
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 12 March 2021
Sec. Renal Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.630820
This article is part of the Research TopicCombating Diabetes and Diabetic Kidney DiseaseView all 13 articles
Background: This study aimed to explore the effects of sodium-glucose co-transporter 2 (SGLT2) on hemoglobin levels in patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease.
Methods: PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, the China National Knowledge Infrastructure database, Wanfang Digital Periodicals Database (WFDP) and the Chinese Biological and Medical database (CBM) were searched for randomized trials of SGLT2 inhibitors in patients with T2DM and chronic kidney disease up to July 25, 2020. A total of four studies that included 19,259 patients were identified.
Results: Compared to control patients, SGLT2 inhibitors were shown to increase hemoglobin levels in patients with T2DM and chronic kidney disease (standard mean difference = 0.70, 95% CI, 0.59–0.82, p < 0.0001).
Conclusion: SGLT2 inhibitors may bring additional benefits to patients with T2DM and chronic kidney disease.
In the past ten years, the incidence of type 2 diabetes mellitus (T2DM) has been increasing (International Diabetes Federation, 2017) which indicates a massive increase in end-stage renal disease on a global scale. One of the most common complications of chronic kidney disease is renal anemia (Sugahara et al., 2017). The presence of anemia significantly increases the risk of micro- and macrovascular complications. Patients who are not properly treated have significantly reduced quality of life and a poor prognosis (Sugahara et al., 2017).
Sodium-glucose co-transporter 2 (SGLT2) inhibitors are a newly approved class of oral hypoglycemic agents that increase the excretion of glucose in the urine by inhibiting the reabsorption of urine glucose in the proximal tubules of the kidney, thereby reducing blood glucose levels, weight and blood pressure (Polidori et al., 2014; Inagaki et al., 2015). In addition, SGLT2 inhibitors also have a protective effect on the kidney (Xu et al., 2017) as evidence has shown that after treatment with SGLT2, hemoglobin levels are increased (Maruyama et al., 2019).
In this study, we aimed to ascertain the effects of SGLT2 inhibitors on the hemoglobin levels in patients with T2DM and chronic kidney disease.
The PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, the China National Knowledge Infrastructure (CNKI) database, Wanfang Digital Periodicals database (WFDP), the Chinese Biological and Medical database (CBM) were searched. The following Medical Subject Headings (MeSH) terms and free-text terms were applied: “Sodium-Glucose Transporter 2 Inhibitors”, “Sodium Glucose Transporter 2 Inhibitors”, “SGLT2 Inhibitors”, “SGLT-2 Inhibitors”, “SGLT 2 Inhibitors”, “Gliflozins”, “Renal Insufficiency, Chronic”, “Chronic Renal Insufficiencies”, “Renal Insufficiencies, Chronic”, “Chronic Renal Insufficiency”, “Kidney Insufficiency, Chronic”, “Chronic Kidney Insufficiency”, “Chronic Kidney Insufficiencies”, “Kidney Insufficiencies, Chronic”, “Chronic Kidney Diseases”, “Chronic Kidney Disease”, “Disease, Chronic Kidney”, “Diseases, Chronic Kidney”, “Kidney Disease, Chronic”, “Kidney Diseases, Chronic”, “Chronic Renal Diseases”, “Chronic Renal Disease”, “Disease, Chronic Renal”, “Diseases, Chronic Renal”, “Renal Disease, Chronic”, “Renal Diseases, Chronic”. All publications up to July 25, 2020 were selected without the restriction of origins, countries, languages or article types.
Published articles that were included in the analysis were required to meet the following criteria: 1) the eligible subjects were men and women with T2DM and chronic kidney disease; 2) interventions involved treatment with SGLT2 inhibitors alone or with other hypoglycemic agents; 3) studies compared placebo control or standard of care; 4) outcomes reported changes in hemoglobin levels from baseline; 5) studies that were randomized controlled trials (RCTs); 6) studies with follow-up times of 12 weeks or longer. Observational studies, non-randomized trials and uncontrolled trials were excluded from the analysis.
Two investigators extracted the following data independently from eligible publications: first author, publication year, study design, inclusion criteria, sample size, patient characteristics, interventions (types and doses of SGLT2 inhibitors), comparison (placebo control or standard care), follow-up duration and outcomes (changes in hemoglobin levels from baseline). The unit of hemoglobin levels was uniformly converted into g/l. Discrepancies were resolved by the discussion between two investigators. The Cochrane risk-of-bias tool was adopted to assess randomization, masking of treatment allocation, blinding, adherence and withdrawals for each of the RCTs (Higgins et al., 2011).
Data analysis was performed using Stata version 12.0 software. The effect sizes on scores were presented as the standard mean difference (SMD) and 95% confidence intervals (CIs). The Chi-squared test based on Q-statistic and I2 statistics was used to estimate the heterogeneity (I2 ≤ 25%, low heterogeneity; 25% < I2 < 50%, moderate heterogeneity; I2 ≥ 50%, high heterogeneity) (Higgins et al., 2003). A fixed-effects model was used to pool the results when heterogeneity was ≤50%, while a random-effects model was used when heterogeneity was >50% (Mantel and Haenszel, 1959; DerSimonian and Laird, 1986). A sensitivity analysis was performed to reveal the influence of a single study on the overall pooled estimates by deleting one study in each turn. Publication bias was evaluated using the Begg’s and Egger’s tests (Begg and Mazumdar, 1994; Egger et al., 1997). p values (<0.05) were considered to represent statistically significant publication bias.
A total of 579 references were retrieved and finally, four studies (Yale et al., 2013; Yale et al., 2014; Wanner et al., 2018; Takashima et al., 2018) met the inclusion criteria for the meta-analysis (Figure 1). The sample size included 19,259 patients with T2DM and chronic kidney disease in this meta-analysis (Table 1 Characteristics of the included studies). Three of the studies were multi-center, double-blind, placebo-controlled trials (Yale et al., 2013; Yale et al., 2014; Wanner et al., 2018) in which white people took up the majority of patients and one was a single-center, open-label, parallel-group trial conducted in Japan (Takashima et al., 2018). The types of SGLT2 inhibitors included canagliflozin (100 mg/300 mg) and enpagliflozin (10 mg/25 mg). Follow-up duration ranged from 12 to 164 weeks. All included studies were evaluated in terms of the risk of bias using the Cochrane risk of bias tool and the details are illustrated in Figure 2 (Risk of bias in the included studies).
With the exception of one open-label study, the other three studies on random sequence generation were fully considered. The studies were all double-blind trials but there was no further explanation on the details of allocation concealment. There were no incomplete outcomes and selective reporting in the four studies. Based on the characteristics, we believe that the included studies had a low risk of bias.
Four studies (Four publications) investigated a total of 19,259 participants (experimental group: 9,668, control group: 9,591) and reported hemoglobin levels. There was high heterogeneity (I2 = 91.7%, p < 0.0001) and so the random-effects model was used. The pooled effect size showed significant differences in hemoglobin levels (SMD = 0.70, 95% CI, 0.59–0.82, p < 0.0001) in favor of the experimental groups compared to the control groups (Figure 3 Meta-analysis and forest plot of hemoglobin levels for experimental group compared with the control group).

FIGURE 3. Meta-analysis and forest plot of hemoglobin levels in the experimental and the control groups.
We further analyzed the effect of SGLT2 inhibitors on hematocrit. Two publications investigated 515 participants (experimental group: 277, control group: 238) reported Hematocrit. There was no heterogeneity (I2 = 0, p = 0.767); thus, the fixed-effects model was used. The pooled effect size showed a significant difference in Hematocrit (SMD = 0.72, 95%CI 0.55–0.90, p < 0.05) in favor of experimental group, compared with the control group (Figure 4 Meta-analysis and forest plot of Hematocrit for experimental group compared with control group).
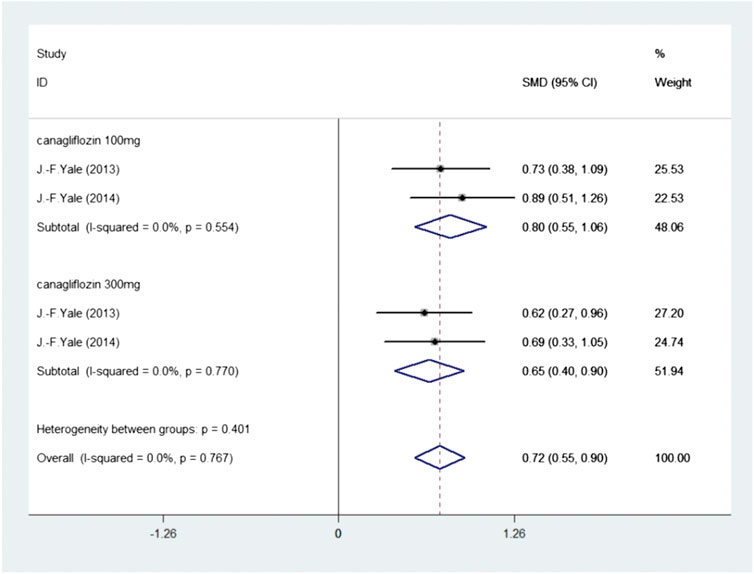
FIGURE 4. Meta-analysis and forest plot of Hematocrit for experimental group compared with control group.
To explore the sources of heterogeneity, we conducted a subgroup analysis of the type and dosage of SGLT2 inhibitors and eGFR. According to the type and dosage of the drug, treatments were divided into four subgroups (canagliflozin 100 mg, canagliflozin 300 mg, empagliflozin 10 mg and empagliflozin 25 mg). Subgroups were divided as to eGFR based on 30 ≤ eGFR <60 ml/min/1.73 m2 and eGFR ≥60 ml/min/1.73 m2. The results are summarized in Figure 5. All of the results in the subgroups were statistically significant compared to those in the control group, however, heterogeneity was not significantly reduced following subgroup analysis.
A sensitivity analysis was conducted by sequentially removing one study to observe the influence of each of the included studies on the overall pooled SMD. No single study was found to significantly influence the overall pooled SMD indicating that the results were stable.
In assessing publication bias, a funnel plot for the four studies analyzed was constructed. The shape of the funnel plot was symmetrical indicating the absence of publication bias. No significant bias was observed using the Begg’s rank correlation test (Z = 1.4, p = 0.161 (>0.05)) and Egger’s linear regression test (t = 0.78, p = 0.451 (>0.05)).
This article conducted a meta-analysis of four randomized controlled studies to explore the effects of SGLT2 inhibitors on hemoglobin levels in diabetic patients with chronic kidney disease. The results showed that the hemoglobin levels of patients after treatment with SGLT2 inhibitors increased from baseline and the differences were statistically significant. The hematocrit levels of patients after treatment with SGLT2 inhibitors increased from baseline and the differences were statistically significant. Whether it was different type and dosage of the drug (canagliflozin 100 mg, canagliflozin 300 mg, empagliflozin 10 mg and empagliflozin 25 mg), or different eGFR (30 ≤ eGFR <60 ml/min/1.73 m2 and eGFR ≥60 ml/min/1.73 m2), the differences were statistically significant.
Most of the observations from previous reports in the literature and meta-analysis demonstrate the effects of SGLT2 on blood sugar levels, cardiovascular events and renal outcomes. There are very few studies that have analyzed the effects of SGLT2 inhibitors on hemoglobin levels or have performed meta-analyses of these effects across multiple RCTs.
SGLT2 inhibitors protect patients with T2DM and chronic kidney disease through several different mechanisms. First, in diabetic patients, upregulation of SGLT2 increases the reabsorption of sodium and glucose by the proximal tubules, SGLT2 inhibitors lower blood sugar by blocking the glucose reabsorption of SGLT2 in the proximal renal tubules. Second, SGLT2 inhibitors also have a certain effect on renal hemodynamics. SGLT2 inhibitors block the reabsorption of glucose and sodium in the proximal tubules and increase the transport of sodium to the macula densa, thereby restoring impaired tubuloglomerular feedback. Thus, SGLT2 inhibitors can alleviate glomerular filtration in the early stage of diabetic nephropathy, reduce albuminuria, and delay the decline of renal function for a long time (Cherney et al., 2014; Škrtić and Cherney, 2015). Besides, the protective effects of SGLT2 inhibitors are also manifested in the reduction of blood pressure, weight loss, osmotic diuresis, reduction of inflammation, fibrosis, and proliferation of proximal renal tubular cells (Panchapakesan et al., 2013).
The mechanisms by which SGLT2 inhibitors improve hemoglobin levels in patients with diabetes and chronic kidney disease are not fully understood. It has been reported that SGLT2 inhibitors have diuretic-like effects and reduce plasma volume (Lambers Heerspink et al., 2013). It has also been reported that in diabetic patients with normal renal function, SGLT2 inhibitors can reduce the load caused by excessive glucose reabsorption in the proximal tubules, and can improve renal tubular interstitial hypoxia and restore fibroblasts to produce erythropoietin (EPO) causing hemoglobin levels to increase (Lambers Heerspink et al., 2013). In diabetic patients with chronic kidney disease, SGLT2 inhibitors can also increase hemoglobin levels by promoting the production of EPO. Studies have also shown that SGLT2 inhibitors can upregulate AMPK and SIRT1 (Swe et al., 2019; Packer 2020), thereby inhibiting HIF-1α and activating HIF-2α (Treins et al., 2006; Dioum et al., 2009; Lim et al., 2010). HIF-2α is the isoform responsible for the synthesis of EPO (Eckardt and Kurtz, 2005). The increase in hematocrit may be due to the decrease in plasma volume caused by SGLT2 inhibitor-related diuresis and natriuresis, or it may be due to increased erythropoiesis after the treatment of SGLT2 inhibitor. The increase in hematocrit during treatment with SGLT2 inhibitors may indicate the improvement of hypoxia and oxidative stress in the tubular interstitial area of the renal cortex, as well as the recovery of EPO production by interstitial fibroblast-like cells. SGLT2 inhibitors also inhibit hepcidin, which may lead to increased iron bioavailability and utilization and increased red blood cell production (Ghanim et al., 2020). These effects on erythropoiesis suggest that SGLT2 inhibitors may reduce the incidence of anemia. The post-hoc analysis of the CREDENCE trial by Megumi Oshima et al. found that the risk of anemia or the risk of starting anemia treatment in the anemia group of patients with type 2 diabetes and chronic kidney disease was significantly lower than that of the placebo group (Oshima et al., 2020). In the exploratory analysis of EMPA-REG test data, the increase in hematocrit during empagliflozin treatment was closely related to beneficial cardiovascular outcomes (Inzucchi et al., 2018). Studies have shown that increased expression of HIF-2α in cardiomyocytes can protect mitochondrial integrity and prevent experimental ischemic damage (Bautista et al., 2009; Mastrocola et al., 2016). For the same blood flow, a higher hematocrit is expected to deliver more oxygen to the tissue (Testani et al., 2010). It has been suggested that the increase in hematocrit may contribute to the cardioprotective effect of these drugs by increasing the oxygen-carrying capacity (Ferrannini et al., 2016; Lytvyn et al., 2017).
Several limitations of this study should be noted. Firstly, a total of four articles were included in the analysis which is a small sample size, however, the total number of patients included was nor large. Secondly, the majority of the subjects were Caucasian and so the applicability of the data to other races including Asians requires further investigation. Thirdly, although we concluded that hemoglobin levels increased after treatment with SGLT2 inhibitors, we did not observe differences in the effects of different types of SGLT2 inhibitors on hemoglobin levels, the relationship between the increase in hemoglobin level and the duration of medication. Fourthly, among the populations included in the study, some had eGFR ≥ 60 ml/min/1.73 m2. This meant that a small number of patients with normal renal function may have been included. In addition, the population included in the study did not have obvious renal anemia before SGLT2 inhibitors treatment. Therefore, for patients with significant renal anemia, the benefits of SGLT2 inhibitors need to be further investigated.
In summary, patients with T2DM and chronic kidney disease have increased hemoglobin and hematocrit levels after treatment with SGLT2 inhibitors. SGLT2 inhibitors may bring additional benefits to patients with T2DM and chronic kidney disease.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
WQ and LY designed the study. TX and BT identified and acquired reports of trials, WQ extracted the data and performed all data analyses. XL contributed to data interpretation. WQ drafted the report and all other authors critically reviewed and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bautista, L., Castro, M. J., López-Barneo, J., and Castellano, A. (2009). Hypoxia inducible factor-2alpha stabilization and maxi-K+ channel beta1-subunit gene repression by hypoxia in cardiac myocytes: role in preconditioning. Circ. Res. 104, 1364–1372. doi:10.1161/CIRCRESAHA.108.190645
Begg, C. B., and Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. doi:10.2307/2533446
Cherney, D. Z., Perkins, B. A., Soleymanlou, N., Maione, M., Lai, V., Lee, A., et al. (2014). Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597. doi:10.1161/CIRCULATIONAHA.113.005081
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. doi:10.1016/0197-2456(86)90046-2
Dioum, E. M., Chen, R., Alexander, M. S., Zhang, Q., Hogg, R. T., Gerard, R. D., et al. (2009). Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science 324, 1289–1293. doi:10.1126/science.1169956
Eckardt, K. U., and Kurtz, A. (2005). Regulation of erythropoietin production. Eur. J. Clin. Invest. 35 Suppl 3, 13–19. doi:10.1111/j.1365-2362.2005.01525.x
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi:10.1136/bmj.315.7109.629
Ferrannini, E., Mark, M., and Mayoux, E. (2016). CV protection in the EMPA-REG outcome trial: a “thrifty substrate” hypothesis. Diabetes Care 39, 1108–1114. doi:10.2337/dc16-0330
Ghanim, H., Abuaysheh, S., Hejna, J., Green, K., Batra, M., Makdissi, A., et al. (2020). Dapagliflozin suppresses hepcidin and increases erythropoiesis. J. Clin. Endocrinol. Metab. 105, dgaa057. doi:10.1210/clinem/dgaa057
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi:10.1136/bmj.327.7414.557
Inagaki, N., Kondo, K., Yoshinari, T., and Kuki, H. (2015). Efficacy and safety of canagliflozin alone or as add-on to other oral antihyperglycemic drugs in Japanese patients with type 2 diabetes: a 52-week open-label study. J. Diabetes Investig. 6, 210–218. doi:10.1111/jdi.12266
International Diabetes Federation (2017). IDF diabetes atlas. 8th ed Available at: http://diabetesatlas.org/IDF_Diabetes_Atlas_8e_interactive_EN/ (Accessed October 05, 2020).
Inzucchi, S. E., Zinman, B., Fitchett, D., Wanner, C., Ferrannini, E., Schumacher, M., et al. (2018). How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 41, 356–363. doi:10.2337/dc17-1096
Lambers Heerspink, H. J., de Zeeuw, D., Wie, L., Leslie, B., and List, J. (2013). Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 15, 853–862. doi:10.1111/dom.12127
Lim, J. H., Lee, Y. M., Chun, Y. S., Chen, J., Kim, J. E., and Park, J. W. (2010). Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol. Cell 38, 864–878. doi:10.1016/j.molcel.2010.05.023
Lytvyn, Y., Bjornstad, P., Udell, J. A., Lovshin, J. A., and Cherney, D. Z. I. (2017). Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation 136, 1643–1658. doi:10.1161/CIRCULATIONAHA.117.030012
Mantel, N., and Haenszel, W. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22, 719–748. doi:10.1093/jnci/22.4.719
Maruyama, T., Takashima, H., Oguma, H., Nakamura, Y., Ohno, M., Utsunomiya, K., et al. (2019). Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol. Ther. 21, 713–720. doi:10.1089/dia.2019.0212
Mastrocola, R., Collino, M., Penna, C., Nigro, D., Chiazza, F., Fracasso, V., et al. (2016). Maladaptive modulations of NLRP3 inflammasome and cardioprotective pathways are involved in diet-induced exacerbation of myocardial ischemia/reperfusion injury in mice. Oxid. Med. Cell. Longev. 2016, 3480637. doi:10.1155/2016/3480637
Oshima, M., Neuen, B. L., Jardine, M. J., Bakris, G., Edwards, R., Levin, A., et al. (2020). Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: a post-hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol. 8, 903–914. doi:10.1016/S2213-8587(20)30300-4
Packer, M. (2020). Interplay of adenosine monophosphate-activated protein kinase/sirtuin-1 activation and sodium influx inhibition mediates the renal benefits of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes: a novel conceptual framework. Diabetes Obes. Metab. 22, 734–742. doi:10.1111/dom.13961
Panchapakesan, U., Pegg, K., Gross, S., Komala, M. G., Mudaliar, H., Forbes, J., et al. (2013). Effects of SGLT2 inhibition in human kidney proximal tubular cells--renoprotection in diabetic nephropathy?. PLoS One 8, e54442. doi:10.1371/journal.pone.0054442
Polidori, D., Mari, A., and Ferrannini, E. (2014). Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves model-based indices of beta cell function in patients with type 2 diabetes. Diabetologia 57, 891–901. doi:10.1007/s00125-014-3196-x
Škrtić, M., and Cherney, D. Z. (2015). Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 24, 96–103. doi:10.1097/MNH.0000000000000084
Sugahara, M., Tanaka, T., and Nangaku, M. (2017). Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int. 92, 306–312. doi:10.1016/j.kint.2017.02.035
Swe, M. T., Thongnak, L., Jaikumkao, K., Pongchaidecha, A., Chatsudthipong, V., and Lungkaphin, A. (2019). Dapagliflozin not only improves hepatic injury and pancreatic endoplasmic reticulum stress, but also induces hepatic gluconeogenic enzymes expression in obese rats. Clin. Sci. (Lond) 133, 2415–2430. doi:10.1042/CS20190863
Takashima, H., Yoshida, Y., Nagura, C., Furukawa, T., Tei, R., Maruyama, T., et al. (2018). Renoprotective effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, in type 2 diabetes patients with chronic kidney disease: a randomized open-label prospective trial. Diab. Vasc. Dis. Res. 15, 469–472. doi:10.1177/1479164118782872
Testani, J. M., Chen, J., McCauley, B. D., Kimmel, S. E., and Shannon, R. P. (2010). Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation 122, 265–272. doi:10.1161/CIRCULATIONAHA.109.933275
Treins, C., Murdaca, J., Van Obberghen, E., and Giorgetti-Peraldi, S. (2006). AMPK activation inhibits the expression of HIF-1alpha induced by insulin and IGF-1. Biochem. Biophys. Res. Commun. 342, 1197–1202. doi:10.1016/j.bbrc.2006.02.088
Wanner, C., Lachin, J. M., Inzucchi, S. E., Fitchett, D., Mattheus, M., George, J., et al. (2018). Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 137, 119–129. doi:10.1161/CIRCULATIONAHA.117.028268
Xu, L., Li, Y., Lang, J., Xia, P., Zhao, X., Wang, L., et al. (2017). Effects of sodium-glucose co-transporter 2 (SGLT2) inhibition on renal function and albuminuria in patients with type 2 diabetes: a systematic review and meta-analysis. PeerJ 5, e3405. doi:10.7717/peerj.3405
Yale, J. F., Bakris, G., Cariou, B., Nieto, J., David-Neto, E., Yue, D., et al. (2014). Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes. Metab. 16, 1016–1027. doi:10.1111/dom.12348
Keywords: meta-analysis, SGLT2 inhibitors, type 2 diabetes, chronic kidney disease, hemoglobin
Citation: Qu W, Yao L, Liu X, Xu T and Tian B (2021) Effects of Sodium-Glucose Co-transporter 2 Inhibitors on Hemoglobin Levels: A Meta-analysis of Randomized Controlled Trials. Front. Pharmacol. 12:630820. doi: 10.3389/fphar.2021.630820
Received: 18 November 2020; Accepted: 25 January 2021;
Published: 12 March 2021.
Edited by:
Keizo Kanasaki, Faculty of Medicine, Shimane University, JapanReviewed by:
Keiichiro Matoba, Jikei University School of Medicine, JapanCopyright © 2021 Qu, Yao, Liu, Xu and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yao, bGl5YW9fY211QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.