- 1Shenzhen Public Service Platform for Clinical Application of Medical Imaging, Shenzhen Key Laboratory for Drug Addiction and Medication Safety, Department of Ultrasound, Peking University Shenzhen Hospital, Shenzhen, China
- 2National Institute on Drug Dependence and Beijing Key Laboratory of Drug Dependence, Peking University, Beijing, China
Persistent and stable drug memories lead to a high rate of relapse among addicts. A number of studies have found that intervention in addiction-related memories can effectively prevent relapse. Deep brain stimulation (DBS) exhibits distinct therapeutic effects and advantages in the treatment of neurological and psychiatric disorders. In addition, recent studies have also found that the substantia nigra pars reticulata (SNr) could serve as a promising target in the treatment of addiction. Therefore, the present study aimed to investigate the effect of DBS of the SNr on the reinstatement of drug-seeking behaviors. Electrodes were bilaterally implanted into the SNr of rats before training of methamphetamine-induced conditioned place preference (CPP). High-frequency (HF) or low-frequency (LF) DBS was then applied to the SNr during the drug-free extinction sessions. We found that HF DBS, during the extinction sessions, facilitated extinction of methamphetamine-induced CPP and prevented drug-primed reinstatement, while LF DBS impaired the extinction. Both HF and LF DBS did not affect locomotor activity or induce anxiety-like behaviors of rats. Finally, HF DBS had no effect on the formation of methamphetamine-induced CPP. In conclusion, our results suggest that HF DBS of the SNr could promote extinction and prevent reinstatement of methamphetamine-induced CPP, and the SNr may serve as a potential therapeutic target in the treatment of drug addiction.
Introduction
Persistent and stable drug memories are considered a major contributor to the intense craving and relapse in drug addiction, which are difficult to eliminate (Hyman and Malenka, 2001; Kauer and Malenka, 2007). Even after extinction, when being re-exposed to drug-associated cues, the original drug memories would be reactivated and cause drug-seeking behaviors, leading to a high relapse rate among addicts (Conklin and Tiffany, 2002; Milton and Everitt, 2012b; Chen et al., 2019b). It has been found that extinction combined with other interventions, such as the retrieval–extinction procedure, can facilitate elimination of drug memories and prevent relapse (He et al., 2011; Xue et al., 2012; Xue et al., 2014; Luo et al., 2015; Liu et al., 2019), which provides a new avenue for the treatment of addiction (Milton and Everitt, 2012a).
Deep brain stimulation (DBS) is an FDA-approved therapy for essential tremor (Schuurman et al., 2000; Opri et al., 2020), Parkinson’s disease (Rosin et al., 2011; Okun, 2012; Katz et al., 2015), idiopathic dystonia (Kleiner-Fisman et al., 2007; Elkaim et al., 2019), and severe obsessive-compulsive disorder (Figee et al., 2013; Wu et al., 2021) and exhibits potential therapeutic effects in the treatment of some other neurological and psychiatric disorders, such as depression (Kennedy et al., 2011; Holtzheimer et al., 2017; Crowell et al., 2019), anorexia nervosa (Lipsman et al., 2013; Lipsman et al., 2017), and addiction (Luigjes et al., 2012; Creed et al., 2015). In addition, unlike pharmacotherapy, DBS has the advantages of adjusting stimulus parameters and starting and stopping stimulation at any time based on the condition of patients, and it also produces minimal side effects when used in clinical application (Kringelbach et al., 2007).
Preclinical and clinical studies have indicated that DBS may be effective in the treatment of cocaine (Creed et al., 2015), morphine (Martinez-Rivera et al., 2016), and heroin (Chen et al., 2019a) addiction. For example, studies have proven that high-frequency (HF) DBS of the nucleus accumbens suppresses seeking behavior and reinstatement of cocaine and methamphetamine (Vassoler et al., 2008; Muller et al., 2013; Vassoler et al., 2013; Batra et al., 2017). However, research has also found that HF DBS of the nucleus accumbens could decrease natural reward-seeking behaviors (Guercio et al., 2015). Meanwhile, it has also been found that DBS can exert distinct effects via different stimulus parameters (Schor and Nelson, 2019). Thus, proper targets and parameters of DBS in the treatment of addiction are yet to be identified (Wang et al., 2018).
Substantia nigra pars reticulata (SNr) is a part of the basal ganglia which is involved in various brain functions such as sleep and motivation (Liu et al., 2020; Lai et al., 2021) and diseases including PD (Du et al., 2018; Willard et al., 2019; Sitzia et al., 2020) and seizures (Wicker et al., 2019; Chen et al., 2020). The most dominant neuronal cells in the SNr are GABAergic neurons, and previous studies have found that the SNr serves as a superb DBS target for the treatment of PD-related symptoms (Chastan et al., 2009; Valldeoriola et al., 2019). Evidence also suggests that PD and addiction share certain common mechanisms which involve the striatum (Villalba and Smith, 2013), the major input areas of the SNr (Van Den Berge et al., 2017), making it possible to apply DBS of the SNr to the treatment of addiction. A recent study has also found that GABA neurons in the SNr play important roles in opioid reward and relapse, and activation of SNr GABA neurons decreased heroin-primed reinstatement (Galaj et al., 2020). Thus, the SNr has great potential to be an effective target of addiction treatment.
Here, we investigated the impacts of HF and low-frequency (LF) DBS of the SNr on extinction of methamphetamine-induced conditioned place preference (CPP) and methamphetamine-primed reinstatement in rats. We also examined the effects on locomotor ability, anxiety-like behaviors, and formation of methamphetamine-induced place preference.
Materials and Methods
Animals
Male Sprague-Dawley rats (260–280 g), purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., were housed five per cage prior to the implantation of electrodes. All rats were given access to freely available food and water with a reverse 12/12 h light/dark cycle. All procedures were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Biomedical Ethics Committee for Animal Use and Protection of Peking University.
Implanting the Stimulating Electrodes
After a period of adaptation, the rats were anesthetized with isoflurane and placed in a stereotaxic apparatus. Stainless steel bipolar electrodes were bilaterally implanted into the SNr at the following coordinates: anterior/posterior, −5.3 mm; medial/lateral, 2.3 mm; and dorsal/ventral, −8.2 mm. Electrodes were secured to the skull with anchoring screws and dental acrylic cement. The rats were housed individually after the surgery and allowed 3–5 days of recovery before behavioral experiments.
Conditioned Place Preference
The CPP procedure in a three-chamber apparatus was performed using an unbiased, counterbalanced protocol as described previously (Liang et al., 2017).
Baseline preference was assessed by placing the rats in the center chamber of the CPP apparatus and allowing them to explore all three chambers freely for 15 min. Rats that showed a strong unconditioned preference for either of the side chambers (i.e., >540 s) were excluded from the experiments. Then the rats were trained for eight consecutive days with alternating injections of methamphetamine (1 mg/kg, i. p.) or saline (1 ml/kg, i. p.) and were confined to the conditioning chambers for 45 min after each injection before being returned to their home cages. The test for the expression of methamphetamine-induced CPP was identical to the initial baseline preference assessment and was performed on the following day after training. After the establishment of CPP, all rats were divided into sham and HF (or LF) DBS groups in an unbiased random manner.
DBS was continuously delivered for 60 min before the extinction sessions. This duration of stimulation was selected based on previous studies showing that 60 min of DBS is sufficient to produce behavioral changes in rats (Martinez-Rivera et al., 2016; Fakhrieh-Asl et al., 2020). A total of six or nine extinction sessions were performed for HF DBS or LF DBS, respectively, until the rats showed no obvious place preference for either chamber. Similar to the expression test, the rats were allowed to move freely between compartments during each extinction session. On the last day, all of the rats received an injection of methamphetamine (1 mg/kg) without DBS and were tested immediately for CPP. The time spent (in seconds) in the methamphetamine-paired chamber minus the time spent in the saline-paired chamber was calculated as the index of the CPP score.
In the experiment of investigating the effect of HF DBS on CPP formation, rats were divided into sham or HF DBS groups based on the baseline preference before training. During the methamphetamine-pairing trials in training, the rats received 60-min sham or HF DBS in their home cages and were then given an injection of methamphetamine (1 mg/kg) and placed into the drug-paired chamber for 45 min. During the saline-pairing trials, the rats received an injection of saline and were placed into the saline-paired chamber for 45 min. The procedure for the test of expression of methamphetamine-induced CPP was identical to that described above.
Deep Brain Stimulation
Monophasic square pulses were delivered to the SNr using a current-based stimulator through a cable connected to the implanted electrodes. The stimulation parameters were HF (130 Hz) or LF (20 Hz) pulse frequencies, 150 μA pulse amplitude, and 100 μs pulse width (Martinez-Rivera et al., 2016). In sham DBS experiments, the rats were connected to the external cable but did not receive electrical stimulation.
Elevated Plus Maze
The elevated plus maze was used to determine anxiety-like behavior as previously described (Xue et al., 2015; Fang et al., 2018). The elevated plus maze consisted of four arms set in a plus-shaped configuration. The apparatus was elevated 70 cm above the floor. The two open arms were 50 cm long and 10 cm wide. The two closed arms were 50 cm long and 10 cm wide with 40-cm-high walls. All rats received a 60-min DBS (sham, HF, or LF) in their home cages, and then each rat was placed in the central zone of the elevated plus maze with its head facing an open arm. The rat was allowed to freely explore the elevated plus maze for 5 min under dim illumination. The number of entries into and time (in seconds) spent on the open arms were recorded.
Open Field Test
The open field test apparatus consisted of a square arena that was 75 cm long, 75 cm wide, and 40 cm high, which was divided into 25 equal squares on the floor of the arena. All rats received a 60-min DBS (sham, HF, or LF) in their home cages, and then each individual rat was placed in the center of the arena and allowed to freely explore for 5 min. The number of crossings (i.e., entering the adjacent square with all four paws) was considered as the index of locomotor activity.
Histology
The animals were anesthetized and transcardially perfused with 0.01 M phosphate buffer solution, followed by 4% paraformaldehyde in 0.2 M phosphate buffer. The brain was extracted, post-fixed overnight at 4°C, and cryoprotected in 30% sucrose in 0.2 M phosphate buffer. The cannula placements were confirmed in 25-μm-thick sections using Nissl staining by light microscopy. Rats with misplaced cannulae were excluded from the statistical analysis.
Statistical Analysis
The paired t test was used to compare the baseline and the test of the CPP scores from the methamphetamine conditioning phase. Two-way repeated measures ANOVA was applied to analyze the differences in CPP scores of extinction sessions between the sham and HF/LF DBS groups. The unpaired t test was used to compare the differences in CPP scores of methamphetamine conditioning or drug-primed reinstatement between the sham DBS and DBS groups. One-way ANOVA was performed to measure the locomotor activity and anxiety-like behaviors of rats between the sham, LF, and HF DBS groups. Data are shown as mean ± SEM, and the statistical analyses and plotting of the graphs were performed using GraphPad Prism 8 (GraphPad Software, California, United States).
Results
High-Frequency Deep Brain Stimulation of the Substantia Nigra Pars Reticulata Facilitated Extinction and Prevented the Reinstatement of Methamphetamine-Induced Conditioned Place Preference
To examine the effect of SNr DBS on extinction and drug-primed reinstatement of methamphetamine-induced CPP, rats were first trained for 8 days with regard to conditioned place preference. After the rats acquired a preference for methamphetamine, DBS was delivered to the SNr for 60 min before each extinction test, and at the end of extinction, an injection of methamphetamine was given to evaluate the reinstatement of drug-seeking behavior (Figures 1A,B). Rats with misplacement of electrodes were excluded from the study.
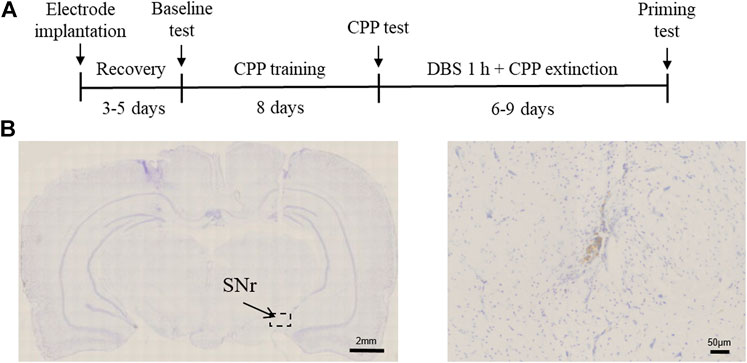
FIGURE 1. Experimental design and electrode implantation site. (A) Experimental timeline. (B) Nissl’s staining of the SNr DBS site.
As shown in Figure 2, two groups of rats exhibited significant preference for the drug-paired side after methamphetamine conditioning (paired t test: Sham DBS: t7 = 3.308, p < 0.05; HF DBS: t7 = 2.868, p < 0.05). Sham or HF DBS was then delivered to the SNr during the drug-free extinction phase, and a two-way repeated measures ANOVA showed overall significant differences in the CPP scores across DBS (F(1, 14) = 5.122, p < 0.05) and extinction sessions (F(3.112, 43.57) = 3.089, p < 0.05) but not DBS × extinction session interactions (F(5, 70) = 0.4994, p = 0.7756), which suggests that HF DBS of the SNr facilitated the extinction of methamphetamine-seeking behavior.
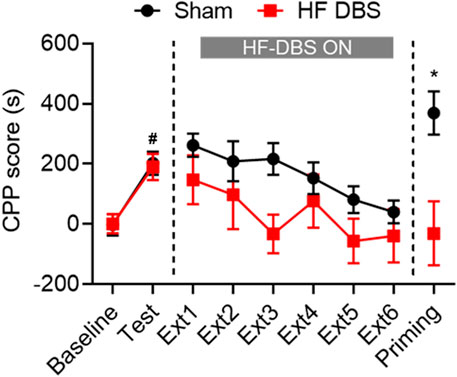
FIGURE 2. HF DBS of the SNr facilitated extinction and prevented the reinstatement of methamphetamine-induced CPP. Methamphetamine (1 mg/kg) induced a significant preference for the drug-paired side in both groups, and sham DBS or HF DBS (130 Hz, 150 μA, 100 μs) was then applied to the SNr during each of the drug-free extinction sessions (15 min). HF DBS caused a significant decrease in CPP scores compared with the sham DBS. After full extinction, a priming injection of methamphetamine (1 mg/kg) was given to the sham DBS and HF DBS groups, and only the sham DBS group, but not the HF DBS group, showed reinstatement of drug-seeking behavior. Data are shown as mean ± SEM. #p < 0.05 compared with baseline (sham and HF DBS), *p < 0.05 compared with HF DBS. Sham DBS: n = 8; HF DBS: n = 8.
Following the last extinction test, all rats received a priming injection of methamphetamine and were tested for reinstatement of drug-seeking behavior. Rats in the HF DBS group showed no significant preference for the methamphetamine-paired side, while those in the sham DBS group exhibited a dramatic increase in CPP scores compared with the HF DBS group (unpaired t test, t14 = 2.178, p < 0.05). Therefore, HF DBS of the SNr blocked the methamphetamine-primed reinstatement of the extinguished drug-seeking behavior.
Low-Frequency Deep Brain Stimulation of the Substantia Nigra Pars Reticulata Impaired the Extinction of Methamphetamine-Induced Conditioned Place Preference and Had No Effect on Methamphetamine-Primed Reinstatement
Since studies have proven that LF DBS generally has different effects compared with HF DBS on the excitability of the stimulated brain region (Kringelbach et al., 2007; Wang et al., 2018), we examined the effects of LF DBS on the extinction of methamphetamine-induced CPP. As shown in Figure 3, after conditioning, the rats showed an overall preference for the drug-paired side (paired t test: Sham DBS: t6 = 4.003, p < 0.01; LF DBS: t5 = 11.17, p < 0.0001). The two groups of rats both underwent extinction until the methamphetamine-seeking behavior of the sham DBS rats was fully extinguished. A two-way repeated measures ANOVA revealed that rats that received LF DBS of the SNr before the extinction sessions exhibited overall significantly higher CPP scores during extinction across DBS (F(1, 11) = 6.473, p < 0.05) and extinction sessions (F(3.737, 41.11) = 5.041, p < 0.01) but not DBS × extinction session interactions (F(8, 88) = 0.5160, p = 0.8415) compared with the sham DBS group. Then all rats received an injection of methamphetamine for the drug-priming test, and the unpaired t test revealed no significant difference in the CPP scores between the sham DBS and LF DBS groups (t11 = 1.374, p = 0.1969). Thus, LF DBS of the SNr impaired the extinction of methamphetamine-induced CPP and produced no effect on reinstatement.
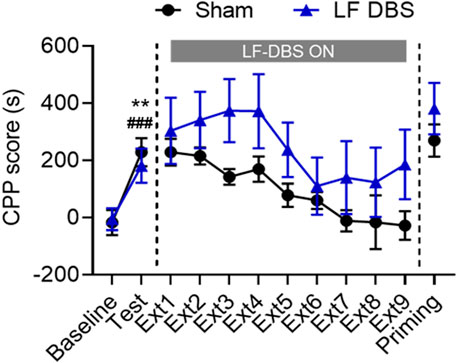
FIGURE 3. LF DBS of the SNr impaired the methamphetamine-induced CPP extinction and had no effect on methamphetamine-primed reinstatement. After methamphetamine conditioning (1 mg/kg), sham DBS or LF DBS (20 Hz, 150 μA, 100 μs) was delivered into the SNr during each of the drug-free extinction sessions (15 min). LF DBS significantly impaired the extinction sessions compared with the sham DBS. A priming injection of methamphetamine (1 mg/kg) was given to the sham DBS and LF DBS groups, and both groups of rats exhibited significant drug-seeking behaviors. Data are shown as mean ± SEM. **p < 0.01 (sham DBS) and ###p < 0.0001 compared with baseline (LF DBS). Sham DBS: n = 7; LF DBS: n = 6.
Deep Brain Stimulation of the Substantia Nigra Pars Reticulata Did Not Affect Locomotor Activity and Anxiety-Like Behavior
To rule out the possibility that SNr DBS may have adverse effects on locomotor activity and induce anxiety-like behavior, we used the open field test and the elevated plus maze test to measure these behaviors. HF DBS or LF DBS was delivered into the SNr for 60 min before the tests. One-way ANOVA showed that there was no significant difference in the distance traveled (F(2, 15) = 0.1107, p = 0.8959) and the time in the central zone (F(2, 15) = 0.02149, p = 0.9788) between the HF DBS, LF DBS, and sham DBS groups in the open field test (Figures 4A,B). Also, no significant difference was found in the open-arm time (F(2, 15) = 0.04105, p = 0.9599) and entries (F(2, 15) = 0.2453, p = 0.7856) between the HF DBS, LF DBS, and sham DBS groups in the elevated plus maze test (Figures 4C,D). Therefore, SNr DBS had no effect on locomotor activity and did not induce anxiety-like behavior in rats.
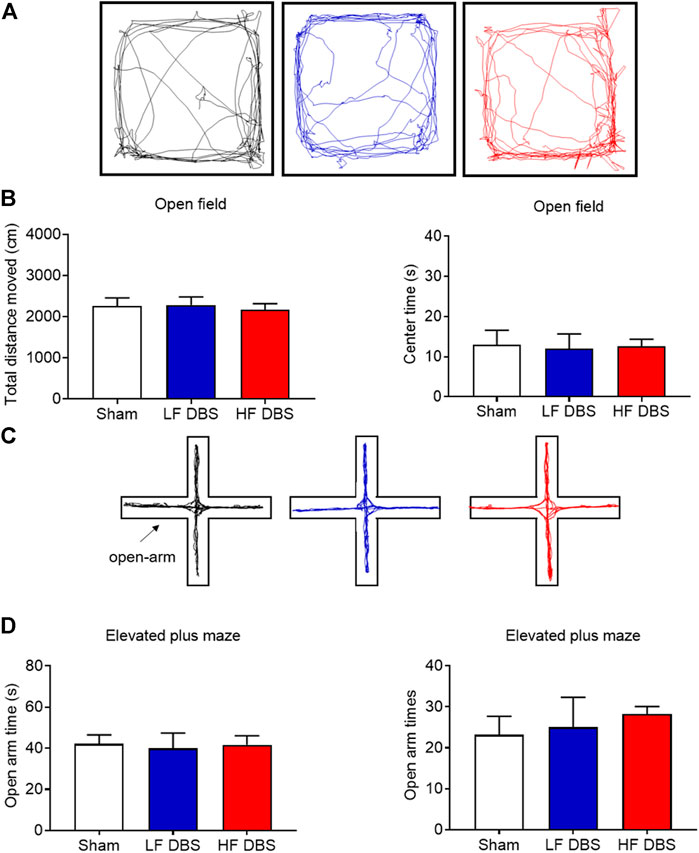
FIGURE 4. DBS of the SNr did not affect locomotor activity and anxiety-like behavior. (A) Representative activity traces of sham, LF, and HF DBS groups in the open field test. (B) Total distance traveled and time in the central zone in the open field test. (C) Representative activity traces of sham, LF, and HF DBS rats in the elevated plus maze test. (D) Time and entries in the open arms of the elevated plus maze test. Data are shown as mean ± SEM. n = 6 for all groups.
High-Frequency Deep Brain Stimulation of the Substantia Nigra Pars Reticulata Had No Effect on the Formation of Methamphetamine-Induced Place Preference
Finally, we investigated the effect of HF DBS of the SNr on the rewarding effects of methamphetamine. As shown in Figure 5, rats received HF DBS of the SNr before the CPP training, and the paired t test showed that both sham (t7 = 3.494, p < 0.05) and HF (t7 = 4.859, p < 0.01) DBS groups formed a significant preference for the drug-paired side, and there was no significant difference in the CPP scores between the two groups (unpaired t test, t14 = 1.272, p = 0.2241), indicating that HF DBS of the SNr in the conditioning phase had no effect on the rewarding effects of methamphetamine.
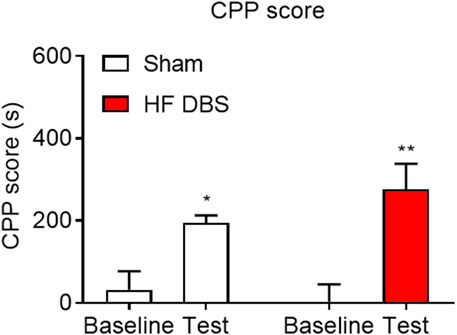
FIGURE 5. HF DBS of the SNr had no effect on the formation of methamphetamine-induced place preference. Both sham DBS and HF DBS groups exhibited a significant preference for the drug-paired side after the methamphetamine conditioning (1 mg/kg), while no difference was found in the CPP scores between the two groups. Data are shown as mean ± SEM. *p < 0.05 (sham DBS) and **p < 0.01 (HF DBS) compared with baseline. Sham DBS: n = 8; HF DBS: n = 8.
Discussion
Our data demonstrated that HF and LF DBS of the SNr produced distinct effects on the extinction of methamphetamine-induced CPP. HF DBS of the SNr facilitated the extinction of methamphetamine-induced CPP and blocked drug-primed reinstatement, while LF DBS suppressed extinction. It is worth noting that HF DBS of the SNr did not affect the reinforcing properties of methamphetamine. These findings suggest that the SNr could be a potential DBS target for the treatment of addiction, although proper stimulation parameters and phases need to be chosen.
The SNr is the ventral part of the substantia nigra. Recent evidence has implied that abnormalities of the substantia nigra are involved in the pathophysiology of addiction (Sharpe et al., 2014; Cassidy et al., 2020), and acute methamphetamine administration could induce neuronal death in the substantia nigra (Sabrini et al., 2020). The substantia nigra also plays a crucial role in the relapse to drug seeking (Hyman et al., 2006; Madsen et al., 2012; Pelloux et al., 2018). However, there is still a lack of sufficient evidence on the exact role of the SNr in addiction, and whether intervention in the SNr can be applied in addiction treatment needs further verification.
On the other hand, the SNr is the convergence region of the striatal output pathways, which comprises striatonigral neurons in the direct pathway and striatopallidal neurons in the indirect pathway (Deniau et al., 2007; Phillips et al., 2020). Evidence suggests that the D1-expressing medium spiny neurons of the direct pathway in the striatum project to the SNr GABA neurons and exhibit D1-mediated presynaptic facilitation (Chuhma et al., 2011). Numerous studies have confirmed that the direct pathway is crucial to drug-seeking behaviors (Cui et al., 2014; Volkow and Morales, 2015; Yager et al., 2019; Salery et al., 2020), and inhibition of the activity of striatal neurons in the direct pathway could suppress cue-induced cocaine-seeking behaviors without affecting the formation of cocaine addiction (Yager et al., 2019). Thus, modulating the activity of the SNr may regulate addiction by affecting striatum activity. Studies also found that HF DBS of the SNr produced negative changes in the cerebral blood volume (CBV) in the striatum, and it also evoked positive CBV changes in multiple basal ganglia nuclei as well as the zona incerta and the ventral tegmental area (Van Den Berge et al., 2017), while existing evidence proved that these brain regions play a crucial role in addiction (Hikida et al., 2010; Mahler et al., 2014; Shen et al., 2014). Therefore, electrical stimulation of the SNr may affect addiction by modulating the neural activity of related brain regions.
In the present study, we attempted to investigate the beneficial effects of DBS of the SNr in the treatment of methamphetamine addiction by using the free access CPP extinction paradigm in rats. The stimulation parameters used in previous studies are mainly LF (10–40 Hz) and HF (100–400 Hz) stimulation, while the medium-frequency (40–60 Hz) stimulation has almost no effect on modulating the functional connectivity of the SNr (Creed et al., 2015; Martinez-Rivera et al., 2016; Van Den Berge et al., 2017; Fakhrieh-Asl et al., 2020). Our results indicated that HF DBS of the SNr promoted extinction and subsequently blocked the drug-primed reinstatement. However, we also found that LF DBS of the SNr suppressed the extinction. Besides, HF DBS of the SNr had no effect on the development of methamphetamine-induced CPP. These results suggest that the stages of addiction and the stimulation parameters should be considered when using SNr DBS in the treatment of addiction. On the other hand, DBS has the ability to modulate the synaptic plasticity, which may also contribute to strengthening the extinction memory and suppressing the subsequent reinstatement of drug-seeking behaviors (Kauer and Malenka, 2007; Creed et al., 2015; Lee et al., 2016; Ni et al., 2018). The mechanism of DBS that promotes extinction may be that HF DBS causes long-term potentiation of the SNr and leads to a decrease in the activity of the dorsal striatum, which plays a critical role in the extinction of the addiction memory, in line with the previous findings (Martinez-Rivera et al., 2016; Yager et al., 2019).
Despite the efficacy of DBS in the treatment of a variety of diseases, the underlying mechanisms of these effects remain unclear. Indeed, it is a limitation of the present study that we did not investigate the mechanisms of the effects of DBS of the SNr on extinction and reinstatement of methamphetamine-induced CPP. Studies have found that DBS could enhance the transmission from the stimulation target and activate surrounding fiber pathways simultaneously, leading to a complex pattern of excitatory and inhibitory effects (Miocinovic et al., 2013). Furthermore, consistent with our findings, different frequencies of DBS could produce distinct effects. Acute LF DBS of the nucleus accumbens combined with the dopamine D1 receptor antagonist SCH23390 effectively abolishes the behavioral sensitization of cocaine (Creed et al., 2015). LF DBS of the dorsal ventral striatum strengthens the morphine extinction memory, whereas HF DBS of the dorsal ventral striatum impairs extinction training and the subsequent extinction memory (Martinez-Rivera et al., 2016). Additionally, HF DBS of the OFC prevents the development of morphine place preference and blocks the drug-primed reinstatement of morphine-seeking behavior (Fakhrieh-Asl et al., 2020).
In conclusion, we have found that HF DBS of the SNr facilitated the extinction of methamphetamine-induced CPP and blocked methamphetamine-primed reinstatement, while LF DBS of the SNr impaired extinction. Meanwhile, HF DBS of the SNr neither affected locomotor activity nor caused anxiety-like behaviors. Moreover, it had no effect on the formation of methamphetamine-induced CPP. Our findings may provide potential targets and options for the future clinical application of DBS in the treatment of addiction.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Biomedical Ethics Committee for Animal Use and Protection of Peking University.
Author Contributions
LZ, YC (Shenzhen), JS, and YS designed the study. LZ, SM, WC, EH, GZ, YL, and ZD performed the experiments. LZ, SM, WC, YC (Beijing), and YX analyzed the data and prepared the manuscript together. All the authors have read and approved the final version of the manuscript.
Funding
This work was supported by the Shenzhen Science and Technology Innovation Committee (Grant no. JCYJ20170412171856582), the Shenzhen Development and Reform Commission (Grant no. XMHT20190104001), the National Natural Science Foundation of China (Grant nos. U1802283, 81821092, and 81901352), and the Beijing Municipal Science and Technology Commission (Grant no. Z181100001518005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Batra, V., Tran, T. L. N., Caputo, J., Guerin, G. F., Goeders, N. E., and Wilden, J. (2017). Intermittent Bilateral Deep Brain Stimulation of the Nucleus Accumbens Shell Reduces Intravenous Methamphetamine Intake and Seeking in Wistar Rats. Jns 126 (4), 1339–1350. doi:10.3171/2016.4.JNS152524
Cassidy, C. M., Carpenter, K. M., Konova, A. B., Cheung, V., Grassetti, A., Zecca, L., et al. (2020). Evidence for Dopamine Abnormalities in the Substantia Nigra in Cocaine Addiction Revealed by Neuromelanin-Sensitive MRI. Am. J. Psychiatry 177 (11), 1038–1047. doi:10.1176/appi.ajp.2020.20010090
Chastan, N., Westby, G. W. M., Yelnik, J., Bardinet, E., Do, M. C., Agid, Y., et al. (2009). Effects of Nigral Stimulation on Locomotion and Postural Stability in Patients with Parkinson's Disease. Brain 132 (Pt 1), 172–184. doi:10.1093/brain/awn294
Chen, B., Xu, C., Wang, Y., Lin, W., Wang, Y., Chen, L., et al. (2020). A Disinhibitory Nigra-Parafascicular Pathway Amplifies Seizure in Temporal Lobe Epilepsy. Nat. Commun. 11 (1), 923. doi:10.1038/s41467-020-14648-8
Chen, L., Li, N., Ge, S., Lozano, A. M., Lee, D. J., Yang, C., et al. (2019a). Long-term Results after Deep Brain Stimulation of Nucleus Accumbens and the Anterior Limb of the Internal Capsule for Preventing Heroin Relapse: An Open-Label Pilot Study. Brain Stimulation 12 (1), 175–183. doi:10.1016/j.brs.2018.09.006
Chen, Y.-Y., Zhang, L.-B., Li, Y., Meng, S.-Q., Gong, Y.-M., Lu, L., et al. (2019b). Post-retrieval Extinction Prevents Reconsolidation of Methamphetamine Memory Traces and Subsequent Reinstatement of Methamphetamine Seeking. Front. Mol. Neurosci. 12, 157. doi:10.3389/fnmol.2019.00157
Chuhma, N., Tanaka, K. F., Hen, R., and Rayport, S. (2011). Functional Connectome of the Striatal Medium Spiny Neuron. J. Neurosci. 31 (4), 1183–1192. doi:10.1523/JNEUROSCI.3833-10.2011
Conklin, C. A., and Tiffany, S. T. (2002). Applying Extinction Research and Theory to Cue-Exposure Addiction Treatments. Addiction 97 (2), 155–167. doi:10.1046/j.1360-0443.2002.00014.x
Creed, M., Pascoli, V. J., and Lüscher, C. (2015). Refining Deep Brain Stimulation to Emulate Optogenetic Treatment of Synaptic Pathology. Science 347 (6222), 659–664. doi:10.1126/science.1260776
Crowell, A. L., Riva-Posse, P., Holtzheimer, P. E., Garlow, S. J., Kelley, M. E., Gross, R. E., et al. (2019). Long-Term Outcomes of Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression. Am. J. Psychiatry 176 (11), 949–956. doi:10.1176/appi.ajp.2019.18121427
Cui, Y., Ostlund, S. B., James, A. S., Park, C. S., Ge, W., Roberts, K. W., et al. (2014). Targeted Expression of μ-opioid Receptors in a Subset of Striatal Direct-Pathway Neurons Restores Opiate Reward. Nat. Neurosci. 17 (2), 254–261. doi:10.1038/nn.3622
Deniau, J. M., Mailly, P., Maurice, N., and Charpier, S. (2007). The Pars Reticulata of the Substantia Nigra: a Window to Basal Ganglia Output. Prog. Brain Res. 160, 151–172. doi:10.1016/S0079-6123(06)60009-5
Du, G., Lewis, M. M., Sica, C., He, L., Connor, J. R., Kong, L., et al. (2018). Distinct Progression Pattern of Susceptibility MRI in the Substantia Nigra of Parkinson's Patients. Mov Disord. 33 (9), 1423–1431. doi:10.1002/mds.27318
Elkaim, L. M., Alotaibi, N. M., Sigal, A., Alotaibi, H. M., Lipsman, N., Kalia, S. K., et al. (2019). Deep Brain Stimulation for Pediatric Dystonia: a Meta‐analysis with Individual Participant Data. Dev. Med. Child. Neurol. 61 (1), 49–56. doi:10.1111/dmcn.14063
Fakhrieh-Asl, G., Sadr, S. S., Karimian, S. M., and Riahi, E. (2020). Deep Brain Stimulation of the Orbitofrontal Cortex Prevents the Development and Reinstatement of Morphine Place Preference. Addict. Biol. 25 (4), e12780. doi:10.1111/adb.12780
Fang, Q., Li, Z., Huang, G.-D., Zhang, H.-H., Chen, Y.-Y., Zhang, L.-B., et al. (2018). Traumatic Stress Produces Distinct Activations of GABAergic and Glutamatergic Neurons in Amygdala. Front. Neurosci. 12, 387. doi:10.3389/fnins.2018.00387
Figee, M., Luigjes, J., Smolders, R., Valencia-Alfonso, C.-E., van Wingen, G., de Kwaasteniet, B., et al. (2013). Deep Brain Stimulation Restores Frontostriatal Network Activity in Obsessive-Compulsive Disorder. Nat. Neurosci. 16 (4), 386–387. doi:10.1038/nn.3344
Galaj, E., Han, X., Shen, H., Jordan, C. J., He, Y., Humburg, B., et al. (2020). Dissecting the Role of GABA Neurons in the VTA versus SNr in Opioid Reward. J. Neurosci. 40 (46), 8853–8869. doi:10.1523/JNEUROSCI.0988-20.2020
Guercio, L. A., Schmidt, H. D., and Pierce, R. C. (2015). Deep Brain Stimulation of the Nucleus Accumbens Shell Attenuates Cue-Induced Reinstatement of Both Cocaine and Sucrose Seeking in Rats. Behav. Brain Res. 281, 125–130. doi:10.1016/j.bbr.2014.12.025
He, Y.-Y., Xue, Y.-X., Wang, J.-s., Fang, Q., Liu, J.-F., Xue, L.-F., et al. (2011). PKMζ Maintains Drug Reward and Aversion Memory in the Basolateral Amygdala and Extinction Memory in the Infralimbic Cortex. Neuropsychopharmacology 36 (10), 1972–1981. doi:10.1038/npp.2011.63
Hikida, T., Kimura, K., Wada, N., Funabiki, K., and Nakanishi, S. (2010). Distinct Roles of Synaptic Transmission in Direct and Indirect Striatal Pathways to Reward and Aversive Behavior. Neuron 66 (6), 896–907. doi:10.1016/j.neuron.2010.05.011
Holtzheimer, P. E., Husain, M. M., Lisanby, S. H., Taylor, S. F., Whitworth, L. A., McClintock, S., et al. (2017). Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression: a Multisite, Randomised, Sham-Controlled Trial. The Lancet Psychiatry 4 (11), 839–849. doi:10.1016/S2215-0366(17)30371-1
Hyman, S. E., and Malenka, R. C. (2001). Addiction and the Brain: the Neurobiology of Compulsion and its Persistence. Nat. Rev. Neurosci. 2 (10), 695–703. doi:10.1038/35094560
Hyman, S. E., Malenka, R. C., and Nestler, E. J. (2006). Neural Mechanisms of Addiction: the Role of Reward-Related Learning and Memory. Annu. Rev. Neurosci. 29, 565–598. doi:10.1146/annurev.neuro.29.051605.113009
Katz, M., Luciano, M. S., Carlson, K., Luo, P., Marks, W. J., Larson, P. S., et al. (2015). Differential Effects of Deep Brain Stimulation Target on Motor Subtypes in Parkinson's Disease. Ann. Neurol. 77 (4), 710–719. doi:10.1002/ana.24374
Kauer, J. A., and Malenka, R. C. (2007). Synaptic Plasticity and Addiction. Nat. Rev. Neurosci. 8 (11), 844–858. doi:10.1038/nrn2234
Kennedy, S. H., Giacobbe, P., Rizvi, S. J., Placenza, F. M., Nishikawa, Y., Mayberg, H. S., et al. (2011). Deep Brain Stimulation for Treatment-Resistant Depression: Follow-Up after 3 to 6 Years. Am. J. Psychiatry 168 (5), 502–510. doi:10.1176/appi.ajp.2010.10081187
Kleiner-Fisman, G., Lin Liang, G. S., Moberg, P. J., Ruocco, A. C., Hurtig, H. I., Baltuch, G. H., et al. (2007). Subthalamic Nucleus Deep Brain Stimulation for Severe Idiopathic Dystonia: Impact on Severity, Neuropsychological Status, and Quality of Life. J. Neurosurg. 107 (1), 29–36. doi:10.3171/JNS-07/07/0029
Kringelbach, M. L., Jenkinson, N., Owen, S. L. F., and Aziz, T. Z. (2007). Translational Principles of Deep Brain Stimulation. Nat. Rev. Neurosci. 8 (8), 623–635. doi:10.1038/nrn2196
Lai, Y.-Y., Kodama, T., Hsieh, K.-C., Nguyen, D., and Siegel, J. M. (2021). Substantia Nigra Pars Reticulata-Mediated Sleep and Motor Activity Regulation. Sleep 44 (1). doi:10.1093/sleep/zsaa151
Lee, J., Finkelstein, J., Choi, J. Y., and Witten, I. B. (2016). Linking Cholinergic Interneurons, Synaptic Plasticity, and Behavior during the Extinction of a Cocaine-Context Association. Neuron 90 (5), 1071–1085. doi:10.1016/j.neuron.2016.05.001
Liang, J., Li, J.-L., Han, Y., Luo, Y.-X., Xue, Y.-X., Zhang, Y., et al. (2017). Calpain-GRIP Signaling in Nucleus Accumbens Core Mediates the Reconsolidation of Drug Reward Memory. J. Neurosci. 37 (37), 8938–8951. doi:10.1523/JNEUROSCI.0703-17.2017
Lipsman, N., Lam, E., Volpini, M., Sutandar, K., Twose, R., Giacobbe, P., et al. (2017). Deep Brain Stimulation of the Subcallosal Cingulate for Treatment-Refractory Anorexia Nervosa: 1 Year Follow-Up of an Open-Label Trial. The Lancet Psychiatry 4 (4), 285–294. doi:10.1016/S2215-0366(17)30076-7
Lipsman, N., Woodside, D. B., Giacobbe, P., Hamani, C., Carter, J. C., Norwood, S. J., et al. (2013). Subcallosal Cingulate Deep Brain Stimulation for Treatment-Refractory Anorexia Nervosa: a Phase 1 Pilot Trial. The Lancet 381 (9875), 1361–1370. doi:10.1016/S0140-6736(12)62188-6
Liu, D., Li, W., Ma, C., Zheng, W., Yao, Y., Tso, C. F., et al. (2020). A Common Hub for Sleep and Motor Control in the Substantia Nigra. Science 367 (6476), 440–445. doi:10.1126/science.aaz0956
Liu, J. F., Tian, J., and Li, J. X. (2019). Modulating Reconsolidation and Extinction to Regulate Drug Reward Memory. Eur. J. Neurosci. 50 (3), 2503–2512. doi:10.1111/ejn.14072
Luigjes, J., van den Brink, W., Feenstra, M., van den Munckhof, P., Schuurman, P. R., Schippers, R., et al. (2012). Deep Brain Stimulation in Addiction: a Review of Potential Brain Targets. Mol. Psychiatry 17 (6), 572–583. doi:10.1038/mp.2011.114
Luo, Y. X., Xue, Y. X., Liu, J. F., Shi, H. S., Jian, M., Han, Y., et al. (2015). A Novel UCS Memory Retrieval-Extinction Procedure to Inhibit Relapse to Drug Seeking. Nat. Commun. 6, 7675. doi:10.1038/ncomms8675
Madsen, H. B., Brown, R. M., Short, J. L., and Lawrence, A. J. (2012). Investigation of the Neuroanatomical Substrates of Reward Seeking Following Protracted Abstinence in Mice. J. Physiol. 590 (10), 2427–2442. doi:10.1113/jphysiol.2011.225219
Mahler, S. V., Vazey, E. M., Beckley, J. T., Keistler, C. R., McGlinchey, E. M., Kaufling, J., et al. (2014). Designer Receptors Show Role for Ventral Pallidum Input to Ventral Tegmental Area in Cocaine Seeking. Nat. Neurosci. 17 (4), 577–585. doi:10.1038/nn.3664
Martínez-Rivera, F. J., Rodriguez-Romaguera, J., Lloret-Torres, M. E., Do Monte, F. H., Quirk, G. J., and Barreto-Estrada, J. L. (2016). Bidirectional Modulation of Extinction of Drug Seeking by Deep Brain Stimulation of the Ventral Striatum. Biol. Psychiatry 80 (9), 682–690. doi:10.1016/j.biopsych.2016.05.015
Milton, A. L., and Everitt, B. J. (2012b). The Persistence of Maladaptive Memory: Addiction, Drug Memories and Anti-relapse Treatments. Neurosci. Biobehavioral Rev. 36 (4), 1119–1139. doi:10.1016/j.neubiorev.2012.01.002
Milton, A. L., and Everitt, B. J. (2012a). Wiping Drug Memories. Science 336 (6078), 167–168. doi:10.1126/science.1221691
Miocinovic, S., Somayajula, S., Chitnis, S., and Vitek, J. L. (2013). History, Applications, and Mechanisms of Deep Brain Stimulation. JAMA Neurol. 70 (2), 163–171. doi:10.1001/2013.jamaneurol.45
Müller, U. J., Voges, J., Steiner, J., Galazky, I., Heinze, H.-J., Möller, M., et al. (2013). Deep Brain Stimulation of the Nucleus Accumbens for the Treatment of Addiction. Ann. N.Y. Acad. Sci. 1282, 119–128. doi:10.1111/j.1749-6632.2012.06834.x
Ni, Z., Kim, S. J., Phielipp, N., Ghosh, S., Udupa, K., Gunraj, C. A., et al. (2018). Pallidal Deep Brain Stimulation Modulates Cortical Excitability and Plasticity. Ann. Neurol. 83 (2), 352–362. doi:10.1002/ana.25156
Okun, M. S. (2012). Deep-brain Stimulation for Parkinson's Disease. N. Engl. J. Med. 367 (16), 1529–1538. doi:10.1056/NEJMct1208070
Opri, E., Cernera, S., Molina, R., Eisinger, R. S., Cagle, J. N., Almeida, L., et al. (2020). Chronic Embedded Cortico-Thalamic Closed-Loop Deep Brain Stimulation for the Treatment of Essential Tremor. Sci. Transl. Med. 12 (572), eaay7680. doi:10.1126/scitranslmed.aay7680
Pelloux, Y., Hoots, J. K., Cifani, C., Adhikary, S., Martin, J., Minier-Toribio, A., et al. (2018). Context-induced Relapse to Cocaine Seeking after Punishment-Imposed Abstinence Is Associated with Activation of Cortical and Subcortical Brain Regions. Addict. Biol. 23 (2), 699–712. doi:10.1111/adb.12527
Phillips, R. S., Rosner, I., Gittis, A. H., and Rubin, J. E. (2020). The Effects of Chloride Dynamics on Substantia Nigra Pars Reticulata Responses to Pallidal and Striatal Inputs. Elife 9. doi:10.7554/eLife.55592
Rosin, B., Slovik, M., Mitelman, R., Rivlin-Etzion, M., Haber, S. N., Israel, Z., et al. (2011). Closed-loop Deep Brain Stimulation Is superior in Ameliorating Parkinsonism. Neuron 72 (2), 370–384. doi:10.1016/j.neuron.2011.08.023
Sabrini, S., Russell, B., Wang, G., Lin, J., Kirk, I., and Curley, L. (2020). Methamphetamine Induces Neuronal Death: Evidence from Rodent Studies. Neurotoxicology 77, 20–28. doi:10.1016/j.neuro.2019.12.006
Salery, M., Trifilieff, P., Caboche, J., and Vanhoutte, P. (2020). From Signaling Molecules to Circuits and Behaviors: Cell-type-specific Adaptations to Psychostimulant Exposure in the Striatum. Biol. Psychiatry 87 (11), 944–953. doi:10.1016/j.biopsych.2019.11.001
Schor, J. S., and Nelson, A. B. (2019). Multiple Stimulation Parameters Influence Efficacy of Deep Brain Stimulation in Parkinsonian Mice. J. Clin. Invest. 129 (9), 3833–3838. doi:10.1172/JCI122390
Schuurman, P. R., Bosch, D. A., Bossuyt, P. M. M., Bonsel, G. J., van Someren, E. J. W., de Bie, R. M. A., et al. (2000). A Comparison of Continuous Thalamic Stimulation and Thalamotomy for Suppression of Severe Tremor. N. Engl. J. Med. 342 (7), 461–468. doi:10.1056/NEJM200002173420703
Sharpe, A. L., Varela, E., Bettinger, L., and Beckstead, M. J. (2014). Methamphetamine Self-Administration in Mice Decreases GIRK Channel-Mediated Currents in Midbrain Dopamine Neurons. Int. J. Neuropsychopharmacol. 18 (5), pyu073. doi:10.1093/ijnp/pyu073
Shen, H.-W., Gipson, C. D., Huits, M., and Kalivas, P. W. (2014). Prelimbic Cortex and Ventral Tegmental Area Modulate Synaptic Plasticity Differentially in Nucleus Accumbens during Cocaine-Reinstated Drug Seeking. Neuropsychopharmacology 39 (5), 1169–1177. doi:10.1038/npp.2013.318
Sitzia, G., Mantas, I., Zhang, X., Svenningsson, P., and Chergui, K. (2020). NMDA Receptors Are Altered in the Substantia Nigra Pars Reticulata and Their Blockade Ameliorates Motor Deficits in Experimental Parkinsonism. Neuropharmacology 174, 108136. doi:10.1016/j.neuropharm.2020.108136
Valldeoriola, F., Muñoz, E., Rumià, J., Roldán, P., Cámara, A., Compta, Y., et al. (2019). Simultaneous Low-Frequency Deep Brain Stimulation of the Substantia Nigra Pars Reticulata and High-Frequency Stimulation of the Subthalamic Nucleus to Treat Levodopa Unresponsive Freezing of Gait in Parkinson's Disease: A Pilot Study. Parkinsonism Relat. Disord. 60, 153–157. doi:10.1016/j.parkreldis.2018.09.008
Van Den Berge, N., Albaugh, D. L., Salzwedel, A., Vanhove, C., Van Holen, R., Gao, W., et al. (2017). Functional Circuit Mapping of Striatal Output Nuclei Using Simultaneous Deep Brain Stimulation and fMRI. Neuroimage 146, 1050–1061. doi:10.1016/j.neuroimage.2016.10.049
Vassoler, F. M., Schmidt, H. D., Gerard, M. E., Famous, K. R., Ciraulo, D. A., Kornetsky, C., et al. (2008). Deep Brain Stimulation of the Nucleus Accumbens Shell Attenuates Cocaine Priming-Induced Reinstatement of Drug Seeking in Rats. J. Neurosci. 28 (35), 8735–8739. doi:10.1523/JNEUROSCI.5277-07.2008
Vassoler, F. M., White, S. L., Hopkins, T. J., Guercio, L. A., Espallergues, J., Berton, O., et al. (2013). Deep Brain Stimulation of the Nucleus Accumbens Shell Attenuates Cocaine Reinstatement through Local and Antidromic Activation. J. Neurosci. 33 (36), 14446–14454. doi:10.1523/JNEUROSCI.4804-12.2013
Villalba, R. M., and Smith, Y. (2013). Differential Striatal Spine Pathology in Parkinson's Disease and Cocaine Addiction: a Key Role of Dopamine? Neuroscience 251, 2–20. doi:10.1016/j.neuroscience.2013.07.011
Volkow, N. D., and Morales, M. (2015). The Brain on Drugs: From Reward to Addiction. Cell 162 (4), 712–725. doi:10.1016/j.cell.2015.07.046
Wang, T. R., Moosa, S., Dallapiazza, R. F., Elias, W. J., and Lynch, W. J. (2018). Deep Brain Stimulation for the Treatment of Drug Addiction. Neurosurg. Focus 45 (2), E11. doi:10.3171/2018.5.FOCUS18163
Wicker, E., Beck, V. C., Kulick-Soper, C., Kulick-Soper, C. V., Hyder, S. K., Campos-Rodriguez, C., et al. (2019). Descending Projections from Thesubstantia Nigra Pars Reticulatadifferentially Control Seizures. Proc. Natl. Acad. Sci. USA 116, 27084–27094. doi:10.1073/pnas.1908176117
Willard, A. M., Isett, B. R., Whalen, T. C., Mastro, K. J., Ki, C. S., Mao, X., et al. (2019). State Transitions in the Substantia Nigra Reticulata Predict the Onset of Motor Deficits in Models of Progressive Dopamine Depletion in Mice. Elife 8. doi:10.7554/eLife.42746
Wu, H., Hariz, M., Visser-Vandewalle, V., Zrinzo, L., Coenen, V. A., Sheth, S. A., et al. (2021). Deep Brain Stimulation for Refractory Obsessive-Compulsive Disorder (OCD): Emerging or Established Therapy? Mol. Psychiatry 26 (1), 60–65. doi:10.1038/s41380-020-00933-x
Xue, Y.-X., Luo, Y.-X., Wu, P., Shi, H.-S., Xue, L.-F., Chen, C., et al. (2012). A Memory Retrieval-Extinction Procedure to Prevent Drug Craving and Relapse. Science 336 (6078), 241–245. doi:10.1126/science.1215070
Xue, Y.-X., Xue, L.-F., Liu, J.-F., He, J., Deng, J.-H., Sun, S.-C., et al. (2014). Depletion of Perineuronal Nets in the Amygdala to Enhance the Erasure of Drug Memories. J. Neurosci. 34 (19), 6647–6658. doi:10.1523/JNEUROSCI.5390-13.2014
Xue, Y.-X., Zhu, Z.-Z., Han, H.-B., Liu, J.-F., Meng, S.-Q., Chen, C., et al. (2015). Overexpression of Protein Kinase Mζ in the Prelimbic Cortex Enhances the Formation of Long-Term Fear Memory. Neuropsychopharmacology 40 (9), 2146–2156. doi:10.1038/npp.2015.56
Keywords: deep brain stimulation, substantia nigra pars reticulata, methamphetamine, extinction, relapse
Citation: Zhang L, Meng S, Chen W, Chen Y, Huang E, Zhang G, Liang Y, Ding Z, Xue Y, Chen Y, Shi J and Shi Y (2021) High-Frequency Deep Brain Stimulation of the Substantia Nigra Pars Reticulata Facilitates Extinction and Prevents Reinstatement of Methamphetamine-Induced Conditioned Place Preference. Front. Pharmacol. 12:705813. doi: 10.3389/fphar.2021.705813
Received: 06 May 2021; Accepted: 10 June 2021;
Published: 30 June 2021.
Edited by:
Di Wen, Hebei Medical University, ChinaReviewed by:
Xiangdong Tang, Sichuan University, ChinaTi-Fei Yuan, Shanghai Jiao Tong University, China
Hongxian Shen, Central South University, China
Copyright © 2021 Zhang, Meng, Chen, Chen, Huang, Zhang, Liang, Ding, Xue, Chen, Shi and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Shi, c2hpeXVAcGt1c3poLmNvbQ==; Jie Shi, c2hpamllQGJqbXUuZWR1LmNu; Yun Chen, Y2hlbnl1bkBwa3VzemguY29t;
†These authors have contributed equally to this work
 Libo Zhang
Libo Zhang Shiqiu Meng
Shiqiu Meng Wenjun Chen
Wenjun Chen Yun Chen2
Yun Chen2 Yanxue Xue
Yanxue Xue Jie Shi
Jie Shi