
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 10 June 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.678631
Objectives: Chinese herb medicine (CHM) is one of the most popular complementary and alternative therapies, which has been widely used to treat Refractory Mycoplasma Pneumoniae Pneumonia (RMPP). However, the effect and safety of CHM remain controversial. Hence, we conducted this meta-analysis to evaluate whether CHM combination therapy could bring benefits to children and adolescents with RMPP.
Methods: Seven databases were used for data searching through November 11, 2020 following the PRISMA checklist generally. Review Manager 5.3, Trial sequential analysis 0.9.5.10 Beta software and Stata16.0 were applied to perform data analyses. Mean difference or risk ratio was adopted to express the results, where a 95% confidence interval (CI) was applied.
Results: In general, this research enrolled 17 trials with 1,451 participants. The overall pooled results indicated that CHM was beneficial for children and adolescents with RMPP by improving the clinical efficacy rate [RR = 1.20, 95% CI (1.15, 1.25), p < 0.00001], shortening antipyretic time [MD = −2.60, 95% CI (−3.06, −2.13), p < 0.00001], cough disappearance time [MD = −2.77, 95% CI (−3.12, −2.42), p < 0.00001], lung rale disappearance time [MD = −2.65, 95% CI (−3.15, −2.15), p < 0.00001], lung X-ray infiltrates disappearance time [MD = −2.75, 95% CI (−3.33, −2.17), p < 0.00001], reducing TNF-α level [MD = −5.49, 95% CI (−7.21, −3.77), p < 0.00001]. Moreover, subgroup results suggested that removing heat-phlegm and toxicity therapy had more advantages in shortening antipyretic time, cough disappearance time, lung X-ray infiltrates disappearance time and reducing TNF-α level. Meanwhile promoting blood circulation therapy seemed to be better at relieving lung rale. However, regarding adverse events, the two groups displayed no statistical difference [RR = 0.97, 95% CI (0.60, 1.57), p = 0.91].
Conclusion: Despite of the apparently positive results in relieving clinical symptoms, physical signs and reducing inflammation, it is premature to confirm the efficacy of CHM in treating RMPP because of the limitation of quality and the number of the included studies. More large-scale, double-blind, well-designed, randomized controlled trials are needed in future research.
As a significant pathogen, Mycoplasma pneumoniae (M. pneumoniae) corresponds to 10–40% of community-acquired pneumonia (CAP) in children and adolescence (Waites et al., 2017; Gao et al., 2019; Kurkela et al., 2019). Most children and adolescents have mild symptoms and M. pneumoniae infection is perceived to be self-limited. However, after being treated by macrolide antibiotics for no less than 7 days, some children and adolescents still show severe symptoms and/or progressively worsening radiological findings, which can be diagnosed as refractory M. pneumoniae pneumonia (RMPP) (Chen et al., 2015). Compared to common M. pneumoniae pneumonia (MPP), RMPP commonly presented with persistent or repeated high fever, severe cough, multiple complications involving atelectasis, pleural effusion, myocarditis, hemolytic anemia, encephalitis, and even multiple organ dysfunction, damaging the health of children and adolescents (Zhai et al., 2020). In recent years, the incidence of RMPP has gradually increased, especially in Asia (Guo et al., 2020; Lee et al., 2020), which is becoming a critical issue for pediatricians.
The pathogenesis of RMPP is multifactorial, including macrolide resistance (Zhao et al., 2020), mixed infection (Zhou et al., 2020), excessive inflammatory reaction (Li et al., 2019a), immunity dysfunction (Yu et al., 2017), and blood hypercoagulability (Huang et al., 2021). The first line antibiotics used for RMPP are macrolides. However, high presence of macrolide resistance has been reported in RMPP (Zhang et al., 2018; Zhao et al., 2020). Although tetracyclines and fluoroquinolones are second-line antibiotics, side effects limit their use in children (Tsai et al., 2020). Corticosteroids can reduce inflammatory responses and have been confirmed to be an effective treatment for RMPP (Kim et al., 2019), but its resistance has been reported in some RMPP cases with more severe presentations (Yan et al., 2016). Intravenous immunoglobulin (IVIG) is another therapy, but the uniform standard for the starting time, dose, and duration has not been established (Yang et al., 2017). Hence, the exploration of an effective and safe pharmacological strategy for children and adolescents with RMPP is highly significant.
Chinese herb medicine (CHM) is a prevailing alternative and complementary therapy (Wang, 2020), and previous reviews have demonstrated that CHM displayed obvious advantages in MPP (Li et al., 2019c; Zhang et al., 2020). In our study, we aimed to focus on the role of CHM in RMPP, as the condition is more critical and treatment is more difficult. Traditional Chinese Medicine (TCM) theory classifies RMPP into the category of “pneumonia with dyspneic cough”. The key pathogenesis of RMPP is asthenia in origin and excess in superficiality. Asthenia in origin include Qi deficiency and Yin deficiency. Excess in superficiality include phlegm heat, pathogenic toxin, and blood stasis. Based on the theory above, classic TCM therapies involves removing heat-phlegm and toxicity, promoting blood circulation, invigorating Qi and consolidating superficies, supplementing Qi and nourishing Yin (Liu and Ma, 2017; Yan et al., 2019). As we all know, CHM has the characteristics of multiple components, targets and pathways, including inhibiting M. pneumoniae, modulating immunity, repairing damaged epithelial cells, improving microcirculation, and alleviating adverse effects of Western medicine (WM) (Tan and Jiang, 2018). For instance, Yupingfeng granule, a kind of Chinese medicine prescription, could reduce inflammation and regulate immune function to benefit RMPP children and adolescents (Wang et al., 2018). Zhou reported the therapy of promoting blood circulation could ameliorate symptoms of children and adolescents with RMPP by anti-inflammatory, improving lung microcirculation and promoting tissue repair (Zhou, 2018). Therefore, we conducted this meta-analysis with the aim to evaluate the effect and safety of CHM and compare the clinical efficacy of different TCM therapies in RMPP.
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher et al., 2015) were followed to perform this systematic review, which was registered in PROSPERO (CRD42020218609). A completed PRISMA checklist was exhibited in Supplementary File S1.
Seven databases, such as PubMed, embase, Cochrane Library, China National Knowledge Infrastructure database (CNKI), VIP database for Chinese Technical Periodicals (VIP), Wanfang Data and China Biomedical Medicine database (CBM), were searched from inception to November 11, 2020. Search terms were used as follow: “refractory mycoplasma pneumoniae pneumonia”, “RMPP”, “traditional Chinese medicine”, “children”, “adolescents”, “randomized controlled trial” etc. Details of all databases of search strategies were shown in Supplementary Table S1. Reference lists and conference proceedings were also checked manually for additional relevant data. We contacted relevant corresponding authors for missing or unreported information when needed. No restrictions were applied on language or publication date.
Studies meeting the following conditions were included for further analysis: 1) Study design: prospective, randomized controlled trials (RCTs); 2) Participants: all the enrolled children and adolescents within 18 years old were required to meet any proper diagnostic criteria of RMPP, such as “Expert consensus on the diagnosis and treatment of Mycoplasma pneumoniae pneumonia in children” (Chen et al., 2015); 3) Interventions and Comparisons: patients receiving CHM (no restriction on dosage, formula, and form) combined with WM in intervention groups, while WM alone as the control; 4) Outcomes: at least one primary outcome of interest with reliable and available data.
Cases meeting the following conditions were excluded: 1) case-control or cohort trials, quasi-randomized studies; 2) cell or animal experiments, case reports, meta-analyses, reviews; 3) studies with poor design, unavailable data, or unreported target outcomes; 4) children and adolescents who received other joint interventions such as acupuncture, cupping, moxibustion, massage, acupoint application.
The primary outcomes were clinical efficacy rate, antipyretic time, and cough disappearance time. The secondary outcomes included the disappearance time of lung rale and lung X-ray infiltrates, inflammation index and adverse effects.
From each study, the collected data are: 1) study characteristics, including publication year, location, as well as the name of the first author; 2) participants information, such as age, gender, sample size, course of disease, and diagnosis standards; 3) details of interventions, including treatment protocols, TCM therapies, and duration; 4) outcome measures.
The Cochrane risk of bias tool (Higgins et al., 2011) was adopted to the evaluation of the selected studies’ methodological quality based on considerations below: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The bias risk assessment was independently conducted by two investigators with three potential responses: low, high and unclear. A third author was also invited to resolve any conflict.
Review Manager 5.3 was applied for statistical analysis. The risk ratio (RR) with 95% confidence intervals (CI) was used to express the results of dichotomous data. Mean difference (MD) or standardized mean difference (SMD) with a 95% CI was adopted for continuous data. Heterogeneous results could not be avoided due to the use of different herbal medicines in all selected studies. For this reason, summary effect measures were calculated by the random-effects method. Statistical heterogeneity was quantified through the I-squared (I2) statistic, whereby an I2 > 50% was deemed substantial heterogeneity (Higgins et al., 2003). Subgroups analyses were performed and categorized according to types of WM and different TCM therapies described in each study, respectively, which could not only reduce heterogeneity to some extent but also compare the clinical efficacy of different TCM therapies in RMPP. The differences at a statistically significant level were defined by p < 0.05. Publication bias was assessed through a funnel plot and calculated by Egger’s tests, when at least ten studies were available. Sensitivity analyses were conducted by changing the random-effects method to a fixed-effect model and deleting each study in sequence to evaluate the stability of the results and explore the sources of heterogeneity.
Some positive findings from meta-analysis might be caused by a random error (by chance) rather than the true effects of the intervention and these results might often increase the likelihood of overestimation (Type I errors) or underestimation (Type II errors) (Pereira and Ioannidis, 2011; Afshari et al., 2017), which could be resolved by Trial sequential analysis (TSA) (Kang, 2021). Therefore, we performed TSA by using TSA 0.9.5.10 Beta software to reduce the false-positive results caused by random errors and determine whether more RCTs were needed to obtain robust conclusions. The error rate was set as 5% for type I and 10% for type II.
A total of 425 articles were retrieved with the predefined search strategy. 299 studies remained after we removed duplicates. By screening titles and abstracts, 244 articles were excluded, and 55 articles were yielded to conduct a full-text evaluation. Among them, 38 articles were excluded, which included non-RCTs (n = 13); trials with inappropriate interventions (n = 13); trials in which outcomes did not meet the inclusion criteria (n = 8); studies lack of adequate drug information (n = 2); articles without access to full text (n = 2). Finally, 17 RCTs (Qian, 2011; Chen, 2014; Meng, 2015; Tian, 2016; Wang and Wu, 2016; Yuan et al., 2016; Lian, 2017; Sun et al., 2017; Tan and Yang, 2017; Zhong et al., 2017; Wang et al., 2018; Wang, 2018; Yang, 2019; He et al., 2020a; He et al., 2020b; Zhang, 2020a; Zhang, 2020b) were eligible for meta-analysis (Figure 1). A list of excluded studies by reading full-text was provided in Supplementary File S2.
All studies were single-center and parallel-design RCTs conducted in China between 2009 and 2019. A total of 1,451 participants were recruited, with 726 in intervention groups and 725 in control groups. WM in our analysis could be divided into two categories: nine studies (Chen, 2014; Meng, 2015; Tian, 2016; Yuan et al., 2016; Sun et al., 2017; Zhong et al., 2017; Wang et al., 2018; He et al., 2020a; Zhang, 2020b) using macrolide antibiotics, and the other eight (Qian, 2011; Wang and Wu, 2016; Lian, 2017; Tan and Yang, 2017; Wang, 2018; Yang, 2019; He et al., 2020b; Zhang, 2020a) using macrolide antibiotics joint with corticosteroids. Ten studies (Qian, 2011; Chen, 2014; Tian, 2016; Yuan et al., 2016; Lian, 2017; Wang, 2018; Yang, 2019; He et al., 2020a; He et al., 2020b; Zhang, 2020a) administrated CHM to participants in decoction form, three (Wang and Wu, 2016; Sun et al., 2017; Wang et al., 2018) in granule, three (Meng, 2015; Tan and Yang, 2017; Zhang, 2020b) in injection and one (Zhong et al., 2017) in oral liquid. TCM therapies were described in all studies, nine (Qian, 2011; Yuan et al., 2016; Lian, 2017; Sun et al., 2017; Tan and Yang, 2017; Zhong et al., 2017; He et al., 2020a; He et al., 2020b; Zhang, 2020b) with removing heat-phlegm and toxicity therapy, four (Chen, 2014; Tian, 2016; Wang, 2018; Zhang, 2020a) with promoting blood circulation therapy and the rest (Wang and Wu, 2016; Sun et al., 2017; Wang et al., 2018; Yang, 2019) with invigorating Qi and consolidating superficies therapy. The treatment duration ranged from one week to four weeks. Table 1 listed the characteristics of selected studies. Supplementary Tables S3, S4 provided detailed information on CHM.
The results of methodological bias were presented in Figure 2. All included studies mentioned the randomized allocation. Ten clearly described the randomization method, including random number table (Yuan et al., 2016; Lian, 2017; Sun et al., 2017; Tan and Yang, 2017; Zhong et al., 2017; Wang et al., 2018; Yang, 2019; He et al., 2020a) or computer-generated numbers (Tian, 2016) or ballot (He et al., 2020b), which were considered as the low risk, while seven (Qian, 2011; Chen, 2014; Meng, 2015; Wang and Wu, 2016; Wang, 2018; Zhang, 2020a; Zhang, 2020b) did not provide the detailed randomization methodology. Allocation concealment was unclear and none of them were double-blinded. It was easy for participants to identify which group they were in, since the packages of CHM and WM were different. All articles had complete data and consistent outcomes as described in method section. And other biases were unclear.
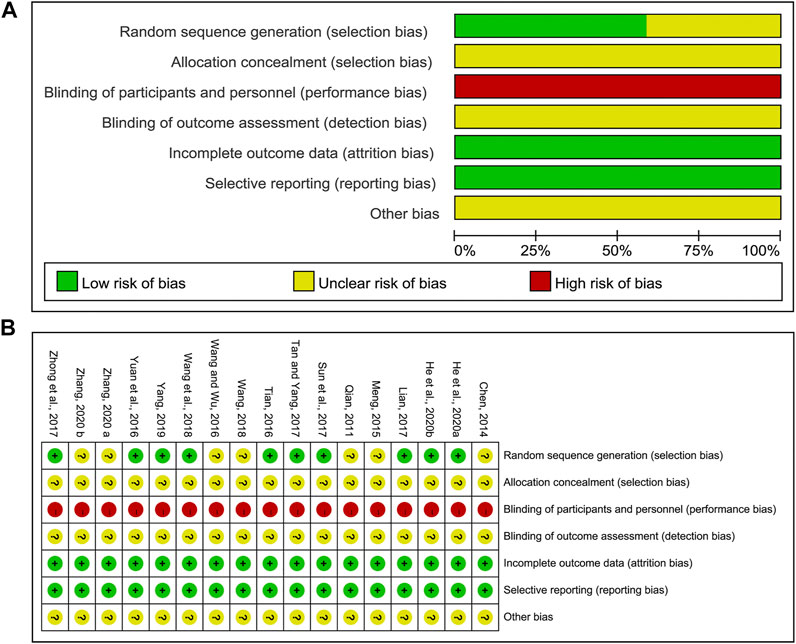
FIGURE 2. Risk of methodological bias of the included studies. (A) Risk of bias graph. (B) Risk of bias summary.
Fourteen studies (1,209 patients) (Qian, 2011; Chen, 2014; Meng, 2015; Tian, 2016; Yuan et al., 2016; Lian, 2017; Tan and Yang, 2017; Zhong et al., 2017; Wang, 2018; Yang, 2019; He et al., 2020a; He et al., 2020b; Zhang, 2020a; Zhang, 2020b) reported clinical efficacy rate. The pooled data indicated that combination of CHM and WM improved clinical efficacy significantly [RR = 1.20, 95% CI (1.15, 1.25), p < 0.00001; I2 = 0%] (Figure 3A). Subgroup analyses (Table 2) showed there was no difference in this result when classified by the type of WM. In terms of TCM differentiation, three therapies obtained the similar clinical efficacy rate.
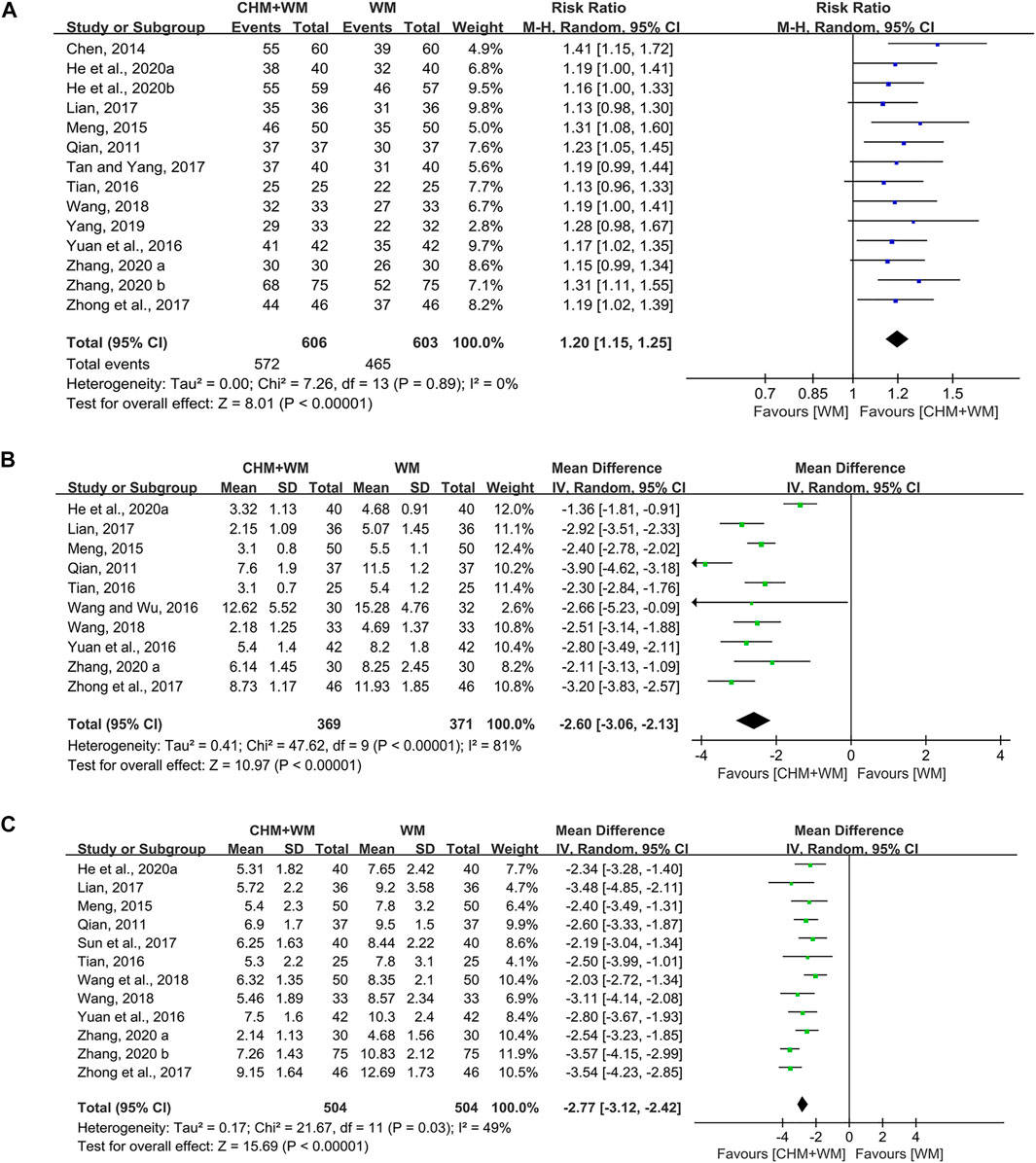
FIGURE 3. Forest plots of primary outcomes. (A) Clinical efficacy rate. (B) Antipyretic time. (C) Cough disappearance time.
Ten studies (740 patients) (Qian, 2011; Meng, 2015; Tian, 2016; Wang and Wu, 2016; Yuan et al., 2016; Lian, 2017; Zhong et al., 2017; Wang, 2018; He et al., 2020a; Zhang, 2020a) mentioned antipyretic time. Overall analyses revealed that CHM combinated with WM showed more advantages in shortening antipyretic time than WM alone [MD = −2.60, 95% CI (−3.06, −2.13), p < 0.00001, I2 = 81%] (Figure 3B). In subgroup analyses (Table 2), we found that this outcome might be associated with TCM therapy, as removing heat-phlegm and toxicity therapy brought more benefits compared with promoting blood circulation therapy and invigorating Qi and consolidating superficies therapy.
Twelve studies (1,008 patients) (Qian, 2011; Meng, 2015; Tian, 2016; Yuan et al., 2016; Lian, 2017; Sun et al., 2017; Zhong et al., 2017; Wang, 2018; Wang et al., 2018; He et al., 2020a; Zhang, 2020a; Zhang, 2020b) were identified. A significant difference was found between groups, since the cough disappearance time in children and adolescents treated with CHM and WM was shorter than those treated with WM alone [MD = −2.77, 95% CI (−3.12, −2.42), p < 0.00001, I2 = 49%] (Figure 3C). Results of subgroup analyses (Table 2) yielded that removing heat-phlegm and toxicity therapy might work faster than the two others.
Ten studies (858 patients) (Qian, 2011; Yuan et al., 2016; Lian, 2017; Sun et al., 2017; Zhong et al., 2017; Wang, 2018; Wang et al., 2018; He et al., 2020a; Zhang, 2020a; Zhang, 2020b) evaluated this outcome measure. Meta-analysis showed that intervention with CHM and WM resulted in a greater decrease than WM alone [MD = −2.65, 95% CI (−3.15, −2.15), p < 0.00001, I2 = 72%] (Figure 4A). Categorized by TCM therapy, the subgroup analyses (Table 2) indicated that promoting blood circulation was the most advantageous in shortening lung rale disappearance time.
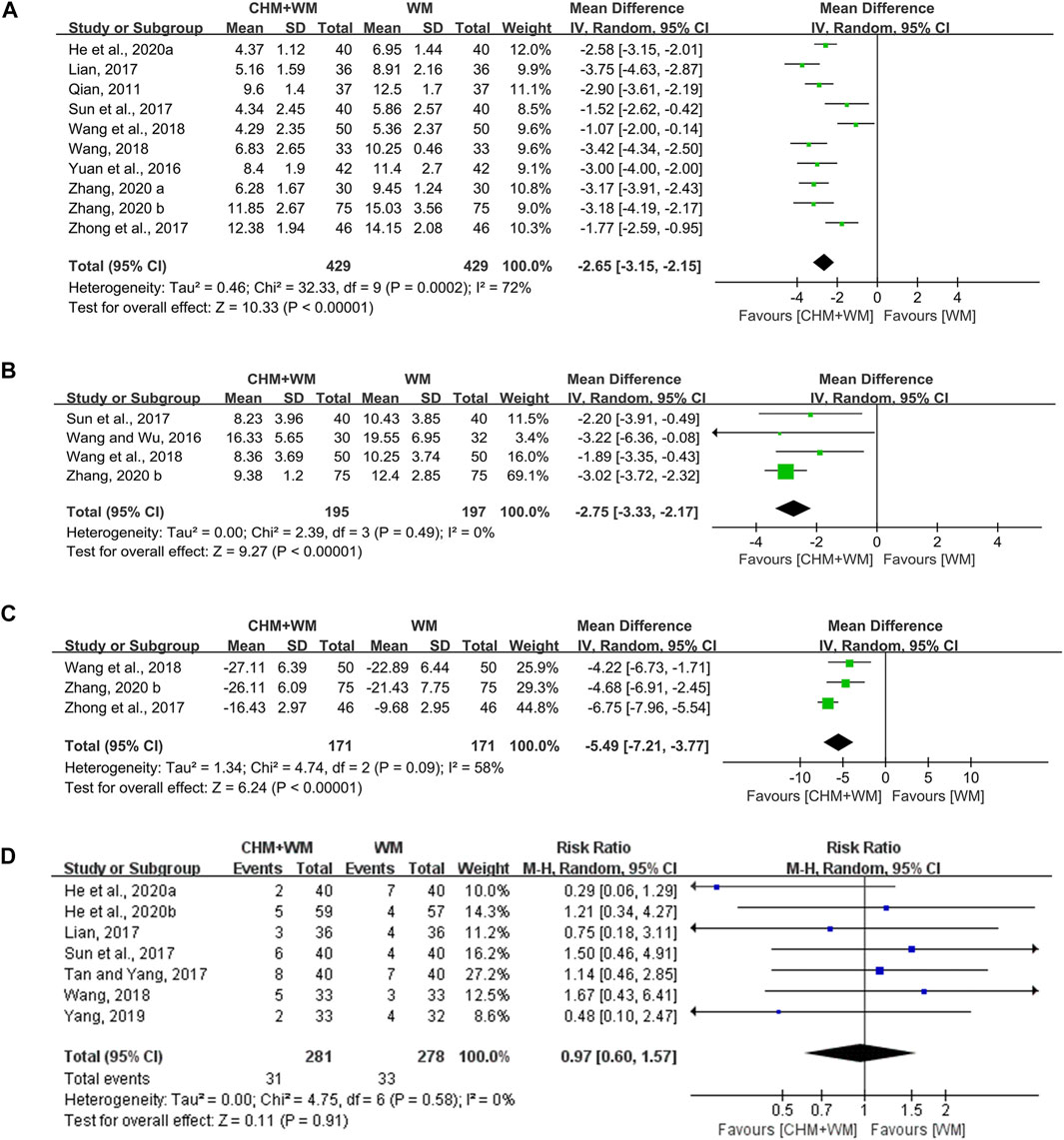
FIGURE 4. Forest plots of secondary outcomes. (A) Lung rale disappearance time. (B) Lung X-ray infiltrates disappearance time. (C) TNF-α. (D) Adverse effects.
The lung X-ray infiltrates disappearance time was assessed in four studies (392 patients) (Wang and Wu, 2016; Sun et al., 2017; Wang et al., 2018; Zhang, 2020b). Statistically significant differences between the groups were noted, which demonstrated that CHM combined with WM was superior [MD = −2.75, 95% CI (−3.33, −2.17), p < 0.00001, I2 = 0%] (Figure 4B). In subgroup analysis (Table 2), TCM therapy of removing heat-phlegm and toxicity was more beneficial than invigorating Qi and consolidating superficies.
Three studies (342 patients) (Zhong et al., 2017; Wang et al., 2018; Zhang, 2020b) mentioned the changes in tumor necrosis factor-α (TNF-α). The analysis result exhibited significant difference between two groups [MD = −5.49, 95% CI (−7.21, −3.77), p < 0.00001, I2 = 58%] (Figure 4C), making it clear that CHM combined with WM was remarkably better than WM alone in reducing inflammation. In addition, the level of TNF-α seemed to be lower in the TCM therapy of removing heat-phlegm and toxicity than invigorating Qi and consolidating superficies (Table 2).
One study (Wang et al., 2018) reported no adverse reactions during treatment. One (Chen, 2014) reported minor adverse reactions in two groups, but the number of cases was not shown. Seven studies (Lian, 2017; Sun et al., 2017; Tan and Yang, 2017; Wang, 2018; Yang, 2019; He et al., 2020a; He et al., 2020b) reported the number of adverse events and details were shown in Supplementary Table S2. All reactions were at a mild level, posing no influence on the therapy. No difference was represented between two groups [RR = 0.97, 95% CI (0.60, 1.57), p = 0.91; I2 = 0%] (Figure 4D). The adverse reactions such as nausea, vomit, diarrhea, stomach ache, and rash were found in both groups, indicating those symptoms might be labeled in the direction of macrolide antibiotics and corticosteroids.
Meta-regression analysis was attempted to explain the heterogeneity in antipyretic time and lung rale disappearance time. Neither type of WM (regression coefficient β = −0.677, SE = 0.487, p = 0.207) nor TCM therapy (regression coefficient β = 0.498, SE = 0.457, p = 0.312) had an association with antipyretic time. In terms of lung rale disappearance time, the type of WM (regression coefficient β = −1.027, SE = 0.341, p = 0.02) was more relevant than TCM therapy (regression coefficient β = 0.528, SE = 0.225, p = 0.051), indicating type of WM might be the source of heterogeneity.
Funnel plots and Egger’s test were performed for the assessment of publication bias. The funnel plot (Figure 5A) revealed a slight asymmetry and Egger’s test (t = 3.05, p = 0.01) indicated possible publication bias in clinical efficacy rate. To further confirm whether the bias had an impact on clinical efficacy rate, trim and fill method was performed. One study was added and the corrected RR was 1.493 [95% CI (1.349, 1.642)], remaining significant. Visual assessment of funnel plots (Figures 5B–D) and Egger’s test showed no publication bias for antipyretic time (t = −1.02, p = 0.338), cough disappearance time (t = 0.71, p = 0.495) and lung rale disappearance time (t = 0.30, p = 0.771).
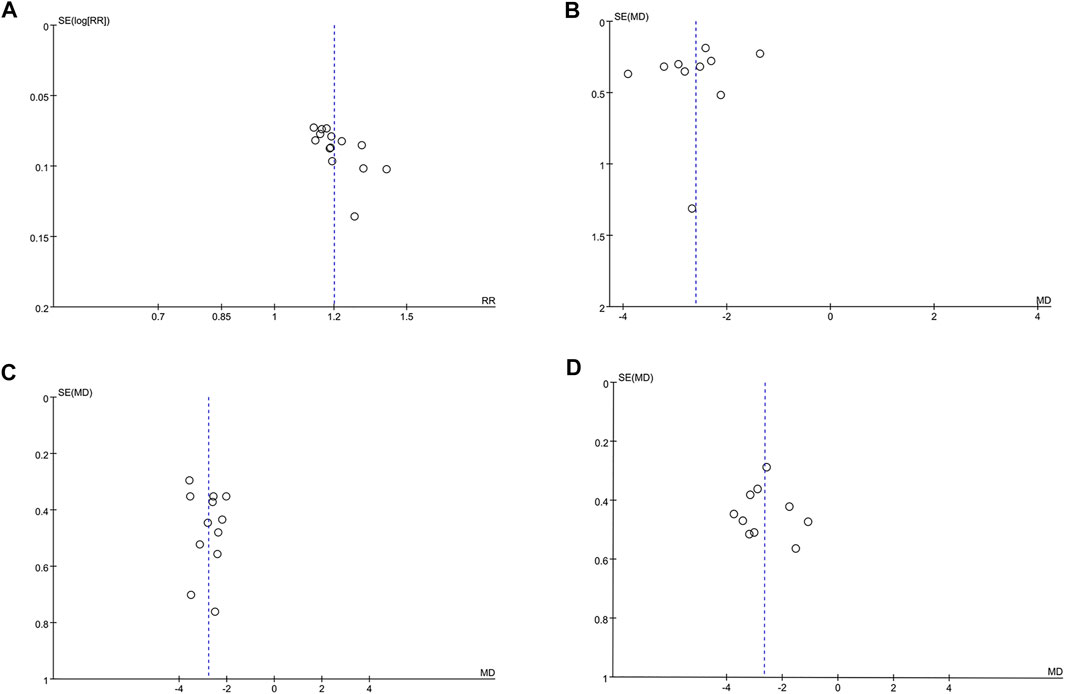
FIGURE 5. The funnel plots for assessing publication bias. (A) Clinical efficacy rate. (B) Antipyretic time. (C) Cough disappearance time. (D) Lung rale disappearance time.
All results showed good consistency whether in the fixed-effects or random-effects model. The overall results did not alter after deleting one study each time. For further verification, we implemented sensitivity analyses of clinical efficacy rate, antipyretic time, cough disappearance time and lung rale disappearance time by STATA 16.0. Supplementary Figure S1 indicated that the outcomes were not reversed by removing any study, which had relatively good stability.
When we deleted one study each time and reanalyzed the rest, we found the heterogeneity still existed except for TNF-α results of Zhong et al. (2017). The heterogeneity dropped from 58 to 0% after this study was excluded. Therefore, this RCT may be a source of considerable heterogeneity, in which azithromycin with intravenous injection was applied while trials of Wang et al. (2018) and Zhang (2020b) exerted azithromycin orally.
The required information size (RIS), defined as the number of events or patients from the included studies necessary to accept or reject the statistical hypothesis (Wetterslev et al., 2009), is important to increase the quality of meta-analysis. Thus, We applied TSA boundaries to calculate the RIS and assess the robustness of our results. Relative risk reduction (RRR) was derived from the meta-analysis. In Figure 6, the Z-score curve (blue line) is found to cross the RIS boundary (vertical red line), TSA boundary (red polylines) and conventional boundary (dotted black lines), which indicated that the conclusion on clinical efficacy rate was robust with the existing evidence.
The main findings of our meta-analysis showed that the combination of CHM and WM could more significantly improve the clinical efficacy rate, relieve clinical symptoms (such as antipyretic time, cough disappearance time, lung rale disappearance time, lung X-ray infiltrates disappearance time) and decrease TNF-α level. Additionally, compared with WM, CHM therapies brought no increase in adverse reactions.
Since different regimens might result in the high heterogeneity. We performed subgroup analysis based on types of WM and different TCM therapies to obtain more reliable conclusion. The combination of CHM and WM showed obvious superiority whether compared with macrolide antibiotics or macrolide antibiotics and corticosteroids in the subgroup analysis. We also found that different TCM therapies had the similar clinical efficacy rate, but they targeted at different symptoms, which provided foundation for the application of CHM in RMPP.
In the process of transforming MPP into RMPP, the monocytes and macrophages secreted large amounts of TNF-α, triggering a series of inflammatory reactions, immune disorders, thereby causing damage to organs. Some studies have demonstrated that TNF-α played an important role in the development of RMPP, which could be used as reference index for the prognosis judgment (Ding et al., 2018; Fan et al., 2019; Li et al., 2019a). As shown in our review, removing heat-phlegm and toxicity therapy had more advantages in shortening antipyretic time, cough disappearance time, lung X-ray infiltrates disappearance time and reducing TNF-α level. This might be due to anti-inflammation and anti-pathogen of Chinese herbs in the included RCTs, such as Scutellaria baicalensis Georgi., Ephedra sinica Stapf, Prunus armeniaca L., and Glycyrrhiza uralensis Fisch (Figure 7A). In TCM theory, they had the effects of clearing away heat and toxin, resolving phlegm and cough, indicating they could relieve post-infectious symptoms caused by M. pneumoniae. From the pharmacological point of view, baicalein, baicalin, wogonin, wogonoside and oroxylin A, the main active ingredients of Scutellaria baicalensis Georgi, were reported to inhibit the production of the inflammatory cytokines (such as interleukin-1β, interleukin-6, interleukin-8 and TNF-α), the molecular mechanisms of which included downregulation of toll-like receptors and inhibition of inflammation-associated pathways such as MAPK, Akt, NF-κB, and JAK-STAT (Liao et al., 2021). Meanwhile, baicalin have been proved to inhibit pathogen directly and reduce the expression of M. pneumoniae in a concentration-dependent manner (Meng et al., 2020). Additionally, Glycyrrhizic Acid contained in Glycyrrhiza uralensis Fisch. was thought to reduces cytokine secretion via blocking NF-κB activation and inhibiting the migration and infiltration of neutrophils, thereby attenuating inflammation in an acute lung injury mouse model (Lee et al., 2019).
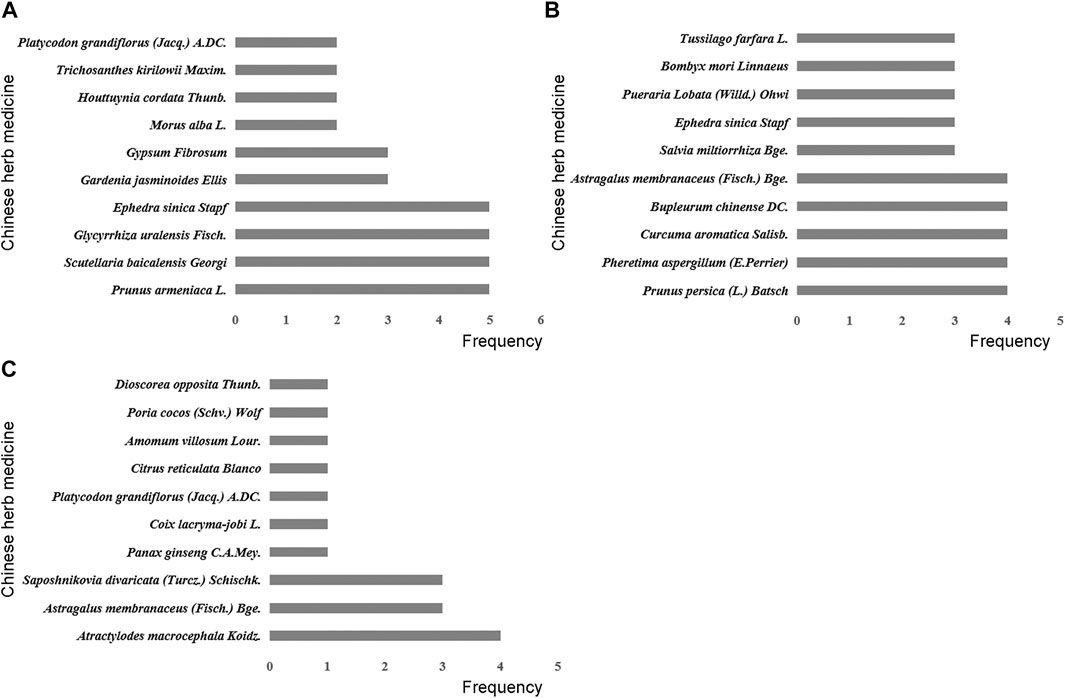
FIGURE 7. Frequency distribution of Chinese herb medicines in different traditional Chinese therapies. (A) Removing heat-phlegm and toxicity therapy. (B) Promoting blood circulation therapy. (C) Invigorating Qi and consolidating superficies therapy.
The correlation of blood hypercoagulation with RMPP has been substantially demonstrated. It is attributed to the possible involvement of severe hypercoagulability and vascular endothelial cell injury, which results from an excessive inflammatory response, in lung injury (Huang et al., 2021). Based on TCM theory, “promoting blood circulation” was an important method for treating RMPP, which seemed to be better at relieving lung rale in our meta-analyses. The more frequently used Chinese herbs in this therapy contained Prunus persica (L.) Batsch and Pheretima aspergillum (E.Perrier) (Figure 7B), which have been documented as potent agents for the treatment of cough for nearly 2000 years. Modern pharmacological studies have found that Prunus persica (L.) Batsch and Pheretima aspergillum (E.Perrier) could ameliorate blood microcirculation through influencing hemorheological changes and platelet aggregation, which was vital for repairing tissue damage and improving respiratory function (Xu et al., 2015; Huang et al., 2018). Besides, an animal study suggested that the inflammatory response could be hindered by amygdalin, an active ingredient from Prunus persica (L.) Batsch, which is achieved through the downregulated expression of TNF-α, interleukin-6, interleukin-1β, and other pro-inflammatory cytokines (Lv et al., 2017).
T-lymphocyte subsets interact with each other and participate in the cellular immunity, so as to maintain the normal immune function of human body. Previous studies have reported T-lymphocyte subsets were closely related to the progression of RMPP, especially CD4+ T cells (Li et al., 2019b; Liu et al., 2020). In TCM, invigorating Qi and consolidating superficies herbs including Atractylodes macrocephala Koidz. and Astragalus membranaceus (Fisch.) Bge (Figure 7C). were expected to regulate immune system. For example, An animal study reported that Atractylodes macrocephala Koidz. water extract administered orally could increase the CD4+ T cell population in the spleen of mice stimulated with lipopolysaccharide (Kwak et al., 2018). According to an vitro study, polysaccharides derived from Atractylodes macrocephala Koidz. could exert immunoregulatory activities via regulating the proportions of CD8+ and CD4+ T cells (Sun et al., 2015). Astragaloside IV, which was known as the chief ingredient of Astragalus membranaceus (Fisch.) Bge., was proved to enhance immune cell proliferation and maintain homeostasis, and it could also reduce the expression of interleukin-4 to ameliorate airway inflammation (Huang et al., 2014).
Our review has presented consistent findings with a meta-analysis published in 2017, which demonstrated that CHM combined with WM could improve clinical efficacy compared with WM alone in children and adolescents with RMPP (Sheng and Huang, 2017). However, only eight RCTs (576 patients) with poor quality were included in the previous meta-analysis, and only clinical efficacy rate was evaluated, which made the conclusions unconvinced. Our research has unique strengths. First, we evaluated multiple outcome measures including some objective figures detected by machines to make our meta-analysis more comprehensive and credible. Second, dialectical diagnosis and treatment is the basic method of the TCM. Although children and adolescents suffer from the same disease, like RMPP, different syndrome types lead to different TCM therapies. Thus, we performed subgroup analysis based on different regimens, which could not only reduce heterogeneity but also compare the clinical efficacy of different TCM therapies in RMPP, providing good guidance for clinical practice.
Apart from efficacy, the safety of CHM in the treatment of RMPP is also an important issue worthy of consideration by clinicians. A total of nine studies in this meta-analysis reported safety, and no serious adverse events were observed. However, our results suggested that CHM had no advantage in alleviating the side effects, which was consistent with previous studies (Li, 2019). In addition, the description of adverse reactions in the instructions for Yupingfeng granule and Shedan Chuanbei oral liquid are “unclear” (Supplementary Table S4), indicating that there is no reliable clinical evidence to support their safety. Therefore, we considered the safety of CHM in the treatment of RMPP remained uncertain, which should be investigated in the further research.
This review is subject to some limitations. First, all included studies were performed in China and failed to describe the blinding method or allocation concealment. The poor quality of methodology might contribute to an exaggerated curative effect and decreased reliability of the evidence, thereby lowering the credibility and generalization of the results. Second, given the small sample size and limited number of studies in certain outcomes, the results might be insufficient to ensure a significant difference. Third, although sensitivity analysis and TSA demonstrated the reliability of our results, heterogeneity was observed in some results, such as antipyretic time and lung rale disappearance time. This might correlate with CHM intervention approaches, such as CHM composition, dose, and different forms administration. Thus, these results with substantial heterogeneity should be interpreted with caution. Last, drug safety is a key factor in clinical applications, but only nine RCTs described adverse events. Therefore, the safety of CHM in RMPP should be validated in future.
The validity of the conclusion in meta-analysis was highly dependent on the quality of the RCTs included, thus more high-quality studies evaluating the efficacy and safety of CHM for RMPP are needed. Based on the above limitations, some recommendations are suggested for further studies: 1) Clinical trials should be registered in advance on clinical trials registry platform. 2) Methodological quality of future trials including randomization, allocation concealment, and blinding should be improved by using random number tables and opaque envelopes,etc. 3) The safety assessments of CHM need more attention. 4) Clinical trials of CHM should standardize syndrome differentiation and treatment, in order to investigate the clinical application of CHM better.
In conclusion, CHM therapies together with WM seemed to be more beneficial for children and adolescents with RMPP in terms of relieving clinical symptoms, physical signs and reducing inflammation. However, due to the limitation of quality and the number of the included studies, we should take a cautious attitude toward these findings. Meanwhile, it is worth mentioning that the safety of CHM should be interpreted with caution. More RCTs with large samples and rigorous designs should be carried out to confirm the overall effect and further explore the optimal CHM therapies. In addition, more experimental studies are needed to provide evidence of CHM in treating RMPP at cellular and animal model levels and explore possible mechanisms.
XL and BY conceived and designed the review. XL and XS searched the database, extracted data and cross-checked with HK. SP, ZY, and JW performed the statistical analysis. XL drafted the paper. BY revised the manuscript critically.
This work was supported by the National Key Research and Development Program (2017YFC1703201) and the Project of State Administration of Traditional Chinese Medicine, China (2019XZZX-ek003)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Yujie Shang and Jinghong Liang for helpful comments, which have significantly improved the quality of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.678631/full#supplementary-material
Afshari, A., Wetterslev, J., and Smith, A. F. (2017). Can Systematic Reviews with Sparse Data Be Trusted? Anaesthesia 72 (1), 12–16. doi:10.1111/anae.13730
Cao, L. F. (2010). Status and Progress of Diagnosis and Treatment of Children and Adolescents with Refractory Mycoplasma Pneumoniae Pneumonia. J. Clin. Pediatr. 28 (01), 94–97. doi:10.3969/j.issn.1000-3606.2010.01.028
Chen, C. Q. (2014). Clinical Observation on the Treatment of Infantile Refractory Mycoplasma Pneumoniae Pneumonia with Integrated Traditional Chinese and Western Medicine. J. Med. Theor. Pract 27 (16), 2193–2194. doi:10.19381/j.issn.1001-7585.2014.16.060
Chen, Z. M., Shang, X. Y., Zhao, S. Y., Xin, D. L., Xu, B. P., Zheng, Y. J., et al. (2015). Expert Consensus on the Diagnosis and Treatment of Mycoplasma Pneumoniae Pneumonia in Children and Adolescents (2015 Edition). Chin. J. Appl. Clin. Pediatr. 30 (17), 1304–1308. doi:10.3760/cma.j.issn.2095-428X.2015.17.006
Ding, Y., Chu, C., Li, Y., Li, G., Lei, X., Zhou, W., et al. (2018). High Expression of HMGB1 in Children with Refractory Mycoplasma Pneumoniae Pneumonia. BMC Infect. Dis. 18 (1), 439. doi:10.1186/s12879-018-3346-8
Fan, H., Lu, B., Yang, D., Zhang, D., Shi, T., and Lu, G. (2019). Distribution and Expression of IL-17 and Related Cytokines in Children with Mycoplasma Pneumoniae Pneumonia. Jpn. J. Infect. Dis. 72 (6), 387–393. doi:10.7883/yoken.JJID.2019.113
Gao, L. W., Yin, J., Hu, Y. H., Liu, X. Y., Feng, X. L., He, J. X., et al. (2019). The Epidemiology of Paediatric Mycoplasma Pneumoniae Pneumonia in North China: 2006 to 2016. Epidemiol. Infect. 147, e192. doi:10.1017/s09-502688-19000839
Guo, X. J., Liu, J., Shen, Q. Y., Zhou, Q. X., Cao, Y. H., Li, W. P., et al. (2020). Epidemiological Analysis of Refractory Mycoplasma Pneumoniae Pneumonia in Children and Adolescents in Weifang City, Shandong Province from 2016 to 2019. Chin. Trop. Med. 20 (09), 893–896. doi:10.13604/j.cnki.46-1064/r.2020.09.22
He, W. C., Zhang, X. J., Zhang, W. J., and Zhang, Y. Q. (2020a). Effect of Integrated Traditional Chinese and Western Medicine on Serum Inflammatory Factors and Immune Function in Children and Adolescents with Refractory Mycoplasma Pneumonia. World J. Integr. Tradit West. Med. 15 (06), 1148–1151+1159. doi:10.13935/j.cnki.sjzx.200641
He, W. C., Zhang, X. J., Zhang, W. J., and Zhang, Y. Q. (2020b). Observation on the Effect of Qingrejiedu Decoction Combined with Erythromycin in the Treatment of Refractory Mycoplasma Pneumonia. Mod. J. Integr. Tradit West. Med. 29 (20), 2198–2202+2207. doi:10.3969/j.issn.1008-8849.2020.20.007
Higgins, J. P. T., Altman, D. G., Gotzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Huang, J. W., Gao, H. W., and Duan, J. F. (2018). Research Progress on the Chemical Constituents and Pharmacological Effects of Earthworm. Guid J. Tradit Chin. Med. Pharm. 24 (12), 104–107. doi:10.13862/j.cnki.cn43-1446/r.2018.12.034
Huang, X., Li, D., Liu, F., Zhao, D., Zhu, Y., and Tang, H. (2021). Clinical Significance of D-Dimer Levels in Refractory Mycoplasma Pneumoniae Pneumonia. BMC Infect. Dis. 21 (1), 14. doi:10.1186/s12879-020-05700-5
Huang, X., Tang, L., Wang, F., and Song, G. (2014). Astragaloside IV Attenuates Allergic Inflammation by Regulation Th1/Th2 Cytokine and Enhancement CD4+CD25+Foxp3 T Cells in Ovalbumin-Induced Asthma. Immunobiology 219 (7), 565–571. doi:10.1016/j.imbio.2014.03.005
Kang, H. (2021). Trial Sequential Analysis: Novel Approach for Meta-Analysis. Anesth. Pain Med. 16 (2), 138–150. doi:10.17085/apm.21038
Kim, H. S., Sol, I. S., Li, D., Choi, M., Choi, Y. J., Lee, K. S., et al. (2019). Efficacy of Glucocorticoids for the Treatment of Macrolide Refractory Mycoplasma Pneumonia in Children: Meta-Analysis of Randomized Controlled Trials. BMC Pulm. Med. 19 (1), 251. doi:10.1186/s12890-019-0990-8
Kurkela, S., Puolakkainen, M., Hokynar, K., Nieminen, T., Saxen, H., Mannonen, L., et al. (2019). Mycoplasma Pneumoniae Outbreak, Southeastern Finland, 2017-2018: Molecular Epidemiology and Laboratory Diagnostic Lessons. Eur. J. Clin. Microbiol. Infect. Dis. 38 (10), 1867–1871. doi:10.1007/s10096-019-03619-7
Kwak, T.-K., Jang, H.-S., Lee, M.-G., Jung, Y.-S., Kim, D.-O., Kim, Y.-B., et al. (2018). Effect of Orally AdministeredAtractylodes macrocephalaKoidz Water Extract on Macrophage and T Cell Inflammatory Response in Mice. Evidence-Based Complement. Altern. Med. 2018, 1–12. doi:10.1155/2018/4041873
Lee, E., Kim, C. H., Kim, C.-H., Lee, Y. J., Kim, H.-B., Kim, B.-S., et al. (2020). Annual and Seasonal Patterns in Etiologies of Pediatric Community-Acquired Pneumonia Due to Respiratory Viruses and Mycoplasma Pneumoniae Requiring Hospitalization in South Korea. BMC Infect. Dis. 20 (1), 132. doi:10.1186/s12879-020-4810-9
Lee, S. A., Lee, S. H., Kim, J. Y., and Lee, W. S. (2019). Effects of Glycyrrhizin on Lipopolysaccharide-Induced Acute Lung Injury in a Mouse Model. J. Thorac. Dis. 11 (4), 1287–1302. doi:10.21037/jtd.2019.04.14
Li, C. C., Shang, Y. X., Shen, X. Z., Chen, Z. M., and Zhao, S. Y. (2013). Guidelines for the Management of Community-Acquired Pneumonia in Children and Adolescents (2013 Revision). Chin. J. Pediatr. 51 (10), 745–752. doi:10.3760/cma.j.issn.0578-1310.2013.10.006
Li, G., Fan, L., Wang, Y., Huang, L., Wang, M., Zhu, C., et al. (2019a). High Co-expression of TNF-α and CARDS Toxin Is a Good Predictor for Refractory Mycoplasma Pneumoniae Pneumonia. Mol. Med. 25 (1), 38. doi:10.1186/s10020-019-0105-210.1186/s10020-019-0105-2
Li, N., Mu, Y. P., Chen, J., and Li, B. (2019b). Value of Absolute Counts of Lymphocyte Subsets in the Early Prediction of Refractory Mycoplasma Pneumoniae Pneumonia in Children. Zhongguo Dang Dai Er Ke Za Zhi 21 (6), 511–516. doi:10.7499/j.issn.1008-8830.2019.06.003
Li, Q., Li, Z.-Y., Zhang, J., Guo, W.-N., Xu, X.-M., Sun, F.-X., et al. (2019c). Xiyanping Plus Azithromycin Chemotherapy in Pediatric Patients with Mycoplasma Pneumoniae Pneumonia: A Systematic Review and Meta-Analysis of Efficacy and Safety. Evidence-Based Complement. Altern. Med. 2019, 1–9. doi:10.1155/2019/2346583
Li, S. F. (2019). Clinical Observation on the Treatment of Infantile Refractory Mycoplasma Pneumoniae Pneumonia. J. Guangming Chin. Med. 34 (17), 2712–2713. doi:10.3969/j.issn.1003-8914.2019.17.048
Lian, H. J. (2017). 36 Cases of Infantile Refractory Mycoplasma Pneumonia Treated by Qingre Huatan Quyu Decoction Combined with Conventional Western Medicine. Tradit Chin. Med. Res. 30 (06), 36–38. doi:10.3969/j.issn.1001-6910.2017.06.15
Liao, H., Ye, J., Gao, L., and Liu, Y. (2021). The Main Bioactive Compounds of Scutellaria Baicalensis Georgi. For Alleviation of Inflammatory Cytokines: A Comprehensive Review. Biomed. Pharmacother. 133, 110917. doi:10.1016/j.biopha.2020.110917
Liu, H. W., and Ma, R. (2017). Expert Consensus on the Diagnosis and Treatment of Mycoplasma Pneumoniae Pneumonia with Integrated Traditional Chinese and Western Medicine (Formulated in 2017). Chin. J. Pract. Pediatr. 32 (12), 881–885. doi:10.19538/j.ek2017120601
Liu, Y. M., Li, X. H., Tang, Y., and Li, M. (2020). Correlation Analysis between Refractory Mycoplasma Pneumonia and Immune Function Indexes. Chin. Med. Eng. 28 (06), 99–101. doi:10.19338/j.issn.1672-2019.2020.06.028
Lv, J., Xiong, W., Lei, T., Wang, H., Sun, M., Hao, E., et al. (2017). Amygdalin Ameliorates the Progression of Atherosclerosis in LDL Receptor-Deficient Mice. Mol. Med. Rep. 16 (6), 8171–8179. doi:10.3892/mmr.2017.7609
Meng, X. H. (2015). Clinical Observation of Integrative Medicines in the Treatment of Children and Adolescents with Refractory Mycoplasma Pneumonia. J. Pediatr. Tradit Chin. Med. 11 (06), 43–45.
Meng, Y. L., Xu, J. Y., Wang, X. X., Xu, H. X., Wang, X., Cai, X. J., et al. (2020). Study on Baicalin against Mycoplasma Pneumoniae. Chin. Arch. Tradit Chin. Med. 38 (02), 158–161+279. doi:10.13193/j.issn.1673-7717.2020.02.039
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 4 (1), 1. doi:10.1186/2046-4053-4-1
Pereira, T. V., and Ioannidis, J. P. A. (2011). Statistically Significant Meta-Analyses of Clinical Trials Have Modest Credibility and Inflated Effects. J. Clin. Epidemiol. 64 (10), 1060–1069. doi:10.1016/j.jclinepi.2010.12.012
Qian, Y. L. (2011). Combination of Traditional Chinese and Western Medicine in Treating Refractory Mycoplasma Pneumoniae Pneumonia in Children and Adolescents. Chin. J. Exp. Tradit Med. Formulae 17 (21), 268–270. doi:10.3969/j.issn.1005-9903.2011.21.075
Sheng, G. L., and Huang, P. Y. (2017). Meta-analysis on the Treatment of Infantile Refractory Mycoplasma Pneumoniae Pneumonia with Integrated Traditional Chinese and Western Medicine. Good Health All 11 (4), 10.
Sun, W. J., Chen, X., Guo, J. G., and Bi, C. B. (2017). Clinical Observation of Yupingfeng Granules in Adjuvant Treatment of Infantile Refractory Mycoplasma Pneumonia. J. Clin. Pulm. Med. 22 (08), 1393–1396. doi:10.3969/j.issn.1009-6663.2017.08.010
Sun, W., Meng, K., Qi, C., Yang, X., Wang, Y., Fan, W., et al. (2015). Immune-enhancing Activity of Polysaccharides Isolated from Atractylodis Macrocephalae Koidz. Carbohydr. Polym. 126, 91–96. doi:10.1016/j.carbpol.2015.03.034
Tan, D., and Jiang, Z. Y. (2018). Research Progress on Mechanism of Traditional Chinese Medicine in Treating Mycoplasma Pneumonia in Children and Adolescents. Chin. Arch. Tradit Chin. Med. 36 (06), 1403–1406. doi:10.13193/j.issn.1673-7717.2018.06.030
Tan, Z. F., and Yang, M. (2017). Yanhuning Injection Adjuvant Treatment of Children and Adolescents with Refractory Mycoplasma Pneumoniae Pneumonia and its Effects on Immune Function and Cytokines. Pract. J. Card. Cereb Pneum Vasc Dis 25 (07), 48–52. doi:10.3969/j.issn.1008-5971.2017.07.011
Tian, H. Y. (2016). Clinical Observation on the Treatment of 50 Cases of Infantile Refractory Mycoplasma Pneumoniae Pneumonia with Integrated Traditional Chinese and Western Medicine. Health all (04), 71.
Tsai, T. A., Tsai, C. K., Kuo, K. C., and Yu, H. R. (2020). Rational Stepwise Approach for Mycoplasma Pneumoniae Pneumonia in Children and Adolescents. J. Microbiol. Immunol. Infect. S1684-1182 (20), 30247–30254. doi:10.1016/j.jmii.2020.10.002
Waites, K. B., Xiao, L., Liu, Y., Balish, M. F., and Atkinson, T. P. (2017). Mycoplasma Pneumoniae from the Respiratory Tract and beyond. Clin. Microbiol. Rev. 30 (3), 747–809. doi:10.1128/cmr.00114-16
Wang, H. F., Zhou, W. W., and Wang, Q. (2018). Study on the Effect and Mechanism of Yupingfeng Granule Combined with Azithromycin in the Treatment of Refractory Mycoplasma Pneumonia in Children and Adolescents. Chin. Mod. Doc 56 (16), 90–92+96.
Wang, H. L., and Wu, L. C. (2016). Observation on the Effect of Yupingfeng Granule in the Treatment of Refractory Mycoplasma Pneumonia in Children and Adolescents. J. Jining Med. Univ. 39 (06), 409–411+415. doi:10.3969/j.issn.1000-9760.2016.06.008
Wang, S. W. (2020). Progress in the Treatment of Mycoplasma Pneumoniae Pneumonia in Children and Adolescents with Integrated Chinese and Western Medicine. Contemp. Med. Symp. 18 (13), 201–203. doi:10.3969/j.issn.2095-7629.2020.13.126
Wang, Z. K. (2018). Clinical Effect and Safety of Integrated Traditional Chinese and Western Medicine in the Treatment of Refractory Mycoplasma Pneumoniae Pneumonia in Children and Adolescents. Shenzhen J. Integr. Tradit Chin. West. Med. 28 (06), 32–33. doi:10.16458/j.cnki.1007-0893.2018.06.015
Wetterslev, J., Thorlund, K., Brok, J., and Gluud, C. (2009). Estimating Required Information Size by Quantifying Diversity in Random-Effects Model Meta-Analyses. BMC Med. Res. Methodol. 9, 86. doi:10.1186/1471-2288-9-86
Xu, X. H., Li, T., Wang, Y. T., and Lu, J. J. (2015). Research Progress of Peach Kernel. Chin. Herb Med. 46 (17), 2649–2655. doi:10.7501/j.issn.0253-2670.2015.17.023
Yan, Y. B., Ding, Y., and Yan, X. Y. (2019). Primary Study on Syndrome Differentiation and Treatment of Refractory Mycoplasma Pneumonia in Children and Adolescents and the Connection between Chinese and Western. Lishizhen Med. Medic Res. 30 (10), 2466–2467. doi:10.3969/j.issn.1008-0805.2019.10.052
Yan, Y., Wei, Y., Jiang, W., and Hao, C. (2016). The Clinical Characteristics of Corticosteroid-Resistant Refractory Mycoplasma Pneumoniae Pneumonia in Children and Adolescents. Sci. Rep. 6, 39929. doi:10.1038/srep39929
Yang, H.-J., Song, D. J., and Shim, J. Y. (2017). Mechanism of Resistance Acquisition and Treatment of Macrolide-Resistant Mycoplasma Pneumoniae Pneumonia in Children. Korean J. Pediatr. 60 (6), 167–174. doi:10.3345/kjp.2017.60.6.167
Yang, X. (2019). Clinical Observation on the Treatment of Infantile Persistent and Refractory Mycoplasma Pneumonia with Lung and Spleen Deficiency Syndrome with Jiawei Shenlingbaizhu Powder. Hunan Univ Tradit Chin Med.
Yu, J.-L., Song, Q.-F., Xie, Z.-W., Jiang, W.-H., Chen, J.-H., Fan, H.-F., et al. (2017). iTRAQ-Based Quantitative Proteomics Study in Patients with Refractory Mycoplasma Pneumoniae Pneumonia. Jpn. J. Infect. Dis. 70 (5), 571–578. doi:10.7883/yoken.JJID.2016.355
Yuan, P. B., Chen, Q. E., Guan, J. W., Chen, J. J., and Ye, G. L. (2016). Observation on Curative Effect of Combination of Traditional Chinese and Western Medicine in Treating Refractory Mycoplasma Pneumoniae Pneumonia. J. Pract. Tradit Chin. Med. 32 (11), 1088–1089. doi:10.3969/j.issn.1004-2814.2016.11.034
Zhai, Y.-Y., Wu, S.-Z., Yang, Y., Yang, L.-Y., Xu, J.-X., Huang, Z.-H., et al. (2020). An Analysis of 20 Clinical Cases of Refractory Mycoplasma Pneumonia in Children. Ann. Palliat. Med. 9 (5), 2592–2599. doi:10.21037/apm-19-497
Zhang, B. (2020a). Clinical Effect and Safety of Integrated Traditional Chinese and Western Medicine in the Treatment of Refractory Mycoplasma Pneumoniae Pneumonia in Children and Adolescents. Chin. Baby (16), 92.
Zhang, C. L. (2020b). A Study on the Application Value of Asarum Injection in Adjuvant Treatment of Children and Adolescents's RMPP. J. Qiannan Med. Univ. Natl. 33 (02), 119–122.
Zhang, G.-M., Huang, Z.-Y., Sun, R., Ye, S.-L., and Feng, Q. (2020). Xiao'er Xiaoji Zhike Oral Liquid Combined with Azithromycin for Mycoplasma Pneumoniae Pneumonia in Children: A Systematic Review and Meta-Analysis. Evidence-Based Complement. Altern. Med. 2020, 1–13. doi:10.1155/2020/9740841
Zhang, X., Chen, Z., Gu, W., Ji, W., Wang, Y., Hao, C., et al. (2018). Viral and Bacterial Co-infection in Hospitalised Children with Refractory Mycoplasma Pneumoniae Pneumonia. Epidemiol. Infect. 146 (11), 1384–1388. doi:10.1017/s0950268818000778
Zhao, F., Liu, J., Xiao, D., Liu, L., Gong, J., Xu, J., et al. (2020). Pathogenic Analysis of the Bronchoalveolar Lavage Fluid Samples with Pediatric Refractory Mycoplasma Pneumoniae Pneumonia. Front. Cel. Infect. Microbiol. 10, 553739. doi:10.3389/fcimb.2020.553739
Zhong, H. P., Wang, K. F., and Liu, S. P. (2017). Clinical Observation of Shedan Chuanbei Liquid in Treating Refractory Mycoplasma Pneumonia in Children and Adolescents. Pharmacol. Clin. Chin. Medic 33 (02), 192–194. doi:10.13412/j.cnki.zyyl.2017.02.054
Zhou, Y., Wang, J., Chen, W., Shen, N., Tao, Y., Zhao, R., et al. (2020). Impact of Viral Coinfection and Macrolide-Resistant Mycoplasma Infection in Children with Refractory Mycoplasma Pneumoniae Pneumonia. BMC Infect. Dis. 20 (1), 633. doi:10.1186/s12879-020-05356-1
Keywords: refractory Mycoplasma pneumoniae pneumonia, Chinese herbal medicine, complementary and alternative medicine, meta-analysis, effectiveness
Citation: Ling X, Sun X, Kong H, Peng S, Yu Z, Wen J and Yuan B (2021) Chinese Herbal Medicine for the Treatment of Children and Adolescents With Refractory Mycoplasma Pneumoniae Pneumonia: A Systematic Review and a Meta-Analysis. Front. Pharmacol. 12:678631. doi: 10.3389/fphar.2021.678631
Received: 10 March 2021; Accepted: 24 May 2021;
Published: 10 June 2021.
Edited by:
Shuai Ji, Xuzhou Medical University, ChinaReviewed by:
Chan-Young Kwon, Kyung Hee University, South KoreaCopyright © 2021 Ling, Sun, Kong, Peng, Yu, Wen and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Yuan, eXVhbmJpbjY4MzU4QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.