
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol., 12 April 2021
Sec. Drugs Outcomes Research and Policies
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.635165
This article is part of the Research TopicTherapeutic Drug Monitoring in Solid Organ TransplantationView all 9 articles
This review summarizes how possible age-related changes in tacrolimus and cyclosporine pharmacokinetics and pharmacodynamics may influence drug dosing and monitoring in the elderly, and highlights how micro-sampling may be useful in this cohort in the future. Advancing biological age leads to physiological changes that can affect drug absorption, distribution, metabolism and excretion, as well as immune system responsiveness. Some studies have shown that elderly recipients may have higher dose-adjusted exposure and/or lower clearance of the calcineurin inhibitors, suggesting that doses may need to be lowered in elderly recipients. Only one study has examined how aging effects drug target enzyme activity and demonstrated that age does not correlate with the calcineurin inhibitor half-maximal inhibitory concentration. Several studies have shown elderly kidney transplant recipients have increased risk of both morbidity and mortality, compared to younger adults due to increased susceptibility to immunosuppressant side effects, particularly cardiovascular disease, infection and malignancy. Current immunosuppressant dosing and monitoring protocols often make no adjustments for age. Lower maintenance immunosuppressant targets in elderly recipients may decrease patient susceptibility to drug side effects, however, further studies are required and appropriate targets need to be established. Blood draw by micro-sampling may be useful for drug monitoring in this cohort in the future, as blood collection is minimally invasive and less painful than venepuncture. Micro-sampling could also make further pharmacokinetic, pharmacodynamics and outcome studies in the elderly more feasible.
Kidney transplantation is first-line treatment for younger patients with end stage renal disease as compared to dialysis, it provides both an increased life expectancy and improved quality of life (Chadban et al., 2012; Dreyer et al., 2015; Registry, 2017; Procurement and Transplantation Network, 2020; Scuderi et al., 2020). Transplantation in the elderly is becoming commonplace, with 19% of kidney transplants in Europe in 2017 (Registry, 2017), and 22% in the USA in 2019 (Procurement and Transplantation Network, 2020) performed in patients aged 65 years and older. A rise in the prevalence of transplantation in the elderly has been attributed to an aging population (Petzinger et al., 2006), increased use of expanded criteria donor kidneys (Dreyer et al., 2015), and improvements in transplant outcomes (Miura et al., 2009).
For the purposes of this review, we define elderly as 65 years of age or older (World Health Organisation, 2015). We acknowledge that this definition may seem inappropriate and outdated as patients are now transplanted well into their 70’s. The drug pharmacokinetics and pharmacodynamics of a 65 year old renal transplant recipient does not necessarily reflect a patient in their 70’s or 80’s. However, currently there are few studies reporting kidney transplant outcomes in recipients aged over 70 years which in part, may be due to the recent and rapid increase in the number of transplants in this age group.
Life-long adherence to immunosuppressant therapy is critical to support long-term graft survival and improve quality of life in the elderly. The intricate balance between under- and over-immunosuppression is increasingly complex in this cohort, due to changes in drug pharmacokinetics and pharmacodynamics (Boesmueller et al., 2011; Krenzien et al., 2015; Karpe et al., 2017). When compared to younger adults, there seems to be greater variability in drug responsiveness (Karpe et al., 2017). Presently, the elderly are the most likely age group to die with a functioning graft (Aymanns et al., 2010; Le Meur, 2015; Registry, 2017); the three main causes of death namely infection, malignancy and cardiovascular events, may be partly attributed to immunosuppressant medication usage (Turnheim, 2003; Barten et al., 2007; Le Meur, 2015; Milone, 2016).
Calcineurin inhibitors, tacrolimus and cyclosporine, are pivotal to mainstay immunosuppressant regimens in kidney transplant recipients (Chadban et al., 2012). Both agents have a narrow therapeutic window and display large pharmacokinetic variability. Therapeutic drug monitoring (TDM) is performed regularly in patients receiving these agents allowing drug doses to be adjusted to maintain drug blood concentrations within a desired therapeutic range (Chadban et al., 2012). TDM currently utilizes venepuncture sampling which requires a larger volume of blood and greater patient discomfort as some elderly patients have difficulty with venous access due to cardiovascular disease or poor venous access.
This review summarizes how possible age-related changes in calcineurin inhibitor pharmacokinetics and pharmacodynamics may influence drug dosing and monitoring in the elderly, and highlights how micro-sampling may be useful in this cohort in the future.
Advancing biological age leads to physiological changes that can affect drug absorption, distribution, metabolism and excretion. For some drugs, there may be a slight decline in absorption due to a reduction in the surface area of the intestinal epithelium and splanchnic blood flow, a delay in gastric emptying, and an increase in gastric pH (Zaghloul et al., 1987; Staatz and Tett, 2005). However, the extent of absorption of drugs that traverse biological membranes by passive diffusion is largely unchanged in the elderly (Hutchison and O'Brien 2007) and may actually improve for basic drugs due to raised gastric pH.
Typically, the elderly have an increased fat percentage, and a reduction in lean muscle mass and total body water, compared to younger adults (Staatz et al., 2002; Boesmueller et al., 2011). Consequently, there is an increase in volume of distribution for lipophilic drugs such as the calcineurin inhibitors and a decrease for hydrophilic drugs (Staatz and Tett, 2005; Barraclough et al., 2012). Drug binding to plasma proteins may be altered in the elderly (Zaghloul et al., 1987; Falck et al., 2008). A reduction in serum albumin can cause a decrease in total drug concentration and an increase in free drug fraction, with no corresponding long-term effect on free drug concentration. This is an important consideration when interpreting TDM results for immunosuppressant drugs, as total rather than the unbound drug concentration is measured (Falck et al., 2008; Karpe et al., 2017).
Liver size and hepatic blood flow can decrease in the elderly affecting drug metabolism (Yatscoff et al., 1998; Krenzien et al., 2015). Drugs with a high liver extraction ratio, may have reduced clearance due to reduced hemoperfusion; while drugs with a low liver extraction ratio, such as calcineurin inhibitors, are often less affected as their clearance is more dependent on the activity of metabolic enzymes (Zaghloul et al., 1987). Drug metabolism via Phase-I CYP3A-mediated reactions may decline with age (Yatscoff et al., 1998; Steinebrunner et al., 2014) although the extent of any decline is often patient- and drug-specific (Steinebrunner et al., 2014). Renal excretion of drugs is frequently significantly reduced in elderly patients with the glomerular filtration rate declining by approximately 1 ml/min/1.73 m2 from a person’s 20’s onwards (Staatz and Tett, 2005; Barraclough et al., 2012). It is unclear if biliary excretion and enterohepatic recirculation of drugs is affected by age.
As drug clearance is generally reduced due to a decline in kidney function, and potential decline in liver function, steady-state drug concentrations may be higher in elderly patients at any given drug dose. Lipophilic drugs may be exposed to the eliminating organs of the body more slowly, as fat acts as a reservoir keeping drug out of the circulation (Falck et al., 2008), increasing the likelihood of drug accumulation with repeat dosing (Staatz and Tett, 2005). This increases the risk of toxicity {Staatz et al., 2002).
A number of membrane transporters effect drug entry into sites such as the blood brain barrier, proximal renal tubules, hepatocytes and the gut (Petzinger et al., 2006; Liang et al., 2015; Nigam, 2015; Stieger and Hagenbuch 2016). More research is required into the effects of ageing on membrane transporter expression and activity. To date studies have shown that mRNA levels of the xenobiotic-processing genes (which code for transporter expression) were significantly lower in aged mice (Miura et al., 2009) and aging reduces P-glycoprotein levels in the blood-brain barrier in healthy adults (Dambrin et al., 2000). When considering transporter effects in the elderly, the presence and impact of multiple comorbidities and concomitant medications can further complicate the situation.
Patient response to medicines is dependent on factors including homeostatic mechanisms, receptor density and affinity, and signal transduction pathways (Hämmerlein et al., 1998; Hutchison and O'Brien 2007; Corsonello et al., 2010). Generally, in the elderly there is a progressive reduction in the body’s homeostatic mechanisms, such that after a drug is administered, it takes longer for counter-regulatory mechanisms to return the body to its original state (Turnheim, 2003; Barten et al., 2007; Milone, 2016). Receptor number and responsiveness also declines with age, however, this does not necessarily change drug sensitivity or effectiveness. Instead, it can take longer for a drug to reach maximum effect, and the effect can be stronger in the elderly. Resultantly, the elderly are more likely to suffer drug side effects when dosed to the same target concentration as younger adults (Turnheim, 2003; Aymanns et al., 2010).
Immunosenescence, a reduction in the robustness of the immune system, is known to occur in the elderly (Turnheim, 2003) and is associated with a loss in the homeostatic equilibrium of the peripheral T-cell pool and a decrease in antibody response (Heinbokel et al., 2013; Le Meur, 2015). A shrinking thymus leads to a progressive decline in the naïve T-cell count and an accumulation of memory T-cells (Albright and Albright 2003; Klinger and Banasik 2015). Overabundant memory T-cells are unable to respond effectively to new immunological challenges (Albright and Albright 2003; Klinger and Banasik 2015). The increase in memory T-cells also causes a decline in naïve B-cells and a reduction in B-cell antibody response (Gibson et al., 2009; Krenzien et al., 2015; Le Meur, 2015). This leads to a reduction in the turn-over of mature B-cells which impacts both antibody specificity and the amount of plasma cells in the bone marrow (Albright and Albright 2003; Heinbokel et al., 2013). Immunosenescence impacts elderly kidney transplant patients by increasing their risk of malignancies, infections, atherosclerosis, autoimmune disorders, and neuro-degeneration disorders (Boesmueller et al., 2011; Heinbokel et al., 2013).
Tacrolimus and cyclosporine block calcineurin enzymatic activity preventing activated T lymphocytes from producing interleukin-2 (IL-2) (Karpe et al., 2017). By reducing IL-2 transcription, the activity of other T- and B-cells which are essential for the growth and development of the immune system are suppressed (Morris and Knechtle 2014; Milone, 2016). Blocking the calcineurin pathway also affects non-immune cells including neurons, skeletal and cardiac myoctyes leading to off-target side effects (Milone, 2016). Usage of calcineurin inhibitors is associated with a range of adverse effects (Muntean and Lucan 2013). The balance between drug effect and drug side-effects is extremely delicate, and patients may experience side-effects when dosed to a standard target concentration.
There is large variability in the pharmacokinetics of tacrolimus and cyclosporine which can be attributed to a number of factors including cytochrome P450 genotype, drug-drug interactions, patient hematocrit, patient weight, time post-transplant and patient hepatic function (Han et al., 2013; Brooks et al., 2016). Both agents have generally poor, but highly variable oral bioavailability (Han et al., 2013; Milone, 2016; Andreu et al., 2017) with absorption reduced by intestinal P-glycoprotein efflux transportation and pre-systemic metabolism by cytochrome P450 3A (CYP3A) (Andreu et al., 2017). Tacrolimus binds extensively to erythrocytes (approximately 99%) and in plasma is primarily bound to alpha-1-acid glycoprotein and albumin (Staatz and Tett 2005; Milone, 2016; Andreu et al., 2017). Cyclosporine also binds extensively to erythrocytes (Milone, 2016) and is primarily bound to lipoproteins in plasma. Once absorbed, both agents are extensively metabolized in the liver by the CYP3A system. Tacrolimus biotransformation results in generation of more than 15 metabolites, with >95% of this agent excreted by the biliary route (Staatz et al., 2002) and <1% excreted as unchanged drug in the urine and faeces (Barraclough et al., 2012). Cyclosporine biotransformation results in generation of more than 25 metabolites with >90% of this agent excreted by the biliary system, and only 6% excreted, mostly as metabolites, by the kidneys (Milone, 2016).
Numerous mixed effects modelling-based studies have examined the pharmacokinetics of calcineurin inhibitors in adult kidney transplant recipients with several investigating age as a covariate (Staatz et al., 2002; Han et al., 2013; Brooks et al., 2016). To date, no studies in adult recipients have reported patient age to be a significant covariate in its own right, however several studies have found that patient factors which can change with age (e.g. weight, kidney function, hematocrit), are important. Notably the sample size of elderly recipients included in each study was generally very low. Currently, four studies have specifically examined the pharmacokinetics of calcineurin inhibitors in elderly renal transplant recipients. Findings are summarized in Table 1, critique follows.
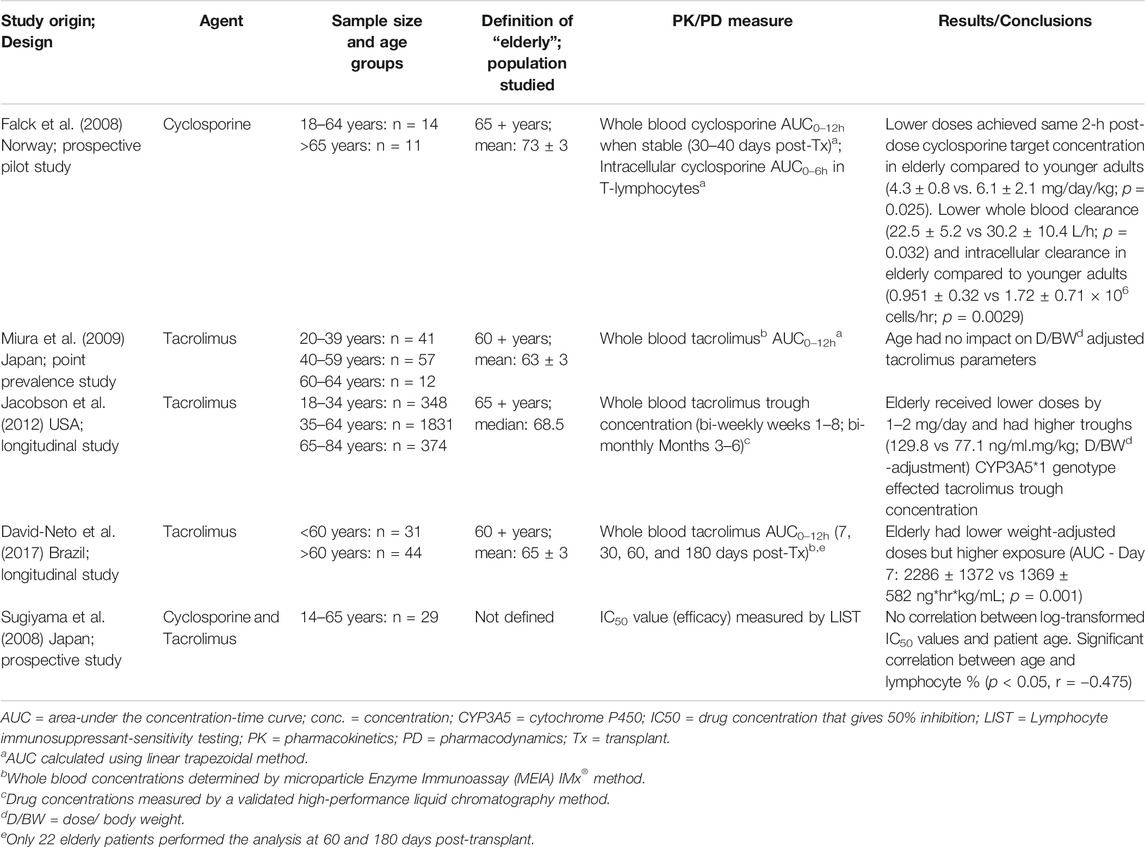
TABLE 1. A summary of pharmacokinetic and pharmacodynamic studies of calcineurin inhibitors in the elderly.
Falck et al examined the pharmacokinetics of cyclosporine in a prospective, pilot study involving 11 elderly (>65 years) and 14 younger adult kidney transplant recipients (Falck et al., 2008). Elderly patients achieved the same 2-hour post-dose cyclosporine target concentration as younger adults with lower weight-adjusted doses (4.3 ± 0.8 vs. 6.1 ± 2.1 mg/kg/day; p = 0.025). This finding supports the idea that drug clearance decreases with advancing age. Falck et al. also found that elderly recipients had a higher intracellular to whole blood cyclosporine area-under-the-concentration-time curve (AUC) ratio (AUCINTRA/AUCWHOLE BLOOD 0.035 ± 0.014 vs. 0.024 ± 0.008, p = 0.024), compared to younger adults, however, whole blood concentrations were similar across the cohorts (Falck et al., 2008). The authors proposed that a larger proportion of cyclosporine may be located at the site of action (within the T-lymphocyte), and suggested that drug target concentrations could be safely lowered in the elderly (Falck et al., 2008). Miura et al examined the pharmacokinetics of tacrolimus in 12 elderly kidney transplant recipients aged 60 years and over, by comparing their drug exposure to younger adult recipients in a point-prevalence study. Age did not influence dose- and weight-adjusted tacrolimus maximum concentration (Cmax), trough concentration (C0) or area-under-the-concentration-time-curve from 0 to 12 hours post-dose (AUC0-12 hour) (Miura et al., 2009). Jacobson et al conducted a retrospective, multi-centre study examining 374 elderly renal transplant recipients over the first 6 months after transplantation (Jacobson et al., 2012). They found that elderly patients (mean: 68.5 years) had higher dose-normalized tacrolimus trough concentrations than younger adults (Jacobson et al., 2012). Concentrations, normalized for both dose and body weight, were also more than 50% higher in elderly recipients compared to younger adults (Jacobson et al., 2012). A limitation to this finding is that full drug exposure was not measured, instead, only trough values were compared. However, these findings were supported by a similar study by David-Neto et al who also showed that elderly recipients (mean age: 65 years; n = 44) achieved higher target exposure (trough concentrations) and lower estimated total body clearance, with a lower normalized tacrolimus dose, compared to younger adult recipients (n = 31) (David-Neto et al., 2017).
With so few studies specifically designed to examine the elderly, it is still difficult to quantify the exact impact aging has on the pharmacokinetics of calcineurin inhibitors. No studies have yet compared robust elderly to more frail elderly patients. Studies to date indicate that calcineurin inhibitor doses, when lowered in elderly kidney transplant recipients, achieve the same level of immunosuppressant drug exposure as younger adults. This is possibly due to a decline in drug clearance and increased volume of distribution with advancing age.
In vitro studies have shown that calcineurin activity is inversely correlated to cytokine expression (particularly IL-2) in transplant recipients taking cyclosporine and tacrolimus (Dambrin et al., 2000). The partial inhibition observed in vivo may explain why there is a reduction in the responsiveness of the immune system in patients taking a calcineurin inhibitor, but not always enough to prevent graft rejection (Yatscoff et al., 1998). The relationship between tacrolimus blood concentrations and cytokine suppression in whole blood lymphocytes (Dambrin et al., 2000) is not yet reliably correlated (Steinebrunner et al., 2014). A study by Bremer and researchers showed that tacrolimus concentrations 1.5 hour post-dose resulted in strong cytokine inhibition, but cytokine suppression responses varied considerably before, and 1.5 hour after dosing, which may be attributed to different sensitivities to tacrolimus (Bremer et al., 2017). These findings suggest that there is a relationship between immunosuppressant exposure and cytokine expression, however, currently, extent of calcineurin inhibition has not been correlated to graft outcomes (Dambrin et al., 2000).
The only study to consider the effect of aging on calcineurin inhibitor pharmacodynamics was by Sugiyama et al. Age did not correlate with the calcineurin inhibitor concentrations that give 50% inhibition of peripheral-blood mononuclear cell-blastogenesis (IC50) values (p > 0.05), and hence, it was inferred that the pharmacological efficacy of immunosuppressant medicines is unlikely to be influenced by patient characteristics such as age (Sugiyama et al., 2008). This study did not examine elderly recipients (mean age 35 years ± 13; range 14–65 years), and does not fit with what is currently known about the elderly and immunosenesence.
Current studies examining graft survival in elderly kidney transplant recipients are outlined in Table 2. Overall, the studies summarized in Table 2 show that elderly recipients are more likely to suffer graft loss than younger adults; with death with a functioning graft being the most common cause of graft loss in this group.
Overall, elderly kidney transplant recipients have increased risk of both morbidity and mortality, compared to younger adults (Meier-Kriesche et al., 2000; Ojo et al., 2000). Ojo and researchers reported that renal transplant recipients aged over 65 years are seven times more likely to die with a functioning graft compared to young adults (aged 18–29 years) (Impedovo et al., 2013). This finding has since been supported by Wu et al (Wu et al., 2005), Impedovo et al (Impedovo et al., 2013), Hatamizadeh et al (Hatamizadeh et al., 2013), and Karim et al (Karim et al., 2014), who all showed that elderly recipients had worse survival, compared to youngers adults.
This heightened mortality risk in elderly recipients may be due to infection, a well-documented side effect of calcineurin inhibitors (Lehner et al., 2015). Meier-Kriesche and researchers showed that the risk of death due to infection increases 5-fold in elderly renal transplant recipients, compared to recipients aged 30–39 years (Meier-Kriesche et al., 2000). However, mortality risk can also be attributed to other side effects such as cardiovascular disease and malignancy (Cameron, 2000; Shi et al., 2015). Furthermore, risk may be further compounded by the increased chance of recipients having multiple comorbidities (Wu et al., 2005), as well drug-drug interactions (from medicines used for these comorbidities) (Huang et al., 2009; Boesmueller et al., 2011). Consequently, from the level of current evidence, it is thought that lower calcineurin inhibitor targets in elderly recipients may reduce the side effect potential without impacting patient and graft survival, however, studies have yet to confirm this (Danovitch et al., 2007; Dreyer et al., 2015; Cossart et al., 2019).
While TDM helps minimize problems associated with use of calcineurin inhibitors, many patients still experience rejection, infection and drug side-effects when drug exposure is in the therapeutic range (Lemaitre et al., 2015). Trough drug measurement may miss between-patient differences in drug absorption and whole blood concentrations may not fully characterize the pharmacological effect of these agents on intra-lymphocyte calcineurin activity because only unbound drug interacts with receptor sites (Wang 2007).
Current TDM methods rely on venepuncture for collection of blood samples. This process can be time-consuming, invasive and cumbersome as well as disruptive of the morning routine critical to medication adherence (Martial et al., 2016; Cossart et al., 2017). Attending venepuncture collection centers involves travel and cost for patients.
Alternative microsampling methods of blood collection based on finger-prick blood draw are currently being investigated (KDIGO, 2009). Such methods require less blood, are minimally invasive (Parker et al., 2016) and may allow patients to take blood samples at home. In dried blood spot sampling (DBS) a small volume of capillary blood is blotted onto a piece of marked filter paper and dried for future analysis (Nys et al., 2017; Edelbroek et al., 2009). In volumetric absorptive sampling (VAM) an absorbent polymeric tip is used to collect a small volume of blood by capillary action (Spooner et al., 2015; Delahaye et al., 2017; Kip et al., 2017). VAM collection has an advantage over DBS sampling in that drug concentration is measured independent of hematocrit which can influence blood viscosity and the accuracy of sample analysis (Spooner et al., 2015; Nys et al., 2017). A finger-prick sample contains capillary, arteriolar and venous blood, whereas a venepuncture sample contains only venous blood. Studies demonstrate that calcineurin inhibitor concentrations measured from finger prick samples strongly correlate with venous blood concentration (Jain et al., 1995; Webb et al., 2005; Kita and Mano 2017). New methods have been studied in other special populations i.e. paediatrics, and shown to be advantageous (Nys et al., 2017). Elderly kidney transplant recipients would be an ideal group to both test and validate microsampling protocols as these methods are likely to be beneficial – only a small amount of blood is required, minimally invasive, sampling convenience (e.g. conducted at home), and sample stability (Spooner et al., 2015; Martial et al., 2016).
Current immunosuppressant dosing and monitoring protocols make no adjustments for age (Shi et al., 2015). Elderly renal transplant recipients are predominantly excluded from clinical trials (Krenzien et al., 2015) making an evidence-based decision on immunosuppressant medicine dosing difficult (Montero et al., 2016). Furthermore, inter-patient pharmacokinetic variability increases with age due to physiological changes (Singh and Bajorek 2014), making the applicability of results from the younger adult transplant population to elderly patients more difficult. Elderly patients are also more likely to have multiple comorbidities resulting in polypharmacy and drug-drug interactions (Akhtar and Ramani, 2015), which only further highlights the importance of achieving drug doses within the target range.
Only one study has examined the impact of an altered calcineurin dosing protocol on graft outcomes in older adults. Badowski et al lowered the tacrolimus dosing targets in a cohort of 88 patients aged over 60 years (group 1 target concentration: 10–12 ng/ml; group 2 target concentration: 8–10 ng/ml) and found that the reduction in tacrolimus target drug concentration improved graft survival, without increasing the risk of acute rejection (p = 0.006) (Badowski et al., 2009). Patients were followed-up for approximately 2 years post-transplant (group 1: 23.8 ± 14.2 months; group 2: 21.3 ± 11.8 months). Further studies are required to quantify how lowered immunosuppressant doses or target concentrations may support graft and patient survival and potentially reduce the development of drug side effects in elderly and frail elderly transplant recipients.
The intricate balance between under- and over-immunosuppression becomes increasingly more complex in elderly transplant recipients, due to changes in pharmacokinetics and pharmacodynamics. Overall findings suggest that calcineurin inhibitor doses may need to be lowered in elderly recipients. However, only one study to date has examined the impact of aging on effectiveness of calcineurin inhibitor activity. Immunosenescence is known to occur in the elderly and likely impacts elderly kidney transplant patients by increasing their risk of malignancies, infections, artherosclerosis, autoimmune disorders, and neuro-degeneration disorders. However, the evidence around age-related pharmacology, particularly in immunosuppression, is sparse. More studies are needed to link drug exposure to outcomes in this cohort. There is also a need to validate micro-sampling for TDM of calcineurin inhibitors in the elderly as micro-sampling has shown to be promising in other special populations. In the future, micro-sampling based TDM may better support monitoring of drug exposure and long-term patient outcomes in the elderly and allow for further pharmacokinetic and pharmacokinetic studies in this cohort.
1) Conception and design: All authors. 2) Collection and assembly of data: AC and CS. 3) Data analysis and interpretation: All authors. 4) Manuscript writing: All authors. 5) Final approval of manuscript: All authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akhtar, S., and Ramani, R. (2015). Geriatric pharmacology. Anesthesiol Clin. 33 (3), 457–469. doi:10.1016/j.anclin.2015.05.004
Albright, J. F., and Albright, J. W. (2003). Aging of adaptive/acquired immunity. in Aging, immunity, and infection. Totowa, NJ, United States: Humana Press.
Andreu, F., Colom, H., Elens, L., van Gelder, T., van Schaik, R. H. N., Hesselink, D. A., et al. (2017). A new CYP3A5*3 and CYP3A4*22 cluster influencing tacrolimus target concentrations: a population approach. Clin. Pharmacokinet. 56, 963–975. doi:10.1007/s40262-016-0491-3
Aymanns, C., Keller, F., Maus, S., Hartmann, B., and Czock, D. (2010). Review on pharmacokinetics and pharmacodynamics and the aging kidney. Cjasn 5, 314–327. doi:10.2215/cjn.03960609
Badowski, M., Gurk-Turner, C., Cangro, C., Weir, M., Philosophe, B., Klassen, D., et al. (2009). The impact of reduced immunosuppression on graft outcomes in elderly renal transplant recipients. Clin. Transpl. 23, 930–937. doi:10.1111/j.1399-0012.2009.01028.x
Barraclough, K. A., Staatz, C. E., Johnson, D. W., Lee, K. J., McWhinney, B. C., et al. (2012). Kidney transplant outcomes are related to tacrolimus, mycophenolic acid and prednisolone exposure in the first week. Transpl. Int. 25, 1182–1193. doi:10.1111/j.1432-2277.2012.01553.x
Barten, M. J., Tarnok, A., Garbade, J., Bittner, H. B., Dhein, , S, et al. (2007). Pharmacodynamics of T-cell function for monitoring immunosuppression. Cell Proliferation 40, 50–63.
Boesmueller, C., Biebl, M., Scheidl, S., Oellinger, R., Margreiter, C., et al. (2011). Long-term outcome in kidney transplant recipients over 70 years in the eurotransplant senior kidney transplant program: a single center experience. Transplantation 92, 210–216. doi:10.1097/tp.0b013e318222ca2f
Bremer, S., Vethe, N. T., Skauby, M., Kasbo, M., Johansson, E. D., et al. (2017). NFAT-regulated cytokine gene expression during tacrolimus therapy early after renal transplantation. Br. J. Clin. Pharmacol. 83, 2494–2502. doi:10.1111/bcp.13367
Brooks, E., Tett, S. E., Isbel, N. M., and Staatz, C. E. (2016). Population pharmacokinetic modelling and bayesian estimation of tacrolimus exposure: is this clinically useful for dosage prediction yet?. Clin. Pharmacokinet. 55, 1295–1335. doi:10.1007/s40262-016-0396-1
Chadban, S. J., Barraclough, K. A., Campbell, S. B., Clark, C. J., Coates, P. T., et al. (2012). KHA-CARI guideline: KHA-CARI adaptation of the KDIGO clinical practice guideline for the care of kidney transplant recipients. Nephrology 17, 204–214. doi:10.1111/j.1440-1797.2011.01559.x
Corsonello, A., Pedone, C., and Incalzi, R. (2010). Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Cmc 17, 571–584. doi:10.2174/092986710790416326
Cossart, A. R., Staatz, C. E., Campbell, S. B., Isbel, N. M., and Cottrell, W. N. (2017). Investigating barriers to immunosuppressant medication adherence in renal transplant patients. Nephrology 24 (1), 102–110. doi:10.1111/nep.13214
Cossart, A. R., Cottrell, W. N., Campbell, S. B., Isbel, N. M., and Staatz, C. E. (2019). Characterizing the pharmacokinetics and pharmacodynamics of immunosuppressant medicines and patient outcomes in elderly renal transplant patients. Transl. Androl. Urol. 8, S198–S213. doi:10.21037/tau.2018.10.16
Dambrin, C., Klupp, J., and Morris, R. E. (2000). Pharmacodynamics of immunosuppressive drugs. Curr. Opin. Immunol. 12, 557–562. doi:10.1016/s0952-7915(00)00138-2
Danovitch, G. M., Gill, J., and Bunnapradist, S. (2007). Immunosuppression of the elderly kidney transplant recipient. Transplantation 84, 285–291. doi:10.1097/01.tp.0000275423.69689.dc
David-Neto, E., Romano, P., Kamada Triboni, A. H., Ramos, F., Agena, F., et al. (2017). Longitudinal pharmacokinetics of tacrolimus in elderly compared with younger recipients in the first 6 months after renal transplantation, Transplantation 101, 1365–1372. doi:10.1097/tp.0000000000001369
Delahaye, L., Janssens, B., and Stove, C. (2017). Alternative sampling strategies for the assessment of biomarkers of exposure. Curr. Opin. Toxicol. 4, 43–51. doi:10.1016/j.cotox.2017.05.003
Doyle, S. E., Matas, A. J., Gillingham, K., and Rosenberg, M. E. (2000). Predicting clinical outcome in the elderly renal transplant recipient. Kidney Int. 57, 2144–2150. doi:10.1046/j.1523-1755.2000.00066.x
Dreyer, G. J., Hemke, A. C., Reinders, M. E. J., and de Fijter, J. W. (2015). Transplanting the elderly: balancing aging with histocompatibility. Transplant. Rev. 29, 205–211. doi:10.1016/j.trre.2015.08.003
Edelbroek, P. M., Heijden, J. v. d., and Stolk, L. M. L. (2009). Dried blood spot methods in therapeutic drug monitoring: methods, assays, and pitfalls. Ther. Drug Monit. 31, 327–336. doi:10.1097/ftd.0b013e31819e91ce
Falck, P., Åsberg, A., Byberg, K.-T., Bremer, S., Bergan, S., et al. (2008). Reduced elimination of cyclosporine A in elderly (>65 years) kidney transplant recipients. Transplantation 86, 1379–1383. doi:10.1097/tp.0b013e31818aa4b6
Gibson, K. L., Wu, Y.-C., Barnett, Y., Duggan, O., Vaughan, R., et al. (2009). B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 8, 18–25. doi:10.1111/j.1474-9726.2008.00443.x
Hämmerlein, A., Derendorf, H., and Lowenthal, D. T. (1998). Pharmacokinetic and pharmacodynamic changes in the elderly. Clinical implications. Clin. Pharmacokinet. 35, 49–64. doi:10.2165/00003088-199835010-00004
Han, K., Pillai, V. C., and Venkataramanan, R. (2013). Population pharmacokinetics of cyclosporine in transplant recipients. AAPS J. 15, 901–912. doi:10.1208/s12248-013-9500-8
Hatamizadeh, P., Molnar, M. Z., Streja, E., Lertdumrongluk, P., Krishnan, M., et al. (2013). Recipient-related predictors of kidney transplantation outcomes in the elderly, Clin. Transpl. 27, 436–443. doi:10.1111/ctr.12106
Heinbokel, T., Elkhal, A., Liu, G., Edtinger, K., and Tullius, S. G. (2013). Immunosenescence and organ transplantation. Transplant. Rev. 27, 65–75. doi:10.1016/j.trre.2013.03.001
Huang, E., Segev, D. L., and Rabb, H. (2009). Kidney transplantation in the elderly. Semin. Nephrol. 29, 621–635. doi:10.1016/j.semnephrol.2009.07.011
Hutchison, L. C., and O’Brien, C. E. (2007). Changes in pharmacokinetics and pharmacodynamics in the elderly patient. J. Pharm. Pract. 20, 4–12. doi:10.1177/0897190007304657
Impedovo, S. V., Ditonno, P., Ricapito, V., Bettocchi, C., Gesualdo, L., et al. (2013). Advanced age is not an exclusion criterion for kidney transplantation. Transplant. Proc. 45, 2650–2653. doi:10.1016/j.transproceed.2013.08.003
Jacobson, P. A., Schladt, D., Oetting, W. S., Leduc, R., Guan, W., et al. (2012). Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am. J. Transpl. 12, 3326–3336. doi:10.1111/j.1600-6143.2012.04232.x
Jain, A. B., Pinna, A., Fung, J. J., Warty, V., Singhal, A. K., et al. (1995). Capillary blood versus arterial or venous blood for tacrolimus monitoring in liver transplantation', Transplantation, 60: 512–514. doi:10.1097/00007890-199509000-00020
Karim, A., Farrugia, D., Cheshire, J., Mahboob, S., Begaj, I., et al. (2014). Recipient age and risk for mortality after kidney transplantation in england', Transplantation 97, 832–838. doi:10.1097/01.tp.0000438026.03958.7b
Karpe, K. M., Talaulikar, G. S., and Walters, G. D. (2017). Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst. Rev. 7, CD006750. doi:10.1002/14651858.CD006750.pub2
KDIGO (2009). KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transpl. 9 (Suppl. 3), S1–S155. doi:10.1111/j.1600-6143.2009.02834.x
Kip, A. E., Kiers, K. C., Rosing, H., Schellens, J. H. M., and Beijnen, J. H. (2017). Volumetric absorptive microsampling (VAMS) as an alternative to conventional dried blood spots in the quantification of miltefosine in dried blood samples. J. Pharm. Biomed. Anal. 135, 160–166. doi:10.1016/j.jpba.2016.12.012
Kita, K., and Mano, Y. (2017). Application of volumetric absorptive microsampling device for quantification of tacrolimus in human blood as a model drug of high blood cell partition. J. Pharm. Biomed. Anal. 143, 168–175. doi:10.1016/j.jpba.2017.05.050
Klinger, M., and Banasik, M. (2015). Immunological characteristics of the elderly allograft recipient. Transplant. Rev. 29, 219–223. doi:10.1016/j.trre.2015.07.002
Krenzien, F., ElKhal, A., Quante, M., Rodriguez Cetina Biefer, H., Hirofumi, U., et al. (2015). A rationale for age-adapted immunosuppression in organ transplantation. Transplantation 99, 2258–2268. doi:10.1097/tp.0000000000000842
Le Meur, Y. (2015). What immunosuppression should be used for old-to-old recipients?. Transplant. Rev. 29, 231–236. doi:10.1016/j.trre.2015.08.004
Lehner, L. J., Staeck, O., Halleck, F., Liefeldt, L., and Bamoulid, J. (2015). Need for optimized immunosuppression in elderly kidney transplant recipients. Transplant. Rev. 29, 237–239. doi:10.1016/j.trre.2015.08.001
Lemaitre, F., Blanchet, B., Latournerie, M., Antignac, M., Houssel-Debry, P., et al. (2015). Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: inside the white blood cells. Clin. Biochem. 48, 406–411. doi:10.1016/j.clinbiochem.2014.12.018
Liang, Y., Li, S., and Chen, L. (2015). The physiological role of drug transporters. Protein Cell 6, 334–350. doi:10.1007/s13238-015-0148-2
Martial, L. C., Aarnoutse, R. E., Schreuder, M. F., Henriet, S. S., Bruggemann, R. J., et al. (2016). 'Cost evaluation of dried blood spot home sampling as compared to conventional sampling for therapeutic drug monitoring in children. PLoS One 11, e0167433. doi:10.1371/journal.pone.0167433
Meier-Kriesche, H. U., Ojo, A., Hanson, J., Cibrik, D., Lake, K., et al. (2000). Increased immunosuppressive vulnerability in elderly renal transplant Recipients1,2. Transplantation 69, 885–889. doi:10.1097/00007890-200003150-00037
Milone, M. C. (2016). Personalized immunosuppression in transplantation. Overview of the pharmacology and toxicology of immunosuppressant agents that require therapeutic drug monitoring. (Amsterdam, Netherlands: Elsevier).
Miura, M., Satoh, S., Kagaya, H., Saito, M., Inoue, T., et al. (2009). No impact of age on dose-adjusted pharmacokinetics of tacrolimus, mycophenolic acid and prednisolone 1 month after renal transplantation. Eur. J. Clin. Pharmacol. 65, 1047–1053. doi:10.1007/s00228-009-0721-9
Montero, N., Pérez-Sáez, M. J., Pascual, J., Abramowicz, D., Budde, K., et al. (2016). Immunosuppression in the elderly renal allograft recipient: a systematic review. Transplant. Rev. 30, 144–153. doi:10.1016/j.trre.2016.05.001
Morris, P., and Knechtle, S. J. (2014). “Calcineurin inhibitors,” in Kidney transplantation - principles and practice. Editors J. C. Mejia, B. Amit, and R. Shapiro (London United Kingdom: Elsevier).
Muntean, A., and Lucan, M. (2013). Immunosuppression in kidney transplantation. Clujul Med. 86, 177–180.
Nigam, S. K. (2015). What do drug transporters really do? Nat. Rev. Drug Discov. 14, 29–44. doi:10.1038/nrd4461
Nys, G., Kok, M. G. M., Servais, A.-C., and Fillet, M. (2017). Beyond dried blood spot: current microsampling techniques in the context of biomedical applications. Trac Trends Anal. Chem. 97, 326–332. doi:10.1016/j.trac.2017.10.002
Ojo, A. O., Hanson, J. A., Wolfe, R. A., Leichtman, A. B., and Agodoa, L. Y. (2000). Long-term survival in renal transplant recipients with graft function, Kidney Int. 57, 307–313. doi:10.1046/j.1523-1755.2000.00816.x
Otero-Raviña, F., Rodríguez-Martínez, M., Gude, F., González-Juanatey, J. R., and Valdés, F. (2005). Renal transplantation in the elderly: does patient age determine the results?, Age Ageing 34, 583–587. doi:10.1093/ageing/afi200
Petzinger, E., and Geyer, J. (2006). Drug transporters in pharmacokinetics. Naunyn Schmiedebergs Arch Pharmacol. 372 (6), 465–475.
Procurement and Transplantation Network (2020). National data - transplants in the U.S. by recipient age. Washington, DC: U.S Department of Health and Human Services
Registry (2017). 'ERA-EDTA Registry annual report 2017. Available at: https://era-edta-reg.org/files/annualreports/pdf/AnnRep2017.pdf (Accessed January 15, 2020).
Sánchez-Guisande, S. L., Dorofaeff, T., Lipman, J., Ballot, D. E., Bandini, R. M., et al. (2016). Is there a role for microsampling in antibiotic pharmacokinetic studies?. Expert Opin. Drug Metab. Toxicol. 12, 601–614. doi:10.1080/17425255.2016.1178238
Schulak, J. A., and Hricik, D. E. (1991). Kidney transplantation in the elderly. Geriatr. Nephrol Urol 1, 105–112. doi:10.1007/bf00577145
Scuderi, C. E., Parker, S. L., Jacks, M., John, G., McWhinney, B., et al. (2020). Kidney transplant recipient's perceptions of blood testing through microsampling and venepuncture. Bioanalysis 12, 873–881. doi:10.4155/bio-2020-0057
Shi, Y. Y., Hesselink, D. A., and van Gelder, T. (2015). Pharmacokinetics and pharmacodynamics of immunosuppressive drugs in elderly kidney transplant recipients. Transplant. Rev. 29, 224–230. doi:10.1016/j.trre.2015.04.007
Singh, S., and Bajorek, B. (2014). Defining “elderly” in clinical practice guidelines for pharmacotherapy. Pharm. Pract. 12, 489. doi:10.4321/s1886-36552014000400007
Spooner, N., Denniff, P., Michielsen, L., De Vries, R., Ji, Q. C., et al. (2015). A device for dried blood microsampling in quantitative bioanalysis: overcoming the issues associated blood hematocrit. Bioanalysis 7, 653–659. doi:10.4155/bio.14.310
Staatz, C. E., Willis, C., Taylor, P. J., and Tett, S. E. (2002). Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin. Pharmacol. Ther. 72, 660–669. doi:10.1067/mcp.2002.129304
Staatz, C. E., and Tett, S. E. (2005). Pharmacokinetic considerations relating to tacrolimus dosing in the elderly. Drugs & Aging 22, 541–557. doi:10.2165/00002512-200522070-00001
Steinebrunner, N., Sandig, C., Sommerer, C., Hinz, U., Giese, T., et al. (2014). Pharmacodynamic monitoring of nuclear factor of activated T cell-regulated gene expression in liver allograft recipients on immunosuppressive therapy with calcineurin inhibitors in the course of time and correlation with acute rejection episodes--a prospective study. Ann. Transpl. 19, 32–40. doi:10.12659/AOT.889809
Stieger, B., and Hagenbuch, B. (2016). Recent advances in understanding hepatic drug transport. F1000Res 5 (5), 2465. doi:10.12688/f1000research.9466.1
Sugiyama, K., Isogai, K., Toyama, A., Satoh, H., Saito, K., Nakagawa, Y., et al. (2008). Pharmacodynamic parameters of immunosuppressive drugs are not correlated with age, duration of dialysis, percentage of lymphocytes or lymphocyte stimulation index in renal transplant recipients, Biol. Pharm. Bull. 31, 2146. doi:10.1248/bpb.31.2146
Turnheim, K. (2003). When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp. Gerontol. 38, 843–853. doi:10.1016/s0531-5565(03)00133-5
Wang, L. (2007). Changes in pharmacokinetics in the elderly and therapeutic drug monitoring of antiarrhythmic drugs. Life Sci. J. 4, 1–7.
Webb, N. J. A., Roberts, D., Preziosi, R., and Keevil, B. G. (2005). Fingerprick blood samples can be used to accurately measure tacrolimus levels by tandem mass spectrometry. Pediatr. Transpl. 9, 729–733. doi:10.1111/j.1399-3046.2005.00367.x
World Health Organisation (2015). World report on ageing and Health. Luxemborg City, Luxemborg: National Library, 1–260.
Wu, C., Evans, I., Joseph, R., Shapiro, R., Tan, H., et al. (2005). Comorbid conditions in kidney transplantation: association with graft and patient survival. Jasn 16, 3437–3444. doi:10.1681/asn.2005040439
Yatscoff, R. W., Aspeslet, L. J., and Gallant, H. L. (1998). Pharmacodynamic monitoring of immunosuppressive drugs. Clin. Chem. 44, 428–432. doi:10.1093/clinchem/44.2.428
Keywords: kidney, transplantation, calcineurin inbibitors, elderly, immunosuppression, pharmacokinetics, pharmacodynamics, therapeutic drug monitoring
Citation: Cossart AR, Isbel NM, Scuderi C, Campbell SB and Staatz CE (2021) Pharmacokinetic and Pharmacodynamic Considerations in Relation to Calcineurin Usage in Elderly Kidney Transplant Recipients. Front. Pharmacol. 12:635165. doi: 10.3389/fphar.2021.635165
Received: 29 November 2020; Accepted: 12 February 2021;
Published: 12 April 2021.
Edited by:
Sandor Kerpel-Fronius, Semmelweis University, HungaryReviewed by:
Filippo Drago, University of Catania, ItalyCopyright © 2021 Cossart, Isbel, Scuderi, Campbell and Staatz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amelia R. Cossart, YW1lbGlhLmNvc3NhcnRAdXFjb25uZWN0LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.