- 1“Stejarul” Research Centre for Biological Sciences, National Institute of Research and Development for Biological Sciences, Piatra Neamt, Romania
- 2Research Centre for Optimal Health, School of Life Sciences, College of Liberal Arts and Sciences, University of Westminster, London, United Kingdom
- 3Pharmacognosy and Phytotherapy, UCL School of Pharmacy, London, United Kingdom
Chemical methods are the most important and widely used traditional plant identification techniques recommended by national and international pharmacopoeias. We have reviewed the successful use of different chemical methods for the botanical authentication of 2,386 commercial herbal products, sold in 37 countries spread over six continents. The majority of the analyzed products were reported to be authentic (73%) but more than a quarter proved to be adulterated (27%). At a national level, the number of products and the adulteration proportions varied very widely. Yet, the adulteration reported for the four countries, from which more than 100 commercial products were purchased and their botanical ingredients chemically authenticated, was 37% (United Kingdom), 31% (Italy), 27% (United States), and 21% (China). Simple or hyphenated chemical analytical techniques have identified the total absence of labeled botanical ingredients, substitution with closely related or unrelated species, the use of biological filler material, and the hidden presence of regulated, forbidden or allergenic species. Additionally, affecting the safety and efficacy of the commercial herbal products, other low quality aspects were reported: considerable variability of the labeled metabolic profile and/or phytochemical content, significant product-to-product variation of botanical ingredients or even between batches by the same manufacturer, and misleading quality and quantity label claims. Choosing an appropriate chemical technique can be the only possibility for assessing the botanical authenticity of samples which have lost their diagnostic microscopic characteristics or were processed so that DNA cannot be adequately recovered.
Introduction
Herbal products are being sold under many and diverse commercial descriptions in the international marketplace, including herbal drugs, botanical drugs, botanicals, phytomedicines, traditional medicines (TMs), herbal medicines (HMs), traditional herbal medicines products (THMPs), natural health products (NHPs), dietary supplements (DSs), plant food supplements (PFSs), nutraceuticals (NCs) and food supplements (FSs) (Ichim, 2019), the differences being mainly due to the prevailing national legislation under which they are marketed (Simmler et al., 2018). Herbal products are commercialized as medicines or foods, according to their officially declared intended final use by their manufacturers operating under various regulatory frameworks, and they are purchased, and subsequently used and consumed, for their medicinal claims (herbal medicines) or their expected health benefits (food supplements) (Thakkar et al., 2020). In the United Kingdom, for example, plant products are regulated under two main criteria, the first being what is claimed, i.e. if a manufacturer claims a medicinal effect, the product will automatically fall under medicines legislation; the second consideration being the activity of the plant in vivo, if it has shown to have a strong medicinal or pharmacological action then it is deemed a medicine regardless of the claims, the most notable plant in this category being Hypericum perforatum L. (St John’s Wort). Whereas in the United States most plant products are regulated as food supplements (botanicals) and in Germany the majority are considered medicines. Unfortunately, these marketing differences, due to significant differences between the regulatory approaches across jurisdictions (Low et al., 2017), are further contributing to their poor regulation on the international market.
Accidental contamination or the deliberate use of filler or substitute species (Shanmughanandhan et al., 2016) leads inherently to non-authentic, adulterated products (Simmler et al., 2018). The adulteration of commercial herbal products is an internationally widespread problem, as it has been reported for many countries from all inhabited continents (Ichim, 2019; Ichim et al., 2020). Moreover, large percentages of adulterated products have been reviewed, irrespective of the formal category of herbal products, being affected food and dietary supplements and medicines altogether (Ichim and de Boer, 2021), including products used in centuries or even millennia-old Ayurveda (Revathy et al., 2012; Seethapathy et al., 2019) and Asian traditional medicine systems (Masada, 2016; Xu et al., 2019). The substantial proportion of adulterated commercial herbal products described appears to be independent of the methods used for their analysis, traditional pharmacopoeial methods being employed, such as macroscopic inspection (van der Valk et al., 2017), microscopy (Ichim et al., 2020), chemical techniques (Li et al., 2008; Upton et al., 2020), or even the more recently developed DNA-based ones, such as the rapidly technologically evolving DNA barcoding and metabarcoding (Ichim, 2019; Grazina et al., 2020).
On the global market, herbal products are sold in an extremely diverse variety of forms, from single ingredient, unprocessed, raw, whole plants to multi-species, highly processed extracts. Therefore, the successful authentication of commercial herbal products reported by peer reviewed studies are a valuable and useful source of information which provide the necessary practicalities, including their strengths and the limitations, of employing the right methods for a specific type of product along the length of its value chain (Booker et al., 2012). Such analyses of peer-reviewed authentication reports focused exclusively on commercial herbal products have concluded that, microscopy, a traditional pharmacopoeial identification method, is cost-efficient and can cope with mixtures and impurities but it has limited applicability for highly processed commercial samples e.g. extracts (Ichim et al., 2020). On the other hand, DNA-based identification, only recently adopted by the first two national Pharmacopoeias (Pharmacopoeia Committee of P. R. China, 2015; British Pharmacopoeia Commission, 2018), facilitate simultaneous multi-taxa identification by using the DNA of different origins extracted from complex mixtures and matrices but false-negatives can be expected if the DNA has been degraded or lost during post-harvest processing or manufacturing (Raclariu et al., 2018a; Ichim, 2019; Grazina et al., 2020). In this respect, our review adds the much needed peer-reviewed, systematically searched information, about the successful use of chemical identification for the authentication of commercial herbal products. While doing so, our review also provides some missing pieces of the commercial herbal products’ authenticity puzzle.
Methods
Databases
Search Strategy
Four databases were systematically searched for peer reviewed records following the PRISMA guidelines (Moher et al., 2009) using combinations of relevant keywords, Boolean operators and wildcards: [(“herbal product” OR “herbal medicine” OR “traditional medicine” OR “food supplement” OR “dietary supplement” OR “herbal supplement” OR nutraceutical) AND (authentic* OR contaminat* OR substitut*)] for Web of Science, PubMed, Scopus, and [(“herbal product” OR “herbal medicine” OR “food supplement” OR “dietary supplement” OR “herbal supplement” OR nutraceutical) AND (authentication OR contamination OR substitution)] for ScienceDirect. The option “search alert” was activated for all four databases, to receive weekly updates after the literature search was performed. Furthermore, we used cross-referencing to identify additional peer-reviewed publications.
Selection Process and Criteria
Identification: 10,497 records were identified through database searching (WoS = 1,317, PubMed = 3,253, Scopus = 5,446, and ScienceDirect = 481), and 196 additional records from cross-referencing and the weekly updates from the four databases. Screening: after the duplicates had been removed, 2,326 records were collected and their abstracts screened. After screening, 1,745 records were excluded for not reporting data relevant for the chemical authentication of herbal products. Eligibility: 581 full-text articles were assessed and screened based on the following eligibility criteria: 1) The reported products had to be “herbal products”; the full wide range of commercial names was searched for and accepted for being included in our analysis. 2) The analyzed products had to be “commercial”; keywords such as “purchased”, “bought”, were accepted. Our analysis excluded samples which were obtained “cost-free”, a “gift” or “donated” by a person, institution or company. 3) The products had to be clearly allocated to a “country” or “territory” (e.g., European Union). 4) The conclusion “authentic”/“adulterated” had to be drawn by the authors of the analyzed studies. 5) The products had to be analyzed with a “chemical” method or techniques.
The set of retrieved full-text articles was further reduced by 446 that did not meet all eligibility criteria. Included: 135 records.
Results
Different chemical methods have been successfully employed for the botanical authentication of 2,386 commercial herbal products, sold in 37 countries spread on six continents. The majority of the analyzed products were reported to be authentic (73%) but more than a quarter proved to be adulterated (27%), when the botanical identity of their content was compared with the label stated ingredients (Table 1).

TABLE 1. The authenticity of the chemically authenticated commercial herbal products at global level.
The herbal products were purchased from 37 countries scattered over six continents: Europe (n = 20), Asia (n = 9), North America (n = 3), Australia (n = 2), South America (n = 2), and Africa (n = 1) (Supplementary Table S1). The numbers of reported samples were geographically heterogeneous, at continental level the highest number of commercial herbal products was reported for Asia (n = 877), North America (n = 767), Europe (n = 573), followed distantly by South America (n = 86), Australia (n = 25) and Africa (n = 5). The proportion of adulterated products varies significantly among continents, being highest in Africa (60%), South America (57%), Australia (44%), and lower in Europe (28%), North America (27%), and Asia (25%). The adulteration percentage of the last three continents enumerated is close to the global one (27%) which can be influenced also by the significantly higher number of commercial products analyzed and reported, compared with the samples analyzed from the other three continents.
The distribution of commercial samples among the 37 countries is highly heterogeneous as well (Table 2). More than 100 commercial products were reported for four countries, i.e. United States (n = 746), China (n = 491) followed distantly by United Kingdom (n = 123) and Italy (n = 119). Another seventeen countries are well represented (n ≥ 10) by the successfully analyzed samples, while the other sixteen countries have even fewer (n < 10) products reported.
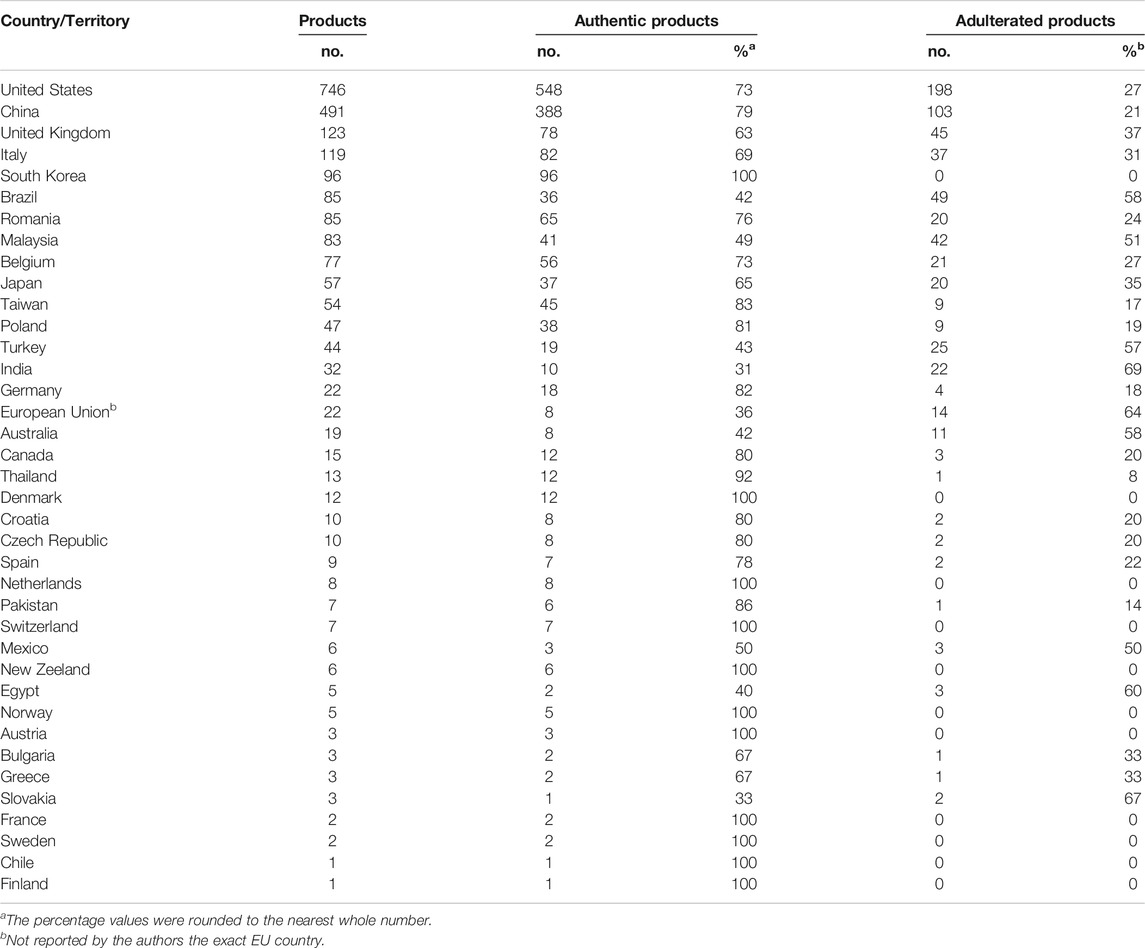
TABLE 2. The distribution and authenticity of the chemically authenticated commercial herbal products at national level.
In twelve countries, out of the total of thirty-seven, all the analyzed commercial herbal products (100%) were reported as authentic, albeit, for eight of them, less than 10 samples were reported. Notably, the botanical identity of the samples purchased from South Korea (n = 96) and Denmark (n = 12) matched the labeled information. The adulterated proportion in the remaining twenty-five countries varied widely, from 8% up to as much as 80%. From the countries where more than 10 samples from their marketplace have been chemically authenticated and non-authenticated products have been reported, the majority of the commercial products was adulterated, being the highest in India (69%), followed closely by Australia (58%), Brazil (58%), Turkey (57%) and Malaysia (51%). Noticeably, the adulteration percentage of the four countries with more than 100 commercial products reported is 37% (United Kingdom), 31% (Italy), 27% (United States) and the lowest is reported for China (21%).
Sampling Heterogeneity and Unavoidable Bias
The authentication raw data were all retrieved from peer-reviewed articles, the vast majority of them after they were indexed in the four major international databases which were systematically searched for while some other few articles were identified after cross-referencing. Although no limiting criteria (e.g. publication year, or language) was used, the authentication data reported in journals with limited-impact and international visibility might be underrepresented in the retrieved data. Moreover, the access of researchers from the economically depressed economies to high-impact journals, and especially to the OA journals, is a further limiting factor for publicly communicating the authentication results relevant for a certain country. On the other hand, as it was previously mentioned as possible bias, also the countries with a functional consumer safety system might be underrepresented as the authentication results of the commercial samples screened by the respective institutions will be published in internal bulletins or protocols, rather than in peer-reviewed journals (Ichim et al., 2020).
Discussion
The chemical identification methods have confirmed that a substantial proportion (27%) of herbal products from the international market place is adulterated: on average, more than one in each four products sold in the 37 countries included in our analysis was proved to be non-authentic regarding their botanical identity. This adulteration percentage, revealed by employing many and very diverse chemical analytical methods, almost matches the figure obtained after the use of DNA-based techniques were assessed for their use for the authentication of commercial herbal products in a comparable number of countries: 27% (Ichim, 2019). Indeed, this percentage was obtained after almost a triple number of commercial herbal products (n = 5,957) were analyzed and their results reviewed recently. Notably, the microscopic authentication of commercial herbal products have reported a much higher adulteration rate (41%) but the number of analyzed samples was considerably much smaller (n = 508) which can be a possible bias of this finding (Ichim et al., 2020).
As it was previously reported by many peer-reviewed reports (Hoban et al., 2018; Seethapathy et al., 2019; Amritha et al., 2020; Anthoons et al., 2021; Palhares et al., 2021), irrespective of the authentication method, adulterated commercial HPs are geographically present across all continents (Supplementary Table S1). Moreover, this highly relevant category of commercial products was found to not comply with the labeled botanical ingredients in proportions almost identical (26 ± 2%), irrespective if they are traditionally used as herbal medicines, as commonly found in Asia, or overwhelmingly consumed as food supplements as in Europe or North America. These two main categories of herbal products commercialized in the global marketplace have many types of value chains (Booker et al., 2012), with some different stakeholders and entities along their shorter or more complex trade chains. Nevertheless, the end-users of both systems seem to be equally affected by non-authentic, accidental contamination or fraudulent substitution of labeled botanical ingredients and even the addition of compounds in an attempt to fool quality control testing e.g. as in adding food dyes to H. perforatum in order to achieve higher UV spectroscopy readings (Booker et al., 2018). Indeed, although monographs for herbal raw materials (e.g., Ph. Eur, USP) allow a minor presence of foreign organic matter (Parveen et al., 2016), the adulteration patters documented by employing different chemical methods, are very diverse and most of them are made possible only by the intentional, economically motivated and fraudulent actions of onerous producers or traders.
The total absence of labeled botanical ingredients and/or their extracts from the commercial herbal products tested was detected by using chemical methods. Commercial samples devoid of labeled botanical ingredient species (Carlson and Thompson, 1998; Ardila et al., 2015; Geng et al., 2019; Zhu et al., 2019) or not even substituted with their related species (Wan et al., 2016). An easy way to increase the profit margin of the products was the use of cheaper plant material as it was the use of other plant parts than the ones recommended, labeled and expected by the product’s users, senna (Senna alexandrina Mill.) stems substituted with leaves and midribs (Kojima et al., 2000), Panax ginseng C.A.Mey roots with other plant parts (leaf or stem) (Govindaraghavan, 2017), or Panax notoginseng Burkill F.H.Chen roots with flowers (Liu et al., 2015). Another similar deceptive adulteration strategy was the reported use of extracts obtained from plant parts other than the recommended ones, such as the decoction of the stem bark to substitute the genuine “jatoba” sap products (Hymenaea stigonocarpa Hayne, Hymenaea martiana Hayne) and the adulteration of Aquilariae Lignum Resinatum (Aquilaria sinensis (Lour.) Spreng) products with cheap resin (e.g. rosin) (Qu et al., 2017). The economically motivated adulteration includes also the use of unlabeled filler species as the DNA of species such as rice (Oryza sativa L.), soybean (Glycine max (L.) Merr.) and wheat (Triticum spp.) was previously identified in commercial herbal products (Newmaster et al., 2013; Ivanova et al., 2016). Yet, the TLC alone was able to detect the fraudulent use of soybean oil as filler in “copaiba” (Copaifera multijuga Hayne) oil-resin products (Barbosa et al., 2009).
The detection of unlabeled species with allergenic potential and known or suspected toxicity was previously reported by the use of DNA-based authentication techniques (Newmaster et al., 2013; Speranskaya et al., 2018). The same potential was shown by the phytochemical analyses which have been able to unmask the presence of unwanted and hazardous botanic ingredients, such as species that should have been notified to authorities (e.g. Ilex paraguariensis A. St-Hil., Epimedium spp., Tribulus terrestris L.), or forbidden toxic plants (e.g. Aristolochia fangchi Y.C.Wu exL.D.Chow and S.M.Hwang) (Deconinck et al., 2019) or even health hazardous contaminations, with Digitalis lanata Ehrh. added to plantain (Plantago major L.) products (Slifman et al., 1998). Moreover, as peanut allergy is a major public health concern and can be severe or even life-threatening (Gray, 2020), chemical methods have proved able to detect adulteration with the peanut skin extract of grape seed-containing herbal products (Vitis vinifera L.) from Australia (Govindaraghavan, 2019) and United States (Villani et al., 2015).
All the intentional adulteration practices documented and reported repeatedly till now (Li et al., 2008; Ichim, 2019; Xu et al., 2019; Ichim et al., 2020; Upton et al., 2020) can be evidenced by peer-reviewed reports referring to the top selling herbal products containing highly valued or widely used medicinal species across countries and cultures. The prices of ginseng herbal medicines and supplements vary widely based on the species, quality, and purity of the ginseng, and this provides a strong driver for intentional adulteration (Ichim and de Boer, 2021). Indeed, several chemical methods were able to identify ginseng products totally or partially devoid of the labeled P. ginseng plant material (Mihalov et al., 2000; Yang et al., 2016) and prove that, in most cases, labeled Panax species were substituted with other Panax species (Li et al., 2010; Yu et al., 2014; Dong et al., 2020), but also the substitution of ginseng root with leaves, stems or flowers (Liu et al., 2015; Govindaraghavan, 2017). Notably, chemical analysis was even able to detect the adulteration and substitution of wild with cultivated ginseng (Zhao et al., 2015) as well as a white ginseng products (P. ginseng) not composed of 6 years old ginseng radix only (Li et al., 2010).
Studies carried out at UCL School of Pharmacy, London have consistently shown that product adulteration is commonplace, with 25–40% of products typically being found to be of poor quality or adulterated, and especially with products obtained via the internet. Although with products that have been registered as Traditional Herbal Medicines under the Traditional Herbal Medicinal Products Directive (THMPD), no adulteration has so far been found and these products have shown to be of acceptable quality (Booker et al., 2016a; Booker et al., 2016b; Booker et al., 2018). This does not necessarily mean that all non-registered products (e.g. food supplements) are of poor quality but the problem being that it is difficult for the general public to be able to reliably discern high quality products from inferior ones. Organic certification provides some assurances regarding traceability, including origin, cultivation methods and manufacturing practices and so until more formal regulations are introduced for these food supplement products, buying organic may be the best option.
The many cases of substituted or adulterated herbal products purchased from a very high number of national marketplaces, where the labeled botanical ingredients did not match the chemically identified ones are, unfortunately, accompanied by other low-quality issues which additionally affect the safety and potential efficacy of commercial herbal products. As many as forty-one peer reviewed research articles, which have reported a case of adulteration among analyzed commercial samples, have also reported other quality issues which further lower the overall quality expected by their users and consumers. Additionally, another nineteen studies reported quality issues of the tested products without identifying any proof for their botanical identity adulteration. For the majority of herbal products reported, considerable variability of their labeled metabolic profile and/or content, such as the alkaloid content of “ma-huang” (Ephedra sinica Stapf) products (Gurley, 1998) or Menispermi Rhizoma (Menispermum dauricum DC) products (Liu et al., 2013b), selected triterpene glycosides and phenolic constituents in black cohosh (A. racemosa) products (Jiang et al., 2006) or the PAC content of cranberry products (Turbitt et al., 2020). Furthermore, aside of significant product-to-product variability, the marked differences of the content of individual flavonoids/flavonolignans in milk thistle (Silybum marianum (L.) Gaertn.) products have revealed quality difference also between different batches by the same manufacturers (Fenclova et al., 2019).
The peer-reviewed authentication results and the methods which were successfully employed to analyze commercial herbal products and significantly contribute to a better understanding of authenticity issues affecting the herbal industry and provides an as close-to-reality possible picture of the commercial herbal products’ authenticity as well as examples of techniques to be efficiently and accurately used for their authentication.
It is clear that chemical analysis alone can only identify existing problems. In order to prevent these problems from arising in the first place, better governance needs to be implemented along all stages of the supply chain. Regulation can help with this process but resources are scarce and real progress on quality is more achievable through having closer and more focused co-operation between the regulators and the producers, manufacturers and retailers of herbal products.
Author Contributions
MI performed the literature systematic search and analyzed the results. MI and AB wrote the manuscript together.
Funding
This publication was supported by the National Core Program funded by the Romanian Ministry of Research and Innovation, project number 25N/February 11, 2019, BIODIVERS 19270401 (for MCI).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.666850/full#supplementary-material.
References
Abdel Kawy, M. A, Haggag, E. G., Abdel Motaal, A. A, and Eissa, N. A (2012). Quality control of certain slimming herbal products present in the Egyptian market. Life Sci. J. 9, 2273–2285.
Abourashed, E. A., and Khan, I. A (2001). High‐performance liquid chromatography determination of hydrastine and berberine in dietary supplements containing goldenseal. J. Pharm. Sci. 90, 817–822. doi:10.1002/jps.1035
Abubakar, B. M., Salleh, F. M., Shamsir Omar, M. S, and Wagiran, A (2018). Assessing product adulteration of Eurycoma longifolia (Tongkat Ali) herbal medicinal product using DNA barcoding and HPLC analysis. Pharm. Biol. 56, 368–377. doi:10.1080/13880209.2018.1479869
Ahmed, R., Ali, Z., Wu, Y, Kulkarni, S., Avery, M., Choudhary, M, et al. (2011). Chemical characterization of a CommercialCommiphora wightiiResin sample and chemical profiling to assess for authenticity. Planta Med. 77, 945–950. doi:10.1055/s-0030-1250674
Amritha, N., Bhooma, V., and Parani, M. (2020). Authentication of the market samples of Ashwagandha by DNA barcoding reveals that powders are significantly more adulterated than roots. J. Ethnopharmacology 256, 112725. doi:10.1016/j.jep.2020.112725
Anthoons, B., Karamichali, I., Schrøder-Nielsen, A., Drouzas, A. D., de Boer, H., and Madesis, P. (2021). Metabarcoding reveals low fidelity and presence of toxic species in short chain-of-commercialization of herbal products. J. Food Compost. Anal. 97, 103767. doi:10.1016/j.jfca.2020.103767
Ardila, J. A., Funari, C. S., Andrade, A. M., Cavalheiro, A. J., and Carneiro, R. L (2015). Cluster analysis of commercial samples ofBauhiniaspp. using HPLC-UV/PDA and MCR-ALS/PCA without peak alignment procedure. Phytochem. Anal. 26, 367–373. doi:10.1002/pca.2571
Avula, B., Navarrete, A., Joshi, V. C., and Khan, I. A. (2006). Quantification of parthenolide in Tanacetum species by LC-UV/LC-MS and microscopic comparison of Mexican/US feverfew samples. Pharmazie 61, 590–594.
Avula, B., Cohen, P. A., Wang, Y.-H., Sagi, S., Feng, W., Wang, M., et al. (2014). Chemical profiling and quantification of monacolins and citrinin in red yeast rice commercial raw materials and dietary supplements using liquid chromatography-accurate QToF mass spectrometry: chemometrics application. J. Pharm. Biomed. Anal. 100, 243–253. doi:10.1016/j.jpba.2014.07.039
Avula, B., Wang, Y.-H., Rumalla, C. S., Smillie, T. J., and Khan, I. A. (2012). Simultaneous determination of alkaloids and flavonoids from aerial parts of Passiflora species and dietary supplements using UPLC-UV-MS and HPTLC. Nat. Product. Commun. 7, 1177–1180. doi:10.1177/1934578x1200700918
Barbosa, K. D. S., Yoshida, M., and Scudeller, V. V. (2009). Detection of adulterated copaiba (Copaifera multijuga Hayne) oil-resins by refractive index and thin layer chromatography. Rev. Bras. Farmacogn. 19, 57–60. doi:10.1590/S0102-695X2009000100013
Beltrame, F. L., Ferroni, D. C., Alves, B. R. V., Pereira, A. V., and Esmerino, L. A. (2009). Avaliação da qualidade das amostras comercias de Baccharis trimera L. (Carqueja) vendidas no Estado do Paraná. Acta Sci. Health Sci. 31, 37–43. doi:10.4025/actascihealthsci.v31i1.3050
Betz, J. M., White, K. D., and Marderosian, A. H. D. (1995). Gas chromatographic determination of yohimbine in commercial yohimbe products. J. AOAC Int. 78, 1189–1194. doi:10.1093/jaoac/78.5.1189
Bilia, A. R., Flamini, G., Taglioli, V., Morelli, I., and Vincieri, F. F. (2002). GC-MS analysis of essential oil of some commercial Fennel teas. Food Chem. 76, 307–310. doi:10.1016/S0308-8146(01)00277-1
Booker, A., Agapouda, A., Frommenwiler, D. A., Scotti, F., Reich, E., and Heinrich, M. (2018). St John's wort ( Hypericum perforatum ) products - an assessment of their authenticity and quality. Phytomedicine 40, 158–164. doi:10.1016/j.phymed.2017.12.012
Booker, A., Frommenwiler, D., Reich, E., Horsfield, S., and Heinrich, M. (2016a). Adulteration and poor quality of Ginkgo biloba supplements. J. Herbal Med. 6, 79–87. doi:10.1016/j.hermed.2016.04.003
Booker, A., Jalil, B., Frommenwiler, D., Reich, E., Zhai, L., Kulic, Z., et al. (2016b). The authenticity and quality of Rhodiola rosea products. Phytomedicine 23, 754–762. doi:10.1016/j.phymed.2015.10.006
Booker, A., Johnston, D., and Heinrich, M. (2012). Value chains of herbal medicines-Research needs and key challenges in the context of ethnopharmacology. J. Ethnopharmacology 140, 624–633. doi:10.1016/j.jep.2012.01.039
Booker, A., Suter, A., Krnjic, A., Strassel, B., Zloh, M., Said, M., et al. (2014). A phytochemical comparison of saw palmetto products using gas chromatography and 1H nuclear magnetic resonance spectroscopy metabolomic profiling. J. Pharm. Pharmacol. 66, 811–822. doi:10.1111/jphp.12198
British Pharmacopoeia Commission (2018). DNA barcoding as a tool for botanical identification of herbal drugs. London, United Kingdom: British Pharmacopoeia Commission.
Brock, C., Whitehouse, J., Tewfik, I., and Towell, T. (2013). Identity issues surrounding American skullcap (Scutellaria lateriflora) and an optimised high performance liquid chromatography method to authenticate commercially available products. J. Herbal Med. 3, 57–64. doi:10.1016/j.hermed.2013.02.001
Budeč, M., Bošnir, J., Racz, A., Lasić, D., Brkić, D., Mosović Ćuić, A., et al. (2019). Verification of authenticity of Ginkgo biloba L. leaf extract and its products present on the Croatian market by analysis of quantity and ratio of ginkgo flavone glycosides (quercitin, kaempferol, and isorhamnetin) to terpene trilactones to the effect of. Acta Clin. Croat. 58, 672–692. doi:10.20471/acc.2019.58.04.15
Carlson, M., and Thompson, R. D. (1998). Liquid chromatographic determination of methylxanthines and catechins in herbal preparations containing guaraná. J. AOAC Int. 81, 691–701. doi:10.1093/jaoac/81.4.691
Cassinese, C., Combarieu, E. D., Falzoni, M., Fuzzati, N., Pace, R., and Sardone, N. (2007). New Liquid Chromatography method with ultraviolet detection for analysis of anthocyanins and anthocyanidins in Vaccinium myrtillus fruit dry extracts and commercial preparations. J. AOAC Int. 90, 911–919. doi:10.1093/jaoac/90.4.911
Chandra, A., Li, Y., Rana, J., Persons, K., Hyun, C., Shen, S., et al. (2011). Qualitative categorization of supplement grade Ginkgo biloba leaf extracts for authenticity. J. Funct. Foods 3, 107–114. doi:10.1016/j.jff.2011.03.004
Chatzinasiou, L., Booker, A., MacLennan, E., Mackonochie, M., and Heinrich, M. (2019). Turmeric (Curcuma longa L.) products: what quality differences exist?. J. Herbal Med. 17-18, 100281. doi:10.1016/j.hermed.2019.100281
Choi, J. Y., Hong, J. H., Dang, Y. M., Jamila, N., Khan, N., Jo, C. H., et al. (2018). Identification markers of adulteration in Korean red ginseng (Panax ginseng) products using high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS). Anal. Lett. 51, 2588–2601. doi:10.1080/00032719.2018.1443340
Cieśla, Ł. M., and Waksmundzka-Hajnos, M. (2010). Application of thin-layer chromatography for the quality control and screening the free radical scavenging activity of selected pharmacuetical preparations containing salvia officinalis L. extract. Acta Pol. Pharm. - Drug Res. 67, 481–485. Available at: https://europepmc.org/article/med/20873415 (Accessed January 30, 2021).
Custers, D., Van Praag, N., Courselle, P., Apers, S., and Deconinck, E. (2017). Chromatographic fingerprinting as a strategy to identify regulated plants in illegal herbal supplements. Talanta 164, 490–502. doi:10.1016/j.talanta.2016.12.008
Czigle, S., Tóth, J., Jedlinszki, N., Háznagy-Radnai, E., Csupor, D., and Tekeľová, D. (2018). Ginkgo biloba food supplements on the European market - adulteration patterns revealed by quality control of selected samples. Planta Med. 84, 475–482. doi:10.1055/a-0581-5203
Deconinck, E., Custers, D., and De Beer, J. O. (2015). Identification of (antioxidative) plants in herbal pharmaceutical preparations and dietary supplements. Methods Mol. Biol. 1208, 181–199. doi:10.1007/978-1-4939-1441-8_14
Deconinck, E., De Leersnijder, C., Custers, D., Courselle, P., and De Beer, J. O. (2013). A strategy for the identification of plants in illegal pharmaceutical preparations and food supplements using chromatographic fingerprints. Anal. Bioanal. Chem. 405, 2341–2352. doi:10.1007/s00216-012-6649-4
Deconinck, E., Vanhamme, M., Bothy, J. L., and Courselle, P. (2019). A strategy based on fingerprinting and chemometrics for the detection of regulated plants in plant food supplements from the Belgian market: two case studies. J. Pharm. Biomed. Anal. 166, 189–196. doi:10.1016/j.jpba.2019.01.015
Demirezer, L., Büyükkaya, A., Ucakturk, E., Kuruüzüm-Uz, A., Güvenalp, Z., and Palaska, E. (2014). Adulteration determining of pharmaceutical forms of Ginkgo biloba extracts from different international manufacturers. Rec. Nat. Prod. 8, 394–400.
Dias, E. G. E., Valenzuela, V. C. T., Alves, M. R., Duarte, M. G. R., and Garcia, E. F. (2013). Qualidade e autenticidade de folhas de chapéu-de-couro (Echinodorus grandiflorus) oriundas de fornecedores de São Paulo. Rev. Bras. Plantas Med. 15, 250–256. doi:10.1590/S1516-05722013000200013
Dong, F., Lin, J., You, J., Ji, J., Xu, X., Zhang, L., et al. (2020). A chemometric modeling-free near infrared barcode strategy for smart authentication and geographical origin discrimination of Chinese ginseng. Spectrochimica Acta A: Mol. Biomol. Spectrosc. 226, 117555. doi:10.1016/j.saa.2019.117555
Farias, K. d. S., Auharek, S. A., Cunha-Laura, A. L., De Souza, J. M. E., Damasceno-Junior, G. A., Toffoli-Kadri, M. C., et al. (2017). Adulteration and contamination of commercial sap of Hymenaea species. Evidence-Based Complement. Altern. Med. 2017, 1. doi:10.1155/2017/1919474
Fatima, K., Mahmud, S., Yasin, H., Asif, R., Qadeer, K., and Ahmad, I. (2020). Authentication of various commercially available crude drugs using different quality control testing parameters. Pak. J. Pharm. Sci. 33, 1641–1657.
Fenclova, M., Novakova, A., Viktorova, J., Jonatova, P., Dzuman, Z., Ruml, T., et al. (2019). Poor chemical and microbiological quality of the commercial milk thistle-based dietary supplements may account for their reported unsatisfactory and non-reproducible clinical outcomes. Sci. Rep. 9, 11118. doi:10.1038/s41598-019-47250-0
Ferrante, L. M. S. d., Mayer, B., Vasconcelos, E. C., and Oliveira, C. M. R. d. (2007). GC/FID-based authentication of Baccharis trimera: a quality control study of products commercialized in Curitiba and metropolitan region (Brazil). Rev. Bras. Farmacogn. 17, 356–360. doi:10.1590/s0102-695x2007000300009
Frommenwiler, D. A., Maire-Widmer, V., Upton, R., Nichols, J., Heubl, G., and Reich, E. (2017). Qualitative and quantitative characterization of two licorice root species (Glycyrrhiza glabra L. and Glycyrrhiza uralensis Fisch.) by HPTLC, validated by HPLC and DNA sequencing. JPC - J. Planar Chromatogr. - Mod. TLC 30, 467–473. doi:10.1556/1006.2017.30.6.2
Frommenwiler, D. A., Reich, E., Sudberg, S., Sharaf, M. H. M., Bzhelyansky, A., and Lucas, B. (2016). St. John's wort versus counterfeit st. John's wort: an HPTLC study. J. AOAC Int. 99, 1204–1212. doi:10.5740/jaoacint.16-0170
Gallo, E., Giocaliere, E., Benemei, S., Bilia, A. R., Karioti, A., Pugi, A., et al. (2012). Anything to declare? Possible risks for patients' health resulting from undeclared plants in herbal supplements. Br. J. Clin. Pharmacol. 73, 482–483. doi:10.1111/j.1365-2125.2011.04115.x
Gardana, C., Scialpi, A., Fachechi, C., and Simonetti, P. (2020). Identification of markers for the authentication of cranberry extract and cranberry-based food supplements. Heliyon 6, e03863. doi:10.1016/j.heliyon.2020.e03863
Gardana, C., Scialpi, A., Fachechi, C., and Simonetti, P. (2018). Near-infrared spectroscopy and chemometrics for the routine detection of bilberry extract adulteration and quantitative determination of the anthocyanins. J. Spectrosc. 2018, 1. doi:10.1155/2018/4751247
Geng, P., Chen, P., Sun, J., McCoy, J.-A. H., and Harnly, J. M. (2019). Authentication of black cohosh (Actaea racemosa) dietary supplements based on chemometric evaluation of hydroxycinnamic acid esters and hydroxycinnamic acid amides. Anal. Bioanal. Chem. 411, 7147–7156. doi:10.1007/s00216-019-02082-9
Gilroy, C. M., Steiner, J. F., Byers, T., Shapiro, H., and Georgian, W. (2003). Echinacea and truth in labeling. Arch. Intern. Med. 163, 699–704. doi:10.1001/archinte.163.6.699
Govindaraghavan, S. (2019). Adulteration of commercial grape seed extracts and other proanthocyanidins (PACs)-rich herbal extracts: multi-compound HPLC profile patterns provide key to detection. Fitoterapia 134, 389–403. doi:10.1016/j.fitote.2019.03.014
Govindaraghavan, S. (2017). Multiple ginsenosides ratios pattern - a pointer to identify Panax ginseng root extracts adulterated with other plant parts?. Fitoterapia 121, 64–75. doi:10.1016/j.fitote.2017.06.024
Gray, C. L. (2020). Current controversies and future prospects for peanut allergy prevention, diagnosis and therapies. Jaa 13, 51–66. doi:10.2147/JAA.S196268
Grazina, L., Amaral, J. S., and Mafra, I. (2020). Botanical origin authentication of dietary supplements by DNA‐based approaches. Compr. Rev. Food Sci. Food Saf. 19, 1080-1109. doi:10.1111/1541-4337.12551
Gurley, B. J., Wang, P., and Gardner, S. F. (1998). Ephedrine-type alkaloid content of nutritional supplements containing Ephedra sinica (Ma-huang) as determined by high performance liquid chromatography. J. Pharm. Sci. 87, 1547–1553. doi:10.1021/js9801844
Guzelmeric, E., Ristivojević, P., Vovk, I., Milojković-Opsenica, D., and Yesilada, E. (2017). Quality assessment of marketed chamomile tea products by a validated HPTLC method combined with multivariate analysis. J. Pharm. Biomed. Anal. 132, 35–45. doi:10.1016/j.jpba.2016.09.030
Harkey, M. R., Henderson, G. L., Gershwin, M. E., Stern, J. S., and Hackman, R. M. (2001). Variability in commercial ginseng products: an analysis of 25 preparations. Am. J. Clin. Nutr. 73, 1101–1106. doi:10.1093/ajcn/73.6.1101
He, K., Pauli, G. F., Zheng, B., Wang, H., Bai, N., Peng, T., et al. (2006). Cimicifuga species identification by high performance liquid chromatography-photodiode array/mass spectrometric/evaporative light scattering detection for quality control of black cohosh products. J. Chromatogr. A 1112, 241–254. doi:10.1016/j.chroma.2006.01.004
Hoban, C. L., Musgrave, I. F., Coghlan, M. L., Power, M. W. P., Byard, R. W., Nash, C., et al. (2018). Adulterants and contaminants in psychotropic herbal medicines detected with mass spectrometry and Next-Generation DNA sequencing. Pharm. Med. 32, 429–444. doi:10.1007/s40290-018-0252-8
Ichim, M. C., and de Boer, H. J. (2021). A review of authenticity and authentication of commercial ginseng herbal medicines and food supplements. Front. Pharmacol. 11, 2185. doi:10.3389/fphar.2020.612071
Ichim, M. C., Häser, A., and Nick, P. (2020). Microscopic authentication of commercial herbal products in the globalized market: potential and limitations. Front. Pharmacol. 11, 867. doi:10.3389/fphar.2020.00876
Ichim, M. C. (2019). The DNA-based authentication of commercial herbal products reveals their globally widespread adulteration. Front. Pharmacol. 10, 1227. doi:10.3389/fphar.2019.01227
Intharuksa, A., Kitamura, M., Peerakam, N., Charoensup, W., Ando, H., Sasaki, Y., et al. (2020). Evaluation of white Kwao Krua (Pueraria candollei Grah. ex Benth.) products sold in Thailand by molecular, chemical, and microscopic analyses. J. Nat. Med. 74, 106–118. doi:10.1007/s11418-019-01351-2
Ivanova, N. V., Kuzmina, M. L., Braukmann, T. W. A., Borisenko, A. V., and Zakharov, E. V. (2016). Authentication of herbal supplements using Next-Generation Sequencing. PLoS One 11, e0156426. doi:10.1371/journal.pone.0156426
Jamila, N., Choi, J. Y., Hong, J. H., Nho, E. Y., Khan, N., Jo, C. H., et al. (2016). Identification and quantification of adulteration in Garcinia cambogia commercial products by chromatographic and spectrometric methods. Food Additives & Contaminants: A 33, 1751–1760. doi:10.1080/19440049.2016.1244733
Jiang, B., Kronenberg, F., Nuntanakorn, P., Qiu, M.-H., and Kennelly, E. J. (2006). Evaluation of the botanical authenticity and phytochemical profile of black cohosh products by high-performance liquid chromatography with selected ion monitoring liquid Chromatography−Mass spectrometry. J. Agric. Food Chem. 54, 3242–3253. doi:10.1021/jf0606149
Jiang, P., Lu, Y., and Chen, D. (2016). Authentication of Schisandra chinensis and Schisandra sphenanthera in Chinese patent medicines. J. Pharm. Biomed. Anal. 131, 263–271. doi:10.1016/j.jpba.2016.08.040
Jiao, P., Jia, Q., Randel, G., Diehl, B., Weaver, S., and Milligan, G. (2010). Quantitative 1H-NMR spectrometry method for quality control of aloe vera products. J. AOAC Int. 93, 842–848. doi:10.1093/jaoac/93.3.842
Jing, Y., Lai, Y., Chen, H., Li, M., Zhou, J., and Lan, Z. (2019). Study on the identification of Pinelliae rhizoma and Pinelliae pedatisectae rhizoma based on the characteristic component triglochinic acid. RSC Adv. 9, 11774–11780. doi:10.1039/c9ra01626k
Juang, L.-J., and Sheu, S.-J. (2005). Chemical identification of the sources of commercial fructus chebulae. Phytochem. Anal. 16, 246–251. doi:10.1002/pca.823
Kamal, A., Haggag, E., Abdelhady, M., Youssif, K., and Sleem, A. (2017). Quality control of herbal products in the Egyptian market used for some gastro-intestinal tract disorders. J. Adv. Pharm. Res. 1, 43–57. doi:10.21608/aprh.2016.859
Karioti, A., Giocaliere, E., Guccione, C., Pieraccini, G., Gallo, E., Vannacci, A., et al. (2014). Combined HPLC-DAD-MS, HPLC-MSn and NMR spectroscopy for quality control of plant extracts: the case of a commercial blend sold as dietary supplement. J. Pharm. Biomed. Anal. 88, 7–15. doi:10.1016/j.jpba.2013.07.040
Kesanakurti, P., Thirugnanasambandam, A., Ragupathy, S., and Newmaster, S. G. (2020). Genome skimming and NMR chemical fingerprinting provide quality assurance biotechnology to validate Sarsaparilla identity and purity. Sci. Rep. 10, 1–11. doi:10.1038/s41598-020-76073-7
Kite, G. C., Howes, M.-J. R., Leon, C. J., and Simmonds, M. S. J. (2003). Liquid chromatography/mass spectrometry of malonyl-ginsenosides in the authentication of ginseng. Rapid Commun. Mass. Spectrom. 17, 238–244. doi:10.1002/rcm.899
Kojima, T., Kishi, M., Sekita, S., and Satake, M. (2000). Origin of sennosides in dietary supplements containing senna stem. J. Food Hyg. Soc. Jpn. 41, 303–306. doi:10.3358/shokueishi.41.303
Kressmann, S., Müller, W. E., and Blume, H. H. (2002). Pharmaceutical quality of differentGinkgo bilobabrands. J. Pharm. Pharmacol. 54, 661–669. doi:10.1211/0022357021778970
Lee, J. (2016). Anthocyanin analyses of Vaccinium fruit dietary supplements. Food Sci. Nutr. 4, 742–752. doi:10.1002/fsn3.339
Lee, J. (2014). Marketplace analysis demonstrates quality control standards needed for black raspberry dietary supplements. Plant Foods Hum. Nutr. 69, 161–167. doi:10.1007/s11130-014-0416-y
Li, J., Pan, L., Naman, C. B., Deng, Y., Chai, H., Keller, W. J., et al. (2014). Pyrrole alkaloids with potential cancer chemopreventive activity isolated from a goji berry-contaminated commercial sample of African mango. J. Agric. Food Chem. 62, 5054–5060. doi:10.1021/jf500802x
Li, L., Luo, G.-A., Liang, Q.-L., Hu, P., and Wang, Y.-M. (2010). Rapid qualitative and quantitative analyses of Asian ginseng in adulterated American ginseng preparations by UPLC/Q-TOF-MS. J. Pharm. Biomed. Anal. 52, 66–72. doi:10.1016/j.jpba.2009.12.017
Li, S., Han, Q., Qiao, C., Song, J., Cheng, C. L., and Xu, H. (2008). Chemical markers for the quality control of herbal medicines: an overview. Chin. Med. 3, 7. doi:10.1186/1749-8546-3-7
Lin, C., Liu, F., Zhang, R., Liu, M., Zhu, C., Zhao, J., et al. (2019). High-Performance Thin-Layer Chromatographic fingerprints of triterpenoids for distinguishing between Isodon lophanthoides and Isodon lophanthoides var. gerardianus. J. AOAC Int. 102, 714–719. doi:10.5740/jaoacint.18-0305
Lin, W.-N., Lu, H.-Y., Lee, M.-S., Yang, S.-Y., Chen, H.-J., Chang, Y.-S., et al. (2010). Evaluation of the cultivation age of dried ginseng radix and its commercial products by using 1H-NMR fingerprint analysis. Am. J. Chin. Med. 38, 205–218. doi:10.1142/S0192415X10007762
Lin, Y.-K., Ho, Y.-L., Zhao, Y., and Chang, Y.-S. (2015). Quality assessment of Fritillariae Thunbergii Bulbus sold in Taiwan markets using a validated HPLC-UV method combined with hierarchical clustering analysis. J. Food Drug Anal. 23, 130–135. doi:10.1016/j.jfda.2014.06.004
Liu, F.-J., Jiang, Y., Li, P., Liu, Y.-D., Yao, Z.-P., Xin, G.-Z., et al. (2020). Untargeted metabolomics coupled with chemometric analysis reveals species-specific steroidal alkaloids for the authentication of medicinal Fritillariae Bulbus and relevant products. J. Chromatogr. A 1612, 460630. doi:10.1016/j.chroma.2019.460630
Liu, P., Yu, H.-S., Zhang, L.-J., Song, X.-B., Kang, L.-P., Liu, J.-Y., et al. (2015). A rapid method for chemical fingerprint analysis of Pan Panax notoginseng powders by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Chin. J. Nat. Medicines 13, 471–480. doi:10.1016/S1875-5364(15)30042-X
Liu, Y., Song, X., Yan, R., Li, T., Chai, X., Qi, A., et al. (2013a). Development and validation of a UPLC-DAD-MS method for characterization and quantification of alkaloids in Menispermi Rhizoma and its preparations. J. Food Drug Anal. 21, 206–218. doi:10.1016/j.jfda.2013.05.012
Liu, Y., Song, X., Yan, R., Li, T., Chai, X., Qi, A., et al. (2013b). Development and validation of a UPLC-DAD-MS method for characterization and quantification of alkaloids in Menispermi Rhizoma and its preparations. J. Food Drug Anal. 21, 206–218. doi:10.1016/j.jfda.2013.05.012
Low, T. Y., Wong, K. O., Yap, A. L. L., De Haan, L. H. J., and Rietjens, I. M. C. M. (2017). The regulatory framework across international jurisdictions for risks associated with consumption of botanical food supplements. Compr. Rev. Food Sci. Food Saf. 16, 821–834. doi:10.1111/1541-4337.12289
Lucio-Gutiérrez, J. R., Delgado-Montemayor, C., Coello-Bonilla, J., Waksman-Minsky, N., and Saucedo, A. L. (2019). Selective 1D-TOCSY and chemometrics to evaluate authenticity of Turnera diffusa and related botanical extracts. Phytochemistry Lett. 30, 62–68. doi:10.1016/j.phytol.2019.01.011
Mannino, G., Di Stefano, V., Lauria, A., Pitonzo, R., and Gentile, C. (2020). Vaccinium macrocarpon (Cranberry)-based dietary supplements: variation in mass uniformity, proanthocyanidin dosage and anthocyanin profile demonstrates quality control standard needed. Nutrients 12, 992. doi:10.3390/nu12040992
Martins, A. R., Soares, M. K. M., Redher, V. L. G., Bajay, M. M., Villela, P. M. S., Zucchi, M. I., et al. (2014). Use of anatomical, chemical, and molecular genetic characteristics in the quality control of medicinal species: a case study of Sarsaparilla (Smilax spp.). Econ. Bot. 68, 410–425. doi:10.1007/s12231-014-9287-2
Masada, S. (2016). Authentication of the botanical origin of Western herbal products using Cimicifuga and Vitex products as examples. J. Nat. Med. 70, 361–375. doi:10.1007/s11418-016-1006-0
Masada-Atsumi, S., Kumeta, Y., Takahashi, Y., Hakamatsuka, T., and Goda, Y. (2014). Evaluation of the botanical origin of black cohosh products by genetic and chemical analyses. Biol. Pharm. Bull. 37, 454–460. doi:10.1248/bpb.b13-00844Available at:http://www.ncbi.nlm.nih.gov/pubmed/24583864(Accessed December 5, 2018).
Mihalov, J. J., Marderosian, A. D., and Pierce, J. C. (2000). DNA identification of commercial ginseng samples. J. Agric. Food Chem. 48, 3744–3752. doi:10.1021/jf000011b
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 6, e1000097. doi:10.1371/journal.pmed.1000097
Muensritharam, L., Tolieng, V., Chaichantipyuth, C., Petsom, A., and Nhujak, T. (2008). Capillary zone electrophoresis for separation and analysis of hydroxycitric acid and hydroxycitric acid lactone: application to herbal products of Garcinia atroviridis Griff. J. Pharm. Biomed. Anal. 46, 577–582. doi:10.1016/j.jpba.2007.11.008
Newmaster, S. G., Grguric, M., Shanmughanandhan, D., Ramalingam, S., and Ragupathy, S. (2013). DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 11, 222. doi:10.1186/1741-7015-11-222
Palhares, R. M., Baratto, L. C., Scopel, M., Mügge, F. L. B., and Brandão, M. G. L. (2021). Medicinal plants and herbal products from Brazil: how can we improve quality?. Front. Pharmacol. 11, 2412. doi:10.3389/fphar.2020.606623
Pan, H., Yao, C., Yao, S., Yang, W., Wu, W., and Guo, D. (2020). A metabolomics strategy for authentication of plant medicines with multiple botanical origins, a case study of Uncariae Rammulus Cum Uncis. J. Sep. Sci. 43, 1043–1050. doi:10.1002/jssc.201901064
Parveen, A., Wang, Y.-H., Fantoukh, O., Alhusban, M., Raman, V., Ali, Z., et al. (2020). Development of a chemical fingerprint as a tool to distinguish closely related Tinospora species and quantitation of marker compounds. J. Pharm. Biomed. Anal. 178, 112894. doi:10.1016/j.jpba.2019.112894
Parveen, I., Gafner, S., Techen, N., Murch, S., and Khan, I. (2016). DNA barcoding for the identification of botanicals in herbal medicine and dietary supplements: strengths and limitations. Planta Med. 82, 1225–1235. doi:10.1055/s-0042-111208
Pawar, R. S., Sagi, S., and Leontyev, D. (2020). Analysis of bitter orange dietary supplements for natural and synthetic phenethylamines by LC-MS/MS. Drug Test. Anal. 12, 1241–1251. doi:10.1002/dta.2871
Politi, M., Zloh, M., Pintado, M. E., Castro, P. M. L., Heinrich, M., and Prieto, J. M. (2009). Direct metabolic fingerprinting of commercial herbal tinctures by nuclear magnetic resonance spectroscopy and mass spectrometry. Phytochem. Anal. 20, 328–334. doi:10.1002/pca.1131
Preto, M. S. M., Tavares, M. I. B., Sebastião, P. J. O., and Azeredo, R. B. V. (2013). Determination of herb authenticity by low-field NMR. Food Chem. 136, 1272–1276. doi:10.1016/j.foodchem.2012.09.045
Qu, L., Chen, J.-b., Zhang, G.-J., Sun, S.-q., and Zheng, J. (2017). Chemical profiling and adulteration screening of Aquilariae Lignum Resinatum by Fourier transform infrared (FT-IR) spectroscopy and two-dimensional correlation infrared (2D-IR) spectroscopy. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 174, 177–182. doi:10.1016/j.saa.2016.11.008
Qu, L., Chen, J.-b., Zhou, Q., Zhangjun, G.-j., Sun, S.-q., and Sun, Y.-z. (2016). Identification of authentic and adulterated Aquilariae Lignum Resinatum by Fourier transform infrared (FT-IR) spectroscopy and two-dimensional correlation analysis. J. Mol. Struct. 1124, 216–220. doi:10.1016/j.molstruc.2016.01.056
Raclariu, A. C., Heinrich, M., Ichim, M. C., and de Boer, H. (2018a). Benefits and limitations of DNA barcoding and metabarcoding in herbal product authentication. Phytochem. Anal. 29, 123–128. doi:10.1002/pca.2732
Raclariu, A. C., Paltinean, R., Vlase, L., Labarre, A., Manzanilla, V., Ichim, M. C., et al. (2017). Comparative authentication of Hypericum perforatum herbal products using DNA metabarcoding, TLC and HPLC-MS. Sci. Rep. 7. doi:10.1038/s41598-017-01389-w
Raclariu, A. C., Ţebrencu, C. E., Ichim, M. C., Ciupercǎ, O. T., Brysting, A. K., and de Boer, H. (2018b). What's in the box? Authentication of Echinacea herbal products using DNA metabarcoding and HPTLC. Phytomedicine 44, 32–38. doi:10.1016/j.phymed.2018.03.058
Raman, V., Avula, B., Galal, A. M., Wang, Y.-H., and Khan, I. A. (2013). Microscopic and UPLC-UV-MS analyses of authentic and commercial yohimbe (Pausinystalia johimbe) bark samples. J. Nat. Med. 67, 42–50. doi:10.1007/s11418-012-0642-2
Raman, V., Budel, J. M., Zhao, J., Bae, J.-Y., Avula, B., Osman, A. G., et al. (2018). Microscopic characterization and HPTLC of the leaves, stems and roots of Fadogia agrestis - an African folk medicinal plant. Revista Brasileira de Farmacognosia 28, 631–639. doi:10.1016/j.bjp.2018.07.006
Raman, V., Sagi, S., Galal, A. M., Avula, B., Viljoen, A., and Khan, I. A. (2015). Adulteration in commercial buchu dietary supplements: analyses of commercial and authentic buchu samples and comparative studies of Agathosma betulina and Agathosma crenulata by microscopy and HPTLC. South Afr. J. Bot. 100, 122–131. doi:10.1016/j.sajb.2015.05.012
Rasmussen, B., Cloarec, O., Tang, H., Stærk, D., and Jaroszewski, J. (2006). Multivariate analysis of integrated and full-resolution 1H-nmr spectral data from complex pharmaceutical preparations: st. John’s wort. Planta Med. 72, 556–563. doi:10.1055/s-2006-931567
Revathy, S., Rathinamala, R., and Murugesan, M. (2012). Authentication methods for drugs used in Ayurveda, Siddha and Unani systems of medicine : an overview. Int. J. Pharm. Sci. Res. 3, 2352–2361.
Rumalla, C. S., Avula, B., Shukla, Y. J., Wang, Y.-H., Pawar, R. S., Smillie, T. J., et al. (2008). Chemical fingerprint ofHoodiaspecies, dietary supplements, and related genera by using HPTLC. J. Sep. Sci. 31, 3959–3964. doi:10.1002/jssc.200800441
Saslis-Lagoudakis, C. H., Bruun-Lund, S., Iwanycki, N. E., Seberg, O., Petersen, G., Jäger, A. K., et al. (2015). Identification of common horsetail (equisetum arvense L.; equisetaceae) using thin layer chromatography versus DNA barcoding. Sci. Rep. 5, 1–12. doi:10.1038/srep11942
Scotti, F., Löbel, K., Booker, A., and Heinrich, M. (2019). St. John’s wort (Hypericum perforatum) products - how variable is the primary material? Front. Plant Sci. 9, 1973. doi:10.3389/fpls.2018.01973
Seethapathy, G. S., Raclariu-Manolica, A.-C., Anmarkrud, J. A., Wangensteen, H., and de Boer, H. J. (2019). DNA metabarcoding authentication of Ayurvedic herbal products on the European market raises concerns of quality and fidelity. Front. Plant Sci. 10, 68. doi:10.3389/fpls.2019.00068
Seethapathy, G. S., Tadesse, M., Urumarudappa, S. K. J., Gunaga, S. S., Vasudeva, R., Malterud, K. E., et al. (2018). Authentication of Garcinia fruits and food supplements using DNA barcoding and NMR spectroscopy. Sci. Rep. 8, 10561. doi:10.1038/s41598-018-28635-z
Shanmughanandhan, D., Ragupathy, S., Newmaster, S. G., Mohanasundaram, S., and Sathishkumar, R. (2016). Estimating herbal product authentication and adulteration in India using a vouchered, DNA-based biological reference material library. Drug Saf. 39, 1211–1227. doi:10.1007/s40264-016-0459-0
Simmler, C., Graham, J. G., Chen, S.-N., and Pauli, G. F. (2018). Integrated analytical assets aid botanical authenticity and adulteration management. Fitoterapia 129, 401–414. doi:10.1016/j.fitote.2017.11.017
Slifman, N. R., Obermeyer, W. R., Aloi, B. K., Musser, S. M., Correll, W. A., Cichowicz, S. M., et al. (1998). Contamination of botanical dietary supplements byDigitalis lanata. N. Engl. J. Med. 339, 806–811. doi:10.1056/nejm199809173391204
Soares, F. P., Almeida, F. S., Miranda, C. C., Carvalho, P. H. L., Romero, N. R., and Bandeira, M. A. M. (2016). Avaliação da qualidade de amostras comerciais de leite de janaguba (Himatanthus drasticus (Mart.) Plumel) em Fortaleza - ceará. Rev. Bras. Plantas Med. 18, 399–407. doi:10.1590/1983-084X/15_198
Sogame, M., Naraki, Y., Sasaki, T., Seki, M., Yokota, K., Masada, S., et al. (2019). Quality assessment of medicinal product and dietary supplements containing Vitex agnus-castus by HPLC fingerprint and quantitative analyses. Chem. Pharm. Bull. 67, 527–533. doi:10.1248/cpb.c18-00725
Song, J., Fang, G., Zhang, Y., Deng, Q., and Wang, S. (2010). Fingerprint analysis of ginkgo biloba leaves and related health foods by high-performance liquid chromatography/electrospray ionization-mass spectrometry. J. AOAC Int. 93, 1798–1805. doi:10.1093/jaoac/93.6.1798
Speranskaya, A. S., Khafizov, K., Ayginin, A. A., Krinitsina, A. A., Omelchenko, D. O., Nilova, M. V., et al. (2018). Comparative analysis of Illumina and Ion Torrent high-throughput sequencing platforms for identification of plant components in herbal teas. Food Control 93, 315–324. doi:10.1016/J.FOODCONT.2018.04.040
Sun, J., and Chen, P. (2011). A flow-injection mass spectrometry fingerprinting method for authentication and quality assessment of Scutellaria lateriflora-based dietary supplements. Anal. Bioanal. Chem. 401, 1577–1584. doi:10.1007/s00216-011-5246-2
Sun, J., and Chen, P. (2012). Ultra high-performance liquid chromatography with high-resolution mass spectrometry analysis of African mango (Irvingia gabonensis) seeds, extract, and related dietary supplements. J. Agric. Food Chem. 60, 8703–8709. doi:10.1021/jf302703u
Thakkar, S., Anklam, E., Xu, A., Ulberth, F., Li, J., Li, B., et al. (2020). Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul. Toxicol. Pharmacol. 114, 104647. doi:10.1016/j.yrtph.2020.104647
Thongkhao, K., Pongkittiphan, V., Phadungcharoen, T., Tungphatthong, C., Urumarudappa, S. K. J., Pengsuparp, T., et al. (2020). Differentiation of Cyanthillium cinereum, a smoking cessation herb, from its adulterant Emilia sonchifolia using macroscopic and microscopic examination, HPTLC profiles and DNA barcodes. Sci. Rep. 10, 14753. doi:10.1038/s41598-020-71702-7
Tian, R.-t., Xie, P.-s., and Liu, H.-p. (2009). Evaluation of traditional Chinese herbal medicine: chaihu (Bupleuri Radix) by both high-performance liquid chromatographic and high-performance thin-layer chromatographic fingerprint and chemometric analysis. J. Chromatogr. A 1216, 2150–2155. doi:10.1016/j.chroma.2008.10.127
Tombul, A. G., Orhan, N., Sezik, E., and Ergun, F. (2012). Morphologic, anatomical, and chromatographic studies on Eucalyptus (L’Hér.) samples from the market. Fabad J. Pharm. Sci. 37, 79–87.
Turbitt, J. R., Colson, K. L., Killday, K. B., Milstead, A., and Neto, C. C. (2020). Application of1H‐NMR‐based metabolomics to the analysis of cranberry (Vaccinium macrocarpon) supplements. Phytochem. Anal. 31, 68–80. doi:10.1002/pca.2867
Upton, R., David, B., Gafner, S., and Glasl, S. (2020). Botanical ingredient identification and quality assessment: strengths and limitations of analytical techniques. Phytochem. Rev. 19, 1157–1177. doi:10.1007/s11101-019-09625-z
Urumarudappa, S. K. J., Gogna, N., Newmaster, S. G., Venkatarangaiah, K., Subramanyam, R., Saroja, S. G., et al. (2016). DNA barcoding and NMR spectroscopy-based assessment of species adulteration in the raw herbal trade of Saraca asoca (Roxb.) Willd, an important medicinal plant. Int. J. Leg. Med. 130, 1457–1470. doi:10.1007/s00414-016-1436-y
van der Valk, J. M. A., Leon, C. J., and Nesbitt, M. (2017). Macroscopic authentication of Chinese materia medica (CMM) : a UK market study of seeds and fruits. J. Herbal Med. 8, 40–51. doi:10.1016/j.hermed.2017.03.007
Vejayan, J., Iman, V., Foong, S.-L., and Ibrahim, H. (2013). Protein markers useful in authenticating Eurycoma longifolia contained herbal aphrodisiac products. Mjs 32, 15–23. doi:10.22452/mjs.vol32no1.4
Vejayan, J., Mohamed, A. N., Zulkifli, A. A., Yahya, Y. A. C., Munir, N., and Yusoff, M. M. (2018). Marker to authenticate Eurycoma longifolia (Tongkat Ali) containing aphrodisiac herbal products. Curr. Sci. 115, 886–894. doi:10.18520/cs/v115/i5/886-894
Viapiana, A., Struck-Lewicka, W., Konieczynski, P., Wesolowski, M., and Kaliszan, R. (2016). An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Matricaria chamomilla L. commercial samples. Front. Plant Sci. 7, 1561. doi:10.3389/fpls.2016.01561
Villani, T. S., Reichert, W., Ferruzzi, M. G., Pasinetti, G. M., Simon, J. E., and Wu, Q. (2015). Chemical investigation of commercial grape seed derived products to assess quality and detect adulteration. Food Chem. 170, 271–280. doi:10.1016/j.foodchem.2014.08.084
Waidyanatha, S., Pierfelice, J., Cristy, T., Mutlu, E., Burback, B., Rider, C. V., et al. (2020). A strategy for test article selection and phytochemical characterization of Echinacea purpurea extract for safety testing. Food Chem. Toxicol. 137, 111125. doi:10.1016/j.fct.2020.111125
Walkowiak, A., Ledziński, Ł., Zapadka, M., and Kupcewicz, B. (2019). Detection of adulterants in dietary supplements with Ginkgo biloba extract by attenuated total reflectance Fourier transform infrared spectroscopy and multivariate methods PLS-DA and PCA. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 208, 222–228. doi:10.1016/j.saa.2018.10.008
Wallace, E. D., Oberlies, N. H., Cech, N. B., and Kellogg, J. J. (2018). Detection of adulteration in Hydrastis canadensis (goldenseal) dietary supplements via untargeted mass spectrometry-based metabolomics. Food Chem. Toxicol. 120, 439–447. doi:10.1016/j.fct.2018.07.033
Wallace, E. D., Todd, D. A., Harnly, J. M., Cech, N. B., and Kellogg, J. J. (2020). Identification of adulteration in botanical samples with untargeted metabolomics. Anal. Bioanal. Chem. 412, 4273–4286. doi:10.1007/s00216-020-02678-6
Wan, J., Liu, M., Jiang, H.-Y., Yang, J., Du, X., Li, X.-N., et al. (2016). Bioactive ent-kaurane diterpenoids from Isodon serra. Phytochemistry 130, 244–251. doi:10.1016/j.phytochem.2016.05.014
Wang, M., Avula, B., Wang, Y.-H., Zhao, J., Avonto, C., Parcher, J. F., et al. (2014a). An integrated approach utilising chemometrics and GC/MS for classification of chamomile flowers, essential oils and commercial products. Food Chem. 152, 391–398. doi:10.1016/j.foodchem.2013.11.118
Wang, M., Zhao, J., Avula, B., Wang, Y.-H., Avonto, C., Chittiboyina, A. G., et al. (2014b). High-Resolution gas chromatography/mass spectrometry method for characterization and quantitative analysis of ginkgolic acids inGinkgo bilobaPlants, extracts, and dietary supplements. J. Agric. Food Chem. 62, 12103–12111. doi:10.1021/jf503980f
Wang, Y.-H., Meng, Y., Zhai, C., Wang, M., Avula, B., Yuk, J., et al. (2019). The chemical characterization of Eleutherococcus senticosus and Ci-Wu-jia tea using UHPLC-UV-QTOF/MS. Ijms 20, 475. doi:10.3390/ijms20030475
Weber, H. A., Zart, M. K., Hodges, A. E., White, K. D., Barnes, S. M., Moody, L. A., et al. (2003). Method validation for determination of alkaloid content in goldenseal root powder. J. AOAC Int. 86, 476–483. doi:10.1093/jaoac/86.3.476
Wen, T., Wei, D. P., Long, F. Y., Zeng, X. Y., and Kang, J. C. (2016). Multigene phylogeny and HPLC analysis reveal fake Ophiocordyceps sinensis in markets. Mycosphere 7, 853–867. doi:10.5943/mycosphere/7/6/16
Wohlmuth, H., Savage, K., Dowell, A., and Mouatt, P. (2014). Adulteration of Ginkgo biloba products and a simple method to improve its detection. Phytomedicine 21, 912–918. doi:10.1016/j.phymed.2014.01.010
Wu, T., Annie Bligh, S. W., Gu, L.-h., Wang, Z.-t., Liu, H.-p., Cheng, X.-m., et al. (2005). Simultaneous determination of six isoflavonoids in commercial Radix Astragali by HPLC-UV. Fitoterapia 76, 157–165. doi:10.1016/j.fitote.2004.11.006
Wu, W.-R., Cheng, C.-S., Cheng, Q.-Q., Lao, C.-C., Cui, H., Tang, Z.-Y., et al. (2020). Novel SNP markers on ginsenosides biosynthesis functional gene for authentication of ginseng herbs and commercial products. Chin. J. Nat. Medicines 18, 770–778. doi:10.1016/S1875-5364(20)60017-6
Xu, M., Huang, B., Gao, F., Zhai, C., Yang, Y., Li, L., et al. (2019). Assesment of adulterated traditional Chinese medicines in China: 2003-2017. Front. Pharmacol. 10, 1446. doi:10.3389/fphar.2019.01446
Yang, W., Qiao, X., Li, K., Fan, J., Bo, T., Guo, D.-a., et al. (2016). Identification and differentiation of Panax ginseng, Panax quinquefolium, and Panax notoginseng by monitoring multiple diagnostic chemical markers. Acta Pharmaceutica Sinica B 6, 568–575. doi:10.1016/j.apsb.2016.05.005
Yao, C., Yang, W., Si, W., Pan, H., Qiu, S., Wu, J., et al. (2016). A strategy for establishment of practical identification methods for Chinese patent medicine from systematic multi-component characterization to selective ion monitoring of chemical markers: shuxiong tablet as a case study. RSC Adv. 6, 65055–65066. doi:10.1039/c6ra10883k
Ye, F., Wang, H., Jiang, S., Wu, J., Shao, J., Cheng, X., et al. (2004). Quality evaluation of commercial extracts of Scutellaria baicalensis. Nutr. Cancer 49, 217–222. doi:10.1207/s15327914nc4902_14
Yoshida, N., Numano, M., Nagasaka, Y., Ueda, K., Tsuboi, H., Tanimoto, T., et al. (2015). Study on health hazards through medicines purchased on the Internet: a cross-sectional investigation of the quality of anti-obesity medicines containing crude drugs as active ingredients. BMC Complement. Altern. Med. 15, 430. doi:10.1186/s12906-015-0955-2
Yu, C., Wang, C.-Z., Zhou, C.-J., Wang, B., Han, L., Zhang, C.-F., et al. (2014). Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. J. Pharm. Biomed. Anal. 99, 8–15. doi:10.1016/j.jpba.2014.06.031
Yu, N., Wei, Y.-l., Zhu, Y., Zhu, N., Wang, Y.-l., Zhang, H.-p., et al. (2018). Integrated approach for identifying and evaluating the quality of Marsdenia tenacissima in the medicine market. PLoS One 13, e0195240. doi:10.1371/journal.pone.0195240
Yuk, J., Patel, D. N., Isaac, G., Smith, K., Wrona, M., Olivos, H.-J., et al. (2016). Chemical profiling of ginseng species and ginseng herbal products using UPLC/QTOF-MS. J. Braz. Chem. Soc. 27, 1476–1483. doi:10.5935/0103-5053.20160189
Zhao, H., Xu, J., Ghebrezadik, H., and Hylands, P. J. (2015). Metabolomic quality control of commercial Asian ginseng, and cultivated and wild American ginseng using 1H NMR and multi-step PCA. J. Pharm. Biomed. Anal. 114, 113–120. doi:10.1016/j.jpba.2015.05.010
Zhao, J., Avula, B., Joshi, V., Techen, N., Wang, Y.-H., Smillie, T., et al. (2011). NMR fingerprinting for analysis ofHoodiaSpecies andHoodiaDietary products. Planta Med. 77, 851–857. doi:10.1055/s-0030-1250583
Zhu, L., Fang, L., Li, Z., Xie, X., and Zhang, L. (2019). A HPLC fingerprint study on Chaenomelis Fructus. BMC Chem. 13, 7. doi:10.1186/s13065-019-0527-5
Zhu, S., Bai, Y., Oya, M., Tanaka, K., Komatsu, K., Maruyama, T., et al. (2011). Genetic and chemical diversity of Eleutherococcus senticosus and molecular identification of Siberian ginseng by PCR-RFLP analysis based on chloroplast trnK intron sequence. Food Chem. 129, 1844–1850. doi:10.1016/J.FOODCHEM.2011.05.128
Keywords: chemical marker, natural product, herbal product, food supplement, herbal medicine, authentication, adulteration, contamination
Citation: Ichim MC and Booker A (2021) Chemical Authentication of Botanical Ingredients: A Review of Commercial Herbal Products. Front. Pharmacol. 12:666850. doi: 10.3389/fphar.2021.666850
Received: 11 February 2021; Accepted: 09 March 2021;
Published: 15 April 2021.
Edited by:
Marcello Locatelli, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Santhosh Kumar J. Urumarudappa, Chulalongkorn University, ThailandSubramanyam Ragupathy, University of Guelph, Canada
Copyright © 2021 Ichim and Booker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mihael Cristin Ichim, Y2ljaGltQGhvdG1haWwuY29t; Anthony Booker, YS5ib29rZXJAd2VzdG1pbnN0ZXIuYWMudWs=
 Mihael Cristin Ichim
Mihael Cristin Ichim Anthony Booker
Anthony Booker