
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 10 May 2021
Sec. Renal Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.675406
This article is part of the Research TopicInteractions between Podocytes, Mesangial Cells, and Glomerular Endothelial Cells in Glomerular DiseasesView all 11 articles
Background: Treatment for adult patients with refractory idiopathic membranous nephropathy (RIMN) by conventional immunosuppressive regimens is not satisfactory. This study aims to evaluate the effectiveness of Chinese herbal medicine, Shulifenxiao formula, as a promising regimen.
Methods: A total of 31 RIMN patients resistant to corticosteroid or immunosuppressive agents were retrospectively analyzed. Shulifenxiao treatment lasted a minimum of 12°months in all patients and extended to 24°months in 11 patients. The primary outcomes [the complete remission (CR) and partial remission (PR)] and secondary outcomes (the serum creatinine and estimated glomerular filtration rate (eGFR) levels) were measured at 6, 12, 18, and 24°months.
Results: The data provided an average follow-up of 21 ± 9.16°months from baseline. The remission was attained in 25/31 patients (80.7%: CR 29.0% and PR 51.6%) at 12°months and in 10/11 patients (90.9%: CR 54.6% and PR 36.4%) at 24°months, respectively. Proteinuria reduced from 6.02 g/d at baseline to 0.98 g/d at 12°months (p < 0.001) and to 0.27 g/d at 24°months (p = 0.003); serum albumin increased from 28 g/L to 37.2 g/L at 12°months (p < 0.001) and to 41.3 g/L at 24°months (p = 0.003); eGFR improved from 100.25 ml/min/1.73 m2 to 118.39 ml/min/1.73 m2 at 6°months (p < 0.001) and finally to 111.62 ml/min/1.73 m2at 24°months (p = 0.008). Only two patients developed subsequent relapse.
Conclusion: Shulifenxiao formula as a clinical cocktail therapy serves as an alternative therapeutic option for steroid and immunosuppressant-resistant RIMN patients, with a favourable safety profile, though further studies are warranted.
Clinical Trial registration:http://www.chictr.org.cn, Chinese Clinical Trials Registry [ChiCTR1800019351].
Membranous nephropathy (MN) is one of the most common causes of nephrotic syndrome in adults. Approximately 75% of MN cases are idiopathic (Glassock, 2010). Over the past few years, most MN patients have received glucocorticoid in combination with an alkylating agent, or received calcineurin inhibitor, according to the 2012 guidelines of kidney disease improving global outcomes (KDIGO). These regimens significantly improve the clinical remission rate and renal survival rate of idiopathic membranous nephropathy (IMN). Nevertheless, nearly 30% of patients fail to respond to the conventional immunosuppressive therapies and eventually develop end-stage renal disease (ESRD) or complications related to nephrotic syndrome (Du Buf-Vereijken et al., 2005; Couser, 2017). Therefore, refractory idiopathic MN (RIMN) is proposed to refer to MN cases resistant to steroids and general immunosuppressive agents (Iida et al., 2000; Chen et al., 2013; Sengupta et al., 2013). Treatment of RIMN is a significant challenge because traditional immunosuppressive agents appear to be unsuccessful and show obvious side effects. Alternative therapeutic agents to treat RIMN are an urgent need.
Based on the traditional theories of Traditional Chinese Medicine (TCM), TCM treatment has been done on humans for thousands of years with using the formula of natural herbal medicines. Up to now, TCM has accumulated rich experience in treating various kidney diseases and holds great potential for providing effective treatments for MN. Recently, existing preclinical and clinical evidence has supported the effectiveness of TCM in treating IMN. TCM treatment reduces proteinuria, improves serum albumin, and thereby prevents the progress of IMN and avoids side effects caused by long-term applications of immunosuppressive agents (Chen et al., 2013; Zhang et al., 2014; Xiao et al., 2018). Combination of TCM and Western medicine has proven to improve the outcome of MN.
Despite this, very few high-quality studies testing the efficacy of TCM in adult RIMN patients are available. This may be due to the difficulty in formulating a control group. So many of the current studies are small single arm studies or case reports, with the observation periods ranging from half a year to one year (Chen, 2016; Zhang et al., 2017). Therefore, for RIMN patients, the possible benefits of TCM remain undetermined.
Medicinal “Shulifenxiao” is an herbal treatment for qi-deficiency, a damp-heat syndrome of RIMN. It has been used for nearly 30 years as an empirical treatment, first devised by Professor Liu Yuning. We have found “Shulifenxiao” formula has a significant clinical effect on the treatment of MN, and so in 2014, we started the clinical data collect of MN patients receiving “Shulifenxiao” treatment to establish a database for MN analysis. Here, we conducted a retrospective study aimed to evaluate the efficacy, recurrence, and safety of the TCM Shulifenxiao formula in patients with RIMN who are resistant to conventional immunosuppressive regimens.
This was a retrospective case-series study. The outpatient records of adult patients with histologically proven membranous nephropathy in Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine (Beijing, China) from January 2014 to June 2017 were screened (n = 157) (Figure 1). Only patients with the following criteria were included: 1) Histologically proven idiopathic membranous nephropathy; 2) Patients who did not respond to corticosteroid and immunosuppressive agents and remained in nephrotic syndrome (proteinuria≥3.5 g/d, serum albumin<30 g/L) even after 6°months of regular immunosuppressive therapy, such as prednisone (PRED), cyclophosphamide (CTX), cyclosporine A (CsA), tacrolimus (TAC), and mycophenolate mofetil (MMF); 3) eGFR > 30 ml/min/1.73 m2. 4) The follow-up period >1°year with an interval between visits of <3°months or >4°visit times per year.
31 patients were found to be eligible for the retrospective analysis.
All eligible patients were treated with a consecutive Shulifenxiao formula (a formula of TCM), which is made of seven Chinese herbs. Medicines was provided by the pharmacy department of Dongzhimen Hospital, and was orally administered 30 min after breakfast and supper (200°ml, bid). All patients were treated with angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor antagonist (ARB). If the patient's blood pressure remained >130/80 mmhg, appropriate calcium channel blockers were added to stabilize blood pressure. Treatment lasted for a minimum of 12°months in all patients. Clinical and laboratory data at 6°months prior to Shulifenxiao treatment, baseline, 6th, 12th, 18th, and 24th°months after Shulifenxiao treatment were analyzed. All adverse events recorded during follow-up were also analyzed.
The primary outcomes were attainment of complete and partial remission and changes in proteinuria and serum albumin. Complete remission (CR) was defined as a reduction of proteinuria to <0.3 g/d and serum albumin >35 g/L; Partial remission (PR) was defined as level of proteinuria decline to <3.5 g/day but minimum >0.3 g/day and at least 50% reduction from baseline with a serum albumin concentration of at least 30 g/L; Nonremission (NR) was defined as a reduction of proteinuria <50% or proteinuria >3.5 g/d. Relapse was defined as an increased proteinuria >3.5 g/d in consecutive analyses in patients with CR or PR. Any relapse was considered as an endpoint of the study. If no relapse was observed prior to the latest visit, June 30, 2018, was designated as the endpoint. Secondary outcomes included changes in serum creatinine and eGFR levels at each time point.
Statistical analysis was performed with SPSS 20.0 (version 20.0; SPSS, Chicago, IL, United States). Values are given as mean ± standard deviation for normal distribution variables, and median with interquartile range for abnormal distribution continuous variables. The t-test was used for comparisons of the means of two normal distributed quantitative variables. One-way analysis of variance (ANOVA) was used for comparisons of the means of multiple normal distributed quantitative variables. For anomaly distributed qualitative variables, a paired nonparametric test was used. A p value < 0.05 was considered to be statistically significant.
Characteristics of the 31 patients at baseline are listed in Table 1. All patients were treated with Shulifenxiao formula for a minimum of 12°months. Of these, 17 patients had complete clinical and laboratory data for 18°months of treatment, and 11 patients had complete data of 24°months of treatment. The average follow-up time was 21 ± 9.16°months. Other diseases associated with MN include 11 patients with hypertension, 1 with diabetes, 6 with hyperlipidemia, 1 with hyperuricemia, and 2 with coronary heart disease. The previous immunosuppressive agents used in the 31 patients were shown in Table 2.
The partial and complete remission rates increased gradually with the extension of treatment time (Figure 2A). The remission rate was 45.2% (14/31; CR 6.5% and PR 38.7%) at 6°months, 80.7% at 12°months (25/31; CR 29.0% and PR 51.6%), and 90.9% at 24°months (10/11; CR 54.6% and PR 36.4%). The average length from treatment initiation to PR was 7.32 ± 3.61°months, while to CR was 12.38 ± 4.73°months. Notably, two (6.45%) patients presented relapse within 24°months. One patient who achieved CR at 6°months relapsed at 12°months (due to a urinary tract infection). Another PR patient relapsed at 24°months (due to an upper respiratory infection), which indicated the end of the follow-up.
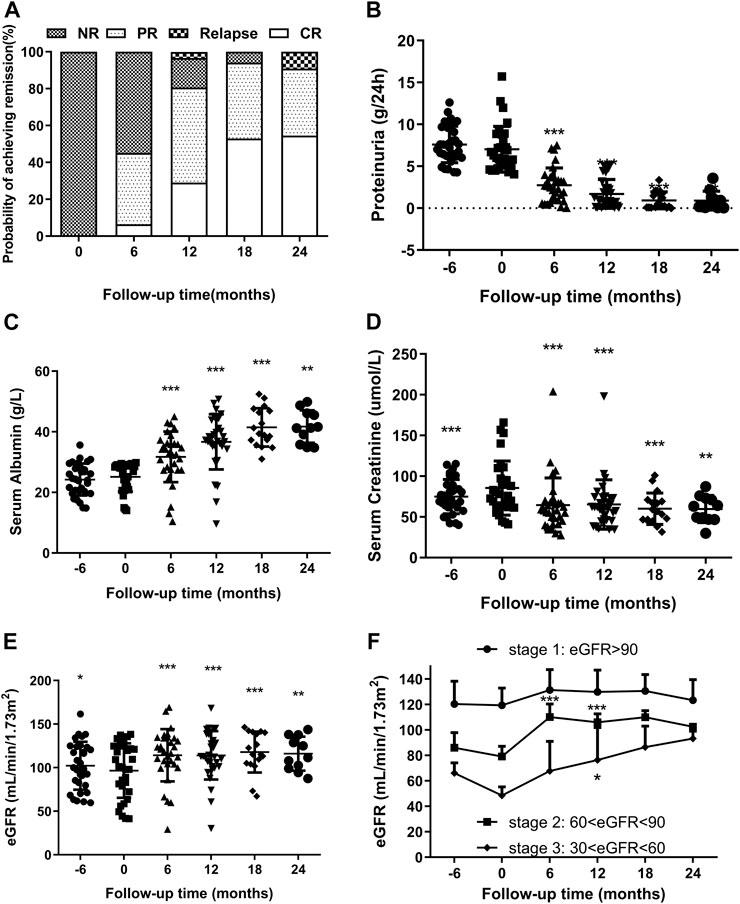
FIGURE 2. (A). Remission and relapse rates of RIMN paitients with follow-up time (in months after starting Shulifenxiao formula therapy). (B,C) Changes of proteinuria and serum albumin levels (median with inter-quartile ranges) with follow-up time in RIMN patients (−6°months means 6°months before Shulifenxiao therapy). The values of each time point were compared to the baseline (0°months), and the p values were evaluated by paired non-parametric test. *p values < 0.05, **p values < 0.01, ***p values < 0.001. (D,E) The changes of serum creatinine and eGFR levels (median with interquartile ranges) with follow-up time in RIMN patients. (F). The changes of eGFR levels (mean ± standard deviation) in RIMN patients of three stages with follow-up time (−6°months means 6°months before Shulifenxiao therapy). The values of each time point were compared to one’s own baseline. The p values of (D,E) were evaluated by a paired non-parametric test, and the p values of (F) were evaluated by one-way ANOVA test.*p values < 0.05, **p values < 0.01, ***p values < 0.001.
The changes in proteinuria and serum albumin levels in RIMN patients are illustrated in Figures 2B,C. The proteinuria levels decreased drastically from 6.02 (5.13–8.80)°g/d at baseline to 0.98 (0.27–2.41)°g/d at 12°months (p < 0.001) and further to 0.27 (0.1–1.34)°g/d at 24°months (p = 0.003). Meanwhile, serum albumin gradually increased from 28 (21.8–28.9)°g/L at baseline to 37.2 (35.2–43.7)°g/L at 12°months (p < 0.001) and to 41.3 (35.8–46.1)°g/L at 24°months (p = 0.003). Levels of both test indices showed a significant difference between baseline and different post-treatment time points.
It is well known that MN has a high spontaneous remission rate (Saito et al., 2017), and it can be concluded that some patients achieve spontaneous remission. In order to exclude such potential spontaneous remission cases, we reviewed the proteinuria and serum albumin levels 6°months prior to the TCM treatment. Data showed that there were no significant changes in these levels in the prior 6°months (p > 0.05).
Another important finding of this study was the reduction in serum creatinine. Although the level of serum creatinine increased from 6°months prior to TCM treatment (p < 0.001), it decreased remarkably from 78 (61.997.2)°g/L at baseline to 58 (45.23–68)°g/L at 6°months (p < 0.001) and continued to fluctuate smoothly until reaching 63 (47–73)°g/L at 24°months (p = 0.003) (Figure 2D). Simulaneously, eGFR significantly increased from 100.25 (72.37–124.70) at baseline to 118.39 (104.09–133.86)°ml/min/1.73 m2 at 6°months (p < 0.001) and finally to 111.62 (99.01–137.83)°ml/min/1.73 m2 at 24°months (p = 0.008) (Figure 2E).
Next, we observed changes in eGFR values. To the best of our knowledge, according to the KDOQI clinical guidelines, chronic kidney disease (CKD) can be classified into different stages (Levey 2003): Stage 1: kidney damage with normal or increased GFR (≥90 ml/min/1.73 m)2; Stage 2: kidney damage with mild decreased GFR (60–89 ml/min/1.73 m)2; Stage 3: moderately decreased GFR (30–59 ml/min/1.73 m)2; Stage 4: severely decreased GFR (15–29 ml/min/1.73 m)2; Stage 5: kidney failure with GFR <15 ml/min/1.73 m2 or dialysis required. As shown in Figure 2F, although there was no significant difference from baseline (p > 0.05), eGFR values in stage 1 were still on the rose after treatment. Interestingly, the eGFR values of the stage 2 group were statistically enhanced from 79.14 ± 8.63 ml/min/1.73 m2 at baseline to 110.16 ± 11.14 ml/min/1.73 m2 at 6°months, and reached 106.02 ± 7.22 ml/min/1.73 m2 at 12°months, and a significant difference was observed compared to baseline (p < 0.001). A similar trend was observed in the group of CKD stage 3: the eGFR values increased from 48.58 ± 7.34 ml/min/1.73 m2 at baseline to 67.85 ± 25.27 ml/min/1.73 m2 at 6°months (p = 0.167), and to 76.39 ± 27.92 ml/min/1.73 m2 at 12 months (p = 0.04). Since there were fewer than 5 cases in stages 2 and 3 during the 18th and 24th°month, we did not compare them. From the above results, it can be seen that Shulifenxiao formula treatment can improve the glomerular filtration rate, especially in patients with low eGFR.
Over the follow-up time of 31 patients, liver function, routine blood tests, and blood pressure in all the patients indicated no significant changes from baseline after the intervention (Table 3). No severe adverse events were reported. Only one patient developed nausea, and another patient developed vomiting, but both were transient and the symptoms resolved after 2 weeks.
The treatment for adult patients with RIMN who are resistant to standard immunosuppressive therapies remains a therapeutic challenge. The efficacy and safety profile of conventional treatments like steroids, CTX, CsA, TAC is not always satisfactory as revealed by recent studies. W. Chen et al. (Chen et al., 2013) reported a benefit of combining TAC with prednisone in treating 14 adults Chinese RIMN patients, that remission was attained in 11/14 patients (78.6%: CR 35.7% and PR 42.9%) at the end of follow-up. T. Saito et al. (Saito et al., 2017) treated 19 patients with Mizoribine (MZR) in combination with prednisolone, and CR was attained in only 10/19 (52.6%) patients after more than 2°years of treatment. F.B. Cortazar et al. (Cortazar et al., 2017) reported a higher remission rate in a study combining low-dose rituximab with oral cyclophosphamide, and an accelerated prednisone taper in treating 15 consecutive IMN patients (including 8 RIMN patients), in which 100% of patients achieved PR and 93% of patients achieved CR at a median time of 2 and 13°months respectively. Regardless of their fair or good efficacy, these therapies have serious adverse events, sometimes with fatal consequences. Therefore, in China, many RIMN patients turn to traditional Chinese medicine (TCM) for alternative therapy. In this study, we retrospectively reviewed the efficacy and safety of Shulifenxiao formula in 31 adult RIMN patients who did not response to 6°months conventional immunosuppressant therapies.
In this study, we excluded cases with spontaneous remission potentials by confirming no changes in the proteinuria, serum albumin levels of all patients from 6°months prior TCM treatment to the baseline. After Shulifenxiao treatment, a significant decrease in proteinuria from 6.02 g/d at baseline to 0.27 g/d at 24°months and an upward trend in serum albumin level was achieved. Previous studies have suggested that patients with proteinuria of 0.3–1.0 g/d showed a favourable prognosis almost equal to CR (Shiiki et al., 2004; Saito et al., 2014), and patients with PR had a similar average GFR decline as those with CR (Troyanov et al., 2004). From this viewpoint, we can consider that most participants of this study achieved remission within 2°years of treatment, which is cross verified with the results in the remission rate. Obviously, Shulifenxiao formula has shown a promising effect in inducing remission in the refractory patients and relatively low relapse rate.
Persistence of nephrotic syndrome in MN portends a poor prognosis, with a considerably high risk of a GFR decline rate of 10 ml/min/1.73 m2, and a 29% ESRD risk rate (Troyanov et al., 2004), therefore long-term immunosuppressive therapies are used. However, these therapies do not always induce remission and may cause significant adverse effects. The calcineurin inhibitor (CsA and TAC) directly affects renal function and cause nephrotoxicity and hypertension. Therefore, under these treatments, improvement of proteinuria and protection of renal function seems to be a trade-off process. Notably, Shulifenxiao formula appeared to show significant benefits in increasing eGFR levels, especially in CKD stage 2: The eGFR values increased from 79.14 to 110.16 ml/min/1.73 m2 at 6°months (p < 0.001). These findings suggest that Shulifenxiao formula can improve renal function by significantly increasing eGFR, which is important for long-term prognosis.
In the present study, no severe adverse events were observed. Liver function, routine blood tests and blood pressure in all patients indicated no significant changes between baseline and post-treatment time points. Only two patients developed nausea or vomiting, both of which were mild and easily controlled. This is clearly an exciting outcome for RIMN patients who have to take long-term immunosuppressants, most of which are associated with serious adverse events. Compared to these conventional drugs, Shulifenxiao formula seems to be a safe and beneficial solution for long-term use.
The mechanism of action for herbal medicines have been studied, and their effects are mainly related to anti-inflammation, antioxidation, antifibrosis, immune system regulation, anticoagulation, and improvement of metabolic disturbance (Zhang et al., 2014; Ren et al., 2017; Wojcikowski et al., 2006). There are 7 Chinese herbal medicines in the Shulifenxiao formula (Table 4). One such component is Saponin in Astragalus which can improve immunity, protect vascular endothelium, inhibit mesangial cell multiplication, encourage the metabolism of body liquid, improve hemorheological targets, protect podocytes, and reduce renal interstitial fibrosis and glomerular sclerosis (Wang et al., 2017). Forsythin in Forsythia can effectively block the TLR4 signaling pathway and further by blocking the expression of inflammatory factors induced by lipopolysaccharide (Toba et al., 1991). Amygdalin in Bitter Apricot can increase the activity of type I collagenase in renal fibroblast cells in order to reduce the expression of type I collagen protein, that would further inhibit the proliferation of human renal fibroblast cells or would promote renal fibroblast apoptosis (Zhao and el al., 2020). Houttuyfonate in Houttuynia cordata has effective antibacterial, antiviral, enhance the body's immunity, diuresis (Good for peeing), and lower blood pressure (Wang et al., 2010). Smilax saponins in Magnolia Officinalis has the Anti-inflammatory, analgesic, antibacterial, antioxidant (Wang, 2014). Hirudin in Leech has the anticoagulation, anti-thrombosis, to reduce the deposition of fibrin-related antigen in the glomerulus in order to reduce the proliferation of mesangial cells and glomerular sclerosis (Ren et al., 2019) (Table 4). Cocktail therapy contains a combination of different medicines, taken by HIV/AIDS patients to improve health. Chinese officials currently administer cocktail therapy for treatment of HIV and COVID-19 (Schimt et al., 1996). The superior antiproteinuric and renoprotective effects of Shulifenxiao formula on RIMN may be related to multifactorial effects. These studies suggest that the Shulifenxiao formula as clinical cocktail therapy has the potential to reduce proteinuria and attenuate kidney injury through these mechanisms (Figure 3).
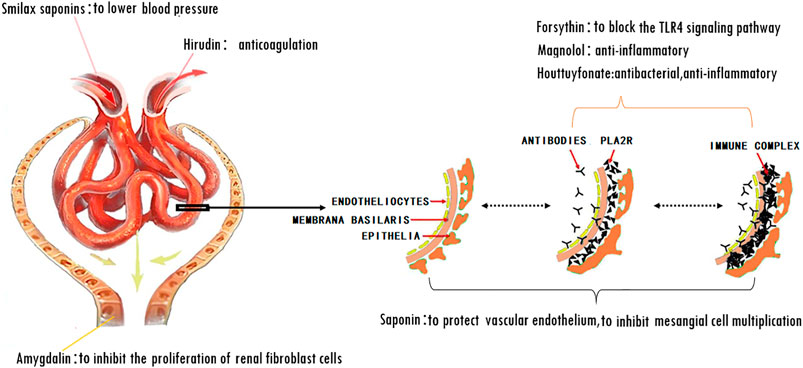
FIGURE 3. Shulifenxiao formula as clinical cocktail therapy has the potential to reduce proteinuria and attenuate kidney injury through these mechanisms.
Our study has the advantages and innovation as this is the first report or information about “Herbal Medicine “Shulifenxiao” therapy for Nephrotic Syndrome of Refractory Idiopathic Membranous Nephropathy” in the world. Most of the previous clinical studies of TCM were the research of IMN and they compared the differences of the efficacy and safety between TCM and traditional immunosuppressant. There were few studies of RIMN. Our study focusses on RIMN, and we strictly selected the patients with nephrotic syndrome after 6 months of treatment with a standardized immunosuppressant regimen.
The major limitation of this study is that it is a retrospective single-centre study, with a limited number of patients, and lacking a parallel control group. As a matter of fact, In China, before most MN patients came to our hospital for the herbal medicine therapy, patients suffering from MN have previously received conventional treatment such as immunosuppressant or hormone therapy. Some patients cannot sustain the side effects of immunologic therapy or ineffective treatment options, and so they prefer to accept to the herbal medicine treatment. Based on real-world conditions and patients’ preference for herbal treatment, all patients were treated with traditional herbal medicine, with no patients treated with immunosuppressant or hormones therapy only. This made it difficult to formulate a control group. Therefore, the statistical results must be interpreted with caution in this setting. Meanwhile, randomized controlled trials with long-term follow-up are needed in the future to establish Shulifenxiao formula as an evidence-based treatment option for RIMN patients.
In this study, patients with refractory membranous nephropathy were treated with Shulifenxiao cocktail therapy. After treatment, it was observed that patient urine protein content decreased, plasma albumin levels increased, clinical remission rate was significantly increased, and patient's renal function was improved.
Shulifenxiao formula as clinical cocktail therapy serves as an alternative therapeutic option for steroid and general immunosuppressant-resistant RIMN patients, with a favourable safety profile, though studies with larger sample sizes and longer follow-ups are warranted. When seeking new RIMN treatment, we believe herbal medicine therapy should be considered.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Consent to publish from the participant to report individual patient data has been obtained from each participant.
YL and WL conceived the trial and obtained research funding. HC and WL designed the study and wrote the study protocol. HC and FF collected clinical data and conducted the statistical analysis and wrote the manuscript, BL contributed to the refinement of the study protocol. WL, YL, and FF critically revised the manuscript. All authors read and approved the final manuscript.
This study was supported by National Natural Science Foundation of China (Grant Nos. 81774278 and 81570656).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.675406/full#supplementary-material
ACEI, angiotensin converting enzyme inhibitors; ALT, alanine aminotransferase; ANOVA, one-way analysis of variance; ARB, angiotensin II receptor antagonist; AST, aspartate transaminase; CKD, chronic kidney disease; CR, complete remission; CsA, cyclosporine A; CTX, cyclophosphamide; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; IMN, idiopathic membranous nephropathy; KDIGO, kidney disease improving global outcomes; MMF, mycophenolate mofetil; MN, membranous nephropathy; MZR, mizoribine; NR, nonremission; PR, partial remission; PRED, prednisone; RIMN, refractory idiopathic membranous nephropathy; SBP, systolic blood pressure; TAC, tacrolimus; TCM, traditional Chinese medicine.
Chen, W., Liu, Q., Ji, Y., Liu, Z., Yu, X., Liao, Y., et al. (2013). Outcomes of Tacrolimus Therapy in Adults with Refractory Membranous Nephrotic Syndrome: a Prospective, Multicenter Clinical Trial. Am. J. Med. Sci. 345 (2), 81–87. doi:10.1097/maj.0b013e31824ce676
Chen, X. (2016). Clinical Observation on Curative Effect of Primary Nephrotic Syndrome Treated with Jiawei Fangji Huangqi Tang and its Effects on Serum IL-10 and TNF-α. J. Traditional Med. Sci. Tech. 4, 393–395.
Chen, Y., Deng, Y., Ni, Z., Chen, N., Chen, X., Shi, W., et al. (2013). Efficacy and Safety of Traditional Chinese Medicine (Shenqi Particle) for Patients with Idiopathic Membranous Nephropathy: a Multicenter Randomized Controlled Clinical Trial. Am. J. Kidney Dis. 62 (6), 1068–1076. doi:10.1053/j.ajkd.2013.05.005
Cortazar, F. B., Leaf, D. E., Owens, C. T., Laliberte, K., Pendergraft, W. F., and Niles, J. L. (2017). Combination Therapy with Rituximab, Low-Dose Cyclophosphamide, and Prednisone for Idiopathic Membranous Nephropathy: a Case Series. BMC Nephrol. 18, 44. doi:10.1186/s12882-017-0459-z
Couser, W. G. (2017). Primary Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 12, 983–997. doi:10.2215/cjn.11761116
Du Buf-Vereijken, P. W. G., Branten, A. J. W., and Wetzels, J. F. M. (2005). Idiopathic Membranous Nephropathy: Outline and Rationale of a Treatment Strategy. Am. J. Kidney Dis. 46, 1012–1029. doi:10.1053/j.ajkd.2005.08.020
Glassock, R. J. (2010). The Pathogenesis of Idiopathic Membranous Nephropathy: A 50-Year Odyssey. Am. J. Kidney Dis. 56 (1), 157–167. doi:10.1053/j.ajkd.2010.01.008
Iida, H., Naito, T., Sakai, N., and Aoki, S. (2000). Effect of Cyclosporine Therapy on Idiopathic Membranous Nephropathy Presented with Refractory Nephrotic Syndrome. Clin. Exp. Nephrol. 4 (1), 81–85. doi:10.1007/s101570050068
Ren, X. D., Zhang, Y. W., Wang, X. P., et al. (2017). Effects of Dangguibuxue Decoction on Rat Glomerular Mesangial Cells Cultured under High Glucose Conditions. Bmc Complement. Altern. Med. 17 (1), 283. doi:10.1186/s12906-017-1774-4
Ren, S.-H., Liu, Z.-J., Cao, Y., Hua, Y., Chen, C., Guo, W., et al. (2019). A Novel Protease-Activated Receptor 1 Inhibitor from the Leech Whitmania Pigra. Chin. J. Nat. Med. 17 (8), 591–599. doi:10.1016/S1875-5364(19)30061-5
Saito, T., Iwano, M., Iwano, M., Matsumoto, K., Mitarai, T., Yokoyama, H., et al. (2017). Mizoribine Therapy Combined with Steroids and Mizoribine Blood Concentration Monitoring for Idiopathic Membranous Nephropathy with Steroid-Resistant Nephrotic Syndrome. Clin. Exp. Nephrol. 21, 961–970. doi:10.1007/s10157-016-1340-2
Saito, T., Iwano, M., Iwano, M., Matsumoto, K., Mitarai, T., Yokoyama, H., et al. (2014). Significance of Combined Cyclosporine−prednisolone Therapy and Cyclosporine Blood Concentration Monitoring for Idiopathic Membranous Nephropathy with Steroid-Resistant Nephrotic Syndrome: a Randomized Controlled Multicenter Trial. Clin. Exp. Nephrol. 18, 784–794. doi:10.1007/s10157-013-0925-2
Schmit, J. C., Cogniaux, J., Hermans, P., Van Vaeck, C., Sprecher, S., Van Remoortel, B., et al. (1996). Multiple drug resistance to nucleoside analogues and nonnucleoside reverse transcriptase inhibitors in an efficiently replicating human immunodeficiency virus type 1 patient strain. J. Infect. Dis. 174 (5), 962–968. doi:10.1093/infdis/174.5.962
Sengupta, U., Kumar, V., Yadav, A. K., et al. (2013). Infusion of Autologous Bone Marrow Mononuclear Cells Leads to Transient Reduction in Proteinuria in Treatment Refractory Patients with Idiopathic Membranous Nephropathy. BMC Nephrol. 14 (1), 262. doi:10.1186/1471-2369-14-262
Shiiki, H., Saito, T., Saito, T., Nishitani, Y., Mitarai, T., Yorioka, N., et al. (2004). Prognosis and Risk Factors for Idiopathic Membranous Nephropathy with Nephrotic Syndrome in Japan. Kidney Int. 65, 1400–1407. doi:10.1111/j.1523-1755.2004.00518.x
Toba, T., Nagashima, S., and Adachi, S. (1991). Is Lactose Really Present in Plants? J. Sci. Food Agric. 54, 305–308. doi:10.1002/jsfa.2740540217
Troyanov, S., Wall, C. A., Scholey, J. W., Miller, J. A., and Cattran, D. C. Toronto Glomerulonephritis Registry Group (2004). Idiopathic Membranous Nephropathy: Definition and Relevance of a Partial Remission. Kidney Int. 66, 1199–1205. doi:10.1111/j.1523-1755.2004.00873.x
Wang, L., Cui, X., Cheng, L., Yuan, Q., Li, T., Li, Y., et al. (2010). Adverse Events to Houttuynia Injection: A Systematic Review. J. Evidence-Based Med. 3 (3), 168–176. doi:10.1111/j.1756-5391.2010.01091.x
Wang, L., et al. (2014). Natural Product Agonists of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ): a Review. Biochem. Pharmacol. 92 (1), 73–89. doi:10.1016/j.bcp.2014.07.018
Wang, T., Xuan, X., Li, M., Gao, P., Zheng, Y., Zang, W., et al. (2017). Retraction Note: Astragalus Saponins Affect Proliferation, Invasion and Apoptosis of Gastric Cancer BGC-823 Cells. Diagn. Pathol. 12, 67. doi:10.1186/s13000-017-0657-9
Wojcikowski, K., Johnson, D. W., and Gobe, G. (2006). Herbs or Natural Substances as Complementary Therapies for Chronic Kidney Disease: Ideas for Future Studies. J. Lab. Clin. Med. 147, 160–166. doi:10.1016/j.lab.2005.11.011
Xiao, J., Yang, Y., Zhu, Y., Qin, Y., Li, Y., Fu, M., et al. (2018). Efficacy and Safety of Traditional Chinese Medicine on Nonerosive Reflux Disease: A Meta-Analysis of Randomized Controlled Trials. Evidence-Based Complement. Altern. Med. 2018, 1–13. doi:10.1155/2018/1505394
Zhang, H., Zhao, T., Gong, Y., Dong, X., Zhang, W., Sun, S., et al. (2014). Attenuation of Diabetic Nephropathy by Chaihuang-Yishen Granule through Anti-inflammatory Mechanism in Streptozotocin-Induced Rat Model of Diabetics. J. Ethnopharmacology 151, 556–564. doi:10.1016/j.jep.2013.11.020
Zhang, K., Dong, P., Yuxia, Q. I., et al. (2017). Clinical Observation on Jiawei Fangjihuangqitang on the Treatment of Primary Nephrotic Syndrome in Adults. Beijing: China Health Standard Management.
Zhang, L., Li, P., Xing, C.-y., Zhao, J.-y., He, Y.-n., Wang, J.-q., et al. (2014). Efficacy and Safety of Abelmoschus Manihot for Primary Glomerular Disease: A Prospective, Multicenter Randomized Controlled Clinical Trial. Am. J. Kidney Dis. 64 (1), 57–65. doi:10.1053/j.ajkd.2014.01.431
Keywords: Chinese herbal medicine, nephrotic syndrome, refractory membranous nephropathy, retrospective analysis, shulifenxiao formula
Citation: Cui H, Fu FQ, Liu B, Liu WJ and Liu YN (2021) Herbal Medicine “Shulifenxiao” Formula for Nephrotic Syndrome of Refractory Idiopathic Membranous Nephropathy. Front. Pharmacol. 12:675406. doi: 10.3389/fphar.2021.675406
Received: 03 March 2021; Accepted: 22 April 2021;
Published: 10 May 2021.
Edited by:
John D. Imig, Medical College of Wisconsin, United StatesReviewed by:
Jonathan P. Troost, University of Michigan, United StatesCopyright © 2021 Cui, Fu, Liu, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Jing Liu, bGl1d2VpamluZy0xOTc3QGhvdG1haWwuY29t; Yu Ning Liu, eXVuaW4xOTQ2QHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.