
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 18 June 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.700498
This article is part of the Research Topic Biomolecules Against Coronaviruses: Molecular Aspects, Multi-omics and Systems Pharmacology View all 7 articles
 Haibo Hu1†
Haibo Hu1† Kun Wang1†
Kun Wang1† Li Wang1†
Li Wang1† Yanjun Du2
Yanjun Du2 Juan Chen3
Juan Chen3 Yongchun Li4
Yongchun Li4 Chuanbo Fan1
Chuanbo Fan1 Ning Li1
Ning Li1 Ying Sun1
Ying Sun1 Shenghao Tu3
Shenghao Tu3 Xuechao Lu1*
Xuechao Lu1* Zhaoshan Zhou1*
Zhaoshan Zhou1* Huantian Cui5*
Huantian Cui5*Combination therapy using Western and traditional Chinese medicines has shown notable effects on coronavirus disease 2019 (COVID-19). The He-Jie-Shen-Shi decoction (HJSS), composed of Bupleurum chinense DC., Scutellaria baicalensis Georgi, Pinellia ternata (Thunb.) Makino, Glycyrrhiza uralensis Fisch. ex DC., and nine other herbs, has been used to treat severe COVID-19 in clinical practice. The aim of this study was to compare the clinical efficacies of HJSS combination therapy and Western monotherapy against severe COVID-19 and to study the potential action mechanism of HJSS. From February 2020 to March 2020, 81 patients with severe COVID-19 in Wuhan Tongji Hospital were selected for retrospective cohort study. Network pharmacology was conducted to predict the possible mechanism of HJSS on COVID-19-related acute respiratory distress syndrome (ARDS). Targets of active components in HJSS were screened using the Traditional Chinese Medicine Systems Pharmacology (TCMSP) and PharmMapper databases. The targets of COVID-19 and ARDS were obtained from GeneCards and Online Mendelian Inheritance in Man databases. The key targets of HJSS in COVID-19 and ARDS were obtained based on the protein–protein interaction network (PPI). Kyoto Encyclopedia of Genes and Genomes analysis (KEGG) was conducted to predict the pathways related to the targets of HJSS in COVID-19 and ARDS. A “herb-ingredient-target-pathway” network was established using Cytoscape 3.2.7. Results showed that the duration of the negative conversion time of nucleic acid was shorter in patients who received HJSS combination therapy. HJSS combination therapy also relieved fever in patients with severe COVID-19. Network pharmacology analysis identified interleukin (IL) 6, tumor necrosis factor (TNF), vascular endothelial growth factor A (VEGFA), catalase (CAT), mitogen-activated protein kinase (MAPK) 1, tumor protein p53 (TP53), CC-chemokine ligand (CCL2), MAPK3, prostaglandin-endoperoxide synthase 2 (PTGS2), and IL1B as the key targets of HJSS in COVID-19-related ARDS. KEGG analysis suggested that HJSS improved COVID-19-related ARDS by regulating hypoxia-inducible factor (HIF)-1, NOD-like receptor, TNF, T cell receptor, sphingolipid, PI3K-Akt, toll-like receptor, VEGF, FoxO, and MAPK signaling pathways. In conclusion, HJSS can be used as an adjuvant therapy on severe COVID-19. The therapeutic mechanisms may be involved in inhibiting viral replication, inflammatory response, and oxidative stress and alleviating lung injury. Further studies are required to confirm its clinical efficacies and action mechanisms.
Since its outbreak in 2019, coronavirus disease 2019 (COVID-19) has become a global pandemic (Jin et al., 2020). Current epidemiological studies have shown more than 130 million people worldwide have been diagnosed (Boban et al., 2021). The pandemic has caused more than 2.9 million deaths worldwide, and the mortality cases continue to rise (Novelli et al., 2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, infects host cells by binding with angiotensin-converting enzyme 2 (ACE2) mainly in the respiratory system, thereby impairing it, as indicated by fever, coughing, shortness of breath, and chest tightness, among other symptoms (Farsalinos et al., 2020; Gao et al., 2020). ACE2 is also expressed on enterocytes, cardiomyocytes, renal proximal tubules, and neurocytes (Xu et al., 2020). Some patients with SARS-CoV-2 infection have also exhibited multi-system symptoms including diarrhea, acute cardiac injury, abnormal renal function, and myalgia (Adapa et al., 2020; Asadi-Pooya et al., 2020; Nishiga et al., 2020; Villapol et al., 2020). Patients with severe COVID-19 tend to develop acute respiratory distress syndrome (ARDS) and multiple organ failure (MOF), which are the major fatal events of COVD-19 (Li et al., 2020; Iwasaki et al., 2021). Clinical studies have further shown that recovered patients can develop sequelae such as interstitial lung disease and hyposmia, which can have long-term impacts on the quality of life (Mihai et al., 2020; Meng et al., 2020).
To date, no effective drugs have been developed to treat COVID-19 (Stasi et al., 2020). Preliminary clinical studies have demonstrated that remdesivir could improve lower respiratory tract infection; however, the safety and efficacy of remdesivir still need to be established (Beigel et al., 2020; Spinner et al., 2020; Wang F. et al., 2020; Wang Y. et al., 2020). Although several vaccines against SARS-CoV-2 infection have passed their phase III trials and some countries have a good vaccination rate, the uncertainty of long-term immunity after vaccination and the potential to develop severe side effects such as anaphylactic shock and thrombosis are also important considerations when opting for vaccination. (Zhu et al., 2020). Given the limitations of current therapeutics and the severity of the disease, the development of new therapies for COVID-19 are urgently needed (Ren et al., 2020; Kwok et al., 2021).
Traditional Chinese medicine (TCM) has gained abundant therapeutic knowledge from treating various diseases for thousands of years. TCM-based treatments for SARS, Middle East respiratory syndrome, and H7N9 have achieved significant therapeutic effects (Zhang et al., 2020). TCM-based treatments have achieved favorable results during the outbreak of COVID-19 early in 2020 (Cui et al., 2020). A meta-analysis indicated that combining the Lian-Hua-Qing-Wen granule and conventional treatment showed a higher overall effective rate on COVID-19 compared with the conventional treatment alone (Liu et al., 2021). “Fei Yan No. 1” could significantly improve the clinical outcomes and reduce the duration of the negative conversion time of nucleic acid in patients with COVID-19 (Ai et al., 2020). Tanreqing Capsule treatment also reduced the duration of the negative conversion time of nucleic acid and increased CD3+ T cell production in patients with COVID-19 (Zhang et al., 2021). The Qingfei Paidu decoction combined with Western medicine improved the blood test results, inflammatory factors, and multi-organ biochemical indices in patients with COVID-19 (Chen et al., 2020; Yang et al., 2020). A retrospective cohort study showed that individualised Chinese medicine could improve the level of D-dimer and reduce the mortality in patients with COVID-19 (Shu et al., 2021).
He-Jie-Shen-Shi decoction (HJSS) has been used for the treatment of severe COVID-19 in clinical practice. It is composed of Bupleurum chinense DC., Scutellaria baicalensis Georgi, Pinellia ternata (Thunb.) Makino, Glycyrrhiza uralensis Fisch. ex DC., Codonopsis pilosula (Franch.) Nannf., Poria cocos (Schw.) Wolf, Alisma plantago-aquatica subsp. orientale (Sam.) Sam., Atractylodes macrocephala Koidz., Neolitsea cassia (L.) Kosterm., Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf, Pyrrosia lingua (Thunb.) Farw., Plantago asiatica L. and Benincasa hispida (Thunb.) Cogn.
Here, the therapeutic effects of HJSS on patients with severe COVID-19 were evaluated in a retrospective cohort study and the possible mechanisms were predicted using network pharmacology.
This study was approved by the Institutional Ethics Board of Wuhan Tongji Hospital (Approval No. TJ-C20200112). Informed consent was waived since this was a retrospective study. Consecutive patients hospitalized from February 9th to March 31st, 2020, in Wuhan Tongji Hospital were enrolled.
All patients (age range, 18–75) who were diagnosed with severe COVID-19 according to the Protocol for Diagnosis and Treatment of COVID-19 guidelines (4th–6th editions) issued by the National Health Commission of China (General Office of The National Health and Health Commission, 2020) were included in the study (Supplementary Table S1).
1) Pregnant or lactating women; 2) severe primary diseases such as severe combined immune deficiency disease, congenital pulmonary airway malformation, congenital heart disease, and bronchopulmonary dysplasia; 3) psychiatric or neurological disorder; 4) transfer to other hospitals; and 5) other factors such as self-withdrawal during treatment and incomplete clinical data records (Supplementary Table S1).
HJSS granules were provided by the Wuhan Tongji Hospital (Tianjin, China). The components of HJSS are shown in Table 1. The herb granules were fully dissolved in 200 ml hot water (>70°C).
12 principal components in HJSS, including atractylenolide III, saikosaponin A, saikosaponin D, lobetyolin, baicalin, pachymic acid, liquiritin, L-arginine, chlorogenic acid, geniposidic acid, cinnamic acid and alisol B-23-acetate were used as the reference standards according to the quality control methods in Pharmacopoeia of People’s Republic of China (2020 edition) issued by Chinese Pharmacopoeia Commission. Briefly, 5 mg of each reference standard was dessolved in 5 ml methanol and incubated at 50°C for 30 min to obtain the stock solution of each reference standard (1 mg/ml). Then, 100 μL of stock solution was dessolved in 900 μL methanol to obtain the test solutions of reference standards. Besides, 300 μL of HJSS were fully dessolved in 900 μL methanol and centrifuged at 13,000 rpm for 10 min and the supernatant was collected to obtain the test solution of HJSS.
Ultra performance liquid chromatography (UPLC; ACQUITY UPLC®, United States) coupled with Xevo G2 quadrupole-time-of-flight (Q-TOF) mass spectrometer (MS; Waters Corp., Milford, MA, United States) systems were used as the quality control of HJSS. 2 μL of each test solution was injected onto an ACQUITY UPLC BEH C18 Column (2.1 × 100 mm, 1.7 μm; column temperature: 50°C; flow rate: 0.3 ml/min). Mobile phase A was 0.1% formic acid aqueous solution and mobile phase B was acetonitrile contained 0.1% formic acid. The mobile phase conditions were: 0 min, 5% B; 1 min, 10% B; 6 min 60% B; 6.5 min 100% B; 10 min 100% B; 10.1 min 5% B; 13 min 5% B.
A Q-TOF MS equipped with an electrospray ionization (ESI) source was used for both positive and negative ionization scan modes (50–1,200 Da). The scan time was 0.2 s.
The detailed parameters of MS were: capillary voltage of 3,000 V (positive mode) and 2,200 V (negative mode), desolvation temperature at 350°C, sample cone voltage of 40 V, extraction cone voltage of 4 V, source temperature of 100°C, cone gas flow of 40 L/h and desolvation gas flow of 800 L/h (both positive and negative modes).
Of the 109 patients assessed for eligibility, 28 were excluded, including one pregnant woman, seven cases with severe primary diseases, four cases with psychiatric or neurological disorder, seven cases that had transferred to other hospitals, and nine cases with incomplete clinical data records. There were no significant differences in baseline characteristics between 28 excluded patients and 81 included patients. A final total of 81 patients were included and divided into the general treatment (GT) and HJSS + GT groups. Baseline data were collected.
The GT group received GT including oxygen inhalation therapy (high-flow nasal cannula oxygen therapy and mechanical ventilation if necessary), parenteral nutrition, antiviral therapy (arbidol, 200 mg, per os three times daily) and other treatments based on the Protocol for Diagnosis and Treatment of COVID-19 guidelines (4th–6th editions). The HJSS + GT group received GT and HJSS (per os twice daily).
Clinical characteristics, respiratory rate (RR), blood oxygen saturation (SpO2), ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2), blood laboratory tests, and chest computed tomography (CT) images taken during hospitalization were collected to observe the effects of HJSS and GT on severe COVID-19. Clinical characteristics included the duration of the negative conversion time of nucleic acid and the duration of fever and cough. Blood laboratory tests included routine blood tests and C-reactive protein (CRP) (Figure 1).
Propensity score matching (PSM) was used to compensate for differences in baseline characteristics between GT and HJSS + GT groups. Propensity scores were calculated by logistic regression using demographic and clinical characteristics of the study population (age, sex, diabetes, hypertension, coronary artery disease, and chronic kidney disease). A caliper of width equal to 0.02 of the standard deviation of the logit of the propensity score was applied. To better match GT and HJSS + GT groups, we used the 1:1 ratio matching method. Covariate balance before and after propensity score matching was assessed using standardized differences. A standardized difference of <0.1 was considered negligible, and balance was assumed to be met.
The Shapiro-Wilks test was used to test for normality. For continuous variables that conform to a normal distribution, we used the mean and standard deviation to describe the data distribution, and two-sample t test was used to compare the differences between GT and HJSS + GT groups. For continuous variables that do not satisfy a normal distribution, we used median and interquartile range to describe the data distribution, and Wilcoxon rank-sum test was used to compare the differences between GT and HJSS + GT groups. For categorical variables, frequency and percentage were used to describe the data distribution, and the chi-square test or Fisher’s exact test were used to compare the differences between GT and HJSS + GT groups. Considering that the baseline values of dependent variables may have some impact on the comparison between the groups, we defined the differences of dependent variables, and intergroup comparisons for the differences of dependent variables were analyzed using the two-sample t test or Wilcoxon rank-sum test.
For all analysis, we used SPSS version 20.0 (SPSS, Inc., Chicago, IL, United States). A p-value < 0.05 was considered statistically significant. Curve fitting was carried out using the GraphPad Prism five graphical package (GraphPad Software, Inc., La Jolla, CA, United States).
A search of the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP: https://tcmspw.com/tcmsp.php) was performed to generate candidate ingredients of HJSS using the following keywords: “Bupleurum chinense DC,.” “Scutellaria baicalensis Georgi,” “Pinellia ternata (Thunb.) Makino,” “Glycyrrhiza uralensis Fisch. ex DC.,” “Codonopsis pilosula (Franch.) Nannf.,”“Poria cocos (Schw.) Wolf,” “Alisma plantago-aquatica subsp. orientale (Sam.) Sam.,”“Atractylodes macrocephala Koidz.,” “Neolitsea cassia (L.) Kosterm.” “Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf,” “Pyrrosia lingua (Thunb.) Farw.,” “Plantago asiatica L.” and “Benincasa hispida (Thunb.) Cogn.” To obtain the active ingredients of HJSS, these ingredients were further screened for oral bioavailability (OB ≥ 30%) and drug-like criteria (DL ≥ 0.18).
The chemical ingredients of HJSS obtained from TCMSP were input into the PubChem database (https://pubchem.ncbi.nlm.nih.gov) for 3D structure identification. The 3D structures of the ingredients were then uploaded to the PharmMapper platform server (http://www.lilab-ecust.cn/pharmmapper/) to generate relevant targets for the active ingredients. The UniProt database (https://www.uniprot.org/) was then used to standardize the target proteins per the active ingredients and obtain the gene names per the targets.
The Online Mendelian Inheritance in Man (OMIM) (http://omim.org) and GeneCards (https://www.genecards.org) databases were searched for relevant targets using the keyword “Acute Respiratory Distress Syndrome (ARDS)” and “COVID-19”. The Venny online interactive platform version 2.1 (http://bioinfogp.cnb.csic.es/tools/venny/) was used to intersect the targets of HJSS, ARDS, and COVID-19 to further isolate treatment targets and obtain the potential targets of HJSS in COVID-19-related ARDS (Yang et al., 2020).
The protein–protein interaction (PPI) of potential targets of HJSS in COVID-19-related ARDS were studied via the STRING database. In addition, Cytoscape 3.7.2 (http://www.cytoscape.org/) was used to construct the PPI network. The interactions between the proteins in the PPI network were analyzed using the MCODE plugin on Cytoscape 3.7.2. Proteins with many interactions showed larger numbers and widths of connections. The top ten highly interactive proteins were then identified as the key targets (Yang et al., 2020).
The intersecting targets of HJSS, ARDS, and COVID-19 were imported into the KOBAS 3.0 database (http://kobas.cbi.pku.edu.cn) for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. The enriched KEGG terms were identified and analyzed based on p-value correction <0.05 (Bonferroni step down). Cytoscape 3.7.2 was then used to establish the “herb-ingredient-target-pathway” network.
Atractylenolide III, saikosaponin A, saikosaponin D, lobetyolin, baicalin, pachymic acid, liquiritin, L-arginine, chlorogenic acid, geniposidic acid, cinnamic acid and alisol B-23-acetate were identified as the main components in HJSS. The detailed information of these compounds were shown in Figure S1. The typical based peak intensity (BPI) chromatograms and the characteristic fragment ions of these compounds were shown in Supplementary Figure S2 and Table S2 respectively (Supplementary meterial).
As shown in Table 2, no significant differences were observed in the baseline characteristics between the GT and HJSS + GT groups. After HJSS and GT treatment, the differences of clinical indicators between GT and HJSS + GT groups were shown in Table 3. For full study population, patients in HJSS + GT group were found to have a significantly lower leukocyte count compared with patients in GT group (p = 0.03). In addition, patients in HJSS + GT group showed a shorter duration of the negative conversion time of nucleic acid (23.09 days vs. 20.13 days, p = 0.03) and fever (15.79 days vs. 12.36 days, p = 0.03) compared with patients in GT group. We used the 1:1 PSM to generate a more balanced subsample of 32 patients in GT and HJSS + GT groups respectively. As shown in Figure 2, PSM eliminated the differences in covariate distributions, and all the standardized differences after matching were less than 0.1. For propensity score-matched subsample, we observed the consistent results for leukocyte count (p = 0.01), duration of the negative conversion time of nucleic acid (23.34 days vs. 21.03 days, p = 0.04) and fever (15.75 days vs. 12.53 days, p = 0.02) differences. Chest CT showed multiple ground-glass opacities and consolidation before HJSS and GT treatment. These chest CT features were significantly improved after both HJSS + GT and GT treatment (Figure 3).
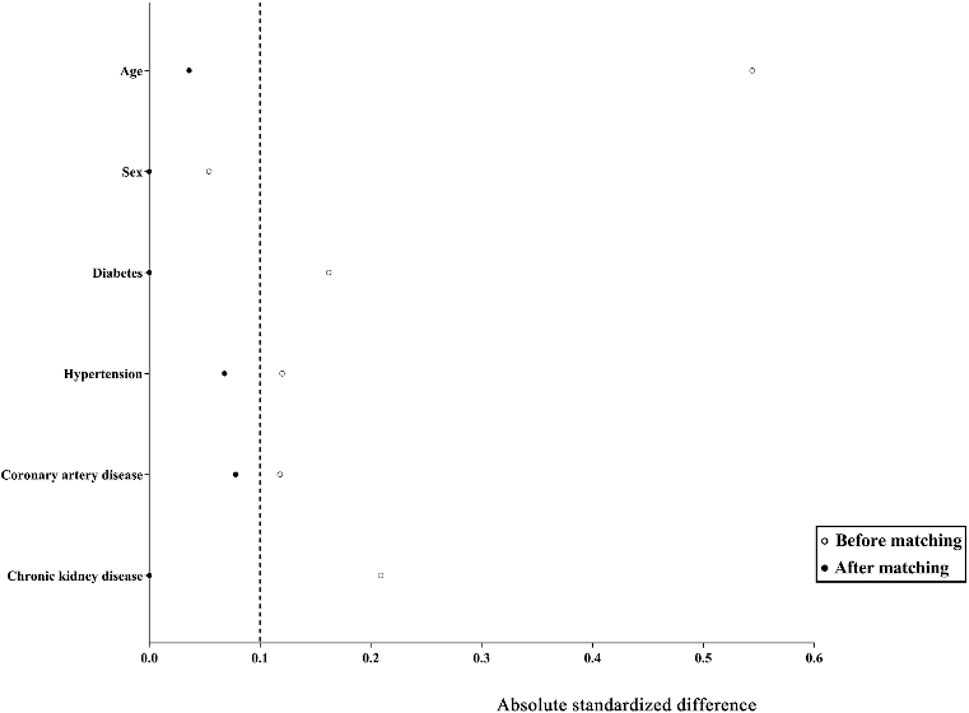
FIGURE 2. Absolute standardized differences comparing characteristics of patients in GT and HJSS + GT groups before matching and after 1:1 propensity score matching. The absolute standardized differences <0.1 show adequate matching. Y axis represented the selected characteristics. X axis of the scatterplot represented whether the status was before matching or after matching.
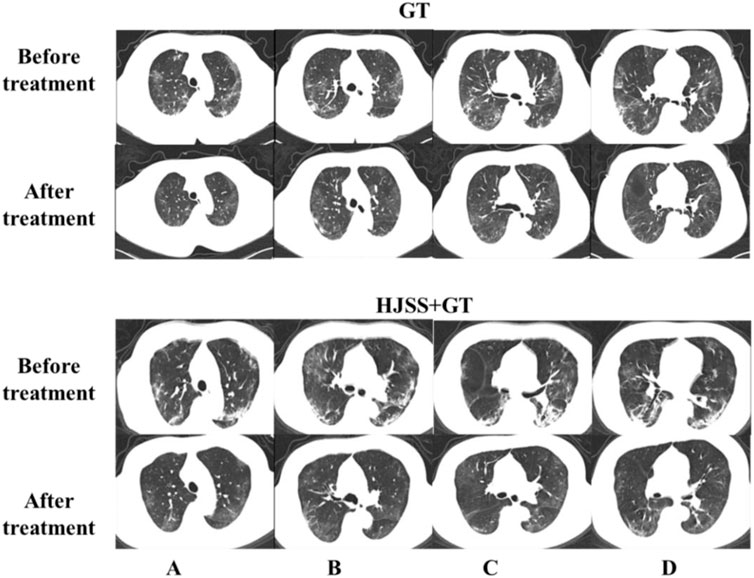
FIGURE 3. Chest CT scan before and after GT and HJSS treatment. Bilateral pulmonary infiltrate was clearly absorbed after GT and HJSS treatment. (A–D) represent different slices. GT group: A 66-year-old female received GT for 15 days. HJSS + GT group: A 59-year-old female received HJSS and GT for 12 days.
A total of 208 active ingredients were obtained from the screening (OB ≥ 30% and DL ≥ 0.18), including Shiwei [Pyrrosia lingua (Thunb.) Farw.] 6, Cheqianzi (Plantago asiatica L.) 9, Yiyiren (Coix lacryma-jobi var. ma-yuen (Rom.Caill.) Stapf) 9, Dongguaren [Benincasa hispida (Thunb.) Cogn.] 2, Guizhi (Neolitsea cassia (L.) Kosterm.) 5, Baizhu (Atractylodes macrocephala Koidz.) 7, Zexie [Alisma plantago-aquatica subsp. orientale (Sam.) Sam.] 10, Fuling (Poria cocos (Schw.)Wolf) 15, Dangshen [Codonopsis pilosula (Franch.) Nannf.] 21, Gancao (Glycyrrhiza uralensis Fisch. ex DC.) 92, Banxia (Pinellia ternata (Thunb.) Makino) 13, Huangqin (Scutellaria baicalensis Georgi) 24, and Chaihu (Bupleurum chinense DC.) 17 (Figure 4). The detailed information of active ingredients was shown in Supplementary Table S3 (Supplementary meterial).
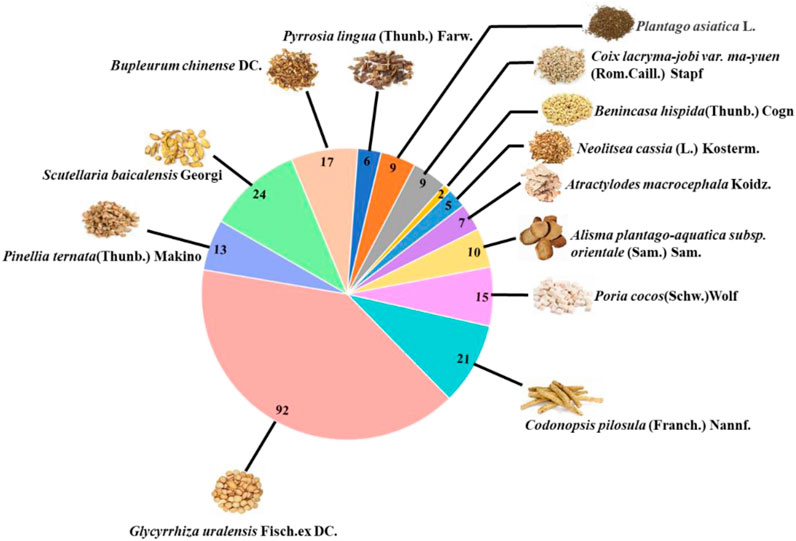
FIGURE 4. The number of active ingredients of each herb in HJSS obtained from TCMSP (OB ≥ 30%, DL ≥ 0.18).
We retrieved 169 targets from the PharmMapper platform server, based on these 208 active ingredients. Meanwhile, we obtained 659 COVID-19 targets and 3473 ARDS targets from the OMIM and GeneCards databases. By intersecting the targets of the active herb ingredients with the disease targets, we identified 28 potential targets for HJSS in COVID-19-related ARDS (Figure 5). The PPI of the 28 potential targets of HJSS in COVID-19-related ARDS were analyzed using the STRING database, and the PPI network was constructed using Cytoscape 3.7.2. The PPI network showed 181 interaction edges obtained with the 28 targets. The top 10 targets with the highest interactions were interleukin (IL) 6, tumor necrosis factor (TNF), vascular endothelial growth factor A (VEGFA), catalase (CAT), mitogen-activated protein kinase (MAPK) 1, tumor protein p53 (TP53), CC-chemokine ligand (CCL2), MAPK3, prostaglandin-endoperoxide synthase 2 (PTGS2), and IL1B. Thus, these proteins were identified as the key targets of HJSS in COVID-19-related ARDS (Figure 6).
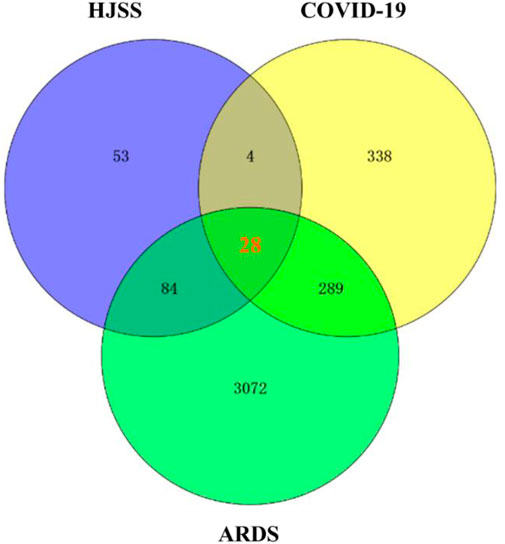
FIGURE 5. After intersecting the targets of HJSS, COVID-19 and ARDS, 28 potential targets of HJSS on COVID-19-related ARDS were obtained (Venn diagram).
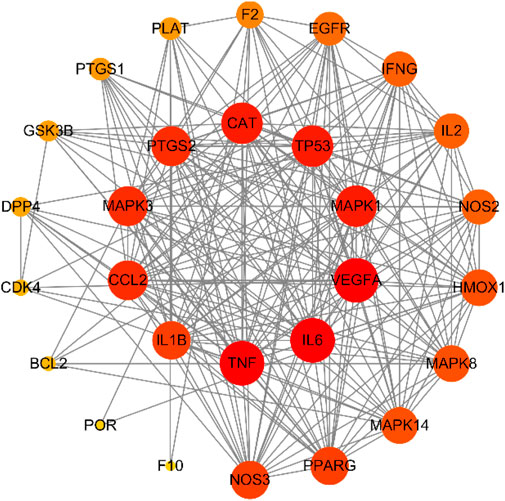
FIGURE 6. PPI network of 28 potential targets obtained from venn diagram. Nodes in red indicate more interactions with other targets, while nodes in yellow indicate less interactions with other targets.
According to the KEGG pathway analysis of 28 potential targets, 181 KEGG terms were enriched and the top twenty pathways with highest significance (lowest p value) were showed in Figure 7. Potential mechanistic pathways of HJSS in COVID-19-related ARDS were primarily related to the HIF-1, NOD-like receptor, TNF, T cell receptor, sphingolipid, PI3K-Akt, toll-like receptor, VEGF, FoxO, and MAPK signaling pathways. Furthermore, the herbs, ingredients, targets, and pathways of the HJSS therapeutic mechanism in COVID-19-related ARDS were imported into Cytoscape 3.7.2 to generate the “herb-ingredient-target-pathway” network. In the “herb-ingredient-target-pathway” network diagram, the orange rhombic nodes represent herbs, the yellow rectangle nodes represent active ingredients, the green rectangle nodes represent targets, and the blue rectangle nodes represent pathways (Figure 8).
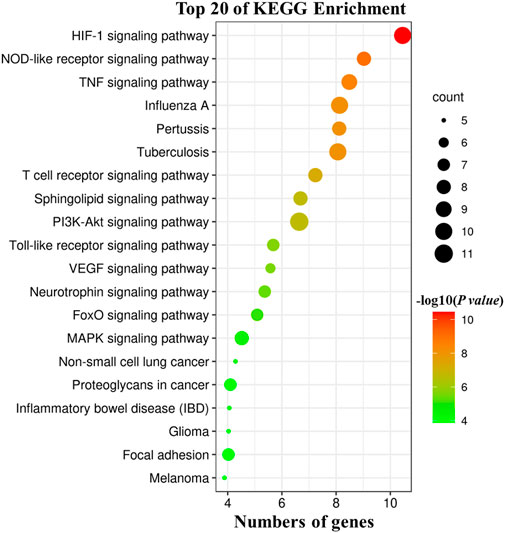
FIGURE 7. KEGG pathway analysis of 28 potential targets. The size of bubbles indicates the numbers of associated genes, the larger bubble indicates more gene counts. Color coding scale indicates the significance of KEGG terms, the deeper red indicates lower p values [higher -log10 (p value)].
In this study, we compared the clinical efficacies of HJSS + GT and GT on severe COVID-19. Our results showed that HJSS + GT shortened the duration of the negative conversion time of nucleic acid and relieved fever. The negative conversion time of nucleic acid is the most direct diagnostic indicator of recovery from COVID-19. Fever is a primary clinical outcome in severe COVID-19 (Bolay et al., 2020). Accelerating the treatment of fever consequently lowers inflammatory levels. Routine blood testing is an easy and efficient way to understand the severity of COVID-19 (Ferrari et al., 2020; Lu et al., 2021). Our results showed lower total lymphocyte count and higher level of CRP in patients with severe COVID-19. In COVID-19, the total lymphocyte count is decreased because during infection, SARS-CoV-2 enters the lymph nodes, spleen, and other immune system organs to replicate and destroys the lymphocytes thereafter (Kim et al., 2020). CRP is also an important laboratory test index reflecting the level of inflammatory response (Felger et al., 2020). Our results also showed lower leukocyte count in HJSS + GT group compared with GT group. However, the difference in leukocyte count may be cause by the individual differences of patients in each group according to the reference range. Furthermore, most patients also exhibited higher RR, lower SpO2 and PaO2/FiO2 and cough, chest CT scans revealed multiple ground-glass opacities and consolidation in severe COVID-19, which fulfill the diagnostic criteria for severe COVID-19 (Li et al., 2020; Pan et al., 2020).
In addition, we screened the potential targets of HJSS on COVID-19 and ARDS using network pharmacology. Most severe patients with COVID-19 develop ARDS, which is a major event that can lead to death in COVID-19 (Torres et al., 2020). The key targets of HJSS identified were IL6, TNF, VEGFA, CAT, MAPK1, TP53, CCL2, MAPK3, PTGS2, IL1B—chemokines and pro-inflammatory highly involved in the progression of COVID-19. CCL2 could be released by multiple organs and could contribute to the inflammatory process during viral infection (Kempuraj et al., 2020). CCL2 could be released from the lungs after SARS-CoV-2 infection and could induce the activation and migration of macrophages to the lungs, initiating an inflammatory response in the lungs (Fuentes et al., 2019). The overproduction of these cytokines and chemokines could trigger hypoxia and lung injury through the impairment of the pulmonary alveoli, further decreasing the capacity of the airway lumen (Ci et al., 2012). Moreover, excessive pro-inflammatory cytokines could lead to an inflammatory cytokine storm, triggering MOF (Wang H. et al., 2020; Wang J. et al., 2020; Karki et al., 2021). MAPK could be activated by pro-inflammatory cytokines to subsequently upregulate the gene expression of pro-inflammatory cytokines and contribute to the progression of the inflammatory response (Li et al., 2015). The effects of HJSS on reducing the duration of fever may in turn decrease chemokine and pro-inflammatory cytokine levels and inhibit MAPK activation. P53 is closely related to the modulation of the cell cycle, DNA repair, cell differentiation, and apoptosis (Engeland et al., 2017; Hafner et al., 2019). Studies have also shown that SARS-CoV-2 could induce the apoptosis of lymphocytes by activating the P53 signaling pathway (Xiong et al., 2020; Bordoni et al., 2021). VEGFA belongs to the PDGF/VEGF growth factor family, which participates in the progression of endothelial cell proliferation and cell migration and is necessary for angiogenesis under physiological and pathological conditions (Zhang et al., 2019). Compared with milder COVID-19 cases, the serum levels of VEGF were significantly higher in severe COVID-19 cases. The increase in VEGF levels could promote vascular permeability and contribute to lung injury (Zhang et al., 2020). Studies showed that the overproduction of reactive oxygen species (ROS) could be observed in ARDS, triggering oxidative stress and exacerbating lung injury (Gong et al., 2020). CAT is an antioxidative enzyme involved in the elimination of ROS. Since CAT activity is decreased in ARDS, promoting its expression could alleviate lung injury (Lei et al., 2021).
KEGG analysis also showed that the HIF-1, NOD-like receptor, sphingolipid, PI3K-Akt, FoxO, and T cell receptor signaling pathways may be related to the mechanisms of HJSS on COVID-19-related ARDS. HIF-1 is an important transcriptional factor induced under anoxia (Simko et al., 2017). PI3K catalyzes the transformation of PIP2 to PIP3, further triggering the phosphorylation and activation of Akt (Delaloge et al., 2017). The activation of the HIF-1 and PI3K-Akt pathways could induce the inflammatory response and angiogenesis (Palazon et al., 2014; Hou et al., 2016). NOD-like receptor, FoxO, and T cell receptor are important modulators of the inflammatory response (He et al., 2014; Hammami et al., 2018; Liu et al., 2019). As an important component of biological membranes, sphingolipids participate in various kinds of biological processes such as cell survival, differentiation, apoptosis, and migration (Hwang et al., 2017). The dysfunction in the sphingolipid pathway could disrupt the balance between cell survival and apoptosis (Hannun and Obeid, 2018). Thus, modulating the sphingolipid signaling pathway could alleviate acute lung injury (Lin et al., 2011). Based on the chest CT and network pharmacology studies, the protective effect of HJSS in lung injury may be involved in the regulation of CAT activity and the sphingolipid signaling pathway.
This is a retrospective cohort and potential mechanistic study evaluating the efficacy and potential mechanism of HJSS on severe COVID-19 patients. Our data indicated that HJSS could be used as an adjuvant therapy on severe COVID-19. Compared with GT, HJSS + GT shortened the duration of the negative conversion time of nucleic acid. A shorter negative conversion time of nucleic acid could relieve pressure on the healthcare system, which is beneficial during this pandemic. Besides, we performed propensity score matched analysis to ascertain the robustness of the result. In network pharmacology study, we screened the potential targets of HJSS on severe COVID-19 by intersecting the targets of the active herb ingredients in HJSS with the targets of COVID-19 and ARDS.
Some limitations in this study should also be realized. Due to the small sample size of this retrospective study, further prospective, multi-center, double-blind and randomized controlled trials are required to evaluate the effect of HJSS in a larger patient population. The therapeutic mechanisms of HJSS still need to be verified. Virus-induced ARDS animal models could be used in further studies to validate the effects and mechanisms of HJSS on severe COVID-19. Component analyses such as high-performance liquid chromatography could also reveal the main components of HJSS. The main components could then be purified and studied in animal (virus-induced ARDS) and cell models (pseudoviral infection and virus-induced inflammation).
In conclusion, HJSS can be used as an adjuvant therapy on severe COVID-19. The therapeutic mechanisms may be involved in inhibiting viral replication, inflammatory response, and oxidative stress and alleviating lung injury. Further studies are required to confirm its clinical efficacies and action mechanisms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Ethics Board of Wuhan Tongji Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HC, HH, KW, and LW wrote the manuscript. HH, XL, ST, and YD conducted retrospective cohort study. HC, JC, NL, YS and finished network pharmacology. ZZ, CF, YL, and XL provided technical guidance for the whole work. All authors contributed to the article and approved the submitted version.
This work was supported by Shandong Science and Technology Development Project of TCM (2017-323, 2019-0596), Qingdao Guidance Plan of Medical Science (2020-WJZD049, 2020-WJZD053) and Qingdao Scientific Plan of TCM (2015-zyy012, 2019-zyy001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer HW declared a past co-authorship with several of the authors NL, LW, and HC to the handling editor.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.700498/full#supplementary-material
Adapa, S., Chenna, A., Balla, M., Merugu, G. P., Koduri, N. M., Daggubati, S. R., et al. (2020). COVID-19 Pandemic Causing Acute Kidney Injury and Impact on Patients with Chronic Kidney Disease and Renal Transplantation. J. Clin. Med. Res. 12, 352–361. doi:10.14740/jocmr4200
Ai, Z., Zhou, S., Li, W., Wang, M., Wang, L., Hu, G., et al. (2020). "Fei Yan No. 1" as a Combined Treatment for COVID-19: An Efficacy and Potential Mechanistic Study. Front. Pharmacol. 11, 581277. doi:10.3389/fphar.2020.581277
Asadi-Pooya, A. A., and Simani, L. (2020). Central Nervous System Manifestations of COVID-19: A Systematic Review. J. Neurol. Sci. 413, 116832. doi:10.1016/j.jns.2020.116832
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al. (2020). Remdesivir for the Treatment of Covid-19 - Final Report. N. Engl. J. Med. 383, 1813–1826. doi:10.1056/NEJMoa2007764
Boban, M. (2021). Novel Coronavirus Disease (COVID‐19) Update on Epidemiology, Pathogenicity, Clinical Course and Treatments. Int. J. Clin. Pract. 75, e13868. doi:10.1111/ijcp.13868
Bolay, H., Gül, A., and Baykan, B. (2020). COVID‐19 Is a Real Headache!. Headache: J. Head Face Pain 60, 1415–1421. doi:10.1111/head.13856
Bordoni, V., Tartaglia, E., Sacchi, A., Fimia, G. M., Cimini, E., Casetti, R., et al. (2021). The Unbalanced p53/SIRT1 axis May Impact Lymphocyte Homeostasis in COVID-19 Patients. Int. J. Infect. Dis. 105, 49–53. doi:10.1016/j.ijid.2021.02.019
Chen, J., Wang, Y.-k., Gao, Y., Hu, L.-S., Yang, J.-w., Wang, J.-r., et al. (2020). Protection against COVID-19 Injury by Qingfei Paidu Decoction via Anti-viral, Anti-inflammatory Activity and Metabolic Programming. Biomed. Pharmacother. 129, 110281. doi:10.1016/j.biopha.2020.110281
Ci, X., Chu, X., Wei, M., Yang, X., Cai, Q., and Deng, X. (2012). Different Effects of Farrerol on an OVA-Induced Allergic Asthma and LPS-Induced Acute Lung Injury. PloS one 7, e34634. doi:10.1371/journal.pone.0034634
Cui, H. T., Li, Y. T., Guo, L. Y., Liu, X. G., Wang, L. S., Jia, J. W., et al. (2020). Traditional Chinese Medicine for Treatment of Coronavirus Disease 2019: a Review. Tradit Med. Res. 5, 65–73. doi:10.12032/TMR20200222165
Delaloge, S., and DeForceville, L. (2017). Targeting PI3K/AKT Pathway in Triple-Negative Breast Cancer. Lancet Oncol. 18, 1293–1294. doi:10.1016/S1470-2045(17)30514-4
Engeland, K. (2017). Cell Cycle Arrest through Indirect Transcriptional Repression by P53: I Have a DREAM. Cell Death Differ 25, 114–132. doi:10.1038/cdd.2017.172
Farsalinos, K., Angelopoulou, A., Alexandris, N., and Poulas, K. (2020). COVID-19 and the Nicotinic Cholinergic System. Eur. Respir. J. 56, 2001589. doi:10.1183/13993003.01589-2020
Felger, J. C., Haroon, E., Patel, T. A., Goldsmith, D. R., Wommack, E. C., Woolwine, B. J., et al. (2020). What Does Plasma CRP Tell Us about Peripheral and central Inflammation in Depression?. Mol. Psychiatry 25, 1301–1311. doi:10.1038/s41380-018-0096-3
Ferrari, D., Motta, A., Strollo, M., Banfi, G., and Locatelli, M. (2020). Routine Blood Tests as a Potential Diagnostic Tool for COVID-19. Clin. Chem. Lab. Med. 58, 1095–1099. doi:10.1515/cclm-2020-0398
Fuentes, N., Cabello, N., Nicoleau, M., Chroneos, Z. C., and Silveyra, P. (2019). Modulation of the Lung Inflammatory Response to Ozone by the Estrous Cycle. Physiol. Rep. 7, e14026. doi:10.14814/phy2.14026
Gao, Z., Xu, Y., Sun, C., Wang, X., Guo, Y., Qiu, S., et al. (2021). A Systematic Review of Asymptomatic Infections with COVID-19. J. Microbiol. Immunol. Infect. 54, 12–16. doi:10.1016/j.jmii.2020.05.001
Gong, X., Qiu, J., Qiu, G., and Cai, C. (2020). Adrenomedullin Regulated by miRNA-574-3p Protects Premature Infants with Bronchopulmonary Dysplasia. Biosci. Rep. 40, BSR20191879. doi:10.1042/BSR20191879
Hafner, A., Bulyk, M. L., Jambhekar, A., and Lahav, G. (2019). The Multiple Mechanisms that Regulate P53 Activity and Cell Fate. Nat. Rev. Mol. Cel Biol. 20, 199–210. doi:10.1038/s41580-019-0110-x
Hammami, A., Abidin, B. M., Heinonen, K. M., and Stäger, S. (2018). HIF-1α Hampers Dendritic Cell Function and Th1 Generation during Chronic Visceral Leishmaniasis. Sci. Rep. 8, 3500. doi:10.1038/s41598-018-21891-z
Hannun, Y. A., and Obeid, L. M. (2018). Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cel Biol. 19, 175–191. doi:10.1038/nrm.2017.107
He, Z., He, X., Chen, Z., Ke, J., He, X., Yuan, R., et al. (2014). Activation of the mTORC1 and STAT3 Pathways Promotes the Malignant Transformation of Colitis in Mice. Oncol. Rep. 32, 1873–1880. doi:10.3892/or.2014.3421
Hou, Y., Nie, Y., Cheng, B., Tao, J., Ma, X., Jiang, M., et al. (2016). Qingfei Xiaoyan Wan, a Traditional Chinese Medicine Formula, Ameliorates Pseudomonas Aeruginosa-Induced Acute Lung Inflammation by Regulation of PI3K/AKT and Ras/MAPK Pathways. Acta Pharmaceutica Sinica B 6, 212–221. doi:10.1016/j.apsb.2016.03.002
Hwang, S., Gustafsson, H. T., O’Sullivan, C., Bisceglia, G., Huang, X., Klose, C., et al. (2017). Serine-Dependent Sphingolipid Synthesis Is a Metabolic Liability of Aneuploid Cells. Cel Rep. 21, 3807–3818. doi:10.1016/j.celrep.2017.11.103
Iwasaki, M., Saito, J., Zhao, H., Sakamoto, A., Hirota, K., and Ma, D. (2021). Inflammation Triggered by SARS-CoV-2 and ACE2 Augment Drives Multiple Organ Failure of Severe COVID-19: Molecular Mechanisms and Implications. Inflammation 44, 13–34. doi:10.1007/s10753-020-01337-3
Jin, Y., Yang, H., Ji, W., Wu, W., Chen, S., Zhang, W., et al. (2020). Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 12, 372. doi:10.3390/v12040372
Karki, R., Sharma, B. R., Tuladhar, S., Williams, E. P., Zalduondo, L., Samir, P., et al. (2021). Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 184, 149–168. doi:10.1016/j.cell.2020.11.025
Kempuraj, D., Selvakumar, G. P., Ahmed, M. E., Raikwar, S. P., Thangavel, R., Khan, A., et al. (2020). COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist 26, 402–414. doi:10.1177/1073858420941476
Kim, K.-D., Hwang, I., Ku, K. B., Lee, S., Kim, S.-J., and Kim, C. (2020). Progress and Challenges in the Development of COVID-19 Vaccines and Current Understanding of SARS-CoV-2- Specific Immune Responses. J. Microbiol. Biotechnol. 30, 1109–1115. doi:10.4014/jmb.2006.06006
Kwok, K. O., Li, K.-K., Wei, W. I., Tang, A., Wong, S. Y. S., and Lee, S. S. (2021). Influenza Vaccine Uptake, COVID-19 Vaccination Intention and Vaccine Hesitancy Among Nurses: A Survey. Int. J. Nurs. Stud. 114, 103854. doi:10.1016/j.ijnurstu.2020.103854
Lei, L., Guo, Y., Lin, J., Lin, X., He, S., Qin, Z., et al. (2021). Inhibition of Endotoxin-Induced Acute Lung Injury in Rats by Bone Marrow-Derived Mesenchymal Stem Cells: Role of Nrf2/HO-1 Signal axis in Inhibition of NLRP3 Activation. Biochem. Biophysical Res. Commun. 551, 7–13. doi:10.1016/j.bbrc.2021.03.009
Li, K., Li, Y., Ma, Z., and Zhao, J. (2015). Crocin Exerts Anti-inflammatory and Anti-catabolic Effects on Rat Intervertebral Discs by Suppressing the Activation of JNK. Int. J. Mol. Med. 36, 1291–1299. doi:10.3892/ijmm.2015.2359
Li, K., Wu, J., Wu, F., Guo, D., Chen, L., Fang, Z., et al. (2020). The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Invest. Radiol. 55, 327–331. doi:10.1097/RLI.0000000000000672
Li, X., and Ma, X. (2020). Acute Respiratory Failure in COVID-19: Is it "typical" ARDS?. Crit. Care 24, 198. doi:10.1186/s13054-020-02911-9
Lin, W.-C., Lin, C.-F., Chen, C.-L., Chen, C.-W., and Lin, Y.-S. (2011). Inhibition of Neutrophil Apoptosis via Sphingolipid Signaling in Acute Lung Injury. J. Pharmacol. Exp. Ther. 339, 45–53. doi:10.1124/jpet.111.181560
Liu, M., Gao, Y., Yuan, Y., Yang, K., Shi, S., Tian, J., et al. (2021). Efficacy and Safety of Herbal Medicine (Lianhuaqingwen) for Treating COVID-19: A Systematic Review and Meta-Analysis. Integr. Med. Res. 10, 100644. doi:10.1016/j.imr.2020.100644
Liu, P., Lu, Z., Liu, L., Li, R., Liang, Z., Shen, M., et al. (2019). NOD-like Receptor Signaling in Inflammation-Associated Cancers: From Functions to Targeted Therapies. Phytomedicine 64, 152925. doi:10.1016/j.phymed.2019.152925
Lu, G., and Wang, J. (2020). Dynamic Changes in Routine Blood Parameters of a Severe COVID-19 Case. Clinica Chim. Acta 508, 98–102. doi:10.1016/j.cca.2020.04.034
Meng, X., Deng, Y., Dai, Z., and Meng, Z. (2020). COVID-19 and Anosmia: A Review Based on Up-To-Date Knowledge. Am. J. Otolaryngol. 41, 102581. doi:10.1016/j.amjoto.2020.102581
Mihai, C., Dobrota, R., Schröder, M., Garaiman, A., Jordan, S., Becker, M. O., et al. (2020). COVID-19 in a Patient with Systemic Sclerosis Treated with Tocilizumab for SSc-ILD. Ann. Rheum. Dis. 79, 668–669. doi:10.1136/annrheumdis-2020-217442
Nishiga, M., Wang, D. W., Han, Y., Lewis, D. B., and Wu, J. C. (2020). COVID-19 and Cardiovascular Disease: from Basic Mechanisms to Clinical Perspectives. Nat. Rev. Cardiol. 17, 543–558. doi:10.1038/s41569-020-0413-9
Novelli, G., Biancolella, M., Mehrian-Shai, R., Erickson, C., Godri Pollitt, K. J., Vasiliou, V., et al. (2020). COVID-19 Update: the First 6 Months of the Pandemic. Hum. Genomics 14, 48. doi:10.1186/s40246-020-00298-w
Palazon, A., Goldrath, A. W., Nizet, V., and Johnson, R. S. (2014). HIF Transcription Factors, Inflammation, and Immunity. Immunity 41, 518–528. doi:10.1016/j.immuni.2014.09.008
Pan, F., Ye, T., Sun, P., Gui, S., Liang, B., Li, L., et al. (2020). Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 295, 715–721. doi:10.1148/radiol.2020200370
Ren, J.-l., Zhang, A.-H., and Wang, X.-J. (2020). Traditional Chinese Medicine for COVID-19 Treatment. Pharmacol. Res. 155, 104743. doi:10.1016/j.phrs.2020.104743
Shu, Z., Chang, K., Zhou, Y., Peng, C., Li, X., Cai, W., et al. (2021). Add-On Chinese Medicine for Coronavirus Disease 2019 (ACCORD): A Retrospective Cohort Study of Hospital Registries. Am. J. Chin. Med. 49, 543–575. doi:10.1142/S0192415X21500257
Simko, V., Iuliano, F., Sevcikova, A., Labudova, M., Barathova, M., Radvak, P., et al. (2017). Hypoxia Induces Cancer-Associated cAMP/PKA Signalling through HIF-Mediated Transcriptional Control of Adenylyl Cyclases VI and VII. Sci. Rep. 7, 10121. doi:10.1038/s41598-017-09549-8
Spinner, C. D., Gottlieb, R. L., Criner, G. J., Arribas López, J. R., Cattelan, A. M., Soriano Viladomiu, A., et al. (2020). Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19. JAMA 324, 1048–1057. doi:10.1001/jama.2020.16349
Stasi, C., Fallani, S., Voller, F., and Silvestri, C. (2020). Treatment for COVID-19: An Overview. Eur. J. Pharmacol. 889, 173644. doi:10.1016/j.ejphar.2020.173644
Torres Acosta, M. A., and Singer, B. D. (2020). Pathogenesis of COVID-19-Induced ARDS: Implications for an Ageing Population. Eur. Respir. J. 56, 2002049. doi:10.1183/13993003.02049-2020
Villapol, S. (2020). Gastrointestinal Symptoms Associated with COVID-19: Impact on the Gut Microbiome. Translational Res. 226, 57–69. doi:10.1016/j.trsl.2020.08.004
Wang, F., Nie, J., Wang, H., Zhao, Q., Xiong, Y., Deng, L., et al. (2020). Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 221, 1762–1769. doi:10.1093/infdis/jiaa150
Wang, H., Zhang, Y., Mo, P., Liu, J., Wang, H., Wang, F., et al. (2020). Neutrophil to CD4+ Lymphocyte Ratio as a Potential Biomarker in Predicting Virus Negative Conversion Time in COVID-19. Int. Immunopharmacology 85, 106683. doi:10.1016/j.intimp.2020.106683
Wang, J., Jiang, M., Chen, X., and Montaner, L. J. (2020). Cytokine Storm and Leukocyte Changes in Mild versus Severe SARS‐CoV‐2 Infection: Review of 3939 COVID‐19 Patients in China and Emerging Pathogenesis and Therapy Concepts. J. Leukoc. Biol. 108, 17–41. doi:10.1002/JLB.3COVR0520-272R
Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., et al. (2020). Remdesivir in Adults with Severe COVID-19: a Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. The Lancet 395, 1569–1578. doi:10.1016/S0140-6736(20)31022-9
Xiong, Y., Liu, Y., Cao, L., Wang, D., Guo, M., Jiang, A., et al. (2020). Transcriptomic Characteristics of Bronchoalveolar Lavage Fluid and Peripheral Blood Mononuclear Cells in COVID-19 Patients. Emerging Microbes & Infections 9, 761–770. doi:10.1080/22221751.2020.1747363
Xu, H., Zhong, L., Deng, J., Peng, J., Dan, H., Zeng, X., et al. (2020). High Expression of ACE2 Receptor of 2019-nCoV on the Epithelial Cells of Oral Mucosa. Int. J. Oral Sci. 12, 8. doi:10.1038/s41368-020-0074-x
Yang, L., Li, N., Hu, H. B., Yin, B., Zhao, G. J., Wang, F. C., et al. (2020). Can Yin-Chai-Xiao-Du Decoction Be Useful of COVID-19? the Mechanism Research Based on Network Pharmacology. Tradit Med. Res. 5, 188–200. doi:10.12032/TMR2020060118
Yang, L., Li, Y. T., Miao, J., Wang, L., Fu, H., Li, Q., et al. (2020). Network Pharmacology Studies on the Effect of Chai-Ling Decoction in Coronavirus Disease 2019. Tradit Med. Res. 5, 145–159. doi:10.12032/TMR20200324170
Yang, R., Liu, H., Bai, C., Wang, Y., Zhang, X., Guo, R., et al. (2020). Chemical Composition and Pharmacological Mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In Silico and Experimental Study. Pharmacol. Res. 157, 104820. doi:10.1016/j.phrs.2020.104820
Zhang, J., Tecson, K. M., and McCullough, P. A. (2020). Endothelial Dysfunction Contributes to COVID-19-Associated Vascular Inflammation and Coagulopathy. Rev. Cardiovasc. Med. 21, 315–319. doi:10.31083/j.rcm.2020.03.126
Zhang, Q., Lu, S., Li, T., Yu, L., Zhang, Y., Zeng, H., et al. (2019). ACE2 Inhibits Breast Cancer Angiogenesis via Suppressing the VEGFa/VEGFR2/ERK Pathway. J. Exp. Clin. Cancer Res. 38, 173. doi:10.1186/s13046-019-1156-5
Zhang, X.-Y., Huang, H.-J., Zhuang, D.-L., Nasser, M. I., Yang, M.-H., Zhu, P., et al. (2020). Biological, Clinical and Epidemiological Features of COVID-19, SARS and MERS and AutoDock Simulation of ACE2. Infect. Dis. Poverty 9, 99. doi:10.1186/s40249-020-00691-6
Zhang, X., Xue, Y., Chen, X., Wu, J.-m., Su, Z.-j., Sun, M., et al. (2021). Effects of Tanreqing Capsule on the Negative Conversion Time of Nucleic Acid in Patients with COVID-19: A Retrospective Cohort Study. J. Integr. Med. 19, 36–41. doi:10.1016/j.joim.2020.10.002
Zhu, F.-C., Li, Y.-H., Guan, X.-H., Hou, L.-H., Wang, W.-J., Li, J.-X., et al. (2020). Safety, Tolerability, and Immunogenicity of a Recombinant Adenovirus Type-5 Vectored COVID-19 Vaccine: a Dose-Escalation, Open-Label, Non-randomised, First-In-Human Trial. The Lancet 395, 1845–1854. doi:10.1016/S0140-6736(20)31208-3
Keywords: coronavirus disease 2019, acute respiratory distress syndrome, he-jie-shen-shi decoction, retrospective cohort study, network pharmacology
Citation: Hu H, Wang K, Wang L, Du Y, Chen J, Li Y, Fan C, Li N, Sun Y, Tu S, Lu X, Zhou Z and Cui H (2021) He-Jie-Shen-Shi Decoction as an Adjuvant Therapy on Severe Coronavirus Disease 2019: A Retrospective Cohort and Potential Mechanistic Study. Front. Pharmacol. 12:700498. doi: 10.3389/fphar.2021.700498
Received: 26 April 2021; Accepted: 09 June 2021;
Published: 18 June 2021.
Edited by:
Jen-Tsung Chen, National University of Kaohsiung, TaiwanReviewed by:
Chan Kam Wa, The University of Hong Kong, Hong KongCopyright © 2021 Hu, Wang, Wang, Du, Chen, Li, Fan, Li, Sun, Tu, Lu, Zhou and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuechao Lu, aG9zcGl0YWxicmVhdGhpbmdAMTYzLmNvbQ==; Zhaoshan Zhou, emhvdXpoYW9zaGFuMjAyMUAxNjMuY29t; Huantian Cui, MTc2MjMxNjQxMUBxcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.