- 1Division of Nephrology, Department of Medicine, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
- 3Division of Ultrasound, West China Hospital, Sichuan University, Chengdu, China
Background: Hyperuricemia is very common in patients with chronic kidney disease (CKD); the role of hyperuricemia in the occurrence and progression of kidney disease remains an interesting and unresolved issue for nephrologists, and whether urate-lowering therapy (ULT) is warranted in CKD patients is still in controversy. To summarize and compare the clinical outcomes and adverse events (AEs) of three common ULT drugs, we performed a systematic review and network meta-analysis of randomized clinical trials (RCTs).
Method: PubMed, MEDLINE, Clinical Trials.gov, EMBASE, and the Cochrane Central Register of Controlled Trials electronic databases were searched. The network meta-analysis was performed using the “gemtc 0.8-7” and its dependent packages in R software. The primary outcome was the change of renal function and uric acid; creatinine, proteinuria, blood pressure, and adverse events were assessed as the secondary outcomes.
Results: 16 RCTs involving 1,943 patients were included in the final network analysis. Febuxostat, allopurinol, and benzbromarone were not found to exert superior effects over placebo upon renoprotective effect. With respect to lowering urate, the three drugs showed to be statistically superior to placebo, while febuxostat could better lower urate than allopurinol (MD: −1.547; 95% CrI: −2.473 to −0.626). It is also indicated that febuxostat was superior to placebo at controlling blood pressure, while no differences were observed when allopurinol and benzbromarone were compared to placebo. These results are stable in subgroup analysis.
Conclusion: There is insufficient evidence to support the renoprotective effects of the three urate-lowering agents in CKD patients with hyperuricemia; febuxostat shows a tendency to be superior to allopurinol on lowering the decline of eGFR and increment of proteinturia, but the difference does not reach a statistical significance. Regarding its urate-lowering effect, febuxostat appears to be a satisfactory alternative to allopurinol and benzbromarone, and can control blood pressure better.
Introduction
Chronic kidney disease (CKD) is a public health problem that causes substantial morbidity and mortality because of its potential to progress to end-stage renal disease and promote high risk of cardiovascular events (Collaboration, 2020). Progression of CKD can result in decreased quality of life, increased medical expenses, and end-stage renal failure. Therefore, it is imperative to identify therapies that can slow the deterioration of kidney function.
Hyperuricemia is very common in CKD patients because of reduced urinary excretion of uric acid (Reginato et al., 2012). Hyperuricemia was also reported to promote development and progression of CKD (Jalal et al., 2013; Uchida et al., 2015). However, whether hyperuricemia is an indirect marker of impaired kidney function or plays a causative role in progression of kidney disease, or both, remains an interesting and unresolved issue for nephrologists (Sampson et al., 2017). The role of uric acid in the development of CKD and whether urate-lowering therapy (ULT) is warranted for its treatment are controversial issues (Tiku et al., 2018; Sato et al., 2019; Steiger et al., 2020).
Xanthine oxidase inhibitors are considered the primary class of ULT for patients with CKD. Although there are concerns over use of allopurinol (it induces fatal hypersensitive reactions and nephrotoxicity), it is still recommended as first-line therapy (Sato et al., 2019). However, these concerns may lead to allopurinol underdosing, resulting in poor control of hyperuricemia, and fewer studies were conducted on the use of allopurinol at sufficient doses (≥300 mg per day) (Stamp et al., 2011; Sato et al., 2019). An alternative, novel, and potent nonpurine selective xanthine oxidase inhibitor is febuxostat. It is well tolerated (Tiku et al., 2018) and does not require dose modification. Furthermore, it has been increasingly studied and was shown to exert satisfactory urate-lowering and renoprotective effects (Shibagaki et al., 2014). Another drug used for ULT, benzbromarone, is extensively prescribed in South America and Asia (Lee et al., 2008). Determining whether these common drugs used as ULT exert renoprotective effects when compared with placebo or usual therapy has been the subject of prior meta-analyses (Bose et al., 2014; Kanji et al., 2015; Liu et al., 2018; Zeng et al., 2018). However, direct comparisons of allopurinol, febuxostat, and benzbromarone remain scarce.
To summarize and compare the clinical outcomes and adverse events (AEs) associated with these three common drugs, we performed a systematic review and network meta-analysis of relevant randomized clinical trials (RCTs).
Materials and Methods
Data Sources and Search Strategy
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement extension for network meta-analysis (Hutton et al., 2015) and PRISMA guidelines (Moher et al., 2009). PubMed, MEDLINE, Clinical Trials.gov, EMBASE, and Cochrane Central Register of Controlled Trials electronic databases were comprehensively searched up to June 1, 2020. Search terms are shown in Supplementary Table 1.
Selection Criteria
All non-RCTs were excluded and language was restricted to English. Selected RCTs were those that 1) included CKD patients aged 18 years or older with hyperuricemia and who were being treated with the specified interventions (allopurinol, febuxostat, benzbromarone, placebo, or usual therapy); and 2) reported changes in renal function through measurement of the estimated glomerular filtration rate (eGFR), creatinine, or proteinuria. Considering that the definition of hyperuricemia has not reached a consensus at the current stage (Bardin and Richette, 2014), it was permissible to define hyperuricemia differently in different studies, but only studies with the mean serum urate level >7 mg/dl at baseline were included into our study. Two independent authors (XL and DHL) screened titles and abstracts in duplicate to ascertain potential eligibility. The exclusion criteria were studies 1) with follow-up time <3 months, 2) that included patients with end-stage renal disease, and 3) that included patients with kidney transplants. Potential eligible articles identified by either author subsequently underwent full-text review. Reasons for excluding articles were simultaneously recorded. Subsequently, three authors (XL, DHL, and YXQ) evaluated the full text of each article to determine whether it should be included. All selected articles were imported into EndNote and utilized for final analyses.
Outcomes
Primary outcomes were changes in eGFR, uric acid, creatinine, proteinuria, and blood pressure, while AEs were assessed as secondary outcomes.
Data Extraction and Quality Assessment
Data were collected in duplicate (XL and DHL), and primary authors were contacted when additional clarification was required. Concrete data points included study information (authors, year of publication, country, study type, sample size, interventions, and comparison arms) and features of study subjects (age, sex, inclusion and exclusion criteria, and clinical outcomes). Two authors (XL and DUL) reciprocally evaluated the extracted data, while another author (YXQ) resolved all disagreements when a consensus was not reached. Two authors (XL and DUL) independently evaluated the quality of each pair of comparison using the Cochrane risk-of-bias tool (Higgins et al., 2011; Supplementary Table 1).
Data Synthesis and Analysis
Network meta-analysis of different interventions was performed using “gemtc 0.8-7” and its dependent packages in R software (version 3.6.3, The R Foundation, https://www.r-project.org). A multiple treatment comparison was conducted using a Bayesian network framework with a Monte Carlo Markov chain (MCMC) model (Jansen et al., 2008). We simultaneously conducted four MCMC models, and the number of simulations was set up to 5,000, with the number of iterations set up to 20,000. To evaluate the overall heterogeneity of the model, we used parameter I (Reginato et al., 2012) to calculate the deviation of the size of the heterogeneity. Mean differences (MDs) with 95% credibility intervals (CrIs) were calculated for continuous variables. For binary variables, odds ratios (ORs) with 95% CrIs were logarithmically converted into MDs with 95% CrIs.
For pairwise meta-analysis, MDs and ORs were calculated with 95% confidence intervals. p < 0.05 was considered statistically different. Heterogeneity was examined using the Q-test and I statistic (Reginato et al., 2012). A random-effects model was used when studies were heterogeneous (p < 0.1 or I2 > 50%). Otherwise, the fixed-effects model was applied. All pairwise meta-analyses were conducted in R with the “meta 4.15-1” package.
Results
Literature Search Outcomes and Study Features
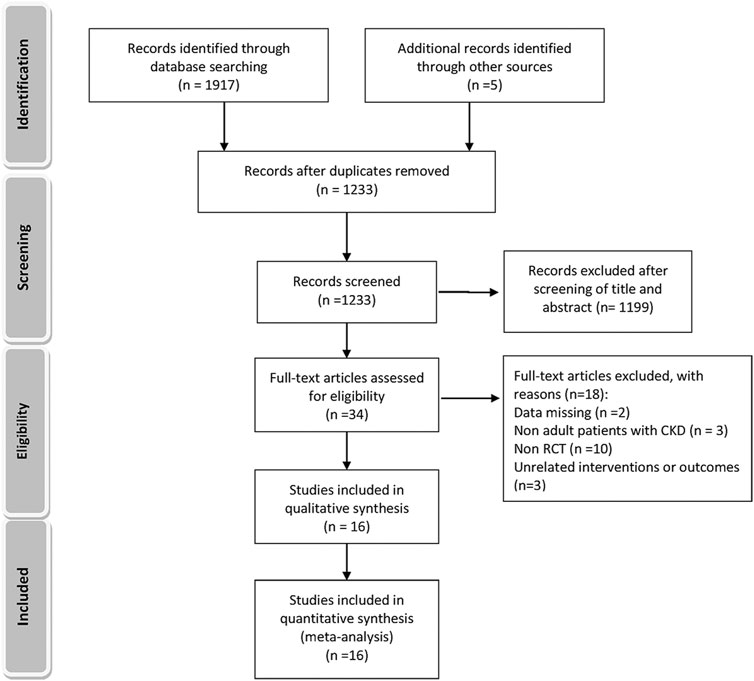
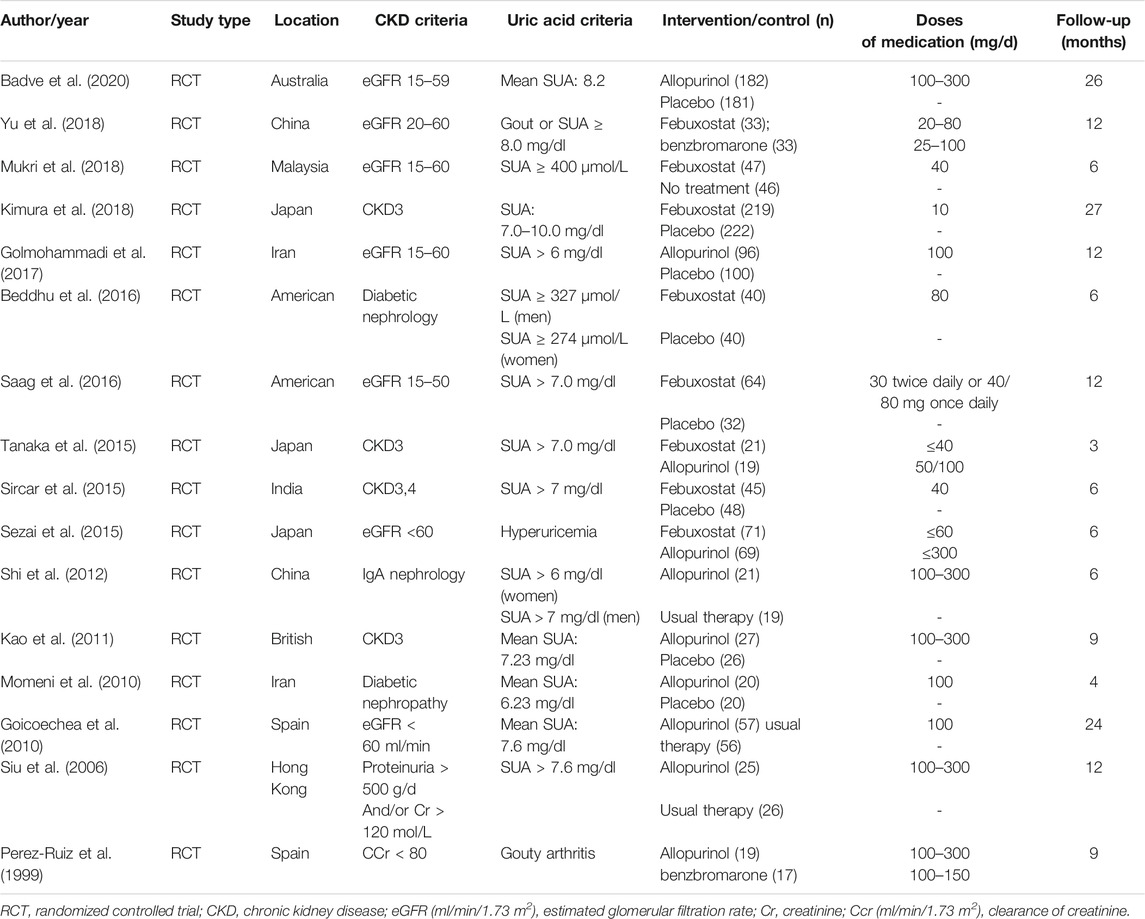
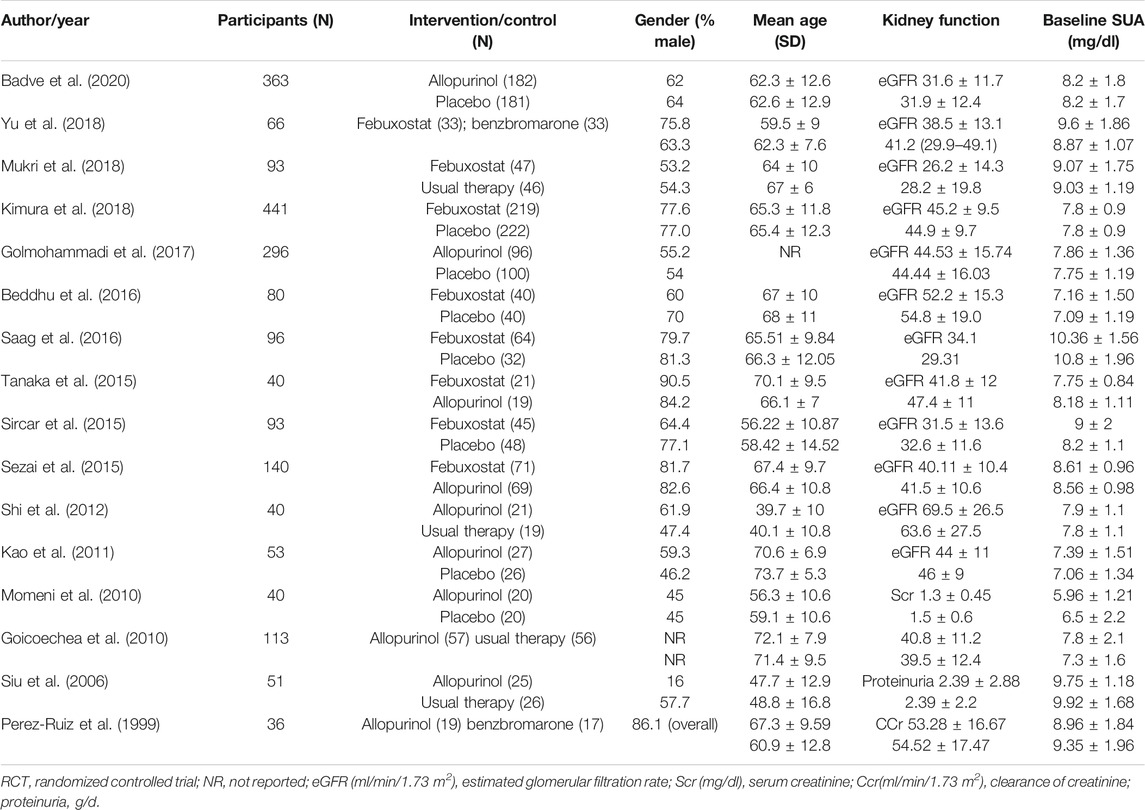
A total of 1,233 titles were identified during the initial search, 1,202 of which were excluded upon screening of titles and abstracts. Following a full-text review of 31 studies, 16 RCTs involving 1,943 patients were included in the final network analysis (Figure 1) (Yu et al., 2018; Mukri et al., 2018; Kimura et al., 2018; Saag et al., 2016; Tanaka et al., 2015; Sircar et al., 2015; Sezai et al., 2015; Shi et al., 2012; Kao et al., 2011; Momeni et al., 2010; Goicoechea et al., 2010; Siu et al., 2006; Perez-Ruiz et al., 1999; Badve et al., 2020; Golmohammadi et al., 2017; Beddhu et al., 2016). Details on the 16 RCTs and the features of patients are summarized in Table 1 and Table 2, respectively. All studies included patients with CKD and hyperuricemia, except for one (Momeni et al., 2010) in which patients with normal uric acid levels were not excluded; therapeutic outcomes of febuxostat vs. benzbromarone, allopurinol, placebo, or usual therapy, and allopurinol vs. benzbromarone, or placebo/usual therapy were reported in one (Yu et al., 2018), two (Sezai et al., 2015; Tanaka et al., 2015), five (Sircar et al., 2015; Beddhu et al., 2016; Saag et al., 2016; Kimura et al., 2018; Mukri et al., 2018), one (Perez-Ruiz et al., 1999), and seven (Siu et al., 2006; Goicoechea et al., 2010; Momeni et al., 2010; Kao et al., 2011; Shi et al., 2012; Golmohammadi et al., 2017; Badve et al., 2020) studies, respectively. Relationships between different therapies are presented in network plots (Figure 2). The area of each circle represents the numbers of included patients, and the thickness of lines connecting them shows the number of articles comparing the two connected therapies.

FIGURE 2. Network plots for relations between different therapies. The width of lines is proportional to the number of studies comparing every pair of interventions. The size of each circle is proportional to the sample size of each therapy.
Outcomes
Uric Acid
Sixteen studies with 1,672 patients (271 patients were lost to follow-up or death) provided data on ULT. Network meta-analysis demonstrated that febuxostat, allopurinol, and benzbromarone were statistically superior to placebo at lowering urate, while febuxostat was associated with superior improvement in uric acid levels compared with allopurinol (MD: −1.547; 95% CrI: −2.473 to −0.626) (Figure 3A, I = 3%) (Reginato et al., 2012). No significant differences were found when benzbromarone was compared with febuxostat and allopurinol (Figure 3A). When we only included studies with patients with CKD and hyperuricemia (Yu et al., 2018; Mukri et al., 2018; Kimura et al., 2018; Saag et al., 2016; Tanaka et al., 2015; Sircar et al., 2015; Sezai et al., 2015; Shi et al., 2012; Kao et al., 2011; Goicoechea et al., 2010; Siu et al., 2006; Perez-Ruiz et al., 1999; Badve et al., 2020; Golmohammadi et al., 2017; Beddhu et al., 2016) (Reginato et al., 2012) (Figure 3B, I = 4%), or patients with eGFR < 60 ml/min/1.73 m2 and hyperuricemia (Yu et al., 2018; Mukri et al., 2018; Kimura et al., 2018; Saag et al., 2016; Tanaka et al., 2015; Sircar et al., 2015; Sezai et al., 2015; Kao et al., 2011; Goicoechea et al., 2010; Badve et al., 2020; Golmohammadi et al., 2017) (Reginato et al., 2012) (Figure 3C, I = 5%), results were consistent.
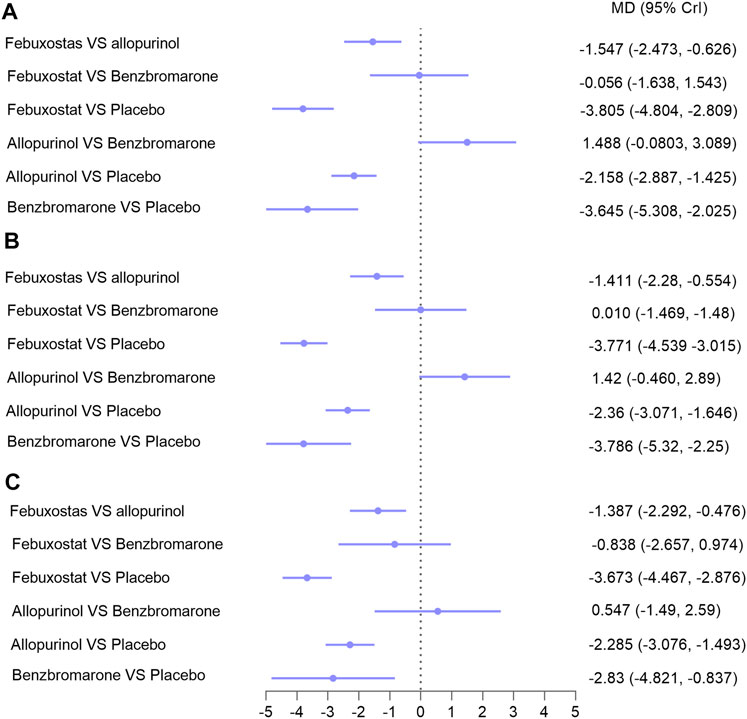
FIGURE 3. Network meta-analysis results for lowering uric acid in (A) patients with CKD; (B) patients with hyperuricemia and CKD; (C) patients with hyperuricemia and eGFR < 60 ml/min/1.73 m2. Abbreviations: MD, mean difference; CrIs, credible intervals; CKD, chronic kidney disease; eGFR (ml/min/1.73 m2), estimated glomerular filtration rate.
Subgroup analysis was performed based on follow-up time (6 months). Data on patients with over 6 months of follow-up were available in nine studies with 1,150 patients. The difference between febuxostat and allopurinol was not statistically significant in these patients (Supplementary Figure 1b, indirect comparison); this result had low certainty of evidence and lacked credibility because of a lack of head-to-head comparison studies. The remaining results were consistent with those described above (Supplementary Figures 1a,b; I ≤10%) (Reginato et al., 2012).
Progression of Chronic Kidney Disease
Indicators of CKD progression, including the change in eGFR, proteinuria, and serum creatinine, were analyzed. Regarding eGFR, 14 trials with 1,594 patients were available for assessment (Yu et al., 2018; Mukri et al., 2018; Kimura et al., 2018; Saag et al., 2016; Tanaka et al., 2015; Sircar et al., 2015; Sezai et al., 2015; Shi et al., 2012; Kao et al., 2011; Goicoechea et al., 2010; Perez-Ruiz et al., 1999; Badve et al., 2020; Golmohammadi et al., 2017; Beddhu et al., 2016). Febuxostat, allopurinol, and benzbromarone did not exert superior effects on improving eGFR over placebo in overall patients (Figure 4, I = 5%) (Reginato et al., 2012), or in patients with follow-up time over or less than 6 months (Supplementary Figure 2). Similarly, no differences were found between the three interventions regarding improvement of eGFR (Figure 4). Similar results were found with serum creatinine (seven studies with 620 patients; Supplementary Figures 3a–c) and proteinuria (five studies with 290 patients; Supplementary Figure 3d). However, febuxostat tended to be superior to allopurinol on lowering the decline of both eGFR (Figure 4) and proteinuria (Supplementary Figure 3d), and the difference did not reach statistical significance.
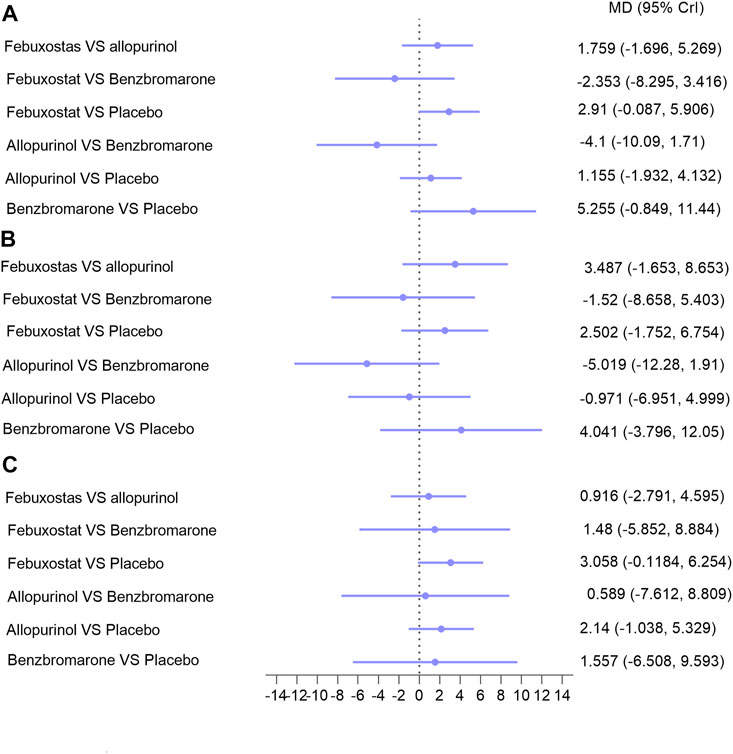
FIGURE 4. Network meta-analysis results for lowering eGFR decline in (A) patients with CKD; (B) patients with hyperuricemia and CKD; (C) patients with hyperuricemia and eGFR < 60 ml/min/1.73 m2. Abbreviations: MD, mean difference; CrIs, credible intervals; CKD, chronic kidney disease; eGFR (ml/min/1.73 m2), estimated glomerular filtration rate.
Blood Pressure
Blood pressure analysis included data from nine studies with 1,045 patients (Kimura et al., 2018; Tanaka et al., 2015; Sircar et al., 2015; Kao et al., 2011; Momeni et al., 2010; Goicoechea et al., 2010; Siu et al., 2006; Badve et al., 2020; Beddhu et al., 2016). Overall, network meta-analysis indicated that febuxostat was superior to placebo with respect to control systolic blood pressure, while allopurinol and benzbromarone were not found to have superior effects than placebo (Figure 5A, I2 = 7%, Reginato et al., 2012, respectively). Notably, febuxostat was associated with better control of systolic blood pressure than allopurinol in patients with eGFR < 60 ml/min/1.73 m2 and hyperuricemia (MD: −6.555, 95% CrI: −12.42 to −0.69) (Figure 5A). No differences were found among these interventions in terms of controlling diastolic blood pressure (Figure 5B).
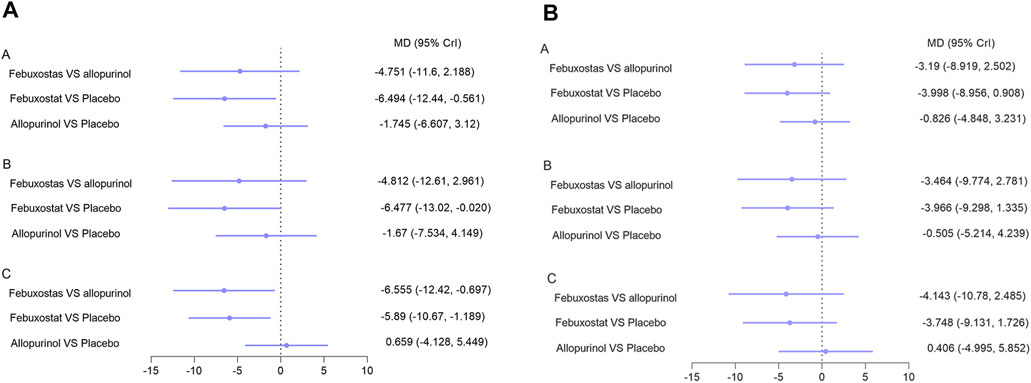
FIGURE 5. Network meta-analysis results for lowering (A) systolic blood pressure and (B) diastolic blood pressure. Note: A: patients with CKD; B: patients with hyperuricemia and CKD; C: patients with hyperuricemia and eGFR < 60 ml/min/1.73 m2. Abbreviations: MD, mean difference; CrI, credible intervals; CKD, chronic kidney disease; eGFR (ml/min/1.73 m2), estimated glomerular filtration rate.
Adverse Events
AEs including deterioration of kidney function (eight studies with 1,200 patients), liver dysfunction (seven studies with 939 patients), cardiovascular events (six studies with 1,141 patients), gastrointestinal symptoms (eight studies with 1,270 patients), and hypersensitivity (eight studies with 1,117 patients) were analyzed. There are no differences found in these AEs among the four interventions (Supplementary Figures 4, 5); febuxostat showed a tendency to be safer with respect to hypersensitivity, renal impairment, and liver dysfuction than allopurinol, but the difference was not statistically significant (Supplementary Figure 4).
Sensitivity Analysis, Subgroup Analysis, and Risk of Bias
Sensitivity analysis was performed by excluding studies that included patients without hyperuricemia and studies with eGFR ≥ 60 ml/min/1.73 m2. Furthermore, a subgroup analysis was performed based on follow-up time (≤6 or >6 months). As described above, sensitivity and the subgroup analysis did not affect our findings to an appreciable degree, highlighting the robustness of our study. The risk of bias for each study is shown in Supplementary Table 1. Overall, the risk of participant or investigator blinding was relatively high, while the risks of other parameters were low or unclear.
Discussion
It is well established that reduced kidney function is accompanied by increased serum urate levels resulting from decreased kidney clearance (Reginato et al., 2012). However, it is increasingly suspected that rising urate levels play a role in the pathogenesis and progression of CKD, and are not simply a marker of kidney disease (Uchida et al., 2015). Whether urate-lowering agents have the capacity to slow the progression of CKD has not been fully elucidated, and studies examining differences in urate-lowering agents such as febuxostat, allopurinol, and benzbromarone are very limited.
This is the first systematic review and network meta-analysis to investigate differences in effectiveness of three commonly used drugs at promoting renal protection and urate reduction in patients with CKD and hyperuricemia. We found that febuxostat can lower serum uric acid and control blood pressure better than allopurinol and benzbromarone. However, the three agents were found to not exert renoprotective effects, including improvement in eGFR, creatinine, or proteinuria. Similar observations were made in subgroup analyses stratified by the CKD stage and follow-up time.
Our findings are consistent with select RCTs in which ULT was shown to have no obvious effects on renal function (Perez-Ruiz et al., 1999; Shi et al., 2012; Beddhu et al., 2016; Saag et al., 2016; Kimura et al., 2018; Yu et al., 2018; Badve et al., 2020). A recent randomized, double-blinded, placebo-controlled study across 31 centers published in the New England Journal of Medicine also concluded that ULT with allopurinol did not improve the decline in eGFR compared with placebo in patients with CKD (Badve et al., 2020). Interestingly, in a prospective cohort study, allopurinol and febuxostat also failed to exert renoprotective effects, even though hyperuricemia was found to be an independent risk factor for CKD advancement (Oh et al., 2019). Conversely, ULT was shown to improve renal function in meta-analyses by Zeng et al. (2018) and Kanji et al. (2015). However, Zeng et al. included a retrospective analysis, and an evaluation in the febuxostat group that lacked data on eGFR was included in error. The study by Kanji et al. analyzed trials with short follow-up time (less than 2 months), and they incorporated Chinese databases, resulting in several non-English RCTs. Notably, febuxostat was also shown to be effective at reducing the risk of CKD progression in CKD populations with hyperuricemia in certain prospective or retrospective cohort studies (Chou et al., 2018; Liu et al., 2019; Yang, 2020); in our analysis, febuxostat showed a tendency to be superior to allopurinol on lowering the decline of eGFR and increment of proteinturia, although the difference is not statistically significant. These conflicting results highlight the need for large, double-blind RCTs to study the differences in renoprotective effects of urate-lowering agents.
We also found that febuxostat was superior at lowering uric acid and controlling blood pressure. Inadequate dosing of allopurinol may explain its poor effectiveness, as the dose of allopurinol in most included studies was less than 300 mg/d (Table 1) because of concerns over possible fetal side effects. Febuxostat, a more recent xanthine oxidase inhibitor (approved by the Food and Drug Administration in 2009), may be a reasonable choice for hyperuricemic patients with CKD as it is cost-effective and well tolerated (Gandhi et al., 2015; Tiku et al., 2018). Furthermore, febuxostat was proven to be more potent for lowering urate, and dose adjustment is not required in CKD patients (Shibagaki et al., 2014; Hira et al., 2015). Therefore, febuxostat may be more effective for lowering urate. Benzbromarone is not recommended for patients with eGFR < 30 ml/min (Richette et al., 2016) and was withdrawn from the United States and several European countries (Wang et al., 2017). Only two studies with 102 patients provided data on benzbromarone. Therefore, the evidence had low certainty and lacked credibility.
Compared with previous meta-analyses (Kim et al., 2017; Liu et al., 2018; Lin et al., 2019; Hu and Brown, 2020), the present report had several strengths. The available evidence was searched comprehensively, only RCTs with follow-up time over 3 months were included, and heterogeneity was very low (≤10%). Additionally, inclusion criteria were restricted to patients with CKD and hyperuricemia. Allopurinol, febuxostat, and benzbromarone were compared directly rather than with placebo only or with placebo/other agents collectively. Futhermore, we performed subgroup analyses according to the CKD stage and follow-up time. Indicators of renal function, including eGFR, creatinine, and proteinuria, were comprehensively evaluated, and AEs (including deterioration of renal or liver function, cardiovascular events, gastrointestinal symptoms, and hypersensitivity) were evaluated. Overall, differences in the effects of febuxostat, allopurinol, and benzbromarone on lowering urate, renal protection, blood pressure control, and AEs in hyperuricemic patients with CKD were compared for the first time, and the number of included RCTs and patients was relatively substantial.
This study also had limitations. First, the methodological quality of trials was suboptimal, as allocation concealment was unclear in most studies because of unexhaustive description of methods, and double-blinding was rated as a high risk in one-third of the trials analyzed. Second, evaluation of renal function focused on eGFR (14 trials with 1,594 patients), creatinine (seven studies with 620 patients), and proteinuria (five studies with 290 patients), while evaluation of progression to end-stage renal disease was insufficient (no data). Finally, while our findings have a reference value, they should not be applied to patients with only CKD or hyperuricemia, as the patients included in our study suffered from both hyperuricemia and CKD.
Conclusion
There is insufficient evidence to support the renoprotective effects of the three urate-lowering agents in CKD patients with hyperuricemia. Regarding its urate-lowering effect, febuxostat appears to be a satisfactory alternative to allopurinol and benzbromarone. It is more effective at lowering serum uric acid and controlling blood pressure in hyperuricemic patients with CKD. Interestingly, febuxostat shows a tendency to be superior to allopurinol on lowering the decline of eGFR and increment of proteinturia, although the difference does not reach a statistical significance; large, double-blind RCTs that study differences in the renoprotective effects of different urate-lowering agents are necessary.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
XL: study design, literature search, data collection, data analysis, data interpretation, figures, tables, and writing. YQ: study design, literature search, data collection, data analysis, data interpretation, and writing. DL: study design, literature search, data collection, data analysis, and data interpretation. JT: study design, literature search, data collection, data analysis, and data interpretation. XL: study design, literature search, data collection, data analysis, and data interpretation. WQ: study design, data analysis, data interpretation, and checking of all works.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Richard Robins, from Liwen Bianji, Edanz Editing China, for editing the English text of a draft of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.690557/full#supplementary-material
References
Badve, S. V., Pascoe, E. M., Tiku, A., Boudville, N., Brown, F. G., Cass, A., et al. Effects of Allopurinol on the Progression of Chronic Kidney Disease. N. Engl. J. Med. 2020; 382: 2504–2513. doi:10.1056/NEJMoa1915833
Bardin, T., and Richette, P. Definition of Hyperuricemia and Gouty Conditions. Curr. Opin. Rheumatol. 2014; 26: 186–191. doi:10.1097/bor.0000000000000028
Beddhu, S., Filipowicz, R., Wang, B., et al. (2016). A Randomized Controlled Trial of the Effects of Febuxostat Therapy on Adipokines and Markers of Kidney Fibrosis in Asymptomatic Hyperuricemic Patients with Diabetic Nephropathy. Can. J. kidney Health Dis. 3. 2054358116675343. doi:10.1177/2054358116675343
Bose, B., Badve, S. V., Hiremath, S. S., Boudville, N., Brown, F. G., Cass, A., et al. (2014). Effects of Uric Acid-Lowering Therapy on Renal Outcomes: a Systematic Review and Meta-Analysis. Nephrol. Dial. Transpl. 29, 406–413. doi:10.1093/ndt/gft378
Chou, H.-W., Chiu, H.-T., Tsai, C.-W., Ting, I.-W., Yeh, H.-C., Huang, H.-C., et al. (2018). Comparative Effectiveness of Allopurinol, Febuxostat and Benzbromarone on Renal Function in Chronic Kidney Disease Patients with Hyperuricemia: a 13-year Inception Cohort Study. Nephrol. Dial. Transpl. 33, 1620–1627. doi:10.1093/ndt/gfx313
Collaboration, G. C. K. D. (2020). Global, Regional, and National burden of Chronic Kidney Disease, 1990-2017: a Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733. doi:10.1016/S0140-6736(20)30045-3
Gandhi, P. K., Gentry, W. M., Ma, Q., and Bottorff, M. B. (2015). Cost-effectiveness Analysis of Allopurinol versus Febuxostat in Chronic Gout Patients: a U.S. Payer Perspective. Jmcp 21, 165–175. doi:10.18553/jmcp.2015.21.2.165
Goicoechea, M., de Vinuesa, S. G., Verdalles, U., Ruiz-Caro, C., Ampuero, J., Rincón, A., et al. (2010). Effect of Allopurinol in Chronic Kidney Disease Progression and Cardiovascular Risk. Cjasn 5, 1388–1393. doi:10.2215/cjn.01580210
Golmohammadi, S., Almasi, A., Manouchehri, M., Omrani, H. R., and Zandkarimi, M. R. (2017). Allopurinol against Progression of Chronic Kidney Disease. Iran J. Kidney Dis. 11, 286–293.
Higgins, J. P. T., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hira, D., Chisaki, Y., Noda, S., Araki, H., Uzu, T., Maegawa, H., et al. (2015). Population Pharmacokinetics and Therapeutic Efficacy of Febuxostat in Patients with Severe Renal Impairment. Pharmacology 96, 90–98. doi:10.1159/000434633
Hu, A. M., and Brown, J. N. (2020). Comparative Effect of Allopurinol and Febuxostat on Long-Term Renal Outcomes in Patients with Hyperuricemia and Chronic Kidney Disease: a Systematic Review. Clin. Rheumatol. 39, 3287–3294. doi:10.1007/s10067-020-05079-3
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 162, 777–784. doi:10.7326/M14-2385
Jalal, D. I., Chonchol, M., Chen, W., and Targher, G. (2013). Uric Acid as a Target of Therapy in CKD. Am. J. Kidney Dis. 61, 134–146. doi:10.1053/j.ajkd.2012.07.021
Jansen, J. P., Crawford, B., Bergman, G., and Stam, W. (2008). Bayesian Meta-Analysis of Multiple Treatment Comparisons: an Introduction to Mixed Treatment Comparisons. Value in Health 11, 956–964. doi:10.1111/j.1524-4733.2008.00347.x
Kanji, T., Gandhi, M., Clase, C. M., and Yang, R. (2015). Urate Lowering Therapy to Improve Renal Outcomes in Patients with Chronic Kidney Disease: Systematic Review and Meta-Analysis. BMC Nephrol. 16, 58. doi:10.1186/s12882-015-0047-z
Kao, M. P., Ang, D. S., Gandy, S. J., Nadir, M. A., Houston, J. G., Lang, C. C., et al. Allopurinol Benefits Left Ventricular Mass and Endothelial Dysfunction in Chronic Kidney Disease. Jasn 2011; 22: 1382–1389. doi:10.1681/ASN.2010111185
Kim, S., Kim, H.-J., Ahn, H.-S., Oh, S. W., Han, K. H., Um, T.-H., et al. (2017). Renoprotective Effects of Febuxostat Compared with Allopurinol in Patients with Hyperuricemia: A Systematic Review and Meta-Analysis. Kidney Res. Clin. Pract. 36, 274–281. doi:10.23876/j.krcp.2017.36.3.274
Kimura, K., Hosoya, T., Uchida, S., Inaba, M., Makino, H., Maruyama, S., et al. (2018). Febuxostat Therapy for Patients with Stage 3 CKD and Asymptomatic Hyperuricemia: A Randomized Trial. Am. J. Kidney Dis. 72, 798–810. doi:10.1053/j.ajkd.2018.06.028
Lee, M.-H. H., Graham, G. G., Williams, K. M., and Day, R. O. (2008). A Benefit-Risk Assessment of Benzbromarone in the Treatment of Gout. Drug Saf. 31, 643–665. doi:10.2165/00002018-200831080-00002
Lin, T.-C., Hung, L. Y., Chen, Y.-C., Lo, W.-C., Lin, C. H., Tam, K.-W., et al. (2019). Effects of Febuxostat on Renal Function in Patients with Chronic Kidney Disease. Medicine (Baltimore) 98, e16311. doi:10.1097/MD.0000000000016311
Liu, X., Wang, H., Ma, R., Shao, L., Zhang, W., Jiang, W., et al. (2019). The Urate-Lowering Efficacy and Safety of Febuxostat versus Allopurinol in Chinese Patients with Asymptomatic Hyperuricemia and with Chronic Kidney Disease Stages 3-5. Clin. Exp. Nephrol. 23, 362–370. doi:10.1007/s10157-018-1652-5
Liu, X., Zhai, T., Ma, R., Luo, C., Wang, H., and Liu, L. (2018). Effects of Uric Acid-Lowering Therapy on the Progression of Chronic Kidney Disease: a Systematic Review and Meta-Analysis. Ren. Fail. 40, 289–297. doi:10.1080/0886022X.2018.1456463
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Plos Med. 6, e1000097. doi:10.1371/journal.pmed.1000097
Momeni, A., Shahidi, S., Seirafian, S., Taheri, S., and Kheiri, S. Effect of Allopurinol in Decreasing Proteinuria in Type 2 Diabetic Patients. Iran J. Kidney Dis. 2010; 4: 128–132.
Mukri, M. N. A., Kong, W. Y., Mustafar, R., Shaharir, S. S., Shah, S. A., Abdul Gafor, A. H., et al. Role of Febuxostat in Retarding Progression of Diabetic Kidney Disease with Asymptomatic Hyperuricemia: A 6-months Open-Label, Randomized Controlled Trial. EXCLI J. 2018; 17: 563–575. doi:10.17179/excli2018-1256
Oh, T. R., Choi, H. S., Kim, C. S., Bae, E. H., Ma, S. K., Sung, S.-A., et al. (2019). Hyperuricemia Has Increased the Risk of Progression of Chronic Kidney Disease: Propensity Score Matching Analysis from the KNOW-CKD Study. Sci. Rep. 9, 6681. doi:10.1038/s41598-019-43241-3
Perez-Ruiz, F., Calabozo, M., Fernandez-Lopez, M. J., Herrero-Beites, A., Ruiz-Lucea, E., Garcia-Erauskin, G., et al. Treatment of Chronic Gout in Patients with Renal Function Impairment an Open, Randomized, Actively Controlled Study. JCR: J. Clin. Rheumatol. 1999; 5: 49–55. doi:10.1097/00124743-199904000-00003
Reginato, A. M., Mount, D. B., Yang, I., and Choi, H. K. (2012). The Genetics of Hyperuricaemia and Gout. Nat. Rev. Rheumatol. 8, 610–621. doi:10.1038/nrrheum.2012.144
Richette, P., Doherty, M., Pascual, E., Barskova, V., Becce, F., Castañeda-Sanabria, J., et al. (2016). 2016 Updated EULAR Evidence-Based Recommendations for the Management of Gout. Ann. Rheum. Dis. 2017 76, 29–42. doi:10.1136/annrheumdis-2016-209707
Saag, K. G., Whelton, A., Becker, M. A., MacDonald, P., Hunt, B., and Gunawardhana, L. Impact of Febuxostat on Renal Function in Gout Patients with Moderate-To-Severe Renal Impairment. Arthritis Rheumatol. 2016; 68: 2035–2043. doi:10.1002/art.39654
Sampson, A. L., Singer, R. F., and Walters, G. D. (2017). Uric Acid Lowering Therapies for Preventing or Delaying the Progression of Chronic Kidney Disease. Cochrane Database Syst. Rev. 10, CD009460. doi:10.1002/14651858.CD009460.pub2
Sato, Y., Feig, D. I., Stack, A. G., Kang, D.-H., Lanaspa, M. A., Ejaz, A. A., et al. (2019). The Case for Uric Acid-Lowering Treatment in Patients with Hyperuricaemia and CKD. Nat. Rev. Nephrol. 15, 767–775. doi:10.1038/s41581-019-0174-z
Sezai, A., Soma, M., Nakata, K.-i., Osaka, S., Ishii, Y., Yaoita, H., et al. Comparison of Febuxostat and Allopurinol for Hyperuricemia in Cardiac Surgery Patients with Chronic Kidney Disease (NU-FLASH Trial for CKD). J. Cardiol. 2015; 66: 298–303. doi:10.1016/j.jjcc.2014.12.017
Shi, Y., Chen, W., Jalal, D., Li, Z., Chen, W., Mao, H., et al. Clinical Outcome of Hyperuricemia in IgA Nephropathy: a Retrospective Cohort Study and Randomized Controlled Trial. Kidney Blood Press. Res. 2012; 35: 153–160. doi:10.1159/000331453
Shibagaki, Y., Ohno, I., Hosoya, T., and Kimura, K. (2014). Safety, Efficacy and Renal Effect of Febuxostat in Patients with Moderate-To-Severe Kidney Dysfunction. Hypertens. Res. 37, 919–925. doi:10.1038/hr.2014.107
Sircar, D., Chatterjee, S., Waikhom, R., Golay, V., Raychaudhury, A., Chatterjee, S., et al. Efficacy of Febuxostat for Slowing the GFR Decline in Patients with CKD and Asymptomatic Hyperuricemia: A 6-Month, Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Kidney Dis. 2015; 66: 945–950. 2015/08/04. DOI: doi:10.1053/j.ajkd.2015.05.017
Siu, Y.-P., Leung, K.-T., Tong, M. K.-H., and Kwan, T.-H. Use of Allopurinol in Slowing the Progression of Renal Disease through its Ability to Lower Serum Uric Acid Level. Am. J. Kidney Dis. 2006; 47: 51–59. doi:10.1053/j.ajkd.2005.10.006
Stamp, L. K., O'Donnell, J. L., Zhang, M., James, J., Frampton, C., Barclay, M. L., et al. (2011). Using Allopurinol above the Dose Based on Creatinine Clearance Is Effective and Safe in Patients with Chronic Gout, Including Those with Renal Impairment. Arthritis Rheum. 63, 412–421. doi:10.1002/art.30119
Steiger, S., Ma, Q., and Anders, H.-J. (2020). The Case for Evidence-Based Medicine for the Association between Hyperuricaemia and CKD. Nat. Rev. Nephrol. 16, 422. doi:10.1038/s41581-020-0288-3
Tanaka, K., Nakayama, M., Kanno, M., Kimura, H., Watanabe, K., Tani, Y., et al. Renoprotective Effects of Febuxostat in Hyperuricemic Patients with Chronic Kidney Disease: a Parallel-Group, Randomized, Controlled Trial. Clin. Exp. Nephrol. 2015; 19: 1044–1053. doi:10.1007/s10157-015-1095-1
Tiku, A., Badve, S. V., and Johnson, D. W. (2018). Urate-Lowering Therapy for Preventing Kidney Disease Progression: Are We There yet? Am. J. Kidney Dis. 72, 776–778. doi:10.1053/j.ajkd.2018.07.022
Uchida, S., Chang, W. X., Ota, T., Tamura, Y., Shiraishi, T., Kumagai, T., et al. (2015). Targeting Uric Acid and the Inhibition of Progression to End-Stage Renal Disease-A Propensity Score Analysis. PLoS One 10, e0145506. doi:10.1371/journal.pone.0145506
Wang, H., Peng, Y., Zhang, T., Lan, Q., Zhao, H., Wang, W., et al. (2017). Metabolic Epoxidation Is a Critical Step for the Development of Benzbromarone-Induced Hepatotoxicity. Drug Metab. Dispos 45, 1354–1363. doi:10.1124/dmd.117.077818
Yang, A. Y. (2020). Comparison of Long-Term Efficacy and Renal Safety of Febuxostat and Allopurinol in Patients with Chronic Kidney Diseases. Cp 58, 21–28. doi:10.5414/CP203466
Yu, H., Liu, X., Song, Y., Cheng, J., Bao, H., Qin, L., et al. Safety and Efficacy of Benzbromarone and Febuxostat in Hyperuricemia Patients with Chronic Kidney Disease: A Prospective Pilot Study. Clin. Exp. Nephrol. 2018; 22: 1324–1330. doi:10.1007/s10157-018-1586-y
Keywords: hyperuricemia, chronic kidney disease, febuxostat, allopurinol, benzbromarone
Citation: Liu X, Qiu Y, Li D, Tan J, Liang X and Qin W (2021) Effectiveness of Drug Treatments for Lowering Uric Acid on Renal Function in Patients With Chronic Kidney Disease and Hyperuricemia: A Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 12:690557. doi: 10.3389/fphar.2021.690557
Received: 03 April 2021; Accepted: 30 June 2021;
Published: 03 August 2021.
Edited by:
Marlies Ostermann, Guy’s and St Thomas’ NHS Foundation Trust, United KingdomReviewed by:
Davide Viggiano, University of Campania Luigi Vanvitelli, ItalyMarco Allinovi, Careggi University Hospital, Italy
Copyright © 2021 Liu, Qiu, Li, Tan, Liang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Qin, cWlud2VpaHhAc2N1LmVkdS5jbg==
 Xiang Liu
Xiang Liu Yuxuan Qiu2,3
Yuxuan Qiu2,3 Duohui Li
Duohui Li Xiuping Liang
Xiuping Liang Wei Qin
Wei Qin