- 1Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium
- 2Department of Microbiology, Immunology and Transplantation, UZ Leuven, Leuven, Belgium
Objectives: This research aims to evaluate the methodological quality of budget impact analyses for orphan drugs and to provide suggestions for future analyses.
Methods: Conference abstracts and peer-reviewed literature on budget impact analyses were collected through searches of Pubmed and Embase. ISPOR good practice guidelines were used as a methodological standard for budget impact analyses. Examined parameters encompassed: perspective, target population, data sources, intervention and comparator(s), time horizon, scope of costs, discounting, validation, assumptions and sensitivity analysis.
Results: Seventy studies on individual orphan drugs and 21 studies on a combination of orphan drugs analyzing budget impact were identified. Overall, analyses considered a third-party payer perspective, reported periodic budget impacts over a one-to-five-year time horizon, and did not apply discounting. A dynamically fluctuating population and costs beyond drug costs were accounted for in 18.7% and 51.7% of studies, respectively. Input data were retrieved from published literature, clinical trials, registries, claims databases, expert opinions, historical data and market research. Assumptions were mostly made about population size and intervention/comparator(s) market uptake, but these assumptions were rarely justified and their impact was insufficiently explored through sensitivity analyses. Budget impact results were rarely validated.
Conclusion: Existing budget impact analyses for orphan drugs are concise, vary greatly and are of substandard methodological quality. To eliminate possible bias in future budget impact analyses, future studies should adhere to national or ISPOR good practice guidelines on budget impact analysis.
Introduction
With health care expenditure trends surpassing economic growth rates globally (OECD, 2019; WHO, 2019), concerns about financial sustainability have surged. The third major contributor to total health expenditure worldwide is pharmaceutical spending (OECD, 2019), which augmented from US$ 390.2 billion in 2001 to US$ 1.2 trillion in 2018 and is expected to reach US$ 1.52 trillion by 2023 (IQVIA, 2020a; IQVIA, 2020b). Scientific innovation and technological advances in research have been conducive to pharmaceutical growth (OECD, 2019). Furthermore, the late 20th century was accentuated by incentivization of drug development for rare diseases (FDA, 1983; Official Journal of the European Communities, 2000); in 1999, the European Commission (EC) adopted the Orphan Regulation for drugs used in the prevention, diagnosis and treatment of chronically debilitating and life-threatening diseases with low prevalence of five in 10,000 (Official Journal of the European Communities, 2000). As a result, orphan drug share of European drug market increased from 3.1% to 7.2% between 2010 and 2017. Moreover, orphan drug expenditure grew 16% compared to only 3% growth in total pharmaceutical expenditure from 2001 to 2017 (Mestre-Ferrandiz et al., 2019). This rise in orphan drug expenditure instigated funding challenges revealing inequity in access to orphan drugs amongst lower-income and higher-income EU countries (Szegedi et al., 2018). Hence, orphan drug reimbursement extrema of 29.4% and 92.8% were captured in, respectively, Poland and France (Szegedi et al., 2018). Additionally, public pharmaceutical expenditure on orphan drugs ranged from 2.25% to 6.51% in, respectively, the Czech Republic and Belgium (Szegedi et al., 2018). It has been of political priority for many countries to address the ascending pressure on health care budgets in order to maintain market access of qualitative interventions within their fiscal constraints.
Prior to a health intervention being publicly available, it is subject to a country-specific reimbursement process that relies on rigorous health technology assessment (HTA) (Drummond et al., 2007; Denis et al., 2010b; Nicod et al., 2019). One pillar in this assessment is budget impact analysis (BIA), a method that measures affordability of a health intervention in a specific healthcare system (Sullivan et al., 2014). To obtain a comprehensive profile of the intervention, BIA is recommended conjointly with cost-effectiveness analysis (CEA), which estimates the value of an intervention opposed to an alternative (Garattini and van de Vooren, 2011; Sullivan et al., 2014; Mauskopf and Earnshaw, 2017; Pearson, 2018). This is crucial for interventions such as orphan drugs that otherwise fail cost-effectiveness criteria because of their expensive nature and limited evidence-based performance (Drummond et al., 2007; Nicod et al., 2019). In contrast, annual health expenditures of orphan drugs in Europe are generally low (Schlander et al., 2018), with a maximum per capita spending of €20.23 in France (Hutchings et al., 2014), and are forecasted to stabilize below the market growth rate at 4–5% of total pharmaceutical expenditures (Schey et al., 2011). However, a recent publication could not refute a possible slignificant impact of orphan drugs on future pharmaceutical budgets (Gombocz and Vogler, 2020).
In this light, BIA has been regarded as an important measure of affordability and has increasingly been integrated in reimbursement assessments worldwide (Foroutan et al., 2018). However, this realization was preceded by debates on BIA value within HTA, making it a rather new analysis that is in continuous development (Niezen et al., 2009; Foroutan et al., 2020). Some studies point out the inaccuracy of BIA with often an overestimation of budget impacts (Geenen et al., 2020a; Geenen et al., 2020b). To aid in framing structural elements of BIA, several studies, as well as the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) in 2007 and 2014, released good practice guidelines on BIA (Trueman et al., 2001; Mauskopf et al., 2007; Garattini and van de Vooren, 2011; Sullivan et al., 2014; Silva et al., 2017).
Likewise, several jurisdictions have specific requirements for BIA often adapted from the ISPOR guidelines and interpreted to fit their principles (Marshall et al., 2008; Ferreira-Da-Silva et al., 2012; Neyt et al., 2015; Mauskopf and Earnshaw, 2016; Foroutan et al., 2018; Ghabri et al., 2018; Ghabri and Mauskopf, 2018; HIQA, 2018; Foroutan et al., 2019). In spite of these standardizing efforts, BIA are often found to be simplistic, incomplete, inconsistent and poorly designed (Mauskopf et al., 2005; Orlewska and Gulacsi, 2009; van de Vooren et al., 2014; Faleiros et al., 2016). This inaccurate and non-transparent portrayal of BIA complicates inter- and intranational BIA comparison but could especially lead to misinformed resource allocation while potentially disadvantaging the availability of unconventional treatments like orphan drugs (Orlewska and Mierzejewski, 2004; Marshall et al., 2008; Ferreira-Da-Silva et al., 2012; Neyt et al., 2015; Ghabri et al., 2018; Ghabri and Mauskopf, 2018; HIQA, 2018; Foroutan et al., 2019).
In this study, we systematically identify BIA of orphan drugs in the literature with the aim of assessing the methodological quality of the reported analyses. Based on this review, recommendations are proposed to improve reporting of BIAs for orphan drugs.
Methods
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Figure 1) (Liberati et al., 2009). A systematic search of peer-reviewed literature, ISPOR conference abstracts and gray literature was conducted in January 2020. PubMed and Embase were scanned for studies describing “budget impact analysis” and “orphan drugs”. A specific protocol and search strategy for every database was specified and is documented in Supplementary Material.
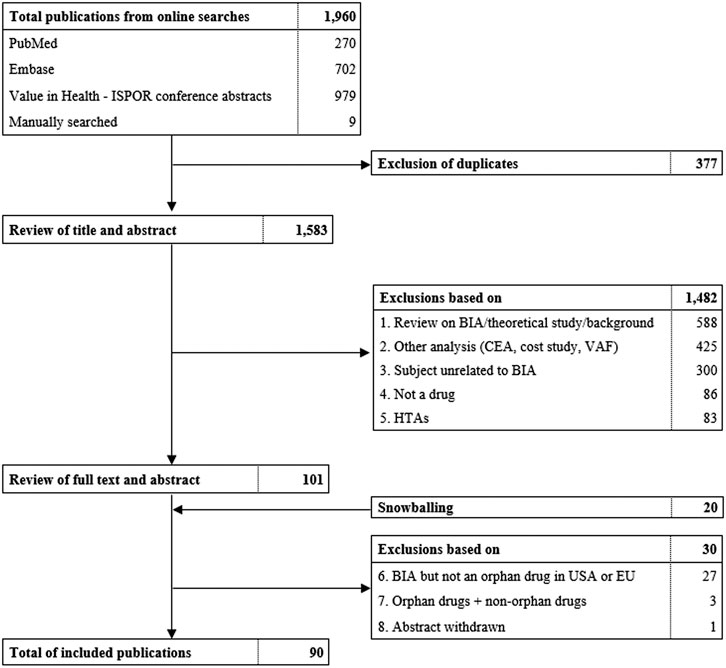
FIGURE 1. Flowchart of literature search and selection of publications. ISPOR, International Society for Pharmacoeconomics and Outcomes Research, BIA, budget impact analysis. CEA, Cost-effictiveness analysis. VAF, value assessment framework. HTA, health technology assessment.
Study Selection
Duplicate removal and filtering of studies was conducted in rayyan.qcri.org, a systematic review web tool. Two reviewers were involved in determining exclusion and inclusion criteria and final selection of studies. The first reviewer screened title and abstracts followed by a second round of examining abstracts and full texts. In case of uncertainties, the second reviewer decided on whether the study was included. This selection process was documented in a PRISMA flowchart (Figure 1).
Eligibility Criteria
Studies analyzing the budget impact of one individual orphan drug or a combination of/multiple orphan drugs were considered. A study was included when the examined drug was labeled ‘orphan’ in the EU or the US for the indication described in the study. Language, country or year of publication were not considered exclusion criteria. Orphanet, European Medicines Agency (EMA) and U.S. Food and Drug Administration (FDA) official websites were used for verification of orphan drug status as of February 2020.
Selection of Parameters and Data Retrieval
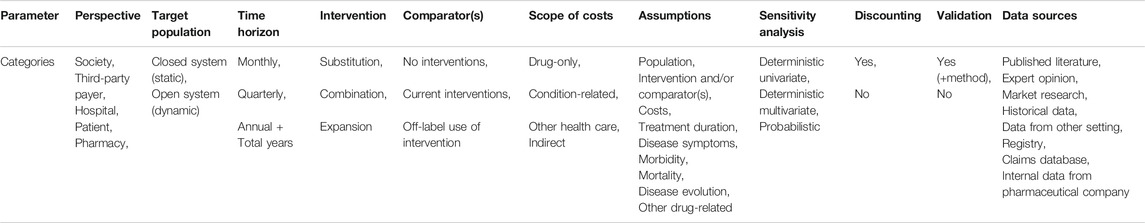
We adopted ISPOR good practice guidelines as quality standards (Mauskopf et al., 2007; Sullivan et al., 2014). Nine parameters were regarded as quality measures of the studies and consisted of; perspective, time horizon, intervention and comparator(s), target population, data sources, scope of costs, sensitivity analysis, discounting and validation.
Different publications concur that these parameters are essential for BIA (Trueman et al., 2001; Orlewska and Mierzejewski, 2004; Marshall et al., 2008; Orlewska and Gulacsi, 2009; Garattini and van de Vooren, 2011; Ferreira-Da-Silva et al., 2012; Sullivan et al., 2014; van de Vooren et al., 2014; Neyt et al., 2015; Faleiros et al., 2016; Mauskopf and Earnshaw, 2016, Mauskopf and Earnshaw, 2017; Silva et al., 2017; Ghabri et al., 2018; HIQA, 2018). Each parameter was subdivided into predefined categories (Table 1) that were attributed to a study if applicable. Data on categories for every study were summarized in a data extraction sheet in Excel (Supplementary Tables S2 and S3).
Analysis
A descriptive analysis of all studies was carried out.
Results
From the total of 1,960 publications initially identified, 90 were included in this systematic review (Figure 1; Supplementary Tables S2 and S3). Thirty-six (40%) studies originated from Europe, 27 (30%) from North-America, 12 (13%) from South-America and 15 (17%) from Asia. The budget impact of individual orphan drugs was analyzed in 69 studies, of which 12 (17%) were full text and 57 (83%) were conference abstracts. Our review covered a variety of orphan drugs targeting cancers (57%), blood disorders (13%), heart diseases (4%), endocrine diseases (7%), inflammation and immune disorders (3%), lung diseases (4%), genetic aberrations (6%), metabolic disorders (1%) and other diseases (4%). Of these drugs, 10% were designated for the treatment of ultra-rare diseases. Furthermore, BIAs of a combination of orphan drugs were identified in 21 studies, of which eight (38%) were available in full text and 13 (62%) as abstract.
Individual Orphan Drugs
The characteristics of BIAs of individual orphan drugs are presented in Supplementary Table S2 and summarized in the following sections.
Perspective
One study considered a societal perspective and all other studies considered the budget holder perspective. With respect to the latter, one study calculated budget impact from the patient perspective, three studies considered the pharmacy perspective and the remaining studies considered the third-party payer perspective. Five studies considered two perspectives instead of one and five studies did not report the perspective of their analysis.
Target Population
In 17 (24.6%) studies, an open system with a dynamically changing population was considered. Thirty-seven (53.6%) studies utilized a closed system and described a static population. In one article (1.4%) both an open system and a closed system were described from different perspectives. Fourteen (20.3%) articles did not report on the dynamics of the eligible population.
Data Sources
All studies reported at least one or more data sources. Eligible patients and population size were based on; expert opinions (7.2%), published literature (26.1%), historical data (20.2%), registries (14.5%), market research (4.3%), internal data from pharmaceutical company (10.1%), claims database (15.9%) and data from other setting (11.6%).
Costs were retrieved from; expert opinions (11.6%), registries (30.4%), internal data from pharmaceutical company (14.5%), published literature (14.5%), data from other setting (23.2%) and claims databases (15.9%).
Intervention or comparator(s) (characteristics, treatment duration, resource use, market effects) were sourced from; expert opinions (18.8%), registries (7.2%), published literature (17.3%), internal data from pharmaceutical company (36.2%), data from other setting (10.1%), claims databases (2.9%) and market research (5.8%).
In one study (1.4%), discounting relied on data from another setting.
Three studies (4.3%) mentioned sources, without specification of the concerned inputs, such as; internal data from pharmaceutical company (4.3%), published literature (1.4%), claims database (1.4%), data from other setting (2.9%) and expert opinion (1.4%).
Four studies (5.8%) did not report on data sources.
Intervention and Comparator(s)
The majority of studies compared an orphan drug with one or more current interventions. In two studies, the orphan drug was compared to no intervention, as no current treatment was available. In five studies, a comparator was not specified or not reported. Introduction of the new intervention was mostly perceived as an additional alternative to current disease interventions in 44 studies, thus expanding the market. In 13 studies the new intervention was assumed to impact the market by substitution of the current treatments and in seven studies by combination with the current interventions. Some studies described the new intervention as influencing the market in multiple ways, on one hand by expansion and substitution, on the other hand by expansion and combination.
Scope of Costs
Drug-only costs were depicted in all studies. Of 47 studies that considered condition-related costs, three studies also addressed indirect costs of productivity loss.
Time Horizon
Apart from one study describing a 60-year horizon and seven references not reporting on a time horizon, all other references examined budget impact over a time horizon ranging from one to five years. In nine studies a one-year horizon was described whereas four references adopted a two-year time horizon. Twenty-eight references and two references respectively marked a three-year horizon and a four-year horizon. A five-year time horizon was considered in 20 references.
Furthermore, 48 studies reported their budget impact annually, seven studies reported monthly numbers and five references jointly reported monthly and annual calculations. In nine studies one net budget impact value over the total time horizon was mentioned.
Assumptions
Assumptions were made about the target population (size) in 56 studies, followed by assumptions about intervention and/or comparator(s) in 47 studies and costs in seven studies. The following assumptions were made: Treatment duration in six studies, morbidity in four studies, mortality in three studies and disease evolution in three studies. Discounting was assumed in one study and other drug-related assumptions in 24 studies. In ten studies budget impact assumptions were not reported.
Discounting
In 55 studies no information on discounting was found. ‘No discounting’ was explicitly mentioned in ten studies. In three studies, an annual discount rate was applied to the budget calculations. One study reported results with and without discounting.
Sensitivity Analysis
Deterministic univariate sensitivity analyses were performed in 14 studies. One study reported multivariate sensitivity analysis/scenario analysis while three other studies presented both univariate and multivariate sensitivity analyses. A probabilistic sensitivity analysis was carried out in one study. The use of a sensitivity analysis was mentioned in thirteen studies without further specification. Thirty-seven studies did not report any sensitivity analysis.
Validation
Four studies validated their results with either stakeholder opinions or comparison to a similar study. All remaining 65 studies did not corroborate their analysis.
Combination of Orphan Drugs
The characteristics of BIAs for combination of orphan drugs are presented in Supplementary Table S3 and summarized in the following sections.
Perspective
In four studies budget impact was calculated from a hospital perspective. Pharmacy perspective was adopted alone in one study and in combination with hospital perspective in another study. In eight studies, third-party payer perspective was used and in another eight studies, the perspective was unspecified.
Target Population
This criterion was largely not applicable for combination of orphan drugs studies, apart from two studies describing a closed system population and one study an open system population.
Data Sources
Eligible patients and population size were retrieved from; registries (9.5%), published literature (19%), claims databases (33.3%), historical data (9.5%) and data from other setting (9.5%).
Costs were derived from published literature (38.1%), claims databases (52.4%), market research (9.5%), internal data from pharmaceutical company (19%), historical data (14.3%), registries (19%) and data from other setting (33.3%).
Intervention or comparator(s) (characteristics, treatment duration, resource use, market effects) were sourced from; registries (71.4%), claims databases (19.0%), published literature (19.0%), historical data (9.5%), internal data from pharmaceutical company (9.5%), market research (9.5%) and data from other setting (14.3%).
One study (4.8%) decided on discounting rate through published literature and one study (4.8%) did not report any data sources.
Scope of Costs
Drug-only costs were considered in all studies.
Time Horizon
In five studies, a one-year horizon was considered for budget impact analysis. One study used a two-year horizon, four studies used a five-year horizon and two studies used a six-year horizon. In one study a seven-year horizon was considered.
One other study considered a nine-year horizon, two studies a ten-year horizon and two other studies a 12-year horizon consisting of a six-year retrospective and a four-year prospective analysis. One study calculated their budget impact based on a 17-year time horizon, one on a 20-year horizon consisting of both ten-year retrospective and prospective analyses, and one on a 21-year horizon consisting of a 13-year retrospective and eight-year prospective analysis.
Annual calculations were reported consistently except for one study reporting monthly values, another one reporting one net value and one study reporting annual and monthly values.
Assumptions
One study made assumptions about costs and three studies about the target population. Ten studies made assumptions about the interventions (market effects) and one study about treatment duration. Eleven studies made other drug-related assumptions whereas nine studies did not mention any assumptions.
Discounting
Majority of studies did not report on discounting except for three that explicitly mentioned ‘no discounting’ and one applying a 3.5% discount rate on their drug costs.
Sensitivity Analysis
A total of six studies reported on sensitivity analyses; three studies conducted deterministic univariate sensitivity analysis, two studies accounted for structural uncertainty through deterministic multivariate sensitivity analyses and one study combined univariate and multivariate sensitivity analyses.
Validation
Five studies validated their data inputs through comparison with reliable data sources. Nine studies compared their budget impact outcomes with a similar study in the same or different region; of these nine studies, two also performed a statistical validation. Seven studies did not report on validation of their analysis.
Discussion
In this review, the methodological quality of BIAs for orphan drugs was systematically assessed based on expert-composed ISPOR good practice guidelines. Majority of our studies covered orphan drugs targeting cancer or blood disorders and primarily originated from Europe and North-America. By mapping out the geographic distribution of BIAs, our review suggests that BIAs are more frequently performed in high-income countries and the Anglosphere than in low- and middle-income countries. We determined that as per ISPOR recommendations, most studies take on a third-party payer perspective, time-horizons are mainly set to one-to-five years while budget impacts are reported periodically, discounting is scarcely applied and overall, data is collected from experts, literature, historical or epidemiological data, national registries, claims databases, market research or pharmaceutical companies. Contrary to ISPOR recommendations, numerous studies do not appropriately account for a dynamically changing population, although a static population may be considered when the population is very defined, scope of costs is often limited to drug-only costs, and analyses are rarely validated. Furthermore, all studies require several assumptions, however these assumptions were seldomly justified nor sufficiently tested by sensitivity analyses. This perceived shortage of sensitivity analyses, may be subject to the numerous BIAs depicted in forms of abstracts which merely capture summarized analyses. Compared to full text BIAs, abstracts described fewer sensitivity analyses, however, in both text types these analyses were often limited when included. Additionally, high-income countries generally performed more and elaborated sensitivity analyses than low- and middle-income countries. Our review shows that most assumptions are made of population size and intervention/comparator(s) market uptake. Due to lack of readily available data and sources specifically on orphan drugs, resorting to assumptions is disadvantageous but inevitable. A sensitivity analysis is essential to investigate the influence of assumptions on structural aspects or variable inputs of the BIA. Moreover, sensitivity analyses allow a more comprehensive prediction of budget impact which should be a staple for orphan drugs considering their data scarcity. This review corroborates precedent findings (Mauskopf et al., 2005; Orlewska and Gulacsi, 2009; van de Vooren et al., 2014; Faleiros et al., 2016) on substandard quality and heterogeneity of BIAs. Multiple factors might be at the cause of the low quality of existing BIAs. One factor is that BIA remains a fairly new method. Not until the nineties did jurisdictions begin requesting BIAs as part of reimbursements and the first methodological guidelines were written only at the end of that decade (Mauskopf, 1998; Sullivan et al., 2014). Since then, the concept of a BIA has evolved with expanded, detailed and updated recommendations were published lastly by the health expert community of ISPOR in 2014 (Sullivan et al., 2014).
Nonetheless, our review assessed only ten and five studies of individual and a combination of orphan drugs, respectively, that were released prior to these ISPOR guidelines (Supplementary Tables S2 and S3).
This implies that, even with updated ISPOR guidelines available, majority of studies still fail to meet methodological quality standards. Another contributor to improper quality might be that, in the past, there have been counterarguments on the usefulness of BIAs due to the close proximity of the technique to CEA (Niezen et al., 2009). However, BIA allows to gage how the total budget in a certain health demographic changes after the introduction of an intervention making this a powerful tool to predict affordability (Garattini and van de Vooren, 2011; Sullivan et al., 2014; Mauskopf and Earnshaw, 2017; Pearson, 2018). Hence, BIAs have become, commensurate with economic evaluations, established as a prerequisite for reimbursement applications in many jurisdictions (Pearson, 2018). As orphan drugs are taking up an emergent share in pharmaceutical expenditure but health care resources remain scarce, correctly predicting budgetary impact is fundamental (Mestre-Ferrandiz et al., 2019). This could facilitate and sustain market access of orphan drugs, which may otherwise be neglected, in a certain health setting.
Recommendations for Future Budget Impact Analyses for Orphan Drugs
Future analyses should be rigorously assessed on adherence to guidelines of methodology and reporting of a budget impact (i.e. national guidelines or ISPOR good practice guidelines) before it is handed to the appropriate budget holder and/or published. We offer a concise template (Table 1) that is required for a comprehensive and correct budget impact analysis and to which budget holders can refer to when reviewing a reimbursement application. All relevant perspectives, being one or more budget holders, should be adopted in the analysis. A dynamic target population is one that accounts for influx and efflux of patients throughout the selected time frame. Costs should not be restricted to drug-costs but also consider condition-related costs such as administration and adverse event costs. To determine the influence of uncertainties on budget impact, assumptions should consistently be accompanied by exhaustive sensitivity analyses. Validation after conducting a budget analysis should be urged and could be done by consulting budget holders and corroborating model parameters. Recently, a study by (Geenen et al., 2020a) proposed a novel method of BIA that could be useful for validation. Additionally, all inputs and formulas should be validated by a second budget impact expert. Comparing the outcomes from earlier analyses with the current budget impact of orphan drugs could deliver clarity on future design or adjustments to budget impact analyses. Furthermore, methodological choices in a BIA should continuously be motivated. Finally, an active effort toward facilitating BIAs should be made by systematically collecting and transparently publishing the required data on orphan drugs.
Strengths and Limitations
This review is the first to methodologically assess the quality of BIAs specifically for orphan drugs. The primary limitation of the current literature on BIAs of orphan drugs is that the majority of studies are reported in the form of conference abstracts, which provide limited information about the objectives, design and results of the study. However, as the domain is still evolving, adding abstracts to this review allowed us to analyze the quality of most recent data on BIA for orphan drugs. This review thus includes 83% and 62% abstract-only BIAs for individual and a combination of orphan drugs, respectively. It should also be noted that BIAs directly submitted to reimbursement agencies were not studied.
Conclusion
With the rise of orphan drug expenditure, budget impact analysis forms an important tool to assess affordability of adopting a new orphan drug in a certain health setting. This research finds that budget impact analyses on orphan drugs are of poor-quality and have low methodological adherence to standardized good practice guidelines. Continued improvement of the validity of these analyses should be prioritized. Future studies should be directed toward; multiple perspectives, an open population, costs beyond drug-only costs, exhaustive sensitivity analyses and validation of their budget impact framework.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
KA, IH, KC, and SS contributed to conception and design of the study. KA acquired the necessary data for the review that was analyzed and interpreted by all authors. KA drafted the manuscript under supervision of IH, KC, and SS. All authors critically revised the manuscript, read and approved the submitted version. Funding was obtained by SS.
Funding
This research was funded by grant G0B9819N received from the Research Foundation of Flanders (FWO, Fonds voor Wetenschappelijk Onderzoek). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
SS has previously conducted research about market access of orphan drugs sponsored by the Belgian Health Care Knowledge Center and by Genzyme (now Sanofi), and he has participated in an orphan drug roundtable sponsored by Celgene. SS is a member of the ISPOR Rare Disease Special Interest Group’s Challenges in Research and Health Technology Assessment of Rare Disease Technologies Working Group, the International Working Group on Orphan Drugs, and the Innoval Working Group on Ultra-Rare Disorders.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Pantea Kiani for the initiation of this review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.630949/full#supplementary-material.
References
Al Jedai, A., AL-Mudaiheem, H., Al daamah, S., Al kasim, F., Wasri, A., Shaheen, N., et al. (2019). PRO17 Budget impact analysis of emicizumab in pediatric hemophilia A patients with inhibitors in Kingdom of Saudi Arabia. Value Health 22, S843–S844. doi:10.1016/j.jval.2019.09.2348
Alexandre, R. F., Squiassi, H. B., and Santana, C. F. (2017). PCN58 Budget impact analysis of axitinib in the treatment of metastatic renal cell carcinoma after the failure of one prior systemic therapy in the brazilian private health system. Value Health 20 (5), A97.
Alonso Martinez, C., Fernández-Polo, A., Jiménez-Lozano, I., Garau, M., Cabañas, M. J., Cañete, C., et al. (2018). Economic impact of orphan drugs used in paediatric patients attending hospital outpatient pharmacy and day hospital. Eur. J. Hosp. Pharm. 25, A6. doi:10.1136/ejhpharm-2018-eahpconf.13
Alva, M. E., Campioni, M., Zamora, J., and Giannopoulou, A. (2015). Budget impact analysis of carfilzomib for the treatment of relapsed refractory multiple myeloma (MM) in Mexico. Value Health 18 (7), A818. doi:10.1016/j.jval.2015.09.243
Alva, M. E., Ortíz, M., and Flores, B. (2018). Pcn77–budget impact analysis of Blinatumomab for the treatment of pediatric patients with philadelphia negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia in Mexico. Value Health 21. doi:10.1016/j.jval.2018.09.159
Appukkuttan, S., Duchesneau, E., Zichlin, M. L., Bhak, R. H., Yaldo, A., Gharibo, M., et al. (2019). Budget impact analysis of the introduction of copanlisib for treatment of relapsed follicular lymphoma in the United States. J. Manag. Care Specialty Pharm. 25 (4), 1–12. doi:10.18553/jmcp.2019.18259
Avxentyev, N. A., Frolov, M. Y., and Makarov, A. S. (2018). Pcn90 - cost-effectiveness and budget impact analysis of nilotinib for the second line tratment of chronic mieloid leukemia in the Russian federation. Value Health 21. S29. doi:10.1016/j.jval.2018.09.172
Barbieri, M., Zamboni, W., Pippo, L., Madaan, P., and Campana, C. (2014). Ambrisentan for the treatment of pulmonary arterial hypertension: a budget impact analysis in the Italian context. Expert Opin. Orphan Drugs 2 (10), 989–997. doi:10.1517/21678707.2014.948416
Bergamelli Ramos, N., Palhares, R., Yamamoto, L., Paladini, L., Pegoretti Rosa, B., Nardi, E. P., et al. (2019). PCN116 Budget-impact analysis of the introduction of Regorafenib in Brazilian supplementary healthcare system as second-line treatment for patients with advanced hepatocellular carcinoma previously treated with Sorafenib. Value Health 22, S77.
Bhardwaj, T., Li, B., Hewitt, K., and Jaszewski, B. (2008). Pcn13 budget impact analysis of sorafenib in the treatment of hepatocellular carcinoma in Canada. Value Health 11 (3). doi:10.1016/s1098-3015(10)70193-5
Bharmal, M., Kearney, M., Zheng, Y., and Phatak, H. (2019). Budget impact model of avelumab in patients with metastatic merkel cell carcinoma in the US. Clinicoecon Outcomes Res. 11, 349–359. doi:10.2147/ceor.S202642
Brosa, M., Betoret, I., and Tapias, G. (2011). Budget impact analysis of somatuline autogel in the management of acromegaly in Spain. Value in Health 14 (7), A412. doi:10.1016/j.jval.2011.08.983
Brosa, M., García Del Muro, X., Mora, J., Villacampa, A., Pozo-Rubio, T., Cubells, L., et al. (2015). Orphan drugs revisited: cost-effectiveness analysis of the addition of mifamurtide to the conventional treatment of osteosarcoma. Expert Rev. Pharmacoecon Outcomes Res. 15 (2), 331–340. doi:10.1586/14737167.2015.972378
Carlton, R., Regan, T., and Narayanan, S. (2018). The budget impact of deflazacort for the treatment of duchenne muscular dys trophy (DMD). Value Health 21, S250. doi:10.1016/j.jval.2018.04.1693
Cristino, J., Fikkert, V., Flament, A., Vingerhoedt, S., and Qian, Y. (2015). The budget impact of denosumab in the treatment of giant cell tumor of the bone (GCTB) in Belgium. Value Health 18 (7), A441. doi:10.1016/j.jval.2015.09.1084
Demir, O., Tetik, E., Cheynel, J., and Yildiz, L. (2018). PSY54 Budget impact analysis of Nintedanib in idiopathic pulmonary fibrosis in Turkey. Value Health 21, S444–S445. doi:10.1016/j.jval.2018.09.2630
Denis, A., Mergaert, L., Fostier, C., Cleemput, I., and Simoens, S. (2010a). Budget impact analysis of orphan drugs in Belgium: estimates from 2008 to 2013. J. Med. Econ. 13 (2), 295–301. doi:10.3111/13696998.2010.491427
Denis, A., Mergaert, L., Fostier, C., Cleemput, I., and Simoens, S. (2010b). A comparative study of European rare disease and orphan drug markets. Health Policy 97 (2-3), 173–179. doi:10.1016/j.healthpol.2010.05.017
Derkach, E. V., Rebrova, O. Y., and Fedyaeva, V. K. (2016). Budget impact analysis of Obinutuzumab and ibrutinib in patients with chronic lymphocytic leukemia in Russia. Value Health 19 (7), PA582. doi:10.1016/j.jval.2016.09.1356
Divino, V., DeKoven, M., Kleinrock, M., Wade, R. L., and Kaura, S. (2016a). Orphan drug expenditures in the United States: a historical and prospective analysis, 2007-18. Health Aff. (Millwood) 35 (9), 1588–1594. doi:10.1377/hlthaff.2016.0030
Divino, V., DeKoven, M., Kleinrock, M., Wade, R. L., Kim, T., and Kaura, S. (2016b). Pharmaceutical expenditure on drugs for rare diseases in Canada: a historical (2007-13) and prospective (2014-18) MIDAS sales data analysis. Orphanet J. Rare Dis. 11 (1), 68. doi:10.1186/s13023-016-0450-y
Djambazov, S., Slavchev, G., Encheva-Malinova, M., Pavlova, Y., Varbanova, V., Velchev, M., et al. (2018). Budget impact analysis of inotuzumab ozogamicin for the treatment of adults with relapsed or refractory b-cell precursor acute lymphoblastic leukemia in Bulgaria. Value in Health 21, S26. doi:10.1016/j.jval.2018.09.153
Drummond, M. F., Wilson, D. A., Kanavos, P., Ubel, P., and Rovira, J. (2007). Assessing the economic challenges posed by orphan drugs. Int. J. Technol. Assess. Health Care 23 (1), 36–42. doi:10.1017/S0266462307051550
Faleiros, D. R., Álvares, J., Almeida, A. M., de Araújo, V. E., Andrade, E. I. G., Godman, B. B., et al. (2016). Budget impact analysis of medicines: updated systematic review and implications. Expert Rev. Pharmacoeconomics Outcomes Res. 16 (2), 257–266. doi:10.1586/14737167.2016.1159958
Fan, Y., Park, S., Corman, S., and Juday, T. (2018). Budgetary impact of treating idiopathic pulmonary fibrosis patients with nintedanib. J. Manag. Care Specialty Pharm. 24 (10), S72. doi:10.18553/jmcp.2018.24.10-a.s1
FDA (1983). Oprhan drug act [online]. Available at: https://www.govinfo.gov/content/pkg/STATUTE-96/pdf/STATUTE-96-Pg2049.pdf (Accessed April 5, 2020).
Ferreira-Da-Silva, A. L., Ribeiro, R. A., Santos, V. C. C., Elias, F. T. S., d’Oliveira, A. L. P., and Polanczyk, C. A. (2012). Guidelines for budget impact analysis of health technologies in Brazil. Cad. Saúde Pública 28 (7), 1223–1238. doi:10.1590/s0102-311x2012000700002
Flostr, S., Rodriguez, I., Maddox, B., Finch, L., Belulaj, S., et al. (2016). Is the orphanage filling up? Projecting the growth and budget impact of orphan drugs in Europe. Value Health 19 (7), A599. doi:10.1016/j.jval.2016.09.1454
Fontanet, M., Obach, M., Pastor, M., Gasol, M., Guarga, L., Delgadillo, J., et al. (2018). Budgetary impact of orphan drugs in the Catalan healthcare service. Value Health 21, S444. doi:10.1016/j.jval.2018.09.2626
Foroutan, N., Tarride, J. E., Xie, F., Jameel, B., Mills, F., and Levine, M. (2020). Stakeholders' feedback on the proposed recommendations for updating the patented medicine prices review board (pmprb) budget impact analysis guidelines. J. Popul. Ther. Clin. Pharmacol. 27 (1), e1–e24. doi:10.15586/jptcp.v27i1.651
Foroutan, N., Tarride, J. E., Xie, F., and Levine, M. (2018). A methodological review of national and transnational pharmaceutical budget impact analysis guidelines for new drug submissions. Clinicoecon Outcomes Res. 10, 821–854. doi:10.2147/CEOR.S178825
Foroutan, N., Tarride, J. E., Xie, F., Mills, F., and Levine, M. (2019). A comparison of pharmaceutical budget impact analysis (BIA) recommendations amongst the Canadian patented medicine prices review board (PMPRB), public and private payers. Pharmacoecon Open 3 (4), 437–451. doi:10.1007/s41669-019-0139-y
Forte, L., Malmberg, C., Mann, K., Jupe, K., Chow, G., Lech, R. A., et al. (2019). PRO78 the current and future costs of orphan drugs in Canada - a public payer budget impact analysis. Value Health 22, S855. doi:10.1016/j.jval.2019.09.2408
Fust, K., Maschio, M., Pastor, L., Kohli, M., Weinstein, M. C., Singh, S., et al. (2017). A budget impact model of the addition of telotristat ethyl treatment in patients with uncontrolled carcinoid syndrome. Value Health 20 (9), A548–A549.
Garattini, L., and van de Vooren, K. (2011). Budget impact analysis in economic evaluation: a proposal for a clearer definition. Eur. J. Health Econ. 12 (6), 499–502. doi:10.1007/s10198-011-0348-5
Gea, E., Gil, E., Barral, N., Gonzalez, V., Soler, A., and Avellanet, M. (2013). Orphan drugs and rare diseases in Andorra. Int. J. Clin. Pharm. 35 (5), 963. doi:10.1007/s11096-013-9801-0
Geenen, J. W., Belitser, S. V., Vreman, R. A., van Bloois, M., Klungel, O. H., Boersma, C., et al. (2020a). A novel method for predicting the budget impact of innovative medicines: validation study for oncolytics. Eur. J. Health Econ. 21 (6), 845–853. doi:10.1007/s10198-020-01176-x
Geenen, J. W., Jut, M., Boersma, C., Klungel, O. H., and Hovels, A. M. (2020b). Affordability of oncology drugs: accuracy of budget impact estimations. J. Mark Access Health Policy 8 (1), 1697558. doi:10.1080/20016689.2019.1697558
Germanyuk, T., Kryvoviaz, O., Toziuk, O., Balicka, O., Ivko, T., Polyshchuk, Y., et al. (2017). Dwarfism: accessibility of somatropin therapy for patients with growth hormone deficiency and impact of its cost on the state budget in Ukraine. Asian J. Pharmaceutics 11 (4), S794–S797. doi:10.22377/ajp.v11i04.1717
Ghabri, S., Autin, E., Poullie, A. I., and Josselin, J. M. (2018). The French National Authority for Health (HAS) guidelines for conducting budget impact analyses (BIA). Pharmacoeconomics 36 (4), 407–417. doi:10.1007/s40273-017-0602-5
Ghabri, S., and Mauskopf, J. (2018). The use of budget impact analysis in the economic evaluation of new medicines in Australia, England, France and the United States: relationship to cost-effectiveness analysis and methodological challenges. Eur. J. Health Econ. 19 (2), 173–175. doi:10.1007/s10198-017-0933-3
Gombocz, M., and Vogler, S. (2020). Public spending on orphan medicines: a review of the literature. J. Pharm. Policy Pract 13, 66. doi:10.1186/s40545-020-00260-0
Hajimiri, S. H., Nazemi, N., and Kebriaeezadeh, A. (2019). PRO92 an analaysis of orphan medicines expenditure in Iran, 2018. Value Health 22, S857. doi:10.1016/j.jval.2019.09.2422
Heemstra, H. E., Hensen, M., and Meijboom, M. J. (2010). Budget impact of orphan drugs in Denmark compared to other European countries. Value Health 13 (7), A414. doi:10.1016/S1098-3015(11)72718-8
HIQA (2018). Guidelines for the budget impact analysis of health technologies in Ireland [online]. Available at: https://www.hiqa.ie/sites/default/files/2018-01/HIQA_BIA_Guidelines_2018_0.pdf (Accessed April 5, 2020).
Hollmann, S., Painter, C., Hogan, A., Morten, P., Goyert, N., Vieira, J., et al. (2018a). Pcn72–budget impact analysis of tisagenlecleucel for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma in England. Value Health 21, S26. doi:10.1016/j.jval.2018.09.154
Hollmann, S., Painter, C., Hogan, A., Wickstead, R. M., Goyert, N., Patel, S., et al. (2018b). Pcn68–budget impact analysis of tisagenlecleucel for the treatment of paediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukaemia in England. Value Health 21, S25. doi:10.1016/j.jval.2018.09.150
Hutchings, A., Schey, C., Dutton, R., Achana, F., and Antonov, K. (2014). Estimating the budget impact of orphan drugs in Sweden and France 2013-2020. Orphanet J. Rare Dis. 9, 22. doi:10.1186/1750-1172-9-22
IQVIA (2020a). Global spending on medicines in 2010, 2018, and a forecast for 2023 (in billion U.S. dollars) [Online]. Available at: https://www.statista.com/statistics/280572/medicine-spending-worldwide/ (Accessed March 25, 2020).
IQVIA (2020b). Revenue of the worldwide pharmaceutical market from 2001 to 2018 (in billion U.S. dollars) [Online]. Available at: https://www.statista.com/statistics/263102/pharmaceutical-market-worldwide-revenue-since-2001/ (Accessed March 25, 2020).
Jain, G., Neves, C., Willis, A., Mendivil, J., and Devercelli, G. (2019). A budget impact model for lanadelumab treatment for the prevention of hae attacks among patients with type I or II HAE: estimates of yearly savings for a U.S. Commercial health plan. J. Manag. Care Specialty Pharm. 25, S38. doi:10.18553/jmcp.2019.25.3-a.s1
Kanters, T. A., Steenhoek, A., and Hakkaart, L. (2014). Orphan drugs expenditure in The Netherlands in the period 2006-2012. Orphanet J. Rare Dis. 9 (1), 154. doi:10.1186/s13023-014-0154-0
Kawalec, P., Holko, P., Krzyzanowska, A., and Glogowski, C. (2010). Pcv35 the budget impact analysis of ambrisentan in 2nd line treatment of adult patients with idiopathic, familiar or associated with connective tissue disease pulmonary hypertension of iii nyha stage. Value in Health 13 (7). doi:10.1016/s1098-3015(11)72378-6
Kim, Y., and Oh, R. (2017). Trend analysis of listing and budget impact for orphan drugs in Korea. Value Health 20 (9), A567. doi:10.1016/j.jval.2017.08.954
Klimes, J., Dolezal, T., Vocelka, M., Suchanková, E., and Kruntoradova, K. (2012). PCN134 expenditures and availability of orphan drugs in the Czech Republic: seven year Experience (2004-2010). Value Health 15 (7), A434.
Knoth, R., Ortendahl, J., Wang, E., Li, X., Khilfeh, I., and Adejoro, O. (2018). C6 budget impact of introducing lenvatinib for the systemic treatment of unresectable hepatocellular carcinoma. J. Manag. Care Specialty Pharm. 24 (10), S22. doi:10.18553/jmcp.2018.24.10-a.s1
Kohli, M., Maschio, M., Joish, V., Frech, F., and Lapuerta, P. (2017). Budget impact of telotristat ethyl in the treatment of patients with uncontrolled carcinoid syndrome. J. Manag. Care Specialty Pharm. 23, S39. doi:10.18553/jmcp.2017.23.10-a.s1
Kolbin, A., Vilum, I., Balykina, Y., and Proskurin, M. (2018). Pcn83 - the use of Obinutuzumab in treatment of refractory and relapsing follicular lymphoma. Value Health 21, S28. doi:10.1016/j.jval.2018.09.165
Kulikov, A., Yagudina, R., and Protsenko, M. V. (2015). Budget impact analysis of dasatinib as a second-line therapy in patients with chronic myelogenous leukemia (CML) in the Russian federation. Value Health 18 (7), A441–A442. doi:10.1016/j.jval.2015.09.1085
Lee, K. W., Niskanen, L., Olson, F., Bornheimer, R., Maamari, R., and Neary, M. P. (2018). Budget impact of pasireotide LAR for the treatment of Cushing's disease from a Finnish societal perspective. Value Health 21, S250–S251. doi:10.1016/j.jval.2018.04.1694
Li, Q., Wehler, E., Kamat, S., Moghadam, R., and Kowal, S. (2018). Pharmacy budget impact of orphan drugs for chronic rare conditions among adult patients. J. Manag. Care Specialty Pharm. 24, S106–S107.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700. doi:10.1136/bmj.b2700
Lin, H., Merkel, M., Pelligra, C., and Shah, A. (2019). PBI18 Evaluating the budget impact of patisiran, the first approved RNAi therapeutic, for treating the polyneuropathy of HATTR amyloidosis. Value Health 22, S50. doi:10.1016/j.jval.2019.04.101
Logviss, K., Krievins, D., and Purvina, S. (2016). Impact of orphan drugs on Latvian budget. Orphanet J. Rare Dis. 11 (1), 59. doi:10.1186/s13023-016-0434-y
Lorenzoni, V., Triulzi, I., and Turchetti, G. (2018). Budget impact analysis of the use of extended half-life recombinant factor VIII (efmoroctocog alfa) for the treatment of congenital haemophilia a: the Italian National Health System perspective. BMC Health Serv. Res. 18 (1), 596. doi:10.1186/s12913-018-3398-x
Marshall, D. A., Douglas, P. R., Drummond, M. F., Torrance, G. W., Macleod, S., and Manti, O. (2008). Guidelines for conducting pharmaceutical budget impact analyses for submission to public drug plans in Canada. Pharmacoeconomics 26 (6), 477–495. doi:10.2165/00019053-200826060-00003
Masoura, P., Murphy, D. R., Chatzikou, M., Alexopoulos, S. T., and Magestro, M. (2013). Budget impact analysis of deferasirox in the treatment of non transfusion dependent thalassemia in Greece. Value Health 16 (7), A378–A379. doi:10.1016/j.jval.2013.08.323
Mauskopf, J. A., Earnshaw, S., and Mullins, C. D. (2005). Budget impact analysis: review of the state of the art. Expert Rev. Pharmacoecon Outcomes Res. 5 (1), 65–79. doi:10.1586/14737167.5.1.65
Mauskopf, J. A., Sullivan, S. D., Annemans, L., Caro, J., Mullins, C. D., Nuijten, M., et al. (2007). Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices--budget impact analysis. Value Health 10 (5), 336–347. doi:10.1111/j.1524-4733.2007.00187.x
Mauskopf, J., and Earnshaw, S. (2016). A methodological review of US budget-impact models for new drugs. Pharmacoeconomics 34 (11), 1111–1131. doi:10.1007/s40273-016-0426-8
Mauskopf, J., and Earnshaw, S. (2017). “Introduction to budget-impact analysis,” in Budget-impact analysis of health care interventions (Durham, USA: Springer Nature).
Mauskopf, J. (1998). Prevalence-based economic evaluation. Value Health 1 (4), 251–259. doi:10.1046/j.1524-4733.1998.140251.x
McMullen, S., Buckley, B., Hall, E., Kendter, J., and Johnston, K. (2017). Budget impact analysis of prolonged half-life recombinant FVIII therapy for hemophilia in the United States. Value Health 20 (1), 93–99. doi:10.1016/j.jval.2016.09.2396
Mestre-Ferrandiz, J., Palaska, C., Kelly, T., Hutchings, A., and Parnaby, A. (2019). An analysis of orphan medicine expenditure in Europe: is 171 it sustainable? Orphanet J. Rare Dis. 14 (1), 287. doi:10.1186/s13023-019-1246-7
Morginstin, T., Hammerman, A., Triki, N., and Weinreb, B. (2019). Budget-impact of drugs for orphan diseases (orphan drugs) in the Israeli health basket: a longitudinal analysis. Israel J. Health Policy Res. 8, 69. doi:10.1186/s13584-019-0336-2
Mucha, J., Stelmachowski, J., Walczak, J., Pieniazek, I., Labak-Klimasara, M., and Obrzut, G. (2015). Budget impact analysis of dasatynib in treatment of adult patients with philadelphia chromosome positive (Ph+) acutely mbphoblastic leukemia (ALL) with resistance or intolerance to prior therapy in Poland. Value Health 18 (7), A663. doi:10.1016/j.jval.2015.09.2412
Nalysnyk, L., Sugarman, R., Cele, C., Uyei, J., and Ward, A. (2018). Budget impact analysis of Eliglustat for the treatment of Gaucher disease type 1 in the United States. J. Manag. Care Spec. Pharm. 24 (10), 1002–1008. doi:10.18553/jmcp.2018.24.10.1002
Naranjo, M., Alva, M. E., and Muñoz, I. (2017). Budget impact analysis comparing Blinatumomab in the treatment of adults with philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (all) with flag-ida and hyper CVAD. Value in Health 20 (9), A549. doi:10.1016/j.jval.2017.08.856
Neyt, M., Cleemput, I., Sande, S. V., and Thiry, N. (2015). Belgian guidelines for budget impact analyses. Acta Clin. Belg. 70 (3), 175–180. doi:10.1179/2295333714Y.0000000118
Nicod, E., Annemans, L., Bucsics, A., Lee, A., Upadhyaya, S., and Facey, K. (2019). HTA programme response to the challenges of dealing with orphan medicinal products: process evaluation in selected European countries. Health Policy 123 (2), 140–151. doi:10.1016/j.healthpol.2017.03.009
Niezen, M. G., de Bont, A., Busschbach, J. J., Cohen, J. P., and Stolk, E. A. (2009). Finding legitimacy for the role of budget impact in drug reimbursement decisions. Int. J. Technol. Assess. Health Care 25 (1), 49–55. doi:10.1017/S0266462309090072
Official Journal of the European Communities (2000). REGULATION (EC) No 141/2000 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 1999 on orphan medicinal products. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32000R0141&from=EN.
Organisation for Economic Cooperation and Development (2019). Health at a glance 2019: OECD indicators. Paris: OECD Publishing. doi:10.1787/4dd50c09-en
Orlewska, E., and Gulacsi, L. (2009). Budget-impact analyses A critical review of published studies. Pharmacoeconomics 27 (10), 807–827. doi:10.2165/11313770-000000000-00000
Orlewska, E., and Mierzejewski, P. (2004). Proposal of polish guidelines for conducting financial analysis and their comparison to existing guidance on budget impact in other countries. Value Health 7 (1), 1–10. doi:10.1111/j.1524-4733.2004.71257.x
Paiva, H., and Asano, E. (2016). Budget impact analysis of the incorporation of ibrutinib for the treatment of relapsed/refractory chronic lymphocytic leukemia in the Brazilian private health care system. Value Health 19 (3), A143. doi:10.1016/j.jval.2016.03.1551
Paladini, L., Pepe, C., Clark, O. A. C., Tencer, T., and Khan, Z. (2012). Economic evaluation of azacitidine for the treatment of myelodysplastic syndromes (MDS) in the brazilian public health care system (SUS). Value in Health 15 (4), A212–A213. doi:10.1016/j.jval.2012.03.1148
Pearson, S. D. (2018). The ICER value framework: integrating cost effectiveness and affordability in the assessment of health care value. Value Health 21 (3), 258–265. doi:10.1016/j.jval.2017.12.017
Pham, H. A., Milev, S., Li, S., Zou, D., Hu, Y., Heeg, B., et al. (2019). Budget impact of glasdegib in combination with low-dose cytarabine for the treatment of first-line acute myeloid leukemia in the United States. Blood 134, 5852. doi:10.1182/blood-2019-122709
Pribylova, L., Pasztor, B., Vesela, S., Vyhnankova, M., Doleckova, J., Duba, J., et al. (2016). New approach to budget impact analysis-ibrutinib in treatment of relapsed/refractory CLL patients in the Czech Republic. Value Health 19 (7), A719. doi:10.1016/j.jval.2016.09.2132
Pyadushkina, E., Avxentyeva, M., and Frolov, M. (2016). PSY34 the budget impact analysis of thrombopoietin receptor agonists for the treatment of idiopathic thrombocytopenic purpura in adults. Value Health 19 (7), A347–A766. doi:10.1016/j.jval.2016.09.1348
Pyadushkina, E., Derkach, E. V., and Boyarskaya, T. (2018). Pcn73 - budget impact of ixazomib in combination with lenalidomide and dexamethasone in relapsed or refractory multiple myeloma in Russia. Value Health 21, S26. doi:10.1016/j.jval.2018.09.155
Ramos Santana, E., Merino Alonso, J., De Leon Gil, A., Suárez González, M., Hernández Rojas, S., Morales Barrios, J. A., et al. (2018). Budgetary impact of ultra-rare diseases in a third-level hospital. Eur. J. Hosp. Pharm. 25, A15. doi:10.1136/ejhpharm-2018-eahpconf.33
Rose, D. B., Nellesen, D., Neary, M. P., and Cai, B. (2017). Budget impact of everolimus for the treatment of progressive, well-differentiated, non-functional neuroendocrine tumors of gastrointestinal or lung origin that are advanced or metastatic. J. Med. Econ. 20 (4), 395–404. doi:10.1080/13696998.2016.1273228
Salazar, A., Aguirre, A., and Herrera, M. (2017). PSY38 Economic evaluation of daratumumab for the treatment of patients with double refractory multiple myeloma in Mexico. Value Health 20 (5), A215. doi:10.1016/j.jval.2017.05.005
Sanon, M., Parthan, A., Taylor, D. C., Coombs, J., Paolantonio, M., and Sasane, M. (2012). PCN27 Clinical benefit and economic impact of three-years of adjuvant imatinib in kit+ gastrointestinal stromal tumors (GIST). Value in Health 15, A1–A256. doi:10.1016/j.jval.2012.03.1144
Savova, A., Kamusheva, M., Georgieva, S., Stoimenova, A., and Petrova, G. (2014). Budget impact analysis of chronic myeloid leukemia treatment in Bulgaria. Biotechnol. Biotechnological Equipment 27 (1), 3595–3598. doi:10.5504/bbeq.2012.0075
Schenkel, B., appanavar, A., O'Day, K., and Queener, M. (2015). Budget impact analysis of ibrutinib for patients with previously treated chronic lymphocytic leukemia. J. Manag. Care Specialty Pharm. 21, S25. doi:10.18553/jmcp.2015.21.10.S1
Schenkel, B., Bozkaya, D., and O'Day, K. (2016). Budget impact analysis of ibrutinib for patients with first-line chronic lymphocytic leukemia. J. Manag. Care Specialty Pharm. 22, S30. doi:10.18553/jmcp.2016.22.10-a.s1
Schey, C., Milanova, T., and Hutchings, A. (2011). Estimating the budget impact of orphan medicines in Europe: 2010 - 2020. Orphanet J. Rare Dis. 6, 62. doi:10.1186/1750-1172-6-62
Schlander, M., Adarkwah, C. C., and Gandjour, A. (2015). Budget impact analysis of drugs for ultra-orphan non-oncological diseases in Europe. Expert Rev. Pharmacoecon Outcomes Res. 15 (1), 171–179. doi:10.1586/14737167.2015.965156
Schlander, M., Dintsios, C. M., and Gandjour, A. (2018). Budgetary impact and cost drivers of drugs for rare and ultrarare diseases. Value Health 21 (5), 525–531. doi:10.1016/j.jval.2017.10.015
Schultz, N. M., and Malone, D. C. (2014). A probabilistic budget impact analysis of cystic fibrosis therapy on health plan pharmacy budgets. Value Health 17 (3), A226. doi:10.1016/j.jval.2014.03.1319
Senbetta, M., appanavar, A., McKenzie, R. S., Ellis, L., and O'Day, K. (2014). Ibrutinib therapy for patients with relapsed or refractory mantle cell lymphoma: a budget impact analysis from a U.S. payer perspective. J. Clin. Oncol. 32 (15). doi:10.1200/jco.2014.32.15_suppl.e19553
Serpik, V. G., and Yagudina, R. (2015). PCN73 budget impact analysis or pharmacological therapy of chronic myeloid leukemia (cml) with nilotinib as the second-line treatment in Russian federation. Value Health 18 (7), A441–A442. doi:10.1016/j.jval.2015.09.1089
Silva, M. T., Silva, E. N. D., and Pereira, M. G. (2017). Budget impact analysis. Epidemiol. Serv. Saude 26 (2), 421–424. doi:10.5123/S1679-49742017000200020
Soto Molina, H., Diaz Martinez, J. P., Escobar Juarez, Y., Rodriguez-Mendoza, M. M., and Frias Gasga, A. E. (2017). Brentuximab vedotin (adcetris®) economic evaluation on patient's treatment with relapse or refractory hodgkin lymphoma. Value Health 20 (5), A97.
Soto Molina, H., Diaz-Alvarez, O., and Sandoval-Avila, M. (2018). Budget impact analysis of Bosentan for treatment of pediatric patients with pulmonary arterial hypertension. Value Health 21, S58. doi:10.1016/j.jval.2018.04.353
Stellato, D., Gerbasi, M., Ndife, B., Ghate, S., Moynahan, A., and Mishra, D. (2018). C11 budget impact of dabrafenib and trametinib in combination as adjuvant treatment of BRAF V600E/K mutation-positive melanoma in a commercially insured U.S. Population. J. Manag. Care Specialty Pharm. 24 (10), S24. doi:10.18553/jmcp.2018.24.10-a.s1
Sujkowska, G., Jagodzinska-Kalinowska, K., and Matusewicz, W. (2015). The availability and expenditure of orphan medicines in Poland. Value Health 18 (3), A304. doi:10.1016/j.jval.2015.03.1770
Sullivan, S. D., Mauskopf, J. A., Augustovski, F., Jaime Caro, J., Lee, K. M., and Minchin, M. (2014). Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health 17 (1), 5–14. doi:10.1016/j.jval.2013.08.2291
Szegedi, M., Zelei, T., Arickx, F., Bucsics, A., Cohn-Zanchetta, E., Furst, J., et al. (2018). The European challenges of funding orphan medicinal products. Orphanet J. Rare Dis. 13 (1), 184. doi:10.1186/s13023-018-0927-y
Tafazzoli, A., Kempster, J., Pavilack, M., Deger, K., Ma, W., and Olufade, T. (2018). Budget impact analysis of moxetumomab for treatment of patients with relapsed or refractory hairy cell leukemia in the United States. J. Manag. Care Specialty Pharm. 24 (10 A), S30. doi:10.18553/jmcp.2018.24.10-a.s1
Tremblay, G., Said, Q., Roy, A. N., Cai, B., Ashton Garib, S., Hearnden, J., et al. (2019). Budget impact of Eltrombopag as first-line treatment for severe aplastic anemia in the United States. Clinicoecon Outcomes Res. 11, 673–681. doi:10.2147/ceor.S226323
Tritaki, G., Souliotis, K., Bouros, D., Papageorgiou, I., Diamantopoulos, A., Rinciog, C., et al. (2016). Pharmacoeconomic assessment of nintedanib for the treatment of idiopathic pulmonary fibrosis in the Greek healthcare system. Value Health 19 (7), A580. doi:10.1016/j.jval.2016.09.1345
Trueman, P., Drummond, M., and Hutton, J. (2001). Developing guidance for budget impact analysis. Pharmacoeconomics 19, 609–621. doi:10.2165/00019053-200119060-00001
Truong, H. L., Nellesen, D., Ludlam, W. H., and Neary, M. P. (2014). Budget impact of pasireotide for the treatment of Cushing's disease, a rare endocrine disorder associated with considerable comorbidities. J. Med. Econ. 17 (4), 288–295. doi:10.3111/13696998.2013.877470
van de Vooren, K., Duranti, S., Curto, A., and Garattini, L. (2014). A critical systematic review of budget impact analyses on drugs in EU countries. Appl. Health Econ. Health Policy 12 (1), 33–40. doi:10.1007/s40258-013-0064-7
Venturini, F., Adami, S., Alberti, C., and Scroccaro, G. (2008). PSY8 Budget impact analysis of deferasirox for the treatment of chronic iron overload in patients with betathalassaemia in Veneto region Italy. Value Health 11 (6), A630–A631. doi:10.1016/S1098-3015(10)67060-X
Villa, S., Castaman, G., and Pradelli, L. (2017). PSY28 idelvion for the treatment of hemophilia B: a budget impact analysis in the Italian setting. Value Health 20 (9), A549. doi:10.1016/j.jval.2017.08.852
Wehler, E. A., Kowal, S., Hernandez-Garduno, A., Danese de los Santos, L., de Anda, J. A., Anaya, P., et al. (2015a). PCN16 estimating the budget impact of switching from Bortezomib intravenous (iv) to Bortezomib subcutaneous (sq) in the treatment of relapsed/refractory multiple myeloma (mm) in Mexico. Value Health 18 (7), A818. doi:10.1016/j.jval.2015.09.244
Wehler, E. A., Kowal, S., Obando, C. A., Muschett, D., de Anda, J. A., Anaya, P., et al. (2015b). PCN17 A budget impact model estimating the financial impact of increased use of generic Bortezomib intravenous (iv) in the treatment of relapsed/refractory multiple myeloma (mm) in Venezuela. Value Health 18 (7), A818. doi:10.1016/j.jval.2015.09.24310.1016/j.jval.2015.09.245
Weidlich, D., Hicks, M. D., Floros, L., Patel, J., Charbonneau, C., and Sung, A. H. (2018). Pin18 - Estimating the budget and clinical impact of introducing isavuconazole for the treatment of patients with possible invasive aspergillosis in the United Kingdom. Value Health 21, S223–S224. doi:10.1016/j.jval.2018.09.1337
Whalen, J. D., Srivastava, B., Ozer-Stillman, I., Gray, L., Price, L., and Magestro, M. (2013). Budget impact of everolimus for tuberous sclerosis complex (TSC) related angiomyolipoma (AML): United Kingdom perspective. Value Health 16 (7), A620. doi:10.1016/j.jval.2013.08.1813
WHO (2019). Global spending on health: a world in transition [Online]. Available at: https://www.who.int/health_financing/documents/health-expenditure-report-2019.pdf?ua=1 (Accessed April 4, 2020). Geneva: World Health Organization.
Xu, F., Chen, W., and Liu, C. (2017). PCN64 Cost-effectiveness and budget impact analysis of imatinib as first-line treatment of chronic myeloid leukemia in China. Value Health 20 (5), A98. doi:10.1016/j.jval.2017.05.005
Yagudina, R., Kulikov, A., and Babiy, V. V. (2015a). PSY27 budget impact analysis of blood clotting factor concentrates in the treatment of Von Willebrand disease. Value Health 18 (7), A663. doi:10.1016/j.jval.2015.09.2412
Yagudina, R., Kulikov, A., and Pochuprina, A. (2015b). PSY29 budget impact analysis of canacinumab in the treatment of patients with muckle–wells syndrome in the Russian federation. Value Health 18 (7), A663. doi:10.1016/j.jval.2015.09.2413
Yang, H., Han, S., Chai, X., Wu, E., Abikoff, C., Hao, Y., et al. (2018). C27 budget impact associated with the introduction of tisagenlecleucel for the treatment of relapsed or refractory diffuse large B-cell lymphoma. J. Manag. Care Specialty Pharm. 24 (10), S29. doi:10.18553/jmcp.2018.24.10-a.s1
Keywords: budget impact analyses, orphan drugs, guidelines, quality assesment, systematic reviews
Citation: Abdallah K, Huys I, Claes K and Simoens S (2021) Methodological Quality Assessment of Budget Impact Analyses for Orphan Drugs: A Systematic Review. Front. Pharmacol. 12:630949. doi: 10.3389/fphar.2021.630949
Received: 18 November 2020; Accepted: 25 January 2021;
Published: 21 April 2021.
Edited by:
Brian Godman, University of Strathclyde, United KingdomReviewed by:
Luis Laranjeira, Eli Lilly, PortugalWania Cristina Da Silva, Federal University of Minas Gerais, Brazil
Copyright © 2021 Abdallah, Huys, Claes and Simoens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khadidja Abdallah, a2hhZGlkamEuYWJkYWxsYWhAa3VsZXV2ZW4uYmU=
 Khadidja Abdallah
Khadidja Abdallah Isabelle Huys
Isabelle Huys Kathleen Claes2
Kathleen Claes2