- 1Department of Pharmacy Practice, Faculty of Pharmacy, Universiti Teknologi MARA (UiTM), Bandar Puncak Alam, Malaysia
- 2Department of Pharmacy Practice, Institute of Pharmaceutical Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 3School of Pharmacy, Monash University, Subang Jaya, Malaysia
- 4Faculty of Pharmacy, Universiti Teknologi MARA (UiTM), Pulau Pinang, Malaysia
- 5Vector-Borne Diseases Research Group (VERDI), Pharmaceutical and Life Sciences CoRe, Universiti Teknologi MARA (UiTM), Shah Alam, Malaysia
Background. Infertility is an emerging health issue for men. Comparative efficacy of different pharmacological interventions on male infertility is not clear. The aim of this review is to investigate the efficacy of various pharmacological interventions among men with idiopathic male infertility. All randomized control trials evaluating the effectuality of interventions on male infertility were included for network meta-analysis (NMA) from inception to 31 April 2020, systematically performed using STATA through the random effect model. The protocol was registered at PROSPERO (CRD42020152891).
Results. The outcomes of interest were semen and hormonal parameters. Treatment effects (p < 0.05) were estimated through WMD at the confidence interval of 95%. Upon applying exclusion criteria, n=28 RCTs were found eligible for NMA. Results from NMA indicated that consumption of supplements increases sperm concentration levels [6.26, 95% CI 3.32, 9.21] in comparison to SERMs [4.97, 95% CI 1.61, 8.32], hormones [4.14, 95% CI 1.83, 6.46], and vitamins [0.15, 95% CI −20.86, 21.15)] with placebo, whereas the use of SERMs increased percentage sperm motility [6.69, 95% CI 2.38, 10.99] in comparison to supplements [6.46, 95% CI 2.57, 10.06], hormones [3.47, 95% CI 0.40, 6.54], and vitamins [−1.24, 95% CI −11.84, 9.43] with placebo. Consumption of hormones increased the sperm morphology [3.71, 95% CI, 1.34, 6.07] in contrast to supplements [2.22, 95% CI 0.12, 4.55], SERMs [2.21, 95% CI −0.78, 5.20], and vitamins [0.51, 95% CI −3.60, 4.62] with placebo. Supplements boosted the total testosterone levels [2.70, 95% CI 1.34, 4.07] in comparison to SERMs [1.83, 95% CI 1.16, 2.50], hormones [0.40, 95% CI −0.49, 1.29], and vitamins [−0.70, 95% CI −6.71, 5.31] with placebo. SERMs increase the serum FSH levels [3.63, 95% CI 1.48, 5.79] better than hormones [1.29, 95% CI −0.79, 3.36], vitamins [0.03, 95% CI −2.69, 2.76], and supplements [−4.45, 95% CI −7.15, −1.76] in comparison with placebo.
Conclusion. This review establishes that all interventions had a significantly positive effect on male infertility. Statistically significant increased sperm parameters were noted in combinations of zinc sulfate (220 mg BID), clomiphene citrate (50 mg BID), and testosterone undecanoate and CoQ10; tamoxifen citrate and FSH were shown to improve the hormonal profile in infertile males.
Systematic Review Registration: PROSPERO, identifier [CRD42020152891].
Introduction
Numerous studies have been published reporting male infertility and a drop in sperm quality (concentration, motility, and morphology) or dismissing the same (Carlsen et al., 1992; Auger et al., 1995; Fisch et al., 1996; Marimuthu et al., 2003; Lackner et al., 2005; Kumar and Singh, 2015). Retrospective data analysis indicates that overall sperm parameters have declined in some parts of the world with geographical variations in semen quality (Auger and Jouannet, 1997; Jørgensen et al., 2001; Swan, 2006; Kumar and Singh, 2015). These geographical variations in semen characteristics could be due to environmental, nutritional, socioeconomic, or other unknown causes (Fisch and Goluboff, 1996) and this coincides with ever-increasing male genital tract abnormalities including testicular cancer and cryptorchidism in various countries (Giwercman and Skakkebaek, 1992; Bussen et al., 2004; Kumar and Singh, 2015).
Inability to achieve clinical conception despite 1 year of unprotected intercourse with the same partner is categorized as human infertility by the World Health Organization (WHO) (Zhao et al., 2016; Buhling et al., 2019; Cao et al., 2019; Hosseini et al., 2019; Wang et al., 2020). A total of 15% (48.5 million) couples worldwide are affected by any kind of infertility, and male infertility dominates the trend with 70% of all cases. Higher male infertility rates are seen in the population of Central Europe, Eastern Europe, and Africa (Zhao et al., 2016; Buhling et al., 2019; Cao et al., 2019; Hosseini et al., 2019; Wang et al., 2020).
The etiology of male factor infertility is multifactorial, primarily based on reduced and poor sperm quality. Concentration, morphology, and motility are three primary endpoints to assess the sperm quality (Buhling et al., 2019). Other factors include genetics (chromosomal aberrations, gene mutations, congenital anomalies, and polymorphisms), morphology of the reproductive system (testicular cancer, aplasia of the germinal cells, alteration in reproductive hormone, varicocele, defects in the transport of sperm, and bilateral sperm ducts), addictive disorders (alcoholism, smoking, and drug addiction), environmental factors (cigarette, smoking, exposure to certain chemicals, and nutritional deficiency), and alteration in spermatogenesis due to pathophysiological conditions (infectious diseases, pregnancy-related infections, urogenital infections, genitourinary dysplasia, immune system-related factor, abnormal levels of biochemical components of seminal plasma, and presence of antiserum antibodies (ASAs)) (Zhao et al., 2016; Buhling et al., 2019; Cao et al., 2019; Hosseini et al., 2019; Wang et al., 2020). These factors can lead to numerous abnormalities of the reproductive system and capacity for fertilization. Moreover, infertility can have severe damaging impacts on social, psychological, and economic well-being and health of the couples (Zhao et al., 2016; Buhling et al., 2019; Cao et al., 2019; Hosseini et al., 2019; Wang et al., 2020). Hormones, selective estrogen receptor modulator, antioxidants, vitamins, and enzymes are some of the treatment protocols for male infertility these days. Although several micronutrient supplementation products are available in the market that promises to improve the spermiogram, there are only a few existing evidence based data that address male factor infertility and micronutrient treatment options (Hamada et al., 2012; Cannarella et al., 2019; Cardoso et al., 2019; Hosseini et al., 2019; Wang et al., 2020).
To date, many systematic reviews and meta-analyses revealed the impact of different interventions in male infertility (Kamischke and Nieschlag, 1999; Boivin, 2003; Lafuente et al., 2013; Giahi et al., 2016; Hosseini et al., 2019; Smits et al., 2019; Wang et al., 2019; Garolla et al., 2020). Though systematic reviews and pairwise meta-analysis are vital tools for decision makers for contriving guidelines and clinical protocols, they produce partial information, because amongst several accessible interventions merely few are examined in head-to-head comparisons (Greco et al., 2015; Tonin et al., 2017). To overcome these issues, WHO introduced network meta-analysis (NMA). One of the several advantages of NMA is its ability to compare interventions quantitatively which otherwise are not directly comparable (Greco et al., 2015). This NMA will facilitate clinicians to mold interventions according to their choice to achieve desired therapeutic outcomes in male infertility, with maximum utilization of resources available.
This study compares effectiveness of multiple pharmacological groups including hormones (follicle stimulating hormone, testosterone undecanoate), selective estrogen receptor modulators (SERMs) (tamoxifen citrate and clomiphene citrate), supplements (coenzyme Q10, carnitine, L-Carnitine, zinc sulfate, fish oil, and Profertil), vitamins (vitamins A, D, and E and folic acid), and enzymes (kallikrein) alone and in combination through NMA. We choose to use sperm concentration, sperm motility, and sperm morphology as primary outcomes for male infertility related complications (Kumar and Singh, 2015; Buhling et al., 2019). Other secondary outcomes include serum total testosterone and serum follicle stimulating hormones (FSH).
Methodology
Seven electronic databases (PubMed, Scopus, Cochrane library, Embase, EBSCOhost, Ovid, and Google scholar) were searched for data sources and strategies from inception till 31 April 2020. Medical subject headings [MeSH] and text terms were included for search terms in this review. The strategic search terms were as follows: “infertility” or “subfertility” or “subfertile” and “azoospermia” or “oligospermia” or “oligozoospermia” or “oligoasthenoteratozoospermia” or “genital disease” or “genitalia” or “genital” or “low sperm count” or “semen.” Details of search strategies used for each database are provided in the Supplementary Material.
Inclusion and Exclusion Criteria
The inclusion criteria were set according to the PICOT framework (population, intervention, comparison, outcomes, and time), as follows (Table 1). Studies which were included comprise of the following:
1) Randomized control trials or cluster-randomized controlled trials.
2) Different interventions evaluating the efficacy of men infertility issues.
3) Those directed at male patients (≥18 years) with infertility only.
4) Reporting sperm concentration, sperm motility, and sperm morphology as primary clinical outcome (alone or in combination with any of the other clinical outcomes, such as serum total testosterone and serum FSH).
5) Those performed in inpatient, outpatient primary care or hospital settings.
6) Original study published in a peer-reviewed journal.
7) Article in English language.
Studies conducted using the observational study designs mentioned were excluded:
1) Observational studies.
2) Cohort studies.
3) Cross-sectional studies.
4) Nonrandomized control trials.
5) Expert opinions.
6) Case reports/series.
7) Editorials
8) Abstracts from conferences.
9) Review articles.
10) Studies involving animals.
Study Selection
Two reviewers MNS and TMK screened titles and abstracts extracted from various databases using the well-defined selection criteria. Appropriate articles were then screened individually by the reviewers to access their inclusion eligibility. Resolution of disagreement was primarily through discussion.
Data Extraction and Synthesis
MNS extracted the data from the studies included using standardized procedure. TMK independently reviewed the data for proper extraction. Details about title, publication year, authors, design of the study, country and study settings, sample size, age of the patients, gender of the patients, follow-up duration, interventions given, criteria for study inclusion and exclusion, and outcome of the study were extracted from each included study. In this review, changes from baseline to end of the intervention were summarized for both intervention and control groups as a result for the outcome measures.
Risk of Bias Assessment
MNS and TMK evaluated the risk of bias (ROB) of the included studies using the Cochrane ROB tool. For RCTs, each ROB item was ranked as “low risk” if it was suspected that a bias would seriously alter the result; it would be “unclear” if it was expected that a bias would raise some uncertainty about the results; or it would be “high risk” if it was prospective that a bias would completely alter the result. Discussion was used to resolve disagreements among reviewers.
Data Analysis
Meta-analysis (MA) and NMA were executed by using Review Manager 5.3 and STATA 14. Comparative efficacies for all interventions were estimated through calculation of mean difference using the random effect model. The assessed significance of results p-values were set to be < 0.05 with 95% confidence interval (CI).
Subgroup analyses were executed for primary and secondary clinical outcomes for different interventions to clarify the heterogeneity among the studies. Robustness of the study results was analyzed through subgroup analysis of the baseline values for sperm concentration, motility and morphology levels, intervention duration, influence of studies on primary clinical outcomes, and geographical areas of the performed studies. Moreover, forest plot was generated to study pairwise comparison for the study of treatment effect in NMA. In addition, treatment effect and mean difference were used to generate league tables to evaluate all the effects (direct and indirect) among interventions.
Study Protocol Registration
The protocol was registered at PROSPERO (CRD42020152891).
Results
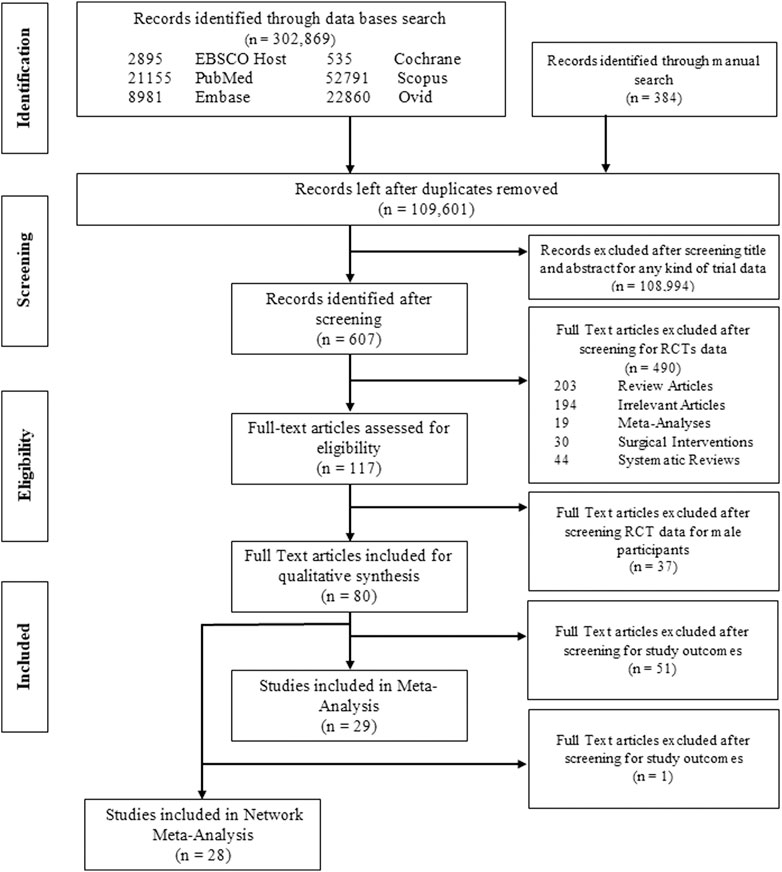
In total, 302,869 articles were extracted from the electronic database searches; after confiscating duplications (n = 193,268), the final count was reduced to 109,601. Evaluation of title and abstract excluded 108,994 studies not fulfilling inclusion criteria. 607 studies remained and full-text assessment was done from which 80 studies (n = 9529 participants) were finally included for qualitative synthesis, whereas quantitative analysis (MA) was conducted for 29 studies, details of which are presented in PRISMA flow diagram (Figure 1). Reasons for omission after full-text assessment are presented in Supplementary Table 1.
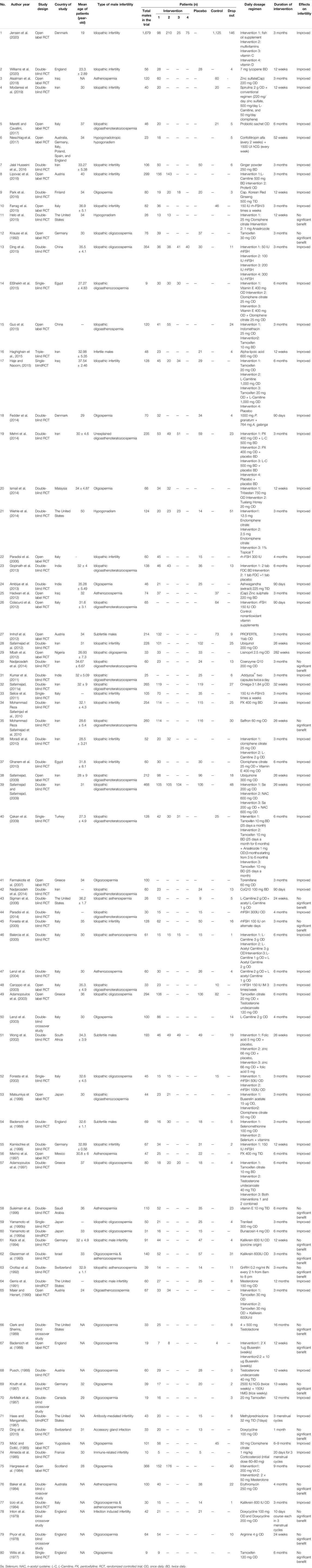
Among all 80 selective articles, 57 RCTs were blinded while the remaining 23 were not blinded. Among the studies included in this review, 13 were conducted in Iran (Safarinejad, 2009; Safarinejad and Safarinejad, 2009; Moradi et al., 2010; Safarinejad, 2011a; Safarinejad, 2011b; Nadjarzadeh et al., 2011; Safarinejad et al., 2011; Safarinejad et al., 2012; Mehni et al., 2014; Nadjarzadeh et al., 2014; Haghighian et al., 2015; Hosseini et al., 2016; Modarresi et al., 2019), 13 in Italy (Izzo et al., 1984; Foresta et al., 2002; Caroppo et al., 2003; Lenzi et al., 2003; Lenzi et al., 2004; Balercia et al., 2005; Foresta et al., 2005; Paradisi et al., 2006; Selice et al., 2011; Colacurci et al., 2012; Paradisi et al., 2014; Farrag et al., 2015; Maretti and Cavallini, 2017), 6 in the United States of America (Haas and Manganiello, 1987; Clark and Sherins, 1989; Gerris et al., 1991; Sigman et al., 2006; Wiehle et al., 2014; Helo et al., 2015), 6 in England (Willis et al., 1977; Pryor et al., 1978; Inton et al., 1979; Badenoch et al., 1988; Scott et al., 1998; Williams et al., 2020), and 4 from Austria (Pusch, 1988; Maier and Hienert, 1990; Imhof et al., 2012; Lipovac et al., 2016) and Germany (Knuth et al., 1987; Krause et al., 1992; Keck et al., 1994; Kamischke et al., 1998) while 3 were from India (Kumar et al., 2011; Ambiye et al., 2013; Gopinath et al., 2013), Iraq (Hadwan et al., 2012; Haje and Naoom, 2015; Alsalman et al., 2018), Japan (Yamamoto et al., 1995a; Yamamoto et al., 1995b; Matsumiya et al., 1998), and Greece (Adamopoulos et al., 1997; Adamopoulos et al., 2003; Farmakiotis et al., 2007). Two were from Egypt (Ghanem et al., 2010; ElSheikh et al., 2015), Denmark (Fedder et al., 2014; Jensen et al., 2020), China (Ding et al., 2015; Guo et al., 2015), and Switzerland (Comhaire et al., 1986; Crottaz et al., 1992) and n = 1 from Turkey (Çakan et al., 2009), Australia (Baker et al., 1984), Malaysia (Ismail et al., 2014), Nigeria (Mbah et al., 2012), France (Almeida et al., 1985), Saudi Arabia (Suleiman et al., 1996), South Africa (Wong et al., 2002), Finland (Park et al., 2016), Yugoslavia (Mićić and Dotlić, 1985), Canada (AinMelk et al., 1987), Israel (Glezerman et al., 1993), Mexico (Merino et al., 1997), and Scotland (Hargreave et al., 1984). Moreover, one multiple center study was conducted in Australia, Germany, Italy, Poland, Spain, and United Kingdom (Nieschlag et al., 2017).
Characteristics of Participants
Eighty studies (n = 9529 patients) fulfilled the selection criteria of this review. The participants claimed infertility issue (WHO criteria) for at least 12 months or tested to have sperm count less than 20 million per ml. Sample size of the included studies ranged from n = 9 to n = 1,679; details are presented in Table 2
Risk of Bias
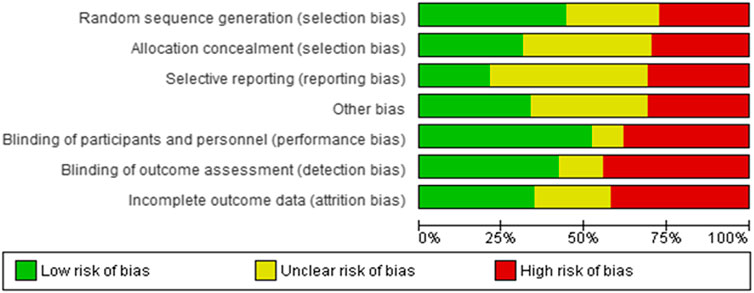
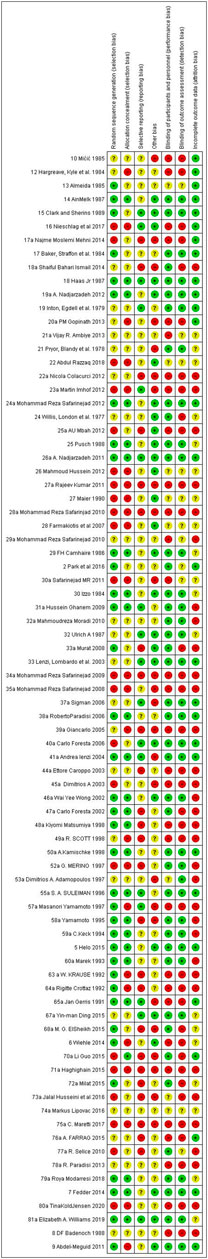
Figures 2 and 3 present ROB for all the included RCTs. Cochrane ROB tool was used to generate the graphs. More than 20% of the studies were categorized as free of attrition bias, reporting bias and other sources of bias. Performance bias and selection bias were granted in only 40 and 35% of the studies, respectively.
Primary Outcomes
Sperm concentration (106/ml), sperm motility (%), and sperm morphology (%) were the three primary clinical outcomes discussed in this study. A total of n = 29 studies were included for quantitative synthesis (Mićić and Dotlić, 1985; AinMelk et al., 1987; Pusch, 1988; Krause et al., 1992; Keck et al., 1994; Kamischke et al., 1998; Foresta et al., 2002; Wong et al., 2002; Adamopoulos et al., 2003; Caroppo et al., 2003; Lenzi et al., 2004; Paradisi et al., 2006; Safarinejad, 2009; Çakan et al., 2009; Ghanem et al., 2010; Safarinejad, 2011b; Selice et al., 2011; Colacurci et al., 2012; Hadwan et al., 2012; Imhof et al., 2012; Safarinejad et al., 2012; Nadjarzadeh et al., 2014; Paradisi et al., 2014; Ding et al., 2015; Farrag et al., 2015; Haje and Naoom, 2015; Lipovac et al., 2016; Alsalman et al., 2018; Jensen et al., 2020). All the studies were randomized control trials and encompass data on the stated outcomes involving interventions grouped under categories like hormones, selective estrogen receptor modulators, supplements, vitamins, and enzymes (Supplementary Figures 1–3).
Sperm Concentration
Pairwise MA results were in the favor of the SERMs (Mićić and Dotlić, 1985; AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009; Ghanem et al., 2010; Haje and Naoom, 2015) where the levels of sperm concentration increased to 6.00 million per mL [95% CI 5.27, 6.72; p = 0.43] followed by supplements [5.99; 95% CI 2.83, 9.15; p < 0.00001; I2 = 91%] (Wong et al., 2002; Lenzi et al., 2004; Safarinejad, 2009; Nadjarzadeh et al., 2011; Hadwan et al., 2012; Imhof et al., 2012; Safarinejad et al., 2012; Nadjarzadeh et al., 2014; Haje and Naoom, 2015; Lipovac et al., 2016; Alsalman et al., 2018; Jensen et al., 2020) (Supplementary Figure 4).
Subgroup analysis of studies examining the effect of SERMs revealed (Mićić and Dotlić, 1985; AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009; Ghanem et al., 2010; Haje and Naoom, 2015) maximum effect with clomiphene citrate [6.91; 95% CI 5.62, 8.20; p = 0.95; I2 = 0%] (Mićić and Dotlić, 1985; Ghanem et al., 2010), followed by tamoxifen citrate [5.61; 95% CI 4.75, 6.46; p = 0.50; I2 = 0%] (AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009; Haje and Naoom, 2015) (Supplementary Figure 5).
Moreover, the subgroup analysis for supplements (Wong et al., 2002; Lenzi et al., 2004; Safarinejad, 2009; Nadjarzadeh et al., 2011; Hadwan et al., 2012; Imhof et al., 2012; Safarinejad et al., 2012; Nadjarzadeh et al., 2014; Haje and Naoom, 2015; Lipovac et al., 2016; Alsalman et al., 2018; Jensen et al., 2020) revealed that studies involving zinc sulfate (Wong et al., 2002; Hadwan et al., 2012; Alsalman et al., 2018) had large effect [11.49; 95% CI -6.42, 29.39; p = 0.001; I2 = 81%] followed by Profertil [10.90; 95% CI 7.98, 13.82] (Imhof et al., 2012) and CoQ10 [6.53; 95% CI 1.88, 11.17; p < 0.00001; I2 = 94%] (Safarinejad, 2009; Nadjarzadeh et al., 2011; Safarinejad et al., 2012; Nadjarzadeh et al., 2014), respectively. Statistically significant results were seen in CoQ10 (Supplementary Figure 6).
Sperm Motility
Pairwise MA results were in the favor of the SERMs (Mićić and Dotlić, 1985; AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009; Ghanem et al., 2010; Haje and Naoom, 2015) where the percentage of sperm motility was increased [6.62; 95% CI 3.69, 9.54; p = 0.005; I2 = 63%] followed by supplements (Wong et al., 2002; Lenzi et al., 2004; Safarinejad, 2009; Nadjarzadeh et al., 2011; Hadwan et al., 2012; Imhof et al., 2012; Safarinejad et al., 2012; Nadjarzadeh et al., 2014; Haje and Naoom, 2015; Lipovac et al., 2016; Alsalman et al., 2018; Jensen et al., 2020) with a standard mean difference of 6.51 [95% CI 3.15, 9.86; p < 0.00001; I2 = 96%] (Supplementary Figure 7).
Subgroup analysis revealed that among SERMs (Mićić and Dotlić, 1985; AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009; Ghanem et al., 2010; Haje and Naoom, 2015) large effects were made by studies which involved clomiphene citrate [8.17; 95% CI 425, 12.10; p = 0.79; I2 = 0%] (Mićić and Dotlić, 1985; Ghanem et al., 2010) followed by tamoxifen citrate [6.26; 95% CI 2.62, 9.90; p = 0.002; I2 = 71%] (AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009; Haje and Naoom, 2015) (Supplementary Figure 8).
Moreover, the subgroup analysis for supplements (Wong et al., 2002; Lenzi et al., 2004; Safarinejad, 2009; Nadjarzadeh et al., 2011; Hadwan et al., 2012; Imhof et al., 2012; Safarinejad et al., 2012; Nadjarzadeh et al., 2014; Haje and Naoom, 2015; Lipovac et al., 2016; Alsalman et al., 2018; Jensen et al., 2020) revealed that studies involving zinc sulfate (Wong et al., 2002; Hadwan et al., 2012; Alsalman et al., 2018) had large effect [16.78; 95% CI 14.27, 19.29; p = 0.76; I2 = 0%] followed by Profertil [7.00; 95% CI -1.50, 15.50] (Imhof et al., 2012) and CoQ10 [6.97; 95% CI 1.94, 12.01; p < 0.00001; I2 = 98%] (Safarinejad, 2009; Nadjarzadeh et al., 2011; Safarinejad et al., 2012; Nadjarzadeh et al., 2014), respectively (Supplementary Figure 9).
Sperm Morphology
Pairwise MA results were in the favor of the hormones (Pusch, 1988; Kamischke et al., 1998; Foresta et al., 2002; Adamopoulos et al., 2003; Caroppo et al., 2003; Paradisi et al., 2006; Selice et al., 2011; Colacurci et al., 2012; Paradisi et al., 2014; Ding et al., 2015; Farrag et al., 2015) where the percentage increase in sperm morphology was maximum [3.68; 95% CI 0.97, 6.39; p < 0.00001; I2 = 83%], followed by supplements [1.93; 95% CI 0.43, 3.43; p < 0.00001; I2 = 89%] (Wong et al., 2002; Lenzi et al., 2004; Safarinejad, 2009; Nadjarzadeh et al., 2011; Hadwan et al., 2012; Imhof et al., 2012; Safarinejad et al., 2012; Nadjarzadeh et al., 2014; Haje and Naoom, 2015; Lipovac et al., 2016; Alsalman et al., 2018; Jensen et al., 2020) (Supplementary Figure 10).
Subgroup analysis suggested that among hormones favourable effect was made by testosterone [12.24; 95% CI 1.00, 23.49; p = 0.01; I2 = 83%] (Pusch, 1988; Adamopoulos et al., 2003), followed by FSH at a dose range of ≥200–300 IU [6.53; 95% CI -0.54, 5.59; p = 0.99; I2 = 0%] (Paradisi et al., 2006; Paradisi et al., 2014; Ding et al., 2015) (Supplementary Figure 11).
Moreover, the subgroup analysis for supplements revealed that Profertil has large effect [14.50; 95% CI 7.31, 21.69] (Imhof et al., 2012) followed by zinc sulfate [4.23; 95% CI -2.39, 10.84; p < 0.0001; I2 = 86% ] (Wong et al., 2002; Hadwan et al., 2012; Alsalman et al., 2018) (Supplementary Figure 12).
Secondary Outcomes
Serum total testosterone (ng/ml) and serum FSH (mIU/ml) were two secondary clinical outcomes discussed in this study. Total n = 12 studies were included (AinMelk et al., 1987; Pusch, 1988; Krause et al., 1992; Kamischke et al., 1998; Paradisi et al., 2006; Safarinejad, 2009; Çakan et al., 2009; Selice et al., 2011; Safarinejad et al., 2012; Paradisi et al., 2014; Helo et al., 2015; Jensen et al., 2020). All the studies were randomized control trials involving interventions grouped under categories like hormones, selective estrogen receptor modulators, supplements, vitamins, and enzymes (Supplementary Figures 13 and 14).
Total Serum Testosterone
Pairwise MA results were in the favor of the supplements (Safarinejad and Safarinejad, 2009; Safarinejad, 2011a; Jensen et al., 2020) where the total serum testosterone concentration was increased [2.74; 95% CI 1.81, 3.68; p = 0.78; I2 = 0%] followed by SERMs [1.50; 95% CI 1.20, 1.79; p < 0.00001; I2 = 92%] (AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009) (Supplementary Figure 15).
Subgroup analysis revealed that among supplements (Safarinejad and Safarinejad, 2009; Safarinejad, 2011a; Jensen et al., 2020) coenzyme Q10 34,39 showed better results in increasing total serum testosterone concentration [2.77; 95% CI 1.83, 3.71; p = 0.76; I2 = 0%]. None of remaining supplements showed substantial effects (Supplementary Figure 16).
Moreover, the subgroup analysis for SERMs (AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009) showed that all the total serum testosterone concentration effect was due to tamoxifen citrate [1.50; 95% CI 1.20, 1.79; p < 0.00001; I2 = 92%] (AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009); none of the studies reported clomiphene citrate (Supplementary Figure 17).
Total Serum Follicle Stimulating Hormone
Pairwise MA results were in the favor of the SERMs (AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009) where the total serum FSH concentration was increased [3.66; 95% CI 1.27, 6.05; p < 0.00001; I2 = 90%] followed by hormones [1.24; 95% CI -0.29, 2.77; p < 0.00001; I2 = 89%] (Supplementary Figure 18).
Subgroup analysis revealed that among SERMS studies only tamoxifen citrate 1.05 [95% CI 0.40, 1.71; p < 0.0001; I2 = 84%] (AinMelk et al., 1987; Krause et al., 1992; Çakan et al., 2009) effect was reported. None of the studies reported clomiphene citrate (Supplementary Figure 19).
Moreover, the subgroup analysis for hormones (Pusch, 1988; Kamischke et al., 1998; Paradisi et al., 2006; Selice et al., 2011; Paradisi et al., 2014; Helo et al., 2015) revealed that studies involving FSH in dose <200–50 IU) (Kamischke et al., 1998; Selice et al., 2011) showed better results in increasing total serum FSH concentration [2.74; 95% CI -0.00, 5.48; p = 0.0009; I2 = 91%] followed by studies involving FSH in dose ≥200–300IU [0.94; 95% CI -0.82, 2.70; p = 0.03; I2 = 80%] (Paradisi et al., 2006; Paradisi et al., 2014) (Supplementary Figure 20).
Network Meta-Analysis
To assess the effect of intervention on primary and secondary parameters, twenty-eight studies (Mićić and Dotlić, 1985; AinMelk et al., 1987; Pusch, 1988; Krause et al., 1992; Kamischke et al., 1998; Foresta et al., 2002; Wong et al., 2002; Adamopoulos et al., 2003; Caroppo et al., 2003; Lenzi et al., 2003; Paradisi et al., 2006; Safarinejad, 2009; Çakan et al., 2009; Ghanem et al., 2010; Nadjarzadeh et al., 2011; Selice et al., 2011; Colacurci et al., 2012; Hadwan et al., 2012; Imhof et al., 2012; Safarinejad et al., 2012; Nadjarzadeh et al., 2014; Paradisi et al., 2014; Ding et al., 2015; Farrag et al., 2015; Haje and Naoom, 2015; Lipovac et al., 2016; Alsalman et al., 2018; Jensen et al., 2020) were included for primary outcomes and seventeen studies (AinMelk et al., 1987; Pusch, 1988; Krause et al., 1992; Kamischke et al., 1998; Paradisi et al., 2006; Safarinejad, 2009; Çakan et al., 2009; Selice et al., 2011; Safarinejad et al., 2012; Paradisi et al., 2014; Helo et al., 2015; Jensen et al., 2020) were included for secondary outcomes. NMA was executed to compare the effects of intervention on primary and secondary parameters. The network plots exhibit the association of all available evidences. The thickness of the lines denotes the number of trials and the size of node represents the sample size (Figure 4).
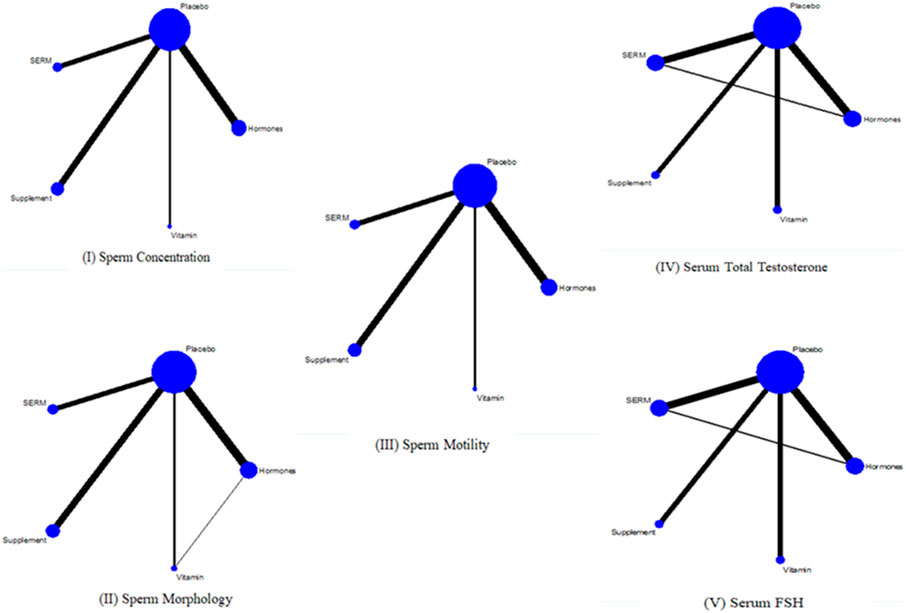
FIGURE 4. Network plots I, II, and III are for primary outcomes whereas IV and V are network plots for secondary outcomes.
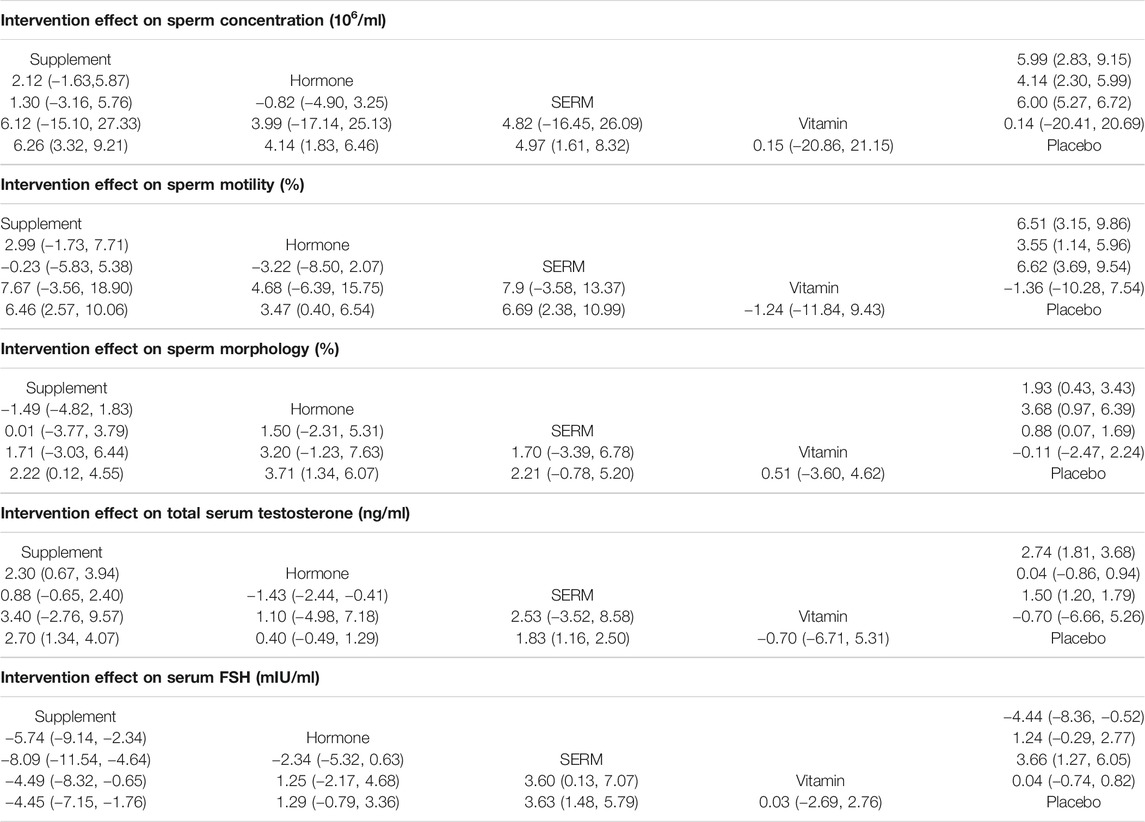
With respect to primary outcomes (sperm concentration, sperm morphology, and sperm motility) when the reference arm was set as a placebo in the analysis, the sperm concentration was found to be considerably higher for supplement 6.26 [CI 95% 3.32, 9.21; p = 0.00] followed by SERMs 4.97 [CI 95% 1.61, 8.32; p = 0.004] and hormone 4.14 [CI 95% 1.83, 6.45; p = 0.00] groups versus the placebo group. On the other hand, the sperm morphology was significantly increased in hormone 3.71 [CI 95% 1.34, 6.07; p = 0.002] followed by supplement 2.22 [CI 95% −0.12, 4.55; p = 0.63] and SERMs 2.21 [CI 95% -0.78, 5.20; p = 0.15] groups versus the placebo group. Moreover, increasing trends in sperm motility were observed in SERMs 6.69 [CI 95% 2.38, 10.99; p = 0.002] trailed by supplement 6.46 [CI 95% 2.86, 10.06; p = 0.00] and hormone 3.47 [CI 95% 0.40, 6.54; p = 0.027] groups versus the placebo group (Table 3).
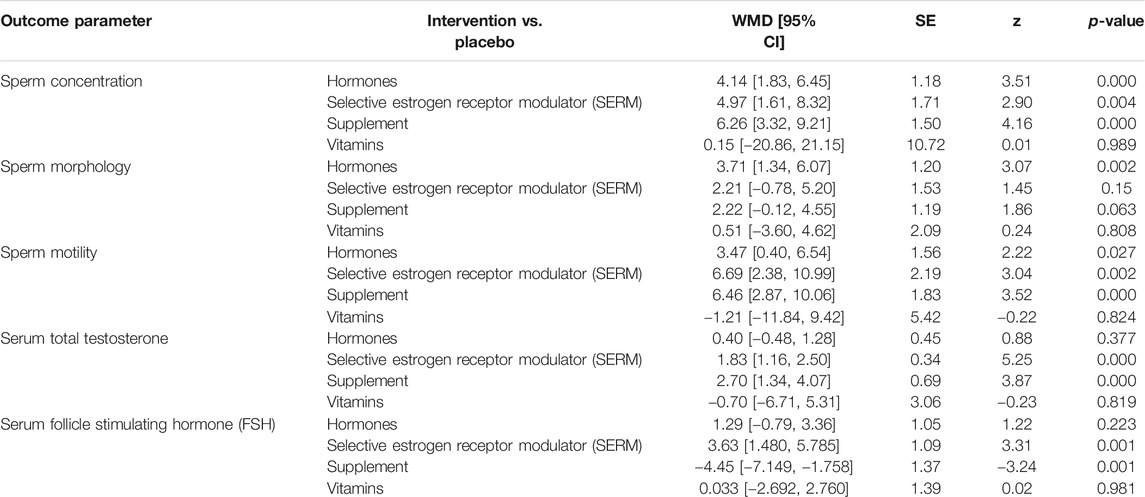
TABLE 3. Network meta-analysis for impact various pharmacological group interventions on primary and secondary clinical outcomes of male infertility.
For secondary outcomes (serum total testosterone and serum FSH) by setting the reference arm as placebo in the analysis serum total testosterone was significantly increased in supplement 2.70 [CI 95% 1.33, 4.07; p = 0.00] followed by SERMS 1.82 [CI 95% 1.15, 2.49; p = 0.00] and hormone 0.40 [CI 95% -0.48, 1.28; p = 0.377] whereas serum FSH was significantly increased in SERMs 3.63 [CI 95% 1.48, 5.78; p = 0.001] followed by hormones 1.28 [CI 95% −0.78, 3.36; p = 0.223] and vitamins 0.033 [CI 95% −2.69, 2.76; p = −2.692] groups versus the placebo group (Table 3).
League table was created by using NMA to elaborate all probable pairwise comparisons between any two of the four interventions and customary pairwise meta-analysis (Table 3). It was obvious from the NMA that all of four interventions show analogous efficacy in increasing primary outcomes (sperm concentration, sperm morphology, and sperm motility) and secondary outcomes (serum total testosterone and serum FSH) (Table 4).
For primary outcomes, supplements were found statistically significant in increasing the sperm concentration 6.26 [CI 95%, 3.32, 9.21; p = 0.00] followed by SERM 4.97 [CI 95%, 1.61, 8.32; p = 0.004] and hormones 4.14 [CI 95%, 1.83, 6.46; p = 0.00]. Sperm motility was significantly increased by SERM 6.69 [CI 95%, 2.38, 10.99; p = 0.002] followed by supplements 6.46 [CI 95%, 2.87, 10.06; p = 0.00] and hormones 3.47 [CI 95%, 0.40, 6.54; p = 0.027]. Moreover, hormones proved to be statistically significant in improving the sperm morphology 3.71 [CI 95%, 1.34, 6.07; p = 0.002], followed by supplements 2.22 [CI 95%, 0.12, 4.55; p = 0.063] and SERMS 2.21 [CI 95%, −0.78, 5.20; p = 0.15] (Figure 5).
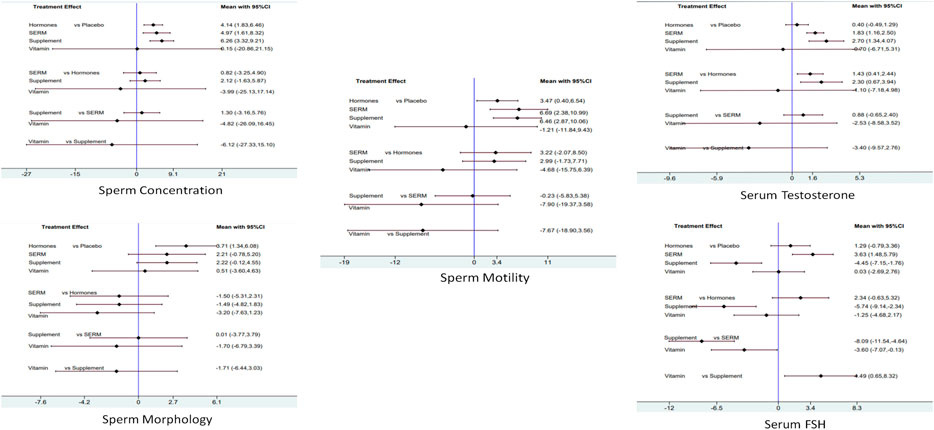
FIGURE 5. Network meta-analysis estimates of change in primary and secondary clinical outcomes of infertile male patients. Sperm concentration, sperm morphology, sperm motility, serum total testosterone, and serum FSH.
For secondary outcomes, supplements were found statistically significant in increasing serum total testosterone concentration 2.70 [CI 95%, 1.34, 4.07; p = 0.00] followed by SERMS 1.83 [CI 95%, 1.16, 2.50; p = 0.00] and hormones 0.40 [CI 5%, −0.49, 1.29; p = 0.37]. Moreover, SERMS proved to be statistically significant in increasing the serum FSH concentration 3.63 [CI 95%, 1.48, 5.79; p = 0.001], followed by hormones 1.29 [CI 95%, −0.79, 3.36; p = 0.223] and vitamins 0.03 [CI 95%, −2.69, 2.76; p = −2.692] (Figure 5).
Strengths and Limitations
Numerous curbs are associated with this study. Due to lack of resources, non-English studies were not reviewed as it was difficult to translate them to other languages. Combination of data from non-English literature might alter the significance of the current analysis of various male infertility interventions. Secondly, there were few studies with different subgroups making it difficult to get a perfect picture of the overall comparison. In addition, heterogeneity among the one‐on‐one and pairwise comparison was in excess of 70%. However, subgroup analysis and removal of poor quality studies from NMA resolved this issue to some extent but it is still a limitation in this study. Also, population of interest, intervention, comparators, and outcomes were the same across all the studies included in the NMA. Therefore, the chance of clinical heterogeneity is at very minimal to negligible level ruling out statistical heterogeneity. Lastly, due to diversified types of the male infertility along with interventions, all such interventions were classified into five categories, i.e., supplements, hormones, SERMs, vitamins, and enzymes. Along with all the limitations, our NMA is the first study estimating and establishing comparison among all available interventions regarding male infertility and this comprises a very significant aspect of this work.
Discussion
This review is the first of its kind to present NMA on the comparative effect of numerous interventions from different pharmacological groups in managing males with infertility, conducted worldwide, in diverse health care settings under different experimental practices.
The studies included in this review encompass different RCTs conducted using moieties from different pharmacological groups including hormones (FSH, testosterone, and anastrozole), selective estrogen receptor modulators (SERMs) (clomiphene citrate and tamoxifen citrate), supplements (zinc sulfate, CoQ10, carnitine, L-Carnitine, fish oil, and Profertil), vitamins (folic acid, vitamin C, vitamin D, and vitamin E), and enzymes (kallikrein). It is evident from the studies that concomitant administration of supplements, hormones, and SERMS in a patient with male infertility can enhance the production of healthy motile sperms through maintaining adequate serum FSH and testosterone levels. The average duration of clinical outcome was reported to be six months. However, it should be noted that no major side effects were reported from any of the included studies. Common side effects were mild GIT disorders, occasional rashes, nervousness, and drowsiness and in few cases there were hot flashes with increased appetite.
The overall analysis on primary outcomes revealed that supplements, SERMs, and hormones increased sperm concentration 6.26 [95% CI 3.32, 9.21], sperm motility 6.69 [95% CI 2.38, 10.99], and sperm morphology, respectively, superior to other included interventions and placebo. Similar findings were reported by Manish Kuchakulla et al. and Rossella Cannarella et al. that supplements improve fertility, sperm concentration and sperm motility but do not effects sperm morphology, which was also proved through multiple RCTs (Fallah et al., 2018; Cannarella et al., 2020; Kuchakulla et al., 2020). Specifically, zinc sulfate proved to be the best in increasing sperm concentration 11.49 [95% CI −6.42, 29.39] and sperm motility 16.78 [95% CI 14.27, 19.29] among all supplement interventions. This effect of zinc sulfate was due to its ability of increasing low and high molecular weight ligands in the semen (Ahmadi et al., 2016). Among all SERMS interventions, clomiphene citrate was the best in increasing sperm concentration 6.91 [95% CI 5.62, 8.20] and sperm motility 8.17 [95% CI 4.25, 12.10]. Clomiphene citrate exerts its effect through raising the endogenous serum FSH, LH and testosterone levels and initiating gametogenesis (Patankar et al., 2007). Meanwhile, testosterone was the best in increasing sperm morphology among all hormones 12.24 [95% CI 1.00, 23.49]. Studies suggested that increased serum testosterone levels lead to better morphology due to its role in pathogenesis of teratozoospermia (Tang et al., 2012).
In addition to primary outcomes, the results of NMA showed statistically noteworthy effect of supplements, hormones, SERMs, and vitamins on secondary outcomes as well. Total serum testosterone levels were significantly enhanced by supplements 2.70 [95% CI 1.34, 4.07] followed by SERMs 1.83 [95% CI 1.16, 2.50], hormones 0.40 [95% CI −0.49, 1.29], and vitamins −0.70 [95% CI -6.71, 5.31] in comparison to placebo. These are in agreement with previous reports (Thakur et al., 2015; Lo et al., 2018). CoQ10 2.77 [95% CI 1.83, 3.71] and tamoxifen citrate 1.50 [95% CI 1.20, 1.79] showed the best results in improving serum total testosterone levels among all supplements and SERMs, respectively (AinMelk et al., 1987; Krause et al., 1992; Safarinejad, 2009; Çakan et al., 2009; Safarinejad et al., 2012). CoQ10 supplementation was found to ameliorate the reduction in testosterone induced by chemicals mainly by neutralizing the generated free radicals (Banihani, 2018). However, tamoxifen citrate stimulated release of LH and FSH through negative feedback of estrogen at the hypothalamus and pituitary which in turn increases the testosterone biosynthesis and stimulates spermatogenesis (Rambhatla et al., 2016).
Serum FSH concentration was majorly increased by SERMs 3.63 [95% CI 1.48, 5.79] followed by hormones, vitamins, placebo, and supplements. Hormones also increase the serum FSH levels 1.29 [95% CI −0.79, 3.36] followed by a minor increase by vitamins 0.03 [95% CI −2.69, 2.76]. Supplements failed to increase serum FSH levels but rather decreased the serum FSH level significantly −4.45 [95% CI −7.15, −1.76] as compared to placebo. The reason involves understanding of the role of serum FSH in sperm production. FSH, along with testosterone, is necessary for maintaining normal sperm count and function in males. Normal spermatogenesis yields low levels of FSH whereas compromised spermatogenesis can yield high serum FSH levels. As discussed above, supplements are the best in improving sperm concentration through normal spermatogenesis resulting in decreased levels of serum FSH (Orlowski and Sarao, 2018). Tamoxifen citrate 3.66 [95% CI 1.27, 6.05] and FSH 2.74 [95% CI −0.00, 5.48] were the best in increasing the serum FSH levels among all SERMs and hormones, respectively (AinMelk et al., 1987; Krause et al., 1992; Kamischke et al., 1998; Çakan et al., 2009; Selice et al., 2011).
It is evident from the NMA of this review that concomitant use of supplements, SERMs, and hormones was associated with additional clinical benefits beyond sperm concentration, sperm motility, and sperm morphology and these include improvement in sex life and conception (Ring et al., 2016). Strict adherence to therapy along with healthy diet could be clinically significant in reducing male infertility and increasing the chances of conception among couples. Finding of our NMA shows that concomitant use of supplements, hormones, and SERMs irrespective of their types has shown significant increase in sperm concentration, sperm motility, sperm morphology, serum total testosterone, and serum FSH in males’ infertility. Furthermore, considering the type of treatment, combination of zinc sulfate, clomiphene citrate, and testosterone undecanoate can be used to increase the sperm parameters (sperm concentration, sperm motility, and sperm morphology) and combination of CoQ10, tamoxifen citrate, and FSH can be used to improve the hormonal profile in infertile males. The verdict of this NMA will enable policy makers to formulate or choose the different accessible interventions, keeping in view the anticipated advantageous outcomes and existing healthcare assets.
Among all RCTs included, the selection, detection, and performance bias were observed to be <35%. This could perhaps influence the NMA results; therefore, it is recommended to infer our NMA results with caution. Due to lack of methodical content elaboration and varied nature of interventions, it is very difficult to conclude which type of intervention will be the most effective. This is a common issue exclusive in complex interventions in which the description of methods is insufficient to extract data which contribute to success of the regimen. Publishing a separate protocol of study is recommended to empower the readers and investigators to better recognize and comprehend study element, enabling them to be replicated in future.
For better outcome reporting, additional investigation is desirable to evaluate the male infertility interventions with respect to time, frequency, and contents. In addition, this reading endows imperative insights for future research focusing on a couture intervention and economic cost investigation in delivering such interventions, and, hence, designing cost effective interventions. The outcomes of this read will assist policy makers in assortment of suitable interventions keeping in view the paramount utility of the available resources.
Conclusion
It is observed that supplements appeared to be the best in increasing sperm concentration [6.26, 95% CI 3.32, 9.21; p = 0.00] and serum total testosterone levels [2.70, 95% CI 1.34, 4.07; p = 0.00]. On the other hand, hormones and SERMs intervention groups showed better sperm morphology [3.71, 95% CI 1.34, 6.07; p = 0.002), sperm motility [6.69, 95% CI 2.38, 10.99; p = 0.002], and serum FSH level [3.63, 95% CI 1.48, 5.79; p = 0.001], respectively.
Clinical Implications
This is perhaps the first paper to compare the one-on-one comparison among the interventions and controls used to improve the sperm morphology and count. In addition, this paper has also compared the groupwise and overall effect of the intervention using network meta-analysis which will serve as an ideal approach to optimize the therapy based on the effect size and might be useful in optimizing the cost of therapy as well. This study is of significant value for healthcare providers and policy makers in selecting perfect blend of interventions for male infertility patients keeping in view of the existing health resources. This review establishes that all interventions had a significantly positive effect on male infertility (sperm count, sperm motility, sperm morphology, serum testosterone, and FSH). Statistically significant increased sperm parameters (sperm concentration, sperm motility, and sperm morphology) were noted in combinations of zinc sulfate (220 mg BID), clomiphene citrate (50 mg BID), and testosterone undecanoate and CoQ10; tamoxifen citrate and FSH were shown to improve the hormonal profile in infertile males. There is a need for the future experimental studies on these interventions with significant effect size so that a better pharmacotherapy can be planned to improve the outcome of therapy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Authors’ Contributions
MS: conceptualization, methodology, software, data curation, validation, formal analysis, investigation, resources, writing, visualization, and funding acquisition. TKhan: conceptualization, methodology, software, data curation, validation, formal analysis, resources, writing, visualization, supervision, and project administration. CN: conceptualization, validation, resources, supervision, and project administration. QY: conceptualization, validation, resources, supervision, and project administration. AB: methodology, resources, and supervision. MK: conceptualization, validation, resources, supervision, and project administration.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.638628/full#supplementary-material
References
Adamopoulos, D. A., Nicopoulou, S., Kapolla, N., Karamertzanis, M., and Andreou, E. (1997). The Combination of Testosterone Undecanoate with Tamoxifen Citrate Enhances the Effects of Each Agent Given Independently on Seminal Parameters in Men with Idiopathic Oligozoospermia. Fertil. Sterility 67, 756–762. doi:10.1016/s0015-0282(97)81379-9
Adamopoulos, D. A., Pappa, A., Billa, E., Nicopoulou, S., Koukkou, E., and Michopoulos, J. (2003). Effectiveness of Combined Tamoxifen Citrate and Testosterone Undecanoate Treatment in Men with Idiopathic Oligozoospermia. Fertil. Sterility 80, 914–920. doi:10.1016/s0015-0282(03)01123-3
Ahmadi, S., Bashiri, R., Ghadiri-Anari, A., and Nadjarzadeh, A. (2016). Antioxidant Supplements and Semen Parameters: An Evidence Based Review. Ijrm 14, 729–736. doi:10.29252/ijrm.14.12.729
AinMelk, Y., Belisle, S., Carmel, M., Jean-Pierre, T., and Tamoxifen, T. (1987). Tamoxifen Citrate Therapy in Male Infertility. Fertil. sterility 48, 113–117. doi:10.1016/s0015-0282(16)59299-1
Almeida, M. D., Feneux, D., Rigaud, C., and Jouannet, P. (1985). Steroid Therapy for Male Infertility Associated with Antisperm Antibodies. Results of a Small Randomized Clinical Trial. Int. J. Androl. 8, 111–117. doi:10.1111/j.1365-2605.1985.tb00824.x
Alsalman, A. R. S., Almashhedy, L. A., and Hadwan, M. H. (2018). Effect of Oral Zinc Supplementation on the Thiol Oxido-Reductive index and Thiol-Related Enzymes in Seminal Plasma and Spermatozoa of Iraqi Asthenospermic Patients. Biol. Trace Elem. Res. 184, 340–349. doi:10.1007/s12011-017-1215-8
Ambiye, V. R., Langade, D., Dongre, S., Aptikar, P., Kulkarni, M., and Dongre, A. (2013). Clinical Evaluation of the Spermatogenic Activity of the Root Extract of Ashwagandha (Withania Somnifera) in Oligospermic Males: a Pilot Study. Evid. Based Complement. Alternat Med. 2013, 571420. doi:10.1155/2013/571420
Auger, J., and Jouannet, P. (1997). Evidence for regional differences of semen quality among fertile French men. Federation Francaise des Centres d'Etude et de Conservation des Oeufs et du Sperme humains. Hum. Reprod. 12, 740–745. doi:10.1093/humrep/12.4.740
Auger, J., Kunstmann, J. M., Czyglik, F., and Jouannet, P. (1995). Decline in Semen Quality Among fertile Men in Paris during the Past 20 Years. N. Engl. J. Med. 332, 281–285. doi:10.1056/nejm199502023320501
Badenoch, D. F., Waxman, J., Boorman, L., Sidhu, B., Moore, H. D., Holt, W. V., et al. (1988). Administration of a Gonadotropin Releasing Hormone Analogue in Oligozoospermic Infertile Males. Eur. J. Endocrinol. 117, 265–267. doi:10.1530/acta.0.1170265
Baker, H. W. G., Straffon, W. G. E., McGowan, M. P., Burger, H. G., Kretser, D. M., and Hudson, B. (1984). A Controlled Trial of the Use of Erythromycin for Men with Asthenospermia. Int. J. Androl. 7, 383–388. doi:10.1111/j.1365-2605.1984.tb00795.x
Balercia, G., Regoli, F., Armeni, T., Koverech, A., Mantero, F., and Boscaro, M. (2005). Placebo-controlled Double-Blind Randomized Trial on the Use of L-Carnitine, L-Acetylcarnitine, or Combined L-Carnitine and L-Acetylcarnitine in Men with Idiopathic Asthenozoospermia. Fertil. sterility 84, 662–671. doi:10.1016/j.fertnstert.2005.03.064
Banihani, S. (2018). Effect of Coenzyme Q10 Supplementation on Testosterone. Biomolecules 8, 172. doi:10.3390/biom8040172
Boivin, J. (2003). A Review of Psychosocial Interventions in Infertility. Soc. Sci. Med. 57, 2325–2341. doi:10.1016/s0277-9536(03)00138-2
Buhling, K., Schumacher, A., Eulenburg, C. z., and Laakmann, E. (2019). Influence of Oral Vitamin and mineral Supplementation on Male Infertility: a Meta-Analysis and Systematic Review. Reprod. BioMedicine Online 39, 269–279. doi:10.1016/j.rbmo.2019.03.099
Bussen, S., Sütterlin, M., Steck, T., and Dietl, J. (2004). Semen Parameters in Patients with Unilateral Testicular Cancer Compared to Patients with Other Malignancies. Arch. Gynecol. Obstet. 269, 196–198. doi:10.1007/s00404-003-0493-x
Çakan, M., Aldemir, M., Topcuoglu, M., and Altuğ, U. (2009). Role of Testosterone/estradiol Ratio in Predicting the Efficacy of Tamoxifen Citrate Treatment in Idiopathic Oligoasthenoteratozoospermic Men. Urol. Int. 83, 446–451. doi:10.1159/000251186
Cannarella, R., Condorelli, R. A., Mongioì, L. M., Barbagallo, F., Calogero, A. E., and La Vignera, S. (2019). Effects of the Selective Estrogen Receptor Modulators for the Treatment of Male Infertility: a Systematic Review and Meta-Analysis. Expert Opin. Pharmacother. 20, 1517–1525. doi:10.1080/14656566.2019.1615057
Cannarella, R., La Vignera, S., Condorelli, R., Mongioì, L., and Calogero, A. (2020). FSH Dosage Effect on Conventional Sperm Parameters: a Meta-Analysis of Randomized Controlled Studies. Asian J. Androl. 22, 309. doi:10.4103/aja.aja_42_19
Cao, D., Ren, Z., Lu, D., Liu, L., Xu, P., Zhang, Q., et al. (2019). Association between CYP1A1 Rs4646903 T> C Genetic Variations and Male Infertility Risk: A Meta-Analysis. Medicine 98 (31). doi:10.1097/md.0000000000016543
Cardoso, J. P., Cocuzza, M., and Elterman, D. (2019). Optimizing Male Fertility: Oxidative Stress and the Use of Antioxidants. World J. Urol. 37, 1029–1034. doi:10.1007/s00345-019-02656-3
Carlsen, E., Giwercman, A., Keiding, N., and Skakkebaek, N. E. (1992). Evidence for Decreasing Quality of Semen during Past 50 Years. Bmj 305, 609–613. doi:10.1136/bmj.305.6854.609
Caroppo, E., Niederberger, C., Vizziello, G. M., and D'Amato, G. (2003). Recombinant Human Follicle-Stimulating Hormone as a Pretreatment for Idiopathic Oligoasthenoteratozoospermic Patients Undergoing Intracytoplasmic Sperm Injection. Fertil. sterility 80, 1398–1403. doi:10.1016/s0015-0282(03)02202-7
Clark, R. V., and Sherins, R. J. (1989). Treatment of Men with Idiopathic Oligozoospermic Infertility Using the Aromatase Inhibitor, Testolactone Results of a Double-Blinded, Randomized, Placebo-Controlled Trial with Crossover. J. Androl. 10, 240–247. doi:10.1002/j.1939-4640.1989.tb00094.x
Colacurci, N., Monti, M. G., Fornaro, F., Izzo, G., Izzo, P., Trotta, C., et al. (2012). Recombinant Human FSH Reduces Sperm DNA Fragmentation in Men with Idiopathic Oligoasthenoteratozoospermia. J. Androl. 33, 588–593. doi:10.2164/jandrol.111.013326
Comhaire, F. H., Rowe, P. J., and Farley, T. M. M. (1986). The Effect of Doxycycline in Infertile Couples with Male Accessory Gland Infection: a Double Blind Prospective Study. Int. J. Androl. 9, 91–98. doi:10.1111/j.1365-2605.1986.tb00871.x
Crottaz, B., Senn, A., Reymond, M. J., Rey, F., Germond, M., and Gomez, F. (1992). Follicle-stimulating Hormone Bioactivity in Idiopathic Normogonadotropic Oligoasthenozoospermia: Double-Blind Trial with Gonadotropin-Releasing hormone**Supported by grant No 3.866-0.86 of the Swiss National Science Foundation and by Hoechst Pharma AG, Zürich, Switzerland. Fertil. sterility 57, 1034–1043. doi:10.1016/s0015-0282(16)55022-5
Ding, Y.-m., Zhang, X.-j., Li, J.-P., Chen, S.-s., Zhang, R.-t., Tan, W.-l., et al. (2015). Treatment of Idiopathic Oligozoospermia with Recombinant Human Follicle-Stimulating Hormone: a Prospective, Randomized, Double-Blind, Placebo-Controlled Clinical Study in Chinese Population. Clin. Endocrinol. 83, 866–871. doi:10.1111/cen.12770
ElSheikh, M. G., Hosny, M. B., Elshenoufy, A., Elghamrawi, H., Fayad, A., and Abdelrahman, S. (2015). Combination of Vitamin E and Clomiphene Citrate in Treating Patients with Idiopathic Oligoasthenozoospermia: A Prospective, Randomized Trial. Andrology 3, 864–867. doi:10.1111/andr.12086
Fallah, A., Mohammad-Hasani, A., and Colagar, A. H. (2018). Zinc Is an Essential Element for Male Fertility: A Review of Zn Roles in Men's Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil 19, 69–81.
Farmakiotis, D., Farmakis, C., Rousso, D., Kourtis, A., Katsikis, I., and Panidis, D. (2007). The Beneficial Effects of Toremifene Administration on the Hypothalamic-Pituitary-Testicular axis and Sperm Parameters in Men with Idiopathic Oligozoospermia. Fertil. sterility 88, 847–853. doi:10.1016/j.fertnstert.2006.12.038
Farrag, A., Sagnella, F., Pappalardo, S., Costantini, A., Lisi, F., Carfagna, P., et al. (2015). The Use of R-hFSH in Treatment of Idiopathic Male Factor Infertility before ICSI. Eur. Rev. Med. Pharmacol. Sci. 19, 2162–2167.
Fedder, M. D., Jakobsen, H. B., Giversen, I., Christensen, L. P., Parner, E. T., and Fedder, J. (2014). An Extract of Pomegranate Fruit and Galangal Rhizome Increases the Numbers of Motile Sperm: a Prospective, Randomised, Controlled, Double-Blinded Trial. PloS one 9. doi:10.1371/journal.pone.0108532
Fisch, H., and Goluboff, E. T. (1996). Geographic Variations in Sperm Counts: a Potential Cause of Bias in Studies of Semen Quality. Fertil. Sterility 65, 1044–1046. doi:10.1016/s0015-0282(16)58284-3
Fisch, H., Goluboff, E. T., Olson, J. H., Feldshuh, J., Broder, S. J., and Barad, D. H. (1996). Semen Analyses in 1,283 Men from the United States over a 25-year Period: No Decline in Quality. Fertil. sterility 65, 1009–1014. doi:10.1016/s0015-0282(16)58278-8
Foresta, C., Bettella, A., Garolla, A., Ambrosini, G., and Ferlin, A. (2005). Treatment of Male Idiopathic Infertility with Recombinant Human Follicle-Stimulating Hormone: a Prospective, Controlled, Randomized Clinical Study. Fertil. sterility 84, 654–661. doi:10.1016/j.fertnstert.2005.03.055
Foresta, C., Bettella, A., Merico, M., Garolla, A., Ferlin, A., and Rossato, M. (2002). Use of Recombinant Human Follicle-Stimulating Hormone in the Treatment of Male Factor Infertility. Fertil. sterility 77, 238–244. doi:10.1016/s0015-0282(01)02966-1
Garolla, A., Petre, G. C., Francini-Pesenti, F., De Toni, L., Vitagliano, A., Di Nisio, A., et al. (2020). Food Supplements in the Treatment of Male Infertility: A Critical Review on Their Formulations and Use.
Gerris, J., Comhaire, F., Hellemans, P., Peeters, K., and Schoonjans, F. (1991). Placebo-controlled Trial of High-Dose Mesterolone**Schering N.V., Brussels, Belgium. Treatment of Idiopathic Male Infertility. Fertil. sterility 55, 603–607. doi:10.1016/s0015-0282(16)54193-4
Ghanem, H., Shaeer, O., and El-Segini, A. (2010). Combination Clomiphene Citrate and Antioxidant Therapy for Idiopathic Male Infertility: a Randomized Controlled Trial. Fertil. Sterility 93, 2232–2235. doi:10.1016/j.fertnstert.2009.01.117
Giahi, L., Mohammadmoradi, S., Javidan, A., and Sadeghi, M. R. (2016). Nutritional Modifications in Male Infertility: a Systematic Review Covering 2 Decades. Nutr. Rev. 74, 118–130. doi:10.1093/nutrit/nuv059
Giwercman, A., and Skakkebaek, N. E. (1992). The Human Testis-An Organ at Risk? Int. J. Androl. 15, 373–375. doi:10.1111/j.1365-2605.1992.tb01351.x
Glezerman, M., Lunenfeld, E., Potashnik, G., Huleihel, M., Soffer, Y., and Segal, S. (1993). Efficacy of Kallikrein in the Treatment of Oligozoospermia and Asthenozoospermia: a Double-Blind trial*†*Presented at the Annual Meeting of the Israel Fertility Association, Tel Aviv, April 21 to 22, 1993.†Supported by a grant from AFB-Parexel, Clinical Research Division, Frankfurt Main, Germany. Fertil. sterility 60, 1052–1056. doi:10.1016/s0015-0282(16)56409-7
Gopinath, P., Kalra, B., Saxena, A., Malik, S., Kochhar, K., Kalra, S., et al. (2013). Fixed Dose Combination Therapy of Antioxidants in Treatment of Idiopathic Oligoasthenozoospermia: Results of a Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Infertil Fetal Med. 4, 6–13.
Greco, T., Biondi-Zoccai, G., Saleh, O., Pasin, L., Cabrini, L., Zangrillo, A., et al. (2015). The Attractiveness of Network Meta-Analysis: a Comprehensive Systematic and Narrative Review. Heart Lung Vessel 7, 133–142.
Guo, L., Jing, J., Feng, Y.-M., and Yao, B. (2015). Tamoxifen Is a Potent Antioxidant Modulator for Sperm Quality in Patients with Idiopathic Oligoasthenospermia. Int. Urol. Nephrol. 47, 1463–1469. doi:10.1007/s11255-015-1065-2
Haas, G. G., and Manganiello, P. (1987). A Double-Blind, Placebo-Controlled Study of the Use of Methylprednisolone in Infertile Men with Sperm-Associated immunoglobulins**Presented in Part at the Forty-First Annual Meeting of the American Fertility Society, Chicago, Illinois, September 28 to October 2, 1985.††Supported in Part by National Institute of Health grant 19908. Fertil. sterility 47, 295–301. doi:10.1016/s0015-0282(16)50009-0
Hadwan, M. H., Almashhedy, L. A., and Alsalman, A. R. S. (2012). Oral Zinc Supplementation Restore High Molecular Weight Seminal Zinc Binding Protein to normal Value in Iraqi Infertile Men. BMC Urol. 12, 32. doi:10.1186/1471-2490-12-32
Haghighian, H. K., Haidari, F., Mohammadi-Asl, J., and Dadfar, M. (2015). Randomized, Triple-Blind, Placebo-Controlled Clinical Trial Examining the Effects of Alpha-Lipoic Acid Supplement on the Spermatogram and Seminal Oxidative Stress in Infertile Men. Fertil. sterility 104, 318–324. doi:10.1016/j.fertnstert.2015.05.014
Haje, M., and Naoom, K. (2015). Combined Tamoxifen and L-Carnitine Therapies for the Treatment of Idiopathic Male Infertility Attending Intracytoplasmic Sperm Injection: a Randomized Controlled Trial. Int. J. Infertil Fetal Med. 6, 20–24. doi:10.5005/jp-journals-10016-1096
Hamada, A. J., Montgomery, B., and Agarwal, A. (2012). Male Infertility: a Critical Review of Pharmacologic Management. Expert Opin. Pharmacother. 13, 2511–2531. doi:10.1517/14656566.2012.740011
Hargreave, T. B., Kyle, K. F., Baxby, K., Rogers, A. C. N., Scott, R., Tolley, D. A., et al. (1984). Randomised Trial of Mesterolone versus Vitamin C for Male Infertility. Br. J. Urol. 56, 740–744. doi:10.1111/j.1464-410x.1984.tb06160.x
Helo, S., Ellen, J., Mechlin, C., Feustel, P., Grossman, M., Ditkoff, E., et al. (2015). A Randomized Prospective Double‐Blind Comparison Trial of Clomiphene Citrate and Anastrozole in Raising Testosterone in Hypogonadal Infertile Men. J. Sex. Med. 12, 1761–1769. doi:10.1111/jsm.12944
Hosseini, B., Nourmohamadi, M., Hajipour, S., Taghizadeh, M., Asemi, Z., Keshavarz, S. A., et al. (2019). The Effect of omega-3 Fatty Acids, EPA, And/or DHA on Male Infertility: a Systematic Review and Meta-Analysis. J. dietary supplements 16, 245–256. doi:10.1080/19390211.2018.1431753
Hosseini, J., Mardi Mamaghani, A., Hosseinifar, H., Sadighi Gilani, M. A., Dadkhah, F., and Sepidarkish, M. (2016). The Influence of Ginger (Zingiber Officinale) on Human Sperm Quality and DNA Fragmentation: A Double-Blind Randomized Clinical Trial. Ijrm 14, 533–540. doi:10.29252/ijrm.14.8.533
Imhof, M., Lackner, J., Lipovac, M., Chedraui, P., and Riedl, C. (2012). Improvement of Sperm Quality after Micronutrient Supplementation. e-SPEN J. 7, e50–e53. doi:10.1016/j.eclnm.2011.11.002
Inton, R. A., Egdell, L. M., Andrews, B. E., Clarke, S. K. R., and Richmond, S. J. (1979). A Double-Bljnd Cross-Over Study of the Effect of Doxycycline on Mycoplasma Infection and Infertility. BJOG:An Int. J. O&G 86, 379–383. doi:10.1111/j.1471-0528.1979.tb10614.x
Ismail, S. B., Bakar, M. B., Nik Hussain, N. H., Norhayati, M. N., Sulaiman, S. A., Jaafar, H., et al. (2014). Comparison on the Effects and Safety of Tualang Honey and Tribestan in Sperm Parameters, Erectile Function, and Hormonal Profiles Among Oligospermic Males. Evid. Based Complement. Alternat Med. 2014, 126138. doi:10.1155/2014/126138
Izzo, P. L., Canale, D., Bianchi, B., Meschini, P., Esposito, G., Menchini Fabris, G. F., et al. (1984). The Treatment of Male Subfertility with Kallikrein. Andrologia 16, 156–161. doi:10.1111/j.1439-0272.1984.tb00256.x
Jensen, T. K., Priskorn, L., Holmboe, S. A., Nassan, F. L., Andersson, A.-M., Dalgård, C., et al. (2020). Associations of Fish Oil Supplement Use with Testicular Function in Young Men. JAMA Netw. Open 3, e1919462. doi:10.1001/jamanetworkopen.2019.19462
Jørgensen, N., Andersen, A. G., Eustache, F., Irvine, D. S., Suominen, J., Petersen, J. H., et al. (2001). Regional Differences in Semen Quality in Europe. Hum. Reprod. 16 (5), 1012–1019.
Kamischke, A., Behre, H. M., Bergmann, M., Simoni, M., Schafer, T., and Nieschlag, E. (1998). Recombinant Human Follicle Stimulating Hormone for Treatment of Male Idiopathic Infertility: a Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Hum. Reprod. 13, 596–603. doi:10.1093/humrep/13.3.596
Kamischke, A., and Nieschlag, E. (1999). Analysis of Medical Treatment of Male Infertility. Hum. Reprod. 14, 1–23. doi:10.1093/humrep/14.suppl_1.1
Keck, C., Behre, H. M., Jockenhovel, F., and Nieschlag, E. (1994). Ineffectiveness of Kallikrein in Treatment of Idiopathic Male Infertility: a Double-Blind, Randomized, Placebo-Controlled Trial. Hum. Reprod. 9, 325–329. doi:10.1093/oxfordjournals.humrep.a138501
Knuth, U. A., Hönigl, W., Bals-Pratsch, M., Schleicher, G., and Nieschlag, E. (1987). Treatment of Severe Oligospermia with Human Chorionic Gonadotropin/human Menopausal Gonadotropin: a Placebo-Controlled, Double Blind Trial. J. Clin. Endocrinol. Metab. 65, 1081–1087. doi:10.1210/jcem-65-6-1081
Krause, W., Holland-moritz, H., and Schramm, P. (1992). Treatment of Idiopathic Oligozoospermia with Tamoxifen - a Randomized Controlled Study. Int. J. Androl. 15, 14–18. doi:10.1111/j.1365-2605.1992.tb01110.x
Kuchakulla, M., Soni, Y., Patel, P., Parekh, N., and Ramasamy, R. (2020). A Systematic Review and Evidence-Based Analysis of Ingredients in Popular Male Fertility Supplements. Urology 136, 133–141. doi:10.1016/j.urology.2019.11.007
Kumar, N., and Singh, A. (2015). Trends of Male Factor Infertility, an Important Cause of Infertility: A Review of Literature. J. Hum. Reprod. Sci. 8, 191. doi:10.4103/0974-1208.170370
Kumar, R., Shamsi, M., Dada, R., Saxena, V., and Venkatesh, S. (2011). Herbo-mineral Supplementation in Men with Idiopathic Oligoasthenoteratospermia : A Double Blind Randomized Placebo-Controlled Trial. Indian J. Urol. 27, 357. doi:10.4103/0970-1591.85440
Lackner, J., Schatzl, G., Waldhör, T., Resch, K., Kratzik, C., and Marberger, M. (2005). Constant Decline in Sperm Concentration in Infertile Males in an Urban Population: Experience over 18 Years. Fertil. sterility 84, 1657–1661. doi:10.1016/j.fertnstert.2005.05.049
Lafuente, R., González-Comadrán, M., Solà, I., López, G., Brassesco, M., Carreras, R., et al. (2013). Coenzyme Q10 and Male Infertility: a Meta-Analysis. J. Assist. Reprod. Genet. 30, 1147–1156. doi:10.1007/s10815-013-0047-5
Lenzi, A., Lombardo, F., Sgrò, P., Salacone, P., Caponecchia, L., Dondero, F., et al. (2003). Use of Carnitine Therapy in Selected Cases of Male Factor Infertility: a Double-Blind Crossover Trial. Fertil. sterility 79, 292–300. doi:10.1016/s0015-0282(02)04679-4
Lenzi, A., Sgrò, P., Salacone, P., Paoli, D., Gilio, B., Lombardo, F., et al. (2004). A Placebo-Controlled Double-Blind Randomized Trial of the Use of Combined L-Carnitine and L-Acetyl-Carnitine Treatment in Men with Asthenozoospermia. Fertil. sterility 81, 1578–1584. doi:10.1016/j.fertnstert.2003.10.034
Lipovac, M., Bodner, F., Imhof, M., and Chedraui, P. (2016). Comparison of the Effect of a Combination of Eight Micronutrients versus a Standard Mono Preparation on Sperm Parameters. Reprod. Biol. Endocrinol. 14, 84. doi:10.1186/s12958-016-0219-0
Lo, E. M., Rodriguez, K. M., Pastuszak, A. W., and Khera, M. (2018). Alternatives to Testosterone Therapy: a Review. Sex. Med. Rev. 6, 106–113. doi:10.1016/j.sxmr.2017.09.004
Maier, U., and Hienert, G. (1990). Tamoxifen and Kallikrein in Therapy of Oligoasthenozoospermia: Results of a Randomized Study. Eur. Urol. 17, 223–225. doi:10.1159/000464043
Maretti, C., and Cavallini, G. (2017). The Association of a Probiotic with a Prebiotic (Flortec, Bracco) to Improve the Quality/quantity of Spermatozoa in Infertile Patients with Idiopathic Oligoasthenoteratospermia: a Pilot Study. Andrology 5, 439–444. doi:10.1111/andr.12336
Marimuthu, P., Kapilashrami, M. C., Misro, M. M., and Singh, G. (2003). Evaluation of Trend in Semen Analysis for 11 Years in Subjects Attending a Fertility Clinic in India. Asian J. Androl. 5, 221–225.
Matsumiya, K., Kitamura, M., Kishikawa, H., Kondoh, N., Fujiwara, Y., Namiki, M., et al. (1998). A Prospective Comparative Trial of a Gonadotropin-Releasing Hormone Analogue with Clomiphene Citrate for the Treatment of Oligoasthenozoospermia. Int. J. Urol. 5, 361–363. doi:10.1111/j.1442-2042.1998.tb00367.x
Mbah, A. U., Ndukwu, G. O., Ghasi, S. I., Shu, E. N., Ozoemena, F. N., Mbah, J. O., et al. (2012). Low-Dose Lisinopril in Normotensive Men with Idiopathic Oligospermia and Infertility: A 5-Year Randomized, Controlled, Crossover Pilot Study. Clin. Pharmacol. Ther. 91, 582–589. doi:10.1038/clpt.2011.265
Mehni, N. M., Ketabchi, A. A., and Hosseini, E. (2014). Combination Effect of Pentoxifylline and L-Carnitine on Idiopathic Oligoasthenoteratozoospermia. Iranian J. Reprod. Med. 12, 817.
Merino, G., Chéquer, J. C. M., Barahona, E., Bermúdez, J. A., Morán, C., and Carranza-lira, S. (1997). Effects of Pentoxifylline on Sperm Motility in Normogonadotropic Asthenozoospermic Men. Arch. Androl. 39, 65–69. doi:10.3109/01485019708987903
Mićić, S., and Dotlić, R. (1985). Evaluation of Sperm Parameters in Clinical Trial with Clomiphene Citrate of Oligospermic Men. J. Urol. 133, 221–222.
Modarresi, R., Aminsharifi, A., and Foroughinia, F. (2019). Impact of Spirulina Supplementation on Semen Parameters in Patients with Idiopathic Male Infertility: A Pilot Randomized Trial. Urol. J. 16, 78–82. doi:10.22037/uj.v0i0.4122
Moradi, M., Moradi, A., Alemi, M., Ahmadnia, H., Abdi, H., Ahmadi, A., et al. (2010). Safety and Efficacy of Clomiphene Citrate and L-Carnitine in Idiopathic Male Infertility: a Comparative Study. Urol. J. 7, 188–193.
Nadjarzadeh, A., Sadeghi, M. R., Amirjannati, N., Vafa, M. R., Motevalian, S. A., Gohari, M. R., et al. (2011). Coenzyme Q10 Improves Seminal Oxidative Defense but Does Not Affect on Semen Parameters in Idiopathic Oligoasthenoteratozoospermia: a Randomized Double-Blind, Placebo Controlled Trial. J. Endocrinol. Invest. 34, e224–8. doi:10.3275/7572
Nadjarzadeh, A., Shidfar, F., Amirjannati, N., Vafa, M. R., Motevalian, S. A., Gohari, M. R., et al. (2014). Effect of Coenzyme Q10 Supplementation on Antioxidant Enzymes Activity and Oxidative Stress of Seminal Plasma: a Double-Blind Randomised Clinical Trial. Andrologia 46, 177–183. doi:10.1111/and.12062
Nieschlag, E., Bouloux, P-M. G., Stegmann, B. J., Shankar, R. R., Guan, Y., Tzontcheva, A., et al. (2017). An Open-Label Clinical Trial to Investigate the Efficacy and Safety of Corifollitropin Alfa Combined with hCG in Adult Men with Hypogonadotropic Hypogonadism. Reprod. Biol. Endocrinol. 15, 17. doi:10.1186/s12958-017-0232-y
Orlowski, M., and Sarao, M. S. (2018). Physiology, Follicle Stimulating Hormone. Follicle Stimulating Horm. 6.
Paradisi, R., Busacchi, P., Seracchioli, R., Porcu, E., and Venturoli, S. (2006). Effects of High Doses of Recombinant Human Follicle-Stimulating Hormone in the Treatment of Male Factor Infertility: Results of a Pilot Study. Fertil. sterility 86, 728–731. doi:10.1016/j.fertnstert.2006.02.087
Paradisi, R., Natali, F., Fabbri, R., Battaglia, C., Seracchioli, R., and Venturoli, S. (2014). Evidence for a Stimulatory Role of High Doses of Recombinant Human Follicle-Stimulating Hormone in the Treatment of Male-Factor Infertility. Andrologia 46, 1067–1072. doi:10.1111/and.12194
Park, H. J., Choe, S., and Park, N. C. (2016). Effects of Korean Red Ginseng on Semen Parameters in Male Infertility Patients: a Randomized, Placebo-Controlled, Double-Blind Clinical Study. Chin. J. Integr. Med. 22, 490–495. doi:10.1007/s11655-015-2139-9
Patankar, S. S., Kaore, S. B., Sawane, M. V., Mishra, N. V., and Deshkar, A. M. (2007). Effect of Clomiphene Citrate on Sperm Density in Male Partners of Infertile Couples. Indian J. Physiol. Pharmacol. 51, 195–198.
Pryor, J. P., Blandy, J. P., Evans, P., Saintonge, D. M. C. D., and Usherwood, M. (1978). Controlled Clinical Trial of Arginine for Infertile Men with Oligozoospermia. Br. J. Urol. 50, 47–50. doi:10.1111/j.1464-410x.1978.tb02765.x
Pusch, H. (1988). Oral Treatment of Oligozoospermia with Testosterone‐Undecanoate: Results of a Double‐Blind‐Placebo‐Controlled Trial/Orale Behandlung der Oligozoospermie mit Testosteronundecanoat: Ergebnisse einer Plazebokontrollierten Doppelblindstudie. Andrologia 21, 76–82.
Rambhatla, A., Mills, J. N., and Rajfer, J. (2016). The Role of Estrogen Modulators in Male Hypogonadism and Infertility. Rev. Urol. 18, 66–72. doi:10.3909/riu0711
Ring, J., Lwin, A., and Köhler, T. (2016). Current Medical Management of Endocrine-Related Male Infertility. Asian J. Androl. 18, 357. doi:10.4103/1008-682x.179252
Safarinejad, M. R. (2011). Effect of omega-3 Polyunsaturated Fatty Acid Supplementation on Semen Profile and Enzymatic Anti-oxidant Capacity of Seminal Plasma in Infertile Men with Idiopathic Oligoasthenoteratospermia: a Double-Blind, Placebo-Controlled, Randomised Study. Andrologia 43, 38–47. doi:10.1111/j.1439-0272.2009.01013.x
Safarinejad, M. R. (2011). Effect of Pentoxifylline on Semen Parameters, Reproductive Hormones, and Seminal Plasma Antioxidant Capacity in Men with Idiopathic Infertility: a Randomized Double-Blind Placebo-Controlled Study. Int. Urol. Nephrol. 43, 315–328. doi:10.1007/s11255-010-9826-4
Safarinejad, M. R. (2009). Efficacy of Coenzyme Q10 on Semen Parameters, Sperm Function and Reproductive Hormones in Infertile Men. J. Urol. 182, 237–248. doi:10.1016/j.juro.2009.02.121
Safarinejad, M. R., and Safarinejad, S. (2009). Efficacy of Selenium And/or N-Acetyl-Cysteine for Improving Semen Parameters in Infertile Men: a Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 181, 741–751. doi:10.1016/j.juro.2008.10.015
Safarinejad, M. R., Safarinejad, S., Shafiei, N., and Safarinejad, S. (2012). Effects of the Reduced Form of Coenzyme Q 10 (Ubiquinol) on Semen Parameters in Men with Idiopathic Infertility: a Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 188, 526–531. doi:10.1016/j.juro.2012.03.131
Safarinejad, M. R., Shafiei, N., and Safarinejad, S. (2011). A Prospective Double-Blind Randomized Placebo-Controlled Study of the Effect of Saffron (Crocus Sativus Linn.) on Semen Parameters and Seminal Plasma Antioxidant Capacity in Infertile Men with Idiopathic Oligoasthenoteratozoospermia. Phytother. Res. 25, 508–516. doi:10.1002/ptr.3294
Scott, R., MacPherson, A., Yates, R., Hussain, B., and Dixon, J. (1998). The Effect of Oral Selenium Supplementation on Human Sperm Motility. BJU Int. 82, 76–80. doi:10.1046/j.1464-410x.1998.00683.x
Selice, R., Garolla, A., Pengo, M., Caretta, N., Ferlin, A., and Foresta, C. (2011). The Response to FSH Treatment in Oligozoospermic Men Depends on FSH Receptor Gene Polymorphisms. Int. J. Androl. 34, 306–312. doi:10.1111/j.1365-2605.2010.01086.x
Sigman, M., Glass, S., Campagnone, J., and Pryor, J. L. (2006). Carnitine for the Treatment of Idiopathic Asthenospermia: a Randomized, Double-Blind, Placebo-Controlled Trial. Fertil. sterility 85, 1409–1414. doi:10.1016/j.fertnstert.2005.10.055
Smits, R. M., Mackenzie‐Proctor, R., Yazdani, A., Stankiewicz, M. T., Jordan, V., and Showell, M. G. (2019). Antioxidants for Male Subfertility. Cochrane Database of Systematic Reviews (London, United Kingdom: Cochrane Library).
Suleiman, S. A., Ali, M. E., Zaki, Z. M., el-Malik, E. M., and Nasr, M. A. (1996). Lipid Peroxidation and Human Sperm Motility: Protective Role of Vitamin E. J. Androl. 17, 530–537.
Swan, S. H. (2006). Semen Quality in fertile US Men in Relation to Geographical Area and Pesticide Exposure. Int. J. Androl. 29, 62–68. doi:10.1111/j.1365-2605.2005.00620.x
Tang, W. H., Jiang, H., Ma, L. L., Hong, K., Zhong, Q., Yang, C. S., et al. (2012). [Relationship of Sperm Morphology with Reproductive Hormone Levels in Infertile Men]. Zhonghua Nan Ke Xue 18, 243–247.
Thakur, A. S., Littarru, G. P., Funahashi, I., Painkara, U. S., Dange, N. S., and Chauhan, P. (2015). Effect of Ubiquinol Therapy on Sperm Parameters and Serum Testosterone Levels in Oligoasthenozoospermic Infertile Men. J. Clin. Diagn. Res. 9, BC01–3. doi:10.7860/JCDR/2015/13617.6424
Tonin, F. S., Rotta, I., Mendes, A. M., and Pontarolo, R. (2017). Network Meta-Analysis: a Technique to Gather Evidence from Direct and Indirect Comparisons. Pharm. Pract. (Granada) 15. doi:10.18549/pharmpract.2017.01.943
Wang, M., Wang, Q., Du, Y., Jiang, H., and Zhang, X. (2020). Vitamins Combined with Traditional Chinese Medicine for Male Infertility: A Systematic Review and Meta‐analysis, Andrology 8, 1038–1050. doi: doi:10.1111/andr.12787
Wang, R., Danhof, N. A., Tjon-Kon-Fat, R. I., Eijkemans, M. J., Bossuyt, P. M., Mochtar, M. H., et al. (2019). Interventions for Unexplained Infertility: a Systematic Review and Network Meta‐analysis. Cochrane Database Syst. Rev. 9 (9), CD012692. doi:10.1002/14651858.cd012692.pub2
Wiehle, R. D., Fontenot, G. K., Wike, J., Hsu, K., Nydell, J., and Lipshultz, L. (2014). Enclomiphene Citrate Stimulates Testosterone Production while Preventing Oligospermia: a Randomized Phase II Clinical Trial Comparing Topical Testosterone. Fertil. sterility 102, 720–727. doi:10.1016/j.fertnstert.2014.06.004
Williams, E. A., Parker, M., Robinson, A., Pitt, S., and Pacey, A. A. (2020). A Randomized Placebo-Controlled Trial to Investigate the Effect of Lactolycopene on Semen Quality in Healthy Males. Eur. J. Nutr. 59, 825–833. doi:10.1007/s00394-019-02091-5
Willis, K. J., London, D. R., Bevis, M. A., Butt, W. R., Lynch, S. S., and Holder, G. (1977). Hormonal Effects of Tamoxifen in Oligospermic Men. J. Endocrinol. 73, 171–178. doi:10.1677/joe.0.0730171
Wong, W. Y., Merkus, H. M. W. M., Thomas, C. M. G., Menkveld, R., Zielhuis, G. A., and Steegers-Theunissen, R. P. M. (2002). Effects of Folic Acid and Zinc Sulfate on Male Factor Subfertility: a Double-Blind, Randomized, Placebo-Controlled Trial. Fertil. sterility 77, 491–498. doi:10.1016/s0015-0282(01)03229-0
Yamamoto, M., Hibi, H., and Miyake, K. (1995). Comparison of the Effectiveness of Placebo and α-blocker Therapy for the Treatment of Idiopathic Oligozoospermia*. Fertil. Sterility 63, 396–400. doi:10.1016/s0015-0282(16)57375-0
Yamamoto, M., Hibi, H., and Miyake, K. (1995). New Treatment of Idiopathic Severe Oligozoospermia with Mast Cell Blocker: Results of a Single-Blind study**Supported by a grant-in-aid for Scientific Research (07671716) from the Ministry of Education, Science and Culture of Japan, Tokyo, Japan. Fertil. Sterility 64, 1221–1223. doi:10.1016/s0015-0282(16)57992-8
Keywords: male infertility, meta-analysis, network meta-analysis, coenzyme Q10, follicle stimulating hormone, selective estrogen receptor modulator
Citation: Shahid MN, Khan TM, Neoh CF, Lean QY, Bukhsh A and Karuppannan M (2021) Effectiveness of Pharmacological Intervention Among Men with Infertility: A Systematic Review and Network Meta-Analysis. Front. Pharmacol. 12:638628. doi: 10.3389/fphar.2021.638628
Received: 07 December 2020; Accepted: 09 July 2021;
Published: 16 August 2021.
Edited by:
Viviane De Maertelaer, Université Libre de Bruxelles, BelgiumReviewed by:
Fathi M. Sherif, University of Tripoli, LibyaSandro La Vignera, University of Catania, Italy
Copyright © 2021 Shahid, Khan, Neoh, Lean, Bukhsh and Karuppannan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Nabeel Shahid, bmFiZWVsc2hhaGlka0Bob3RtYWlsLmNvbQ==; Tahir Mehmood Khan, dGFoaXIua2hhbkB1dmFzLmVkdS5waw==
 Muhammad Nabeel Shahid
Muhammad Nabeel Shahid Tahir Mehmood Khan
Tahir Mehmood Khan Chin Fen Neoh
Chin Fen Neoh Qi Ying Lean
Qi Ying Lean Allah Bukhsh
Allah Bukhsh Mahmathi Karuppannan
Mahmathi Karuppannan