- 1Department of Dermatology, The 305 Hospital of PLA, Xicheng, China
- 2Capital Medical University, Beijing Chaoyang Hospital, Beijing, China
Introduction: The population of young women who suffered from female pattern hair loss (FPHL) or female androgenic alopecia (AGA) is gradually increasing. Platelet-rich plasma is a novel and promising therapeutic method as a nonsurgical treatment for FPHL.
Objective: To summarize different preparation methods of PRP and treatment regimes in FPHL, qualitatively evaluate the current observations, and quantitively analyze the efficacy of PRP in FPHL treatment.
Methods: Six databases, MEDLINE, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials, LILACS, and CNKI, were searched with terms “platelet-rich plasma,” synonyms for AGA and FPHL. Meta-analysis was conducted with enrolled observational studies and randomized controlled trials separately.
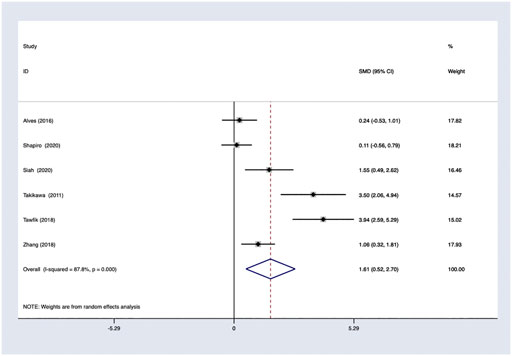
Results: We evaluated 636 studies and 12 trials from all searched databases. A total of 42 studies of 1,569 cases, including 776 female participants covering 16 randomized controlled trials and 26 observational trials, were included for qualitative synthesis study and systematic review. PRP showed positive efficacy in treating FPHL in hair density compared to the control groups with odds ratio (OR) 1.61, 95% CI 0.52–2.70, and compared to baseline with OR 1.11, 95% CI 0.86–1.37.
Conclusion: PRP showed excellent efficiency as a novel therapy of FPHL through hair density evaluation. Further studies are needed to define standardized protocols, and large-scale randomized trials still need to be conducted to confirm its efficacy.
Introduction
Female pattern hair loss (FPHL), with an alternative name female androgenic alopecia (AGA), is the most common type of hair loss affecting 6.0% of Chinese women, among which 3.6% were under 40-year-old (Wang et al., 2010). FPHL is characterized by progressive follicular miniaturization and the accompanying conversion of terminal follicles into vellus-like follicles (Trueb, 2002). Patients may present with hair density reduction, hair thinning, and widening of the area especially on the center of the scalp (Olsen, 2001). Those changes may lead to serious psychological impacts (van Zuuren et al., 2012) on one’s self-esteem, interpersonal relationships, and the social status (Cash, 2001). Although multiple nonsurgical therapeutic methods like topical minoxidil, oral finasteride, and low-level laser comb had been introduced to FPHL treatment, more large-scale–randomized controlled trials still need to be investigated to confirm their efficacy (van Zuuren et al., 2016). Despite decreased number of more actively proliferating progenitor cells, the current studies have presented an unaltered number of hair follicle stem cells in a hair loss scalp (Garza et al., 2011). Hence the application of autologous stem cells including autologous micro-grafts enriched of human follicle cells (HF-MSCs) as well as platelet-rich plasma has been explored and gradually been introduced to clinical use (Gentile et al., 2019).
Platelet-rich plasma (PRP) is a preparation of an enriched platelet autologous plasma in which the concentration of platelet is above normal contained in the whole blood (Wu et al., 2016). Platelets are able to secrete growth factors, adhesion molecules, and chemokines. After being activated, those effective factors interact with the local environment and promote cell differentiation, proliferation, and regeneration (Weibrich et al., 2002; Davì and Patrono, 2007; Sánchez-González et al., 2012). Published data also highlighted that PRP contains major growth factors, including basic fibroblast growth factor (bFCF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), transforming growth factor-β (TGF-β), and insulin-like growth factor-1 (IGF-1) (Cervelli et al., 2012). In the past decade, PRP has been studied and widely used in alopecia, acne scarring, skin rejuvenation, chronic wounds, and vitiligo (Hesseler and Shyam, 2019). Comparing with traditional nonsurgical therapies and the surgical approaches such as hair transplantation, PRP is believed as a promising treatment of AGA with lower cost and fewer adverse effects. However, only a limited number of publications focus on the utilization of PRP in AGA treatment, and few studies or reviews about the application of PRP in FPHL have been performed.
In this study, we summarized different PRP preparation methods, reviewed the various treatment regime in FPHL that are reported in the previous studies, qualitatively evaluated the current observations, and quantitively analyzed the efficacy of PRP in FPHL treatment. This work may offer a reference to the clinical workers and associated FPHL patients.
Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (http://www.prisma-statement.org) and Meta-analysis Of Observation Studies in Epidemiology (MOOSE) statement (Stroup et al., 2000).
Literature Screening
Two investigators independently conducted a systematic search of studies published before October 29, 2020, using the databases MEDLINE via PubMed, embase, Web of Science via Ovid, Cochrane Central Register of Controlled Trials (CENTRAL), LILACS, and CNKI. The strategy of search terms was composed of at least one term from two search blocks: the term “platelet-rich plasma” and synonyms for androgenic alopecia (AGA) and female pattern hair loss (FPHL). All synonyms were found based on MESH and ENTRÉE (Supplementary Material S1).
Study Selection
Original studies include observational studies (i.e., case series, cross-sectional, case-control, and cohort) and randomized trials of platelet-rich plasma (PRP), and AGA in woman patients in English, German, Swedish, Norwegian, Spanish, Danish, Turkish, and Chinese were all eligible for inclusion. Exclusion criteria were reviews, studies only included male patients, abstracts, unpublished studies, and lack of raw data. Conference reports were also excluded for insufficient details for analysis. Two reviewers screened the titles and abstracts of the identified studies. If the information provided in the abstracts was not sufficient to access the eligibility, a full-text evaluation was conducted. All observational studies and randomized trials will be recruited for qualitative analysis, and only randomized controlled trials will be analyzed quantitively and enrolled in the meta-analysis in this work. Two authors also evaluated the quality of the included studies independently. Any disagreement was resolved through discussion or decided by a third person.
Data Extraction
Two reviewers completed the data extraction work independently with the following information: author, year of publication, journal name, country, methods of PRP preparation, treatment regime, and the number of cases/controls (total, exposed, nonexposed). In case of disagreement, a third person conducted a further assessment.
Endpoint Definition
The efficacy of PRP was evaluated by an increase in hair density, increment of hair count, improvement in the hair-pull test, satisfaction of patients from the questionaries, and changes of hair thickness compared with photos taken before and after the treatment sections. Given that various test methods were taken through the studies we included, only the most widely used methods would be set at the endpoints for all-pooled studies.
To access the safety of PRP, all-side effects, including local injection pain, headache, increasing scalp sensitivity, and any allergic effects, would be recorded.
Data Analysis
We conducted the meta-analysis with Stata statistical software 15.0 (Metrika Consulting, Stockholm, Sweden) using the “metan” command. A random-effect analysis with the method of Dorsmanin and Laird (Higgins et al., 2020) was taken, given the considerable heterogeneity. We chose 95% confidence intervals (CI) and I (Trueb, 2002) to access statistical heterogeneity (Higgins et al., 2003; Cook and Reed, 2015): 30–60% as moderate heterogeneity, 50–90% substantial heterogeneity, greater than 75% considerable heterogeneity (Higgins et al., 2020). Funnel plots and Egger tests were performed for publication bias assessment with asymmetry in the funnel plot and a p-value that was less than 0.1. Eggers’s test would overrule visual inspection if results diverged between the two methods above.
Clinical heterogeneity was measured by stratification, for example, subgroup analysis of the study types, i.e., randomized controlled trials and observational trials. The observational trials were further stratified into case series, cohort studies, cross-sessional studies, and case-control studies. We also conducted a subgroup analysis based on different endpoint definitions, e.g., hair density, hair count, and increment in hair density. Subgroup analysis with different PRP preparation methods and PRP concentrations were only performed with sufficient data.
The quality of each study was evaluated based on Joanna Briggs Institute Critical Appraisal tools for case-series studies (Munn et al., 2020), Newcastle-Ottawa Scale (Stang, 2010) for cohort studies and case-control studies, and Cochrane Risk of Bias Tool for Randomized Controlled Trials (Higgins et al., 2011). Three groups of study quality were defined: high, middle, and low. Rovis was used for the presentation of the quality of each study (McGuinness and Higgins, 2020).
Results
Literature Search
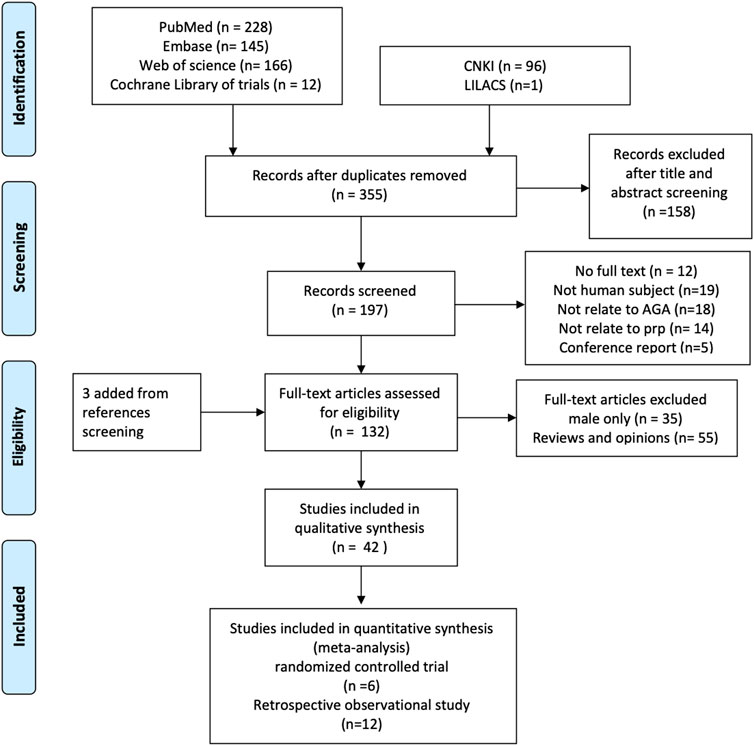
We found 636 literature and 12 trials from databases and three additional references by reference screening. Six hundred and three studies were excluded for reasons including duplicated, male patients only, and not in human subjects (Figure 1).
Although only the PRP effect on a female would be evaluated in this study and given only a few pure women studies were found, studies involved in both female and male patients would also be pooled in this work. A total of 42 studies, 1,569 cases including 776 female participants covering 16 randomized controlled trials and 26 observational trials, were included for qualitative synthesis study and systematic review.
Study Characters
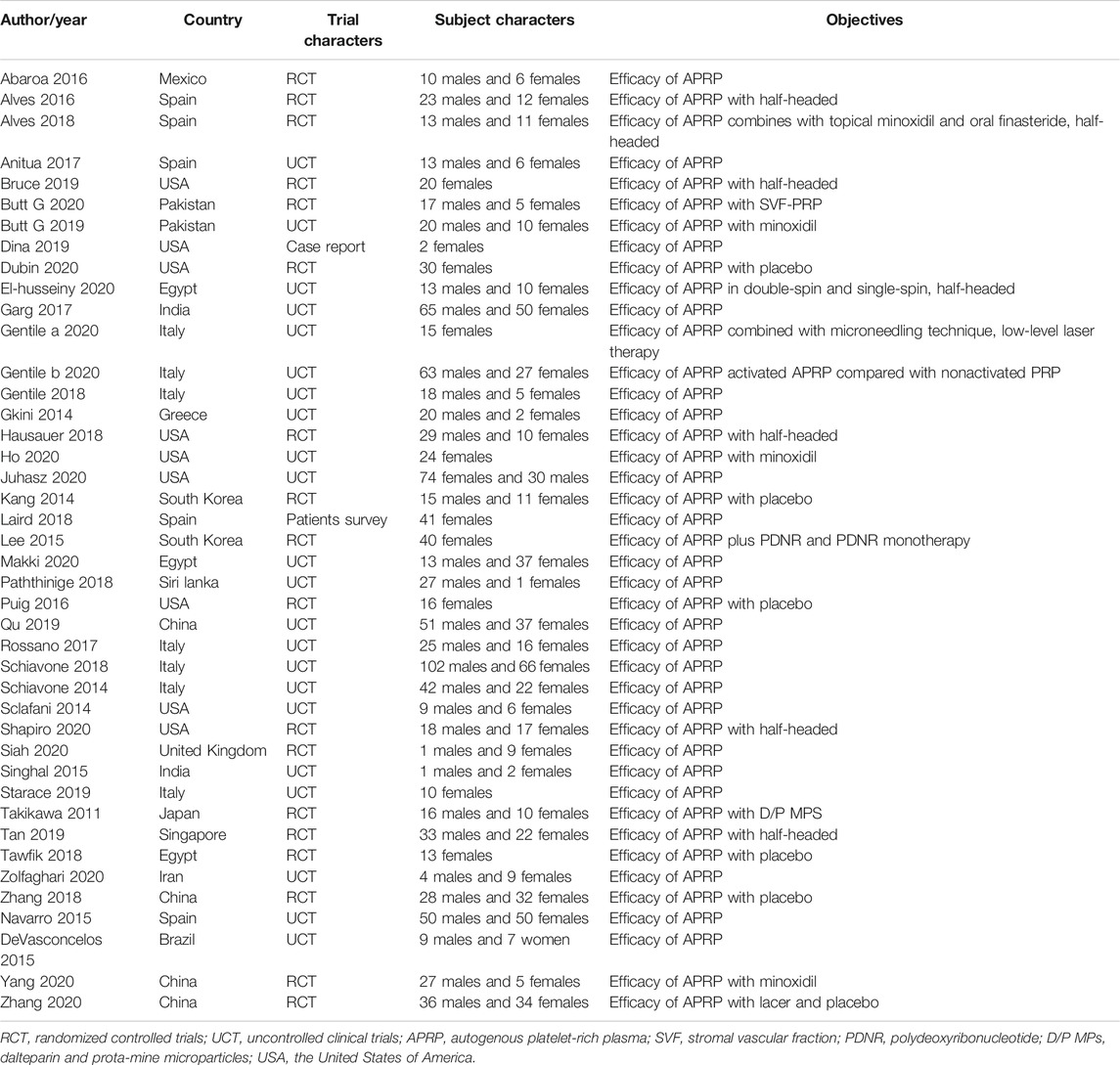
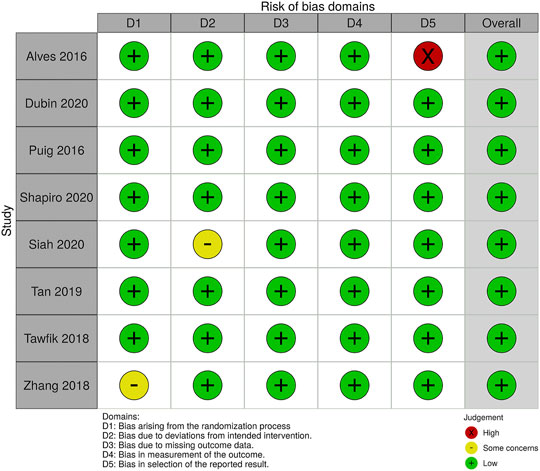
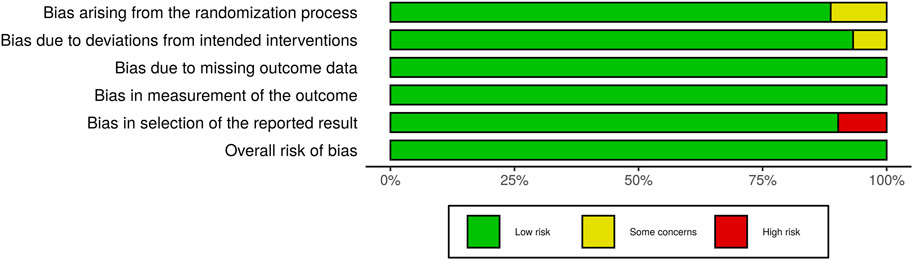
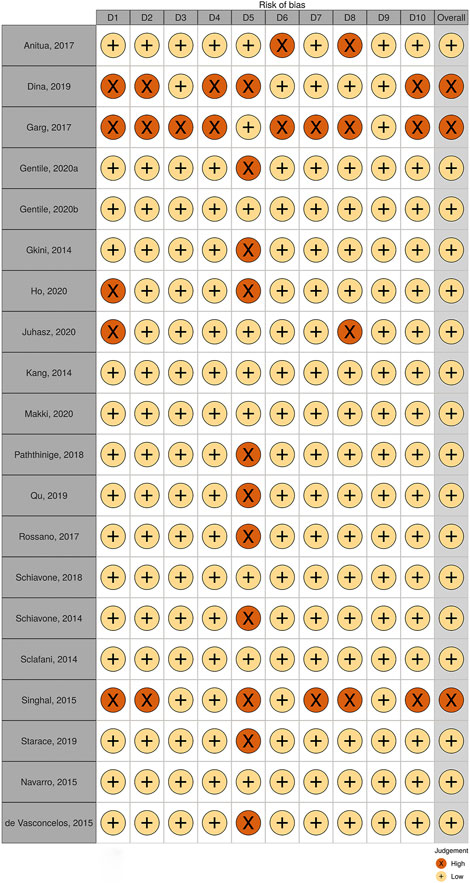
Studies with various group settings were enrolled (Table 1). Eight out of 16 randomized controlled trials investigated the effect of PRP comparing with placebo, in which 6 of which were done in half-headed comparation. Four studies evaluated the differences of the effect of PRP combination therapy with topical minoxidil, oral finasteride, laser, and polydeoxyribonucleotide (PDRN), while the other four accessed the different efficacy from treating strategy, i.e., PRP concentration, centrifugation protocol, treatment sessions, and treatment doses. The risk of bias of all-eight–randomized placebo-controlled trials was graded by two reviewers (Figures 2, 3).
Observation studies including 21 case series, 2 case reports, one patient survey, one treatment protocol, and one treatment experience were enrolled, while one case series containing only one female patient was abandoned. Quality of the total 20 case series was accessed, three of which were excluded for high risk after evaluation (Figure 4).
Study Subjects
Among all 42 studies, we only found seven studies that recruited only female participants. The mean age of the total-enrolled patients was above 18 years old and between 25 and 35 years old. Most female patients had a history of AGA for at least 3–5 years with type I to III FPHL on Ludwig scale (Harrison, 2017) or 1–5 on Sinclair score system (Kasprzak et al., 2019). Patients who had previous hair transplantation, a drug-taking history that could cause hair loss or any inflammations, scars, or erythema on the scale were excluded. Laboratory tests, including hemoglobin, platelet count, serum ferritin, liver function, thyroid function, and female hormone profile, were checked for excluding any other autoimmune or systematic diseases that could cause hair loss. Acute or chronic infections should also be tested before using PRP.
Platelet-Rich Plasma Preparation
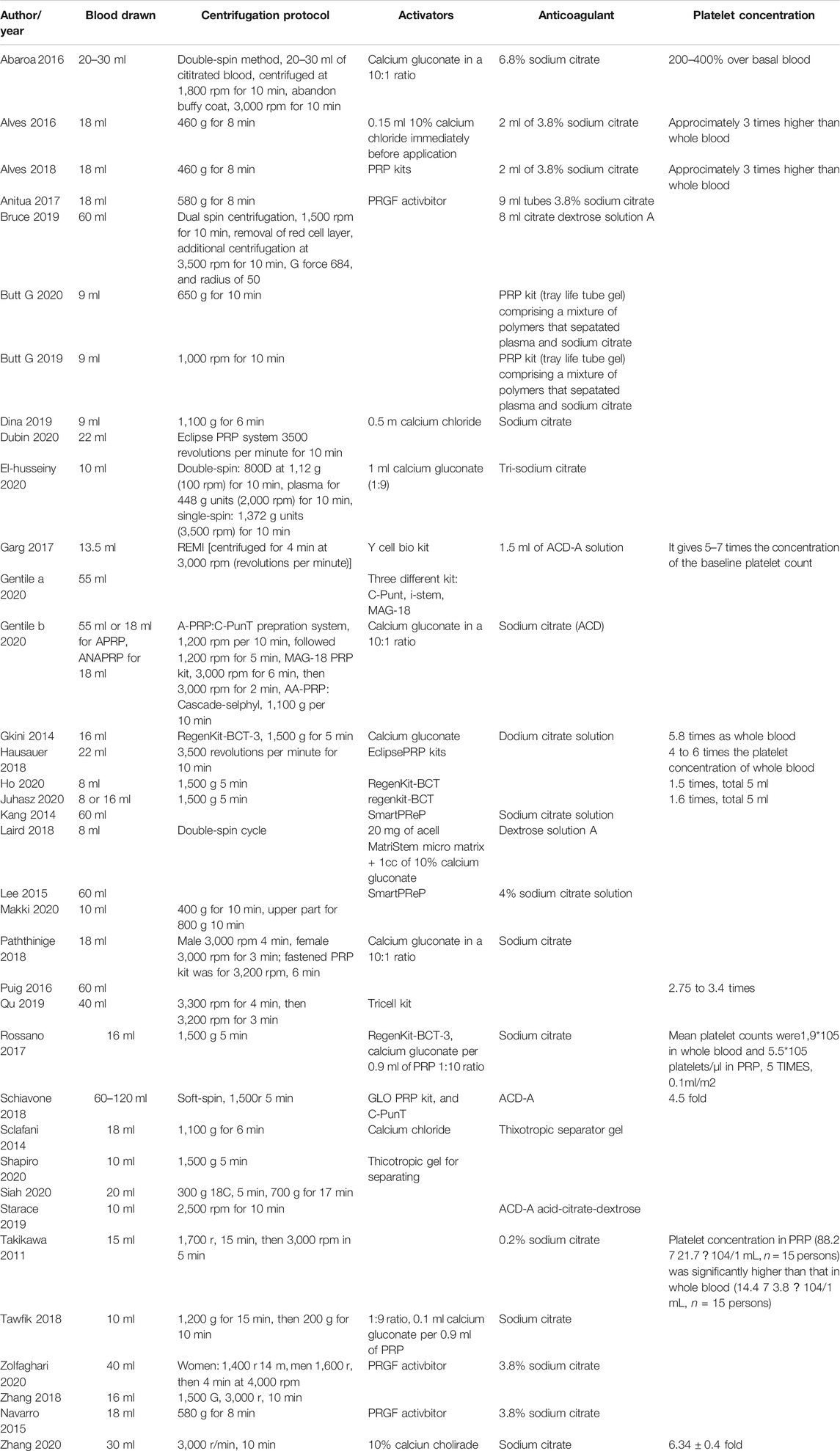
The methods of PRP preparation vary in studies (Table 2). PRP kits were commonly used from a different company and centrifugation protocol. The choices of activators and anticoagulation vary depending on PRP kits and study purposes. In all-enrolled studies, calcium gluconate in a 10:1 ratio and sodium citrate were mainly added as activators and anticoagulants, respectively. Moreover, platelet concentration differs from 1.5 times to seven-fold as whole blood according to the PRP preparation protocols.
Treatment Strategy
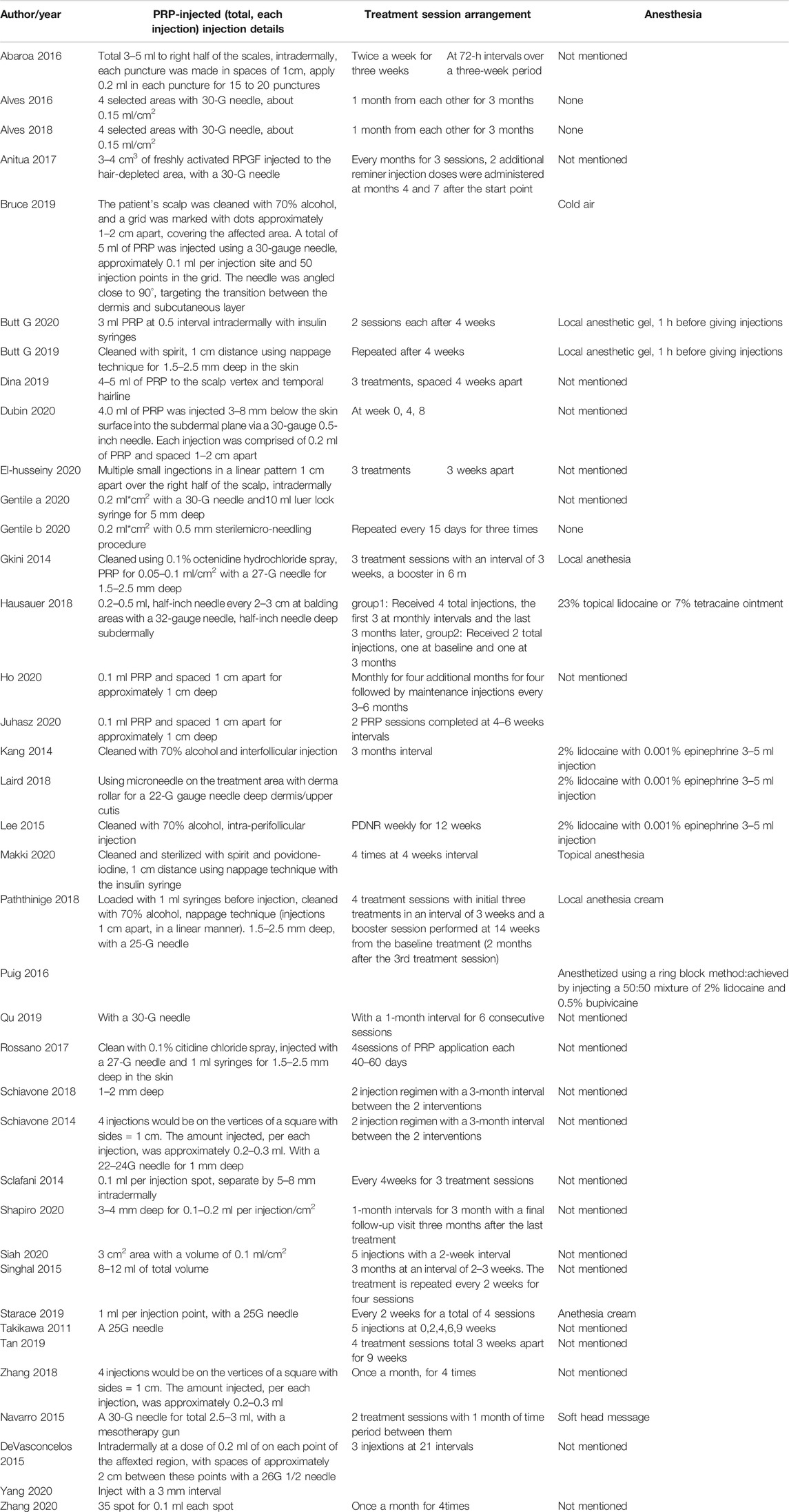
Despite all studies performed intradermal injections on the local alopecia area for AGA treatment, the whole procedure diverged (Table 3). 70% alcohol, spirit, or 0.1% octenidine hydrochloride spray were used for cleaning the local skin, and local anesthesia with the help of 2% lidocaine with 0.001% epinephrine were commonly used. two studies used anesthesia cream, one used soft head message, and one conducted cold air anesthesia before injections.
The majority of studies selected 22- to 30-G gauge needles with insulin syringes to perform the procedure, while sterilemicro-needling and DHN1 mesotherapy gun were also used in independent studies. Nappage technique (Modarressi, 2013) was taken by most studies with 1 to 3 cm distance between each injection point. 1cc PRP was injected within each grided injection point. The depth of intradermal injections was approximately 1.5–2.5 mm deep, but 0.5 mm deep for the sterilemicro-needling procedure. Intrafollicular injections and intra-perifollicular injections were also carried in several studies.
For treatment schedules, normally 3–5 treatment sessions with 4–6 intervals were performed. And the time of follow-up was commonly from 12 weeks to 9 months depending on the growth period of hairs (Lee et al., 2020).
Outcome Evaluation Methods and Adverse Effects
In addition to Ludwig and Sinclair scale, endpoints evaluation methods included biopsy with ki-67 immunochemistry stain, photographic evaluation, hair density, hair count, global photographs, and phototrichogram analysis (Ueki et al., 2003), physician global assessment score (PhGAS), patient global assessment score (PaGAS), and pull test. The satisfaction questionnaires and scales were taken from the perspective of patients and other observers were also used to evaluate the efficacy of PRP in some of the recruited studies (Table 4).
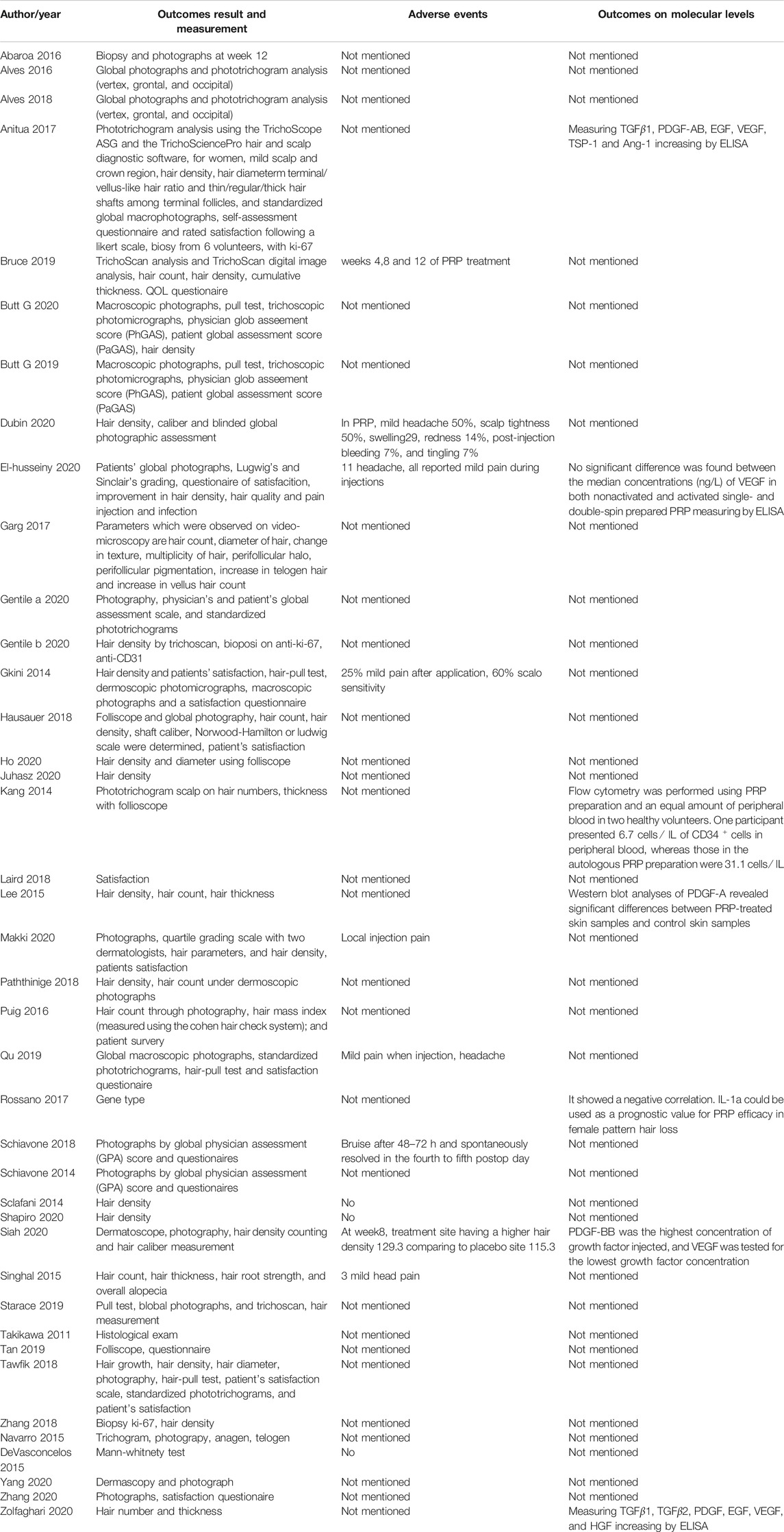
TABLE 4. Outcomes measurement and adverse events in enrolled studies, and the evaluation of growth factors.
All-included studies showed positive responses and an improvement compared with the baseline; a few pooled patients reported adverse effects, including bruise and mild pain on injection sites after 48–72 h, which would resolve spontaneously in the 4th or 5th postoperation day. Mild headache and scalp sensitivity were also reported in a few studies.
Labs
Apart from clinical evaluation, 7 out of 42 studies also did an assessment on platelet-rich growth factors (PRGF) (Table 4). 6 out of 7 focused on hair follicles regenerative factors including VEGF, PDGF, IGF, TGF-β, and HGF through enzyme-linked immunosorbent assay (ELISA), Western blot for mRNA expression, flow cytometry on CD34+, and animal models. One study reveals a negative correlation between individual genetic inflammatory profile and efficacy of PRP, which was different from PRP in males (Rossano et al., 2017).
Subgroup Meta-Analysis
PRP efficacy compared to placebo. Only endpoints evaluated in the same measurement methods would be pooled in the meta-analysis. Using the random-effects model, PRP showed positive efficacy in the treatment of FPHL in hair density comparing to the control groups with odds ratio [OR] 1.61; 95% CI 0.52–2.70 (Figure 5).
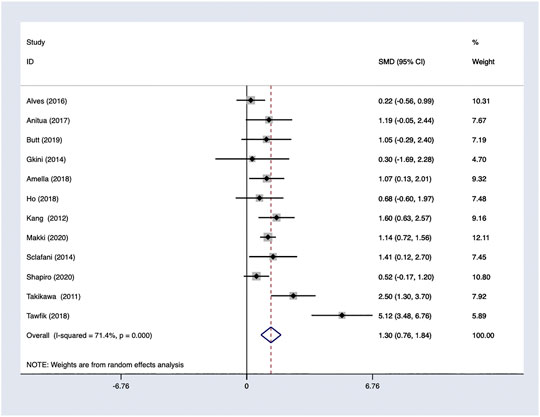
PRP efficacy compared to the baseline. Only studies with high quality would be pooled in the analysis. Using a random-effects model, PRP showed effectiveness and improvements on hair density comparing to baseline with OR 1.11; 95% CI 0.86–1.37 (Figure 6).
Meta-analysis with other evaluation methods was not conducted due to limited studies.
Heterogeneity
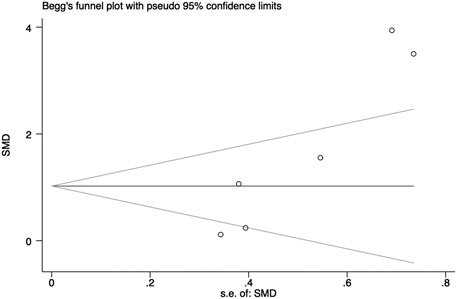
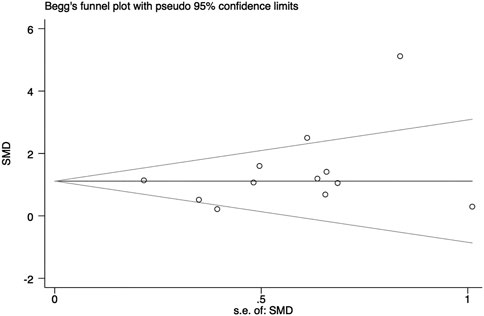
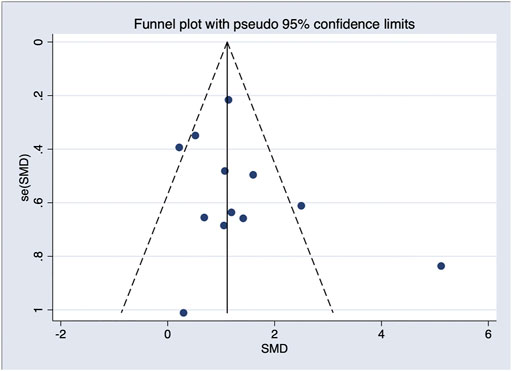
In general, a meta-analysis of PRP efficacy comparing to the placebo had considerable statistical heterogeneity (I (Trueb, 2002) >75%), while a smaller substantial heterogeneity exists when comparing to the baseline. Visual inspection of funnel plots and Eggers tests were consistently in agreement with publication bias (Figure 7). Funnel plot that comparing with placebo suggested a tendency toward lack of large studies with both positive and negative results, two of the enrolled small studies showed little association (Figure 8). For the funnel plot in the baseline of the comparison group, a tendency toward a lack of small studies with negative and positive results was indicated (Figure 9).
Discussion
Our study demonstrated an effective response and relative safety for the application of PRP in the treatment of FPHL with the meta-analysis for RCT and observation studies. Especially for those patients with negative response to the tropical use of minoxidil, PRP offered an alternative treatment option. We only used hair density as an endpoint to perform meta-analysis for the diversity of endpoint settings among the enrolled studies, while hair density is the most commonly used one. This result is in accordance with previous systemic review (Torabi et al., 2020).
The major strengths of the study suggested a positive efficacy of PRP for female AGA and elucidated different PRP preparing methods and treatment regimes. Although there were several systemic reviews (Torabi et al., 2020) and meta-analysis on the application of PRP in AGA treatment (Evans et al., 2020), one mainly focus on female patients remain scarce.
In the author’s opinion, the need for larger scale randomized–controlled trials and extensive meta-analysis precedes a considerable heterogeneity challenge. Heterogeneity anticipated was mainly because of female’s nationalities and races, the difference treatment regime, PRP preparation methods, injection details, and PRP concentration. Subgroup analysis according to the different patient’s races and nationalities were not included in this study for the limited-published data and only a few studies included races information. Lower heterogeneity showed when studies of low quality were abandoned suggesting methodological heterogeneity contributed. Although efforts had been made to accommodate this issue, limited number of studies just based on women patients impeded methods like subgroup analysis, which resulted in a challenge to interpret the efficacy of PRP on female patients. As some studies measured patients of both gender but without separated data for female patients, we cannot prove strong evidence for the treating efficacy.
Selection bias is possibly addressed by group settings among studies. The prevalence of AGA is known to vary from the races (Paik et al., 2001; Gan and Sinclair, 2005; Khumalo et al., 2007). Enrolled studies were conducted by different countries, while all-pooled female participants were diverse in races. Subgroups regarding to ethnicity were not included in this study for the limited-enrolled studies. Whether divergences exist in the efficacy of PRP through races remain unclear. Besides, the majority of randomized studies were set in half-headed. Patients received PRP on half-scalp and placebo on the other half. Both injected spots showed improvement of hair growth or hair density. Although PRP-injected site showed a more obvious effect, the improvement of hair density on the placebo-injected sites may result in a smaller difference between PRP and placebo. Besides, whether PRP had a growth effect on the opposite side of the scalp remains obscure.
In aggregate, our study showed an efficacy of PRP in the therapy of FPHL through hair density evaluation. Although the mechanism of action on hair follicles is still under debate, it has proved to be a promising option for FPHL treatment. Given that current treatments differ from methodology and treatment technique, further studies are needed to define standardized protocols and large-scale–randomized trials still need to be conducted to confirm its efficacy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
SZ and FQ contributed equally to this work. SZ and FQ did the conceptualization, CZ and YH screening the literatures, FQ and YG evaluated the quality of the included studies, SZ made the decision when any disagreement occurs. SZ and XY extracted the data. FQ and CZ did the meta-analysis and formal analysis, SZ and FQ wrote the original draft, SZ and YH reviewed and editing the draft. YH and CZ did the supervision of the whole work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.642980/full#supplementary-material.
References
Cash, T. F. (2001). The psychology of hair loss and its implications for patient care. Clin. Dermatol. 19 (2), 161–166. doi:10.1016/s0738-081x(00)00127-9
Cervelli, V., Scioli, M. G., Gentile, P., Doldo, E., Bonanno, E., Spagnoli, L. G., et al. (2012). Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cell Transl. Med. Mar. 1 (3), 206–220. doi:10.5966/sctm.2011-0052
Cook, D. A., and Reed, D. A. (2015). Appraising the quality of medical education research methods. Acad. Med. 90 (8), 1067–1076. doi:10.1097/ACM.0000000000000786
Davì, G., and Patrono, C. (2007). Platelet activation and atherothrombosis. N. Engl. J. Med. 357:(24), 2482–2494. doi:10.1056/NEJMra071014
Evans, A. G., Mwangi, J. M., Pope, R. W., Ivanic, M. G., Botros, M. A., Glassman, G. E., et al. (2020). Platelet-rich plasma as a therapy for androgenic alopecia: a systematic review and meta-analysis. J. Dermatol. Treat. 26, 1–14. doi:10.1080/09546634.2020.1770171
Gan, D. C. C., and Sinclair, R. D. (2005). Prevalence of male and female pattern hair loss in Maryborough. J. Invest. Dermatol. Symp. Proc. 10 (3), 184–189. doi:10.1111/j.1087-0024.2005.10102.x
Garza, L. A., Yang, C.-C., Zhao, T., Blatt, H. B., Lee, M., He, H., et al. (2011). Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J. Clin. Invest. 121 (2), 613–622. doi:10.1172/JCI44478
Gentile, P., Scioli, M. G., Bielli, A., De Angelis, B., De Sio, C., De Fazio, D., et al. (2019). Platelet-rich plasma and micrografts enriched with autologous human follicle mesenchymal stem cells improve hair re-growth in androgenetic alopecia. biomolecular pathway analysis and clinical evaluation. Biomedicines 7 (2), 27. doi:10.3390/biomedicines7020027
Harrison, J. (2017). Advances in aesthetic therapies: plasma-rich protein procedure for the treatment of alopecia. Plast. Surg. Nurs. 37 (2), 52–55. doi:10.1097/psn.0000000000000182
Hesseler, M. J., and Shyam, N. (2019). Platelet-rich plasma and its utility in medical dermatology: a systematic review. J. Am. Acad. Dermatol. 81 (3), 834–846. doi:10.1016/j.jaad.2019.04.037
Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2020). Cochrane handbook for systematic reviews of interventions. version 6.1 (updated September 2020). Cochrane.
Higgins, J. P. T., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Kasprzak, M., Sicińska, J., and Sinclair, R. (2019). The trichoscopy derived Sinclair scale: enhancing visual assessment through quantitative trichoscopy. Australas. J. Dermatol. 60 (2), 134–136. doi:10.1111/ajd.12964
Khumalo, N. P., Jessop, S., Gumedze, F., and Ehrlich, R. (2007). Hairdressing and the prevalence of scalp disease in African adults. Br. J. Dermatol. 157 (5), 981–988. doi:10.1111/j.1365-2133.2007.08146.x
Lee, J., Rabbani, C. C., Gao, H., Steinhart, M. R., Woodruff, B. M., Pflum, Z. E., et al. (2020) Hair-bearing human skin generated entirely from pluripotent stem cells. Nature582 (7812), 399–404. doi:10.1038/s41586-020-2352-3
McGuinness, L. A., and Higgins, J. P. T. (2020). Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 12 (1), 55–61. doi:10.1002/jrsm.1411
Modarressi, A. (2013). Platlet rich plasma (PRP) improves fat grafting outcomes. World J. Plast. Surg. 2 (1), 6–13. doi:10.1097/prs.0000000000005676
Munn, Z., Barker, T. H., Moola, S., Tufanaru, C., Stern, C., McArthur, A., et al. (2020). Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid. Synth. 18 (10), 2127–2133. doi:10.11124/JBISRIR-D-19-00099
Olsen, E. A. (2001). Female pattern hair loss. J. Am. Acad. Dermatol. 45 (3 Suppl. l), S70–S80. doi:10.1067/mjd.2001.117426
Paik, J.-H., Yoon, J.-B., Sim, W.-Y., Kim, B.-S., and Kim, N.-I. (2001). The prevalence and types of androgenetic alopecia in Korean men and women. Br. J. Dermatol. 145 (1), 95–99. doi:10.1046/j.1365-2133.2001.04289.x
Rossano, F., Di Martino, S., Iodice, L., Di Paolo, M., Misso, S., Tomeo, R., et al. (2017). Correlation between individual inflammation genetic profile and platelet rich plasma efficacy in hair follicle regeneration: a pilot study reveals prognostic value of IL-1a polymorphism. Eur. Rev. Med. Pharmacol. Sci. 21 (22), 5247–5257. doi:10.26355/eurrev_201711_13848
Sánchez-González, D. J., Méndez-Bolaina, E., and Trejo-Bahena, N. I. (2012). Platelet-rich plasma peptides: key for regeneration. Int. J. Peptides 12, 1. doi:10.1155/2012/532519
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of observational studies in epidemiology. a proposal for reporting. JAMA 283 (15), 2008–2012. doi:10.1001/jama.283.15.2008
Torabi, P., Behrangi, E., Goodarzi, A., and Rohaninasab, M. (2020). A systematic review of the effect of platelet-rich plasma on androgenetic alopecia of women. Dermatol. Ther. 33, e13835. doi:10.1111/dth.13835
Trueb, R. M. (2002). Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 37 (8–9), 981–990. doi:10.1016/s0531-5565(02)00093-1
Ueki, R., Tsuboi, R., Ogawa, H., and Inaba, Y. (2003). Phototrichogram analysis of Japanese female subjects with chronic diffuse hair loss. J. Invest. Dermatol. Symp. Proc. 8 (1), 116–120. doi:10.1046/j.1523-1747.2003.12184.x
van Zuuren, E. J., Fedorowicz, Z., and Carter, B. (2012). Evidence-based treatments for female pattern hair loss: a summary of a Cochrane systematic review. Br. J. Dermatol. Nov 167 (5), 995–1010. doi:10.1111/j.1365-2133.2012.11166.x
van Zuuren, E. J., Fedorowicz, Z., and Schoones, J. (2016). Interventions for female pattern hair loss. Cochrane Database Syst. Rev. 13 (5), CD007628. doi:10.1002/14651858.CD007628.pub4
Wang, T. L., Zhou, C., Shen, Y. W., Wang, X. Y., Ding, X. L., Tian, S., et al. (2010). Prevalence of androgenetic alopecia in China: a community-based study in six cities. Br. J. Dermatol. Apr 162 (4), 843–847. doi:10.1111/j.1365-2133.2010.09640.x
Weibrich, G., Kleis, W. K. G., Hafner, G., and Hitzler, W. E. (2002). Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J. Craniomaxillofac. Surg. 30 (2), 97–102. doi:10.1054/jcms.2002.0285
Keywords: platelet-rich plasma, female androgenic alopecia, female pattern hair loss, systematic review, meta-analysis
Citation: Zhou S, Qi F, Gong Y, Zhang C, Zhao S, Yang X and He Y (2021) Platelet-Rich Plasma in Female Androgenic Alopecia: A Comprehensive Systematic Review and Meta-Analysis. Front. Pharmacol. 12:642980. doi: 10.3389/fphar.2021.642980
Received: 18 December 2020; Accepted: 19 March 2021;
Published: 06 May 2021.
Edited by:
Pietro Gentile, University of Rome Tor Vergata, ItalyReviewed by:
Kurt Neumann, Independent Researcher, Kerékteleki, HungaryRaja Ahsan Aftab, Taylor’s University, Malaysia
Copyright © 2021 Zhou, Qi, Gong, Zhang, Zhao, Yang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanling He, aGV5YW5saW5nZGVybWF0b2xvZ3lAZ21haWwuY29t
†These authors have contributed equally to this work
 Shuying Zhou
Shuying Zhou Fei Qi2†
Fei Qi2†