.00923.x
Frenel, J. S., Le Tourneau, C., O'Neil, B., Ott, P. A., Piha-Paul, S. A., Gomez-Roca, C., et al. (2017). Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results from the Phase Ib KEYNOTE-028 Trial. J. Clin. Oncol. 35 (36), 4035–4041. doi:10.1200/JCO.2017.74.5471
Friedman, C. F., Proverbs-Singh, T. A., and Postow, M. A. (2016). Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2 (10), 1346–1353. doi:10.1001/jamaoncol.2016.1051
Greer, B. E., Koh, W. J., Abu-Rustum, N. R., Apte, S. M., Campos, S. M., Chan, J., et al. (2010). Cervical Cancer. J. Natl. Compr. Canc Netw. 8 (12), 1388–1416. doi:10.6004/jnccn.2010.0104
Gu, L., Khadaroo, P. A., Su, H., Kong, L., Chen, L., Wang, X., et al. (2019). The Safety and Tolerability of Combined Immune Checkpoint Inhibitors (Anti-PD-1/pd-L1 Plus Anti-CTLA-4): a Systematic Review and Meta-Analysis. BMC cancer. 19 (1), 559. doi:10.1186/s12885-019-5785-z
Gubens, M. A., Sequist, L. V., Stevenson, J. P., Powell, S. F., Villaruz, L. C., Gadgeel, S. M., et al. (2019). Pembrolizumab in Combination with Ipilimumab as Second-Line or Later Therapy for Advanced Non-small-cell Lung Cancer: KEYNOTE-021 Cohorts D and H. Lung Cancer. 130, 59–66. doi:10.1016/j.lungcan.2018.12.015
Haratani, K., Hayashi, H., Chiba, Y., Kudo, K., Yonesaka, K., Kato, R., et al. (2018). Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non-small-cell Lung Cancer. JAMA Oncol. 4 (3), 374–378. doi:10.1001/jamaoncol.2017.2925
Hasan Ali, O., Diem, S., Markert, E., Jochum, W., Kerl, K., French, L. E., et al. (2016). Characterization of Nivolumab-Associated Skin Reactions in Patients with Metastatic Non-small Cell Lung Cancer. Oncoimmunology 5 (11), e1231292. doi:10.1080/2162402X.2016.1231292
Herbst, R. S., Baas, P., Kim, D. W., Felip, E., Pérez-Gracia, J. L., Han, J. Y., et al. (2016). Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-small-cell Lung Cancer (KEYNOTE-010): a Randomised Controlled Trial. Lancet 387 (10027), 1540–1550. doi:10.1016/S0140-6736(15)01281-7
Hodi, F. S., O'Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., et al. (2010). Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 363 (8), 711–723. doi:10.1056/NEJMoa1003466
Hofmann, L., Forschner, A., Loquai, C., Goldinger, S. M., Zimmer, L., Ugurel, S., et al. (2016). Cutaneous, Gastrointestinal, Hepatic, Endocrine, and Renal Side-Effects of Anti-PD-1 Therapy. (Oxford, England : 1990). Eur. J. Cancer. 60, 190–209. doi:10.1016/j.ejca.2016.02.025
Hwang, S. J., Carlos, G., Wakade, D., Byth, K., Kong, B. Y., Chou, S., et al. (2016). Cutaneous Adverse Events (AEs) of Anti-programmed Cell Death (PD)-1 Therapy in Patients with Metastatic Melanoma: A Single-Institution Cohort. J. Am. Acad. Dermatol. 74 (3), 455–e1.e451. doi:10.1016/j.jaad.2015.10.029
Imafuku, K., Yoshino, K., Ymaguchi, K., Tsuboi, S., Ohara, K., and Hata, H. (2017). Nivolumab Therapy before Vemurafenib Administration Induces a Severe Skin Rash. J. Eur. Acad. Dermatol. Venereol. 31 (3), e169–e171. doi:10.1111/jdv.13892
Joseph, R. W., Cappel, M., Goedjen, B., Gordon, M., Kirsch, B., Gilstrap, C., et al. (2015). Lichenoid Dermatitis in Three Patients with Metastatic Melanoma Treated with Anti-PD-1 Therapy. Cancer Immunol. Res. 3 (1), 18–22. doi:10.1158/2326-6066.CIR-14-0134
Kanameishi, S., Otsuka, A., Nonomura, Y., Fujisawa, A., Endo, Y., and Kabashima, K. (2016). Idiopathic Thrombocytopenic Purpura Induced by Nivolumab in a Metastatic Melanoma Patient with Elevated PD-1 Expression on B Cells. Ann. Oncol. 27 (3), 546–547. doi:10.1093/annonc/mdv580
Kattan, J. G., Farhat, F. S., Chahine, G. Y., Nasr, F. L., Moukadem, W. T., Younes, F. C., et al. (2008). Weekly Docetaxel, Zoledronic Acid and Estramustine in Hormone-Refractory Prostate Cancer (HRPC). Invest. New Drugs. 26 (1), 75–79. doi:10.1007/s10637-007-9074-3
Kinoshita, Y., and Saeki, H. (2017). A Review of Toxic Epidermal Necrolysis Management in Japan. Allergol. Int. 66 (1), 36–41. doi:10.1016/j.alit.2016.06.001
Kostal, M., Blaha, M., Lanska, M., Koštálová, M., Bláha, V., Štepánová, E., Malý, J., et al. (2012). Beneficial Effect of Plasma Exchange in the Treatment of Toxic Epidermal Necrolysis: a Series of Four Cases. J. Clin. Apher. 27 (4), 215–220. doi:10.1002/jca.21213
Kumar, V., Chaudhary, N., Garg, M., Floudas, C. S., Soni, P., and Chandra, A. B. (2017). Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 8, 49. doi:10.3389/fphar.2017.00049
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Cowey, C. L., Lao, C. D., et al. (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 373 (1), 23–34. doi:10.1056/NEJMoa1504030
Lipowicz, S., Sekula, P., Ingen-Housz-Oro, S., Liss, Y., Sassolas, B., Dunant, A., et al. (2013). Prognosis of Generalized Bullous Fixed Drug Eruption: Comparison with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Br. J. Dermatol. 168 (4), 726–732. doi:10.1111/bjd.12133
Logan, I. T., Zaman, S., Hussein, L., and Perrett, C. M. (2020). Combination Therapy of Ipilimumab and Nivolumab-Associated Toxic Epidermal Necrolysis (TEN) in a Patient with Metastatic Melanoma: A Case Report and Literature Review. J. Immunother. 43 (3), 89–92. doi:10.1097/CJI.0000000000000302
Macdonald, J. B., Macdonald, B., Golitz, L. E., LoRusso, P., and Sekulic, A. (2015). Cutaneous Adverse Effects of Targeted Therapies: Part II: Inhibitors of Intracellular Molecular Signaling Pathways. J. Am. Acad. Dermatol. 72 (2), 221–228. quiz 237-228. doi:10.1016/j.jaad.2014.07.033
Maloney, N. J., Ravi, V., Cheng, K., Bach, D. Q., and Worswick, S. (2020). Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis-like Reactions to Checkpoint Inhibitors: a Systematic Review. Int. J. Dermatol. 59 (6), e183–e188. doi:10.1111/ijd.14811
Martínez, P., and Del Campo, J. M. (2017). Pembrolizumab in Recurrent Advanced Cervical Squamous Carcinoma. Immunotherapy 9 (6), 467–470. doi:10.2217/imt-2016-0119
Michot, J. M., Bigenwald, C., Champiat, S., Collins, M., Carbonnel, F., Postel-Vinay, S., et al. (2016). Immune-related Adverse Events with Immune Checkpoint Blockade: a Comprehensive Review. Eur. J. Cancer. Oxford, England : 1990 54, 139–148. doi:10.1016/j.ejca.2015.11.016
Min Lee, C. K., Li, S., Tran, D. C., Zhu, G. A., Kim, J., Kwong, B. Y., et al. (2018). Characterization of Dermatitis after PD-1/pd-L1 Inhibitor Therapy and Association with Multiple Oncologic Outcomes: A Retrospective Case-Control Study. J. Am. Acad. Dermatol. 79 (6), 1047–1052. doi:10.1016/j.jaad.2018.05.035
Minkis, K., Garden, B. C., Wu, S., Pulitzer, M. P., and Lacouture, M. E. (2013). The Risk of Rash Associated with Ipilimumab in Patients with Cancer: a Systematic Review of the Literature and Meta-Analysis. J. Am. Acad. Dermatol. 69 (3), e121–8. doi:10.1016/j.jaad.2012.12.963
Mockenhaupt, M., Viboud, C., Dunant, A., Naldi, L., Halevy, S., Bouwes Bavinck, J. N., et al. (2008). Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study. J. Invest. Dermatol. 128 (1), 35–44. doi:10.1038/sj.jid.5701033
Naidoo, J., Page, D. B., Li, B. T., Connell, L. C., Schindler, K., Lacouture, M. E., et al. (2015). Toxicities of the Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Antibodies. Ann. Oncol. 26 (12), 2375–2391. doi:10.1093/annonc/mdv383
Narita, Y. M., Hirahara, K., Mizukawa, Y., Kano, Y., and Shiohara, T. (2011). Efficacy of Plasmapheresis for the Treatment of Severe Toxic Epidermal Necrolysis: Is Cytokine Expression Analysis Useful in Predicting its Therapeutic Efficacy? J. Dermatol. 38 (3), 236–245. doi:10.1111/j.1346-8138.2010.01154.x
Nayar, N., Briscoe, K., and Fernandez Penas, P. (2016). Toxic Epidermal Necrolysis-like Reaction with Severe Satellite Cell Necrosis Associated with Nivolumab in a Patient with Ipilimumab Refractory Metastatic Melanoma. J. Immunother. 39 (3), 149–152. doi:10.1097/CJI.0000000000000112
Nishijima, T. F., Shachar, S. S., Nyrop, K. A., and Muss, H. B. (2017). Safety and Tolerability of PD-1/pd-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist 22 (4), 470–479. doi:10.1634/theoncologist.2016-0419
O'Kane, G. M., Labbé, C., Doherty, M. K., Young, K., Albaba, H., and Leighl, N. B. (2017). Monitoring and Management of Immune-Related Adverse Events Associated with Programmed Cell Death Protein-1 Axis Inhibitors in Lung Cancer. Oncologist 22 (1), 70–80. doi:10.1634/theoncologist.2016-0164
Ohlmann, C. H., Kohlmorgen, S., Sahi, D., Engelmann, U., and Heidenreich, A. (2007). Lethal Course after Chemotherapy with Docetaxel. Acute Liver Failure with Accompanying Erythema Multiforme Major. Urologe A. 46 (10), 1425–1427. doi:10.1007/s00120-007-1367-9
Okazaki, T., Tanaka, Y., Nishio, R., Mitsuiye, T., Mizoguchi, A., Wang, J., et al. (2003). Autoantibodies against Cardiac Troponin I Are Responsible for Dilated Cardiomyopathy in PD-1-Deficient Mice. Nat. Med. 9 (12), 1477–1483. doi:10.1038/nm955
Pedoeem, A., Azoulay-Alfaguter, I., Strazza, M., Silverman, G. J., and Mor, A. (2014). Programmed Death-1 Pathway in Cancer and Autoimmunity. Clin. Immunol. 153 (1), 145–152. doi:10.1016/j.clim.2014.04.010
Pfaendler, K. S., and Tewari, K. S. (2016). Changing Paradigms in the Systemic Treatment of Advanced Cervical Cancer. Am. J. Obstet. Gynecol. 214 (1), 22–30. doi:10.1016/j.ajog.2015.07.022
Quach, H. T., Dewan, A. K., Davis, E. J., Ancell, K. K., Fan, R., Ye, F., et al. (2019). Association of Anti-programmed Cell Death 1 Cutaneous Toxic Effects with Outcomes in Patients with Advanced Melanoma. JAMA Oncol. 5 (6), 906–908. doi:10.1001/jamaoncol.2019.0046
Reschke, R., Mockenhaupt, M., Simon, J. C., and Ziemer, M. (2019). Severe Bullous Skin Eruptions on Checkpoint Inhibitor Therapy - in Most Cases Severe Bullous Lichenoid Drug Eruptions. J. Dtsch Dermatol. Ges. 17 (9), 942–948. doi:10.1111/ddg.13876
Ribas, A., Puzanov, I., Dummer, R., Schadendorf, D., Hamid, O., Robert, C., et al. (2015). Pembrolizumab versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Melanoma (KEYNOTE-002): a Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 16 (8), 908–918. doi:10.1016/S1470-2045(15)00083-2
Robert, C., Long, G. V., Brady, B., Dutriaux, C., Maio, M., Mortier, L., et al. (2015). Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 372 (4), 320–330. doi:10.1056/NEJMoa1412082
Robert, C., Schachter, J., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2015). Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 372 (26), 2521–2532. doi:10.1056/NEJMoa1503093
Roujeau, J. C., and Stern, R. S. (1994). Severe Adverse Cutaneous Reactions to Drugs. N. Engl. J. Med. 331 (19), 1272–1285. doi:10.1056/NEJM199411103311906
Sanlorenzo, M., Vujic, I., Daud, A., Algazi, A., Gubens, M., Luna, S. A., et al. (2015). Pembrolizumab Cutaneous Adverse Events and Their Association with Disease Progression. JAMA Dermatol. 151 (11), 1206–1212. doi:10.1001/jamadermatol.2015.1916
Schachter, J., Ribas, A., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2017). Pembrolizumab versus Ipilimumab for Advanced Melanoma: Final Overall Survival Results of a Multicentre, Randomised, Open-Label Phase 3 Study (KEYNOTE-006). Lancet 390 (10105), 1853–1862. doi:10.1016/S0140-6736(17)31601-X
Tie, Y., Ma, X., Zhu, C., Mao, Y., Shen, K., Wei, X., et al. (2017). Safety and Efficacy of Nivolumab in the Treatment of Cancers: A Meta-Analysis of 27 Prospective Clinical Trials. Int. J. Cancer. 140 (4), 948–958. doi:10.1002/ijc.30501
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F., et al. (2012). Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 366 (26), 2443–2454. doi:10.1056/NEJMoa1200690
Tumeh, P. C., Harview, C. L., Yearley, J. H., Shintaku, I. P., Taylor, E. J., Robert, L., et al. (2014). PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 515 (7528), 568–571. doi:10.1038/nature13954
Vivar, K. L., Deschaine, M., Messina, J., Divine, J. M., Rabionet, A., Patel, N., et al. (2017). Epidermal Programmed Cell Death-Ligand 1 Expression in TEN Associated with Nivolumab Therapy. J. Cutan. Pathol. 44 (4), 381–384. doi:10.1111/cup.12876
Wang, Y., and Li, G. (2019). PD-1/PD-L1 Blockade in Cervical Cancer: Current Studies and Perspectives. Front. Med. 13 (4), 438–450. doi:10.1007/s11684-018-0674-4
Weber, J. S., D'Angelo, S. P., Minor, D., Hodi, F. S., Gutzmer, R., Neyns, B., et al. (2015). Nivolumab versus Chemotherapy in Patients with Advanced Melanoma Who Progressed after Anti-CTLA-4 Treatment (CheckMate 037): a Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 16 (4), 375–384. doi:10.1016/S1470-2045(15)70076-8
Weber, J. S., Hodi, F. S., Wolchok, J. D., Topalian, S. L., Schadendorf, D., Larkin, J., et al. (2017). Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 35 (7), 785–792. doi:10.1200/JCO.2015.66.1389
Yang, W., Li, S., and Yang, Q. (2019). Risk of Dermatologic and Mucosal Adverse Events Associated with PD-1/pd-L1 Inhibitors in Cancer Patients: A Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 98 (20), e15731. doi:10.1097/MD.0000000000015731
Keywords: severe bullous skin reactions, literature review, case report, toxic epidermal necrolysis, cervical cancer, PD-1
Citation: Li X, Qu L-X, Ren Y-M and Hu C (2021) Case Report: A Case Report and Literature Review on Severe Bullous Skin Reaction Induced by anti-PD-1 Immunotherapy in a Cervical Cancer Patient. Front. Pharmacol. 12:707967. doi: 10.3389/fphar.2021.707967
Received: 11 May 2021; Accepted: 12 August 2021;
Published: 24 August 2021.
Edited by:
Halina Was, Military Institute of Medicine, PolandReviewed by:
Maja Mockenhaupt, University of Freiburg Medical Center, GermanyNiti Mittal, Pt. B.D. Sharma Postgraduate Institute of Medical Sciences, India
Pinaki Chakravarty, Tezpur Medical College and Hospital, India
Copyright © 2021 Li, Qu, Ren and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Li, bGl4aWFuZzE5OTAwMTA5QHNpbmEuY29t
†These authors have contributed equally to this work.
 Xiang Li
Xiang Li Li-Xin Qu1†
Li-Xin Qu1†