- 1Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Pulmonary and Critical Care University of Miami Miller School of Medicine Miami, Miami, FL, United States
- 3Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Microbiology, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran
- 5Non-Communicable Diseases Research Center, Alborz University of Medical Sciences,Karaj, Iran
Background: The incidence of Mycobacterium avium complex (MAC) increases as immunosuppressed conditions become more common. MAC's standard treatment regimen includes a macrolide, ethambutol, and a rifamycin, among which rifampin and rifabutin are the most commonly used. Although current guidelines recommend initial therapy for MAC with rifampin, it has been theorized to be less efficacious than rifabutin.
Methods: We reviewed the relevant scientific literature published up to February 18, 2020. Statistical analyses were performed with Comprehensive Meta-Analysis Software Version 2.0 (Biostat, Englewood, NJ). The pooled frequency with 95% confidence intervals (CI) was assessed using a random-effect model. We considered P <0.05 as statistically significant for publication bias.
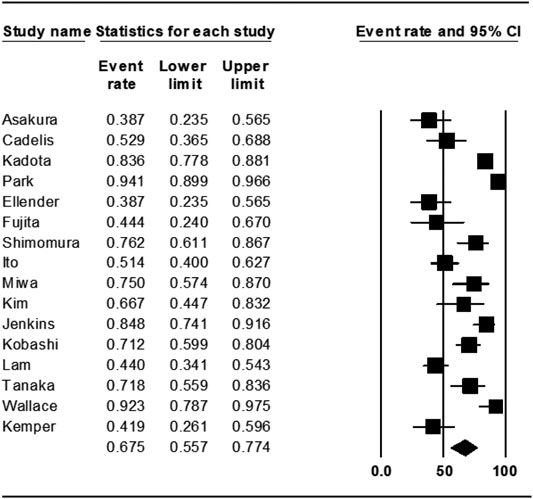
Results: After reviewing 3665 records, we identified 24 studies that satisfied the inclusion criteria. Among these studies, 8 had rifabutin in their regimens (rifabutin group) and 16 had rifampin in their regimens (rifampin group). The estimated pooled treatment success rate was found to be 54.7% (95% CI 41.0-67.0%) in rifabutin groups and 67.5% (95% CI 55.7-77.4%) in rifampin groups. There was no evidence of publication bias among the included studies (Egger’s test p-value was 0.7).
Conclusion: In this study, it was shown that in comparison to Rifabutin, rifampin has similar treatment success rates in treating MAC. In order to determine the exact preference of each of these drugs, double-blind clinical trial studies are recommended.
Introduction
Nontuberculous mycobacteria (NTM) are bacteria in the Mycobacterium genus but exclude Mycobacterium tuberculosis complex and M. leprae (Tortoli, 2014; Shahraki et al., 2015). There is an increasing interest in NTM disease due to the association of NTM infection with immunocompromised states, such as human immunodeficiency virus (HIV), and underlying lung diseases, such as bronchiectasis, chronic obstructive pulmonary disease, and cystic fibrosis (Mirsaeidi et al., 2014a).
Mycobacterium avium complex (MAC) is the most common species isolated worldwide but is associated with treatment failure rates of 18–40% (Nasiri et al., 2020). The standard of the care treatment regimen for MAC consists of a macrolide, ethambutol, and a rifamycin. The most commonly used rifamycins are rifampin and rifabutin. Current guidelines recommend initial therapy for MAC with rifampin. Rifabutin is traditionally reserved for severe systemic or recurrent disease (Kim et al., 2019). Rifampin has been preferred for pulmonary MAC due to the reduced tolerance of rifabutin in the elderly who are more likely to have underlying chronic lung diseases such as bronchiectasis and chronic obstructive pulmonary disease (Griffith et al., 2007). Rifabutin is generally well-tolerated in younger HIV populations that are more likely to have disseminated MAC (Crabol et al., 2016). Thus, rifampin is used for pulmonary MAC and rifabutin for disseminated cases by convention. Rifabutin also has less severe drug-drug interaction, which is paramount for those on antiretroviral therapies (Horne et al., 2011).
Despite these differences, there remains uncertainty if one rifamycin is superior for the treatment of MAC. This has led to a considerable variation in practice. Analyzing observational and controlled trials, we herein report a meta-analysis comparing the treatment success rates of rifampin versus rifabutin for pulmonary and disseminated MAC.
Methods
Search Strategy
We searched Pubmed/Medline and Embase for studies published up to February 18, 2020. The search strategies were based on (Mycobacterium avium or MAC) and (rifabutin or rifampin). This combination of terms was used for searching article title, abstract, or keywords. In Medline and Embase, the relevant MeSH and Emtree terms were also used, respectively. Only studies written in English were selected. This study was conducted and reported according to the PRISMA guidelines (Moher et al., 2009). The study did not require Institutional Review Board approval.
Study Selection
The records found through database searching were merged, and the duplicates were removed using EndNote X7 (Thomson Reuters, New York, NY, United States). Two reviewers independently screened the records by title, abstract, and full-text to exclude those not related to the current study. Included studies met the following inclusion criteria: 1) patients were diagnosed with MAC using the criteria suggested by ATS/IDSA (Griffith et al., 2007); 2) all study patients were treated with rifampin or rifabutin-containing regimens; and 3) the treatment outcomes were addressed. We defined treatment success as the achievement of culture conversion and completion of the planned treatment without relapse while on treatment. Studies with insufficient information about treatment outcomes were excluded. Conference abstracts, editorials, and reviews were also excluded.
Data Extraction and Quality Assessment
Two reviewers designed a data extraction form. These reviewers extracted the data from all eligible studies, and differences were resolved by consensus. The following data were extracted: first author name; year of publication; study duration, type of study (RCTS, cohorts, etc.), country/ies where the study was conducted; the number of patients with MAC; age; HIV/AIDS status; treatment protocols (treatment regimens, and duration of treatment), and treatment outcome. The methodological quality of the eligible studies was assessed according to the Cochrane-based criteria (Higgins et al., 2019).
Data Analysis
Statistical analyses were performed with Comprehensive Meta-Analysis Software Version 2.0 (Biostat, Englewood, NJ). The pooled success treatment rate with 95% confidence intervals (CI) was assessed using a random-effect model. Since prevalence would be affected by the spectrum of populations included, we expected to find significant heterogeneity across the studies. Thus, an a priori decision was made to select the random-effects model because this would give more consistent estimates. The between-study heterogeneity was assessed by Cochran’s Q and the I2 statistic. I2 values of 25, 50, and 75% were considered to represent low, moderate, and high heterogeneity, respectively (Higgins and Thompson, 2002). To minimize heterogeneity, subgroup analyses stratified by study design, disease type, number of drugs used, and treatment length were performed. Publication bias was assessed statistically using Egger’s test (p < 0.05 was considered indicative of statistically significant publication bias).
Results
Study Selection
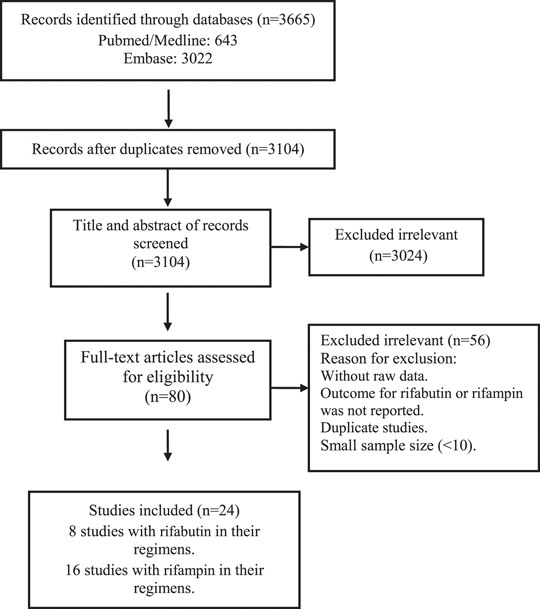
The studies included and excluded through the review process are summarized in Figure 1. A total of 3,665 records were found in the initial search; after removing duplicate articles, the titles and abstracts of 3,104 references were screened. Of these, 80 articles were selected for a full-text review. After the full-text review, 24 studies that described the treatment outcomes of rifabutin vs rifampin-containing regimens were chosen for the meta-analysis.
Characteristics of Included Studies
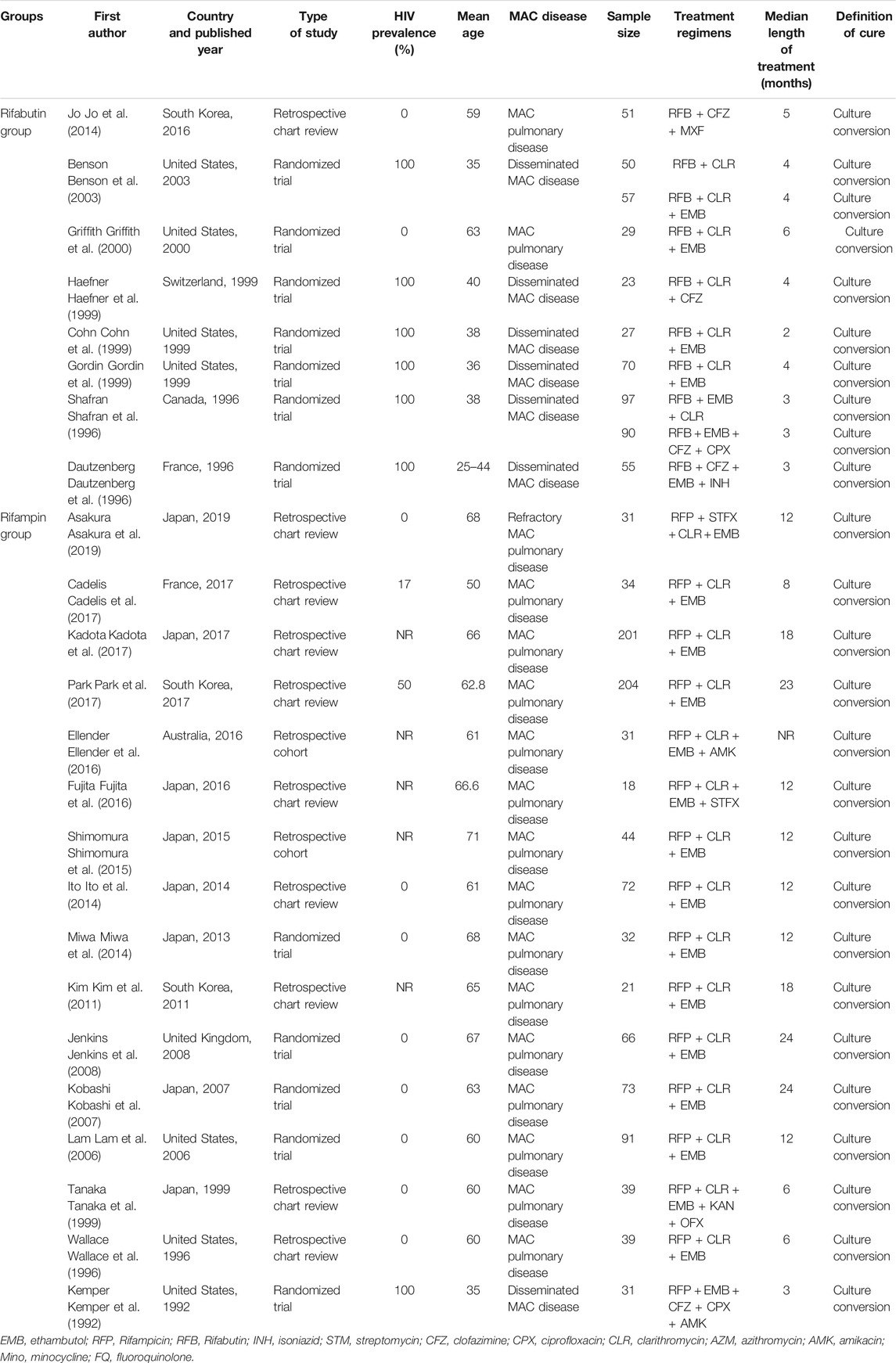
The characteristics of the included studies are described in Table 1. The study period ranged from 1992 to 2017. The 24 studies comprised 12 RCTs, ten retrospective chart review studies, and two retrospective cohort studies. Eight studies were conducted in Japan, 7 in the United States, 3 in the Republic of Korea, 2 in France, 1 in Canada, 1 in the United Kingdom, 1 in Australia, and 1 in Switzerland. The mean age of the patients ranged from 35 to 71 years. Eight studies used rifabutin in their regimens (rifabutin group), and 16 studies used rifampin in their regimens (rifampin group). The median duration of treatment ranged from 2 to 24 months. Studies sample size range from 18 to 204, with a total number of 1,576 patients. All studies used the definition of treatment success suggested by the ATS/IDSA.
Quality Assessment
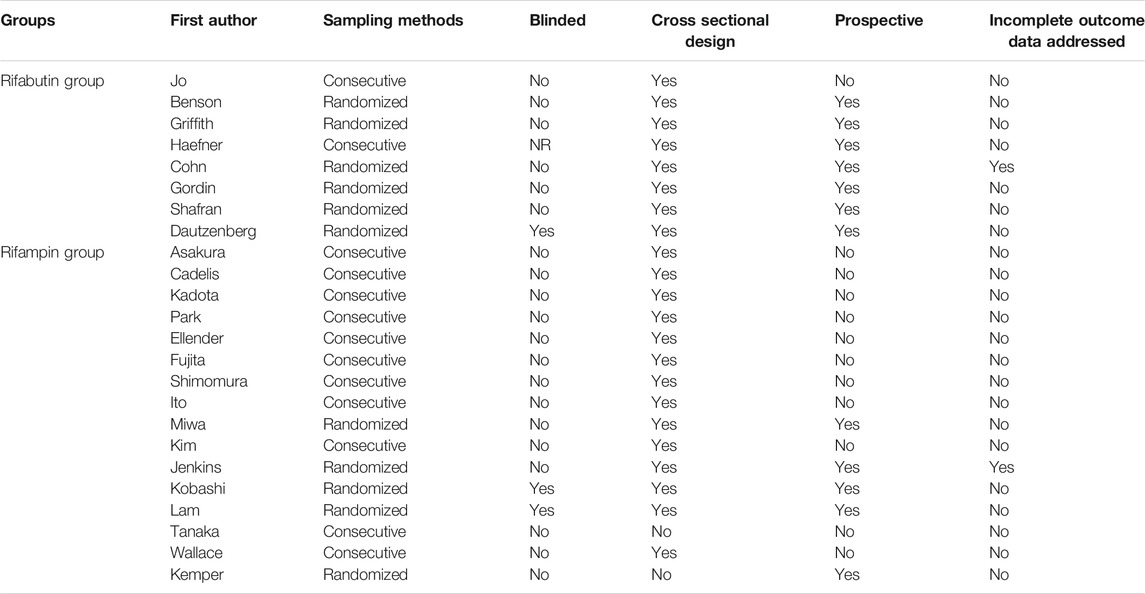
Based on the Cochrane-based tool (Table 2), the included studies had a low risk of bias. The RCTs had a low risk of bias in random sequence generation, incomplete outcome data, and selective reporting. However, blinding of outcome assessment was only fulfilled in 2 studies, and blinding of the participants and study personnel were not reported in 1 of them (Higgins and Thompson, 2002). All except two studies report the treatment outcome, and all of them include detailed follow-up data after treatment.
Treatment Success
The treatment outcomes of 1,576 patients from 24 studies were assessed, and all patients met the criteria for treatment success. A total of 549 patients were identified for evaluating rifabutin-based regimens, and 1,027 patients were identified for evaluation of rifampin-based regimens.
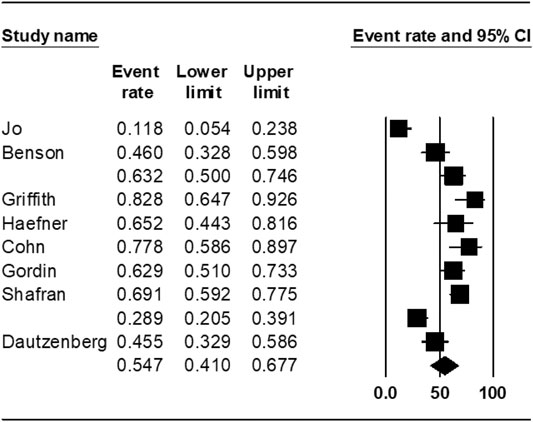
The pooled treatment success rate was found to be 54.7% [95% CI 41.0–67.0%] in rifabutin-groups (Figure 2). The heterogeneity of the effect estimate (I2) was 88% of the variance, and the p-value (Cochran Q test) was <0.001. There was no evidence of publication bias (Egger’s test p-value was 0.7).
The treatment outcomes of rifampin-containing regimens from 16 studies were also assessed, and the weighted proportion of treatment success among included patients was 67.5% (95% CI 55.7–77.4%). The heterogeneity of the effect estimate (I2) was 90% of the variance, and the p-value (Cochran Q test) was <0.001 (Figure 3). There was no evidence of publication bias (Egger’s test p-value was 0.7).
Subgroup Analysis
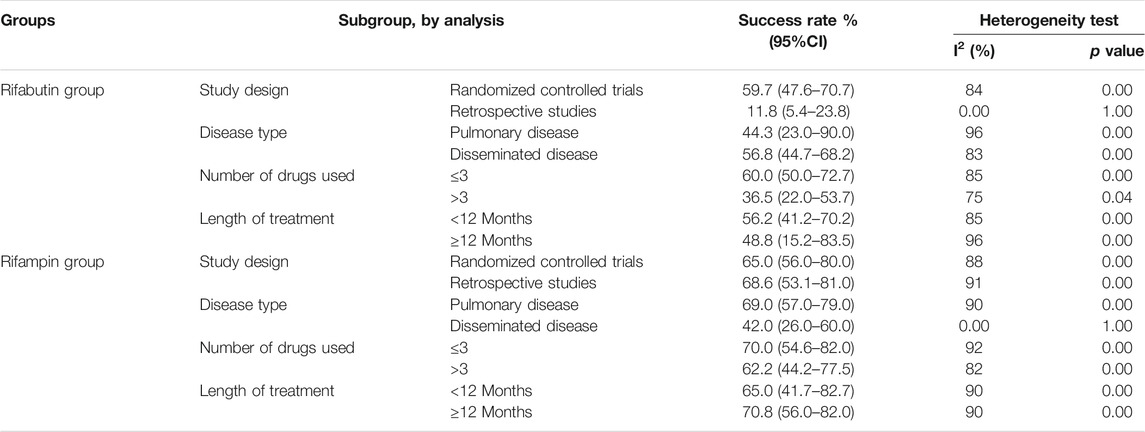
The subgroup analysis based on treatment regimens is shown in Table3. In the subgroup analyses within the rifabutin-containing regimens, patients’ treatment success rate from the RCTs was higher than that of the other observational studies. The treatment success rate was 60.0% (95% CI, 50.0–72.7) when ≤3 drugs were used, and 36.5% (95% CI, 22.0–53.7) when >3 drugs were used. The treatment success rate for the patients with the pulmonary disease was 44.3% (95% CI, 23.0–90.0), while that with the disseminated disease was 56.8% (95% CI, 44.7–68.2). The result of the Egger test showed no evidence of publication bias (p-value = 0.8).
Discussion
This study suggests that rifampin is not inferior to rifabutin and may lead to better treatment success rates for MAC. However, there was a significant variation in treatment success rates. The treatment success rate for rifampin was 64.2% (95% CI 55.1–73.3%) compared to 55.2% (95% CI 44.4–66.1%) for rifabutin.
Rifampin has been theorized to be less efficacious than rifabutin due to its effect on the metabolism of other antibiotics (Mirsaeidi et al., 2014b). A study in 1996 found a rifabutin regimen was superior to a rifampin regimen for MAC bacteremia (Shafran et al., 1996). Rifampin is a more potent inducer for the cytochrome (CYP) enzyme system (i.e., strong inducer for CYP3A4 and CYP2C19; moderate inducer for CYP2B6, CYP2C8, and CYP2C9; weak inducer for CYP1A2) while rifabutin only induces CYP3A4 and to a lesser extent (Shulha et al., 2019). Tuloup and colleagues indicated that rifabutin, contrary to rifampin, does not appear likely to cause severe drug-drug interactions, even with sensitive CYP substrates (Tuloup et al., 2021).
Rifampin has been shown to decrease peak serum concentrations of key antibiotics often used in MAC treatment, including clarithromycin, azithromycin, and moxifloxacin (van Ingen et al., 2012). Boorgula et al. in their study found that rifampin monotherapy failed after only 4 days of treatment, and by day 26 of the trial, all MAC population were resistant to rifampin (Boorgula et al., 2021).
Our study results indicate that the pharmacokinetic effect of rifampin is clinically overstated and does not lead to less efficacy compared to rifabutin for the treatment of MAC.
According to our findings, rifampin may be superior to rifabutin for MAC treatment. One explanation for our results is the increased age of patients with human immunodeficiency virus (HIV) in recent decades. Rifabutin has traditionally been recommended for disseminated cases mostly seen in HIV patients, as they were younger with increased ability to tolerate the medication and less potential for interference with CYP enzymes while on antiretroviral therapies (Cowman et al., 2019; Currier and Gandhi, 2021). 22 of 24 studies included in our analysis were published within the last 20 years capturing the present population of aging patients with HIV. Rifabutin is known to have decreased tolerability in these older populations (Finch et al., 2002). With the advent of newer antiretroviral therapies, patients with HIV have experienced increased longevity (Life expectancy of indivi, 2008). As a result, rifabutin regimens are being used in aging populations with decreased tolerability leading to truncation of therapy and treatment failure in MAC. This phenomenon was observed in our study in the shorter duration of rifabutin treatment in the HIV groups.
Limitations of this study are attributable to the potential for confounding variables. The other drugs in the treatment regimen, such as macrolide, can affect as confounders on the treatment’s success rate; however, there was not enough data to separately analyze and discuss the effect of other drugs. Therefore, further investigation such as large, multicenter randomized controlled trials for comparison between Rifampin and Rifabutin success rate is needed to validate the findings. Furthermore, it might be possible to separately discuss the results in HIV and non-HIV subgroups in such trials.
The prevalence of concomitant HIV in the rifampin studies was much less than in the rifabutin studies. HIV was reported in 3 out of 16 studies in the rifampin group (13% of patients) than 6 out of 8 studies in the rifabutin group (85% of patients). One would expect lower treatment success rates in HIV patients due to dysfunctional T lymphocytes, a condition not readily conducive to eradicating MAC. Disseminated MAC is also more prevalent in HIV populations and may increase difficulty with the treatment and the eradication of the bacteria. Other variables center on the lack of consistent dosing protocols and pharmacodynamic parameters among the studies included. Poor treatment success of MAC has been attributed to seldomly met pharmacodynamic indices (van Ingen et al., 2012). The success of rifamycins has been associated with peak concentrations to minimum inhibitory concentration (MIC) ratios, or area under the curve to MIC ratios, which are not reported. Lastly, as half of the included studies were not randomized, there is a risk for type 1 error. Rifabutin may have been employed for more severe cases as is recommended, which would lead to the errant finding that rifampin is a superior treatment (Finch et al., 2002).
The results of this meta-analysis encourage the development of large, randomized controlled trials that can compare the effect of rifampin versus rifabutin in HIV and non-HIV subgroups and disseminated and non-disseminated MAC disease. It should also be noted that double-blind clinical trial studies are necessary to determine the exact preference of each of these drugs.
Conclusions
This study demonstrates that the treatment success rates of rifampin for MAC are comparable to that of Rifabutin. Our findings also suggest that the pharmacokinetic interactions of rifampin may be overestimated in the clinical setting. Large randomized control trials comparing rifampin versus rifabutin in different patient subgroups are required to analyze further and corroborate these findings. This data can help create a unified clinical practice guideline for MAC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
Conception and design of the study: MJN, MM Acquisition of data: BH, TA, MG, BA, MD, FK Analysis and/or interpretation of data: MJN Drafting and revision of the manuscript: BA, MM, MJN, MM.
Funding
This study was financially supported by the Research Department of the School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant number: 26842).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Asakura, T., Suzuki, S., Fukano, H., Okamori, S., Kusumoto, T., Uwamino, Y., et al. (2019). Sitafloxacin-Containing Regimen for the Treatment of Refractory Mycobacterium avium Complex Lung Disease. Open Forum Infect. Dis. 6 (4), ofz108. doi:10.1093/ofid/ofz108
Benson, C. A., Williams, P. L., Currier, J. S., Holland, F., Mahon, L. F., MacGregor, R. R., et al. (2003). A Prospective, Randomized Trial Examining the Efficacy and Safety of Clarithromycin in Combination with Ethambutol, Rifabutin, or Both for the Treatment of Disseminated Mycobacterium avium Complex Disease in Persons with Acquired Immunodeficiency Syndrome. Clin. Infect. Dis. 37 (9), 1234–1243. doi:10.1086/378807
Boorgula, G. D., Jakkula, L. U. M. R., Gumbo, T., Jung, B., and Srivastava, S. (2021). Comparison of Rifamycins for Efficacy against Mycobacterium avium Complex and Resistance Emergence in the Hollow Fiber Model System. Front. Pharmacol. 12, 593. doi:10.3389/fphar.2021.645264
Cadelis, G., Ducrot, R., Bourdin, A., and Rastogi, N. (2017). Predictive Factors for a One-Year Improvement in Nontuberculous Mycobacterial Pulmonary Disease: An 11-year Retrospective and Multicenter Study. Plos Negl. Trop. Dis. 11 (8), e0005841. doi:10.1371/journal.pntd.0005841
Cohn, D. L., Fisher, E. J., Peng, G. T., Hodges, J. S., Chesnut, J., Child, C. C., et al. (1999). A Prospective Randomized Trial of Four Three-Drug Regimens in the Treatment of Disseminated Mycobacterium avium Complex Disease in AIDS Patients: Excess Mortality Associated with High-Dose Clarithromycin. Terry Beirn Community Programs for Clinical Research on AIDS. Clin. Infect. Dis. 29 (1), 125–133. doi:10.1086/520141
Cowman, S., van Ingen, J., Griffith, D. E., and Loebinger, M. R. (2019). Non-Tuberculous Mycobacterial Pulmonary Disease. Eur. Respir. J. 54 (1), 1900250. doi:10.1183/13993003.00250-2019
Crabol, Y., Catherinot, E., Veziris, N., Jullien, V., and Lortholary, O. (2016). Rifabutin: Where Do We Stand in 2016? J. Antimicrob. Chemother. 71 (7), 1759–1771. doi:10.1093/jac/dkw024
Currier, J. S., and Gandhi, R. (2021). Mycobacterium avium Complex (MAC) Infections in Persons with HIV. Waltham, MA: Post TW.
Dautzenberg, B., Olliaro, P., Ruf, B., Esposito, R., Opravil, M., Hoy, J. F., et al. (1996). Rifabutin versus Placebo in Combination with Three Drugs in the Treatment of Nontuberculous Mycobacterial Infection in Patients with AIDS. Clin. Infect. Dis. 22 (4), 705–708. doi:10.1093/clinids/22.4.705
Ellender, C. M., Law, D. B., Thomson, R. M., and Eather, G. W. (2016). Safety of IV Amikacin in the Treatment of Pulmonary Non-Tuberculous Mycobacterial Disease. Respirology 21 (2), 357–362. doi:10.1111/resp.12676
Finch, C. K., Chrisman, C. R., Baciewicz, A. M., and Self, T. H. (2002). Rifampin and Rifabutin Drug Interactions: An Update. Arch. Intern. Med. 162 (9), 985–992. doi:10.1001/archinte.162.9.985
Fujita, K., Fujita, M., Ito, Y., Hirai, T., Mio, T., Watanabe, K., et al. (2016). Preliminary Evaluation of a Sitafloxacin-Containing Regimen for Relapsed or Refractory Pulmonary Mycobacterium avium Complex Disease. Open Forum Infect. Dis. 3 (3), ofw147. doi:10.1093/ofid/ofw147
Gordin, F. M., Sullam, P. M., Shafran, S. D., Cohn, D. L., Wynne, B., Paxton, L., et al. (1999). A Randomized, Placebo-Controlled Study of Rifabutin Added to a Regimen of Clarithromycin and Ethambutol for Treatment of Disseminated Infection with Mycobacterium avium Complex. Clin. Infect. Dis. 28 (5), 1080–1085. doi:10.1086/514748
Griffith, D. E., Aksamit, T., Brown-Elliott, B. A., Catanzaro, A., Daley, C., Gordin, F., et al. (2007). An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 175 (4), 367–416. doi:10.1164/rccm.200604-571ST
Griffith, D. E., Brown, B. A., Cegielski, P., Murphy, D. T., and Wallace, R. J. (2000). Early Results (At 6 Months) with Intermittent Clarithromycin-Including Regimens for Lung Disease Due to Mycobacterium avium Complex. Clin. Infect. Dis. 30 (2), 288–292. doi:10.1086/313644
Haefner, M., Funke-Kissling, P., Pfyffer, G. E., L?thy, R., and Opravil, M. (1999). Clarithromycin, Rifabutin and Clofazimine for Treatment of Disseminated Mycobacterium avium Complex Disease in AIDS Patients. Clin. Drug Invest. 17 (3), 171–178. doi:10.2165/00044011-199917030-00001
Higgins, J. P., and Thompson, S. G. (2002). Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Horne, D. J., Spitters, C., and Narita, M. (2011). Experience with Rifabutin Replacing Rifampin in the Treatment of Tuberculosis. Int. J. Tuberc. Lung Dis. 15 (11), 1485–1489. doi:10.5588/ijtld.11.0068i
Ito, Y., Hirai, T., Fujita, K., Kubo, T., Maekawa, K., Ichiyama, S., et al. (2014). The Influence of Environmental Exposure on the Response to Antimicrobial Treatment in Pulmonary Mycobacterial Avium Complex Disease. BMC Infect. Dis. 14 (1), 522. doi:10.1186/1471-2334-14-522
Jenkins, P. A., Campbell, I. A., Banks, J., Gelder, C. M., Prescott, R. J., and Smith, A. P. (2008). Clarithromycin vs Ciprofloxacin as Adjuncts to Rifampicin and Ethambutol in Treating Opportunist Mycobacterial Lung Diseases and an Assessment of Mycobacterium Vaccae Immunotherapy. Thorax 63 (7), 627–634. doi:10.1136/thx.2007.087999
Jo, K. W., Kim, S., Lee, J. Y., Lee, S. D., Kim, W. S., Kim, D. S., et al. (2014). Treatment Outcomes of Refractory MAC Pulmonary Disease Treated with Drugs with Unclear Efficacy. J. Infect. Chemother. 20 (10), 602–606. doi:10.1016/j.jiac.2014.05.010
Kadota, J. I., Kurashima, A., and Suzuki, K. (2017). The Clinical Efficacy of a Clarithromycin-Based Regimen for Mycobacterium avium Complex Disease: A Nationwide post-marketing Study. J. Infect. Chemother. 23 (5), 293–300. doi:10.1016/j.jiac.2017.01.007
Kemper, C. A., Meng, T. C., Nussbaum, J., Chiu, J., Feigal, D. F., Bartok, A. E., et al. (1992). Treatment of Mycobacterium avium Complex Bacteremia in AIDS with a Four-Drug Oral Regimen. Rifampin, Ethambutol, Clofazimine, and Ciprofloxacin. The California Collaborative Treatment Group. Ann. Intern. Med. 116 (6), 466–472. doi:10.7326/0003-4819-116-6-466
Kim, E. Y., Chi, S. Y., Oh, I. J., Kim, K. S., Kim, Y. I., Lim, S. C., et al. (2011). Treatment Outcome of Combination Therapy Including Clarithromycin for Mycobacterium avium Complex Pulmonary Disease. Korean J. Intern. Med. 26 (1), 54–59. doi:10.3904/kjim.2011.26.1.54
Kim, H. J., Lee, J. S., Kwak, N., Cho, J., Lee, C. H., Han, S. K., et al. (2019). Role of Ethambutol and Rifampicin in the Treatment of Mycobacterium avium Complex Pulmonary Disease. BMC Pulm. Med. 19 (1), 212. doi:10.1186/s12890-019-0982-8
Kobashi, Y., Matsushima, T., and Oka, M. (2007). A Double-Blind Randomized Study of Aminoglycoside Infusion with Combined Therapy for Pulmonary Mycobacterium avium Complex Disease. Respir. Med. 101 (1), 130–138. doi:10.1016/j.rmed.2006.04.002
Lam, P. K., Griffith, D. E., Aksamit, T. R., Ruoss, S. J., Garay, S. M., Daley, C. L., et al. (2006). Factors Related to Response to Intermittent Treatment of Mycobacterium avium Complex Lung Disease. Am. J. Respir. Crit. Care Med. 173 (11), 1283–1289. doi:10.1164/rccm.200509-1531OC
Life Expectancy of Individuals on Combination Antiretroviral Therapy in High-Income Countries: a Collaborative Analysis of 14 Cohort Studies. Lancet, 2008. 372(9635): p. 293–299.doi:10.1016/S0140-6736(08)61113-7
Mirsaeidi, M., Farshidpour, M., Allen, M. B., Ebrahimi, G., and Falkinham, J. O. (2014). Highlight on Advances in Nontuberculous Mycobacterial Disease in North America. Biomed. Res. Int. 2014, 919474. doi:10.1155/2014/919474
Mirsaeidi, M., Farshidpour, M., Ebrahimi, G., Aliberti, S., and Falkinham, J. O. (2014). Management of Nontuberculous Mycobacterial Infection in the Elderly. Eur. J. Intern. Med. 25 (4), 356–363. doi:10.1016/j.ejim.2014.03.008
Miwa, S., Shirai, M., Toyoshima, M., Shirai, T., Yasuda, K., Yokomura, K., et al. (2014). Efficacy of Clarithromycin and Ethambutol for Mycobacterium avium Complex Pulmonary Disease. A Preliminary Study. Ann. Am. Thorac. Soc. 11 (1), 23–29. doi:10.1513/AnnalsATS.201308-266OC
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 151 (4), 264–269. doi:10.7326/0003-4819-151-4-200908180-00135
Nasiri, M. J., Ebrahimi, G., Arefzadeh, S., Zamani, S., Nikpor, Z., and Mirsaeidi, M. (2020). Antibiotic Therapy success Rate in Pulmonary Mycobacterium avium Complex: a Systematic Review and Meta-Analysis. Expert Rev. Anti Infect. Ther. 18 (3), 263–273. doi:10.1080/14787210.2020.1720650
Park, S., Jo, K. W., Lee, S. D., Kim, W. S., and Shim, T. S. (2017). Clinical Characteristics and Treatment Outcomes of Pleural Effusions in Patients with Nontuberculous Mycobacterial Disease. Respir. Med. 133, 36–41. doi:10.1016/j.rmed.2017.11.005
Shafran, S. D., Singer, J., Zarowny, D. P., Phillips, P., Salit, I., Walmsley, S. L., et al. (1996). A Comparison of Two Regimens for the Treatment of Mycobacterium avium Complex Bacteremia in AIDS: Rifabutin, Ethambutol, and Clarithromycin versus Rifampin, Ethambutol, Clofazimine, and Ciprofloxacin. Canadian HIV Trials Network Protocol 010 Study Group. N. Engl. J. Med. 335 (6), 377–383. doi:10.1056/NEJM199608083350602
Shahraki, A. H., Heidarieh, P., Bostanabad, S. Z., Khosravi, A. D., Hashemzadeh, M., Khandan, S., et al. (2015). Multidrug-Resistant Tuberculosis" May Be Nontuberculous Mycobacteria. Eur. J. Intern. Med. 26 (4), 279–284. doi:10.1016/j.ejim.2015.03.001
Shimomura, H., Ono, A., Imanaka, K., Majima, T., Masuyama, H., Sato, T., et al. (2015). Retrospective Investigation of Combination Therapy with Clarithromycin and Levofloxacin for Pulmonary Mycobacterium avium Complex Disease. J. Pharm. Health Care Sci. 1, 24. doi:10.1186/s40780-015-0025-4
Shulha, J. A., Escalante, P., and Wilson, J. W. (2019). Pharmacotherapy Approaches in Nontuberculous Mycobacteria Infections. Mayo Clin. Proc. 94 (8), 1567–1581. doi:10.1016/j.mayocp.2018.12.011
T. P. J. Higgins, J. Chandler, M. Cumpston, T. Li, M. J. Page, and V. A. Welch (Editors) (2019). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. London: Cochrane. Available from: www.training.cochrane.org/handbook (Accessed January 1, 2020).
Tanaka, E., Kimoto, T., Tsuyuguchi, K., Watanabe, I., Matsumoto, H., Niimi, A., et al. (1999). Effect of Clarithromycin Regimen for Mycobacterium avium Complex Pulmonary Disease. Am. J. Respir. Crit. Care Med. 160 (3), 866–872. doi:10.1164/ajrccm.160.3.9811086
Tortoli, E. (2014). Microbiological Features and Clinical Relevance of New Species of the Genus Mycobacterium. Clin. Microbiol. Rev. 27 (4), 727–752. doi:10.1128/CMR.00035-14
Tuloup, V., France, M., Garreau, R., Bleyzac, N., Bourguignon, L., Tod, M., et al. (2021). Model-based Comparative Analysis of Rifampicin and Rifabutin Drug-Drug Interaction Profile. Antimicrob. Agents Chemother. 65 (9), e0104321. doi:10.1128/AAC.01043-21
van Ingen, J., Egelund, E. F., Levin, A., Totten, S. E., Boeree, M. J., Mouton, J. W., et al. (2012). The Pharmacokinetics and Pharmacodynamics of Pulmonary Mycobacterium avium Complex Disease Treatment. Am. J. Respir. Crit. Care Med. 186 (6), 559–565. doi:10.1164/rccm.201204-0682OC
Keywords: Mycobacterium avium complex, rifabutin, rifampin, meta-analysis, systematic rewiew
Citation: Hajikhani B, Nasiri MJ, Adkinson BC, Azimi T, Khalili F, Goudarzi M, Dadashi M, Murthi M and Mirsaeidi M (2021) Comparison of Rifabutin-Based Versus Rifampin-Based Regimens for the Treatment of Mycobacterium avium Complex: A meta-Analysis Study. Front. Pharmacol. 12:693369. doi: 10.3389/fphar.2021.693369
Received: 13 April 2021; Accepted: 24 August 2021;
Published: 07 September 2021.
Edited by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyReviewed by:
Paulo J. G. Bettencourt, Catholic University of Portugal, PortugalPatrícia Paiva Corsetti, Federal University of Alfenas, Brazil
Copyright © 2021 Hajikhani, Nasiri, Adkinson, Azimi, Khalili, Goudarzi, Dadashi, Murthi and Mirsaeidi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Javad Nasiri, bWoubmFzaXJpQGhvdG1haWwuY29t; Mehdi Mirsaeidi, bXNtMjQ5QG1lZC5taWFtaS5lZHU=
 Bahareh Hajikhani
Bahareh Hajikhani Mohammad Javad Nasiri
Mohammad Javad Nasiri Brian C. Adkinson2
Brian C. Adkinson2 Farima Khalili
Farima Khalili Mehdi Goudarzi
Mehdi Goudarzi Masoud Dadashi
Masoud Dadashi Mukunthan Murthi
Mukunthan Murthi Mehdi Mirsaeidi
Mehdi Mirsaeidi