
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 14 September 2021
Sec. Drugs Outcomes Research and Policies
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.707499
Objective: To systematically review and compare the efficacy and posttreatment resistance of ceftazidime-avibactam therapy and ceftazidime-avibactam-based combination therapy in patients with Gram-negative pathogens.
Methods: PubMed, Embase, Web of Science, CNKI, and Wanfang Data databases were searched from their inception up to March 31, 2021, to obtain studies on ceftazidime-avibactam therapy versus ceftazidime-avibactam-based combination therapy in patients with carbapenem-resistant Gram-negative pathogens. The primary outcome was mortality rate, and the second outcomes were microbiologically negative, clinical success, and the development of resistance after ceftazidime-avibactam treatment.
Results: Seventeen studies representing 1,435 patients (837 received ceftazidime-avibactam-based combination therapy and 598 received ceftazidime-avibactam therapy) were included in the meta-analysis. The results of the meta-analysis showed that no statistically significant difference was found on mortality rate (Petos odds ratio (OR) = 1.03, 95% confidence interval (CI) 0.79–1.34), microbiologically negative (OR = 0.99, 95% CI 0.54–1.81), and clinical success (OR =0.95, 95% CI 0.64–1.39) between ceftazidime-avibactam-based combination therapy and ceftazidime-avibactam therapy. Although there was no difference in posttreatment resistance of ceftazidime-avibactam (OR = 0.65, 95% CI 0.34–1.26) in all included studies, a trend favoring the combination therapy was found (according to the pooled three studies, OR = 0.18, 95% CI 0.04–0.78).
Conclusions: The current evidence suggests that ceftazidime-avibactam-based combination therapy may not have beneficial effects on mortality, microbiologically negative, and clinical success to patients with carbapenem-resistant Gram-negative pathogens. A trend of posttreatment resistance occurred more likely in ceftazidime-avibactam therapy than the combination therapy. Due to the limited number of studies that can be included, additional high-quality studies are needed to verify the above conclusions.
Infections caused by carbapenem-resistant Gram-negative bacilli (CRGN) are challenging and associated with a high mortality rate (CDC, 2019); it spreads rapidly in the past decade causing a huge public health burden (Bassetti et al., 2017; Avendano et al., 2020; Zhen et al., 2021). Until now, several antimicrobial agents have been developed to fight against these superbugs, among which, ceftazidime-avibactam (CAZ-AVI) is a novel antibiotic combination, consisting of a third-generation cephalosporin (ceftazidime) and a synthetic β-lactamase inhibitor (avibactam). The special chemical structure allows avibactam to bond closely to and inactivate class A, class C, and some class D β-lactamases, but not class B metallo-β-lactamases (Zhanel et al., 2013).
Previous in vitro studies (Gaibani et al., 2017; Romanelli et al., 2020) demonstrated that the CAZ-AVI activity was enhanced after combining other agents. However, the results of combination therapy are inconclusive in real-world experiences (Tumbarello et al., 2015; Sousa et al., 2018). Additionally, phase 3 randomized clinical programs have assessed the efficacy of CAZ-AVI compared with other therapies (Carmeli et al., 2016; Wagenlehner et al., 2016) but not with CAZ-AVI-based combination therapy. In 2015, CAZ-AVI was first approved for marketing by the US Food and Drug Administration (FDA) for the treatment of adults (age >18) with serious infections including complicated intra-abdominal infection (cIAI) (along with metronidazole) and complicated urinary tract infection (cUTI) (Administration USFaD., 2015). This scenario occurred only when there were limited or no other therapeutic options. Subsequently, it was approved by the European Medicines Agency (EMA) in 2016 (Shirley, 2018), by the National Medical Products Administration in China in 2019 (14) and in other countries (15, 16). A wealth of CAZ-AVI-treating CRGN studies in real-world practice has been reported. There has been a dispute on whether monotherapy therapy would be inferior to combination therapy (15). Also, this debate occurred in the posttreatment CAZ-AVI scenario (Shields et al., 2018; Tumbarello et al., 2019; Ackley et al., 2020; Castón et al., 2020; Iannaccone et al., 2020; Tumbarello et al., 2021).
Therefore, a meta-analysis aiming to systematically compare the mortality rate, microbiologically negative, clinical success, and the development of resistance between CAZ-AVI therapy and CAZ-AVI-based combination therapy was conducted.
The meta-analysis was reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). The PRISMA checklist was provided as Supplementary Material.
Multiple databases, including PubMed, Embase, Web of Science, CNKI, and Wanfang Data, were searched for the studies on CAZ-AVI therapy and CAZ-AVI-based combination therapy for the treatment of infections caused by CRGN (up to March 31, 2021). The search keywords were “ceftazidime avibactam” and “carbapenem resistant. The search strategy was provided as Supplementary Material. The references of relevant articles (both original research and review) were also inspected. The article search and reference inspection were performed by two researchers independently screening the titles and abstracts and examining the full texts and reference lists according to the inclusion and exclusion criteria listed below and (Bassetti et al., 2017) cross-checking each other’s studies.
Inclusion criteria:
i) All types of clinical studies were included: both randomized controlled trials (RCTs) and observational studies (retrospective and prospective).
ii) Studies with availability separate data on CAZ-AVI therapy and any CAZ-AVI-based combination therapy.
iii) Studies reported more than ten adult patients (age >18) with infections caused by CRGN.
iv) No language restrictions on the articles: eligible studies published in any languages were all included.
Exclusion criteria:
i) In vitro studies, reviews, or case reports (n < 10).
ii) Data on CAZ-AVI therapy or CAZ-AVI-based combination regimen were not reported.
In case of insufficient data provided in the eligible articles, corresponding authors were contacted for more information.
The primary outcome was the mortality rate at an appropriate time point (14, 30, and 90 days, in hospital), and the secondary outcomes were microbiologically negative, clinical success, and the development of resistance after CAZ-AVI treatments. Two researchers independently extracted and cross-checked the studies to ensure the selected studies were unique based on the following information of the studies: the first author, published year, country, study design, patient demographics (age, sex, and number of patients), bacteria, carbapenemase, the severity of illness, comorbidity index, combination agents, and infection type.
The Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool (Sterne et al., 2016a) was used to evaluate the risk of bias in observational studies (11 cohort studies and 1 case-cohort study). The tool includes seven bias domains: confounding domain, domain of selection of participants, classification domain, deviations domain, missing data, measurement domain, and domain of selection of the reported result. The risk of bias for individual studies was accessed by two researchers answering signal questions according to a hypothetical randomized trial. According to the design of the study, the Agency for Healthcare Research and Quality (AHRQ) tool (Rostom et al., 2004) was applied to evaluate the residual five studies (two case series and three cross-sectional studies). Each study was assessed independently by two researchers, and if there was a dispute, a third researcher was employed to resolve the dispute.
The definitions were based on studies included in this meta-analysis. Combination therapy was defined as one or more agents combined with CAZ-AVI for at least 24 h of treatment. Clinical success was defined as the resolution or remarkable improvement of the symptoms and signs of infection caused by CRGN. Microbiologically negative was defined as negative culture from blood or specific sites at the end of the treatment. CRGN was defined as resistance to any carbapenem using an automated or manual methodology.
RevMan 5.3 software was used to perform the meta-analysis. Pooled odds ratios (ORs) or Peto ORs and 95% confidence intervals (CIs) were computed in the analysis. I2 and Q statistics were used to evaluate the heterogeneity. I2 > 50% and p < 0.1 are assumed as clinical heterogeneity. A fixed-effects model was performed for the meta-analysis. Subgroup analyses were performed according to the endpoint of mortality. Sensitivity analyses were performed as well. p-value less than 0.05 was considered significant.
The database search described in Database Search generated 17 eligible articles (Sousa et al., 2018; Kuang et al., 2020; Guimarães et al., 2019; Rathish et al., 2021; Shields et al., 2018; Tumbarello et al., 2019; Ackley et al., 2020; Iannaccone et al., 2020; Castón et al., 2020; Tumbarello et al., 2021; Zhu et al., 2021; King et al., 2017; Temkin et al., 2017; De la Calle et al., 2019; Chen et al., 2020; Jiang et al., 2020; Jorgensen et al., 2020). Specifically, 1,106 references were found by searching for the keywords: “ceftazidime avibactam” and “carbapenem resistant.” After removing the duplicates, 667 potentially relevant articles were left. The 620 records containing only titles and abstracts were then excluded. The remaining 47 studies were further screened using the inclusion and exclusion criteria and returned 17 articles. The details of the database search flow are shown in the study selection flowchart (Figure 1). We used the PRISMA2020 tool (R package and ShinyApp for making PRISMA2020 flow diagrams) (Haddaway et al., 2021) to generate the flow diagram (Figure 1).
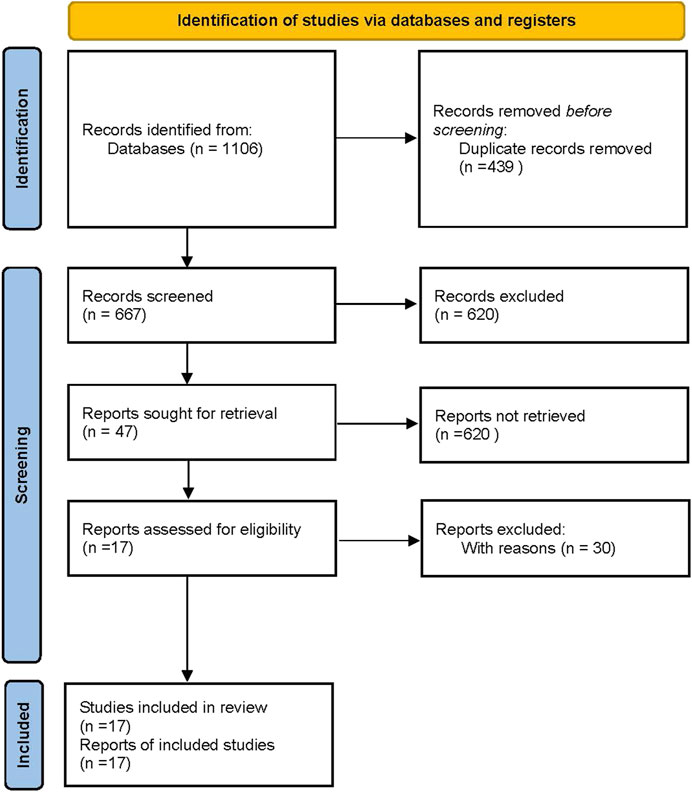
FIGURE 1. Flow diagram of meta-analysis generated by PRISMA 2020 (Haddaway et al., 2021).
The basic information of the 17 articles included in the meta-analysis was summarized (Table 1). There are 11 cohort studies, one case-cohort study, two case series, and three case-sectional studies and all of them were retrospective observational. In total, 1,435 patients were included in this analysis, and the number of patients for different studies ranged from 10 (Chen et al., 2020) to 577 (Tumbarello et al., 2021). The studies were published between 2017 and 2021. Four studies were from the USA (King et al., 2017; Shields et al., 2018; Ackley et al., 2020; Jorgensen et al., 2020), three were from Spain (Sousa et al., 2018; De la Calle et al., 2019; Castón et al., 2020), four were from China (Chen et al., 2020; Jiang et al., 2020; Kuang et al., 2020; Zhu et al., 2021), three were from Italy (Tumbarello et al., 2019; Iannaccone et al., 2020; Tumbarello et al., 2021), one was from Brazil (Guimarães et al., 2019), one was from India (Rathish et al., 2021), and one was from Europe and Australia (Temkin et al., 2017). In terms of the pathogens, most of the studies reported carbapenem-resistant Enterobacteriaceae (CRE) with carbapenem-resistant Klebsiella pneumoniae (CKP) as the most frequent, while one study included both CKP and carbapenem-resistant Pseudomonas aeruginosa (CRPA) (Temkin et al., 2017) and one study reported CRE, CRPA, and Acinetobacter baumannii (AB) (Kuang et al., 2020). As for infection types, most studies demonstrated more than one type of infection, while two were only bloodstream infections (BSI) (Tumbarello et al., 2019; Iannaccone et al., 2020), and one was pneumonia (Jiang et al., 2020). Reagents that have been combined with CAZ-AVI therapy include aminoglycosides (AMG), polymyxin (PM), tigecycline (TGC), colistin (COL), amikacin (AMK), imipenem (IMP), gentamicin (GEN), ciprofloxacin (CIP), meropenem (MEM), fosfomycin (FOS), carbapenems (CAP), fluoroquinolone (FLU), minocycline (MIN), and sulfamethoxazole-trimethoprim (SUL-TRI). Patients were treated by CAZ-AVI and observed for at least 72 h in 12 studies (Temkin et al., 2017; Shields et al., 2018; De la Calle et al., 2019; Tumbarello et al., 2019; Ackley et al., 2020; Castón et al., 2020; Chen et al., 2020; Jiang et al., 2020; Jorgensen et al., 2020; Kuang et al., 2020; Tumbarello et al., 2021; Zhu et al., 2021), for at least 48 h in three studies (Sousa et al., 2018; Guimarães et al., 2019; Rathish et al., 2021), and for at least 24 h in one study (King et al., 2017). There is only one study in which the treatment time was not reported (Iannaccone et al., 2020).
Robvis tool (McGuinness and Higgins, 2021) was applied for the publication quality (risk-of-bias) assessment. The risk-of-bias assessment results for each study (Figure 2A) and each domain (Figure 2B) are displayed. Ten studies (King et al., 2017; Shields et al., 2018; De la Calle et al., 2019; Tumbarello et al., 2019; Ackley et al., 2020; Castón et al., 2020; Chen et al., 2020; Jorgensen et al., 2020; Kuang et al., 2020; Rathish et al., 2021) have a critical overall risk of bias (judged by one study was at critical risk of bias in at least one domain (Sterne et al., 2016b)) and two moderates (Sousa et al., 2018; Tumbarello et al., 2021). The quality of the other five studies assessed by the AHRQ tool is all poor quality (Table 2).
Out of the 17 included eligible studies, three studies (Shields et al., 2018; Jiang et al., 2020; Zhu et al., 2021) did not extract separate data on mortality rates from CAZ-AVI therapy alone or in combination with other drugs. Given the fact that most studies’ overall risk of bias was critical, a subgroup meta-analysis was not performed based on the risk-of-bias levels but on mortality at an appropriate time point (14, 30, and 90 days, in hospital). Sensitivity analyses were performed according to the study design, comorbidity index, illness severity, bacteria, infection type, and carbapenemase. There was no statistically significant difference in the overall mortality rates between CAZ-AVI therapy alone and ceftazidime-avibactam-based combination therapy (Peto OR = 1.03, 95% CI 0.79–1.34; I2 = 0%) (Figure 3).
For the 14-day mortality, the Peto OR was 0.90 (95% CI 0.32–2.50; I2 = 45%) with no significant difference, but there were only three articles (Sousa et al., 2018; Kuang et al., 2020; Guimarães et al., 2019) and 106 patients included. Moderate clinical heterogeneity (I2 = 45%; p = 0.16) was observed for one report (Kuang et al., 2020), which may be due to the pathogen complexity (including CRE, CRPA, and AB). By excluding this study, I2 was 0%, and the Peto OR was 0.50 (95% CI 0.15–1.64). Six studies (Sousa et al., 2018; Kuang et al., 2020; Tumbarello et al., 2019; Castón et al., 2020; Tumbarello et al., 2021; Jorgensen et al., 2020) were used to assess the 30-day mortality rate, contributing to 63.0% of the weight. No significant heterogeneity existed among these studies (I2 = 0%; p = 0.93), and there was no statistically significant difference between CAZ-AVI therapy and combination therapy (Peto OR = 0.96, 95% CI 0.69–1.33; I2 = 0%). The Peto OR of 90-day mortality assessment was 1.74 (95% CI 0.79–3.82; I2 = 0%), thus exhibiting no significant difference. Three studies (De la Calle et al., 2019; Ackley et al., 2020; Chen et al., 2020) consisting of 139 patients were included in this analysis. For the in-hospital mortality assessment, four studies (King et al., 2017; Temkin et al., 2017; Iannaccone et al., 2020; Rathish et al., 2021) were included with Peto OR = 1.01 (95% CI 0.55–1.86; I2 = 0%).
Ten studies (King et al., 2017; Temkin et al., 2017; Shields et al., 2018; Sousa et al., 2018; De la Calle et al., 2019; Ackley et al., 2020; Jiang et al., 2020; Kuang et al., 2020; Rathish et al., 2021; Zhu et al., 2021) were included in the clinical success analysis, in which only one (case series) study (Temkin et al., 2017) did not define clinical success, and the other nine studies defined clinical success as resolution or remarkable improvement of the symptoms and different signs of infection at some point (such as the endpoint) (Sousa et al., 2018; De la Calle et al., 2019; Ackley et al., 2020), absence of recurrent infections (Shields et al., 2018; De la Calle et al., 2019; Ackley et al., 2020), or resistance to CAZ-AVI (Rathish et al., 2021). Sensitivity analyses were performed according to the study design, definitions of clinical success, illness severity, and carbapenemase. However, no statistical difference was observed based on a pooled OR of 0.95 (95% CI 0.64–1.39; I2 = 0%) (Figure 4).
Five studies (King et al., 2017; Temkin et al., 2017; Sousa et al., 2018; Castón et al., 2020; Chen et al., 2020) reported the outcomes of microbiologically negative, including a total of 212 patients. Out of these studies, three studies defined the microbiologically negative as sterilization of the index culture at the end of treatment or an appropriate point (7 days) (King et al., 2017; Sousa et al., 2018; Castón et al., 2020), while the other two studies had no definitions (Temkin et al., 2017; Chen et al., 2020). Sensitivity analyses were performed according to the study design, definitions of microbiologically negative, illness severity, and carbapenemase, and there was no significant difference. The pooled OR of the five studies was 0.99 (95% CI 0.54–1.81; I2 = 0%) (Figure 5).
Six studies (Shields et al., 2018; Tumbarello et al., 2019; Ackley et al., 2020; Iannaccone et al., 2020; Castón et al., 2020; Tumbarello et al., 2021) assessed the development of resistance after CAZ-AVI treatment, in which 967 patients (642 in CAZ-AVI-based combination therapy and 325 in CAZ-AVI therapy) were included. Sensitivity analyses were performed by the study design, the illness severity, comorbidity index, and infection type. Heterogeneity was either low or absent, and no difference was observed (Figure 6). Klebsiella pneumoniae carbapenemase- (KPC-) producing isolates were reported in all the six studies, with one study (Shields et al., 2018) reporting both KPC-2 and KPC-3 and another study (Ackley et al., 2020) reporting parts of the isolates as KPC-producing bacteria. In our analysis, there was no significant difference (OR = 0.65, 95% CI 0.34–1.26; I2 = 0%). However, when we pooled only three studies (Tumbarello et al., 2019; Ackley et al., 2020; Iannaccone et al., 2020) including 266 participants, the pooled OR was 0.18 (95% CI 0.04–0.78; I2 = 0%) favoring CAZ-AVI-based combination therapy. Therefore, we observed a posttreatment resistance trend in CAZ-AVI alone therapy (Figure 6,p-value = 0.2). Further study with larger samples would need to be conducted to confirm this assumption.
The mortality, clinical success, microbiologically negative, and posttreatment resistance between CAZ-AVI therapy alone and in combination with other agents were compared. The results indicate that CAZ-AVI concomitant therapy may not exhibit beneficial effects on mortality, clinical success, and microbiologically negative to patients infected with CRGN. This is consistent with the two previous meta-analyses (one reported only mortality (Fiore et al., 2020), and the other one reported mortality and microbiologically negative (Onorato et al., 2019)). However, a trend of posttreatment resistance is likely to occur in the CAZ-AVI therapy based on the pooled three studies, OR = 0.18, 95% CI 0.04–0.78).
Four subgroups were analyzed on data regarding mortality, and the discrepancies were not significant, which is consistent with previous studies reported by Fiore (Fiore et al., 2020) and Onorato (Onorato et al., 2019). Gutiérrez-Gutiérrez et al. (Gutiérrez-Gutiérrez et al., 2017) reported no difference observed in overall mortality rates between patients who received antibiotics concurrent with other agents (colistin plus tigecycline most common) and monotherapy (any but not CAZ-AVI) treating carbapenemase-producing Enterobacteriaceae (CPE) infections. However, in the high-mortality-score patients, combination therapy was related to lower mortality compared with monotherapy. Another study conducted by Tumbarello (Tumbarello et al., 2015) demonstrated that combination therapy including meropenem improved survival rates in severity illness patients with KPC-Kp infections. Here, we performed sensitivity analyses according to the severity of illness, and no significant difference was observed.
Clinical success, which was first evaluated in our meta-analysis, showed no difference in the 10 studies (Sousa et al., 2018; Kuang et al., 2020; Rathish et al., 2021; Shields et al., 2018; Ackley et al., 2020; Zhu et al., 2021; King et al., 2017; Temkin et al., 2017; De la Calle et al., 2019; Jiang et al., 2020) included (OR = 0.95, 95% CI 0.64–1.39; I2 = 0%). Results of microbiologically negative were evaluated in five studies (Sousa et al., 2018; Castón et al., 2020; King et al., 2017; Temkin et al., 2017; Chen et al., 2020) and showed no discrepancies (OR = 0.99, 95% CI 0.54–1.81; I2 = 0%). Out of the five studies, two studies (Temkin et al., 2017; Chen et al., 2020) did not define the microbiologically negative. Besides, to the best of our knowledge, this was the first meta-analysis to systematically evaluate the development of resistance after CAZ-AVI treatment alone or in combination with other agents. CAZ-AVI was first approved by the US FDA (Tumbarello et al., 2019) in 2015, and subsequently, rapidly emerging resistance was first reported by Humphries et al. (Humphries et al., 2015). In their study, an old woman with infections caused by KPC-producing KP (KPC-3) received salvage therapy and developed resistance to CAZ-AVI after 9 days of treatment (Humphries et al., 2015). In our meta-analysis, six studies (Shields et al., 2018; Tumbarello et al., 2019; Ackley et al., 2020; Iannaccone et al., 2020; Castón et al., 2020; Tumbarello et al., 2021) reported posttreatment resistance of CAZ-AVI, and only one study (Shields et al., 2018) provided detailed information on the potential mechanism of CAZ-AVI resistance that both KPC-2- and KPC-3-producing CRE were included (Shields et al., 2018). Interestingly, resistance emerged only from KPC-3-producing isolates. This is consistent with the study reported by Humphries et al. (Humphries et al., 2015). Further study is needed to unravel the potential resistance mechanism of CAZ-AVI posttreatment. Additionally, although there was no significant difference (OR = 0.65, 95% CI 0.34–1.26; I2 = 0%) in CAZ-AVI resistance between treatment in combination and alone, combination therapy was more likely associated with lower resistance when we pooled three studies (Tumbarello et al., 2019; Ackley et al., 2020; Iannaccone et al., 2020) (OR = 0.18, 95% CI 0.04–0.78; I2 = 0%). Further studies with a larger sample size are needed to test this assumption. Given that CAZ-AVI concomitant with other antibiotics (such as TGC or COL) may result in potential side effects or toxicity (Sousa et al., 2018), decisions regarding CAZ-AVI-based combination therapy should be made according to the traits of patients.
This study has several limitations. Firstly, all studies included were retrospective observational design studies, with no RCTs or prospective observational studies included. Secondly, the overall risk of bias of most studies was critical. Thirdly, the sample sizes of several subgroups were small. Fourth, due to the fact that the data on the type of infection between CAZ-AVI therapy alone or in combination with other agents were not available, subgroup analysis was not performed according to the infection type.
In summary, studies for CAZ-AVI single therapy or CAZ-AVI combination therapy in patients with CRGN infections were analyzed. The outcomes including mortality, clinical success, microbiologically negative, and posttreatment resistance were compared, and no significant differences were observed between the two therapies. To the best of the authors’ knowledge, this is the first meta-analysis study accessing the resistance between CAZ-AVI treatment alone and in combination. However, given the low quality of the evidence and limited samples in some subgroups, definitive conclusions cannot be made. RCTs and large prospective observational studies are needed to evaluate these issues in future studies.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
JZ and HZ designed the study. JZ and SL contributed to manuscript writing and revised and supervised the project. DL and FF contributed to literature searches. HY and XH checked the data. All authors approved the manuscript.
This work was supported by the National Natural Science Foundation of China (81702064).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.707499/full#supplementary-material
Ackley, R., Roshdy, D., Meredith, J., Minor, S., Anderson, W. E., Capraro, G. A., et al. (2020). Meropenem-vaborbactam Versus Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 64 (5), e02313. doi:10.1128/aac.02313-19
Administration USFaD (2015). Drug Trials Snapshot: AVYCAZ (cIAI). Available at: https://wwwfdagov/drugs/drug-approvals-and-databases/drug-trials-snapshot-avycaz-ciai. Accessed July 31, 2021.
Avendano, E. E., Raman, G., Chan, J., and McCann, E. (2020). Burden of Carbapenem Non-susceptible Infections in High-Risk Patients: Systematic Literature Review and Meta-Analysis. Antimicrob. Resist. Infect. Control. 9 (1), 193. doi:10.1186/s13756-020-00858-8
Bassetti, M., Poulakou, G., Ruppe, E., Bouza, E., Van Hal, S. J., and Brink, A. (2017). Antimicrobial Resistance in the Next 30 years, Humankind, Bugs and Drugs: A Visionary Approach. Intensive Care Med. 43 (10), 1464–1475. doi:10.1007/s00134-017-4878-x
Carmeli, Y., Armstrong, J., Laud, P. J., Newell, P., Stone, G., Wardman, A., et al. (2016). Ceftazidime-avibactam or Best Available Therapy in Patients with Ceftazidime-Resistant Enterobacteriaceae and Pseudomonas aeruginosa Complicated Urinary Tract Infections or Complicated Intra-abdominal Infections (REPRISE): A Randomised, Pathogen-Directed, Phase 3 Study. Lancet Infect. Dis. 16 (6), 661–673. doi:10.1016/s1473-3099(16)30004-4
Castón, J. J., Gallo, M., García, M., Cano, A., Escribano, A., Machuca, I., et al. (2020). Ceftazidime-avibactam in the Treatment of Infections Caused by KPC-Producing Klebsiella pneumoniae: Factors Associated with Clinical Efficacy in a Single-center Cohort. Int. J. Antimicrob. Agents. 56 (3), 106075. doi:10.1016/j.ijantimicag.2020.106075
CDC (2019). Antibiotic Resistance Threats in the United States 2019. Available at: https://wwwcdcgov/drugresistance/pdf/threats-report/2019-ar-threats-report-508pdf. Accessed April 15, 2021.
Chen, W., Sun, L., Guo, L., Cao, B., Liu, Y., Zhao, L., et al. (2020). Clinical Outcomes of Ceftazidime-Avibactam in Lung Transplant Recipients with Infections Caused by Extensively Drug-Resistant Gram-Negative Bacilli. Ann. Transl Med. 8 (3), 39. doi:10.21037/atm.2019.10.40
De la Calle, C., Rodríguez, O., Morata, L., Marco, F., Cardozo, C., García-Vidal, C., et al. (2019). Clinical Characteristics and Prognosis of Infections Caused by OXA-48 Carbapenemase-Producing Enterobacteriaceae in Patients Treated with Ceftazidime-Avibactam. Int. J. Antimicrob. Agents. 53 (4), 520–524. doi:10.1016/j.ijantimicag.2018.11.015
Fiore, M., Alfieri, A., Di Franco, S., Pace, M. C., Simeon, V., Ingoglia, G., et al. (2020). Ceftazidime-avibactam Combination Therapy Compared to Ceftazidime-Avibactam Monotherapy for the Treatment of Severe Infections Due to Carbapenem-Resistant Pathogens: A Systematic Review and Network Meta-Analysis. Antibiotics (Basel) 9 (7), 388. doi:10.3390/antibiotics9070388
Gaibani, P., Lewis, R. E., Volpe, S. L., Giannella, M., Campoli, C., Landini, M. P., et al. (2017). In Vitro Interaction of Ceftazidime-Avibactam in Combination with Different Antimicrobials Against KPC-Producing Klebsiella pneumoniae Clinical Isolates. Int. J. Infect. Dis. 65, 1–3. doi:10.1016/j.ijid.2017.09.017
Guimarães, T., Nouér, S. A., Martins, R. C. R., Perdigão Neto, L. V., Martins, W. M. B. S., Narciso Barbosa, A. C., et al. (2019). Ceftazidime-avibactam as Salvage Therapy for Infections Caused by Enterobacteriales Coresistant to Carbapenems and Polymyxins. Antimicrob. Agents Chemother. 63 (10), e00528. doi:10.1128/aac.00528-19
Gutiérrez-Gutiérrez, B., Salamanca, E., de Cueto, M., Hsueh, P. R., Viale, P., Paño-Pardo, J. R., et al. (2017). Effect of Appropriate Combination Therapy on Mortality of Patients with Bloodstream Infections Due to Carbapenemase-Producing Enterobacteriaceae (INCREMENT): A Retrospective Cohort Study. Lancet Infect. Dis. 17 (7), 726–734. doi:10.1016/S1473-3099(17)30228-1
Haddaway, N. R., Page, M. J., Pritchared, C. C., and McGuinness, L. A. (2021). PRISMA2020: R Package and ShinyApp for Producing PRISMA 2020 Compliant Flow diagrams(Version 0.0.2). Zenodo.
Humphries, R. M., Yang, S., Hemarajata, P., Ward, K. W., Hindler, J. A., Miller, S. A., et al. (2015). First Report of Ceftazidime-Avibactam Resistance in a KPC-3-Expressing Klebsiella pneumoniae Isolate. Antimicrob. Agents Chemother. 59 (10), 6605–6607. doi:10.1128/aac.01165-15
Iannaccone, M., Boattini, M., Bianco, G., Corcione, S., Cavallo, R., and Costa, C. (2020). Ceftazidime-avibactam Susceptible to Resistant KPC-Producing Enterobacterales Bloodstream Infections: An Observational Study. J. Chemother. 32 (3), 160–162. doi:10.1080/1120009x.2019.1709363
Jiang, T., Deng, F., and Yun, B. (2020). Efficacy of Ceftazidime-Avibactam Therapy in Patients with Pneumonia Infections Caused by Carbapemen-Resistant Klesiella Pneumoniae. Jiangsu Med. J. 46 (12), 1286–1288. doi:10.19460/j.cnki.0253-3685.2020.12.025
Jorgensen, S. C. J., Trinh, T. D., Zasowski, E. J., Lagnf, A. M., Bhatia, S., Melvin, S. M., et al. (2020). Evaluation of the INCREMENT-CPE, Pitt Bacteremia and qPitt Scores in Patients with Carbapenem-Resistant Enterobacteriaceae Infections Treated with Ceftazidime-Avibactam. Infect. Dis. Ther. 9 (2), 291–304. doi:10.1007/s40121-020-00288-4
King, M., Heil, E., Kuriakose, S., Bias, T., Huang, V., El-Beyrouty, C., et al. (2017). Multicenter Study of Outcomes with Ceftazidime-Avibactam in Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 61 (7), e00449. doi:10.1128/aac.00449-17
Kuang, H., Zhong, C., Wang, Y., Ye, H., Ao, K., Zong, Z., et al. (2020). Clinical Characteristics and Outcomes of Patients with Multidrug-Resistant Gram-Negative Bacterial Infections Treated with Ceftazidime/avibactam. J. Glob. Antimicrob. Resist. 23, 404–407. doi:10.1016/j.jgar.2020.10.023
McGuinness, L. A., and Higgins, J. P. T. (2021). Risk-of-bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-Of-Bias Assessments. Res. Synth. Methods 12 (1), 55–61. doi:10.1002/jrsm.1411
Onorato, L., Di Caprio, G., Signoriello, S., and Coppola, N. (2019). Efficacy of Ceftazidime/avibactam in Monotherapy or Combination Therapy Against Carbapenem-Resistant Gram-Negative Bacteria: A Meta-Analysis. Int. J. Antimicrob. Agents 54 (6), 735–740. doi:10.1016/j.ijantimicag.2019.08.025
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Rathish, B., Wilson, A., Warrier, A., Prakash, S., Babu, R., and Joy, S. (2021). Clinical Outcomes in Carbapenem-Resistant Enterobacteriaceae Infections Treated with Ceftazidime-Avibactam: A Single-center Observational Study. Cureus 13 (2), e13081. doi:10.7759/cureus.13081
Romanelli, F., De Robertis, A., Carone, G., Dalfino, L., Stufano, M., Del Prete, R., et al. (2020). In Vitro Activity of Ceftazidime/avibactam Alone and in Combination with Fosfomycin and Carbapenems Against KPC-Producing Klebsiella pneumoniae. New Microbiol. 43 (3), 136–138.
Rostom, A. D. C., and Cranney, A. (2004). Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US). (Evidence Reports/Technology Assessments, No. 104. Appendix D. Quality Assessment Forms. Available at: https://www.ncbi.nlm.nih.gov/books/NBK35156/.
Shields, R. K., Nguyen, M. H., Chen, L., Press, E. G., Kreiswirth, B. N., and Clancy, C. J. (2018). Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance Among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 62 (5). e02497. doi:10.1128/aac.02497-17
Shirley, M. (2018). Ceftazidime-avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 78 (6), 675–692. doi:10.1007/s40265-018-0902-x
Sousa, A., Pérez-Rodríguez, M. T., Soto, A., Rodríguez, L., Pérez-Landeiro, A., Martínez-Lamas, L., et al. (2018). Effectiveness of Ceftazidime/avibactam as Salvage Therapy for Treatment of Infections Due to OXA-48 Carbapenemase-Producing Enterobacteriaceae. J. Antimicrob. Chemother. 73 (11), 3170–3175. doi:10.1093/jac/dky295
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: A Tool for Assessing Risk of Bias in Non-randomised Studies of Interventions. BMJ 355, i4919. doi:10.1136/bmj.i4919
Sterne, J. A. C. H. J., Elbers, R. G., and Reeves, B. C.The development group for ROBINS-I (2016). Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I): Detailed Guidance. Available at: http://wwwriskofbiasinfo (Accessed October 12, 2016).
Temkin, E., Torre-Cisneros, J., Beovic, B., Benito, N., Giannella, M., Gilarranz, R., et al. (2017). Ceftazidime-avibactam as Salvage Therapy for Infections Caused by Carbapenem-Resistant Organisms. Antimicrob. Agents Chemother. 61 (2), 61. doi:10.1128/aac.01964-16
Tumbarello, M., Trecarichi, E. M., Corona, A., De Rosa, F. G., Bassetti, M., Mussini, C., et al. (2019). Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients with Infections Caused by Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae. Clin. Infect. Dis. 68 (3), 355–364. doi:10.1093/cid/ciy492
Tumbarello, M., Trecarichi, E. M., De Rosa, F. G., Giannella, M., Giacobbe, D. R., Bassetti, M., et al. (2015). Infections Caused by KPC-Producing Klebsiella pneumoniae: Differences in Therapy and Mortality in A Multicentre Study. J. Antimicrob. Chemother. 70 (7), 2133–2143. doi:10.1093/jac/dkv086
Tumbarello, M., Raffaelli, F., Giannella, M., Mantengoli, E., Mularoni, A., Venditti, M., et al. (2021). Ceftazidime-Avibactam Use for Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae Infections: A Retrospective Observational Multicenter Study. Clin. Infect. Dis. 2021, ciab176 doi:10.1093/cid/ciab176
Wagenlehner, F. M., Sobel, J. D., Newell, P., Armstrong, J., Huang, X., Stone, G. G., et al. (2016). Ceftazidime-avibactam Versus Doripenem for the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis: RECAPTURE, A Phase 3 Randomized Trial Program. Clin. Infect. Dis. 63 (6), 754–762. doi:10.1093/cid/ciw378
Zhanel, G. G., Lawson, C. D., Adam, H., Schweizer, F., Zelenitsky, S., Lagacé-Wiens, P. R., et al. (2013). Ceftazidime-avibactam: A Novel Cephalosporin/β-Lactamase Inhibitor Combination. Drugs 73 (2), 159–177. doi:10.1007/s40265-013-0013-7
Zhen, X., Stålsby Lundborg, C., Sun, X., Zhu, N., Gu, S., and Dong, H. (2021). Economic Burden of Antibiotic Resistance in China: A National Level Estimate for Inpatients. Antimicrob. Resist. Infect. Control. 10 (1), 5. doi:10.1186/s13756-020-00872-w
Keywords: ceftazidime-avibactam therapy, combination therapy, carbapenem-resistant gram-negative pathogen, efficacy, meta-analysis
Citation: Li D, Fei F, Yu H, Huang X, Long S, Zhou H and Zhang J (2021) Ceftazidime-Avibactam Therapy Versus Ceftazidime-Avibactam-Based Combination Therapy in Patients With Carbapenem-Resistant Gram-Negative Pathogens: A Meta-Analysis. Front. Pharmacol. 12:707499. doi: 10.3389/fphar.2021.707499
Received: 10 May 2021; Accepted: 16 August 2021;
Published: 14 September 2021.
Edited by:
Ileana Mardare, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Sunita Nair, Consultant, Mumbai, IndiaCopyright © 2021 Li, Fei, Yu, Huang, Long, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Zhou, emhvdWhhbzUxMUBxcS5jb20=; Jie Zhang, emhhbmdqaWVzcHBoQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.