- 1Dermatological Department, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Geriatric Department, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Vitiligo is the most common depigmenting disorder characterized by white patches in the skin. The pathogenetic origin of vitiligo revolves around autoimmune destruction of melanocytes in which, for instance, oxidative stress is responsible for melanocyte molecular, organelle dysfunction and melanocyte specific antigen exposure as well as melanocyte cell death and thus serves as an important contributor for vitiligo progression. In recent years, natural products have shown a wide range of pharmacological bioactivities against many skin diseases, and this review focuses on the effects and mechanisms of natural compounds against vitiligo models. It is showed that some natural compounds such as flavonoids, phenols, glycosides and coumarins have a protective role in melanocytes and thereby arrest the depigmentation, and, additionally, Nrf2/HO-1, MAPK, JAK/STAT, cAMP/PKA, and Wnt/β-catenin signaling pathways were reported to be implicated in these protective effects. This review discusses the great potential of plant derived natural products as anti-vitiligo agents, as well as the future directions to explore.
Introduction
Pathogenesis of Vitiligo
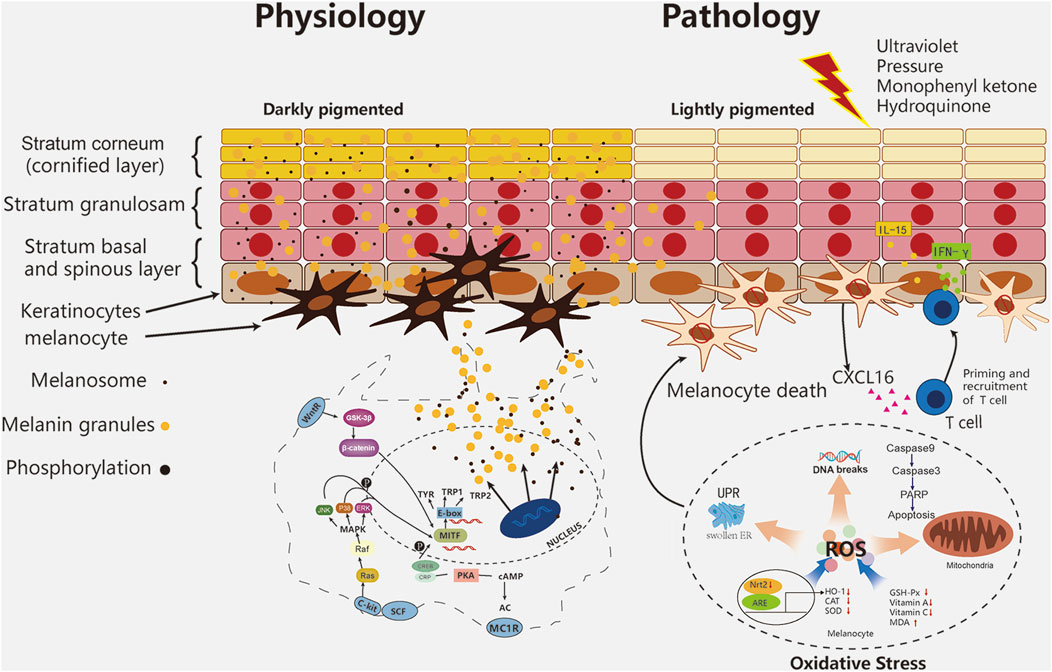
Vitiligo is a chronic autoimmune destruction of melanocytes, leading to the pigment loss on the surface of skin and mucosa and then the gradual expansion of decolorized skin plaque (Seneschal et al., 2021). The severity of the disease affects about 1% of humans (Whitton et al., 2015). Clinically, vitiligo is divided into segmental vitiligo (SV), non-segmental vitiligo (NSV) and mixed vitiligo (MV) (Ezzedine et al., 2015). NSV is the most common type of vitiligo. Its clinical features are clear boundary, reticular, different size, different distribution, depigmentation and milky white. SV is a piece or several pieces, along the skin area dominated by a certain cutaneous ganglion segment, generally unilateral, accounting for 5–16% of all vitiligo cases (Speeckaert et al., 2020). MV includes the combination of SV and then bilateral NSV plaque after a period of time (Ezzedine et al., 2015). The debilitating nature of vitiligo leads to poor quality of life and mental health (Patel et al., 2017). Dermal exposure to UV light from depigmented skin increases the risk of skin irritation and cancer (Ahluwalia et al., 2017).
The main theories of the pathogenesis of vitiligo are: 1) oxidative stress theory, 2) autoimmune theory, 3) neural theory and 4) biochemical theory. The autoimmune theory is the most accepted one (Rodrigues et al., 2017). More than half of the 40 susceptibility genes revealed by genome-wide analysis are involved in immunoregulatory activities (Jin et al., 2016). Strong evidence shows that oxidative stress is a key factor in the occurrence and development of diseases (Denat et al., 2014). Several endogenous and exogenous stimuli are related to the occurrence of diseases. Endogenous factors include melanin synthesis, proliferation, differentiation, cell metabolism, immune response and apoptosis (Al-Shobaili and Rasheed 2015). Exogenous stimuli include exposure to the environment (e.g., cytotoxic chemicals, trauma, UV exposure, monophenones and other phenolics), other diseases (severe infections, neurological disorders, malignancies, calcium imbalance), and pharmaceutical applications (e.g., certain hormones, vaccination, drugs) (Xie et al., 2016). These all induce oxidative stress in melanocytes, which may be important in activating autoimmune responses associated with vitiligo (Abdel-Malek et al., 2020; Chen X. et al., 2019; Xie et al., 2016). The decrease of the level and activity of antioxidant enzymes (such as catalase and glutathione peroxidase) and the imbalance of Pro oxidation/anti oxidation balance are also the reasons for the production and accumulation of ROS (Wang Y. et al., 2019). Nrf2-ARE regulates cellular protective genes related to oxidative stress (Jian et al., 2011). In vitiligo melanocytes, Nrf2 has nuclear translocation and decreased transcriptional activity, resulting in decreased HO-1 expression and abnormal redox balance (Jian et al., 2014). Due to the deficiency of antioxidant function, melanocytes in vitiligo are particularly sensitive to ROS accumulation, which leads to DNA damage, protein oxidation/breakage, mitochondrial dysfunction, endoplasmic reticulum abnormalities and lipid peroxidation (Bickers and Athar 2006; Chen J. et al., 2021). Increased ROS levels even modified tyrosinase (Tyr) and other melanin proteins into new antigens (Rodrigues et al., 2017). Oxidative stress leads to the accumulation of misfolded proteins in the lumen of the endoplasmic reticulum (ER), which in turn activates the unfolded protein response (UPR) to restore cellular homeostasis and maintain cell survival. The disturbance of endoplasmic reticulum Ca2+ triggered by oxidative stress may also induce UPR and apoptosis (Carreras-Sureda et al., 2018). Under continuous cell pressure, UPR promotes autoimmune response through apoptosis cascade, and then activates CD8+ T cells to produce adaptive immune response, and T cells release interferon-γ (INF-γ), It binds to receptors on keratinocytes, further releases and presents inflammatory cytokines such as CXC-L16 and IL-15, and further recruits T cells to the skin through a positive feedback loop (Bergqvist and Ezzedine 2021). The recruitment of CD8+and T cells induced by cytokines and chemokines ensure the final destruction of epidermal melanocytes (Figure 1). After naive T cells are activated by antigen-presenting cells, a small subset of these precursor cells eventually develop into several subsets of memory T cells, including effector memory T (TEM) cells, tissue resident memory T (TRM) cells and central memory T (TCM) cells. TRM play a major role in vitiligo recurrence (Chen and Shen 2020). In the process, melanocytes, fibroblasts, innate lymphoid cells, natural killer cells, and keratinocytes collectively contribute to the pathogenesis of vitiligo (Seneschal et al., 2021).
Vitiligo Therapy
However, there is no clear treatment for local and systemic vitiligo. The most widely used therapy is local steroid and narrowband ultraviolet B monotherapy (Grimes and Nashawati 2017), they are not effective in all patients and are expensive, not easily accepted, and are associated with side effects (Huo et al., 2014). Local corticosteroids, calcineurin inhibitors and phototherapy are still the basis of treatment, and long-term use of steroids can lead to decreased immunity (Whitton et al., 2016). Among the numerous treatments currently available, including medical, physical, or surgical approaches, each modality has its disadvantages and side effects (Yamaguchi et al., 2007).
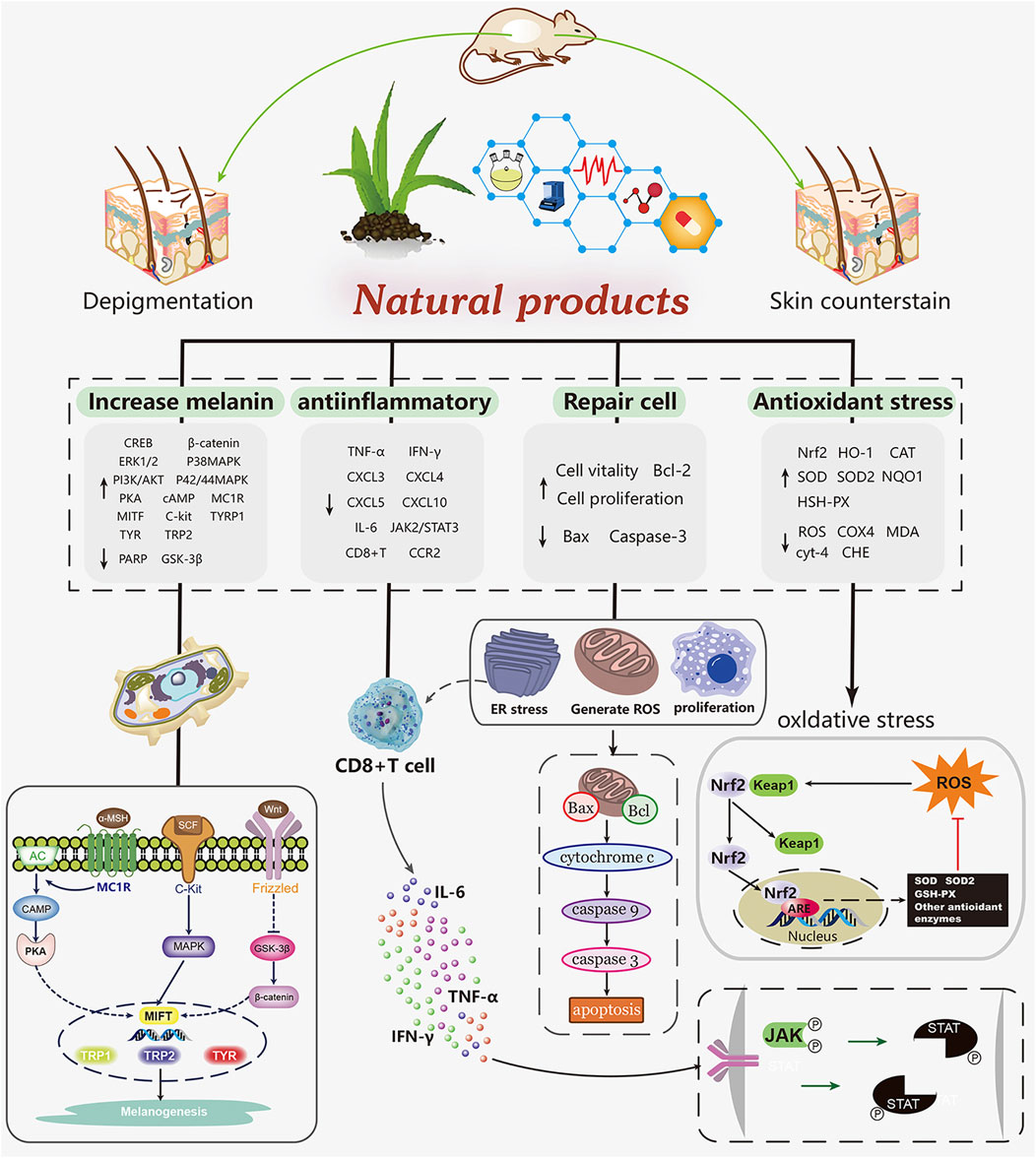
In vivo and in vitro experiments, natural products have been shown to promote melanin production and prevent melanin from being destroyed in a network way. It mainly includes scavenging free radicals (NOS) to alleviate the damage of melanocytes caused by oxidative stress, activating melanogenesis related pathways, increasing the expression of tyrosinase gene, reducing the expression of chemokines and inflammatory cytokines, preventing the migration of CD8 + T cells. This paper reviews several natural drugs for the treatment of vitiligo. Among the natural products that we screened, 16 compounds (such as baicalein, quercetin, paeonol. etc) exert antioxidant effects to protect melanocytes by scavenging free radicals, activating the Nrf2/HO-1 pathway, while maintaining normal cell morphology, slowing down apoptosis and preventing injury. Four compounds (vitexin, baicalin, EGCG and berberine) achieved repigmentation of vitiligo skin lesions via anti-inflammatory effects, the process of which involved the activation of the JAK/STAT pathway as well as the inhibition of autoimmunity caused by the migration of immune cells such as CD8 + T. In mammals, there are three major melanocyte specific enzymes catalyzing melanin biosynthesis: tyrosinase (TYR), tyrosinase associated protein 1 (TRP-1) and TRP-2. TRP-1 and Trp-2 are downstream functional proteins of TYR (Yin et al., 2018). Microphthalmia associated transcription factor (MITF) is a transcription factor important for melanogenesis genes (Hearing 1999). Previous studies summarized three main pathways of melanin biosynthesis regulated by tyrosinase: MAPK, cAMP/PKA, Wnt/β - catenin signaling pathway, and reviewed the effects of several natural products on melanin synthesis and tyrosinase activity (Niu and Aisa 2017; Pillaiyar et al., 2017). Among the compounds we screened, 37 compounds (such as quercetin, afzelin, puerarin, geniposide, etc) promote melanocyte generation through the above pathways (Figure 2).
Flavonoids
Baicalein
Baicalein is a flavonoid extracted from the roots of Scutellaria baicalensis Georgi that has been extensively applied in Traditional Medicine in Asia (Figure 3A) (Kim et al., 2014). It has been reported to have anti-cytotoxic, anti-inflammatory, and anti-tumor effects (Huang et al., 2005; Hwang et al., 2005; Yarla et al., 2016). In addition, the antioxidant effects of baicalein have received special attention during the past decades, including reducing the levels of reactive oxygen species (ROS) generated by chemical agents (Chiu et al., 2010; Choi et al., 2016; Zhao et al., 2018) or ultraviolet radiation (Wang et al., 2018). Baicalein has strong antioxidant properties, partly because compounds scavenge ROS by oxidative consumption of the three 5, 6, 7-position OH–groups in its structure (de Oliveira et al., 2015), and it form stable semiquinone radicals, which also underlie its powerful antioxidant activity (Gao et al., 1999). The apoptosis of PIG1 cells induced by H2O2 may be mediated by mitochondrial pathway. In the in vitro model of H2O2-induced oxidative stress of PIG1, baicalein protected PIG1 cells from H2O2-induced oxidative stress and apoptosis, maintained mitochondrial membrane potential, released cytochrome c, and decreased the Bax/Bcl-2 ratio. The mechanism mainly involves the activation of mitochondrial dependent caspase and the regulation of p38MAPK pathway. Baicalein at the concentration of 40 µM had the strongest protective effect on melanocytes (Liu et al., 2012). In human vitiligo melanocytes (PIG3V) induced by hydrogen peroxide, baicalein increased the expression of Nrf2 and its downstream gene HO-1 in PIG3V cells, promoted the translocation of Nrf2 from cytoplasm to nucleus, indicating that the protective effect of baicalein on melanocytes depends on Nrf2 signaling pathway (Ma et al., 2018). Baicalein also has antioxidant effect on keratinocytes (Wang et al., 2018), therefore, the development of baicalein topical preparation for the treatment of vitiligo may be a feasible method.
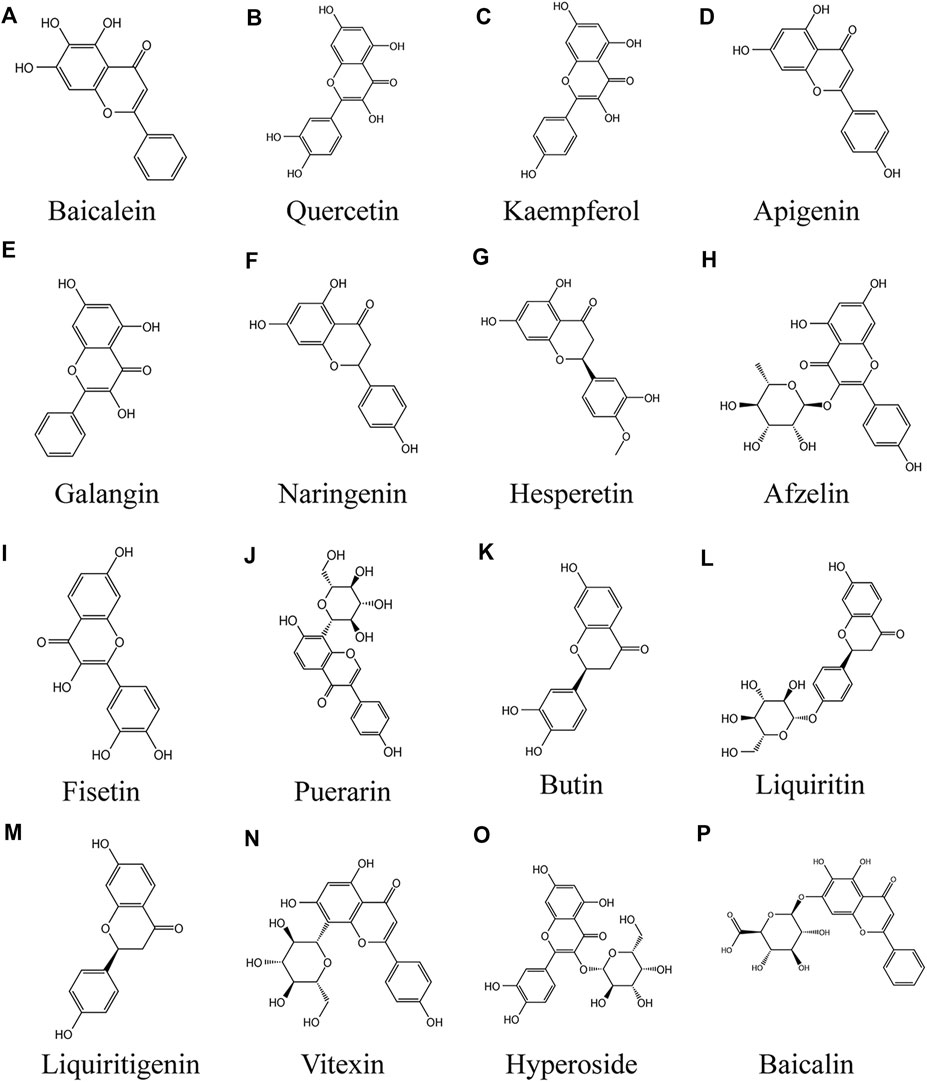
FIGURE 3. Anti vitiligo flavonoids (A) Baicalein, (B) Quercetin, (C) Kaempferol, (D) Apigenin, (E) Galangin, (F) Naringenin, (G) Hesperetin, (H) Afzelin, (I) Fisetin, (J) Puerarin, (K) Butin, (L) Liquiritin, (M) Liquiritigenin, (N) Vitexin, (O) Hyperoside, (P) Baicalin.
Quercetin
Quercetin is a kind of polyhydroxy flavonoid, which is chemically named 3,3,4,5,7-pentahydroxyflavone (Figure 3B). It has high content in apple, onion and green tea, and also exists in Asparagus racemosus Willd., Ficus ingens (Miq.) Miq., Coriandrum sativum L. and Capparis spinosa L. It is one of the most consumed flavonoids in people's daily diet (Alvarez-Arellano et al., 2020). Quercetin has a variety of biological activities, such as antioxidant and scavenging free radicals (Wang et al., 2021e), anti-cancer, anti-aging (Zhu et al., 2015), anti-inflammatory (Rauf et al., 2018; Cui et al., 2019) anti-virus and immune regulation (Zhu et al., 2015; Alvarez-Arellano et al., 2020). It has a very important clinical significance in the treatment of bacterial infection, viral infection, hyperlipidemia and immune system diseases, especially for those caused by increased oxidative stress Cell damage and even mitochondrial dysfunction related diseases have potential therapeutic effects. Quercetin treatment of cultured melanoma cells or NHEM promote melanin synthesis and tyrosinase activity. In cell experiment, treatment of HMVII cells with quercetin at different doses (1, 5, 10, 20 μm) and for different times (1, 3, 5, 7 days) resulted in a dose and time-dependent increase in melanin content. The mechanism of action may be reflected by the genomic mechanism of new messenger RNA and protein synthesis (Nagata et al., 2004). Interestingly, the triple combination of GT extract/quercetin/folic acid prevented H2O2-induced cell damage in a synergistic manner, suggesting that effective antioxidant combinations should be studied to combat ROS types. Among them, quercetin and GT extract had strong protective effect on H2O2 induced cell death, and 100 μm quercetin had the most significant protective effect (Jeong et al., 2005). Cuiping Guan et al. observed endoplasmic reticulum expansion and configuration changes in cells treated with H2O2 and NaOH/H2O2. Quercetin alleviated the increase of ROS level induced by H2O2, and weakened the inhibition of tyrosinase expression by hydrogen peroxide. The mechanism may be to prevent oxidative stress damage, and tyrosinase is effectively exported from endoplasmic reticulum (Guan et al., 2015). Different nanoparticles, such as transporter, solid lipid nanoparticles, nanostructured lipid carriers, liposomes, nano emulsions and polymer nanoparticles, maximize the ideal properties and/or therapeutic activity of quercetin (Nasr and Al-Karaki 2020), which provides a feasibility for the external treatment of vitiligo with quercetin.
Kaempferol
Kaempferol (Figure 3C) is a kind of flavonoids, which mainly comes from the rhizome of Kaempferia galanga L. (Liu Z. Q. et al., 2021). It widely exists in all kinds of fruits, vegetables and beverages, hazelnut, tea, propolis, broccoli and grapefruit (Calderón-Montaño et al., 2011). Kaempferol can be used to combat cardiovascular disease, cancer outbreak, immune dysfunction, diabetes, oxidative stress and other diseases (Imran et al., 2019). Kaempferol has no obvious cytotoxic effect on B16F10 cells at the concentration of 16–32 μm for 24 h (Wang et al., 2017). Kaliziri is an extract from Baccharoides anthelmintica (L.) Moench, which has showed relatively good therapeutic effects for vitiligo (Maimaiti et al., 2017). Kaempferol is one of the main active components of Baccharoides anthelmintica (L.) Moench extract (Tuerxuntayi et al., 2014). After treatment with 1, 5, 10 and 20 μm kaempferol for 7 days, melanin content in hmvii cells increased significantly. It enhanced tyrosinase activity in HMVII cells and mouse buccal hair follicles by inducing tyrosinase protein expression (Takekoshi et al., 2014). Based on the SDTNBI method and experimental verification, kaempferol markedly increased tyrosinase activity and melanin biosynthesis gene expression in B16F10 cells, and effectively promoted melanin synthesis (Wang et al., 2017).
Apigenin
Apigenin (4′, 5,7-trihydroxyflavone; (Figure 3D) natural plant flavone, widely exists in common fruits and vegetables. It is considered to be a flavonoid with biological activity (Liu et al., 2017). Compared with other flavonoids, apigenin is relatively non-toxic and non-mutagenic, and has significant effects on normal cells and cancer cells (Patel et al., 2007). It has demonstrated a variety of pharmacological effects, including antidepressant (Li et al., 2015; Zhang X. et al., 2019), anti-inflammatory, liver protection, antithrombotic, anticancer, anti-aging (Lim et al., 2015), anti-oxidant (Wang J. et al., 2020). Apigenin significantly increased the activities of glutathione peroxidase (GSH PX), catalase (CAT) and superoxide dismutase (SOD), in a dose-dependent manner, and significantly inhibited the level of malondialdehyde (MDA), a biomarker of oxidative stress, in a H2O2 induced cell line PIG3V. Interestingly, apigenin markedly increased protein expression levels of Nrf2 and its downstream NQO1 and HO-1 in a dose-dependent manner, but had no effect on Nrf2 knockout cells. The results showed that apigenin protects melanocytes from oxidative damage dependent Nrf2 pathway (Zhang et al., 2020). In the dopamine (DA)—induced melanocyte model, 10 μm apigenin treatment significantly reduced ROS aggregation, reduced Da induced melanocyte apoptosis, and inhibited caspase-3 and PARP activities, which may be involved in the anti-apoptotic effect of apigenin. The mechanisms include inhibition of JNK, p38MAPK and Akt (Lin M. et al., 2011). In vitro, HMVII cells were treated with apigenin at 1, 5, 10 and 20 μm for 7 days, and the melanin content increased significantly (Takekoshi et al., 2014). So far, there is little evidence that apigenin promote adverse metabolic reactions in vivo when ingesting nutrition related amounts (Shukla and Gupta 2010; Takekoshi et al., 2014; Weng et al., 2016). Apigenin's local drug delivery system transports apigenin to local skin tissue instead of penetrating into blood circulation (Li et al., 1996). Therefore, apigenin may be a relatively safe method for the treatment of vitiligo.
Galangin
Galangin (GA, 3,5,7-trihydroxyflavone; Figure 3E) is an important natural active flavone, which is mainly extracted from the roots of Alpinia officinarum Hance, it has long been used as herbs and spices in South Africa and Asia (Yang et al., 2018). GA has been reported to possess a variety of biological activities, including antibacterial (Skiba et al., 2016), antiviral, anti-inflammatory (Cushnie et al., 2007), anti-obesity (Ma et al., 2019) and antioxidant (Sinha et al., 2014), it is reported that these effects are exerted by regulating NF - κ B, Nrf2 and cAMP/CREB signaling pathways (Yang C.-C. et al., 2020). 4.25 mg/kg GA significantly increased the number of basal melanocytes and melanoepidermal cells in shaving area of mice with hydroquinone induced vitiligo, and promote the melanin hair follicles to increase, the mechanism is to prevent oxidative stress by reducing cholinesterase (CHE) activity and MDA content and increase the expression of TYR protein. Malondialdehyde (MDA) is the final product of lipid peroxidation, which is considered as a specific indicator of oxidative stress (Huo et al., 2014). However, GA metabolism is fast and its bioavailability is low. 90% of GA is metabolized in 1 h and is metabolized in 2 h in hepatocytes. Therefore, it is necessary to modify GA by methylation to slow down its metabolism and improve its bioavailability (Fang et al., 2019).
Naringenin and Hesperetin
Naringenin (Figure 2F) and hesperetin (Figure 3G) are two main flavonoids identified from Citrus × limon (L.) Osbeck extract (Singh et al., 2020). The flavonoids in sweet orange peel include flavonoid glycosides, flavones and flavonols, among which flavanones exist in the form of glycosides (hesperidin and naringenin) or aglycones (hesperidin and naringenin) (Ramful et al., 2010). Citrus flavonoids have many biological activities, such as anti-tumor, anti-oxidation and anti-inflammatory (Salehi et al., 2019). It was found that flavonoids from navel orange peel extract had antioxidant activity (Long et al., 2021). Citrus products stimulate cell melanin production and tyrosinase expression, thereby preventing skin damage caused by ultraviolet light (Chiang et al., 2011). Huang et al. (2012) used 20 μg/ml citrus extract for melanin synthesis experiment. Citrus are rich in hesperidin, neohesperidin or naringin, and acid hydrolytic extracts of these three species of Citrus promote melanin synthesis. In this experiment, 50 μm hesperetin increased the expression of β-catenin, induced the rapid phosphorylation of p38MAPK, ERK and Akt, and activated the downstream transcription factor CREB phosphorylation in less than 1 h. Hesperidin stimulates melanogenesis by activating CREB and MAPKs in Wnt/β-catenin pathway (Huang et al., 2012). Naringenin enhanced tyrosinase activity of B16 mouse melanocytes in a time-dependent manner and increased melanin content in a concentration dependent manner, reaching the maximum at 100 μM. The mechanism included increasing the expression level of melanin producing enzyme (TYRP1 and DCT) and MITF (Ohguchi et al., 2006). Another study also confirmed that naringenin up-regulated tyrosinase activity of B16-F10 cells in a concentration dependent manner. Naringenin up regulated the expression of MITF by increasing the expression of β-catenin and the phosphorylation of Akt or GSK3, and then increased the activity of tyrosinase, so as to improve the melanin synthesis of B16-F10 cells, rather than through cAMP pathway (Huang et al., 2011). These results suggest that hesperetin induced melanogenesis in cell models may contribute to the development of topical beauty agents.
Afzelin
Afzelin (3-O-α-l-rhamnopyranoside; Figure 3H) is a flavonoid isolated from Thesium chinense Turcz. and widely distributed in Korea and China (Li G.-H. et al., 2021). Previous studies have shown that afzelin has antibacterial, anticancer and anti-inflammatory effects (Satthakarn et al., 2015). Afzelin markedly alleviated ultraviolet induced oxidative stress in human skin, damage of mitochondrial membrane potential and mitochondrial permeability (Shin et al., 2013). Afzelin showed strong anti-oxidant activity in DPPH radical scavenging experiment (Kim et al., 2008). Many studies have shown that afzelin could effectively treat skin diseases (Lee et al., 2014). In the experiment of melanogenesis induced by afzelin in human epidermal melanocytes, 100 μm afzelin increased the protein levels of TRP-1 and TYR by up regulating MITF, but did not increase the protein levels of TRP-2. The mechanism is p38MAPK phosphorylation, which up regulates MITF, and is independent of cAMP/PKA pathway (Jung et al., 2016).
Fisetin
Fisetin (3,30,40,7-tetrahydroxyflavone; Figure 3I) is a dietary flavone, which exists in a variety of vegetables and fruits, including apples, strawberries, grapes, cucumbers and onions (Khan et al., 2018). Studies have found that fisetin has anti allergic, anti-arthritis and neuroprotective effects (Ahmad et al., 2017; Ahmad et al., 2019). New data also showed that fisetin had anti-cancer activity (Jie et al., 2015; Khan and Mukhtar 2015; Ding et al., 2020). In particular, it has recently been found that it inhibited inflammation and antioxidant stress (Silva et al., 2007; Ahmad et al., 2019). Interestingly, fisetin has a two-way regulatory effect on melanin production. Takekoshi et al. first reported that fisetin could promote Tyr activity and melanin content of human melanoma cells (Takekoshi et al., 2014). However, Shon et al. found that fisetin inhibited the melanin content in and out of mouse B16F10 melanoma cells mediated by α - MSH(Shon et al., 2016). A recent study found that the difference of dual effects of fisetin was related to its concentration, because high concentration of fisetin inhibited the synthesis of melanin in zebrafish larvae (400 μm) and B16F10 melanoma cells (40 μm). When the concentration of fisetin exceeded 25 μm, the inhibitory activity increased slightly, and 200 μ m had the highest inhibitory rate on mushroom tyrosinase activity in vitro. Surprisingly, 5 μm fisetin slightly increased the content of spontaneous melanin and extracellular melanin, fisetin (at 20 μm) markedly increased the content and release of melanin in B16F10 cells by up regulating the expression of TYR and MITF, and promoted the melanin synthesis of zebrafish larvae. The mechanism is that fistein inhibits GSK-3 β, which activates β - Catenin, which leads to melanogenesis by activating MITF and tyrosinase (Molagoda et al., 2020). Therefore, low dose of fisetin may be an effective drug in the treatment of vitiligo.
Puerarin
Puerarin (7,40-dihydroxyisoflavone-8b-glucopyranoside; Figure 3J) is an isoflavonoid derivative isolated from the root of the traditional Chinese medicine Pueraria lobata (Willd.) Ohwi (Zhang 2019). Puerarin exhibits a wide range of antioxidant activities in cardiovascular diseases, diabetes, obesity, osteoporosis, and other diseases (Xu et al., 2021; Chang et al., 2021; Liu Y. et al., 2021; Xiao et al., 2020). In addition, puerarin has anti-inflammatory, anti-viral and other pharmacological activities (Wang Z.-K. et al., 2020; Wang H. X. et al., 2021). Puerarin exhibited obvious pharmacological activities against vitiligo in vitro and in vivo. Park et al. found that puerarin could increase the melanin content of melanocytes in vitro, and topical application could improve the melanin content of mouse skin tissue, the mechanism is via activation of the cAMP pathway, followed by elevation of MITF, tyrosinase, Trp-2, and Bcl-2 to increase melanocyte survival and melanin content (Park et al., 2014). In the 4-benzyloxyphenol-induced vitiligo mouse model, after one week of Puerarin Treatment, HE staining of the skin at the depigmented sites showed increased hair follicles, which were surrounded by a large number of melanocytes. Puerarin at 40 μmol/L significantly increased the melanin content of human melanocytes by decreasing the phosphorylation of ERK in the cells to promote TRP-1 and MITF expression, which led to an increase in melanin content (Ding et al., 2019).
Butin
Butin (7, 30, 40-trihydroxydihydroflavone, BUT; (Figure 3K) flavonoid with antioxidant activity, isolated from Alpinia officinarum Hance, Dalbergia odorifera T.C.Chen (Duan et al., 2017). BUT exhibited a wide range of pharmacological activities for the treatment of aging, diabetes, liver diseases, and cancer (Zhang et al., 2011). In the hydroquinone-induced vitiligo mouse model, (4.25, 42.5 mg/kg) butin increased the melanin content in the skin lesions by increasing the expression of Tyr and TRP-1 protein, reducing the serum cholinesterase activity and malondialdehyde content. Besides, BUT promoted the proliferation of basal melanocytes (Huo et al., 2017). A recent study found that butin induced melanin production both in vivo and in vitro when the concentration was 40 μmol/L, while tyrosinase activity peaked. Meanwhile, in a H2O2-induced zebrafish model, butin reduced the levels of reactive oxygen species in vivo (Lai et al., 2021).
Liquiritin and Liquiritigenin
Liquiritigenin (LQ; Figure 3L) and liquiritigenin (LQG; Figure 3M) are flavonoids extracted from Glycyrrhiza uralensis Fisch. ex DC. (Du et al., 2021; Mou et al., 2021). They have many biological activities, such as antiviral, anti-inflammatory, anti-oxidation, anti-tumor and so on (Pastorino et al., 2018). LQ and LQG were used to treat mouse melanoma B16-F1 cells and human melanoma hmvii cells with different doses for 72 h. The results showed that both natural drugs significantly increased the content of melanin in melanocytes in a dose-dependent manner, and had no effect on cell viability. Interestingly, both LQ and LQG could significantly up regulate tyrosinase activity and the expression of MITF and its downstream TRP-1 and Trp-2. Further studies have found that 50 μm LQ and LQG trigger melanin synthesis through p38 phosphorylation and activation of PKA/CREB signaling pathway (Uto et al., 2019).
Vitexin
Vitexin (apigenin-8-C-β-D-glucopyranoside; Figure 3N) is a natural flavonoid present in various medicinal plants such as: Crataegus L., Vigna Savi, Passiflora cristalina Vanderpl. & Zappi, Mimosa L., bamboo, etc (He et al., 2016). It has many pharmacological activities, anti-inflammatory, antiviral, anticancer, antihypertensive (Ding et al., 2021; Chen Y. et al., 2021). At the same time, Vitexin is an antioxidant (Ożarowski and Karpiński 2020). In H2O2-induced human melanocyte PIG1, Vitexin inhibited hydrogen peroxide induced apoptosis and promoted cell proliferation by activating the MAPK-Nrf2/ARE pathway, including decreasing IL-1β. The expression of IL-17A, Bax, caspase-3 and ROS, up-regulated the expression of p53, Bcl-2, Nrf2, HO-1, NQO-1, SOD (Li et al., 2020).
Hyperoside
Hyperoside (quercetin-3-O-galactoside, Hyp; Figure 3O) belongs to flavonol glycosides isolated from Rhododendron brachycarpum D. Don ex g. don, Abelmoschus manihot (L.) Medik. Rhododendron L.(Zhou et al., 2021). Studies have shown that Hyp possesses antioxidant, anticancer, antifibrotic, antiallergic, anti-inflammatory and other pharmacological activities (Huang et al., 2020). Hyperoside markly increased the proliferation of melanocytes in a dose - and time-dependent manner in vitro. In the H2O2-induced melanocyte model, Hyp protected melanocytes from oxidative damage by regulating the PI3K/Akt pathway, inhibiting p38 phosphorylation and suppressing mitochondrial apoptotic signaling, which included upregulation of the Bcl-2/Bax ratio and expression of Akt, and downregulation of caspase 3, p38 (Yang et al., 2016).
Baicalin
Baicalin (7-glucuronic acid-5,6-dihydroxy-flavone, BA; Figure 3P) is a kind of small molecular flavonoids extracted from Scutellaria baicalensis Georgi (Fan et al., 2021). BA has a variety of pharmacological activities, such as antioxidant stress, regulation of immunity, regulation of lipid metabolism disorders, anti-inflammatory and improve cell apoptosis (Xin et al., 2020). Recent studies have found that baicalin mediates antioxidant stress by activating Nrf2 signaling pathway (Wang X. et al., 2021). In the 40% monobenzone cream-induced vitiligo model, BA intraperitoneal injection inhibited the infiltration of leukocytes and CD8 + T cells in vitiligo lesions, increased the tyrosinase activity in the lesion area, reduced the expression of chemokine CXCL10 and its receptor CXCR3, and reduced the expression of inflammatory factors in serum samples, including IL-6 and TNF- α, IFN- γ And IL-13 (Zhu et al., 2019). The results of this study are obviously exciting. The infiltration of active CD8 + T cells occurs around the lesions of vitiligo, which is an important reason for the immune destruction of melanocytes (Wu et al., 2013). In vivo experiments, BA could inhibit the infiltration of immune T cells, remove inflammatory factors to slow down the appearance of leukoplakia, and reduce the area of decolorized spots. So it is a potential drug for the treatment of vitiligo.
Polyphenol
Epigallocatechin-3-Gallate
Epigallocatechin-3-gallate (EGCG, Figure 4A) belongs to catechin polyphenols and is one of the main bioactive substances in Camellia sinensis (L.) Kuntze (Huang et al., 2021). It has many pharmacological effects, including anti-inflammatory, anti-atherosclerotic and anti-cancer effects (Mereles and Hunstein 2011), it's also an antioxidant (Kalaiselvi et al., 2013). Katiyar et al. suggested that EGCG could be a topical preparation to resist UVB-induced ROS-related inflammatory skin diseases, photocarcinogenesis and photoaging (Katiyar et al., 1999). In a model of monobenzone-stimulated vitiligo in mice, EGCG delayed the time to depigmentation, the area of depigmentation and reduced the incidence of hyperpigmentation in the dorsal skin of mice. The underlying mechanism was the inhibition of CD8 + T cell migration and inflammatory cytokine expression. Meanwhile, EGCG decreased the expression of IFN-γ, TNF-α, and IL-6 in serum. 5%EGCG cream is the optimal concentration for the treatment of vitiligo (Zhu et al., 2014). IFN - γ, which plays a key role in vitiligo pathogenesis, feedback through crosstalk to promote CD8 + T cell recruitment to the skin (Harris et al., 2012). High levels of CXC chemokines induced by IFN - γ such as CXCL9, CXCL10 and CXCL11 were also found in patient serum, being the most highly expressed genes in the transcriptional profile of skin lesions from vitiligo patients (Rashighi et al., 2014). Excitingly, EGCG inhibited IFN - γ - induced phosphorylation activation of JAK2, STAT1 and STAT3 in human melanocytes and significantly suppressed the levels of ICAM-1, CXCL10 and MCP-1 in a dose-dependent manner. In primary cultured human melanocytes. EGCG reduced the expression of CXCR3, CCR2, and CD11a in purified CD8 + T cells derived from the CD4 + T leukemia cell line Jurkat and peripheral blood monoclonal cells (PBMCs) in a dose-dependent character (Ning et al., 2015).
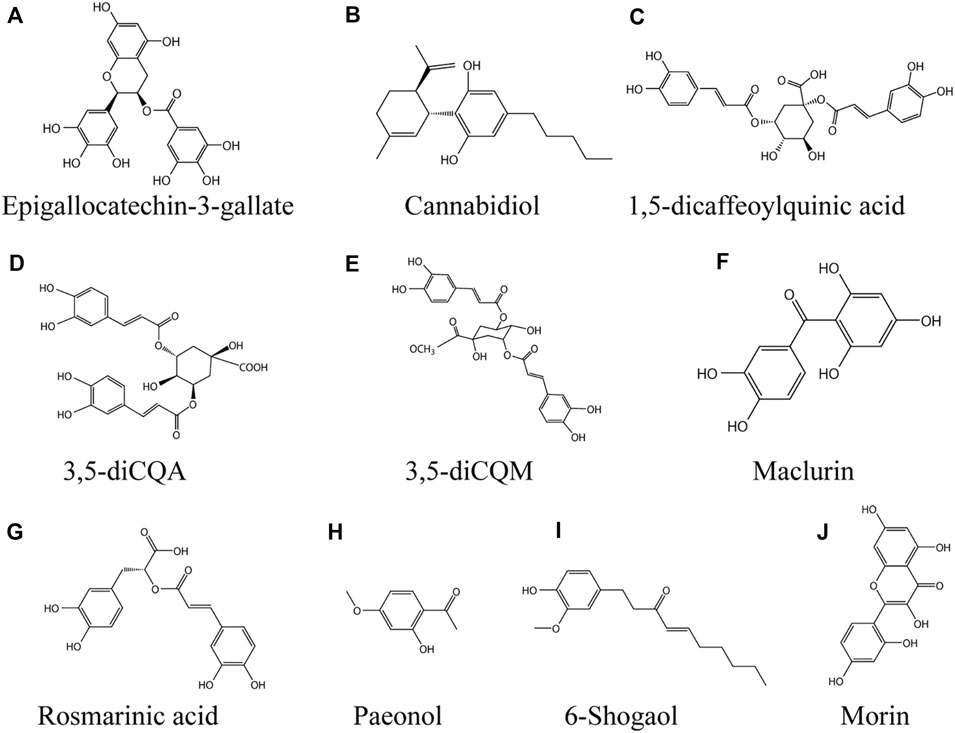
FIGURE 4. Phenolic compounds against vitiligo (A) EGCG, (B) Cannabidiol, (C) 1,5-dicQA, (D) 3,5-diCQA, (E) 3,5-diCQM, (F) Maclurin, (G) Rosmarinic acid, (H) Paeonol, (I) 6-Shogaol, (J) Morin.
Cannabidiol
Cannabinediol (CBD; (Figure 4B) non-psychoactive compound extracted from Cannabis sativa L., is an open pyran ring analogue. CBD has anti-inflammatory, antioxidant and anti-apoptotic effects (Iuvone et al., 2009). In addition, it has immunomodulatory properties (Mechoulam and Hanus 2002). In recent years, it has attracted great attention due to its potential in the treatment of various pathological diseases, including skin and cosmetic diseases (Baswan et al., 2020). CBD increaseed melanin content by binding to CB1 receptor of human epidermal melanocytes. The mechanism is to up regulate the expression of MITF by activating p42/44MAPK and p38MAPK signaling pathways, and then promote melanogenesis (Hwang et al., 2017). The safety of CBD transdermal drug delivery system is also under development (Paudel et al., 2010).
1,5-Dicaffeoylquinic Acid
1,5-dicaffeoylquinic acid (1,5-dicQA; Figure 4C) is a natural polyphenol widely found in Baccharoides anthelmintica (L.) Moench and Helianthus annuus L. (Cheevarungnapakul et al., 2019; Dogra et al., 2020). 1, 5-diCQA has a wide range of pharmacological effects, such as antioxidant, neuroprotective, antifibrotic and other biological activities (Cao et al., 2010). Plant seeds containing 1, 5-diCQA are used in traditional medicine to treat vitiligo (Tuerxuntayi et al., 2014). A previous study found that 1,5-dicqa exerts antioxidant activity through reducing intracellular ROS level and Nrf2 dependent pathway (Cao et al., 2010). 1, 5-diCQA inhibited Aβ42-induced neurotoxicity by activating PI3K/Akt, decreasing GSK3 β level and regulating Bcl-2/Bax ratio (Xiao et al., 2011). B16 cells treated with 0, 5, 50 or 100 μM 1, 5-dicQA significantly up-regulated the transcription levels of MITF, TYR, TRP1 and TRP2 in a dose-dependent manner without cytotoxicity. The mechanism is to activate p38 MAPK, ERK MAPK and PKA signaling pathway to promote the melanin synthesis of B16 cells. In conclusion, these studies suggest that 1,5-dicQA may be an effective drug for the treatment of hypopigmented skin diseases (Mamat et al., 2018).
3,5-diCQA
3,5-caffeoylquinic acid (3,5-diCQA; Figure 4D) is a polyphenolic compound extracted from the root of Cichorium intybus L. (Legrand et al., 2016), it also accumulated in Achillea millefolium L. and Artemisia dracunculus L. 3,5-diCQA has high free radical scavenging activity (Bernard et al., 2020). Phenolic compounds are beneficial to human health, especially their anti-inflammatory and antioxidant properties (Miguel et al., 2020). Plant polyphenols help the skin resist the damage caused by sunlight (Perez-Sanchez et al., 2016). 3,5-diCQA increased the content of melanin in melanocytes in a dose-dependent manner, the possible mechanism is that 3,5-diCQA promoted the phosphorylation of Akt and GSK-3 β, leading to the accumulation of β - Catenin in the cytoplasm. Subsequently, β - Catenin transferred to the nucleus and bound to LEF, which increased the protein expression of downstream MITF, TYR, TRP1 and TRP2. Therefore, 3,5-diCQA may restore skin pigmentation under the loss of antioxidant enzymes or melanocyte dysfunction (Mamat et al., 2017).
3,5-diCQM
3,5-dicaffeoylquinic acids (3,5-diCQM; Figure 4E) is a sort of natural phenolic acids condensed from quinic acid and caffeic acid by esterification. It is widely found in plants such as Gloriosa superba L., Inula helenium L., Xanthium strumarium subsp. strumarium, Crataegus azarolus L. and other fruit trees (Bernard et al., 2020). Caffeoylquinic acid derivatives have been used in traditional medicine in the east to treat a variety of diseases and show a diversity of pharmacological effects, such as liver protection (Basnet et al., 1996), anti-microbial (Zhu et al., 2004), anti-inflammatory (Góngora et al., 2002). In addition, the antioxidant activity of caffeoylquinic acid derivatives has been reported (Lin Y.-L. et al., 2011; Jang and Koh 2019). In the experiment of B16F10 melanoma cells treated with 0–50 μm 3, 5-dicCQM, the results showed that 3,5-diCQM could induce pigmentation. 3,5-diCQM drives melanogenesis in a dose-dependent manner, and the molecular mechanism underlying its ability to induce pigmentation is through activation of the p38 signaling pathway, phosphorylation and activation of CREB, and a cAMP/PKA dependent signaling pathway, which in turn upregulates the transcription factor MITF, thereby activating tyrosinase activity (Kim et al., 2015).
Maclurin
Maclurin [(3,4-dihydroxyphenyl) -(2,4,6-trihydroxyphenyl; Figure 4F) methanone] is a natural phenolic compound that belongs to the benzophenone family and is found in Morus alba L., Garcinia mangostana L. Previous studies have found that maclurin has pharmacological activities such as anticancer, antioxidant and anti-skin aging (Lee 2018; Lee et al., 2018; Mi Moon et al., 2019). The content of melanin in melanocytes was increased by maclurin in a dose-dependent manner, by a mechanism involving the activation of the cAMP/PKA/CREB signaling pathway, which in turn increased tyrosinase, MITF and their downstream TRP-1 and Trp-2 protein content, while also involving the activation of p38 MAPK and p44/42 MAPK pathways. In vitro, maclurin significantly attenuated UVB induced ROS production, inhibited hydrogen peroxide induced reduction of melanin and decreased cell survival (Hwang et al., 2019).
Rosmarinic Acid
Rosmarinic acid (a-o-caffeoyl-3,4-dihydroxyphenyl lacticacid; Figure 4G) is a natural phenolic compound, which exists in many Labiatae plants, such as Perilla L., Rosmarinus officinalis L., Prunella vulgaris L. (Zhang et al., 2021). Rosmarinic acid has anti-inflammatory, anti-oxidation, anti-cancer and other pharmacological activities (Wang L. et al., 2019; Zhang et al., 2021). Incubation of 50 μm rosmarinic acid with B16 melanoma cells for 48 h resulted in a significant increase in melanin content and tyrosinase protein expression, the mechanism being that rosmarinic acid induces melanin synthesis by activating the PKA/CREB signaling pathway via phosphorylation (Shomirzoeva et al., 2019). Recently, ultradeformable liposomes (UL) have been developed by scientific researchers, which greatly increased the skin permeation ability of rosmarinic acid by UL containing oleic acid, exhibiting potential as a formulation for development for external use (Subongkot et al., 2021).
Paeonol
Paeonol (Pae; 2′-hydroxy-4-methoxyacetophenone; Figure 4H) is a natural phenolic compound extracted from the Paeonia × suffruticosa Andrews (Miao et al., 2021). Paeonol has been used in traditional Chinese medicine as an anti-inflammatory and antipyretic drug with cardiovascular, anti-inflammatory, neuroprotective, antitumour and other pharmacological activities (Zhang L. et al., 2019; Tsai et al., 2020). Paeonol alleviated UVB-induced skin photoaging by activating Nrf2 and the antioxidant response element (Sun et al., 2018). Paeonol exhibits anti-inflammatory, anti-allergic activity in animal models of atopic dermatitis and psoriasis (Meng et al., 2017; Meng et al., 2019). In H2O2-induced PIG1 oxidative stress model of normal human epidermal melanocytes, paeonol inhibited hydrogen peroxide induced decrease in cell viability in a dose-dependent manner. 20 μM paeonol increased the enzymatic activities of SOD, CAT, GSH-Px and the expression of HO-1, NQO1, and SOD2 by activating the Nrf2 signaling pathway, but paeonol was unable to affect melanogenesis in PIG1 cells and acted only as a protective agent (Guo and Zhang 2021).
6-Shogaol
6-shogaol (Figure 4I), a natural phenolic compound, is the major active ingredient in Zingiber officinale Roscoe. Studies have found that 6-shogao possesses antiemetic, anti-inflammatory, antioxidant, and anticancer activities, as well as cardiovascular and neuroprotective effects (Kou et al., 2018; Chen et al., 2020; Yadav and Jang 2020). Jin et al. found that 6-shogaol exerts cellular antioxidant activity by mediating Nrf2 signaling through activation of the JNK pathway (Kim and Jang 2016). Feng et al. found that 6-shogaol inhibited UVB induced inflammation and oxidative stress in keratinocytes (Chen F. et al., 2019). Pretreatment of HEMn-MPs with 5 µM 6-shogaol for 6 h protected melanocytes from rhododendrol-induced cytotoxicity and maintained their original cell viability. In the oxidative stress-induced HEMn-MPs model, cells pretreated with 6-shogao maintained the initial cellular morphology, and 6-shogao significantly attenuated H2O2 induced oxidative stress and death and melanogenesis inhibition in melanocytes (Yang L. et al., 2020).
Morin
Morin (2′,3,4′,5,7-pentahydroxyflavone; Figure 4J) is a natural polyphenol found in onion, fig, guava leaves, apple and other Moraceae families such as Psidium guajava L., Maclura pomifera (Raf.) C.K.Schneid., as well as the medicinal plant Alpinia officinarum Hance (Lotito and Frei 2006). Studies have found that Morin has antitumor, antihypertensive, antioxidant, anti-inflammatory, antidiabetic, neuroprotective, antibacterial and other pharmacological effects (Caselli et al., 2016; Heeba et al., 2021). 50 μM Morin significantly upregulated the expression of MITF, as well as its downstream TRP-1 and Trp-2 to increase melanin production in B16F10 mouse melanoma cells, and the mechanisms include the activation of ERK and p38 signaling pathways via the phosphorylated MAPK pathway (Shin et al., 2021). Excitingly, long-term doses of oral Morin did not exhibit any toxicity (Cho et al., 2006).
Glycosides
Geniposide
Geniposide (GP; Figure 5A) (C17H24O10) is a sort of iridoid glycoside extracted from Gardenia jasminoides J. Ellis fruits, which widely exists in nearly 40 species of plants in various families, especially Rubiaceae (Shan et al., 2017). In terms of biological activity, GP has been found to have a variety of pharmacological effects, such as anti-diabetes (Zhang et al., 2016), neuroprotective (Zhao et al., 2017), anti-inflammatory, antioxidant, etc (Zhou et al., 2019). GP is also an important component in many traditional Chinese herbal medicines for the treatment of vitiligo, such as Eucommia ulmoides Oliv. and Rehmannia glutinosa (Gaertn.) DC. (Zhou et al., 2019). In HEMn or HEKn models induced by norepinephrine (NE), GP significantly abolished the inhibitory effect of NE on HEMn melanogenesis in the presence of recombinant SCF. The binding of NE to α 1-adrenoceptor in melanocytes decreased cAMP level, resulting in decreased intracellular calcium uptake associated with c-kit production. The mechanism of GP promoting melanogenesis was through activating GLP-1R/c-kit receptor signal to enhance the expression of c-kit receptor, so as to eliminate the depigmentation caused by norepinephrine (Wen-Jun et al., 2008). In the melanocytes induced by H2O2, GP increased the activities of SOD and CAT, reduced the accumulation of ROS, thus increased the antioxidant capacity of melanocytes and inhibited melanocyte apoptosis by upregulating the Bcl-2/Bax ratio, while increasing cell viability. This process involves activation of PI3K/Akt signaling pathway (Zhou et al., 2019).
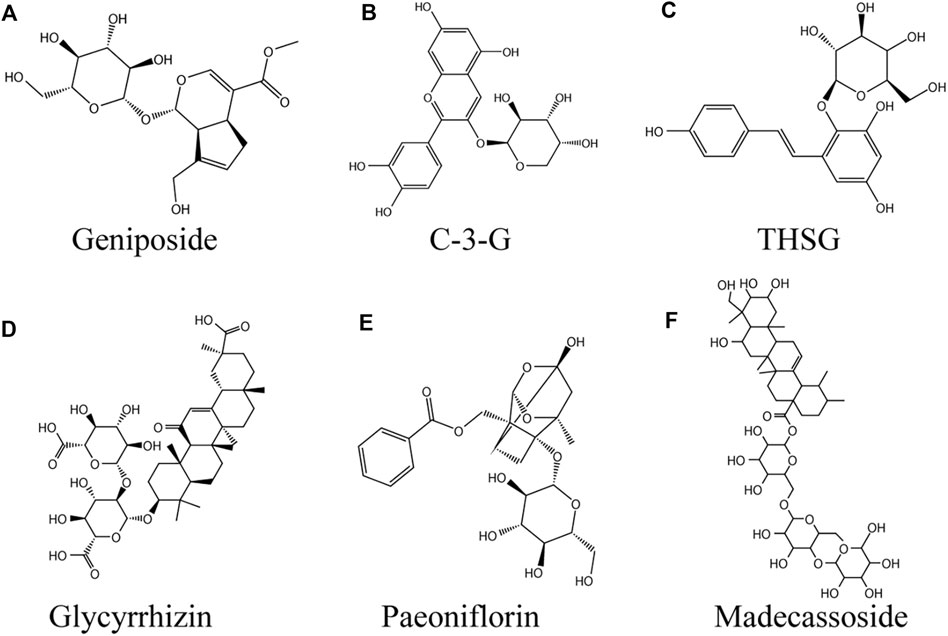
FIGURE 5. Glycosides against vitiligo (A) Geniposide, (B) C-3-G, (C) THSG, (D) Glycyrrhizin, (E) Paeoniflorin, (F) Madecassoside.
Cyanidin-3-o-β-Glucopyranoside
Cyanidin-3-o-β-glucopyranoside (C-3-G; Figure 5B) is a kind of anthocyanin flavonoids widely found in vegetables and fruits. Chinese Myrica cerifera L. is a rich source of C-3-G, which is one of the most common anthocyanins (Sun et al., 2013). Anthocyanins are beneficial to human body. Known pharmacological actions include anti-inflammatory, anti-fatigue, anti-oxidation, anti-tumor and so on (Cui et al., 2018). In addition to the ability to significantly reduce ROS mediated oxidative damage, C-3-G inhibited UVA induced damage to primary human dermal fibroblasts (HDFS) by inducing autophagy (Wu et al., 2019). In one study, TVM-A12 human melanoma cells were incubated with 5 μm C-3-G, and the cells return to the differentiation state from the proliferation state. In addition, two human melanoma cell lines A-375 and M14 also obtained dendritic phenotypes after C-3-G treatment. At the concentration used, the cells treated with C-3-G showed no apoptosis or necrosis. At the same time, C-3-G significantly up-regulated the expression of tyrosinase, thereby inducing the content of melanin in TVM-A12 cells, which was mediated by up regulating cAMP pathway. It is particularly encouraging that the active concentration of C-3-G is comparable to food intake (range of μm) and has no toxicity. (Serafino et al., 2004).
2, 3, 5, 4′-Tetrahydroxystilbene-2-O-β-D-Glucoside
2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-D-glucoside (THSG; Figure 5C) is a water-soluble active ingredient in Reynoutria multiflora (Thunb.) Moldenke (He et al., 2015). Numerous studies have found that THSG exerts antioxidant effects by protecting cells from oxidative damage caused by H2O2 through increasing SOD activity, reducing MDA content, and inhibiting ROS production (Yang et al., 2014; Wu et al., 2020). In hairless skin model, the extract of Reynoutria multiflora could up regulate SOD in a dose-dependent manner, thereby reducing UVB-induced oxidative damage, suggesting that the extract of Reynoutria multiflora contains anti skin photoaging agent (Hwang et al., 2006). At the first time, it was proved to be an effective tyramine Acid enzyme activators and melanogenesis stimulants (Guan et al., 2008). THSG increased tyrosinase activity in a dose-dependent manner at concentrations ranging from 1 to 10 μg/ml (Jiang et al., 2009). Guan et al. (2008) reached a similar conclusion. The mechanism was that THSG directly activated AC or inhibited PDE, increased cAMP level in cytoplasm, and mediated the activation of MITF/CREB. P38MAPK had a regulatory effect on melanin formation and the induced expression of tyrosinase and MITF in B16 cells induced by THSG (Jiang et al., 2009).
Glycyrrhizin
Glycyrrhizin (GLC; (Figure 5D) natural triterpene saponin, is one of the major chemical components extracted from Glycyrrhiza Tourn. ex L. and has been widely used in Asia, Europe, the Middle East (Cai et al., 2017). Studies have shown that GLC has antiallergic, immunomodulatory, antiulcer, anticancer, antioxidant, and antiviral effects (Wang G. et al., 2021). Jung et al. were the first to find that GLC induced melanin production in B16 melanoma cells in a dose-dependent manner by upregulating the tyrosinase and Trp-2 genes (Jung et al., 2001). Li et al. also verified this conclusion. Further studies revealed that GLC increases melanin production in melanocytes by three pathways, 1) activating activator protein-1 (AP-1) and CRE promoter to activate p42/44 MAPK signaling, 2) activating cAMP signaling, and 3) decreasing GSK3β phosphorylation while inducing CREB phosphorylation (Lee et al., 2005). Pretreatment with 1 mM GLC significantly inhibited H2O2-induced melanocyte apoptosis, and GR protected melanocytes from oxidative stress by reducing ROS production in cells via activation of the Nrf2/HO-1 pathway (Mou et al., 2019). Clinical study observations suggest that treatment with oral GLC in combination with UVB improves active generalized vitiligo (Mou et al., 2016).
Paeoniflorin
Paeoniflorin (C23H28O11, PF; (Figure 5E) monoterpene glycoside, is a major active ingredient isolated from the roots of Paeonia veitchii Lynch or Paeonia lactiflora Pall., which has been applied for 1,200 years in China (Li J. et al., 2021). In vivo and in vitro, paeoniflorin has a wide range of pharmacological activities, including anti-inflammatory, antioxidant, immunomodulatory, analgesic, anticonvulsant, antithrombotic, neuroprotective, cardioprotective, hepatoprotective, antitumor and antidepressant effects (Zhou Y.-X. et al., 2020). In the 40% monobenzone-induced vitiligo mouse model, the number of hair follicles and melanin content in the skin of paeoniflorin treated for 10 days were significantly higher than those of the model group. In vitro, 10 μg/ml paeoniflorin can stimulate the synthesis of melanin, and its mechanism is to increase the protein level of MITF and its downstream TRP-1 by promoting the phosphorylation of ERK and CREB (Hu M. et al., 2020). Interestingly, PF protected H2O2-induced PIG1 and PIG3V cells from oxidative stress by activating JNK/Nrf2/HO-1 signaling pathway, including decreased ROS level and increased SOD, CAT, Nrf2 and HO-1 expression. PF at a concentration of 50 μM, the cell viability was significantly increased (Yuan et al., 2020).
Cistanche deserticola Polysaccharide
Cistanche deserticola polysaccharide (CDP) is the main active component isolated from Cistanche deserticola Ma. It is widely used in anti-virus and anti-tumor in North Africa, Arab and Asian countries (Gu et al., 2016). In addition, CDP also has the pharmacological activities of liver injury protection, lipid balance, anti-aging, regulating immune function and antioxidant (Liu et al., 2018). CDP promoted the formation of melanin in vivo and in vitro. CDP promoted melanogenesis by activating MAPK signaling pathway and up regulating the expression of MITF and its downstream genes Tyr, TRP1 and Trp2, including increasing the phosphorylation levels of p38, JNK and ERK proteins. In vivo, CDP promotes melanin production in zebrafish. Notably, CDP protected HEM and B16F10 cells from oxidative stress induced by H2O2 and significantly inhibited apoptosis induced by oxidative stress (Hu Y. et al., 2020).
Madecassoside
Madecassoside (MADE; (Figure 5F) natural triterpenoid saponin, is isolated from Centella asiatica (L.) Urb (Zhou J. et al., 2020). Studies have found that asiatic acid has a wide range of pharmacological activities, such as anti-apoptotic, anti-inflammatory, and anti-oxidative (Peng et al., 2020). In the H2O2-induced oxidative stress model, MADE reduced the shrinkage of dendrites of melanocytes affected by oxidative stress in a dose-dependent manner, maintained cell morphology, improved mitochondrial swelling, and exerted antioxidant activity by increasing autophagy by upregulating the levels of LC3-II and LC3-I in melanocytes. Therefore, MADE is a promising natural product against vitiligo (Ling et al., 2017).
Coumarin
Psoralidin
Psoralidin (PL; Figure 6A), a natural coumarin isolated from Cullen corylifolium (L.) Medik. seeds, is structurally similar to coumestrol (Cao et al., 2019). It also exists naturally in various plants, such as lemon, lime and parsnip divaricata. PL is beneficial to diabetic complications, oxidative stress, obesity, osteoporosis, apoptosis, autophagy and cell proliferation (Sharifi-Rad et al., 2020). PL is used in the traditional Uyghur medicinal materials for color restoration (Wang et al., 2014; Niu et al., 2016; Pei et al., 2016), and several psoralen compounds, such as 8-MOP and 5-MOP, isolated from the same plant (Zang et al., 2019), are used in ultraviolet color restoration therapy (Dincer Rota et al., 2021). In vitro experiments showed that PL could improve the activity of tyrosinase (Shi et al., 2018). A recent clinical controlled study found that treatment of vitiligo lesions with psoralen in combination with NBUVB had higher efficacy compared with NBUVB alone, and there were no serious complications (Zabolinejad et al., 2020). The mechanism may provide an antioxidant effect by regulating the PI3K/Akt signaling pathway, affecting the downstream GSK3 β/β - Catenin, and the Nrf2/HO-1 axis (Zhai et al., 2018).
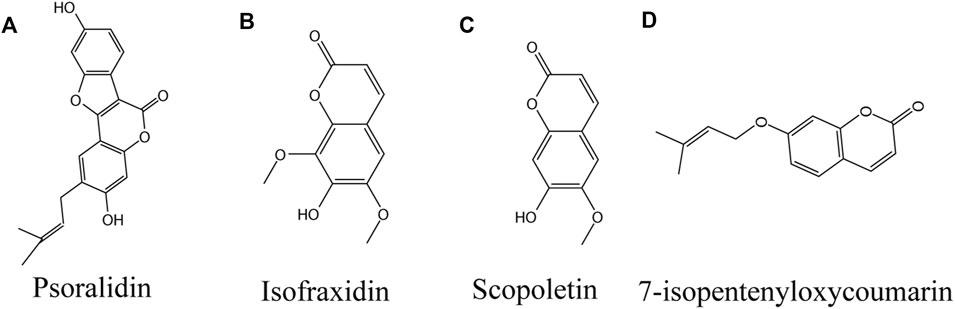
FIGURE 6. Coumarins against vitiligo (A) Psoralidin, (B) Isofraxidin, (C) Scopoletin, (D) 7-isopentenyloxycoumarin.
Isofraxidin
Isofraxidin (Figure 6B) is a natural coumarin isolated from Artemisia capillaris Thunb. (Yim et al., 2017b). Isofraxidin related derivatives have been found to have anti-inflammatory, antioxidant, neuroprotective and other biological activities (Lau et al., 2019; Majnooni et al., 2020). Yim et al. emphasized that isofraxidin may be a new type of pigmentation agent. At the dose of 12.5 μm and 25 μm, zebrafish treated with isofraxidin showed higher melanin synthesis and tyrosinase activity in vivo experiments. In vitro, 25 μm isofraxidin significantly increased the melanin content in B16F10 cells by up regulating MITF and tyrosinase gene expression (Yim et al., 2017a).
Scopoletin
Scopoletin (SP; Figure 6C) is a phenolic coumarin isolated from Evolvulus alsinoides (L.) L., which has many pharmacological effects (Zhang F. et al., 2019). It has been isolated from Gramineae, Liliaceae, Musaceae, Compositae, Convolvulaceae and Leguminosae (Tal and Robeson 1986; Gnonlonfin et al., 2011). SP has been reported to have anti-inflammatory effect (Chaingam et al., 2020) and antioxidant effect (Usman et al., 2020), When B16F10 melanoma cells were treated with 0–50 μm SP, it was found that SP to induce melanin synthesis in a dose-dependent manner by increasing the expression of MITF and tyrosinase via increasing CREB phosphorylation (Ahn et al., 2014). When zebrafish embryos were exposed to the compound for 2 days, the increase of pigment was detected. In vitro, SP at concentrations of 0–50 μmol/L enhanced melanogenesis in vivo and in vitro by increasing melanin content and Tyr and MITF expression (Heriniaina et al., 2018).
7-Isopentenyloxycoumarin
7-isopentenoxycoumarin (Figure 6D), a natural pentenoxyumbelliferone derivative widely found in Rutaceae and umbelliferaceae, is a floral extract of Amaranthus retroflexus L., which is used as a food product in southern and central Italy (Fiorito et al., 2017). Studies have shown that 7-isopentenyloxycoumarins have antifungal, antioxidant, anticancer, neuroprotective, and anti-inflammatory properties (Preziuso et al., 2020). Fiorito et al. found that 7-isopentenoxycoumarin significantly induced melanogenesis at a dose of 40 μm, with the highest induction of melanin at 72 h, and excitingly the induction was 6-fold greater than that of the control group, and the mechanism of action was to increase melanogenesis by elevating MITF and its downstream genes tyrosinases, TRP-1 and Trp-2. Interestingly, 7-isopentenoxycoumarin interacted with the ERβ (ER-β) Binding may also be involved in melanin biosynthesis, as this receptor antagonist inhibited melanogenesis (Fiorito et al., 2018).
Other Compounds
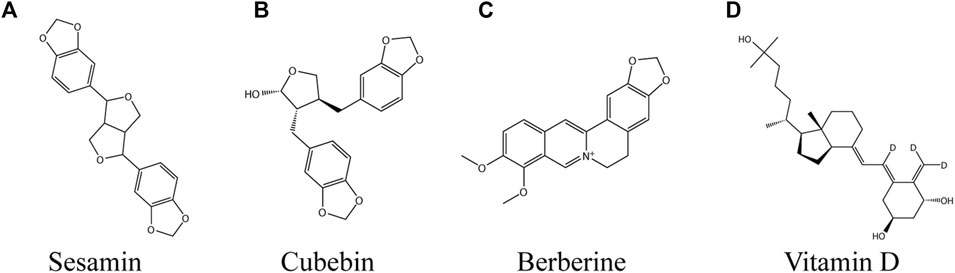
Sesamin
Sesamin (Figure 7A), a kind of lignan found in oil and Sesamum indicum L. seed, has been found to have a variety of biological activities and is beneficial to human body (Udomruk et al., 2020). The high antioxidant activity of sesamin has been reported (Pathak et al., 2014). In addition, its anti-nociceptive and anti-inflammatory activity was also reported (Jeng et al., 2005; Monteiro et al., 2014). It increased the content of tocotrienoll in the skin, so as to reduce sunburn and tumor incidence rate (Yamada et al., 2008), reduced the skin erythema caused by UVB, improved skin inflammation, protected skin from wrinkle formation and light damage (Lin et al., 2019). In the concentration range of 1–10 μM, sesamin increased melanin production in a dose-dependent manner. The mechanism is that sesamin up regulated CREB gene by activating cAMP/PKA signaling pathway, then up regulated the expression of tyrosinase and MITF, and finally induced melanin synthesis (Jiang et al., 2011). The currently developed transdermal drug delivery system will successfully transform lignin into an external preparation for the treatment of vitiligo in the future (Nguyen et al., 2015).
Cubebin
Cubebin (Figure 7B) is a compound extracted from the seeds of Piper cubeba L. f. (Godoy de Lima et al., 2018). It exhibits various pharmacological activities, such as trypanosomiasis, anti-Mycobacterium (Silva et al., 2007), analgesic, anti-inflammatory (Souza et al., 2004) and vasodilator (Somani et al., 2017). In vitro, cubebin showed a concentration time-dependent melanogenesis activity in B16 cells. The mechanism is to promote melanin synthesis by increasing the phosphorylation level of p38 MAPK, which in turn increases the expression of MITF and tyrosinase (Hirata et al., 2007).
Berberine
Berberine (BBR, (Figure 7C) natural isoquinoline alkaloid, is a major compound isolated from the Chinese herb Coptis chinensis Franch. (Wang et al., 2021d), and studies have found that BBR has pharmacological activities against cancer, hypolipidemic, cardiovascular, anti-inflammatory, and antioxidant stress (Zhao et al., 2021). Wei et al. studied the potential medicinal value of berberine in vitiligo. At 0.1–5.0 μM BBR induced melanocyte proliferation in a time-dependent manner. At the same time, BBR inhibited the oxidative damage induced by H2O2 by down regulating the activities of CAT and SOD and reducing the accumulation of ROS in PIG1 cells, 5 μM BBR inhibited the cleavage of PARP and the apoptosis of melanocytes induced by H2O2 by down regulating the ratio of Bax/Bcl-2. The antioxidant effect of BBR depends on Nrf2-ARE pathway, and the process involves up regulation of HO-1, SOD and NQO-1 protein expression, and the protective effect is obviously reduced after Nrf2 gene is knocked out. In addition, BBR enhanced the expression of MITF in melanocytes induced by oxidative stress, thereby increasing the production of melanin. Finally, BBR inhibited H2O2-induced upstream NF-κB activation and expression of IL-6 and IL-8 (Jiang et al., 2019). BBR could protect melanocytes from oxidative stress through anti-oxidation and anti-inflammatory. It is a potential natural drug against vitiligo.
Vitamin D
Vitamin D (1,25-dihy-droxyvitamin D3, Figure 7D) is a cyclopentane phenanthrene compound, which is an essential vitamin for human body, it mainly comes from daily meat (Dominguez et al., 2021). Vitamin D has been used in autoimmune diseases, cancer and osteoporosis (Sintov et al., 2014). Vitamin D deficiency leads to the excessive production of ROS in mitochondria and damages the antioxidant system (Latham et al., 2021). Previous studies have confirmed that vitamin D compounds regulate the proliferation, differentiation, migration and apoptosis of melanocytes and affect T-cell mediated peripheral immune response (Birlea et al., 2008; Kawakami et al., 2014). In H2O2-induced oxidative stress models in PIG1 and PIG3V cells, vitamin D activated WNT/β-Catenin signaling pathway exerted antioxidant activity, the mechanism included the promotion of GSK3β inactivation, increased β-catenin nuclear translocation and activation of the downstream Nrf2/ARE pathway. In addition, vitamin D passed through up regulation of MITF in a β-catenin pathway dependent manner promoted melanocyte proliferation and protected melanocytes from H2O2-induced apoptosis by suppressing caspase3 expression (Tang et al., 2018).
Conclusion and Prospective
In traditional medicine, there are many traditional herbal medicines (Kwon et al., 2018; Xu et al., 2017; Moreira et al., 2012) for the treatment of pigment deficiency. Their extracts significantly improved the activity of tyrosinase (Xu et al., 2017). However, their specific components and mechanism of action are still unclear. Vitiligo is an autoimmune skin disease in which melanocytes are destroyed by autoreactive CD8+ T cells, resulting in cutaneous leukoplakia. Depigmented mouse skin lesions with autoimmune features were fitted to a monobenzone-induced vitiligo model (Zhu et al., 2013). Unfortunately, among all natural drugs mentioned in this review, only four natural products (such as Vitexin, baicalin, EGCG and berberine) currently exhibit anti depigmenting pharmacological activity in this model. We mentioned earlier that oxidative stress may be a driver of autoimmune destruction and that intense and persistent oxidative stress leads to apoptosis, damage, and antigen exposure of melanocytes, which in turn trigger autoimmune destruction. Therefore, recently, researchers have paid more attention to the mechanism of action of natural products against oxidative stress in melanocytes. Some natural drugs (such as baicalein, Vitexin, maclurin, etc.) inhibited the damaging effects of H2O2-induced oxidative stress on melanocytes, even counterstaining depigmented mouse skin lesions. Therefore, it is reasonable to speculate that some of these members contribute to protection against autoimmune destruction. 37 natural products can elevate melanin expression by elevating tyrosinase activity in an in vitro melanocyte model in vivo, but their contribution to intervening in autoimmune destruction is similarly unknown. The above mentioned vitiligo models are all inducible and cannot fully replicate the disease characteristics of human vitiligo, thus increasing the difficulty of developing new drugs. Genetically edited mice have not been widely promoted (Riding et al., 2019). Several natural drugs have been reported for their use in the clinic. For example: observations from clinical studies have shown that oral Glycyrrhizin Combined with UVB therapy improves active systemic vitiligo, but also exposes minor side effects (Mou et al., 2016). In clinical studies, the overall recolor rate of PUVA (psoralen plus UVA) was only 44% (Bishnoi and Parsad 2018). However, due to the lack of large clinical research, they still have a long way to go before they can be promoted as anti-vitiligo agents (Maleš et al., 2019). A systematic review study showed that vitiligo does not generally produce benefi cial outcomes with the use of topical antioxidants, but the added effect of oral intake cannot be ignored (Speeckaert et al., 2018). Developing new natural products against autoimmune destruction will be a hotspot in the future and also provide a new direction to elucidate the pathogenesis of vitiligo. Vitiligo treatment serves two purposes: 1) Controlling vitiligo disease progression during the active phase and 2) increasing melanocyte production during the stationary phase. We propose the hypothesis that in the future natural drugs with anti-oxidant and anti-autoimmune properties are used in vitiligo progression phase and natural drugs with improved tyrosinase activity are used in stability phase.
In conclusion, strong evidence that natural products can prevent or treat vitiligo is still lacking. Appropriately designed clinical trials are needed to further understand the efficacy of natural products against vitiligo. As an auxiliary means of phototherapy, plant derived compounds with antioxidant properties are becoming an attractive choice for the treatment of vitiligo (Dell'Anna et al., 2007). Natural products (NPs) extracted from plants show the effect of increasing the expression of melanin, and have less side effects on human body, which is of great significance for us to continue to develop natural drugs (Yin et al., 2017).
Author Contributions
Concepts: YP. Wrote the paper: YP, SW, YH, and JL. Designed the figures: JZ, YP, and YY. Reviewed manuscript: JG and QN. All authors commented on the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant nos. 81804066, 82074443, 81873310), and by Xinglin Scholar Research Promotion Project of Chengdu University of TCM (Grant nos. QNXZ2019017, and QNXZ2020003) and “Hundred Talents Program” of the Hospital of Chengdu University of Traditional Chinese Medicine (Grant nos. 20-B01, 20-Q03, and 20-Q05).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
HO-1, heme oxygenase-1; Nrf2, The nuclear factor erythroid 2-like factor 2; NE, Norepinephrine; GCLM, g-glutamylcystine ligase modulatory subunit; GCLC, g-glutamylcystine ligase catalytic subunit; Cyt-4, cytochrome c; ROS, reactive oxygen species; TYR, tyrosinase; TRP-1, tyrosinase-related protein 1; TRP-2, tyrosinase-related protein 2; MITF, microphthalmia-associated transcription factor; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; cAMP, intracellular cyclic adenosine monophosphate; GSK-3β, glycogen synthase kinase 3β; cRE, cAMP-response element; cREB, cAMP-response element binding protein; PKA, protein kinase A; α-MSH, α melanocyte stimulating hormone; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; c-Kit, proto-oncogene; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon-γ; ARE, antioxidant response element; SOD, Superoxide dismutase; CAT, catalase; HEMn, normal human epidermal melanocytes; HEKn, normal human epidermal keratinocytes
References
Abdel-Malek, Z. A., Jordan, C., Ho, T., Upadhyay, P. R., Fleischer, A., and Hamzavi, I. (2020). The enigma and Challenges of Vitiligo Pathophysiology and Treatment. Pigment Cel Melanoma Res 33 (6), 778–787. doi:10.1111/pcmr.12878
Ahluwalia, J., Correa-Selm, L. M., and Rao, B. K. (2017). Vitiligo: Not Simply a Skin Disease. Skinmed 15 (2), 125–127. doi:10.1080/14725843.2017.1282416
Ahmad, A., Ali, T., Park, H. Y., Badshah, H., Rehman, S. U., and Kim, M. O. (2017). Neuroprotective Effect of Fisetin against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Mol. Neurobiol. 54 (3), 2269–2285. doi:10.1007/s12035-016-9795-4
Ahmad, A., Ali, T., Rehman, S. U., and Kim, M. O. (2019). Phytomedicine-Based Potent Antioxidant, Fisetin Protects CNS-Insult LPS-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment. J. Clin. Med. 8 (6). doi:10.3390/jcm8060850
Ahn, M.-J., Hur, S.-J., Kim, E.-H., Lee, S. H., Shin, J. S., Kim, M.-K., et al. (2014). Scopoletin fromCirsium setidensIncreases Melanin Synthesis via CREB Phosphorylation in B16F10 Cells. Korean J. Physiol. Pharmacol. 18 (4), 307–311. doi:10.4196/kjpp.2014.18.4.307
Al-Shobaili, H. A., and Rasheed, Z. (2015). Oxidized Tyrosinase: A Possible Antigenic Stimulus for Non-segmental Vitiligo Autoantibodies. J. Dermatol. Sci. 79 (3), 203–213. doi:10.1016/j.jdermsci.2015.06.009
Alvarez-Arellano, L., Salazar-García, M., and Corona, J. C. (2020). Neuroprotective Effects of Quercetin in Pediatric Neurological Diseases. Molecules 25 (23). doi:10.3390/molecules25235597
Basnet, P., Matsushige, K., Hase, K., Kadota, S., and Namba, T. (1996). Four Di-O-caffeoyl Quinic Acid Derivatives from Propolis. Potent Hepatoprotective Activity in Experimental Liver Injury Models. Biol. Pharm. Bull. 19 (11), 1479–1484. doi:10.1248/bpb.19.1479
Baswan, S. M., Klosner, A. E., Glynn, K., Rajgopal, A., Malik, K., Yim, S., et al. (2020). Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Ccid 13, 927–942. doi:10.2147/ccid.s286411
Bergqvist, C., and Ezzedine, K. (2021). Vitiligo: A Focus on Pathogenesis and its Therapeutic Implications. J. Dermatol. 48 (3), 252–270. doi:10.1111/1346-8138.15743
Bernard, G., Alves Dos Santos, H., Etienne, A., Samaillie, J., Neut, C., Sahpaz, S., et al. (2020). MeJA Elicitation of Chicory Hairy Roots Promotes Efficient Increase of 3,5-diCQA Accumulation, a Potent Antioxidant and Antibacterial Molecule. Antibiotics (Basel) 9 (10). doi:10.3390/antibiotics9100659
Bickers, D. R., and Athar, M. (2006). Oxidative Stress in the Pathogenesis of Skin Disease. J. Invest. Dermatol. 126 (12), 2565–2575. doi:10.1038/sj.jid.5700340
Birlea, S., Costin, G.-E., and Norris, D. (2008). Cellular and Molecular Mechanisms Involved in the Action of Vitamin D Analogs Targeting Vitiligo Depigmentation. Cdt 9 (4), 345–359. doi:10.2174/138945008783954970
Bishnoi, A., and Parsad, D. (2018). Clinical and Molecular Aspects of Vitiligo Treatments. Int. J. Mol. Sci. 19 (5). doi:10.3390/ijms19051509
Cai, X., Wang, X., Li, J., and Chen, S. (2017). Protective Effect of Glycyrrhizin on Myocardial Ischemia/reperfusion Injury-Induced Oxidative Stress, Inducible Nitric Oxide Synthase and Inflammatory Reactions through High-Mobility Group Box 1 and Mitogen-Activated Protein Kinase Expression. Exp. Ther. Med. 14 (2), 1219–1226. doi:10.3892/etm.2017.4617
Calderón-Montaño, J. M., Burgos-Morón, E., Pérez-Guerrero, C., and López-Lázaro, M. (2011). A Review on the Dietary Flavonoid Kaempferol. Mini Rev. Med. Chem. 11 (4), 298–344.
Cao, H.-j., Li, C.-r., Wang, L.-y., Ziadlou, R., Grad, S., Zhang, Y., et al. (2019). Effect and Mechanism of Psoralidin on Promoting Osteogenesis and Inhibiting Adipogenesis. Phytomedicine 61, 152860. doi:10.1016/j.phymed.2019.152860
Cao, X., Xiao, H., Zhang, Y., Zou, L., Chu, Y., and Chu, X. (2010). 1, 5-Dicaffeoylquinic Acid-Mediated Glutathione Synthesis through Activation of Nrf2 Protects against OGD/reperfusion-induced Oxidative Stress in Astrocytes. Brain Res. 1347, 142–148. doi:10.1016/j.brainres.2010.05.072
Carreras-Sureda, A., Pihán, P., and Hetz, C. (2018). Calcium Signaling at the Endoplasmic Reticulum: fine-tuning Stress Responses. Cell Calcium 70, 24–31. doi:10.1016/j.ceca.2017.08.004
Caselli, A., Cirri, P., Santi, A., and Paoli, P. (2016). Morin: A Promising Natural Drug. Cmc 23 (8), 774–791. doi:10.2174/0929867323666160106150821
Chaingam, J., Juengwatanatrakul, T., Yusakul, G., Kanchanapoom, T., and Putalun, W. (2020). HPLC-UV-based Simultaneous Determination of Canthin-6-One Alkaloids, Quassinoids,and Scopoletin: the Active Ingredients in Eurycoma Longifolia Jack and Eurycoma Harmandiana Pierre, and Their Anti-inflammatory Activities. J. AOAC Int. 104, 802–810. doi:10.1093/jaoacint/qsaa141
Chang, X., Zhang, T., Liu, D., Meng, Q., Yan, P., Luo, D., et al. (2021). Puerarin Attenuates LPS-Induced Inflammatory Responses and Oxidative Stress Injury in Human Umbilical Vein Endothelial Cells through Mitochondrial Quality Control. Oxid Med. Cel Longev 2021, 6659240. doi:10.1155/2021/6659240
Cheevarungnapakul, K., Khaksar, G., Panpetch, P., Boonjing, P., and Sirikantaramas, S. (2019). Identification and Functional Characterization of Genes Involved in the Biosynthesis of Caffeoylquinic Acids in Sunflower (Helianthus Annuus L.). Front. Plant Sci. 10, 968. doi:10.3389/fpls.2019.00968
Chen, F., Tang, Y., Sun, Y., Veeraraghavan, V. P., Mohan, S. K., and Cui, C. (2019a). 6-shogaol, a Active Constiuents of Ginger Prevents UVB Radiation Mediated Inflammation and Oxidative Stress through Modulating NrF2 Signaling in Human Epidermal Keratinocytes (HaCaT Cells). J. Photochem. Photobiol. B: Biol. 197, 111518. doi:10.1016/j.jphotobiol.2019.111518
Chen, J., Li, S., and Li, C. (2021a). Mechanisms of Melanocyte Death in Vitiligo. Med. Res. Rev. 41 (2), 1138–1166. doi:10.1002/med.21754
Chen, L., and Shen, Z. (2020). Tissue-resident Memory T Cells and Their Biological Characteristics in the Recurrence of Inflammatory Skin Disorders. Cell Mol Immunol 17 (1), 64–75. doi:10.1038/s41423-019-0291-4
Chen, T. C., Yen, C. K., Lu, Y. C., Shi, C. S., Hsieh, R. Z., Chang, S. F., et al. (2020). The Antagonism of 6-shogaol in High-Glucose-Activated NLRP3 Inflammasome and Consequent Calcification of Human Artery Smooth Muscle Cells. Cell Biosci 10, 5. doi:10.1186/s13578-019-0372-1
Chen, X., Guo, W., Chang, Y., Chen, J., Kang, P., Yi, X., et al. (2019b). Oxidative Stress-Induced IL-15 Trans-presentation in Keratinocytes Contributes to CD8+ T Cells Activation via JAK-STAT Pathway in Vitiligo. Free Radic. Biol. Med. 139, 80–91. doi:10.1016/j.freeradbiomed.2019.05.011
Chen, Y., Wang, B., Yuan, X., Lu, Y., Hu, J., Gao, J., et al. (2021b). Vitexin Prevents Colitis-Associated Carcinogenesis in Mice through Regulating Macrophage Polarization. Phytomedicine 83, 153489. doi:10.1016/j.phymed.2021.153489
Chiang, H.-M., Lin, J.-W., Hsiao, P.-L., Tsai, S.-Y., and Wen, K.-C. (2011). Hydrolysates of Citrus Plants Stimulate Melanogenesis Protecting against UV-Induced Dermal Damage. Phytother. Res. 25 (4), 569–576. doi:10.1002/ptr.3302
Chiu, W.-T., Shen, S.-C., Chow, J.-M., Lin, C.-W., Shia, L.-T., and Chen, Y.-C. (2010). Contribution of Reactive Oxygen Species to Migration/invasion of Human Glioblastoma Cells U87 via ERK-dependent COX-2/PGE2 Activation. Neurobiol. Dis. 37 (1), 118–129. doi:10.1016/j.nbd.2009.09.015
Cho, Y.-M., Onodera, H., Ueda, M., Imai, T., and Hirose, M. (2006). A 13-week Subchronic Toxicity Study of Dietary Administered Morin in F344 Rats. Food Chem. Toxicol. 44 (6), 891–897. doi:10.1016/j.fct.2005.12.002
Choi, E.-O., Jeong, J.-W., Park, C., Hong, S. H., Kim, G.-Y., Hwang, H.-J., et al. (2016). Baicalein Protects C6 Glial Cells against Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis through Regulation of the Nrf2 Signaling Pathway. Int. J. Mol. Med. 37 (3), 798–806. doi:10.3892/ijmm.2016.2460
Cui, H. X., Chen, J. H., Li, J. W., Cheng, F. R., and Yuan, K. (2018). Protection of Anthocyanin from Myrica Rubra against Cerebral Ischemia-Reperfusion Injury via Modulation of the TLR4/NF-Κb and NLRP3 Pathways. Molecules 23 (7). doi:10.3390/molecules23071788
Cui, S., Wu, Q., Wang, J., Li, M., Qian, J., and Li, S. (2019). Quercetin Inhibits LPS-Induced Macrophage Migration by Suppressing the iNOS/FAK/paxillin Pathway and Modulating the Cytoskeleton. Cell Adhes. Migration 13 (1), 1–12. doi:10.1080/19336918.2018.1486142
Cushnie, T. P. T., Hamilton, V. E. S., Chapman, D. G., Taylor, P. W., and Lamb, A. J. (2007). Aggregation of Staphylococcus aureus Following Treatment with the Antibacterial Flavonol Galangin. J. Appl. Microbiol. 103 (5), 1562–1567. doi:10.1111/j.1365-2672.2007.03393.x
de Oliveira, M. R., Nabavi, S. F., Habtemariam, S., Erdogan Orhan, I., Daglia, M., and Nabavi, S. M. (2015). The Effects of Baicalein and Baicalin on Mitochondrial Function and Dynamics: A Review. Pharmacol. Res. 100, 296–308. doi:10.1016/j.phrs.2015.08.021
Dell'Anna, M. L., Mastrofrancesco, A., Sala, R., Venturini, M., Ottaviani, M., Vidolin, A. P., et al. (2007). Antioxidants and Narrow Band-UVB in the Treatment of Vitiligo: a Double-Blind Placebo Controlled Trial. Clin. Exp. Dermatol. 32 (6), 631–636. doi:10.1111/j.1365-2230.2007.02514.x
Denat, L., Kadekaro, A. L., Marrot, L., Leachman, S. A., and Abdel-Malek, Z. A. (2014). Melanocytes as Instigators and Victims of Oxidative Stress. J. Invest. Dermatol. 134 (6), 1512–1518. doi:10.1038/jid.2014.65
Dincer Rota, D., Aksoy Sarac, G., Arca, E., and Onder, M. (2021). Comparison of the Efficacy of Broad-Band Targeted UVB Phototherapy and Topical Psoralen with Targeted UVA Phototherapy in Localized Vitiligo. Dermatol. Ther. 34 (1), e14562. doi:10.1111/dth.14562
Ding, G., Xu, X., Li, D., Chen, Y., Wang, W., Ping, D., et al. (2020). Fisetin Inhibits Proliferation of Pancreatic Adenocarcinoma by Inducing DNA Damage via RFXAP/KDM4A-dependent Histone H3K36 Demethylation. Cell Death Dis 11 (10), 893. doi:10.1038/s41419-020-03019-2
Ding, T., Zhao, T., Li, Y., Liu, Z., Ding, J., Ji, B., et al. (2021). Vitexin Exerts Protective Effects against Calcium Oxalate crystal-induced Kidney Pyroptosis In Vivo and In Vitro. Phytomedicine 86, 153562. doi:10.1016/j.phymed.2021.153562
Ding, X., Mei, E., Hu, M., Zhou, C., Li, X., Cai, L., et al. (2019). Effect of Puerarin on Melanogenesis in Human Melanocytes and Vitiligo Mouse Models and the Underlying Mechanism. Phytotherapy Res. 33 (1), 205–213. doi:10.1002/ptr.6218
Dogra, N. K., Kumar, S., and Kumar, D. (2020). Vernonia Anthelmintica (L.) Willd.: An Ethnomedicinal, Phytochemical, Pharmacological and Toxicological Review. J. Ethnopharmacology 256, 112777. doi:10.1016/j.jep.2020.112777
Dominguez, L. J., Farruggia, M., Veronese, N., and Barbagallo, M. (2021). Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for its Treatment. Metabolites 11 (4), 255. doi:10.3390/metabo11040255
Du, Y., Luo, M., Du, Y., Xu, M., Yao, Q., Wang, K., et al. (2021). Liquiritigenin Decreases Aβ Levels and Ameliorates Cognitive Decline by Regulating Microglia M1/M2 Transformation in AD Mice. Neurotox Res. 39 (2), 349–358. doi:10.1007/s12640-020-00284-z
Duan, J., Guan, Y., Mu, F., Guo, C., Zhang, E., Yin, Y., et al. (2017). Protective Effect of Butin against Ischemia/reperfusion-Induced Myocardial Injury in Diabetic Mice: Involvement of the AMPK/GSK-3β/Nrf2 Signaling Pathway. Sci. Rep. 7, 41491. doi:10.1038/srep41491
Ezzedine, K., Eleftheriadou, V., Whitton, M., and van Geel, N. (2015). Vitiligo. The Lancet 386 (9988), 74–84. doi:10.1016/s0140-6736(14)60763-7
Fan, Z., Cai, L., Wang, S., Wang, J., and Chen, B. (2021). Baicalin Prevents Myocardial Ischemia/Reperfusion Injury through Inhibiting ACSL4 Mediated Ferroptosis. Front. Pharmacol. 12, 628988. doi:10.3389/fphar.2021.628988
Fang, D., Xiong, Z., Xu, J., Yin, J., and Luo, R. (2019). Chemopreventive Mechanisms of Galangin against Hepatocellular Carcinoma: A Review. Biomed. Pharmacother. 109, 2054–2061. doi:10.1016/j.biopha.2018.09.154
Fiorito, S., Epifano, F., Palmisano, R., Genovese, S., and Taddeo, V. A. (2017). A Re-investigation of the Phytochemical Composition of the Edible Herb Amaranthus Retroflexus L. J. Pharm. Biomed. Anal. 143, 183–187. doi:10.1016/j.jpba.2017.05.051
Fiorito, S., Epifano, F., Preziuso, F., Cacciatore, I., di Stefano, A., Taddeo, V. A., et al. (2018). Natural Oxyprenylated Coumarins Are Modulators of Melanogenesis. Eur. J. Med. Chem. 152, 274–282. doi:10.1016/j.ejmech.2018.04.051
Gao, Z., Huang, K., Yang, X., and Xu, H. (1999). Free Radical Scavenging and Antioxidant Activities of Flavonoids Extracted from the Radix of Scutellaria Baicalensis Georgi. Biochim. Biophys. Acta (Bba) - Gen. Subjects 1472 (3), 643–650. doi:10.1016/s0304-4165(99)00152-x
Gnonlonfin, B. G., Gbaguidi, F., Gbenou, J. D., Sanni, A., and Brimer, L. (2011). Changes in Scopoletin Concentration in Cassava Chips from Four Varieties during Storage. J. Sci. Food Agric. 91 (13), 2344–2347. doi:10.1002/jsfa.4465
Godoy de Lima, R., Barros, M. T., and da Silva Laurentiz, R. (2018). Medicinal Attributes of Lignans Extracted from Piper Cubeba: Current Developments. ChemistryOpen 7 (2), 180–191.
Góngora, L., Giner, R. M., Máñez, S., del Carmen Recio, M., Schinella, G., and Rios, J. L. (2002). Effects of Caffeoyl Conjugates of Isoprenyl-Hydroquinone Glucoside and Quinic Acid on Leukocyte Function. Life Sci. 71 (25), 2995–3004. doi:10.1016/s0024-3205(02)02167-7
Grimes, P. E., and Nashawati, R. (2017). Depigmentation Therapies for Vitiligo. Dermatol. Clin. 35 (2), 219–227. doi:10.1016/j.det.2016.11.010
Gu, C., Yang, X., and Huang, L. (2016). Cistanches Herba: A Neuropharmacology Review. Front. Pharmacol. 7, 289. doi:10.3389/fphar.2016.00289
Guan, C., Xu, W., Hong, W., Zhou, M., Lin, F., Fu, L., et al. (2015). Quercetin Attenuates the Effects of H2O2 on Endoplasmic Reticulum Morphology and Tyrosinase export from the Endoplasmic Reticulum in Melanocytes. Mol. Med. Rep. 11 (6), 4285–4290. doi:10.3892/mmr.2015.3242
Guan, S., Su, W., Wang, N., Li, P., and Wang, Y. (2008). A Potent Tyrosinase Activator from Radix Polygoni Multiflori and its Melanogenesis Stimulatory Effect in B16 Melanoma Cells. Phytother. Res. 22 (5), 660–663. doi:10.1002/ptr.2358
Guo, S., and Zhang, Q. (2021). Paeonol Protects Melanocytes against Hydrogen Peroxide-Induced Oxidative Stress through Activation of Nrf2 Signaling Pathway. Drug Dev. Res. doi:10.1002/ddr.21793
Harris, J. E., Harris, T. H., Weninger, W., John Wherry, E., Hunter, C. A., and Turka, L. A. (2012). A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-γ for Autoreactive CD8+ T-Cell Accumulation in the Skin. J. Invest. Dermatol. 132 (7), 1869–1876. doi:10.1038/jid.2011.463
He, H., Wang, S., Tian, J., Chen, L., Zhang, W., Zhao, J., et al. (2015). Protective Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-Glucoside in the MPTP-Induced Mouse Model of Parkinson's Disease: Involvement of Reactive Oxygen Species-Mediated JNK, P38 and Mitochondrial Pathways. Eur. J. Pharmacol. 767, 175–182. doi:10.1016/j.ejphar.2015.10.023
He, M., Min, J.-W., Kong, W.-L., He, X.-H., Li, J.-X., and Peng, B.-W. (2016). A Review on the Pharmacological Effects of Vitexin and Isovitexin. Fitoterapia 115, 74–85. doi:10.1016/j.fitote.2016.09.011
Hearing, V. J. (1999). Biochemical Control of Melanogenesis and Melanosomal Organization. J. Invest. Dermatol. Symp. Proc. 4 (1), 24–28. doi:10.1038/sj.jidsp.5640176
Heeba, G. H., Rabie, E. M., Abuzeid, M. M., Bekhit, A. A., and Khalifa, M. M. (2021). Morin Alleviates Fructose-Induced Metabolic Syndrome in Rats via Ameliorating Oxidative Stress, Inflammatory and Fibrotic Markers. Korean J. Physiol. Pharmacol. 25 (3), 177–187. doi:10.4196/kjpp.2021.25.3.177
Heriniaina, R. M., Dong, J., Kalavagunta, P. K., Wu, H.-L., Yan, D.-S., and Shang, J. (2018). Effects of Six Compounds with Different Chemical Structures on Melanogenesis. Chin. J. Nat. Medicines 16 (10), 766–773. doi:10.1016/s1875-5364(18)30116-x
Hirata, N., Naruto, S., Ohguchi, K., Akao, Y., Nozawa, Y., Iinuma, M., et al. (2007). Mechanism of the Melanogenesis Stimulation Activity of (−)-cubebin in Murine B16 Melanoma Cells. Bioorg. Med. Chem. 15 (14), 4897–4902. doi:10.1016/j.bmc.2007.04.046
Hu, M., Chen, C., Liu, J., Cai, L., Shao, J., Chen, Z., et al. (2020a). The Melanogenic Effects and Underlying Mechanism of Paeoniflorin in Human Melanocytes and Vitiligo Mice. Fitoterapia 140, 104416. doi:10.1016/j.fitote.2019.104416
Hu, Y., Huang, J., Li, Y., Jiang, L., Ouyang, Y., Li, Y., et al. (2020b). Cistanche Deserticola Polysaccharide Induces Melanogenesis in Melanocytes and Reduces Oxidative Stress via Activating NRF2/HO‐1 Pathway. J. Cel Mol Med 24 (7), 4023–4035. doi:10.1111/jcmm.15038
Huang, J., Tong, X., Zhang, L., Zhang, Y., Wang, L., Wang, D., et al. (2020). Hyperoside Attenuates Bleomycin-Induced Pulmonary Fibrosis Development in Mice. Front. Pharmacol. 11, 550955. doi:10.3389/fphar.2020.550955
Huang, K., Li, C., Gao, F., Fan, Y., Zeng, F., Meng, L., et al. (2021). Epigallocatechin-3-Gallate Promotes the In Vitro Maturation and Embryo Development Following IVF of Porcine Oocytes. Dddt 15, 1013–1020. doi:10.2147/dddt.s295936
Huang, W. H., Lee, A. R., Chien, P. Y., and Chou, T. C. (2005). Synthesis of Baicalein Derivatives as Potential Anti-aggregatory and Anti-inflammatory Agents. J. Pharm. Pharmacol. 57 (2), 219–225. doi:10.1211/0022357055371
Huang, Y.-C., Yang, C.-H., and Chiou, Y.-L. (2011). Citrus Flavanone Naringenin Enhances Melanogenesis through the Activation of Wnt/β-Catenin Signalling in Mouse Melanoma Cells. Phytomedicine 18 (14), 1244–1249. doi:10.1016/j.phymed.2011.06.028
Huang, Y. C., Liu, K. C., and Chiou, Y. L. (2012). Melanogenesis of Murine Melanoma Cells Induced by Hesperetin, a Citrus Hydrolysate-Derived Flavonoid. Food Chem. Toxicol. 50 (3-4), 653–659. doi:10.1016/j.fct.2012.01.012
Huo, S.-X., Liu, X.-M., Ge, C.-H., Gao, L., Peng, X.-M., Zhao, P.-P., et al. (2014). The Effects of Galangin on a Mouse Model of Vitiligo Induced by Hydroquinone. Phytother. Res. 28 (10), 1533–1538. doi:10.1002/ptr.5161
Huo, S.-X., Wang, Q., Liu, X.-M., Ge, C.-H., Gao, L., Peng, X.-M., et al. (2017). The Effect of Butin on the Vitiligo Mouse Model Induced by Hydroquinone. Phytother. Res. 31 (5), 740–746. doi:10.1002/ptr.5794
Hwang, I. K., Yoo, K.-Y., Kim, D. W., Jeong, S. J., Won, C.-K., Moon, W.-K., et al. (2006). An Extract of Polygonum Multiflorum Protects against Free Radical Damage Induced by Ultraviolet B Irradiation of the Skin. Braz. J. Med. Biol. Res. 39 (9), 1181–1188. doi:10.1590/s0100-879x2006000900005
Hwang, J.-M., Tseng, T.-H., Tsai, Y.-Y., Lee, H.-J., Chou, F.-P., Wang, C.-J., et al. (2005). Protective Effects of Baicalein on Tert-Butyl Hydroperoxide-Induced Hepatic Toxicity in Rat Hepatocytes. J. Biomed. Sci. 12 (2), 389–397. doi:10.1007/s11373-005-1572-8
Hwang, Y. S., Oh, S. W., Park, S. H., Lee, J., Yoo, J. A., Kwon, K., et al. (2019). Melanogenic Effects of Maclurin Are Mediated through the Activation of cAMP/PKA/CREB and P38 MAPK/CREB Signaling Pathways. Oxid Med. Cel Longev 2019, 9827519. doi:10.1155/2019/9827519
Hwang, Y. S., Kim, Y.-J., Kim, M. O., Kang, M., Oh, S. W., Nho, Y. H., et al. (2017). Cannabidiol Upregulates Melanogenesis through CB1 Dependent Pathway by Activating P38 MAPK and P42/44 MAPK. Chemico-Biological Interactions 273, 107–114. doi:10.1016/j.cbi.2017.06.005
Imran, M., Rauf, A., Shah, Z. A., Saeed, F., Imran, A., Arshad, M. U., et al. (2019). Chemo-preventive and Therapeutic Effect of the Dietary Flavonoid Kaempferol: A Comprehensive Review. Phytotherapy Res. 33 (2), 263–275. doi:10.1002/ptr.6227
Iuvone, T., Esposito, G., De Filippis, D., Scuderi, C., and Steardo, L. (2009). Cannabidiol: a Promising Drug for Neurodegenerative Disorders?. CNS Neurosci. Ther. 15 (1), 65–75. doi:10.1111/j.1755-5949.2008.00065.x
Jang, Y., and Koh, E. (2019). Antioxidant Content and Activity in Leaves and Petioles of Six Sweet Potato (Ipomoea Batatas L.) and Antioxidant Properties of Blanched Leaves. Food Sci. Biotechnol. 28 (2), 337–345. doi:10.1007/s10068-018-0481-3
Jeng, K.-C. G., Hou, R. C. W., Wang, J.-C., and Ping, L.-I. (2005). Sesamin Inhibits Lipopolysaccharide-Induced Cytokine Production by Suppression of P38 Mitogen-Activated Protein Kinase and Nuclear Factor-Κb. Immunol. Lett. 97 (1), 101–106. doi:10.1016/j.imlet.2004.10.004
Jeong, Y.-M., Choi, Y.-G., Kim, D.-S., Park, S.-H., Yoon, J.-A., Kwon, S.-B., et al. (2005). Cytoprotective Effect of green tea Extract and Quercetin against Hydrogen Peroxide-Induced Oxidative Stress. Arch. Pharm. Res. 28 (11), 1251–1256. doi:10.1007/bf02978208
Jian, Z., Li, K., Liu, L., Zhang, Y., Zhou, Z., Li, C., et al. (2011). Heme Oxygenase-1 Protects Human Melanocytes from H2O2-Induced Oxidative Stress via the Nrf2-ARE Pathway. J. Invest. Dermatol. 131 (7), 1420–1427. doi:10.1038/jid.2011.56
Jian, Z., Li, K., Song, P., Zhu, G., Zhu, L., Cui, T., et al. (2014). Impaired Activation of the Nrf2-ARE Signaling Pathway Undermines H2O2-Induced Oxidative Stress Response: a Possible Mechanism for Melanocyte Degeneration in Vitiligo. J. Invest. Dermatol. 134 (8), 2221–2230. doi:10.1038/jid.2014.152
Jiang, W., Li, S., Chen, X., Zhang, W., Chang, Y., He, Y., et al. (2019). Berberine Protects Immortalized Line of Human Melanocytes from H2O2-Induced Oxidative Stress via Activation of Nrf2 and Mitf Signaling Pathway. J. Dermatol. Sci. 94 (1), 236–243. doi:10.1016/j.jdermsci.2019.03.007
Jiang, Z., Li, S., Liu, Y., Deng, P., Huang, J., and He, G. (2011). Sesamin Induces Melanogenesis by Microphthalmia-Associated Transcription Factor and Tyrosinase Up-Regulation via cAMP Signaling Pathway. Acta Biochim. Biophys. Sinica 43 (10), 763–770. doi:10.1093/abbs/gmr078
Jiang, Z., Xu, J., Long, M., Tu, Z., Yang, G., and He, G. (2009). 2, 3, 5, 4'-Tetrahydroxystilbene-2-O-Beta-D-Glucoside (THSG) Induces Melanogenesis in B16 Cells by MAP Kinase Activation and Tyrosinase Upregulation. Life Sci. 85 (9-10), 345–350. doi:10.1016/j.lfs.2009.05.022
Jie, Q., Kodithuwakku, N. D., Yuan, X., He, G., Chen, M., Xu, S., et al. (2015). Anti-allergic and Anti-inflammatory Properties of a Potent Histamine H1 Receptor Antagonist, Desloratadine Citrate Disodium Injection, and its Anti-inflammatory Mechanism on EA.Hy926 Endothelial Cells. Eur. J. Pharmacol. 754, 1–10. doi:10.1016/j.ejphar.2015.02.016
Jin, Y., Andersen, G., Yorgov, D., Ferrara, T. M., Ben, S., Brownson, K. M., et al. (2016). Genome-wide Association Studies of Autoimmune Vitiligo Identify 23 New Risk Loci and Highlight Key Pathways and Regulatory Variants. Nat. Genet. 48 (11), 1418–1424. doi:10.1038/ng.3680
Jung, E., Kim, J. H., Kim, M. O., Jang, S., Kang, M., Oh, S. W., et al. (2016). Afzelin Positively Regulates Melanogenesis through the P38 MAPK Pathway. Chemico-Biological Interactions 254, 167–172. doi:10.1016/j.cbi.2016.06.010
Jung, G.-D., Yang, J.-Y., Song, E.-S., and Park, J.-W. (2001). Stimulation of Melanogenesis by Glycyrrhizin in B16 Melanoma Cells. Exp. Mol. Med. 33 (3), 131–135. doi:10.1038/emm.2001.23
Kalaiselvi, P., Rajashree, K., Bharathi Priya, L., and Padma, V. V. (2013). Cytoprotective Effect of Epigallocatechin-3-Gallate against Deoxynivalenol-Induced Toxicity through Anti-oxidative and Anti-inflammatory Mechanisms in HT-29 Cells. Food Chem. Toxicol. 56, 110–118. doi:10.1016/j.fct.2013.01.042
Katiyar, S. K., Matsui, M. S., Elmets, C. A., and Mukhtar, H. (1999). Polyphenolic Antioxidant (-)-Epigallocatechin-3-Gallate from Green Tea Reduces UVB-Lnduced Inflammatory Responses and Infiltration of Leukocytes in Human Skin. Photochem. Photobiol. 69 (2), 148–153. doi:10.1111/j.1751-1097.1999.tb03267.x
Kawakami, T., Ohgushi, A., Hirobe, T., and Soma, Y. (2014). Effects of 1,25-dihydroxyvitamin D3 on Human Epidermal Melanocytes and Melanoblasts. J. Dermatol. Sci. 76 (1), 72–74. doi:10.1016/j.jdermsci.2014.07.005
Khan, H., Marya, S., Amin, S., Kamal, M. A., and Patel, S. (2018). Flavonoids as Acetylcholinesterase Inhibitors: Current Therapeutic Standing and Future Prospects. Biomed. Pharmacother. 101, 860–870. doi:10.1016/j.biopha.2018.03.007
Khan, N., and Mukhtar, H. (2015). Dietary Agents for Prevention and Treatment of Lung Cancer. Cancer Lett. 359 (2), 155–164. doi:10.1016/j.canlet.2015.01.038
Kim, H. J., Kim, J. S., Woo, J.-T., Lee, I.-S., and Cha, B.-Y. (2015). Hyperpigmentation Mechanism of Methyl 3,5-Di-Caffeoylquinate through Activation of P38 and MITF Induction of Tyrosinase. Acta Biochim. Biophys. Sin 47 (7), 548–556. doi:10.1093/abbs/gmv040
Kim, J.-K., and Jang, H.-D. (2016). 6-shogaol Attenuates H2O2-Induced Oxidative Stress via Upregulation of Nrf2-Mediated γ-glutamylcysteine Synthetase and Heme Oxygenase Expression in HepG2 Cells. Food Sci. Biotechnol. 25 (1), 319–327. doi:10.1007/s10068-016-0045-3
Kim, J. K., Kim, Y. S., Kim, Y., Uddin, M. R., Kim, Y. B., Kim, H. H., et al. (2014). Comparative Analysis of Flavonoids and Polar Metabolites from Hairy Roots of Scutellaria Baicalensis and Scutellaria Lateriflora. World J. Microbiol. Biotechnol. 30 (3), 887–892. doi:10.1007/s11274-013-1498-7
Kim, S. K., Kim, H. J., Choi, S. E., Park, K. H., Choi, H. K., and Lee, M. W. (2008). Anti-oxidative and Inhibitory Activities on Nitric Oxide (NO) and Prostaglandin E2 (COX-2) Production of Flavonoids from Seeds of Prunus Tomentosa Thunberg. Arch. Pharm. Res. 31 (4), 424–428. doi:10.1007/s12272-001-1174-9
Kou, X., Wang, X., Ji, R., Liu, L., Qiao, Y., Lou, Z., et al. (2018). Occurrence, Biological Activity and Metabolism of 6-shogaol. Food Funct. 9 (3), 1310–1327. doi:10.1039/c7fo01354j
Kwon, S.-J., Kim, Y.-M., Jang, H.-J., and Seo, Y.-K. (2018). Synergistic Effect of rice Bran Extract and Extremely Low-Frequency Electromagnetic fields on Dermal Papilla/melanocytes in Melanogenesis. Bioelectromagnetics 39 (8), 595–603. doi:10.1002/bem.22151
Lai, Y., Feng, Q., Zhang, R., Shang, J., and Zhong, H. (2021). The Great Capacity on Promoting Melanogenesis of Three Compatible Components in Vernonia Anthelmintica (L.) Willd. Int. J. Mol. Sci. 22 (8). doi:10.3390/ijms22084073
Latham, C. M., Brightwell, C. R., Keeble, A. R., Munson, B. D., Thomas, N. T., Zagzoog, A. M., et al. (2021). Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 12, 660498. doi:10.3389/fphys.2021.660498
Lau, K. M., Yue, G. G., Chan, Y. Y., Kwok, H. F., Gao, S., Wong, C. W., et al. (2019). A Review on the Immunomodulatory Activity of Acanthopanax Senticosus and its Active Components. Chin. Med. 14, 25. doi:10.1186/s13020-019-0250-0
Lee, J., Jung, E., Park, J., Jung, K., Park, E., Kim, J., et al. (2005). Glycyrrhizin Induces Melanogenesis by Elevating a cAMP Level in B16 Melanoma Cells. J. Invest. Dermatol. 124 (2), 405–411. doi:10.1111/j.0022-202x.2004.23606.x
Lee, J., Kim, Y. S., and Park, D. (2007). Rosmarinic Acid Induces Melanogenesis through Protein Kinase A Activation Signaling. Biochem. Pharmacol. 74 (7), 960–968. doi:10.1016/j.bcp.2007.06.007
Lee, S., So, Y.-J., Shin, M., Cho, J., and Lee, J. (2014). Antibacterial Effects of Afzelin Isolated from Cornus Macrophylla on Pseudomonas aeruginosa, a Leading Cause of Illness in Immunocompromised Individuals. Molecules 19 (3), 3173–3180. doi:10.3390/molecules19033173
Lee, S. Y. (2018). Synergistic Effect of Maclurin on Ginsenoside Compound K Induced Inhibition of the Transcriptional Expression of Matrix Metalloproteinase-1 in HaCaT Human Keratinocyte Cells. J. Ginseng Res. 42 (2), 229–232. doi:10.1016/j.jgr.2017.11.003
Lee, Y. J., Jung, O., Lee, J., Son, J., Cho, J. Y., Ryou, C., et al. (2018). Maclurin Exerts Anti-cancer Effects on PC3 Human Prostate Cancer Cells via Activation of P38 and Inhibitions of JNK, FAK, AKT, and C-Myc Signaling Pathways. Nutr. Res. 58, 62–71. doi:10.1016/j.nutres.2018.07.003
Legrand, G., Delporte, M., Khelifi, C., Harant, A., Vuylsteker, C., Mörchen, M., et al. (2016). Identification and Characterization of Five BAHD Acyltransferases Involved in Hydroxycinnamoyl Ester Metabolism in Chicory. Front. Plant Sci. 7, 741. doi:10.3389/fpls.2016.00741
Li, B., Pinch, H., and Birt, D. R. (1996). Influence of Vehicle, Distant Topical Delivery, and Biotransformation on the Chemopreventive Activity of Apigenin, a Plant Flavonoid, in Mouse Skin. Pharm. Res. 13 (10), 1530–1534. doi:10.1023/a:1016083613916
Li, G.-H., Fang, K.-L., Yang, K., Cheng, X.-P., Wang, X.-N., Shen, T., et al. (2021a). Thesium Chinense Turcz.: An Ethnomedical, Phytochemical and Pharmacological Review. J. Ethnopharmacology 273, 113950. doi:10.1016/j.jep.2021.113950
Li, J., Ren, S., Li, M., Bi, J., Yang, G., and Li, E. (2021b). Paeoniflorin Protects against Dextran Sulfate Sodium (DSS)-induced Colitis in Mice through Inhibition of Inflammation and Eosinophil Infiltration. Int. Immunopharmacology 97, 107667. doi:10.1016/j.intimp.2021.107667
Li, R., Zhao, D., Qu, R., Fu, Q., and Ma, S. (2015). The Effects of Apigenin on Lipopolysaccharide-Induced Depressive-like Behavior in Mice. Neurosci. Lett. 594, 17–22. doi:10.1016/j.neulet.2015.03.040
Li, X.-S., Tang, X.-Y., Su, W., and Li, X. (2020). Vitexin Protects Melanocytes from Oxidative Stress via Activating MAPK-Nrf2/ARE Pathway. Immunopharmacology and Immunotoxicology 42 (6), 594–603. doi:10.1080/08923973.2020.1835952
Lim, H., Park, H., and Kim, H. P. (2015). Effects of Flavonoids on Senescence-Associated Secretory Phenotype Formation from Bleomycin-Induced Senescence in BJ Fibroblasts. Biochem. Pharmacol. 96 (4), 337–348. doi:10.1016/j.bcp.2015.06.013
Lin, M., Lu, S.-s., Wang, A.-x., Qi, X.-y., Zhao, D., Wang, Z.-h., et al. (2011a). Apigenin Attenuates Dopamine-Induced Apoptosis in Melanocytes via Oxidative Stress-Related P38, C-Jun NH2-terminal Kinase and Akt Signaling. J. Dermatol. Sci. 63 (1), 10–16. doi:10.1016/j.jdermsci.2011.03.007
Lin, T. Y., Wu, P. Y., Hou, C. W., Chien, T. Y., Chang, Q. X., Wen, K. C., et al. (2019). Protective Effects of Sesamin against UVB-Induced Skin Inflammation and Photodamage In Vitro and In Vivo. Biomolecules 9 (9). doi:10.3390/biom9090479
Lin, Y.-L., Lu, C.-K., Huang, Y.-J., and Chen, H.-J. (2011b). Antioxidative Caffeoylquinic Acids and Flavonoids from Hemerocallis Fulva Flowers. J. Agric. Food Chem. 59 (16), 8789–8795. doi:10.1021/jf201166b
Ling, Y., Gong, Q., Xiong, X., Sun, L., Zhao, W., Zhu, W., et al. (2017). Protective Effect of Madecassoside on H2O2-Induced Oxidative Stress and Autophagy Activation in Human Melanocytes. Oncotarget 8 (31), 51066–51075. doi:10.18632/oncotarget.17654
Liu, B., Jian, Z., Li, Q., Li, K., Wang, Z., Liu, L., et al. (2012). Baicalein Protects Human Melanocytes from H2O2-Induced Apoptosis via Inhibiting Mitochondria-dependent Caspase Activation and the P38 MAPK Pathway. Free Radic. Biol. Med. 53 (2), 183–193. doi:10.1016/j.freeradbiomed.2012.04.015
Liu, R., Ji, P., Liu, B., Qiao, H., Wang, X., Zhou, L., et al. (2017). Apigenin Enhances the Cisplatin Cytotoxic Effect through P53-Modulated Apoptosis. Oncol. Lett. 13 (2), 1024–1030. doi:10.3892/ol.2016.5495
Liu, Y., Qiu, Y., Chen, Q., Han, X., Cai, M., and Hao, L. (2021a). Puerarin Suppresses the Hepatic Gluconeogenesis via Activation of PI3K/Akt Signaling Pathway in Diabetic Rats and HepG2 Cells. Biomed. Pharmacother. 137, 111325. doi:10.1016/j.biopha.2021.111325
Liu, Y., Wang, H., Yang, M., Liu, N., Zhao, Y., Qi, X., et al. (2018). Cistanche Deserticola Polysaccharides Protects PC12 Cells against OGD/RP-induced Injury. Biomed. Pharmacother. 99, 671–680. doi:10.1016/j.biopha.2018.01.114
Liu, Z. Q., Yao, G. L., Zhai, J. M., Hu, D. W., and Fan, Y. G. (2021b). Kaempferol Suppresses Proliferation and Induces Apoptosis and DNA Damage in Human Gallbladder Cancer Cells through the CDK4/CDK6/cyclin D1 Pathway. Eur. Rev. Med. Pharmacol. Sci. 25 (3), 1311–1321.
Long, X., Zeng, X., Yan, H., Xu, M., Zeng, Q., Xu, C., et al. (2021). Flavonoids Composition and Antioxidant Potential Assessment of Extracts from Gannanzao Navel Orange (Citrus Sinensis Osbeck Cv. Gannanzao) Peel. Nat. Product. Res. 35 (4), 702–706. doi:10.1080/14786419.2019.1593162
Lotito, S., and Frei, B. (2006). Consumption of Flavonoid-Rich Foods and Increased Plasma Antioxidant Capacity in Humans: Cause, Consequence, or Epiphenomenon?. Free Radic. Biol. Med. 41 (12), 1727–1746. doi:10.1016/j.freeradbiomed.2006.04.033
Lu, W., Zhao, Y., Kong, Y., Zhang, W., Ma, W., Li, W., et al. (2018). Geniposide Prevents H2 O2 -induced Oxidative Damage in Melanocytes by Activating the PI3K-Akt Signalling Pathway. Clin. Exp. Dermatol. 43 (6), 667–674. doi:10.1111/ced.13409
Ma, J., Li, S., Zhu, L., Guo, S., Yi, X., Cui, T., et al. (2018). Baicalein Protects Human Vitiligo Melanocytes from Oxidative Stress through Activation of NF-E2-Related Factor2 (Nrf2) Signaling Pathway. Free Radic. Biol. Med. 129, 492–503. doi:10.1016/j.freeradbiomed.2018.10.421
Ma, Y. L., Zhao, F., Yin, J. T., Liang, C. J., Niu, X. L., Qiu, Z. H., et al. (2019). Two Approaches for Evaluating the Effects of Galangin on the Activities and mRNA Expression of Seven CYP450. Molecules 24 (6), 1171. doi:10.3390/molecules24061171
Maimaiti, Z., Turak, A., and Aisa, H. A. (2017). Two New Compounds from the Seeds of Vernonia Anthelmintica. J. Asian Nat. Prod. Res. 19 (9), 862–868. doi:10.1080/10286020.2016.1269760
Majnooni, M. B., Fakhri, S., Shokoohinia, Y., Mojarrab, M., Kazemi-Afrakoti, S., and Farzaei, M. H. (2020). Isofraxidin: Synthesis, Biosynthesis, Isolation, Pharmacokinetic and Pharmacological Properties. Molecules 25 (9), 2040. doi:10.3390/molecules25092040
Maleš, Ž., Drvar, D. L., Duka, I., and Žužul, K. (2019). Application of Medicinal Plants in Several Dermatovenerological Entities. Acta Pharm. 69 (4), 525–531.
Mamat, N., Lu, X. Y., Kabas, M., and Aisa, H. A. (2018). Potential Anti-vitiligo Properties of Cynarine Extracted from Vernonia Anthelmintica (L.) Willd. Int. J. Mol. Med. 42 (5), 2665–2675. doi:10.3892/ijmm.2018.3861
Mamat, N., Dou, J., Lu, X., Eblimit, A., and Haji Akber, A. (2017). Isochlorogenic Acid A Promotes Melanin Synthesis in B16 Cell through the β-catenin Signal Pathway. Acta Biochim. Biophys. Sin (Shanghai) 49 (9), 800–807. doi:10.1093/abbs/gmx072
Mechoulam, R., and Hanus, L. (2002). Cannabidiol: an Overview of Some Chemical and Pharmacological Aspects. Part I: Chemical Aspects. Chem. Phys. Lipids 121 (1-2), 35–43. doi:10.1016/s0009-3084(02)00144-5
Meng, Y., Liu, Z., Zhai, C., Di, T., Zhang, L., Zhang, L., et al. (2019). Paeonol Inhibits the Development of 1-chloro-2,4-dinitrobenzene-induced A-topic D-ermatitis via M-ast and T C-ells in BALB/c M-ice. Mol. Med. Rep. 19 (4), 3217–3229. doi:10.3892/mmr.2019.9985
Meng, Y., Wang, M., Xie, X., Di, T., Zhao, J., Lin, Y., et al. (2017). Paeonol Ameliorates Imiquimod-Induced Psoriasis-like Skin Lesions in BALB/c Mice by Inhibiting the Maturation and Activation of Dendritic Cells. Int. J. Mol. Med. 39 (5), 1101–1110. doi:10.3892/ijmm.2017.2930
Mereles, D., and Hunstein, W. (2011). Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls Than Promises?. Ijms 12 (9), 5592–5603. doi:10.3390/ijms12095592
Mi Moon, K., Young Kim, C., Yeul Ma, J., and Lee, B. (2019). Xanthone-related Compounds as an Anti-browning and Antioxidant Food Additive. Food Chem. 274, 345–350. doi:10.1016/j.foodchem.2018.08.144
Miao, J., Zhong, J., Lan, J., Ye, S., Ye, P., Li, S., et al. (2021). Paeonol Attenuates Inflammation by Confining HMGB1 to the Nucleus. J. Cel Mol Med 25 (6), 2885–2899. doi:10.1111/jcmm.16319
Miguel, S., Legrand, G., Duriot, L., Delporte, M., Menin, B., Michel, C., et al. (2020). A GDSL Lipase-like from Ipomoea Batatas Catalyzes Efficient Production of 3,5-diCQA when Expressed in Pichia pastoris. Commun. Biol. 3 (1), 673. doi:10.1038/s42003-020-01488-x
Molagoda, I. M. N., Karunarathne, W., Park, S. R., Choi, Y. H., Park, E. K., Jin, C. Y., et al. (2020). GSK-3beta-Targeting Fisetin Promotes Melanogenesis in B16F10 Melanoma Cells and Zebrafish Larvae through Beta-Catenin Activation. Int. J. Mol. Sci. 21 (1). doi:10.3390/ijms21010312
Monteiro, É., Chibli, L., Yamamoto, C., Pereira, M., Vilela, F., Rodarte, M., et al. (2014). Antinociceptive and Anti-inflammatory Activities of the Sesame Oil and Sesamin. Nutrients 6 (5), 1931–1944. doi:10.3390/nu6051931
Moreira, C. G., Horinouchi, C. D. S., Souza-Filho, C. S., Campos, F. R., Barison, A., Cabrini, D. A., et al. (2012). Hyperpigmentant Activity of Leaves and Flowers Extracts of Pyrostegia venusta on Murine B16F10 Melanoma. J. Ethnopharmacology 141 (3), 1005–1011. doi:10.1016/j.jep.2012.03.047
Mou, K., Pan, W., Han, D., Wen, X., Cao, F., Miao, Y., et al. (2019). Glycyrrhizin Protects Human Melanocytes from H2O2-induced O-xidative D-amage via the Nrf2-dependent I-nduction of HO-1. Int. J. Mol. Med. 44 (1), 253–261. doi:10.3892/ijmm.2019.4200
Mou, K. H., Han, D., Liu, W. L., and Li, P. (2016). Combination Therapy of Orally Administered Glycyrrhizin and UVB Improved Active-Stage Generalized Vitiligo. Braz. J. Med. Biol. Res. 49 (8). doi:10.1590/1414-431x20165354
Mou, S. Q., Zhou, Z. Y., Feng, H., Zhang, N., Lin, Z., Aiyasiding, X., et al. (2021). Liquiritin Attenuates Lipopolysaccharides-Induced Cardiomyocyte Injury via an AMP-Activated Protein Kinase-dependent Signaling Pathway. Front. Pharmacol. 12, 648688. doi:10.3389/fphar.2021.648688
Nagata, H., Takekoshi, S., Takeyama, R., Homma, T., and Yoshiyuki Osamura, R. (2004). Quercetin Enhances Melanogenesis by Increasing the Activity and Synthesis of Tyrosinase in Human Melanoma Cells and in normal Human Melanocytes. Pigment Cel Res 17 (1), 66–73. doi:10.1046/j.1600-0749.2003.00113.x
Nasr, M., and Al-Karaki, R. (2020). Nanotechnological Innovations Enhancing the Topical Therapeutic Efficacy of Quercetin: A Succinct Review. Cdd 17 (4), 270–278. doi:10.2174/1567201817666200317123224
Nguyen, D.-V., Li, F., Li, H., Wong, B. S., Low, C. Y., Liu, X.-Y., et al. (2015). Drug Permeation through Skin Is Inversely Correlated with Carrier Gel Rigidity. Mol. Pharmaceutics 12 (2), 444–452. doi:10.1021/mp500542a
Ning, W., Wang, S., Dong, X., Liu, D., Fu, L., Jin, R., et al. (2015). Epigallocatechin-3-gallate (EGCG) Suppresses the Trafficking of Lymphocytes to Epidermal Melanocytes via Inhibition of JAK2: Its Implication for Vitiligo Treatment. Biol. Pharm. Bull. 38 (11), 1700–1706. doi:10.1248/bpb.b15-00331
Niu, C., and Aisa, H. A. (2017). Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 22 (8), 1303. doi:10.3390/molecules22081303
Niu, C., Pang, G. X., Li, G., Dou, J., Nie, L. F., Himit, H., et al. (2016). Synthesis and Biological Evaluation of Furocoumarin Derivatives on Melanin Synthesis in Murine B16 Cells for the Treatment of Vitiligo. Bioorg. Med. Chem. 24 (22), 5960–5968. doi:10.1016/j.bmc.2016.09.056
Ohguchi, K., Akao, Y., and Nozawa, Y. (2006). Stimulation of Melanogenesis by the Citrus Flavonoid Naringenin in Mouse B16 Melanoma Cells. Biosci. Biotechnol. Biochem. 70 (6), 1499–1501. doi:10.1271/bbb.50635
Ożarowski, M., and Karpiński, T. M. (2020). Extracts and Flavonoids of Passiflora Species as Promising Anti-inflammatory and Antioxidant Substances. Curr. Pharm. Des. doi:10.2174/1381612826666200526150113
Park, W.-s., Kwon, O., Yoon, T.-J., and Chung, J. H. (2014). Anti-graying Effect of the Extract of Pueraria Thunbergiana via Upregulation of cAMP/MITF-M Signaling Pathway. J. Dermatol. Sci. 75 (2), 153–155. doi:10.1016/j.jdermsci.2014.05.003
Pastorino, G., Cornara, L., Soares, S., Rodrigues, F., and Oliveira, M. B. P. P. (2018). Liquorice (Glycyrrhiza Glabra ): A Phytochemical and Pharmacological Review. Phytotherapy Res. 32 (12), 2323–2339. doi:10.1002/ptr.6178
Patel, D., Shukla, S., and Gupta, S. (2007). Apigenin and Cancer Chemoprevention: Progress, Potential and Promise (Review). Int. J. Oncol. 30 (1), 233–245. doi:10.3892/ijo.30.1.233
Patel, S., Rauf, A., Khan, H., Meher, B. R., and Hassan, S. S. U. (2017). A Holistic Review on the Autoimmune Disease Vitiligo with Emphasis on the Causal Factors. Biomed. Pharmacother. 92, 501–508. doi:10.1016/j.biopha.2017.05.095
Pathak, N., Rai, A. K., Kumari, R., and Bhat, K. V. (2014). Value Addition in Sesame: A Perspective on Bioactive Components for Enhancing Utility and Profitability. Pharmacogn Rev. 8 (16), 147–155. doi:10.4103/0973-7847.134249
Paudel, K. S., Hammell, D. C., Agu, R. U., Valiveti, S., and Stinchcomb, A. L. (2010). Cannabidiol Bioavailability after Nasal and Transdermal Application: Effect of Permeation Enhancers. Drug Develop. Ind. Pharm. 36 (9), 1088–1097. doi:10.3109/03639041003657295
Pei, T., Zheng, C., Huang, C., Chen, X., Guo, Z., Fu, Y., et al. (2016). Systematic Understanding the Mechanisms of Vitiligo Pathogenesis and its Treatment by Qubaibabuqi Formula. J. Ethnopharmacology 190, 272–287. doi:10.1016/j.jep.2016.06.001
Peng, L. Y., Shi, H. T., Yuan, M., Li, J. H., Song, K., Huang, J. N., et al. (2020). Madecassoside Protects against LPS-Induced Acute Lung Injury via Inhibiting TLR4/NF-Κb Activation and Blood-Air Barrier Permeability. Front. Pharmacol. 11, 807. doi:10.3389/fphar.2020.00807
Pérez-Sánchez, A., Barrajón-Catalán, E., Herranz-López, M., Castillo, J., and Micol, V. (2016). Lemon Balm Extract ( Melissa Officinalis , L.) Promotes Melanogenesis and Prevents UVB-Induced Oxidative Stress and DNA Damage in a Skin Cell Model. J. Dermatol. Sci. 84 (2), 169–177. doi:10.1016/j.jdermsci.2016.08.004
Pillaiyar, T., Manickam, M., and Jung, S.-H. (2017). Recent Development of Signaling Pathways Inhibitors of Melanogenesis. Cell Signal. 40, 99–115. doi:10.1016/j.cellsig.2017.09.004
Preziuso, F., Genovese, S., Marchetti, L., Sharifi-Rad, M., Palumbo, L., Epifano, F., et al. (2020). 7-Isopentenyloxycoumarin: What Is New across the Last Decade. Molecules 25 (24), 5923. doi:10.3390/molecules25245923
Ramful, D., Bahorun, T., Bourdon, E., Tarnus, E., and Aruoma, O. I. (2010). Bioactive Phenolics and Antioxidant Propensity of Flavedo Extracts of Mauritian Citrus Fruits: Potential Prophylactic Ingredients for Functional Foods Application. Toxicology 278 (1), 75–87. doi:10.1016/j.tox.2010.01.012
Rashighi, M., Agarwal, P., Richmond, J. M., Harris, T. H., Dresser, K., Su, M.-W., et al. (2014). CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Sci. Translational Med. 6 (223), 223ra23. doi:10.1126/scitranslmed.3007811
Rauf, A., Imran, M., Khan, I. A., Ur-Rehman, M., Gilani, S. A., Mehmood, Z., et al. (2018). Anticancer Potential of Quercetin: A Comprehensive Review. Phytotherapy Res. 32 (11), 2109–2130. doi:10.1002/ptr.6155
Riding, R. L., Richmond, J. M., and Harris, J. E. (2019). Mouse Model for Human Vitiligo. Curr. Protoc. Immunol. 124 (1), e63. doi:10.1002/cpim.63
Rodrigues, M., Ezzedine, K., Hamzavi, I., Pandya, A. G., and Harris, J. E. (2017). New Discoveries in the Pathogenesis and Classification of Vitiligo. J. Am. Acad. Dermatol. 77 (1), 1–13. doi:10.1016/j.jaad.2016.10.048
Salehi, B., Fokou, P. V. T., Sharifi-Rad, M., Zucca, P., Pezzani, R., Martins, N., et al. (2019). The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals (Basel) 12 (1), 11. doi:10.3390/ph12010011
Satthakarn, S., Hladik, F., Promsong, A., and Nittayananta, W. (2015). Vaginal Innate Immune Mediators Are Modulated by a Water Extract of Houttuynia Cordata Thunb. BMC Complement. Altern. Med. 15, 183. doi:10.1186/s12906-015-0701-9
Seneschal, J., Boniface, K., D’Arino, A., and Picardo, M. (2021). An Update on Vitiligo Pathogenesis. Pigment Cel Melanoma Res. 34 (2), 236–243. doi:10.1111/pcmr.12949
Serafino, A., Sinibaldi Vallebona, P., Lazzarino, G., Tavazzi, B., Rasi, G., Pierimarchi, P., et al. (2004). Differentiation of Human Melanoma Cells Induced by Cyanidin‐3‐ O ‐β‐glucopyranoside. FASEB j. 18 (15), 1940–1942. doi:10.1096/fj.04-1925fje
Shan, M., Yu, S., Yan, H., Guo, S., Xiao, W., Wang, Z., et al. (2017). A Review on the Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology of Geniposide, a Natural Product. Molecules 22 (10), 1689. doi:10.3390/molecules22101689
Sharifi-Rad, J., Kamiloglu, S., Yeskaliyeva, B., Beyatli, A., Alfred, M. A., Salehi, B., et al. (2020). Pharmacological Activities of Psoralidin: A Comprehensive Review of the Molecular Mechanisms of Action. Front. Pharmacol. 11, 571459. doi:10.3389/fphar.2020.571459
Shi, M., Zhang, Y., Song, M., Sun, Y., Li, C., and Kang, W. (2018). Screening the Marker Components in Psoralea Corylifolia L. With the Aids of Spectrum-Effect Relationship and Component Knock-Out by UPLC-MS(2). Int. J. Mol. Sci. 19 (11), 3439. doi:10.3390/ijms19113439
Shin, S., Ko, J., Kim, M., Song, N., and Park, K. (2021). Morin Induces Melanogenesis via Activation of MAPK Signaling Pathways in B16F10 Mouse Melanoma Cells. Molecules 26 (8), 2150. doi:10.3390/molecules26082150
Shin, S. W., Jung, E., Kim, S., Kim, J. H., Kim, E. G., Lee, J., et al. (2013). Antagonizing Effects and Mechanisms of Afzelin against UVB-Induced Cell Damage. PLoS One 8 (4), e61971. doi:10.1371/journal.pone.0061971
Shomirzoeva, O., Li, J., Numonov, S., Mamat, N., Shataer, D., Lu, X., et al. (2019). Chemical Components of Hyssopus Seravshanicus: Antioxidant Activity, Activations of Melanogenesis and Tyrosinase, and Quantitative Determination by UPLC-DAD. Nat. Product. Res. 33 (6), 866–870. doi:10.1080/14786419.2017.1408105
Shon, M.-S., Kim, R.-H., Kwon, O. J., Roh, S.-S., and Kim, G.-N. (2016). Beneficial Role and Function of Fisetin in Skin Health via Regulation of the CCN2/TGF-β Signaling Pathway. Food Sci. Biotechnol. 25 (S1), 133–141. doi:10.1007/s10068-016-0110-y
Shukla, S., and Gupta, S. (2010). Apigenin: a Promising Molecule for Cancer Prevention. Pharm. Res. 27 (6), 962–978. doi:10.1007/s11095-010-0089-7
Silva, M. L. A., Coímbra, H. S., Pereira, A. C., Almeida, V. A., Lima, T. C., Costa, E. S., et al. (2007). Evaluation of Piper Cubeba Extract, (-)-cubebin and its Semi-synthetic Derivatives against Oral Pathogens. Phytother. Res. 21 (5), 420–422. doi:10.1002/ptr.2088
Singh, B., Singh, J. P., Kaur, A., and Singh, N. (2020). Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 132, 109114. doi:10.1016/j.foodres.2020.109114
Sinha, R., Srivastava, S., Joshi, A., Joshi, U. J., and Govil, G. (2014). In-vitro Anti-proliferative and Anti-oxidant Activity of Galangin, Fisetin and Quercetin: Role of Localization and Intermolecular Interaction in Model Membrane. Eur. J. Med. Chem. 79, 102–109. doi:10.1016/j.ejmech.2014.04.002
Sintov, A. C., Yarmolinsky, L., Dahan, A., and Ben-Shabat, S. (2014). Pharmacological Effects of Vitamin D and its Analogs: Recent Developments. Drug Discov. Today 19 (11), 1769–1774. doi:10.1016/j.drudis.2014.06.008
Skiba, M. A., Szendzielorz, K., Mazur, B., and Król, W. (2016). The Inhibitory Effect of Flavonoids on Interleukin-8 Release by Human Gastric Adenocarcinoma (AGS) Cells Infected with Cag PAI (+) Helicobacter pylori. cejoi 3 (3), 229–235. doi:10.5114/ceji.2016.63119
Somani, G. S., Nahire, M. S., Parikh, A. D., Mulik, M. B., Ghumatkar, P. J., Laddha, K. S., et al. (2017). Neuroprotective Effect of Cubebin: A Dibenzylbutyrolactone Lignan on Scopolamine-Induced Amnesia in Mice. Indian J. Med. Res. 146 (2), 255–259. doi:10.4103/ijmr.IJMR_156_14
Souza, G. H. B., da Silva Filho, A. A., de Souza, V. A., Pereira, A. C., de A. Royo, V., e Silva, M. L. A., et al. (2004). Analgesic and Anti-inflammatory Activities Evaluation of (-)-O-Acetyl, (-)-O-Methyl, (-)-O-Dimethylethylamine Cubebin and Their Preparation from (-)-cubebin. Il Farmaco 59 (1), 55–61. doi:10.1016/j.farmac.2003.07.012
Speeckaert, R., Dugardin, J., Lambert, J., Lapeere, H., Verhaeghe, E., Speeckaert, M. M., et al. (2018). Critical Appraisal of the Oxidative Stress Pathway in Vitiligo: a Systematic Review and Meta-Analysis. J. Eur. Acad. Dermatol. Venereol. 32 (7), 1089–1098. doi:10.1111/jdv.14792
Speeckaert, R., Lambert, J., Bulat, V., Belpaire, A., Speeckaert, M., and van Geel, N. (2020). Autoimmunity in Segmental Vitiligo. Front. Immunol. 11, 568447. doi:10.3389/fimmu.2020.568447
Subongkot, T., Ngawhirunpat, T., and Opanasopit, P. (2021). Development of Ultradeformable Liposomes with Fatty Acids for Enhanced Dermal Rosmarinic Acid Delivery. Pharmaceutics 13 (3), 404. doi:10.3390/pharmaceutics13030404
Sun, C., Huang, H., Xu, C., Li, X., and Chen, K. (2013). Biological activities of extracts from Chinese bayberry (Myrica rubra Sieb. et Zucc.): a review. Plant Foods Hum. Nutr. 68 (2), 97–106. doi:10.1007/s11130-013-0349-x
Sun, Z., Du, J., Hwang, E., and Yi, T.-H. (2018). Paeonol Extracted from Paeonia Suffruticosa Andr. Ameliorated UVB-Induced Skin Photoaging via DLD/Nrf2/ARE and MAPK/AP-1 Pathway. Phytotherapy Res. 32 (9), 1741–1749. doi:10.1002/ptr.6100
Takekoshi, S., Nagata, H., and Kitatani, K. (2014). Flavonoids Enhance Melanogenesis in Human Melanoma Cells. Tokai J. Exp. Clin. Med. 39 (3), 116–121.
Tal, B., and Robeson, D. J. (1986). The Metabolism of Sunflower Phytoalexins Ayapin and Scopoletin. Plant Physiol. 82 (1), 167–172. doi:10.1104/pp.82.1.167
Tang, L., Fang, W., Lin, J., Li, J., Wu, W., and Xu, J. (2018). Vitamin D Protects Human Melanocytes against Oxidative Damage by Activation of Wnt/β-Catenin Signaling. Lab. Invest. 98 (12), 1527–1537. doi:10.1038/s41374-018-0126-4
Tsai, C. F., Su, H. H., Chen, K. M., Liao, J. M., Yao, Y. T., Chen, Y. H., et al. (2020). Paeonol Protects against Myocardial Ischemia/Reperfusion-Induced Injury by Mediating Apoptosis and Autophagy Crosstalk. Front. Pharmacol. 11, 586498. doi:10.3389/fphar.2020.586498
Tuerxuntayi, A., Liu, Y. Q., Tulake, A., Kabas, M., Eblimit, A., and Aisa, H. A. (2014). Kaliziri Extract Upregulates Tyrosinase, TRP-1, TRP-2 and MITF Expression in Murine B16 Melanoma Cells. BMC Complement. Altern. Med. 14, 166. doi:10.1186/1472-6882-14-166
Udomruk, S., Kaewmool, C., Phitak, T., Pothacharoen, P., and Kongtawelert, P. (2020). Sesamin Promotes Neurite Outgrowth under Insufficient Nerve Growth Factor Condition in PC12 Cells through ERK1/2 Pathway and SIRT1 Modulation. Evid. Based Complement. Alternat Med. 2020, 9145458. doi:10.1155/2020/9145458
Usman, H., Ullah, M. A., Jan, H., Siddiquah, A., Drouet, S., Anjum, S., et al. (2020). Interactive Effects of Wide-Spectrum Monochromatic Lights on Phytochemical Production, Antioxidant and Biological Activities of Solanum Xanthocarpum Callus Cultures. Molecules 25 (9), 2201. doi:10.3390/molecules25092201
Uto, T., Ohta, T., Yamashita, A., Fujii, S., and Shoyama, Y. (2019). Liquiritin and Liquiritigenin Induce Melanogenesis via Enhancement of P38 and PKA Signaling Pathways. Medicines 6 (2), 68. doi:10.3390/medicines6020068
Wang, G., Hiramoto, K., Ma, N., Yoshikawa, N., Ohnishi, S., Murata, M., et al. (2021a). Glycyrrhizin Attenuates Carcinogenesis by Inhibiting the Inflammatory Response in a Murine Model of Colorectal Cancer. Int. J. Mol. Sci. 22 (5), 2609. doi:10.3390/ijms22052609
Wang, H. X., Zeng, M. S., Ye, Y., Liu, J. Y., and Xu, P. P. (2021b). Antiviral Activity of Puerarin as Potent Inhibitor of Influenza Virus Neuraminidase. Phytotherapy Res. 35 (1), 324–336. doi:10.1002/ptr.6803
Wang, J., Wang, S., Sun, S., Lu, Y., Gao, K., Guo, C., et al. (2020a). Apigenin-7-O-β-D-(-6"-p-coumaroyl)-glucopyranoside Treatment Elicits a Neuroprotective Effect through GSK-3β Phosphorylation-Mediated Nrf2 Activation. Aging 12 (23), 23872–23888. doi:10.18632/aging.104050
Wang, J. Y., Chen, H., Wang, Y. Y., Wang, X. Q., Chen, H. Y., Zhang, M., et al. (2017). Network Pharmacological Mechanisms of Vernonia Anthelmintica (L.) in the Treatment of Vitiligo: Isorhamnetin Induction of Melanogenesis via Up-Regulation of Melanin-Biosynthetic Genes. BMC Syst. Biol. 11 (1), 103. doi:10.1186/s12918-017-0486-1
Wang, L., Yang, H., Wang, C., Shi, X., and Li, K. (2019a). Rosmarinic Acid Inhibits Proliferation and Invasion of Hepatocellular Carcinoma Cells SMMC 7721 via PI3K/AKT/mTOR Signal Pathway. Biomed. Pharmacother. 120, 109443. doi:10.1016/j.biopha.2019.109443
Wang, X., Yu, J.-y., Sun, Y., Wang, H., Shan, H., and Wang, S. (2021c). Baicalin Protects LPS-Induced Blood-Brain Barrier Damage and Activates Nrf2-Mediated Antioxidant Stress Pathway. Int. Immunopharmacology 96, 107725. doi:10.1016/j.intimp.2021.107725
Wang, Y.-F., Liu, Y.-N., Xiong, W., Yan, D.-M., Zhu, Y., Gao, X.-M., et al. (2014). A UPLC-MS/MS Method for In Vivo and In Vitro Pharmacokinetic Studies of Psoralenoside, Isopsoralenoside, Psoralen and Isopsoralen from Psoralea Corylifolia Extract. J. Ethnopharmacology 151 (1), 609–617. doi:10.1016/j.jep.2013.11.013
Wang, Y.-s., Cho, J.-G., Hwang, E.-S., Yang, J.-E., Gao, W., Fang, M.-z., et al. (2018). Enhancement of Protective Effects of Radix Scutellariae on UVB-Induced Photo Damage in Human HaCaT Keratinocytes. Appl. Biochem. Biotechnol. 184 (4), 1073–1093. doi:10.1007/s12010-017-2611-4
Wang, Y., Li, S., and Li, C. (2019b). Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity. Med. Sci. Monit. 25, 1017–1023. doi:10.12659/msm.914898
Wang, Y., Liu, H., Zheng, M., Yang, Y., Ren, H., Kong, Y., et al. (2021d). Berberine Slows the Progression of Prediabetes to Diabetes in Zucker Diabetic Fatty Rats by Enhancing Intestinal Secretion of Glucagon-like Peptide-2 and Improving the Gut Microbiota. Front. Endocrinol. (Lausanne) 12, 609134. doi:10.3389/fendo.2021.609134
Wang, Y., Quan, F., Cao, Q., Lin, Y., Yue, C., Bi, R., et al. (2021e). Quercetin Alleviates Acute Kidney Injury by Inhibiting Ferroptosis. J. Adv. Res. 28, 231–243. doi:10.1016/j.jare.2020.07.007
Wang, Z.-K., Chen, R.-R., Li, J.-H., Chen, J.-Y., Li, W., Niu, X.-L., et al. (2020b). Puerarin Protects against Myocardial Ischemia/reperfusion Injury by Inhibiting Inflammation and the NLRP3 Inflammasome: The Role of the SIRT1/NF-Κb Pathway. Int. Immunopharmacology 89 (Pt B), 107086. doi:10.1016/j.intimp.2020.107086
Wen-Jun, L., Hai-Yan, W., Wei, L., Ke-Yu, W., and Rui-Ming, W. (2008). Evidence that Geniposide Abrogates Norepinephrine-Induced Hypopigmentation by the Activation of GLP-1r-dependent C-Kit Receptor Signaling in Melanocyte. J. Ethnopharmacology 118 (1), 154–158. doi:10.1016/j.jep.2008.03.021
Weng, L., Guo, X., Li, Y., Yang, X., and Han, Y. (2016). Apigenin Reverses Depression-like Behavior Induced by Chronic Corticosterone Treatment in Mice. Eur. J. Pharmacol. 774, 50–54. doi:10.1016/j.ejphar.2016.01.015
Whitton, M. E., Pinart, M., Batchelor, J., Leonardi-Bee, J., González, U., Jiyad, Z., et al. (2015). Interventions for Vitiligo. Cochrane Database Syst. Rev., CD003263. doi:10.1002/14651858.cd003263.pub5
Whitton, M., Pinart, M., Batchelor, J. M., Leonardi-Bee, J., Gonzalez, U., Jiyad, Z., et al. (2016). Evidence-based Management of Vitiligo: Summary of a Cochrane Systematic Review. Br. J. Dermatol. 174 (5), 962–969. doi:10.1111/bjd.14356
Wu, J., Zhou, M., Wan, Y., and Xu, A. (2013). CD8+ T Cells from Vitiligo Perilesional Margins Induce Autologous Melanocyte Apoptosis. Mol. Med. Rep. 7 (1), 237–241. doi:10.3892/mmr.2012.1117
Wu, S., Hu, Y., Bai, W., Zhao, J., Huang, C., Wen, C., et al. (2019). Cyanidin‐3‐o‐glucoside Inhibits UVA‐induced Human Dermal Fibroblast Injury by Upregulating Autophagy. Photodermatol. Photoimmunol Photomed. 35 (5), 360–368. doi:10.1111/phpp.12493
Wu, T. Y., Lin, J. N., Luo, Z. Y., Hsu, C. J., Wang, J. S., and Wu, H. P. (2020). 2,3,4',5-Tetrahydroxystilbene-2-O-beta-D-Glucoside (THSG) Activates the Nrf2 Antioxidant Pathway and Attenuates Oxidative Stress-Induced Cell Death in Mouse Cochlear UB/OC-2 Cells. Biomolecules 10 (3), 465. doi:10.3390/biom10030465
Xiao, H. B., Cao, X., Wang, L., Run, X. Q., Su, Y., Tian, C., et al. (2011). 1,5-dicaffeoylquinic Acid Protects Primary Neurons from Amyloid β 1-42-induced Apoptosis via PI3K/Akt Signaling Pathway. Chin. Med. J. (Engl) 124 (17), 2628–2635. doi:10.3760/cma.j.issn.0366-6999.2011.17.012
Xiao, L., Zhong, M., Huang, Y., Zhu, J., Tang, W., Li, D., et al. (2020). Puerarin Alleviates Osteoporosis in the Ovariectomy-Induced Mice by Suppressing Osteoclastogenesis via Inhibition of TRAF6/ROS-dependent MAPK/NF-κB Signaling Pathways. Aging 12 (21), 21706–21729. doi:10.18632/aging.103976
Xie, H., Zhou, F., Liu, L., Zhu, G., Li, Q., Li, C., et al. (2016). Vitiligo: How Do Oxidative Stress-Induced Autoantigens Trigger Autoimmunity?. J. Dermatol. Sci. 81 (1), 3–9. doi:10.1016/j.jdermsci.2015.09.003
Xin, L., Gao, J., Lin, H., Qu, Y., Shang, C., Wang, Y., et al. (2020). Regulatory Mechanisms of Baicalin in Cardiovascular Diseases: A Review. Front. Pharmacol. 11, 583200. doi:10.3389/fphar.2020.583200
Xu, D.-X., Guo, X.-X., Zeng, Z., Wang, Y., and Qiu, J. (2021). Puerarin Improves Hepatic Glucose and Lipid Homeostasis In Vitro and In Vivo by Regulating the AMPK Pathway. Food Funct. 12 (6), 2726–2740. doi:10.1039/d0fo02761h
Xu, P., Su, S., Tan, C., Lai, R.-S., and Min, Z.-S. (2017). Effects of Aqueous Extracts of Ecliptae Herba, Polygoni Multiflori Radix Praeparata and Rehmanniae Radix Praeparata on Melanogenesis and the Migration of Human Melanocytes. J. Ethnopharmacology 195, 89–95. doi:10.1016/j.jep.2016.11.045
Yadav, A. K., and Jang, B. C. (2020). Anti-Survival and Pro-apoptotic Effects of 6-Shogaol on SW872 Human Liposarcoma Cells via Control of the Intrinsic Caspase Pathway, STAT-3, AMPK, and ER Stress. Biomolecules 10 (10). doi:10.3390/biom10101380
Yamada, Y., Obayashi, M., Ishikawa, T., Kiso, Y., Ono, Y., and Yamashita, K. (2008). Dietary Tocotrienol Reduces UVB-Induced Skin Damage and Sesamin Enhances Tocotrienol Effects in Hairless Mice. J. Nutr. Sci. Vitaminol 54 (2), 117–123. doi:10.3177/jnsv.54.117
Yamaguchi, Y., Brenner, M., and Hearing, V. J. (2007). The Regulation of Skin Pigmentation. J. Biol. Chem. 282 (38), 27557–27561. doi:10.1074/jbc.r700026200
Yang, B., Yang, Q., Yang, X., Yan, H.-B., and Lu, Q.-P. (2016). Hyperoside Protects Human Primary Melanocytes against H2O2-Induced Oxidative Damage. Mol. Med. Rep. 13 (6), 4613–4619. doi:10.3892/mmr.2016.5107
Yang, C.-C., Hsiao, L.-D., and Yang, C.-M. (2020a). Galangin Inhibits LPS-Induced MMP-9 Expression via Suppressing Protein Kinase-dependent AP-1 and FoxO1 Activation in Rat Brain Astrocytes. Jir 13, 945–960. doi:10.2147/jir.s276925
Yang, C. C., Lin, C. C., Hsiao, L. D., and Yang, C. M. (2018). Galangin Inhibits Thrombin-Induced MMP-9 Expression in SK-N-SH Cells via Protein Kinase-dependent NF-Κb Phosphorylation. Int. J. Mol. Sci. 19 (12), 4084. doi:10.3390/ijms19124084
Yang, L., Yang, F., Teng, L., and Katayama, I. (2020b). 6-Shogaol Protects Human Melanocytes against Oxidative Stress through Activation of the Nrf2-Antioxidant Response Element Signaling Pathway. Int. J. Mol. Sci. 21 (10), 3537. doi:10.3390/ijms21103537
Yang, X.-P., Liu, T.-Y., Qin, X.-Y., and Yu, L.-C. (2014). Potential protection of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-Glucoside against Staurosporine-Induced Toxicity on Cultured Rat hippocampus Neurons. Neurosci. Lett. 576, 79–83. doi:10.1016/j.neulet.2014.05.045
Yarla, N. S., Bishayee, A., Sethi, G., Reddanna, P., Kalle, A. M., Dhananjaya, B. L., et al. (2016). Targeting Arachidonic Acid Pathway by Natural Products for Cancer Prevention and Therapy. Semin. Cancer Biol. 40-41, 48–81. doi:10.1016/j.semcancer.2016.02.001
Yim, S. H., Tabassum, N., Kim, W. H., Cho, H., Lee, J. H., and Batkhuu, G. J. (2017b). Isolation and Characterization of Isofraxidin 7-O-(6'-O-P-Coumaroyl)-β-Glucopyranoside from Artemisia Capillaris Thunberg: A Novel, Nontoxic Hyperpigmentation Agent that Is Effective In Vivo. Evid. Based Complement. Alternat Med. 2017, 1401279. doi:10.1155/2017/1401279
Yim, S. H., Tabassum, N., Kim, W. H., Cho, H., Lee, J. H., Batkhuu, G. J., et al. (2017a). Isolation and Characterization of Isofraxidin 7-O-(6'-O-P-Coumaroyl)-β-Glucopyranoside from Artemisia Capillaris Thunberg: A Novel, Nontoxic Hyperpigmentation Agent that Is Effective In Vivo. Evid. Based Complement. Alternat Med. 2017, 1401279. doi:10.1155/2017/1401279
Yin, L., Pang, G., Niu, C., Habasi, M., Dou, J., and Aisa, H. A. (2018). A Novel Psoralen Derivative-MPFC Enhances Melanogenesis via Activation of P38 MAPK and PKA Signaling Pathways in B16 Cells. Int. J. Mol. Med. 41 (6), 3727–3735. doi:10.3892/ijmm.2018.3529
Yin, L., Niu, C., Liao, L. X., Dou, J., Habasi, M., and Aisa, H. A. (2017). An Isoxazole Chalcone Derivative Enhances Melanogenesis in B16 Melanoma Cells via the Akt/GSK3β/β-Catenin Signaling Pathways. Molecules 22 (12), 2077. doi:10.3390/molecules22122077
Yuan, J., Lu, Y., Wang, H., Feng, Y., Jiang, S., Gao, X. H., et al. (2020). Paeoniflorin Resists H(2)O(2)-Induced Oxidative Stress in Melanocytes by JNK/Nrf2/HO-1 Pathway. Front. Pharmacol. 11, 536. doi:10.3389/fphar.2020.00536
Zabolinejad, N., Maleki, M., Salehi, M., Ashrafi, Z., Molkara, S., and Layegh, P. (2020). Psoralen and Narrowband UVB Combination Provides Higher Efficacy in Treating Vitiligo Compared with Narrowband UVB Alone: A Randomised Clinical Trial. Australas. J. Dermatol. 61 (1), e65–e69. doi:10.1111/ajd.13184
Zang, D., Niu, C., and Aisa, H. A. (2019). Amine Derivatives of Furocoumarin Induce Melanogenesis by Activating Akt/GSK-3β/β-Catenin Signal Pathway. Drug Des. Develop. Ther. 13, 623–632. doi:10.2147/DDDT.S180960
Zhai, Y., Wang, Q., Li, Y., Cui, J., Feng, K., Kong, X., et al. (2018). The Higher Osteoprotective Activity of Psoralidin In Vivo Than Coumestrol Is Attributed by its Presence of an Isopentenyl Group and through Activated PI3K/Akt axis. Biomed. Pharmacother. 102, 1015–1024. doi:10.1016/j.biopha.2018.03.166
Zhang, B., Wang, J., Zhao, G., Lin, M., Lang, Y., Zhang, D., et al. (2020). Apigenin Protects Human Melanocytes against Oxidative Damage by Activation of the Nrf2 Pathway. Cell Stress and Chaperones 25 (2), 277–285. doi:10.1007/s12192-020-01071-7
Zhang, F., Zhang, Y., Yang, T., Ye, Z. Q., Tian, J., Fang, H. R., et al. (2019a). Scopoletin Suppresses Activation of Dendritic Cells and Pathogenesis of Experimental Autoimmune Encephalomyelitis by Inhibiting NF-Κb Signaling. Front. Pharmacol. 10, 863. doi:10.3389/fphar.2019.01037
Zhang, L., Li, D.-c., and Liu, L.-f. (2019b). Paeonol: Pharmacological Effects and Mechanisms of Action. Int. Immunopharmacology 72, 413–421. doi:10.1016/j.intimp.2019.04.033
Zhang, L. (2019). Pharmacokinetics and Drug Delivery Systems for Puerarin, a Bioactive Flavone from Traditional Chinese Medicine. Drug Deliv. 26 (1), 860–869. doi:10.1080/10717544.2019.1660732
Zhang, R., Lee, I. K., Piao, M. J., Kim, K. C., Kim, A. D., Kim, H. S., et al. (2011). Butin (7,3′,4′-Trihydroxydihydroflavone) Reduces Oxidative Stress-Induced Cell Death via Inhibition of the Mitochondria-dependent Apoptotic Pathway. Ijms 12 (6), 3871–3887. doi:10.3390/ijms12063871
Zhang, W., Cheng, C., Sha, Z., Chen, C., Yu, C., Lv, N., et al. (2021). Rosmarinic Acid Prevents Refractory Bacterial Pneumonia through Regulating Keap1/Nrf2-Mediated Autophagic Pathway and Mitochondrial Oxidative Stress. Free Radic. Biol. Med. 168, 247–257. doi:10.1016/j.freeradbiomed.2021.03.038
Zhang, X., Bu, H., Jiang, Y., Sun, G., Jiang, R., Huang, X., et al. (2019c). The Antidepressant Effects of Apigenin Are Associated with the Promotion of Autophagy via the mTOR/AMPK/ULK1 Pathway. Mol. Med. Rep. 20 (3), 2867–2874. doi:10.3892/mmr.2019.10491
Zhang, Y., Ding, Y., Zhong, X., Guo, Q., Wang, H., Gao, J., et al. (2016). Geniposide Acutely Stimulates Insulin Secretion in Pancreatic β-cells by Regulating GLP-1 receptor/cAMP Signaling and Ion Channels. Mol. Cell Endocrinol. 430, 89–96. doi:10.1016/j.mce.2016.04.020
Zhao, C., Zhang, H., Li, H., Lv, C., Liu, X., Li, Z., et al. (2017). Geniposide Ameliorates Cognitive Deficits by Attenuating the Cholinergic Defect and Amyloidosis in Middle-Aged Alzheimer Model Mice. Neuropharmacology 116, 18–29. doi:10.1016/j.neuropharm.2016.12.002
Zhao, L., Li, H., Gao, Q., Xu, J., Zhu, Y., Zhai, M., et al. (2021). Berberine Attenuates Cerebral Ischemia-Reperfusion Injury Induced Neuronal Apoptosis by Down-Regulating the CNPY2 Signaling Pathway. Front. Pharmacol. 12, 609693. doi:10.3389/fphar.2021.609693
Zhao, W.-Z., Wang, H.-T., Huang, H.-J., Lo, Y.-L., and Lin, A. M.-Y. (2018). Neuroprotective Effects of Baicalein on Acrolein-Induced Neurotoxicity in the Nigrostriatal Dopaminergic System of Rat Brain. Mol. Neurobiol. 55 (1), 130–137. doi:10.1007/s12035-017-0725-x
Zhou, J., Chen, F., Yan, A., and Xia, X. (2020a). Madecassoside Protects Retinal Pigment Epithelial Cells against Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis through the Activation of Nrf2/HO-1 Pathway. Biosci. Rep. 40 (10), 4347. doi:10.1042/bsr20194347
Zhou, J., Zhang, S., Sun, X., Lou, Y., Bao, J., and Yu, J. (2021). Hyperoside Ameliorates Diabetic Nephropathy Induced by STZ via Targeting the miR-499-5p/APC axis. J. Pharmacol. Sci. 146 (1), 10–20. doi:10.1016/j.jphs.2021.02.005
Zhou, Y.-X., Gong, X.-H., Zhang, H., and Peng, C. (2020b). A Review on the Pharmacokinetics of Paeoniflorin and its Anti-inflammatory and Immunomodulatory Effects. Biomed. Pharmacother. 130, 110505. doi:10.1016/j.biopha.2020.110505
Zhou, Y. X., Zhang, R. Q., Rahman, K., Cao, Z. X., Zhang, H., and Peng, C. (2019). Diverse Pharmacological Activities and Potential Medicinal Benefits of Geniposide. Evid. Based Complement. Alternat Med. 2019, 4925682. doi:10.1155/2019/4925682
Zhu, X., Zhang, H., and Lo, R. (2004). Phenolic Compounds from the Leaf Extract of Artichoke (Cynara scolymusL.) and Their Antimicrobial Activities. J. Agric. Food Chem. 52 (24), 7272–7278. doi:10.1021/jf0490192
Zhu, Y., Tchkonia, T., Pirtskhalava, T., Gower, A. C., Ding, H., Giorgadze, N., et al. (2015). The Achilles' Heel of Senescent Cells: from Transcriptome to Senolytic Drugs. Aging Cell 14 (4), 644–658. doi:10.1111/acel.12344
Zhu, Y., Wang, S., Lin, F., Li, Q., and Xu, A. (2014). The Therapeutic Effects of EGCG on Vitiligo. Fitoterapia 99, 243–251. doi:10.1016/j.fitote.2014.08.007
Zhu, Y., Wang, S., and Xu, A. (2013). A Mouse Model of Vitiligo Induced by Monobenzone. Exp. Dermatol. 22 (7), 499–501. doi:10.1111/exd.12184
Keywords: vitiligo, oxidative stress, natural product, melanocytes, flavonoids, phenols, glycosides
Citation: Pang Y, Wu S, He Y, Nian Q, Lei J, Yao Y, Guo J and Zeng J (2021) Plant-Derived Compounds as Promising Therapeutics for Vitiligo. Front. Pharmacol. 12:685116. doi: 10.3389/fphar.2021.685116
Received: 24 March 2021; Accepted: 13 July 2021;
Published: 11 November 2021.
Edited by:
Gokhan Zengin, Selcuk University, TurkeyReviewed by:
Vuyisile Samuel Thibane, University of South Africa, South AfricaErna Karalija, University of Sarajevo, Bosnia and Herzegovina
Copyright © 2021 Pang, Wu, He, Nian, Lei, Yao, Guo and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Guo, Z3VvamluZzY2QGNkdXRjbS5lZHUuY24=; Jinhao Zeng, emVuZ2ppbmhhb0BjZHV0Y20uZWR1LmNu
 Yaobin Pang
Yaobin Pang Shi Wu
Shi Wu Yingjie He1
Yingjie He1 Yejing Yao
Yejing Yao Jing Guo
Jing Guo