
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 16 April 2021
Sec. Obstetric and Pediatric Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.660123
This article is part of the Research TopicTherapeutic Implications for Pregnant Women with Systemic Autoimmune Diseases and their ChildrenView all 11 articles
 Maddalena Larosa1,2,3
Maddalena Larosa1,2,3 Nathalie Costedoat-Chalumeau2,3,4
Nathalie Costedoat-Chalumeau2,3,4 Gaëlle Guettrot-Imbert2,3
Gaëlle Guettrot-Imbert2,3 Veronique Le Guern2,3
Veronique Le Guern2,3 Nathalie Morel2,3
Nathalie Morel2,3 Diogo Jesus5,6
Diogo Jesus5,6 Luca Iaccarino1
Luca Iaccarino1 Luís Inês5,7
Luís Inês5,7 Andrea Doria1*
Andrea Doria1*Introduction: Systemic Lupus Erythematosus (SLE) mainly occurs during childbearing age. Remission or low disease activity state (LDAS) before conception are recommended by experts to achieve a favourable lupus pregnancy outcome but little is known on the best way to evaluate remission or activity status during pregnancy.
Objectives: We tested SLE-disease activity score (SLE-DAS) in the first trimester as predictor of maternal flares and obstetrical complications in 2nd and 3rd trimester in a cohort of SLE pregnant women.
Patients and Methods: Inclusion criteria were: 1) women ≥ 18 years; 2) affected with SLE (SLICC 2012); 3) enrolled in two referral centers (Italy and France) 4) with an ongoing singleton pregnancy at 12 weeks (only one pregnancy per patient). Disease activity was assessed at first trimester of pregnancy, using SLE-pregnancy disease activity index (SLEPDAI) and retrospectively applying SLE-DAS. Maternal lupus flares at 2nd and 3rd trimester were defined by the SELENA-SLEDAI Flare Index (SFI). Adverse pregnancy outcome (APO) included: fetal and neonatal death, placental insufficiency with premature delivery <37 weeks, and small for gestational age (SGA) (≤3rd percentile).
Results: We included 158 pregnant patients affected with SLE. At first trimester the median SLEPDAI (IQR) was 2 (0–4) and the median SLE-DAS (IQR) 1.32 (0.37–2.08). At least one flare occurred in 25 (15.8%) women during the 2nd and 3rd trimester. APO occurred in 19 (12.0%) patients. A significant correlation between SLE-DAS and SLEPDAI was found in this cohort (Spearman’s ρ = 0.97, Figure 1). At multivariate analysis, both SLE-DAS and SLEPDAI predicted maternal flares (adjOR = 1.2; 95% CI = 1.0–1.3, p = 0.02; adjOR 1.3, 95% CI = 1.1–1.6 per unit increase, p = 0.01, respectively). SLE-DAS and SLEPDAI were associated with APO at univariate analysis (p = 0.02).
Conclusions: SLE-DAS was highly correlated with SLEPDAI and its use in the first trimester predicted maternal flares in the 2nd and 3rd trimester, making SLE-DAS a reliable instrument to measure SLE activity during pregnancy.
It is well known that Systemic Lupus Erythematosus (SLE) mainly affects women in childbearing age, with a ratio of female-to-male close to 9:1 (Pons-Estel et al., 2017). Therefore, pregnancy is common in SLE patients, who are at risk for complications such as maternal flares, preeclampsia (PE), foetal loss, and preterm birth (Larosa et al., 2019).
During the last few decades, the outcome of pregnancy in SLE has consistently improved, possibly due to preconception counseling (Andreoli et al., 2017). Risk stratification before conception is pivotal to achieve a favourable outcome (Andreoli et al., 2017) and it should assess SLE remission, autoantibodies (Abs) profile, treatment and previous pregnancy morbidity (Andreoli et al., 2017). Indeed, active/flaring SLE in the 6–12 months before conception is a major risk factor for flares during pregnancy and/or puerperium, as well as for foetal morbidity (i.e. intra-uterine growth restriction-IUGR-, pregnancy loss and preterm delivery) (Andreoli et al., 2017).
In the past decades, efforts has been made to prevent damage accrual in SLE patients by developing treat-to-target (T2T) strategies (Gatto et al., 2019; Jesus et al., 2019a) which primarily pursue the achievement of a durable remission or at least a low disease activity state (LDAS) (Zen et al., 2015; Franklyn et al., 2016; Fischer-Betz and Specker, 2017; Polachek et al., 2017; Ugarte-Gil et al., 2017; van Vollenhoven et al., 2017). Nevertheless, the best definition of such conditions is still under debate and not widely shared.
Recently, Jesus et al. (2019a) developed a new score to measure disease activity, named SLE Disease Activity Score (SLE-DAS) (Jesus et al., 2019a). This score encompasses 17 items and includes some continuous variables (Jesus et al., 2019a). When comparing the performance of this instrument with the SLE disease Activity Index 2000 (SLEDAI-2K) (Gladman et al., 2002), the Authors found that SLE-DAS has a higher accuracy in measuring SLE disease activity, a better sensitivity-to-change and a higher predictive value for damage accrual (Jesus et al., 2019b). Other advantages are that SLE-DAS is a continuous measure of disease activity and includes two important features absent in the SLEDAI-2K, i.e. haemolytic anaemia and lupus enteritis (Jesus et al., 2019a).
During pregnancy, some maternal physiological changes can occur and consequently mislead the evaluation of disease activity. Hence, scoring SLE activity by applying the SLEDAI-2K could be biased. For this reason, Buyon et al. proposed a modification of SLEDAI-2K, which is named SLEPDAI (Buyon et al., 1999). Nonetheless, this score has never been validated.
Since no data on SLE-DAS applicability during pregnancy are available, we aimed to evaluate SLE-DAS in the first trimester as predictor of maternal flares and obstetrical complications.
This study was carried out at two referral centers for rare systemic and autoimmune diseases: Rheumatology Unit, University of Padova, Italy (from 2002 to July 2019), and Internal Medicine Department, Cochin Hospital, Paris, France (from 2014 to July 2019, by using all pregnancies from the prospective GR2 study clinicaltrial.gov NCT02450396).
Inclusion criteria were 1) women ≥18 years; 2) affected with SLE (SLICC 2012 criteria) (Petri et al., 2012); 3) with an ongoing singleton pregnancy at 12 weeks (only one pregnancy per patient).
Patients underwent at least one visit in the first trimester, and were followed up according to current clinical practice until the end of pregnancy. This project adheres to the principles of the Declaration of Helsinki and was approved by the local ethics committees.
Demographic, clinical and laboratory findings were collected at first trimester by assessing the following variables: age, disease duration (years), skin colour, multiparity, associated anti-phospholipid syndrome (APS) (Miyakis et al., 2006) and SLE manifestations.
Serological and other laboratory data were assessed at first trimester according to standard tests including anti-double stranded DNA (anti-dsDNA), C3 and/or C4 serum levels, and 24-hour (h) proteinuria. Anti-phospholipid (aPL) Abs were also tested including anti-cardiolipin (aCL) IgM and IgG, anti-ß2 glycoprotein-1 (anti-ß2GP1) IgM and IgG, and lupus anticoagulant (LAC) according to standard definitions (Miyakis et al., 2006) and recommendations (Moore, 2014).
Women were considered on therapy when they were taking at least one of the following drugs: hydroxychloroquine (HCQ), prednisone, immunosuppressants (IS), low dose aspirin (LDA) and low molecular weight heparin (LMWH).
Disease activity was assessed at first trimester by applying SLEPDAI (Buyon et al., 1999) and by retrospectively applying SLE-DAS using the online free calculator available at http://sle-das.eu/ (Jesus et al., 2019a).
Maternal flares in the 2nd and 3rd trimester of gestation were assessed according to SELENA-SLEDAI flare index (SFI) (Petri et al., 2005); flares were subdivided into mild/moderate and severe.
APO were defined with a binary composite score obtained when any of the following event occurred: an otherwise unexplained intrauterine foetal death (IUFD) >12 weeks; a neonatal death within the first 7 days after birth; placental insufficiency (IUGR, PE/eclampsia -E-, Hemolysis, Elevated Liver enzymes, Low Platelets count -HELLP-syndrome, and/or placental abruption) leading to a premature delivery before 37 weeks; small for gestational age (SGA): birth weight ≤3rd percentile according the AUDIPOG curve (Mamelle et al., 2001). Definitions of PE and HELLP are summarized in Supplementary Material.
We described patients’ characteristics applying mean ± standard deviation (SD) and median with interquartile range (IQR) for parametric and non-parametric continuous variables, respectively. APO’s and maternal flares’ incidences was reported by confidence intervals (CI) set at 95%. The following variables were assessed at first trimester by univariate analysis:
- Continuous: age at pregnancy (years), disease duration (years), SLEPDAI and SLE-DAS scores, prednisone dosage (mg/day);
- Categorical/binary: associated APS, previous renal involvement, serological features including anti-dsDNA, hypocomplementemia (low C3 and/or C4), aPL profile (LAC, IgG/IgM anti-aCL, IgG/IgM anti-beta2GPI, triple positive aPL), active 24 h proteinuria (>0.5 g/day); concomitant treatment including HCQ, prednisone, IS, LDA, and/or LMWH.
Comparison of continuous variables with a parametric and non-parametric distribution was performed using T-test and Wilcoxon’s rank-sum test, respectively. Pearson’s chi-square (or Fisher’s exact test when appropriate) was used to evaluate bivariate associations between categorical variables at univariate analyses. A correlation between SLEPDAI and SLE-DAS in the first trimester was assessed according to Spearman’s correlation test, considering the non-parametric distribution of these scores. Multivariate analysis was performed according to a logistic regression model. The choice of independent variables was based on current knowledge and significant variables at univariate analysis (p < 0.1). Two separate multivariate analyses were performed for the outcomes of maternal flares and APO, respectively. Significance at logistic regression analysis was set at 5%. Models’ performance was evaluated using the Hosmer–Lemeshow (HL) goodness-of-fit test and area under the ROC curve (AUC). All statistics were conducted using R software version package 1.3.1073.
A total of 158 pregnancies in 158 patients affected with SLE were collected (107 enrolled at Internal Medicine Department, Cochin Hospital, and 51 at the Rheumatology Department, University of Padova). Mean ± SD age was 31.9 ± 4.6 years, and 89 (56.3%) women were multiparous.
The majority were White (n = 124, 78.5%), followed by Black (n = 21, 13.3%), Asian (n = 11, 6.9%) and other origin (n = 2, 1.3%).
Previous SLE manifestations before pregnancy occurred as following: articular involvement in 125 (79.1%) women, mucocutaneous in 108 (68.4%), renal and haematological in 59 (37.3%) respectively, serositis in 36 (22.8%) and neuropsychiatric (NPSLE) in 7 (4.4%).
Median SLEPDAI (IQR) was 2 (0–4) and median SLE-DAS (IQR) was 1.32 (0.37–2.08).
We observed 153 (96.8%) live births: their mean birth weight ±SD was 2,946 ± 583 g (2 missing data), and the median delivery term (IQR) was at 38 WG (36–39) (3 missing data). Four (2.5%) IUFD occurred and a medical termination of pregnancy was performed in 1 (0.6%) case.
At least one flare occurred in 25 (15.8%, 95% CI 9.9–20.9) patients during the 2nd and 3rd trimester: 23 (14.6%) women experienced mild/moderate flares and 2 (1.3%) severe renal flares. Overall, we observed that 10 (6.3%) patients had at least one articular flare, 7 (4.4%) mucocutaneous, 5 (3.2%) renal, 3 (1.8%) hematological and serositis flares, respectively. No NPSLE flares occurred in this study (Table 1).
Nineteen (12.0%; 95% CI: 7.8–18.0) pregnancies were complicated by obstetrical events (Table 2). Four patients (2.5%) had an IUFD and 13 (8.2%) preterm deliveries due to placental insufficiency. In addition, 3 pregnancies (2.0%, 7 missing data) ended with SGA births. No neonatal deaths occurred.
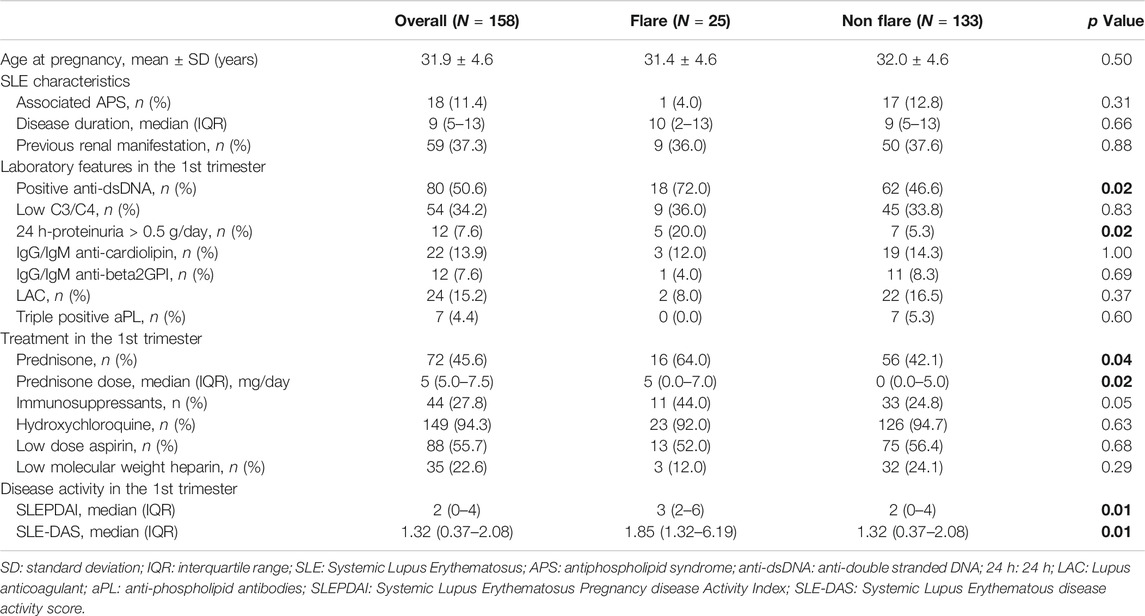
TABLE 2. Univariate analysis: Maternal flares in the 2nd and 3rd trimester. Bold values refer to significative p values (p < 0.05).
At univariate analysis, both SLE-DAS and SLEPDAI scores in the first trimester were associated with maternal flares in 2nd and 3rd trimester of gestation (p = 0.01 for both). In addition, anti-dsDNA (p = 0.02) and active 24 h-proteinuria (p = 0.02) were also found to be associated with maternal flares. Regarding treatment, the use of prednisone and prednisone dosage (p = 0.04 and p = 0.02, respectively) (Table 2). There was a high and significant correlation between SLEPDAI and SLE-DAS (rho = 0.97, p < 0.01) in the first trimester.
At univariate analysis, SLE-DAS (p = 0.02), SLEPDAI (p = 0.02) and anti-dsDNA (p = 0.01) were associated with APO (Table 3).
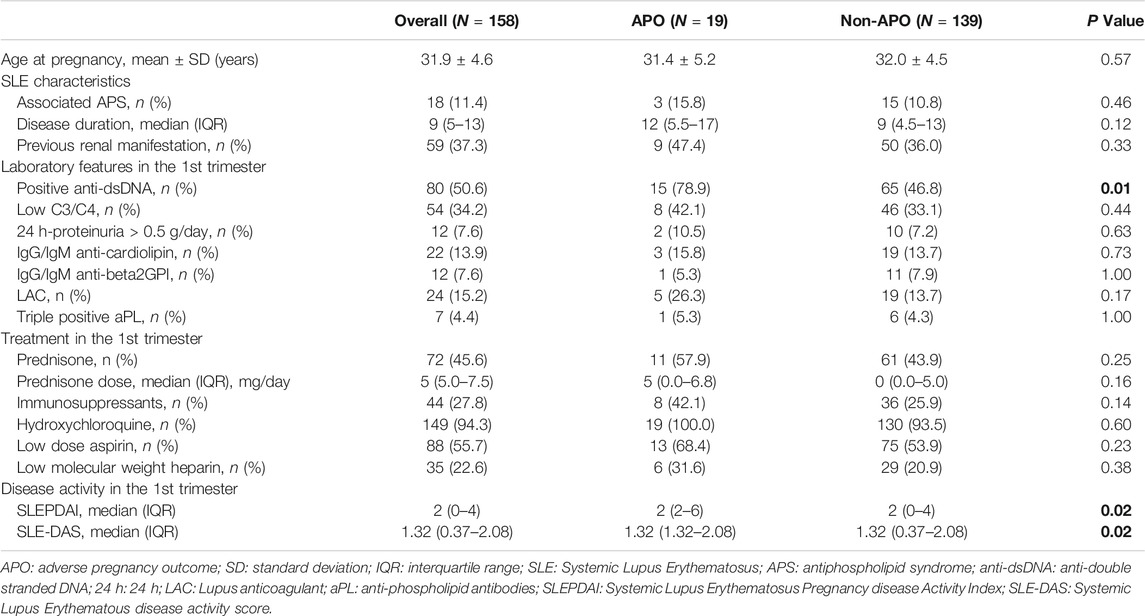
TABLE 3. Univariate analysis: Adverse pregnancy outcome (APO). Bold values refer to significative p values (p < 0.05).
Regarding maternal flares, since both anti-dsDNA and active 24 h-proteinuria are components of SLEPDAI and SLE-DAS, we did not include these two variables into our logistic regression models in order to avoid collinearity issues. As we found a high correlation between SLEPDAI and SLE-DAS in the first trimester, two different logistic regression models were performed using SLEPDAI and SLE-DAS as explanatory variables (Table 4).
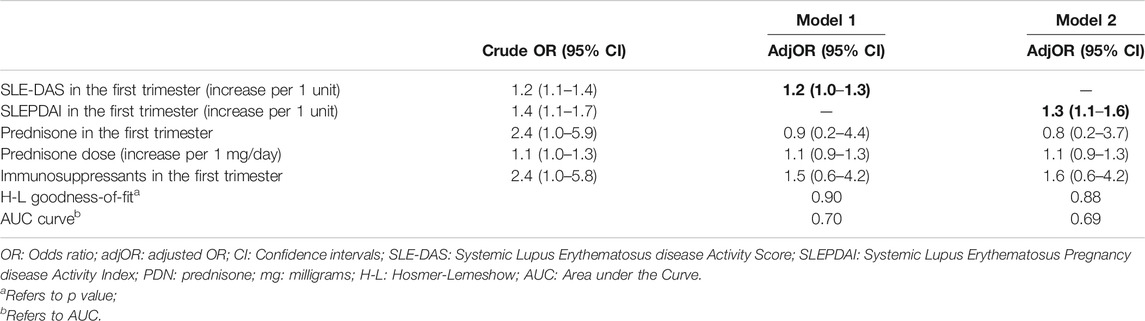
TABLE 4. Multivariate logistic regression for maternal flares. Bold values refer to significative p values (p < 0.05).
SLE-DAS in the first trimester was predictor of maternal flares in the 2nd and 3rd trimester (adjusted-adj-OR:1.2; 95% CI:1.0–1.3; p = 0.02). Also, SLEPDAI resulted predictor of maternal flares in pregnancy (adjOR = 1.3; 95% CI:1.1–1.6; p = 0.01).
We did not perform a multivariate analysis for APO as only anti-dsDNA, SLEPDAI and SLE-DAS resulted statistically associated at univariate analysis; therefore, due to collinearity issues a multivariate analysis was not assessed.
In this study of 158 pregnant women affected with SLE we analyzed disease activity in the 1st trimester assessed by SLEPDAI and SLE-DAS. Although 59 (37.3%) patients had had a previous renal involvement, SLE activity during the 1st trimester was mild: median (IQR) SLEPDAI of 2 (0–4) and median (IQR) SLE-DAS of 1.32 (0.37–2.08). Even though remission/LDAS state cut-off definitions in pregnant patients have not been validated yet, the low scores observed in disease activity indexes in our study, suggest that most patients were not active in the first trimester.
Twenty-five (15.8%) women experienced at least one flare in the 2nd and 3rd trimester. Among them, the majority of our patients had mild/moderate flares (n = 23, 14.6%), and only 2 women (1.3%) developed severe flares. This is in line with the PROMISSE study which reported mild/moderate flares occurred in 22.3% and severe flares in 5.5% of SLE patients during 385 prospective pregnancies (Buyon et al., 2015).
APO occurred in 12% of patients, which is also in keeping with the PROMISSE study where these complications occurred in 19% of cases (Buyon et al., 2015). Finally, a high rate of live births was found (96.8%), probably due to the high rate of LDAS/remission state observed in the first trimester of pregnancy in our study. Hence, our results strengthen that remission or LDAS in the early pregnancy is important to avoid fetal complications during pregnancies (Andreoli et al., 2017).
A high correlation between SLE-DAS and SLEPDAI in the 1st trimester was observed in our cohort (ρ = 0.97, p < 0.01). This is in line with previous findings (Jesus et al., 2019a) who reported a high correlation between the SLE-DAS and SLEDAI-2K. SLEPDAI (Buyon et al., 1999) is a non-validated score adapted to pregnancy that includes the 24 items from the SELENA-SLEDAI with modifications of 15 of them, to avoid wrongly attribution of some physiological or pathological pregnancy changes to SLE (Buyon et al., 1999; Andreoli et al., 2019). Even if SLEDAI-2K and SELENA-SLEDAI are slightly different, correlation between SLE-DAS and both scores was therefore expected.
In multivariate analyses, both SLE-DAS and SLEPDAI at first trimester of pregnancy were independent predictors of SLE flares in the 2nd and 3rd trimester (adjOR, 95% CI = 1.2 (1.0–1.3); adjOR 95% CI = 1.3 (1.1–1.6). In addition, performance of both scores was assessed by AUC and Goodness-of-fit analysis which proved that SLE-DAS model performs slightly better than SLEPDAI model (Table 4). Thus, the high correlation between SLE-DAS and SLEPDAI, the predictive value of SLE-DAS for lupus flares, as well as the aforementioned performance models’ tests prove that SLE-DAS is an appropriate instrument for monitoring SLE disease activity during pregnancy.
Both SLE-DAS and SLEPDAI were associated with APO at univariate analysis (p = 0.02), but since anti-dsDNA is already included in both scores, no multivariate analysis could be done. Although our patients had mainly inactive mild SLE, our results suggest that active SLE may still be associated with poor obstetrical prognosis. Such association between disease activity and APO has been already observed in elderly cohorts in which patients had a more active SLE (Clowse et al., 2005). These findings has led to current EULAR recommendations which pinpoint the importance of a stable mild/inactive disease when pregnancy is planned (Andreoli et al., 2017).
Our study has some limitations. First, we applied SLE-DAS retrospectively. This was unavoidable since SLE-DAS was validated in 2019 (Jesus et al., 2019a). Second, in keeping with current recommendations (Andreoli et al., 2017), most pregnancies occurred in patients in clinical remission or LDAS, therefore limiting the possibility to demonstrate a stronger association between high disease activity and APO. The strengths of this study also need to be remarked: first, this a quite large multicentric cohort from 2 referral centers in SLE; second, we acknowledged the role of SLE-DAS as a very simple tool to define disease activity in pregnant patients.
In conclusion, SLE-DAS in the first trimester may be a useful instrument to predict maternal flares in the 2nd and 3rd trimester.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The studies involving human participants were reviewed and approved by Azienda Ospedaliera-Università degli Studi di Padova and CPP Ile de France VI. The patients/participants provided their written informed consent to participate in this study.
ML contributed to the conception and design of the work, the follow-up of patients, acquisition, analysis and interpretation of data and she drafted the work; NC-C contributed to the conception and design of the work, the follow-up of patients, and the revision of the manuscript for important intellectual content; GG-I, VG, NM, and LI followed up patients; DJ and LI helped in revising the work; AD contributed to the conception and design of the work, interpreted the data and revised the manuscript for important intellectual content. All the authors approved the final version of the manuscript and gave their agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.660123/full#supplementary-material.
Andreoli, L., Bertsias, G. K., Agmon-Levin, N., Brown, S., Cervera, R., Costedoat-Chalumeau, N., et al. (2017). EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 76, 476–485. doi:10.1136/annrheumdis-2016-209770
Andreoli, L., Gerardi, M. C., Fernandes, M., Bortoluzzi, A., Bellando-Randone, S., Brucato, A., et al. (2019). Disease activity assessment of rheumatic diseases during pregnancy: a comprehensive review of indices used in clinical studies. Autoimmun. Rev. 18, 164–176. doi:10.1016/j.autrev.2018.08.008
Buyon, J. P., Kim, M. Y., Guerra, M. M., Laskin, C. A., Petri, M., Lockshin, M. D., et al. (2015). Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann. Intern. Med. 163, 153–163. doi:10.7326/m14-2235
Buyon, J. P., Kalunian, K. C., Ramsey-Goldman, R., Petri, M. A., Lockshin, M. D., Ruiz-Irastorza, G., et al. (1999). Assessing disease activity in SLE patients during pregnancy. Lupus 8, 677–684. doi:10.1191/096120399680411272
Clowse, M. E. B., Magder, L. S., Witter, F., and Petri, M. (2005). The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum. 52, 514–521. doi:10.1002/art.20864
Fischer-Betz, R., and Specker, C. (2017). Pregnancy in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract. Res. Clin. Rheumatol. 31, 397–414. doi:10.1016/j.berh.2017.09.011
Franklyn, K., Lau, C. S., Navarra, S. V., Louthrenoo, W., Lateef, A., Hamijoyo, L., et al. (2016). Definition and initial validation of a lupus low disease activity state (LLDAS). Ann. Rheum. Dis. 75, 1615–1621. doi:10.1136/annrheumdis-2015-207726
Gatto, M., Zen, M., Iaccarino, L., and Doria, A. (2019). New therapeutic strategies in systemic lupus erythematosus management. Nat. Rev. Rheumatol. 15, 30–48. doi:10.1038/s41584-018-0133-2
Gladman, D. D., Ibañez, D., and Urowitz, M. B. (2002). Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 29, 288–291.
Jesus, D., Matos, A., Henriques, C., Zen, M., Larosa, M., Iaccarino, L., et al. (2019a). Derivation and validation of the SLE Disease Activity Score (SLE-DAS): a new SLE continuous measure with high sensitivity for changes in disease activity. Ann. Rheum. Dis. 78, 365–371. doi:10.1136/annrheumdis-2018-214502
Jesus, D., Rodrigues, M., Matos, A., Henriques, C., Pereira da Silva, J. A., and Inês, L. S. (2019b). Performance of SLEDAI-2K to detect a clinically meaningful change in SLE disease activity: a 36-month prospective cohort study of 334 patients. Lupus 28, 607–612. doi:10.1177/0961203319836717
Larosa, M., Del Ross, T., Calligaro, A., Favaro, M., Zanatta, E., Iaccarino, L., et al. (2019). Clinical outcomes and predictors of maternal and fetal complications in pregnancies of patients with systemic lupus erythematosus. Expert Rev. Clin. Immunol. 15, 617–627. doi:10.1080/1744666x.2019.1601557
Mamelle, N., Cochet, V., and Claris, O. (2001). Definition of fetal growth restriction according to constitutional growth potential. Neonatology 80, 277–285. doi:10.1159/000047157
Miyakis, S., Lockshin, M. D., Atsumi, T., Branch, D. W., Brey, R. L., Cervera, R., et al. (2006). International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 4, 295–306. doi:10.1111/j.1538-7836.2006.01753.x
Moore, G. (2014). Recent guidelines and recommendations for laboratory detection of lupus anticoagulants. Semin. Thromb. Hemost. 40, 163–171. doi:10.1055/s-0033-1364185
Petri, M., Orbai, A. M., Alarcón, G. S., Gordon, C., Merrill, J. T., Fortin, P. R., et al. (2012). Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686. doi:10.1002/art.34473
Petri, M., Kim, M. Y., Kalunian, K. C., Grossman, J., Hahn, B. H., Sammaritano, L. R., et al. (2005). Combined oral contraceptives in women with systemic lupus erythematosus. N. Engl. J. Med. 353, 2550–2558. doi:10.1056/nejmoa051135
Polachek, A., Gladman, D. D., Su, J., and Urowitz, M. B. (2017). Defining low disease activity in systemic lupus erythematosus. Arthritis Care Res. 69, 997–1003. doi:10.1002/acr.23109
Pons-Estel, G. J., Ugarte-Gil, M. F., and Alarcón, G. S. (2017). Epidemiology of systemic lupus erythematosus. Expert Rev. Clin. Immunol. 13, 799–814. doi:10.1080/1744666x.2017.1327352
Ugarte-Gil, M. F., Wojdyla, D., Pons-Estel, G. J., Catoggio, L. J., Drenkard, C., Sarano, J., et al. (2017). Remission and Low Disease Activity Status (LDAS) protect lupus patients from damage occurrence: data from a multiethnic, multinational Latin American Lupus Cohort (GLADEL). Ann. Rheum. Dis. 76, 2071–2074. doi:10.1136/annrheumdis-2017-211814
van Vollenhoven, R., Voskuyl, A., Bertsias, G., Aranow, C., Aringer, M., Arnaud, L., et al. (2017). A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann. Rheum. Dis. 76, 554–561. doi:10.1136/annrheumdis-2016-209519
Keywords: systemic lupus erythematosus, SLE-DAS, pregnancy, maternal flares, adverse obstetrical outcome
Citation: Larosa M, Costedoat-Chalumeau N, Guettrot-Imbert G, Le Guern V, Morel N, Jesus D, Iaccarino L, Inês L and Doria A (2021) SLE-DAS in the First Trimester of Gestation Predicts Maternal Lupus Flares Later in Pregnancy. Front. Pharmacol. 12:660123. doi: 10.3389/fphar.2021.660123
Received: 28 January 2021; Accepted: 18 March 2021;
Published: 16 April 2021.
Edited by:
Maria Gerosa, University of Milan, ItalyReviewed by:
Giacomo Emmi, Università degli Studi di Firenze, ItalyCopyright © 2021 Larosa, Costedoat-Chalumeau, Guettrot-Imbert, Le Guern, Morel, Jesus, Iaccarino, Inês and Doria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Doria, YWRvcmlhQHVuaXBkLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.