- 1Department of Neonatology, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Pharmacology, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai, China
Objective: The aim of the study was to observe the clinical efficacy and safety of intravenous and oral sequential treatment with voriconazole for Candida central nervous system (CNS) infection in premature infants.
Methods: The study included retrospective analysis of the clinical data of six premature infants with Candida CNS infection admitted to the neonatology department in Shanghai Children’s Hospital between November 2016 and November 2019. By reviewing the characteristics of voriconazole based on the literature, it showed that infants without gastrointestinal dysfunction could be effectively treated by intravenous and oral sequential therapy with voriconazole (both 7 mg/kg/dose, every 12 h). Clinical manifestations, the time required for the cerebrospinal fluid (CSF), blood culture, nonspecific infection markers such as platelets and C-reactive protein (CRP) to turn normal, and drug-related side effects were observed and recorded in the process of treatment. All data were statistically analyzed by T test and Mann–Whitney U test.
Results: A total of six premature infants were diagnosed with Candida CNS infection, two cases were diagnosed by a positive CSF culture and four cases were clinically diagnosed. Blood culture was positive for Candida in five cases. Among the 6 patients, 4 cases were Candida albicans and 2 cases were Candida parapsilosis. All the six cases were cured. After 3–5 days of treatment, symptoms such as lethargy, apnea, and feeding intolerance were improved and disappeared; a repeated blood culture turned negative in 3–7 days; CSF returned to normal in 15 ± 9 days on an average. Brain abscess, meningeal inflammation, and other infectious lesions were cleared on cranial magnetic resonance imaging (MRI) after treatment. The average total course of voriconazole was 61 ± 29 days, and the average oral treatment was 28 ± 15 days. No Candida recurrence was found during the treatment, and no drug-related side effects such as skin rash, liver and kidney function impairment, or visual abnormalities were found. The white blood cells, CSF glucose/plasma glucose ratio, and protein in CSF were significantly improved after the treatment (p < 0.05). No statistically significant difference was identified in the liver and kidney function indexes (p > 0.05).
Conclusion: Voriconazole is a relatively safe and effective alternative treatment for Candida CNS infection in preterm infants. No severe drug-related side effects were detected.
Introduction
Invasive fungal infection (IFI) has been identified as the third leading cause of late-onset sepsis (LOS) in neonatal intensive care unit (NICU) (Leibovitz, 2012; Zhuang et al., 2014). Premature infants (gestational age <32 weeks), very low birth weight infants (Xia et al., 2014), and patients with compromised immunologic function are especially susceptible to this infection. Multiple tissues and organs, such as the central nervous system, could be involved, which may significantly increase the mortality of neonates and lead to neurodevelopmental impairment (NDI) (Adams-Chapman et al., 2013). Therefore, it is particularly important to choose effective antifungal drugs timely in order to improve the outcome and reduce mortality in newborns, especially premature infants.
However, the choice of antifungal drugs is limited in newborns. Due to refractory fungal infections and the emergence of antifungal-resistant strains, the optimal antifungal therapy for fungal meningitis in newborn is still unknown. The purpose of this study was to analyze clinical data, review-related articles, and seek evidence for clinical management.
Objects and Methods
Objects
There were six premature infants diagnosed with Candida CNS infection in the neonatology department of Shanghai Children’s Hospital from November 2016 to November 2019. According to Chinese guidelines for definition of IFI in severe patients, identification of fungus in CSF culture is the golden standard for confirmed diagnosis. The criteria for clinical diagnosis are as follows: 1) at least one risk factor; 2) one major clinical feature (evidence of specific imaging changes in the central nervous system) or two minor clinical features (presence of corresponding symptoms, signs of central nervous system infection, and supporting laboratory evidence, such as increased cell counts or abnormal biochemistry of CSF); and 3) microbial evidence of fungal infection was found in the blood culture. Among the 6 cases, 2 cases were confirmed as Candida CNS infection with positive CSF culture and 4 cases were clinically diagnosed with positive blood culture but negative CSF culture.
Methods
Data collection: Clinical data of premature infants with Candida CNS infection were collected retrospectively. General data such as personal history, birth history, clinical manifestations, examination, treatment, and outcome were recorded and analyzed.
Antifungal therapy: According to culture and drug sensitivity, 4 cases were treated with intravenous fluconazole (12 mg/kg/dose, once daily) initially but failed (two positive repeated blood cultures). Treatment was then changed to intravenous voriconazole (7 mg/kg/dose, every 12 h). 2 cases were given intravenous voriconazole once diagnosed. Oral sequential treatment with voriconazole was given at the same dose after clinical situation improved, the blood culture turned negative, and CSF returned to normal. The oral voriconazole was discontinued until the clinical symptoms and signs of infection disappeared and infection lesions in cranial imaging were cleared (Pappas et al., 2016). In this study, blood culture was repeated every 3 days, until it came back negative twice. Lumbar puncture was repeated after 3 days of treatment for the first time, and then every 1 week until CSF was back to normal. Complete blood count (CBC) was monitored every 3 days initially, and then weekly after it turned to normal. Electrolytes and the liver and kidney function were repeated weekly to rule out the side effects. Cranial MRI was performed at the beginning of the disease, before discharge and before discontinuing oral treatment.
Follow-up: Patients were followed up monthly from discharge to corrected age (CA) of 6 months, then every 2–3 months from CA of 6–12 months, and every 3–6 months from CA of 12–24 months. The number of follow-up visits was adjusted according to the results, including the Medical Behavioral Neurological Assessment (NBNA), the Gesell Developmental Scales (GDS), the Bayley-III Infant Assessment Scale, cranial MRI, brainstem auditory evoked potentials, retinopathy, and vision screening. The patients who did not come for follow-up clinic were assessed by the Age and Developmental Process Questionnaire (ADPQ) by phone call to the guardians.
Statistical methods: SPSS 22.0 statistical software was used for data analysis. The measurement data of normal distribution were expressed as the mean ± standard deviation (SD). Due to the limited number of cases, paired T-test was performed for the data with normal distribution before and after voriconazole treatment, and Mann–Whitney U test was performed for the data with non-normal distribution. p < 0.05 was considered statistically significant.
Results
General Data
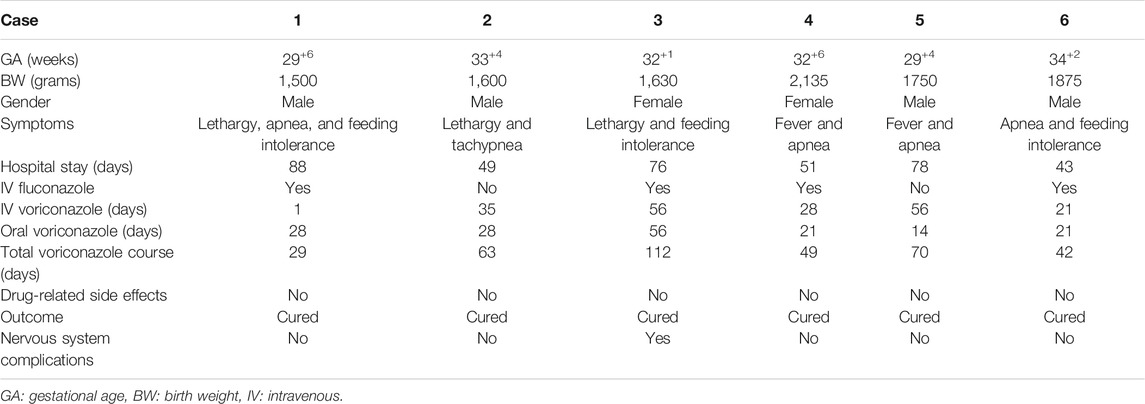
In this study, a total of six premature infants were diagnosed with Candida CNS infection, among which two cases were confirmed by positive CSF culture and four cases were clinically diagnosed with positive blood culture but negative CSF culture. The average gestational age was 32 ± 2 weeks and the average birth weight was 1748 ± 229 g. The average age of diagnosis was 19 ± 10 days and the average hospital stay was 64 ± 19 days. Among these six cases, four were male and two were female. Five cases were delivered by cesarean section and one case was born vaginally. Parenteral nutrition was provided in five cases. Broad-spectrum antibiotics were given in three cases. Three cases received noninvasive respiratory support. One case received pulmonary surfactant. One case was treated with prophylactic fluconazole. Clinical manifestations at onset of the disease included recurrent apneas (4/6), feeding intolerance (3/6), lethargy (3/6), fever (2/6), and tachypnea (1/6) (see Table 1).
Laboratory Results
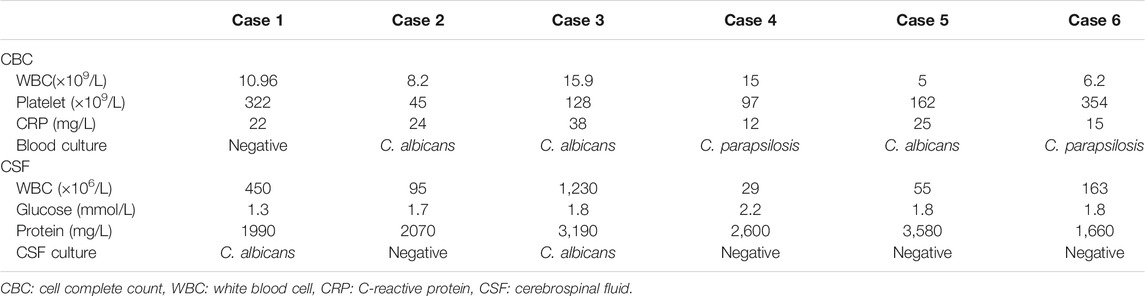
1) CSF was positive in two cases and blood culture was positive in five cases, among which CSF and blood culture were both positive for the same fungal strain in one case. All the six cases grew Candida, four cases were C. albicans and two cases were C. parapsilosis. Fluconazole and voriconazole were both sensitive in all cases by in vitro drug sensitivity test.
2) White blood cell counts in CSF were increased in all six cases, accompanied with decreased glucose and elevated protein.
3) CRP was increased in all six cases and thrombocytopenia was identified in three cases (See Table 2).
Imaging
Cranial MRI was performed in all six cases during hospitalization. Four cases showed brain abscess, among which three cases presented with multiple scattered miliary nodules (Figure 1A) and one case presented with brain parenchymal lesions with multiple abscesses and granuloma formation (Figure 2A). Two cases presented with meningeal inflammation.
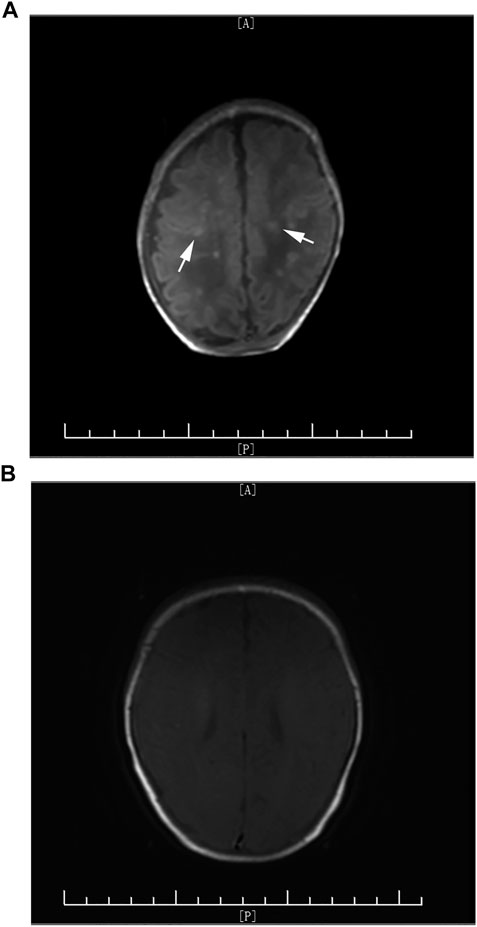
FIGURE 1. (A) Arrows indicate multiple scattered high-signal nodules in T1WI before voriconazole treatment. (B) Clearance of high-signal nodules before treatment was discontinued.

FIGURE 2. (A) Multiple nodular and circle-enhanced signals in T2-FLAIR at 1 week after infection. (B) Clearance of enhanced signals before treatment was discontinued. (C) Arrows indicate paraventricular white matter injury in T1WI before treatment was discontinued.
Clinical Efficacy
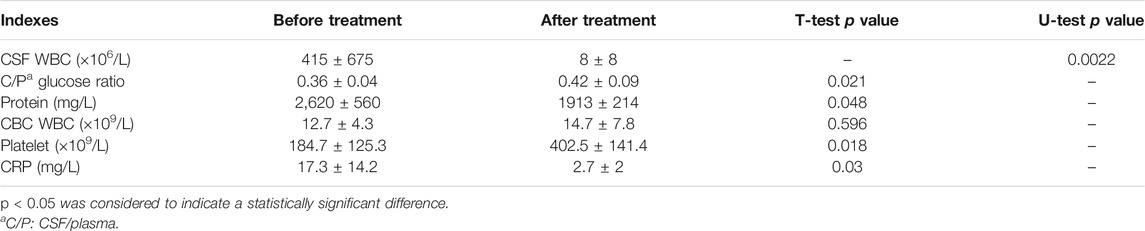
Intravenous and oral sequential voriconazole treatment was given in all six cases; among them, four cases were treated with fluconazole initially but failed (2 positive repeated blood cultures). The average time of voriconazole treatment was 61 ± 29 days, including 33 ± 21 days for intravenous treatment and 28 ± 15 days for oral treatment (see Table 1). Blood culture turned negative after 3–7 days of treatment with voriconazole. Platelet, CRP, and CSF turned to a normal range after 6 ± 1 day, 7 ± 1 day, and 15 ± 9 days, respectively. The difference was statistically significant (p < 0.05) (See Table 3).
Safety Evaluation
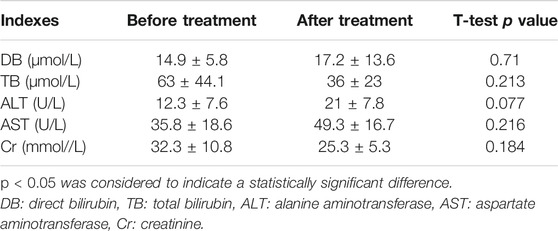
All patients had no drug-related side effects during treatment. No significant changes were observed in the liver and kidney functions before and after the treatment (p > 0.05) (see Table 4).
Outcome
One case was discharged early by the request of guardians due to economic issue of the family. The symptoms had already improved before discharge, but CSF was still abnormal and cranial MRI showed multiple miliary abscesses. Other patients were discharged after symptoms disappeared and blood culture and CSF returned to normal. Abnormal signals of MRI were improved but not cleared before discharge. Oral sequential treatment of voriconazole was continued after discharge for all the patients. Fungal meningitis was cured in all six cases ultimately.
Follow-Up
One case failed to come to follow-up clinic and brain MRI was not repeated; therefore, follow-ups through phone call were provided. It seems there are no significant neurodevelopment problems with this infant according to ADPQ. The other five cases were followed up in the outpatient clinic of our hospital. Brain MRI was repeated before discontinuing oral voriconazole (Figure 1B, Figure 2B). Although the infection lesions in the brain were cleared, impaired white matter was identified in one case (Figure 2C). Meanwhile, the patient showed nervous system underdevelopment, mainly in fine and gross motor development. Rehabilitation had been provided. The other four infants who were followed up had no significant neurodevelopment sequela; their growth and intellectual development were basically normal. Brainstem evoked potential, retinal screening, and visual examination were all normal in 6 cases.
Discussion
IFI is one of the important causes of increased morbidity and mortality in premature infants. In addition to immature autoimmune system, the risk factors of IFI in premature infants include small gestational age, very low birth weight, skin or gastrointestinal colonization, necrotizing enterocolitis (NEC), prolonged course of broad-spectrum antibiotics, deep vein catheterization, H2 blockers, and parenteral nutrition (Arsenault and Bliss, 2015). Studies have shown that neutropenia is also an independent risk factor for IFI in premature infants (Ramy et al., 2018). In our study, all the patients were premature infants, and five of them received parenteral nutrition.
Related studies have shown that in China, the incidence of IFI in NICU is 2.42 in 1,000 admissions; among them, 73.5% were premature infants and 75.5% were low birth weight infants (Chen et al., 2017). The most common pathogen is Candida, including C. albicans and C. parapsilosis, which is consistent with foreign reports (Chow et al., 2012). C. albicans is more likely to lead to central nervous system infection (Chang et al., 2017). In our study, 6 cases were all Candida infections, 4 cases were C. albicans and 2 cases were C. parapsilosis, in line with the current studies. Another study of 11 Chinese NICUs (Xia et al., 2014) showed that invasive Candida incidence (ICI) accounted for 0.74% of the total discharges of premature infants, among which 22.4% could develop fungal meningitis, and the mortality rate of ICI was 19.3%.
However, early diagnosis of fungal CNS infection is difficult. First of all, the typical clinical symptoms are rare, such as fever, seizures, and other specific signs of meningitis. Apneas, feeding intolerance, lethargy, and other nonspecific manifestations of infection are commonly seen. Second, the diagnosis of CNS infection is still based on positive CSF culture, but the positive rate is relatively low and it needs a long time for fungi to grow. Meanwhile, there are some reports that indicate that several cases with normal CSF results were clinically diagnosed with fungal CNS infection (Zhuang et al., 2014). Other laboratory indicators, such as (1,3)-β-glucan (BDG) (Litvintseva et al., 2014), decreased platelet count (Yang and Mao, 2018), and elevated CRP, are helpful in early diagnosis of infection in premature infants but cannot directly point to fungal infection. Therefore, cranial imaging examination plays a more and more important role in the diagnosis of fungal meningitis. Meningeal inflammation, brain abscess, diversification of cerebral infarction, cerebral thrombosis, and demyelinating change could be seen on cranial MRI (Pappas et al., 2016). Mao et al. (2011) reported that multiple micro-abscesses on cranial MRI is an important characteristic of Candida meningitis in neonates, and multiple high-signal lesions on early DWI combined with systemic fungal infection can help with early diagnosis of Candida meningitis and differentiation with infection by bacteria or other pathogens. Imaging provides important evidence and evaluation of clinical diagnosis, severity, course of treatment, and prognosis. In our study, cranial MRI was performed in all six cases. Multiple scattered miliary nodules of brain abscess (Figure 1A) were seen in three cases, extensive brain parenchymal lesions with multiple abscesses associated with granuloma formation was seen in one case (Figure 2A), and the other two cases only presented with meningeal inflammation. The case with most severe MRI presentation (Figure 2A) needed the longest time for CSF to return to normal (21 days vs. average 15 days) and the longest course of voriconazole treatment (112 days vs. average 61 days). Although the radiographic inflammatory lesions were cleared, the later-on cerebral white matter injury in this case still leads to nervous system underdevelopment during follow-up. It indicates that the severity of cranial MRI in cases with fungal CNS infection may have a predictive value for long-term neurodevelopmental outcome.
Timely and adequate choice of medication is particularly important to treat fungal meningitis. Amphotericin B and amphotericin B liposomes are still the recommended first-line drugs, although side effects such as hypokalemia, myocardial injury, and acute kidney injury (AKI) are not rarely seen. Chen et al. (2019) reported a meta-analysis to evaluate efficacy and safety of echinocandins vs. amphotericin B in children and neonates, which revealed that the amphotericin B group had a higher risk of treatment discontinuation because of adverse effects. Fluconazole had a good effect in the past, but with increased prophylactic treatment, there has been emergence of drug-resistant strains. In our study, initial fluconazole treatment in all four cases failed, even though it was sensitive according to the in vitro susceptibility test. This could be due to weak MIC interpretive breakpoints for fluconazole and Candida. The clinical data supporting the breakpoints were not from infections involving strains with elevated fluconazole MICs (Pfaller et al., 2006). Polyene and pyrimidine are also not suitable for neonates due to possible severe side effects. The European guidelines for diagnosis and management of Candida diseases (Hope et al., 2012) also warned that prolonged treatment of micafenxin may increase the incidence of liver tumors in rats. Therefore, we took voriconazole, the second generation of triazole antifungal agent, as our choice.
Voriconazole is a first-line drug for the treatment of invasive Aspergillus which also can treat candidiasis. It has been widely used in the antifungal treatment in children and adults with a good efficacy. Huang et al. (2014) reported four premature infants with invasive fungal infection treated with voriconazole (4 mg/kg/dose, every 12 h). Two cases showed a mild increase of ALT during the treatment, which was reversible after the discontinuation of voriconazole. (Celik et al., 2013) successfully cured 12 out of 17 neonatal fungal sepsis cases with voriconazole, and no severe or irreversible side effects were detected. Altuncu E et al. (Altuncu et al., 2015) reported two premature infants diagnosed with C. parapsilosis who were successfully treated with voriconazole (8 mg/kg/day) after failure of amphotericin B treatment. One of them was an infant with extremely low birth weight and no side effects were found during treatment. Until now, data of voriconazole treatment for children less than 2 years old are still limited. Regarding the dosage, a Chinese single-center study in recent years (Liu et al., 2017) pointed out that for most Asian children aged under 2 year, 5–7 mg/kg/dose, twice daily is recommended for initial intravenous treatment. Recently, a Japanese study (Mori et al., 2015) about pharmacokinetics and safety of voriconazole showed that the removal of voriconazole is significantly faster in children than in adults. It indicates that a higher recommended dosage may be acceptable in pediatric patients. In the meantime, children tolerated voriconazole well as same as adults. The main side effects included photophobia and abnormal hepatic function. In our study, six premature infants with Candida CNS infection received intravenous and oral sequential voriconazole treatment (7 mg/kg/dose, every 12 h). No impaired liver or renal function was found during the treatment. No abnormality was found in retina and visual examination. According to the study of Zhuang et al. (2014), fluconazole (5 cases) and amphotericin B (2 cases) were used to treat fungal meningitis in premature infants. The average time for platelet, CRP, and CSF to turn normal was 5.7, 9.5, and 24.2 days, respectively. The average time for clinical symptoms to disappear was 18.4 days. Compared with this study, our study seems to indicate that voriconazole is more superior due to less time needed for infection markers to turn normal, although the sample size was too small. Another retrospective study (Wu et al., 2020) about the antifungal regimen for Candida parapsilosis showed that the therapeutic effects of voriconazole were superior to fluconazole.
The optimal course of treatment for Candida meningitis is still not clear. Prolonged duration is usually needed, often more than 3 weeks. In our study, the average time of whole antifungal therapy was 61 ± 29 days and the average time of oral sequential treatment was 28 ± 15 days. Oral sequential treatment could shorten hospital stay and decrease medical cost. The evaluation of neurodevelopment is very important for patients with fungal meningitis. Long-term follow-up is needed. Rehabilitation should be started as soon as possible if there is any evidence of NDI.
In conclusion, intravenous and oral sequential therapy of voriconazole could be used as an alternative treatment for Candida CNS infection. However, continuous therapeutic drug monitoring (TDM) should be implemented due to lack of voriconazole pharmacokinetics in patients less than 2 year old and variation of bioavailability associated with metabolic enzymes, age, and drug interactions (Mori et al., 2015; Hu et al., 2018). Further prospective studies with a larger sample size are needed.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Shanghai Children’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
YZ and CY contributed to the conception and design of this study. YZ drafted the manuscript and revised it critically. XG, ZL, and DW contributed to acquisition, analysis, and interpretation of data. All authors read the manuscript and gave the permission to be published.
Funding
This study was funded by the Clinical Pharmacy Innovation Research Institute of Shanghai Jiaotong University School of Medicine (2019) (No. CXYJY2019MS003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors want to thank Gang Qiu and Cheng Cai for their help with this study.
References
Adams-Chapman, I., Bann, C. M., Das, A., Goldberg, R. N., Stoll, B. J., Walsh, M. C., et al. (2013). Neurodevelopmental Outcome of Extremely Low Birth Weight Infants with Candida Infection. J. Pediatr. 163 (4), 961–e3. doi:10.1016/j.ipeds.2013.04.03410.1016/j.jpeds.2013.04.034
Altuncu, E., Bilgen, H., Soysal, A., and Ozek, E. (2015). Successful Treatment of Candida parapsilosis Fungemia in Two Preterms with Voriconazole. Case Rep. Pediatr. 2015, 1–3. doi:10.1155/2015/402137
Arsenault, A. B., and Bliss, J. M. (2015). Neonatal Candidiasis: New Insights into an Old Problem at a Unique Host-Pathogen Interface. Curr. Fungal Infect. Rep. 9 (4), 246–252. doi:10.1007/s12281-015-0238-x
Celik, I. H., Demirel, G., Oguz, S. S., Uras, N., Erdeve, O., and Dilmen, U. (2013). Compassionate Use of Voriconazole in Newborn Infants Diagnosed with Severe Invasive Fungal Sepsis. Eur. Rev. Med. Pharmacol. Sci. 17 (6), 729–734.
Chang, S., Huang, W., and Liu, X. (2017). Clinical Characteristics of Fungemia in Premature Infants. Chin. J. Infect. Control. 16 (9), 829–832. doi:10.3969/j.issn.1671-9638.2017.09.009
Chen, H., Cui, J., and Tang, H., and (2017). Epidemiological Changes in Invasive Fungal Infection in a Neonatal Intensive Case Unit. Chin. J. Perinat Med. 20 (8), 577–582. doi:10.3760/cma.j.issn.1007-9408.2017.08.007
Chen, Y.-H., Cheng, I.-L., Lai, C.-C., and Tang, H.-J. (2019). Echinocandins vs. Amphotericin B against Invasive Candidiasis in Children and Neonates: A Meta-Analysis of Randomized Controlled Trials. Int. J. Antimicrob. Agents 53 (6), 789–794. doi:10.1016/j.ijantimicag.2019.02.019
Chow, B. D., Linden, J. R., and Bliss, J. M. (2012). Candida Parapsilosisand the Neonate: Epidemiology, Virulence and Host Defense in a Unique Patient Setting. Expert Rev. Anti-infective Ther. 10 (8), 935–946. doi:10.1586/eri.12.74
Hope, W. W., Castagnola, E., Groll, A. H., Roilides, E., Akova, M., Arendrup, M. C., et al. (2012). ESCMID* *This Guideline Was Presented in Part at ECCMID 2011. European Society for Clinical Microbiology and Infectious Diseases. Guideline for the Diagnosis and Management of Candida Diseases 2012: Prevention and Management of Invasive Infections in Neonates and Children Caused by Candida Spp. Clin. Microbiol. Infect. 18 (Suppl. 7), 38–52. doi:10.1111/1469-0691.12040
Hu, L., Dai, T.-t., Zou, L., Li, T.-m., Ding, X.-s., and Yin, T. (2018). Therapeutic Drug Monitoring of Voriconazole in Children from a Tertiary Care center in China. Antimicrob. Agents Chemother. 62 (12), e00955–18. doi:10.1128/AAC.00955-18
Huang, J., Hua, S., and Feng, Z. (2014). Four Premature Infants with Invasive Fungal Infection Treated with Voriconazole. Chin. J. Perinat Med. 17 (2), 126–127. doi:10.3760/cma.j.issn.1007-9408.2014.02.012
Leibovitz, E. (2012). Strategies for the Prevention of Neonatal Candidiasis. Pediatr. Neonatal. 53 (2), 83–89. doi:10.1016/j/pedneo.2012.01.004
Litvintseva, A. P., Lindsley, M. D., Gade, L., Smith, R., Chiller, T., Lyons, J. L., et al. (2014). Utility of (1-3)-β-D-Glucan Testing for Diagnostics and Monitoring Response to Treatment during the Multistate Outbreak of Fungal Meningitis and Other Infections. Clin. Infect. Dis. 58 (5), 622–630. doi:10.1093/cid/cit808
Liu, L., Zhou, X., Wu, T., Jiang, H., Yang, S., and Zhang, Y. (2017). Dose Optimisation of Voriconazole with Therapeutic Drug Monitoring in Children: a Single-centre Experience in China. Int. J. Antimicrob. Agents 49 (4), 483–487. doi:10.1016/j.ijantimicag.2016.11.028
Mao, J., Li, J., Chen, D., Zhang, J., Du, Y. N., Wang, Y. J., et al. (2011). [Value of MRI in the Diagnosis of Cerebral Abscess Caused by Candida Albicans in Premature Infants]. Zhongguo Dang Dai Er Ke Za Zhi 13 (8), 621–626.
Mori, M., Kobayashi, R., Kato, K., Maeda, N., Fukushima, K., Goto, H., et al. (2015). Pharmacokinetics and Safety of Voriconazole Intravenous-To-Oral Switch Regimens in Immunocompromised Japanese Pediatric Patients. Antimicrob. Agents Chemother. 59 (2), 1004–1013. doi:10.1128/AAC.04093-14
Pappas, P. G., Kauffman, C. A., Andes, D. R., Clancy, C. J., Marr, K. A., Ostrosky-Zeichner, L., et al. (2016). Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 62 (4), e1–e50. doi:10.1093/cid/civ933
Pfaller, M. A., Diekema, D. J., and Sheehan, D. J. (2006). Interpretive Breakpoints for Fluconazole and Candida Revisited: a Blueprint for the Future of Antifungal Susceptibility Testing. Cmr 19 (2), 435–447. doi:10.1128/CMR.19.2.435-447.2006
Ramy, N., Hashim, M., Abou Hussein, H., Sawires, H., Gaafar, M., and El Maghraby, A. (2018). Role of Early Onset Neutropenia in Development of Candidemia in Premature Infants. J. Trop. Pediatr. 64 (1), 51–59. doi:10.1093/tropej/fmx02910.1093
Wu, Y., Wei, D., Gong, X., Shen, Y., Zhu, Y., Wang, J., et al. (2020). Initial Use of Voriconazole Positively Affects Outcome of Candida Parapsilosis Bloodstream Infection: a Retrospective Analysis. Transl Pediatr. 9 (4), 480–486. doi:10.21037/tp-20-37
Xia, H., Wu, H., Xia, S., Zhu, X., Chen, C., Qiu, G., et al. (2014). Invasive Candidiasis in Preterm Neonates in China. Pediatr. Infect. Dis. J. 33 (1), 106–109. doi:10.1097/INF.0000000000000009
Yang, Y.-C., and Mao, J. (2018). Value of Platelet Count in the Early Diagnosis of Nosocomial Invasive Fungal Infections in Premature Infants. Platelets 29 (1), 65–70. doi:10.1080/09537104.2017.129381010.1080/09537104.2017.1293810
Keywords: voriconazole, premature infants, oral sequential therapy, invasive fungal infection, candida, CNS infection
Citation: Zhu Y, Gong X, Li Z, Wang D and Yan C (2021) Clinical Analysis of Intravenous and Oral Sequential Treatment With Voriconazole for Candida Central Nervous System Infection in Six Premature Infants. Front. Pharmacol. 12:631293. doi: 10.3389/fphar.2021.631293
Received: 19 November 2020; Accepted: 02 June 2021;
Published: 24 June 2021.
Edited by:
Venkata Kashyap Yellepeddi, The University of Utah, United StatesCopyright © 2021 Zhu, Gong, Li, Wang and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chongbing Yan, eWFuY2JAc2hjaGlsZHJlbi5jb20uY24=
 Yingying Zhu1
Yingying Zhu1 Xiaohui Gong
Xiaohui Gong Chongbing Yan
Chongbing Yan