- 1Department of Translational Medicine, University of Piemonte Orientale, Novara, Italy
- 2Respiratory Unit, S. Andrea Hospital, Vercelli, Italy
- 3Pharmacology, Faculty of Medicine, Catholic University of the Sacred Heart, Roma, Italy
- 4ASST Spedali Civili di Brescia, Department of Emergency, Brescia, Italy
Cough variant asthma (CVA), a common asthma phenotype characterized by nonproductive cough and bronchial hyperreactivity (BHR), is usually detected by bronchial provocation tests (BPTs) which are time-consuming, expensive, and unsafe. The primary study objective was to provide proof of concept for the use of fractional exhaled nitric oxide (FENO), eosinophil count percentage in induced sputum (sEOS%), forced expiratory flow between 25 and 75% of forced vital capacity (FEF25–75%) % predicted value, and FEF25–75% z-scores as surrogate markers predicting BHR in young adults with suspected CVA; the secondary objective was to compare the diagnostic performance of the various techniques. Three hundred and ten subjects (median age 24 years) were included in a cross-sectional study. Subjects were characterized as BHR positive (POS) (n = 147) or BHR negative (NEG) (n = 163) according to methacholine BPT. Classification accuracies were expressed as areas under the receiver operator characteristic curves (AUC). Compared with BHR NEG, FEF25–75% % predicted value and FEF25–75% z-scores were lower in the BHR POS group (p < 0.001), whereas FENO (p < 0.001) and sEOS% were higher (p < 0.001). AUC values for detecting BHR were as follows: FENO, 0.98 (SD = 0.02); sEOS%, 0.98 (SD = 0.02); FEF25–75% % pred, 0.93 (SD = 0.05); FEF25–75% z scores, 0.92 (SD = 0.05). Optimal cutoff values (OCV) for BHR prediction were as follows: FENO, 32.7 ppb (sensitivity = 0.93, specificity = 0.96), sEOS%, 3.80% (sensitivity = 0.94, specificity = 0.94), FEF25–75% % predicted value, 80.0% (sensitivity = 0.90, specificity = 0.87), and FEF25–75% z-score, −0.87 (sensitivity = 0.89, specificity = 0.87). Non-invasive/semi-invasive airway inflammatory or small airway functional measures might be used as surrogate markers predicting BHR in young adults with suspected CVA.
Introduction
Asthma is characterized by chronic airway inflammation, bronchial hyperreactivity (BHR), and episodes of bronchoconstriction clinically presenting as variable and recurring cough, dyspnea, and wheezing (ginasthma, 2020). Asthma is a heterogeneous disease, including a broad spectrum of diseases described as various phenotypes (Haldar et al., 2008). Cough variant asthma (CVA), a frequent asthma phenotype, is characterized by a cough as a prevalent symptom and BHR (Corrao et al., 1979). The presence of BHR is generally detected with bronchial provocation tests (BPTs), a positive response to bronchodilators or both (Irwin et al., 2006; Achilleos, 2016). BPTs are the gold standard, but expensive, time consuming, and unsafe as they are potentially able to induce severe bronchospasm (Coates et al., 2017). Simpler, safer, and more rapid predictive methods would be relevant to clinical practice (Bao et al., 2018) as they would facilitate the identification of those patients with suspected CVA who need to be referred for BHT.
Fractional exhaled nitric oxide (FENO) is a non-invasive, standardized, safe, simple, and well-accepted surrogate marker of airway inflammation (Jatakanon et al., 1998b; ElHalawani et al., 2003; Berkman et al., 2005; Malerba et al., 2008). FENO is elevated in subjects with atopic asthma (Ricciardolo et al., 2004) and correlates with sputum eosinophilia before and after glucocorticoid treatment (Jatakanon et al., 1998b; Malerba et al., 2008), and with bronchoalveolar lavage (BAL) eosinophil counts (Lim et al., 1999); a strong correlation between FENO concentrations and BHR has been observed in children with asthma (Ciprandi et al., 2010). FENO levels were also found correlated with BHR in apprentices exposed to occupational risk for asthma (Tossa et al., 2010).
Increasing evidence shows that inflammation of small airways (<2 mm diameter) and surrounding alveolar tissue and small airway function play a pivotal pathophysiological role in cough exacerbation, nocturnal attacks, and exercise-induced wheeze (Van Der Wiel et al., 2013). Forced expiratory flow between 25 and 75% of forced vital capacity (FEF25–75%) has been found to correlate with functional imaging assessment of small airway function (Jain et al., 2005) and proposed as an early marker for peripheral airway airflow limitation (<2 mm) (McFadden and Linden, 1972; Terra Filho et al., 1986; Perez et al., 2013) and eosinophilic inflammation (Malerba et al., 2016). Using computed tomography airway morphometric analysis, (Niimi et al., 2000), several studies have shown a good correlation between FEF25–75% and the High-Resolution computed tomography finding of air trapping. Recently, FEF25–75% has been shown to be feasible parameter for identifying small airway dysfunction early in CVA patients (Yuan et al., 2019). Moreover, in a cross sectional study, reduced FEF25–75% was associated with increased frequency of respiratory symptoms, greater healthcare utilization and higher levels of biomarkers of distal airway inflammation, including FENO and sputum eosinophils (Riley et al., 2015).
Eosinophil differential count in induced sputum (sEOS%), a semi-invasive technique, is a standardized, recommended, evidence-based, direct measure of airway inflammation and its use is reported into the most relevant guidelines (Pin et al., 1992). sEOS% counts increase during asthma exacerbations (Pizzichini et al., 1999) and, similarly to FENO concentrations, decrease after treatment with corticosteroids (Jatakanon et al., 1998a). The primary objective of this study was to provide proof of concept for the use of various noninvasive/semi-invasive inflammatory or functional measures, including FENO, sEOS% and FEF25–75%, as surrogate markers predicting BHR in a cohort of young adults with suspected CVA and maintained lung function as reflected by normal forced expiratory volume in 1 s percentage of predicted (FEV1%) values; secondary study objective was to compare their diagnostic performance. We chose to study subjects aged from 18 to 45 years to minimize the impact of confounding factors related to airways aging and possible co-morbidities associated with older ages on study outcomes.
Methods
Subjects and Study Design
We performed a cross-sectional study of data collected from 310 adult subjects aged from 18 to 45 years referred for cough to the Respiratory Medicine Unit of the Department of Internal Medicine, University of Brescia and to the Department of Translational Medicine, University of Piemonte Orientale, Respiratory Unit of Vercelli’s Hospital, Italy, in an out-subject setting from January 2016 to March 2018.
Inclusion criteria were as follow: suspected CVA with cough as a predominant symptom, chest tightness, dyspnea or wheezing with nocturnal awakenings for >3 weeks; normal chest X-ray; maintained lung function as reflected by FEV1% > 80% of predicted values with spirometric measurement. Subjects were excluded if they met the following criteria: upper respiratory infection during the previous 6 weeks, use of systemic and/or inhaled corticosteroids during the previous 6 weeks, current or past history of smoking, any significant medical condition, a prior asthma diagnosis and the usual contraindications to methacholine challenge tests. No subject was under antihistamines and no subject had symptoms of allergic rhinitis at the time of the inclusion. The study was approved by the Local Center Ethics Committees (N 0770-2016 and N 035 -2017) and all the subjects gave their written informed consent. Recruited subjects underwent the following procedures: clinical examination; symptom evaluation; skin prick testing; pulmonary function tests; methacholine challenge test; FENO measurement; sputum induction, and sEOS% count. Interventions were performed in the following order to reduce the effect of bronchoconstriction on FENO: FENO measurement, spirometry, methacholine challenge and sputum induction (American Thoracic Society, European Respiratory Society, 2005).
This study was conducted in agreement with the STROBE statement for observational studies (von Elm et al., 2008).
Skin Prick Test
Allergy was assessed by skin prick test positivity to the most common respiratory allergens as stated by the European Academy of Allergy and Clinical Immunology (Anonymous, 1989).
Pulmonary Function Tests
Pulmonary function measures were obtained using a pneumotachograph with a volume integrator (CAD/Net system 1070; Medical Graphics Corporation, St. Paul, Minn., United statesSA), following American Thoracic Society criteria (Clausen et al., 1997). Spirometric parameters were expressed as percent of predicted values and z-scores. Predicted values and z-scores were derived using prediction equations from the Global Lung Function Initiative (GLI-2012; http://www.lungfunction.org/) (Quanjer et al., 1993; Quanjer et al., 2012). Only pre-bronchodilator data were included in the study data analysis.
Bronchial Provocation Test
A methacholine challenge test was performed as a dose-response curve by increasing (doubling) doses of methacholine chlorohydrate every 3 min according to international guidelines (Pizzichini et al., 1999). Results were expressed as cumulative doses of methacholine provoking a 20% fall in FEV1 (PD20 FEV1). A methacholine challenge test result was considered positive if the PD20 FEV1 was <16.00 mg/ml (Crapo et al., 2000).
FENO
FENO was determined with a high-resolution chemiluminescence NO analyser (Ecomedics AG Analyzer CLD88; Dürnten, Switzerland), with detection limit of 0.06 ppb and measurement range reaching 100 ppb. FENO was measured at a flow rate of 50 ml/s as per ATS/ERS guidelines. Measurements were obtained in accordance with the ATS recommendations for on-line measurement of FENO in adults (American Thoracic Society, European Respiratory Society, 2005).
Sputum Induction
After baseline FEV1 and FVC measurements, subjects were pre-treated with inhaled salbutamol (200 μg by metered-dose inhaler) and 10 min later were asked to inhale a hypertonic (4.5%) nebulized sterile saline solution for three periods of 5 min each at most by means of an ultrasonic nebulizer (Ultraneb 2000; DeVilbiss, Somerset, PA, USA). Nebulization was discontinued if one of the following symptoms occurred: wheezing, chest tightness or moderate-to-severe dyspnea. Sputum was processed as previously reported (Malerba et al., 2006). The cut-off for an abnormal result was considered a sEOS% value > 3% of total non-squamous cells (Balbi et al., 2007).
Statistical Analysis
In an opportunistic sample of 310 young adults with CVA, we aimed to provide a proof of concept for the use of FENO, sEOS%, FEF25–75% predicted value, and FEF25–75% z scores as surrogate markers predicting BHR. Subject characteristics have been summarized according to BHR status. The normality Shapiro Wilk test was performed for assessing data distribution. Normally distributed data were expressed as mean and the standard deviation (SD); nonparametric data were expressed as median and interquartile range (25th to 75th percentiles); categorical data were expressed as percentage and absolute numbers. Wilcoxon rank sum test or t-test, depending on data distribution, was performed for continuous data between-group comparisons; Pearson chi-square test or Fisher-exact test, whatever appropriate, was used for categorical variable between-group comparisons. Correlations between PD20 FEV1, sEOS% and FENO were expressed as Spearman Rho correlation coefficient. FEF25–75% was also expressed as a z-score using the regression equation and variance derived from a normal population assessed in our laboratory. FEF25–75% z-score was calculated as the difference between the measured and predicted FEF25–75% value divided by the reference SD (Jones et al., 2003). A Z score that equaled zero indicated the subject's pulmonary function was at the predicted value, whereas Z scores of 1 and −1 indicated pulmonary function that was 1 SD above and below the predicted values, respectively.
The predictive accuracies for each BHR predictor have been estimated via logistic regression models. The estimates have been adjusted by gender and age and validated performing a 10 fold repeated cross-validation procedure. Receiver operating characteristic (ROC) curves, sensitivity and specificity values with relative standard deviations computed across iterations have been reported. ROC curves for the leading predictors have also been reported.
Optimal cut off values (OCV) for BHR prediction were estimated as the values combining the best sensitivity and specificity for BHR POS detection. The Youden index (J), a main summary statistic of the ROC curve, was used as a measure of model quality (Youden, 1950). Statistical analysis has been performed using R 3.2.5 (R Core Team., 2018), together with caret (Kuhn, 2008) and pROC packages (Robin et al., 2011).
Results
Baseline Characteristics of Subjects Studied
Clinical data from 310 subjects were included in the analysis. Subjects were divided in two groups based on methacholine BPT results. Subjects with positive BPT were categorized as BHR POS (n = 147); if BPT was negative, subjects were identified as BHR NEG (n = 163).
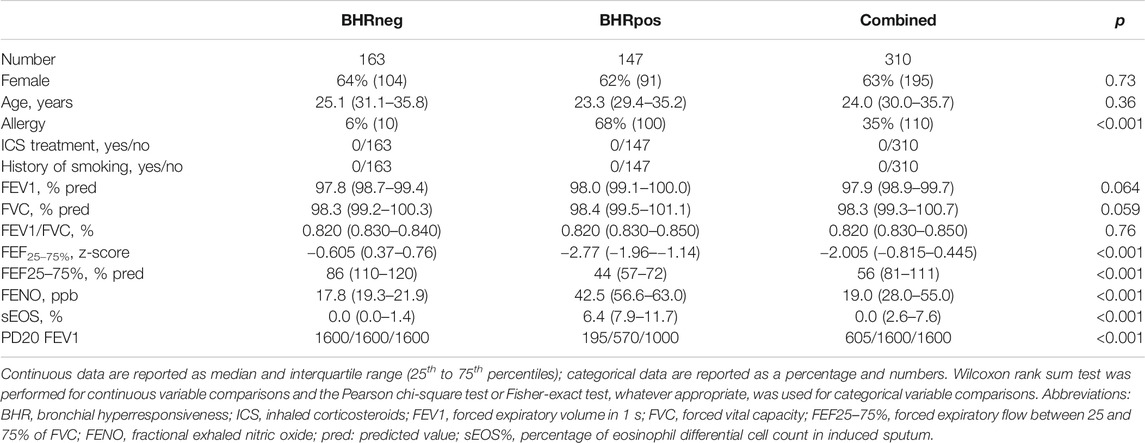
There was no between-group difference in age (p = 0.36) and gender (p = 0.73), whereas allergy was more prevalent in BHR POS (68%) than in BHR NEG (6%) (p < 0.001) (Table 1). One hundred ten subjects (35%) were sensitized to perennial and/or pollen allergens. Subjects were all ex smokers or non-smokers.
Functional and Inflammatory Biomarkers
In all subjects, FVC, FEV1 and FEV1/FVC values were within normal reference ranges showing no significant differences between BHR POS and BHR NEG group (Table 1).
By contrast, median FEF25–75% z-score (p < 0.001) and FEF25–75% % predicted value (p < 0.001) were both significantly lower in the BHR POS than BHR NEG group (Table 1), suggesting that small airways disease was present in the former.
FENO (p < 0.001) and sEOS% median values (p < 0.001) were elevated in the BHR POS group compared with BHR NEG group (Table 1). Mean PD20 FEV1 was 642 ± 489 μg. FENO concentrations were correlated with PD20 FEV1 values (rho = −0.88; p < 0.001). FENO and sEOS% values were highly correlated (rho = 0.886 p < 0.001).
Diagnostic Accuracy of Single Measurements for BHR Prediction
Logistic regression analysis showed that measurement of FENO, sEOS%, FEF25–75% z-score, and FEF25–75% % predicted values were able to predict BHR with high accuracy, sensitivity and specificity (Table 2). FENO and sEOS% were the best performing techniques as reflected by their AUC values (0.98 for both) (Figure 1), and sensitivity and specificity values, which were above 0.92 (Table 2). AUCs for FEF25–75% % pred and FEF25–75% z-score were >0.91, with sensitivity and specificity values > 0.86 (Figure 1; Table 2).
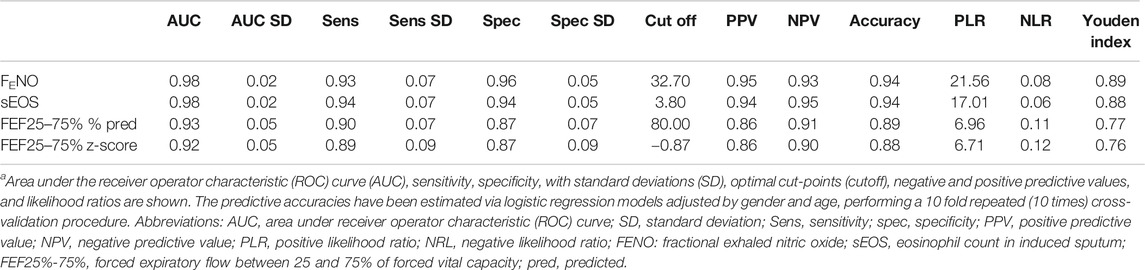
TABLE 2. BHR predictive accuracies of FENO measurement, percentage of sputum eosinophil cell counts, FEF25%–75% of predicted value, and FEF25%–75% z-score in 310 subjects with suspected cough variant asthma.a
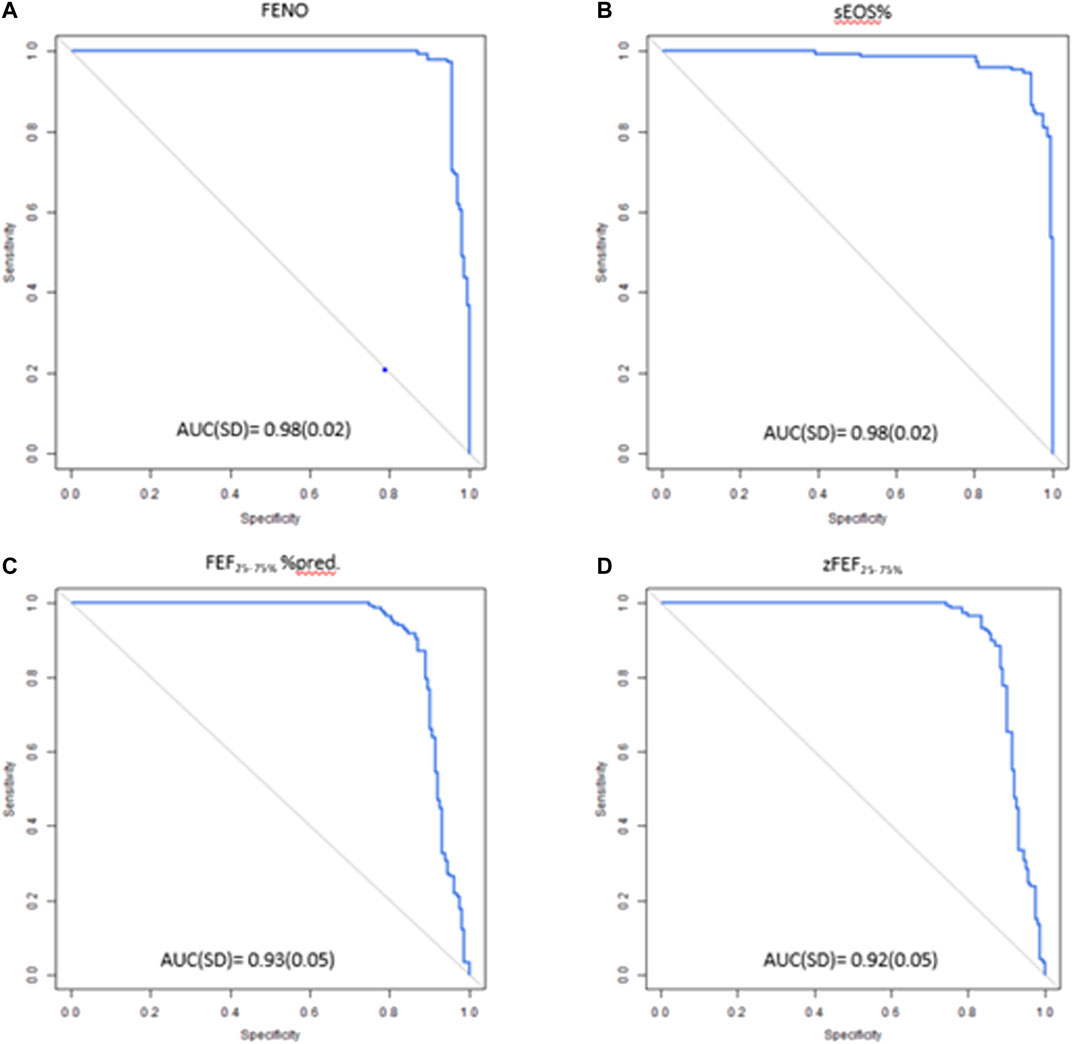
FIGURE 1. ROC curves of (A) FENO (B) sEOS% (C) FEF25–75%%pred (D) zFEF25–75% in predicting positive BHR. ROC, receiver operating characteristic; FENO, fractional exhaled nitric oxide; sEOS%, percentage of eosinophils in induced sputum; FEF25–75%, forced expiratory flow at 25–75% of forced vital capacity: AUC, Area under the curve; SD standard deviation.
OCV for BHR prediction were as follows: FENO, 32.7 ppb (sensitivity = 0.93, specificity = 0.96), sEOS%, 3.80% (sensitivity = 0.94, specificity = 0.94), FEF25–75% % predicted, 80.0% (sensitivity = 0.90, specificity = 0.87), and FEF25–75% z-score, -0.87 (sensitivity = 0.89, specificity = 0.87). Youden J values of various techniques for predicting BHR are shown in Table 1.
Discussion
Our study provided proof of concept that non-invasive/semi-invasive measures reflecting airway inflammation or small-airway function might be used as surrogate markers of BHR in young adults with suspected CVA and normal lung function. Predictive models were of high quality as reflected by values of Youden’s index (J), the maximum potential effectiveness of a biomarker which combines sensitivity and specificity (Youden, 1950), ranging from 0.76 to 0.89. We confirmed the potential utility of FENO and FEF25–75% predicted value measurement in predicting the presence of BHR in subjects with suspected CVA and extend this observation to eosinophil counts in induced sputum. Of note, in our study, the discriminant abilities of the various methods were remarkably higher than those reported in previous studies (Schleich et al., 2012; Bao et al., 2018; Chen et al., 2019), This discrepancy might reflect differences in study population characteristics, including age, ethnicity, and lung function, across various studies.
We observed that young adults with suspected CVA and normal lung function having FENO >37.2 ppb, sEOS% >3.8%, FEF25–75% % pred <80% and zFEF25–75% <-0.87% have elevated probability of being BHR POS and classification accuracy >91% (98% for both FENO and sEOS%). These findings support a close relationship between airway inflammation and peripheral airway function.
FENO is a surrogate marker of airway inflammation, particularly useful in patients with atopic eosinophilic asthma (Berkman et al., 2005; Gibson, 2009; Dweik et al., 2011). FeNO was proposed to be able also to predict ICS responsiveness in chronic cough although supported by few studies and without a strong evidence (Song et al., 2017b).
In individuals with chronic cough (Maniscalco et al., 2015; Chen et al., 2019), FENO is able to distinguish CVA and eosinophilis bronchitis from other causes of chronic cough. A recent meta-analysis pointed out that Fractional exhaled nitric oxide (FeNO) has moderate diagnostic accuracy for predicting cough variant asthma (CVA) with high specificity in patients with chronic cough (Song et al., 2017a). We observed a strong correlation between FENO and sEOS% values consistent with previous reports on the ability of FENO to reflect eosinophilic airway inflammation (Cirillo et al., 2013; Ricciardolo, 2014). In the present study, FENO showed a remarkable predictive ability to detect BHR (AUC: 0.98; sensitivity: 0.93; specificity: 0.96; PPV: 0.95; NPV: 0.93) at a cutoff value of >32.7 ppb, higher than that reported in previous studies at FENO cutoff values of 43 ppb (AUC: 0.79; sensitivity: 0.72; specificity: 0.82; PPV: 0.66; NPV: 0.5) (Bao et al., 2018), 34 ppb (AUC: 0.62; PPV: 0.88; NPV: 0.62) (Schleich et al., 2012), and 25 ppb (AUC: 0.65; PPV: 0.83; NPV: 0.49) (Bougard et al., 2020), and others (Song et al., 2017a; Chen et al., 2019). These discrepancies might be explained, at least partly, by population study differences, including ethnicity (all Caucasian population vs. all Chinese population) (Bao et al., 2018), mean age (24 years vs. 43 (Bao et al., 2018) or 41 (Schleich et al., 2012) or 51 (Bougard et al., 2020) years), smoking habit (nonsmokers vs. 6–19% (Bao et al., 2018) or 34% (Schleich et al., 2012) current smokers), atopy (35 vs. 93% (Schleich et al., 2012), not reported in reference 6), symptoms (suspected CVA vs. suspected asthma with negative bronchodilator reversibility test (Schleich et al., 2012), and inhaled corticosteroid (ICS) treatment (Bougard et al., 2020). Atopy seems to paly a robust role as in a recent paper the diagnostic accuracy of FENO for predicting CVA in chronic cough in patients with atopy was clearly higher than in patients without (Chen et al., 2019). Based on our data, FENO might have a greater predictive value in distinguishing subjects with or without BHR in young adults with undifferentiated cough and normal lung function. In this population, the high BHR predictive accuracy of FENO measurement (AUC: 0.98; PPV: 0.95; NPP: 0.93; PLR: 21.56; NLR: 0.08) suggest that BPT should be limited to individuals with FENO > 32.7 ppb. In this perspective FENO could be used as a rule-in test for CVA as previously suggested (Song et al., 2017a).
Measurement of percentage of eosinophil cell counts in induced sputum as a candidate for predicting BHR is novel. In the present study, at a cutoff value of 3.8%, this method showed higher accuracy and BHR predicting capacity (AUC: 0.98; PPV: 0.94; NPV: 0.95) than peripheral blood eosinophil cell counts at a cutoff value of 3.5% (AUC: 0.76; PPV: 58.9; NPV: 85.1) (Bao et al., 2018). Apart from variations in study populations, this inconsistency might derive from methodological differences, as measurement of eosinophil cell counts in induced sputum is a direct and likely more accurate measure of airway inflammation than peripheral blood eosinophils. Along with FENO, sEOS% showed the highest discriminative capacity to identify BHR POS individuals under our experimental conditions. However, measurement of sEOS% is not available in all centers as it requires trained and experienced staff for sputum induction, processing and analysis.
Classification above a threshold value of 70% is considered significant (Bijlsma et al., 2006). In the present study, FEF25–75% measures showed significant discriminative capabilities as reflected by AUCs >0.91 with PPV >0.85 and NPV >0.89 at OCV. FEF25–75% has been found to correlate with functional imaging assessment of small airway function (Jain et al., 2005). Small airways disease plays a relevant role in asthma pathophysiology (Van Der Wiel et al., 2013; van der Wiel et al., 2014). Airway wall thickening induced by inflammation, airway narrowing, and enhanced airway muscular tone contribute to small airway dysfunctions and poorly controlled asthma (van der Palen et al., 2013). Measurement of FEF25–75% to detect small airway dysfunction in asthmatic subjects with normal FEV1 values could be a useful diagnostic tool (Malerba et al., 2016). Our findings, showing that the FEF25–75% % predicted values in BHR POS individuals are lower than those observed in BHR NEG individuals, confirm the presence of small airway disease in subjects with FVC, FEV1, and FEV1/FVC values within normal limits. Compared with FENO at cutoff of 32.7 ppb (AUC: 0.98; sensitivity: 0.93), FEF25–75% % predicted value showed a lower discriminative capacity at a cutoff of 80% (AUC: 0.93), but a similar sensitivity (0.90) using 80% as cutoff value. If inflammatory measures such as FENO and sEOS% are not available, a FEF25–75% > 80% predicted value could help rule out CVA diagnosis in young adults with cough and aid clinicians in diagnosing BHR positive subjects referring to BPT only subjects with FEF25–75% < 80% predicted values.
Strengths of our study are represented by assessment and comparison of both small airway function and inflammatory potential surrogate markers of BHR, including measurement of eosinophil counts in induced sputum, and inclusion of a large cohort of young adults with suspected CVA and normal lung function as reflected by FEV1 values.
Inclusion of a relatively homogeneous study population consisting of non-smoker, steroid-naïve, individuals with a median age of 24 years represents a strength, but, at the same time, a study limitation as it precludes the assessment of the impact of confounding factors, including age, ethnicity, smoking habit, comorbidities, and ICS treatment on study outcomes. These findings cannot be generalized. FENO concentration reliably reflects central airway inflammation, but is not generally considered to reflect peripheral airway inflammation for which estimating alveolar NO concentration (CANO) by measurement of FENO at different flow rates could be more useful (Lehtimäki et al., 2020). Other study limitations include the lack of internal validation of results with training and testing datasets and external validation, the higher prevalence of atopy in BHR POS than in BHR NEG subjects (68 vs. 6%, respectively), which may have influenced the outcomes of FENO and sEOS% diagnostic performance, the lack of assessment of air pollution exposure potential impact and objective measures of smoking status, and the absence of post-bronchodilator data. However, the latter is not usually required in individuals with FEV1 >80% predicted value. As our study was conducted in young adults, results and their interpretation should be limited to this age group. Further research is warranted to determine if these results can be generalized to older individuals with asthma.
Finally, assessment of small airway function was limited to FEF25–75%. However, other functional measure, including nitrogen washout and plethysmography, are not routinely used in clinical practice.
In conclusion, our study shows elevated capacities of noninvasive/semi-invasive methods, particularly FENO and sEOS%, in predicting BHR in young adults with suspected CVA and normal lung function, and points out the importance of the target population choice in determining their diagnostic performances. External validation of these research outcomes in independent cohorts is required before translating this approach into clinical decision-making.
Data Availability Statement
The datasets presented in this article are not readily available because available upon reasonable request; Requests to access the datasets should be directed tobWFyaW8ubWFsZXJiYUB1bml1cG8uaXQ=.
Ethics Statement
The study was approved by the Brescia Local Center Ethics Committees (N 0770-2016 and N 035-2017). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, MM.; methodology, DA.; formal analysis, DA.; investigation, AR and BR.; data curation, AR and PM.; writing—original draft preparation, AR and PM; writing—review and editing, supervision MM.
Funding
This study was (partially) funded by the AGING Project – Department of Excellence – DIMET, Università del Piemonte Orientale.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Achilleos, A. (2016). Evidence-based evaluation and management of chronic cough. Med. Clin. North America. 100 (5), 1033–1045. doi:10.1016/j.mcna.2016.04.008
American Thoracic Society, European Respiratory Society (2005). ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 171 (8), 912–930. doi:10.1164/rccm.200406-710ST
Anonymous (1989). Skin tests used in type I allergy testing position paper. Sub-committee on skin tests of the European Academy of Allergology and clinical Immunology. Allergy, 44, 11–59.
Balbi, B, Pignatti, P, Corradi, M, Baiardi, P, Bianchi, L, Brunetti, G, et al. (2007). Bronchoalveolar lavage, sputum and exhaled clinically relevant inflammatory markers: Values in healthy adults. Eur Respir J 30 (4), 112306. doi:10.1183/09031936.00112306
Bao, W., Zhang, X., Lv, C., Bao, L., Yin, J., Huang, Z., et al. (2018). The value of fractional exhaled nitric oxide and forced mid-expiratory flow as predictive markers of bronchial hyperresponsiveness in adults with chronic cough. J. Allergy Clin. Immunol. Pract. 6 (4), 1313–1320. doi:10.1016/j.jaip.2017.09.026
Berkman, N., Avital, A., Breuer, R., Bardach, E., Springer, C., and Godfrey, S. (2005). Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax. 60 (5), 383–388. doi:10.1136/thx.2004.031104
Bijlsma, S., Bobeldijk, I., Verheij, E. R., Ramaker, R., Kochhar, S., Macdonald, I. A., et al. (2006). Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal. Chem. 78 (2), 567–574. doi:10.1021/ac051495j
Bougard, N., Nekoee, H., Schleich, F., Guissard, F., Paulus, V., Donneau, A. F., et al. (2020). Assessment of diagnostic accuracy of lung function indices and FeNO for a positive methacholine challenge. Biochem. Pharmacol. 179, 113981. doi:10.1016/j.bcp.2020.113981
Chen, L. C., Zeng, G. S., Wu, L. L., Zi, M., Fang, Z. K., Fan, H. Z., et al. (2019). Diagnostic value of FeNO and MMEF for predicting cough variant asthma in chronic cough patients with or without allergic rhinitis. J Asthma. 58 (3), 326–333. doi:10.1080/02770903.2019.1694035
Ciprandi, G., Tosca, M. A., and Capasso, M. (2010). Exhaled nitric oxide in children with allergic rhinitis and/or asthma: a relationship with bronchial hyperreactivity. J Asthma. 47 (10), 1142–1147. doi:10.3109/02770903.2010.527026
Cirillo, I., Ricciardolo, F. L., Medusei, G., Signori, A., and Ciprandi, G. (2013). Exhaled nitric oxide may predict bronchial hyperreactivity in patients with allergic rhinitis. Int. Arch. Allergy Immunol. 160 (3), 322–328. doi:10.1159/000341675
Clausen, J. L., Coates, A. L., and Quanjer, P. H. (1997). Measurement of lung volumes in humans: review and recommendations from an ATS/ERS workshop. Eur. Respir. J. 10 (6), 1205–1206. doi:10.1183/09031936.97.10061205
Coates, A. L., Wanger, J., Cockcroft, D. W., Culver, B. H., Carlsen, K. H., Diamant, Z., et al. (2017). ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J. 49 (5), 1601526. doi:10.1183/13993003.01526-2016
Corrao, W. M., Braman, S. S., and Irwin, R. S. (1979). Chronic cough as the sole presenting manifestation of bronchial asthma. N. Engl. J. Med. 300 (12), 633–637. doi:10.1056/nejm197903223001201
Crapo, R. O., Casaburi, R., Coates, A. L., Enright, P. L., Hankinson, J. L., Irvin, C. G., et al. (2000). Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 161 (1), 309–329. doi:10.1164/ajrccm.161.1.ats11-99
Dweik, R. A., Boggs, P. B., Erzurum, S. C., Irvin, C. G., Leigh, M. W., Lundberg, J. O., et al. (2011). An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 184 (5), 602–615. doi:10.1164/rccm.9120-11ST
ElHalawani, S. M., Ly, N. T., Mahon, R. T., and Amundson, D. E. (2003). Exhaled nitric oxide as a predictor of exercise-induced bronchoconstriction. Chest. 124 (2), 639–643. doi:10.1378/chest.124.2.639
Gibson, P. G. (2009). Using fractional exhaled nitric oxide to guide asthma therapy: design and methodological issues for ASthma TReatment ALgorithm studies. Clin. Exp. Allergy. 39 (4), 478–490. doi:10.1111/j.1365-2222.2009.03226.x
ginasthma (2020). Global Strategy for asthma management and prevention. Available from: www.ginasthma.org. (Accessed March 9, 2020).
Haldar, P., Pavord, I. D., Shaw, D. E., Berry, M. A., Thomas, M., Brightling, C. E., et al. (2008). Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 178 (3), 218–224. doi:10.1164/rccm.200711-1754oc
Irwin, R. S., Baumann, M. H., Bolser, D. C., Boulet, L.-P., Braman, S. S., Brightling, C. E., et al. (2006). Diagnosis and management of cough executive summary. Chest. 129 (1), 1S–23S. doi:10.1378/chest.129.1_suppl.1s
Jain, N., Covar, R. A., Gleason, M. C., Newell, J. D., Gelfand, E. W., and Spahn, J. D. (2005). Quantitative computed tomography detects peripheral airway disease in asthmatic children. Pediatr Pulmonol. 40 (3), 211–218. doi:10.1002/ppul.20215
Jatakanon, A., Lim, S., Chung, K. F., and Barnes, P. J. (1998a). An inhaled steroid improves markers of airway inflammation in patients with mild asthma. Eur Respir J. 12 (5), 1084–1088. doi:10.1183/09031936.98.12051084
Jatakanon, A., Lim, S., Kharitonov, S. A., Chung, K. F., and Barnes, P. J. (1998b). Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 53 (2), 91–95. doi:10.1136/thx.53.2.91
Jones, M. H., Howard, J., Davis, S., Kisling, J., and Tepper, R. S. (2003). Sensitivity of spirometric measurements to detect airway obstruction in infants. Am. J. Respir. Crit. Care Med. 167 (9), 1283–1286. doi:10.1164/rccm.200204-339oc
Kuhn, M. (2008). Building Predictive Models in R Using the caret Package. J. Stat Software. 28 (5), 5. doi:10.18637/jss.v028.i05
Lehtimäki, L., Karvonen, T., and Högman, M. (2020). Clinical values of nitric oxide parameters from the respiratory system. Curr. Med. Chem. 27 (42), 7189–7199. doi:10.2174/0929867327666200603141847
Lim, S., Jatakanon, A., John, M., Gilbey, T., O'connor, B. J., Chung, K. F., et al. (1999). Effect of inhaled budesonide on lung function and airway inflammation. Assessment by various inflammatory markers in mild asthma. Am. J. Respir. Crit. Care Med. 159 (1), 22–30. doi:10.1164/ajrccm.159.1.9706006
Malerba, M., Radaeli, A., Olivini, A., Damiani, G., Ragnoli, B., Sorbello, V., et al. (2016). Association of FEF25-75% impairment with bronchial hyperresponsiveness and airway inflammation in subjects with asthma-like symptoms. Respiration. 91 (3), 206–214. doi:10.1159/000443797
Malerba, M., Ragnoli, B., Radaeli, A., and Tantucci, C. (2008). Usefulness of exhaled nitric oxide and sputum eosinophils in the long-term control of eosinophilic asthma. Chest. 134 (4), 733–739. doi:10.1378/chest.08-0763
Malerba, M., Ricciardolo, F., Radaeli, A., Torregiani, C., Ceriani, L., Mori, E., et al. (2006). Neutrophilic inflammation and IL-8 levels in induced sputum of alpha-1-antitrypsin PiMZ subjects. Thorax. 61 (2), 129–133. doi:10.1136/thx.2005.043471
Maniscalco, M., Faraone, S., Sofia, M., Molino, A., Vatrella, A., and Zedda, A. (2015). Extended analysis of exhaled and nasal nitric oxide for the evaluation of chronic cough. Respir. Med. 109 (8), 970–974. doi:10.1016/j.rmed.2015.05.016
McFadden, E. R., and Linden, D. A. (1972). A reduction in maximum mid-expiratory flow rate. A spirographic manifestation of small airway disease. Am. J. Med. 52 (6), 725–737. doi:10.1016/0002-9343(72)90078-2
Niimi, A., Matsumoto, H., Amitani, R., Nakano, Y., Mishima, M., Minakuchi, M., et al. (2000). Airway wall thickness in asthma assessed by computed tomography. relation to clinical indices. Am. J. Respir. Crit. Care Med. 162 (4), 1518–1523. doi:10.1164/ajrccm.162.4.9909044
Perez, T., Chanez, P., Dusser, D., and Devillier, P. (2013). Small airway impairment in moderate to severe asthmatics without significant proximal airway obstruction. Respir. Med. 107 (11), 1667–1674. doi:10.1016/j.rmed.2013.08.009
Pin, I., Gibson, P. G., Kolendowicz, R., Girgis-Gabardo, A., Denburg, J. A., Hargreave, F. E., et al. (1992). Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax 47 (1), 25–29. doi:10.1136/thx.47.1.25
Pizzichini, M. M. M., Pizzichini, E., Clelland, L., Efthimiadis, A., Pavord, I., Dolovich, J., et al. (1999). Prednisone-dependent asthma: inflammatory indices in induced sputum. Eur. Respir. J. 13 (1), 15–21. doi:10.1183/09031936.99.13101599
Quanjer, P. H., Stanojevic, S., Cole, T. J., Baur, X., Hall, G. L., Culver, B. H., et al. (2012). Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur. Respir. J. 40 (6), 1324–1343. doi:10.1183/09031936.00080312
Quanjer, P. H., Tammeling, G. J., Cotes, J. E., Pedersen, O. F., Peslin, R., and Yernault, J.-C. (1993). Lung volumes and forced ventilatory flows. Eur. Respir. J. 6 (Suppl. 16), 5–40. doi:10.1183/09041950.005s1693
R Core Team (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation.
Ricciardolo, F. L. M. (2014). Revisiting the role of exhaled nitric oxide in asthma. Curr. Opin. Pulm. Med. 20 (1), 53–59. doi:10.1097/MCP.0000000000000006
Ricciardolo, F. L. M., Sterk, P. J., Gaston, B., and Folkerts, G. (2004). Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 84 (3), 731–765. doi:10.1152/physrev.00034.2003
Riley, M. C., Wenzel, S. E., Castro, M., Erzurum, S. C., Chung, K. F., Fitzpatrick, A. M., et al. (2015). Clinical implications of having reduced mid forced expiratory flow rates (FEF25-75), independently of FEV1, in adult patients with asthma. PLoS One. 10 (12), 145476. doi:10.1371/journal.pone.0145476
Robin, X., Turck, N., Hainard, A., Tiberti, N., Lisacek, F., Sanchez, J. C., et al. (2011). pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 12 (1), 77. doi:10.1186/1471-2105-12-77
Schleich, F. N., Asandei, R., Manise, M., Sele, J., Seidel, L., and Louis, R. (2012). Is FENO50 useful diagnostic tool in suspected asthma? Int. J. Clin. Pract. 66 (2), 158–165. doi:10.1111/j.1742-1241.2011.02840.x
Song, W.-J., Kim, H. J., Shim, J.-S., Won, H.-K., Kang, S.-Y., Sohn, K.-H., Kim, B.-K., Jo, E.-J., Kim, M.-H., Kim, S.-H., Park, H.-W., Kim, S.-S., Chang, Y.-S., Morice, A. H., Lee, B.-J., and Cho, S.-H. (2017a). Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variant asthma and eosinophilic bronchitis in adults with chronic cough: A systematic review and meta-analysis. Journal of Allergy and Clinical Immunology 140 (3), 701–709. doi:10.1016/j.jaci.2016.11.037
Song, W.-J., Won, H.-K., Moon, S.-D., Chung, S.-J., Kang, S.-Y., Sohn, K.-H., et al. (2017b). Could fractional exhaled nitric oxide test be useful in predicting inhaled corticosteroid responsiveness in chronic cough? A systematic review. J. Allergy Clin. Immunol. Pract. 5, 135–143. doi:10.1016/j.jaip.2016.07.017
Terra Filho, M., Vargas, F. S., Cukier, A., Fiss, E., Romeiro Neto, M., and Croce, J. (1986). [Forced mid-expiratory flow rate (FEF 25-75%): a critical analysis of its value in recognizing diseases of the small airways]. Allergol. Immunopathol (Madr). 14 (3), 199–203.
Tossa, P., Paris, C., Zmirou-Navier, D., Demange, V., Acouetey, D. S., Michaely, J. P., et al. (2010). Increase in exhaled nitric oxide is associated with bronchial hyperresponsiveness among apprentices. Am. J. Respir. Crit. Care Med. 182 (6), 738–744. doi:10.1164/rccm.200903-0415OC
van der Palen, J., Ginko, T., Kroker, A., van der Valk, P., Goosens, M., Padullés, L., et al. (2013). Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opin. Drug Deliv. 10 (8), 1023–1031. doi:10.1517/17425247.2013.808186
van der Wiel, E., Postma, D. S., van der Molen, T., Schiphof-Godart, L., ten Hacken, N. H. T., and van den Berge, M. (2014). Effects of small airway dysfunction on the clinical expression of asthma: a focus on asthma symptoms and bronchial hyper-responsiveness. Allergy. 69 (12), 1681–1688. doi:10.1111/all.12510
Van Der Wiel, E., Ten Hacken, N. H. T., Postma, D. S., and Van Den Berge, M. (2013). Small-airways dysfunction associates with respiratory symptoms and clinical features of asthma: a systematic review. J. Allergy Clin. Immunol. 131 (3), 646–657. doi:10.1016/j.jaci.2012.12.1567
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., and Vandenbroucke, J. P. (2008). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61 (4), 344–349. doi:10.1016/j.jclinepi.2007.11.008
Youden, W. J. (1950). Index for rating diagnostic tests. Cancer 3 (1), 32–35. doi:10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3
Keywords: bronchial hyperreactivity, fractional exhaled nitric oxide, forced expiratory flow, sputum eosinophils, cough variant asthma, bronchial provocation tests
Citation: Malerba M, Ragnoli B, Azzolina D, Montuschi P and Radaeli A (2021) Predictive Markers of Bronchial Hyperreactivity in a Large Cohort of Young Adults With Cough Variant Asthma. Front. Pharmacol. 12:630334. doi: 10.3389/fphar.2021.630334
Received: 17 November 2020; Accepted: 25 March 2021;
Published: 19 April 2021.
Edited by:
Mauro Maniscalco, Fondazione Salvatore Maugeri (IRCCS), ItalyReviewed by:
Massimo Corradi, University of Parma, ItalyHelen Petsky, Queensland Children’s Medical Research Institute, Australia
Copyright © 2021 Malerba, Ragnoli, Azzolina, Montuschi and Radaeli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Malerba, bWFyaW8ubWFsZXJiYUB1bml1cG8uaXQ=; Paolo Montuschi, cGFvbG8ubW9udHVzY2hpQHVuaWNhdHQuaXQ=; Alessandro Radaeli, YWxlc3NhbmRyby5yYWRhZWxpQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Mario Malerba
Mario Malerba Beatrice Ragnoli
Beatrice Ragnoli Danila Azzolina
Danila Azzolina Paolo Montuschi
Paolo Montuschi Alessandro Radaeli
Alessandro Radaeli