
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 20 July 2021
Sec. Drugs Outcomes Research and Policies
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.652759
Background: To update the efficacy and safety data of monoclonal antibodies for the treatment of neuromyelitis optica spectrum disorders (NMOSD) and explore the differences in the effect of treatment between patients seropositive and seronegative for AQP4-IgG. Methods: PubMed, Embase, and the Cochrane Library published up to July 2020 were searched for randomized controlled trials (RCTs) of monoclonal antibodies treatment (mAb) in patients with NMOSD. The primary outcome was the hazard ratio (HR) for relapse. The secondary outcomes included Expanded Disability Status Scale (EDSS) changes from baseline, adverse events (AEs), and serious adverse events (SAEs). A random-effects model was applied for the effect of heterogeneity among trials. Results: We included 603 patients (monoclonal antibody group, n=382, and control group, n=221) from seven RCTs. There were fewer relapses in the mAb group (HR=0.32, 95% CI: 0.23-0.46, p<0.001), as well as in the AQP4-IgG-seropositive patients (HR=0.18, 95% CI: 0.10–0.32, p<0.001), but not in AQP4-IgG-seronegative NMOSD. Similar results were observed when considering satralizumab only. The mAb had no impact on the changes in EDSS scores from baseline (WMD=−0.21, 95% CI: −0.50-0.09, p=0.176). The mAb did not lead to a higher frequency of AEs (OR=1.18, 95% CI: 0.70–1.98, p=0.529) or SAEs (OR=0.99, 95% CI: 0.63–1.56, p=0.975) compared with the control group. Conclusions: Compared to the control arm, monoclonal antibody therapy showed a significantly better outcome in restraining the HR for relapse among patients with NMOSD but insignificant effects in NMOSD patients with seronegative APQ4-IgG. The safety profile in each arm had no significant difference.
Neuromyelitis optica spectrum disorders (NMOSD) are devastating autoantibody-induced inflammatory diseases of the central nervous system that primarily affect the spinal cord, optic nerves, and brainstem, causing paralysis and blindness (Sellner et al., 2010; Kremer et al., 2014; Trebst et al., 2014; Wingerchuk et al., 2015). NMOSD encompasses a group of syndromes typically characterized by optic neuritis and/or acute myelitis, often in association with serum IgG autoantibodies directed against aquaporin-4 (AQP4-IgG), an astrocytic water channel (Papadopoulos and Verkman, 2012). The reported incidence and prevalence of NMOSD are dependent on geographical location and ethnicity; Asians and those of African ancestry are at increased risk, with high mortality rates reported in the latter (Wingerchuk, 2009; Sellner et al., 2010; Pittock and Lucchinetti, 2016; Mealy et al., 2018). Women are more affected than men, with a ratio of about 9:1 in adults and 3:1 in children (Wingerchuk et al., 2015). The median age of NMOSD onset is the late 30s (Sellner et al., 2010; Trebst et al., 2014).
The key point of the treatment of patients with NMOSD is to prevent relapse and reduce attack severity to lower the irreversible neurological impairments resulting from the successive attacks (Sellner et al., 2010; Wingerchuk et al., 2015). Several novel biological agents, including rituximab, eculizumab, inebilizumab, satralizumab, and tocilizumab, were applied in clinical trials to assess their effects on preventing NMOSD relapse. Azathioprine is effective for the management of relapses and disability in patients with NMOSD, but the adverse events are frequent and might limit its use (Espiritu and Pasco, 2019). Similar conclusions were reached for rituximab (Damato et al., 2016; Huang et al., 2019) and mycophenolate mofetil (Huang et al., 2019). Nevertheless, those conclusions are based on meta-analyses that included a wide variety of study types. To date, only one meta-analysis based on randomized controlled trials (RCTs) was published on the topic of NMOSD treatment (Xue et al., 2020), but it only included four RCTs, each investigating a different monoclonal antibody agent. Since the publication of that meta-analysis (Xue et al., 2020), three additional RCTs regarding the efficacy and safety of monoclonal antibody agents were completed and published (Tahara et al., 2020; Traboulsee et al., 2020; Zhang et al., 2020).
Therefore, the aim of this meta-analysis was to update the efficacy and safety data of monoclonal antibodies for the treatment of NMOSD and explore the differences in the effect of treatment between patients seropositive and seronegative for AQP4-IgG.
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Selcuk, 2019). We started by searching relevant articles according to the PICOS principle (Aslam and Emmanuel, 2010), followed by screening on the basis of the inclusion criteria: 1) Population: NMOSD; 2) Interventions: using monoclonal antibody therapy; 3) Comparison: different treatment or placebo; 4) Study: RCTs; and 5) Language: English. PubMed, Embase, and the Cochrane Library were searched for available papers published up to July 2020, using the MeSH terms “Neuromyelitis Optica” as well as relevant key words such as “monoclonal antibody” and “randomized controlled trial.”
The study characteristics (authors, year of publication, site of the study population, blinding methodology, and age of the patients), treatment parameters (sample size, median follow-up time, treatment, and control agents), and outcomes (hazard ratio (HR) for relapse, Expanded Disability Status Scale (EDSS) changes from baseline, adverse events (AEs), and serious adverse events (SAEs)) were extracted by two authors, independently. The discrepancies were solved by discussion.
For outcome assessments, the HRs of relapse and their 95% confidence intervals (CIs) were extracted from the Kaplan-Meier survival curves in each study. To account for more conservative results, studies that reported relative risks (RRs) were not considered in this pooled analysis. EDSS score changes from baseline were reported in four of the trials, and the number of patients in each arm, as well as the mean change with its standard deviation, were used for analysis. The numbers of cases of AEs and SAEs in each arm were used to conclude the safety results.
The level of evidence of all RCTs was assessed independently by two authors according to the Cochrane Handbook and NOS criteria (Lo et al., 2014; Higgins et al., 2019). Discrepancies in the assessment were resolved through discussion until a consensus was reached.
All analyses were performed using the STATA SE 14.0 software (StataCorp, College Station, Texas, United States). The effects and corresponding 95% CIs were used to compare the outcomes. Weighted mean differences (WMDs) were used for the changes in EDSS. AEs and SAEs were analyzed in terms of odds ratios (ORs). Statistical heterogeneity among the studies was calculated using Cochran’s Q test and the I2 index (Higgins et al., 2019). To avoid the effect of heterogeneity between each study, regardless of the results of Cochran’s Q test and the I2 index, random effect models were applied for the pooled analyses. We did not assess potential publication bias by funnel plots and Egger’s test because the number of studies included in every meta-analysis was smaller than 10, in which case the funnel plots and Egger’s test could yield misleading results and are not recommended (Higgins et al., 2011; Higgins et al., 2019).
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Figure 1 shows the study selection process. A total of 1,440 records were initially identified. After removing the duplicates, 1,050 were screened, and 636 were excluded. The 414 remaining full-text papers were assessed for eligibility and 407 were excluded (no accessible full-text, n = 13; study aim/design, n = 140; population, n = 202; exposures, n = 47; and meta-analyses, n = 5).
Finally, seven RCTs were selected, encompassing 382 patients with the study drug and 221 controls (Nikoo et al., 2017; Cree et al., 2019; Pittock et al., 2019; Yamamura et al., 2019; Tahara et al., 2020; Traboulsee et al., 2020; Zhang et al., 2020) (Table 1). There were five double-blind RCTs and two open-label ones. One study examined inebilizumab (Cree et al., 2019), two examined rituximab (Nikoo et al., 2017; Tahara et al., 2020), one examined eculizumab (Pittock et al., 2019), two examined satralizumab (Yamamura et al., 2019; Traboulsee et al., 2020), and one examined tocilizumab (Zhang et al., 2020). Five studies used a placebo as control (Cree et al., 2019; Pittock et al., 2019; Yamamura et al., 2019; Tahara et al., 2020; Traboulsee et al., 2020), while two used azathioprine (Nikoo et al., 2017; Zhang et al., 2020).
For studies that reported the HR of relapse for all NMOSD patients (Cree et al., 2019; Yamamura et al., 2019; Traboulsee et al., 2020; Zhang et al., 2020). The pooled analysis showed a significantly better outcome in the treatment group (HR = 0.32, 95% CI: 0.23–0.46, p < 0.001; I2 = 0.0%, Pheterogeneity = 0.575). Subgroup analysis for AQP4-IgG-seropositive and negative patients indicated a positive association between treatment and favorable outcome among patients with AQP4-IgG-seropositive NMOSD (HR = 0.18, 95% CI: 0.10–0.32, p < 0.001; I2 = 35.9%, Pheterogeneity = 0.197), whereas the association with AQP4-IgG-seronegative NMOSD was not significant (HR = 0.85, 95% CI: 0.34–2.12, p = 0.729; I2 = 0.0%, Pheterogeneity = 0.529) (Figure 2 and Supplementary Table 1).
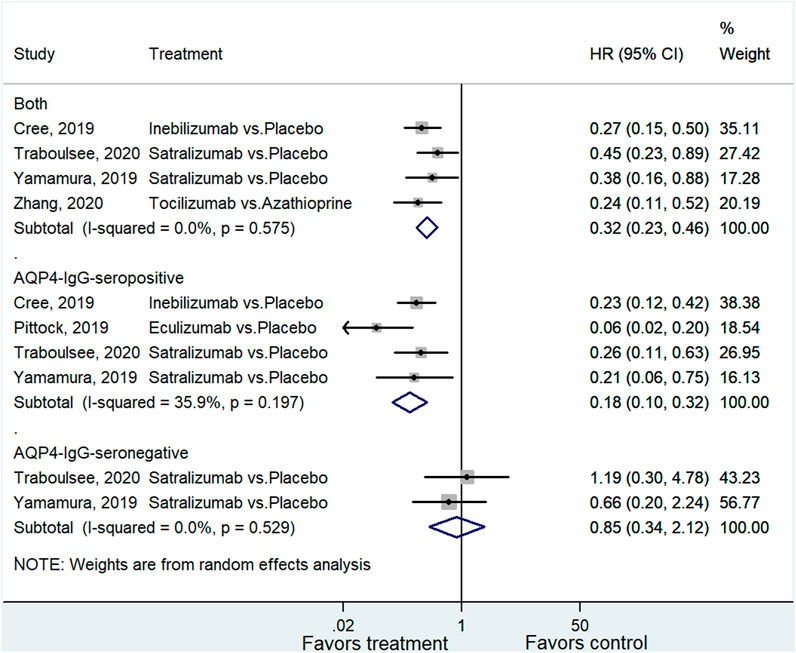
FIGURE 2. Forest plot of HR for relapse in all NMOSD, AQP4-IgG-seropositive NMOSD, and AQP4-IgG-seronegative NMOSD patients.
Two studies examined satralizumab on the relapse of NMOSD (Yamamura et al., 2019; Traboulsee et al., 2020). The pooled analysis showed a significantly better outcome in the satralizumab group (HR = 0.42, 95% CI: 0.25–0.72, p = 0.001; I2 = 0.0%, Pheterogeneity = 0.761). Subgroup analysis for AQP4-IgG-seropositive and negative patients indicated a positive association between treatment and favorable outcome among patients with AQP4-IgG-seropositive NMOSD (HR = 0.24, 95% CI: 0.12–0.50, p < 0.001; I2 = 0.0%, Pheterogeneity = 0.785), whereas the association with AQP4-IgG-seronegative NMOSD was not significant (HR = 0.85, 95% CI: 0.34–2.12, p = 0.719; I2 = 0.0%, Pheterogeneity = 0.529) (Figure 3 and Supplementary Table 1).
Four studies reported the changes in EDSS (Nikoo et al., 2017; Pittock et al., 2019; Tahara et al., 2020; Traboulsee et al., 2020). The treatment had no impact on the changes in EDSS scores from baseline (WMD = −0.21, 95% CI: −0.50–0.09, p = 0.176; I2 = 47.0%, Pheterogeneity = 0.129) (Figure 4 and Supplementary Table 1).
Six studies reported the AEs (Cree et al., 2019; Pittock et al., 2019; Yamamura et al., 2019; Tahara et al., 2020; Traboulsee et al., 2020; Zhang et al., 2020). The treatment did not lead to a higher frequency of AEs compared with the control group (OR = 1.18, 95%CI: 0.70–1.98, p = 0.529; I2 = 6.9%, Pheterogeneity = 0.372) (Figure 5 and Supplementary Table 1). Six studies reported the SAEs (Cree et al., 2019; Pittock et al., 2019; Yamamura et al., 2019; Tahara et al., 2020; Traboulsee et al., 2020; Zhang et al., 2020). The treatment did not lead to a higher frequency of SAEs compared with the control group (OR = 0.99, 95% CI: 0.63–1.56, p = 0.975; I2 = 0.0%, Pheterogeneity = 0.671) (Figure 6 and Supplementary Table 1).
Four studies had a low risk of bias for each of the items of the Cochrane tool (Cree et al., 2019; Pittock et al., 2019; Yamamura et al., 2019; Tahara et al., 2020). Nikoo et al. (2017) had an unclear risk of bias regarding allocation concealment and high risks of bias for blinding of participants and personnel and blinding of outcome assessment. Traboulsee et al. (2020) carried an unclear risk of bias for other biases. Zhang et al. (2020) had a high risk of bias for blinding of participants and personnel. The details were summarized in Figure 7.
The sensitivity analysis showed that none of the studies significantly affected the results of the above analyses (Figures 8A–E).
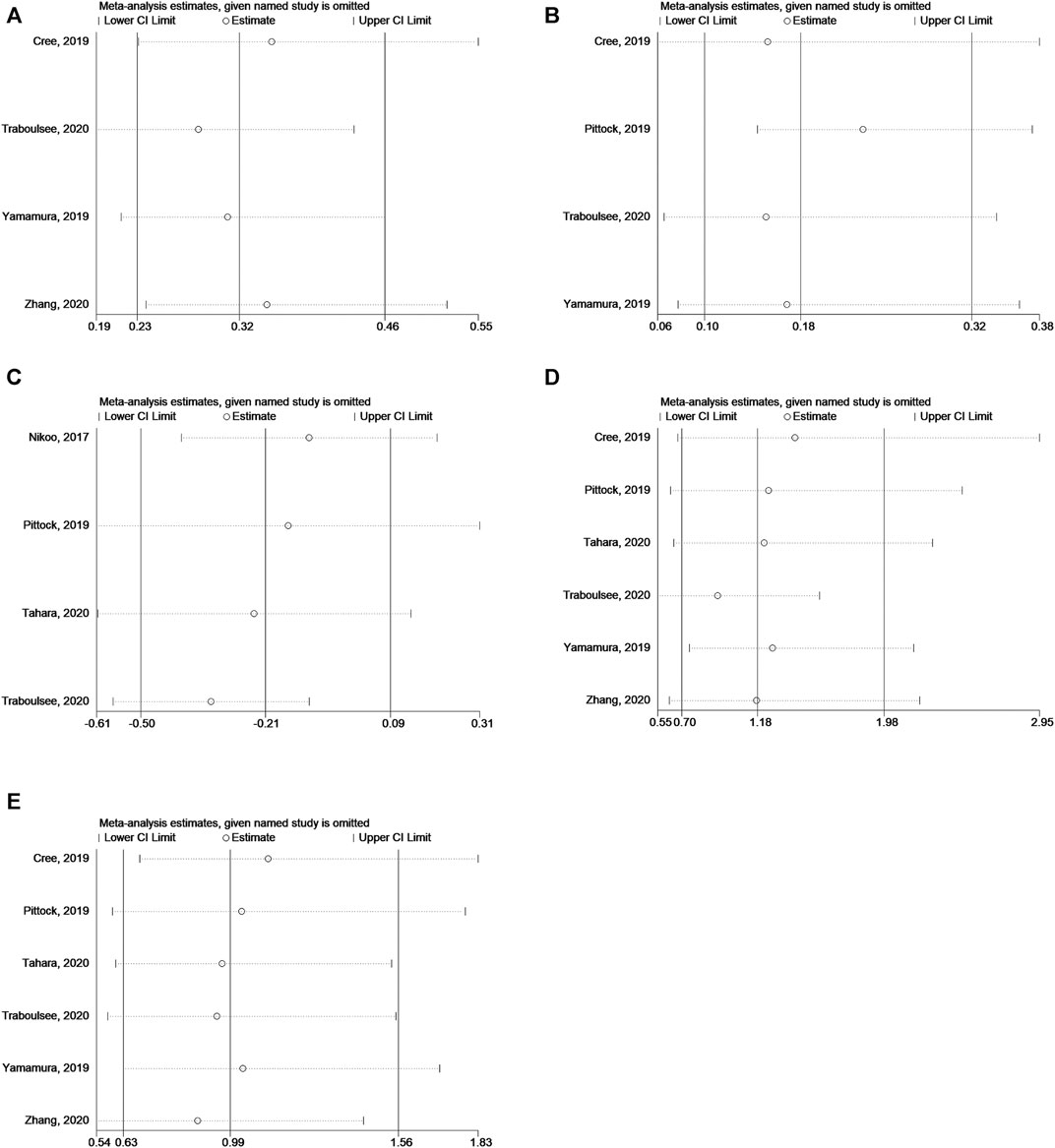
FIGURE 8. (A) Sensitivity analysis for HR of relapse in NMOSD patients. (B) Sensitivity analysis for HR of relapse in NMOSD patients with AQP4-IgG-seropositive. (C) Sensitivity analysis for EDSS score change in NMOSD patients. (D) Sensitivity analysis for odds of AEs. (E) Sensitivity analysis for odds of SAEs.
Monoclonal antibodies can be used for the management of NMOSD, but evidence from meta-analyses is rare. Therefore, this meta-analysis aimed to update the efficacy and safety data of monoclonal antibodies for the treatment of NMOSD and explore the differences in the effect of treatment between patients seropositive and seronegative for AQP4-IgG. The results indicate that compared to the control arm, monoclonal antibody therapy showed a significantly better outcome in restraining the HR for relapse among patients with NMOSD. The effects in patients with seronegative APQ4-IgG NMOSD patients were not significant. The safety profile in each arm had no significant difference.
In NMOSD, the poor outcomes are due to the repeated relapses that lead to progressive neurologic impairment (Birnbaum and Kerr, 2008; Jacob et al., 2009; Jiao et al., 2013; Hinson et al., 2016). Two previous meta-analyses of rituximab for NMOSD showed that rituximab could decrease the relapse events of NMOSD and alleviate the neurological disability of the patients (Damato et al., 2016; Huang et al., 2019). A meta-analysis of four RCTs showed that the monoclonal antibodies decreased the annualized relapse rate, on-trial relapse risk, EDSS score, and the occurrence of SAEs compared with the control treatment (Xue et al., 2020). In addition, the benefits were observed for AQP-4-positive patients. When adding three additional RCTs, similar results are still observed. Those effects are mainly attributable to B cell depletion (De Romeuf et al., 2008).
Azathioprine has been shown to be effective for the management of relapses and disability in patients with NMOSD, but the adverse events are frequent and might limit its use (Espiritu and Pasco, 2019). Despite this efficacy, the monoclonal antibodies were still more effective than azathioprine and mycophenolate mofetil, as for the comparison between the monoclonal antibodies and the placebo. In addition, the monoclonal antibodies had a tolerability profile that was more advantageous than for azathioprine. Similar results were observed for mycophenolate mofetil (Huang et al., 2019). Therefore, monoclonal antibodies are probably a better option than other immunomodulatory drugs for the management of NMOSD. Nevertheless, their costs are high, and cost-benefit analyses should be performed. In addition, two meta-analyses of rituximab raised cautions regarding its use as a first-line agent in NMOSD because of the tolerability profile (Damato et al., 2016; Huang et al., 2019). Of note, those meta-analyses examined all kinds of study design together, leading to high heterogeneity. In the present meta-analysis, the AE and SAE profile were not significantly worse than that of the control arm, but they should be taken with caution because of the two studies that used azathioprine as a control group, probably increasing the numbers of safety events in the control arm. Depleting B cells carries a theoretical risk of increased cancers and infection (Radaelli et al., 2016; Hauser et al., 2017), which should be examined in future studies.
Of note, NMOSD encompasses a group of syndromes that share optic neuritis and/or acute myelitis as their manifestation, but the patients can be seropositive or seronegative for AQP4-IgG, supporting the heterogeneity of the conditions included in NMOSD (Papadopoulos and Verkman, 2012), and particularly in AQP4-IgG-negative NMOSD (Wingerchuk et al., 2015). Clinically, patients with AQP4-IgG-positive or -negative NMOSD cannot be distinguished (Wingerchuk et al., 2007; Sepulveda et al., 2016; Weinshenker and Wingerchuk, 2017). Previous studies (Cree et al., 2019; Pittock et al., 2019; Yamamura et al., 2019; Traboulsee et al., 2020), as the present meta-analysis, support the use of monoclonal antibodies for AQP4-IgG-positive NMOSD. Still, monoclonal antibody therapy was tried in AQP4-IgG-negative NMOSD (Yamamura et al., 2019; Traboulsee et al., 2020). Traboulsee et al. (2020) included both AQP-IgG-negative and positive patients in an attempt to encompass the whole spectrum of NMOSD, and they reported no benefit of satralizumab in such patients. Such results are supported by the SAkuraStar trial by Yamamura et al. (2019). Still, they attributed this lack of efficacy to the small sample size (the proportion of negative patients was capped to represent their frequency in the population (Wingerchuk et al., 2007; Sepulveda et al., 2016)) and to the heterogeneity of AQP4-IgG-negative NMOSD (Wingerchuk et al., 2015). In addition, their study was not powered to examine efficacy in AQP4-IgG-negative NMOSD patients (Traboulsee et al., 2020). Studies that will include more patients with AQP4-IgG-negative NMOSD or studies that will better characterize the pathogenesis of AQP4-IgG-negative NMOSD could provide more definitive answers about the management of such patients.
Importantly, all safety data included in the present meta-analysis and reported by the included studies are short-term safety data. The long-term adverse events associated with monoclonal antibody therapy vary according to the target of the antibody used but generally include infections, cancers, autoimmune diseases, and organ-specific toxicity (e.g., cardiotoxicity and lung toxicity) (Hansel et al., 2010; Lu et al., 2020). Among the antibodies included in the meta-analysis, rituximab is the one with the longest market life. The late-onset AEs of rituximab include neutropenia, immune compromise, infections, leukoencephalopathy, viral reactivation, intestinal perforation, and pneumonitis (Ram et al., 2009). For the other antibodies included here, fewer data are available. Tocilizumab might increase the risk of infection and cancer (Jones and Panova, 2018). Real-life studies are necessary to determine the long-term safety of these antibodies.
This study has limitations. Although we included all newly published RCTs in our meta-analysis, the number of RCTs is still limited. Nevertheless, the sensitivity analysis showed robust results of monoclonal antibody therapy in the treatment of patients with NMOSD. In addition to the small number of RCTs, the seven RCTs covered five different monoclonal antibodies, and the controls were either a placebo or a comparator drug. The drug mechanism of each monoclonal antibody therapy may vary significantly, which will inevitably increase the heterogeneity of the meta-analysis. Therefore, we applied the random-effect model in all pooled analyses to minimize this effect.
In conclusion, compared with the control arm, monoclonal antibody therapy showed a significantly better outcome in restraining the HR for relapse among patients with NMOSD, despite the fact that the effects in patients with NMOSD with seronegative APQ4-IgG were not significant. The safety profile in each arm had no significant difference, which indicates a satisfying safety outcome. More RCTs are needed for each monoclonal antibody individually. A network meta-analysis with abundant studies comparing different monoclonal antibodies is encouraged.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
FK and JW carried out the studies, participated in collecting data, and drafted the article. HZ and HC, performed the statistical analysis and participated in its design. JH and LL participated in acquisition, analysis, or interpretation of data and draft the article. All authors read and approved the final article.
This study was funded by the Shenzhen Municipal Commission of Health and Family Planning (SZFZ2018013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank all study participants who were enrolled in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.652759/full#supplementary-material
Aslam, S., and Emmanuel, P. (2010). Formulating a Researchable Question: A Critical Step for Facilitating Good Clinical Research. Indian J. Sex. Transm. Dis. 31, 47–50. doi:10.4103/0253-7184.69003
Birnbaum, J., and Kerr, D. (2008). Optic Neuritis and Recurrent Myelitis in a Woman with Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 4, 381–386. doi:10.1038/ncprheum0818
Cree, B. A. C., Bennett, J. L., Kim, H. J., Weinshenker, B. G., Pittock, S. J., Wingerchuk, D. M., et al. (2019). Inebilizumab for the Treatment of Neuromyelitis Optica Spectrum Disorder (N-MOmentum): a Double-Blind, Randomised Placebo-Controlled Phase 2/3 Trial. The Lancet 394, 1352–1363. doi:10.1016/s0140-6736(19)31817-3
Damato, V., Evoli, A., and Iorio, R. (2016). Efficacy and Safety of Rituximab Therapy in Neuromyelitis Optica Spectrum Disorders. JAMA Neurol. 73, 1342–1348. doi:10.1001/jamaneurol.2016.1637
De Romeuf, C., Dutertre, C.-A., Le Garff-Tavernier, M., Fournier, N., Gaucher, C., Glacet, A., et al. (2008). Chronic Lymphocytic Leukaemia Cells Are Efficiently Killed by an Anti-CD20 Monoclonal Antibody Selected for Improved Engagement of FcγRIIIA/CD16. Br. J. Haematol. 140, 635–643. doi:10.1111/j.1365-2141.2007.06974.x
Espiritu, A. I., and Pasco, P. M. D. (2019). Efficacy and Tolerability of Azathioprine for Neuromyelitis Optica Spectrum Disorder: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 33, 22–32. doi:10.1016/j.msard.2019.05.011
Hansel, T. T., Kropshofer, H., Singer, T., Mitchell, J. A., and George, A. J. T. (2010). The Safety and Side Effects of Monoclonal Antibodies. Nat. Rev. Drug Discov. 9, 325–338. doi:10.1038/nrd3003
Hauser, S. L., Bar-Or, A., Comi, G., Giovannoni, G., Hartung, H.-P., Hemmer, B., et al. (2017). Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 376, 221–234. doi:10.1056/nejmoa1601277
Higgins, J. P. T., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised trialsThe Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. London: Cochrane Collaboration. (updated July 2019).
Hinson, S. R., Lennon, V. A., and Pittock, S. J. (2016). Autoimmune AQP4 Channelopathies and Neuromyelitis Optica Spectrum Disorders. Handb Clin. Neurol. 133, 377–403. doi:10.1016/b978-0-444-63432-0.00021-9
Huang, W., Wang, L., Zhang, B., Zhou, L., Zhang, T., and Quan, C. (2019). Effectiveness and Tolerability of Immunosuppressants and Monoclonal Antibodies in Preventive Treatment of Neuromyelitis Optica Spectrum Disorders: A Systematic Review and Network Meta-Analysis. Mult. Scler. Relat. Disord. 35, 246–252. doi:10.1016/j.msard.2019.08.009
Jacob, A., Matiello, M., Weinshenker, B. G., Wingerchuk, D. M., Lucchinetti, C., Shuster, E., et al. (2009). Treatment of Neuromyelitis Optica with Mycophenolate Mofetil: Retrospective Analysis of 24 Patients. Arch. Neurol. 66, 1128–1133. doi:10.1001/archneurol.2009.175
Jiao, Y., Fryer, J. P., Lennon, V. A., Jenkins, S. M., Quek, A. M. L., Smith, C. Y., et al. (2013). Updated Estimate of AQP4-IgG Serostatus and Disability Outcome in Neuromyelitis Optica. Neurology 81, 1197–1204. doi:10.1212/wnl.0b013e3182a6cb5c
Jones, G., and Panova, E. (2018). New Insights and Long-Term Safety of Tocilizumab in Rheumatoid Arthritis. Ther. Adv. Musculoskelet. 10, 195–199. doi:10.1177/1759720x18798462
Kremer, L., Mealy, M., Jacob, A., Nakashima, I., Cabre, P., Bigi, S., et al. (2014). Brainstem Manifestations in Neuromyelitis Optica: a Multicenter Study of 258 Patients. Mult. Scler. 20, 843–847. doi:10.1177/1352458513507822
Lo, C. K., Mertz, D., and Loeb, M. (2014). Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 14, 45. doi:10.1186/1471-2288-14-45
Lu, R. M., Hwang, Y. C., Liu, I. J., Lee, C. C., Tsai, H. Z., Li, H. J., et al. (2020). Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 27, 1. doi:10.1186/s12929-019-0592-z
Mealy, M. A., Kessler, R. A., Rimler, Z., Reid, A., Totonis, L., Cutter, G., et al. (2018). Mortality in Neuromyelitis Optica Is Strongly Associated with African Ancestry. Neurol. Neuroimmunol. Neuroinflamm. 5, e468. doi:10.1212/nxi.0000000000000468
Nikoo, Z., Badihian, S., Shaygannejad, V., Asgari, N., and Ashtari, F. (2017). Comparison of the Efficacy of Azathioprine and Rituximab in Neuromyelitis Optica Spectrum Disorder: a Randomized Clinical Trial. J. Neurol. 264, 2003–2009. doi:10.1007/s00415-017-8590-0
Papadopoulos, M. C., and Verkman, A. (2012). Aquaporin 4 and Neuromyelitis Optica. Lancet Neurol. 11, 535–544. doi:10.1016/s1474-4422(12)70133-3
Pittock, S. J., Berthele, A., Fujihara, K., Kim, H. J., Levy, M., Palace, J., et al. (2019). Eculizumab in Aquaporin-4-Positive Neuromyelitis Optica Spectrum Disorder. N. Engl. J. Med. 381, 614–625. doi:10.1056/nejmoa1900866
Pittock, S. J., and Lucchinetti, C. F. (2016). Neuromyelitis Optica and the Evolving Spectrum of Autoimmune Aquaporin-4 Channelopathies: a Decade Later. Ann. N.Y. Acad. Sci. 1366, 20–39. doi:10.1111/nyas.12794
Radaelli, M., Moiola, L., Sangalli, F., Esposito, F., Barcella, V., Ferrè, L., et al. (2016). Neuromyelitis Optica Spectrum Disorders: Long-Term Safety and Efficacy of Rituximab in Caucasian Patients. Mult. Scler. 22, 511–519. doi:10.1177/1352458515594042
Ram, R., Ben-Bassat, I., Shpilberg, O., Polliack, A., and Raanani, P. (2009). The Late Adverse Events of Rituximab Therapy - Rare but There!. Leuk. Lymphoma 50, 1083–1095. doi:10.1080/10428190902934944
Selcuk, A. A. (2019). A Guide for Systematic Reviews: PRISMA. Turk Arch. Otorhinolaryngol. 57, 57–58. doi:10.5152/tao.2019.4058
Sellner, J., Boggild, M., Clanet, M., Hintzen, R. Q., Illes, Z., Montalban, X., et al. (2010). EFNS Guidelines on Diagnosis and Management of Neuromyelitis Optica. Eur. J. Neurol. 17, 1019–1032. doi:10.1111/j.1468-1331.2010.03066.x
Sepúlveda, M., Armangué, T., Sola-Valls, N., Arrambide, G., Meca-Lallana, J. E., Oreja-Guevara, C., et al. (2016). Neuromyelitis Optica Spectrum Disorders. Neurol. Neuroimmunol. Neuroinflamm. 3, e225. doi:10.1212/nxi.0000000000000225
Tahara, M., Oeda, T., Okada, K., Kiriyama, T., Ochi, K., Maruyama, H., et al. (2020). Safety and Efficacy of Rituximab in Neuromyelitis Optica Spectrum Disorders (RIN-1 Study): a Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 19, 298–306. doi:10.1016/s1474-4422(20)30066-1
Traboulsee, A., Greenberg, B. M., Bennett, J. L., Szczechowski, L., Fox, E., Shkrobot, S., et al. (2020). Safety and Efficacy of Satralizumab Monotherapy in Neuromyelitis Optica Spectrum Disorder: a Randomised, Double-Blind, Multicentre, Placebo-Controlled Phase 3 Trial. Lancet Neurol. 19, 402–412. doi:10.1016/s1474-4422(20)30078-8
Trebst, C., Jarius, S., Jarius, S., Berthele, A., Paul, F., Schippling, S., et al. (2014). Update on the Diagnosis and Treatment of Neuromyelitis Optica: Recommendations of the Neuromyelitis Optica Study Group (NEMOS). J. Neurol. 261, 1–16. doi:10.1007/s00415-013-7169-7
Weinshenker, B. G., and Wingerchuk, D. M. (2017). Neuromyelitis Spectrum Disorders. Mayo Clinic Proc. 92, 663–679. doi:10.1016/j.mayocp.2016.12.014
Wingerchuk, D. M., Banwell, B., Bennett, J. L., Cabre, P., Carroll, W., Chitnis, T., et al. (2015). International Consensus Diagnostic Criteria for Neuromyelitis Optica Spectrum Disorders. Neurology 85, 177–189. doi:10.1212/wnl.0000000000001729
Wingerchuk, D. M., Lennon, V. A., Lucchinetti, C. F., Pittock, S. J., and Weinshenker, B. G. (2007). The Spectrum of Neuromyelitis Optica. Lancet Neurol. 6, 805–815. doi:10.1016/s1474-4422(07)70216-8
Wingerchuk, D. M. (2009). Neuromyelitis Optica: Effect of Gender. J. Neurol. Sci. 286, 18–23. doi:10.1016/j.jns.2009.08.045
Xue, T., Yang, Y., Lu, Q., Gao, B., Chen, Z., and Wang, Z. (2020). Efficacy and Safety of Monoclonal Antibody Therapy in Neuromyelitis Optica Spectrum Disorders: Evidence from Randomized Controlled Trials. Mult. Scler. Relat. Disord. 43, 102166. doi:10.1016/j.msard.2020.102166
Yamamura, T., Kleiter, I., Fujihara, K., Palace, J., Greenberg, B., Zakrzewska-Pniewska, B., et al. (2019). Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. N. Engl. J. Med. 381, 2114–2124. doi:10.1056/nejmoa1901747
Zhang, C., Zhang, M., Qiu, W., Ma, H., Zhang, X., Zhu, Z., et al. (2020). Safety and Efficacy of Tocilizumab versus Azathioprine in Highly Relapsing Neuromyelitis Optica Spectrum Disorder (TANGO): an Open-Label, Multicentre, Randomised, Phase 2 Trial. Lancet Neurol. 19, 391–401. doi:10.1016/s1474-4422(20)30070-3
Keywords: neuromyelitis optica, inebilizumab, rituximab, eculizumab, satralizumab, tocilizumab, meta-analysis
Citation: Kong F, Wang J, Zheng H, Cai H, Hua J and Li L (2021) Monoclonal Antibody Therapy in Neuromyelitis Optica Spectrum Disorders: a Meta-analysis of Randomized Control Trials. Front. Pharmacol. 12:652759. doi: 10.3389/fphar.2021.652759
Received: 13 January 2021; Accepted: 30 June 2021;
Published: 20 July 2021.
Edited by:
Jean-Marie Boeynaems, Université libre de Bruxelles, BelgiumReviewed by:
Kurt Neumann, Independent researcher, Budapest, HungaryCopyright © 2021 Kong, Wang, Zheng, Cai, Hua and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanxin Kong, YmFueGlhQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.