- 1Department of Emergency and Critical Care Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 3Department of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Effective treatments for coronavirus disease 2019 (COVID-19) are urgently needed. The real role of corticosteroid use in COVID-19 has long been of interest and is disputable.
Methods: We aimed to quantitatively reevaluate the efficacy of corticosteroids on COVID-19. Databases were searched for eligible meta-analyses/systematic reviews with available outcome data. For each association, we estimated the summary effect size with fixed- and random-effects models, 95% confidence intervals, and 95% prediction intervals. Heterogeneity, Egger’s test, evidence of small-study effects and excess significance bias, and subgroup analyses were rigorously evaluated.
Results: Intended outcomes of 12 eligible studies were mortality, clinical improvement, hospitalization, mechanical ventilation (MV), adverse events (AEs), intensive care unit (ICU) stay, hospital stay, virus clearance time (VCT), and negative conversion. Corticosteroid administration was associated with a 27% risk reduction in MV [hazard ratio (HR): 0.73 (0.64–0.83)] and a 20% reduction in mortality of critically ill/severe COVID-19 patients [HR: 0.80 (0.65–0.98)]. Interestingly, shorter ICU stays and, conversely, potentially longer hospital stays, a longer VCT, and a longer time to negative conversion were associated with corticosteroid use. There was no significant impact of different corticosteroid doses on mortality. Only one study showed slightly excess significant bias. Caution should be applied given the weak nature of the evidence, and it has been confirmed by sensitivity analyses too.
Conclusion: This umbrella study found benefits from corticosteroids on MV and especially the mortality of critically ill/severe patients with shorter ICU stays but prolonged hospital stays and VCT. The benefits and harms should be reevaluated and balanced before corticosteroids are cautiously prescribed in clinical practice.
Introduction
At the end of 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV-2), broke out in Wuhan, China (Zhou et al., 2020); by March 11, 2020, the outbreak was declared a pandemic of coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) (Zhang et al., 2020). To date, COVID-19 has affected millions worldwide, while no pronouncedly effective treatment has been established (Holshue et al., 2020).
The immune response is acknowledged to be a key factor in COVID-19, of which the first phase is characterized by fever, cough, and high viral loads; the next stage is marked by lung inflammation and respiratory failure accompanied by a decreased viral load; and the last stage shows an uncontrolled hyperinflammatory response, subsequent pulmonary fibrosis, and multiorgan dysfunction with a high mortality risk (Tay et al., 2020). As the main immunomodulatory agent used for clinical management, corticosteroids are thought to be a candidate for COVID-19 treatment (Ho et al., 2003; Tay et al., 2020). Both benefits and poor outcomes have been reported in the previous literature (Budhathoki et al., 2020; Cano et al., 2020; Cheng et al., 2020; Lu et al., 2020; Pasin et al., 2020; Pei et al., 2020; Sarkar et al., 2020; Siemieniuk et al., 2020; Sterne et al., 2020; Tlayjeh et al., 2020; van Paassen et al., 2020; Ye et al., 2020), and rigorous data on its efficacy are still limited, which potentially will stimulate clinical research and address this controversy. The United Kingdom–based Randomized Evaluation on COVID-19 Therapy (RECOVERY) trial reported reliable findings from 6,425 patients who were randomized to receive 6 mg/d dexamethasone or standard of care (SOC). Dexamethasone resulted in a mortality reduction of 2.8% compared with SOC [22.9 vs. 25.7%, relative risk (RR): 0.83, 95% confidence interval (95% CI): 0.75–0.93], and more benefits could be found for the mortality of patients receiving invasive mechanical ventilation [29.3 vs. 41.4%, respectively, RR: 0.64, 95% CI: 0.51–0.81] (Horby et al., 2020). The results of the meta-analysis were also controversial since a previous meta-analysis demonstrated that COVID-19 patients who used corticosteroids were more likely to develop critical illness and had a higher mortality and longer hospital stays (Wang et al., 2020). Another meta-analysis showed a significant reduction in short-term mortality [odds ratio (OR): 0.72, 95% CI: 0.57–0.87] and the need to receive mechanical ventilation (MV) compared to SOC, although the data were sparse to draw firm conclusions (van Paassen et al., 2020). To our knowledge, there has been little attempt to summarize the data from existing secondary studies.
Considering the great impact of the pandemic and the inconsistency in these findings, this study aimed to comprehensively summarize the emerging secondary evidence (meta-analyses with/without systematic reviews) being reported worldwide. We sought to (i) determine the association between outcomes of interest and corticosteroid use; (ii) better understand the strength of the data and the extent of potential bias in the claimed results via the umbrella method; (iii) show reliable evidence by summarizing abundant sample sizes, totally analyzing the heterogeneity and performing subgroup analyses; and (iv) provide a potential effective treatment for COVID-19.
Methods
We rigorously conducted this umbrella meta-analysis following prior guidelines (Aromataris et al., 2015) (Supplementary Appendix File A1). The protocol has been registered on the INPLASY website (https://inplasy.com/register/) (ID: INPLASY202110116, doi: 10.37766/inplasy 2021.1.0116) (Supplementary Appendix File A2).
Search Strategy
We searched PubMed, EMBASE, Cochrane Library, and preprint platforms for related systematic reviews and/or meta-analyses from database inception to December 1, 2020, with no language restrictions. The search keywords used were as follows: COVID-19, corticosteroids, systematic review, and meta-analysis. Detailed search information can be found in Supplementary Appendix File A3. A manual search of reference lists from the retrieved studies was also performed.
Selection Criteria
Articles were initially reviewed through titles and abstracts. Then, full texts of potentially eligible studies were examined by two authors, and any discrepancies were resolved with a discussion involving a third author (Binghao Zhao) who is familiar with COVID-19 epidemiology and evidence-based medicine.
The eligible criteria were as follows:
Population: Hospitalized patients with confirmed COVID-19.
Intervention: Reasonable corticosteroid use (methylprednisolone, dexamethasone, prednisone, corticoids, and steroids) during the hospital stay.
Comparison: No corticosteroid use, including SOC and placebo.
Outcomes: Mortality, MV, hospital stay, virus clearance time (VCT), intensive care unit (ICU) stay, adverse events (AEs), clinical improvement, hospitalization, and negative conversion. Eligible studies should report at least one of the intended outcomes.
Study design: Meta-analysis with/without a systematic review with available data. Associations with intended outcomes of eligible secondary studies should include at least two primary studies, except network meta-analyses.
Only studies with the most complete information could be included. We excluded single-arm meta-analyses; meta-analyses involving pregnant or pediatric patients with different presentations of COVID-19 in these populations; and meta-analyses involving organ transplant recipients or inflammatory or rheumatologic patients using long-term corticosteroids.
Data Extraction
Data extraction was performed independently by two authors, and any disagreements were resolved by discussion with a third author. For each eligible article, we recorded the first author, publication year, number of included studies, number of participants, comparisons, study design, quality assessment methods, subgroup data, searching and registration information, pooled risk estimates [RR, OR, hazard ratio (HR), incident RR, mean difference (MD), and weighted MD (WMD)], and 95% CI. For each primary study from the eligible meta-analyses, the first author, number of cases and subjects, maximally adjusted risk estimate, and 95% CI were extracted for further analysis if available.
Assessment of Small-Study Effects
Egger’s regression asymmetry test was conducted to help identify small-study effects (Sterne et al., 2011). Although small-study effects can indicate publication bias and other reporting biases, they can also reflect genuine chance, heterogeneity, or other causes for the differences between small and large studies. We calculated the standard error (SE) of the study with the largest sample size of each meta-analysis to determine whether larger estimates of effect size were predicted by small studies compared to large studies. If the p value for Egger’s test was <0.10 and the largest study had a smaller effect size than the summary effect size, criteria for the existence of small-study effects were fulfilled (Sterne et al., 2011).
Evidence of Excess Significance Bias
An excess significance test was conducted to investigate whether the observed number of studies (O) with nominally significant results (p < 0.05, “positive” results) was larger than the expected number of significant results (E) (Ioannidis and Trikalinos, 2007). In each meta-analysis, E is calculated from the sum of the statistical power estimates for each component study. We used the effect size of the largest study in a meta-analysis and the pooled results based on fixed- and random-effects models as plausible effect sizes to estimate the power of each component study (Ioannidis, 2013). The estimate from the largest study should be closer to the true estimate than the results of less precise studies with small-study effects. The statistical power was computed by using a noncentral t distribution (Lubin and Gail, 1990). The excess significance test was regarded as positive if the p value was <0.10, given that O > E.
Reviewing the Current Evidence
Statistically significant associations between corticosteroid use and outcomes of interest were categorized into four levels: strong, highly suggestive, suggestive, and weak using specific criteria. For strong evidence, p < 10–6, number of cases >1,000, I2 < 50%, p < 0.05 of the largest study in the meta-analysis, and 95% prediction interval (PI) excludes the null value, small-study effects (p > 0.1 for Egger’s test), and excess significance bias (p > 0.1). For highly suggestive evidence, p < 10–6, number of cases >1,000, and p < 0.05 of the largest study in the meta-analysis. For suggestive evidence, p < 10–3 and number of cases >1,000. For weak evidence, the sole criterion was p < 0.05 (Kalliala et al., 2017). When p > 0.05, there was no association. For the number of cases with unknown outcomes, the quality was judged according to the total population; for both cases and total populations with unknown outcomes, the quality was weak regardless of whether p < 0.05.
Statistical Analysis
For each meta-analysis, we also estimated the summary effect size and its 95% CI using both fixed-effects and random-effects models (Kalliala et al., 2017). After addressing the uncertainty of the estimated summary effect in the random-effects model and the heterogeneity between the studies, the 95% CI was calculated to predict the expected effect size range in the new original studies (Riley et al., 2011). For the largest dataset of each meta-analysis, we calculated the SE of the effect size and determined whether the SE was less than 0.10. For binary variables, HRs and 95% CIs were used to pool the results from each meta-analysis together based on the extracted RR, OR, HR, and incident RR because the influence of time on mortality and MV could be properly considered, and there were still heterogeneity and missing information about sample size across the involved populations of each study. For continuous variables, MDs and WMDs were used. We used the I2 statistic and derived p values of the Cochran’s Q statistic to assess heterogeneity among studies (Riley et al., 2011). When I2 was greater than 50 or 75%, the heterogeneity was considered to be substantial or considerable, respectively. We also calculated the 95% CI of I2 to assess the uncertainty around the heterogeneity estimates (Ioannidis et al., 2007). Sensitivity analyses were performed to detect the source of heterogeneity and other bias if necessary, hence, to check the robustness of these findings.
Data analyses were performed using Stata (version 14.0) and R software (version 3.5.3) with the “forest” public packages. A two-sided p value <0.05 was considered statistically significant except for in the special cases described above.
Results
Literature Search
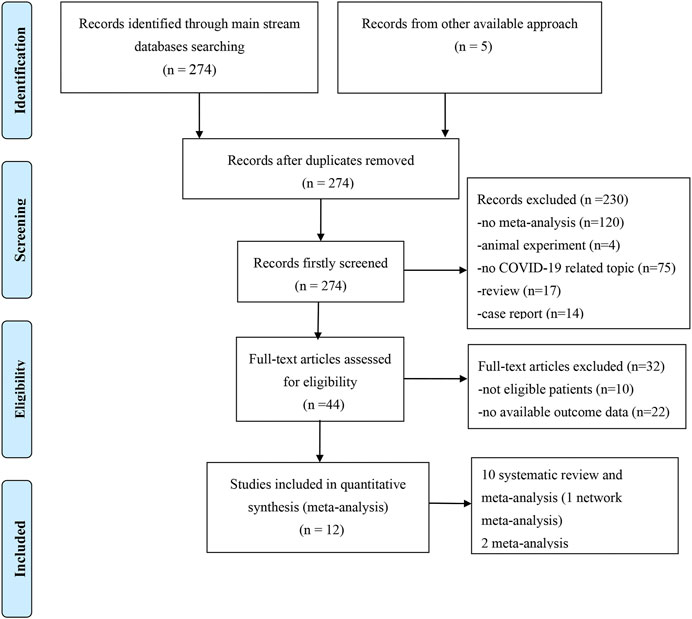
We obtained 279 records from the electronic database search (274 from PubMed, EMBASE, and Cochrane Library; 5 from the manual search of the reference list). After excluding the five duplicated studies, 230 studies were removed by screening titles and abstracts and 32 were removed after assessment of the full text. Ultimately, 12 meta-analyses with/without systematic reviews were included for further analysis (Figure 1). No studies from preprint platforms were included, considering the lack of a strict peer-review process.
Characteristics of the Included Studies
The main characteristics of the included studies are summarized in Table 1. Among 12 eligible studies, all of them (Budhathoki et al., 2020; Cano et al., 2020; Cheng et al., 2020; Lu et al., 2020; Pasin et al., 2020; Pei et al., 2020; Sarkar et al., 2020; Siemieniuk et al., 2020; Sterne et al., 2020; Tlayjeh et al., 2020; van Paassen et al., 2020; Ye et al., 2020) reported mortality; six studies (Budhathoki et al., 2020; Cheng et al., 2020; Pasin et al., 2020; Siemieniuk et al., 2020; Tlayjeh et al., 2020; van Paassen et al., 2020) reported MV; five studies (Budhathoki et al., 2020; Cheng et al., 2020; Lu et al., 2020; Sarkar et al., 2020; Siemieniuk et al., 2020) reported the outcome duration of hospital stay; two studies (Cheng et al., 2020; Sarkar et al., 2020) reported VCT; two studies (Budhathoki et al., 2020; Cheng et al., 2020) reported clinical improvement; one study (Cheng et al., 2020) reported ICU stay duration; one study (Sterne et al., 2020) reported AE outcomes; one study (Budhathoki et al., 2020) reported negative conversion time; and one study (Budhathoki et al., 2020) reported hospitalization. Notably, Budhathoki et al. (Budhathoki et al., 2020) conducted subgroup analyses on invasive MV and non-invasive MV. Cano et al. (Cano et al., 2020), Cheng et al. (Cheng et al., 2020), Pasin et al. (Pasin et al., 2020), Sterne et al. (Sterne et al., 2020), Tlayjeh et al. (Tlayjeh et al., 2020), and Ye et al. (Ye et al., 2020) analyzed mortality for severe vs. non-severe COVID-19, for high dose vs. low dose of corticosteroids, for MV requirement vs. no MV requirement, and for critically ill COVID-19. Cheng et al. (Cheng et al., 2020) reported VCT for severe and non-severe COVID-19. The drug dose was based on defined cutoffs: 15 mg/d dexamethasone, 400 mg/d hydrocortisone, and 1 mg/kg methylprednisolone or equivalent corticosteroids, as described in the primary studies (Annane et al., 2017). Patients who needed MV were assigned to critically ill/severe COVID-19, and patients with no MV or no oxygen requirement were assigned to non-severe COVID-19. The study number of each comparison varied from 1 to 27, and Sarkar et al. (Sarkar et al., 2020) conducted the largest review incorporating the most patients. Among the 12 articles, 10 (Budhathoki et al., 2020; Cano et al., 2020; Cheng et al., 2020; Lu et al., 2020; Pei et al., 2020; Sarkar et al., 2020; Siemieniuk et al., 2020; Tlayjeh et al., 2020; van Paassen et al., 2020; Ye et al., 2020) were systematic reviews and meta-analyses, 2 (Pasin et al., 2020; Sterne et al., 2020) were meta-analyses, and 1 (Siemieniuk et al., 2020) was a network meta-analysis; three studies (Pasin et al., 2020; Siemieniuk et al., 2020; Sterne et al., 2020) involved only randomized controlled trials (RCTs), while the others included not only RCTs but also observational studies or case series. All studies applied accurate quality assessment tools to evaluate the studies’ quality; however, only two studies (Pasin et al., 2020; Sterne et al., 2020) provided clear registration information. All eligible meta-analyses used summary-level data based on the published literature, and none provided or used individual participant data. The publication year was 2020.
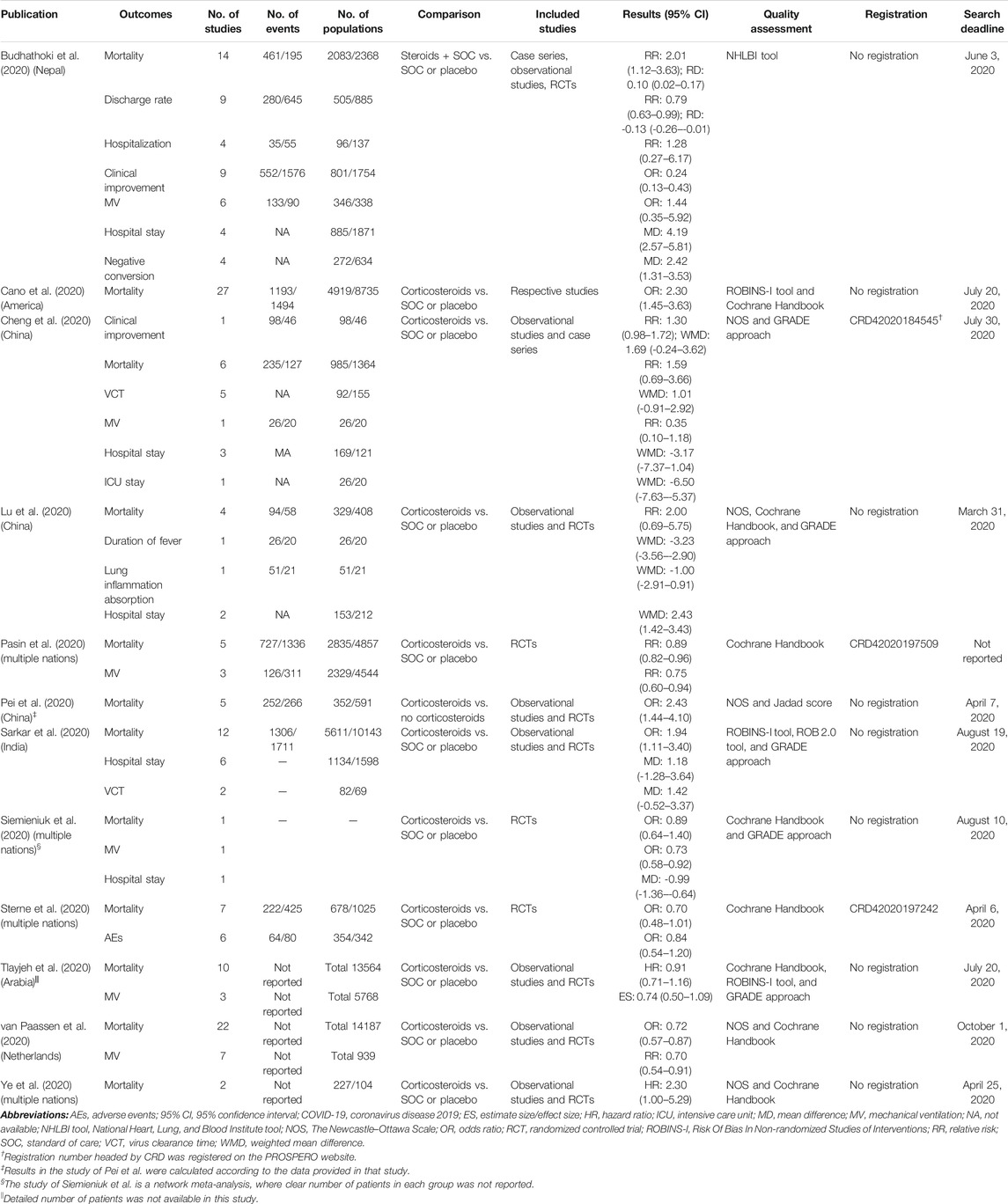
TABLE 1. Main characteristics of included meta-analyses and/or systematic reviews that evaluate systematic corticosteroid use on COVID-19.
Summary of the Main Results of the Outcomes
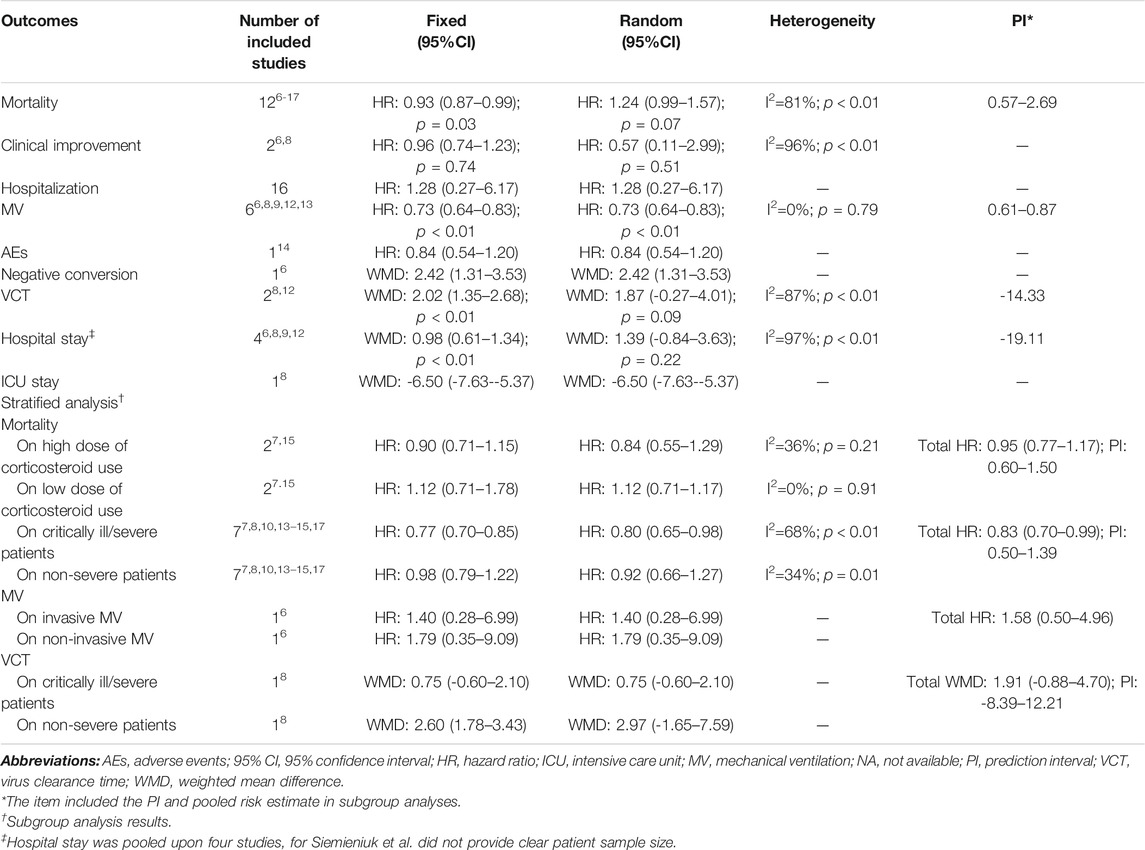
The 12 meta-analyses with 12 kinds of outcomes and 34 comparisons were summarized and reperformed using both fixed- and random-effects models (Table 2). Both fixed and random p values <0.05 were found in 13 comparisons that incorporated outcomes of mortality, discharge rate, clinical improvement, hospital stay, negative conversion, and MV. Four comparisons (discharge rate, mortality, MV) under the random-effects model illustrated significant benefits from corticosteroids, and nine comparisons (mortality, clinical improvement, hospital stay, negative conversion) with the random-effects model illustrated harms. The largest study with the smallest SE for each comparison suggested that 25 of 34 comparisons were significant at a p value <0.05. After excluding null values of 95% CI, no association between corticosteroid use and outcomes of interest was found. Ten comparisons (37%, 10/27) of outcomes revealed low heterogeneity (I2 ≤ 50%), 5 (18.5%, 5/27) revealed substantial heterogeneity (50% < I2 ≤ 75%), and 12 (44.4%, 12/27) showed considerable heterogeneity estimates (I2 > 75%) (Table 3).
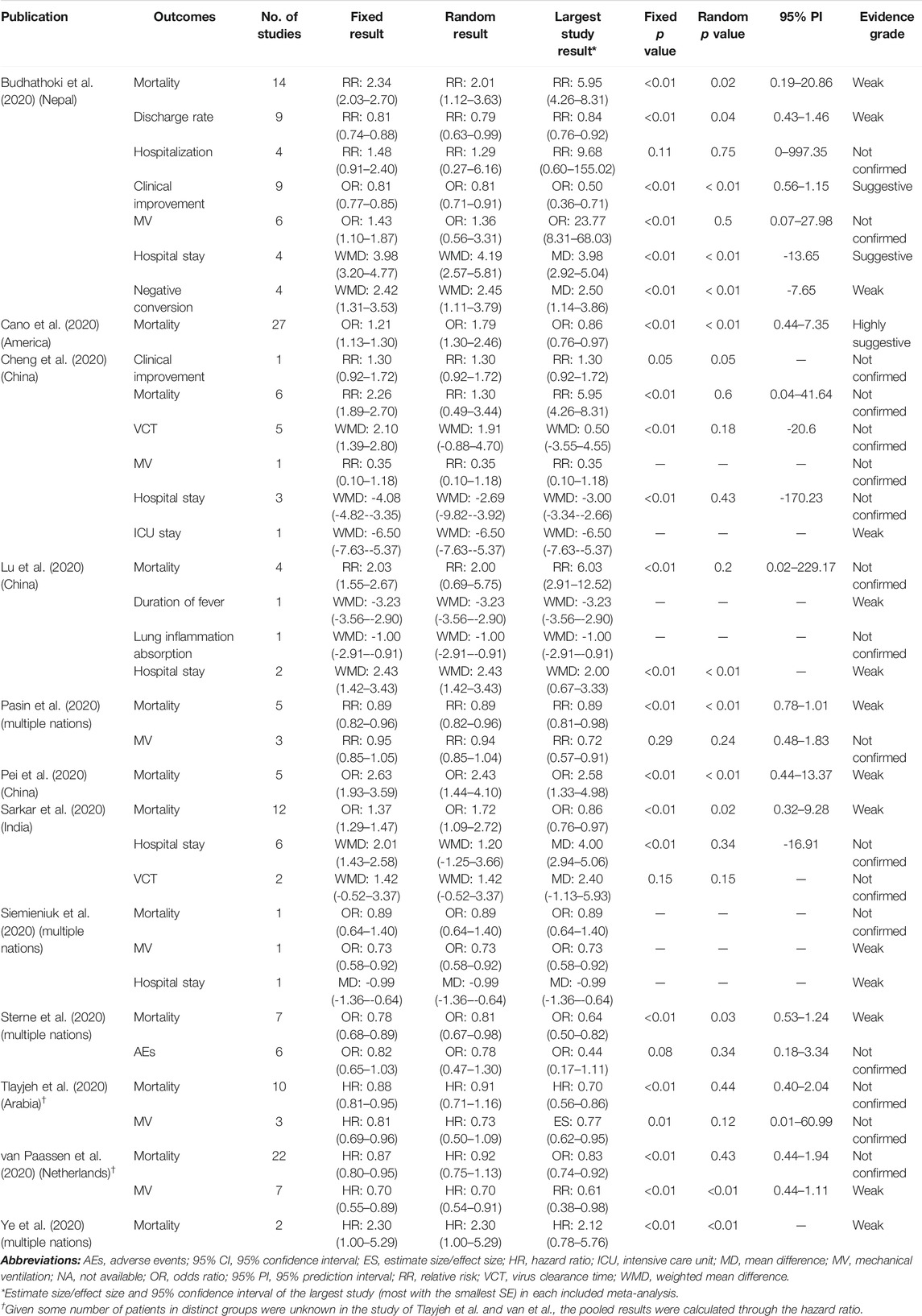
TABLE 2. Descriptions of the risk estimates of 34 primary comparisons included in umbrella meta-analysis.
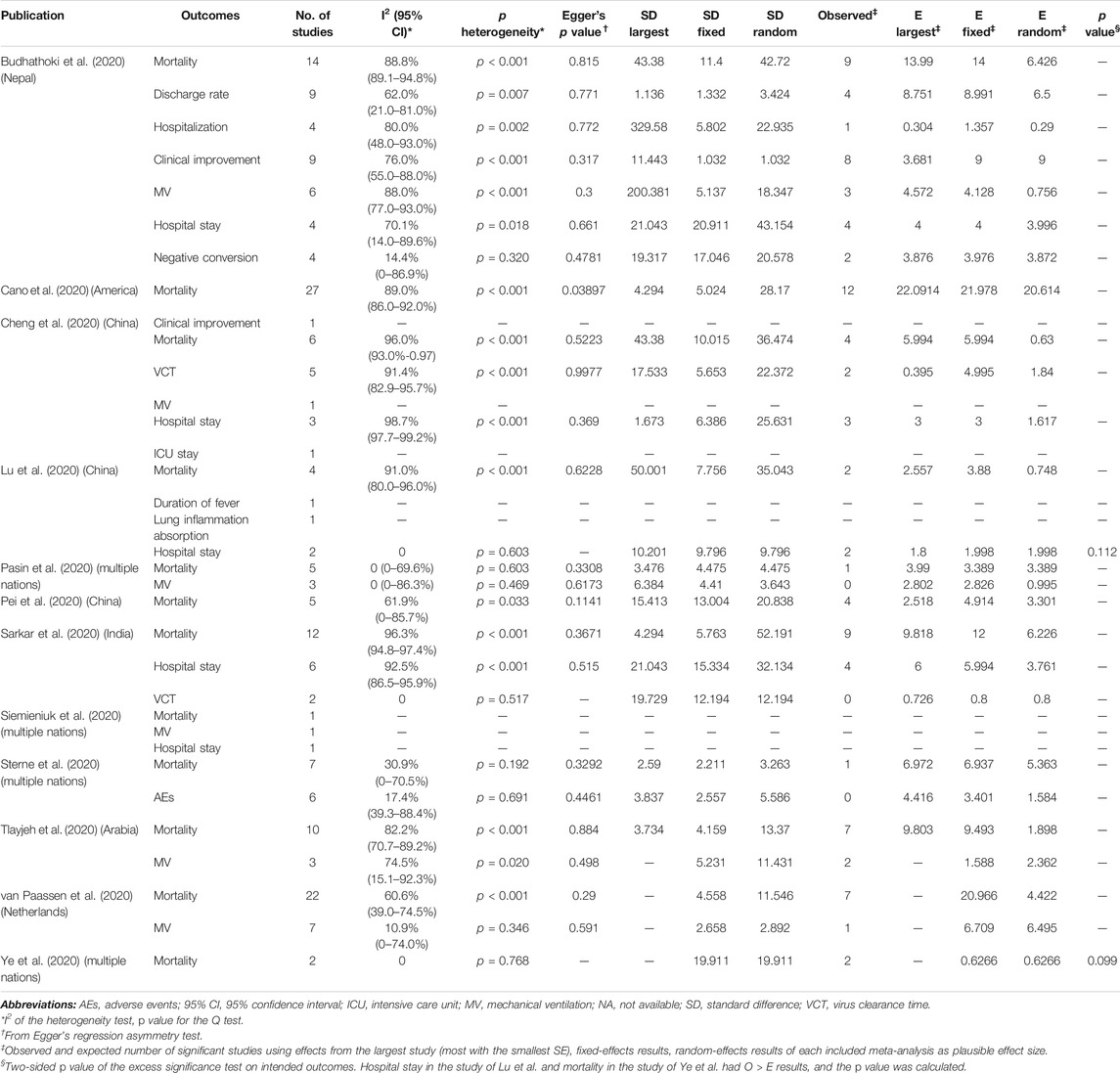
TABLE 3. Evaluation of bias and heterogeneity of 34 primary comparisons evaluating systematic corticosteroid use on COVID-19.
Small-Study Effects
With the exception of Cano et al. (Cano et al., 2020), who analyzed corticosteroid use on mortality, there was no evidence for the exhibition of small-study effects according to Egger’s test (Table 3). However, there were only five comparisons including no less than 10 studies, which provided enough statistical power to identify the small-study effects through Egger’s test.
Excessive Significance Bias
O and E for the largest study, summary effect size on fixed effects, and summary effect size on random effects were rigorously calculated. Only O > all three kinds of plausible E with p < 0.10 was thought to have excessive significance bias. O > E was found by Lu et al. (Lu et al., 2020) for hospital stay and Ye et al. (Ye et al., 2020) for mortality, p = 0.112 and p = 0.099, respectively. Therefore, only mortality reported by Ye et al. (Ye et al., 2020) showed evidence of excessive significance bias, although the effect was slight (Table 3).
Pooled Results From Each Eligible Study
We extracted subgroup outcomes from the eligible studies, and there were critically ill/severe and non-severe patients. Eight meta-analyses (Budhathoki et al., 2020; Cano et al., 2020; Cheng et al., 2020; Pasin et al., 2020; Siemieniuk et al., 2020; Sterne et al., 2020; Tlayjeh et al., 2020; Ye et al., 2020) reported 22 subgroup analyses, and only four comparisons had significant results on the primary effect size, effect size on fixed models, and effect size on random models. Four comparisons of mortality were significant at p < 0.05 in the random-effects model: one suggested no benefits from corticosteroids for severe COVID-19 and the other three showed a controversial status regarding the MV-required and no MV/oxygen–required patients (Table 4).
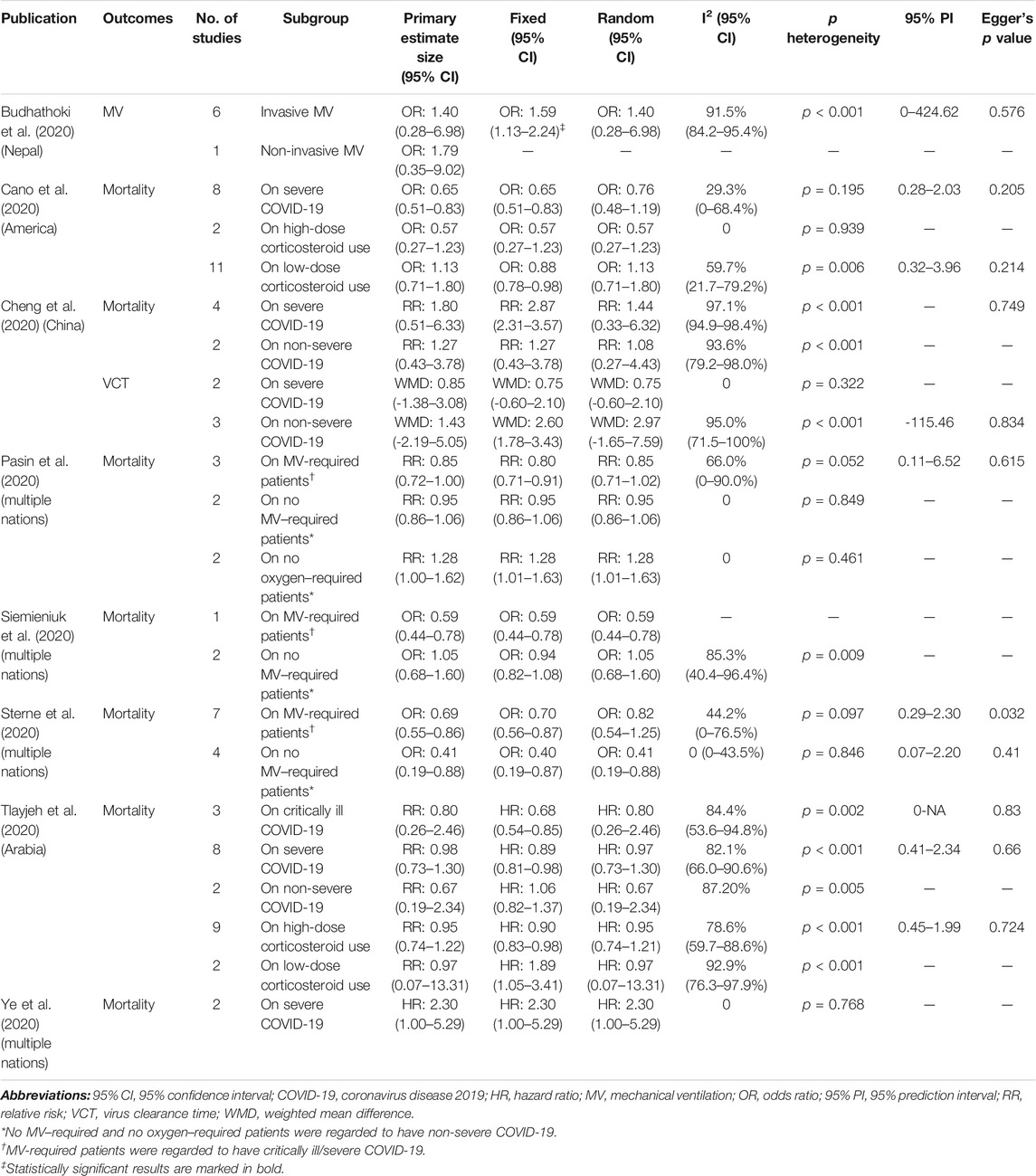
TABLE 4. Description of available subgroup results on outcomes of interest reported by included meta-analysis.
By synthesizing all outcomes of interest together, corticosteroid use was associated with a 27% risk reduction of MV (HR: 0.73, 95% CI: 0.64–0.83; p < 0.01 for random-effects models) with low evidence of heterogeneity and with a 20% risk reduction of mortality of critically ill/severe patients (HR: 0.80, 95% CI: 0.65–0.98 for random-effects models) with substantial heterogeneity. Interestingly, we also observed a shortened ICU stay; however, there was a signal of prolonged negative conversion, VCT, and hospital stay in COVID-19 patients who used corticosteroids (Figures 2–4, Table 5, and Supplementary Appendix Figures A1–A3). There was no significant impact of different corticosteroid doses on mortality (HR: 0.84, 95% CI: 0.55–1.29 for high dose; HR: 1.12, 95% CI: 0.71–1.17 for low dose), and a significant impact on other outcomes of interest was not detected.
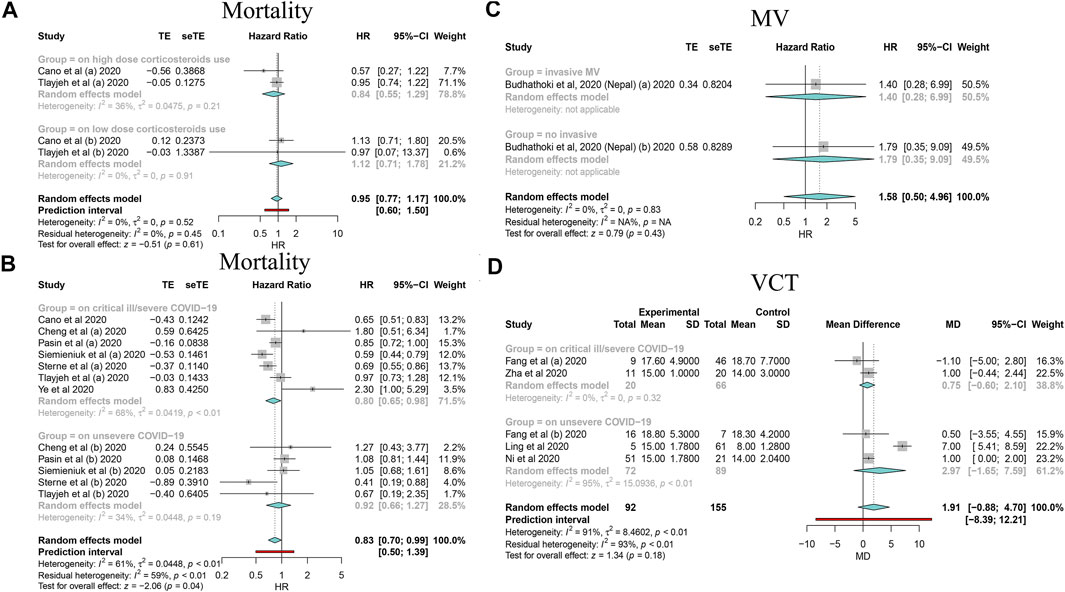
FIGURE 2. Forest plots based on random effects showing subgroup analysis results on the association between corticosteroid use and mortality, MV, and VCT. (A) Subgroup relating to corticosteroid use dose; (B) subgroup relating to COVID-19 severity; (C) subgroup relating to MV type; (D) subgroup relating to COVID-19 severity.
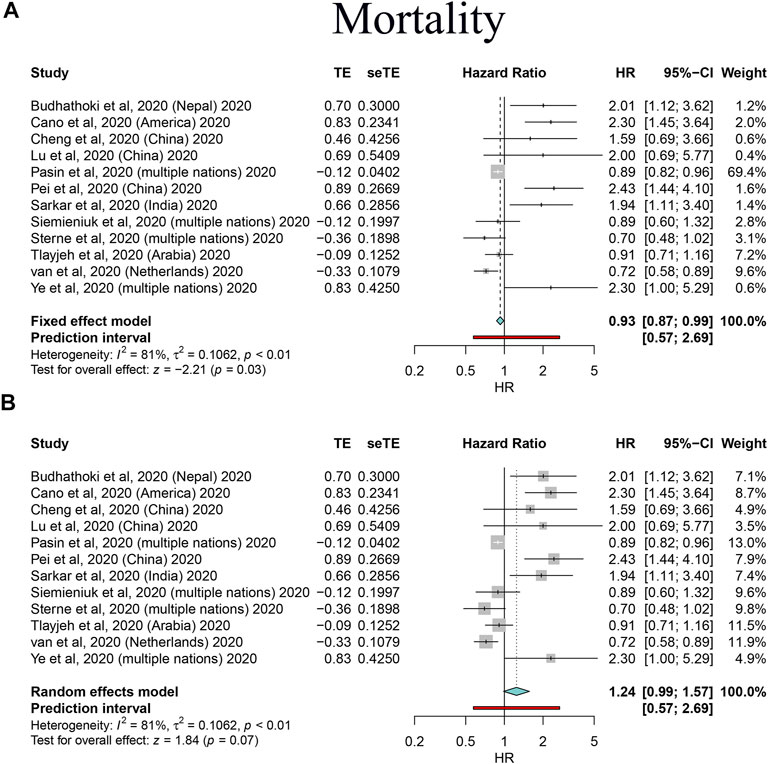
FIGURE 3. Forest plots based on both fixed and random effects showing main results on the association between corticosteroid use and mortality. (A) Results based on the fixed-effects model; (B) results based on the random-effects model.
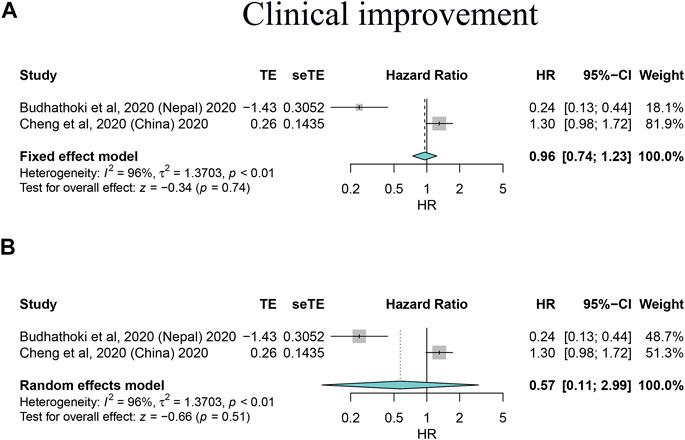
FIGURE 4. Forest plots based on both fixed and random effects showing main results on the association between corticosteroid use and clinical improvement. (A) Results based on the fixed-effects model; (B) results based on the random-effects model.
Great heterogeneity was ascertained in several analyses; thus, we performed sensitivity analyses to control inconsistency and check other sources of bias by omitting heterogeneous studies. We found corticosteroid use was associated with increased mortality in total patients not only in the fixed-effects model (HR: 2.13, 95% CI: 1.69–2.69) but also in the random-effects models (HR: 2.13, 95% CI: 1.69–2.69); however, the use was strongly associated with reduced mortality in critically ill/severe COVID-19 patients (HR: 0.76, 95% CI: 0.68–0.84 for fixed-effects models, and HR: 0.74, 95% CI: 0.63–0.88 for random-effects models), and evidence of negative correlation was even consolidated by sensitivity analyses after omitting studies; prolonged hospital stay still existed and was confirmed (WMD: 3.39, 95% CI: 2.77–4.01 for fixed-effects models, and HR: 3.54, 95% CI: 2.34–4.74 for random-effects models). Overall, our main findings were further consolidated by the sensitivity analyses that also helped to control across-study heterogeneity to some extent (Table 6).
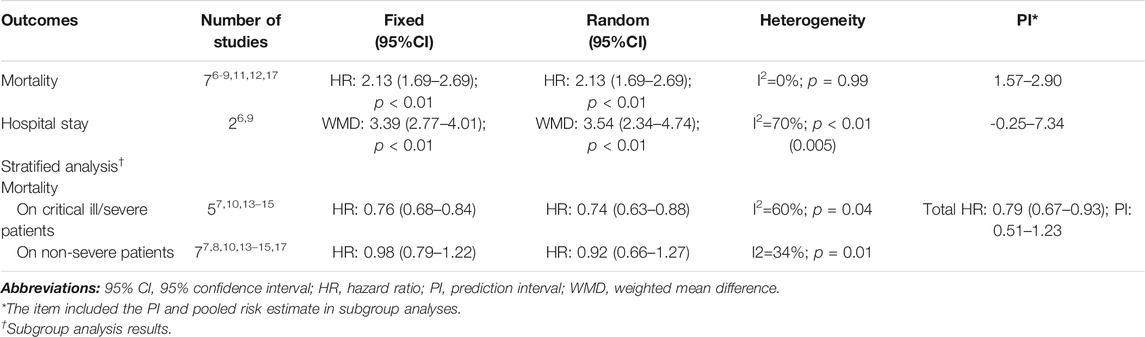
TABLE 6. Sensitivity analyses on the summarized pooled outcomes and subgroup analysis results by omitting studies of great heterogeneity.
Grading the Evidence
In this part, the effect size p value for each comparison was mainly focused on the random effects. Comparing corticosteroid to no corticosteroid use, 14 comparisons (41.2%) were supported by weak evidence, 2 (5.9%) were supported by suggestive evidence, and 1 (2.9%) was supported by highly supported evidence (Table 2). The others were not confirmed. Based on the evidence grade, most comparisons were based on weak evidence; therefore, the interpretation should be made with caution.
Discussion
As available and affordable immunomodulators, corticosteroid use and the interaction with COVID-19 outcomes of interest are of major interest in the administration of these drugs to COVID-19 patients. This timely umbrella meta-analysis mainly found benefits from corticosteroids on a 27% MV risk reduction and a 20% mortality risk reduction of critically ill/severe COVID-19 patients. Surprisingly, corticosteroid use was also significantly associated with a decreased ICU stay time; however, it seemed to lead to a prolonged hospital stay and a longer VCT and negative conversion time. The dose of corticosteroids had little impact on mortality. Corticosteroids were less correlated with other outcomes, including total mortality, clinical improvement, hospitalization, and AEs. Considering the weak evidence, a precise association should be further estimated in large-scale trials.
In asymptomatic or mild COVID-19, an effective immune response could be elicited; however, in severe SARS-CoV-2 infection, the immune response is always not satisfactory and leads to progressive pulmonary damage in the form of acute respiratory distress syndrome (ARDS) or hyperinflammatory status and subsequent multiorgan dysfunction (Azkur et al., 2020; Tay et al., 2020). During this process, a damage-associated molecular pattern is constructed, which further triggers the release of an array of proinflammatory cytokines and chemokines, including IL-6, interferon-γ–induced protein 10, macrophage inflammatory proteins 1α and 1β, and monocyte chemoattractant protein 1. As more monocytes, macrophages, and T cells assemble, aggressive inflammation is promoted (Huang et al., 2020). Therefore, corticosteroids can serve as a key factor to eliminate the inflammatory microenvironment.
Corticosteroids result in lower mortality among critically ill/severe patients, which is consistent with the real situation of patients in clinical practice (Shang et al., 2020). The results of the RECOVERY trial suggest that the risk of death was reduced by 12.1% among patients prescribed a low dose of dexamethasone and receiving invasive MV at randomization (Horby et al., 2020). However, the RECOVERY trial is limited by methodological flaws such as the absence of stratification and incomplete information associated with mortality, which caused heterogeneity at the randomization level (Normand, 2020). In our study, those patients were assigned to critically ill/severe COVID-19 patients. Dramatically, a large number of ongoing clinical trials involving corticosteroids in critically ill patients with COVID-19 quit recruitment after these findings were publicly available because an equipoise for withholding corticosteroids was no longer present. These real-world results from different clinical and geographic settings suggest that, without compelling contraindications, a rigorous corticosteroid regimen can be considered for critically ill/severe patients with COVID-19 as a component of SOC.
However, the findings contrast with outcomes reported for the administration of corticosteroids among patients with influenza, whose mortality and hospital-acquired infections were improved with corticosteroid administration (Lansbury et al., 2020). In the current study, it was impossible to pinpoint whether the distinct populations were critically ill at the time of randomization. Patients might represent a spectrum of illnesses among patients requiring supplementary oxygen by nasal prongs to those with non-invasive MV supported by a form of high-flow oxygen or positive pressure delivered by a mask (Docherty et al., 2020). Different MV or oxygen–requiring types contributed to the heterogeneity among patients. Another problem is that corticosteroid-induced complications could not be evaluated robustly given the limitations of the available data and various definitions/assessment methods across studies. However, the synthesized AEs were likely to be comparable in both patients randomized to corticosteroids and no corticosteroids based on this study (HR: 0.84, 95% CI: 0.54–1.20).
An interesting finding is that corticosteroid use is associated with a decreased ICU stay time as well as a prolonged hospital stay, VCT, and negative conversion. The decreased ICU stay time suggested that corticosteroids seemed to be effective in critically ill/severe patients with COVID-19, which was also addressed in the current study. Agents of corticosteroids are thought to have higher lung tissue-to-plasma ratios and accordingly can be used in lung injury models (Annane et al., 2017). These agents administrated in the ICU to cure ARDS of critically ill patients were associated with a shorter ICU stay (Annane et al., 2017). Early after the outbreak of COVID-19, the WHO recommended against the routine use of systematic corticosteroids for COVID-19 patients due to acknowledged AEs and a prolonged VCT. Reviewing the AEs, they were found to downregulate immunity, which can explain the prolonged hospital stay and negative conversion (Felsenstein et al., 2020). Similar results in a retrospective study suggested corticosteroids were associated with delayed MERS coronavirus RNA clearance (Arabi et al., 2018). Additionally, studies also showed corticosteroid use was associated with longer throat viral RNA detectability and acute respiratory syndrome coronavirus 2 shedding in mild-to-moderate COVID-19 patients, and clinical benefits on these patients were limited (Jamaati et al., 2021; Tang et al., 2021). Immune response and CD8+ T cell infiltration were involved in the COVID-19 pathological process, and reduction of CD4+ T cells, CD8+ T cells, and NK cells was observed on day 7 after corticosteroid use (Du et al., 2020). The dysregulated immune response and suppressive immune cells, therefore, may contribute to retarding virus shedding and a longer VCT and negative conversion. With the exception of the elusive status of corticosteroids on COVID-19, there are considerable benefits for ARDS, and a meta-analysis showed that the mixed use of hydrocortisone and methylprednisolone offered a reduced time to extubation, a shorter hospital stay, and less mortality with an increase in MV-free days and ICU-free days (Hashimoto et al., 2017). The proposed doses for methylprednisolone are 1–2 mg/kg, followed by the same daily dose at an infusion rate of 10 ml/h daily with a gradual taper in this setting (Meduri et al., 2018; Villar et al., 2020). Another study supported that methylprednisolone at 2 mg/kg is superior than dexamethasone at 6 mg/kg with lower MV and reduced hospital stay due to hypothesized higher lung tissue-to-plasma ratios in methylprednisolone (Ranjbar et al., 2021). An individual patient analysis investigating corticosteroid use on early and late ARDS demonstrated improved survival and a decreased duration of MV (Meduri et al., 2016). Based on current and previous evidence, the Society of Critical Medicine/European Society of Intensive Care Medicine guidelines recommend the early prescription of corticosteroids for moderate-to-severe ARDS (Annane et al., 2017); similarly, the Chinese National Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (available at http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml) published by the Chinese National Health Committee set the initial recommendations for methylprednisolone in patients with clinically progressive deterioration. Other international societies and organizations, such as the American Thoracic Society, the Infectious Disease Society of America, the National Institute of Health of the United States, the Surviving Sepsis Campaign, and the WHO, have also mentioned recommendations for corticosteroids in COVID-19 patients (Cano et al., 2020; Lane and Fauci, 2021).
Implications and Limitations
To our best knowledge, this is the first umbrella meta-analysis that summarizes the current secondary and quantitative evidence. This study expands the strength of the data and the comprehensive synthesis results, extends the understanding of the role of corticosteroid use, and potentially offers a high-level reference for the management of COVID-19. This study also has several limitations: (1) Most of the incorporated evidence was estimated as weak, and there was considerable heterogeneity among patients involved in each meta-analysis. The performed sensitivity analyses consolidated the robustness of such findings and strongly supported benefits from corticosteroid use on critically ill/severe patients, and we still recommend coming umbrella reviews based on meta-analyses incorporating high-quality RCTs. And the implications of the current evidence need to be combined with the actual clinical situation or validated in high-quality trials. (2) The optimal dose and duration of treatment could not be assessed in this analysis; however, no clear evidence of the effects of different doses of corticosteroids associated with COVID-19 mortality was found in this study. Discrepancies derived from corticosteroid type and administration methods were difficult to address. These problems needed to be further investigated in original studies advent. (3) Some results were limited by missing outcome data, and the definitions of the outcomes of interest were not consistent across the primary studies in each meta-analysis. In the future, the one-dose-fits-all strategy is unlikely to be endorsed, and precise stratification analyses are needed to investigate the proper dose or duration and its real efficacy in pediatrics. It would be interesting to analyze the cost-effectiveness status of wide corticosteroid use on COVID-19 due to its available role in such a pandemic.
Conclusion
The current umbrella synthesis indicates that corticosteroid use is associated with reduced MV and mortality in critically ill/severe COVID-19 patients, and corticosteroid use leads to a decreased ICU stay but possibly a prolonged hospital stay, a longer VCT, and a longer time to negative conversion. There was no clear evidence of the impact of different corticosteroid doses on mortality. Considering the inconsistency in these results, the benefits and harms of corticosteroids should be reevaluated. Since corticosteroids are affordable and accessible in health care, corticosteroid use may be recommended in critically ill patient care without steadily increasing the total mortality under the pressure of such a rapid global outbreak.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Author Contributions
LZ had full access to all of the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors prepared the concept and design, performed acquisition, analysis, and interpretation of data, and read and approved the final manuscript. BC, JM, and LZ drafted the manuscript and performed statistical analysis. BC, JM, YY, TS, and BZ critically revised the manuscript for important intellectual content. BC and LZ supervised the work.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC, Grant number: 81660015) and the Key Research and Development Program of Jiangxi Province (Grant number: 20171BBG70018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank original data from previous studies and the authors who are glad to share these data in publications. The authors thank efforts from medical practitioners fighting against the COVID-19 pandemic.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.670170/full#supplementary-material
Abbreviations
AEs, adverse events; ARDS, acute respiratory distress syndrome; 95% CI, 95% confidence interval; COVID-19, coronavirus disease 2019; E, expected number of studies; HR, hazard ratio; ICU, intensive care unit; MD, mean difference; MV, mechanical ventilation; O, observed number of studies; OR, odds ratio; RCTs, randomized controlled trials; RR, relative risk; SE, standard error; SOC, standard of care; VCT, virus clearance time; WHO, World Health Organization; WMD, weighted mean difference.
References
Annane, D., Pastores, S. M., Arlt, W., Balk, R. A., Beishuizen, A., Briegel, J., et al. (2017). Critical Illness-Related Corticosteroid Insufficiency (CIRCI): a Narrative Review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med. 43 (12), 1781–1792. doi:10.1007/s00134-017-4914-x
Annane, D., Pastores, S. M., Rochwerg, B., Arlt, W., Balk, R. A., Beishuizen, A., et al. (2017). Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 43 (12), 1751–1763. doi:10.1007/s00134-017-4919-5
Arabi, Y. M., Mandourah, Y., Al-Hameed, F., Sindi, A. A., Almekhlafi, G. A., Hussein, M. A., et al. (2018). Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 197 (6), 757–767. doi:10.1164/rccm.201706-1172oc
Aromataris, E., Fernandez, R., Godfrey, C. M., Holly, C., Khalil, H., and Tungpunkom, P. (2015). Summarizing Systematic Reviews. Int. J. Evid. Based Healthc. 13 (3), 132–140. doi:10.1097/xeb.0000000000000055
Azkur, A. K., Akdis, M., Azkur, D., Sokolowska, M., Veen, W., Brüggen, M. C., et al. (2020). Immune Response to SARS‐CoV‐2 and Mechanisms of Immunopathological Changes in COVID‐19. Allergy 75 (7), 1564–1581. doi:10.1111/all.14364
Budhathoki, P., Shrestha, D. B., Rawal, E., and Khadka, S. (2020). Corticosteroids in COVID-19: Is it Rational? A Systematic Review and Meta-Analysis. SN Compr. Clin. Med. 2 (12), 2600–2620. doi:10.1007/s42399-020-00515-6
Cano, E. J., Fuentes, X. F., Campioli, C. C., O'Horo, J. C., Saleh, O. A., Odeyemi, Y., et al. (2020). Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes: Systematic Review and Meta-Analysis. Chest 159(3), 1019-1040. doi:10.1016/j.chest.2020.10.054
Cheng, W., Li, Y., Cui, L., Chen, Y., Shan, S., Xiao, D., et al. (2020). Efficacy and Safety of Corticosteroid Treatment in Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Pharmacol. 11, 571156. doi:10.3389/fphar.2020.571156
Docherty, A. B., Harrison, E. M., Green, C. A., Hardwick, H. E., Pius, R., Norman, L., et al. (2020). Features of 20 133 UK Patients in Hospital with Covid-19 Using the ISARIC WHO Clinical Characterisation Protocol: Prospective Observational Cohort Study. BMJ 369, m1985. doi:10.1136/bmj.m1985
Du, R.-H., Liang, L.-R., Yang, C.-Q., Wang, W., Cao, T.-Z., Li, M., et al. (2020). Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARS-CoV-2: a Prospective Cohort Study. Eur. Respir. J. 55 (5), 2000524. doi:10.1183/13993003.00524-2020
Felsenstein, S., Herbert, J. A., McNamara, P. S., and Hedrich, C. M. (2020). COVID-19: Immunology and Treatment Options. Clin. Immunol. 215, 108448. doi:10.1016/j.clim.2020.108448
Hashimoto, S., Sanui, M., Egi, M., Ohshimo, S., Shiotsuka, J., Seo, R., et al. (2017). The Clinical Practice Guideline for the Management of ARDS in Japan. J. Intensive Care 5, 50. doi:10.1186/s40560-017-0222-3
Ho, J. C., Ooi, G. C., Mok, T. Y., Chan, J. W., Hung, I., Lam, B., et al. (2003). High-dose Pulse versus Nonpulse Corticosteroid Regimens in Severe Acute Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 168 (12), 1449–1456. doi:10.1164/rccm.200306-766oc
Holshue, M. L., DeBolt, C., Lindquist, S., Lofy, K. H., Wiesman, J., Bruce, H., et al. (2020). First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 382 (10), 929–936. doi:10.1056/nejmoa2001191
Horby, P., Horby, P., Lim, W. S., Emberson, J. R., Mafham, M., Bell, J. L., et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N. Engl. J. Med. 2020 384, 693-704. doi:10.1056/NEJMoa2021436
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. The Lancet 395 (10223), 497–506. doi:10.1016/s0140-6736(20)30183-5
Ioannidis, J. P. A. (2013). Clarifications on the Application and Interpretation of the Test for Excess Significance and its Extensions. J. Math. Psychol. 57 (5), 184–187. doi:10.1016/j.jmp.2013.03.002
Ioannidis, J. P. A., Patsopoulos, N. A., and Evangelou, E. (2007). Uncertainty in Heterogeneity Estimates in Meta-Analyses. BMJ 335 (7626), 914–916. doi:10.1136/bmj.39343.408449.80
Ioannidis, J. P., and Trikalinos, T. A. (2007). An Exploratory Test for an Excess of Significant Findings. Clin. trials 4 (3), 245–253. doi:10.1177/1740774507079441
Jamaati, H., Hashemian, S. M., Farzanegan, B., Malekmohammad, M., Tabarsi, P., Marjani, M., et al. (2021). No Clinical Benefit of High Dose Corticosteroid Administration in Patients with COVID-19: A Preliminary Report of a Randomized Clinical Trial. Eur. J. Pharmacol. 897, 173947. doi:10.1016/j.ejphar.2021.173947
Kalliala, I., Markozannes, G., Gunter, M. J., Paraskevaidis, E., Gabra, H., Mitra, A., et al. (2017). Obesity and Gynaecological and Obstetric Conditions: Umbrella Review of the Literature. BMJ 359, j4511. doi:10.1136/bmj.j4511
Lane, H. C., and Fauci, A. S. (2021). Research in the Context of a Pandemic. N. Engl. J. Med. 384 (8), 755–757. doi:10.1056/nejme2024638
Lansbury, L. E., Rodrigo, C., Leonardi-Bee, J., Nguyen-Van-Tam, J., and Shen Lim, W. (2020). Corticosteroids as Adjunctive Therapy in the Treatment of Influenza. Crit. Care Med. 48 (2), e98–e106. doi:10.1097/ccm.0000000000004093
Lu, S., Zhou, Q., Zhou, Q., Huang, L., Shi, Q., Zhao, S., et al. (2020). Effectiveness and Safety of Glucocorticoids to Treat COVID-19: A Rapid Review and Meta-Analysis. Ann. Transl Med. 8 (10), 627. doi:10.21037/atm-20-3307
Lubin, J. H., and Gail, M. H. (1990). On Power and Sample Size for Studying Features of the Relative Odds of Disease. Am. J. Epidemiol. 131 (3), 552–566. doi:10.1093/oxfordjournals.aje.a115530
Meduri, G. U., Bridges, L., Shih, M. C., Marik, P. E., Siemieniuk, R. A. C., and Kocak, M. (2016). Prolonged Glucocorticoid Treatment Is Associated with Improved ARDS Outcomes: Analysis of Individual Patients' Data from Four Randomized Trials and Trial-Level Meta-Analysis of the Updated Literature. Intensive Care Med. 42 (5), 829–840. doi:10.1007/s00134-015-4095-4
Meduri, G. U., Siemieniuk, R. A. C., Ness, R. A., and Seyler, S. J. (2018). Prolonged Low-Dose Methylprednisolone Treatment Is Highly Effective in Reducing Duration of Mechanical Ventilation and Mortality in Patients with ARDS. J. Intensive Care 6, 53. doi:10.1186/s40560-018-0321-9
Normand, S. T. (2020). The RECOVERY Platform. N. Engl. J. Med. 384, 757-758. doi:10.1056/NEJMe2025674
Pasin, L., Navalesi, P., Zangrillo, A., Kuzovlev, A., Likhvantsev, V., Hajjar, L. A., et al. (2020). Corticosteroids for Patients with Coronavirus Disease 2019 (COVID-19) with Different Disease Severity: A Meta-Analysis of Randomized Clinical Trials. J. Cardiothorac. Vasc. Anesth. 35 (2), 578–584. doi:10.1053/j.jvca.2020.11.057
Pei, L., Zhang, S., Huang, L., Geng, X., Ma, L., Jiang, W., et al. (2020). Antiviral Agents, Glucocorticoids, Antibiotics, and Intravenous Immunoglobulin in 1142 Patients with Coronavirus Disease 2019: a Systematic Review and Meta-Analysis. Pol. Arch. Intern. Med. 130 (9), 726–733. doi:10.20452/pamw.15543
Ranjbar, K., Moghadami, M., Mirahmadizadeh, A., Fallahi, M. J., Khaloo, V., Shahriarirad, R., et al. (2021). Methylprednisolone or Dexamethasone, Which One Is Superior Corticosteroid in the Treatment of Hospitalized COVID-19 Patients: a Triple-Blinded Randomized Controlled Trial. BMC Infect. Dis. 21 (1), 337. doi:10.1186/s12879-021-06045-3
Riley, R. D., Higgins, J. P. T., and Deeks, J. J. (2011). Interpretation of Random Effects Meta-Analyses. BMJ 342, d549. doi:10.1136/bmj.d549
Sarkar, S., Khanna, P., and Soni, K. D. (2020). Are the Steroids a Blanket Solution for COVID‐19? A Systematic Review and Meta‐analysis. J. Med. Virol. 93, 1538–1547. doi:10.1002/jmv.26483
Shang, L., Zhao, J., Hu, Y., Du, R., and Cao, B. (2020). On the Use of Corticosteroids for 2019-nCoV Pneumonia. The Lancet 395 (10225), 683–684. doi:10.1016/s0140-6736(20)30361-5
Siemieniuk, R. A., Bartoszko, J. J., Ge, L., Zeraatkar, D., Izcovich, A., Kum, E., et al. (2020). Drug Treatments for Covid-19: Living Systematic Review and Network Meta-Analysis. BMJ 370, m2980. doi:10.1136/bmj.m2980
Sterne, J. A. C., Sterne, J. A. C., Murthy, S., Diaz, J. V., Slutsky, A. S., Villar, J., et al. (2020). Association between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients with COVID-19: A Meta-Analysis. JAMA 324 (13), 1330–1341. doi:10.1001/jama.2020.17023
Sterne, J. A. C., Sutton, A. J., Ioannidis, J. P. A., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 343, d4002. doi:10.1136/bmj.d4002
Tang, X., Feng, Y. M., Ni, J. X., Zhang, J. Y., Liu, L. M., Hu, K., et al. (2021). Early Use of Corticosteroid May Prolong SARS-CoV-2 Shedding in Non-intensive Care Unit Patients with COVID-19 Pneumonia: A Multicenter, Single-Blind, Randomized Control Trial. Respiration 100 (2), 116–126. doi:10.1159/000512063
Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A., and Ng, L. F. P. (2020). The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 20 (6), 363–374. doi:10.1038/s41577-020-0311-8
Tlayjeh, H., Mhish, O. H., Enani, M. A., Alruwaili, A., Tleyjeh, R., Thalib, L., et al. (2020). Association of Corticosteroids Use and Outcomes in COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Infect. Public Health 13 (11), 1652–1663. doi:10.1016/j.jiph.2020.09.008
van Paassen, J., Vos, J. S., Hoekstra, E. M., Neumann, K. M. I., Boot, P. C., and Arbous, S. M. (2020). Corticosteroid Use in COVID-19 Patients: a Systematic Review and Meta-Analysis on Clinical Outcomes. Crit. Care 24 (1), 696. doi:10.1186/s13054-020-03400-9
Villar, J., Ferrando, C., Martínez, D., Ambrós, A., Muñoz, T., Soler, J. A., et al. (2020). Dexamethasone Treatment for the Acute Respiratory Distress Syndrome: a Multicentre, Randomised Controlled Trial. Lancet Respir. Med. 8 (3), 267–276. doi:10.1016/S2213-2600(19)30417-5
Wang, Y., Ao, G., Qi, X., and Zeng, J. (2020). The Influence of Corticosteroid on Patients with COVID-19 Infection: A Meta-Analysis. Am. J. Emerg. Med. 43, 267-269. doi:10.1016/j.ajem.2020.06.040
Ye, Z., Wang, Y., Colunga-Lozano, L. E., Prasad, M., Tangamornsuksan, W., Rochwerg, B., et al. (2020). Efficacy and Safety of Corticosteroids in COVID-19 Based on Evidence for COVID-19, Other Coronavirus Infections, Influenza, Community-Acquired Pneumonia and Acute Respiratory Distress Syndrome: a Systematic Review and Meta-Analysis. CMAJ 192 (27), E756–e767. doi:10.1503/cmaj.200645
Zhang, N., Shi, N., Li, S., Liu, G., Han, Y., Liu, L., et al. (2020). A Retrospective Study on the Use of Chinese Patent Medicine in 24 Medical Institutions for COVID-19 in China. Front. Pharmacol. 11, 574562. doi:10.3389/fphar.2020.574562
Keywords: corticosteroids, coronavirus, COVID-19, critically ill/pulmonary fibrosis manifested, umbrella meta-analysis
Citation: Cheng B, Ma J, Yang Y, Shao T, Zhao B and Zeng L (2021) Systemic Corticosteroid Administration in Coronavirus Disease 2019 Outcomes: An Umbrella Meta-Analysis Incorporating Both Mild and Pulmonary Fibrosis–Manifested Severe Disease. Front. Pharmacol. 12:670170. doi: 10.3389/fphar.2021.670170
Received: 24 February 2021; Accepted: 30 April 2021;
Published: 26 May 2021.
Edited by:
Lon J. Van Winkle, Rocky Vista University, United StatesReviewed by:
Sung Hwi Hong, Severance Hospital, South KoreaRamy Abou Ghayda, Case Western Reserve University, United States
Copyright © 2021 Cheng, Ma, Yang, Shao, Zhao and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linxiang Zeng, emVuZ2xpbnhpYW5nMTk3MkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Bin Cheng
Bin Cheng Jinxiu Ma2†
Jinxiu Ma2† Binghao Zhao
Binghao Zhao Linxiang Zeng
Linxiang Zeng