
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 28 May 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.652412
 Zhe Chen1
Zhe Chen1 Qingyang Shi1
Qingyang Shi1 Yingying Peng2,3
Yingying Peng2,3 Yongjie Chen4
Yongjie Chen4 Lujia Cao1
Lujia Cao1 Bo Pang1
Bo Pang1 Zhaochen Ji1
Zhaochen Ji1 Chunxiang Liu1*
Chunxiang Liu1* Junhua Zhang1*
Junhua Zhang1*Background:Mycoplasma pneumoniae pneumonia (MPP) causes flu-like symptoms in children, increasing the burden on the health and education systems. In China, traditional Chinese medicine oral liquids (TCMOLs) combined with azithromycin (TCMOLs + Azithromycin) is commonly used to treat MPP in children. However, TCMOLs with the optimal clinical applicability remain unknown. Here, we evaluated the clinical effectiveness and safety of TCMOLs + Azithromycin in children with MPP.
Methods: We searched PubMed, Embase, Cochrane Library, Ovid, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Data Knowledge Service Platform, and VIP information resource integration service platform databases for eligible randomized controlled trials (RCTs) published from database inception to October 2020. Two reviewers independently performed data extraction and risk of bias assessment. After Bayesian random effect modeling and surface under the cumulative ranking curve (SUCRA) scoring, we ranked each intervention. We assessed heterogeneity using multivariate meta-regression for potential modifiers and used the Grading of Recommendations, Assessment, Development, and Evaluation to rate pooled evidence’s certainty.
Results: In the 63 included RCTs with 6,410 children, five different TCMOLs were combined with azithromycin. TCMOLs + Azithromycin had significantly better primary outcomes than did azithromycin alone. Of all TCMOLs, Xiaoer Xiaoji Zhike (XEXJZK)+Azithromycin showed the best effectiveness with respect to the response rate (odds ratio [OR] = 6.5, 95% credible interval [CrI] = 4.3–10; low certainty) and pulmonary rales disappearance time (mean difference [MD] = −2.1, 95% CrI: −2.9 to −1.2; low certainty) with SUCRA 85 and 80%, respectively. Pudilan Xiaoyan + Azithromycin showed the highest effectiveness with respect to cough disappearance time (MD = −2.6, 95% CrI: −3.4 to −1.7; very low certainty) and fever disappearance time (MD = −1.8, 95% CrI: −2.3 to −1.3; very low certainty) with SUCRA 87 and 87%, respectively. The difference in the adverse effects between TCMOLs + Azithromycin and azithromycin alone was nonsignificant.
Conclusion: Of the different TCMOLs, XEXJZK may be the best option to combine with azithromycin to treat children with MPP. However, our results should be interpreted with caution due to the low certainty of evidence. In general, TCMOLs’ safety remains unclear because of a lack of evidence. More high-quality RCTs are needed to further evaluate efficacy and safety of these TCMOLs.
Mycoplasma pneumoniae (MP) is a main causative agent underlying lower respiratory tract infections in the children and adolescents, at regional and epidemic levels globally (Principi et al., 2001; Jain et al., 2015; Kutty et al., 2019). Among children and adolescents with community-acquired pneumonia, MP pneumonia (MPP) is noted in 10–40% of cases, all at an annually increasing incidence (Korppi et al., 2004; Biondi et al., 2014; Jain et al., 2015). When prolonged course or aggravated infection occurs, MPP affects the nervous, digestive, and cardiovascular system and results in extrapulmonary manifestations (Narita, 2009; Narita, 2010; Flateau et al., 2013; Langley et al., 2016). Although MPP is usually a benign self-limiting disease with a mild and moderate degree of severity, it may lead to severe infection and warrant hospitalization.
MP is a small self-replicating bacterium, which lacks a cell wall and has a very small genome (Razin et al., 1998). Due to the biological structure of MP, antibiotics that act on the MP ribosome and inhibit protein synthesis are used for clinical therapy (Waites et al., 2009). Macrolides, tetracyclines, and fluoroquinolones are considered highly effective against MP infection. Of these, macrolides are the firstline antibiotic used in pediatric MPP treatment (Li et al., 2009).
Macrolide therapy administered in the early stages of MPP shortens the hospitalization and symptom duration (Shah et al., 2012; Lee et al., 2018). Of these macrolides, azithromycin is the firstline medication demonstrates good tolerance and compliance (Shan et al., 2017; Wang and Yang, 2018). Although short-term azithromycin use is effective and safe, its long-term use may lead to drug resistance and increased the incidence of adverse events (AEs) of skin, respiratory, digestive, and nervous system (Smith et al., 2015; Yang et al., 2018). Moreover, the increasing use of azithromycin in Asia, Europe, and North America has resulted in increased MP drug resistance—representing a new challenge (Pereyre et al., 2016). No high-quality evidence on the long-term use of antibiotics for treating MPP in children has been reported thus far (Gardiner et al., 2015), hinting at the potential utility of the alternative medicine.
As traditional Chinese medicine (TCM) continues to evolve, its usefulness in MPP management and treatment are being studied (Liu et al., 2010; Yang et al., 2017). TCM acts through “multiple prescriptions, multiple targets, and multiple pathways” (Sun et al., 2020); hence, it can not only alleviate MPP symptoms but also negate azithromycin drug resistance and prevent the associated AEs to a certain extent (Duan et al., 2019).
For treating upper respiratory tract infections in children, Chinese physicians incline to use combination therapy with TCM (Shi et al., 2019). The TCM oral liquids (TCMOLs) have been approved by China’s National Medical Products Administration for use in children with MPP. A real-world study in a Chinese hospital reported that the combination therapy of TCMOLs and antibiotics is used widely in China (Ren et al., 2016). TCMOLs have been used not only for MPP but also for other acute upper respiratory infections (Gao et al., 2015). In addition, TCMOLs may also have anti-inflammatory, immunoregulation, and IgM response–suppressive effects (Guo et al., 2018; Shi et al., 2020). Although some studies have reported on the effects of TCMOLs, the TCMOL with highest efficacy against MPP in children has not been identified. Therefore, here, we conducted a network meta-analysis to assess the efficacy and safety of the different TCMOLs in combination with azithromycin.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the PRISMA-extension for network meta-analysis to report our results (Moher et al., 2009; Hutton et al., 2015; Shamseer et al., 2015). Our protocol is also registered in PROSPERO (number: CRD42020197026).
We only included randomized controlled trials (RCTs), without restrictions on their language and publication type.
We included the studies on ≤15-year-old patients with a clinical diagnosis of MPP. No restriction on the patients’ sex, disease course, geography, and race were implemented. However, only the studies in which the clinical diagnostic criteria followed clinical guidelines or expert consensus were considered (Jiang et al., 2014; Ni, 2019; Lu and Zhang 2020).
We only considered combinations of various TCMOLs with azithromycin as well as azithromycin alone. As mentioned, the use of TCMOLs is approved by the National Medical Products Administration and can be looked found on the Chinese government’s website. In all studies, the TCMOL administration mode was oral, but no restriction was placed on azithromycin administration mode, dose, and duration.
1. Response rate. We calculated response rate as [(number of total patients−number of invalid patients)/number of total patients] × 100%. Patients with unchanged or worsening symptoms (e.g., fever, cough, pulmonary rales, and pulmonary shadows in x-ray) were considered invalid. The timeframe of response rate was 2 weeks (14 days).
2. Cough disappearance time. The disappearance of cough was defined as the comprehensive score of day cough symptom score + night cough symptom score being ≤1 for at least 24 h (Lai, 2016). Cough disappearance time was defined as the time interval between treatment initiation and cough disappearance.
3. Fever disappearance time. The disappearance of fever was defined as three or more consecutive measurements of normal body temperature (36–37.3°C) taken 7 h apart maintained over at least 24 h. Fever disappearance time was defined as the time interval between treatment initiation and fever disappearance.
4. Pulmonary rales disappearance time. The disappearance of pulmonary rales was defined as no pulmonary rales being found over at least 24 h. Pulmonary rales disappearance time was defined as the time interval between treatment initiation and pulmonary rales disappearance.
1. Related clinical indicators: Length of hospitalization and disappearance time of pulmonary shadows in x-ray.
2. Inflammatory cytokine: Changes from baseline of absolute tumor necrosis factor (TNF) α, absolute interleukin (IL) 6, and absolute C-reaction protein (CRP).
3. Safety: AEs and adverse incidence rates (ARs).
We excluded the studies having missing outcomes, having obvious errors, or having no full-text articles available. We also excluded studies on patients with the clinical diagnosis of non-MPP (e.g., severe MPP or refractory MPP) and the studies comparing the effects of non-azithromycin (e.g., clarithromycin, erythromycin, roxithromycin, or combinations of azithromycin and other non-azithromycin antibiotics). Studies on patients with other organ disorder or serious diseases were also excluded.
We searched China National Knowledge Infrastructure (CNKI), Wanfang Data Knowledge Service Platform, VIP information resource integration service platform, PubMed, Embase, Cochrane Library, Ovid, and Web of Science databases for randomized controlled studies on the use of different TCMOLs combined with azithromycin in children with MPP—published from database inception to October 2020. The search was performed using a combination of MeSH terms and free words. The complete search strategy is given in Supplementary File S1. We also searched the reference list of the included studies and clinical trial registries to find more relevant RCTs (Supplementary File S1).
Two researchers (ZC and ZJ) screened and filtered the candidate articles independently according to the criteria. First, they screened the titles and abstracts, and then, they read the full-text to make a final decision. We set a uniform standard to less the measurement bias for data extraction. The extracted information was as follows:
1) Study characteristics: authors, publication year and country.
2) Basic information of the included populations: total number of patients, treatment duration, sex, age, and illness course.
3) Details in the importance of interventions: medication names and usage, intervention types, and comparison types.
4) Information and definitions of outcomes: response rate, fever disappearance time, cough disappearance time, pulmonary rales disappearance time, and number of any AEs.
If there was disagreement among the reviewers, a third researcher (CL) made the final decision on the inclusion of the study.
Two researchers (LC and YP) independently used the Cochrane risk of bias tool to evaluate the included RCTs’ quality (Higgins et al., 2011). Seven terms were included in the evaluation: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. For each term, a judgment was formed with high, unclear, and low risk of bias. The study with all low risk of bias across the terms was labeled as having overall low risk of bias. The study with at least one high risk of bias across the terms was labeled as having overall high risk of bias. Others were labeled as having overall some concerns risk of bias. In case a disagreement occurred here, a third researcher resolved it through discussion or consultation.
We measured dichotomous outcomes (response rate and AR) as odds ratios (ORs) and continuous outcomes (fever, cough, pulmonary rales, and pulmonary shadow disappearance time; hospitalization length; and change in all inflammatory cytokines from baseline) as mean differences (MDs).
We performed a network meta-analysis with combined direct and indirect comparisons using the Bayesian random method on the basis of the consistency assumption. Between-study heterogeneity was set with a vague prior. The MDs and log (OR) values were derived from the posterior distribution of the model. The medians and their corresponding 95% credible intervals (CrIs) were also reported.
The models were optimized using the Markov chain Monte Carlo (MCMC) method with weighted sample size running in four chains. The iterations were set as least 200,000 to obtain model convergence. The convergence was assessed using the Brooks–Gelman–Rubin method with potential scale reduction factor up to 1. The ranking of interventions was evaluated based on the surface under the cumulative ranking curve (SUCRA).
We also conducted univariate meta-regressions to detect potential modifiers. Here, five regressors were considered: male proportion, age, treatment duration, illness course, and drug delivery mode. We used predictive mean matching imputation to address some missing data in the regressors and rated the cumulative evidence using the Grading of Recommendations, Assessment, Development, and Evaluation framework and the expanded version for network meta-analysis (Guyatt et al., 2011; Puhan et al., 2014; Brignardello-Petersen et al., 2018). First, we rated the direct comparisons according to the four domains of risk of bias, heterogeneity, indirectness, and publication bias. The rating of indirect comparisons was derived from the rating of the direct comparisons that contributed to the indirect evidence. The rating of network results was combined from the direct and indirect evidence with further considerations of imprecision and incoherence.
The publication bias was assessed by comparison-adjusted funnel plot with Begg’s rank test.
All estimates were calculated using R (version 3.6.2) along with the MCMC engine JAGS (version 3.4.0). The Cochrane tool RevMan (version 5.3) was used for plotting a risk of bias graph.
The database search afforded 543 articles; after screening and removal of duplicates, 286 articles remained. Finally, after screening the titles, abstracts and full-texts, 63 RCTs were included in the final network analysis (Figure 1).
The 63 RCTs (Supplementary File S2) included 6,410 children with MPP (n = 3,209 [intervention group] and 3,201 [comparison group]). All patients were Chinese, and most of them were infants, toddlers, and preschoolers (median age = 5.84 years)—with median disease course and treatment duration of 5.6 and 13 days, respectively.
Five combined interventions were included: azithromycin alone, with Pudilan Xiaoyan oral liquid (PDLXY; PDLXY + Azithromycin), with Shuanghuanlian oral liquid (SHL; SHL + Azithromycin), with Xiaoer Feire Kechuan oral liquid (XEFRKC; XEFRKC + Azithromycin), or with Xiaoer Xiaoji Zhike oral liquid (XRXJZK; XRXJZK + Azithromycin). Summary of all included studies and detailed chemical characterizations of TCMOLs are listed in Supplementary Tables S1, S2.
Regarding primary outcomes, 93.65, 57.14, 60.32, and 46.03% of the included RCTs reported response rate, cough disappearance time, fever disappearance time, and pulmonary rales disappearance time, respectively. Regarding safety, 46.03% of the included RCTs reported the number of AEs. Complete information is listed in Supplementary Table S3.
All the included RCTs used “random assignment,” and 23 of the RCTs mentioned specific methods, with most of them using the random number tables. In most RCTs, allocation concealment and blinding were not reported: only two RCTs mentioned the blinding of participants and personnel, and only one reported the blinding of outcome assessment. There were no missing data in all included RCTs. Thirty-five of the RCTs did not mention the selective reporting outcomes. Other risks of biases were unclear. The complete evaluation of the risk of bias is presented in Supplementary Figures S1, S2.
Fifty-nine RCTs reported the response rates for four TCMOLs combined with azithromycin for treating MPP in 5,940 children. The network plot is presented in Figure 2A.
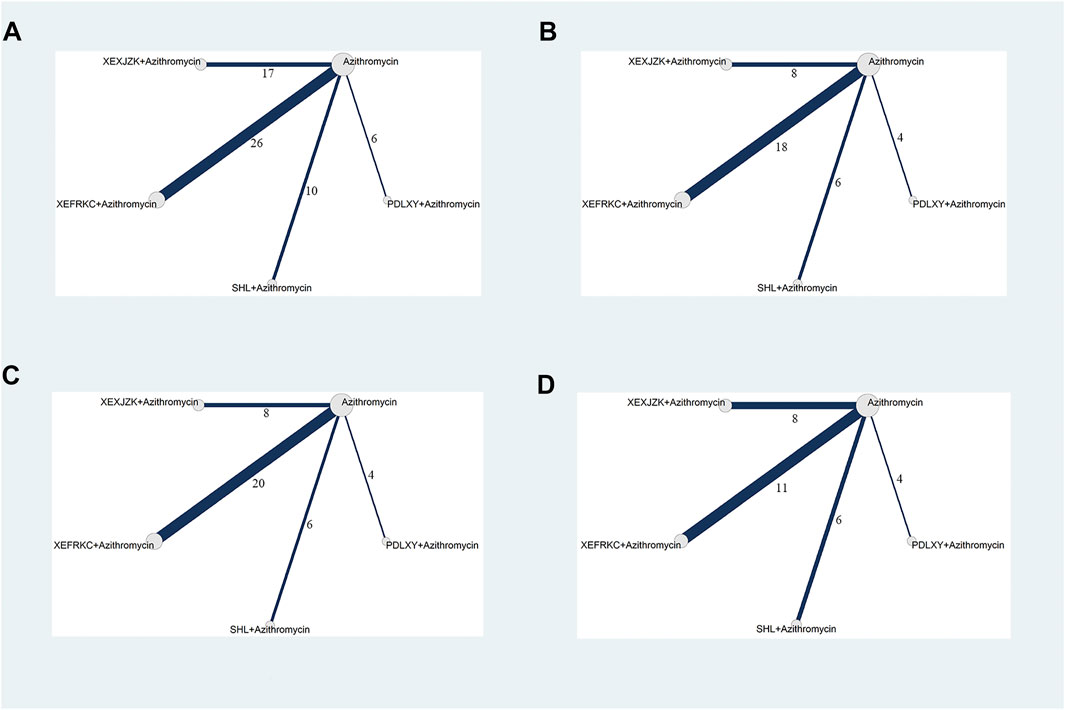
FIGURE 2. Network Plots of Primary Outcomes. PDLXY: Pudilan Xiaoyan oral liquid; SHL: Shuanghuanlian oral liquid; XEFRKC: Xiaoer Feire Kechuan oral liquid; XRXJZK: Xiaoer Xiaoji Zhike oral liquid. (A) response rate; (B) cough disappearance time; (C) fever disappearance time; (D) pulmonary rales disappearance time.
The four interventions demonstrated significant differences compared with azithromycin alone: PDLXY + Azithromycin (OR = 4.3, 95% CrI: 2.3–7.8), SHL + Azithromycin (OR = 5.4, 95% CrI: 3.3–9.2), XEFRKC + Azithromycin (OR = 5.1, 95% CrI: 3.8–7.0), and XEXJZK + Azithromycin (OR = 6.5, 95% CrI: 4.3–10; Table 1 and Supplementary Figure S3A). The network analysis demonstrated no significant differencesw between PDLXY + Azithromycin, SHL + Azithromycin, XEFRKC + Azithromycin, and XEXJZK + Azithromycin outcomes (Table 1).
Regarding the response rate, XEXJZK + Azithromycin had evidence with low certainty of evidence but the best SUCRA value (85%). SHL + Azithromycin, XEFRKC + Azithromycin, and PDLXY + Azithromycin showed low certainty of evidence (SUCRA = 64, 59, and 40%, respectively; Table 2 and Supplementary Figure S4A).
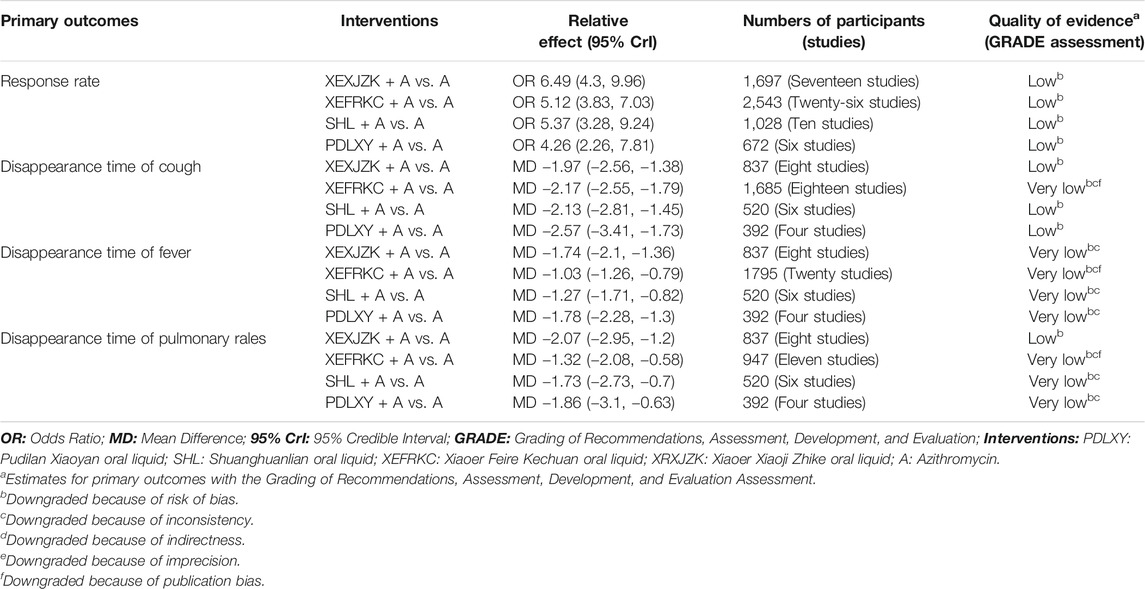
TABLE 2. Grading of recommendations, assessment, development, and evaluation of primary outcomes of TCMOLs + Azithromycin compared with azithromycin.
Thirty-six RCTs reported cough disappearance time for four TCMOLs combined with azithromycin for treating MPP in 3,434 children. The network plot is presented in Figure 2B.
The four interventions showed significant differences compared with azithromycin alone: PDLXY + Azithromycin (MD = −2.6, 95% CrI: −3.4 to −1.7), SHL + Azithromycin (MD = −2.1, 95% CrI: −2.8 to −1.5), XEFRKC + Azithromycin (MD = −2.2, 95% CrI: −2.6 to −1.8), and XEXJZK + Azithromycin (MD = −2, 95% CrI: −2.6 to −1.4; Table 1 and Supplementary Figure S3B). The network analysis showed no significant differences between PDLXY + Azithromycin, SHL + Azithromycin, XEFRKC + Azithromycin, and XEXJZK + Azithromycin (Table 1).
For cough disappearance time, XEXJZK + Azithromycin showed evidence with low certainty of evidence and a 61% SUCRA. For PDLXY + Azithromycin and SHL + Azithromycin, evidence quality demonstrated low certainty (SUCRA: 87 and 58%, respectively), whereas for XEFRKC + Azithromycin, it demonstrated very low certainty (SUCRA: 44%; Table 2 and Supplementary Figure S4B).
Thirty-eight RCTs reported fever disappearance time for four TCMOLs combined with azithromycin for treating MPP in 3,544 children. The network plot is presented in Figure 2C.
The four interventions exhibited significant differences compared with azithromycin alone: PDLXY + Azithromycin (MD = −1.8, 95% CrI: −2.3 to −1.3), SHL + Azithromycin (MD = −1.3, 95% CrI: −1.7 to −0.82), XEFRKC + Azithromycin (MD = −1.0, 95% CrI: −1.3 to −0.79), and XEXJZK + Azithromycin (MD = −1.7, 95% CrI: −2.1 to −1.4; Table 3 and Supplementary Figure S3C). The network analysis showed significant differences among PDLXY + Azithromycin, SHL + Azithromycin, and XEXJZK + Azithromycin and between XEZJZK + Azithromycin and PDLXY + Azithromycin compared with XEFRKC + Azithromycin (Table 3).
For fever disappearance time, PDLXY + Azithromycin, XEXJZK + Azithromycin, SHL + Azithromycin, and XEFRKC + Azithromycin showed very low certainty of evidence (SUCRA: 87, 85, 49, and 29%, Respectively; Table 2 and Supplementary Figure S4C).
Twenty-nine RCTs reported pulmonary rales disappearance time for four TCMOLs combined with azithromycin for treating MPP in 2,696 children. The network plot is presented in Figure 2D.
The four interventions demonstrated significant differences compared with azithromycin alone: PDLXY + Azithromycin (MD = −1.9, 95% CrI: −3.1 to −0.63), SHL + Azithromycin (MD = −1.7, 95% CrI: −2.7 to −0.7), XEFRKC + Azithromycin (MD = −1.3, 95% CrI: −2.1 to −0.58), and XEXJZK + Azithromycin (MD = −2.1, 95% CrI: −2.9 to −1.2; Table 3 and Supplementary Figure S3D). The network analysis indicated no significant differences between PDLXY + Azithromycin, SHL + Azithromycin, XEFRKC + Azithromycin, and XEXJZK + Azithromycin (Table 3).
For pulmonary rales disappearance time, XEXJZK + Azithromycin showed evidence with low certainty but the best SUCRA value (80%). PDLXY + Azithromycin, SHL + Azithromycin, and XEFRKC + Azithromycin showed very low certainty of evidence (SUCRA: 68, 62, and 39%, respectively; Table 2 and Supplementary Figure S4D).
Based on the SUCRA of the primary outcomes, we use the biplot to combine the SUCRA of response rate with the SUCRAs of cough, fever, and pulmonary rales disappearance times so as to find the best optional interventions.
The results of three biplots further confirmed that XEXJZK + Azithromycin had the highest SUCRA value at 37.4, 72.25, and 68% among the eligible interventions (Figure 3 and Supplementary Table S4).
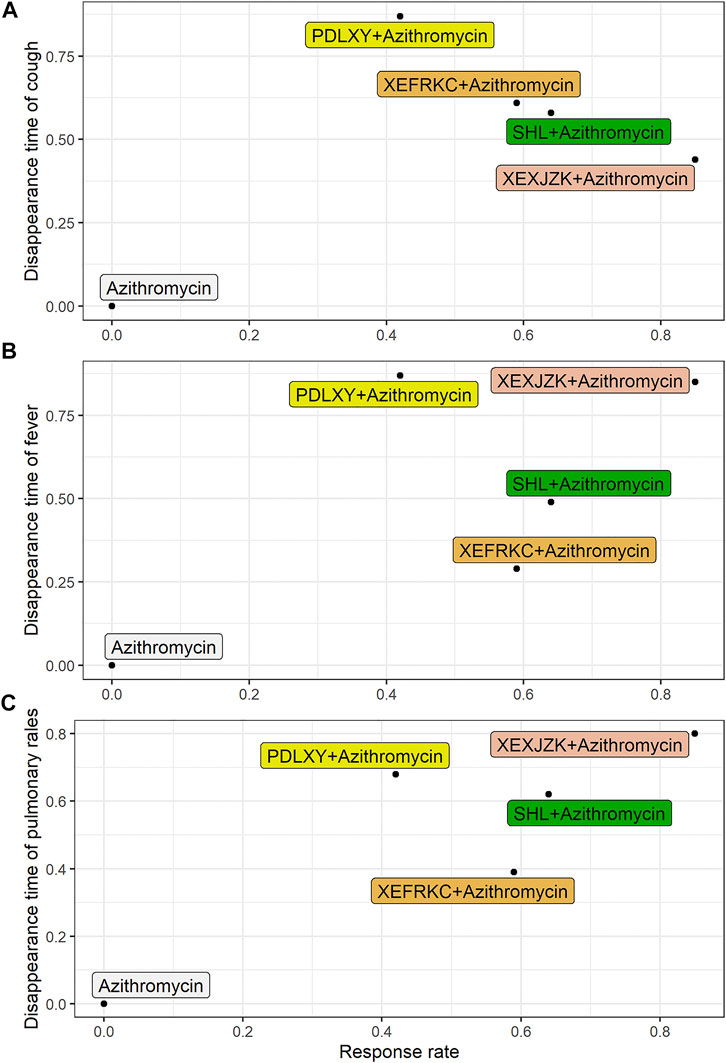
FIGURE 3. Biplot of Primary Outcomes. PDLXY: Pudilan Xiaoyan oral liquid; SHL: Shuanghuanlian oral liquid; XEFRKC: Xiaoer Feire Kechuan oral liquid; XRXJZK: Xiaoer Xiaoji Zhike oral liquid. (A) cough disappearance time + response rate; (B) fever disappearance time + response rate; (C) pulmonary rales disappearance time + response rate.
Twenty-nine RCTs reported hospitalization length after MPP treatment with three TCMOLs combined with azithromycin in 2,696 children. The network plot is illustrated in Supplementary Figure S5A.
The three interventions demonstrated significant differences compared with azithromycin (Supplementary Figure S6A). Moreover, the network analysis showed significant differences between SHL + Azithromycin and XEFRKC + Azithromycin (Supplementary Table S5).
According to SUCRA, SHL + Azithromycin (SUCRA: 92%) was superior to others, followed by XEXJZK + Azithromycin (SUCRA: 71%; Supplementary Figure S7A).
Seven RCTs reported disappearance time of pulmonary shadows in x-ray after MPP treatment with three TCMOLs combined with azithromycin in 551 children. The network plot is presented in Supplementary Figure S5B.
The three interventions demonstrated a significant difference compared with azithromycin (Supplementary Figure S6B). The network analysis showed significant differences between XEFRKC + Azithromycin and XEXJZK + Azithromycin (Supplementary Table S5).
According to SUCRA, XEXJZK + Azithromycin (SUCRA: 94%) was superior to others, followed by SHL + Azithromycin (SUCRA: 69%; Supplementary Figure S7B).
Twelve RCTs reported IL-6 changes after MPP treatment with three TCMOLs combined with azithromycin in 1,281 children. The network plot is given in Supplementary Figure S8A.
Only two interventions indicated significant differences compared with azithromycin (Supplementary Figure S9A). The network analysis showed no significant differences among the interventions (Supplementary Table S6).
According to SUCRA, SHL + Azithromycin (SUCRA: 85%) was superior to others, followed by XEFRKC + Azithromycin (SUCRA: 59%; Supplementary Figure S10A).
Ten RCTs reported TNF-α changes after MPP treatment with four TCMOLs combined with azithromycin in 1,145 children. The network plot is shown in Supplementary Figure SB.
Only one intervention demonstrated a significant difference compared with azithromycin alone (Supplementary Figure S9B). The network analysis showed no significant difference among all three interventions (Supplementary Table S6).
According to SUCRA, SHL + Azithromycin (SUCRA: 94%) was superior to others, followed by XEFRKC + Azithromycin (SUCRA: 59%; Supplementary Figure S10B).
Nine RCTs reported CRP changes after MPP treatment with three TCMOLs combined with azithromycin in 1,087 children. The network plot is shown in Supplementary Figure S8C.
Only one intervention indicated significant differences compared with azithromycin alone (Supplementary Figure S9C). The network analysis demonstrated no significant difference among the four interventions (Supplementary Table S7).
According to SUCRA, XEFRKC + Azithromycin (SUCRA: 70%) was superior to others, followed by XEZJZK + Azithromycin (SUCRA: 66%; Supplementary Figure S10C).
Regarding safety, 46.03, 38.1, 26.98, 26.98, and 14.29% of RCTs reported the number of AEs, ARs, abdominal pain and diarrhea incidence, nausea and vomiting incidence, and skin rash incidence.
Thirty-nine RCTs reported 158 and 224 AEs after MPP treatment with TCMOLs + Azithromycin and azithromycin alone, respectively (PDLXY + Azithromycin [4 trials, 9 AEs], SHL + Azithromycin [8 trials, 31 AEs], XEFRKC + Azithromycin [10 trials, 54 AEs], and XEXJZK + Azithromycin [17 trials, 64 AEs]; Supplementary Table S8).
Twenty-four RCTs reported ARs after MPP treatment with four TCMOLs combined with azithromycin. The network plot is shown in Supplementary Figure S11.
In terms of ARs and abdominal pain and diarrhea incidence, the difference between four interventions and azithromycin alone was nonsignificant (Supplementary Figures S12A,B). In terms of nausea and vomiting incidence and skin rash incidence, only one intervention demonstrated a significant difference compared with azithromycin alone (Supplementary Figures S12C,D).
Meta-regression was used to analyze the five covariates of primary outcomes and further explore potential confounding factors that may affect outcomes: gender proportion, age, treatment duration, disease course, and drug delivery mode. The meta-regression results showed no obvious modifiers in the primary outcomes, indicating that the results were relatively stable (Supplementary Table S9 and Supplementary Figure S13).
Begg’s test was used to identify the possible publication bias related to the different interventions and the impact of small sample studies. Different interventions were marked with different colored points. The results demonstrated asymmetry distribution in the funnel plot of the response rate (p = 0.0019), indicating potential publication bias.
Finally, distributions in the funnel plot of the disappearance times of cough (p = 0.6047), fever (p = 0.7154), and pulmonary rales (p = 0.6526) were all symmetric (Figure 4).
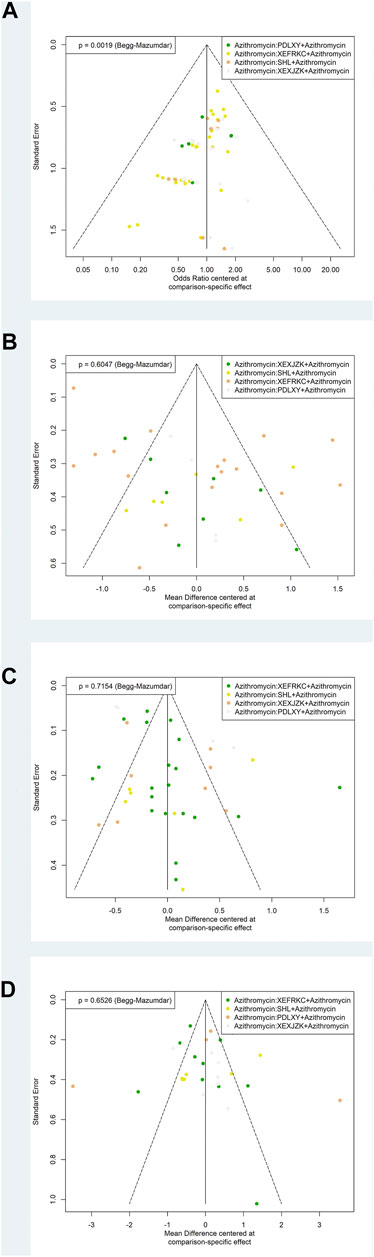
FIGURE 4. Funnel Plots of Primary Outcomes. PDLXY: Pudilan Xiaoyan oral liquid; SHL: Shuanghuanlian oral liquid; XEFRKC: Xiaoer Feire Kechuan oral liquid; XRXJZK: Xiaoer Xiaoji Zhike oral liquid. (A) response rate; (B) cough disappearance time; (C) fever disappearance time; (D) pulmonary rales disappearance time.
Based on the 63 included RCTs on 6,410 children with MPP, we found that all TCMOLs combined with azithromycin demonstrated significantly better outcomes than azithromycin alone did—with respect to cough, fever, and pulmonary rales. Of the different TCMOLs, XEXJZK + Azithromycin showed the highest odds of response rate and shortened pulmonary rales disappearance time. Moreover, PDLXY + Azithromycin demonstrated the largest reductions in the cough and fever disappearance times. Furthermore, XEXJZK + Azithromycin shortened hospitalization length and pulmonary shadow disappearance time. No significant differences were found in terms of AEs; however, the related evidence is insufficient and requires further investigation.
In general, TCMOLs might be effective in relieving cough, fever and pulmonary rales in children with MPP. Specifically, XEXJZK and PDLXY might have the most significant effects. However, we noted that the related certainty of evidence was generally low to very low, suggesting that the research evaluating TCMOLs further is needed.
A previous meta-analysis on TCMOLs combined with azithromycin for treating MPP in children focused only on a single TCMOL’s efficacy and safety (Zhu et al., 2019; Li et al., 2020; Zhang et al., 2020). Although its results could aid clinicians in making clinical decisions, it could not indicate optimal effects of various TCMOLs in clinical practice. Meta-analysis and network meta-analysis of TCM injections (TCMIs) combined with azithromycin in the treatment of MPP in children have also been published (Duan et al., 2019). Their results have some instructive significance for the clinical applications of TCMIs. However, the national policies and the patients’ willingness in China might somewhat limit the TCMIs’ utility in the real-life clinical settings. Therefore, the current study focused on comparing the clinical efficacy and safety of different TCMOLs to select the best interventions, which is helpful for further clinical practice.
The included TCMOLs (PDLXY, XEFRKC, XEXJZK, and SHL) are registered and approved for clinical use by State Food and Drug Administration. Because TCMOLs contains many TCM ingredients, the potential risk of impact in clinical applications may occur.
The safety and potential effects of high-frequency use of TCM ingredients (Scutellaria baicalensis and Isatis tinctoria) and possibly high-risk TCM ingredients (ephedra and areca nut) should be evaluated: I. tinctoria can cause allergic reactions and anaphylactoid reactions, even leading to urticaria, multiple granulomas, and hemolysis (Wang et al., 2012; Zhang, 2016). S. baicalensis can cause skin allergy (Lin et al., 2016); when used in large doses, it can cause maternal toxicity (Tian et al., 2009). Ephedrine alkaloids in ephedra can stimulate the central nervous system and sympathetic nervous system to produce adverse reactions, all of which can affect tissues including the myocardium, bladder smooth muscles, and external urethral sphincter (Odaguchi et al., 2019). In general, ephedra has many adverse events, including cardiovascular and cerebrovascular diseases (stroke, hypertension, palpitation, and heart attack), gastrointestinal diseases (nausea and vomiting), and mental disorders (anxiety) (Schulman, 2003). Areca nut not only has a carcinogenic, mutagenic effect but also has an effect on the reproductive system (Liu et al., 2013). In case of an overdose, the areca nut’s chemical components (alkaloids and tannins) can cause cardiotoxicity and neurotoxicity, even leading to death (Lin et al., 2018; Jia et al., 2020).
Due to a lack of professional knowledge, some patients think that TCM is safe, and thus, they use it uninterrupted over a long term and eventually overdose (Ernst, 2007). Excessive intake of TCM can not only cause an increase in adverse reactions but also affect the effectiveness of clinical treatment (Schulman, 2003). The incidence of adverse events and diseases related to TCM herbs has newly become a serious public problem; thus, cautious clinical use of TCMOLs is advised by experts and by various guidelines.
We used the Bayesian approach to compare various TCMOLs in combination with azithromycin for treating MPP in children. To select the best TCMOL-based interventions and generate a better guide for clinical decision-making based on TCM, we conducted a detailed literature review, which made the included literature more comprehensive and made our results more plausible. The inclusion and exclusion criteria were highly stringent. The interventions in all included studies involved azithromycin alone and in combination with TCMOLs—all reducing the effect of the potential heterogeneity and making the results more precise.
Here, we considered not only response rate but also cough, fever, and pulmonary rales disappearance times as primary outcomes. Because the response rate and other primary outcomes are strongly correlated, we drew a biplot to further improve our results’ accuracy. To eliminate the potential confounding factors and ensure the stability of our results, we performed meta-regression of five covariates that potentially affected the primary outcomes.
Although we applied no limitations on the study population and location, all the included studies were found to be conducted in China; this may have caused risk of bias.
There were also some deficiencies in the methodological quality of these studies. The random sequence generation of some studies was high-risk, and methods of the random sequence generation were not fully mentioned in most studies. Only two trials reported blinding, and none mentioned allocation concealment.
The small sample also made it difficult to detect significant differences between the treatment and control groups.
Regarding the secondary outcomes, few studies reported medical imaging (pulmonary shadows in x-ray) and laboratory testing (inflammatory cytokine and CRP levels). Thus, it was impossible to compare the superiority or inferiority of all the interventions.
There was no direct comparative study on various TCMOLs combined with azithromycin found. Therefore, we used Bayesian network meta-analysis to make the indirect comparison, which may have caused inconsistency between direct and indirect comparisons.
In conclusion, XEXJZK + Azithromycin, with the highest SUCRA value, may be the best treatment option for MPP in children. However, because of the low certainty of evidence, caution should be exercised when interpreting this result. Although the evidence of safety did not show the significant difference between TCMOLs + Azithromycin and azithromycin alone, the adverse effects remained unclear, mainly due to the lack of high-quality evidence. The dissemination of the evidence should also be cautioned due to the limitations. Finally, high-quality RCTs with focus on patient-important outcomes are thus required.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
ZC conceived and designed this study. ZC and QS cowrote the registry and manuscript. ZC and ZJ carried out data extraction independently. QS conducted the data analyses. LC and YP assessed the risk of bias. YC, LC, CL, BP, and JZ revised and reviewed the manuscript. All the authors participated in the writing process of the study.
Supported by Training Program of Innovation Team of Tianjin Higher Education Institution through Tianjin Municipal Education Commission (No. TD13-5047) and Special Support Plan for Talent Development of Tianjin—Young Top Talent Project (No. 201504).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.652412/full#supplementary-material
Biondi, E., McCulloh, R., Alverson, B., Klein, A., Dixon, A., and Ralston, S. (2014). Treatment of Mycoplasma Pneumonia: a Systematic Review. Pediatrics 133, 1081–1090. doi:10.1542/peds.2013-3729
Brignardello-Petersen, R., Bonner, A., Alexander, P. E., Siemieniuk, R. A., Furukawa, T. A., Rochwerg, B., et al. (2018). Advances in the GRADE Approach to Rate the Certainty in Estimates from a Network Meta-Analysis. J. Clin. Epidemiol. 93, 36–44. doi:10.1016/j.jclinepi.2017.10.005
Duan, X., Wang, K., Wu, J., Zhang, D., Liu, X., Ni, M., et al. (2019). Comparative Efficacy of Chinese Herbal Injections Combined with Azithromycin for Mycoplasma Pneumonia in Children: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Pharm. Ther. 44, 675–684. doi:10.1111/jcpt.12855
Ernst, E. (2007). Herbal Medicines: Balancing Benefits and Risks. Novartis Found. Symp. 282, 154–167. doi:10.1002/9780470319444.ch11
Flateau, C., Asfalou, I., Deman, A. L., Ficko, C., Andriamanantena, D., Fontan, E., et al. (2013). Aortic Thrombus and Multiple Embolisms during a Mycoplasma Pneumoniae Infection. Infection 41, 867–873. doi:10.1007/s15010-013-0475-2
Gao, Y., Wei, Y., Lin, H., and Sun, Y. Y. (2015). Analysis on Prescription Application of Xiao’er Feire Kechuan Oral Solution in Tianjin Children’s Hospital in September 2014. Drugs & Clinic 30, 322–325.
Gardiner, S. J., Gavranich, J. B., and Chang, A. B. (2015). Antibiotics for Community-Acquired Lower Respiratory Tract Infections Secondary to Mycoplasma Pneumoniae in Children. Cochrane Database Syst. Rev. 1, Cd004875. doi:10.1002/14651858.CD004875.pub5
Guo, S., Bao, L., Qu, T., Mao, X., Gao, Y., Xu, Y., et al. (2018). Ameliorative Effects of Infantile Feire Kechuan Oral Solution on Mycoplasma Pneumoniae Pneumonia in Infant Mouse and Rat Models. Evid. Based Complement. Alternat Med., 8139040. doi:10.1155/2018/8139040
Guyatt, G., Oxman, A. D., Akl, E. A., Kunz, R., Vist, G., Brozek, J., et al. (2011). GRADE Guidelines: 1. Introduction-GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 64, 383–394. doi:10.1016/j.jclinepi.2010.04.026
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 162, 777–784. doi:10.7326/m14-2385
Jain, S., Williams, D. J., Arnold, S. R., Ampofo, K., Bramley, A. M., Reed, C., et al. (2015). Community-acquired Pneumonia Requiring Hospitalization Among U.S. Children. N. Engl. J. Med. 372, 835–845. doi:10.1056/NEJMoa1405870
Jia, Z., Han, T., Lin, Q., Qu, W., Jia, T., Liu, M., et al. (2020). Toxicity and its Mechanism Study of Arecae Semen Aqueous Extract in Wistar Rats by UPLC-HDMS-Based Serum Metabolomics. Evid. Based Complement. Alternat Med. 2020, 2716325. doi:10.1155/2020/2716325
Jiang, Z. F., Shen, K. L., and Shen, Y. (2014). Zhu Futang Practice of Pediatrics. 8th edition. Beijing: People's Medical Publishing House, 1280–1282.
Korppi, M., Heiskanen-Kosma, T., and Kleemola, M. (2004). Incidence of Community-Acquired Pneumonia in Children Caused by Mycoplasma Pneumoniae: Serological Results of a Prospective, Population-Based Study in Primary Health Care. Respirology 9, 109–114. doi:10.1111/j.1440-1843.2003.00522.x
Kutty, P. K., Jain, S., Taylor, T. H., Bramley, A. M., Diaz, M. H., Ampofo, K., et al. (2019). Mycoplasma Pneumoniae Among Children Hospitalized with Community-Acquired Pneumonia. Clin. Infect. Dis. 68, 5–12. doi:10.1093/cid/ciy419
Lai, K. F. (2016). Guidelines for the Diagnosis and Treatment of Cough (2015). Chin. J. Tuberculosis Respir. Dis. 39, 323–354.
Langley, A., Anooshiravani, N., Kwan, S., Zeller, J., and Pope, E. (2016). Erythema Multiforme in Children and Mycoplasma Pneumoniae Aetiology. J. Cutan. Med. Surg. 20, 453–457. doi:10.1177/1203475416639018
Lee, H., Yun, K. W., Lee, H. J., and Choi, E. H. (2018). Antimicrobial Therapy of Macrolide-Resistant Mycoplasma Pneumoniae Pneumonia in Children. Expert Rev. Anti Infect. Ther. 16, 23–34. doi:10.1080/14787210.2018.1414599
Li, T. L., Pu, F. L., Wu, Y., Zhao, G. Z., Li, H. Z., Ma, Q. X., et al. (2020). Meta-analysis of Xiaoer Xiaoji Zhike Oral Liqud Combined with Azithromycin in Treatment of Mycoplasma Pneumonia in Children. China J. Chin. Materia Med. 45, 2193–2202. doi:10.19540/j.cnki.cjcmm.20200211.503
Li, X., Atkinson, T. P., Hagood, J., Makris, C., Duffy, L. B., and Waites, K. B. (2009). Emerging Macrolide Resistance in Mycoplasma Pneumoniae in Children: Detection and Characterization of Resistant Isolates. Pediatr. Infect. Dis. J. 28, 693–696. doi:10.1097/INF.0b013e31819e3f7a
Lin, H., Zhou, J., Lin, K., Wang, H., Liang, Z., Ren, X., et al. (2016). Efficacy of Scutellaria Baicalensis for the Treatment of Hand, Foot, and Mouth Disease Associated with Encephalitis in Patients Infected with EV71: A Multicenter, Retrospective Analysis. Biomed. Res. Int. 2016, 5697571. doi:10.1155/2016/5697571
Lin, Q., Jia, Z., Xu, X., Xu, S., Han, T., Gao, Y., et al. (2018). Sub-chronic Toxicity Study of Arecae Semen Aqueous Extract in Wistar Rats. J. Ethnopharmacol 215, 176–183. doi:10.1016/j.jep.2017.08.031
Liu, D. J., Zheng, B., Cai, B. H., Zhou, W. M., and Yu, B. X. (2010). Traditional Chinese and Western Medicine Treatment of Mycoplasmal Pneumonia in Children and the Serum Cytokine Changes. Nan Fang Yi Ke Da Xue Xue Bao 30, 626–627.
Liu, D. L., Wang, X. Y., Yang, B., and Zhnag, H. (2013). Review of Pharmacological Effects and Toxicological Information of Arecae Semen. Zhongguo Zhong Yao Za Zhi 38, 2273–2275.
Lu, Q., and Zhang, H. (2020). Expert Consensus on Laboratory Diagnostics and Clinical Practice of Mycoplasma Pneumoniae Infection in Children in China (2019). Chin. J. Pediatr., 366–373. doi:10.3760/cma.j.cn112140-20200304-00176
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Ann. Intern. Med. 151, 264–269. doi:10.7326/0003-4819-151-4-200908180-00135
Narita, M. (2010). Pathogenesis of Extrapulmonary Manifestations of Mycoplasma Pneumoniae Infection with Special Reference to Pneumonia. J. Infect. Chemother. 16, 162–169. doi:10.1007/s10156-010-0044-x
Narita, M. (2009). Pathogenesis of Neurologic Manifestations of Mycoplasma Pneumoniae Infection. Pediatr. Neurol. 41, 159–166. doi:10.1016/j.pediatrneurol.2009.04.012
Ni, X. (2019). Guidelines for Diagnosis and Treatment of Community-Acquired Pneumonia in Children (2019 Version). Chin. J. Clin. Infect. Dis. 12, 6–13.
Odaguchi, H., Hyuga, S., Sekine, M., Nakamori, S., Takemoto, H., Huang, X., et al. (2019). The Adverse Effects of Ephedra Herb and the Safety of Ephedrine Alkaloids-free Ephedra Herb Extract (EFE). Yakugaku Zasshi 139, 1417–1425. doi:10.1248/yakushi.19-00122
Pereyre, S., Goret, J., and Bébéar, C. (2016). Mycoplasma Pneumoniae: Current Knowledge on Macrolide Resistance and Treatment. Front. Microbiol. 7, 974. doi:10.3389/fmicb.2016.00974
Principi, N., Esposito, S., Blasi, F., and Allegra, L. (2001). Role of Mycoplasma Pneumoniae and Chlamydia Pneumoniae in Children with Community-Acquired Lower Respiratory Tract Infections. Clin. Infect. Dis. 32, 1281–1289. doi:10.1086/319981
Puhan, M. A., Schünemann, H. J., Murad, M. H., Li, T., Brignardello-Petersen, R., Singh, J. A., et al. (2014). A GRADE Working Group Approach for Rating the Quality of Treatment Effect Estimates from Network Meta-Analysis. Bmj 349, g5630. doi:10.1136/bmj.g5630
Razin, S., Yogev, D., and Naot, Y. (1998). Molecular Biology and Pathogenicity of Mycoplasmas. Microbiol. Mol. Biol. Rev. 62, 1094–1156. doi:10.1128/mmbr.62.4.1094-1156.1998
Ren, P. Z., Yang, W., Liu, H., Zeng, X. B., Zhuang, Y., et al. (2016). Analysis of Chinese and Western Medicine Characteristics in Patients with Pneumonia Based on Hospital Information System Data. Mod. Chin. Clin. Med. 23, 34–39.
Schulman, S. (2003). Addressing the Potential Risks Associated with Ephedra Use: a Review of Recent Efforts. Public Health Rep. 118, 487–492. doi:10.1093/phr/118.6.48710.1016/s0033-3549(04)50284-4
Shah, S. S., Test, M., Sheffler-Collins, S., Weiss, A. K., and Hall, M. (2012). Macrolide Therapy and Outcomes in a Multicenter Cohort of Children Hospitalized with Mycoplasma Pneumoniae Pneumonia. J. Hosp. Med. 7, 311–317. doi:10.1002/jhm.1904
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. Bmj 350, g7647. doi:10.1136/bmj.g7647
Shan, L. S., Liu, X., Kang, X. Y., Wang, F., Han, X. H., and Shang, Y. X. (2017). Effects of Methylprednisolone or Immunoglobulin when Added to Standard Treatment with Intravenous Azithromycin for Refractory Mycoplasma Pneumoniae Pneumonia in Children. World J. Pediatr. 13, 321–327. doi:10.1007/s12519-017-0014-9
Shi, L., Wu, Q. G., Zhang, J. C., Yang, G. M., Liu, W., and Wang, Z. F. (2020). Mechanism of Shuang-Huang-Lian Oral Liquid for Treatment of Mycoplasmal Pneumonia in Children on Network Pharmacology. Comb. Chem. High Throughput Screen. 23, 955–971. doi:10.2174/1386207323666200514073428
Shi, M. L., Wu, G. L., Xue, D., Ma, Y. J., Liu, J. Y., and Wang, L. (2019). Investigation and Analysis of the Current Situation of Chinese Patent Medicines and Combined Use of Chinese and Western Medicines in Children with Upper Respiratory Tract Infection. Chin. Remedies Clin. 19, 2280–2281.
Smith, C., Egunsola, O., Choonara, I., Kotecha, S., Jacqz-Aigrain, E., and Sammons, H. (2015). Use and Safety of Azithromycin in Neonates: a Systematic Review. BMJ Open 5, e008194. doi:10.1136/bmjopen-2015-008194
Sun, J. H., Sun, F., Yan, B., Li, J. Y., and Xin, L. (2020). Data Mining and Systematic Pharmacology to Reveal the Mechanisms of Traditional Chinese Medicine in Mycoplasma Pneumoniae Pneumonia Treatment. Biomed. Pharmacother. 125, 109900. doi:10.1016/j.biopha.2020.109900
Tian, X. Y., Cheung, L. M., Leung, K., Qi, C., Deng, B., Deng, P. X., et al. (2009). The Effects of Scutellaria Baicalensis Extract on Embryonic Development in Mice. Birth Defects Res. B Dev. Reprod. Toxicol. 86, 79–84. doi:10.1002/bdrb.20186
Waites, K. B., Crabb, D. M., and Duffy, L. B. (2009). Comparative In Vitro Susceptibilities of Human Mycoplasmas and Ureaplasmas to a New Investigational Ketolide, CEM-101. Antimicrob. Agents Chemother. 53, 2139–2141. doi:10.1128/aac.00090-09
Wang, J., and Yang, C. (2018). Clinical Effect of Sequential Therapy with Azithromycin in Children Mycoplasma Pneumoniae Pneumonia. Pak J. Pharm. Sci. 31, 1649–1652.
Wang, Z., Wang, D., Sui, Y., Cui, H., and Yu, Y. (2012). Experimental Study on Anaphylaxis of Qingkailing Injection and its Components on Beagle Dogs. J. Tradit Chin. Med. 32, 641–645. doi:10.1016/s0254-6272(13)60085-0
Yang, A. J., Xi, K., Yao, Y. Q., Ding, Y. X., Yang, L. J., and Cui, H. (2017). Effect of Qingfei Mixture () on Pediatric Mycoplasma Pneumoniae Pneumonia with Phlegm Heat Obstructing Fei (Lung) Syndrome. Chin. J. Integr. Med. 23, 681–688. doi:10.1007/s11655-017-2409-9
Yang, D., Chen, L., and Chen, Z. (2018). The Timing of Azithromycin Treatment Is Not Associated with the Clinical Prognosis of Childhood Mycoplasma Pneumoniae Pneumonia in High Macrolide-Resistant Prevalence Settings. PLoS One 13, e0191951. doi:10.1371/journal.pone.0191951
Zhang, G. M., Huang, Z. Y., Sun, R., Ye, S. L., and Feng, Q. (2020). Xiao'er Xiaoji Zhike Oral Liquid Combined with Azithromycin for Mycoplasma Pneumoniae Pneumonia in Children: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat Med. 2020, 9740841. doi:10.1155/2020/9740841
Zhang, Y. W. (2016). Analysis of Pharmacological Effects and Adverse Reactions of Radix Isatidis. Cardiovasc. Dis. J. integrated traditional Chin. West. Med. 4, 95–96. doi:10.16282/j.cnki.cn11-9336/r.2016.15.067
Zhu, Y. F., Yang, Y. F., Xie, W. W., Lin, G. H., and Chen, L. (2019). Pharmacoeconomic Evaluation of Xiaoer Feire Kechuan Oral Liquid Combined with Azithromycin Compared with Xiaoer Xiaoji Zhike Oral Liquid Combined with Azithromyxin for Mycoplasma Pneumonia in Children. Chin. J. New Drugs 28, 1913–1920.
Keywords: Mycoplasma pneumoniae pneumonia in children, traditional Chinese medicine oral liquids, Azithromycin, network meta-analysis, randomized controlled trial
Citation: Chen Z, Shi Q, Peng Y, Chen Y, Cao L, Pang B, Ji Z, Liu C and Zhang J (2021) Traditional Chinese Medicine Oral Liquids Combined With Azithromycin for Mycoplasma pneumoniae Pneumonia in Children: A Bayesian Network Meta-Analysis. Front. Pharmacol. 12:652412. doi: 10.3389/fphar.2021.652412
Received: 15 January 2021; Accepted: 14 May 2021;
Published: 28 May 2021.
Edited by:
Ke-Wu Zeng, Peking University Health Science Center, ChinaReviewed by:
Jiarui Wu, Beijing University of Chinese Medicine, ChinaCopyright © 2021 Chen, Shi, Peng, Chen, Cao, Pang, Ji, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxiang Liu, bGl1Y2h1bnhpYW5nZWJtQGZveG1haWwuY29t; Junhua Zhang, empodGNtQGZveG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.