- 1Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
Urinary tract infections (UTIs) are one of the most common bacterial infections acquired both in community and hospital. Fluoroquinolones, represented by levofloxacin and ciprofloxacin, are widely used for treatment of UTIs. However, it remains controversial for the comparison between the 2 drugs, which propelled us to conduct the first evidence-based research on this topic. To establish their relative efficacy and safety, we searched Pubmed, embase, and Web of Science for randomized controlled trials (RCTs) for UTIs. A total of 5 RCTs were finally included, involving 2,352 patients and a systematic review and meta-analysis were performed to compare the end-of-therapy and posttherapy clinical success rate, microbial eradication rate and adverse event rate. Jadad score and Review Manager 5.3.0 version were applied respectively to evaluate the study quality and heterogeneity. There was no significant difference between levofloxacin and ciprofloxacin group in end-of-therapy or posttherapy clinical success rate and microbial eradication rate (p > 0.05). As for adverse event rate, the 2 drugs were comparable and both safe for clinical use. Based on one included trial and pharmacological research, we raised hypothesis that levofloxacin was superior to ciprofloxacin for treatment of E. coli-induced chronic bacterial prostatitis (CBP) and it required a further study to prove it.
Introduction
UTIs consists of complicated urinary tract infections (cUTIs) and the uncomplicated urinary tract infections (including prostatitis, pyelonephritis and cystitis). And cUTIs are often associated with abnormal urogenital system structure or function (Foxman, 2010). UTIs are some of the most common bacterial infections, affecting 150 million people each year worldwide. In the United States, the societal costs of these infections, including health care costs and time missed from work, are approximately US$3.5 billion per year (Flores-Mireles et al., 2015). Women are much more sensitive to UTIs (Stamm and Norrby, 2001) and studies have reported that 40∼50% of women worldwide will suffer from UTIs at least once in their lifetime. UTIs are identically harmful for men, especially reproductive function. CBP, a specific type of UTIs, has negative effect on sperm motility and morphology (Rusz et al., 2012). Compared with normal ejaculate, a higher leukocyte count could be observed in CBP patients (Schuppe et al., 2017), which is associated with the pathophysiological changes of sperm damage (La Vignera et al., 2014). Once spread to the accessory gland, UTIs could cause a great decline of total sperm number and bilateral infection is more detrimental (Vicari et al., 2006; La Vignera et al., 2011).
Levofloxacin and ciprofloxacin are antimicrobial agents and are expected to develop a widened use for its underlying effect in neuroinflammation modulating (Zusso et al., 2019), hematopoietic stem cell transplantation (Rambaran and Seifert, 2019), and even inhibition of SARS-CoV-2 replication (Karampela and Dalamaga, 2020). With respect to UTIs, the therapeutic effect of fluoroquinolones has been proved by many studies (Bader et al., 2017; Chu and Lowder, 2018; Bientinesi et al., 2020). Studies show that in Asian countries, 24.1% of patients with UTIs were given fluoroquinolones, second only to cephalosporin antibiotics (34.4%) (Choe et al., 2018a). The guidelines of the Urological Association of Asia list fluoroquinolones as the first choice drug for pyelonephritis (LE:1A,GR:A) (Choe et al., 2018b). Among fluoroquinolones, levofloxacin and ciprofloxacin are most commonly used in the treatment of acute pyelonephritis (AP) and cUTIs (Kranz et al., 2018).
Levofloxacin and ciprofloxacin are both recommended for clinical application in UTIs and, though commonly prescribed, there’s no final conclusion on the comparative merit of the either one. Levofloxacin shows advantage over ciprofloxacin in terms of efficacy, disease reoccurrence and adverse event (Zhang et al., 2012). On the contrary, microbiology evidence shows that the uropathogen is more sensitive to ciprofloxacin (Afriyie et al., 2018; Humphries et al., 2019). Currently, no evidence-based medical research has been published worldwide on this topic, which makes our study the first systematic review and meta-analysis in the world. Our objective was to compare the efficacy and safety of the two drugs in the treatment of UTIs, by performing a meta-analysis of high-quality RCTs that compared levofloxacin and ciprofloxacin.
Materials and Methods
Search Strategy
We performed a systematic literature search using the PubMed, Web of Science and embase databases up to January, 2021. We restricted our search to articles published in English, using the following search string: terms (((((“Cystitis” [Mesh]) OR “Pyelonephritis” [Mesh]) OR “Prostatitis” [Mesh]) OR “Urinary Tract Infections” [Mesh]) AND “Levofloxacin” [Mesh]) AND “Ciprofloxacin” [Mesh]. We also searched the reference lists of all relevant studies included in our meta-analysis. In addition, the reference lists of all eligible studies were reviewed manually. Two investigators (CDH and SYZ) searched and evaluated studies independently. Any induced disagreement was arbitrated by a third investigator (AJZ).
Study Selection Criteria
The study was included if met the following criteria: 1) RCT. 2) Study populations: patients with cUTIs, cystitis, pyelonephritis or bacterial prostatitis. 3) At lease one outcome for efficacy (clinical effective rate and microbial eradication rate) and safety (adverse event rate) were reported between levofloxacin and ciprofloxacin.
The following trials were excluded: non-RCT (reviews, letters, editorial comments, case reports, conference abstracts); Patients with non-UTIs; The intervention did not contain levofloxacin or ciprofloxacin, or combined with other anti-infective drugs; Outcome indicators did not include clinical effective rate, microbial eradication rate or incidence of adverse reactions; Jadad score < 3; pediatric articles, unpublished articles and non-English articles.
Study Quality Assessment
Methodological quality of RCTs were evaluated according to Jadad scoring criteria (Jadad et al., 1996). Jadad scale was used to score the selected literature from three aspects of random allocation, including randomization, blind method and withdrawal and dropout of the study. The quality of the selected article was evaluated by two reviewers (CDH and SYZ) independently. The score of 1∼2 was classified as low quality study, and 3∼5 as high quality study.
Statistical Analyses
Review Manager 5.3.0 (Cochrane Collaboration, Oxford, United Kingdom) was used for statistical analysis and heterogeneity testing. The heterogeneity was assessed through the chi-squared (χ2) test (Cochran’s Q) and inconsistency index (I2) (Higgins and Thompson, 2002). When χ2 p value >0.05 and I2 ≤ 50%, fixed effect model was adopted for analysis. Otherwise, the data with a I2 > 50% or χ2p value ≤ 0.05 was adopted for random effect model analysis. The relative ratio (RR) was used as the pooled statistic to calculate the 95% confidence interval.
Results
Selected RCTs
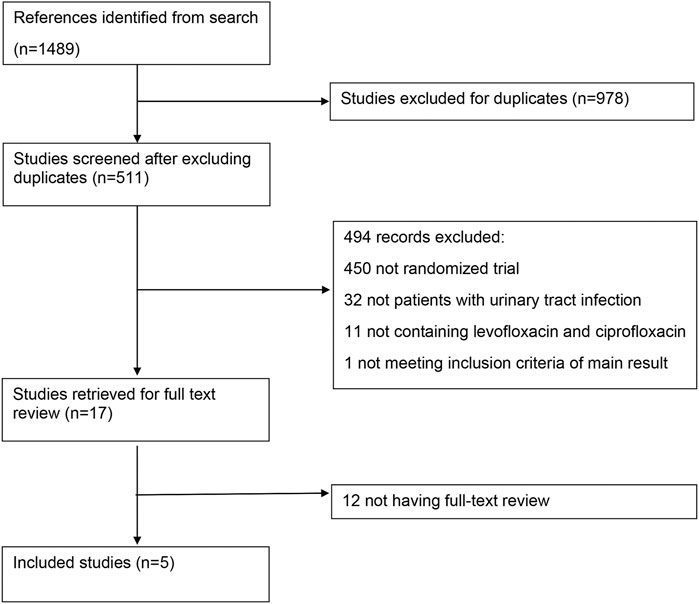
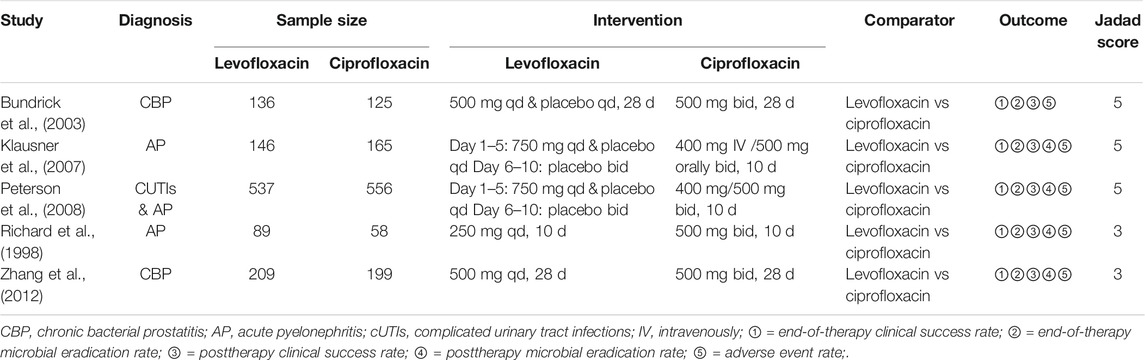
Seventeen full-text literatures were retrieved from 511 records in first screening, and 5 RCT studies were finally included (Figure 1). Table 1 showed basic characteristics of 5 studies chosen for the meta-analysis. The included literature was all of high methodological quality (2 studies with a Jadad score of 3 and 3 studies with a score of 5), among which 2 were AP (Richard et al., 1998; Klausner et al., 2007), 2 were CBP (Bundrick et al., 2003; Zhang et al., 2012), and the rest one was AP and cUTIs (Peterson et al., 2008). Details of 5 included literature could be seen in Supplementary Table S1. The research did not yield studies focusing on cystitis as a result of data absence. A total of 2,352 adult patients, all over 18 years old, were enrolled in multicenter RCTs. Levofloxacin was prescribed once a day at 250∼750 mg, orally or intravenously and patients received ciprofloxacin twice a day with a total dose of 900–1,000 mg orally or a single dose of 400 mg intravenously.
Treatment duration ranged from 5 to 28 days and definition for posttherapy was shown in Supplementary Table S1. We combined the 3 articles concerning AP as AP and cUTIs shared a similar dose and course of antibiotics application. As the population for analysis were various (intent-to-treat, modified intent-to-treat and microbially evaluable, respective definition could be seen in Supplementary Table S1), the pattern adopted by all corresponding articles was chosen to pool the outcome measurement except for adverse event, whose data came from all patients received 1 or more dose of studied drugs.
Part 1. Outcomes for AP
Two studies (Richard et al., 1998; Klausner et al., 2007) reported the rate of clinical improvement, microbial eradication and adverse event in intent-to-treat population (Figure 2). A higher incidence could be found in end-of-therapy (RR: 1.16, 95% CI: 0.93∼1.46, p > 0.05, Figure 2A) and posttherapy clinical effective rate (RR: 1.16, 95% CI: 0.86–1.55, p > 0.05, Figure 2B) for levofloxacin but without a significant divergence. For the absence of posttherapy microbial eradication rate, relevant analysis was unable to conduct. No evidence proved a significant difference with respect to microbial eradication rate at end-of-therapy (RR: 1.12, 95% CI: 0.86∼1.46, p > 0.05, Figure 2C) or adverse event rate (RR: 0.92, 95% CI: 0.45∼1.88, p > 0.05, Figure 2D). And no serious adverse event was reported.
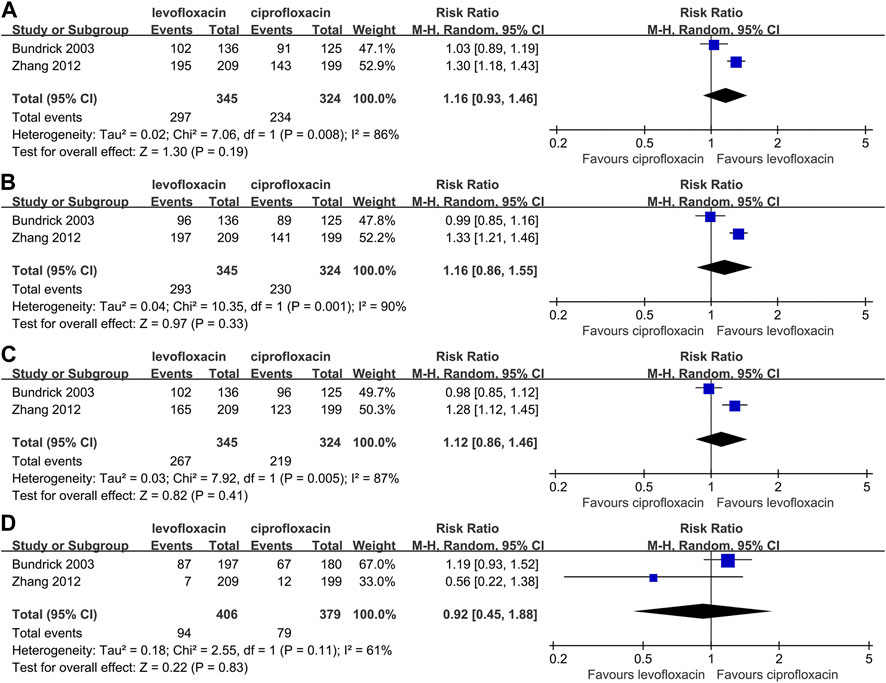
FIGURE 2. Comparison between levofloxacin and ciprofloxacin in acute pyelonephritis; 2(A) end-of-therapy clinical success rate; 2(B) posttherapy clinical success rate; 2(C) microbial eradication rate; 2(D) adverse event rate.
Part 2. Outcomes for CBP
The rate of clinical improvement, microbiological eradication and adverse event were reported by 3 articles (Bundrick et al., 2003; Peterson et al., 2008; Zhang et al., 2012) based on microbiologically evaluable population (Figure 3). As for the clinical effective rate, no matter at end-of-therapy (RR: 1.01, 95%CI: 0.93–1.10, p > 0.05, Figure 3A) or posttherapy (RR: 0.99, 95%CI: 0.95–1.04, p > 0.05, Figure 3B), there was no significant difference between the 2 drugs. What’s more, the statistical difference of end-of-therapy (RR: 0.99, 95% CI: 0.94–1.04, p > 0.05, Figure 3C) or posttherapy (RR: 0.96, 95% CI: 0.92–1.01, p > 0.05, Figure 3D) microbial eradication rate was unsignificant.
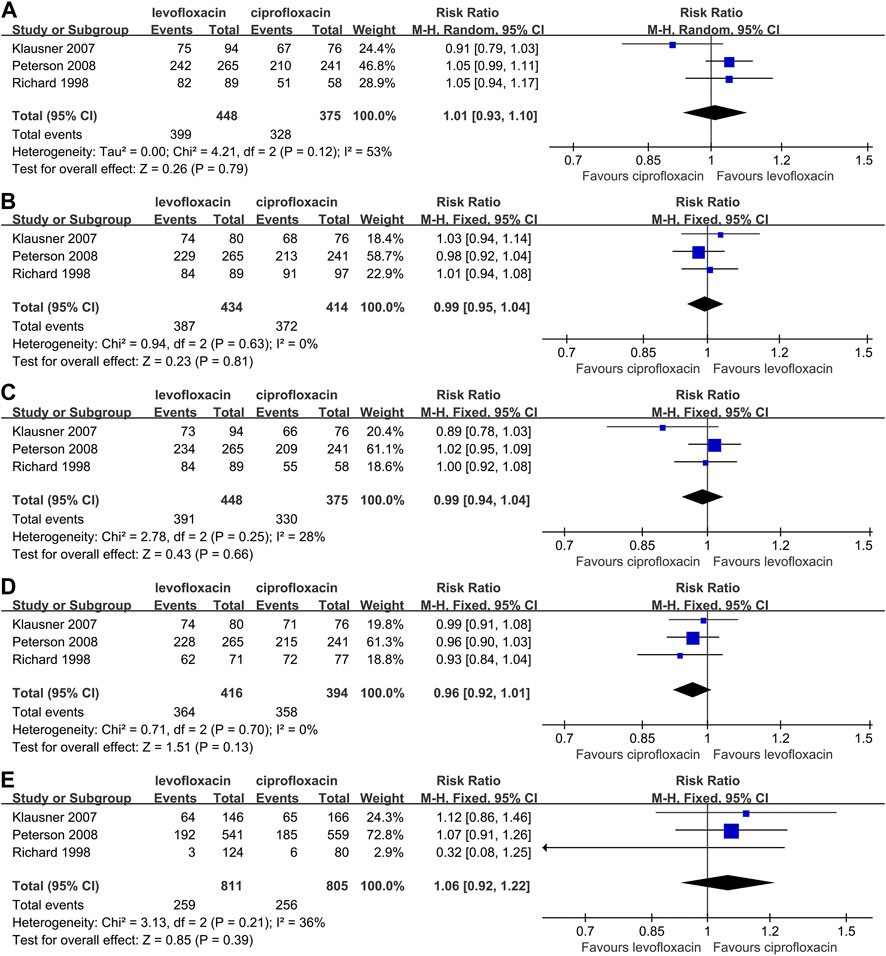
FIGURE 3. Comparison between levofloxacin and ciprofloxacin in chronic bacterial prostatitis; 3(A) end-of-therapy clinical success rate; 3(B) posttherapy clinical success rate; 3(C) end-of-therapy microbial eradication rate; 3(D) posttherapy microbial eradication rate; 3(E) adverse event rate.
When compared directly, the levofloxacin and ciprofloxacin did not show a significant divergence in adverse event rate (RR: 1.06, 95% CI: 0.92∼1.22, p > 0.05, Figure 3E). 5/146 severe adverse event in levofloxacin subject, with one case of urosepsis, and 6/166 in ciprofloxacin were noted (Klausner et al., 2007). The corresponding number is 17/537, 15/556 in another study (Richard et al., 1998) and one was considered related to allergic reaction to levofloxacin. No treatment-related death case was mentioned by all 3 studies.
Discussion
UTIs are becoming a global health issue and induce significant quantity of societal cost (Flores-Mireles et al., 2015). Timely and effective antibiotic treatment, together with nutraceutical, could reduce the long-term damage to reproductive system (Mongioi et al., 2016). In the treatment of UTIs, although the guidelines list levofloxacin and ciprofloxacin as first-line drugs for cUTIs and AP, no evidence-based research has proven the comparative advantage of the either one. Currently, there is no meta-analysis on this issue. Under these conditions, we performed a latest systematic review and pooled analysis of all available trials. All involved trials were RCTs with high level of evidence (mean Jadad score = 3.9), which gave our study enough authority on this topic.
For AP and CBP treatment, the analysis did not show significant statistical difference in terms of end-of-therapy or posttherapy clinical effective rate and microbial eradication rate. The adverse event rate shared a similar finding. As for the treatment of CBP, however, Zhang (Zhang et al., 2012) reported that levofloxacin was with higher efficacy, lower disease reoccurrence and adverse event rate in Chinese patients. The isolated bacteria from urine sample could account for this controversy. The most common uropathogen was E. coli in this trial while Enterococcus faecalis ranked first and E. coli got a fifth place in a similar American study (Bundrick et al., 2003), which did not find the difference between the two drugs. Nowadays, the prevalence of resistant E. coli rose in community-acquired urinary tract infection (Lee et al., 2018). Based on the fact that the minimal inhibitory concentration of levofloxacin for resistant E. coli isolates were lower than that of ciprofloxacin (Becnel Boyd et al., 2009), hypothesis could be raised naturally that bacterial spectrum and corresponding sensitive antibiotics for Chinese patients were not totally consistent with other countries and therefore the dispute arised. But it did not mean that levofloxacin was recommended for E. coli-induced UTIs treatment until other first-line drugs failed to work, based on the fact that gram-negative bacteria was least susceptible to levofloxacin and ciprofloxacin in the sensitivity test both in China and United States (Lu et al., 2012; Bouchillon et al., 2013).
Limited by existing studies, the hypothesis could be hardly confirmed unless RCTs with a large sample performed. But it was consistent with pharmacologic research. Fluoroquinolones exhibit concentration dependent killing (Craig, 1998) and levofloxacin was characterized by a nearly twice renal excretion rate (84%) than ciprofloxacin (43%), possessed with higher urinary bactericidal titers and long lasting time (Naber, 2001). Drusano et al. showed that the prostate/plasma ratio of more than 70% of the subjects exceeded 1.0, indicating that levofloxacin was able to penetrate the prostate and suitable for local infection (Drusano et al., 2000). Wagenlehner et al. reported that the blood concentration of levofloxacin in healthy volunteers was higher than that of ciprofloxacin at a single dose (Wagenlehner et al., 2006). It proved reasonable explanation for the non-inferiority of levofloxacin, whose minimum inhibitory concentration for uropathogen was higher in disk diffusion, compared with ciprofloxacin.
For the adverse event, the 2 drugs were comparable and no noteworthy serious or death case came into publication. The most common side effect were digest tract symptom (flatulence and diarrhea) and central nervous symptom (headache, dizziness and nausea), which was consistent with existing report (Stahlmann and Lode, 2013). No adverse event was considered directly related to treatment except for an allergic reaction case (Peterson et al., 2008). Known severe side effect, such as QT prolongation, seizure and tendon rupture (Stahlmann and Lode, 2013), were not reported by all 5 trials. All mentioned above proved that levofloxacin and ciprofloxacin were both with safety in clinical application.
There were several limitations in our meta-analysis. First, the quantity of available studies were small, resulting in inadequate statistical confidence. RCTs with larger scale were necessary to furtherly explore the answer. Second, the difference in standard course and dose of the 2 drugs could bias the result. Some researchers held the view that as part of the short-course therapy, the course of levofloxacin treatment concluded 5 days sooner than that of ciprofloxacin (5 vs. 10 days), potentially biasing the efficacy assessments in favor of ciprofloxacin (Klausner et al., 2007; Peterson et al., 2008). Third, it was the study design and the inclusion criteria of the individual RCTs that may be responsible for failing to reveal the differences between levofloxacin and ciprofloxacin. Actually, most of these RCTs included were to show noninferiority between agents for drug registration and approval purposes. Therefore, they may fail to show clinical superiority of any antibiotic over another. We have good reasons, though, to believe that the high quality of included RCTs could make up for this shortcoming.
Conclusion
At present, this is the first evidence-based research comparing efficacy and safety between levofloxacin and ciprofloxacin as for urinary tract infection. There is no significant difference between the 2 drugs in end-of-therapy or posttherapy clinical success rate, microbial eradication rate or adverse event rate.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
DC and YS have joint first authorship for this publication. DC, YS and JA were responsible for the searching and screening of articles for this review. YS, YH, and BC were responsible for the writing of the manuscript. DC and LY were responsible for conceptualizing and revising the manuscript. ZC and LL were involved in conceptualizing, proofreading and diagram preparation. All authors gave their final approval for the submission of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant no. 82000721), Post-Doctor Research Project, West China Hospital, Sichuan University (Grant no. 2019HXBH089), Health commission of Sichuan province (20PJ036).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.658095/full#supplementary-material.
References
Afriyie, D. K., Adu, L. B., Dzradosi, M., Amponsah, S. K., Ohene-Manu, P., and Manu-Ofei, F. (2018). Comparative in vitro activity of ciprofloxacin and levofloxacin against isolated uropathogens in Ghana: a pilot study. Pan Afr. Med. J. 30, 194. doi:10.11604/pamj.2018.30.194.15457
Bader, M. S., Loeb, M., and Brooks, A. A. (2017). An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med. 129 (2), 242–258. doi:10.1080/00325481.2017.1246055
Becnel Boyd, L., Maynard, M. J., Morgan-Linnell, S. K., Horton, L. B., Sucgang, R., Hamill, R. J., et al. (2009). Relationships among ciprofloxacin, gatifloxacin, levofloxacin, and norfloxacin MICs for fluoroquinolone-resistant Escherichia coli clinical isolates. Aac 53 (1), 229–234. doi:10.1128/aac.00722-08
Bientinesi, R., Murri, R., and Sacco, E. (2020). Efficacy and safety of levofloxacin as a treatment for complicated urinary tract infections and pyelonephritis. Expert Opin. Pharmacother. 21 (6), 637–644. doi:10.1080/14656566.2020.1720647
Bouchillon, S. K., Badal, R. E., Hoban, D. J., and Hawser, S. P. (2013). Antimicrobial susceptibility of inpatient urinary tract isolates of gram-negative bacilli in the United States: results from the study for monitoring antimicrobial resistance trends (SMART) program: 2009−2011. Clin. Ther. 35 (6), 872–877. doi:10.1016/j.clinthera.2013.03.022
Bundrick, W., Heron, S. P., Ray, P., Schiff, W. M., Tennenberg, A. M., Wiesinger, B. A., et al. (2003). Levofloxacin versus ciprofloxacin in the treatment of chronic bacterial prostatitis: a randomized double-blind multicenter study. Urology 62 (3), 537–541. doi:10.1016/s0090-4295(03)00565-x
Choe, H. S., Lee, S. J., Yang, S. S., Hamasuna, R., Yamamoto, S., Cho, Y. H., et al. (2018a). Summary of the UAA-AAUS guidelines for urinary tract infections. Int. J. Urol. 25 (3), 175–185. doi:10.1111/iju.13493
Choe, H. S., Lee, S. J., Cho, Y. H., Çek, M., Tandoğdu, Z., Wagenlehner, F., et al. (2018b). Aspects of urinary tract infections and antimicrobial resistance in hospitalized urology patients in Asia: 10-Year results of the Global Prevalence Study of Infections in Urology (GPIU). J. Infect. Chemother. 24 (4), 278–283. doi:10.1016/j.jiac.2017.11.013
Chu, C. M., and Lowder, J. L. (2018). Diagnosis and treatment of urinary tract infections across age groups. Am. J. Obstet. Gynecol. 219 (1), 40–51. doi:10.1016/j.ajog.2017.12.231
Craig, W. A. (1998). State‐of‐the‐Art clinical article: pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26 (1), 1–10. doi:10.1086/516284
Drusano, G. L., Preston, S. L., Van Guilder, M., North, D., Gombert, M., Oefelein, M., et al. (2000). A population pharmacokinetic analysis of the penetration of the prostate by levofloxacin. Antimicrob. Agents Chemother. 44 (8), 2046–2051. doi:10.1128/aac.44.8.2046-2051.2000
Flores-Mireles, A. L., Walker, J. N., Caparon, M., and Hultgren, S. J. (2015). Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13 (5), 269–284. doi:10.1038/nrmicro3432
Foxman, B. (2010). The epidemiology of urinary tract infection. Nat. Rev. Urol. 7 (12), 653–660. doi:10.1038/nrurol.2010.190
Higgins, J. P. T., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statist. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Humphries, R. M., Hindler, J. A., Shaffer, K., and Campeau, S. A. (2019). Evaluation of ciprofloxacin and levofloxacin disk diffusion and etest using the 2019EnterobacteriaceaeCLSI breakpoints. J. Clin. Microbiol. 57 (3). doi:10.1128/jcm.01797-18
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J. M., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin. trials 17 (1), 1–12. doi:10.1016/0197-2456(95)00134-4
Karampela, I., and Dalamaga, M. (2020). Could respiratory fluoroquinolones, levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch. Med. Res. 51 (7), 741–742. doi:10.1016/j.arcmed.2020.06.004
Klausner, H. A., Brown, P., Peterson, J., Kaul, S., Khashab, M., Fisher, A. C., et al. (2007). A trial of levofloxacin 750 mg once daily for 5 days versus ciprofloxacin 400 mg and/or 500 mg twice daily for 10 days in the treatment of acute pyelonephritis. Curr. Med. Res. Opin. 23 (11), 2637–2645. doi:10.1185/030079907x233340
Kranz, J., Schmidt, S., Lebert, C., Schneidewind, L., Mandraka, F., Kunze, M., et al. (2018). The 2017 update of the German clinical guideline on epidemiology, diagnostics, therapy, prevention, and management of uncomplicated urinary tract infections in adult patients. Part II: therapy and prevention. Urol. Int. 100 (3), 271–278. doi:10.1159/000487645
La Vignera, S., Condorelli, R. A., Vicari, E., Salmeri, M., Morgia, G., Favilla, V., et al. (2014). Microbiological investigation in male infertility: a practical overview. J. Med. Microbiol. 63 (Pt 1), 1–14. doi:10.1099/jmm.0.062968-0
La Vignera, S., Vicari, E., Condorelli, R., D’Agata, R., and Calogero, A. E. (2011). Hypertrophic-congestive and fibro-sclerotic ultrasound variants of male accessory gland infection have different sperm output. J. Endocrinol. Invest. 34 (10), e330–e335. doi:10.1007/bf03346729
Lee, D. S., Lee, S.-J., and Choe, H.-S. (2018). Community-acquired urinary tract infection byEscherichia coliin the era of antibiotic resistance. Biomed. Res. Int. 2018, 1. doi:10.1155/2018/7656752
Lu, P.-L., Liu, Y.-C., Toh, H.-S., Lee, Y.-L., Liu, Y.-M., Ho, C.-M., et al. (2012). Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009-2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 40 (Suppl. l), S37–S43. doi:10.1016/s0924-8579(12)70008-0
Mongioi, L., Calogero, A. E., Vicari, E., Condorelli, R. A., Russo, G. I., Privitera, S., et al. (2016). The role of carnitine in male infertility. Andrology 4 (5), 800–807. doi:10.1111/andr.12191
Naber, K. G. (2001). Which fluoroquinolones are suitable for the treatment of urinary tract infections? Int. J. Antimicrob. Agents 17 (4), 331–341. doi:10.1016/s0924-8579(00)00362-9
Peterson, J., Kaul, S., Khashab, M., Fisher, A. C., and Kahn, J. B. (2008). A double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urology 71 (1), 17–22. doi:10.1016/j.urology.2007.09.002
Rambaran, K. A., and Seifert, C. F. (2019). Ciprofloxacin vs. levofloxacin for prophylaxis in recipients of hematopoietic stem cell transplantation. J. Oncol. Pharm. Pract. 25 (4), 884–890. doi:10.1177/1078155218787286
Richard, G., Klimberg, I., Fowler, C., Callery-D’Amico, S., and Kim, S. (1998). Levofloxacin versus ciprofloxacin versus lomefloxacin in acute pyelonephritis. Urology 52 (1), 51–55. doi:10.1016/s0090-4295(98)00160-5
Rusz, A., Pilatz, A., Wagenlehner, F., Linn, T., Diemer, T., Schuppe, H. C., et al. (2012). Influence of urogenital infections and inflammation on semen quality and male fertility. World J. Urol. 30 (1), 23–30. doi:10.1007/s00345-011-0726-8
Schuppe, H.-C., Pilatz, A., Hossain, H., Diemer, T., Wagenlehner, F., and Weidner, W. (2017). Urogenital infection as a risk factor for male infertility. Dtsch Arztebl Int. 114 (19), 339–346. doi:10.3238/arztebl.2017.0339
Stahlmann, R., and Lode, H. M. (2013). Risks associated with the therapeutic use of fluoroquinolones. Expert Opin. Drug Saf. 12 (4), 497–505. doi:10.1517/14740338.2013.796362
Stamm, W. E., and Norrby, S. R. (2001). Urinary tract infections: disease panorama and challenges. J. Infect. Dis. 183 (Suppl. 1), S1–S4. doi:10.1086/318850
Vicari, E., La Vignera, S., Castiglione, R., and Calogero, A. E. (2006). Sperm parameter abnormalities, low seminal fructose and reactive oxygen species overproduction do not discriminate patients with unilateral or bilateral post-infectious inflammatory prostato-vesiculo-epididymitis. J. Endocrinol. Invest. 29 (1), 18–25. doi:10.1007/bf03349172
Wagenlehner, F. M. E., Kinzig-Schippers, M., Sörgel, F., Weidner, W., and Naber, K. G. (2006). Concentrations in plasma, urinary excretion and bactericidal activity of levofloxacin (500mg) versus ciprofloxacin (500mg) in healthy volunteers receiving a single oral dose. Int. J. Antimicrob. Agents 28 (6), 551–559. doi:10.1016/j.ijantimicag.2006.07.026
Zhang, Z.-C., Jin, F.-S., Liu, D.-M., Shen, Z.-J., Sun, Y.-H., and Guo, Y.-L. (2012). Safety and efficacy of levofloxacin versus ciprofloxacin for the treatment of chronic bacterial prostatitis in Chinese patients. Asian J. Androl. 14 (6), 870–874. doi:10.1038/aja.2012.48
Keywords: urinary tract infection, fluoroquinolone, levofloxacin, ciprofloxacin, efficacy, safety
Citation: Cao D, Shen Y, Huang Y, Chen B, Chen Z, Ai J, Liu L, Yang L and Wei Q (2021) Levofloxacin Versus Ciprofloxacin in the Treatment of Urinary Tract Infections: Evidence-Based Analysis. Front. Pharmacol. 12:658095. doi: 10.3389/fphar.2021.658095
Received: 25 January 2021; Accepted: 01 March 2021;
Published: 08 April 2021.
Edited by:
Aldo Eugenio Calogero, University of Catania, ItalyReviewed by:
Rossella Cannarella, University of Catania, ItalyPayam Behzadi, Islamic Azad University, ShahreQods, Iran
Copyright © 2021 Cao, Shen, Huang, Chen, Chen, Ai, Liu, Yang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Yang, d3ljbGVmbHVlQDE2My5jb20=; Qiang Wei, d2VpcWlhbmc5MzNAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Dehong Cao
Dehong Cao Yinzhi Shen
Yinzhi Shen Yin Huang1
Yin Huang1 Liangren Liu
Liangren Liu Lu Yang
Lu Yang Qiang Wei
Qiang Wei