- 1The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou, China
- 2Center for Molecular Medicine, University Medical Center Utrecht, Utrecht, Netherlands
- 3Guangdong Provincial Key Laboratory of Clinical Research on Traditional Chinese Medicine Syndrome, Guangzhou, China
- 4Guangdong-Hong Kong-Macau Joint Lab on Chinese Medicine and Immune Disease Research, Guangzhou University of Chinese Medicine, Guangzhou, China
- 5State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Objectives: To evaluate the current evidence whether Chinese medicine compound (CMC) can reduce the serum levels of rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (anti-CCP).
Methods: We comprehensively searched PubMed, Embase, the Cochrane Library, China National Knowledge Infrastructure (CNKI), the Database for Chinese Technical Periodicals (VIP), and Wanfang data. We then performed a systematic review and meta-analysis of all randomized controlled trials (RCTs) assessing the CMC therapy methods. This study is registered with PROSPERO, number CRD42020216284.
Results: In total, 65 studies were eligible for inclusion, including 6099 patients. The result of the meta-analysis showed that compared with common Western medicine therapy, CMC monotherapy or combined with Western medicine was able to reduce serum RF (SMD= −0.85, 95%CI −1.04 to −0.67) and anti-CCP (SMD= −0.56, 95%CI −0.79 to −0.32) levels to some extent. In the efficacy meta-analysis, a greater number of CMC-treated patients achieved the efficacy criteria after a period of treatment, where the relative risk (RR) was 1.20 [1.08, 1.33] for achieving ACR20, 1.57 [1.38, 1.78] for ACR50, and 2.21 [1.72, 2.84] for ACR70. At the same time, there was a statistically significant difference in the effective rate of the patient's TCM symptoms (RR = 1.22, 95%CI 1.19–1.26).
Conclusions: Through this meta-analysis and systematic review, we found that CMC for the treatment of RA is effective in reducing RF and anti-CCP levels and might have better clinical efficacy than Western medicine monotherapy. Some active components are responsible for this efficacy and worth further exploring.
Highlights
1. Through this meta-analysis and systematic review, we found that CMC to treat RA is effective in reducing RF and anti-CCP levels and might have better clinical efficacy than Western medicine monotherapy.
2. Through frequency analysis of the CMC constituent herbs involved in the 65 literatures included in the meta-analysis, we summarized the active ingredients and pharmacological effects of five high-frequency representative Chinese herbs and found that CMC can reduce RF and anti-CCP levels possibly by regulating the immune response and inhibiting B lymphocyte proliferation.
3. CMC may be a potential and efficacious therapeutic adjunct to delay the progression and improve outcomes of RA.
Introduction
As an incurable autoimmune disease, rheumatoid arthritis (RA) can cause cartilage and bone damages as well as disability, and finally lead to poor quality of life. The average prevalence of RA is estimated at 0.5–1.0% globally (Ngian, 1999). In China, the people suffer from RA with an estimated prevalence of 0.42% (Zeng et al., 2013). Although remission is now an achievable goal for the majority of patients owing to advances in early diagnosis, new drugs and improved treatment strategies, the rate of reaching the standard of domestic RA treatment in China is still low at present. Currently, mainstream therapeutic strategy, i.e. Western medical treatment, including disease-modifying anti-rheumatic drugs (DMARDs), biologic agents, glucocorticoids (GC), which are considered to be effective means to rapidly alleviate and control the progression of RA, but the toxic side effects caused by Western medicine long-term use (Rainsford, 1999; Wang et al., 2018), the non-response of some patients to drugs (He et al., 2019), and the expensive price of biologic agents are the main problems faced by Western medical treatment. Therefore, there is still a considerable unmet need in RA treatment, which has led to an increasing number of patients with RA to seek complementary and integrative medicines.
Traditional Chinese Medicine (TCM), the most common complementary and alternative therapeutic approach for Western medicine, also has a long history in the treatment of RA, whether taken internally or externally, monotherapy or in combination, it has obvious therapeutic effects with few side effects. Chinese medicine compound (CMC) is the main form and means of clinical use of TCM, which concentrates the advantages and characteristics of TCM in the treatment of diseases. Under the guidance of the theoretical system that the concept of holism and treatment based on syndrome differentiation, CMC is formulated as a mixture of different kinds of Chinese herbal medicines, including decoction, granule and Chinese patent medicine, etc. In China, it is quite common that DMARDs are combined with various CMC in the treatment of RA. Modern scholars of TCM have carried out many clinical studies on CMC for the treatment of RA, but the literature reported in these clinical studies is of varying quality and results. One study (Yao, 2010) showed that the efficacy of the CMC experimental group was better than that of the control group after two months of treatment, and the difference was statistically significant (p < 0.05). On the contrary, the results of Chen's randomized controlled trial (Chen et al., 2010) found that the total effective rate in the CMC group was lower than that in the Western medicine group. Especially the efficacy in alleviating pain symptoms was inferior to that of the Western medicine group, and the difference was statistically significant (p < 0.05). It is precisely because of the differences in conclusions between studies that we need to conduct a systematic review to objectively evaluate the role and underlying mechanisms of CMC in the treatment of RA.
RF and anti-CCP are serological indicators for the diagnosis of RA. Anti-CCP has higher specificity and sensitivity for RA than RF, which combined detection with RF can compensate for the lack of specificity and sensitivity of RF, and has good diagnostic value for RA (van Venrooij et al., 2008). There are several studies showing that anti-CCP is a sensitive serological indicator of the degree of bone erosion and predict the prognosis of RA patients, thus assisting in the optimal therapeutic management of RA patients (Forslind et al., 2004; Ronnelid et al., 2005; Schoels et al., 2011). So far, no systematic review has been found to describe the efficacy of CMC in reducing RF and CCP levels in RA patients.
The following is a systematic review and meta-analysis of randomized controlled trials (RCTs) of CMC in the treatment of RA, to provide some references for improving RA therapeutic strategy.
Methods
Literature Search and Strategy
According to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA), we searched the PubMed, embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), the database for Chinese Technical Periodicals (VIP) and Wanfang data from the inception dates to September 31, 2020. The keywords used were as follows: Chinese keywords were Chinese pinyin such as “Zhongyi, Zhongyao and Zhongyiyao” (which means “Traditional Chinese Medicine”) and “Leifengshiguanjieyan, Leifengshixingguanjieyan” (which means “rheumatoid arthritis”). while English searches combined subject terms (MeSH) and free words, with a retrieval strategy of “Arthritis, Rheumatoid” or “rheumatoid arthritis” AND “Medicine, Chinese Traditional” or “Chinese medicine” or “herbal medicine” or “Traditional Chinese Medicine”.
Study Selection Criteria
Study Type
We merely included the RCTs that involved CMC to treat RA, regardless of blinding, publication status or language.
Participant Type
Adults (usually over 18 years of age) with a diagnosis of RA either using the 1987 American College of Rheumatology (ACR) classification criteria (Arnett et al., 1988) for RA, or using the 2010 ACR/European League Against Rheumatism (EULAR) classification criteria (Aletaha et al., 2010) for RA, and regardless of gender, age, the severity of disease, duration of disease, etc.
Intervention Measures
All experimental groups were administered orally with any types of CMC, including CMC monotherapy or combined with Western medicine. The control groups received only oral Western medicine treatment.
Major Research Indicators
Primary Outcomes
The primary outcomes include mean serum RF and anti-CCP levels after CMC treatment.
Secondary Outcomes
The secondary outcomes pertained to the clinical efficacy.
1) The efficacy of response of RA to treatment with CMC by the ACR outcome measure ACR20, 50 and 70. The ACR20, 50 and 70 response is defined as at least a 20, 50 and 70% reduction from baseline in the number of both tender and swollen joints and at least a 20, 50 and 70% improvement in three or more of the five remaining ACR core set measures (patient’s assessment of pain, level of disability, C-reactive protein level, global assessment of disease by the patient, and global assessment of disease by the physician) (Felson et al., 1995).
2) Standard of curative effect of TCM symptoms. Refer to the “Guiding Principles for Clinical Research of New Chinese Medicines”: 1) Effective: decrease of integral symptoms scored ≥30%, some relief of TCM symptoms; 2) Ineffective: integral symptoms score lower <30%, no relief or even aggravation of TCM symptoms (Zheng, 2002).
Exclusion Criteria
Exclusion criteria included republished literature; the literature whose research topic is complications of RA; animal experiments, reviews, conference papers, incomplete case reports or important data reports with no reply from the corresponding author(s).
Data Selection
Three authors participated in the data extraction of all the studies included in the review. Two authors (Xuan Tang and Zehao Liu) independently extracted the relevant data from the eligible studies. A third author (Zhihua Yang) resolved any divergence still present after discussion. Data extracted from the selected studies included authors' name, publication year, trial design, characteristics of participants, intervention methods, components of CMC, duration of treatment and endpoint evaluation indicators.
Quality Assessment
Assessment of risk of bias was undertaken for each included study using the Cochrane Collaboration’s risk of bias assessment tool (Green and Higgins, 2008). Seven sources of bias were assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. The evaluation criteria of each item were judged as “low risk of bias”, “unclear risk of bias” and “high risk of bias”.
Statistical Analysis
Extracted data were combined for meta-analysis using R4.0.3 and Stata12.0 software. The dichotomous data was evaluated using the relative risk (RR) and 95% confidence interval (CI), and the continuous data were combined using the standardized mean difference (SMD) and 95% CI. The I-squared [I(Zeng et al., 2013)] statistic was used to assess the heterogeneity across the included studies, as suggested by literature (Higgins et al., 2003). The analysis was carried out using a fixed-effects model according to if I-squared ≤50%. Instead, a random-effects model was adopted when significant heterogeneity (I-squared > 50%) was found. In addition, Egger's test was used to estimate and represent the risk of potential publication bias if the number of included trials reached 10 (Begg and Mazumdar, 1994).
At the same time, we performed descriptive statistics on the frequency of each component of CMC involved in the 65 literature that was finally included in the meta-analysis, in order to explore the high-frequency Chinese medicine for RA treatment and their active ingredients and pharmacological effects, and to find out the potential mechanism that reduces serum RF and anti-CCP levels.
Subgroup Analysis and Investigation of Heterogeneity
Where sufficient studies were available and the data were heterogeneous, we carried out separate meta-analyses for studies according to some factors including intervention duration and intervention measures of the experimental group.
Sensitivity Analysis
We performed a sensitivity analysis to explore heterogeneity and the differences in effect size. After excluding different studies in turn and re-performing the meta-analysis of the remaining studies, we assessed whether the results obtained were significantly different from those before the exclusion so as to assess whether the results of the meta-analysis were robust.
Results
Literature Search Results
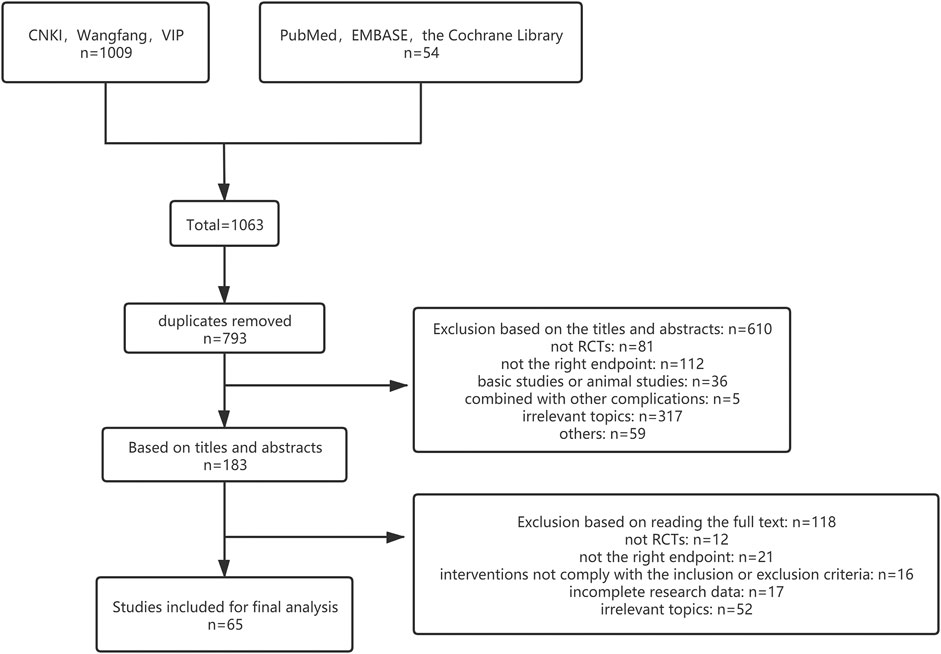
According to the retrieval strategy, 1,063 literature was initially detected, including 471 of CNKI, After removal of duplicates via literature management software across databases, 793 studies were screened. Through reading titles and abstracts, 610 literature was excluded because they did not meet the inclusion criteria. And 118 literature was excluded after examination of the full text. Finally, 65 studies that met the inclusion criteria were included in our meta-analysis. Figure 1 shows the process and consequences of literature screening. The process followed for the selection of eligible studies is described in a flow diagram (Figure 1).
Characteristics of Studies
A total of 65 studies were included in the meta-analysis were conducted in China. These studies, published between 2006 to 2020, included 6,131 patients, 3139 RA patients that received CMC, and 2992 RA patients that received Western medicine monotherapy. In the end, 6,099 patients completed the study. A total of 63 studies (Huang et al., 2006; Li, 2006; Wang et al., 2006; Liu et al., 2007; Luo, 2008; Shen et al., 2008; Li, 2009; Liu et al., 2009; Ma et al., 2009; Xiang et al., 2009; Chen et al., 2010; Yao, 2010; Zhou et al., 2010; Han and Song, 2011; Shen et al., 2011; Yang et al., 2011; Li et al., 2012a; Li, 2013; Liu et al., 2013; Wei et al., 2013; Wei and Liu, 2013; Yang, 2013; Cao and Liu, 2014; He and Xiao, 2014; Niu, 2014; Wang and Tao, 2014; Yu and Chen, 2014; Zhang and Chen, 2014; Li et al., 2015a; Shu et al., 2015; Su et al., 2015; Zhao, 2015; Zheng and Shi, 2015; Chen et al., 2016; Han et al., 2016; Jia et al., 2016; Jiang and Wu, 2016; Liu et al., 2016; Qian et al., 2016; You et al., 2016; Gao and Bu, 2017; Guo et al., 2017; Li et al., 2017; Pang et al., 2017; Wang, 2017; Wang and Tu, 2017; Bian and Wang, 2018; Ge, 2018; Guo et al., 2018; Hu, 2018; Ma et al., 2018; Shi and Yang, 2018; Xu et al., 2018; Zeng and Chen, 2018; Fang et al., 2019; Li et al., 2019; Pang et al., 2019; Song et al., 2019; Yuan et al., 2019; Zhang, 2019; Zhao, 2019; Cao et al., 2020; Jiang and Ma, 2020) and 8 studies (Liu et al., 2009; Chen et al., 2010; Han et al., 2016; Gao and Bu, 2017; Pang et al., 2017; Bian and Wang, 2018; Pang et al., 2019; Li, 2020) explored RF and anti-CCP levels respectively after treatment. In addition, 14 studies (Chen et al., 2010; Shen et al., 2011; Yang, 2013; Cao and Liu, 2014; He and Xiao, 2014; Wang and Tao, 2014; Zhang and Chen, 2014; Li et al., 2015b; Shu et al., 2015; Zhao, 2015; Jiang and Wu, 2016; Qian et al., 2016; Wang and Tu, 2017; Pang et al., 2019; Cao et al., 2020) researched the efficacy of response of ACR20, 50 and 70. Moreover, 33 studies (Huang et al., 2006; Li, 2006; Luo, 2008; Shen et al., 2008; Liu et al., 2009; Xiang et al., 2009; Yao, 2010; Zhou et al., 2010; Liu et al., 2013; Wei et al., 2013; Wei and Liu, 2013; Yang, 2013; He and Xiao, 2014; Li et al., 2015a; Shu et al., 2015; Zheng and Shi, 2015; Chen et al., 2016; Han et al., 2016; Jiang and Wu, 2016; Liu et al., 2016; You et al., 2016; Guo et al., 2017; Li et al., 2017; Wang and Tu, 2017; Bian and Wang, 2018; Ge, 2018; Guo et al., 2018; Ma et al., 2018; Shi and Yang, 2018; Xu et al., 2018; Li et al., 2019; Song et al., 2019; Yuan et al., 2019) reported the effective rate of TCM symptoms.
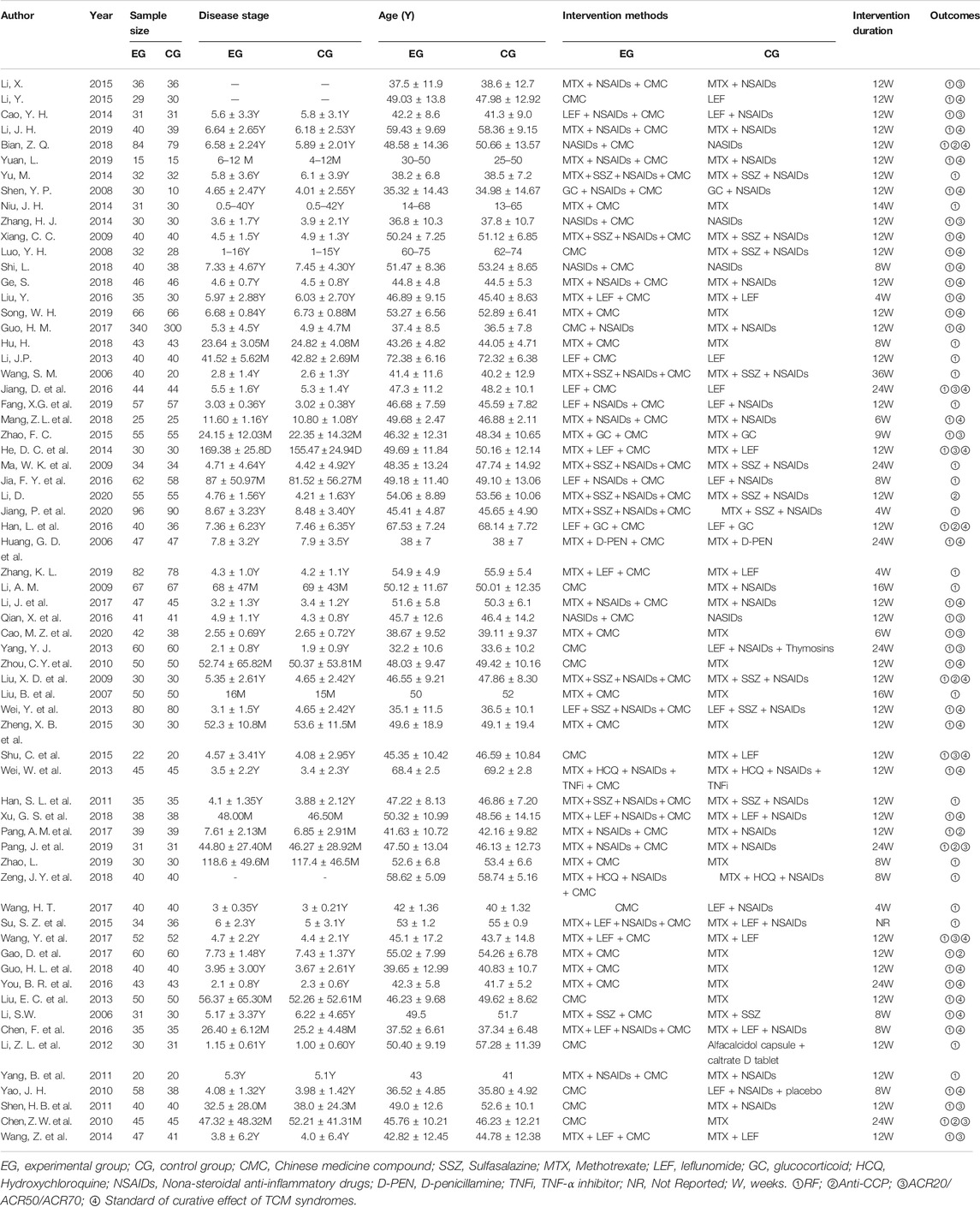
The experimental group received oral CMC or CMC combined with Western medicine as intervention measures, while the control group received conventional Western medicine for the treatment of RA as the positive control. Among all the medications included in the study, CMC included decoction, patent medicines (including capsules, tablets, and pills), and powder. Western medicine mainly included DMARDs such as methotrexate (MTX), leflunomide (LEF), sulfasalazine (SSZ), hydroxychloroquine sulfate (HCQ), as well as nonsteroidal anti-inflammatory drugs (NSAIDs) and GC, etc. There were no statistically significant differences between the basic information about the patients. A summary with baseline characteristics of included patients is shown in Table 1. The details (including dosages, herbs, quality control and mentions) of interventions in above studies were disposed in Supplementary Material 1 and Table 2. All herbs used in are assessable and well documented in Supplementary Material 5 (including their Pinyin Names, Accepted Names, Latin Names, Full Formats and Medicinal Sources).
Quality of the Included Studies
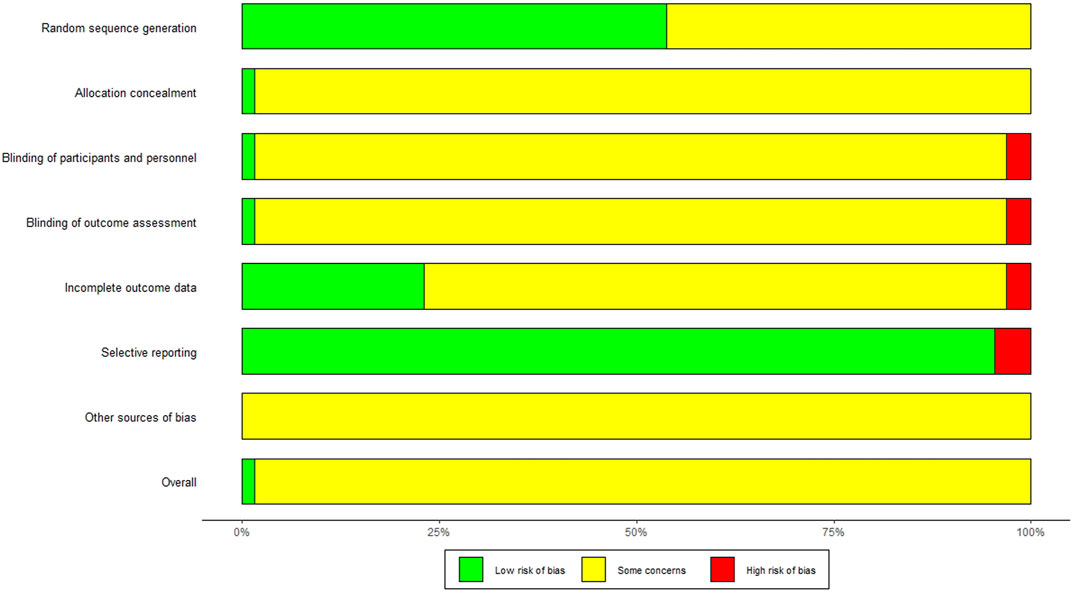
The quality of the trials was assessed and graded according to the criteria described in The Cochrane Handbook 4.2.6. Overall, for most of the included studies, the risk of bias was unclear. In addition, although the random design was mentioned in all studies, only 53.85% (35/65) of the studies described specific methods of random sequence generation. The 35 studies all used the simple random grouping method, regarding random sequence generation of which 32 studies used the random number table method and the other 3 studies selected by draw lots. Except for the study of Zhou (Zhou et al., 2010), the use of allocation concealment was not mentioned in the rest literature. Only one trial (Jiang and Wu, 2016) mentioned the double-blind of patients and personnel, and two studies (Huang et al., 2006; Xu et al., 2018) explicitly mentions the absence of the use of blinding, while the rest literature did not mention the use of blindness. There were 14 studies (Huang et al., 2006; Ma et al., 2009; Li et al., 2012a; He and Xiao, 2014; Li et al., 2015a; Li et al., 2015b; Shu et al., 2015; Chen et al., 2016; Li et al., 2017; Bian and Wang, 2018; Xu et al., 2018; Fang et al., 2019; Li et al., 2019; Pang et al., 2019) described the dropouts, and two trials (Li, 2006; Zhang and Chen, 2014) were not reported the number of people and the reasons for the failure at follow-up. Pre-designed outcomes were reported in most studies, detecting a low risk of reporting bias. The review authors’ judgments about each methodological quality item were presented as percentages across all the included studies in Figure 2.
Effects of Interventions
Primary Outcomes
Serum RF Level After Treatment
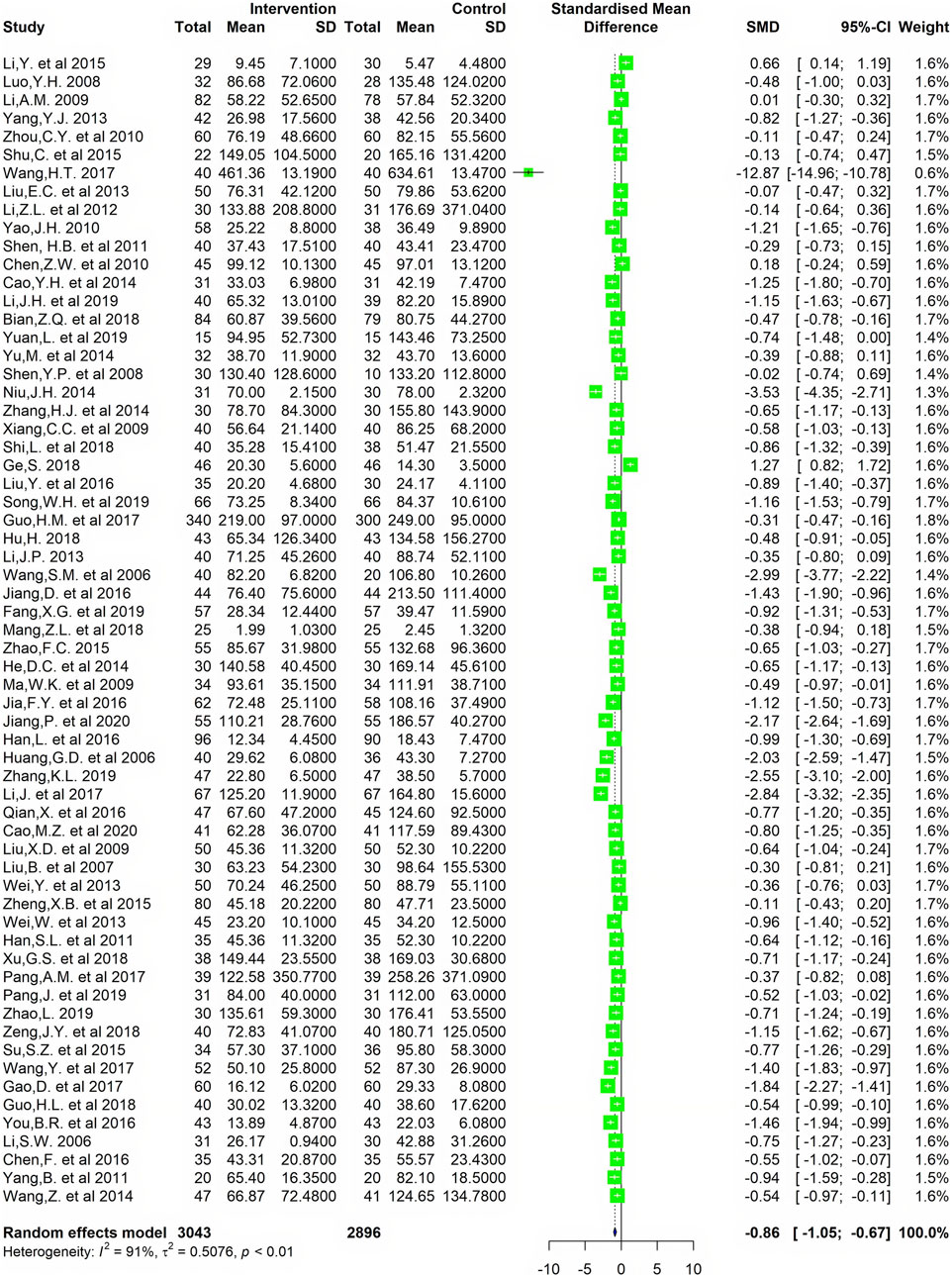
Sixty-three studies including 5,939 patients (3,043 in the experimental group and 2,896 in the control group) provided the serum RF concentration data. The SMD of the RF levels in treatment group (CMC involved) between baseline and follow-up was 2.64 and 95%CI was [2.25, 3.02], while the SMD in control group (no CMC involved) between baseline and follow-up was 2.08 and 95%CI was [1.75, 2.40] (Supplementary Material 2). After treatment, the results showed that CMC had a significant difference in reducing serum RF level compared with the control group. (SMD = -0.86, 95%CI = [−1.05, −0.67]) (Figure 3).
Serum Anti-CCP Level After Treatment
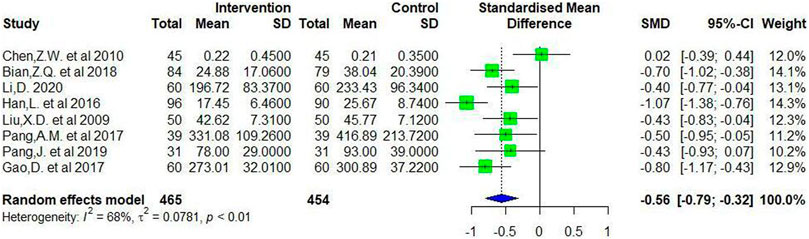
Eight studies have observed changes in serum anti-CCP level before and after treatment and provided relevant data. The SMD of the anti-CCP levels in treatment group (CMC involved) between baseline and follow-up was 2.15 and 95%CI was [1.28, 3.01], while the SMD in control group (no CMC involved) between baseline and follow-up was 1.62 and 95%CI was [0.91,2.33] (Supplementary Material 3). Since a significant heterogeneity was detected [I (Zeng et al., 2013) = 68%, p < 0.01], the randomized effect model was adopted for analysis and the results indicated that CMC could significantly reduce the level of anti-CCP after treatment (SMD = −0.56, 95%CI = [−0.79, −0.32]) (Figure 4).
Secondary Outcomes
ACR Response
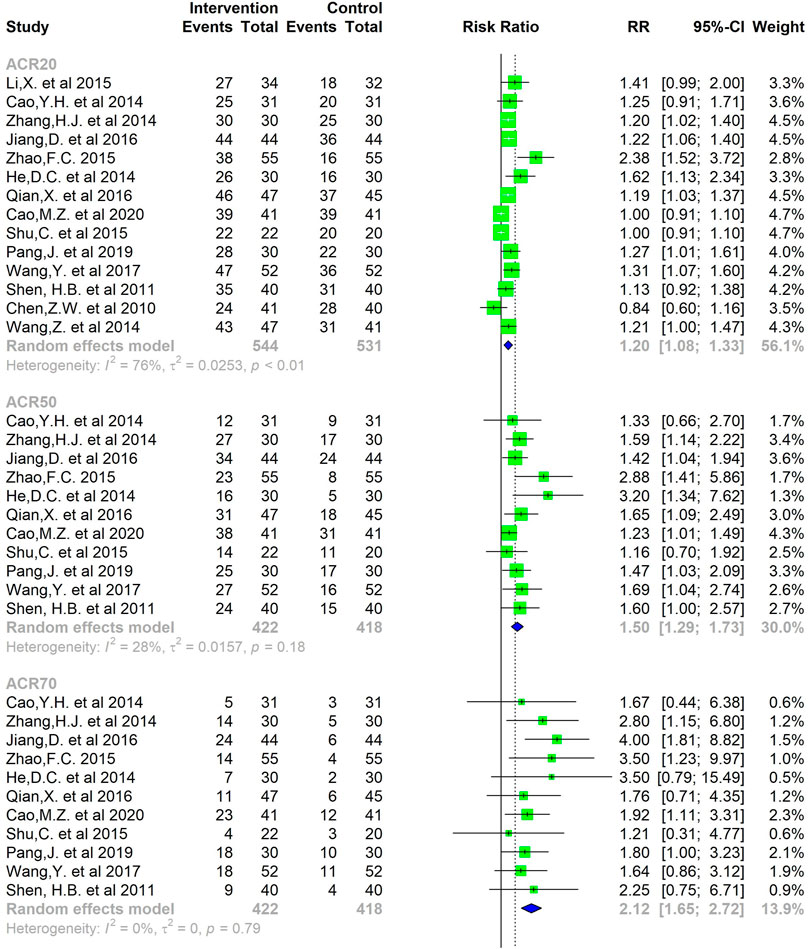
A total of 14 studies reported ACR20 response rates over the treatment time, and 11 of them also reported the proportion of patients who achieved ACR50 and ACR70 response after treatment. The I-squared was 76% and the p-value was <0.01, so a random-effects model was adopted for the meta-analysis of ACR20 response. The results of the pooled analysis suggest that the proportion of RA patients who achieved an ACR20 response after CMC treatment was superior to that of the control group. (SMD = 1.20, 95%CI = [1.08, 1.33]) (Figure 5).
The low risk of heterogeneity was detected among all studies in terms of the ACR50 and ACR70 response of CMC therapy in RA patients. The analysis was performed using a fixed-effects model and the results demonstrated that the RR of achieving ACR50 was 1.50 [1.38, 1.78] and correspondingly the RR of achieving ACR70 was 2.12 [1.65, 2.72] (Figure 5).
Clinical Efficacy of TCM Symptoms
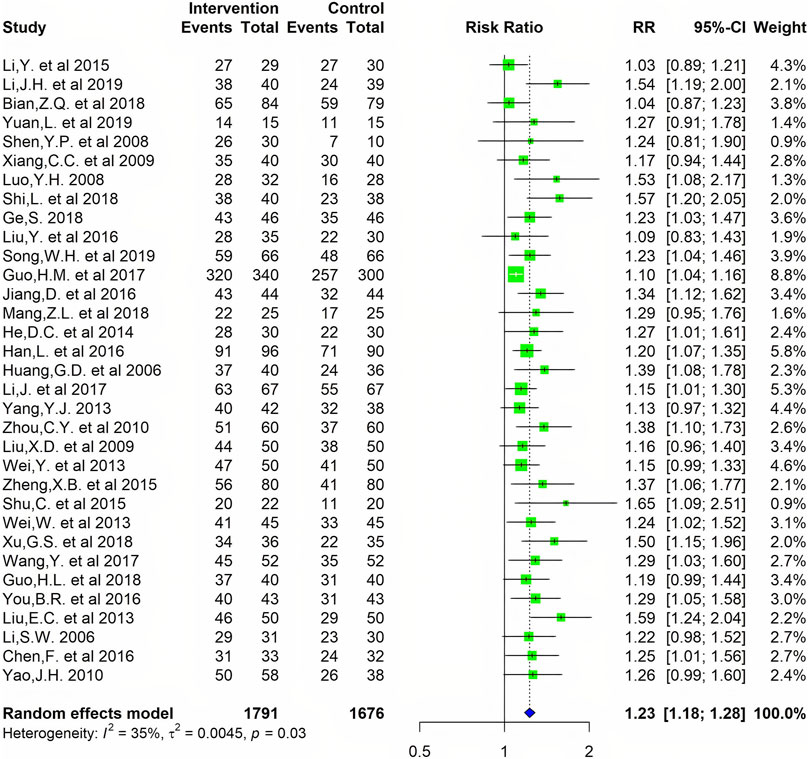
Data on the clinical efficacy of TCM symptoms were available for 33 studies containing a total of 3,467 cases. The meta-analysis yielded a pooled RR of 1.23 (I2 = 35%, 95% CI 1.18–1.28). As shown in Figure 6, CMC has a good effect on patients with RA.
Sensitivity and Subgroup Analysis
Through sensitivity analysis, after excluding different studies in turn, it was found that there was no change in the significance of any of the outcomes. However, in the sensitivity analysis of the primary outcomes, anti-CCP, it was found that I-squared dropped to 43.4% when a study (Han et al., 2016) was excluded. Considering the difference in the average age of the included patients, unlike other studies, this study included elderly patients with RA, aged ≥60 years old. When analyzing the response rate of TCM syndromes, it was found that after a study (Guo et al., 2017) were excluded, I-squared could be reduced to 11.9%. By reviewing the original article, it was found that the sample size of this study was large compared with other studies (total sample size >600), suggesting that the source of heterogeneity may be caused by the difference in sample size between studies.
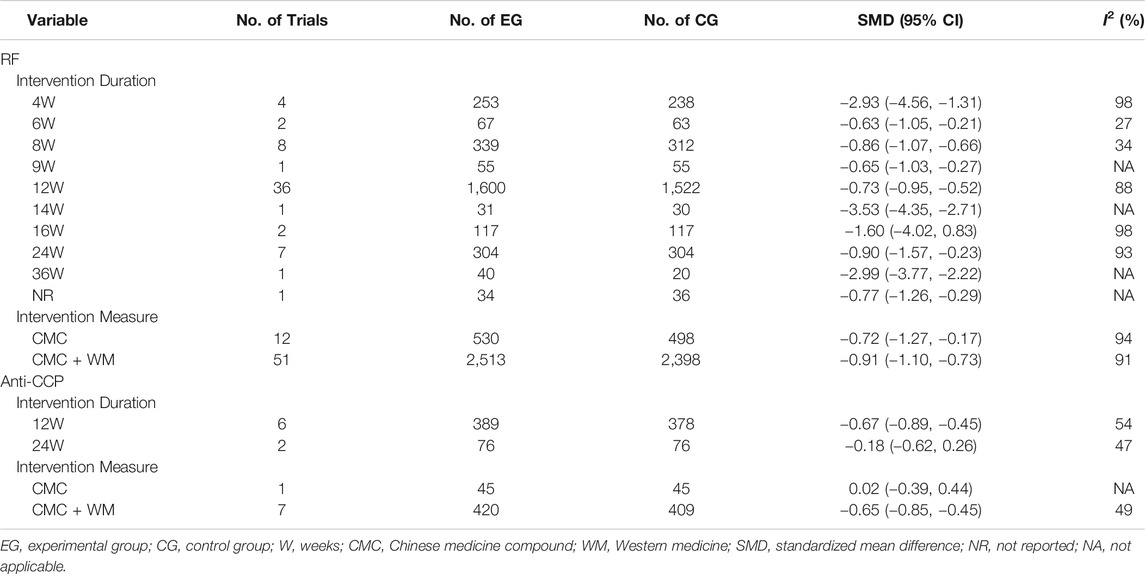
After the heterogeneity test, we conducted a subgroup analysis to explore the source of heterogeneity when the heterogeneity between included studies was found to be significant and not negligible. To assess whether the length of the intervention duration and the difference in intervention measures affect that CMC lower levels of RF and anti-CCP, we conducted a subgroup analysis of the primary outcomes RF and anti-CCP. The subgroup analysis was based on the different number of weeks in terms of intervention duration (4 weeks; 6 weeks; 8 weeks; 9 weeks; 12 weeks; 14 weeks; 16 weeks; 24 weeks; 36 weeks; not reported), and different intervention measures (CMC monotherapy or CMC combined with Western medicine) specified the subgroups. Table 3 summarizes the results of RF and anti-CCP subgroup analysis.
We found that the heterogeneity could not be eliminated after grouping according to the results of subgroup analysis, indicating that differences in intervention duration and intervention measures might not be the underlying source of heterogeneity. Combined with the results of subgroup analysis and sensitivity analysis, we discussed the heterogeneity between studies, considering that the heterogeneity between included studies was mainly related to the following aspects:
1) There were differences in the CMC components as well as the dosage regimens of intervention measures among studies; 2) The average age and disease course of the included participants were different, resulting in varying severity of the disease; 3) Due to the slow-acting of the herbal medicine, it usually takes several months after treatment to show significant therapeutic effects. However, the duration of intervention varies greatly from study to study, which may lead to the heterogeneity of the results.
Publication Bias
Analysis for publication bias was performed using Egger’s test for endpoints of RF. Egger’s asymmetry coefficient, known to be low powered, did detect potential bias in the meta-analysis of RF (p < 0.01). These publication bias may be due to the included studies were all published in Chinese. Moreover, all literature included had positive results, and the articles with negative results were often not able to be published, which caused publication bias to some extent. As described above, the publication bias could not be excluded.
Frequency Distribution Analysis
A total of 71 CMCs were recorded, and 156 single Chinese medicines were obtained, which were sorted by frequency of occurrence from high to low, we listed the Chinese medicine with a frequency of more than 10 times. There were a total of 26 traditional Chinese herbs (Table 4). The top five were Angelica sinensis (Oliv.) Diels [Apiaceae;Radix Angelicae Sinensis] (Danggui), Glycyrrhiza glabra L.[Fabaceae;Radix Glycyrrhizae] (Gancao), Neolitsea cassia (L.) Kosterm.[Lauraceae;Ramulus Cinnamomi] (Guizhi), Paeonia lactiflora Pall.[Paeoniaceae;Radix Paeoniae Alba] (Baishao), and Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. [Apiaceae;Radix Angelicae Pubescentis] (Duhuo).
Discussion
Main Findings
RF and anti-CCP belong to the serum biomarkers involved in the 2010 ACR/EULAR RA classification criteria, and they precede the onset of clinical symptoms and predict a more severe disease course, indicating a pathogenic role in RA (Kay and Upchurch, 2012).
There is a study confirmed that the combined presence of anti-CCP and IgM-RF mediates increased production of proinflammatory cytokine in vitro and this combination is associated with increased systemic inflammation and disease activity in RA (Moura et al., 2012). Accumulating evidences suggest that the presence of high titers of RF in combination with anti-CCP associated with increased disease activity, more aggressive arthritis, worse prognosis and reduced rates of remission in patients with RA (Jónsson et al., 1995; Moeez et al., 2013; Sokolove et al., 2014; Valesini et al., 2015). Moreover, anti-CCP not only has a great diagnostic value for RA in terms of sensitivity and specificity but also seem to be better predictors of poor prognostic features such as progressive joint destruction (Harre et al., 2012). In addition, anti-CCP status predicts response to therapy as well (Burska et al., 2014).
The observations suggest that RF and anti-CCP are capable of promoting a more accurate prognosis and by targeting these two markers will contribute to a better disease management. There is no doubt that underscores the utility of these autoantibodies in RA.
In China, CMC has a long history of treating RA, and several clinical trials have been conducted in recent years on the treatment of RA with CMC. However, to our knowledge, this is the first systematic review and meta-analysis of published RCTs to assess the effect of CMC in reducing the levels of serum RF and anti-CCP in patients with RA. We revealed that 65 RCTs observed the comparison between CMC or CMC combined with Western medicine and Western medicine monotherapy in the treatment of RA in terms of changing the serological markers RF and anti-CCP level. The pooled analysis showed that after treatment, serum RF and anti-CCP levels were decreased more significantly in the experimental group compared to the Western medicine control groups than at baseline. It indicated that CMC in the treatment of RA had a potential role in reducing serum RF and anti-CCP levels. In addition, we found that the CMC group had better ACR response and more effective clinical efficacy of TCM syndromes. The RR of ACR70 was higher than that of ACR20 and ACR50, and the proportion of patients achieving an ACR20 response was not significant than that of ACR50 and ACR70 response. This might be partially explained as high heterogeneity as well as difference in effect estimates observed in ACR20. The study of Chen (Chen et al., 2010) might be the source of the heterogeneity, which was related to the contradiction between the results reported in his study with other studies. In the results of this study, the ACR20 response rate was lower in the experimental group than in the control group, and the difference was not statistically significant. In addition, in the meta-analysis of ACR50, we found that the two studies of Zhao (Zhao, 2015) and He (He and Xiao, 2014) showed a high confidence interval value, indicating lower external validity.
Furthermore, this study summarized the frequency of CMC constituent involved in 65 research literatures and found that the top five from high to low were Angelica sinensis (Oliv.) Diels [Apiaceae;Radix Angelicae Sinensis] (Danggui), Glycyrrhiza glabra L.[Fabaceae;Radix Glycyrrhizae] (Gancao), Neolitsea cassia (L.) Kosterm.[Lauraceae;Ramulus Cinnamomi] (Guizhi), Paeonia lactiflora Pall.[Paeoniaceae;Radix Paeoniae Alba] (Baishao), and Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. [Apiaceae;Radix Angelicae Pubescentis] (Duhuo). The active ingredients and pharmacological effects of the above five high-frequency representative Chinese herb were summarized in Table 5, and as illustrated in this table, most of the main active ingredients of the five Chinese herbs have immunomodulatory effects. The polysaccharides isolated from Angelica sinensis (Oliv.) Diels [Apiaceae;Radix Angelicae Sinensis] (Danggui) can regulate the immune response by regulating expression of Th1 and Th2 related cytokines (Chen et al., 2013). Cinnamaldehyde is isolated from the essential oil produced from the Neolitsea cassia (L.) Kosterm.[Lauraceae;Ramulus Cinnamomi] (Guizhi), and it has been reported to reduce the release of pro-inflammatory cytokines by inhibiting the activation of macrophages and monocytes through suppression of the mitogen-activated protein kinases (MAPKs) signaling pathway (Chao et al., 2008). Total glycoside of paeony (TGP) is extracted from the dried root of Paeonia lactiflora Pall.[Paeoniaceae;Radix Paeoniae Alba] (Baishao). Paeoniflorin (Pae) is the major active component of TGP. Pae can balance the subsets of immune cells through inhibiting abnormal activated cell subsets and restoring regulatory cell subsets by integrating multiple signaling pathways, such as JAK2/STAT3 pathway, MAPKs/NF-κB pathway, PI3K/Akt/mTOR pathway, etc. (Zhang et al., 2019; Zhang and Wei, 2020). It is well known that the pathogenesis of RA is related to B lymphocytes, and RF and anti-CCP are considered to be the results of B lymphocyte activation and differentiation into plasma cells (Cambridge et al., 2003; Dörner et al., 2004). One study confirmed through animal experiments that Pae inhibits the activation and proliferation of B cells by selectively blocking the LPS/TLR4 signaling pathway (Zhang et al., 2015). In addition, another study found that Pae can regulate PI3K/Akt/mTOR pathways mediated by BAFF (B-cell activating factor)/BAFF-R, thereby inhibiting antibody production by B lymphocytes in CIA rats. This may be one of the mechanisms of action of Pae to downregulate antibodies production and treat RA (Li et al., 2012b). Therefore, we figured that the mechanism by which TCM or CMC can reduce serum RF and anti-CCP levels might be related to the regulation of the immune response and inhibition of B lymphocytes proliferation.
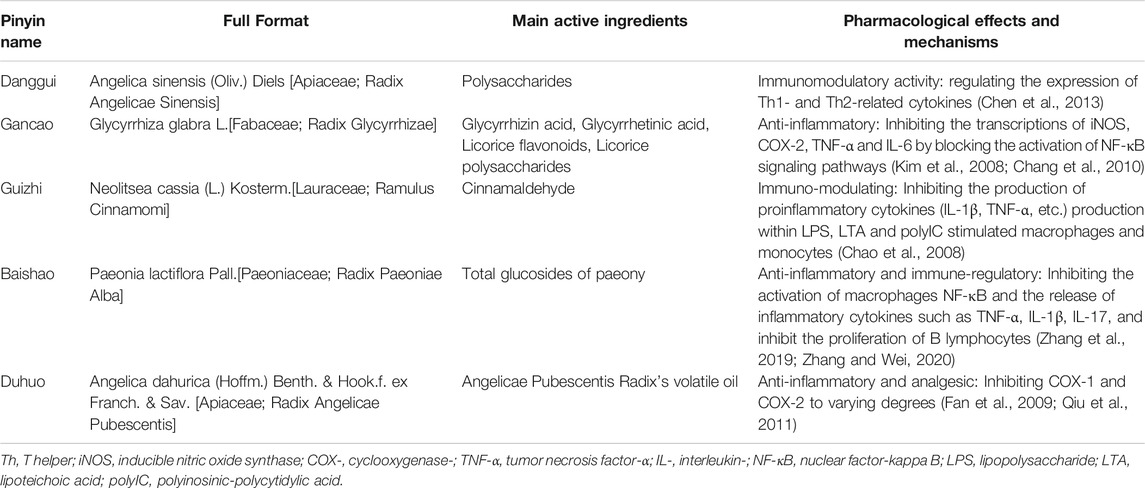
TABLE 5. Main active ingredients, pharmacological effects and mechanisms of the top five Chinese medicine in frequency distribution.
Limitations for Research
There are some limitations in our systematic review. Firstly, since there has been no standardized CMC abroad, the above researches were all completed in China. Moreover, the foundation and clinical effects of CMC in other countries may be inconsistent with these data. With the successful development and proven definitive efficacy and safety of biologicals worldwide, biologicals are now more commonly used in the treatment of RA in many places. However, their use is not common in China as Europe, which is due to the more expensive treatment price and economic cost of biologicals compared to traditional treatment options, and this is also one of the reasons why early, adequate, and full course use of biologicals is difficult for RA patients in many developing countries (Joyce et al., 2014). It follows that among the studies included in the systematic review and meta-analysis, we found that only one (Wei and Liu, 2013) of the interventions used biologicals. Therefore, the conclusions of this systematic review have some limitations. Secondly, the overall quality of the literature included was poor, and the design of the methodological part need to be improved. Most of the included studies had inadequate or missing descriptions of randomization, allocation concealment and blinding method, resulting in an inability to fully assess the internal validity of the trials. Additionally, the sample size of most studies was too small to perform robust analysis, which fundamentally affected the strength of synthesis evidence, and therefore resulting in the limitations and conservatism of this systematic review. Thirdly, due to the different dosage forms used in each study intervention (including decoction, capsule, tablet, pill, etc.), the composition and dosage of CMC administered were also different. These had led to a diversity of intervention measures with no uniform standard, which may lead to heterogeneity. If all CMC intervention measures are combined into the same category of treatment for analysis, the inferred results of meta-analysis would be limited to some extent. Finally, outcome measures and measuring instruments for RF and anti-CCP varied from studies.
Conclusion
In summary, CMC may be an effective treatment to treat RA. In terms of reducing the levels of RF and anti-CCP, CMC (or CMC combined with Western medicine) is more effective than only Western medicine.
Through a systematic review of the potency of CMC to change serum biomarkers levels and the clinical efficacy for the treatment of RA, we found that CMC may be a potential and efficacious therapeutic adjunct to delay the progression and improve outcomes of RA. In the future, RCTs with larger samples and higher quality should be carried out to provide more accurate and complete data to support and verify the potential of TCM compound in the treatment of RA.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
RH and ZW conceived the study, participated in its design and coordination. XT, ZL, and ZY developed the search strategies. XT, ZL, and ZY conducted data collection, analysis and interpretation. XT drafted the paper. RH, ZW, MW, SX, and XC revised it. All authors have approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (No. 81804041), the special project of State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2020ZZ17), Guangdong Provincial Key laboratory of Chinese Medicine for Prevention and Treatment of Refractory Chronic Diseases (2018) (No. 2018B030322012), the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-HongKong-Macau Joint Lab) (No: 2020B1212030006), Natural Science Foundation of Guangdong Province (No. 2021A1515011477, 2021A1515011593), grant from Guangzhou Basic Research Program (No. 202102010256), the China-Sweden Joint Research Base Project on TCM of Guangdong Provincial Hospital of Chinese Medicine (YN2020RD01), as well as grants from Guangdong Provincial Hospital of Chinese Medicine (No. MB2019ZZ07, YN10101906, YN2018ML08, YN2018ZD06).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Professor Bijlsma, EULAR Past-President, for his valuable advice to improve the quality of this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.686360/full#supplementary-material
References
Aletaha, D., Neogi, T., Silman, A. J., Funovits, J., Felson, D. T., Bingham, C. O., et al. (2010). 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum. 62 (9), 2569–2581. doi:10.1002/art.27584
Arnett, F. C., Edworthy, S. M., Bloch, D. A., McShane, D. J., Fries, J. F., Cooper, N. S., et al. (1988). The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheum. 31 (3), 315–324. doi:10.1002/art.1780310302
Begg, C. B., and Mazumdar, M. (1994). Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 50 (4), 1088–1101. doi:10.2307/2533446
Bian, Z. Q., and Wang, Y. D. (2018). Warm Yang Dehumidification, Phlegm Tongluo Prescription Combined with Conventional Therapy to Treat 90 Cases of Cold and Dampness Bizu Rheumatoid Arthritis. Glob. Chin. Med. 11 (03), 447–450.
Burska, A. N., Hunt, L., Boissinot, M., Strollo, R., Ryan, B. J., Vital, E., et al. (2014). Autoantibodies to Posttranslational Modifications in Rheumatoid Arthritis. Mediators Inflamm. 2014, 492873. doi:10.1155/2014/492873
Cambridge, G., Leandro, M. J., Edwards, J. C. W., Ehrenstein, M. R., Salden, M., Bodman-Smith, M., et al. (2003). Serologic Changes Following B Lymphocyte Depletion Therapy for Rheumatoid Arthritis. Arthritis Rheum. 48 (8), 2146–2154. doi:10.1002/art.11181
Cao, M. Z., Wang, Z. L., and Jiang, X. S. (2020). Clinical Efficacy of Modified Duhuo Jisheng Decoction Combined with Methotrexate in Treatment of Patients with Rheumatoid Arthritis of Active Stage. China J. Pharm. Econ. 15 (04), 55–58+62.
Cao, Y. H., and Liu, J. (2014). Clinical Observation of Heat Clearing and Wetting Combined with Western Medicine in the Treatment of Dampness-Heat Type Rheumatoid Arthritis. J. Anhui Univ. Chin. Med. 33 (06), 19–22.
Chang, Y.-l., Chen, C.-l., Kuo, C.-L., Chen, B.-c., and You, J.-s. (2010). Glycyrrhetinic Acid Inhibits ICAM-1 Expression via Blocking JNK and NF-Κb Pathways in TNF-α-Activated Endothelial Cells. Acta Pharmacol. Sin 31 (5), 546–553. doi:10.1038/aps.2010.34
Chao, L. K., Hua, K.-F., Hsu, H.-Y., Cheng, S.-S., Lin, I.-F., Chen, C.-J., et al. (2008). Cinnamaldehyde Inhibits Pro-inflammatory Cytokines Secretion from Monocytes/macrophages through Suppression of Intracellular Signaling. Food Chem. Toxicol. 46 (1), 220–231. doi:10.1016/j.fct.2007.07.016
Chen, F., Min, C. H., Zhou, Y., and Zhang, H. Y. (2016). Clinical Observation of 35 Cases of Rheumatoid Arthritis of Kidney Qi Deficiency and Cold Type Treated by Antshen Juanbi Capsule Combined with Western Medicine. J. Traditional Chin. Med. 57 (12), 1045–1048.
Chen, X.-P., Li, W., Xiao, X.-F., Zhang, L.-L., and Liu, C.-X. (2013). Phytochemical and Pharmacological Studies on Radix Angelica Sinensis. Chin. J. Nat. Medicines 11 (6), 577–587. doi:10.1016/s1875-5364(13)60067-9
Chen, Z., Sun, J., Li, Y. M., and Chen, Y. Q. (2010). Efficacy of Shenshi Qianghuo Dihuang Decoction in Rheumatoid Arthritis: a Randomized Controlled Trial. J. Chin. Integr. Med. 8 (1), 35–39. doi:10.3736/jcim20100107
Dörner, T., Egerer, K., Feist, E., and Burmester, G. R. (2004). Rheumatoid Factor Revisited. Curr. Opin. Rheumatol. 16 (3), 246–253. doi:10.1097/00002281-200405000-00013
Fan, L., Li, L., and He, H. F. (2009). Study on the Anti-inflammatory and Analgesic Pharmacological Effects of Volatile Oil. Anhui Med. Pharm. J. 13 (02), 133–134.
Fang, X. G., Wang, M., Chen, H. Y., Bai, Z. X., Chen, Z. F., and Lan, P. M. (2019). Self-designed Tongluo Powder Combined with Leflumide in the Treatment of Wind-Cold and Rheumatism Type Rheumatoid Arthritis and its Effect on Serum WNT-3 and BMP-2 Levels. Mod. J. Integrated Traditional Chin. West. Med. 28 (13), 1408–1411+1453.
Felson, D. T., Anderson, J. J., Boers, M., Bombardier, C., Furst, D., Goldsmith, C., et al. (1995). American College of Rheumatology Preliminary Definition of Improvement in Rheumatoid Arthritis. Arthritis Rheum. 38 (6), 727–735. doi:10.1002/art.1780380602
Forslind, K., Ahlmen, M., Eberhardt, K., Hafstrom, I., and Svensson, B. (2004). Prediction of Radiological Outcome in Early Rheumatoid Arthritis in Clinical Practice: Role of Antibodies to Citrullinated Peptides (Anti-CCP). Ann. Rheum. Dis. 63 (9), 1090–1095. doi:10.1136/ard.2003.014233
Gao, D., and Bu, S. S. (2017). Clinical Research on Liuwei Dihuang Tang and Siwu Tang Treatment of Rheumatoid Arthritis. ACTA Chin. Med. 32 (06), 1079–1081.
Ge, S. (2018). Observation on the Curative Effect of Combined Chinese and Western Medicine on Rheumatoid Arthritis. J. Pract. Traditional Chin. Med. 34 (11), 1371–1372.
Green, S., and Higgins, J. P. T. (2008). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England: Wiley-Blackwell.
Guo, H. L., Wang, G., and Tian, J. X. (2018). Yukang Pills Combined with Methotrexate Tablets in the Treatment of 40 Cases of Rheumatoid Arthritis. TCM Res. 31 (11), 23–26.
Guo, H. M., Guo, L. W., and Xing, J. Y. (2017). Clinical Observation on 340 Cases of Rheumatoid Arthritis Treated by Ant Tongbi Capsule. World Chin. Med. 12 (08), 1859–1862.
Han, L., Ba, Y., Gu, J. N. T. H., Shi, R., Ba, H. E. G. L., and Wei, R. (2016). Effect of Removing Wind, Dehumidifying and Tonifying Kidney in the Treatment of Senile Rheumatoid Arthritis and the Influence of Antibody Levels against Cyclic Citrulline Polypeptide, Rheumatoid Factor, Erythrocyte Sedimentation Rate, C-Reactive Protein and Keratin. Chin. J. Gerontol. 36 (18), 4558–4560.
Han, S. L., and Song, Y. W. (2011). A Randomized Controlled Study of Eliminating Phlegm, Eliminating Stasis and Eliminating Rheum in the Treatment of Rheumatoid Arthritis. Chin. Arch. Traditional Chin. Med. 29 (12), 2808–2810.
Harre, U., Georgess, D., Bang, H., Bozec, A., Axmann, R., Ossipova, E., et al. (2012). Induction of Osteoclastogenesis and Bone Loss by Human Autoantibodies against Citrullinated Vimentin. J. Clin. Invest. 122 (5), 1791–1802. doi:10.1172/jci60975
He, D. C., and Xiao, J. J. (2014). Clinical Efficacy and Safety of Bizhengning in the Treatment of Rheumatoid Arthritis. Mod. J. Integrated Traditional Chin. West. Med. 23 (28), 3090–3092+3095.
He, D., Liu, Z., Wang, M., Shu, Y., Zhao, S., Song, Z., et al. (2019). Synergistic Enhancement and Hepatoprotective Effect of Combination of Total Phenolic Extracts of Citrus Aurantium L. And Methotrexate for Treatment of Rheumatoid Arthritis. Phytotherapy Res. 33 (4), 1122–1133. doi:10.1002/ptr.6306
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hu, H. (2018). Short-term and Long-Term Effects of Applying Traditional Chinese Medicine Syndrome Differentiation Combined with Methotrexate in the Treatment of Heumatoid Arthritis. J. Sichuan Traditional Chin. Med. 36 (02), 76–79.
Huang, G. D., Li, J. B., Huang, D. F., Huang, Y. H., Xiao, M. Z., Tang, L. J., et al. (2006). Observation on Effect of Centipede Longsnake Decoction Combined with Western Medicine in Treating Rheumatoid Arthritis. Chin. J. Clin. Rehabil. 43, 186–187.
Jia, F. Y., Wang, J., and Yang, Q. B. (2016). A Clinical Study on Treatment of Rheumatoid Arthritis by Eliminating Rheum Bnourishing the Liver and Yishen Decoction. Asia-Pacific Traditional Med. 12 (05), 143–144.
Jiang, D., and Wu, J. (2016). Analysis and Evaluation of the Curative Effect of Self-Prepared Chinese Medicine Combined with Anti-rheumatism Medicine on Active Rheumatoid Arthritis. J. Mod. Med. Health 32 (22), 3507–3510.
Jiang, P., and Ma, L. J. (2020). Clinical Study on the Treatment of Rheumatoid Arthritis by Removing Phlegm and Removing Blood Stasis. China Cont. Med. Edu. 12 (17), 158–160.
Jónsson, T., Arinbjarnarson, S., Thorsteinsson, J., Steinsson, K., Geirsson, Á. J., Jónsson, H., et al. (1995). Raised IgA Rheumatoid Factor (RF) but Not IgM RF or IgG RF Is Associated with Extra-articular Manifestations in Rheumatoid Arthritis. Scand. J. Rheumatol. 24 (6), 372–375. doi:10.3109/03009749509095183
Joyce, A. T., Gandra, S. R., Fox, K. M., Smith, T. W., and Pill, M. W. (2014). National and Regional Dose Escalation and Cost of Tumor Necrosis Factor Blocker Therapy in Biologic-Naïve Rheumatoid Arthritis Patients in US Health Plans. J. Med. Econ. 17 (1), 1–10. doi:10.3111/13696998.2013.856314
Kay, J., and Upchurch, K. S. (2012). ACR/EULAR 2010 Rheumatoid Arthritis Classification Criteria. Rheumatology 51 (Suppl. 6), vi5–vi9. doi:10.1093/rheumatology/kes279
Kim, J.-Y., Park, S. J., Yun, K.-J., Cho, Y.-W., Park, H.-J., and Lee, K.-T. (2008). Isoliquiritigenin Isolated from the Roots of Glycyrrhiza Uralensis Inhibits LPS-Induced iNOS and COX-2 Expression via the Attenuation of NF-Κb in RAW 264.7 Macrophages. Eur. J. Pharmacol. 584 (1), 175–184. doi:10.1016/j.ejphar.2008.01.032
Li, A. M. (2009). Siteng Yin and Siwu Tang Were Used to Treat 160 Cases of Rheumatoid Arthritis. Chin. ethnic folk Med. 18 (10), 51–52.
Li, D. (2020). Effect of Huatan Huoxue Formula on Swollen Joint Count index and Joint Pain Indexin Patients with Phlegm and Blood Stasis Type of Rheumatoid Arthritis. J. Med. Forum 41 (04), 91–94.
Li, J., Rong, B., Jia, J., and Pan, M. Z. (2017). Effect of Modified Juanbitang on Inflammatory Factors and Serum MMP-3OPG and ANKL in Synovial Fluid of Patients with Wind-Cold-Wetness Type Heumatoid Arthritis. Chin. J. Exp. Traditional Med. Formulae 23 (22), 165–170.
Li, J. H., Lin, Y. B., Yu, G. S., Zhang, S. X., Liu, Y. Y., and Xu, H. B. (2019). Clinical Observation on Treating 41 Cases of Rheumatoid Arthritis of Mixed Heat and Cold Type with Guizhi Shaoyao Zhimu Tang Combined with Methotrexate. Rheum. Arthritis 8 (02), 31–34+42.
Li, J. P. (2013). Clinical Analysis of 40 Cases of Rheumatoid Arthritis Treated by Combination of Chinese and Western Medicine. J. Sichuan Traditional Chin. Med. 31 (01), 86–88.
Li, P.-P., Liu, D.-D., Liu, Y.-J., Song, S.-S., Wang, Q.-T., Chang, Y., et al. (2012). BAFF/BAFF-R Involved in Antibodies Production of Rats with Collagen-Induced Arthritis via PI3K-Akt-mTOR Signaling and the Regulation of Paeoniflorin. J. Ethnopharmacology 141 (1), 290–300. doi:10.1016/j.jep.2012.02.034
Li, S. W. (2006). Clinical Observation on Treatment of Rheumatoid Arthritis by Sanbi Decoction. Chin. Arch. Traditional Chin. Med. (09), 1738–1739.
Li, X., Liu, J. F., and Zhao, G. Q. (2015). Clinical Observation on the Treatment of Rheumatoid Arthritis by Adding or Subtracting Judanxi Gout Prescription Combined with Methotrexate. Anhui Med. J. 36 (08), 995–998.
Li, Y., Fan, W. M., Gu, S. F., and Liu, W. H. (2015). Clinical Observation of Kidney-Tonifying Collateral-Activating Blood-Activating Formula in Treating Rheumatoid Arthritis with Mutual junction of Phlegm and Blood Stasis. J. Anhui Univ. Chin. Med. 34 (04), 17–20.
Li, Z. L., Wang, R. S., Shen, J., Xu, L. M., Yue, T., Zhu, Q., et al. (2012). Effect of Tonifying Kidney and Removing Blood Stasis on Bone Metabolism in Patients with Early Rheumatoid Arthritis. J. Traditional Chin. Med. 53 (03), 215–218.
Liu, B., Liu, W., and Wang, Y. (2007). Siwu Decoction Combined with Methotrexate Attenuated and Effective Treatment for Rheumatoid Arthritis. Chin. J. Integr. Med. 27 (1).
Liu, E. C., Liu, Y., Wang, S. H., and Wang, S. F. (2013). Clinical Observation on the Treatment of Rheumatoid Arthritis with Lumbago Capsule. Clin. J. Traditional Chin. Med. 25 (02), 109–111.
Liu, X. D., Zhang, J. L., Ye, L. H., Liu, F. Y., and Chen, Y. (2009). Effect of Wenhua Juanbi Prescription on TNF and IL-1 in Peripheral Blood of Patients with Rheumatoid Arthritis. Chin. J. Integr. Med. 29 (09), 787–790.
Liu, Y., Zhang, H. J., Guo, Y. X., and Meng, Q. L. (2016). Clinical Observation of 35 Cases of Heumatoid Arthritis of Damp Heat Type Treated with Si Miao San He Xuan Bi Tang and Western Medicine. World J. Integrated Traditional West. Med. 11 (6), 800–803.
Luo, Y. H. (2008). 32 Cases of Senile Rheumatoid Arthritis Were Treated with Spleen-Invigorating and Blood-Activating Formula. Shaanxi J. Tradit Chin. Med. (08), 997–999.
Ma, W. K., Zhong, Q., Liu, Z. Q., and Yao, X. M. (2009). Clinical Study on the Treatment of Rheumatoid Arthritis by Sanwu Capsule Combined with Methotrexate and Salazosulphapyridine. J. New Chin. Med. 41 (11), 42–44.
Ma, Z. L., Wang, C. Y., and Liu, B. B. (2018). Clinical Observation of Buyang Huanwu Decoction in Treating Phlegm and Blood Stasis Type Rheumatoid Arthritis. Mod. J. Integrated Traditional Chin. West. Med. 27 (12), 1313–1315.
Moeez, S., John, P., and Bhatti, A. (2013). Anti-citrullinated Protein Antibodies: Role in Pathogenesis of RA and Potential as a Diagnostic Tool. Rheumatol. Int. 33 (7), 1669–1673. doi:10.1007/s00296-012-2635-6
Moura, R. A., Graca, L., and Fonseca, J. E. (2012). To B or Not to B the Conductor of Rheumatoid Arthritis Orchestra. Clinic Rev. Allerg Immunol. 43 (3), 281–291. doi:10.1007/s12016-012-8318-y
Niu, J. H. (2014). Observation on the Curative Effect of Guizhi Peony Zhimu Decoction Combined with Juanyi Decoction and Juanyi Decoction in Treating Rheumatoid Arthritis. Shaanxi J. Tradit Chin. Med. 35 (08), 984–986.
Pang, A. M., Jiang, P., Li, J. X., and Chi, X. W. (2017). Effects of Hebi Formula on Bone Erosion in Early Rheumatoid Arthritis of Liver and Spleen Disorder Type. Chin. J. Traditional Chin. Med. 32 (12), 5682–5685.
Pang, J., Xu, J., Li, L., and Li, Y. L. (2019). Clinical Study of Guikun Fengshi Mixture Combined with Methotrexate in the Treatment of Rheumatoid Arthritis. Pharmacol. Clin. Chin. Mater. Med. 35 (05), 155–158.
Qian, X., Chen, X., Wei, G., Guo, Y. K., and Sun, Z. L. (2016). Clinical Effect of Duhuo Jisheng Tang Combined with Meloxicam in Treatment of Rheumatoid Arthritis. Chin. J. Exp. Traditional Med. Formulae 22 (7), 173–176.
Qiu, J. B., Xu, Q., and Jiang, X. H. (2011). Effects of Ethanol Extract of Angelicae Pubescentis Radix on Cyclooxygenase. China Med. Herald 8 (16), 42–43.
Rainsford, K. D. (1999). Profile and Mechanisms of Gastrointestinal and Other Side Effects of Nonsteroidal Anti-inflammatory Drugs (NSAIDs). Am. J. Med. 107 (6A), 27–35. doi:10.1016/s0002-9343(99)00365-4
Ronnelid, J., Wick, M. C., Lampa, J., Lindblad, S., Nordmark, B., Klareskog, L., et al. (2005). Longitudinal Analysis of Citrullinated Protein/peptide Antibodies (Anti-CP) during 5 Year Follow up in Early Rheumatoid Arthritis: Anti-CP Status Predicts Worse Disease Activity and Greater Radiological Progression. Ann. Rheum. Dis. 64 (12), 1744–1749. doi:10.1136/ard.2004.033571
Schoels, M., Bombardier, C., and Aletaha, D. (2011). Diagnostic and Prognostic Value of Antibodies and Soluble Biomarkers in Undifferentiated Peripheral Inflammatory Arthritis: a Systematic Review. J. Rheumatol. Suppl. 87, 20–25. doi:10.3899/jrheum.101070
Shen, H.-b., Bai, Y.-j., Huo, Z.-j., Li, W.-n., and Tang, X.-p. (2011). Assessment of Clinical Effect of Therapy Combining Disease with Syndrome on Rheumatoid Arthritis. J. Traditional Chin. Med. 31 (1), 39–43. doi:10.1016/s0254-6272(11)60009-5
Shen, Y. P., Chuan, X. W., and Liu, Y. (2008). Effect of Nourishing Yin and Supplementing Qi on Hormone Withdrawal in Rheumatoid Arthritis. Shandong J. Traditional Chin. Med. (02), 95–96.
Shi, L., and Yang, F. (2018). 40 Cases of Rheumatoid Arthritis Were Treated by Renal Differentiation. J. Shaanxi Univ. Traditional Chin. Med. 041 (001), 66–69.
Shu, C., Hua, D. P., and Li, Y. (2015). Clinical Observation on Yishen Qingluo Huoxue Formula in Treatment of Intermingled Phlegm and Blood Stasis Type of Heumatoid Arthritis. Chin. Arch. Traditional Chin. Med. 33 (01), 34–37.
Sokolove, J., Johnson, D. S., Lahey, L. J., Wagner, C. A., Cheng, D., Thiele, G. M., et al. (2014). Rheumatoid Factor as a Potentiator of Anti-citrullinated Protein Antibody-Mediated Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 66 (4), 813–821. doi:10.1002/art.38307
Song, W. H., Li, Q., Wang, F. Z., Tao, W. X., and Guan, Q. C. R. (2019). Effects of Wenyang Bushen Method on the Levels of Serum 25 Hydroxyvitamin D3 in Patients with Plateau Rheumatoid Arthritis. World Chin. Med. 14 (6), 1466–1470.
Su, S. Z., Ye, X. Y., Peng, J. H., and Chen, B. (2015). Clinical Observation on Treating Rheumatoid Arthritis by Bushen Huoxue Therapy. Clin. J. Chin. Med. (13), 18–20.
Valesini, G., Gerardi, M. C., Iannuccelli, C., Pacucci, V. A., Pendolino, M., and Shoenfeld, Y. (2015). Citrullination and Autoimmunity. Autoimmun. Rev. 14 (6), 490–497. doi:10.1016/j.autrev.2015.01.013
van Venrooij, W. J., van Beers, J. J. B. C., and Pruijn, G. J. M. (2008). Anti-CCP Antibody, a Marker for the Early Detection of Rheumatoid Arthritis. Ann. N. Y Acad. Sci. 1143, 268–285. doi:10.1196/annals.1443.013
Wang, H. T. (2017). Clinical Research on Treating Rheumatoid Arthritis by the Buyi Qixue, Qushi Tongluo Therapy. Clin. J. Chin. Med. 9 (27), 62–63.
Wang, S. M., Wang, X., and Gong, Q. (2006). Observation of 40 Cases of Rheumatoid Arthritis Treated by Combination of Traditional Chinese and Western Medicine. J. Sichuan Traditional Chin. Med. (06), 47–48.
Wang, W., Zhou, H., and Liu, L. (2018). Side Effects of Methotrexate Therapy for Rheumatoid Arthritis: A Systematic Review. Eur. J. Med. Chem. 158, 502–516. doi:10.1016/j.ejmech.2018.09.027
Wang, Y., and Tu, S. H. (2017). Clinical Study on Modified Wutou Decoction in Treatment of Heumatoid Arthritis. ACTA Chin. Med. 32 (09), 1716–1719.
Wang, Z., and Tao, X. J. (2014). [Treatment of Rheumatoid Arthritis by Yangxue Tongluo Recipe Combined with Immunosuppressive Agents: a Clinical Observation]. Zhongguo Zhong Xi Yi Jie He Za Zhi 34 (3), 276–278.
Wei, W., and Liu, K. K. (2013). Clinical Observation of Traditional Chinese Medicine Combined with Etanercept in Treatment of Elderly Rheumatoid Arthritis. Chin. Arch. Traditional Chin. Med. 31 (04), 939–941.
Wei, Y., Yang, X. M., and Wang, S. E. (2013). Clinical Observation of 50 Cases of Active Rheumatoid Arthritis Treated by Combination of Chinese and Western Medicine. Sci. Tech. Chin. Traditional Med. 20 (1), 54–55.
Xiang, C. C., Xiong, Q. D., and Wu, J. Y. (2009). 40 Cases of Rheumatoid Arthritis Were Treated with Bushendecoction and Western Medicine. Shaanxi J. Tradit Chin. Med. 30 (12), 1614–1616.
Xu, G. S., Yu, X. F., Kong, M. Z., Chen, J. C., and Qiu, M. S. (2018). Clinical Observation on the Treatment of Rheumatoid Arthritis with Heat and Blood Stasis by Traditional Chinese Medicine Therapy of Clearing Heat and Activating Blood Circulation. Chin. J. Traditional Chin. Med. 33 (03), 1167–1170.
Yang, B., Liang, Q. H., Wu, D., Tang, T., and Peng, W. J. (2011). Clinical Observation of Simiao Pill Combined with Western Medicine in Treating 20 Cases of Active Rheumatoid Arthritis. J. Traditional Chin. Med. 52 (18), 1566–1569.
Yang, Y. J. (2013). Treatment of 42 Cases of Rheumatoid Arthritis with Compound Ma Qian Zisan. China Pharm. 22 (2), 84–85.
Yao, J. H. (2010). Clinical Observation of 58 Cases of Rheumatoid Arthritis Treated by Heat Bi Decoction. J. Traditional Chin. Med. 51 (12), 1086–1088.
You, B. R., Tian, X. W., and Liu, C. J. (2016). Clinical Observation of Liuwei Dihuanghe Siwu Decoction Combined with Methotrexate in the Treatment of Rheumatoid Arthritis. Guiding J. TCM 22 (6), 95–97.
Yu, M., and Chen, Y. Y. (2014). Observation on Effect of Integrated Chinese Medicine and Western Medicine on 32 Pafients with Rheumatoid Arthritis. Intern. Med. China 9 (01), 12–14.
Yuan, L., Wu, J. Y., Tang, J., Chen, Y. G., and Zhang, Z. Y. (2019). Clinical Observation of Baihu Plus Guizhi Decoction Combined with Western Medicine in Treating Rheumatoid Arthritis with Rheumatic Heat Arthralgia Syndrome. J. Liaoning Univ. TCM 21 (12), 168–171.
Zeng, J. Y., and Chen, S. K. (2018). The Clinical Effect of Traditional Chinese Medicine Combined with Antirheumatic Drugs on Rheumatoid Arthritis. Clin. J. Chin. Med. 10 (27), 82–83.
Zeng, X. F., Zhu, S. L., Tan, A. C., and Xie, X. P. (2013). Disease Burden and Quality of Life of Rheumatoid Arthritis in China: A Systematic Review. Chin. J. Evid-based Med. 13 (03), 300–307.
Zhang, H. J., and Chen, T. B. (2014). Clinical Observation of Clearing Damp Analgesic Decoction Combined with Meloxicam in Treatment of Rheumatoid Arthritis. Shaanxi J. Tradit Chin. Med. 10, 1336–1338.
Zhang, J., Li, H., Huo, R., Zhai, T., Li, H., Sun, Y., et al. (2015). Paeoniflorin Selectively Inhibits LPS-Provoked B-Cell Function. J. Pharmacol. Sci. 128 (1), 8–16. doi:10.1016/j.jphs.2015.02.011
Zhang, K. L. (2019). Research on the Clinical Effect of Integrated Chinese and Western Medicinesin Treatment of 47 Cases of Rheumatoid Arthritis. Chin. ethnic folk Med. 28 (11), 83–85.
Zhang, L., and Wei, W. (2020). Anti-inflammatory and Immunoregulatory Effects of Paeoniflorin and Total Glucosides of Paeony. Pharmacol. Ther. 207, 107452. doi:10.1016/j.pharmthera.2019.107452
Zhang, L., Yu, J., Wang, C., and Wei, W. (2019). The Effects of Total Glucosides of Paeony (TGP) and Paeoniflorin (Pae) on Inflammatory-Immune Responses in Rheumatoid Arthritis (RA). Funct. Plant Biol. 46 (2), 107–117. doi:10.1071/fp18080
Zhao, F. C. (2015). Observation on the Curative Effect of Combined Chinese and Western Medicine on Rheumatoid Arthritis. Mod. J. Integrated Traditional Chin. West. Med. 24 (19), 2107–2109.
Zhao, L. (2019). Clinical Efficacy of the Guizhi Shaoyao Zhimu Decoction on Rheumatoid Arthritis of the Fenghan Shibi Type. Clin. J. Chin. Med. 11 (34), 51–53.
Zheng, X. B., and Shi, C. H. (2015). Zhuithmg Tougu Capsules Combined with Methotmxate on the Treatment of Rheumatoid Arthritis for 80 Cases. Chin. Med. Mod. distance Educ. 13 (09), 63–65.
Zheng, X. Y. (2002). Guiding Principles for Clinical Research of New Chinese Medicines. Beijing: China Medical Science and Technology Press.
Keywords: Chinese medicine compound, rheumatoid arthritis, rheumatoid factor, anti-cyclic citrullinated peptide antibodies, meta-analysis
Citation: Tang X, Liu Z, Yang Z, Xu S, Wang M, Chen X, Wen Z and Huang R (2021) The Effect of Chinese Medicine Compound in the Treatment of Rheumatoid Arthritis on the Level of Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Antibodies: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:686360. doi: 10.3389/fphar.2021.686360
Received: 26 March 2021; Accepted: 17 June 2021;
Published: 30 June 2021.
Edited by:
Yihai Wang, Guangdong Pharmaceutical University, ChinaCopyright © 2021 Tang, Liu, Yang, Xu, Wang, Chen, Wen and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Runyue Huang, cnlodWFuZ0BnenVjbS5lZHUuY24=
†These authors have contributed equally to this work
 Xuan Tang
Xuan Tang Zehao Liu1†
Zehao Liu1† Zhihua Yang
Zhihua Yang Maojie Wang
Maojie Wang Xiumin Chen
Xiumin Chen Zehuai Wen
Zehuai Wen Runyue Huang
Runyue Huang