- 1Department of Urology Surgery, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 2School of Graduate Studies, Shandong Academy of Medical Sciences, Shandong First Medical University, Jinan, China
- 3Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 4Department of Medical Imaging, The First People’s Hospital of Pingdu, Qingdao, China
- 5Department of Radiation Oncology, The Fourth People’s Hospital of Jinan, Jinan, China
- 6Department of Radiation Oncology, Qilu Hospital of Shandong University, Jinan, China
- 7Shandong University, Jinan, China
Background/Aims: Hodgkin Lymphoma (HL) has become one of the most treatable cancers, with more than 80% patients in the advanced stage being cured through improvement of therapeutic regimens. Nevertheless, some treatments were accompanied with toxicities.
Methods: In the current study, a network meta-analysis (NMA) was conducted to compare the efficacies and toxicities of different chemotherapy regimens for advanced Hodgkin lymphoma (HL). We reviewed PubMed and EMBASE databases from inception to May 2018, and identified randomized controlled trials (RCTs) in which advanced HL patients received chemotherapy. Fourteen eligible RCTs published between 1992 and 2017 were enrolled in this NMA. These studies included a total of 5,964 HL patients, and assessed at least one of seven different chemotherapy regimens. Direct and indirect evidence was combined to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs), and to establish a surface under the cumulative ranking (SUCRA) curve.
Results: A cluster analysis was performed to evaluate efficacies and toxicities of different regimens. The COPP + ABVD (cyclophosphamide + vincristine + procarbazine + prednisone + doxorubicin + bleomycin + vinblastine + dacarbazine) regimen had the highest SUCRA partial response and overall remission rate values, while the ABVD regimen resulted in the lowest incidences of anemia, thrombocytopenia, neutropenia, and leucopenia.
Conclusion: Cluster analysis revealed that COPP + ABVD had the best efficacy against advanced HL among the seven regimens, and ABVD had the lowest toxicity.
Introduction
Hodgkin lymphoma (HL) is a malignancy of the lymphatic system with characteristics that the presence of Reed-Sternberg cells, although these cells typically account for <1% of cells in the affected tissue (Yung and Linch, 2003). The disease is more common in men than women, and peaks in incidence in young adults and in those whose age was older than 60 years (Townsend and Linch, 2012). HL is rare among children and relatively rare in middle-aged adult, but is the most commonly diagnosed cancer among adolescents in the age range 15–19 (Punnett et al., 2010). Five-year survival for HL patients during the 2000–2004 periods was 85.2% (Shenoy et al., 2011).
Advanced stage HL is usually treated with chemotherapy and radiotherapy (Eichenauer et al., 2011). The MOPP (mechlorethamine + vincristine + procarbazine + prednisone) chemotherapy regimen was developed to treat patients with advanced HL following radiation (Ansell, 2016; Vassilakopoulos and Johnson, 2016), and results in a long-term progression-free survival (PFS) rate of 54% and an overall survival (OS) rate of 48% (Ansell, 2016). The combination of doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) is currently the standard of treatment for HL around the world (Chisesi et al., 2011; Corazzelli et al., 2011). ABVD was considered as a better option for HL therapy when compared to non-cross-resistant alternating regimens (MOPP + ABV [hybrid]: mechlorethamine + vincristine + procarbazine + prednisone + doxorubicin + bleomycin + vinblastine, as it was associated with a higher risk of secondary malignancy (Souza et al., 2009). However, recent findings suggest that the BEACOPP (bleomycin + etoposide + doxorubicin + cyclophosphamide + vincristine + procarbazine + prednisone) regimen is more effective in controlling advanced HL than ABVD (Corazzelli et al., 2011). The dose-dense Stanford V (doxorubicin + vinblastine + mechlorethamine + vincristine + bleomycin + etoposide + prednisone) regimen was developed in the early 1990s, but ultimately failed to improve upon ABVD outcomes (Chisesi et al., 2011; Vassilakopoulos and Johnson, 2016). The German Hodgkin Study Group (GHSG) developed the COPP + ABVD regimen (cyclophosphamide + vincristine + procarbazine + prednisone + doxorubicin + bleomycin + vinblastine + dacarbazine) to improve advanced HL patient outcomes (Vassilakopoulos and Johnson, 2016).
While many chemotherapy regimens are used for treatment of advanced HL, the optimal regimen has not yet been identified. We conducted a systematic review including bothtraditional and network meta-analyses (NMA) to more precisely evaluate the efficacies and toxicities of various HL chemotherapy regimens. And these regimens we choosed which are commonly used in developing countries now and so this results would be of most use to them.
Materials and Methods
Study Retrieval Strategy
The PubMed and EMBASE electronic databases were comprehensively searched from inception to May 2018. The search strategy was combined key words and free words, including the following search terms: “lymphoma,” “chemotherapy regimen,” “doxorubicin,” “bleomycin,” “vinblastine,” “dacarbazine,” “etoposide,” “cyclophosphamide,” “vincristine,” “procarbazine,” “prednisone,” “mechlorethamine,” and “randomized controlled trial” (RCT). We also conducted a manual search to identify additional relevant references (Supplementary Material).
Inclusion and Exclusion Criteria
Studies inclusion criteria were as follows: 1) RCTs; 2) different interventions for treating HL were included in this study such as ABVD: combination of doxorubicin, bleomycin, vinblastine and dacarbazine; BEACOPP: an intensified regimen consisting of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; Stanford V: doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide and prednisone; COPP + ABVD: cyclophosphamide, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine and dacarbazine; MOPP: mechlorethamine, vincristine, procarbazine and prednisone; MOPP + ABV (Hybrid): mechlorethamine, vincristine, procarbazine, prednisone, doxorubicin, bleomycin and vinblastine; MOPP + ABVD (Alternating): mechlorethamine, vincristine, procarbazine and prednisone followed by doxorubicin, bleomycin, vinblastine and dacarbazine; 3) advanced HL patients (untreated advanced HL) were diagnosed by histopathological examination, and aged 16–83 years; 4) outcomes that including CR, PR, ORR, OS, anemia, thrombocytopenia, neutropenia, leukopenia and nausea/vomiting were described; and 5) study was published in English. Exclusion criteria were: 1) patients with severe heart or lung diseases and metabolic diseases; 2) pregnant or lactating patients; 3) patients who received prior radiotherapy or chemotherapy; 4) nodular lymphocyte predominant HL; 5) patients with hepatic or renal dysfunction; 6) studies with insufficient data, such as non-paired studies; 7) non-RCT studies; 8) duplicated publications; 9) meeting reports, systematic reviews (meta-analyses), or summaries and (10) non-English literature, 11) Studies containing targeted protocols.
Data Extraction and Quality Assessment
Data were collected from enrolled studies by two researchers independently using a unified data collection form. Any disagreements in the data extraction process were resolved by discussion. Two researchers evaluated method quality for each RCT according to the Cochrane Collaboration’s tool for assessing bias risk (Higgins et al., 2011). This tool comprises random method, allocation concealment, blinding, attrition, selective reporting and other bias. The assessment designates a value of ‘‘low,’’ ‘‘high,’’ or ‘‘unclear’’ risk of bias by assigning a judgment of “yes,” “no,” or “unclear,” respectively, for each domain. The number of domains deemed “unclear” or “no” is calculated and each study is classified as follows: 1) 0–1 domains, low bias risk; 2) ≥4 domains, high bias risk; 3) 2–3 domains, moderate bias risk (Chung and Lee, 2013). Quality assessments and investigation of publication bias were performed using Review Manager 5 (RevMan 5.2.3, Cochrane Collaboration, Oxford, United Kingdom).
Statistical Analysis
Direct comparisons between different treatment arms were made using a traditional pairwise meta-analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to combine intervention efficacy estimates. Study heterogeneity was examined using Chi-square and I-square tests (Chen et al., 2015). Results were presented as a network plot using R version 3.2.2, with each node indicating an intervention, node sizes representing sample sizes, and the thickness of lines connecting any two nodes representing the number of included studies. Comparisons between different interventions were made using Bayesian NMA. According to non-informative priors, effect sizes and precision were specified in each analysis. After four chains and a 20,000-simulation burn-in phase, convergence and lack of auto-correlation were explored and verified. Direct probability statements were concluded in an additional 50,000-simulation phase (Tu et al., 2012). The node-splitting method was used to select a consistency or inconsistency model, by evaluating the consistency between direct and indirect evidence (Zhu et al., 2015). For the interpretation of ORs, the probability of each intervention being the most effective or safest treatment was calculated by using a Bayesian approach, with probability values estimated by the surface under the cumulative ranking (SUCRA) curve and the rank of each intervention (Salanti et al., 2011; Chaimani et al., 2013). Cluster analysis was used to group the short-term efficacies and toxicities of regimens according to their similarity (Chaimani et al., 2013). R v3.2.1 package gemtc (V.0.6) with the Markov Chain Monte Carlo engine Open BUGS (V.3.4.0) were used for all calculations in this study.
Results
Baseline Characteristics of Included Studies
A total of 1,088 articles studying HL cases treated with at least one of seven chemotherapy regimens were initially identified from electronic databases. We excluded two duplicate studies, 17 letters or reviews, 4 non-human studies, and 109 non-English articles. The remaining 965 studies were evaluated according to the full text. We further excluded 378 non-RCT studies, 377 unrelated to HL, 185 unrelated to chemotherapy, 1 with duplicated contents and 1 due to unavailable or missing data. In total, 14 RCT studies met our meta-analysis inclusion criteria. These studies evaluated seven chemotherapy regimens, including ABVD, BEACOPP, Stanford V, MOPP, COPP + ABVD, MOPP + ABV (hybrid), and MOPP + ABVD (alternating) (Canellos et al., 1992; Somers et al., 1994; Connors et al., 1997; Glick et al., 1998; Diehl et al., 2003; Duggan et al., 2003; Ballova et al., 2005; Gobbi et al., 2005; Federico et al., 2009; Hoskin et al., 2009; Viviani et al., 2011; Gordon et al., 2013; Mounier et al., 2014; Carde et al., 2016) (Supplementary Figure S1). They included 5,964 total Caucasian patients with HL, most of whom received the ABVD regimen. Included RCTs were published between 1992 and 2016. The enrolled studies included 14 study objects from European and American, 13 two-arm trials and 1 was a three-arm trail. Characteristics of included studies are shown in Supplementary Table S1 and the bias assessment with Cochrane Collaboration’s tool is presented in Figure 1. In the assessment of blinding, some studies were considered has having an unclear bias for missing the discussion over the blinding-related results, some had a high risk of bias for the incompleteness of blinding, and there was an unclear risk of other bias as they did not describe patients lost to follow-up. In selective reporting, some studies were characterized as unclear risk as they did not give the explicit description about whether pre-specified outcomes had been shown.
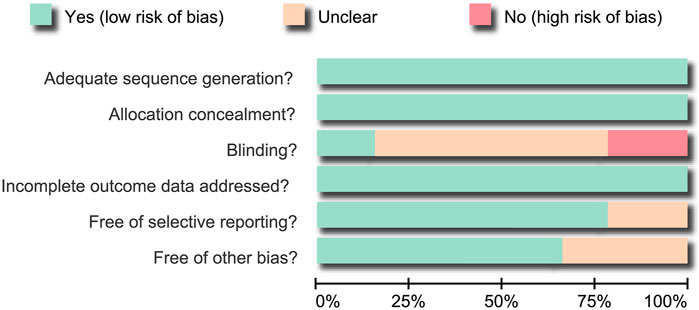
FIGURE 1. Bias risk assessment using the Cochrane Collaboration’s tool. Fourteen eligible randomized controlled trials were analyzed in this NMA.
Pairwise Meta-Analysis to Assess Efficacies and Toxicities of the Seven Chemotherapy Regimens
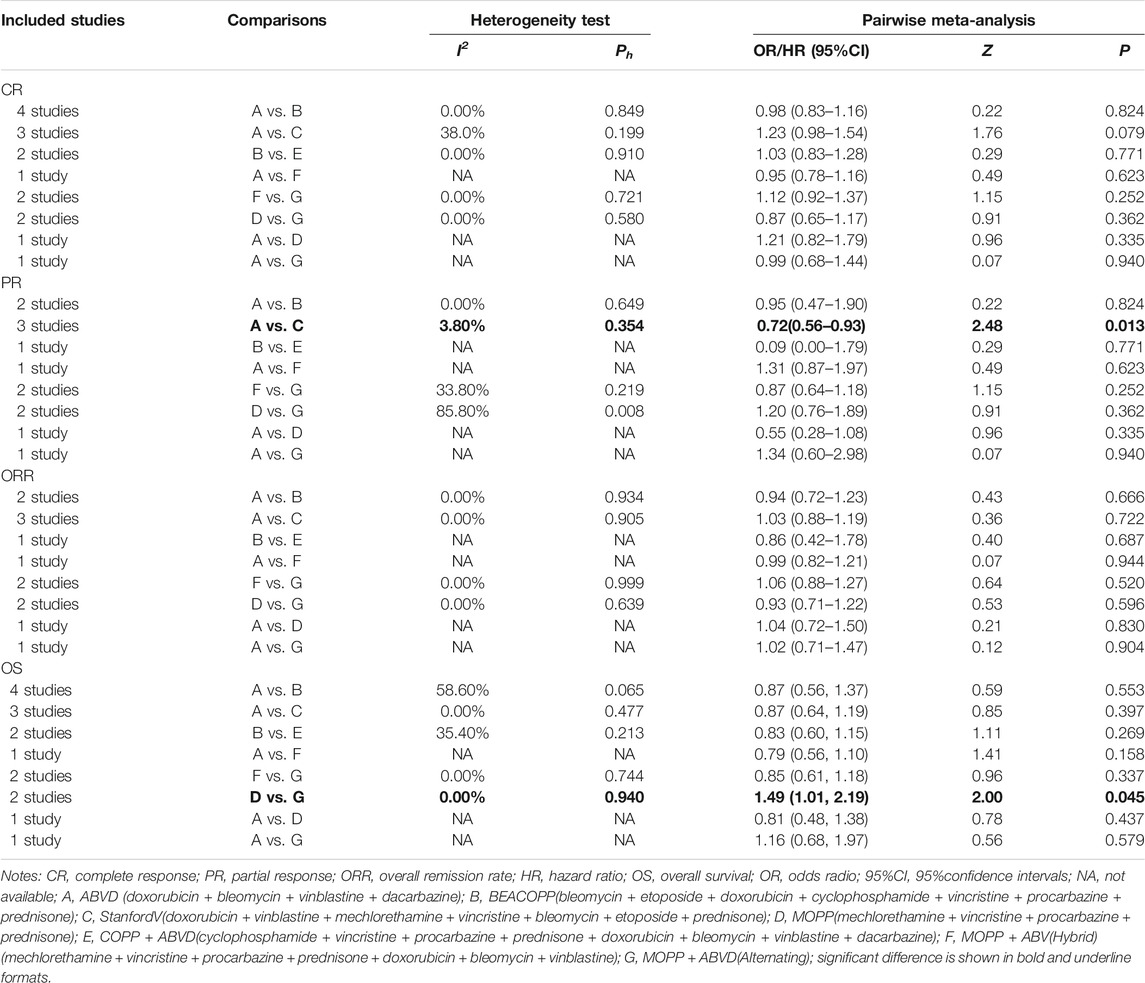
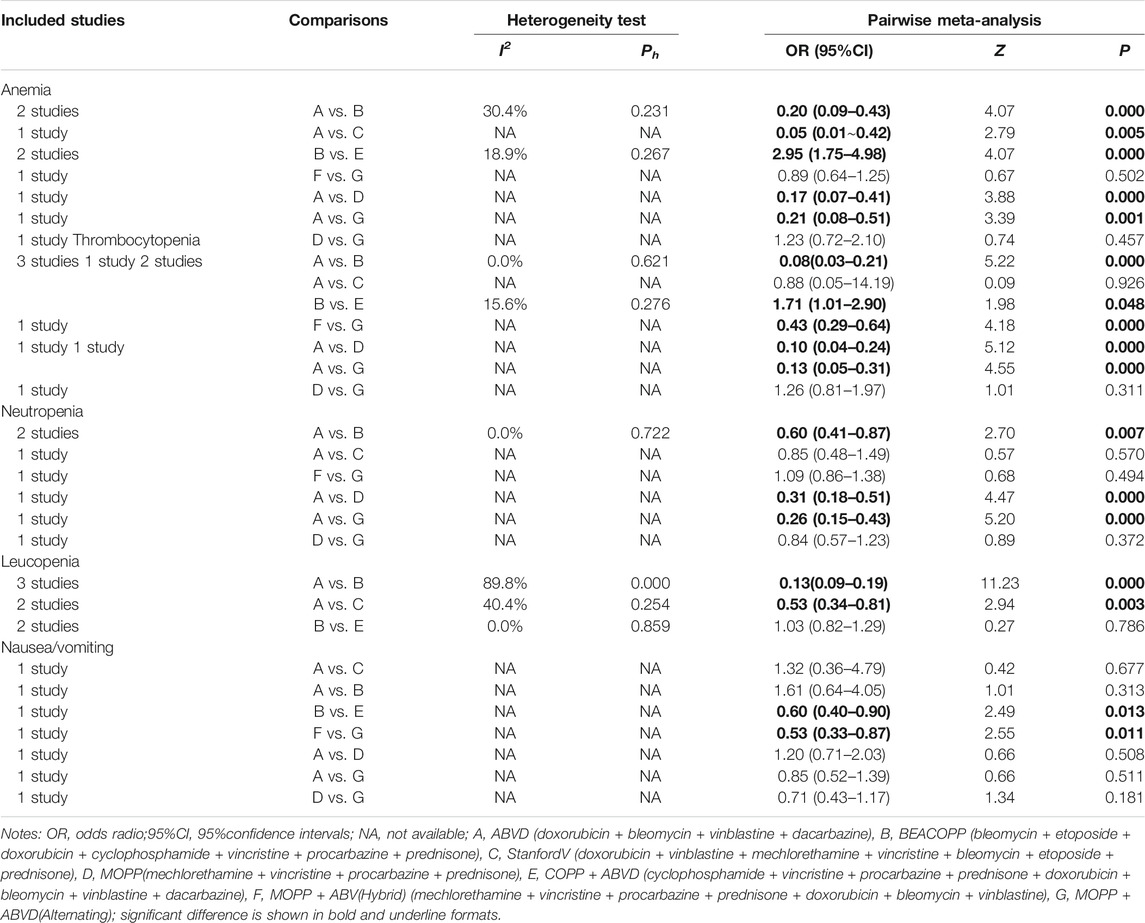
Direct pairwise comparisons of efficacies and toxicities of the seven chemotherapy regimens have carried out. As shown in Table 1, in terms of the short-term efficacy, patients treated with the Stanford V regimen had relatively higher PR rates in the short-term (OR = 0.72, 95%CI = 0.56–0.93) than those treated with the ABVD regimen; about the long-term efficacy, MOPP had lower overall survival (HR = 1.49, 95%CI = 1.01–2.19) compared to patients treated with MOPP + ABVD. As seen in Table 2, incidences of anemia, thrombocytopenia, neutropenia, and leucopenia were higher in BEACOPP-treated patients (anemia: OR = 0.20, 95%CI = 0.09–0.43; thrombocytopenia: OR = 0.08, 95%CI = 0.03–0.21; neutropenia: OR = 0.60, 95%CI = 0.41–0.87; Leucopenia: OR = 0.13, 95%CI = 0.09–0.19) than those treated with the ABVD regimen. The MOPP (anemia: OR = 0.17, 95%CI = 0.07–0.41; thrombocytopenia: OR = 0.10, 95%CI = 0.04–0.24; neutropenia: OR = 0.31, 95%CI = 0.18–0.51) and MOPP + ABVD regimens (anemia: OR = 0.21, 95%CI = 0.08–0.51; thrombocytopenia: OR = 0.13, 95%CI = 0.05–0.31; neutropenia: OR = 0.26, 95%CI = 0.15–0.43) exhibited higher incidences of anemia, thrombocytopenia, and neutropenia, while the Stanford V regimen resulted in higher incidences of anemia and leucopenia (anemia: OR = 0.05, 95%CI = 0.01–0.42; leucopenia: OR = 0.53, 95%CI = 0.34–0.81).
Network Evidence of Seven Chemotherapy Regimens in the Treatment of HL
This NMA included seven chemotherapy regimens, including, ABVD, BEACOPP, Stanford V, COPP + ABVD, MOPP, MOPP + ABV (Hybrid) and MOPP + ABVD (Alternating). It could be observed in Figure 2 that at the aspect of chemotherapy efficacy, as CR and OS, the majority of HL patients received the ABVD regimen followed by MOPP + ABVD regimen and there were more studies focusing on ABVD vs BEACOPP and ABVD vs Stanford V. As shown in Figure 3, at the aspect of toxicity, as anemia, thrombocytopenia, leucopenia and nausea/vomiting, BEACOPP regimen was selected by most of the HL patients; for neutropenia, MOPP + ABVD regimen was selected by the great majority of HL patients and there were more studies focusing on ABVD vs BEACOPP.
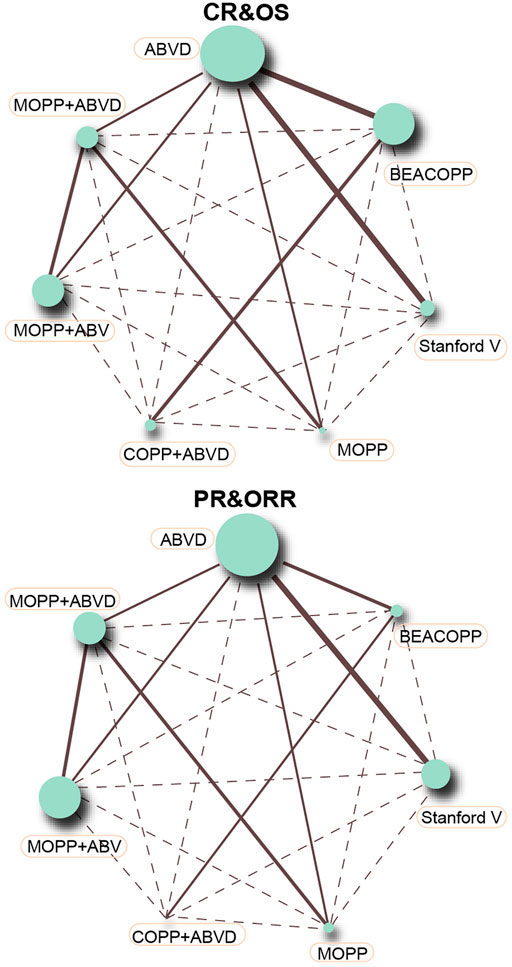
FIGURE 2. CR, PR, ORR, and OS in advanced HL patients treated with different chemotherapy regimens (each node represented an intervention, node sizes implicated sample sizes, and the thickness of lines connecting any two nodes signified the number of included studies).
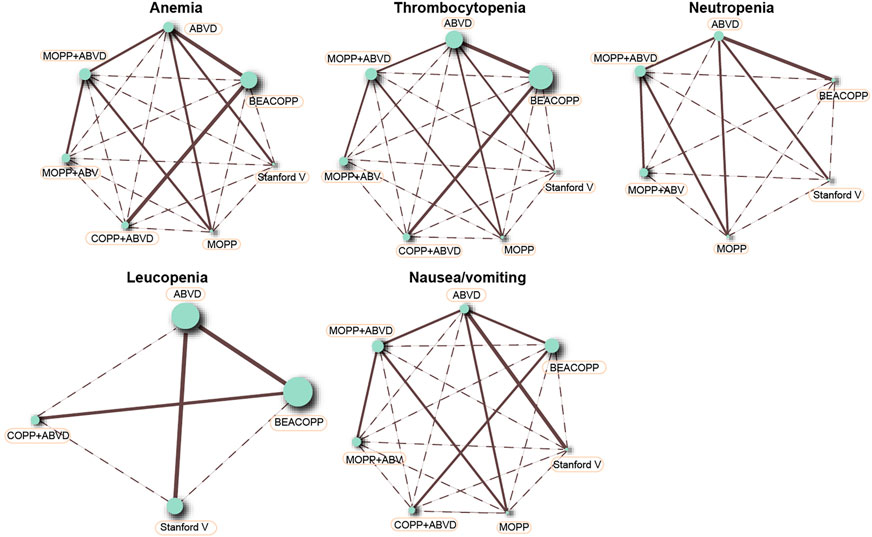
FIGURE 3. Anemia, thrombocytopenia, neutropenia, leucopenia, and nausea/vomiting in advanced HL patients treated with different chemotherapy regimens.
Inconsistency Tests of CR, PR, ORR, and OS Among all Included Studies
The inconsistency tests of CR, PR, ORR, and OS were performed using the node-splitting method. Consistency was shown in direct and indirect evidence of all outcomes, and thus the consistency model was adopted (all p > 0.05) (Figure 4).
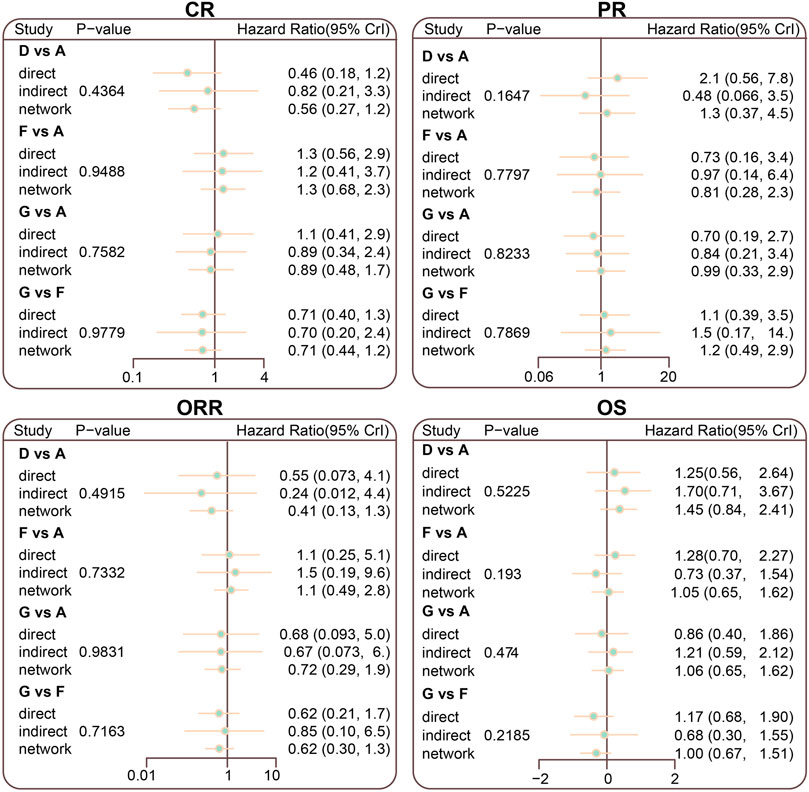
FIGURE 4. Node splitting diagram for CR, PR, ORR, and OS in advanced HL patients treated with different chemotherapy regimens; A = ABVD (doxorubicin + bleomycin + vinblastine + dacarbazine); B = BEACOPP (bleomycin + etoposide + doxorubicin + cyclophosphamide + vincristine + procarbazine + prednisone); C = StanfordV (doxorubicin + vinblastine + mechlorethamine + vincristine + bleomycin + etoposide + prednisone); D = MOPP (mechlorethamine + vincristine + procarbazine + prednisone);E = COPP + ABVD (cyclophosphamide + vincristine + procarbazine + prednisone + doxorubicin + bleomycin + vinblastine + dacarbazine); F = MOPP + ABV (Hybrid) (mechlorethamine + vincristine + procarbazine + prednisone + doxorubicin + bleomycin + vinblastine); G = MOPP + ABVD (Alternating).
Main Results of Network Meta-analyses
The Stanford V regimen resulted in lower CR rates than the ABVD regimen (OR = 0.61, 95% CI = 0.38–0.92). The BEACOPP and Stanford V regimens had higher anemia incidences than the ABVD regimen (OR = 7.13, 95% C I = 1.12–54.77; OR = 31.87, 95% CI = 1.44–1548.37, respectively). Thrombocytopenia occurred more frequently in BEACOPP- and MOPP-treated HL patients than those treated with the ABVD regimen (OR = 17.54, 95% CI = 3.49–136.87; OR = 21.38, 95% CI = 1.53–333.04, respectively). Leucopenia occurred more frequently in BEACOPP-treated patients than ABVD-treated patients (OR = 23.00, 95% CI = 2.70–1.9e+02) (Figure 5; Table 3; Supplementary Table S2).
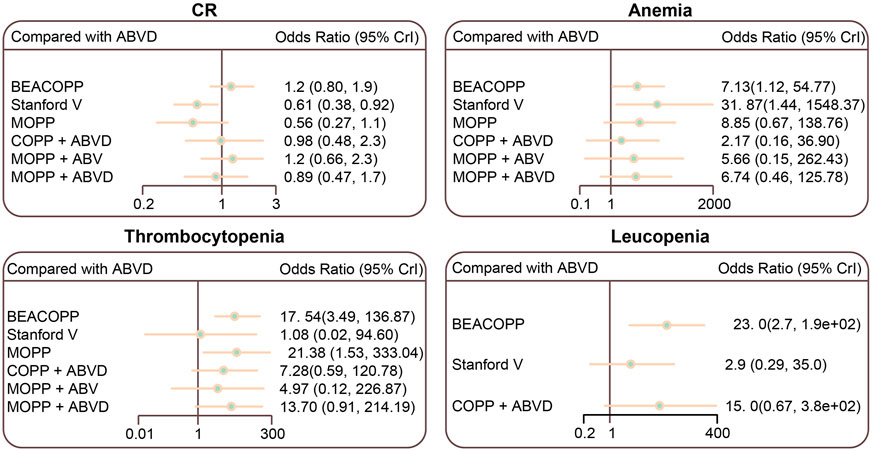
FIGURE 5. Relative relationship forest plots for CR, anemia, thrombocytopenia, and leucopenia in advanced HL patients treated with different chemotherapy regimens. ABVD (doxorubicin + bleomycin + vinblastine + dacarbazine); BEACOPP (bleomycin + etoposide + doxorubicin + cyclophosphamide + vincristine + procarbazine + prednisone); Stanford V (doxorubicin + vinblastine + mechlorethamine + vincristine + bleomycin + etoposide + prednisone); MOPP (mechlorethamine + vincristine + procarbazine + prednisone); COPP (cyclophosphamide + vincristine + procarbazine + prednisone); ABV (Hybrid) (doxorubicin + bleomycin + vinblastine).
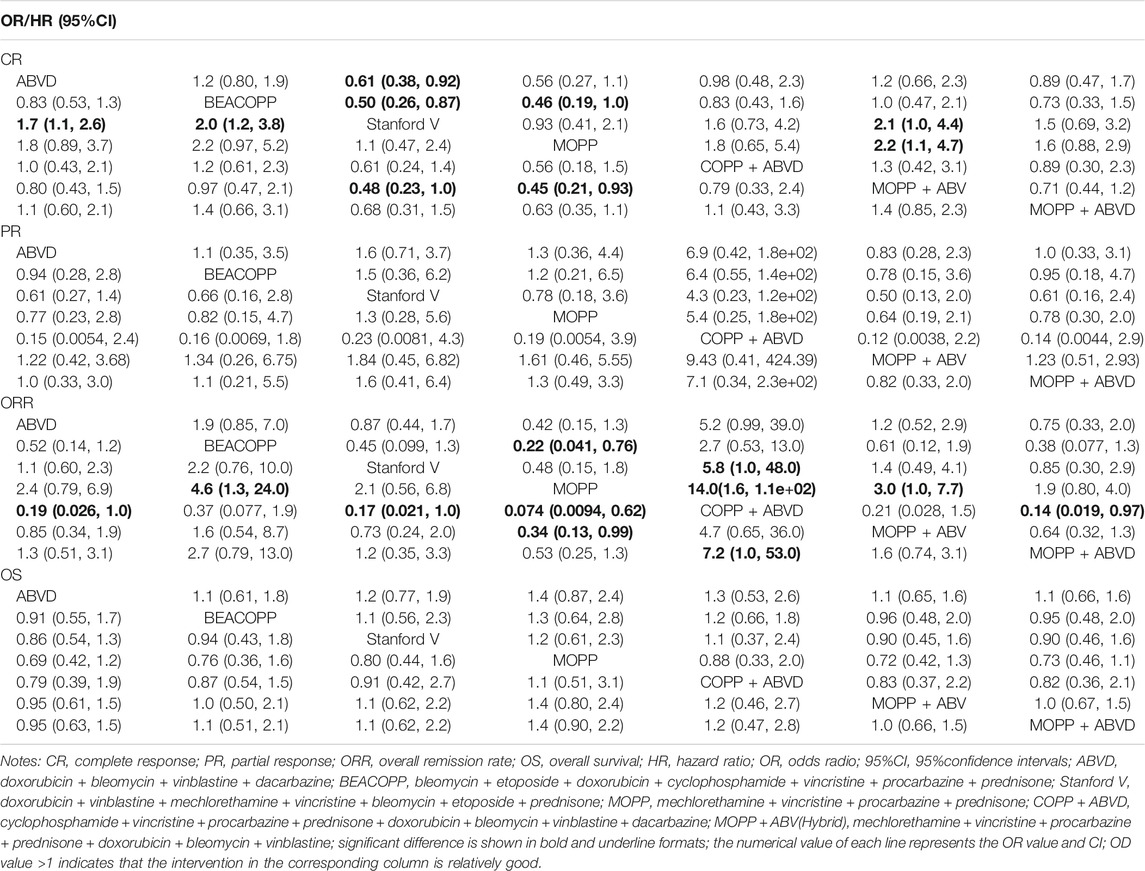
TABLE 3. OR (95%CI) of seven treatment modalities of three endpoints for efficacy in Hodgkin lymphoma.
SUCRA Curves of Chemotherapy Efficacies and Toxicities
The result of SUCRA curve was shown in Table 4, the MOPP + ABV regimen had the highest SUCRA CR value (85.30%), and COPP + ABVD had the highest SUCRA PR (93.47%) and ORR (95.20%) values. The MOPP regimen had the highest SUCRA OS value (74.26%). The ABVD regimen had the highest SUCRA value of anemia (93.81%), thrombocytopenia (90.42%), neutropenia (90.21%) and leucopenia (95.63%), indicating that ABVD has the lowest incidence of anemia, thrombocytopenia, neutropenia and leucopenia. The BEACOPP regimen had the highest SUCRA value of nausea/vomiting (78.03%), indicating that BEACOPP had lowest nausea/vomiting.
SUCRA Value Cluster Analyses
The results of SUCRA value cluster analyses are shown in Figure 6. In chemotherapy efficacy (CR, PR, ORR, and OS), the COPP + ABVD regimen was most effective among the seven regimens. In toxicity (anemia, neutropenia and thrombocytopenia), BEACOPP, MOPP, and MOPP + ABVD had higher toxicities than the other regimens, and ABVD was less toxic.
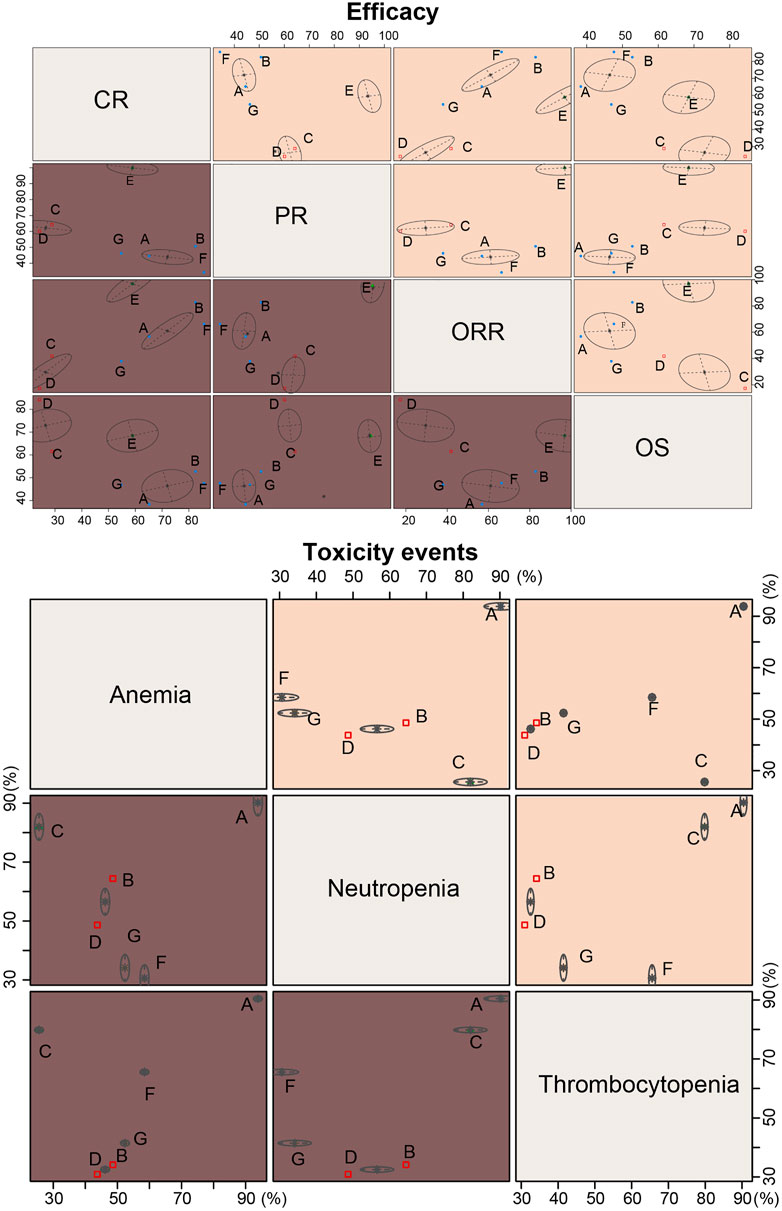
FIGURE 6. Cluster ranking plots based on SUCRA efficacy and toxicity values of seven chemotherapy regimens for HL. A = ABVD (doxorubicin + bleomycin + vinblastine + dacarbazine); B = BEACOPP (bleomycin + etoposide + doxorubicin + cyclophosphamide + vincristine + procarbazine + prednisone); C = Stanford V (doxorubicin + vinblastine + mechlorethamine + vincristine + bleomycin + etoposide + prednisone); D = MOPP (mechlorethamine + vincristine + procarbazine + prednisone);E = COPP + ABVD (cyclophosphamide + vincristine + procarbazine + prednisone + doxorubicin + bleomycin + vinblastine + dacarbazine); F = MOPP + ABV (Hybrid) (mechlorethamine + vincristine + procarbazine + prednisone + doxorubicin + bleomycin + vinblastine); G = MOPP + ABVD (Alternating).
Discussion
Our NMA compared the outcomes of seven chemotherapy regimens across 14 RCTs involving 5,964 HL patients. Our results demonstrated that, compared with other regimens, COPP + ABVD produced the best outcomes, with high SUCRA PR and ORR values. However, the ABVD regimen exhibited the lowest toxicity rates. ABVD is the most widely used regimen for advanced HL patients (Corazzelli et al., 2011). After the MOPP regimen, ABVD was superior to or less toxic than non-cross-resistant alternating regimens (MOPP + ABVD and MOPP + ABV), multidrug regimens (MOPP) and chemoradiotherapy (Stanford V) (Ansell, 2016; Carde et al., 2016). Thus, ABVD is still the standard HL treatment, with a good balance of efficacy and toxicity (Chisesi et al., 2011).
Our results indicated that the MOPP + ABV regimen had the highest SUCRA CR value. At the time the current trial was planned, randomized trials comparing MOPP + ABV (hybrid) treatment with both MOPP to ABVD (sequential) and MOPP + ABVD (alternating) therapy had recently begun. Some trials demonstrated that same effectiveness between the MOPP + ABV (hybrid) and MOPP_+ ABVD (alternating) which are more effective than sequential MOPP to ABVD treatment (Santoro et al., 1987; Connors et al., 1997). Additionally, MOPP + ABV have relationship with greater incidences of acute toxicity, MDS, and leukemia (Duggan et al., 2003).
Besides, our study also showed that the BEACOPP regimen had the lowest incidence of nausea/vomiting in the seven chemotherapy regimens. As the larger the SUCRA value was, the better the rank of the intervention was, BEACOPP was the best regimen for the outcome-nausea/vomiting. Nausea/vomiting were bad outcomes, so the lower incidence they had, the better the regimen was. As an alternative to escalated BEACOPP therapy, patients who have not experienced CR with more standard ABVD chemotherapy can receive an autologous stem cell transplant (Majhail et al., 2006). Our NMA indicated that the BEACOPP, MOPP, and MOPP + ABVD regimens exhibited higher toxicities than the other regimens. Toxicities related to BEACOPP included hematological toxicity and infection, and longer-term risks which including infertility, stem cell injury, and leukemia (Ansell, 2016). The escalated BEACOPP regimen has been proved that it can lead to more haematological toxicities WHO grade III or IV (Skoetz et al., 2017). Six cycles of escalated BEACOPP regimen significantly improves OS compared with ABVD and other regimens (Skoetz et al., 2013). The point of our study is the efficacy between different drugs rather than different doses of the same drug. Besides, the outcome of our article is various, focusing not only on the efficacy but also on the safety.
Furthermore, we used a Bayesian network model to assess direct and indirect evidence inconsistency via the node-splitting method. This method allowed us to eliminate potential errors of the NMA and further compare the seven interventions (Yan et al., 2015). In comparison with the Skoetz N et al. Lancet 2013 study, our study has made some improvements. First, Skoetz N et al. Lancet 2013 study focused on overall survival but our study involved overall survival, complete response, partial response, overall remission rate, anemia, thrombocytopenia, neutropenia, leukopenia and nausea/vomiting. Second, in Skoetz N et al. Lancet 2013 study, compared with overall survival for ABVD, overall survival for each regimen was not differed as presented in their Figure 3. But in our study, we supplemented SUCRA values. From the SUCRA values, we could infer that people receive MOPP regimen have longer term overall survival, followed by COPP + ABVD. Third, we adopted cluster analysis of SUCRA values to find the best regimen from all outcomes and we illustrated that COPP + ABVD was considered as the best regimen based on comprehensive analysis. Last, in our study, SUCRA values were concluded from comparisons among all regimens and the largest SUCRA values indicated the best regimen. While SUCRA values were not mentioned in Skoetz N et al. Lancet 2013 study, so the best regimen could not be suggested. Nevertheless, our study had several limitations. First, the number of included studies was relatively small, and there was no cross-research comparison. Second, patient cohort sizes differed between the seven chemotherapy regimens, which may have biased our NMA results and reduced the accuracy of our findings (Jansen, 2012; Saramago et al., 2012). Third, we could not statistically analyze PFS indicators, since only 4/14 included studies provided PFS indicator information. Forth, nausea/vomiting were excluded from the toxicity analysis because comparisons among different regimens about this outcome were not statistically different. Further toxicity analysis about nausea/vomiting need to be investigated in the future. Finally, treatment-related toxicity, especially pulmonary and cardiac toxicity and infection (Diehl et al., 2003), is more common in older patients. Only 4/14 studies mentioned pulmonary toxicity due to bleomycin treatment, and only 3/14 discussed treatment related mortality rates, which limited our ability to assess treatment-related toxicity.
In summary, we found that the COPP + ABVD regimen had the best efficacy in HL patients, and ABVD with the lowest toxicity. At present, PET-CT is mostly used as the main way to evaluate CR, while our study used CT as the main way to evaluate CR about the included literature, which might affect our final result. Additionally, statistical analysis of PFS indicators cannot be carried out since only 4 of the 14 literatures had PFS indicators. Furthermore, treatment-related toxicity played a vital role among old patients, especially the risk of pulmonary cardiac, toxicity and infection, while only 4 enrolled studies referred to bleomycin due to pulmonary toxicity and others did not mentioned for statistical analysis; only three enrolled studies referred to treatment related mortality rate and others did not mention for statistical analysis. While our study included a large total number of patients, and our results were in agreement with other groups’ findings, our conclusions must be confirmed by additional studies with large sample sizes and broader multivariate analyses.
Author Contributions
FP and YY: Literature retrieval, data extraction, and article writing. BD: Literature retrieval, data extraction, and literature quality evaluation. YY and HG: Data verification and literature quality evaluation. HG: Statistical analysis. XD: Study design and quality supervision. FZ: Study design, statistical analysis, and manuscript modification. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by grants from the Shandong Provincial Natural Science Foundation (Grant nos. ZR2019LZL007 and ZR2017BH117).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to reviewers for critical comments to the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.694545/full#supplementary-material
Supplementary Figure S1 | Flow chart showing literature search and study selection. Fifteen randomized controlled trials met the inclusion criteria and were included in this meta-analysis.
References
Ansell, S. M. (2016). Hodgkin Lymphoma: MOPP Chemotherapy to PD-1 Blockade and beyond. Am. J. Hematol. 91, 109–112. doi:10.1002/ajh.24226
Ballova, V., Rüffer, J. U., Haverkamp, H., Pfistner, B., Müller-Hermelink, H. K., Dühmke, E., et al. (2005). A Prospectively Randomized Trial Carried Out by the German Hodgkin Study Group (GHSG) for Elderly Patients with Advanced Hodgkin's Disease Comparing BEACOPP Baseline and COPP-ABVD (Study HD9elderly). Ann. Oncol. 16, 124–131. doi:10.1093/annonc/mdi023
Canellos, G. P., Anderson, J. R., Propert, K. J., Nissen, N., Cooper, M. R., Henderson, E. S., et al. (1992). Chemotherapy of Advanced Hodgkin's Disease with MOPP, ABVD, or MOPP Alternating with ABVD. N. Engl. J. Med. 327, 1478–1484. doi:10.1056/NEJM199211193272102
Carde, P., Karrasch, M., Fortpied, C., Brice, P., Khaled, H., Casasnovas, O., et al. (2016). Eight Cycles of ABVD versus Four Cycles of BEACOPPescalated Plus Four Cycles of BEACOPPbaseline in Stage III to IV, International Prognostic Score ≥ 3, High-Risk Hodgkin Lymphoma: First Results of the Phase III EORTC 20012 Intergroup Trial. J. Clin. Oncol. 34, 2028–2036. doi:10.1200/JCO.2015.64.5648
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical Tools for Network Meta-Analysis in STATA. PLoS One 8, e76654. doi:10.1371/journal.pone.0076654
Chen, L. X., Li, Y. L., Ning, G. Z., Li, Y., Wu, Q. L., Guo, J. X., et al. (2015). Comparative Efficacy and Tolerability of Three Treatments in Old People with Osteoporotic Vertebral Compression Fracture: a Network Meta-Analysis and Systematic Review. PLoS One 10, e0123153. doi:10.1371/journal.pone.0123153
Chisesi, T., Bellei, M., Luminari, S., Montanini, A., Marcheselli, L., Levis, A., et al. (2011). Long-term Follow-Up Analysis of HD9601 Trial Comparing ABVD versus Stanford V versus MOPP/EBV/CAD in Patients with Newly Diagnosed Advanced-Stage Hodgkin's Lymphoma: a Study from the Intergruppo Italiano Linfomi. J. Clin. Oncol. 29, 4227–4233. doi:10.1200/JCO.2010.30.9799
Chung, J. H., and Lee, S. W. (2013). Assessing the Quality of Randomized Controlled Urological Trials Conducted by Korean Medical Institutions. Korean J. Urol. 54, 289–296. doi:10.4111/kju.2013.54.5.289
Connors, J. M., Klimo, P., Adams, G., Burns, B. F., Cooper, I., Meyer, R. M., et al. (1997). Treatment of Advanced Hodgkin's Disease with Chemotherapy-Ccomparison of MOPP/ABV Hybrid Regimen with Alternating Courses of MOPP and ABVD: a Report from the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 15, 1638–1645. doi:10.1200/JCO.1997.15.4.1638
Corazzelli, G., Russo, F., and Pinto, A. (2011). ABVD versus BEACOPP for Hodgkin's Lymphoma. N. Engl. J. Med. 365, 1545–1546. doi:10.1056/NEJMc1109618
Diehl, V., Franklin, J., Pfreundschuh, M., Lathan, B., Paulus, U., Hasenclever, D., et al. (2003). Standard and Increased-Dose BEACOPP Chemotherapy Compared with COPP-ABVD for Advanced Hodgkin's Disease. N. Engl. J. Med. 348, 2386–2395. doi:10.1056/NEJMoa022473
Duggan, D. B., Petroni, G. R., Johnson, J. L., Glick, J. H., Fisher, R. I., Connors, J. M., et al. (2003). Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin's Disease: Report of an Intergroup Trial. J. Clin. Oncol. 21, 607–614. doi:10.1200/JCO.2003.12.086
Eichenauer, D. A., Engert, A., Dreyling, M., and Group, E. G. W. (2011). Hodgkin's Lymphoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 22 (Suppl. 6), vi55–8. doi:10.1093/annonc/mdr378
Federico, M., Luminari, S., Iannitto, E., Polimeno, G., Marcheselli, L., Montanini, A., et al. (2009). ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J. Clin. Oncol. 27, 805–811. doi:10.1200/JCO.2008.17.0910
Glick, J. H., Young, M. L., Harrington, D., Schilsky, R. L., Beck, T., Neiman, R., et al. (1998). MOPP/ABV Hybrid Chemotherapy for Advanced Hodgkin's Disease Significantly Improves Failure-free and Overall Survival: the 8-year Results of the Intergroup Trial. J. Clin. Oncol. 16, 19–26. doi:10.1200/JCO.1998.16.1.19
Gobbi, P. G., Levis, A., Chisesi, T., Broglia, C., Vitolo, U., Stelitano, C., et al. (2005). ABVD versus Modified stanford V versus MOPPEBVCAD with Optional and Limited Radiotherapy in Intermediate- and Advanced-Stage Hodgkin's Lymphoma: Final Results of a Multicenter Randomized Trial by the Intergruppo Italiano Linfomi. J. Clin. Oncol. 23, 9198–9207. doi:10.1200/JCO.2005.02.907
Gordon, L. I., Hong, F., Fisher, R. I., Bartlett, N. L., Connors, J. M., Gascoyne, R. D., et al. (2013). Randomized Phase III Trial of ABVD versus Stanford V with or without Radiation Therapy in Locally Extensive and Advanced-Stage Hodgkin Lymphoma: an Intergroup Study Coordinated by the Eastern Cooperative Oncology Group (E2496). J. Clin. Oncol. 31, 684–691. doi:10.1200/JCO.2012.43.4803
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hoskin, P. J., Lowry, L., Horwich, A., Jack, A., Mead, B., Hancock, B. W., et al. (2009). Randomized Comparison of the stanford V Regimen and ABVD in the Treatment of Advanced Hodgkin's Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J. Clin. Oncol. 27, 5390–5396. doi:10.1200/JCO.2009.23.3239
Jansen, PJ. P. (2012). Network Meta-Analysis of Individual and Aggregate Level Data. Res. Synth. Methods 3, 177–190. doi:10.1002/jrsm.1048
Majhail, N. S., Weisdorf, D. J., Defor, T. E., Miller, J. S., McGlave, P. B., Slungaard, A., et al. (2006). Long-term Results of Autologous Stem Cell Transplantation for Primary Refractory or Relapsed Hodgkin's Lymphoma. Biol. Blood Marrow Transpl. 12, 1065–1072. doi:10.1016/j.bbmt.2006.06.006
Mounier, N., Brice, P., Bologna, S., Briere, J., Gaillard, I., Heczko, M., et al. et al. (2014). ABVD (8 Cycles) versus BEACOPP (4 Escalated Cycles ≥ 4 Baseline): Final Results in Stage III-IV Low-Risk Hodgkin Lymphoma (IPS 0-2) of the LYSA H34 Randomized Trial. Ann. Oncol. 25, 1622–1628. doi:10.1093/annonc/mdu189
Punnett, A., Tsang, R. W., and Hodgson, D. C. (2010). Hodgkin Lymphoma across the Age Spectrum: Epidemiology, Therapy, and Late Effects. Semin. Radiat. Oncol. 20, 30–44. doi:10.1016/j.semradonc.2009.09.006
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: an Overview and Tutorial. J. Clin. Epidemiol. 64, 163–171. doi:10.1016/j.jclinepi.2010.03.016
Santoro, A., Bonadonna, G., Valagussa, P., Zucali, R., Viviani, S., Villani, F., et al. (1987). Long-term Results of Combined Chemotherapy-Radiotherapy Approach in Hodgkin's Disease: Superiority of ABVD Plus Radiotherapy versus MOPP Plus Radiotherapy. J. Clin. Oncol. 5, 27–37. doi:10.1200/JCO.1987.5.1.27
Saramago, P., Sutton, A. J., Cooper, N. J., and Manca, A. (2012). Mixed Treatment Comparisons Using Aggregate and Individual Participant Level Data. Stat. Med. 31, 3516–3536. doi:10.1002/sim.5442
Shenoy, P., Maggioncalda, A., Malik, N., and Flowers, C. R. (2011). Incidence Patterns and Outcomes for Hodgkin Lymphoma Patients in the United States. Adv. Hematol. 2011, 725219. doi:10.1155/2011/725219
Skoetz, N., Trelle, S., Rancea, M., Haverkamp, H., Diehl, V., Engert, A., et al. (2013). Effect of Initial Treatment Strategy on Survival of Patients with Advanced-Stage Hodgkin's Lymphoma: a Systematic Review and Network Meta-Analysis. Lancet Oncol. 14, 943–952. doi:10.1016/S1470-2045(13)70341-3
Skoetz, N., Will, A., Monsef, I., Brillant, C., Engert, A., and von Tresckow, B. (2017). Comparison of First-Line Chemotherapy Including Escalated BEACOPP versus Chemotherapy Including ABVD for People with Early Unfavourable or Advanced Stage Hodgkin Lymphoma. Cochrane Database Syst. Rev. 5, CD007941. doi:10.1002/14651858.CD007941.pub3
Somers, R., Carde, P., Henry-Amar, M., Tarayre, M., Thomas, J., Hagenbeek, A., et al. (1994). A Randomized Study in Stage IIIB and IV Hodgkin's Disease Comparing Eight Courses of MOPP versus an Alteration of MOPP with ABVD: a European Organization for Research and Treatment of Cancer Lymphoma Cooperative Group and Groupe Pierre-et-Marie-Curie Controlled Clinical Trial. J. Clin. Oncol. 12, 279–287. doi:10.1200/JCO.1994.12.2.279
Souza, E. M., Baiocchi, O. C., Zanichelli, M. A., Alves, A. C., and Oliveira, J. S. (2009). Comparison between Hybrid MOPPABV and ABVD Chemotherapy Protocols for Hodgkin's Lymphoma in Public Hospitals of the Largest South American City: a Retrospective 14-year Study. Ann. Hematol. 88, 633–637. doi:10.1007/s00277-008-0635-0
Townsend, W., and Linch, D. (2012). Hodgkin's Lymphoma in Adults. Lancet 380, 836–847. doi:10.1016/S0140-6736(12)60035-X
Tu, Y. K., Needleman, I., Chambrone, L., Lu, H. K., and Faggion, C. M. (2012). A Bayesian Network Meta-Analysis on Comparisons of Enamel Matrix Derivatives, Guided Tissue Regeneration and Their Combination Therapies. J. Clin. Periodontol. 39, 303–314. doi:10.1111/j.1600-051x.2011.01844.x
Vassilakopoulos, T. P., and Johnson, P. W. (2016). Treatment of Advanced-Stage Hodgkin Lymphoma. Semin. Hematol. 53, 171–179. doi:10.1053/j.seminhematol.2016.05.006
Viviani, S., Zinzani, P. L., Rambaldi, A., Brusamolino, E., Levis, A., Bonfante, V., et al. (2011). ABVD versus BEACOPP for Hodgkin's Lymphoma when High-Dose Salvage Is Planned. N. Engl. J. Med. 365, 203–212. doi:10.1056/NEJMoa1100340
Yan, M., Kumachev, A., Siu, L. L., and Chan, K. K. (2015). Chemoradiotherapy Regimens for Locoregionally Advanced Nasopharyngeal Carcinoma: A Bayesian Network Meta-Analysis. Eur. J. Cancer 51, 1570–1579. doi:10.1016/j.ejca.2015.04.027
Yung, L., and Linch, D. (2003). Hodgkin's Lymphoma. Lancet 361, 943–951. doi:10.1016/S0140-6736(03)12777-8
Zhu, G. Q., Shi, K. Q., Huang, S., Wang, L. R., Lin, Y. Q., Huang, G. Q., et al. (2015). Systematic Review with Network Meta-Analysis: the Comparative Effectiveness and Safety of Interventions in Patients with Overt Hepatic Encephalopathy. Aliment. Pharmacol. Ther. 41, 624–635. doi:10.1111/apt.13122
Keywords: hodgkin lymphoma, chemotherapy, efficacy, randomized controlled trial, network meta-analysis
Citation: Pei F, Yu Y, Dong B, Guan H, Dong X and Zhao F (2021) Efficacies and Toxicities of Seven Chemotherapy Regimens for Advanced Hodgkin Lymphoma. Front. Pharmacol. 12:694545. doi: 10.3389/fphar.2021.694545
Received: 12 July 2021; Accepted: 11 October 2021;
Published: 16 November 2021.
Edited by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyReviewed by:
Laith Naser AL-Eitan, Jordan University of Science and Technology, JordanAnne Beaven, University of North Carolina at Chapel Hill, United States
Copyright © 2021 Pei, Yu, Dong, Guan, Dong and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fen Zhao, emhhb2ZlbjEwMjlAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Fajun Pei1†
Fajun Pei1† Yang Yu
Yang Yu