- 1Forschungsstelle für Fernöstliche Medizin, Department of Vegetation Analysis and Phytodiversity, Albrecht von Haller Institute of Plant Sciences, Georg August University, Göttingen, Germany
- 2Clinic for Gastroenterology and Gastrointestinal Oncology, University Medicine Göttingen, Göttingen, Germany
- 3Clinic, Hann. Münden, Germany
Traditional medicines embody knowledge on medicinal plants that has been accumulated through cultural evolution over millennia. In the latter half of the 20th century, two approaches to medicinal plant research have been established: the “Bench to Bedside” and the “Bedside to Bench” approaches which serve primarily for the development of more efficient therapeutics. Here, we propose a third, novel approach: from “Tradition to Pathogenesis” which aims to understand the pathogenesis of diseases based on the cultural evolution of their respective empirical treatments. We analyse multiple examples of diseases where the acting mechanism of traditional treatments across multiple cultures points to the pathogenesis of the respective disease. E.g., many cultures traditionally treat rheumatism with anti-bacterial botanical drugs, which is at odds with our current understanding that rheumatism is an aseptic inflammation. Furthermore, gastric ailments have traditionally been treated with anti-infectious botanical drugs indicating local infections, as demonstrated by the discovery of Helicobacter pylori as a common cause of gastric ulcer. Understanding traditional treatments can thus help to elucidate the pathogenesis of the disease.
Co-Evolution of Traditional Medicine and Medicinal Plants
Recent anthropological research data have led to the astounding conclusion that traditional herbal medicine has most probably a longer history than mankind: Apes have been observed to use medicinal plants for the treatment of diseases (Huffman, 2001). Moreover, human populations that settled in the same region of Africa use the same plants with very similar indications (Huffman, 2001). One example for this transfer of medicinal knowledge from animals to humans is Vernonia amygdalina Del. Chimpanzees (Pan troglodytes) have been observed on numerous occasions to chew on the bitter pith of this plant as self-medication in case of parasitic nematode infections (Huffman, 2001). Traditional healers of the WaTongwe people of the Mahale Mountains in Tanzania, where the use of V. amygdalina by Chimpanzees has also been observed, use this plant for intestinal parasites, diarrhoea, and stomach upset. Phytochemical research has demonstrated that sesquiterpene lactones in V. amygdalina possess anthelmintic, antiamoebic, antitumor, and antibiotic properties (Huffman, 2001).
We can thus propose a long-term co-evolution between man and his food and medicinal plants, resulting in the adaption of human pharmacology to the bioactive plant metabolites. The fact that already Neanderthals 50.000 years ago used yarrow (Achillea millefolium) and camomile (Matricaria chamomilla) - two plants still registered as medicinal plants in the European Pharmacopoeia - as well as poplar buds (Populus spec.) (Hardy et al., 2012; Weyrich et al., 2017) as medicine, demonstrates that contemporary phytotherapeutic practice goes back to the dawn of man. The accumulated body of knowledge (referred to as “tradition” or “culture”) is transferrable from person to person as humans and their closest relatives are further able to learn successful behaviours. The process of the improvement and distribution of this knowledge can be referred to as “cultural evolution.” The evolutionary pressure that drives this cultural evolution is the survival benefit for tribes with knowledge of effective treatments. Just as for the use of single herbs, their traditional combinations evolved over time. Various prescriptions include the same medicinal plants in different combinations as the individual effects i.e., anti-inflammatory, mucoprotective or microcirculation enhancing, add to the synergistic effect of the whole. This is referred to as multicomponent-multitargeted therapy. These considerations enable us to understand the pathological processes of diseases by analysing the commonalities in the pharmacological properties of traditional medicinal plant drugs used in multiple cultures to treat the disease. This approach constitutes a new possible use of pharmacognosy, a discipline that has for the past century been dominated by two approaches, the “bench to bedside” and the “bedside to bench” approach.
The Two Established Hypothesis of Medicinal Research
Both the “bench to bedside” and the “bedside to bench” approach are based on the application of the ideas of modern biomedicine to the medicinal plants and practices of traditional medicine. In the “bench to bedside” approach, pure chemical compounds are isolated from medicinal plants and tested by high throughput screening for their activity in various in vitro model systems as potential drugs.
The alternative “bedside to bench” approach uses modern biochemistry and pharmacology in order to characterize traditional medicinal plant preparations and their acting mechanisms, and to develop refined extracts. Based on this approach, bioassay guided fractionation can be applied in order to develop advanced herbal medicinal products with improved therapeutic activity (Figure 1).
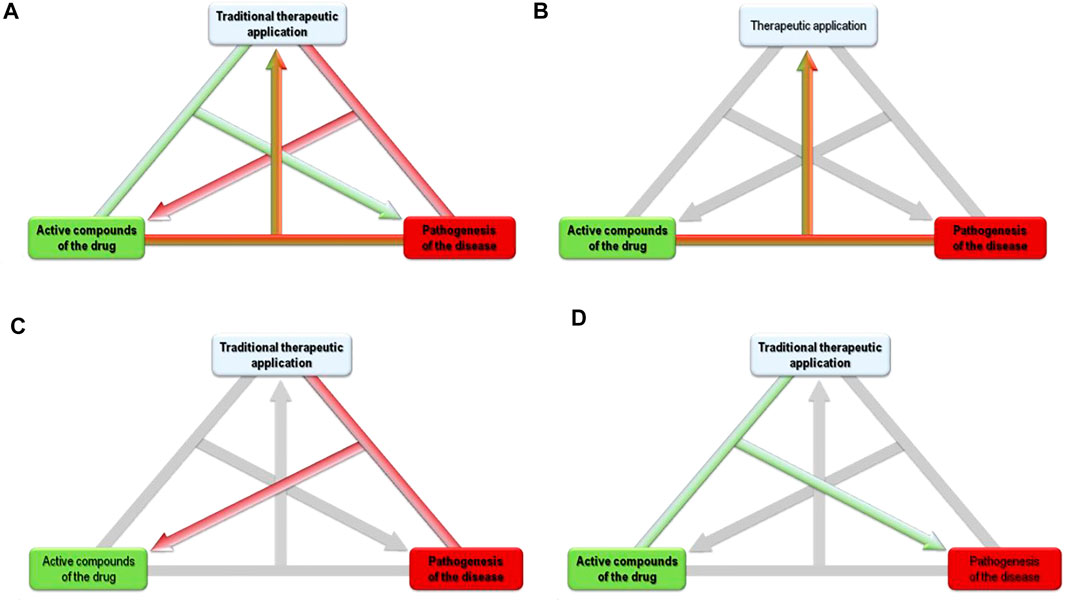
FIGURE 1. (A) From “active compounds of the drug,” to its “traditional therapeutic application,” or “pathogenesis of the disease.” (B) Bench to Bedside: Pathogenesis + Active compounds known → Therapeutic applications unknown. (C) Bedside to Bench: Pathogenesis + Therapeutic application known → Active compounds unknown. (D) Tradition to Pathogenesis: Active compounds + Therapeutic application known → Pathogenesis unknown.
Tradition to Pathogenesis: A Third, Novel Hypothesis of Medicinal Research
Here, we propose a third way of medicinal plant research: We propose that the traditional use of botanical drugs may clarify the pathogenesis of modern diseases. The known bioactivities of the plant constituents and their traditional application might thus help to understand the pathological processes of the treated disease. Figure 1 intends to visualize that if two of the three items “traditional therapeutic application,” “active compounds of the drug” and “pathogenesis of the disease” are known, the third can be researched based on the other two. E.g., in “bench to bedside,” a compound and its pharmacology are known, the research aims to find a fitting therapeutic application. In “bedside to bench,” the traditional therapeutic application and the pathophysiology of the treated disease are known, research aims to find the respective active components (of the plant extract). Our new proposal completes the logical triangle by using the known traditional therapeutic application of the plant - and its known compounds with known activities - for research that aims to find the pathophysiology of the treated disease. This approach can thus be called “Tradition to Pathogenesis” in line with the two previously established approaches. The knowledge of medicinal plants thus helps to understand the shared pathogenesis or association of different diseases and the association between traditional and modern biomedical based pathogenesis.
The idea of predicting the source of evolutionary pressure from an observed adaptation is not new. In 1862, Charles Darwin predicted that the 40 cm long nectary of the Madagascan orchid Angraecum sesquipedale Thouars indicated that there must be a pollinating insect with an equally long proboscis (Darwin, 1862). This was confirmed in 1903 when the sphinx moth Xanthopan morganii praedicta was discovered by W Rothschild and K Jordan. Here, we apply the same line of reasoning to the cultural evolution of medicine for the first time.
Learning From History
Helicobacter pylori as the Causative Agent of Gastric Ulcers
As one example, in the 1980s, it was found that the bacterium Helicobacter pylori can cause peptic ulcers - a discovery honoured with the 2005 Nobel Prize in medicine to JR Warren and BJ Marshall (Marshall et al., 1985). This result could have been anticipated as numerous systems of traditional medicine worldwide treat peptic ulcers with herbal drugs that exert pronounced anti-bacterial properties.
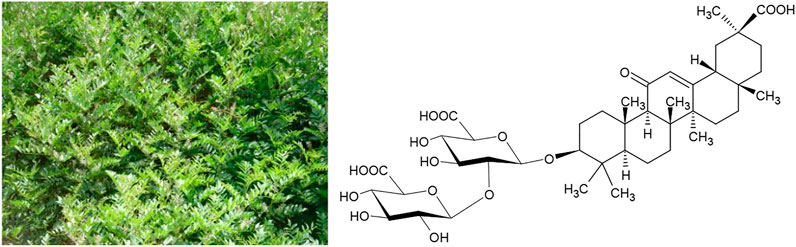
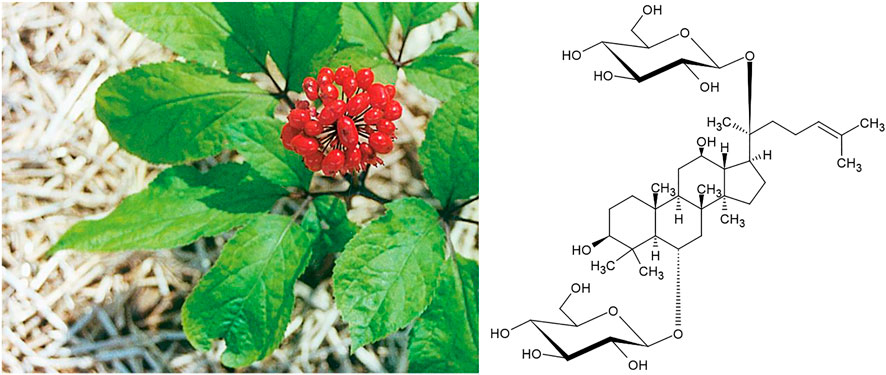
One amongst several such examples are the plant drugs from Glycyrrhiza spec. (G. glabra L. and G. uralensis Fisch.exDC.) that are used as remedies against peptic ulcers from the Atlantic to the Pacific, and the anti-bacterial activity of which is well documented in the literature (Verheijen, 1948). Recent experimental work has verified the effectiveness of Glycyrrhiza spec. extracts against Helicobacter pylori (Asha et al., 2013). Typical active constituents of Glycyrrhiza spec. are triterpene glycosides like saponins such as glycyrrhizic acid (Figure 2).
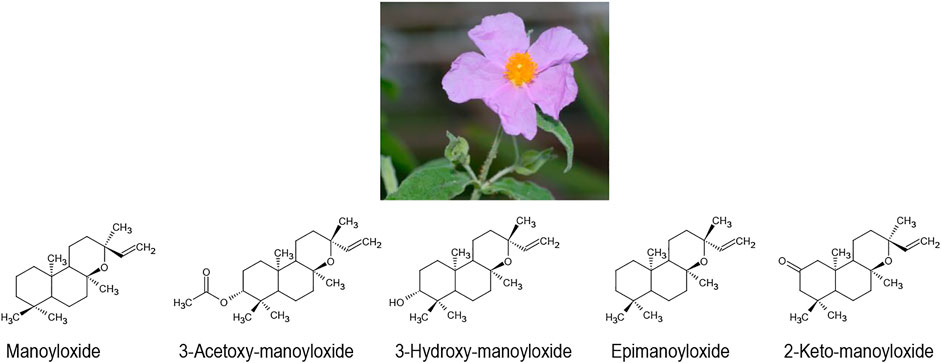
Another famous plant drug against gastric ulcers is the Mediterranean species Cistus creticus L. (including some other species of the same genus). In Cretan traditional medicine, small clumps of labdanum resin, which is collected from the leaves of the plant, are swallowed with Raki as a traditional treatment for gastric ulcer (oral communication, Nyktaris Dimitris, Crete). In Turkish and Italian traditional medicine, tee infusions of the flowers and leaves are used in the same indication and have been successfully tested in animal models (Attaguile et al., 1995; Yesilada et al., 1997). Direct activity of Cistus spec. extracts on Helicobacter pylori cultures in vitro have also been reported (Yesilada et al., 1999). However, in the ancient East Mediterranean, the plant found much wider uses as incense, anti-infective, and for wound treatment (Husemann, 1889; Zohary, 1983). Most recently, a strong activity of the volatile oil phase of the extract against Borrelia burgdorferi in vitro, could be demonstrated (Hutschenreuther et al., 2010; Kuchta et al., 2012; Rauwald et al., 2013). This volatile oil is mainly characterised by manoyloxides such as (manoyloxide, 3-acetoxy-manoyloxide, 3-hydroxy-manoyloxide-epimanoyloxide, 2-keto-manoyloxide), (Figure 3) (Kuchta et al., 2012).
In our previously published research (Rauwald et al., 2019), we were able to show that these manoyloxides are specific for labdanum from Cretan C. creticus. Besides monoterpenes, simple alkanes were dominant in Spanish labdanum - traditionally prepared by hot water extraction of the aerial parts of Cistus ladanifer L., whereas only traces of the major anti-bacterial and anti-viral manoyloxide constituents could be detected (Rauwald et al., 2019). This corresponds with the historical development of the use of labdanum in European traditional herbal medicine: After the Ottoman conquest of Crete in 1645, Western European doctors shifted from Cretan labdanum to Spanish labdanum for the treatment of infectious diseases. Shortly thereafter, labdanum largely fell out of pharmaceutical use in most areas outside of the natural range of C. creticus (Rauwald et al., 2019). Labdanum of Cistus ladanifer L. continues to be used for perfume.
Apart from marker compounds of medicinal plant drugs, we also have “markers” for judging treatment response. The aim of medical treatments has changed from “absence of symptoms” in traditional medicine to “absence of analytical marker compounds or organisms in the human body” today. Consequently, a direct comparison of antibacterial in vitro effects of plant extracts (based on historical documents) with that of mono-molecular antibiotics will often give confusing results. In the case of H. pylori, about one third of the world population test positive for its presence in the stomach, although only a small fraction of these will ever develop gastric ulcer. In the vast majority of cases, H. pylori remains present but inactive. As traditional healers had no means of detecting the bacteria in the human organism, they could only judge the success of their therapy based on the symptoms of their patients. Consequently, the restoration of this inactive, symptom free state was the adaptive peak in the cultural evolution of traditional medicine.
Based on the above example of H. pylori, we have collected data on two other diseases where the apparent mechanism of action of traditional herbal medications across a multitude of cultures should help to clarify the underlying pathogenesis.
Rheumatoid Arthritis and Spirochaeta Infection
One of the most interesting and most consistent correlations between seemingly unrelated traditional indications of medicinal plants in numerous human cultures is the correlation between syphilis and rheumatoid arthritis.
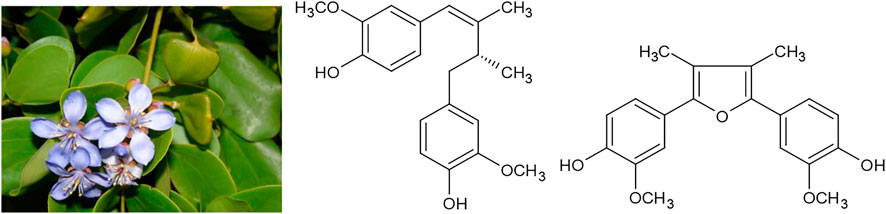
For example, in Japanese Kampo medicine, the main indication for Smilax china L. is syphilis. The same is true for its most common formulation “Hachimitaigeho,” which is further used against numerous infectious and inflammatory diseases of the female reproductive system (Otsuka et al., 2016). In addition, in the Chinese “Bencao Gangmu,” the most extensive and famous compendium of classical Chinese drugs, rheumatoid arthritis is mentioned as a secondary indication (Luo, 2003). Its most significant active components are triterpenes like sarsasapogenin (Figure 4).
We find both indications in Western Herbal Medicine, where the Mesoamerican species of the same genus, mainly Smilax aristolochiifolia Mill. (syn. S. medica Schltdl.&Cham.) and Smilax officinalis Kunth are used. In the Eclectic Medicine Tradition of the United States but also in Europe, these species were used as the preferred herbal remedy for syphilis, especially in the chronic stage of the disease, and also highly recommended for the treatment of rheumatic affections (Felter and Lloyd, 1905; Pereira and Brown, 1855). It is interesting to note that the British pharmacognosist Jonathan Pereira, one of the most famous of his time, already theorised a hidden “venereal origin,” i.e., syphilis infection, as a possible cause of rheumatism (Pereira and Brown, 1855) and Felter and Lloyd (Felter and Lloyd, 1905) also mention “gonorrhoeal rheumatism.”
Another medicinal plant that was intensively used against syphilis is the so called “lignum vitae,” the resin or alcoholic extract of the wood of the Caribbean trees Guaiacum officinale L. or Guaiacum sanctum L. After the epidemic spread of syphilis through Europe in the 15th century, these drugs were imported in large quantities and praised for their effectiveness in the treatment - or at least suppression - of the disease. One of the first patient narratives in the history of medicine were the treaties “De morbo Gallico” (1519) by the German knight and scholar Ulrich von Hutten, who suffered from syphilis himself and described his own treatment (von Hutten, 1533). Among the therapies von Hutten described, Guaiacum resin seems to have been the most effective. Soon, the same drug also found use against rheumatoid arthritis and even as late as 1907, the “British pharmaceutical codex” of the Pharmaceutical Society of Great Britain describes an alkaline solution of Guaiacum resin which “use is empirical in chronic rheumatism, rheumatoid arthritis, and syphilis” (Pharmaceutical Society of Great Britain and Council: University College, 1907). Major components of Guaiacum resin are lignans like dehydroguaialignan and furoguajacin (Figure 5).
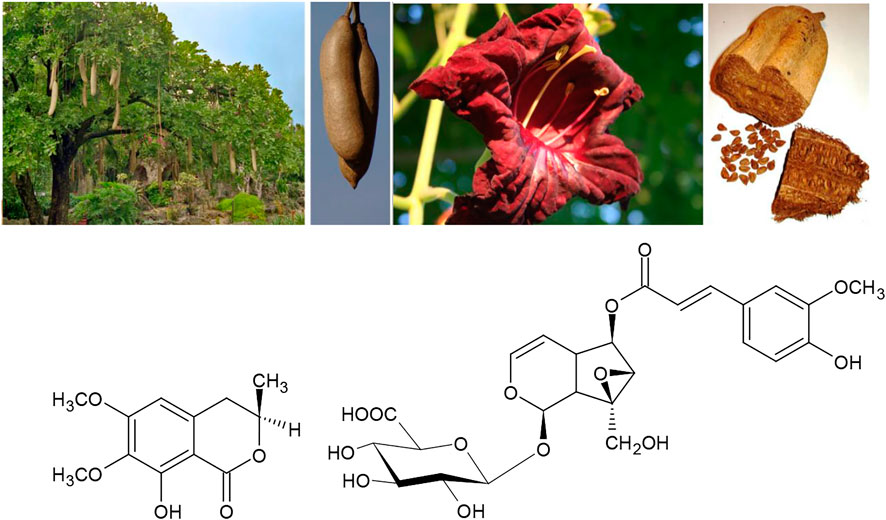
Turning to African traditions, a considerable amount of medicinal knowledge has been documented during the last two centuries. Also in this tradition, an example of the dual use of the same medicinal plant against both, rheumatoid arthritis and syphilis features very prominent: “Both the unripe fruits and the bark of the sausage tree Kigelia africana (Lam.)Benth. are taken as a traditional remedy for syphilis and rheumatism” (Neuwinger, 1996; Orwa et al., 2009). Potentially active constituents are kigelin and minecoside (Figure 6). K. africana has also been shown to interfere with the response of bacteria to quorum sensing autoinducer compounds that inform the microbes about the density of their own population in several types of bacteria. This mechanism therefore facilitates the manipulation of bacterial growth speed making it a promising candidate for developing the ancestral knowledge of Traditional African medicine to a future in the context of integrative medicine (Kahumba et al., 2015).
Bacterial Translocation Through the “Leaky Gut” and the Pathogenesis of “Autoimmune Diseases”
The above described cultural evolution of therapeutic procedures does not only apply to the traditional use of medicinal plants but also to empirical experience in the use of modern medicine. In this context, interesting observations concerning the therapy of chronic diseases of the bone and joints in correlation with potential gastrointestinal infections have been reported.
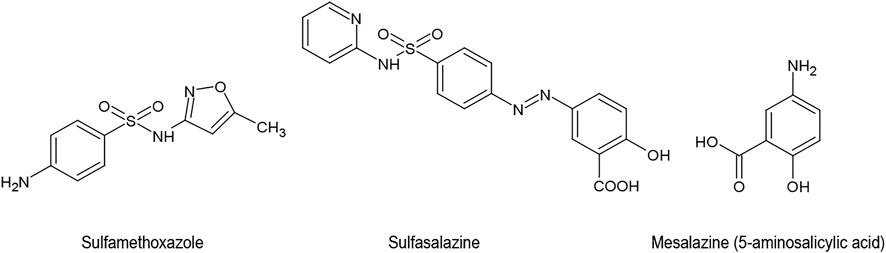
Sulphonamides are a class of antibiotic agents that were developed in Germany during the 1930s. They suppress the enzyme dihydropteroate synthase and inhibit the incorporation of para-aminobenzoic acid into folic acid. The affinity of sulphonamides for the bacterial enzyme is about 10,000 times greater than its affinity for the corresponding mammalian enzyme. However, the sulphonamide sulfamethoxazole, which was introduced to the US in 1961 as a remedy for bacterial infections such as urinary tract infections and bronchitis, soon developed a secondary, purely empirical career in the therapy of alleged “autoimmune diseases” and especially osteoarthritis (Rozin, 2007). These observations are not limited to sulfamethoxazole. The related sulfasalazine is also capable of suppressing clinical symptoms and biochemical signs of rheumatoid arthritis (Neumann et al., 1983; Pullar et al., 1983). Sulfasalazine can further be used for joint-pain associated with inflammatory bowel disease. Its use has however declined because of side effects (Voulgari, 2011). Instead, 5-aminosalicylic acid (mesalazine) is used, which is devoid of the antibacterial sulphonamide group in the molecule (Figure 7).
These empirical developments of the clinical use of chemosynthetic agents - a short term “cultural evolution” if you like - have already repeatedly led to publications of theories proclaiming the bacterial origin of rheumatism (Rozin, 2007; Neumann, 1988). These theories could however not enter the clinical mainstream as bacterial products could not yet be detected in the liquid after joint puncture. In this context of inflammation and intractable bacterial infection, spontaneous bacterial peritonitis in liver cirrhosis is one of the rare cases where standard therapy consists of broad-spectrum antibiotics, even though no bacterium is found in the ascites. For diagnosis, abdominal pain and elevated leucocyte or neutrophil count within the ascites are sufficient. As for Crohn’s disease, NOD2 variants are genetic risk factors for bacterial translocation (Appenrodt et al., 2010).
Bacterial translocation is also of highest interest in relation to the so called “leaky gut” syndrome, which is currently at the centre of the scientific discussion concerning the pathogenesis of diverse “autoimmune diseases.” In pathologic conditions, the permeability of the gut epithelial lining can be compromised allowing the passage of toxins, antigens, and bacteria in the lumen to enter the blood stream creating a “leaky gut.” Commensal bacteria from the gut lumen are able to escape from a “leaky gut” together with their products, inducing inflammation and even systemic tissue damages if translocated into peripheral circulation (Brenchley and Douek, 2012). The increased membrane permeability of the intestinal mucosal barrier appears to further correlate with a host of clinical disorders including: inflammatory and functional bowel disease, food allergies, allergic disorders, rheumatoid arthritis, celiac disease, and chronic dermatological conditions (Porras et al., 2006; Zhou et al., 2009).
All the above hints strongly to an antibacterial therapy regime as a promising treatment approach. Further treatment considerations should include modulation of the intestinal flora or mucosal protection, both of which are available in herbal therapies such as Glycyrrhiza glabra (Asha et al., 2013), Cistus spec. (Attaguile et al., 1995; Yesilada et al., 1997), or the Japanese Kampo prescription Juzentaihoto (Otsuka et al., 2016).
The Cultural Evolution of “Off Label Uses” for Active Constituents
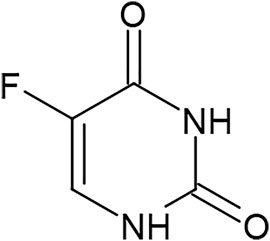
The observation already described by Darwin (Darwin, 1871) that evolutionary processes - replication, mutation, and selection of information - occur throughout all aspects of human culture poses the question whether the historical repurposing of a medicines - as proposed above i.e., for sulphonamides - can be compared with the development of traditional medicine through cultural evolution. This includes many cultural practices such as therapeutic applications and can - also in the case of synthetic drugs - lead to the development of “off label uses” via the described evolutionary processes. Their empirical use can evolve in the same way as for herbal remedies, a form of “short-term cultural evolution” that shall serve here as an introductory example: 5-FU is a chemotherapeutic agent that acts as a wrong base analogue based on its structural similarity with the pyrimidine base cytosine and thymidine (DNA) and uracil (RNA). It has been used as therapy for gastrointestinal cancers since the 60s (Figure 8). Whereas most cases show tumour regression upon 5-FU therapy, some cases react to this agent with - as of yet unexplained - complete tumour remission.
In order to understand these varying response rates, we might look at alternative uses for 5-FU, namely as a mutagenic agent for RNA viruses. The antiviral activity of 5-FU against the lymphocytic choriomeningitis virus was demonstrated in an animal model and has been interpreted to predict a potential efficacy for other arenaviruses, such as Lassa fever (Ruiz-Jarabo et al., 2003). As 5-FU boosts the rate of mutations via incorporation during viral RNA synthesis it is a prime candidate for research into approaches for anti-viral therapy based on the quasi-species model of RNA virus evolution. This effect has been experimentally demonstrated, e.g., for influenza viruses (Pauly and Lauring, 2015). As 5-FU is an anti-mitotic agent which also inhibits thymidylate synthase, it further prevents DNA synthesis. In this context it has been shown that for noncancerous manifestations of human papilloma virus (HPV), a group of DNA viruses of which certain strains cause common warts (verruca vulgaris), local application is effective (Kollipara et al., 2015).
The quasi-species model (Eigen, 1971; Eigen, 1996) proposes that it should be possible to cure viral infections by boosting the mutation rate of the viral genome above the selection rate of the surrounding evolutionary pressure. Above this error threshold (Biebricher and Eigen, 2005), nonsense mutants are generated faster than they can be selected against, resulting in a meltdown of the genetic information that is referred to as “error catastrophe” (Eigen, 2002).
The bench to bedside research on potential therapeutic agents that target the viral “error catastrophe” has not yet resulted in approved drugs for clinical use. However, newer research into human resistance mechanisms against HIV-AIDS indicate that the human immune system itself applies precisely this antiviral strategy in form of the APOBEC3G protein that initiates an increased mutation rate in the viral genome (Malim, 2009). This mechanism appears to be evolutionarily conserved and not just active against HIV but also hepatitis B virus, simple retroviruses, and even endogenous retroelements (Holmes et al., 2007).
Can we therefore assume, that those gastrointestinal tumours which quickly and completely recede under 5-FU might be caused by viral infection at the initial stages?
Could the viral “error catastrophe” or the APOBEC3G system be a potential target of traditional antiviral plant drugs with as of yet unknown mechanisms of action?
Similar reasoning also applies to numerous traditional medicinal plants and their use.
One of the most impressive examples is certainly Artemisia annua L. and its constituent artemisinine. Based on the traditional application of the fresh plant juice in the treatment of malaria as documented in the Zhou Hou Jiu Zu Fang (A Handbook of Formulas for Emergencies), written in 340 AD by the Chinese physician and Daoist philosopher Ge Hong, Tu You-you et al. (You-you, 1982a; You-you et al., 1982b) isolated the active sesquiterpene lactone artemisinine, that has since been marketed worldwide as a treatment for malaria (Plasmodium falciparum). This research was later honoured with the 2015 Nobel Prize in Physiology or Medicine. After their introduction however, artemisinine and its derivatives like artesunate have proven effective in a number of ailments seemingly unrelated to malaria. These “off label uses” include most prominently the use of Artemisia annua and artemisinin for cancer therapy (Efferth, 2017; Efferth, 2006), their activity against viral infections (Efferth et al., 2008) even including hepatitis C virus (HCV) infections (Efferth et al., 2008; Dai et al., 2016), and their ability to attenuate arthritis (Lin et al., 2016). The question, if and how these seemingly unrelated activities are connected and if e.g., viral or protozoal infections might play a role in the development of arthritis might be an interesting starting point for future research.
Galantamine, an alkaloid isolated from Galanthus woronowii Losinsk., has shown a similar broad therapeutic versatility. It was originally developed based on the local use in the treatment of poliomyelitis documented in an observational study in the Caucasus Mountains (Heinrich, 2010). In 1951, Mashkovsky and Kruglikova-Lvov (Mashkovsky and Kruglikova-Lvov, 1951) published the first work that establishes the acetyl-choline esterase inhibiting properties of isolated galantamine. Its indication soon broadened to also include myasthenia gravis and muscular dystrophy, residual poliomyelitis paralysis symptoms, trigeminal neurologica, and other forms of neuritis. The scientific rationale for using cholinesterase inhibitors like galantamine in the management of Alzheimer’s disease is based on the cholinergic hypothesis. Impairment of the central cholinergic system is typically observed in Alzheimer’s patients and is accompanied by a loss of cholinergic neurons in the forebrain and a marked decrease in the activity of choline acetyltransferase. Overall, galantamine represents an example for the successful ethnobotanydriven development of a natural product into a clinically important drug (Heinrich, 2010).
Bone Turnover Related to Improved Testicular Functions
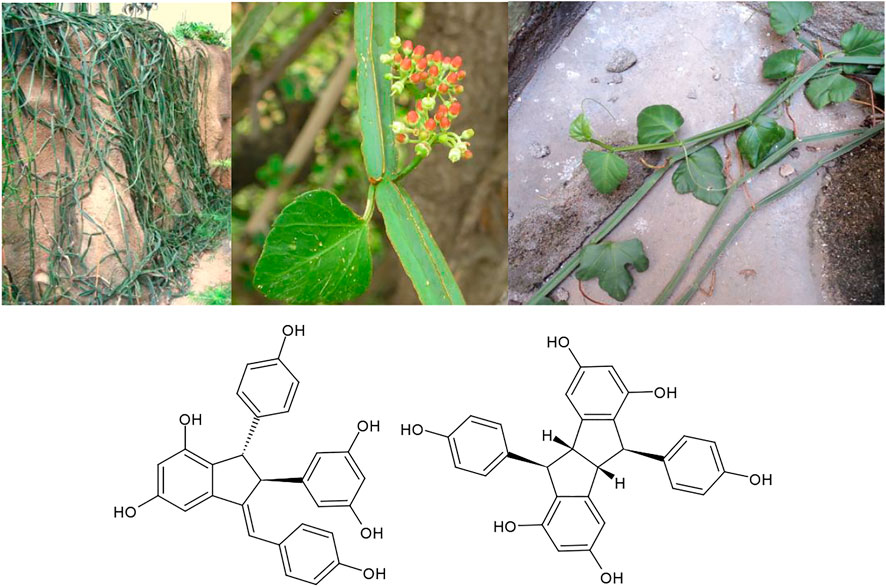
It is very striking that through numerous medicinal traditions around the world, identical plants are used both for osteological ailments like osteoporosis and for accelerating the healing of broken bones, as well as for the treatment of male sexual dysfunctions like infertility and decreasing sperm production. Whereas numerous “aphrodisiac” effects are reported from local medicine worldwide, this specific effect is most strongly correlated with the parallel use of the same plant in osteology. E.g., in Sub-Saharan Africa and in India, Cissus quadrangularis L. extracts are used in both of these indications (Neuwinger, 1996). Typical constituents of these extracts are stilbenoids like quadrangularin A and pallidol (Figure 9). Extracts of C. quadrangularis have been experimentally demonstrated to accelerate the healing of fractured jaw bones in an animal model (Brahmkshatriya et al., 2015), to alleviate bone deterioration in osteotomized rats via p38 MAPK signalling (Kanwar et al., 2015), to up-regulate the matrix mineralization of human osteoblast like SaOS-2 cells (Muthusami et al., 2011), and to enhance biomineralization through up-regulation of MAPK-dependent alkaline phosphatase activity in osteoblasts (Parisuthiman et al., 2009). The validity of the traditional use of the same plant for increasing sperm production has also been experimentally validated (Neuwinger, 1996). Most recently, extracts of C. quadrangularis were demonstrated to prevent quinalphos induced male reproductive toxicity in an animal model (Kokilavani et al., 2014). For the similarly used closely related species Cissus populnea Guill.&Perr. a proliferation effect on the sperm producing TM4 Sertoli cell line was observed (Osibote et al., 2011).
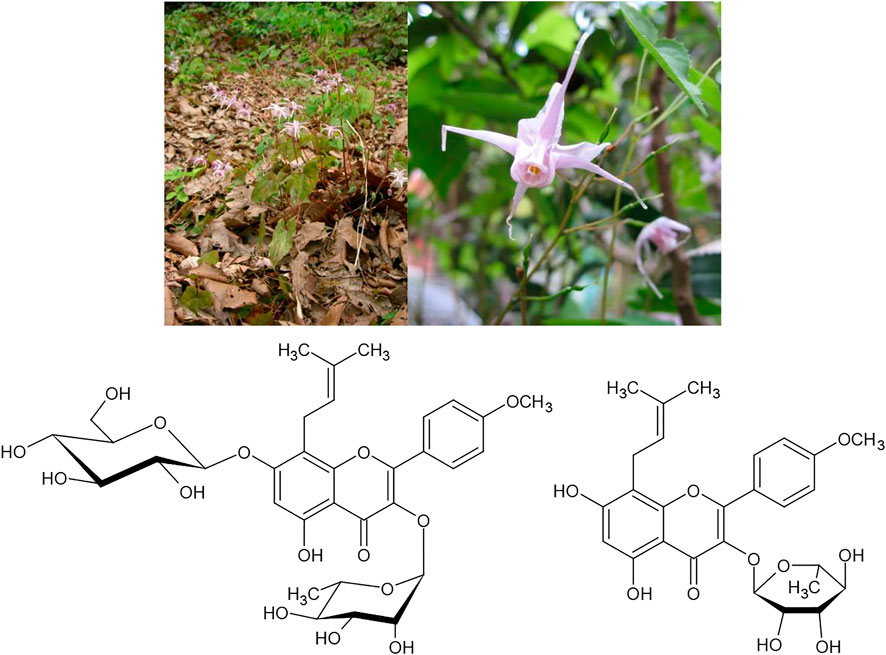
In East Asia, we find several medicinal plant drugs with the same pattern of dual traditional use. Especially for species of the genus Epimedium (e.g., E. grandiflorum C. Morren) numerous uses in both indications are known. A review of its uses in osteoporosis therapy has been published by Zhai et al. (Zhai et al., 2013). It was shown to induce bone neoformation, to reduce osteocyte and osteoclast densities (Burim et al., 2016) and to induce osteogenesis from bone marrow mesenchymal stem cells (Kim et al., 2017). These effects have mainly been attributed to its prenylated flavonol glycoside icariside II, which can enhance the osteogenic differentiation of bone marrow stromal cells (Luo et al., 2015), and icariin, which modulates the process of bone formation via the BMP-2/Smad4 signal transduction pathway (Figure 10) (Liang et al., 2012). Furthermore, in a mouse model, significant increases of testicular weights, sperm counts and sperm motility were observed under treatment with the total flavonoid fraction of the drug (Yuan et al., 2014). In an in vitro model, isolated icariin was shown to promote the proliferation of Sertoli cells by activating the ERK1/2 signal pathway. In a parallel study the prenylflavonoid (i.e., icariin derivatives) fraction of an extract of leaves of the closely related species E. koreaum was shown to exert powerful protective effects on ovariectomy induced osteoporosis in rats by stimulating bone formation and inhibiting bone turnover and bone resorption, suggesting that the extract fraction could be an alternative to hormone replacement therapy for the prevention of postmenopausal osteoporosis (Zhao et al., 2016).
The above discussed case of the application of Epimedium spec. for both osteoporosis and sexual dysfunction reflects the symptom pattern in traditional East Asian medicine systems referred to as “decreased kidney function.”
Yet another East Asian plant drug for which this dichotomy of indications can easily be observed is Panax ginseng C.A.Mey (Figure 11). In both, in vitro and animal models Korean Red Ginseng was shown to prevent radiation-induced bone loss (Kim et al., 2015) and to counteract glucocorticoid-induced osteoporosis (Lee et al., 2013). In a trial with elderly rats, sperm number, germ cell count, Sertoli cell count and Sertoli cell index were significantly restored (Kopalli et al., 2015). In similar experiments, a significant increase of testicle weight was observed (Kim et al., 2010) and testicular damage through 2,3,7, 8-tetrachlorodibenzo-p-dioxin was minimised (Kim et al., 1999).
One possible explanation for the described twofold activity of numerous plant drugs on both the bone and the testicular system may lie in the close ontological relation between the testicular cells and the bone marrow cells in vertebrate ontogenesis (Nayernia et al., 2006). This is especially true for bone marrow cells, in the case of which a direct effect of P. ginseng extracts has been experimentally observed (Kim et al., 2014).
The testicular functions are mainly regulated via androgenic hormones like testosterone (Kopalli et al., 2015), whereas the formation of bone tissue depends on the p38 MAPK signalling (Kanwar et al., 2015). Recent research points to a regulatory connection between the early steps of these two pathways (Jin et al., 2010; Banerjee et al., 2016; Chen et al., 2016). It therefore seems likely that the traditionally used plant extracts interact with pharmacological targets early in the regulatory pathways. However, experimental data in this regard are sparse and much further research is needed.
The presented insights into this field may not only give us a deeper understanding of male reproductive problems but also lead to “new” approaches in the treatment of common osteological diseases like osteoporosis and further to adjuvant treatments for bone fractures. Last but not least, the possibility of prevention of bone loss may be of interest for deep space travel as a pharmaceutical approach to prevent the ensuing bone loss from microgravity.
Conclusion
Based on the above presented series of documented cases of dual use of the same medicinal plant for seemingly unrelated diseases from various systems of traditional medicine worldwide, as well as by theoretical considerations grounded on the principles of biological and cultural evolution, we propose “Tradition to Pathogenesis” as a completely new approach in medicinal plant research: Using the known pharmacological properties of medicinal plants and the documented empirical knowledge of their use, it is possible to gain a new understanding of the pathogenesis of the treated diseases. The study of the “disease symptom patterns” (Chin. Zheng) (Zhao et al., 2015) that are traditionally associated with certain herbal drugs and drug mixtures, may be an important guiding light for future discoveries.
In such traditional medicinal systems like in Traditional Chinese Medicine (TCM), Ayurveda, and Kampo medicine the empirical use of medicinal plants are traditionally not based on the modern knowledge of physiology. However, they contain internally consistent theories of pathogenesis. Anthropologically, these theories of pathogenesis in traditional medicinal systems seem to be based on the accumulation of empirical traditional knowledge over the centuries that was later systematised. Empirical traditional knowledge is - just as any other cultural tradition - subject to cultural evolution. In the context of medicine, this means that successful treatments are remembered and replicated whereas unsuccessful treatment attempts are forgotten or discarded if toxic. However, treatment success depends on 1) human physiology and 2) the pathophysiology of the disease. These two factors form the equivalent of Darwinian evolutionary pressure in the cultural evolution of traditional medicine. Thus - although traditional medicine is not based on the modern knowledge of human physiology, biochemistry or genetics - underlaying information is “imprinted” onto the traditional theories of pathogenesis by cultural evolution. Traditional medical knowledge therefore exhibits an a priori internal structure that corresponds to human pharmacology and physiology, long before these were scientifically understood.
The idea that human cultures undergo a similar evolutionary process as genetic evolution goes back at least to Darwin himself (Darwin, 1871). The first dynamic model of gene-culture co-evolution based on Darwin’s principles was published in 1976 by Feldman and Cavalli-Sforza (Feldman and Cavalli-Sforza, 1976). For example, the above discussed case of the traditional application of Epimedium spec. for both osteoporosis and sexual dysfunction reflects the symptom pattern in traditional East Asian medicine systems referred to as “decreased kidney function”. I.e., these applications of Epimedium spec. have a direct footing in human physiology that was “imprinted” onto the traditional medicine system by cultural evolution. This application of the previously established concept of cultural evolution to traditional medicine and pathophysiology is highlighted in our present work for the first time. It is of note that in modern pathophysiology the suprarenal glands are involved in both bone turnover (cortisol, zona fasciculata) and the production of sexual hormones (zona reticularis).
Whilst the isolation and search for single acting compounds from plants did not lead to a further boost of new chemical drugs, as was seen for salicine, codeine, morphine, digitoxin, irinotecan, vincristine, and taxol, the unlifted treasure lies in the clarification of acting mechanisms of traditional herbal extracts. How the theory of cultural evolution can be applied in order to correlate Traditional to Modern pharmacology is an interesting topic for a wide array of future research. Here, we are focusing on the modern understanding of pathological processes based on traditional views on pathogenesis as a first step towards this aim.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors (KK).
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Prof. Dr. med. Harald Schwörer, clinic for gastroenterology and gastrointestinal oncology, University Medicine Göttingen, is kindly acknowledged for his valuable suggestions concerning both the historical use of sulphonamides in the therapy of rheumatism and the use of antibiotics against bacterial peritonitis. We thank Hans Rausch, Phytochem Referenzsubstanzen, Neu-Ulm, for his extremely helpful suggestions on rheumatism and discussions on H. pylori. We are grateful to Dr. Robert Cameron, Max-Planck-Institute for Solar System Research for his contribution concerning epistemology. The work in Göttingen is supported by the “Förderkreis der Forschungsstelle für Fernöstliche Medizin.”. We acknowledge support by the Open Access Publication Funds of the Göttingen University.
References
Appenrodt, B., Grünhage, F., Gentemann, M. G., Thyssen, L., Sauerbruch, T., and Lammert, F. (2010). Nucleotide-binding Oligomerization Domain Containing 2 (NOD2) Variants Are Genetic Risk Factors for Death and Spontaneous Bacterial Peritonitis in Liver Cirrhosis. Hepatology 51, 1327–1333. doi:10.1002/hep.23440
Asha, M. K., Debraj, D., Prashanth, D., Edwin, J. R., Srikanth, H. S., Muruganantham, N., et al. (2013). In Vitro anti-Helicobacter pylori Activity of a Flavonoid Rich Extract of Glycyrrhiza Glabra and its Probable Mechanisms of Action. J. Ethnopharmacol 145, 581–586. doi:10.1016/j.jep.2012.11.033
Attaguile, G., Caruso, A., Pennisi, G., and Savoca, F. (1995). Gastroprotective Effect of Aqueous Extract of Cistus Incanus L. In Rats. Pharmacol. Res. 31, 29–32. doi:10.1016/1043-6618(95)80043-3
Banerjee, B., Nandi, P., Chakraborty, S., Raha, S., Sen, P. C., and Jana, K. (2016). Resveratrol Ameliorates Benzo(a)pyrene-Induced Testicular Dysfunction and Apoptosis: Involvement of P38 MAPK/ATF2/iNOS Signaling. J. Nutr. Biochem. 34, 17–29. doi:10.1016/j.jnutbio.2016.04.003
Biebricher, C. K., and Eigen, M. (2005). The Error Threshold. Virus. Res. 107, 117–127. doi:10.1016/j.virusres.2004.11.002
Brahmkshatriya, H. R., Shah, K. A., Ananthkumar, G. B., and Brahmkshatriya, M. H. (2015). Clinical Evaluation of Cissus Quadrangularis as Osteogenic Agent in Maxillofacial Fracture: A Pilot Study. Ayu 36, 169–173. doi:10.4103/0974-8520.175542
Brenchley, J. M., and Douek, D. C. (2012). Microbial Translocation across the GI Tract. Annu. Rev. Immunol. 30, 149–173. doi:10.1146/annurev-immunol-020711-075001
Burim, R. A., Sendyk, D. I., Hernandes, L. S., de Souza, D. F., Correa, L., and Deboni, M. C. (2016). Repair of Critical Calvarias Defects with Systemic Epimedium Sagittatum Extract. J. Craniofac. Surg. 27, 799–804. doi:10.1097/SCS.0000000000002451
Chen, Y., Wu, Y., Gan, X., Liu, K., Lv, X., Shen, H., et al. (2016). Iridoid Glycoside from Cornus Officinalis Ameliorated Diabetes Mellitus-Induced Testicular Damage in Male Rats: Involvement of Suppression of the AGEs/RAGE/p38 MAPK Signaling Pathway. J. Ethnopharmacol 194, 850–860. doi:10.1016/j.jep.2016.10.079
Dai, R., Xiao, X., Peng, F., Li, M., and Gong, G. (2016). Artesunate, an Anti-malarial Drug, Has a Potential to Inhibit HCV Replication. Virus Genes 52 (1), 22–28. doi:10.1007/s11262-015-1285-7
Darwin, C. (1862). On the Various Contrivances by Which British and Foreign Orchids Are Fertilised by Insectls. London: John Murray, 197–203.
Darwin, C. (1871). The Descent of Man and Selection in Relation to Sex. in” Photoreproduction of the 1871 Edition (Princeton, New JerseyLondon: Princeton University PressJ. Murray), 60–61.
Efferth, T. (2017). From Ancient Herb to Modern Drug: Artemisia Annua and Artemisinin for Cancer Therapy. Semin. Cancer Biol. 46, 65–83. doi:10.1016/j.semcancer.2017.02.009
Efferth, T. (2006). Molecular Pharmacology and Pharmacogenomics of Artemisinin and its Derivatives in Cancer Cells. Curr. Drug Targets 7 (4), 407–421. doi:10.2174/138945006776359412
Efferth, T., Romero, M. R., Wolf, D. G., Stamminger, T., Marin, J. J., and Marschall, M. (2008). The Antiviral Activities of Artemisinin and Artesunate. Clin. Infect. Dis. 47 (6), 804–811. doi:10.1086/591195
Eigen, M. (2002). Error Catastrophe and Antiviral Strategy. Proc. Natl. Acad. Sci. U S A. 99, 13374–13376. doi:10.1073/pnas.212514799
Eigen, M. (1996). On the Nature of Virus Quasispecies. Trends Microbiol. 4, 216–218. doi:10.1016/0966-842X(96)20011-3
Eigen, M. (1971). Selforganization of Matter and the Evolution of Biological Macromolecules. Naturwissenschaften 58, 465–523. doi:10.1007/BF00623322
Feldman, M. W., and Cavalli-Sforza, L. L. (1976). Cultural and Biological Evolutionary Processes, Selection for a Trait under Complex Transmission. Theor. Popul. Biol. 9 (2), 238–259. doi:10.1016/0040-5809(76)90047-2
Felter, H. W., and Lloyd, J. U.King's American Dispensatory (1905). Cincinatti: Ohio Valley Co., 1729.
Hardy, K., Buckley, S., Collins, M. J., Estalrrich, A., Brothwell, D., Copeland, L., et al. (2012). Neanderthal Medics? Evidence for Food, Cooking, and Medicinal Plants Entrapped in Dental Calculus. Naturwissenschaften 99, 617–626. doi:10.1007/s00114-012-0942-0
Heinrich, M. (2010). Galanthamine from Galanthus and Other Amaryllidaceae-Cchemistry and Biology Based on Traditional Use. Alkaloids Chem. Biol. 68, 157–165. doi:10.1016/s1099-4831(10)06804-5
Holmes, R. K., Malim, M. H., and Bishop, K. N. (2007). APOBEC-mediated Viral Restriction: Not Simply Editing? Trends Biochem. Sci. 32, 118–128. doi:10.1016/j.tibs.2007.01.004
Huffman, M. A. (2001). Self-Medicative Bahavior in the African Great Apes: An Evolutionary Perspective into the Origins of Human Traditional Medicine. BioScience, 51, 651–661. doi:10.1641/0006-3568(2001)051[0651:smbita]2.0.co;2Self-Medicative Behavior in the
Husemann, T. (1889). Ladanum und Laudanum - Ein Beitrag zur Geschichte der Arzneimittel. Archiv der Pharmacie 27 (24), 1075–1132. doi:10.1002/ardp.18892272303
Hutschenreuther, A., Birkemeyer, C., Grötzinger, K., Straubinger, R. K., and Rauwald, H. W. (2010). Growth Inhibiting Activity of Volatile Oil from Cistus Creticus L. Against Borrelia Burgdorferi s.S. In Vitro. Pharmazie 65, 290–295.
Jin, H., Wang, D. Y., Mei, Y. F., Qiu, W. B., Zhou, Y., Wang, D. M., et al. (2010). Mitogen-activated Protein Kinases Pathway Is Involved in Physiological Testosterone-Induced Tissue Factor Pathway Inhibitor Expression in Endothelial Cells. Blood Coagul. Fibrinolysis 21, 420–424. doi:10.1097/MBC.0b013e328337b475
Kahumba, J., Rasamiravaka, T., Okusa, P. N., Bakari, A., Bizumukama, L., Kalonji, J. B., et al. (2015). Traditional African Medicine: From Ancestral Knowledge to a Modern Integrated Future. Science 350 (6262), S61–S63. doi:10.1126/science.350.6262.871-c
Kanwar, J. R., Samarasinghe, R. M., Kumar, K., Arya, R., Sharma, S., Zhou, S. F., et al. (2015). Cissus Quadrangularis Inhibits IL-1β Induced Inflammatory Responses on Chondrocytes and Alleviates Bone Deterioration in Osteotomized Rats via P38 MAPK Signaling. Drug Des. Devel Ther. 9, 2927–2940. doi:10.2147/DDDT.S77369
Kim, D. R., Lee, J. E., Shim, K. J., Cho, J. H., Lee, H. C., Park, S. K., et al. (2017). Effects of Herbal Epimedium on the Improvement of Bone Metabolic Disorder through the Induction of Osteogenic Differentiation from Bone Marrow-Derived Mesenchymal Stem Cells. Mol. Med. Rep. 15, 125–130. doi:10.3892/mmr.2016.6015
Kim, J., Lee, H., Kang, K. S., Chun, K. H., and Hwang, G. S. (2015). Protective Effect of Korean Red Ginseng against Glucocorticoid-Induced Osteoporosis In Vitro and In Vivo. J. Ginseng Res. 39, 46–53. doi:10.1016/j.jgr.2014.06.001
Kim, S. G., Lee, A. J., Bae, S. H., Kim, S. M., Lee, J. H., Kim, M. J., et al. (2014). Total Extract of Korean Red Ginseng Facilitates Human Bone Marrow Hematopoietic colony Formation In Vitro. Blood Res. 49, 177–181. doi:10.5045/br.2014.49.3.177
Kim, W., Hwang, S., Lee, H., Song, H., and Kim, S. (1999). Panax Ginseng Protects the Testis against 2,3,7, 8-Tetrachlorodibenzo-P-Dioxin Induced Testicular Damage in guinea Pigs. BJU Int. 83, 842–849. doi:10.1046/j.1464-410x.1999.00046.x
Kim, Y. H., Kim, G. H., Shin, J. H., Kim, K. S., and Lim, J. S. (2010). Effect of Korean Red Ginseng on Testicular Tissue Injury after Torsion and Detorsion. Korean J. Urol. 51, 794–799. doi:10.4111/kju.2010.51.11.794
Kokilavani, P., Suriyakalaa, U., Elumalai, P., Abirami, B., Ramachandran, R., Sankarganesh, A., et al. (2014). Antioxidant Mediated Ameliorative Steroidogenesis by Commelina Benghalensis L. And Cissus Quadrangularis L. against Quinalphos Induced Male Reproductive Toxicity. Pestic. Biochem. Physiol. 109, 18–33. doi:10.1016/j.pestbp.2014.01.002
Kollipara, R., Ekhlassi, E., Downing, C., Guidry, J., Lee, M., and Tyring, S. K. (2015). Advancements in Pharmacotherapy for Noncancerous Manifestations of HPV. J. Clin. Med. 4, 832–846. doi:10.3390/jcm4050832
Kopalli, S. R., Hwang, S. Y., Won, Y. J., Kim, S. W., Cha, K. M., Han, C. K., et al. (2015). Korean Red Ginseng Extract Rejuvenates Testicular Ineffectiveness and Sperm Maturation Process in Aged Rats by Regulating Redox Proteins and Oxidative Defense Mechanisms. Exp. Gerontol. 69, 94–102. doi:10.1016/j.exger.2015.05.004
Kuchta, K., Grötzinger, K., Birkemeyer, C., and Rauwald, H. W. (2012). Labdanum from Mediterranean Cistus Species: GC-MS Fingerprints and Relative Quantification of Antispirochaetal Manoyloxides, 78.
Lee, J. H., Lee, H. J., Yang, M., Moon, C., Kim, J. C., Bae, C. S., et al. (2013). Effect of Korean Red Ginseng on Radiation-Induced Bone Loss in C3H/HeN Mice. J. Ginseng Res. 37, 435–441. doi:10.5142/jgr.2013.37.435
Liang, W., Lin, M., Li, X., Li, C., Gao, B., Gan, H., et al. (2012). Icariin Promotes Bone Formation via the BMP-2/Smad4 Signal Transduction Pathway in the hFOB 1.19 Human Osteoblastic Cell Line. Int. J. Mol. Med. 30, 889–895. doi:10.3892/ijmm.2012.1079
Lin, Z. M., Yang, X. Q., Zhu, F. H., He, S. J., Tang, W., and Zuo, J. P. (2016). Artemisinin Analogue SM934 Attenuate Collagen-Induced Arthritis by Suppressing T Follicular Helper Cells and T Helper 17 Cells. Sci. Rep. 6, 38115. doi:10.1038/srep38115
Luo, G., Gu, F., Zhang, Y., Liu, T., Guo, P., and Huang, Y. (2015). Icariside II Promotes Osteogenic Differentiation of Bone Marrow Stromal Cells in Beagle Canine. Int. J. Clin. Exp. Pathol. 8, 4367–4377.
Luo, X. (2003). Compendium of Materia Medica, Category of Herbs (IV): A Translation of the "Bencao Gangmu. Beijing: Foreign Languages Press.
Malim, M. H. (2009). APOBEC Proteins and Intrinsic Resistance to HIV-1 Infection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 675–687. doi:10.1098/rstb.2008.0185
Marshall, B. J., Armstrong, J. A., McGechie, D. B., and Glancy, R. J. (1985). Attempt to Fulfil Koch's Postulates for Pyloric Campylobacter. Med. J. Aust. 142, 436–439. doi:10.5694/j.1326-5377.1985.tb113443.x
Mashkovsky, M. D., and Kruglikova-Lvov, R. P. (1951). On the Pharmacology of the New Alklaloid Galantamine. Farmakol. Toxicol. (Moscow) 14, 27–30.
Muthusami, S., Senthilkumar, K., Vignesh, C., Ilangovan, R., Stanley, J., Selvamurugan, N., et al. (2011). Effects of Cissus Quadrangularis on the Proliferation, Differentiation and Matrix Mineralization of Human Osteoblast like SaOS-2 Cells. J. Cel Biochem 112, 1035–1045. doi:10.1002/jcb.23016
Nayernia, K., Lee, J. H., Drusenheimer, N., Nolte, J., Wulf, G., Dressel, R., et al. (2006). Derivation of Male Germ Cells from Bone Marrow Stem Cells. Lab. Invest. 86, 654–663. doi:10.1038/labinvest.3700429
Neumann, V. (1988). Biochemical Aspects of Infection in Rheumatoid Arthritis and Ankylosing Spondylitis. Baillieres Clin. Rheumatol. 2, 259–269. doi:10.1016/s0950-3579(88)80012-8
Neumann, V. C., Grindulis, K. A., Hubball, S., McConkey, B., and Wright, V. (1983). Comparison between Penicillamine and Sulphasalazine in Rheumatoid Arthritis: Leeds-Birmingham Trial. Br. Med. J. (Clin Res. Ed. 287, 1099–1102. doi:10.1136/bmj.287.6399.1099
Neuwinger, H. D. (1996). African Ethnobotany: Poisons and Drugs: Chemistry, Pharmacology, Toxicology. Weinheim, Germany: Chapman & Hall, 252–257.
Orwa, C., Mutua, A., Kindt, R., Kamnadass, R., and Anthony, S. (2009). Agroforestry Database: A Tree Reference and Selection Guide Version 4.0.
Osibote, E., Noah, N., Sadik, O., McGee, D., and Ogunlesi, M. (2011). Electrochemical Sensors, MTT and Immunofluorescence Assays for Monitoring the Proliferation Effects of Cissus Populnea Extracts on Sertoli Cells. Reprod. Biol. Endocrinol. 9, 65. doi:10.1186/1477-7827-9-65
Otsuka, K. (2016). in Kampo: A Clinical Guide to Theory and Practice. Editors G. T. DeSoriano, and N. T. Dawes London, UK: Singing Dragon.
Parisuthiman, D., Singhatanadgit, W., Dechatiwongse, T., and Koontongkaew, S. (2009). Cissus Quadrangularis Extract Enhances Biomineralization through Up-Regulation of MAPK-dependent Alkaline Phosphatase Activity in Osteoblasts. In Vitro Cel Dev Biol Anim 45, 194–200. doi:10.1007/s11626-008-9158-1
Pauly, M. D., and Lauring, A. S. (2015). Effective Lethal Mutagenesis of Influenza Virus by Three Nucleoside Analogs. J. Virol. 89, 3584–3597. doi:10.1128/JVI.03483-14
Pereira, J., and Brown, Green. (1855). The Elements of Materia Medica and Therapeutics. 4th Edition. London: Longman, 290–291.
Pharmaceutical Society of Great Britain, Council: University College, L. (1907). The British Pharmaceutical Codex: An imperial Dispensatory for the Use of Medical Practitioners and Pharmacists. London: Pharmaceutical Society of Great Britain., 1191.
Porras, M., Martín, M. T., Yang, P. C., Jury, J., Perdue, M. H., and Vergara, P. (2006). Correlation between Cyclical Epithelial Barrier Dysfunction and Bacterial Translocation in the Relapses of Intestinal Inflammation. Inflamm. Bowel Dis. 12, 843–852. doi:10.1097/01.mib.0000231571.88806.62
Pullar, T., Hunter, J. A., and Capell, H. A. (1983). Sulphasalazine in Rheumatoid Arthritis: a Double Blind Comparison of Sulphasalazine with Placebo and Sodium Aurothiomalate. Br. Med. J. (Clin Res. Ed. 287, 1102–1104. doi:10.1136/bmj.287.6399.1102
Rauwald, H. W., Liebold, T., Grötzinger, K., Kuchta, K., and Lehmann, J. (2013). On the Antispirochaetal Activity of Manoyloxides and Carvacrol from the Oleoresin Labdanum of Cistus Creticus L. Planta Med. 79, PN53. doi:10.1055/s-0033-1352396
Rauwald, H. W., Liebold, T., Grötzinger, K., Lehmann, J., and Kuchta, K. (2019). Labdanum and Labdanes of Cistus Creticus and C. Ladanifer: Anti-Borrelia Activity and its Phytochemical Profiling✰. Phytomedicine 60, 152977. doi:10.1016/j.phymed.2019.152977
Rozin, A. (2007). Is Osteoarthritis an Infection-Associated Disease and a Target for Chemotherapy? Chemotherapy 53, 1–9. doi:10.1159/000098243
Ruiz-Jarabo, C. M., Ly, C., Domingo, E., and de la Torre, J. C. (2003). Lethal Mutagenesis of the Prototypic Arenavirus Lymphocytic Choriomeningitis Virus (LCMV). Virology 308, 37–47. doi:10.1016/s0042-6822(02)00046-6
Verheijen, H. C.De Invloed van Succus Liquiritiae op de Maagzuursecretie en zijn Werking bij Ulcus Ventriculi (1948). Ned Tijdschr Geneeskd 92, 2910–2912.
von Hutten, U. Aedibus Thomae Bertheleti, MDXXXIII [printed 1533] Cum Privilegio, De Morbo Gallico; Londini.
Voulgari, P. V. (2011). Rheumatological Manifestations in Inflammatory Bowel Disease. Ann. Gastroenterol. 24, 173–180.
Weyrich, L. S., Duchene, S., Soubrier, J., Arriola, L., Llamas, B., Breen, J., et al. (2017). Neanderthal Behaviour, Diet, and Disease Inferred from Ancient DNA in Dental Calculus. Nature 544, 357–361. doi:10.1038/nature21674
Yesilada, E., Gurbuz, I., and Ergun, E. (1997). Effects of Cistus Laurifolius L. Flowers on Gastric and Duodenal Lesions. J. Ethnopharmacol 55, 201–211.
Yesilada, E., Gurbuz, I., and Shibata, H. (1999). Screening of Turkish Anti-ulcerogenic Folk Remedies for Anti-Helicobacter pylori Activity. J. Ethnopharmacol 66, 289–293.
You-you, T., Mu-Yun, N., Yu-Rong, Z., Lan-Na, L., Shu-Lian, C., Mu-Qun, Z., et al. (1982b). Studies on the Constituents of Artemisia Annua Part II. Planta Med. 44 (3), 143–145. doi:10.1055/s-2007-971424
You-you, T., (1982a). Chemical Studies on Qinghaosu (Artemisinine). China Cooperative Research Group on Qinghaosu and its Derivatives as Antimalarials. J. Tradit Chin. Med. 2 (1), 3–8.
Yuan, D., Wang, H., He, H., Jia, L., He, Y., Wang, T., et al. (2014). Protective Effects of Total Flavonoids from Epimedium on the Male Mouse Reproductive System against Cyclophosphamide-Induced Oxidative Injury by Up-Regulating the Expressions of SOD3 and GPX1. Phytother Res. 28, 88–97. doi:10.1002/ptr.4956
Zhai, Y. K., Guo, X., Pan, Y. L., Niu, Y. B., Li, C. R., Wu, X. L., et al. (2013). A Systematic Review of the Efficacy and Pharmacological Profile of Herba Epimedii in Osteoporosis Therapy. Pharmazie 68, 713–722.
Zhao, B. J., Wang, J., Song, J., Wang, C. F., Gu, J. F., Yuan, J. R., et al. (2016). Beneficial Effects of a Flavonoid Fraction of Herba Epimedii on Bone Metabolism in Ovariectomized Rats. Planta Med. 82 (4), 322–329. doi:10.1055/s-0035-1558294
Zhao, X., Zheng, X., Fan, T. P., Li, Z., Zhang, Y., and Zheng, J. (2015). A Novel Drug Discovery Strategy Inspired by Traditional Medicine Philosophies. Science 347 (6219 Suppl. l), S38–S40. doi:10.1126/science.347.6219.337-c
Zhou, Q., Zhang, B., and Verne, G. N. (2009). Intestinal Membrane Permeability and Hypersensitivity in the Irritable Bowel Syndrome. Pain 146 (1-2), 41–46. doi:10.1016/j.pain.2009.06.017
Keywords: traditional medicine, pharmacology, pathogenesis, mechanism of action, Evolution, Cultural evolution
Citation: Kuchta K and Cameron S (2021) Tradition to Pathogenesis: A Novel Hypothesis for Elucidating the Pathogenesis of Diseases Based on the Traditional Use of Medicinal Plants. Front. Pharmacol. 12:705077. doi: 10.3389/fphar.2021.705077
Received: 04 May 2021; Accepted: 27 September 2021;
Published: 25 October 2021.
Edited by:
Michael Heinrich, UCL School of Pharmacy, United KingdomReviewed by:
Abhay Prakash Mishra, University of the Free State, South AfricaChi-Jung Tai, Pingtung Hospital, Taiwan
Copyright © 2021 Kuchta and Cameron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenny Kuchta, a2Vubnkua3VjaHRhQG1lZC51bmktZ29ldHRpbmdlbi5kZQ==; Silke Cameron, c2lsa2UuY2FtZXJvbkBtZWQudW5pLWdvZXR0aW5nZW4uZGU=
 Kenny Kuchta
Kenny Kuchta Silke Cameron
Silke Cameron