
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 30 April 2021
Sec. Experimental Pharmacology and Drug Discovery
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.656774
This article is part of the Research Topic Innovation in Ocular Pharmacology View all 11 articles
 Joanna Dolar-Szczasny1*
Joanna Dolar-Szczasny1* Claudio Bucolo2
Claudio Bucolo2 Sandrine Zweifel3
Sandrine Zweifel3 Adriano Carnevali4
Adriano Carnevali4 Robert Rejdak1
Robert Rejdak1 Wojciech Załuska5
Wojciech Załuska5 Aleksandra Czarnek-Chudzik6
Aleksandra Czarnek-Chudzik6 Mario Damiano Toro1,3,7*
Mario Damiano Toro1,3,7*Purpose: To evaluate the effect of repeated intravitreal bevacizumab injections on blood-aqueous barrier permeability in eyes with neovascular age-related macular degeneration (AMD).
Patients and Methods: Forty-eight consecutive patients with neovascular AMD received 3 intravitreal bevacizumab injections (1 mg) every 30–40 days. Subjects were followed for a period of 4 months and were examined at baseline, 1 day and 1 month after each injection. A control group comprised of 19 neovascular AMD patients waiting to begin anti-vascular endothelial growth factor (VEGF) therapy. Anterior chamber (AC) inflammation was evaluated with biomicroscopy and laser flare photometry.
Results: None of the subjects treated with bevacizumab had detectable ocular inflammation during follow-up. An analysis for variance (ANOVA) of the mixed-effects model has shown neither an effect between treatment and control group (p = 0.921), nor over the time course of the follow-up (p = 0.773). Before treatment, median AC inflammation was 6.7 photons/ms (range: 3.5–18.2 photons/ms). One month after the first, second, and third injections, median laser flare was 6.4, 6.8, and 6.6 photons/ms, respectively, none of which were significantly different from baseline (all p > 0.05). Blood-aqueous barrier permeability did not change between injections and was not different from the control group.
Conclusion: Inflammation induced by intravitreal bevacizumab was not detected by examination or flare photometry. This suggests that monthly bevacizumab dosing seems to be safe. The absence of AC inflammation could also reflect the known anti-inflammatory properties of anti-VEGF agents.
Vascular endothelial growth factor (VEGF) has become the main target for treating neovascular age-related macular degeneration (AMD) in recent years (Plyukhova et al., 2020). As a result, intravitreal injections of anti-VEGF agents are now widely used to halt neovascular AMD progression and, hopefully, improve central visual acuity. Unfortunately, each intraocular injection, even when performed under sterile conditions, carries a risk of vision-threatening complications, including intraocular inflammation, endophthalmitis, intraocular pressure elevation, vitreous hemorrhage, and retinal detachment (Falavarjani and Nguyen, 2013). The most serious of these complications is sterile or infectious endophthalmitis, which can lead to significant visual loss (Dossarps et al., 2015).
Among anti-VEGF agents, bevacizumab is often used intravitreally to handle several retinal diseases (Falavarjani and Nguyen, 2013; Reibaldi et al., 2014; Dossarps et al., 2015; Platania et al., 2015; Plyukhova et al., 2020; Yousef et al., 2020; Toro et al., 2021). Bevacizumab is used in ophthalmology in an off-label fashion and, thus, remains a somewhat controversial treatment option. Because the approved indications by drug regulatory agencies did not include ocular diseases, a concern regarding the safety profile and risk for emerged considering the potential ocular inflammatory response following intravitreal administration. Safety issue using chronic intravitreal bevacizumab dosing regimen has also arisen because most AMD patients require at least three injections during the first year of treatment (Plyukhova et al., 2020). On this regards it could be useful develop a biodegradable deliver system to inject bevacizumab, avoiding a multiple treatment (Conti et al., 1997).
Secondly, with regard to recent reports on more frequent inflammatory reactions after the use of the newly registered anti-VEGF drug-brolucizumab (Baumal et al., 2020), the issue of side effects has become even more topical. At the beginning of 2020, the American Society of Retinal Specialists (ASRS) alerted ophthalmologists to reported cases of ocular inflammation after brolucizumab injections (Beovu Update for ASRS Members, 2020).
A potent ocular inflammatory response is easily visible by slit-lamp examination after intraocular injection. This is especially true of changes in anterior chamber fluid clarity, but slight fluid changes can be overlooked (Falavarjani and Nguyen, 2013). However, a laser flare photometer can detect even subtle blood-ocular barrier changes that may not be detectable with standard ophthalmological clinical examination (Tugal-Tutkun and Herbort, 2010). Laser flare photometry (LFP) is a non-invasive tool and allows anterior chamber flare (from disruption of the blood-ocular barriers) to be objectively, accurately, and reproducibly quantified. Therefore, LFP allows ocular inflammatory responses induced by medications or surgical procedures to be examined and compared (Ladas et al., 2005; Tugal-Tutkun and Herbort, 2010; Orès et al., 2020).
Here, we use LFP to examine the effect of multiple intravitreal bevacizumab injections on ocular inflammation in neovascular AMD patients.
The study protocol was reviewed and approved by the Institutional Review Board (IRB) at Medical University, Lublin, Poland (n° KE-0254/208/2013) on July 11th, 2013. Being the LFP a noninvasive diagnostic tool and according the ongoing regulation, the IRB waived the requirement of informed consent. All study conduct adhered to the tenets of the Declaration of Helsinki.
Consecutive patients diagnosed with neovascular AMD in the Lublin University Department of General Ophthalmology between January and September 2015 were retrospectively considered for inclusion in this cross-sectional analysis. All subjects had AMD with active macular neovascularization (MNV) confirmed with fluorescein angiography (FA), indocyanine green angiography, and optical coherence tomography (OCT). Only patients who required at least three intravitreal bevacizumab injections on a pro re nata treatment regimen and who had received a LFP monitoring during the loading phase, were included. Patients with advanced cataract, a history of uveitis or inflammation, vitreous hemorrhage, neovascular glaucoma, corneal opacities, recent ocular surgery (within 3 months), or prior anti-VEGF injections were excluded.
A control group of active neovascular AMD patients who were waiting to begin anti-VEGF therapy were also included and observed over a 4 months period. The delayed therapy in this group of patients was related to insufficient health availability in our clinic. These patients were informed about alternative medical centers and about the risks of delayed treatment but, due to transportation barriers and reimbursement issues, decided to wait for therapy in our hospital.
The use of intravitreal bevacizumab (Avastin, Genentech, Inc., South San Francisco, CA) as an off-label treatment was approved by the local ethics committee and it is part of clinical routine care in our department. All injections were performed under sterile conditions in our operating room after the patient had signed an informed consent for off-label drug administration. All treatments in this study were carried out as part of routine clinical care for neovascular AMD. After the eye was topically anesthetized with proxymetacaine (0.5%, Alcon-Couvrer nv, Puurs, Belgium), it was disinfected with several drops of 5% povidone iodine (Betadine Ophthalm., Alcon Laboratiries Inc.) placed in the conjunctival sac. Next, a 1.25 mg bevacizumab dose in 0.05 ml was injected into the vitreous cavity. The injected bevacizumab was obtained from a 4 ml vial that contained a 25 mg bevacizumab/ml solution. Sterile tuberculin syringes were used under sterile conditions to generate 0.1 ml (2.5 mg bevacizumab) aliquots of the drug just before intravitreal injections were prepared. The dosages were prepared by the doctor performing whole procedure in the surgical theatre. One day before and 5 days after injection, patients prophylactically used a topical antibiotic. Multiple injections were carried out with a pro renata regimen based on the clinical activity of the disease and the availability of the drug.
All patients were carefully and prospectively examined before, one day and one month after each of the three intravitreal bevacizumab injections during the loading phase. Treated and control subjects were monitored for a range of 4 months.
Ocular inflammation was qualitatively and quantitatively assessed by slit-lamp and fundoscopic examinations and a laser flare photometer, respectively (Kowa FM-500, Kowa Company, Ltd., Tokyo, Japan). All evaluations were made following pupil dilation with topical 1% tropicamide (Polfa-Warsaw SA, Poland). The final flare photometry value was automatically calculated by averaging 5 individual measurements. A total of 7 measurements were obtained, but the highest and lowest measurement values were excluded by the flare meter. All measurements were performed in a darkened room after calibrating the flare meter.
Data are presented as mean, median, standard deviation (SD), minimum value (min) and maximum value (max). Differences between groups were tested for statistical significance using the Mann-Whitney U test. For repeated measure data, a linear mixed-effects model was carried out to estimate differences between study groups and different time points. The two factors were adjusted for the baseline value. An analysis of variance (ANOVA) test of the mixed-effects model and the comparison to baseline for the treatment group, are presented as p-values. Statistical significance was defined as p < 0.05. Statistical analyses were performed using STATISTICA 12 statistical software (StatSoft Polska, Krakow, Poland) and the statistical software R version 4.0.
A total of 48 eyes of 48 patients (20 men, 28 women) were ultimately included in the injection group. Median patient age was 70 years (range: 47–87 years). A total of 19 eyes of 19 patients (8 men, 11 women) were ultimately included in the control group. Median control patient age was 65 years (range: 49–86 years).
None of the 48 patients in the injection group had clinically detectable anterior chamber inflammation during the follow-up period. Before treatment, median LFP measured 6.7 photons/ms (range: 3.5–18.2 photons/ms). This value was not significantly different from baseline one day following the first (median: 6.8 photons/ms, p = 0.738), second (7.1 photons/ms, p = 0.350), or third (6.5 photons/ms, p = 0.882) injection. The same was also true one month following the first (6.4 photons/ms, p = 0.419), second (6.8 photons/ms, p = 0.842), and third (6.6 photons/ms, p = 0.333) injections (Table 1). An ANOVA test of the linear mixed-effects model was carried out with neither a significant difference for treatment-control groups (p = 0.921) nor for the time points (p = 0.773). Mean values of anterior chamber flare were not significantly different between treated women and treated men at any follow-up time point examined (Table 2). Furthermore, there were no significant differences in blood-aqueous barrier permeability between treated (injection group) and untreated (control group) subjects at any time point examined (Table 3).
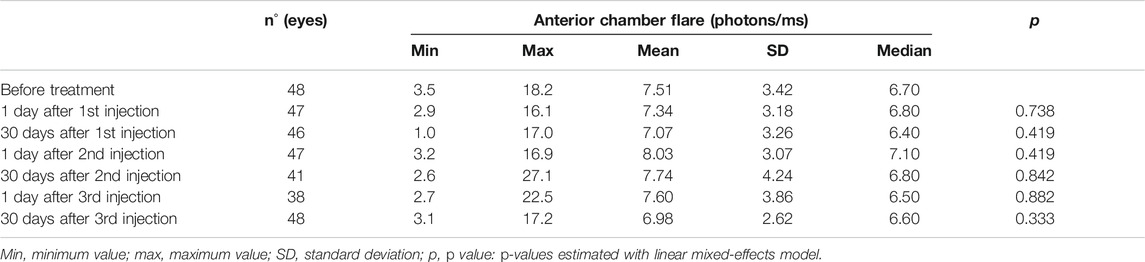
TABLE 1. Anterior chamber flare before and 1 day and 1 month after each intravitreal bevacizumab injection.
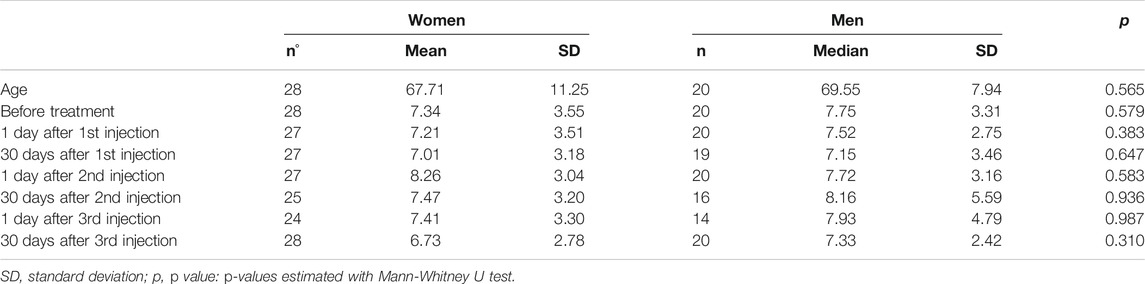
TABLE 2. Anterior chamber flare before and 1 day and 1 month after each intravitreal bevacizumab injection in group of women and men.
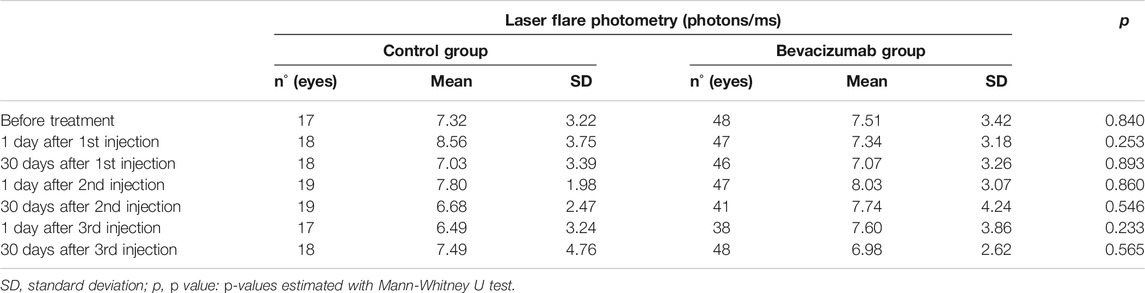
TABLE 3. Anterior chamber inflammation in the control group and in eyes treated with intravitreal bevacizumab.
Vascular endothelial growth factor is a well-known promoter of angiogenesis and has been shown to be involved in the pathogenesis of wet AMD. Although many other methods have been explored, intravitreal anti-VEGF therapy is the only disease-modifying treatment for the retinal neovascular diseases (Plyukhova et al., 2020). The three anti-VEGF agents commonly used to treat retinal neovascular diseases include bevacizumab, ranibizumab, and aflibercept (Plyukhova et al., 2020). Intravitreal injections are simple to perform and the procedure for this treatment has been well established. However, intraocular injections are still considered to be invasive treatment (Avery et al., 2014), particularly for multiple injections. Some concern about intravitreal injection still exists, even though the injection is performed under sterile conditions (Grzybowski et al., 2018). In addition, the topic of inflammatory response has returned with the launch of a new anti-VEGF agent—brolucizumab (Yousef et al., 2020; Toro et al., 2021). For this drug, in phase 3 clinical trials and according to the FDA label, the incidence of ocular inflammation was higher (>4%) than other anti-VEGF agents (<1%) (Dugel, 2017; US Food and Drug Administration, 2019).
The most common treatment, in some countries, for ocular neovascular disease is bevacizumab, even though its use is off-label (Berg et al., 2015). Clinical observations have shown that the pharmacological effect of bevacizumab to handle retinal diseases is as safe and effective as to other anti-VEGF agents (Berg et al., 2015; Martin et al., 2020; Plyukhova et al., 2020). The CATT Research Group performed the largest bevacizumab-ranibizumab comparison study, which included 1,208 patients from 44 centers in the United States. Patients were put on a monthly or as-needed treatment scheme. Endophthalmitis was rare, occurring after 2 of 5,449 injections (0.04%) in 599 patients treated with ranibizumab injections (Martin et al., 2011). A similar incidence (0.07%, 4 of 5,508 injections) was observed in the 586 patients treated with bevacizumab (p = 0.49) (Reibaldi et al., 2019). However, one or more serious systemic adverse events occurred in 31.7% of ranibizumab-treated patients and in 39.9% of bevacizumab-treated patients (p = 0.004) (Martin et al., 2011).
Endophthalmitis is the most threatening complication associated with intravitreal injection (Reibaldi et al., 2019). However, clinical data suggests that most cases following injection were caused by medications contaminated during dose extraction (Merani and Hunyor, 2015).
Another safety concern surrounding intravitreal bevacizumab use, is ocular inflammation, that is known to occur after multiple intravitreal injections. Some reported cases associated with intravitreal injections suggest sterile endophthalmitis (Chong et al., 2010; Williams et al., 2016). These observations raise concerns of treating ophthalmologists regarding the risks and benefits of neovascular AMD treatments. Higher doses of intravitreal anti-VEGF agents have been shown to significantly increase the risk of intraocular inflammation (Rosenfeld et al., 2005). However, one study assessed anterior chamber inflammation after one intravitreal bevacizumab injection in eyes with exudative AMD and found no inflammatory response (Martin et al., 2012). Furthermore, a significant decrease from pre-injection values occurred in anterior chamber flare 7 days after injection. Our results, in accordance with Yeniad et al. (2011) did not show a decrease in ocular inflammation following injection. It may have been that Kiss et al. administered topical steroids following injection (Kiss et al., 2006), as it was commonly done in some centers. Unfortunately, this was not discussed by authors. In another study, a reduction in laser flare was observed two months after bevacizumab injections. However, only 8 patients were included in this analysis (Errera et al., 2014).
Even though we did not observe a decrease in ocular inflammation, we also did not observe an increase in inflammation. This finding is in agreement with pre-clinical in vivo studies that examined the ocular toxicity of four different intravitreal bevacizumab doses. A 5 mg dose of bevacizumab was not toxic to the retina, and only a few inflammatory cells in the vitreous were identified (Manzano et al., 2006; Xu et al., 2010).
The VEGF protein is known to provoke an inflammatory reaction by increasing vascular permeability and activating adhesion of leucocytes to the vascular endothelium (Adamis and Shima, 2005). It is also well-known that eyes with neovascular AMD have markedly increased VEGF levels and higher flare values than normal eyes (Kubota et al., 1994). Indeed, Kubota et al. have shown that flare values in eyes with age-related macular degeneration were 0.28 ± 0.18 mg/ml, being significant in comparison with the control (0.12 ± 0.05 mg/ml) (Kubota et al., 1994). Therefore, our control group was comprised of eyes with neovascular AMD to minimize baseline differences in ocular inflammation. The delayed therapy in this group of patients was related to insufficient health availability in our clinic and not to an unethical decision. Till November 2015 (our study was concluded in September 2015) treatment of patients with wet AMD has been an epidemiological and economical problem in Poland. Since that date special treatment program financed from public funds started and the situation slowly improved (Figurska et al., 2020). Mekjavic et al. have provided a comprehensive overview of the clinical and economic burden of wet-AMD and DME in Central and Eastern Europe and the status quo associated with their management (Jaki Mekjavic et al., 2019). Patients from our control group were fully aware of risks resulting from delayed treatment but, due to transportation barriers and reimbursement issues, decided to wait for injections in our hospital and be monitored for the time pending the therapy.
Prior studies have compared ocular inflammation in patients treated with intravitreal anti-VEGF for various exudative eye diseases (e.g., non-proliferative diabetic retinopathy, macular edema with branch or central retinal vein occlusion). No differences in anterior chamber inflammation were observed (Yeniad et al., 2011). Various anti-VEGF agents (bevacizumab, ranibizumab, and aflibercept) have also been compared. Blaha et al. found a small, but statistically significant, difference between the change in anterior chamber flare 1 day after intravitreal bevacizumab or intravitreal ranibizumab administration (Blaha et al., 2015). However, the small observed difference was not clinically relevant because no evidence of increased cell or are counts has been observed after routine use of these drugs (Blaha et al., 2015).
Recent reports of a new agent-brolucizumab and its possible side-effects have re-ignited interest in the cause of inflammatory reactions after intra-vitreous anti-VEGF drug administration. The mechanism of inflammation during anti-VEGF therapy remains unclear and is currently under investigation. Various theories suggest an immune response to the active molecule of the drug, other protein by-products within the drug or pH changes. One of the possible hypotheses for the pathogenic mechanism of this spectrum of events that is under investigation is the formation of local anti-bodies (Agrawal et al., 2013; Baumal et al., 2020; Haug et al., 2020). Clarification of the pathogenesis of inflammatory reactions after some anti-VEGF drugs is important also for clinical reasons. It is crucial to distinguish non-infectious from infectious intraocular inflammation, a severe vision-threatening condition that requires urgent evaluation and treatment.
In conclusion, our results showed a good safety profile and lack of inflammatory response following multiple intravitreal bevacizumab injections. These observations confirm that multiple intravitreal bevacizumab administrations are safe with no risk for patients with exudative AMD, even though CATT study demonstrated that the proportion of patients with one or more systemic serious adverse events was higher with bevacizumab than ranibizumab. Further prospective studies with an adequate sample size calculation and longitudinal testing are mandatory to confirm our preliminary data.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The study protocol was reviewed and approved by the Institutional Review Board (IRB) at Medical University, Lublin, Poland (n° KE-0254/208/2013), on July 11th, 2013. With the LFP being a noninvasive diagnostic tool and according the ongoing regulation, the IRB waived the requirement of informed consent. All study conduct adhered to the tenets of the Declaration of Helsinki.
Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
SZ is Consultant for Bayer Healthcare Pharmaceuticals, Novartis Pharma AG and Roche Diagnostics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adamis, A. P., and Shima, D. T. (2005). The role of vascular endothelial growth factor in ocular health and disease. Retina 25 (2), 111–118. doi:10.1097/00006982-200502000-00001
Agrawal, S., Joshi, M., and Christoforidis, J. B. (2013). Vitreous inflammation associated with intravitreal anti-VEGF pharmacotherapy. Mediators Inflamm. 2013, 1–6. doi:10.1155/2013/943409
Avery, R. L., Bakri, S. J., Blumenkranz, M. S., Brucker, A. J., Cunningham, E. T., DugelD'Amico, P. U., et al. (2014). Intravitreal injection technique and monitoring. Retina 34 (Suppl. 12), S1–S18. doi:10.1097/iae.0000000000000399
Baumal, C. R., Spaide, R. F., Vajzovic, L., Freund, K. B., Walter, S. D., John, V., et al. (2020). Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology 127 (10), 1345–1359. doi:10.1016/j.ophtha.2020.04.017
Beovu Update for ASRS Members (2020). Available at: https://www.asrs.org/clinical/clinical-updates (Accessed Aug 27th, 2020).
Berg, K., Pedersen, T. R., Sandvik, L., and Bragadóttir, R. (2015). Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 122 (1), 146–152. doi:10.1016/j.ophtha.2014.07.041
Blaha, G. R., Brooks, N. O., Mackel, C. E., Pani, A., Stewart, A. P., Price, L. L., et al. (2015). Changes in flare after intravitreal injection of three different anti-vascular endothelial growth factor medications. Retina 35 (3), 577–581. doi:10.1097/iae.0000000000000334
Conti, B., Bucolo, C., Giannavola, C., Puglisi, G., Giunchedi, P., and Conte, U. (1997). Biodegradable microspheres for the intravitreal administration of acyclovir: in vitro/in vivo evaluation. Eur. J. Pharmaceut. Sci. 5 (5), 287–293. doi:10.1016/S0928-0987(97)00023-7
Chong, D. Y., Anand, R., Williams, P. D., Qureshi, J. A., and Callanan, D. G. (2010). Characterization of sterile intraocular inflammatory responses after intravitreal bevacizumab injection. Retina 30 (9), 1432–1440. doi:10.1097/iae.0b013e3181dc04da
Dossarps, D., Bron, A. M., Koehrer, P., Aho-Glélé, L. S., Creuzot-Garcher, C., Berthon, L., et al. (2015). Endophthalmitis after intravitreal injections: incidence, presentation, management, and visual outcome. Am. J. Ophthalmol. 160 (1), 17–25. doi:10.1016/j.ajo.2015.04.013
Dugel, P. U. (2017). “Expanded week 96 safety outcomes: analysis of pooled data from HAWK & HARRIER studies,” in Presented at the 2020 meeting of the macula society, March 19–22, California, San Diego.
Errera, M. H., Girmens, J. F., Ayello-Scheer, S., Nourry, H., Warnet, J. M., Sahel, J. A., et al. (2014). Correlation between aqueous flare and chorioretinal neovascularization in age-related macular degeneration following intravitreal bevacizumab injections. J. Fr. Ophtalmol. 37 (1), 30–35. doi:10.1016/j.jfo.2013.02.008
Falavarjani, K. G., and Nguyen, Q. D. (2013). Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye 27 (7), 787–794. doi:10.1038/eye.2013.107
Figurska, M., Matysik-Wożniak, A., Adamiec-Mroczek, J., Dolar-Szczasny, J., Misiuk-Hojło, M., Teper, S., et al. (2020). One-year outcomes of the Polish treatment program for the wet form of age-related macular degeneration using intravitreal therapy. Eur. J. Ophthalmol. 30 (3), 586–594. doi:10.1177/1120672119874598
Grzybowski, A., Told, R., Sacu, S., Bandello, F., Moisseiev, E., Loewenstein, A., et al. (2018). 2018 update on intravitreal injections: euretina expert consensus recommendations. Ophthalmologica 239 (4), 181–193. doi:10.1159/000486145
Haug, S. J., Hien, D. L., Uludag, G., Ngoc, T. T. T., Lajevardi, S., Halim, M. S., et al. (2020). Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am. J. Ophthalmol. Case Rep. 18, 100680. doi:10.1016/j.ajoc.2020.100680
Jaki Mekjavic, P., Jurate Balciuniene, V., Ceklic, L., Ernest, J., Jamrichova, Z., Zsolt Nagy, Z., et al. (2019). The burden of macular diseases in central and eastern europe-implications for healthcare systems. Value Health Reg. Issues 19, 1–6. doi:10.1016/j.vhri.2018.11.002
Kiss, C., Michels, S., Prager, F., Weigert, G., Geitzenauer, W., and Schmidt-Erfurth, U. (2006). Evaluation of anterior chamber inflammatory activity in eyes treated with intravitreal bevacizumab. Retina 26 (8), 877–881. doi:10.1097/01.iae.0000237080.10627.b7
Kubota, T., Küchle, M., and Nguyen, N. X. (1994). Aqueous flare in eyes with age-related macular degeneration. Jpn. J. Ophthalmol. 38 (1), 67–70.
Ladas, J. G., Wheeler, N. C., Morhun, P. J., Rimmer, S. O., and Holland, G. N. (2005). Laser flare-cell photometry: methodology and clinical applications. Surv. Ophthalmol. 50 (1), 27–47. doi:10.1016/j.survophthal.2004.10.004
Manzano, R. P. A., Peyman, G. A., Khan, P., and Kivilcim, M. (2006). Testing intravitreal toxicity of bevacizumab (Avastin). Retina 26 (3), 257–261. doi:10.1097/00006982-200603000-00001
Martin, D. F., Martin, D. F., Maguire, M. G., Ying, G. S., Grunwald, J. E., Fine, S. L., et al. (2011). Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 364 (20), 1897–1908. doi:10.1056/NEJMoa1102673
Martin, D. F., Maguire, M. G., Fine, S. L., Ying, G.-s., Jaffe, G. J., Grunwald, J. E., et al. (2012). Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology 119 (7), 1388–1398. doi:10.1016/j.ophtha.2012.03.053
Martin, D. F., Maguire, M. G., Fine, S. L., Ying, G.-s., Jaffe, G. J., Grunwald, J. E., et al. (2020). Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology 127 (4S), S135–S145. doi:10.1016/j.ophtha.2020.01.029
Merani, R., and Hunyor, A. P. (2015). Endophthalmitis following intravitreal anti-vascular endothelial growth factor (VEGF) injection: a comprehensive review. Int. J. Retina Vitreous 1 (1), 9. doi:10.1186/s40942-015-0010-y
Orès, R., Terrada, C., Errera, M. H., Thorne, J. E., Doukhan, R., Cassoux, N., et al. (2020). Laser flare photometry: a useful tool for monitoring patients with juvenile idiopathic arthritis-associated uveitis. Ocul. Immunol. Inflamm., 1–11. doi:10.1080/09273948.2020.1792511
Platania, C. B., Di Paola, L., Leggio, G. M., Romano, G. L., Drago, F., Salomone, S., et al. (2015). Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: a computational approach. Front. Pharmacol. 6, 248. doi:10.3389/fphar.2015.00248
Plyukhova, A. A., Budzinskaya, M. V., Starostin, K. M., Rejdak, R., Bucolo, C., Reibaldi, M., et al. (2020). Comparative safety of bevacizumab, ranibizumab, and aflibercept for treatment of neovascular age-related macular degeneration (AMD): a systematic review and network meta-analysis of direct comparative studies. J. Clin. Med. 9 (5), 1522. doi:10.3390/jcm9051522
Reibaldi, M., Russo, A., Avitabile, T., Uva, M. G., Franco, L., Longo, A., et al. (2014). Treatment of persistent serous retinal detachment in Vogt-Koyanagi-Harada syndrome with intravitreal bevacizumab during the systemic steroid treatment. Retina 34 (3), 490–496. doi:10.1097/iae.0b013e3182a0e446
Reibaldi, M., Avitabile, T., Bandello, F., Longo, A., Bonfiglio, V., Russo, A., et al. (2019). The effectiveness of 0.6% povidone iodine eye drops in reducing the conjunctival bacterial load and needle contamination in patients undergoing anti-VEGF intravitreal injection: a prospective, randomized study. J. Clin. Med. 8 (7), 1031. doi:10.3390/jcm8071031
Rosenfeld, P., Schwartz, S., Blumenkranz, M., Miller, J., Haller, J., Reimann, J., et al. (2005). Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 112 (6), 1048–1053. doi:10.1016/j.ophtha.2005.01.043
Toro, M. D., Brézin, A. P., Burdon, M., Cummings, A. B., Evren Kemer, O., Malyugin, B. E., et al. (2021). Early impact of COVID-19 outbreak on eye care: insights from EUROCOVCAT group. Eur. J. Ophthalmol. 31 (1), 5–9. doi:10.1177/1120672120960339
Tugal-Tutkun, I., and Herbort, C. P. (2010). Laser flare photometry: a noninvasive, objective, and quantitative method to measure intraocular inflammation. Int. Ophthalmol. 30 (5), 453–464. doi:10.1007/s10792-009-9310-2
US Food and Drug Administration (2019). Center for drug evaluation and research. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761125Orig1s000SumR.pdf Published September 25 (AccessedJune 11, 2020).
Williams, P. D., Chong, D., Fuller, T., and Callanan, D. (2016). Noninfectious vitritis after intravitreal injection of anti-vegf agents. Retina 36 (5), 909–913. doi:10.1097/iae.0000000000000801
Xu, W., Wang, H., Wang, F., Jiang, Y., Zhang, X., Wang, W., et al. (2010). Testing toxicity of multiple intravitreal injections of bevacizumab in rabbit eyes. Can. J. Ophthalmol. 45 (4), 386–392. doi:10.3129/i10-024
Yeniad, B., Ayranci, O., Tuncer, S., Kir, N., Ovali, T., Tugal-Tutkun, I., et al. (2011). Assessment of anterior chamber inflammation after intravitreal bevacizumab injection in different ocular exudative diseases. Eur. J. Ophthalmol. 21 (2), 156–161. doi:10.5301/ejo.2010.5239
Yousef, Y. A., ElRimawi, A. H., Nazzal, R. M., Qaroot, A. F., AlAref, A. H., Mohammad, M., et al. (2020). Coats' disease: characteristics, management, outcome, and scleral external drainage with anterior chamber maintainer for stage 3b disease. Medicine 99 (16), e19623. doi:10.1097/md.0000000000019623
Keywords: bevacizumab, neovascular age-related macular degeneration, laser-flare photometry, intraocular inflammation, blood-aqueous barrier integrity
Citation: Dolar-Szczasny J, Bucolo C, Zweifel S, Carnevali A, Rejdak R, Załuska W, Czarnek-Chudzik A and Toro MD (2021) Evaluation of Aqueous Flare Intensity in Eyes Undergoing Intravitreal Bevacizumab Therapy to Treat Neovascular Age-Related Macular Degeneration. Front. Pharmacol. 12:656774. doi: 10.3389/fphar.2021.656774
Received: 21 January 2021; Accepted: 25 February 2021;
Published: 30 April 2021.
Edited by:
Benedetto Falsini, Catholic University of the Sacred Heart, ItalyCopyright © 2021 Dolar-Szczasny, Bucolo, Zweifel, Carnevali, Rejdak, Załuska, Czarnek-Chudzik and Toro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna Dolar-Szczasny, am9hbm5hc3pjemFzbnlAb3AucGw=; Mario Damiano Toro, dG9yby5tYXJpb0BlbWFpbC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.