- 1Centre for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 2Centre for Global Health Research, Brighton and Sussex Medical School, University of Sussex, Brighton, United Kingdom
- 3School of Public Health, Addis Ababa University, Addis Ababa, Ethiopia
- 4Department of Biomedical Sciences, College of Veterinary Medicine and Agriculture, Addis Ababa University, Addis Ababa, Ethiopia
- 5Rye Medical Centre, Rye, United Kingdom
- 6Department of Pharmacology and Clinical Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Background: Podoconiosis and lymphatic filariasis are the most common causes of lower limb lymphoedema in the tropics. Many sufferers experience frequent painful episodes of acute bacterial infection. Plant based traditional medicines are used to treat infections in many countries and are culturally established in Ethiopia. Ethiopian medicinal plants found to have antibacterial and antifungal activities were reviewed with the aim of increasing information about the treatment of wound infections in patients with lymphoedema.
Methods: This study collates data from published articles on medicinal plants with antibacterial and antifungal activities in Ethiopia. A systematic search of Scopus, EMBASE, PUBMED/MEDLINE and Google Scholar was undertaken. The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed. The protocol was registered on PROSPERO with registration number CRD42019127471. All controlled studies of in vitro antibacterial and antifungal activities were considered. All articles containing the descriptors published until June 28, 2019 were included. The outcome was measured as percent inhibition of microbial growth. For quality assessment of individual in vitro studies, OECD guidelines and the WHO-Good Laboratory Practice (GLP) handbook were used.
Results: Seventy-nine studies met the inclusion criteria. A total of 150 plant species and three compounds had been tested against 42 species of bacteria, while 43 plant species had been tested against 22 species of fungus.
Conclusion: Materials derived from several Ethiopian medicinal plants have been shown to have promising activity against a variety of bacteria and fungi. Those derived from Azadiractha indica A. Juss. and Lawsonia inerms L. are the most extensively studied against a wide range of gram-negative and positive bacteria, and fungal species.
Introduction
Lymphoedema is a chronic illness that has a major physical and psychological effect on patients and lowers the quality of patient life substantially. Neglected tropical diseases (NTDs) such as podoconiosis, lymphatic filariasis, and leprosy are the most common causes of lower limb lymphoedema in the tropics. Suffering can be aggravated by frequent painful episodes of acute bacterial limb infection known as “acute attacks” (Ndzeshang et al., 2020). Cellulitis and erysipelas are typical wound complications, with the majority of infections caused by group A, C, or G streptococci and Staphylococcus aureus species (Al-Niaimi and Cox, 2009). However, Aeromonas hydrophila/caviae, Acinetobacter lwoffii, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Shewanella algae, Staphylococcus aureus, Streptococcus pyogenes, Streptococcus dysgalactiae, Staphylococcus haemolyticus, Streptococcus agalactiae and Staphylococcus simulans have recently been found to be involved in colonising wounds of lymphoedematous limbs in patients from Ethiopia (Nigussie et al., 2020b).
Lymphoedema caused by the aforementioned NTDs is progressive if not treated. In the early stages oedema can be reversed overnight, but with disease progression there can be serious impairment and loss of independence. Patients are excluded from society because of their incapacitating and stigmatized impairments which cause significant economic effects, intergenerational poverty, and alienation from society. In Ethiopia, an estimate of 5.6 and 34.9 million peoples are at risk of lymphatic filariasis and podoconiosis respectively (Caprioli et al., 2020). There are 1.5 million cases of podoconiosis across 345 districts of Ethiopia, and the country has the highest prevalence of podoconiosis in the world (Deribe et al., 2020).
The use of medicinal plants has a long history in the treatment of a range of diseases, including infectious diseases, and these days hundreds of thousands of plant species have been tested for their medicinal properties (Górniak et al., 2019). However, the phytochemical and pharmacological activities of many more plants remain to be studied. Plant-derived substances are tolerated and accepted by patients and seem a reliable source of antimicrobial compounds (Górniak et al., 2019).
Medicinal plants are commonly used worldwide as alternative treatments for mental and physical illnesses. Herbal formulated medicines and traditional health practices are considered more affordable and accessible to most rural societies than modern drugs (Jima and Megersa, 2018). The World Health Organization (WHO) estimated that about 65% of the world population use medicinal plants for their primary health care. In addition, approximately 39% of the drugs developed since 1980 have been derived from plants and their derivatives (Nwonuma et al., 2020).
Traditional medicine has long been established in the culture of Ethiopian communities (Lulekal et al., 2014). In rural areas this includes the use of plant-based treatments of inflammation, wounds and infection (Gebremeskel et al., 2018). Records from as far back as the fifteenth century detail traditional medical practices and remedies obtained from oral tales, early medico-religious manuscripts, and traditional pharmacopeia (Jima and Megersa, 2018). The antimicrobial activities of these traditional medicinal plants are based on their secondary metabolites such as alkaloids, terpenoids, flavonoids, tannins and glycosides (Sisay et al., 2019).
Many in vitro antibacterial and antifungal studies have been conducted on the safety and efficacy of Ethiopian medicinal plants used to treat bacterial and fungal infections. However, data on the efficacy and safety of these medicinal plants in the management of wound infection have not yet been summarized. This systematic review draws together up-to-date information on Ethiopian medicinal plants used as antibacterial and antifungal agents that might potentially be used for the management of wound infections in lymphoedema.
The aim of this systematic literature review was to evaluate Ethiopian medicinal plants found to have antibacterial and antifungal properties in vitro studies.
In the context of this review, terms are defined as follows:
“Ethiopian medicinal plants” refer to plants that are found in Ethiopia and have been utilized traditionally for medicinal purposes by societies in Ethiopia and elsewhere.
“In vitro anti-infective activity tests” involve direct culture of the microorganisms in media and application of plant extracts to the media to evaluate their activity.
“Anti-infective agents” refer to agents (medicinal plant extracts, fractions, and/or compounds) that act against infective agents (bacteria, fungi and others) either by inhibiting the agent’s growth or by killing it.
Materials and Methods
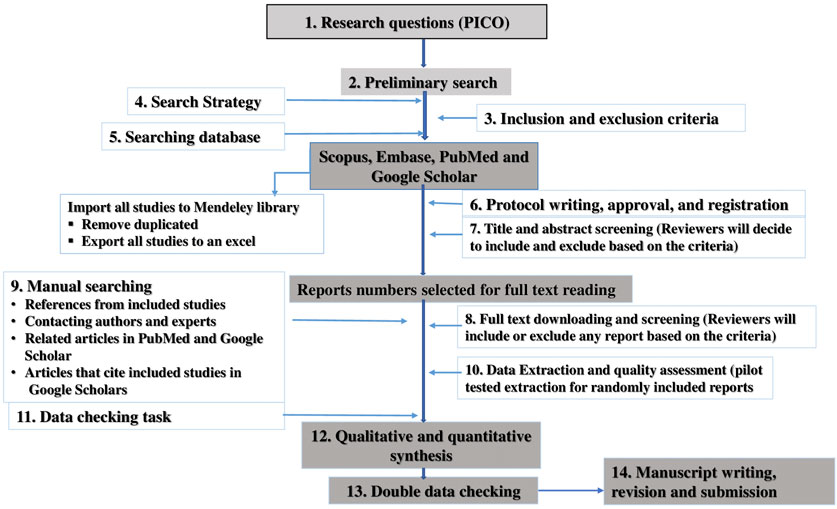
To ensure inclusion of relevant information, the study was undertaken based on the guideline of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (Lulekal et al., 2014) (Figure 1). The protocol for this review was registered on PROSPERO, with registration number CRD42019127471 (Nigussie et al., 2020a).
Study Design
This systematic review considered all controlled in vitro antibacterial and antifungal studies the activities of Ethiopian medicinal plants. The components population, exposure (intervention), comparator and outcome (PICO) of this review are given underneath:
Study subjects: Microorganisms (bacteria and fungi) that were used for anti-infective efficacy tests of medicinal plants.
Intervention: Medicinal plants as whole plants or their adjuncts: seed, root, flower, bud and leaf extracts used in the experimental groups. The intervention products used were manufactured from a single or complex medicinal plant, plant extracts, or plant preparations, regardless of the types of the preparations (extracts, decoctions, tablets, capsules, pills, powders, injections or other types of preparations), but not synthesized compounds. There were no restrictions on dosage form, concentration, frequency of administration, dose, intensity or duration of medicinal plants used.
Comparator: Placebo (no intervention) or conventional (reference) drugs used for treatment of controls.
Outcomes: Outcomes were the rate of response to treatment, such as efficacy of medicinal plants in inhibiting or killing microbial growth (in culture media) in comparison with conventional drugs.
Eligibility Criteria
Inclusion criteria: Published works including theses, articles and proceedings in English until June 2019 that deal with efficacy evaluation of antibacterial and antifungal activities in vitro studies.
Exclusion criteria: Newspapers, clinical trial studies and reviews.
Information Sources
Electronic databases were looked at using a combination of free text keywords and Medical Subject Heading (MeSH) terms related to Ethiopian restorative plants investigated to have anti-inflammatory, anti-infective and wound healing activities. Scopus, Embase, PubMed/Medline and Google Scholar were utilized as sources of data for the search. Grey literature such as thesis, technical reports, working papers, evaluation reports, conference proceedings, patents, and preprints was too included in the review.
Search Strategy
The search approach covered all articles containing descriptors available till June 28, 2019. Only articles composed in English have been used on this study. Structured search methods have been developed using the lexical terms of each database and targeting the “title” and “abstract” fields. We additionally searched manually using the references of already distributed works. The following search terms were used: Ethiopia, medicinal plants, Ethiopian medicinal plants, herbal products, care, management, therapeutic, lymphoedema, lymphedema, swelling, podoconiosis, elephantiasis, bacteria, anti-bacterial, fungi, anti-infective, antimicrobial, anti-fungal and different associated words or phrases.
Selection of Studies
After electronic searching, the records were uploaded to Mendeley. Some studies were screened before being totally selected. All medicinal drug and antifungal studies were screened severally by two investigators (DN and TB by scanning the titles and abstracts of the articles that supported the inclusion criteria. For documents that fitted the inclusion criteria, the investigators scan the whole article to substantiate whether or not it met the criteria. Disagreements were resolved by discussion between the two investigators.
Data Extraction and Management
Two reviewers (DN and TB) independently extracted data using a data extraction form. We performed a standardization exercise before beginning the review to make ensure consistency between the reviewers. The subsequent information were extracted: title, author, year of publication, statistical methods used, study duration, type of microorganism used for the study (clinical isolates or reference strains), concentrations of the plant extracts used for activity evaluation, reference drugs used, minimum inhibitory concentration (MIC) of extracts/fractions (µg/ml, mg/ml), zone of inhibition (mm), concentrations that inhibit microbial growth of the extracts/fractions, type of solvent extracts and fractions used for activity and safety, parts of plant used, extraction type, sources of the plants, place of collection, traditional use, scientific names of the plants, local names of the plants, voucher numbers, types and number of compounds isolated (if any). When individual studies had multiple treatments, groups were combined to avoid the possibility of introducing bias caused by multiple statistical comparisons with one control group (Lulekal et al., 2014).
Outcomes Measured
For the in vitro studies of antibacterial and antifungal activities, the most outcomes measured were the percent of inhibition of growth of microorganisms, minimum inhibitory concentration, and concentration needed to inhibit multiplication of micro-organisms by 50% (IC50).
Assessment of Risk of Bias
Two review authors (DN and TB) independently assessed the risk of bias for each included study. The internal and external validity of each study was evaluated using this tool. For the in vitro antibacterial and antifungal studies, a good practice for pharmaceutical microbiology laboratories guidelines (WHO) was used for quality assessment (WHO, 2009; World Heath Organisation, 2011). Criteria used to assess the quality of in vitro individual studies were reported in Nigussie et al. (2020a).
Then, the risk of bias criteria was judged as “low,” “high” or “unclear.” Studies with a low and medium risk of bias were considered for analysis, whereas high risk of bias studies were omitted from the analysis.
Data Synthesis and Analysis
All studies included for data synthesis were classified into two different experimental models, i.e., antibacterial and antifungal activity studies, in keeping with the kind and purpose of the studies. Heterogeneity was evaluated descriptively from the narrative synthesised data, and potential reasons for heterogeneity were identified by examining individual study and subgroup characteristics. Interventional, methodological and statistical heterogeneity was apparent among the studies. Consequently, statistical pooling of studies for meta-analysis was not possible.
Instead, a narrative (qualitative) overview of the studies was conducted using textual descriptions of studies, grouping, and tabulation. Then, a detail of the characteristics of the studies compared the effect of each plant extract relative to controls, the main parameters measured/analyzed, the quality of included studies and the risk of bias of all studies was described.
Results
Literature Search Results and Description of Study Characteristics
In the search of Ethiopian medicinal plants used for their anti-inflammatory, wound healing or anti-infective activities, a total of 2,330 relevant articles were independently identified by two reviewers for preliminary review from electronic and manual searches. After removal of duplicates by reviewing relevant titles and abstracts, a total of 330 articles on antibacterial and antifungal activities were retrieved for full text review. After a detailed review of each article, 234 articles were excluded and a total of 96 articles were retained: anti-bacterial activity (n = 79) and anti-fungal activity (n = 17) (Figure 1 and Supplementary Tables S1–S3).
Excluded Studies
In this review, we have identified that there were many studies conducted in these areas. However, most of the published articles did not meet the inclusion criteria due to i) Incomplete information: not reported the concentration of plant extracts used, the number of experimental duplicates, the method of outcome measurement, the negative and positive controls used, the sources and quality control of micro-organism, the time at which the outcomes were measured, the sources of the cell lines, and statistical methods used for data analysis; ii) Not relevant studies: clinical trials; newspaper reports; reviews, studies conducted on medicinal plants which are not growing in Ethiopia, activity was not conducted for human pathogens (animal and plant pathogens).
Studies Included for the Antibacterial Activity of Medicinal Plants
Characteristics of the Studies
For the anti-bacterial studies, seventy-nine studies were eligible for data extraction. The year of publication ranged from 2003 to 2019. A total of 76 peer-reviewed full articles and 3 MSc thesis were included. The seventy-nine studies were conducted in Ethiopia (Seshathri and Thiyagarajan, 2011), India (eight), Kenya (seven), Iran (three), Sudan (three), Cameroon and South Africa (two each), China (one), Oman (one), Malaysia (one), Nigeria (one), Pakistan (one), Netherlands (one) and Tunisia (one) (Supplementary Tables S1, S3).
All the studies designs met the inclusion criteria and tests were performed according to the procedures described in the national, regional and international guidelines. The titles of the studies met the objectives stated in the studies. Of the 79 studies, 36 used agar well diffusion techniques with micro-dilution assay (MIC and MBC), and 28 used paper disc diffusion method with micro-dilution assay (MIC and MBC). Two of the studies used agar-well diffusion alone, while nine used microdilution methods for minimum inhibitory concentration and minimum bactericidal assays together with other methods, colorimetric assay and crystal violet assay methods.
A total of 144 plant species and four compounds were tested and all except two plant species were identified and authenticated by a botanist. Out of the 144 plant species 14 of them are found in Ethiopia (Table 1). Leaves were the most used plant parts for anti-bacterial tests (n = 82) (Table 1). All essential oils were extracted by steam distillation with a Clevenger-type apparatus, while maceration and Soxhlet techniques were the most frequently used techniques to extract plant materials.
A total of 25 gram-negative and 17 gram-positive bacteria were tested in the studies. Most of the microorganisms tested were American Type Culture Collection (ATCC) reference microorganisms and some were clinical isolates from samples. Among the gram-negative bacteria, Escherichia coli was tested against more than 70 types of medicinal plants, followed by Pseudomonas aeruginosa, Klebsiella pneumoniae and Salmonella typhi tested, which were tested against thirty-nine, twenty-eight and twenty-two medicinal plants, respectively. Among the gram-positives, Staphylococcus aureus was the most tested bacteria and was tested against sixty-six medicinal plants. Others included Bacillus subtilis (twelve), Streptococcus pyogenes (ten), and Enterococcus faecalis (eight) (Supplementary Table S1).
Twenty-six studies used SPSS statistical software for data analysis. One-way ANOVA was used to test the existence of statistically significant differences between mean zones of inhibition of controls and test substances. However, 45 studies did not report the statistical method used. The rest used the unpaired Student t-test to test the differences between treatment and control arms (Supplementary Table S1).
Main Parameters Analysed
For agar well and paper disc diffusion assay methods, the outcome measured at each test level was the diameter (mm) of the zone of inhibition of the control and experimental tests using a calibrated distance measuring instrument. The time of measurement for all included studies was after 24 h exposure to reference and test substances. For the microdilution methods (MIC and MBC), colour change for colorimetric assays or bacterial growth for non-colorimetric methods (visually identified as clear or turbid solution in test tubes after 24 h treatment) were used.
A wide range of concentrations and different types of units of measurements were used across the studies. Among the medicinal plants tested against the microorganisms, at least one plant constituent was active against bacteria. The units used to describe MIC were µg/ml, µl/ml, mg/ml, µg/disc, % (w/v) and ppm. Similarly, for the measurement of zone of inhibition of bacterial growth, millimetre (mm) and millimetre squared (mm2) were used.
The lowest concentration (8 µg/ml) of plant material that inhibited the growth of microorganisms was reported by (Chaieb et al., 2011). Eleven human pathogenic strains were tested against thymoquinone, a constituent of the black seed of Nigella sativa L. It was shown to inhibit the growth of Bacillus cereus ATCC 14579 and S. epidermidis CIP 106510 at 8 µg/ml and S. aureus ATCC 25923 16 µg/ml (Chaieb et al., 2011). Ameya et al. (2017) reported the minimum inhibitory concentration of the alcoholic extract of Echinops kebericho Mesfin against S. aureus, E. coli and E. faecalis, which ranged from 3.12 to 12.5 µg/ml (Gemechu et al., 2015). Similarly, Oumer et al. reported the MIC values of latex of Aloe trichosantha Berger, which ranged from 10 to 100 µg/ml for bacterial species such as bacillus species, E. coli species, Salmonella species, Shigella species, S. aureus and V. cholerae (Ameya et al., 2017) (Supplementary Table S1).
Minale et al. reported the MIC values of Aloe sinana Reynolds and its compounds, Microdontn, Aloin and Aloinoside ranged from 10 to 50 µg/ml for the leaf latex, 10–25 µg/ml for Aloinoside, 5–200 µg/ml for Microdontn, 10–200 µg/ml for Aloin against gram-negative and gram-positive bacterial species. This was shown to have a strong anti-bacterial activity in comparison to the positive control, ciprofloxacin (Minale et al., 2014). Gadissa et al. tested the essential oils of Blephari scuspidata, Boswellia ogadensis Vollesen and Thymus schimperi Ronniger (Gadisa et al., 2019). The MICs of Blepharis cuspidate against S. aureus (ACCT & MRSA), E. coli (MDR) and K. pneumonia (MDR) were 1.56, 12.5, and 3.12 µl/ml, respectively, which is comparable to ciprofloxacin activity (Gadisa et al., 2019).
Similarly, Thymus schimperi Ronniger was reported to have MICs of 3.12 µl/ml, 6.5 L/ml, and 3.12 µl/ml against S. aureus (ATCC & MRSA), E. coli (ACCT & MDR) and K. pneumoniae (ATCC & MDR), respectively, and the minimum bactericidal concentration (MBC) ranged from 3.12 to 12.5 µl/ml. In addition, Boswellia ogadensis Vollesen was shown to have MIC values of 3.12, 6.25, and 3.12 µl/ml against S. aureus (ATCC & MRSA), E. coli (ACCT & MDR) and K. pneumoniae (ATCC & MDR), respectively, and MBC ranged from 6.25 to 12.5 µl/ml (Gadisa et al., 2019).
The MIC values of Boswellia ogadensis Vollesen and Thymus schimperi Ronniger essential oil combination against S. aureus (ATCC & MRSA), E. coli (ACCT & MDR), K. pneumoniae (ATCC & MDR) were 3.12, 6.25, and 1.56 µl/ml, respectively. The MBC ranged from 1.56 to 25 µl/ml. Similarly, the combination of essential oil of T. Schimper Ronniger and Blepharis cuspidata Lindau showed significant activity against S. aureus (ATCC & MRSA), E. coli (ACCT & MDR) and K. pneumoniae (ATCC & MDR) with MIC of 0.39, 1.56, and 0.39 µl/ml, respectively, and with MBC values ranges from 0.39 to 3.12 µl/ml. The combined activities of essential oils of B. cuspidata Lindau and B. ogadensis Vollesen showed similar activity against S. aureus (ATCC & MRSA), E. coli (ACCT & MDR) and K. pneumoniae (ATCC & MDR) with MICs of 1.56, 6.25, and 0.78 µl/ml, respectively. The MBC ranged from 1.56 to 25 µl/ml. These essential oils were shown to have comparable activity to ciprofloxacin (Gadisa et al., 2019).
Habbal et al. (2011) reported that 50% ethanol extracts of Lawsonia inermis L. demonstrated antibacterial activity against a wide range of gram-negative and positive bacterial strains with the highest antibacterial activity against P. aeruginosa. Nagarajan et al. (2013) reported that ethanol, chloroform, hexane and methane extracts of L. inermis L. showed nearly equal zones of inhibition against B. subtitles, S. aurous, and E. coli at 400 mg/kg comparable to that of tetracycline. Ethanol, methanol, and ethyl acetate extracts of Azadiractha indica A. Juss were reported by Maleki et al. to have a wider zone of inhibition against P. aeruginosa, S. aureus and E. faecalis at 300 mg/ml. The extracts had bactericidal activity against both reference and clinical isolates of S. aureus and P. aeruginosa, and bacteriostatic activity against E. faecalis (Maleki et al., 2018).
The degree of bacterial growth inhibition, as determined by values of diameter of inhibition zone (IZ) of the respective plant extracts, varied among the extracts and microorganisms. The widest inhibition was reported by Bacha et al. (2016) who showed the inhibitory zones of petroleum ether extract (500 mg/ml) of seeds of Nigella sativa L. to be 44 ± 0.31 mm against Bacillus cereus and 40 ± 2.33 mm against B. cereus ATCC 10987 compared to that of gentamycin (29 mm). Wide zones of inhibition were recorded for the petroleum ether extract of stem of Kosteletzkya begonifolia Ulbr. and stem of Leucas marthineensis (Jacq.) R. Br. against E. coli, S. typhimurium, S. aureus and P. aeruginosa at all concentrations, comparable to ciprofloxacin (Tadesse et al., 2016). In another study, acetone extract of Capsicum frutescens L. against ATCC S. aureus at a concentration of 0.1 mg/ml was reported to produce an inhibitory zone of 28 mm (Ameya et al., 2017).
Quality of Included Studies (Bias Analysis)
Critical appraisal of the studies included was done using the checklist for Good In Vitro Method Practices (OECD) and the WHO Good Practice for Microbiology Laboratory. Seven main criteria were used to evaluate the validity of methodological and reporting qualities (details are in the Materials and Methods section) (Supplementary Table S1).
Under the main checklist there were thirty criteria to evaluate the internal validity of the studies. Studies with unacceptable levels of bias were excluded. However, the studies included still had some weaknesses in reporting the status of microbiology facilities, regular equipment, apparatus maintenance and calibration. In addition, there was lack of clarity as to whether the test methods were validated or not; and there was also lack of evidence as to whether the microbiological tests were performed and supervised by an experienced person qualified in microbiology or equivalent, and whether the opinions and interpretations of test results in reports were done by authorized personnel with suitable experience and relevant knowledge.
There was also some methodological weakness. For instance, the number of replicates for each testing condition, including concentration level(s) used for the reference and control item(s), and test items were not specified in some studies. None of the studies reported the applicability domain of the in vitro methods or any limitations or exceptions to the methods. Four studies did not report complete information about the degree of inhibition of bacterial growth and the concentrations by the respective medicinal plants, and one study did not mention the unit of measurement of the zone of inhibition by plant extracts.
We categorized the judgment of bias as “yes,” “no” or “unclear.” A “yes” judgement indicated a low risk of bias; and a “no” judgment indicated high risk of bias; the judgment was “unclear” if insufficient details were reported to assess the risk of bias properly.
Studies Included on Anti-fungal Activity of Medicinal Plants
Characteristics of the Studies
Seventeen studies that evaluated anti-fungal activities of Ethiopian medicinal plants were included. The year of publication of the studies included ranged from 2000 to 2018, and studies were conducted in six different countries, Ethiopia (n = 7), Kenya (n = 1), India (n = 5), Colombia (n = 1), Lithuanian (n = 1), Republic of Korea (n = 1), and Romania (n = 1). Sixteen studies were peer reviewed full articles and one was an MSc thesis. Five studies used microdilution assay, two used agar well diffusion method, and twelve used both methods (Supplementary Table S2).
Medicinal plants claimed to have anti-fungal activities were tested against different fungal species and one species of yeast. These were Candida albicans, Aspergillus species, Trichophyton species, Microscopium species, Penicillium species, Fusarium species, Epidermophyton species and Rhodotorula rubra. Aspergillus species (n = 18) were the most-studied fungi, followed by Trichophyton species (n = 13) and Candida albicans (n = 10).
A total of 42 different species of medicinal plants were tested against different fungi, and all of them identified and authenticated by botanists and with voucher numbers. Out of these, four plant species are endemic to Ethiopia (Table 2).
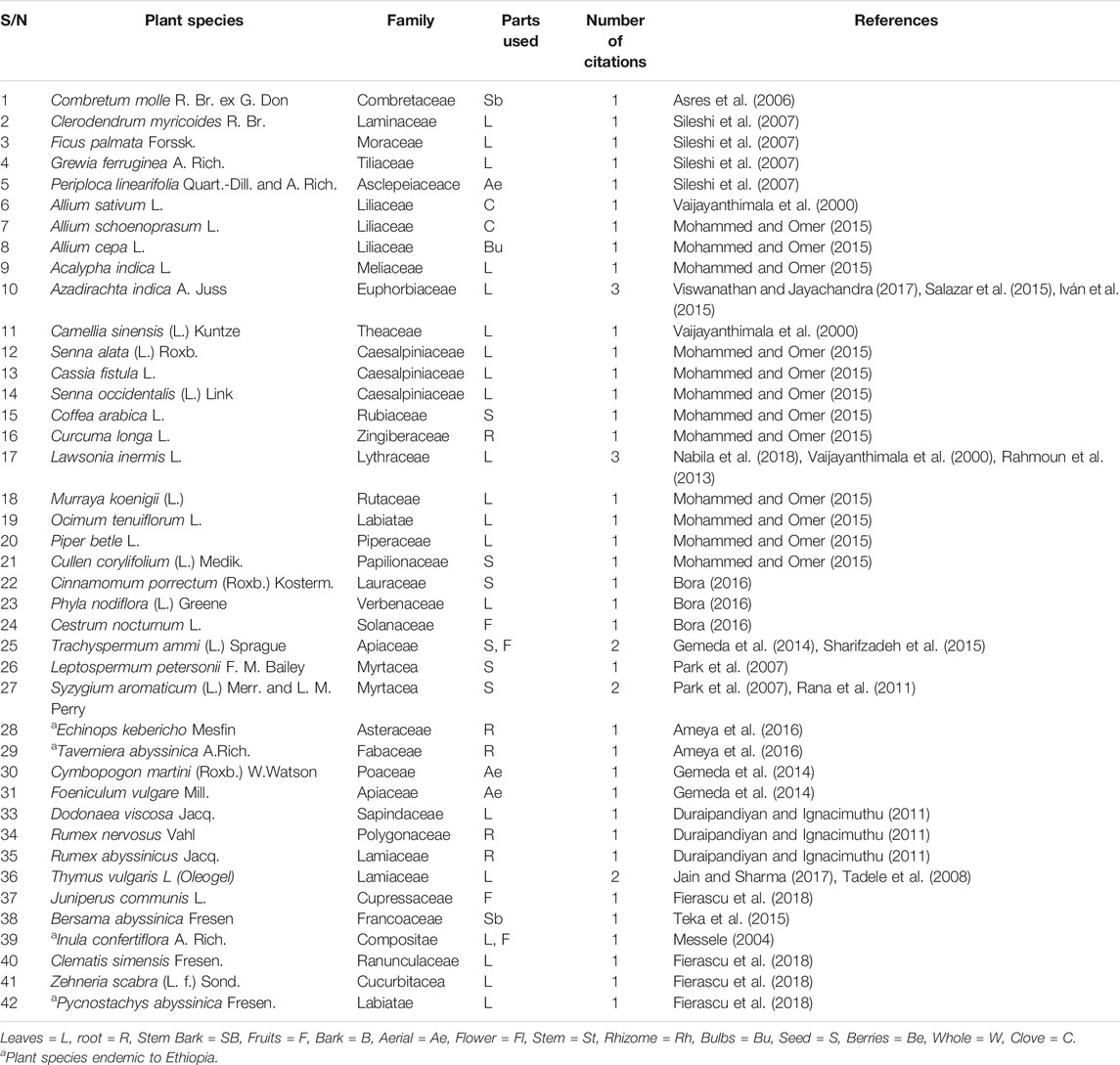
TABLE 2. Summary of common medicinal plants identified from literature search for anti-fungal activity.
Leaves (n = 21), seeds (n = 6), roots (n = 4), stem bark (n = 2), aerial parts (n = 2), cloves (n = 2), bulbs (n = 1), fruits (n = 4), were the plant parts used (Table 2). The maceration technique was the most frequently used method of extraction for plant extracts, followed by Soxhlet. Steam distillation with Clevenger-type apparatus was used for extraction of essential oils. Hydro-alcohol solvents were the most frequently used solvents for the extraction of plant materials followed by aqueous solvents.
Six studies used the agar well diffusion (AWD) method, six the micro dilution (MID) method and five both methods. For both experimental methods, the duration of exposure of the microorganisms to the extracts ranged from 2 to 7 days incubation time; and outcomes were measured after this. Zone of inhibition of fungal growth, turbidity (visually) and anti-fungal activity index (%) were the outcomes measured in the included studies. All the measurements were replicated three times and the results were presented as mean ± SD. One-way ANOVA followed by Tukey’s test was used to compare extraction solvents and the difference in the sensitivity of the test microorganisms.
Main Parameters
The antifungal activity of plant extracts was measured in a similar way as that of the anti-bacterial activity. These were zone of inhibition of fungal growth for the agar well and paper disc diffusion methods and fungal growth which distinguished clear and turbid solutions for the micro-dilution methods, measured after incubation periods.
A wide range of concentrations and units of measurement were used across the studies. For the MIC and minimum fungicidal concentration (MFC) mg/ml, µg/ml and activity index in percent (%), and mm was used for the measurements of ZI in AWD assays.
The activity index of the extracts was determined using the following formula:
Ameya et al. (2016) reported that the methanol extract of Echinops kebericho Mesfin against Aspergillus flavus and Candida albicans had MICs of 6.25 and 3.12 µg/ml, respectively; and the MFC of methanol extract to be 12.5 and 6.25 µg/ml against A. flavus and C. Albicans, respectively. The ethanol extract had MICs of 12.5 and 6.25 µg/ml against A. flavus and C. albicans, respectively with fungicidal activity of 22.92 and 12.50 µg/ml, respectively. The zone of inhibition of the methanol extract against C. albicans and A. flavus were 18.66 ± 0.57 and 20.33 ± 0.57 mm, respectively. In this study, ethanol and methanol extracts of E. kebericho Mesfin were shown to have comparable activity with ketoconazole (Supplementary Table S2).
Kasparaviciene et al. evaluated the activity of oleo-gels formulated with different concentrations of thyme essential oil. The MIC value of 0.25% essential oil of thyme in oleo-gels against C. albicans was 0.05% (Kasparaviciene et al., 2018). In another study, the antifungal activity of T. vulgaris essential oil against dermatophytic fungi was reported by Neetu et al. to have a very strong antifungal activity at low concentrations. The MIC values ranged from 0.05 to 0.1 µl/ml; and the MFC ranged from 0.05 to 2 µg/ml against the dermatophtic fungi (Jain and Sharma, 2017) (Supplementary Tables S2, S3).
The seed extracts of Trachyspermum ammi (L.) Sprague (0.2 mg/ml) and the leaf extract of Cestrum nocturnum L. (0.2 mg/ml) exhibited the widest zones of inhibition, at 38.3 and 31.3 mm, respectively against C. albicans. Similarly, the methanol extract of E. kebericho Mesfin exhibited ZI of 20.33 ± 0.57 mm against C. albicans and 18.6 mm against A. flavus (Supplementary Table S2). Salazar et al showed that leaf and seed oil extracts of neem tree inhibited the growth of Trichophyton menta, Trichophyton rubrum, Epidermophyton floccos and Microsporumcanis. Whereas Simhadri et al reported that the aqueous extract of Azadirachta indica A. Juss. leaves had superior activity against T. rubrum, M. gypseum, E. floccosum, and Candida species (Viswanathan and Jayachandra, 2017).
Quality of Included Studies (Bias Analysis)
Checklists employed for antibacterial studies were also used in the antifungal studies. There were gaps in methodology as well as in reporting and interpreting the outcomes. Validation of the test methods before conducting the experiments was not reported for all included studies, and eight studies did not report the statistical methods used.
There was no evidence that the anti-fungal activity tests were performed or supervised by an experienced person qualified in microbiology or equivalent. Similarly, there was no report on whether the microbiology facilities were fit for purpose or detailed description of the workflow for the microbiology methods and related processes. Furthermore, nine studies did not report the statistical methods used, not expressed an estimate of the uncertainty of the test result on the test report, and limitations of the test were not reported clearly.
Discussion
The purpose of this review was to demonstrate the activities of Ethiopian medicinal plants as antimicrobial agents that might potentially be used for limb care (particularly, of tropical lymphoedema and associated wounds). This section discusses the efficacy of plant extracts and their secondary metabolites investigated as antibacterial and antifungal, and the most frequently used models.
This systematic review identified a total of 96 articles covering two different experimental models, i.e., 79 antibacterial activity and 17 antifungal activity models. Overall, medicinal plant extracts tested for these two conditions in in vitro were shown to have good activity. Despite the heterogeneity of the studies, all plant extracts investigated succeeded in inhibiting bacterial and fungal growth.
In this review of antibacterial activity, a total of 144 medicinal plant species and four compounds were investigated against 25 gram-negative and 17 gram-positive bacteria using agar well diffusion, paper disc diffusion, broth micro/macro-dilution and agar dilution method. A summary of plant species whose extracts and their isolated compounds were shown to have significant in vitro activity against bacteria is the focus for our discussion.
Chaieb et al. reported the MIC of thymoquinone, constituent of N. sativa L., which was shown to have MIC of 32 µg/ml against V. parahaemolyticus ATCC 17802 and E. faecalis ATCC 29212, 16 µg/ml against L. monocytogene ATCC 19115, 8 µg/ml against B. cereus ATCC 14579, S epidermidis CIP 106510, M. luteus NCIMB 8166, S. aureus ATCC 25923 and S. epidermidis CIP 106510 in a broth microdilution assay method. Its activity was shown to be similar to the standard drugs gentamycin and erythromycin (Chaieb et al., 2011). This finding agrees with the report of Kokoska et al., who tested the essential oil of N. sativa L. seed against gram-positive bacteria. Thymoquinone, the main constituent of the essential oil, was shown to have a potent bacteriostatic effect with MIC ranging from 8 to 64 µg/ml in broth microdilution method (Kokoska et al., 2008). However, E. coli ATCC 35218, S. enterica serovar Typhimurium ATCC 14028, and P. aeruginosa ATCC27853 were found to be resistant to this compound (MIC >512 µg/ml) (Kokoska et al., 2008).
Ameya et al. (2016) tested the alcoholic extract of E. kebericho Mesfin against S. aureus, E. coli and E. faecalis and demonstrated significant activity with MIC ranged from 3.12 to 12.5 µg/ml using AWD, while E. coli was found to be resistant. Anwar et al. assessed the antimicrobial activity of latex of Aloe trichosantha A. Berger and its compounds (aloin A/B and aloin-6′-O- acetate A/B), which were effective against E. coli, Salmonella and V. cholerae strains with an average MIC value of 25 µg/ml. The activities of the test substances could be due to changes to cell wall integrity, enzymatic activity and protein inactivation in the microorganisms (Oumer et al., 2014).
Minale et al. performed anti-bacterial activity tests on Aloe sinana Reynolds and its compounds (Microdontin, Aloin and Aloinoside) against 21 strains of bacteria using the disk diffusion method. The leaf latex showed high inhibitory activities against B. pumillus 82, B. subtilis ATCC 6633 and S. aureus ML 267, E. coli K99, E. coli K88, E. coli CD/99/1, E. coli LT37, E. coli 306, E. coli 872, E. coli ROW 7/12, E. coli 3:37C, S. enterica TD 01, S. typhi Ty2, S. boydii D13629, S. dysentery 8, S. flexneri Type 6, S. soneii 1, V. cholerae 85, V. cholerae 293, V. cholerae 1,313 and V. cholerae 1,315 at a concentration of 200 µg/ml, which showed comparable activity to the standard drug ciprofloxacin. Similarly, compounds isolated from Aloe sinana Reynolds were shown to have high activity against E. coli, S. typhi Typ 2, Shigella, S. aureus and V. cholerae, comparable to the reference drug, ciprofloxacin (Minale et al., 2014). The leaf latex’s action was due to the secondary metabolite anthraquinones, which possess a range of functional groups and have the ability to disrupt bacterial cell wall permeability and inhibit nucleic acid synthesis and then cause death of the microorganism (Malmir et al., 2017; Xu et al., 2018).
According to Gadisa et al., combined essential oils of oregano-basil, basil-bergamot, oregano-bergamot and oregano-perilla have significant activity against S. aureus, E. coli, B. subtilis and S. cerevisiae, respectively. The synergistic effect of these essential oils may be due to synergistic or additive interactions between different classes of compounds such as phenols, aldehydes, ketones, alcohols, esters, ethers or hydrocarbons, which might act on the same target or different targets (Gadisa et al., 2019). This finding is consistent with Nasir et al. (Haroun and Al-Kayali, 2016), who postulated that the ability of plant extracts to act synergistically with antibiotics and other plant extracts could be considered a new approach to combat antimicrobial resistance. There is low risk of bacterial resistance in plant extracts and antibiotics combinations, due to the varied modes of action of the compounds present in the extracts, to which the organism had never been exposed before (Haroun and Al-Kayali, 2016).
Antibacterial activity of Lawsonia inermis L. was also reported against wide range of gram-positives and gram-negatives (Annavarapu et al., 2016; Nabila et al., 2018). This could be due to the presence of a compound, 2-hydroxy-1, 4-naphthoquinone. Quinones are the main constituent in the leaves of L. Inermis L. and are known in making complexing irreversibly with nucleophilic amino acids, leading to inactivation of the protein and loss of function in microorganisms. Cell wall adhesions, polypeptides and membrane bound enzymes are the targets in microbial cells (Nabila et al., 2018).
In another anti-microbial study, the leaf and stem bark extracts of Azadirachta indica A. Juss. exhibited significant antibacterial activity against a wide range of bacteria due to the tricyclic diterpenoids isolated from stem bark, and azadirachtins, quercetin and β-sitosterol isolated from the leaves (Raja et al., 2013).
Bacha et al tested 18 plant extracts against E. coli K12 DMS 498, S. aureus DMS 346, B. cereus ATCC 10987, B. cereus, Lab strain and P. aeruginosa 1,117 using AWD and MID methods. The highest ZI was recorded with petroleum ether extract of N. Sativa L against B. cereus and B. cereus ATCC 10987; and mature unripe fruit oil of Aframomum corrorima (A. Braun) P. C. M. Jansen against S. aureus. The activities of petroleum ether extract of seed of N. sativa L against both laboratory isolated and reference strain of B. cereus were greater than the activity of gentamycin sulphate. The oil extract of unripe fruit of A. corrorima (A. Braun) P. C. M. Jansen was shown to have an activity comparable to the reference drug gentamycin sulphate. P. aeruginosa was the most resistant to all the plant extracts tested in this study (Bacha et al., 2016). The antimicrobial activities of extracts of A. corrorima, Nigella sativa L., A. angustifolium (Sonn.) K. Schum. and V. amygdalina (Delile) Sch. Bip. were due to the presence of phenol, tannin, saponin and flavonoids, flavonoids and terpenoids compounds and their combinations (Bacha et al., 2016). The antibacterial activity of flavonoids is well documented and found in almost all parts of the plants, which inhibit the energy metabolism and synthesis of nucleic acids of different microorganisms (Cushnie and Lamb, 2005). Furthermore, tannins were reported to have antibacterial activity against S. aureus, acting by inducing complexation with enzyme or substrates and causes toxicity; and altering the membrane of the microbes (Akiyama, 2001).
Many studies have been carried out to screen medicinal plants for their antifungal activity, and various groups of researchers have initiated antifungal programs for traditionally used plants. Classes of compounds from plant metabolites, such as terpenoids (isoprenoids), saponins, phenolic compounds, flavonoids, coumarins, alkaloids, proteins and peptides showed anti-fungal activity against different fungal species (Aqil et al., 2010; Duraipandiyan and Ignacimuthu, 2011). Under this review, 15 studies were included comprising 42 species of plant extracts against 50 species of fungus using agar well diffusion, disc diffusion, macro/microdilution and agar dilution methods.
Alcoholic extracts (methanol and ethanol) of E. kebericho Mesfin were tested by Ameya et al. against A. flavus and C. albicans using disc diffusion and agar dilution methods, and shown to cause significant inhibition at low concentration, comparable to the reference drug ketoconazole. The alcoholic solvents have the ability to extract phenolic compounds such as flavonoids, anthocyanins and phenolic acids which may contribute to the antifungal activity of the extracts (Gemechu et al., 2015).
Kasparaviciene et al. tested the activity of oleo-gels, formulated with different concentrations of thyme essential oil against C. albicans by broth dilution method, which showed significant activity with MIC value of 0.25%. Thymol was reported the major constituent of the thyme essential oil in this study. The biological activity of thyme essential oil depends on its yield and chemical composition, and the essential oils have several chemical names depending on the main constituents they have, such as thymol, carvacrol, terpineol, and linalool (Kasparaviciene et al., 2018).
Similarly, Jain et al. reported the antifungal activity of T. vulgaris essential oil against T. mentagrophytes MTCC 7687, M. gypseum MTCC 452, M. fulvumMTCC2837, T. rubrum MTCC 296, T. soudanense (isolate) and T. interdigitale (isolate) using macro-dilution method. T. vulgaris L. essential oil was shown to have significant activity against the dermatophytes with MIC ranges from 0.02 to 0.1 μl/ml (Jain and Sharma, 2017). These activities could be due to high content of phenolic compounds and potent vapour activity against dermatophytes (Soković et al., 2009). This finding agrees with the report of Marina et al., which showed the activity of T. vulgaris L. essential oil against Alternaria alternata, Fusarium tricinctum, all Aspergillus species and dermatomycetes at concentration of 0.25 µL/ml and Phomopsis helianthi and Cladosporium cladosporioides at 0.125 µl/ml by macro-dilution method (Soković et al., 2009).
In another study, T. ammi (L.) Sprague seed extract exhibited potent antifungal efficacy, with a maximum MZ of 38.3 mm diameter against C. albicans using the AWD method (Gemeda et al., 2014). This is in agreement with the finding of Sharifzadeh et al., which evaluated T ammi (L.) Sprague essential oil against C. albicans, which were fluconazole-resistant, with MIC values ranging from 300 to 400 µg/ml (Sharifzadeh et al., 2015). The extracts of A. indica was also shown to have antifungal activity, attributable to the terpenoids. The fractions of A. indica A. Juss have complex mixtures of compounds reported to have synergistic and additive effect of against fungus (Salazar et al., 2015).
Conclusion
The present study showed that plant extracts and compounds traditionally used in Ethiopia are promising anti-infective agents. In this review Calpurnia aurea (Aiton) Benth., Croton macrostachyus Hochst. ex Delile, Withania somnifera (L.) DunalAchyranthes aspera L., Datura stramonium L., Solanum incanum L., Verbascum erianthum Benth., Nigella sativa L., Gymnanthemum amygdalinum (Delile) Sch. Bip., Olinia rochetiana A. Juss., Sida rhombifolia L, Bersama abyssinica Fresen. and Azadirachta indica A. Juss are the most studied plants species against bacteria, and Azadirachta indica A. Juss and Lawsonia inerms L. against fungal species. Thymoquinone, a constituent of the black seed of Nigella sativa L., alcoholic extract of Echinop skebericho Mesfin, Aloe sinana Reynolds and its compounds (Microdontin, Aloin and Aloinoside), alcoholic extract of Azadiractha indica A. Juss and Lawsonia inerms L. are the most effective plant materials against gram negative and gram-positive species. In addition, Azadiractha indica A. Juss and Lawsonia inerms L. have activity against a wide range of gram-negative and positive bacterial strains. Similarly, methanol extract of Echinops kebericho Mesfin and oleo-gels formulated with different concentrations of thyme essential oil are the most effective against different fungal species.
Strength and Limitations
This systematic review will provide up-to-date information on Ethiopian medicinal plants used as anti-infective agents that might potentially be used for limb care (lymphoedema and associated wounds). This information could lead to the development of more research on the investigation of the effect of medicinal plants on against infection for future therapeutic use. However, as it will summarizes studies written only in English this could be and which is considered the anticipated one of the limitations of this review. In addition, this study has will considered a wide range of methodological approaches and used different.
Implications for Future Research and Recommendations
It is vital to systematically summarize, and document medicinal plants tested against different disease agents and used traditionally for treatment, and to test further their effectiveness against a range of disease-related pathology such as wounds in patients with lymphoedema. Information about many medicinal plants is fragmented, meaning that systematic compilation and synthesis is important. The findings of this systematic review helped us in identifying and prioritizing medicinal plants species for further investigation to determine their efficacy as alternative therapy to microbial infections associated with lymphoedema.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
DN, GD, EM, TT, MB, BL, and AF were involved in conceptualization and design of the study, and in collection, analysis and interpretation of the data. DN wrote the first draft of the manuscript and DN, GD, EM, TT, MB, BL, and AF critically reviewed the manuscript for intellectual content. All authors have read and approved the final manuscript.
Funding
This research was funded by the National Institute for Health Research (NIHR) Global Health Research Unit on NTDs at BSMS using United Kingdom aid from the United Kingdom Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the United Kingdom Department for Health and Social Care.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank CDT-Africa, Addis Ababa University and the Ethiopian Public Health Institute for organising and sponsoring training on systematic review and meta-analysis; and our special gratitude to Clare Callow, Grit Gansch, and Manuela McDermid for administrative support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.633921/full#supplementary-material
References
Abdelraheim Belal, A. (2017). Antibacterial Activity of Cuminum Cyminum L. Oil on Six Types of Bacteria. Am. J. BioScience 5 (4), 70–73. doi:10.11648/j.ajbio.20170504.13
Abdissa, D., Geleta, G., Bacha, K., and Abdissa, N. (2017). Phytochemical Investigation of Aloe Pulcherrima Roots and Evaluation for its Antibacterial and Antiplasmodial Activities. PLoS One 12 (3), 1–11. doi:10.1371/journal.pone.0173882
Abew, B., Sahile, S., and Moges, F. (2014). In Vitro antibacterial Activity of Leaf Extracts of Zehneria Scabra and Ricinus communis against Escherichia coli and Methicillin Resistance Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 4, 816–820. doi:10.12980/apjtb.4.201414b16
Abreham, B., Rahel, A., Taye, M., Workabebe, D., Awokech, G., and Birtukan, G. (2015). Antimicrobial Activity of Thymus Schimperi Ronninger (Lamiaceae) against Standard and Clinical Isolates of Human Pathogenic Bacteria. J. Med. Plants Res. 9 (11), 379–384. doi:10.5897/jmpr2015.5761
Adedapo, A. A., Jimoh, F. O., Koduru, S., Afolayan, A. J., and Masika, P. J. (2008). Antibacterial and Antioxidant Properties of the Methanol Extracts of the Leaves and Stems of Calpurnia Aurea. BMC Complement Altern. Med. 8, 1–8. doi:10.1186/1472-6882-8-53
Akiyama, H. (2001). Antibacterial Action of Several Tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 48 (4), 487–491. doi:10.1093/jac/48.4.487
Akujobi, C. N., Ofodeme, C. N., and Enweani, C. A. (2010). Determination of Antibacterial Activity of Carica Papaya (Pawpaw) Extracts. Niger. J. Clin. Pract. 13 (1), 55–57.
Al-Niaimi, F., and Cox, N. (2009). Cellulitis and Lymphoedema: a Vicious Cycle. J. Lymphoedema 4 (2), 38–42.
Albejo, B., Endale, M., Kibret, B., and Anza, M. (2015). Phytochemical Investigation and Antimicrobial Activity of Leaves Extract of Vernonia Auriculifera Hiern. J. Pharm. Pharmacogn Res. 3 (6), 141–147.
Amare, A., Hadush, A., Aregawi, H., and Kide, N. (2013). Antibacterial Activity of Oil Extracts of Black Mustard ( Brassica Nigra ) Seeds against Bacteria Isolated from Fresh Juice in Selected Areas of Axum Town. Int. J. Int. Sci. Inn. Tech. 4 (4), 15–18.
Ameya, G., Manilal, A., and Merdekios, B. (2018). In Vitro Antibacterial Activity and Phytochemical Analysis of Nicotiana Tabacum L. Extracted in Different Organic Solvents. Open Microbiol. J. 11 (1), 352–359. doi:10.2174/1874285801711010352
Ameya, G., Aklilu, A., Bisrat, N., Nassir, M., and Negas, A. (2017). In Vitro antimicrobial Activity of Fermented Spices and Capsicum Frutescensagainst Multi Drug Resistance Clinical Isolate and Standard Reference Bacteria. Afr. J Clin Exp Microbiol January 19 (1), 9–17.
Ameya, G., Gure, A., and Dessalegn, E. (2016). Antimicrobial Activity of Echinops Kebericho against Human Pathogenic Bacteria and Fungi. Afr. J. Tradit Complement Altern. Med. 13 (6), 199–203. doi:10.21010/ajtcam.v13i6.29
Amoo, S. O., Finnie, J. F., and Van Staden, J. (2012). Acetylcholinesterase Inhibition, Antioxidant, Antiinflammatory, Antimicrobial and Phytochemical Properties of Huernia hystrix. Phytother. Res. 26 (5), 639–645. doi:10.1002/ptr.3614
Annavarapu, T. R., Renuka, P., Akhil, P., Divya, P., and Devi Priyanka, P. (2016). Evaluation of the Anti-inflammatory Activity of Combination of Ethanol Extracts of Azadirachta indica (Neem) and Lawsonia Inermis (Henna). Asian J. Pharm. Clin. Res. 9 (5), 256–258.
Aqil, F., Zahin, M., Ahmad, I., and Owais, M. (2010). “Antifungal Activity of Medicinal Plant Extracts and Phytocompounds: A Review,” in Combating Fungal Infections. Editors I. Ahmad, M. Owais, M. Shahid, and F. Aqil (Berlin, Heidelberg: Springer), 1–37. doi:10.1007/978-3-642-12173-9_19
Asamenew, G., Bisrat, D., Mazumder, A., and Asres, K. (2011). In Vitro antimicrobial and Antioxidant Activities of Anthrone and Chromone from the Latex of Aloe Harlana Reynolds. Phytother. Res. 25 (12), 1756–1760. doi:10.1002/ptr.3482
Asres, K., Mazumder, A., and Bucar, F. (2006). Antibacterial and Antifungal Activities of Extracts of Combretum Molle. Ethiop Med. J. 44 (3), 269–277.
Ayal, G., Belay, A., and Kahaliw, W. (2019). Evaluation of Wound Healing and Anti-inflammatory Activity of the Leaves of Calpurnia Aurea (ait.) Benth (Fabaceae) in Mice. Wound Med. 25 (1), 100151. doi:10.1016/j.wndm.2019.100151
Bacha, K., Tariku, Y., Gebreyesus, F., Zerihun, S., Mohammed, A., Weiland-Bräuer, N., et al. (2016). Antimicrobial and Anti-Quorum Sensing Activities of Selected Medicinal Plants of Ethiopia: Implication for Development of Potent Antimicrobial Agents. BMC Microbiol. 16 (1), 1–9. doi:10.1186/s12866-016-0765-9
Begashaw, B., Mishra, B., Tsegaw, A., and Shewamene, Z. (2017). Methanol Leaves Extract Hibiscus Micranthus Linn Exhibited Antibacterial and Wound Healing Activities. BMC Complement Altern. Med. 17 (1), 1–12. doi:10.1186/s12906-017-1841-x
Bekele, A. (2015). Antimicrobial Activity of Thymus Schimperi Ronninger (Lamiaceae) against Standard and Clinical Isolates of Human Pathogenic Bacteria. J. Med. Plants Res. 9 (11), 7. doi:10.5897/JMPR2015.5761
Belay, G., Tariku, Y., Kebede, T., and Hymete, A. (2011). Ethnopharmacological Investigations of Essential Oils Isolated from Five Ethiopian Medicinal Plants against Eleven Pathogenic Bacterial Strains. Phytopharmacology 1, 133–143. doi:10.5958/j.0975-4261.3.4.049
Berehe, S., and Boru, A. (2014). Phytochemical Screening and Antimicrobial Activities of Crude Extract of Lepdium Sativum Seeds Growing in Ethiopia. Int. J. Pharm. Sci. Res. 5 (10), 4182–4187. doi:10.13040/IJPSR.0975-8232.5(10).4182-87
Bisht, P., and Rawat, V. (2014). Antibacterial Activity of Withania Somnifera against Gram-Positive Isolates from Pus Samples. Ayu 35 (3), 330–332. doi:10.4103/0974-8520.153757
Bora, L. (2016). Anticandidal Activity of Medicinal Plants and Pseudomonas aeruginosa Strains of Clinical Specimens. J. Microbiol. Immunol. Infect. 49 (2), 276–280. doi:10.1016/j.jmii.2014.10.002
Burt, S. A., and Reinders, R. D. (2003). Antibacterial Activity of Selected Plant Essential Oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 36 (3), 162–167. doi:10.1046/j.1472-765x.2003.01285.x
Caprioli, T., Martindale, S., Mengiste, A., Assefa, D., H/kiros, F., Tamiru, M., et al. (2020). Quantifying the Socio-Economic Impact of Leg Lymphoedema on Patient Caregivers in a Lymphatic Filariasis and Podoconiosis Co-endemic District of ethiopia. Plos Negl. Trop. Dis. 14 (3), e0008058–18. doi:10.1371/journal.pntd.0008058
Chaieb, K., Kouidhi, B., Jrah, H., Mahdouani, K., and Bakhrouf, A. (2011). Antibacterial Activity of Thymoquinone, an Active Principle of Nigella Sativa and its Potency to Prevent Bacterial Biofilm Formation. BMC Complement Altern. Med. 11 (1), 29. doi:10.1186/1472-6882-11-29Available at: http://www.biomedcentral.com/1472-6882/11/29
Chalo, D. M. (2015). Evaluation of Antimicrobial Activity, Toxicity and Phytochemical Screening of Selected Medicinal Plants of Losho, Narok County, Kenya. Nairobi, Kenya: Univeristy of Nairobi.
Cowan, M. M. (1999). Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 12 (4), 564–582. doi:10.1128/cmr.12.4.564Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88925&tool=pmcentrez&rendertype=abstract
Cushnie, T. P. T., and Lamb, A. J. (2005). Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 26 (5), 343–356. doi:10.1016/j.ijantimicag.2005.09.002
Danlami, U. (2016). Phytochemical, Nutritional and Antimicrobial Evaluations of the Aqueous Extract of Brassica Nigra (Brassicaceae) Seeds. American Journal of Applied Chemistry 4 (4), 161. doi:10.11648/j.ajac.20160404.17
Debalke, D., Birhan, M., Kinubeh, A., and Yayeh, M. (2018). Assessments of Antibacterial Effects of Aqueous-Ethanolic Extracts of Sida Rhombifolia’s Aerial Part. Sci. World J. 2018, 8. doi:10.1155/2018/8429809
Delelegn, A., Sahile, S., and Husen, A. (2018). Water Purification and Antibacterial Efficacy of Moringa Oleifera Lam. Agric. Food Secur 7 (1), 1–10. doi:10.1186/s40066-018-0177-1
Deribe, K., Negussu, N., Newport, M. J., Davey, G., and Turner, H. C. (2020). The Health and Economic burden of Podoconiosis in Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 114 (4), 284–292. doi:10.1093/trstmh/traa003
Desta, A. G., Abdelwuhab, M., Tadesse, W. T., and Gurmu, A. E. (2017). In Vitro antibacterial Activities of the Leaf Extracts of Aloe Macrocarpa Tod (Aloaceae). Eur. J. Integr. Med. 12 (April), 74–78. doi:10.1016/j.eujim.2017.04.010
Djeussi, D. E., Noumedem, J. A. K., Ngadjui, B. T., and Kuete, V. (2016). Antibacterial and Antibiotic-Modulation Activity of Six Cameroonian Medicinal Plants against Gram-Negative Multi-Drug Resistant Phenotypes. BMC Complement Altern. Med. 16 (1), 1–10. doi:10.1186/s12906-016-1105-1
Dua, A., Garg, G., Singh, B., and Mahajan, R. (2013). Antimicrobial Properties of Methanolic Extract of Cumin (Cuminum Cyminum) Seeds. Int. J. Res. Ayur. Pharm. 4 (1), 104–107. doi:10.7897/2277-4343.04136
Duraipandiyan, V., Abdullah Al-Harbi, N., Ignacimuthu, S., and Muthukumar, C. (2012). Antimicrobial Activity of Sesquiterpene Lactones Isolated from Traditional Medicinal Plant, Costus Speciosus (Koen ex.Retz.) Sm. BMC Complement Altern. Med. 12 (13), 2–6. doi:10.1186/1472-6882-12-13
Duraipandiyan, V., and Ignacimuthu, S. (2011). Antifungal Activity of Traditional Medicinal Plants from Tamil Nadu , India. Asian Pac. J. Trop. Biomed., 204–215. doi:10.1016/S2221-1691(11)60157-3
Era, O. N. G., and Nyanchoka, T. (2016). Screening for Antibacterial and Antifungal Compounds in Bersama Abyssinica FRESEN. Nairobi, Kenya: Kenyatta University.
Ewansiha, J. U., Garba, S. A. I., Mawak, J. D., and Oyewole, O. A. (2012). Antimicrobial Activity of Cymhopogon Citratus (Lemon Grass) and It’s Phytochemical Properties. Front Sci. [Internet] 2 (6), 214–220. doi:10.5923/j.fs.20120206.l4 Available at: http://irepos.unijos.edu.ng/jspui/bitstream/123456789/439/1/.
Fierascu, I., Ungureanu, C., Avramescu, S. M., Cimpeanu, C., Georgescu, M. I., Fierascu, R. C., et al. (2018). Genoprotective, Antioxidant, Antifungal and Anti-inflammatory Evaluation of Hydroalcoholic Extract of Wild-Growing Juniperus Communis L. (Cupressaceae) Native to Romanian Southern Sub-carpathian hills. BMC Complement. Altern. Med. 18 (3), 1–14. doi:10.1186/s12906-017-2066-8
Gadisa, E., Weldearegay, G., Desta, K., Tsegaye, G., Hailu, S., Jote, K., et al. (2019). Combined Antibacterial Effect of Essential Oils from Three Most Commonly Used Ethiopian Traditional Medicinal Plants on Multidrug Resistant Bacteria. BMC Complement Altern. Med. 19 (1), 1–9. doi:10.1186/s12906-019-2429-4
Gebremeskel, L., Bhoumik, D., Sibhat, G. G., and Tuem, K. B. (2018). In Vivo wound Healing and Anti-inflammatory Activities of Leaf Latex of Aloe Megalacantha baker (Xanthorrhoeaceae). Evidence-Based Complement. Altern. Med. 2018, 1–7. doi:10.1155/2018/5037912
Gemechu, A. B., Abdella, G., and Engda, D. (2015). Antimicrobial Activity of Taverniera Abyssinica A. Rich against Human Pathogenic Bacteria and Fungi. Afr. J. Microbiol. Res. 9 (50), 2385–2390. doi:10.5897/ajmr2015.7507
Gemeda, N., Woldeamanuel, Y., Asrat, D., and Debella, A. (2014). Effect of Cymbopogon Martinii, Foeniculum Vulgare, and Trachyspermum Ammi Essential Oils on the Growth and Mycotoxins Production by Aspergillus Species. Int. J. Food Sci. 2014. doi:10.1155/2014/874135
Geyid, A., Abebe, D., Debella, A., Makonnen, Z., Aberra, F., Teka, F., et al. (2005). Screening of Some Medicinal Plants of Ethiopia for Their Anti-microbial Properties and Chemical Profiles. J. Ethnopharmacology 97 (3), 421–427. doi:10.1016/j.jep.2004.08.021
Girmay, S. (2015). Preliminary Phytochemical Screening and In Vitro Antimicrobial Activity of Datura Stramonium Leaves Extracts Collected from Eastern Ethiopia. Int. Res. J. Biol. Sci. 4 (1), 55–59.
Goji, M., Gebre-Mariam, T., Asres, K., Lemma, H., Gemeda, N., and Yirsaw, K. (2007). Screening of the Antimicrobial Activities of Some Plants Used Traditionally in Ethiopia for the Treatment of Skin Disorders. Ethiop Pharm. J. 24 (2). doi:10.4314/epj.v24i2.35108
Górniak, I., Bartoszewski, R., and Króliczewski, J. (2019). Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 18, 241–272. doi:10.1007/s11101-018-9591-z
Habbal, O., Hasson, S., El-Hag, A., Al-Mahrooqi, Z., Al-Hashmi, N., Al-Bimani, Z., et al. (2011). Antibacterial Activity of Lawsonia Inermis Linn (Henna) against Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 1 (3), 173–176. doi:10.1016/s2221-1691(11)60021-x
Habtamu, A., and Mekonnen, Y. (2017b). Antibacterial Potential of the 80 % Methanol and Chloroform Extracts of Clematis Hirsuta. Afr. J Pharm Pharmacol 11 (16), 204–208. doi:10.5897/AJPP2016.4540
Habtamu, A., and Mekonnen, Y. (2017a). Evaluation of the Antibacterial Activities of Leaf Extracts of Achyranthus Aspera. Afr. J Bacteriol Res 9 (February), 9–14.
Habtamu, A., and Melaku, Y. (2018). Antibacterial and Antioxidant Compounds from the Flower Extracts of Vernonia Amygdalina. Adv. Pharmacol. Sci. 2018, 1–6. doi:10.1155/2018/4083736
Hagos, Z., Teka, M. Z., Brhane, M. Y., Gopalakrishnan, V. K., and Chaithanya, K. K. (2018). In Vitro antibacterial Activities of the Methanolic and Aqueous Extracts of Moringa Stenopetala Leaves. Drug Invent Today 10 (5), 642–650.
Haroun, M. F., and Al-Kayali, R. S. (2016). Synergistic Effect of Thymbra Spicata L. Extracts with Antibiotics against Multidrug- Resistant Staphylococcus aureus and Klebsiella pneumoniae Strains. Iran J. Basic Med. Sci. 19 (11), 1193–1200.
Hassanshahian, M., Bayat, Z., Saeidi, S., and Shiri, Y. (2014). Antimicrobial Activity of Trachyspermum Ammi Essential Oil against Human Bacterial. Int. J. Adv. Biol. Biomed. Res. [Internet] 2 (1), 18–24. Available at: http://www.ijabbr.com.
Hawaze, S., Deti, H., and Suleman, S. (2012). In Vitro antimicrobial Activity and Phytochemical Screening of Clematis Species Indigenous to Ethiopia. Indian J. Pharm. Sci. 74 (1), 29–35. doi:10.4103/0250-474x.102540
Hussien, J., Hymete, A., and Rohloff, J. (2010). Volatile Constituents and Biological Activities ofPycnostachys abyssinicaandPycnostachys Eminiiextracts. Pharm. Biol. 48 (12), 1384–1391. doi:10.3109/13880209.2010.486406
Iván, D., Salazar, O., Alberto, R., Sánchez, H., Sánchez, F. O., Arteaga, M. A., et al. (2015). antifungal activity of neem (Azadirachta indica : meliaceae) extracts against dermatophytes Actividad antifúngica de extractos de neem (Azadirachta indica : Meliaceae) sobre hongos dermatofitos. Acta Biologica Colombiana 20 (3), 201–207. doi:10.15446/abc
Jain, N., and Sharma, M. (2017). Screening of Thymus Vulgaris Essential Oil against Fungi Causing Dermatophytosis in Human Beings. Int. J. Pharm. Pharm. Sci. 9 (10), 236. doi:10.22159/ijpps.2017v9i10.20054
Jima, T. T., and Megersa, M. (2018). Ethnobotanical Study of Medicinal Plants Used to Treat Human Diseases in Berbere District , Bale Zone of Oromia Regional State , South East Ethiopia. Evid Based. Complement Altern Med. 2018, 16. doi:10.1155/2018/8602945
Kahaliw, W., Aseffa, A., Abebe, M., Teferi, M., and Engidawork, E. (2017). Evaluation of the Antimycobacterial Activity of Crude Extracts and Solvent Fractions of Selected Ethiopian Medicinal Plants. BMC Complement Altern. Med. 17 (1), 1–10. doi:10.1186/s12906-017-1563-0
Kalayou, S., Haileselassie, M., Gebre-egziabher, G., Tiku'e, T., Sahle, S., Taddele, H., et al. (2012). In-vitro Antimicrobial Activity Screening of Some Ethnoveterinary Medicinal Plants Traditionally Used against Mastitis, Wound and Gastrointestinal Tract Complication in Tigray Region, Ethiopia. Asian Pac. J. Trop. Biomed. 2, 516–522. doi:10.1016/s2221-1691(12)60088-4
Kasparaviciene, G., Kalveniene, Z., Pavilonis, A., Marksiene, R., Dauksiene, J., and Bernatoniene, J. (2018). Formulation and Characterization of Potential Antifungal Oleogel with Essential Oil of Thyme. Evidence-Based Complement. Altern. Med. 2018, 1–6. doi:10.1155/2018/9431819
Kaushik, P., Goyal, P., Chauhan, A., and Chauhan, G. (2010). In Vitro Evaluation of Antibacterial Potential of Dry FruitExtracts of Elettaria Cardamomum Maton (Chhoti Elaichi). Iran J. Pharm. Res. 9 (3), 287–292.
Kavitha, M., Raja, M., Kamaraj, C., Karthik Raja, R., Balasubramaniam, V., Balasubramani, G., et al. (2017). In Vitro Antimicrobial Activity of Azadirachta indica ( Leaves ) against Fish Pathogenic Bacteria Isolated from Naturally Infected Dawkinsia Filamentosa ( Blackspot Barb ). Med. Aromatic Plants 6 (3).
Kokoska, L., Havlik, J., Valterova, I., Sovova, H., Sajfrtova, M., and Jankovska, I. (2008). Comparison of Chemical Composition and Antibacterial Activity of Nigella Sativa Seed Essential Oils Obtained by Different Extraction Methods. J. Food Prot. 71 (12), 2475–2480. doi:10.4315/0362-028x-71.12.2475
Kongue, M. D. T., Talontsi, F. M., Lamshöft, M., Kenla, T. J. N., Dittrich, B., Kapche, G. D. W. F., et al. (2013). Sonhafouonic Acid, a New Cytotoxic and Antifungal Hopene-Triterpenoid from Zehneria Scabra Camerunensis. Fitoterapia 85 (1), 176–180. doi:10.1016/j.fitote.2013.01.009
Kwak, Y., Kim, S., and Kim, H. (2017). The Antibacterial Effect of Cinnamomum Verum Extract. Biomed. Res. 28 (15), 6667–6670.
Lulekal, E., Rondevaldova, J., Bernaskova, E., Cepkova, J., Asfaw, Z., Kelbessa, E., et al. (2014). Antimicrobial Activity of Traditional Medicinal Plants from Ankober District, North Shewa Zone, Amhara Region, Ethiopia. Pharm. Biol. 52 (5), 614–620. doi:10.3109/13880209.2013.858362
Maleki, L., Sadeghian-rizi, T., Ghannadian, M., Sanati, M. H., and Sadeghi-aliabadi, H. (2018). Antibacterial Activity of Azadirachta indica Leaf Extracts against Some Pathogenic Standards and Clinical Bacterial Isolates. Avicenna J. Clin. Microb. Infec 5 (1), 8–13. doi:10.5812/ajcmi.12987
Malmir, M., Serrano, R., and Silva, O. (2017). “Anthraquinones as Potential Antimicrobial Agents-A Review,” in Antimicrobial Research: Novel Bioknowledge and Educational Programs [Internet]. Editor A. Mendez-Vilas (FORMATEX), 55–61. Available at: http://www.formatex.info/microbiology6/book/55-61.pdf.
Megeressa, M., Bisrat, D., Mazumder, A., and Asres, K. (2015). Structural Elucidation of Some Antimicrobial Constituents from the Leaf Latex of Aloe Trigonantha L.C. Leach. BMC Complement Altern. Med. 15 (1), 1–8. doi:10.1186/s12906-015-0803-4
Meshesha, M., Deyou, T., Tedla, A., and Abdissa, N. (2017). Chemical Constituents of the Roots of Kniphofia Isoetifolia Hochst. And Evaluation for Antibacterial Activity. J. Pharm. Pharmacogn Res. 5 (6), 345–353.
Messele, B. (2004). Studies on Extracts of Some Medicinal Plants Traditionally Used for Dermatological Disorders in Ethiopia A Thesis Submitted to the School of Graduate Studies of the Addis Ababa University in Partial Fulfillment of the Requirement for the Degree of Master. Addis Ababa, Ethiopia: Addsi Ababa University.
Minale, G., Bisrat, D., Asres, K., and Mazumder, A. (2014). In Vitro antimicrobial Activities of Anthrones from the Leaf Latex of Aloe Sinana Reynolds. Int. J. Green. Pharm. 8 (1), 7–12. doi:10.4103/0973-8258.126812
Moglad, E. H. O., Abdalla, O. M., Algadir, H. A., Koko, W. S., and Saadabi, A. M. (2014). In Vitro Antimicrobial Activity and Cytotoxicity of Maerua Oblongifolia. Int. Inv J. Med. Med. Sci. 1 (3), 32–37.
Mohammed, H. A., and Omer, A. F. A. (2015). Antibacterial Activity of Azadirachta indica (Neem) Leaf Extract against Bacterial Pathogens in Sudan. Am. J. Res. Commun. 3 (5), 246–251.
Mulat, M., Chali, K., Tariku, Y., and Bacha, K. (2015). Evaluation for in-vitro Antibacterial Activity of Selected Medicinal Plants against Food-Borne Pathogens. Int. J. Pharm. Sci. Rev. Res. 32 (2), 45–50.
Mummed, B., Abraha, A., Feyera, T., Nigusse, A., and Assefa, S. (2018). In Vitro Antibacterial Activity of Selected Medicinal Plants in the Traditional Treatment of Skin and Wound Infections in Eastern Ethiopia. Biomed. Res. Int. 2018, 8. doi:10.1155/2018/1862401
Mwitari, P. G., Ayeka, P. A., Ondicho, J., Matu, E. N., and Bii, C. C. (2013). Antimicrobial Activity and Probable Mechanisms of Action of Medicinal Plants of Kenya: Withania Somnifera, Warbugia Ugandensis, Prunus Africana and Plectrunthus Barbatus, PLoS ONE 8. doi:10.1371/journal.pone.0065619
Nabila, A., Kheira, H., Zakaria, B., and Noureddine, D. (2018). Antifungal Activity of Lawsonia Inermis Leaf Extract against Dermatophytes Species. Int. J. Biosci. 12 (05), 279–283. doi:10.1016/S2221-1691(11)60157-3
Nagarajan, M., Rajasekaran, S., and Ganesh, K. S. (2013). Antibacterial Activity of Lawsonia Inermis L. Int. J. Mod. Biol. Med. 4 (3), 169–175.
Nasir, M., Tafess, K., and Abate, D. (2015). Antimicrobial Potential of the Ethiopian Thymus Schimperi Essential Oil in Comparison with Others against Certain Fungal and Bacterial Species. BMC Complement. Altern. Med. 15. doi:10.1186/s12906-015-0784-3
Ndzeshang, B. L., Mbiakop, R. T., Nchanji, G. T., Kien, C. A., Amambo, G. N., Abong, R. A., et al. (2020). Clinical, Haematological and Biochemical Profiling of Podoconiosis Lymphoedema Patients Prior to Their Involvement in a Clinical Trial in the Northwest Region of Cameroon. Trans. R. Soc. Trop. Med. Hyg. 114 (12), 954–961. doi:10.1093/trstmh/traa146
Ngeny, L. C., Magiri, E., Mutai, C., Mwikwabe, N., and Bii, C. (2013). Antimicrobial Properies and Toxicity of Hagenia Abyssinica (Bruce) J.F.Gmel, Fuerstia Africana T.C.E. Fries, Asparagus racemosus (Willd.) and Ekebergia Capensis Sparrm. Afr. J Pharmacol Ther 2 (3), 76–82.
Nigussie, D., Legesse, B. A., Davey, G., and Fekadu, A. (2020). Ethiopian Medicinal Plants Used for Inflammatory, Wound Healing or Anti-infective Activities : Protocol for Systematic Literature Review and Meta-Analysis. BMJ OPEN Sci. 4 (e100064), 1–8. doi:10.1136/bmjos-2020-100064
Nigussie, D., Makonnen, E., Legesse, B. A., Fekadu, A., and Davey, G. (2020). Antimicrobial Susceptibility of Bacteria Isolated from the Infected Wounds of Patients with Lymphoedema in East Wollega, Ethiopia. Trans. Rsoc Trop. Med. Hyg. 114 (12), 962–973. doi:10.1093/trstmh/traa143
Njeru, S. N., Obonyo, M. A., Nyambati, S. O., and Ngari, S. M. (2015). Antimicrobial and Cytotoxicity Properties of the Crude Extracts and Fractions of Premna Resinosa (Hochst.) Schauer (Compositae): Kenyan Traditional Medicinal Plant. BMC Complement. Altern. Med. 15 (295), 1–9. doi:10.1186/s12906-015-0811-4
Nwonuma, C. O., Adelani-Akande, T. A., Osemwegie, O. O., Olaniran, A. F., and Adeyemo, T. A. (2020). Preliminary In Vitro Antimicrobial Potential and Phytochemicals Study of Some Medical Plants. F1000Res 8, 81. doi:10.12688/f1000research.17094.3
Obey, J. K., von Wright, A., Orjala, J., Kauhanen, J., and Tikkanen-Kaukanen, C. (2016). Antimicrobial Activity ofCroton macrostachyusStem Bark Extracts against Several Human Pathogenic Bacteria. J. Pathog. 2016 (Mic), 1–5. doi:10.1155/2016/1453428
Omidpanah, S., Vazirian, M., Hosseinkhani, F., Hadjiakhondi, A., and Pirali, M. (2016). Antibacterial Activity of Essential Oil of Trachyspermum Ammi (L) Sprague Ex Turrill against Isolated and Standard Bacteria. Am. J. Essent. Oils Nat. Prod. 4 (2), 5–11.
Oumer, A., Bisrat, D., Mazumder, A., and Asres, K. (2014). A New Antimicrobial Anthrone from the Leaf Latex of Aloe Trichosantha. Nat. Prod. Commun. 9 (7), 949–952. doi:10.1177/1934578x1400900717
Palla, A. H., Khan, N. A., Bashir, S., Ur-Rehman, N., Iqbal, J., and Gilani, A.-H. (2015). Pharmacological Basis for the Medicinal Use of Linum usitatissimum (Flaxseed) in Infectious and Non-infectious Diarrhea. J. Ethnopharmacology 160, 61–68. doi:10.1016/j.jep.2014.11.030
Park, M. J., Gwak, K. S., Yang, I., Choi, W. S., Jo, H. J., Chang, J. W., et al. (2007). Antifungal Activities of the Essential Oils in Syzygium Aromaticum (L.) Merr. Et Perry and Leptospermum Petersonii bailey and Their Constituents against Various Dermatophytes. J. Microbiol. 45 (5), 460–465.
Rahmoun, N., Boucherit-Otmani, Z., Boucherit, K., Benabdallah, M., and Choukchou-Braham, N. (2013). Antifungal Activity of the AlgerianLawsonia Inermis(henna). Pharm. Biol. 51 (1), 131–135. doi:10.3109/13880209.2012.715166
Raja, R. Y., Krishna, K. C., Lokanatha, O., Mamatha, S., and Damodar Reddy, C. (2013). Antimicrobial Activity of Azadirachta Indica ( Neem ) Leaf , Bark and Seed Extracts. Int. J. Res. Phytochem. Pharmacol. 3 (1), 1–4.
Rana, I. S., Rana, A. S., and Rajak, R. C. (2011). Evaluation of Antifungal Activity in Essential Oil of the Syzygium Aromaticum (L.) by Extraction, Purification and Analysis of its Main Component Eugenol. Braz. J. Microbiol. 42, 1269–1277. doi:10.1590/s1517-83822011000400004
Rawat, V., and Bisht, P. (2014). Antibacterial Activity of Withania Somnifera against Gram-Positive Isolates from Pus Samples. Ayu 35 (3), 330. doi:10.4103/0974-8520.153757
Regassa, F., and Araya, M. (2012). In Vitro antimicrobial Activity of Combretum Molle (Combretaceae) against Staphylococcus aureus and Streptococcus Agalactiae Isolated from Crossbred Dairy Cows with Clinical Mastitis. Trop. Anim. Health Prod. 44, 1169–1173. doi:10.1007/s11250-011-0054-4
Romha, G., Admasu, B., Hiwot Gebrekidan, T., Aleme, H., and Gebru, G. (2018). Antibacterial Activities of Five Medicinal Plants in Ethiopia against Some Human and Animal Pathogens. Evidence-Based Complement. Altern. Med. 2018, 1–10. doi:10.1155/2018/2950758
Rondevaldova, J., Leuner, O., Teka, A., Lulekal, E., Havlik, J., Van Damme, P., et al. (2015). In Vitro antistaphylococcal Effects of Embelia Schimperi Extracts and Their Component Embelin with Oxacillin and Tetracycline. Evid Based. Complement Altern Med. 2015. doi:10.1155/2015/175983
Sahalie, N. A., Abrha, L. H., and Tolesa, L. D. (2018). Chemical Composition and Antimicrobial Activity of Leave Extract of Ocimum Lamiifolium (Damakese) as a Treatment for Urinary Tract Infection. Cogent Chem. 4 (1), 1–10. doi:10.1080/23312009.2018.1440894
Salazar, D. O., Alberto, R., Sánchez, H., Sánchez, F. O., Arteaga, M. A., Fernanda, L., et al. (2015). Antifungal Activity of NEEM (Azadirachta indica : Meliaceae) Extracts against Dermatophytes. Acta Biol. Colomb 20 (3), 201–207. doi:10.15446/abc.v20n3.45225
Sánchez, E., García, S., and Heredia, N. (2010). Extracts of Edible and Medicinal Plants Damage Membranes of vibrio Cholerae. Appl. Environ. Microbiol. 76 (20), 6888–6894. doi:10.1128/AEM.03052-09
Seshathri, K., and Thiyagarajan, T. (2011). Antimicrobial Activity of Chewing Sticks of Jimma – Ethiopia against Streptococcus Pyogens. J. Phytol. 3 (8), 34–37.
Sharifzadeh, A., Khosravi, A. R., Shokri, H., and Sharafi, G. (2015). Antifungal Effect of Trachyspermum Ammi against Susceptible and Fluconazole-Resistant Strains of Candida Albican S. J. de Mycologie Médicale 25 (2), 143–150. doi:10.1016/j.mycmed.2015.03.008
Sileshi, A., Gebre-Mariam, T., and Asres, K. (2007). Antibacterial and Antifungal Activities of Extracts of Some Medicinal Plants of Ethiopia. Ethiop Pharm. J. 25 (2), 111–120. doi:10.4314/epj.v25i2.35125
Singh, R. B., Singh, V., Singh, K. R., and Ebibeni, N. (2011). Antimicrobial Activity of Lemongrass (Cymbopogon Citratus) Oil against Microbes of Environmental, Clinical and Food Origin. Int. Res. Pharm. Pharmacol. 1 (9), 228–236.
Sisay, M., Bussa, N., Gashaw, T., and Mengistu, G. (2019). Investigating In Vitro Antibacterial Activities of Medicinal Plants Having Folkloric Repute in Ethiopian Traditional Medicine. J. Evidence-based Integr. Med. 24, 1–9. doi:10.1177/2515690x19886276
Soković, M. D., Vukojević, J., Marin, P. D., Brkić, D. D., Vajs, V., and Van Griensven, L. J. (2009). Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 14 (1), 238–249. doi:10.3390/molecules14010238
Tadeg, H., Mohammed, E., Asres, K., and Gebre-Mariam, T. (2005). Antimicrobial Activities of Some Selected Traditional Ethiopian Medicinal Plants Used in the Treatment of Skin Disorders. J. Ethnopharmacology 100 (1–2), 168–175. doi:10.1016/j.jep.2005.02.031
Tadele, A., Urga, K., Gemeda, N., Lemma, H., and Melaku, D. (2008). Antimicrobial Activity of Topical Formulations Containing Thymus Vulgaris Essential Oil on Major Pathogens Causing Skin Diseases. Ethiop Pharm. J. 110, 103–110. doi:10.4314/epj.v26i2.43041
Tadesse, B., Yinebeb, T., and Ketema, B. (2016). Antibacterial Activity of Selected Medicinal Plants Used in South-Western Ethiopia. Afr. J. Microbiol. Res. 10 (46), 1961–1972. doi:10.5897/ajmr2016.8328
Taye, B., Giday, M., Animut, A., and Seid, J. (2011). Antibacterial Activities of Selected Medicinal Plants in Traditional Treatment of Human Wounds in Ethiopia. Asian Pac. J. Trop. Biomed. 1 (5), 370–375. doi:10.1016/s2221-1691(11)60082-8
Tchana, M. E., Fankam, A. G., Mbaveng, A. T., Nkwengoua, E. T., Seukep, J. A., Tchouani, F. K., et al. (2014). Activities of Selected Medicinal Plants against Multi-Drug Resistant Gram-Negative Bacteria in Cameroon. Afr. Health Sci. 14 (1), 167–172. doi:10.4314/ahs.v14i1.25
Teka, A., Rondevaldova, J., Asfaw, Z., Demissew, S., Van Damme, P., Kokoska, L., et al. (2015). In Vitro antimicrobial Activity of Plants Used in Traditional Medicine in Gurage and Silti Zones, South central Ethiopia. BMC Complement Altern. Med. 15 (1), 1–7. doi:10.1186/s12906-015-0822-1
Teshome, S., and Teshale, C. (2013). Preliminary Phytochemical Screening and Evaluation of the Antibacterial Activity of the Gradient Extracts of Leaves of Clematis Simensis. Int. J. Biotechnol. Res. [Internet] 1 (8), 116–119. Available at: http://academeresearchjournals.org/journal/ijbr.
Umer, S., Tekewe, A., and Kebede, N. (2013). Antidiarrhoeal and Antimicrobial Activity of Calpurnia Aurea Leaf Extract. BMC Complement Altern. Med. [Internet] 13, 21. doi:10.1186/1472-6882-13-21
Vaijayanthimala, J., Anandi, C., Udhaya, V., and Pugalendi, K. V. (2000). Anticandidal Activity of Certain South Indian Medicinal Plants. Phytother. Res. 14 (3), 207–209. doi:10.1002/(sici)1099-1573(200005)14:3<207::aid-ptr564>3.0.co;2-i
Vijayasanthi, M. V. K. (2014). Antimicrobial Activities of Delonix Elata (Bojer Ex Hook.) Raf. And Spathodea campanulata P. Beauv. Afr. J Microbiol Res. 8 (7), 697–701.
Viswanathan, S., and Jayachandra, K. (2017). Antifungal Activity of Various Extracts of Azadirachta indica Leaf - an In-Vitro Study. Int. J. Chemtech Res. 10 (15), 305–311.
WHO (2009). Hand Book of Good Laboratory Practice (GLP)- Quality Practices for Regulated Non-clinical Research and Development. 2nd Edn. Lausanne, Switzerland.
Wodi, M. S. (2016). Phytochemicals Investigation and Antimicrobial Evaluation of Foeniculum Vulgare Leaves. Harer, Ethiopia: Haramaya University.
World Health Organization (2011). Annex 2 WHO Good Practices for Pharmaceutical Microbiology Laboratories. Lausanne, Switzerland. 961. 69–93.
Xu, L., Li, X., Cui, Y., Pang, M., Wang, F., and Qi, J. (2018). Antibacterial Activity of Anthraquinone from Aloe on Spiced Pig Head. IOP Conf. Ser. Mater. Sci. Eng. 275, 1–7. doi:10.1088/1757-899x/275/1/012014
Yeabyo, S., Zenebe Teka, M., Gopalakrishnan, V. K., Hagos, Z., and Krishna Chaithanya, K. (2018). Antibacterial Activity of Root Extracts of Verbascum Sinaiticum against Multidrug-Resistant Enterobacteriaceae Family Gram-Negative and Two Gram-Positive Bacteria. Drug Invention Today 10, 1387–1394.
Yemata, G., Desta, B., and Fetene, M. (2019). In Vitro antibacterial Activity of Traditionally Used Medicinal Plants against Xanthomonas Campestris Pv. Musacearum in Ethiopia. Biodiversitas 20 (2), 555–561. doi:10.13057/biodiv/d200235
Keywords: systematic literature review, antifungal, antibacterial, Ethiopia, medicinal plants
Citation: Nigussie D, Davey G, Tufa TB, Brewster M, Legesse BA, Fekadu A and Makonnen E (2021) Antibacterial and Antifungal Activities of Ethiopian Medicinal Plants: A Systematic Review. Front. Pharmacol. 12:633921. doi: 10.3389/fphar.2021.633921
Received: 26 November 2020; Accepted: 14 May 2021;
Published: 01 June 2021.
Edited by:
Alessandra Durazzo, Council for Agricultural Research and Economics, ItalyReviewed by:
Vivekananda Mandal, Guru Ghasidas Vishwavidyalaya, IndiaWondimeneh Shiferaw, Debre Berhan University, Ethiopia
Copyright © 2021 Nigussie, Davey, Tufa, Brewster, Legesse, Fekadu and Makonnen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dereje Nigussie, ZGVyZV9uaWdAaG90bWFpbC5jb20=
 Dereje Nigussie
Dereje Nigussie Gail Davey
Gail Davey Takele Beyene Tufa
Takele Beyene Tufa Malcolm Brewster
Malcolm Brewster Belete Adefris Legesse
Belete Adefris Legesse Abebaw Fekadu1,2
Abebaw Fekadu1,2 Eyasu Makonnen
Eyasu Makonnen