
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 20 August 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.692566
This article is part of the Research Topic Food/Diet Supplements from Natural Sources: Current Status and Future Challenges from a Pharmacological Perspective View all 17 articles
The traditional use of plants and their preparations in the treatment of diseases as a first medication in the past centuries indicates the presence of active components for specific targets in the natural material. Many of the tested plants in this study have been traditionally used in the treatment of Diabetes mellitus type 2 and associated symptoms in different cultural areas. Additionally, hypoglycemic effects, such as a decrease in blood glucose concentration, have been demonstrated in vivo for these plants. In order to determine the mode of action, the plants were prepared as methanolic and aqueous extracts and tested for their effects on intestinal glucose and fructose absorption in Caco2 cells. The results of this screening showed significant and reproducible inhibition of glucose uptake between 40 and 80% by methanolic extracts made from the fruits of Aronia melanocarpa, Cornus officinalis, Crataegus pinnatifida, Lycium chinense, and Vaccinium myrtillus; the leaves of Brassica oleracea, Juglans regia, and Peumus boldus; and the roots of Adenophora triphylla. Furthermore, glucose uptake was inhibited between 50 and 70% by aqueous extracts made from the bark of Eucommia ulmoides and the fruit skin of Malus domestica. The methanolic extracts of Juglans regia and Peumus boldus inhibited the fructose transport between 30 and 40% in Caco2 cells as well. These findings can be considered as fundamental work for further research regarding the treatment of obesity-correlated diseases, such as Diabetes mellitus type 2.
In the last years, not only did the absolute number of people suffering from Diabetes mellitus increase from 108 Mio in 1980 to 422 Mio in 2014 but also the prevalence of this disease rose from 4.7 to 8.5% among adults during this time period worldwide (WHO, 2019). Apart from the estimated annual cost to the world of US$ 827 billion, Diabetes is associated with premature mortality and decreased quality of life (WHO, 2016). Hyperglycemia, as one of the main symptoms for diagnosis, results from decreased insulin secretion and/or reduced insulin action and can lead to long-term damage and dysfunction of different organs (American Diabetes Associa, 2014). Particularly, the most common kind of the disease, Diabetes mellitus type 2 (American Diabetes Associa, 2014), can be prevented or delayed in its progress by a lifestyle, which includes a healthy diet, regular exercise with moderate intensity, and no tobacco use (WHO, 2019).
With a focus on the diet, not only a decrease of energy intake to reduce obesity (Grundy, 1998) but also the selection of the consumed food can improve the health status. Supplements can be used to ensure the adequate intake of certain nutrients and support patient’s therapy (Ota and Ulrih, 2017). For example, the reduction of glucose, fructose, and saturated long-chain fatty acids and the increased intake of polyunsaturated, omega-3 fatty acids, such as eicosapentaenoic and docosahexaenoic acid, play a very important role in the prevention and improvement of metabolic disorders by alleviating inflammation processes (Sears and Perry, 2015). Regarding the consumption of plant-based food, secondary metabolites from plants showed hypoglycemic properties by affecting different targets, for example, modulation of intracellular insulin signaling pathways, increase in insulin secretion from β-cells, and inhibition of intestinal enzymes and transporters (Hanhineva et al., 2010; Bahadoran et al., 2013; Ota and Ulrih, 2017). In order to treat obesity as one of the main risk factors for Diabetes mellitus type 2 (Smith, 2007), the reduction of nutritional uptake into enterocytes as the last step before energy overload affects the body is an important therapeutic approach. Furthermore, the inhibition of enzymes and transporters leads to increased concentrations of nutrients in distal sections of the small intestine, which initiates the “ileal brake.” This complex mechanism is known to influence the digestive process and ingestive behavior resulting in reduced appetite and food intake (Maljaars et al., 2008). The glucose transport in the small intestine is maintained by the cotransporter SGLT1 (sodium-glucose linked transporter 1) and GLUT2 (glucose transporter 2), which belongs to the GLUT family and facilitates diffusion processes. The intestinal fructose uptake is performed by GLUT5 (glucose transporter 5) and GLUT2 as well (Schreck and Melzig, 2018). Medicinal plants traditionally used in the treatment of diseases have been applied as candidates for drug discovery in the last decades (Fabricant and Farnsworth, 2001). Aspirin, atropine, ephedrine, digoxin, and morphine are examples of effective substances, which owe their discovery to investigative studies of folk medicine (Gilani and Atta-ur-RahmanTrends, 2005). Ethnopharmacology studies show the continuing acceptance of traditionally used plant preparations as a therapy option due to their long-term experience and marginal side effects (Fabricant and Farnsworth, 2001; Gilani and Atta-ur-RahmanTrends, 2005). The plants tested in this study were chosen due to their traditional use in some cultural areas and countries during the last centuries and their hypoglycemic effects, which were discovered in animal or human studies, as listed in Table 1. For example, in Jordan, preparations of Allium sativum, Ceratonia siliqua, Cuminum cyminum, Juglans regia, Nigella sativa, Olea europaea, Sarcopoterium spinosum have been used in the treatment of Diabetes mellitus (Al-Aboudi and Afifi, 2011), whereas in Congo, Brassica oleracea and Citrus limon have been prepared as folk medicine to treat Diabetes mellitus (Katemo et al., 2012). The plants Adenophora triphylla, Cornus officinalis, Crataegus pinnatifida, Eucommia ulmoides, Lycium chinense, Pueraria lobata, and Rosa rugosa belong to the pharmacopoeia of the Traditional Chinese Medicine (Stöger, 2009). Although Adenophora triphylla and Crataegus pinnatifida showed hypoglycemic effects in studies, the traditional use as antidiabetic remedy was not confirmed in English-language literature as shown in Table 1. According to Table 1, some of the plants, such as Olea europaea and Brassica oleracea, were used in different areas of the world supporting the assumption of efficacy in the treatment of the metabolic disorder. The plants used in this study have been already tested in animal experiments and hypoglycemic properties such as a decrease in blood glucose were confirmed as shown in Table 1, but the exact mechanisms still need to be studied. In order to determine the mode of action, methanolic and aqueous plant extracts were screened for their inhibitory activity on intestinal transporters using Caco2 cells, which originally stem from a colorectal adenocarcinoma cell line. After confluence, the cells need 15–21 days to differentiate and fully express intestinal characteristics, such as a brush border membrane with enzymes and transporters. The adenocarcinoma cell line is widely used as a model for intestinal transport studies (Chantret et al., 1988; Hidalgo et al., 1989; Jumarie and Malo, 1991; Sambuy et al., 2005). We have determined the expression of the SGLT1, GLUT2, and GLUT5 transporters in the Caco2 cells during 21 days (Schreck and Melzig, 2019) and adapted the experimental conditions to ensure valuable results.
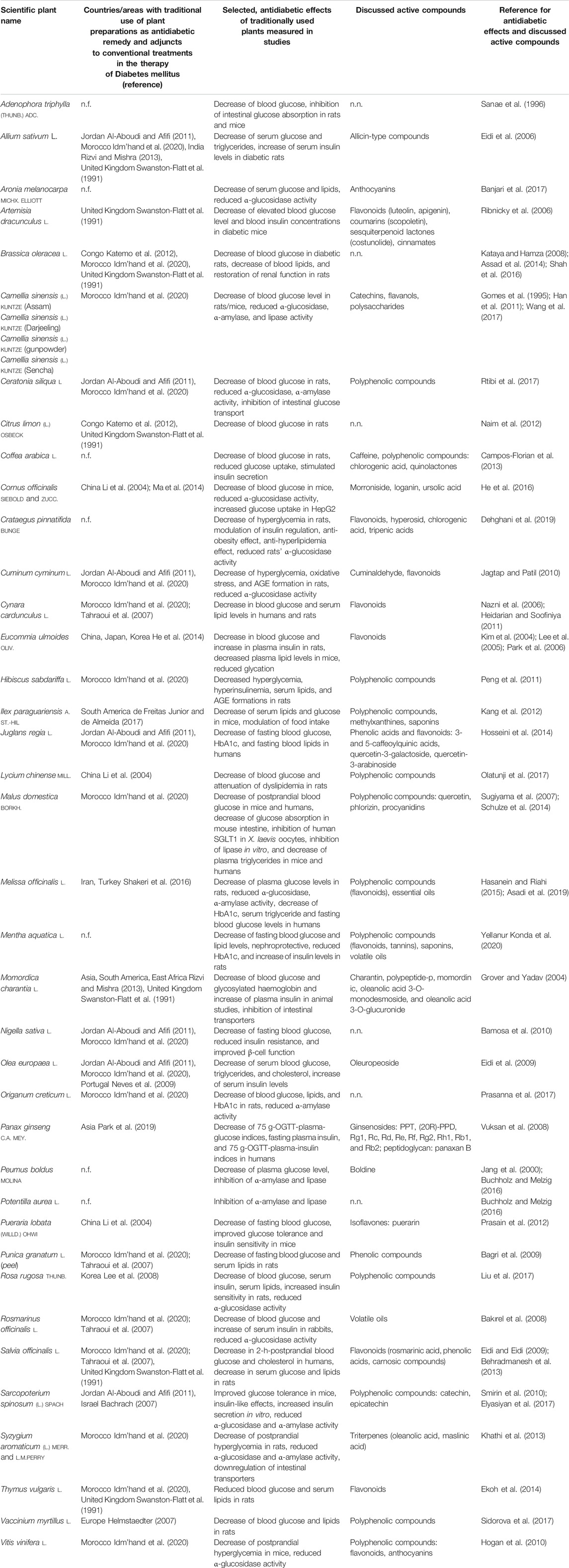
TABLE 1. Selected plants, which are/were traditionally used in specific countries and their antidiabetic effects measured in studies and discussed active compounds (n.f. = not found in English-language literature for this indication; n.n. = not named).
The methanolic and aqueous extract preparations were performed according to Buchholz (2017) with modifications. The parts of the plants, which were used to prepare the extracts, are listed in Tables 2, 3. Except for the fresh fruits of Malus domestica, the botanicals were obtained as dried plant material from the companies. The bulbs of Allium sativum, fruits of Aronia melanocarpa and Vaccinium myrtillus, fruit skin of Punica granatum, the herbal part of Cynara cardunculus and Potentilla aurea, leaves of Mentha aquatica and Olea europaea, roots of Panax ginseng and Sarcopoterium spinosum, and seeds of Nigella sativa were purchased from Kräuter Schulte aktiv Kräuter Drogerie e.K. (Gernsbach, Germany). The following herbal drugs were obtained from Alfred Galke GmbH (Gittelde, Germany): flowers of Hibiscus sabdariffa and Syzygium aromaticum; fruits of Ceratonia siliqua; fruit skin of Citrus limon; leaves of Brassica oleracea,Camellia sinensis, Ilex paraguariensis, Juglans regia, Melissa officinalis, Origanum creticum, Rosmarinus officinalis, and Salvia officinalis; and seeds of Cuminum cyminum and Vitis vinifera (pomace). The plant materials used in Traditional Chinese Medicine, such as the bark of Eucommia ulmoides; flowers of Rosa rugosa; fruits of Cornus officinalis,Crataegus pinnatifida, and Lycium chinense; and the roots of Adenophora triphylla and Pueraria lobata were acquired from Zieten Apotheke (Berlin, Germany). The fruits of the cucurbitaceae Momordica charantia were kindly given by Dr. Serhat Sezai Çiçek (Institute of Pharmacy of Christian-Albrechts-Universitaet Kiel, Germany). The green seeds of Coffea arabica were purchased from Ridders Kaffeerosterei (Berlin, Germany). The leaves of Peumus boldus were gained from Heinrich Klenk GmbH & Co. KG (Schwebheim, Germany), whereas the leaves of Artemisia dracunculus and the fresh fruits of Malus domestica were obtained from a local supermarket. The fresh fruits of Malus domestica were washed with water. The fruit’s skin was cut into small pieces, dried in a drying cabinet at 40°C for 3 days, and freeze-dried for 48 h. The dried plant material was ground with a M20 mill from IKA®-Werke GmbH & Co. KG (Staufen, Germany). 10.0 g of the plant’s powder, adjusted to the drug extract ratio as listed in Tables 2, 3, was extracted in 100 ml. For the preparation of the aqueous extracts, plant material was extracted in water obtained from the ultrapure water system LaboStar from Siemens AG Wasseraufbereitung (Barsbüttel, Germany) for 15 min at 40°C and filtered with a Buechner filter. In some cases, extracts were centrifuged at 4°C first with an Allegra X-30R centrifuge from Beckman Coulter GmbH (Krefeld, Germany) to facilitate the filtration process. The extracts were freeze-dried for at least 48 h with the lyophilization machine Alpha 2-4 LSCplus from Martin Christ Gefriertrocknungsanlagen GmbH (Osterode am Harz, Germany). The powder was stored at −20°C until use. For the preparation of the methanolic extracts, plant material was extracted in methanol purchased from VWR International S.A.S. (Fontenay-sous-Bois, France) for 60 min at 40°C and filtered. In order to obtain a dry powder, they were evaporated to 5 ml with the rotary evaporator RV 10 basic from IKA®-Werke GmbH & Co. KG (Staufen, Germany) and dried in a Petri dish from VWR International GmbH (Darmstadt, Germany) under the exhaust hood overnight. Subsequently, the extracts were freeze-dried to lose residual water and stored at −20°C until use. For application in cell experiments, the aqueous extracts were solved in DPBS (Dulbecco’s phosphate-buffered saline) purchased from Lonza Cologne AG (Cologne, Germany), whereas the extracts made with methanol were first dissolved in DMSO (0.3% V/V final well concentration), which was acquired from Fisher Scientific GmbH (Schwerte, Germany), and filled with DPBS until final volume. Extract solutions were filtered with a 0.8 µm and a 0.2 µm sterile filter purchased from Carl Roth GmbH + Co. KG (Karlsruhe, Germany) and stored at −20°C until use. Three different extract concentrations (1.0, 0.1, or 0.01 mg/ml) were tested in MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] as described in the Supplementary Material and only the highest concentration, which did not affect cell viability, was used in uptake studies. The GLUT2 inhibitor phloretin, which was purchased from Cayman Chemical (Michigan, United States), and the SGLT1 inhibitor phlorizin applied from Carl Roth GmbH + Co. KG (Karlsruhe, Germany) were used with a final well concentration of 100 µM as a positive control for uptake inhibition of glucose.
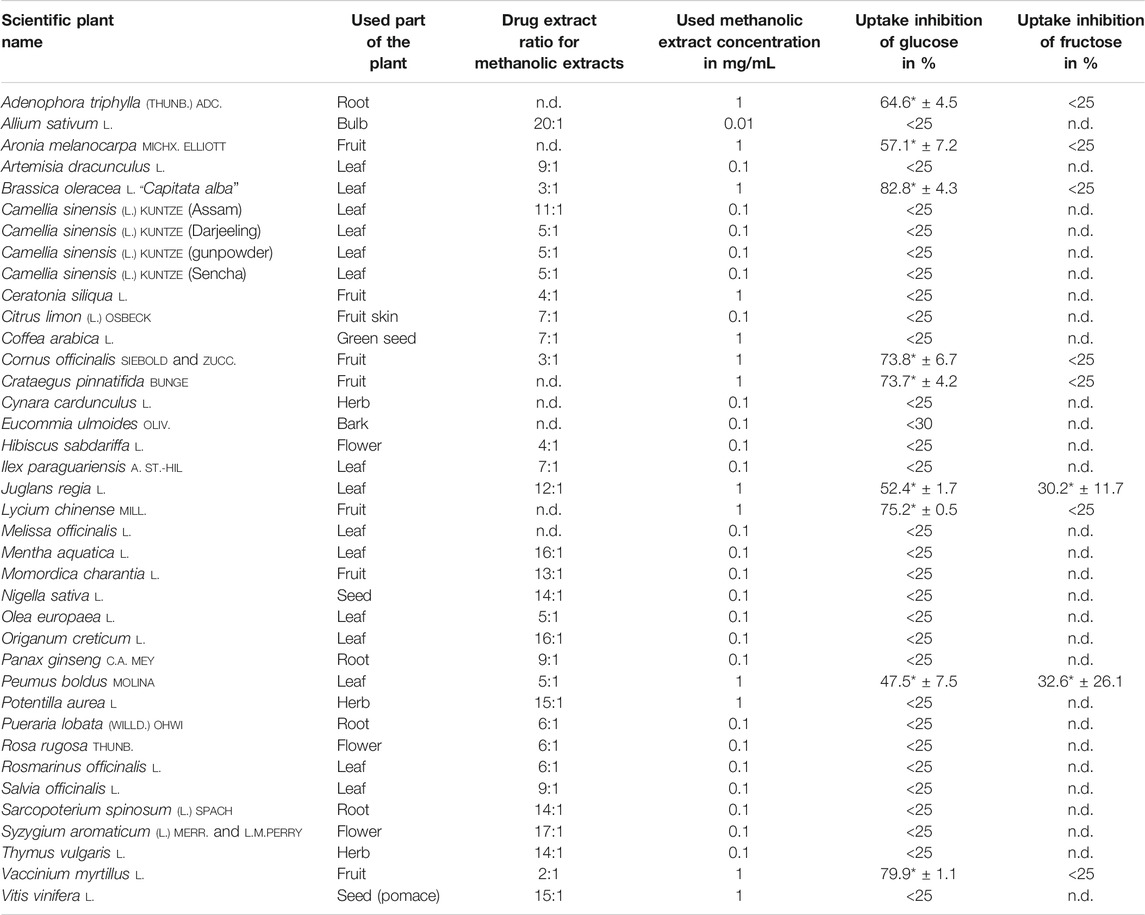
TABLE 2. Results of glucose and fructose uptake studies with methanolic extracts, the used plant part, drug extract ratio, and tested concentration, which did not show any influence on cells in MTT assay. Only the plants that showed the strongest inhibition rates on glucose uptake were tested for fructose uptake inhibition. Regarding methanolic and aqueous extracts, the extract of a plant that showed the stronger inhibition on glucose absorption was repeated and tested for its impact on fructose uptake; mean value ± SD; *significant difference in U-test (n = 2 with 12 replicates each; two-sided; α = 0.05); n.d. = not determined.
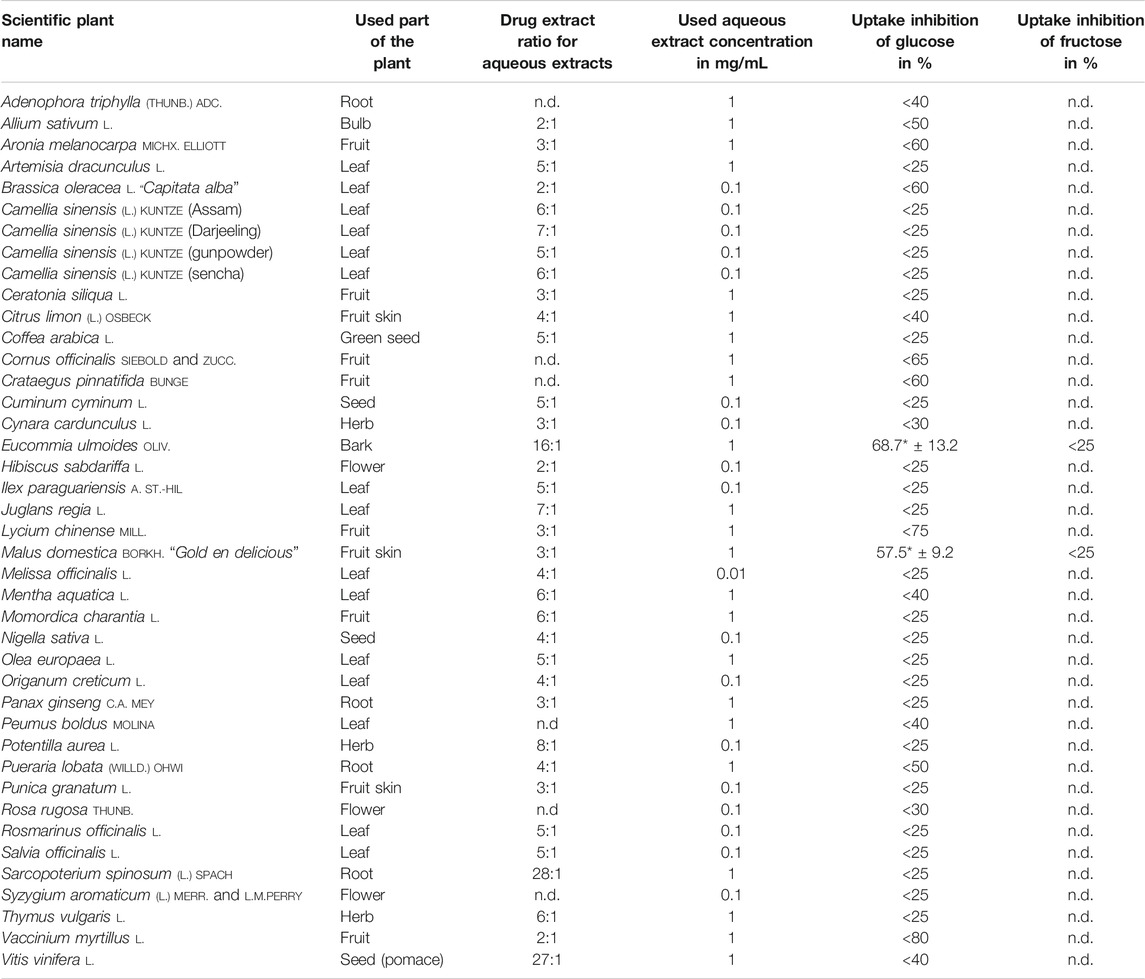
TABLE 3. Results of glucose and fructose uptake studies with aqueous extracts, the used plant part, drug extract ratio, and tested concentration, which did not show any influence on cells in MTT assay. Only the plants that showed the strongest inhibition rates on glucose uptake were tested for fructose uptake inhibition. Regarding methanolic and aqueous extracts, the extract of a plant that showed the stronger inhibition on glucose absorption was repeated and tested for its impact on fructose uptake; mean value ± SD; *significant difference in U-test (n = 2–3; 12 replicates each; two-sided; α = 0.05); n.d. = not determined.
Caco2 cells were purchased from Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany) and seeded in 24-well plates, which were acquired from Greiner Bio-One GmbH (Frickenhausen, Germany), for every experiment. As general medium DMEM (Dulbecco’s modified eagle medium) without phenol red containing 1% UltraGlutamine 1, both acquired from Lonza Cologne AG (Cologne, Germany) and 5 or 20% FBS derived from Bio&SELL GmbH (Nürnberg, Germany) for experiment or mother line, respectively, was used for the handling with Caco2 cells. Cells were washed once with PBS from Lonza Cologne AG (Cologne, Germany) for every medium change and washed twice before Trypsin (TrypLE™ Express without phenol red) from Gibco Life Technologies corporation (NY, United States) was applied to detach the cells for seeding. The cells were incubated at 37°C and 5% CO2-supply during the whole time. Passages 20–35 were used in the experiments. The buffers and solutions for cell culture were prepared with water obtained from the ultrapure water system LaboStar from Siemens AG Wasseraufbereitung (Barsbüttel, Germany).
For uptake studies, cells were seeded in a density of 400,000 cells per well. Confluence appeared 24 h after seeding and was controlled visually. The radiolabeled substrates 1 mCi/ml glucose (specific activity: 30 Ci/mmol) and 1 mCi/ml fructose (specific activity: 5 Ci/mmol) were obtained from Biotrend Chemikalien GmbH (Köln, Germany). Although fructose absorption into cells was determined by using 54 nM solution of radiolabeled substrate as final well concentration, the 2 nM radiolabeled glucose solution was spiked with 0.001 µM non-radiolabeled glucose, which was purchased from Merck KGaA (Darmstadt, Germany). For uptake studies with extracts, cells were used at day three and day fifteen after confluence for glucose and fructose, respectively. On the day of the experiment, cells were washed once with 2 ml buffer containing 20 mM Hepes [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], which was obtained from Carl Roth GmbH + Co. KG (Karlsruhe, Germany) and 150 mM NaCl purchased from Fisher Scientific GmbH (Schwerte, Germany). The pH of the buffer was adjusted with sodium hydroxide from Merck KGaA (Darmstadt, Germany) using a 766 Laboratory pH meter from Knick Elektronische Messgeraete GmbH & Co. KG (Berlin, Germany) to 7.4. After 1 h starving incubation with Hepes-NaCl buffer at 37°C, substrate or substrate-extract solution were added to the cells and incubated at 37°C. After 1 h, the buffer was discarded, the cells were washed once with 1 ml ice-cold Hepes-NaCl buffer, and 500 µL Hepes-NaCl solution containing 1% Triton X 100 was added to each well. The samples were taken from the well plates, added to a sufficient volume of scintillation cocktail in scintillation tubes, both purchased from Hidex (Mainz, Germany), mixed for 20 s using a vortexer from Heidolph Instruments GmbH & Co. KG (Schwabach, Germany), and measured with a β-Counter from Hidex (Mainz, Germany). The plant extracts, which showed the strongest inhibition on intestinal glucose transporters, were repeated and tested for their influence on fructose uptake with a focus on methanolic extracts. Randomly, well plates, which were cultivated and treated under the same conditions as cells used for uptake studies, were tested in Hoechst Assay as described in the Supplementary Material to determine variations depending on the procedure.
All statistical tests were performed using Microsoft Excel. Normal distribution was established via Shapiro–Wilk test manually before using Excel’s tool “Data analysis” for provision of the F-test and the t-tests. If the data was not normally distributed, Mann–Whitney U test was applied for further statistical analysis. IC50 data was calculated with GraphPad Prism 5.0.
In order to prevent decreased metabolic activity due to the use of toxic extracts, methanolic and aqueous plant preparations were tested in MTT assay and the concentration, which did not reduce cell viability significantly, was used for uptake studies as shown in Tables 2, 3. According to the results obtained from Hoechst Assay that showed an average coefficient of variation of 21.7% within 14 randomly tested well plates, uptake inhibition of plant extracts was distinguishable from general fluctuations in the procedure for values >25%. In every experiment, a control without extract was running and served as 100% uptake control. After correction of values by subtracting the blank, uptake inhibition was calculated as the difference between control and sample. The obtained values of monosaccharide uptake inhibition in Caco2 cells treated with methanolic and aqueous extracts are reported in Tables 2, 3, respectively. Only the herbal drugs that showed the strongest inhibition rates on glucose uptake were tested for fructose uptake inhibition. For some of the plants, methanolic and aqueous extracts decreased glucose uptake significantly. However, only the extract of a plant that showed stronger inhibition on glucose absorption was repeated and tested for its impact on fructose uptake as described for the following plants. Additionally, the focus for further studies was on methanolic extracts as they naturally contain fewer monosaccharides, which could interfere with the measurement. The methanolic extracts made from the fruits of Aronia melanocarpa (uptake inhibition: 57.1%),Cornus officinalis (uptake inhibition: 73.8%), Crataegus pinnatifida (uptake inhibition: 73.7%), Lycium chinense (uptake inhibition: 75.2%), and Vaccinium myrtillus (uptake inhibition: 79.9%); the leaves of Brassica oleracea (uptake inhibition: 82.8%), Juglans regia (uptake inhibition: 52.4%), and Peumus boldus (uptake inhibition: 47.5%); and the roots of Adenophora triphylla (uptake inhibition: 64.6%) indicated a strong and reproducible inhibitory potential between 40 and 80% on intestinal glucose transporters as visualized in Figure 1. Seven of these extracts showed higher uptake inhibition rates than the two controls phloretin (uptake inhibition: 60.4%) and phlorizin (uptake inhibition: 55.1%). A queous extracts made from the bark of Eucommia ulmoides (uptake inhibition: 68.7%) and the fruits of Malus domestica (uptake inhibition: 57.5%) inhibited glucose uptake significantly and reproducibly. Additionally, for the methanolic extract of Brassica oleracea and the aqueous extract of Eucommia ulmoides, IC50 was calculated as 0.0904 and 0.4285 mg/ml, respectively. These extracts decreased glucose uptake in a dose-dependent manner. The methanolic extracts of Juglans regia (uptake inhibition: 30.2%) and Peumus boldus (uptake inhibition: 32.6%) inhibited the fructose uptake significantly.
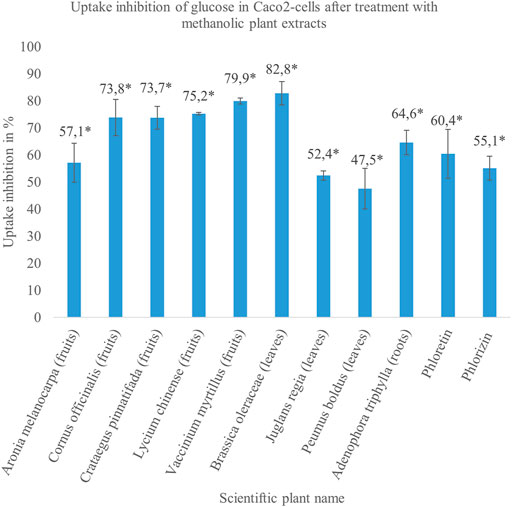
FIGURE 1. The results of the methanolic plant extracts, which reduced glucose uptake between 40 and 80% in treated cells significantly compared to untreated cells in a concentration of 1 mg/ml. The extracts were made from different parts of the plants enclosed in brackets. The solutions of 100 µM phloretin and 100 µM phlorizin were used as positive controls and inhibited glucose uptake in Caco2 cells by approximately 60%. The illustrated results refer to the data in Table 2. Mean value ± SD. * Significant difference in U-test (n = 2 with 12 replicates each; two-sided, α = 0.05).
According to the results of the present study, the observed hypoglycemic effects in animal and/or human studies after treatment with preparations of Adenophora triphylla,Aronia melanocarpa, Brassica oleracea, Cornus officinalis,Crataegus pinnatifida, Eucommia ulmoides, Juglans regia,Lycium chinense, Malus domestica, Peumus boldus, and Vaccinium myrtillus as listed in Table 1 possibly relate to the inhibition of intestinal transporters. The methanolic plant extracts made from the leaves of Juglans regia and Peumus boldus decreased glucose as well as fructose uptake possibly related to inhibition of GLUT2, which is able to transport both monosaccharides. These findings show that the traditional use of Brassica oleracea, Cornus officinalis, Eucommia ulmoides, Juglans regia, Lycium chinense, Malus domestica, and Vaccinium myrtillus and their preparations as antidiabetic remedy and adjuncts to conventional treatments in the therapy of Diabetes mellitus is reasonable. Extracts of these plants show inhibitory activity on intestinal glucose and fructose transporters resulting in lower blood concentrations of the monosaccharides and reduced concomitant health problems.
In literature, there is not much comparable data available, because the inhibition of intestinal transporters by crude plant extracts in Caco2 cells has not been investigated very well (Schreck and Melzig, 2018). Kim et al. determined a moderate inhibition of glucose uptake in Caco2 cells for methanolic extracts of Adenophora triphylla (uptake inhibition: 13.4%) and Cornus officinalis (uptake inhibition: 16.4%), but other results as for Crataegus pinnatifida (no uptake inhibition) are not consistent with our findings (Kim et al., 2011). Zhang et al. confirmed an uptake inhibition of 26.3% for Eucommia ulmoides (Zhang et al., 2015). Manzano et al. only tested specific polyphenol-rich fractions of Malus domestica (Manzano and Williamson, 2010), which showed inhibitory activity on intestinal glucose transporters, but it is not directly comparable with our work. Discrepancies between results from different studies probably depend on different preparations of the extracts, the use of modified substrates, and/or an insufficient validation of the cell model. We determined the expression of relevant transporters over three weeks in Caco2 cells and adapted our experimental settings in accordance with the ensured presence of functional transporters (Schreck and Melzig, 2019). Buchholz et al. examined whether extracts of the traditionally used plants inhibit cleavage of polysaccharides and lipids by affecting lipase and α-amylase activity (Prasain et al., 2012). The methanolic extracts of Aronia melanocarpa, Peumus boldus, and Vaccinium myrtillus showed strong lipase and α-amylase inhibition as well as inhibition of intestinal monosaccharide transporters in our studies. This multi-target effect of the mentioned plant preparations describes a partial, but multiple drug action and is proposed to exceed drugs with single-target effects. Based on synergistic mechanisms, the plants that affect various targets to alleviate monosaccharide uptake show therapeutic advantages concerning higher intended and lower adverse effects (Csermely et al., 2005). Since the plants, especially the fruits, contain sugars themselves, it cannot be totally excluded that a part of the inhibition is a “pseudo-inhibition” and comes from the displacement of the radioactive substrates by non-radioactive monosaccharides during absorption processes. For this reason, the focus of this work was on methanolic extracts that naturally contain fewer monosaccharides. Commonly, the discussed constituents of the plants with inhibitory effects on intestinal transporters are polyphenolic compounds as shown in Table 1. Particularly, the chalcones phlorizin and phloretin (Debnam and Levin, 1975; Zheng et al., 2012; Raja and Kinne, 2015), which are often used as positive controls in transport studies, the flavonoids quercetin, fisetin, and myricetin (Kwon et al., 2007) and the tannins (-)-epigallocatechin gallate, (-)-epigallocatechin, and (-)-epicatechin gallate seem to act as inhibitors of intestinal, monosaccharide transporters (Johnston et al., 2005). Further studies are planned to investigate which fraction of the extracts, or more specifically, which group of compounds are responsible for the inhibition of the intestinal glucose and fructose transporters in this study.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
KS designed the studies, performed experiments, analyzed the data, and drafted the manuscript. MM supervised the performance of the experiments and interpretation of the data. MM proofread the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Yi Gu for screening the plants in the MTT assay according to our protocol. We acknowledge the support from the Open Access Publication Fund of the Freie Universitaet Berlin.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.692566/full#supplementary-material
Al-Aboudi, A., and Afifi, F. U. (2011). Plants Used for the Treatment of Diabetes in jordan: A Review of Scientific Evidence. Pharm. Biol. 49, 221–239. doi:10.3109/13880209.2010.501802
American Diabetes Association (2014). Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 37, 81–90.
Asadi, A., Shidfar, F., Safari, M., Hosseini, A. F., Fallah Huseini, H., Heidari, I., et al. (2019). Efficacy of Melissa Officinalis L. (Lemon Balm) Extract on Glycemic Control and Cardiovascular Risk Factors in Individuals with Type 2 Diabetes: A Randomized, Double-Blind, Clinical Trial. Phytother Res. 33, 651–659. doi:10.1002/ptr.6254
Assad, T., Khan, R. A., and Feroz, Z. (2014). Evaluation of Hypoglycemic and Hypolipidemic Activity of Methanol Extract of brassica Oleracea. Chin. J. Nat. Medicines 12, 648–653. doi:10.1016/s1875-5364(14)60099-6
Bachrach, Z. Y. (2007). Ethnobotanical Studies of Sarcopoterium Spinosum in israel. Isr. J. Plant Sci. 55, 111–114. doi:10.1560/ijps.55.1.111
Bagri, P., Ali, M., Aeri, V., Bhowmik, M., and Sultana, S. (2009). Antidiabetic Effect of Punica Granatum Flowers: Effect on Hyperlipidemia, Pancreatic Cells Lipid Peroxidation and Antioxidant Enzymes in Experimental Diabetes. Food Chem. Toxicol. 47, 50–54. doi:10.1016/j.fct.2008.09.058
Bahadoran, Z., Mirmiran, P., and Azizi, F. (2013). Dietary Polyphenols as Potential Nutraceuticals in Management of Diabetes: A Review. J. Diabetes Metab. Disord. 12, 43. doi:10.1186/2251-6581-12-43
Bakırel, T., Bakırel, U., Keleş, O. Ü., Ülgen, S. G., and Yardibi, H. (2008). In Vivo assessment of Antidiabetic and Antioxidant Activities of Rosemary (Rosmarinus Officinalis) in Alloxan-Diabetic Rabbits. J. Ethnopharmacology 116, 64–73.
Bamosa, A. O., Kaatabi, H., Lebdaa, F. M., Elq, A. M., and Al-Sultanb, A. (2010). Effect of Nigella Sativa Seeds on the Glycemic Control of Patients with Type 2 Diabetes Mellitus. Indian J. Physiol. Pharmacol. 54, 344–354.
Banjari, I., Misir, A., Šavikin, K., Jokić, S., Molnar, M., De Zoysa, H. K. S., et al. (2017). Antidiabetic Effects of Aronia Melanocarpa and its Other Therapeutic Properties. Front. Nutr. 4, 53. doi:10.3389/fnut.2017.00053
Behradmanesh, S., Derees, F., and Rafieian-Kopaei, M. (2013). Effect of Salvia Officinalis on Diabetic Patients. J. Ren. Inj Prev 2, 51–54. doi:10.12861/jrip.2013.18
Buchholz, T. (2017). “Secondary Metabolites as Inhibitors of Selected Digestive Enzymes,” (Freie Universitaet Berlin). Thesis.
Buchholz, T., and Melzig, M. F. (2016). Medicinal Plants Traditionally Used for Treatment of Obesity and Diabetes Mellitus - Screening for Pancreatic Lipase and α-Amylase Inhibition. Phytother. Res. 30, 260–266. doi:10.1002/ptr.5525
Campos-Florian, J., Bardales-Valdivia, J., Caruajulca-Guevara, L., and Cueva-Llanos, D. (2013). Anti-diabetic Effect of Coffea Arabica, in Alloxan-Induced Diabetic Rats. Emirates J. Food Agric. 25, 772.
Chantret, I., Barbat, A., Dussaulx, E., Brattain, M. G., and Zweibaum, A. (1988). Epithelial Polarity, Villin Expression, and Enterocytic Differentiation of Cultured Human colon Carcinoma Cells: A Survey of Twenty Cell Lines. Cancer Res. 48, 1936–1942.
Csermely, P., Ágoston, V., and Pongor, S. (2005). The Efficiency of Multi-Target Drugs: The Network Approach Might Help Drug Design. Trends Pharmacol. Sci. 26, 178–182. doi:10.1016/j.tips.2005.02.007
de Freitas Junior, L. M., and de Almeida, E. B. (2017). Medicinal Plants for the Treatment of Obesity: Ethnopharmacological Approach and Chemical and Biological Studies. Am. J. Transl Res. 9, 2050–2064.
Debnam, E. S., and Levin, R. J. (1975). An Experimental Method of Identifying and Quantifying the Active Transfer Electrogenic Component from the Diffusive Component during Sugar Absorption Measured In Vivo. J. Physiol. 246, 181–196. doi:10.1113/jphysiol.1975.sp010885
Dehghani, S., Mehri, S., and Hosseinzadeh, H. (2019). The Effects of Crataegus Pinnatifida (Chinese Hawthorn) on Metabolic Syndrome: A Review. Iran J. Basic Med. Sci. 22, 460–468. doi:10.22038/IJBMS.2019.31964.7678
Eidi, A., and Eidi, M. (2009). Antidiabetic Effects of Sage (Salvia Officinalis l.) Leaves in normal and Streptozotocin-Induced Diabetic Rats. Diabetes Metab. Syndr. Clin. Res. Rev. 3, 40–44. doi:10.1016/j.dsx.2008.10.007
Eidi, A., Eidi, M., and Darzi, R. (2009). Antidiabetic Effect ofOlea europaeaL. In normal and Diabetic Rats. Phytother. Res. 23, 347–350. doi:10.1002/ptr.2629
Eidi, A., Eidi, M., and Esmaeili, E. (2006). Antidiabetic Effect of Garlic (Allium Sativum l.) in normal and Streptozotocin-Induced Diabetic Rats. Phytomedicine 13, 624–629. doi:10.1016/j.phymed.2005.09.010
Ekoh, S. N., Akubugwo, I. E., Ude, V. C., and Edwin, N. (2014). Anti-hyperglycemic and Anti-hyperlipidemic Effect of Spices (Thymus Vulgaris, Murraya Koenigii, Ocimum Gratissimum and Piper Guineense) in Alloxan-Induced Diabetic Rats. Int. J. Biosciences 4, 179–187.
Elyasiyan, U., Nudel, A., Skalka, N., Rozenberg, K., Drori, E., Oppenheimer, R., et al. (2017). Anti-diabetic Activity of Aerial Parts of Sarcopoterium Spinosum. BMC Complement. Altern. Med. 17, 356. doi:10.1186/s12906-017-1860-7
Fabricant, D. S., and Farnsworth, N. R. (2001). The Value of Plants Used in Traditional Medicine for Drug Discovery. Environ. Health Perspect. 109, 69–75. doi:10.1289/ehp.01109s169
Gilani, A. H., Rahman, U.-A., and Trends, R. (2005). Trends in Ethnopharmacology. J. Ethnopharmacology 100, 43–49. doi:10.1016/j.jep.2005.06.001
Gomes, A., Vedasiromoni, J. R., Das, M., Sharma, R. M., and Ganguly, D. K. (1995). Anti-hyperglycemic Effect of Black tea (Camellia Sinensis) in Rat. J. Ethnopharmacology 45, 223–226. doi:10.1016/0378-8741(95)01223-z
Grover, J. K., and Yadav, S. P. (2004). Pharmacological Actions and Potential Uses of Momordica Charantia: A Review. J. Ethnopharmacology 93, 123–132. doi:10.1016/j.jep.2004.03.035
Grundy, S. M. (1998). Multifactorial Causation of Obesity: Implications for Prevention. Am. J. Clin. Nutr. 67, 563S–572S. doi:10.1093/ajcn/67.3.563s
Han, Q., Yu, Q.-Y., Shi, J., Xiong, C.-Y., Ling, Z.-J., and He, P.-M. (2011). Molecular Characterization and Hypoglycemic Activity of a Novel Water-Soluble Polysaccharide from tea (Camellia Sinensis) Flower. Carbohydr. Polym. 86, 797–805. doi:10.1016/j.carbpol.2011.05.039
Hanhineva, K., Törrönen, R., Bondia-Pons, I., Pekkinen, J., Kolehmainen, M., Mykkänen, H., et al. (2010). Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 11, 1365–1402. doi:10.3390/ijms11041365
Hasanein, P., and Riahi, H. (2015). Antinociceptive and Antihyperglycemic Effects of Melissa Officinalis Essential Oil in an Experimental Model of Diabetes. Med. Princ Pract. 24, 47–52. doi:10.1159/000368755
He, K., Song, S., Zou, Z., Feng, M., Wang, D., Wang, Y., et al. (2016). The Hypoglycemic and Synergistic Effect of Loganin, Morroniside, and Ursolic Acid Isolated from the Fruits of Cornus Officinalis. Phytother. Res. 30, 283–291. doi:10.1002/ptr.5529
He, X., Wang, J., Li, M., Hao, D., Yang, Y., Zhang, C., et al. (2014). Eucommia Ulmoides oliv.: Ethnopharmacology, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. J. Ethnopharmacology 151, 78–92. doi:10.1016/j.jep.2013.11.023
Heidarian, E., and Soofiniya, Y. (2011). Hypolipidemic and Hypoglycemic Effects of Aerial Part of Cynara Scolymus in Streptozotocin-Induced Diabetic Rats. J. Med. Plants Res. 5, 2717–2723.
Helmstaedter, A. (2007). Antidiabetic Drugs Used in Europe Prior to the Discovery of Insulin. Die Pharmazie 62, 717–720.
Hidalgo, I. J., Raub, T. J., and Borchardt, R. T. (1989). Characterization of the Human colon Carcinoma Cell Line (Caco-2) as a Model System for Intestinal Epithelial Permeability. Gastroenterology 96, 736–749. doi:10.1016/s0016-5085(89)80072-1
Hogan, S., Zhang, L., Li, J., Sun, S., Canning, C., and Zhou, K. (2010). Antioxidant Rich Grape Pomace Extract Suppresses Postprandial Hyperglycemia in Diabetic Mice by Specifically Inhibiting Alpha-Glucosidase. Nutr. Metab. 7, 71. doi:10.1186/1743-7075-7-71
Hosseini, S., Jamshidi, L., Mehrzadi, S., Mohammad, K., Najmizadeh, A. R., Alimoradi, H., et al. (2014). Effects of Juglans Regia L. Leaf Extract on Hyperglycemia and Lipid Profiles in Type Two Diabetic Patients: A Randomized Double-Blind, Placebo-Controlled Clinical Trial. J. Ethnopharmacology 152, 451–456. doi:10.1016/j.jep.2014.01.012
Idm’hand, E., Msanda, F., and Cherifi, K. (2020). Ethnopharmacological Review of Medicinal Plants Used to Manage Diabetes in morocco. Clin. Phytoscience 6, 18.
Jagtap, A. G., and Patil, P. B. (2010). Antihyperglycemic Activity and Inhibition of Advanced Glycation End Product Formation by Cuminum Cyminum in Streptozotocin Induced Diabetic Rats. Food Chem. Toxicol. 48, 2030–2036. doi:10.1016/j.fct.2010.04.048
Jang, Y. Y., Song, J. H., Shin, Y. K., Han, E. S., and Lee, C. S. (2000). Protective Effect of Boldine on Oxidative Mitochondrial Damage in Streptozotocin-Induced Diabetic Rats. Pharmacol. Res. 42, 361–371. doi:10.1006/phrs.2000.0705
Johnston, K., Sharp, P., Clifford, M., and Morgan, L. (2005). Dietary Polyphenols Decrease Glucose Uptake by Human Intestinal Caco-2 Cells. FEBS Lett. 579, 1653–1657. doi:10.1016/j.febslet.2004.12.099
Jumarie, C., and Malo, C. (1991). Caco-2 Cells Cultured in Serum-free Medium as a Model for the Study of Enterocytic Differentiation In Vitro. J. Cel. Physiol. 149, 24–33. doi:10.1002/jcp.1041490105
Kang, Y.-R., Lee, H.-Y., Kim, J.-H., Moon, D.-I., Seo, M.-Y., Park, S.-H., et al. (2012). Anti-obesity and Anti-diabetic Effects of Yerba Mate (ilex Paraguariensis) in C57bl/6j Mice Fed a High-Fat Diet. Lab. Anim. Res. 28, 23–29. doi:10.5625/lar.2012.28.1.23
Kataya, H. A. H., and Hamza, A. A. (2008). Red Cabbage (brassica Oleracea) Ameliorates Diabetic Nephropathy in Rats. Evidence-Based Complement. Altern. Med. 5, 281–287. doi:10.1093/ecam/nem029
Katemo, M., Mpiana, P. T., Mbala, B. M., Mihigo, S. O., Ngbolua, K. N., Tshibangu, D. S. T., et al. (2012). Ethnopharmacological Survey of Plants Used against Diabetes in Kisangani City (Dr congo). J. Ethnopharmacology 144, 39–43. doi:10.1016/j.jep.2012.08.022
Khathi, A., Serumula, M. R., Myburg, R. B., Van Heerden, F. R., and Musabayane, C. T. (2013). Effects of Syzygium Aromaticum-Derived Triterpenes on Postprandial Blood Glucose in Streptozotocin-Induced Diabetic Rats Following Carbohydrate challenge. PloS one 8, e81632. doi:10.1371/journal.pone.0081632
Kim, H. K., Baek, S.-S., and Cho, H.-Y. (2011). Inhibitory Effect of Pomegranate on Intestinal Sodium Dependent Glucose Uptake. Am. J. Chin. Med. 39, 1015–1027. doi:10.1142/s0192415x11009378
Kim, H. Y., Moon, B. H., Lee, H. J., and Choi, D. H. (2004). Flavonol Glycosides from the Leaves of Eucommia Ulmoides O. With Glycation Inhibitory Activity. J. ethnopharmacology 93, 227–230. doi:10.1016/j.jep.2004.03.047
Kwon, O., Eck, P., Chen, S., Corpe, C. P., Lee, J. H., Kruhlak, M., et al. (2007). Inhibition of the Intestinal Glucose Transporter Glut2 by Flavonoids. FASEB j. 21, 366–377. doi:10.1096/fj.06-6620com
Lee, M.-K., Kim, M.-J., Cho, S.-Y., Park, S. A., Park, K.-K., Jung, U. J., et al. (2005). Hypoglycemic Effect of Du-Zhong (Eucommia Ulmoides oliv.) Leaves in Streptozotocin-Induced Diabetic Rats. Diabetes Res. Clin. Pract. 67, 22–28. doi:10.1016/j.diabres.2004.05.013
Lee, Y.-H., Jung, M. G., Kang, H. B., Choi, K.-C., Haam, S., Jun, W., et al. (2008). Effect of Anti-histone Acetyltransferase Activity from Rosa Rugosa Thunb. (Rosaceae) Extracts on Androgen Receptor-Mediated Transcriptional Regulation. J. Ethnopharmacology 118, 412–417. doi:10.1016/j.jep.2008.05.006
Li, W. L., Zheng, H. C., Bukuru, J., and De Kimpe, N. (2004). Natural Medicines Used in the Traditional Chinese Medical System for Therapy of Diabetes Mellitus. J. Ethnopharmacology 92, 1–21. doi:10.1016/j.jep.2003.12.031
Liu, L., Tang, D., Zhao, H., Xin, X., and Aisa, H. A. (2017). Hypoglycemic Effect of the Polyphenols Rich Extract from Rose Rugosa Thunb on High Fat Diet and Stz Induced Diabetic Rats. J. Ethnopharmacology 200, 174–181. doi:10.1016/j.jep.2017.02.022
Ma, W., Wang, K.-J., Cheng, C.-S., Yan, G.-q., Lu, W.-L., Ge, J.-F., et al. (2014). Bioactive Compounds from Cornus Officinalis Fruits and Their Effects on Diabetic Nephropathy. J. Ethnopharmacology 153, 840–845. doi:10.1016/j.jep.2014.03.051
Maljaars, P. W. J., Peters, H. P. F., Mela, D. J., and Masclee, A. A. M. (2008). Ileal Brake: A Sensible Food Target for Appetite Control. A Review. Physiol. Behav. 95, 271–281. doi:10.1016/j.physbeh.2008.07.018
Manzano, S., and Williamson, G. (2010). Polyphenols and Phenolic Acids from Strawberry and Apple Decrease Glucose Uptake and Transport by Human Intestinal Caco-2 Cells. Mol. Nutr. Food Res. 54, 1773–1780. doi:10.1002/mnfr.201000019
Naim, M., Amjad, F. M., Sultana, S., Islam, S. N., Hossain, M. A., Begum, R., et al. (2012). Comparative Study of Antidiabetic Activity of Hexane-Extract of Lemon Peel (limon Citrus) and Glimepiride in Alloxan-Induced Diabetic Rats. Bangla Pharma J. 15, 131–134. doi:10.3329/bpj.v15i2.12577
Nazni, P., Vijayakumar, T. P., Alagianambi, P., and Amirthaveni, M. (2006). Hypoglycemic and Hypolipidemic Effect of Cynara Scolymus Among Selected Type 2 Diabetic Individuals. Pakistan J. Nutr. 5, 147–151.
Neves, J. M., Matos, C., Moutinho, C., Queiroz, G., and Gomes, L. R. (2009). Ethnopharmacological Notes about Ancient Uses of Medicinal Plants in Trás-Os-Montes (Northern of Portugal). J. Ethnopharmacology 124, 270–283. doi:10.1016/j.jep.2009.04.041
Olatunji, O. J., Chen, H., and Zhou, Y. (2017). Effect of the Polyphenol Rich Ethyl Acetate Fraction from the Leaves of Lycium Chinensemill. On Oxidative Stress, Dyslipidemia, and Diabetes Mellitus in Streptozotocin-Nicotinamide Induced Diabetic Rats. Chem. biodiversity 14, e1700277. doi:10.1002/cbdv.201700277
Ota, A., and Ulrih, N. P. (2017). An Overview of Herbal Products and Secondary Metabolites Used for Management of Type Two Diabetes. Front. Pharmacol. 8, 436. doi:10.3389/fphar.2017.00436
Park, S. A., Choi, M.-S., Kim, M.-J., Jung, U. J., Kim, H.-J., Park, K.-K., et al. (2006). Hypoglycemic and Hypolipidemic Action of Du-Zhong (Eucommia Ulmoides Oliver) Leaves Water Extract in C57bl/ksj-Db/db Mice. J. Ethnopharmacology 107, 412–417. doi:10.1016/j.jep.2006.03.034
Park, S. J., Nam, J., Ahn, C. W., and Kim, Y. (2019). Anti-diabetic Properties of Different Fractions of Korean Red Ginseng. J. Ethnopharmacology 236, 220–230. doi:10.1016/j.jep.2019.01.044
Peng, C.-H., Chyau, C.-C., Chan, K.-C., Chan, T.-H., Wang, C.-J., and Huang, C.-N. (2011). Hibiscus sabdariffaPolyphenolic Extract Inhibits Hyperglycemia, Hyperlipidemia, and Glycation-Oxidative Stress while Improving Insulin Resistance. J. Agric. Food Chem. 59, 9901–9909. doi:10.1021/jf2022379
Prasain, J. K., Peng, N., Rajbhandari, R., and Wyss, J. M. (2012). The Chinese Pueraria Root Extract (Pueraria Lobata) Ameliorates Impaired Glucose and Lipid Metabolism in Obese Mice. Phytomedicine 20, 17–23. doi:10.1016/j.phymed.2012.09.017
Prasanna, R., Ashraf, E. A., and Essam, M. A. (2017). Chamomile and Oregano Extracts Synergistically Exhibit Antihyperglycemic, Antihyperlipidemic, and Renal Protective Effects in Alloxan-Induced Diabetic Rats. Can. J. Physiol. Pharmacol. 95, 84–92. doi:10.1139/cjpp-2016-0189
Raja, M., and Kinne, R. K. H. (2015). Identification of Phlorizin Binding Domains in Sodium-Glucose Cotransporter Family: Sglt1 as a Unique Model System. Biochimie 115, 187–193. doi:10.1016/j.biochi.2015.06.003
Ribnicky, D. M., Poulev, A., Watford, M., Cefalu, W. T., and Raskin, I. (2006). Antihyperglycemic Activity of Tarralin, an Ethanolic Extract of Artemisia Dracunculus L. Phytomedicine 13, 550–557. doi:10.1016/j.phymed.2005.09.007
Rizvi, S. I., and Mishra, N. (2013). Traditional Indian Medicines Used for the Management of Diabetes Mellitus. J. Diabetes Res. 2013, 712092. doi:10.1155/2013/712092
Rtibi, K., Selmi, S., Grami, D., Amri, M., Eto, B., El-benna, J., et al. (2017). Chemical Constituents and Pharmacological Actions of Carob Pods and Leaves (Ceratonia Siliqua l.) on the Gastrointestinal Tract: A Review. Biomed. Pharmacother. 93, 522–528. doi:10.1016/j.biopha.2017.06.088
Sambuy, Y., De Angelis, I., Ranaldi, G., Scarino, M. L., Stammati, A., and Zucco, F. (2005). The Caco-2 Cell Line as a Model of the Intestinal Barrier: Influence of Cell and Culture-Related Factors on Caco-2 Cell Functional Characteristics. Cell Biol Toxicol 21, 1–26. doi:10.1007/s10565-005-0085-6
Sanae, F., Miyamoto, K.-i., Sawanishi, H., Kizu, H., Tomimori, Y., Sun, J.-n., et al. (1996). Hypoglycaemic Effects ofShokatsu-Cha(Xiao-Ke-Ca) in Streptozotocin-Induced Diabetes Mellitus. Phytother. Res. 10, 127–130. doi:10.1002/(sici)1099-1573(199603)10:2<127::aid-ptr784>3.0.co;2-q
Schreck, K., and Melzig, M. F. (2018). Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells. Molecules (Basel, Switzerland) 23, 2544. doi:10.3390/molecules23102544
Schreck, K., and Melzig, M. F. (2019). The Expression of Selected Intestinal Glucose, Fructose and Long-Chain Fatty Acid Transporters Investigated in Caco-2 Cells. Planta Med. 85, 491.
Schulze, C., Bangert, A., Kottra, G., Geillinger, K. E., Schwanck, B., Vollert, H., et al. (2014). Inhibition of the Intestinal Sodium-Coupled Glucose Transporter 1 (Sglt1) by Extracts and Polyphenols from Apple Reduces Postprandial Blood Glucose Levels in Mice and Humans. Mol. Nutr. Food Res. 58, 1795–1808. doi:10.1002/mnfr.201400016
Sears, B., and Perry, M. (2015). The Role of Fatty Acids in Insulin Resistance. Lipids Health Dis. 14, 121. doi:10.1186/s12944-015-0123-1
Shah, M. A., Sarker, D. M. M. R., and Inamdar, M. (2016). Antidiabetic Potential of brassica Oleracea Var. Italica in Type 2 Diabetic sprague Dawley (Sd) Rats. Int. J. Pharmacognosy Phytochem. Res. 8, 462–469.
Shakeri, A., Sahebkar, A., and JavadiMelissa officinalis, B. l. (2016). Melissa Officinalis L. - A Review of its Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacology 188, 204–228. doi:10.1016/j.jep.2016.05.010
Sidorova, Y., Shipelin, V., Mazo, V., Zorin, S., Petrov, N., and Kochetkova, A. (2017). Hypoglycemic and Hypolipidemic Effect of Vaccinium Myrtillus L. Leaf and Phaseolus Vulgaris L. Seed Coat Extracts in Diabetic Rats. Nutrition 41, 107–112. doi:10.1016/j.nut.2017.04.010
Smirin, P., Taler, D., Abitbol, G., Brutman-Barazani, T., Kerem, Z., Sampson, S. R., et al. (2010). Sarcopoterium Spinosum Extract as an Antidiabetic Agent: In Vitro and In Vivo Study. J. Ethnopharmacology 129, 10–17. doi:10.1016/j.jep.2010.02.021
Smith, S. C. (2007). Multiple Risk Factors for Cardiovascular Disease and Diabetes Mellitus. Am. J. Med. 120, S3–S11. doi:10.1016/j.amjmed.2007.01.002
Stöger, E. A. (2009). Arzneibuch der chinesischen medizin, monographien des arzneibuchs der volksrepublik china 2000 und 2005. Germany: Deutscher Apotheker Verlag Stuttgart.
Sugiyama, H., Akazome, Y., Shoji, T., Yamaguchi, A., Yasue, M., Kanda, T., et al. (2007). Oligomeric Procyanidins in Apple Polyphenol Are Main Active Components for Inhibition of Pancreatic Lipase and Triglyceride Absorption. J. Agric. Food Chem. 55, 4604–4609. doi:10.1021/jf070569k
Swanston-Flatt, S. K., Flatt, P. R., Day, C., and Bailey, C. J. (1991). Traditional Dietary Adjuncts for the Treatment of Diabetes Mellitus. Proc. Nutr. Soc. 50, 641–651. doi:10.1079/pns19910077
Tahraoui, A., El-Hilaly, J., Israili, Z. H., and Lyoussi, B. (2007). Ethnopharmacological Survey of Plants Used in the Traditional Treatment of Hypertension and Diabetes in South-Eastern morocco (Errachidia Province). J. Ethnopharmacology 110, 105–117. doi:10.1016/j.jep.2006.09.011
Vuksan, V., Sung, M.-K., Sievenpiper, J. L., Stavro, P. M., Jenkins, A. L., Di Buono, M., et al. (2008). Korean Red Ginseng (Panax Ginseng) Improves Glucose and Insulin Regulation in Well-Controlled, Type 2 Diabetes: Results of a Randomized, Double-Blind, Placebo-Controlled Study of Efficacy and Safety. Nutr. Metab. Cardiovasc. Dis. 18, 46–56. doi:10.1016/j.numecd.2006.04.003
Wang, X., Liu, Q., Zhu, H., Wang, H., Kang, J., Shen, Z., et al. (2017). Flavanols from the Camellia Sinensis Var. Assamica and Their Hypoglycemic and Hypolipidemic Activities. Acta Pharmaceutica Sinica B 7, 342–346. doi:10.1016/j.apsb.2016.12.007
WHO (2019). Diabetes. Available at: https://www.who.int/en/news-room/fact-sheets/detail/diabetes (Accessed April, , 20).
Yellanur Konda, P., Egi, J. Y., Dasari, S., Katepogu, R., Jaiswal, K. K., and Nagarajan, P. (2020). Ameliorative Effects of Mentha Aquatica on Diabetic and Nephroprotective Potential Activities in Stz-Induced Renal Injury. Comp. Clin. Pathol. 29, 189–199. doi:10.1007/s00580-019-03042-6
Zhang, Y., Zhang, H., Wang, F., Yang, D., Ding, K., and Fan, J. (2015). The Ethanol Extract of Eucommia Ulmoides Oliv. Leaves Inhibits Disaccharidase and Glucose Transport in Caco-2 Cells. J. Ethnopharmacology 163, 99–105. doi:10.1016/j.jep.2015.01.015
Keywords: plant extracts, traditional use, glucose, fructose, uptake inhibition, Diabetes mellitus, Caco2 cells
Citation: Schreck K and Melzig MF (2021) Traditionally Used Plants in the Treatment of Diabetes Mellitus: Screening for Uptake Inhibition of Glucose and Fructose in the Caco2-Cell Model. Front. Pharmacol. 12:692566. doi: 10.3389/fphar.2021.692566
Received: 08 April 2021; Accepted: 15 July 2021;
Published: 20 August 2021.
Edited by:
Michał Tomczyk, Medical University of Bialystok, PolandReviewed by:
Alexander N. Shikov, Saint-Petersburg State Chemical Pharmaceutical Academy, RussiaCopyright © 2021 Schreck and Melzig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias F. Melzig, bWVsemlnQHplZGF0LmZ1LWJlcmxpbi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.