- 1State Key Laboratory of Characteristic Chinese Medicine Resources in Southwest China, Pharmacy College, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Innovative Institute of Chinese Medicine and Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
For decades, chronic diseases including cardiovascular and cerebrovascular diseases (CCVDs) have plagued the world. Meanwhile, we have noticed a close association between CCVDs and vascular lesions, such as hypertension. More focus has been placed on TMPs and natural products with vasodilation and hypotension. TMPs with vasodilatory and hypotensive activities are mainly from Compositae, Lamiaceae, and Orchidaceae (such as V. amygdalina Del., T. procuinbens L., M. glomerata Spreng., K. galanga L., etc.) whereas natural products eliciting vasorelaxant potentials were primarily from flavonoids, phenolic acids and alkaloids (such as apigenin, puerarin, curcumin, sinomenine, etc.). Furthermore, the data analysis showed that the vasodilatory function of TMPs was mainly concerned with the activation of eNOS, while the natural products were primarily correlated with the blockage of calcium channel. Thus, TMPs will be used as alternative drugs and nutritional supplements, while natural products will be considered as potential therapies for CCVDs in the future. This study provides comprehensive and valuable references for the prevention and treatment of hypertension and CCVDs and sheds light on the further studies in this regard. However, since most studies are in vitro and preclinical, there is a need for more in-depth researches and clinical trials to understand the potential of these substances.
Introduction
It is universally acknowledged that the pathogenesis of cardiovascular and cerebrovascular diseases (CCVDs) is complex and long, which is an obstacle to the development of human health. In 2011, the United Nations recognised non-communicable diseases, including CCVDs, as the major health barriers for humans and developed plans to reduce the impact of these diseases (Mensah and Mayosi, 2013). According to WHO, 31% of the world's deaths in 2016 were from CCVDs. These causes included ischaemic heart disease (IHD), ischaemic stroke, haemorrhagic stroke, atrial fibrillation, peripheral arterial disease, aortic aneurysm, atherosclerotic, cardiomyopathy, and myocarditis, hypertensive heart disease, endocarditis, rheumatic heart disease, arrhythmias, etc (Kratz et al., 2017; Roth et al., 2017). Importantly, many common chronic CCVDs, such as heart failure, myocardial infarction, stroke, vascular dementia, and chronic kidney diseases, are closely related to hypertension (Sureda et al., 2017). However, hypertension is often caused by vascular lesions such as vessel wall thickening, vessel stenosis, vessel occlusion and endothelial cell injury (Mathieu et al., 2009; Farias, Pereira and Rosa 2010; Howlett 2014; Singh et al., 2017). For example, hypertension is more likely to cause IHD, stroke, cerebral haemorrhage and cerebral ischaemia in the elder population (Collaboration, 2002). Additionally, diabetic vasculopathy is concerned with vessel wall thickening and endothelial cell damage, which is the main cause of blindness, kidney failure, heart attacks, and stroke (Wimmer et al., 2019). Furthermore, hypertensive patients are also more likely to develop diabetes. (Sowers et al., 2001).
Remarkably, WHO encourages the use of traditional medicinal plants (TMPs) to improve various chronic diseases with increasing risk around the world. TMPs were shown to be beneficial for human health due to the richness of active compounds. Natural products, referring to small molecules derived from TMPs, also improve processes of biological metabolism through regulating the activity of enzymes in the body (Loai and Zohar, 2015; Briguglio et al., 2018). What’s more, TMPs have been used as food supplements to treat chronic diseases without prescription. Currently, TMPs and natural products are playing an indispensable part for improving human health as a complementary alternative therapy, although some side effects have been found. In the past two decades, scientists have explored the vasodilation of TMPs and natural products, and roughly explained their mechanism. These natural resources have also been proved to be potentially effective for the treatment of CCVDs in clinic and drawn greater attention, even in developed countries including the United States and Australia. (Delfan et al., 2014; Liu and Huang, 2016; Shaito et al., 2020). However, the vasodilatory activities, the underlying mechanism and the clinical efficacy of TMPs and natural products, had not been comprehensively over-reviewed.
This article reviews the underlying mechanism and the clinical efficacy of TMPs and natural products with vasodilation, and puts forward a view that TMPs and natural products can be used as adjuvants in the prevention and treatment of CCVDs. We hope this review will lay the foundation for an in-depth investigation on the development and utilization of nature resources in this filed.
Search Strategy
This review aims to provide readers with a brief account of the main achievements in the field of vasodilation-based natural products and plant extracts from TMPs worldwide during the years of 1998–2020. To this end, the literatures from several databases including PubMed, Web of Science and Google Scholar were searched to obtain any studies evaluating TMPs and natural products with vasodilation. The data collected in this review were limited to English articles that primarily focused on the vasodilation and the underlying mechanisms of screening of TMPs and natural products.
Vasodilation Mechanism of TMPs and Natural Products
The vascular tone changes of vascular smooth muscle cells (VSMCs) occurs through electromechanical and chemomechanical coupling and then acting on α-receptor, angiotensin-converting enzymes, angiotensin receptor or potassium/calcium channels. The detailed mechanism of action is as follows (Hill et al., 2001; Cole and Welsh, 2011). Figure 1 shows a brief description of the vascular tone changes of VSMs to support our understanding of vasodilatory mechanisms (Alexander et al., 2019a; Alexander et al., 2019b).
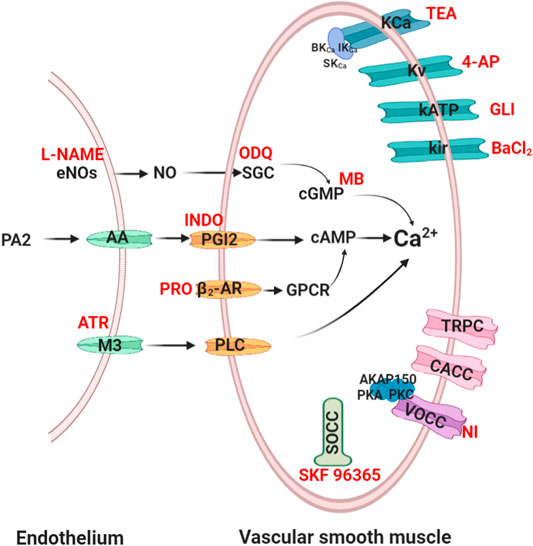
FIGURE 1. Routes of vasodilation mechanisms. Red words denote common blockers of the corresponding pathways. eNOs, endothelial nitric oxide synthase; SGC, soluble guanylyl cyclase; cGMP, cyclic 3′,5′-guanosine monophosphate; PA 2, phospholipase A 2; AA, arachidonic acid; PGI2, prostaglandin 2; cAMP, cyclic adenosine 3′, 5′-monophosphate; β2-AR, β2-adrenoreceptor; PLC, phospholipase C; L-NAME, nitro-L-arginine; ODQ, 1H- [1, 2, 4] oxadiazolo [4, 3-α] quinoxalin-1-one; MB, methylene blue; INDO, indomethacin; PRO, propranolol; ATR, atropine; NI, nifedipine; GLI, glibenclamide; 4-AP, 4-aminopyridine; TEA, tetraethylammonium.
Endothelium-Mediated Vasodilation Mechanism
Endothelial-derived relaxing factor (EDRF), including nitric oxide (NO) and prostacyclin from endothelial cells, plays an important role in vasodilation (Török, 2000; Tokoudagba et al., 2010).
NO Signalling Cascade-Mediated Vasodilation
NO is the main EDRF that induces relaxation of VSMCs. In addition, NO also plays a crucial role in CCVDs such as atherosclerotic disease (Malekmohammad et al., 2020). Endothelial nitric oxide synthase (eNOS) catalyses the production of NO, which diffuses into VSMCs and then enhances cGMP synthesis. cGMP activates dependent protein kinase to cause vasodilation by reducing the intracellular Ca2+ concentration (Bian et al., 2008). Therefore, to validate the eNOS/NO/sGC/cGMP/signalling pathway, L-NAME (non-selective NO inhibitor) is used to inhibit NO synthesis. ODQ (sGC pathways inhibitor) and MB (cGMP pathways inhibitor) are usually used to inhibit the sGC/cGMP pathway (Bian et al., 2008).
PGI2 Signalling Cascade-Mediated Vasodilation
Prostaglandin 2 (PGI2) is also a major EDRF, prostacyclin synthase-catalysed intermediate prostaglandin H2, and catalysed by cyclooxygenase (COX) for arachidonic acid synthesis. PGI2 activates adenylate cyclase (AC) to produce cAMP and then regulates dependent protein kinase to reduce the intracellular Ca2+ concentration (Ameer O. Z. et al., 2010). Indomethacin is commonly used to inhibit COX and PGI2 channels.
Muscarinic Receptor Signalling Cascade-Mediated Vasodilation
M3 receptor is a Gαq-protein-coupled receptor (GPCR) that presents in endothelium. (Walch et al., 2000). Atropine is commonly used to block phospholipase-C signalling pathways by inhibiting M3.
VSMCs Mediated Vasodilation Mechanism
The contraction of VSMCs mainly rely on Ca2+ influx via regulating ion channels (K+/Ca2+) or receptor channels. Ca2+ enter cells mainly through voltage dependent L-type Ca2+ channels (LTCC), store-operated calcium channels (SOCC) and transient receptor potential channels (TRPC). Of course, Ca2+ influx also relies on the regulation of Ca2+-gated Cl channels (CACC) (Brozovich et al., 2016). Moreover, K+ channels also changes of vascular tone by regulating extracellular Ca2+ influx.
β2-Adrenoreceptor-Mediated Vasodilation
β2-adrenoreceptor, a GPCR, only exists on the membrane of VSMCs. This receptor passes through the GPCR/AC pathway, which catalyses the breakdown of ATP into cAMP and subsequently causes vasodilation (Ch'ng et al., 2017). Propranolol, a nonselective β2-adrenergic receptor inhibitor, eventually causes vasoconstriction through inhibiting this channel.
Potassium Ion Channels Mediated Vasodilation
There are mainly four types of K+ channels in VSMCs: voltage-sensitive K+ channels (Kv), ATP-sensitive K+ channels (KATP), inward rectifier-type K+ channels (Kir) and Ca2+ activated K+ channels (KCa). KCa, widely appear in pulmonary artery smooth muscle cells, include large conductance Ca2+-dependent K channels (BK channels), intermediate-conductance Ca2+ activated K+ channel (IKCa) and small-conductance Ca2+ activated K+ channel (SKCa), and the BK channel was associated with the β-1 subunit (Brenner et al., 2000). The four channels were blocked by 4-aminopyridine (4-AP), glibenclamide, BaCl2, and tetraethylammonium (TEA), respectively (Su et al., 2014). Furthermore, other channels such as KCNQ subfamily, EAG (ether-à-go-go or KCNH) subfamily, Ca2+-activated Slo subfamily and Ca2+ and Na-activated SK subfamily were found in VSMCs (Brozovich et al., 2016; Barros et al., 2020).
Calcium Ion Channels Mediated Vasodilation
There are mainly three types of Ca2+ channels in VSMCs membranes, including voltage-operated calcium channels (VOCC) and receptor-operated calcium channels (ROCC). VOCC were regulated by membrane potential-dependent voltage, and ROCC bind to GPCR, SOCC and so on. LTCC, as a predominant VOCC, has been shown to be concerned with the A-kinase anchor protein 150 (AKAP150) and protein kinase C/A (Navedo et al., 2008). SOCC is mediated by the sarcoplasmic reticulum (SR) Ca2+ sensor stromal interaction molecule (STIM) and orai channels (Trebak, 2012). TRPC, including TRPC, TRPM, TRPV, TRPA, TRPP, and TRPML, are nonselective cation channels that carry receptor-operated Ca2+ currents (ROCs) triggered by phospholipase C (PLC)-catalyzed hydrolysis of phosphatidylinositol 4,5-bisphosphate [PI(4, 5)P2] (Mori et al., 2015). These channels may be also stimulated in store-operated manner, via tyrosine kinases, or by lysophospholipids, hypoosmotic solutions, and mechanical stimuli (Chen et al., 2020). Several TRPC, such as TRPC1, TRPC3, TRPC6, and TRP M4 were found to be involved in vasoconstriction and more sensitive than LTCC (Beech, 2005). Additionally, it was indicated that both SOCC and ROCC are concerned with TRPC family (McFadzean and Gibson, 2002). The blockers of VOCC and SOCC are nifedipine, SKF 96365 or Gd3+ respectively in vitro experiments (Xu et al., 2015; Alexander et al., 2019b).
Traditional Medicinal Plants With Vasodilation In Vitro and Antihypertensive Activities In Vivo
This section describes the vasodilatory and antihypertensive effects of plant extracts. Most extracts act on complex pathways for vasodilation or hypotension in rats. Supplementary Table S1 shows the details of studies investigating vasodilation of TMPs. All the discussed TMPs, which were primarily from Composite (17%), were shown in Figure 2A. TMPs mainly displayed vasodilation by acting on eNOS and Ca2+ channels (Figure 3A). Most of TMPs, such as V. amygdalina Del., T. procuinbens L., M. glomerata Spreng., K. galanga L., etc., have significant vasodilatory bioactivities in vitro, but the hypotensive efffect of only 40% of TMPs have been investigated in vivo at present, such as G. procumbens, H. cere, Bidens pilosa Linn, etc (Figure 3A). In addition, some TMPs have significant vasodilation but the mechanistic exploration was insufficient, such as G. procumbens, M glomerata Spreng.
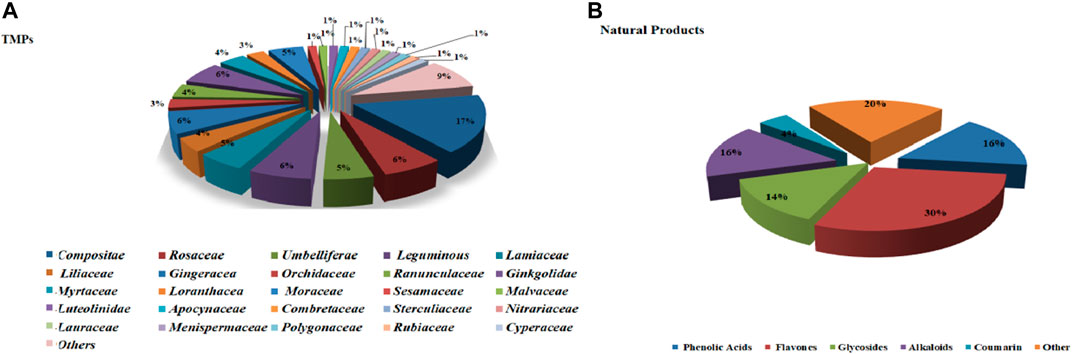
FIGURE 2. The ratio of TMPs and natural products. (A): ratio of TMPs in this article (classified by family); (B): ratio of natural products in this article (classified by compound type).
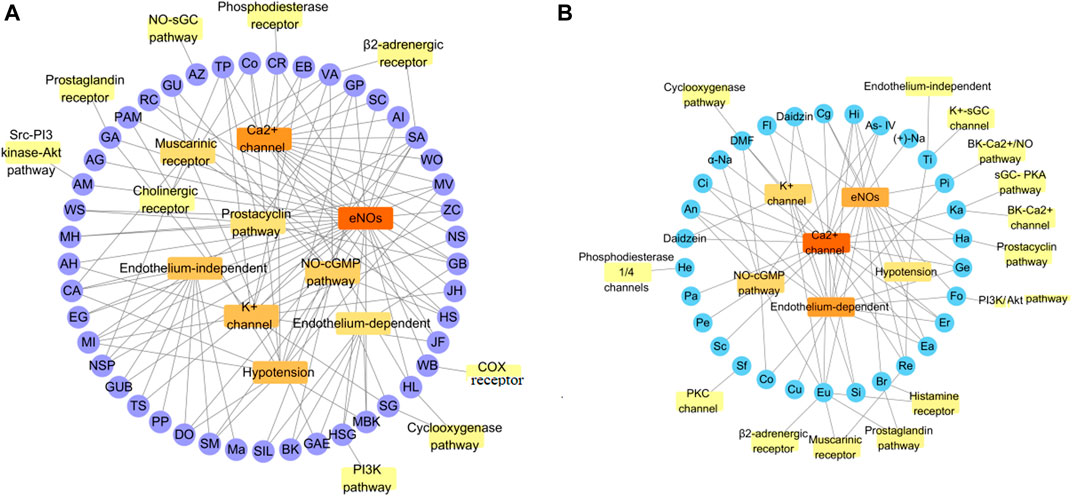
FIGURE 3. Some mechanisms of vasodilation caused by TMPs and natural products. (A) Vasodilation mechanism of TMPs; (B) Vasodilation mechanism of Natural Products. The vasodilatory function of TMPs was mainly concerned with the activation of eNOs, while the natural products were primarily associated with blockage of calcium channel. They also act on other pathways, including sGC-cGMP, potassium channels, muscarinic receptors, cyclooxygenase pathways, prostaglandin I2, etc.
Compositae
Erigeron breviscapus (Vant.) Hand-Mazz.
Erigeron breviscapus (EB), as a traditional Chinese medicine, is commonly used for neuroprotection and vascular protection. EB induced relaxation on U44619 (Ca2+ agonists, EC50 0.354 mg mL−1) pre-contracted aortic rings, which was abolished by glibenclamide (KATP inhibitor) or TEA (KCa channel inhibitor). However, this effect of EB was not affected by endothelial removal or ipiliocin (BKCa inhibitor), BaCl2 (Kir inhibitor) or 4-AP (Kv inhibitor). These results indicated that the vasodilatory activities of EB are mediated by Ca2+ and KATP channels, rather than endothelial cells or K+ channels (Pan et al., 2008).
Vernonia amygdalina Del.
Vernonia amygdalina Del. (VA) is used to treat hypertension in Malaysia. The ethanol extract of VA leaves (VAE) caused the relaxation of Phenylephrine (PHE, 1 μM)-pre-contracted aortic rings (EC50 0.057 mg mL−1). However, its effects were significantly reduced by endothelial removal, TEA, 4-AP, BaCl2, glibenclamide, L-NAME (eNOS blocker), methylene blue (cGMP inhibitor), indomethacin (COX inhibitor), atropine (muscarinic receptor inhibitor) and propranolol (β2-adrenoreceptor inhibitor). Hence, the effect of VAE was related with the NO/cGMP/PGI2 pathways, Ca2+/K+ channels or β2-adrenergic receptor (Ch’ng et al., 2017). Additionally, VA (i.v. 10.0 mg kg−1) ultimately caused a reduction in blood pressure in SD rats (Taiwo et al., 2010).
Tridax procuinbens L.
The water extract of the leaf from Tridax procuinbens (TP) has been shown to reduce blood pressure, but its mechanism remains unclear. The data showed that TP could significantly relieve the contraction caused by PHE (0.1 μM) and K+ (60 mM). TP (10−9–10−5 M) also antagonised the Ca2+-induced vasoconstriction in a Ca2+-free context by high K+. The activity of TP was repressed by BaCl2 and apamin (K+ channels blockers), L-NAME, indomethacin, atropine, propranolol, and methylene blue; however, it was not affected by glibenclamide, TEA, 4-AP or ODQ (guanylyl cyclase inhibitor). Therefore, this action is intervened by endothelial cells and partial ion channels (Salahdeen et al., 2015).
Artemisia herba Alba Asso.
Artemisia herba Alba Asso (AH) is used to treat diabetes and hypertension in Morocco. AH relaxed the contraction elicited by noradrenalin (NE, 1 μM) in endothelium-containing aortas. However, its activity was significantly abolished by L-NAME (100 μM), endothelial removal, methylene blue (10 μM) and ODQ (50 μM) but not atropine (10 μM), TEA (5 mM), indomethacin (10 μM) or glibenclamide (10 μM). It was suggested that the vasodilation of AH occurs mainly through the activation of eNOS/SGC/cGMP pathways rather than K+ channels (Skiker et al., 2010).
Bidens pilosa L.
Previous studies have shown that the antihypertensive effect of Bidens pilosa (BP) extract is closely related to its vasodilatory activity. The extract of BP was shown to induce a concentration-dependent vasorelaxation of rat aortas pre-contracted by K+ (60 mM, inhibition rate 90% at 1.5 mg mL−1) and NE (1 μM, inhibition rate 88% at 1.5 mg mL−1) which was significantly inhibited by indomethacin or pyrilamine maleate (histamine-1 inhibitor) (Nguelefack et al., 2005). Furthermore, the extract of BP leaves (10, 20, and 30 mg kg−1) decreased SBP by 18.26, 42.5 and 30% in normotensive rats and by 25.77, 38.96 and 28.64% in SHR, respectively. Moreover, it also induced hypotension by 27, 34.13 and 18.73% in salt-loaded hypertensive rats, respectively (Dimo et al., 2003). These results indicated that the effect is concerned with vasodilation.
Gynura procumbens L. Merr.
Gynura procumbens (GP) has been shown to decrease blood pressure by inhibiting the angiotensin-converting enzyme. The present experiments showed that the aqueous extract of GP significantly reduced the contraction induced by angiotensin I and II in rat aortic rings, which was blocked by indomethacin (10 μM) or L-NAME (0.1 μM) (Poh et al., 2013). Moreover, the butanoic fraction of GP (2.5–5 mg mL−1) also released the PHE (1 μM)-/K+ (80 mM)-induced contractions. GP (i.v., 10–20 mg kg−1) also reduced mean arterial pressure in anesthetized rats (Hoe et al., 2011). Therefore, it was suggested that GP causes vasodilation through upregulating eNOS levels.
Helichrysum ceres S. Moore.
Ethnomedical evidences suggested that extracts from Helichrysum have anti-inflammatory and anti-allergic activities and are commonly used to treat renal and cardiopulmonary diseases. The researchers found that the ethanolic extract of Helichrysum ceres leaf (HCE) could relax atropine (1 µM)/NE (1 µM)/K+ (20, 80 mM)-induced contractions which was weakened by L-NAME (100 µM) in rat aortic. Furthermore, HCE could cause hypotension in normotensive rats or Dahl salt sensitive hypertensive rats (Musabayane et al., 2008). It was shown that activity of HCE is related to the eNOS levels.
Mikania glomerata Spreng.
Mikania globerata (MG) is mainly used to treat respiratory diseases in Brazil. The aqueous extracts and hydroalcoholic extract (HAE) of MG leaves significantly inhibited the histamine contractions in isolated guinea pig tracheas. HAE also induced relaxation in guinea pig tracheas pre-contracted by histamine (IC50 0.34 mg mL−1), acetylcholine (IC50 0.72 mg mL−1) or K+ (IC50 1.41 mg mL−1). Moreover, the dichloromethane fraction of MG could relax the isolated mesenteric vascular or aorta in rats (Soares de Moura et al., 2002). But the mechanism of MG needs more exploration in the future.
Flos chrysanthemi Indici
The ethyl acetate extract from Flos chrysanthemi Indici (FCE, 9.4–150 mg/L) antagonized vasoconstriction induced by PHE (1 μM)/K+ (60 mM), which was significantly inhibited by endothelium removal, L-NAME (10−4 M), glibenclamide and methylene blue (10−5 M). But this effect of FCE was not affected by TEA, BaCl2, 4-AP, 5-HD or propranolol. In addition, FCE attenuated PHE-induced contraction in calcium-free or potassium-free solutions by upregulated NO levels in rat aortic. Overall, it was showed that FCE is in association with regulation of the NO/cGMP pathway and inhibition of KATP (Jiang et al., 2005).
Others
The hydroalcoholic extract of Senecio nutans sch. Bip and the aqueous extract of Tanacetum vulgare L were shown to relax the PHE/K+/NE-dependent contractions of rat aortic rings in an endothelium-dependent manner (Paredes et al., 2016), (Lahlou et al., 2008). Additionally, the dichloromethane extract of Kaempferia galanga L and the methanol extract from Stevia rebaudiana had shown antihypertensive activity in anaesthetised rats or SHR (Othman et al., 2006; Melis 1995). But the mechanism of these extracts is not sufficiently studied at present.
Rosaceae
Crataegus gracilior J. B. Phipps.
Hawthorn is used worldwide as traditional medicines to treat CCVDS, such as Crataegus gracilior J. B. Phipps (Mexican hawthorn, MH). The aqueous extracts of the leaves and fruits of MH elicited relaxation of aortic rings treated by PHE (1 µM), and its methanol extract (EC50 4.34 mg mL−1) had significant vasodilator effects (Hernández-Pérez et al., 2014). Additionally, the extract of Crataegus leaves and flowers caused relaxation in PHE (10 mM)-mediated rat aorta (IC50 15.1 μg mL−1) or human papillary artery (IC50 19.3 μg mL−1) from patients undergoing coronary artery bypass surgery. In short, the vasodilation of the extract is concerned with the eNOS pathway, but other pathways need to be further explored (Brixius et al., 2006).
Fragaria x Ananassa Duch.
The vasodilation activity of the aqueous extract of Fragaria x ananassa Duch (wild strawberry, WS) leaves attenuated NE-induced (0.1 µM) vasoconstriction in rat aortas which was suppressed by L-NAME or indomethacin. Therefore, similar to the hawthorn aqueous extract, the aqueous extracts could cause endothelium-dependent vasodilation which were correlated with the NO/COX pathways (Hernández-Pérez et al., 2014).
Rubus chingii Hu.
Rubus chingii (RC) is a commonly used traditional Chinese medicine that can improve renal function and treat polyuria. The ethanol extract of RC caused PHE (1 μM)-induced relaxation in rat aortas. However, this effect of RC was significantly attenuated by L-NAME (10 µM), ODQ (10 µM), diltiazem (10 μM, Ca2+ antagonist), wortmannin (0.1 µM, PI3-kinase inhibitor), thapsigargin (1 μM, Ca2+ modulator), Gd3+ (10 μM, Ca2+ modulator), 2-aminoethyl diphenylborinate (75 μM, Ca2+ modulator) and 4-AP (1 mM), rather than TEA (1 mM), glibenclamide (10 µM), indomethacin, atropine, and propranolol. This study indicated that RC induces vasodilation in an endothelium-dependent manner, mainly by activating the Ca2+/eNOS and the NO/sGC/cGMP/KV channels (Su et al., 2014).
Aronia melanocarpa
The high content of phenolic constituents of Aronia melanocarpa (AM) is characterized by a variety of biological activities. Researchers have found that AM juice caused potent endothelium-dependent relaxations in porcine coronary artery rings, which was markedly abolished by L-NAME, PP2 (Src kinase inhibitor) and wortmannin. This result showed that AM juice promotes NO levels in the coronary artery endothelium by activating the Src/PI3 kinase/Akt pathway. Its main active components may be conjugated anthocyanins and chlorogenic acid (Kim et al., 2013).
Sorbus commixta Hedl.
The cortex of this species has been used for antitussive purposes in oriental medicine. The methanol extract of Sorbus commixta cortex (SC) produced relaxation of the PHE-induced aorta which was attenuated by L-NAME, methylene blue, ODQ and endothelial removal except for indomethacin, glibenclamide, TEA, atropine or propranolol. Thus, its vasodilatory activity occurs through activation of the NO/cGMP pathway rather than blockage of KCa or KATP (Kang et al., 2005).
Carum roxburghianum (DC.) Kurz.
Carum roxburghianum (CR) exhibit vasodilatory and cardiac modulatory actions. The crude extract of CR (10–100 mg kg−1) induced decrease arterial blood pressure of rats. Meanwhile, CR also inhibited high K+ (80 mM)-/PHE (1 μM)-induced contractions in isolated rabbit aortas, which was not impaired by L-NAME. Therefore, CR extracts caused vasodilation might through antagonising Ca2+ channels, regulating NO levels and inhibiting phosphodiesterase (Khan et al., 2015).
Umbelliferae
Angelica dahurica Bentham
Angelica dahurica Bentham (ADB) has been used for the treatment of CCVDs in Asia. The methanol extract of ADB (1 mg mL−1) resisted PHE (1 μM)-/K+ (60 mM)-induced contractions in aortic rings but not caffeine (opener of ryanodine-sensitive receptors) in a Ca2+-free context (Lee et al., 2015). But the vasodilatory activity of ADB needs further investigation.
Ligusticum chuanxiong Hort.
Ligusticum chuanxiong (Lc) have been widely used in the treatment of CCVDs in Asian countries. The CHCl3 extract of Lc could inhibit contraction induced by NE in aortic strips and abolished Ca2+-independent contractions evoked by 12-deoxyphorbol 13-isobutyrate in Ca2+-free medium containing EGTA (1 mM). Furthermore, Lc significantly inhibited NE-mediated extracellular regulated protein kinases 1/2 (ERK1/2) activation but not P38 mitogen activated protein kinases (MAPK) (Kim B. et al. 2004).
Bupleurum fruticosum L.
The studies have shown that Bupleurum fruticosum (BF) is beneficial to the heart and circulatory system. The chloroformic extract of the BF roots (0.1 mg mL−1) induced relaxation of rat thoracic aorta induced by NE or caffeine. This activity was blocked by cyclopiazonic acid (Ca2+- ATP blocker) (Testai et al., 2005).
Coriandrum sativum L.
Coriander (Co) is traditionally used for gastrointestinal diseases and CCVDs. Researchers have found that Co could resist K+ (80 mM)-induced contractions in the rabbit jejunum and PHE (1 μM)-/K+ (80 mM)-induced contractions in rabbit aortas. Meanwhile, Co (1–30 mg mL−1) caused hypotension in normal rats (Jabeen et al., 2009).
Leguminous
Glycyrrhiza uralensis Fisch.
Glycyrrhiza uralensis (GU) is used in traditional Chinese medicine that displays a variety of bioactivities. The GU caused relaxation of rat aortic rings treated by PHE (1 µM)/K+ (80 mM) in endothelium-intact aortic rings. The vasodilatory activity of GU was weakened by L-NAME (10 µM), methylene blue (10 μM), indomethacin (10 μM), atropinee (1 µM), propranolol (1 µM), glibenclamide (10 μM) and 4-AP (1 µM) except for TEA (1 mM) or BaCl2 (10 μM) (Tan et al., 2017). Thus, GU may relax blood vessels through activating the NO/sGC/cGMP pathway rather than KCa or Kir.
Albizia inopinata G. P. Lewis.
The ethanol extract of Albizia inopinata G. P. Lewis (AI) could inhibit vasoconstriction induced by K+ (80 and 30 mM) or PHE (1 μM) (IC50 54, 52, and 65 μg mL−1, respectively). Additionally, AI antagonised contractions caused in Ca2+-free medium induced by NE (1 μM) rather than caffeine (20 mM) in rat aortas (Pires et al., 2000). Furthermore, AI leaves abolished PHE (1 μM)/K+(80 mM)-induced vasoconstriction (IC50 65 and 54 μg mL−1, respectively) which was repressed by endothelial removal or L-NAME (10 and 100 μM) but not atropine (1 μM) or indomethacin (10 μM). Moreover, AI leaves (20 mg kg−1) produced a significantly hypotensive effect and reduction in heart rate and cardiac output and total peripheral resistance in SHR (Maciel et al., 2004).
Others
The total alkaloids of Sophora alopecuroids L. (SA, 40 mg L−1) resisted concentrate induced by K+-/Ca2+ in rabbit aortas, while was not significantly affected by removal of endothelium, L-NAME, indomethacin or propranolol (Zhang et al., 2009). Seeds of Securigera securidaca (SS) were used for the improvement of hyperlipidaemia in Iranian medicine. The hydroalcoholic extract of SS could improve vascular endothelium-dependent relaxation and decrease lipid levels and peroxidation in a rat model fed a high-fat diet (Garjani et al., 2009).
Lamiaceae
Mentha x villosa Hubs.
Mentha x villosa (MV) has anti-parasitic and tranquillising action and treatment of gastrointestinal diseases. Essential oil from MV induced significant and dose-dependent hypotensive and bradycardic responses, which were weakened by atropine (2 mg kg−1). Moreover, MV could attenuate PHE (1 μM) (IC50 255 μg mL−1)-, prostaglandin F2-α (10 μM) (IC50 174 μg mL−1)-/K+ (80 mM) (IC50 165 μg mL−1)-induced contractions in rat aortic rings. This effect was abolished by endothelial removal, L-NAME (100 μM) or indomethacin (10 μM) but not atropine (1 μM) (Guedes et al., 2004). The results suggested that activity of MV is primarily associated with endothelial cells, but the role of ion channels is no clear at present.
Ziziphora clinopodioides L.
The CHCl3 extracts of Ziziphora clinopodioides L (ZC) inhibited PHE (1 μM, EC50 0.27 g L−1)-/K+ (60 mM, EC50 0.34 g L−1)-induced contractions in rat aortic rings, which was significantly decreased by 4-AP (1 mM) or endothelial removal, rather than glibenclamide (100 μm), iberiotoxin (10 nm) or thapsigargin (100 nm). In Ca2+-free solution, ZC also significantly inhibited vasoconstriction in K+/PHE pre-contracted rings (Senejoux et al., 2010). It was shown that activity of ZC is mainly associated with endothelial cells and Kv.
Salviae miltiorrhizae Bqe.
The roots of Salvia miltiorrhiza (SM) is used to treat CCVDS, such as angina pectoris and myocardial infarction in TCM. The aqueous extract of SM relaxed the NA-induced aorta which was abolished by L-NAME (100 μM), methylene blue (10 μM) and endothelium removal. Additionally, SM produced hypotensive response in normal rats through regulating release of angiotensin and bradykinin (Kamata et al., 1993). Moreover, SM caused hypotension in albino rats and rabbits, which was abolished by atropine and propranolol. Interestingly, low concentration of this extract, not higher concentration, induced vasodilation of the renal, mesenteric and femoral arteries (Lei and Chiou, 1986).
Orthosiphon stamineus Benth.
Orthosiphon stamineus in folk medicine is used in the treatment of hypertension and kidney stones. The methanolic extract of Orthosiphon stamineus (CF) relieved PHE (1 μM) (EC50 2.21 μg mL−1)-/K+(60 mM) (EC50 3.32 μg mL−1)-induced constriction of rat thoracic aorta which was resisted by L-NAME (10 μM, EC50 17.8 μg mL−1), methylene blue (10 μM, EC50 12.09 μg mL−1), TEA (1 mM, EC50 24.35 μg mL−1), 4-AP (1 mM, EC50 27.09 μg mL−1), BaCl2 (10 μM, EC50 22.41 μg mL−1), glibenclamide (10 μM, EC50 10.24 μg/mL−1) and propranolol (1 μM, EC50 7.76 μg mL−1), rather than indomethacin (10 μM) (EC50 4.62 μg mL−1) or ODQ (10 μM) (EC50 3.87 μg mL−1). Thus, the vasodilatory activity of CF was in relationship with regulation of NO/cGMP pathway, muscarinic/β-adrenergic receptors and blockage of Ca2+ and K+ channels (Yam M. F. et al., 2016).
Agastache mexicana Ssp.
The dichloromethane extract of Agastache mexicana (AM) significantly relaxed NA (0.1 μM)-induced aortic contraction with endothelium (EC50 174 μg mL−1) or without endothelium (EC50 293 μg mL−1) which was significantly inhibited by TEA. In conclusion, this activity is correlated with endothelial cells or KCa (Flores-Flores et al., 2016).
Marrubium vulgare L.
The water extract of Marrubium (Ma) showed a potent inhibition on K+-induced rat aorta contractions and decreased SBP by improving endothelial function in SHR (El Bardai et al., 2003). But mechanisms of Ma needs more exploration in vivo or vitro in the future.
Liliaceae
Allium fistulosum L.
Welsh onion (WO, Allium fistulosum L.) has been consumed to prevent CCVDs. The WO extracts, particularly green-leaf extracts (RG, 30–40 mg mL−1), could resist NE (30 nM)-induced contractions in aortic ring. The activity was abolished by L-NAME (100 μM), TEA (1 mM) and SQ29548 (TXA2-receptor antagonist, 10 μM) (Chen et al., 1999). The hydroalcoholic extract of onion peel relaxed vasoconstriction induced by K+/PHE which was not attenuated by endothelial removal, L-NAME (100 μM), methylene blue (10 μM) and indomethacin (10 μM) in rat thoracic aortas (Naseri et al., 2008). Thus, the activity of WO is independent of the eNOS/cGMP pathway and whether it acts on ion channels needs further investigation.
Allium sativum L.
The extracts of Allium sativum (AS) induce hypotension in hypertensive patients. AS could relieve NE (3 μM)-induced contractions in the aortic ring which was resisted by L-NAME (100 μM) and indomethacin (5 μM) (Takashima et al., 2017). Thus, the application about AS also needs to be supported by more research in CCVDs.
Gingeracea
Alpinia zerumbet (Pers.) Burtt. et Smith.
The hydroalcoholic extract of Alpinia zerumbet leaves (AZ) could resist NE-induced contractions which was not suppressed by indomethacin, 4-AP or glibenclamide except for L-NAME and ODQ (de Moura et al., 2005). The essential oil of AZ (0.01–3,000 mg mL−1) also relieved PHE-induced contractions which was inhibited by L-NAME and endothelial removal but not TEA (500 mM) or indomethacin (10 mM) (Pinto et al., 2009). Thus, the activity of AZ is mainly concerned with endothelial cells.
Curcuma comosa Roxb.
Researchers have found that Curcuma comosa Roxb (CC) prevented the impairment of vascular relaxation by regulating the eNOS and ER-α protein levels in aorta of ovariectomised rats (Intapad et al., 2012). Additionally, CC promoted the phosphorylation of serine 1,177 in eNOS and serine 473 in Akt protein (Intapad et al., 2009). CC also improved the diastolic function of the aorta in hypercholesterolemic rats by activation of HSP70 and BCl-2 levels, improving activity of antioxidant enzymes (Kam et al., 2012).
Curcuma longa L.
The methanolic extract of Curcuma longa (i. v, CL, 10, 20 and 30 mg kg−1) could reduce blood pressure (2.0, 27.1, and 26.7%) and heart rate (5.8, 19.3, and 22.9%) in normal rats. CL (1–1,000 μg ml−1) also relieved PHE (10 μM)/K (80 mM) -induced aortic contraction, which was attenuated by glibenclamide, BaCl2, TEA or 4-AP. In addition, CL inhibited CaCl2 (1–30 mm) induced contraction in Ca2+ free medium. This suggested that CL relaxes blood vessels by blockage of K+ channels (Adaramoye et al., 2009).
Others
The methanol and water extracts of curcuma herbs such as C. kwangsiensis (1 mg mL−1), C. phaeocaulis (1 mg mL−1), C. wenyujin (1 mg mL−1), and C. zedoaria (1 mg mL−1) could relieve prostaglandin F-2α (6 μM)-induced contractions in aortic ring. Additionally, curcuma herbs such as C. zedoaria (3% wt/wt) could lower blood pressure and protect endothelial cells in SHR (Goto et al., 2005). Therefore, curcuma herbs, with acivities of invigorating blood circulation and eliminating stasis according to Chinese Medicine, may have significant potential for the prevention and treatment of CCVDs.
Orchidaceae
Orchis mascula. ex L.
Orchis mascula (OM) is used to treat CCVDS in Pakistan and India. The OM extract inhibited PHE (1 μM)-/K+ (80 mM)-induced contractions in isolated rabbit aortas. OM (10 and 30 mg kg−1) also reduced SBP and improved endothelial function in SHR. Moreover, OM decreased TG, LDL-C levels in tyloxapol and high-fat diet-induced hyperlipidemia (Aziz et al., 2009).
Dendrobium officinale Kimura. et Migo.
Dendrobium officinale (DO) has been found to improve metabolic diseases including hypertension and diabetes mellitus. In addition, the extract of DO (3.1 g kg−1) significantly reduced SBP and mean arterial pressure in the hypertensive rats. Moreover, it reversed thoracic aortic thickening and endothelial cell apoptosis, decreased plasma ET-1/TXB2 levels and upregulated PGI2/NO levels (Liang et al., 2018).
Ranunculaceae
Paeonia suffruticosa Andrew.
The methanolic extract of the root bark of Paeonia suffruticosa (PS) showed a vasodilation in rat aortas pre-contracted by PHE (0.3 μM, IC50 16.8 μg mL−1). PS increased the endothelium and SOD function in rats fed a high-fat diet. Therefore, PM elicited vasorelaxant activity by protecting endothelial cells (Yoo et al., 2006).
Nigella sativa L.
Nigella sativa (NS, 2–14 mg mL−1) extract induced a dose-dependent relaxation in aortic rings treated by PHE (1 μM)/K+ (60 mM) which was abolished by diltiazem, TEA and glibenclamide except for L-NAME, indomethacin or ruthenium red (LTCC inhibitor). This finding was suggested that effect of NS is concerned with activating on K+ channels (Niazmand et al., 2014).
Ginkgo biloba L.
Ginkgo biloba leaf extract (GB) has been clinically used to improve peripheral vascular disease in France and Germany. Researchers have found that GB produced a dose-dependent relaxation in aortic rings treated by NE, which was alleviated by L-NAME (100 μM), TEA (100 μM) and indomethacin (100 μM). In contrast, the effects of GB (3 mg mL−1) was strongly attenuated to 53% in Ca2+-free medium (Kubota et al., 2001), (Nishida and Satoh, 2003). Additionally, GB significantly reduced SBP in rats fed with 8.0% NaCl or SHR and potentiated the relaxation in response to acetylcholine in aortic (Kubota et al., 2006). However, GB was significantly increased serum alanine aminotransferase and hepatic CYP2B protein levels in aged SHR (Tada et al., 2008). It was suggested that the effect of GB was caused by Ca2+ channels inhibition and NO levels promotion, endothelial cells protection in SHR. Notably, terpenoids and flavonoids may be the main active components of GB (Nishida and Satoh, 2003; Seiichiro and Hiroyasu, 2004).
Myrtaceae
Syzygium guineense
The hydroalcohol extract of the leaves of Syzygium guineense (SG) (50, 100, and 150 mg kg−1, orally) reduced systolic/diastolic blood pressure (6.9, 34.0, and 40.8 mmHg)/(5.0, 18.3, and 25.9 mmHg) in 1-kidney-1-clip hypertensive rat model. Moreover, the extract (70 mg kg−1) caused relaxation in aortas pre-contracted by K+ (80 mM), with a maximum relaxation of 56.22%. The relaxation mechanism was concerned with muscarinic or histamine receptors, KATP and COX/NO/cGMP pathway, independent of the endothelium system (Ayele et al., 2010).
Myrciaria cauliflora
Jabuticaba (Myrciaria cauliflora) hydroalcoholic extract (JH, 0.38–1.92 mg mL−1) resisted K+-/PHE-induced aortic ring contractions, which was weakened by TEA, glibenclamide and 4-AP. Therefore, JH might activates the K+/Ca2+ channels to cause vasodilation rather than SR Ca2+-ATPase or endothelium (Andrade et al., 2016).
Pimenta dioica L.
The aqueous extract of Pimenta dioica (EC50 45 mg kg−1) decreased the blood pressure in SHR which was not related to ion concentration (K+,Na+,Ca2+, and Mg2+), α/β-adrenoceptor and cholinergic receptor (Suárez et al., 2000).
Loranthaceae
Loranthus ferrugineus Roxb.
Loranthus ferrugineus Roxb could be successively fractionated by chloroform, ethyl acetate and n-butanol. n-butanol fraction of LFME (NBF-LFME) produced a significant inhibition of PHE-/K+-induced aortic ring contractions. Moreover, NBF-LFME lowered blood pressure more compared with the other fractions in normal rats. Thus, the effects of LFME were attributed to content of terpenoids in the n-butanol fraction (Ameer O. et al., 2010).
Agelanthus dodoneifolius
The ethanolic extract of Agelanthus dodoneifolius (AD, 0.01–10 mg kg−1, i,v.) could decrease the systolic and diastolic blood pressure in normotensive rats but not heart rate. Fruther, the extract was divided into 14 fractions (F1-F14). F4, contained most of the dihydropyranone dodoneine, produced the most effective activation (ED50 160 μg mL−1) in NE-induced aortic rings contractions and reduced systolic and diastolic blood pressure by 56.9 and 81.6%, respectively in SHR (Ouedraogo et al., 2011). But the relationship between hypotensive activity and vasodilation of AD needs further investigation in the future.
Moraceae
Morus bombycis Koidzumi
The ethanol extract of Morus bombycis koidzumi (MBK) exhibited vasodilatory effect (IC50 3.9 μg mL−1) which was abolished by L-NAME or endothelial removal in rat aortas. Moreover, MBK extract (10, 30 and 100 mg kg−1) dose-dependently reduced SBP and attenuated liver lipid peroxidation and DNA-damage in SHR (Oh et al., 2007a). Therefore, the hypotensive activity of MBK is closely related to vasodilation, but the mechanism of vasodilation needs more exploration.
Morus alba L.
The major components of Mulberry leaves (ML) are polyphenols, flavonoids, carbohydrates, proteins and lipids. Researchers have found that ML could produce vasorelaxation of aortas treated by high K+ (60 mM) or PHE (1 μM) in arteries, which was abolished by ruthenium red (Xia et al., 2007). But mechanistic studies are notably imperfect such as endothelial cells and ion channels.
Ficus sycomorus L.
The extracts of Ficus sycomorus leaves (decoction, macerated and ethanol extract) exhibited a significant vasodilation in rat aortas (IC50 1.27, 0.38, and 0.13 mg mL−1, respectively). But the effect of extract was inhibited by L-NAME except for ethanol extract (Ramdé-Tiendrébéogo et al., 2014). Thus, the vasodilatory potential of the extracts needs further exploration in the future.
Humulus lupulus L.
The extracts of Humulus lupulus (HL, 10−9–10−2 g L−1) inhibited NE (0.1 μM)-induced arterial ring contractions in sham-ovariectomised female rats. The vasorelaxation was strongly abolished by L-NAME (100 μM), indomethacin (10 μM) and thapsigargin (100 μM). The data suggested that vasodilation of HL is concerned with regulation of eNOS/COX levels and inhibition of Ca2+ channel (Figard et al., 2008).
Other Sources
Sesamum indicum L.
The petroleum ether soluble fraction of the root extract of Sesamum indicum L (SIL, 180 μg mL−1) significantly inhibited PHE (2 μM)-/K+ (80 mM)-induced contractions (98.13 and 70.19%, respectively) in rat aortas. Additionally, which was abolished by L-NAME (300 μM) or methylene blue (10 μM) rather than propranolol (10 μM), atropine (1 μM), indomethacin (10 μM) or glibenclamide (10 μM). These results revealed that the vasodilation of SIL is chiefly mediated by endothelium-dependent pathway (Suresh Kumar et al., 2008).
Hibiscus sabdariffa L.
The methanolic extract of the calyces of Hibiscus sabdariffa L (HS) inhibited high K+ (80 mM)-/PHE (1 μM)-induced vasoconstriction in SHR. Meanwhile, the activity of HS was eliminated by atropine (1 μM), L-NAME (10 μM) or methylene blue (10 μM) except for indomethacin (10 μM). These results indicated that the effects of HS are likely mediated by the NO/cGMP pathway or Ca2+ channels (Ajay et al., 2007).
Jasminum Spp.
The ethanol extract of Jasmine flower (JF) caused a concentration-dependent relaxation in endothelium-intact rings treated by PHE (10 μM)/K+ (60 mM), which was decreased by L-NAME (3 mM), BaCl2 (1 mM), 4-AP (5 mM) and TEA (1 mM), expect for indomethacin (10 μM), and glibenclamide (10 μM). Additionally, JF inhibited the contraction of PHE after endothelium removal in Ca2+-free medium. The activity of JF was concerned with the NO levels or K+ channels (Yin et al., 2014).
Hancornia speciosa Gomes.
The extract of leaves from Hancornia speciosa Gomes (HSG) produced vasodilatation (pIC50 5.6). This effect was suppressed by endothelial removal, L-NAME (100 μM), indomethacin (10 μM) or wortmannin (0.3 μM). In conclusion, HSG induced vasodilation in rat aortas possibly through PI3K activation (Ferreira et al., 2007).
Pseuderanthemum palatiferum
Water extracts from fresh leaves of Pseuderanthemum palatiferum (PP) caused the relaxation of NE-contracted endothelium-intact aorta rings (EC50 81.0 μg mL−1), removal endothelium (EC50 136.4 μg mL−1). Neither L-NAME nor atropine altered the vasodilation effect of the extract. The vasodilatory effect was partially dependent on endothelium rather than muscarinic receptor (Khonsung et al., 2011).
Terminalia superba Engl.et Diels
The aqueous, the methanolic and the methylene chloride extracts from the stem bark of Terminalia superba (TS) induced vasodilation on K+-/PHE-induced contractions in rat aortic rings. In contrast, the effect of TS was endothelium-dependent which was decreased by L-NAME (Tom et al., 2010).
Guazuma ulmifolia Lam.
The procyanidin fraction of Guazuma ulmifolia bark (GUB, 10 mg kg−1) decreased systolic arterial pressure and heart rate in hypertensive rats which was attenuated by L-NAME (31 mg kg−1). GUB reduced the contractions induced by NE (0.1 μM) in the aortic rings of normotensive (IC50 35.3 ng mL−1) or hypertensive rats (IC50101 ng mL−1). This activity was attenuated by endothelial removal or L-NAME (30 μM) but not indomethacin (10 μM) or atropine (10 μM) (Magos et al., 2008). Thus, the potential of GUB is mainly associated with the endothelial rather than the PGI2 pathway.
Nitraria sibirica Pall.
The extract from the fruits of Nitraria sibirica Pall (NSP, 0.1–10 g L−1) produced vasodilation in PHE (1 μM)-/K+ (60 mM)-induced pre-contracted aortic rings which was significantly suppressed by endothelial removal, L-NAME (100 μM), atropine (1 μM) and charybdotoxin (30 nM, K+ channel blocker) plus apamin (30 nM). Furthermore, NSP (i.v. 1, 5, 10, and 20 mg kg−1) induced hypotensive effect in SHR and Wistar rats (Senejoux et al., 2012). But the relationship between the hypotensive activity and the vasodilation needs further exploration.
Persea americana Mill.
The aqueous extract of Persea americana Mill (PAM) abolished the positive inotropic and chronotropic responses induced by NE (10−10–10−5 M)/Ca2+ (5–40 mM) in guinea pig atrial muscle. However, this effect was markedly inhibited by L-NAME (10 μM). Furthermore, PAM (i.v. 25–400 mg kg−1) significant reduced blood pressure and heart rates in normotensive and hypertensive rats (Ojewole et al., 2007).
Stephania abyssinica Walp.
Stephania abyssinica Walp (SA) is used to treat arterial hypertension in west region of Cameroon. A previous study indicated that aqueous (ASAW) and methanol (MSAW) extracts from fresh leaves of S. abyssinica exhibited vasorelaxation by K+-induced contractions (EC50 0.16, 0.35 mg kg−1) in aortic rings. ASAW (EC50, 0.18 mg kg−1) also resisted contracted by PHE in aortic rings which was affected by TEA, glibenclamide, and propranolol but not L-NAME. These results indicated that the vasodilation of ASAW is mediated by Ca2+/KATP channels (Nguelefack et al., 2015).
Fagopyrum esculentum Moench.
The hot-water extract of Fagopyrum esculentum (FE) evoked a significant vasodilation in rat aortic rings contracted by PHE (1 μM, EC50 2.2 mg mL−1) or K+ (50 μM, EC50 1.9 mg mL−1). Moreover, the acidic fraction of FE (EC50 0.25 mg mL−1) had markedly stronger effect which was weakened by endothelial removal or L-NAME (100 μM). These results suggested that acidic partial vasodilation is mediated by the NO/cGMP pathway (Ushida et al., 2008).
Uncariae ramulus et Uncus.
The alcohol extract of Uncariae ramulus et Uncus displayed a vasodilatory effect. The underlying mechanism consists of Ca2+ channel blockage and endothelium-dependent. The alkaloids and tannins might be the main active components. Additionally, Uncaria sinensis hexane extracts (HEUS), ethyl acetate extracts (EAEUS) and methanol extracts (MEUS) had a protective effect against photothrombotic ischaemic injury in mice. HEUS (10, 50, and 100 mg kg−1) also reduced infarct volume and edema compared with EAEUS and MEUS. However, HEUS did not reduce eNOS levels of brain infarction in mice, suggesting that the protective effect of HEUS is primarily concerned with endothelium (Yuzurihara et al., 2002), (Goto et al., 2000).
Capparis aphylla
The extract of Capparis aphylla (CA) could inhibit PHE (1 μM) (EC50 0.1 mg mL−1)/K+ (80 mM) (EC50 1.22 mg mL−1)-induced contractions in rabbit aortic rings, which was abolished by endothelium removal, L-NAME and atropine. Moreover, CA (3–100 mg kg−1, i.v.) reduced mean arterial pressure in normotensive rats, which was partially blocked by atropine (2 mg kg−1) (Shah and Gilani, 2011). Thus, the activity of CA may be associated with activation of muscarinic receptors or eNOS.
Others
The aqueous extract of Rheum undulatum L. (Ru) could alleviate PHE-induced constriction in rat aortic, which was attenuated by endothelial removal, L-NAME, methylene blue and ODQ. These results suggested that activity is mediated by NO/cGMP pathways (Kim H. H. et al., 2004). The aqueous extract of Echinodorus grandiflorus (EG, 0.1–10 mg) significantly induced renal vasodilation in rabbits treated by NE-construction which was attenuated by L-NAME (100 μM) and methylene blue (20 μM). However, its activity was not affected by charydbotoxin (100 nM, Ca2+ blocker) or glibenclamide (3 μM) (Tibiriçá et al., 2007). The extracts of Maytenus ilicifolia (MI) leaves reduced the mean arterial pressure and heart rate in anaesthetised rats which was significantly reduced by L-NAME, methylene blue, ODQ, TEA, 4-AP and glibenclamide except for atropine, propranolol (Crestani et al., 2009). The extract of Globularia alypum (GA) also relieved diastolic PHE (2–4 ng mL−1)-induced mesenteric artery contractions in rats, which was inhibited by endothelial removal or atropine, but not indomethacin (10 μM) or L-NAME (100 μM) (Chokri et al., 2012). The extract of Gmelina arborea hexane leaves (GAE) produced vasodilatory effects in PHE (1 μM)-induced contractions which was reduced by L-NAME (2 μM), indomethacin (2 μM) and glibenclamide (2 μM) (Wansi et al., 2012).
Natural Products With Vasodilation In Vitro and Antihypertensive Activities In Vivo
This section summarized the vasodilation of natural compounds. Some compounds have significant vasodilation (EC50/IC50 < 10 μM), whose mechanism are related to multi-pathways (Supplementary Table S2). The natural products, mainly flavones (30%), were shown in Figure 2B. Importantly, they showed vasodilation mainly by acting on Ca2+ channels and eNOS (Figure 3B).
Additionally, we briefly analysed the structure-activity relationship of the compounds with remarkable functions, as shown in Figure 4. Among the phenolic acids, hexahydrocurcumin and curcumin had the prominent vasodilatory effects. The difference in bioactivity may be related to the number of double bonds. All compounds, specifically flavones, displayed the most excellent potential. To summarise, the vasodilation of these compounds was related to the number and position of double bonds, carbonyls, phenolic hydroxyl groups and methoxy groups. Furthermore, some compounds, such as chrysin glucoside, tilianin, reticuline and hirsutine also exhibited hypotension in vivo (Figure 3B).
Flavones
Apigenin
Apigenin (Ap) was a flavonoid in the Chinese herbal medicine Flos Chrysanthemi that displays anti-hypertensive and anti-inflammatory activities. Ap markedly reduced rat thoracic aorta contractions induced by pyrogallol or acetylcholine (pD2 6.56, 5.31), which was weakened by L-NAME rather than aminoguanidine or indomethacin. Additionally, Ap significantly reduced blood pressure of in hypertension rat by ameliorating NO levels and nitrite urinary excretion (Jin et al., 2009).
α-Naphthoflavone
α-Naphthoflavone (α-NF) induced relaxation in PHE-induced aortas (EC50 0.95 μM), which was significantly inhibited by endothelial removal, L-NAME or methylene blue. In HUVECs, α-NF also activated NO channels which was blocked by SKF 96365 (Ca2+ channel blockers) and Ni2+ (Ca2+ channel blockers). Thus, the vasodilatory activities of α-NF might be mediated by the Ca2+ channels and the NO/cGMP pathway (Cheng et al., 2003).
Formononetin
Formononetin (Fo), as a methoxylated isoflavone, enhanced NO levels or endothelial cell function and also induced vasodilation by K+ pre-contractions in rat aortas (EC50 107.2 μM). This action was reduced by endothelial removal and L-NAME. Moreover, the vasodilatory activity was concerned with the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway (Li et al., 2018).
Puerarin
Puerarin (Pu) is the main isoflavone found in Pueraria lobata, which has been used for CCVDs. Pu (10−10−10−8 M) induced relaxation in PHE-induced aortas through promoting HO function in endothelial removal. Additionally, Pu caused relaxation of aortic rings contracted with NE (1 μM) (EC50 1.1 μM), which was significantly repressed by iberiotoxin (50 nM, BKCa blocker). Pu also alleviated high glucose-induced endothelium-dependent vascular dysfunction in rat aortic rings (Meng et al., 2009), (Sun et al., 2007). Thus, the activities of Pu are concerned with endothelial cells or BKCa.
Hesperetin
Hesperetin (He) was shown to relieve NE (1 μM)-/K+ (60 mM)-induced contractions (IC50 62.8, 62.2 μM). The effects were not inhibited by endothelial removal, glibenclamide (10 mM), TEA (2 mM) or nifedipine (0.1 μM). Moreover, He inhibited calmodulin-activated PDE1 and PDE4 isolated from bovine aortas (IC50 74, 70 μM) (Orallo et al., 2004).
5, 7-Dimethoxyflavone
In Thai traditional medicine, Kaempferia parviflora is used to treat CCVDs, including hypertension, asthma or diarrhoea. 5, 7-dimethoxyflavone (DMF, 1–100 μM), which was the primary component of Kaempferia parviflora, caused relaxations in aortic rings pre-contracted by methoxamine. This effect was abolished by endothelial removal, L-NAME (300 μM), indomethacin (10 μM), TEA (5 mM), glibenclamide (10 μM), 4-AP (1 mM), BaCl2 (10 μM) or ODQ (10 μM). Therefore, DMF-induced relaxation was mediated through the activation of the NO/cGMP/COX pathways and the inhibition of Ca2+ channels (Tep-Areenan et al., 2010).
Eupatorin
Eupatorin (Eu) is the primary component in Orthosiphon stamineus, used in Malaysia to treat hypertension. Eu produced relaxation of aortic rings pre-contracted by PHE in intact endothelium. This effect was reduced by L-NAME (pD2 4.60), methylene blue (pD2 6.05), ODQ (pD2 5.84), indomethacin (pD2 6.27), TEA (pD2 6.09), 4-AP (pD2 6.34), BaCl2 (pD2 6.47), atropine (pD2 6.36) and propranolol (pD2 6.49). Overall, the effect of Eu was related to activation of the NO/SGC/cGMP pathway, regulation of muscarinic/β-adrenergic receptor and inhibition of K+ channels (Yam M. et al., 2016).
Scutellarin
Scutellarin (Sc), obtained from Erigeron breviscapus Hand. Mazz, has been used to treat CCVDs such as hypertension. Sc aroused relaxation in rat aortic rings pre-contracted by NE (1 μM, IC50 7.7 μM), which was not influenced by TEA (10 mM), glibenclamide (10 μM), atropine (100 nM), propranolol (1 μM), indomethacin (10 μM) or L-NAME (100 μM). Additionally, Sc inhibited the increase of intracellular Ca2+ induced by NE and had no effect on phorbol-12, 13-diacetate-induced contractions in Ca2+-free solution (Pan et al., 2008). It was shown that the activity of Sc may be through acting on ion channels rather than endothelial cells.
Daidzein/Daidzin
Daidzein and daidzin were shown to produce relaxation in aortas pre-contracted by U46619 (100 nM) (IC50 20 and 140 μM, respectively), which was weakened by glibenclamide (1 μM), TEA (100 mM), iberiotoxin (100 nM), 4-AP (1 mM) or BaCl2 (100 μM). Daidzein-induced vasodilation of rat cerebral basilar arteries was partially dependent on BKCa channels in VSMCs (Zhang H.-T. et al., 2010). Therefore, the effects of daidzein and daidzein were concerned with inhibition of K+ channels (Sun et al., 2007).
Kaempferol
Kaempferol (Ka) produced relaxation in pulmonary arterial rings pre-contracted by PHE (1 µM) (pD2 5.03), which was not affected by glibenclamide, BaCl2, 4-AP (1 mM), L-NAME or indomethacin. However, this activity was repressed by TEA (10 mM) and ODQ. Therefore, Ka caused vasodilation through blocking BKCa/LTCC or regulating the sGC/PKA pathway (Mahobiya et al., 2018).
Baicalein/Luteolin
Baicalein and luteolin were shown to significantly alleviate insulin resistance (IR)-induced SBP elevation which were inhibited by bisphenol A diglycidyl ether in rats fed fructose for 12 weeks. Meanwhile, they also reduced excessive vasoconstriction of PHE/K+ in IR animals through elevating NO/ROS (reactive oxygen species) levels (Huang et al., 2004).
Genistein
Genistein (4′,5,7-trihydroxyisoflavone) is naturally occurring flavonoid found in the Leguminosae plant. Studies had shown that genistein elicited vasodilatory, anti-thrombotic and anti-atherosclerotic. This vasodilation was closely related to regulation of eNOS/angiotensin levels or inhibition of K+/Ca2+ channels (Sureda et al., 2017).
Others
Hydroxy-2,3,5-trimethoxy-xanthone (HM-1, EC50 1.42 μM), 1-hydroxy-2,3,4,7-tetramethoxy-xanthone (HM-2, EC50 6.00 μM), 1-hydroxy-2,3,4,5-tetramethoxy-xanthone (HM-3, EC50 5.27 μM), 1,7-dihydroxy-2,3,4,5-tetramethoxy-xanthone (HM-4, EC50 3.09 μM), 1,5-dihydroxy-2,3-dimethoxy-xanthone (HM-5, EC50 3.86 μM) and 1,7-dihydroxy-2,3-dimethoxy-xanthone (HM-7, EC50 5.76 μM) caused vasodilation in coronary arteries pre-contracted by 5-hydroxytryptamine (5-HT, 1 μM). Furthermore, removal of the endothelium decreased the vasodilation of HM-1 and HM-7 but did not affect HM-2, HM-3, HM-4 or HM-5 (Wang et al., 2009). The number of methoxy groups (HM 1, HM 2) and the substitution position of hydroxyl groups (HM 5, HM 7) had significant effects. In addition, Floranol (Fo) was shown to induce vasodilation pre-contracted by PHE (0.1 μM) (IC50 19.9 μM), which was not affected by L-NAME or endothelium removal (Lemos et al., 2006).
Alkaloids
Harmaline
Harmaline (Ha) has shown hypotensive activity in vivo, but the mechanism is no clear at present. Researchers had found that Ha (3, 10, and 30 μM) induce relaxation in aortas pre-contracted by NE/K+, which was significantly suppressed by L-NAME, indomethacin, prazosin (α-adrenoreceptors blocker) or diltiazem. Thus, the effect of Ha was related with activation of the prostacyclin or eNOS pathway (Berrougui et al., 2006).
Reticuline
Reticuline (Re) resisted contractions induced by PHE (1 μM) and K+ (80 mM) (IC50 40 and 240 μM, respectively). Additionally, Re (i.v. 5, 10 and 20 mg kg−1) produced hypotensive effect in normotensive rats, which was attenuated by L-NAME (20 mg kg−1) or atropine (2 mg kg−1). These results suggested that Re reduced blood pressure by activating the muscarinic or eNOS receptors, and blocking Ca2+ channels (Dias et al., 2004).
Sinomenine
Sinomenine acutum Rehder has been used in the treatment of rheumatoid arthritis in China and its extract is also found to have vasodilator activity. Sinomenine (Si, 0.1–10 μM), as the mainly compound from Sinomenine acutum Rehder, produced relaxation in aortic rings pre-contracted by PHE (10 nM) or K+ (40 mM) which was attenuated by glibenclamide. Similarly, Si (1–100 μM) also reduced Ca2+ concentration in aortic smooth muscle (A7r5) cells induced by PHE (1 μM) or K+ (40 mM), which was eliminated by glibenclamide. Moreover, Si (i.v. 2.5–10 mg kg−1) decreased SBP in SHR (Lee et al., 2007). Thus, the activity of Si was concerned with activation of KATP.
(+)-Nantenine
(+)-Nantenine (Na), isolated from Nandina domestica, was widely used for the treatment of asthma, uterine bleeding and diabetes. Na could relieve contractions induced by NE (IC50 6.24 μM) or high K+ (60 mM, IC50 5.23 μM) in rat aortic rings, which was not modified by endothelial removal, glibenclamide (10 μM) or TEA (2 mM) (Orallo and Alzueta, 2001). The activity of Si was concerned with acting on KATP or endothelial cells.
Cassiarin A
The leaves of Cassia siamea were used for the treatment of hypertension in traditional folk medicine. Cassiarin A (Ca), isolated from Cassia siamea, was shown to induce relaxation in mesenteric arteries pre-contracted with PHE (1 μM, EC50 6.4 μM), which was significantly reduced by endothelial removal, L-NAME (100 μM), ODQ (10 μM), TEA (1 mM) and iberiotoxin (100 nM) rather than indomethacin (10 mM), glibenclamide (10 mM) and 4-AP (1 mM). These results suggested that Ca activity is mediated by NO and BKCa channels (Matsumoto et al., 2010).
2-Benzyl-5-Hydroxy-6-Methoxy-3,4-Dihydroisoquinolin-1-One
Isoquinolinone alkaloids have antihypertensive, antiarrhythmic and other activities. Researchers have found that 2-Benzyl-5-hydroxy-6-methoxy-3, 4-dihydroisoquinolin-1-one (ZC2) relax mesenteric artery segments pre-contracted by KCl (pEC50 4.56), PHE (pEC50 5.39) or U46619 (pEC50 4.67), which was not modify by endothelial removal or glibenclamide (10 μM). Thus, ZC2 induced vasodilation through inhibiting VOCC and ROCC (Xu et al., 2012).
Geissoschizine Methyl Ether/Hirsutine
Geissoschizine methyl ether (Ge) and hirsutine resisted NE (10 nM)-induced contractions (EC50 0.74, 10.6 μM) which were abolished by endothelial removal or L-NAME (100 μM). Furthermore, hirsutine reduced the SBP and heart rate of SHR (Yuzurihara et al., 2002). These results were suggested that the vasodilatory activity of NE is closely related to endothelial cells.
Piperine
Piperine (Pi), as main compound in Sahatsatara formula, had been demonstrated to have hypotensive effects in L-NAME-induced endothelial dysfunction rats. Moreover, it relaxed the thoracic aorta and has vascular protection effects against hypertension in rats (Booranasubkajorn et al., 2017).
Uncarialin A
Uncaria rhynchophylla Miq. ex Havil has been used to treat hypertension and epilepsy in clinics. Uncarialin A, separated from the hooks of U. rhynchophylla, resisted PHE-induced contractions (IC50 0.18 M) independent of endothelial cells. Additionally, Uncarialin A reduced concentration of Ca2+ in VSMCs by inhibiting the LTCC subunit α-1c (Cav1.2) (Yun et al., 2020).
8-Oxo-9-Dihydromakomakine
8-Oxo-9-dihydromakomakine (Di), separated from the leaves of Aristotelia chilensis, produced dose-dependent relaxation of aortic rings pre-contracted by PHE (1 μM)/KCl (60 mm), which was inhibited by TEA and BaCl2. It was suggested that the activity of Di is related to reduction intracellular Ca2+ concentration by inhibiting KCa or Kir (Cifuentes et al., 2018).
Curine
Curine could inhibit contractions induced by KCl and Bay K8644 in rat aortas and decreased intracellular Ca2+ concentration in VSMCs. The activity was not abolished by 3-isobutyl-1-methylxanthine (phosphodiesterase inhibitor), dibutyryl cyclic AMP (protein kinase A activator) or 8-br-cyclic GMP (protein activator kinase G) (Medeiros et al., 2011). It was indicated that potential of curine is associated with endothelial cells and ion channels.
Phenolic Acids
Ethyl Rosmarinate
Ethyl rosmarinate (Er), as a major component of Rosmarinus officialis and Hyptis suaveolens, has cardioprotective activities. Er produced relaxation pre-contracted by PHE (10 μM) (pD2 4.42) or K+ (80 mM) (pD2 4.56) in endothelium-denuded rings. However, the effect was not abolished by TEA (5 mM), glibenclamide (10 μM), BaCl2 (1 mM) and ODQ (1 μM) except for 4-AP (1 mM). Er also reduced the contractions induced by deoxyepinephrine (10 µM) and caffeine (20 mM) in a Ca2+-free solution and inhibited extracellular Ca2+ influx. Therefore, the vasodilation of Er was mediated by endothelial cells or Kv (Wicha et al., 2015).
Paeonol
Paeonol (Pa) is the main component of the Chinese herbs Paeonia suffruticosa Andr. and Cynanchum paniculatum (Bunge) Kitagawa. Pa displayed anti-ischemia reperfusion injury, antihypertensive, anti-platelet aggregation, scavenges oxygen free radicals, anti-atherosclerosis and anti-VSMCs proliferation activities. Researchers have found that Pa relaxed PHE-induced isolated rat aortic rings (EC50 290 μM). Additionally, Pa significantly inhibited vasoconstriction induced by angiotensin II, prostaglandin F-2α, 5-HT, dopamine, vasopressin and endothelin-1. Moreover, the activity of Pa was not affected by L-NAME, ODQ, propranolol, glibenclamide, TEA and BaCl2 in the rings. Therefore, the vasodilatory effect of Pa was in relationship with regulation of Ca2+ channels (Li et al., 2010).
Hexahydrocurcumin/Curcumin
Curcumin (Cu) was the main active component of the roots of Curcuma longa L. Hexahydrocurcumin (HCC) was a reduction product of Cu and metabolites in mice or humans. HCC has a wide variety of pharmacological effects, including anti-inflammatory, anti-viral, anti-fungal, anti-oxidant and anti-cancer effects. Moreover, HHC relaxed endothelium-intact aortic rings pre-contracted by PHE (10 μM, EC50 3.87 μM) or K+ (80 mM, EC50 95.12 μM). This agent relieved the CaCl2-induced contractions in K+ solutions and also suppressed the contractions induced by PHE/caffeine in Ca2+-free solutions. Additionally, HHC relieved phobal-12-myristate-13-acetate (PMA, activator of PKC) pre-contracted aortic rings (EC50 93.36 μM). Collectively, it was suggested that the effect of HHC is related to endothelium cells, VOCC, ROCC and PKC-mediated Ca2+ channels (Moohammadaree et al., 2015). Cu (pD2 6.83) also relaxed the aortic rings pre-contracted by PHE (1 μM). Further, Cu reversed the vasodilatory dysfunction induced by high glucose (44 mmol/L) by improving HO-1 function in aortic rings. However, the activities were resisted by protoporphyrin IX zinc (1 μM, inhibitor of HO-1) or methylene blue (1 μM) (Sasaki et al., 2003).
Sodium Ferulate/Ferulic Acid
Ferulic acid (Fa) was the main component of Radix Angelicae Sinensis and Rhizoma Chuanxiong. Studies have shown that sodium ferulate (Sf) or Fa displayed anti-platelet aggregative, anti-inflammatory, antioxidative activities. Sf relaxed the aortic rings pre-contracted with PHE/K+ (pD2 2.7 and 2.6, respectively), which was no affected by TEA, glibenclamide, 4-AP and BaCl2. Sf also inhibited contraction in K+/PE/PMA pre-contracted rings in Ca2+-free solution (Chen G. et al., 2009). Moreover, Fa decreased superoxide anion levels in SHR aortas and improved acetylcholine-induced vasodilation in SHR but not in WKY rats (Suzuki et al., 2007). This activity was related to the methoxy modified 3-position in the benzene ring and 2-propylene (Fukuda et al., 2015).
Ellagic Acid
Ellagic acid (Ea), a polyphenolic compound, has anti-hypertensive, anti-diabetic, anti-oxidantive, anti-inflammatory and anti-hyperlipidaemia effects. Ea relaxed the aortic pre-contracted by PHE (pD2 5.60), which was partially abolished by endothelium removal and L-NAME rather than indomethacin. Therefore, the activity of Ea was related to endothelium cells and Ca2+ channels (Yılmaz and Usta, 2013).
Glycosides
Astragaloside IV
Astragalus membranaceus has been used to treat and prevent CCVDs such as haemorrhagic stroke and viral myocarditis. Astragaloside IV (As-IV), the main component of A. membranaceus, has anti-cardiac hypertrophy, anti-inflammatory and anti-oxidant activities. As-IV could antagonise contractions induced by PHE/K+ in aortic rings from normal rats and SHR. Similarly, As-IV attenuated the vasoconstriction induced by angiotensin II or PHE in presence of perivascular adipose tissue (Zhang et al., 2006). This activity of As-IV was abolished by L-NAME or ODQ rather than 7-nitroindazole (neuronal eNOS inhibitor). Therefore, this vasodilatory was associated with blockage of calcium channel, and activation of NO/cGMP pathway (Zhang et al., 2007). Additionally, As-IV (orally, 40–80 mg kg d−1) improved the expression of eNOS, SOD and GSH-Px in the thoracic aorta and decreased ROS levels in a streptozotocin-induced diabetic rat model. It was suggested that As-IV ameliorates endothelial damages in the thoracic aorta of diabetic rats via regulating levels of ROS and calpain-1 (Nie et al., 2019).
Cornuside
Cornuside (Co, 100 μM) relaxed PHE (3 μM) pre-contracted rat aortas, which was inhibited by endothelial removal, L-NAME and ODQ rather than diltiazem (10 μM), TEA (100 μM), glibenclamide (10 μM), indomethacin (1 μM), atropine (1 μM) or propranolol (1 μM). Co also increased cGMP levels in HUVECs, which was inhibited by L-NAME (10 μM) or ODQ (1 μM). Therefore, Co displayed vasodilatory activity through activating the NO/cGMP pathway (Kang et al., 2007).
Chrysin Glucoside
Chrysin glucoside (Cg) could inhibit NE (1 μM)-induced contractions (IC50 52 μM) in rat aortas, which was inhibited by L-NAME and endothelial removal. Cg (2.5 mg kg−1) significantly increased urine flow, glomerular filtration and electrolyte excretion (Na+/K+) in rats. Additionally, Cg (i.v. 10 mg kg−1) caused an immediate decrease in the mean arterial blood pressure of the anaesthetised rats, which was inhibited by L-NAME (Kang et al., 2007).
Tilianin
Agastache mexicana is used as an antihypertensive drug in Mexico. Tilianin (Ti, 0.002–933 μM), derived from A. mexicana, produced significant relaxation in aortic rings pre-contracted by NE (0.1 μM) or serotonin (5-HT, 100 μM). This relaxation was markedly abolished by endothelium removal, L-NAME (10 μM), TEA (5 mM), 4-AP (0.1 μM) or ODQ (1 μM) but not by indomethacin (10 μM) or atropine (1 μM). Additionally, Ti (50 mg kg−1, orally) significantly reduced systolic and diastolic blood pressure in an SHR (Kang et al., 2007). The vasodilatory activity of Ti was concerned with endothelial cells or K+ channels.
Jujuboside B
Zizyphi Spinosi Semen (ZSS) has protective effects against myocardial ischemic injury and hypertension. Jujuboside B (Ju), obtained from ZSS, reduced the tension of rat aorta with intact endothelium. However, the vasodilatory activity of Ju was attenuated by L-NAME, KN93, EGTA, SKF96365, iberiotoxin and glibenclamide rather than indometacin. Therefore, Ju displays its vasodilation through promoting eNOS levels and inhibiting K+/Ca2+ channels (Zhao et al., 2016).
Glucosyl hesperidin
Researchers have found that hesperidin possessed anti-oxidant, anti-hypertensive. Moreover, glucosyl hesperidin (Gh) has antihypertensive effects and improves lipid metabolism. Gh (50 mg kg d−1, orally) could reduce blood pressure in SHR and enhance endothelium-dependent vasodilation induced by acetylcholine. Additionally, Gh improved endothelial function by inhibiting the expression of nicotinamide adenine dinucleotide phosphate oxidase in SHR aorta (Yamamoto et al., 2008).
Terpenoids
Ent-Trachyloban-14, 15-Dione
Croton zambesicus is used to treat hypertension in Benin. Ent-trachyloban-14, 15-dione (DT10), separated from Croton zambesicus, relieved K+-induced (100 mM) contractions in rat aortas (−logIC50 6.3), which was significantly decreased by L-NAME (−logIC50 5.7). Furthermore, DT10 inhibited K+-evoked contractions in aorta rings and SH-SY5Y (human neuroblastoma cells) (Martinsen et al., 2010).
Sesquiterpenoids
Zerumbone resisted aortas contracted by K+ (60 mM, IC50 16 μM) (Fusi et al., 2015). Sesquiterpenoids [curcumanes A, B, C, and (±) D], which were isolated from Curcuma longa, also have significant vasodilation. They could significant resist KCl-induced aortic contractions in rats (EC50 2.40, 0.91, 6.61, 14.56, and 16.03 μM, respectively) and curcumanes A, B, and C also inhibited PHE-induced aortic ring contractions in rats (EC50 3.37, 0.83, and 4.26 μM, respectively). Additionally, curcumane C promoted the growth of human umbilical vein endothelial cells, which was inhibited by L-NAME. Curcumanes A and B produced vasodilation through regulation of VDCC and ROCC (Qiao et al., 2019: Liu et al., 2019). Therefore, sesquiterpenoids with significant activities should be paid more attention in the prevention and treatment of CCVDs.
Coumarin
Imperatorin/Isoimperatorin
Imperatorin and isoimperatorin were shown to relax rat aorta contractions by PHE. The effect of imperatorin was significantly stronger than that of isoimperatorin. However, this activity was inhibited by endothelial removal and L-NAME (Nie et al., 2009).
(+)-Cis-4′-O-Acetyl-3′-O-Angeloylkhellactone
(+)-cis-4′-O-acetyl-3′-O-angeloylkhellactone (Al) relaxed rat aortas pre-contracted by PHE (1 μM, EC50 17.8 μM) which was diminished by endothelial removal, L-NAME (100 μM) or methylene blue (30 μM). However, this function was not eliminated by indomethacin (30 μM), atropine (0.1 μM), triprolidine (10 μM), TEA (10 mM) and propranolol (3 μM). These results suggested that activity is mediated by Ca2+ channels and the NO/cGMP pathways (Lee et al., 2002).
Others
Perillaldehyde
Perillaldehyde (Pe), is major compound from aqueous extract of Perilla leaves, improves NO levels in VSMCs. Pe (0.01–1 mM) also resisted aorta contraction by prostaglandin F-2α or NE, which was weakened by L-NAME, endothelial removal, propranolol, theophylline, TEA or glibenclamide. Therefore, the vasodilatory effect of Pe was concerned with blockage of Ca2+ channel (Takagi et al., 2005). Further, Pe (150 mg kg−1) reduced aortic atherosclerotic plaques, improved endothelial function, increased tetrahydrobiopterin and NO levels in carotid arteries of mice and rats fed high-fat diet (Yu and Liu 2018).
Cinnamaldehyde
Cinnamaldehyde (Ci), separated from Cinnamomi Cortex, has anti-platelet aggregation by regulating arachidonic acid. Ci (1–1,000 μM) also resisted rat aortas contracted by prostaglandin F-2α (5 μM), NE (0.1 μM) or K+ (60 mM). The activity was not inhibited by indomethacin, propranolol (10 μM), theophylline (100 mM, phosphodiesterase inhibitor), TEA (1 mM) or glibenclamide, instead of L-NAME (100 mM) (Yanaga et al., 2006). Further, Ci inhibited LTCC in mice ventricular myocytes and mesenteric artery SMCs (Buglak et at., 2018), (Alvarez-Collazo et al., 2014). The vasodilatory effect was related to endothelium cells and the Ca2+ channels.
Phthalides
Ligusticum chuanxiong is used for CCVDs to promote the circulation of blood and removed stasis in TCM. Ligustilide (Li), main phthalides of L. chuanxiong, was used to treat CCVDs. Li was shown to relax rat mesenteric arteries pre-treated by KCl, CaCl2, NA or 5-hydroxytryptamine (5-HT). But the activates was not affect by propranolol, glibenclamide, TEA and BaCl2. It was indicated that Li induces vasodilation through regulating VOCC and ROCC rather than endothelial cells (Cao et al., 2006). Further, the recent research found that the activity of phthalide dimers was generally superior to that of monomeric phthalides. Phthalides dimers, such as Chuanxiongdiolides R4 and R6, inhibited KCl-induced (60 mM) vasoconstriction. Moreover, Chuanxiongdiolides R4 and R6 also significantly inhibited the LTCC subunit α-1c (Cav1.2) (Tang et al., 2020).
Isosteviol
Isosteviol (Is, 10 μM) significantly relaxed the vasopressin (10−8 M)-/K+ (100 mM)-induced vasoconstriction in aortic rings, which was resisted by apamin and glibenclamide rather than K+ (30 mM). Therefore, this effect might be attributive to inhibition of KATP or SKCa (Wong et al., 2004).
Piceatannol
Piceatannol (Pi) caused relaxation in aortas pre-contracted by PHE (EC50 2.4 μM). This effect was reduced by endothelial removal, L-NAME, methylene blue, ODQ, 4-AP and TEA rather than indomethacin, atropine, propranolol, nifedipine, BaCl2 or glibenclamide. Moreover, charybdotoxin and iberia toxin (BKCa channel blockers) could also reduce the activity of Pi. Therefore, this vasodilation of Pi may be related to the activation of BKCa and NO pathway (Oh et al., 2007b).
Eudesmin
Eudesmin (Eu) induced relaxation of rat aortic pre-contracted by PHE (IC50 10.69 μg ml−1), which was blocked by endothelial removal, L-NAME, ODQ, indomethacin and diphenhydramine (type 1 histamine receptor), instead of atropine, propranolol and glibenclamide. This effect was mediated by regulation of histamine receptors and NO/prostaglandin pathways (Raimundo et al., 2009).
Brazilin
Studies have found that brazilin (Br) has anti-diabetic, anti-inflammatory, anti-asthmatic, anti-platelet aggregation, anti-tumour and anti-oxidant. Further, Br resisted the NE/K+-induced contraction of aortic rings (EC50 83.51 and 79.79 μM, respectively), which was significantly attenuated by endothelium removal, L-NAME, methylene blue or indomethacin. Thus, the activity of Br was related to inhibition of ERK1/2 phosphorylation or blockage of Ca2+ channels (Yan et al., 2015).
Tanshinone IIA
Tanshinone IIA (Tan IIA) could reduce infarct area and blood pressure in hamsters. Moreover, Tan IIA caused endothelium-dependent relaxation, which was blocked by oestrogen receptor antagonist ICI 182 and 780. Therefore, the activity of Tan IIA was related to oestrogen receptor, NO channels and ERK1/2 phosphorylation (Fan et al., 2011).
Sodium Danshensu
Sodium danshensu (So) has anticoagulation and arrhythmia resistance on myocardial ischaemia-reperfusion injury. Moreover, So (1–3 g L−1) inhibited the PHE-/K+induced contraction of rat aortic, which was partially antagonised by TEA and apamin (SKCa blocker). However, the vasodilatory effect was not abolished by iberiotoxin (BKCa blocker), BaCl2 and glibencalmide (Zhang N. et al., 2010). Thus, the vasodilation of So was concerned with SKCa rather than BKCa, Kir and KATP.
Caracasanamide
Caracasanamide (i.v.) was shown to reduce blood pressure and increase cardiac muscle strength, respiratory rate and tidal volume in rats. Additionally, it also induced vasodilation in rats through acting on cardiac β1-adrenergic receptors (Delle Monache et al., 1993).
Others
Other lignans (saucerneol, saucerneol D and machilin D) exhibited vasodilation in rat aortic treated by PHE (10 μM, EC50 2.2, 12.7 and 17.8 μM, respectively). The activities were significantly inhibited by L-NAME or endothelial removal. Additionally, saucerneol and sacerneol D were shown to significantly reduce left ventricular pressure (Oh et al., 2008).
Clinical Application
We also summarized the clinical applications of TMPs and natural products with vasodilatory activies (Figure 5). TMPs and natural products, listed in Figure 5, were mainly used in the treatment of diabetes, hypertension, hyperlipidaemia, and some encephalopathy, which demonstrated the relationship between the vasodilatory activity and the treatment of CCVDs. Some TMPs, such as M. Vulgare and Nigella sativa, etc, combined with conventional therapeutics improved the efficacy and tolerability of the drugs, and reduced their adverse reactions and side effects. Other TMPs, such as such as Hibiscus sabdariffa L, showed obviously antihypertensive effect. However, the detailed analysis of the drug’s underlying mechanism was not performed. In this present study, the role of TMPs and natural products in the prevention and treatment of CCVDs was positive and encouraging, but there were serious limitations in the druggability studies of this field due to lack of researches on toxicology and pharmacokinetics of TMPs and natural products with vasodilatory actives.
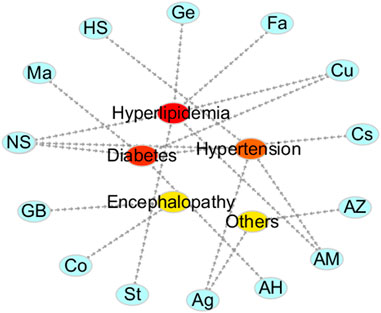
FIGURE 5. Clinical application of some TMPs and natural products. The vasodilating TMPs and natural products are mainly used in the treatment of diabetes, hypertension, hyperlipidemia and some encephalopathy in the clinic.
Diabetes
In addition to the medical treatment with glbenclamide, the patients suffering from Ⅱ diabetes were treated with M. vulgare (Ma, 3 weeks). The results showed that Ma could reduce levels of blood glucose (0.64%), cholesterol (4.16%) and TG (5.78%) in patients. C. obtusifolia lowered blood glucose (15.25%), cholesterol (14.62%) and TG (42.0%), respectively (Herrera-Arellano et al., 2004a). Therefore, it showed significant differences between the hypoglycemic effect produced by C. obtusifolia and M. vulgare. The type II diabetes treatment with curcumin (Cu, 1,000 mg d−1) plus piperine (absorption enhancer, 10 mg d−1) or placebo plus standard care for 12 weeks, significantly reduced the levels of TC (−21.86 vs.−17.06), non-HDL-C (−23.42 vs.−16.84) and lipoprotein (1.50 vs. −0.34), and increased the levels of HDL-C (1.56 vs.−0.22) as compared with the placebo group (Panahi et al., 2017). Nigella sativa (NS) (Kaatabi et al., 2015), Artemisia herba alba asso (AH) (Al-Waili 1986) and Coriandrum sativum (Cs) (Waheed et al., 2006) can significantly reduce fasting blood glucose in type Ⅱ diabetes. Furthermore, NS could significantly reduce fasting blood glucose, glycated hemoglobin and glutathione, but also improve the levels of total antioxidant capacity, SOD, catalase and glutathione.
Hypertension
NS oil (5 mL d−1, 8 weeks) significantly reduced diastolic and SBP in healthy volunteers without adverse effects. But there was no effect on levels of HAA, alanine aminotransferase, alkaline phosphatase, and creatinine (Fallah Huseini et al., 2013). Hibiscus sabdariffa L (HS, 9.6 mg d−1) also significantly reduced SBP in 30–80 year old hypertension patients, and there was no significant difference with captopril (50 mg d−1) (Herrera-Arellano et al., 2004b). Furthermore, aged garlic (Ag, 480/960 mg d−1, 12 weeks) significantly reduced SBP in hypertension patients (Ried et al., 2013).
Hyperlipidemia
NS (Sabzghabaee et al., 2012) (2 g d−1, 4 weeks) and Strawberries (Basu et al., 2014) (St, 5 or 50 g d−1, 12 weeks) significantly reduced serum malondialdehyde, TC, TG, LDL-C or HDL-C in adults. Ferulic acid (Fa, 1 g d−1, 6 weeks) could reduce levels of TC, TG, LDL-C or HDL-C in hyperlipidemia patients (Bumrungpert et al., 2018). AM (300 mg d−1, 2 months) and Cu (1 g d−1, 8 weeks) reduced levels of TC, LDL-C, HDL-C, TG and non- HDL-C in metabolic syndrome (MS) patients (Sikora et al., 2012). Additionally, Genistein (Ge, 54 mg d−1, 6 months) improved brachial artery vasodilation in postmenopausal women with MS, while lowering levels of TC, TG, and homocysteine (Irace et al., 2013). Furthermore, Cu (500 mg·d-1, 10 weeks) reduced body mass index, waist circumference, hip circumference and HDL-C levels in obese girls, but there was no significant difference with control groups (Panahi et al., 2014).
Encephalopathy
Ginkgo biloba extract (GB, 120 mg d−1, 52 weeks) improved cognitive performance in dementia patients and improved ADAS-Cog, GERR and CGIC scores in patients. In addition, GB leaves also improved the prognosis with acute ischemic stroke and increased the NIHSS score in patients. GB leaves (240 mg d−1, 3 months) could improve memory and attention in senile Alzheimer’s disease (Bars et al., 1997; Maurer et al., 1997; Oskouei et al., 2013). Coriander (Co, 4 weeks) also relieved migraine compared with control group by the Akaike criteria (Mansouri et al., 2015).
Conclusion
This review discussed TMPs and natural products with vasodilation in vitro. Their possible mechanisms and clinical application were also summarised. Notably, TMPs with vasodilation are mainly from Compositae while natural products are flavonoids. The vasodilatory function of TMPs and natural products is mainly attributed to regulation of eNOS or Ca2+ channels. Further, we analysed briefly the structure-activity relationship of the compounds with significant vasodilatory effects. The vasodilation of the natural compounds was related to the number and position of double bonds, carbonyls, phenolic hydroxyl groups, and methoxy groups. Overall, these evidences suggested that TMPs and natural products are emerging alternatives for the prevention or treatment of CCVDs such as hypertension.
It was foreseeable that they will receive more attention in the future, although there were still some limitations based on literatures. Firstly, the current thoracic aortic models were usually used as the main mode to investigate the vasodilation, whereas different vessels, such as cerebral artery, abdominal aorta and mesenteric artery, have not sufficiently explored (Pfaltzgraff and Bader 2015). Secondly, the various channel blockers, such as TEA, BaCl2, and 4-AP had been applied to block K+ channels. However, the relationship between the vasodilation of TMPs and natural products and other, such as KCNQ, TRPC, and TACC, had not been adequately investigated. Therefore, the underlying mechanisms, especially in relationship with various types of ion channels such as KCa, should be further explored. Thirdly, the clinical researches on the vasodilatory activity of TMPs and natural products is severely insufficient in this regard, although they exhibited remarkable potential in animal models. In addition, current the clinical studies on TMPs and natural products also have some deficiencies. For example, mechanisms of action usually remain unknown and only a small number of patients had been reported in almost all the related literatures. Finally, the application of TMPs is troublesome owing to difficulties in source identification, active ingredients, quality standard and mechanism study. Generally, natural products always maintain unsatisfactory druggability owing to their poor oral bioavailability, low plasma concentrations and so on. The ingested natural products was either excreted unabsorbably or metabolized rapidly after absorption, such as apigenin (Tang et al., 2017), sinomenine (Chen W. et al., 2009) and Kaempferol (Calderón-Montaño et al., 2011), etc.
However, developments in technologies such as metabolomics, proteomics and genomics will facilitate the application of TMPs and natural products in the treatment of CCVDs (Harvey et al., 2015). The bioavailability of natural products will be greatly improved by amelioration of hydrophilicity or alteration of administration modes. For instance, the curcumin nanoparticles with higher hydrophilicity achieved by loading into sophorolipid micelles had an appreciably higher bioavailability than that of free curcumin crystals (Peng et al., 2018). In addition, compared to oral administration, nasal administered paeonol was absorbed rapidly in rats (Adki and Kulkarni, 2020). The bioavailability of intravenous injection of cinnamaldehyde was also superior to that of oral administration (Zhu et al., 2017), etc.
In brief, we are still optimistic about the prospect of TMPs and natural products. TMPs will be used as alternative drugs and nutritional supplements, while natural products will be considered as potential therapies for CCVDs in the future. This study provides comprehensive and valuable references for the prevention and treatment of hypertension and CCVDs and sheds light on the further studies in this regard. In the next few years, it is necessary to investigate absorption, distribution, metabolism, and excretion of TMPs and natural products with vasodilation in vivo. Also, the activities of the major metabolites of these natural resources should be concerned.
Author Contributions
FT contributed to the drafting of the manuscript. Others were involved in searching, screening the search results, translation, and data collection. Y-ZT, HA, and CP obtained funding, designed, conceived and supervised process, and revised the manuscript. All the authors have read and approved the final manuscript.
Funding
This work was financially supported by the Program for the National Natural Science Foundation of China (Nos. 81703693 and U19A2010), Sichuan Science and Technology Program (No. 2021JDJQ0040), China Postdoctoral Science Foundation (No. 2019M653363), and Xinglin Scholar Research Premotion Project of Chengdu University of TCM (No. QNXZ2018009).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.627458/full#supplementary-material.
Abbreviations
SBP, systolic blood pressure; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; non-HDL-C, non-high-density lipoprotein cholesterol; MS, metabolic syndrome; HAA, horizontal aspartate aminotransferase; ADAS-Cog, Alzheimer’s disease assessment scale-cognitive subscale; GERRI, geriatric evaluation by relative’s rating instrument; CGIC, clinical global impression of change; NIHSS, National Institutes of Health Stroke Scale.
References
Adaramoye, O. A., Anjos, R. M., Almeida, M. M., Veras, R. C., Silvia, D. F., Oliveira, F. A., et al. (2009). Hypotensive and endothelium-independent vasorelaxant effects of methanolic extract from Curcuma longa L. in rats. J. Ethnopharmacol. 124, 457–462. doi:10.1016/j.jep.2009.05.021
Adki, K. M., and Kulkarni, Y. A. (2020). Chemistry, pharmacokinetics, pharmacology and recent novel drug delivery systems of paeonol. Life Sci. 250, 117544. doi:10.1016/j.lfs.2020.117544
Ajay, M., Chai, H. J., Mustafa, A., Gilani, A., and Mustafa, M. (2007). Mechanisms of the anti-hypertensive effect of Hibiscus sabdariffa L. calyces. J. Ethnopharmacol. 109, 388–393. doi:10.1016/j.jep.2006.08.005
Alexander, S. P. H., and Kelly, E., Mathie, A., Peters, J. A., Veale, E. L., Armstrong, J. F., et al. (2019a) THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: introduction and other protein targets. J. pharmacol. 176(Suppl. 1), S1–S20. doi:10.1111/bph.14747
Alexander, S. P. H., Mathie, A., Peters, J. A., Veale, E. L., Striessnig, J., Kelly, , et al. (2019b) The concise guide to pharmacology 2019/20: ion channels. J. Pharmacol. 176(Suppl. 1), S142–S228. doi:10.1111/bph.14749
Alvarez-Collazo, J., Alonso-Carbajo, L., López-Medina, A. I., Alpizar, Y. A., Tajada, S., Nilius, B., et al. (2014). Cinnamaldehyde inhibits L-type calcium channels in mouse ventricular cardiomyocytes and vascular smooth muscle cells. Pflugers Arch. Eur. J. Physiol. 466, 2089–2099. doi:10.1007/s00424-014-1472-8
Al-Waili, N. (1986). Treatment of diabetes mellitus by Artemisia herba-alba extract: preliminary study. Clin. Exp. Pharmacol. Physiol. 13, 569–574. doi:10.1111/j.1440-1681.1986.tb00940.x
Ameer, O., Salman, I., Siddiqui, M., Yam, M., Sriramaneni, R., Sadikun, A., et al. (2010). Cardiovascular activity of the n-butanol fraction of the methanol extract of Loranthus ferrugineus Roxb. Braz. J. Med. Biol. Res. 43, 186–194. doi:10.1590/s0100-879x2010005000002
Ameer, O. Z., Salman, I. M., Siddiqui, M. J. A., Yam, M. F., Sriramaneni, R. N., Mohamed, A. J., et al. (2010). Pharmacological mechanisms underlying the vascular activities of Loranthus serrugineus Roxb. in rat thoracic aorta. J. Ethnopharmacol. 127, 19–25. doi:10.1016/j.jep.2009.09.057
Andrade, D., Borges, L., Torres, I., Conceição, E., and Rocha, M. (2016). Jabuticaba-induced endothelium-independent vasodilating effect on isolated arteries. Arq. Bras. Cardiol. 107, 223–229. doi:10.5935/abc.20160118
Ayele, Y., Urga, K., and Engidawork, E. (2010). Evaluation of in vivo antihypertensive and in vitro vasodepressor activities of the leaf extract of Syzygium guineense (Willd) D.C. Phytother. Res. 24, 1457–1462. doi:10.1002/ptr.3141
Aziz, N., Mehmood, M., Siddiqi, H., Mandukhail, S., Sadiq, F., Maan, W., et al. (2009). Antihypertensive, antidyslipidemic and endothelial modulating activities of Orchis mascula. Hypertens. Res. 32, 997–1003. doi:10.1038/hr.2009.148
Barros, F., de la Peña, P., Domínguez, P., Sierra, M. L., and Pardo, L. A. (2020). The EAG voltage-dependent K(+) channel subfamily: similarities and differences in structural organization and gating. Front. Pharmacol. 11, 411. doi:10.3389/fphar.2020.00411
Bars, L. P. L., Katz, M. M., Berman, N., Itil, T. M., Freedman, A. M., et al. (1997). A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. JAMA 278, 1327–1332. doi:10.1001/jama.278.16.1327
Basu, A., Betts, N. M., Nguyen, A., Newman, E. D., Fu, D., and Lyons, T. J. (2014). Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J. Nutr. 144, 830–837. doi:10.3945/jn.113.188169
Beech, D. J. (2005). Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clin. Exp. Pharmacol. Physiol. 32, 597–603. doi:10.1111/j.1440-1681.2005.04251.x
Berrougui, H., Martín-Cordero, C., Khalil, A., Hmamouchi, M., Ettaib, A., Marhuenda, E., et al. (2006). Vasorelaxant effects of harmine and harmaline extracted from Peganum harmala L. seeds in isolated rat aorta. Pharmacol. Res. 54, 150–157. doi:10.1016/j.phrs.2006.04.001
Bian, K., Doursout, M. F., and Murad, F. (2008). Vascular system: role of nitric oxide in cardiovascular diseases. J. Clin. Hypertens. 10, 304–310. doi:10.1111/j.1751-7176.2008.06632.x
Booranasubkajorn, S., Huabprasert, S., Wattanarangsan, J., Chotitham, P., Jutasompakorn, P., Laohapand, T., et al. (2017). Vasculoprotective and vasodilatation effects of herbal formula (Sahatsatara) and piperine in spontaneously hypertensive rats. Phytomedicine 24, 148–156. doi:10.1016/j.phymed.2016.11.013
Brenner, R., Peréz, G. J., Bonev, A. D., Eckman, D. M., Kosek, J. C., Wiler, S. W., et al. (2000). Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407, 870–876. doi:10.1038/35038011
Briguglio, M., Hrelia, S., Malaguti, M., Serpe, L., Canaparo, R., Dell’Osso, B., et al. (2018) Food bioactive compounds and their interference in drug pharmacokinetic/pharmacodynamic profiles. Pharmaceutics 10, 277. doi:10.3390/pharmaceutics10040277
Brixius, K., Willms, S., Napp, A., Tossios, P., Ladage, D., Bloch, W., et al. (2006). Crataegus special extract WS 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc. Drugs Ther. 20, 177–184. doi:10.1007/s10557-006-8723-7
Brozovich, F. V., Nicholson, C. J., Degen, C. V., Gao, Y. Z., Aggarwal, M., and Morgan, K. G. (2016). Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol. Rev. 68, 476–532. doi:10.1124/pr.115.010652
Buglak, N. E., Jiang, W., and Bahnson, E. S. (2018). Cinnamic aldehyde inhibits vascular smoothmuscle cell proliferation and neointimal hyperplasia in Zucker diabetic fatty rats. Redox Biol. 19, 166–178. doi:10.1016/j.redox.2018.08.013
Bumrungpert, A., Lilitchan, S., Tuntipopipat, S., Tirawanchai, N., and Komindr, S. (2018). Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: a randomized, double-blind, placebo-controlled clinical trial. Nutrients 10, 713. doi:10.3390/nu10060713
Calderón-Montaño, J. M., Burgos-Morón, E., Pérez-Guerrero, C., and López-Lázaro, M. (2011). A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 11, 298–344. doi:10.2174/138955711795305335
Cao, Y. X., Zhang, W., He, J. Y., He, L. C., and Xu, C. B. (2006). Ligustilide induces vasodilatation via inhibiting voltage dependent calcium channel and receptor-mediated Ca2+ influx and release. Vasc. Pharmacol. 45, 171–176. doi:10.1016/j.vph.2006.05.004
Chen, J., Tsai, S., and Chen, H. (1999). Welsh onion (Allium fstulosum L.) extracts alter vascular responses in rat aortae. J. Cardiovasc. Pharmacol. 33, 515–520. doi:10.1097/00005344-199904000-00001
Chen, G., Ye, Y., Li, L., Yang, Y., Qian, A., and Hu, S. (2009). Endothelium-independent vasorelaxant effect of sodium ferulate on rat thoracic aorta. Life Sci. 84, 81–88. doi:10.1016/j.lfs.2008.11.003
Chen, W., Zhou, Y., Kang, J., Li, Q., and Feng, X. (2009). Study on pharmacokinetics and absolute bioavailability of sinomenine in beagle dogs. Zhongguo Zhong Yao Za Zhi 34, 468–471.
Chen, X., Sooch, G., Demaree, I. S., White, F. A., and Obukhov, A. G. (2020) Transient receptor potential canonical (TRPC) channels: then and now. Cells 9, 1983. doi:10.3390/cells9091983
Cheng, Y., Li, C., Lee, C., and Kang, J. (2003). Alpha-naphthoflavone induces vasorelaxation through the induction of extracellular calcium influx and NO formation in endothelium. Naunyn. Schmiedebergs. Arch. Pharmacol. 368, 377–385. doi:10.1007/s00210-003-0820-6
Ch’ng, Y., Loh, Y., Tan, C., Ahmad, M., Asmawi, M., Wan Omar, W., et al. (2017). Vasorelaxant properties of Vernonia amygdalina ethanol extract and its possible mechanism. Pharm. Biol. 55, 2083–2094. doi:10.1080/13880209.2017.1357735
Chokri, A., El Abida, K., Zegzouti, Y., and Ben Cheikh, R. (2012). Endothelium-dependent vascular relaxation induced by Globularia alypum extract is mediated by EDHF in perfused rat mesenteric arterial bed. Can. J. Physiol. Pharmacol. 90, 607–616. doi:10.1139/y2012-035
Cifuentes, F., Palacios, J., Paredes, A., Nwokocha, C. R., and Paz, C. (2018). 8-oxo-9-dihydromakomakine isolated from Aristotelia chilensis induces vasodilation in rat aorta: role of the extracellular calcium influx. Molecules 23, 3050. doi:10.3390/molecules23113050
Cole, W. C., and Welsh, D. G. (2011). Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch. Biochem. Biophys. 510, 160–173. doi:10.1016/j.abb.2011.02.024
Collaboration, P. S. (2002). Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913. doi:10.1016/s0140-6736(02)11911-8
Crestani, S., Rattmann, Y., Cipriani, T., de Souza, L., Iacomini, M., Kassuya, C., et al. (2009). A potent and nitric oxide-dependent hypotensive effect induced in rats by semi-purified fractions from Maytenus ilicifolia. Vascul. Pharmacol. 51, 57–63. doi:10.1016/j.vph.2009.02.005
de Moura, R., Emiliano, A., de Carvalho, L., Souza, M., Guedes, D., Tano, T., et al. (2005). Antihypertensive and endothelium-dependent vasodilator effects of Alpinia zerumbet, a medicinal plant. J. Cardiovasc. Pharmacol. 46, 288–294. doi:10.1097/01.fjc.0000175239.26326.47
Delfan, B., Saki, K., Bahmani, M., Rangsaz, N., Delfan, M., Mohseni, N., et al. (2014). A study on anti-diabetic and anti-hypertension herbs used in Lorestan province, Iran. J. HerbMed Pharmacol. 3, 71–76.
Delle Monache, G., Botta, B., Delle Monache, F., Espinal, R., De Bonnevaux, S., De Luca, C., et al. (1993). Novel hypotensive agents from Verbesina caracasana. 2. Synthesis and pharmacology of caracasanamide. J. Med. Chem. 36, 2956–2963. doi:10.1021/jm00072a016
Dias, K., Da Silva Dias, C., Barbosa-Filho, J., Almeida, R., De Azevedo Correia, N., and Medeiros, I. (2004). Cardiovascular effects induced by reticuline in normotensive rats. Planta Med. 70, 328–333. doi:10.1055/s-2004-818944
Dimo, T., Nguelefack, T., Tan, P., Yewah, M., Dongo, E., Rakotonirina, S., et al. (2003). Possible mechanisms of action of the neutral extract from Bidens pilosa L. leaves on the cardiovascular system of anaesthetized rats. Phytother. Res. 17, 1135–1139. doi:10.1002/ptr.1132
El Bardai, S., Morel, N., Wibo, M., Fabre, N., Llabres, G., Lyoussi, B., et al. (2003). The vasorelaxant activity of marrubenol and marrubiin fromMarrubium vulgare. Planta Med. 69, 75–77. doi:10.1055/s-2003-37042
Fallah Huseini, H., Amini, M., Mohtashami, R., Ghamarchehre, M., Sadeqhi, Z., Kianbakht, S., et al. (2013). Blood pressure lowering effect of Nigella sativa L. seed oil in healthy volunteers: a randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 27, 1849–1853. doi:10.1002/ptr.4944
Fan, G., Zhu, Y., Guo, H., Wang, X., Wang, H., and Gao, X. (2011). Direct vasorelaxation by a novel phytoestrogen tanshinone IIA is mediated by nongenomic action of estrogen receptor through endothelial nitric oxide synthase activation and calcium mobilization. J. Cardiovasc. Pharmacol. 57, 340–347. doi:10.1097/fjc.0b013e31820a0da1
Farias, D., Pereira, A. F., and Rosa, G. (2010). Metabolic syndrome in coronary artery and occlusive vascular diseases: a systematic review. Arq. Bras. Cardiol. 94, 150–78. doi:10.1590/s0066-782x2010000600024
Ferreira, H., Serra, C., Lemos, V., Braga, F., and Cortes, S. (2007). Nitric oxide-dependent vasodilatation by ethanolic extract of Hancornia speciosa via phosphatidyl-inositol 3-kinase. J. Ethnopharmacol. 109, 161–164. doi:10.1016/j.jep.2006.06.009
Figard, H., Girard, C., Mougin, F., Demougeot, C., and Berthelot, A. (2008). Effects of aqueous hop (Humulus lupulus L.) extract on vascular reactivity in rats: mechanisms and influence of gender and hormonal status. Phytomedicine 15, 185–193. doi:10.1016/j.phymed.2007.09.016
Flores-Flores, A., Hernández-Abreu, O., Rios, M. Y., León-Rivera, I., Aguilar-Guadarrama, B., Castillo-España, P., et al. (2016). Vasorelaxant mode of action of dichloromethane-soluble extract from Agastache mexicana and its main bioactive compounds. Pharm. Biol. 54, 2807–2813. doi:10.1080/13880209.2016.1184690
Fukuda, T., Kuroda, T., Kono, M., Hyoguchi, M., Tanaka, M., and Matsui, T. (2015). Augmentation of ferulic acid-induced vasorelaxation with aging and its structure importance in thoracic aorta of spontaneously hypertensive rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 388, 1113–1117. doi:10.1007/s00210-015-1171-9
Fusi, F., Durante, M., Sgaragli, G., Khanh, P., Son, N., Huong, T., et al. (2015). In vitro vasoactivity of zerumbone from Zingiber zerumbet. Planta Med. 81, 298–304. doi:10.1055/s-0034-1396307
Garjani, A., Fathiazad, F., Zakheri, A., Akbari, N., Azarmie, Y., Fakhrjoo, A., et al. (2009). The effect of total extract of Securigera securidaca L. seeds on serum lipid profiles, antioxidant status, and vascular function in hypercholesterolemic rats. J. Ethnopharmacol. 126, 525–532. doi:10.1016/j.jep.2009.09.003
Goto, H., Sakakibara, I., Shimada, Y., Kasahara, Y., and Terasawa, K. (2000). Vasodilator effect of extract prepared from Uncariae ramulus on isolated rat aorta. Am. J. Chin. Med. 28, 197–203. doi:10.1142/s0192415x00000246
Goto, H., Sasaki, Y., Fushimi, H., Shibahara, N., Shimada, Y., and Komatsu, K. (2005). Effect of curcuma herbs on vasomotion and hemorheology in spontaneously hypertensive rat. Am. J. Chin. Med. 33, 449–457. doi:10.1142/s0192415x05003053
Guedes, D., Silva, D., Barbosa-Filho, J., and de Medeiros, I. (2004). Endothelium-dependent hypotensive and vasorelaxant effects of the essential oil from aerial parts of Mentha x villosa in rats. Phytomedicine 11, 490–497. doi:10.1016/j.phymed.2004.04.002
Harvey, A. L., Edrada-Ebel, R., and Quinn, R. J. (2015). The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 14, 111–129. doi:10.1038/nrd4510
Hernández-Pérez, A., Bah, M., Ibarra-Alvarado, C., Rivero-Cruz, J., Rojas-Molina, A., Rojas-Molina, J., et al. (2014). Aortic relaxant activity of Crataegus Gracilior Phipps and identification of some of its chemical constituents. Molecules 19, 20962–20974. doi:10.3390/molecules191220962
Herrera-Arellano, A., Aguilar-Santamaria, L., Garcia-Hernandez, B., Nicasio-Torres, P., and Tortoriello, J. (2004a). Clinical trial of Cecropia obtusifolia and Marrubium vulgare leaf extracts on blood glucose and serum lipids in type 2 diabetics. Phytomedicine 11, 561–566. doi:10.1016/j.phymed.2004.01.006
Herrera-Arellano, A., Flores-Romero, S., Chavez-Soto, M., and Tortoriello, J. (2004b). Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine 11, 375–382. doi:10.1016/j.phymed.2004.04.001
Hill, M. A., Zou, H., Potocnik, S. J., Meininger, G. A., and Davis, M. J. (2001). Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J. Appl. Physiol. 91, 973–983. doi:10.1152/jappl.2001.91.2.973
Hoe, S., Lee, C., Mok, S., Kamaruddin, M., and Lam, S. (2011). Gynura procumbens Merr. decreases blood pressure in rats by vasodilatation via inhibition of calcium channels. Clinics (Sao Paulo) 66, 143–150. doi:10.1590/s1807-59322011000100025
Howlett, J. G. (2014). Nebivolol: vasodilator properties and evidence for relevance in treatment of cardiovascular disease. Can. J. Cardiol. 30, S29–S37. doi:10.1016/j.cjca.2014.03.003
Huang, Y., Wong, C., Lau, C., Yao, X., Tsang, S., Su, Y., et al. (2004). Inhibition of nitric oxide/cyclic GMP-mediated relaxation by purified flavonoids, baicalin and baicalein, in rat aortic rings. Biochem. Pharmacol. 67, 787–794. doi:10.1016/j.bcp.2003.10.002
Intapad, S., Saengsirisuwan, V., Prasannarong, M., Chuncharunee, A., Suvitayawat, W., Chokchaisiri, R., et al. (2012). Long-term effect of phytoestrogens from Curcuma comosa Roxb. on vascular relaxation in ovariectomized rats. J. Agric. Food Chem. 60, 758–764. doi:10.1021/jf203173b
Intapad, S., Suksamrarn, A., and Piyachaturawat, P. (2009). Enhancement of vascular relaxation in rat aorta by phytoestrogens from Curcuma comosa Roxb. Vascul. Pharmacol. 51, 284–290. doi:10.1016/j.vph.2009.07.005
Irace, C., Marini, H., Bitto, A., Altavilla, D., Polito, F., Adamo, E. B., et al. (2013). Genistein and endothelial function in postmenopausal women with metabolic syndrome. Eur. J. Clin. Invest. 43, 1025–1031. doi:10.1111/eci.12139
Jabeen, Q., Bashir, S., Lyoussi, B., and Gilani, A. (2009). Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. J. Ethnopharmacol. 122, 123–130. doi:10.1016/j.jep.2008.12.016
Jiang, H.-D., Cai, J., Xu, J.-H., Zhou, X.-M., and Xia, Q. (2005). Endothelium-dependent and direct relaxation induced by ethyl acetate extract from Flos chrysanthemi in rat thoracic aorta. J. Ethnopharmacol. 101, 221–226. doi:10.1016/j.jep.2005.04.018
Jin, B., Qian, L., Chen, S., Li, J., Wang, H., Bruce, I., et al. (2009). Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur. J. Pharmacol. 616, 200–205. doi:10.1016/j.ejphar.2009.06.020
Kaatabi, H., Bamosa, A. O., Badar, A., Al-Elq, A., Abou-Hozaifa, B., Lebda, F., et al. (2015) Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One 10, e0113486. doi:10.1371/journal.pone.0113486
Kam, T., Wong, C., Kwan, P., Fat-Yiu, W., Chiu, S., Chan, S., et al. (2012). Effects and mechanism of turmeric vasorelaxation of the thoracic aorta in hypercholesterolemic rats. J. Med. Food 15, 190–199. doi:10.1089/jmf.2011.1625
Kamata, K., Iizuka, T., Nagai, M., and Kasuya, Y. (1993). Endothelium-dependent vasodilator effects of the extract from Salviae miltiorrhizae radix. A study on the identification of lithospermic acid B in the extracts. Gen. Pharmacol. 24, 977–981. doi:10.1016/0306-3623(93)90176-x
Kang, D., Lee, J., Choi, D., Sohn, E., Moon, M., and Lee, H. (2005). Vascular relaxation by the methanol extract of Sorbus cortex via NO-cGMP pathway. Biol. Pharm. Bull. 28, 860–864. doi:10.1248/bpb.28.860
Kang, D., Choi, D., Lee, J., Lee, Y., Moon, M., Yang, S., et al. (2007). Endothelial NO/cGMP-dependent vascular relaxation of cornuside isolated from the fruit of Cornus officinalis. Planta Med. 73, 1436–1440. doi:10.1055/s-2007-990243
Khan, M., Khan, A., Najeeb-ur-Rehman, , and Gilani, A. (2015). Blood pressure lowering, vasodilator and cardiac-modulatory potential of Carum roxburghianum seed extract. Clin. Exp. Hypertens. 37, 102–107. doi:10.3109/10641963.2014.913602
Khonsung, P., Panthong, A., Chiranthanut, N., and Intahphuak, S. (2011). Hypotensive effect of the water extract of the leaves of Pseuderanthemum palatiferum. J. Nat. Med. 65, 551–558. doi:10.1007/s11418-011-0540-z
Kim, B., Kim, J., Kim, A., Kim, Y., Lee, Y., Bae, Y., et al. (2004). Ligusticum wallichi-induced vasorelaxation mediated by mitogen-activated protein kinase in rat aortic smooth muscle. J. Ethnopharmacol. 90, 397–401. doi:10.1016/j.jep.2003.11.003
Kim, H. H., Park, S. Y., Ahn, D. K., and Park, S. K. (2004). Vasodilation effects between the water extract of RheumpPalmatum L. and Rheum ndulatum I. In rat thoracie aorta. Korea. J. Herbol. 19, 99. doi:10.1002/ptr.2042
Kim, J., Auger, C., Kurita, I., Anselm, E., Rivoarilala, L., Lee, H., et al. (2013). Aronia melanocarpa juice, a rich source of polyphenols, induces endothelium-dependent relaxations in porcine coronary arteries via the redox-sensitive activation of endothelial nitric oxide synthase. Nitric Oxide 35, 54–64. doi:10.1016/j.niox.2013.08.002
Kratz, J. M., Grienke, U., Scheel, O., Mann, S. A., and Rollinger, J. M (2017) Natural products modulating the hERG channel: heartaches and hope. Natural Prod. Rep. 34, 957–980. doi:10.1039/c7np00014f
Kubota, Y., Tanaka, N., Umegaki, K., Takenaka, H., Mizuno, H., Nakamura, K., et al. (2001). Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci. 69, 2327–2336. doi:10.1016/s0024-3205(01)01303-0
Kubota, Y., Tanaka, N., Kagota, S., Nakamura, K., Kunitomo, M., Umegaki, K., et al. (2006). Effects of Ginkgo biloba extract feeding on salt-induced hypertensive Dahl rats. Biol. Pharm. Bull. 29, 266–269. doi:10.1248/bpb.29.266
Lahlou, S., Tangi, K., Lyoussi, B., and Morel, N. (2008). Vascular effects of Tanacetum vulgare L. leaf extract: in vitro pharmacological study. J. Ethnopharmacol. 120, 98–102. doi:10.1016/j.jep.2008.07.041
Lee, J., Roh, T., Rho, M., Kim, Y., and Lee, H. (2002). Mechanisms of relaxant action of a pyranocoumarin from Peucedanum japonicum in isolated rat thoracic aorta. Planta Med. 68, 891–895. doi:10.1055/s-2002-34934
Lee, P., Chen, W., Liu, I., and Cheng, J. (2007). Vasodilatation induced by sinomenine lowers blood pressure in spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 34, 979–984. doi:10.1111/j.1440-1681.2007.04668.x
Lee, K., Shin, M., Ham, I., and Choi, H. (2015). Investigation of the mechanisms of Angelica dahurica root extract-induced vasorelaxation in isolated rat aortic rings. BMC. Comple. Altern. Med. 15, 395. doi:10.1186/s12906-015-0889-8
Lei, X., and Chiou, G. (1986). Cardiovascular pharmacology of panax notoginseng (burk) F. H. Chen and Salvia miltiorrhiza. Am. J. Chin. Med. 14, 145–152. doi:10.1142/s0192415x86000235
Lemos, V., Côrtes, S., dos Santos, M., Ellena, J., Moreira, M., and Doriguetto, A. (2006). Structure and vasorelaxant activity of floranol, a flavonoid isolated from the roots of Dioclea grandiflora. Chem. Biodivers. 3, 635–645. doi:10.1002/cbdv.200690066
Li, T., Zhong, Y., Tang, T., Luo, J., Cui, H., Fan, R., et al. (2018). Formononetin induces vasorelaxation in rat thoracic aorta via regulation of the PI3K/PTEN/Akt signaling pathway. Drug Des. Devel Ther. 12, 3675–3684. doi:10.2147/dddt.s180837
Li, Y., Bao, J., Xu, J., Murad, F., and Bian, K. (2010). Vascular dilation by paeonol–a mechanism study. Vascul. Pharmacol. 53, 169–176. doi:10.1016/j.vph.2010.07.001
Liang, K., Fang, P., Shi, Q., Su, J., Li, B., Chen, S., et al. (2018). [Antihypertensive effect and mechanism of Dendrobium officinale flos on high-blood pressure rats induced by high glucose and high fat compound alcohol]. Zhongguo Zhong Yao Za Zhi 43, 147–153. doi:10.19540/j.cnki.cjcmm.20171027.020
Liu, C., and Huang, Y. (2016). Chinese herbal medicine on cardiovascular diseases and the mechanisms of action. Front. Pharmacol. 7, 469. doi:10.3389/fphar.2016.00469
Liu, Y., Liu, F., Qiao, M.-M., Guo, L., Chen, M.-H., Peng, C., et al. (2019). Curcumanes A and B, two bicyclic sesquiterpenoids with significant vasorelaxant activity from curcuma longa. Org. Lett. 21, 1197–1201. doi:10.1021/acs.orglett.9b00149
Loai, B., and Zohar, K. (2015). Interactions between CYP3A4 and dietary polyphenols. Oxid. Med. Cell Longev. 2015, 854015. doi:10.1155/2015/854015
Maciel, S., Dias, K., and Medeiros, I. (2004). Calcium mobilization as the endothelium-independent mechanism of action involved in the vasorelaxant response induced by the aqueous fraction of the ethanol extract of Albizia inopinata G. P. Lewis (AFL) in the rat aorta. Phytomedicine 11, 130–134. doi:10.1078/0944-7113-00361
Magos, G., Mateos, J., Páez, E., Fernández, G., Lobato, C., Márquez, C., et al. (2008). Hypotensive and vasorelaxant effects of the procyanidin fraction from Guazuma ulmifolia bark in normotensive and hypertensive rats. J. Ethnopharmacol. 117, 58–68. doi:10.1016/j.jep.2008.01.015
Mahobiya, A., Singh, T., Rungsung, S., Kumar, T., Chandrasekaran, G., Parida, S., et al. (2018). Kaempferol-induces vasorelaxation via endothelium-independent pathways in rat isolated pulmonary artery. Pharmacol. Rep. 70, 863–874. doi:10.1016/j.pharep.2018.03.006
Malek mohammad, K., Sewell, R. D., and Rafieian-Kopaei, M. (2020) Mechanisms of medicinal plant activity on nitric oxide (NO) bioavailability as prospective treatments for atherosclerosis. Current Phar. Design. 9, 301. doi:10.3390/biom9080301
Mansouri, A., Zayeri, F., Baghestani, A. R., Ghorbanifar, Z., Delavar Kasmaei, H., and Sheidaei, A. (2015). Effect of coriander fruit on clinical course of migraine patients: a comparison between random effect and transition models. Horizon Med. Sci. 21, 129–134. doi:10.18869/acadpub.hms.21.2.129
Martinsen, A., Baccelli, C., Navarro, I., Abad, A., Quetin-Leclercq, J., and Morel, N. (2010). Vascular activity of a natural diterpene isolated from Croton zambesicus and of a structurally similar synthetic trachylobane. Vascul. Pharmacol. 52, 63–69. doi:10.1016/j.vph.2009.11.002
Mathieu, P., Poirier, P., Pibarot, P., Lemieux, I., and Després, J. P. (2009). Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension 53, 577–584. doi:10.1161/hypertensionaha.108.110320
Matsumoto, T., Kobayashi, T., Ishida, K., Hirasawa, Y., Morita, H., Honda, T., et al. (2010). Vasodilator effect of cassiarin A, a novel antiplasmodial alkaloid from Cassia siamea, in rat isolated mesenteric artery. Biol. Pharm. Bull. 33, 844–848. doi:10.1248/bpb.33.844
Maurer, K., Ihl, R., Dierks, T., and Frölich, L. (1997). Clinical efficacy of Ginkgo biloba special extract EGb 761 in dementia of the Alzheimer type. J. Psychiatr. Res. 31, 645–655. doi:10.1016/s0022-3956(97)00022-8
McFadzean, I., and Gibson, A. (2002). The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br. J. Pharmacol. 135, 1–13. doi:10.1038/sj.bjp.0704468
Medeiros, M. A., Pinho, J. F., De-Lira, D. P., Barbosa-Filho, J. M., Araújo, D. A., Cortes, S. F., et al. (2011). Curine, a bisbenzylisoquinoline alkaloid, blocks L-type Ca2+ channels and decreases intracellular Ca2+ transients in A7r5 cells. Eur. J. Pharmacol. 669, 100–107. doi:10.1016/j.ejphar.2011.07.044
Melis, M. (1995). Chronic administration of aqueous extract of Stevia rebaudiana in rats: renal effects. J. Ethnopharmacol. 47, 129–134. doi:10.1016/0378-8741(95)01271-e
Meng, X., Ni, C., Zhu, L., Shen, Y., Wang, L., and Chen, Y. (2009). Puerarin protects against high glucose-induced acute vascular dysfunction: role of heme oxygenase-1 in rat thoracic aorta. Vascul. Pharmacol. 50, 110–115. doi:10.1016/j.vph.2008.11.003
Mensah, G. A., and Mayosi, B. M. (2013). The 2011 United Nations high-level meeting on non-communicable diseases: the Africa agenda calls for a 5-by-5 approach. South Afr. Med. J. 103, 77–79. doi:10.7196/samj.6347
Moohammadaree, A., Changtam, C., Wicha, P., Suksamrarn, A., Tocharus, J., and Tocharus, C. (2015). Mechanisms of vasorelaxation induced by hexahydrocurcuminin isolated rat thoracic aorta. Phytother Res. 29, 1806–1813. doi:10.1002/ptr.5448
Mori, M. X., Itsuki, K., Hase, H., Sawamura, S., Kurokawa, T., Mori, Y., et al. (2015). Dynamics of receptor-operated Ca(2+) currents through TRPC channels controlled via the PI(4,5)P2-PLC signaling pathway. Front. Pharmacol. 6, 22. doi:10.3389/fphar.2015.00022
Musabayane, C., Kamadyaapa, D., Gondwe, M., Moodley, K., and Ojewole, J. (2008). Cardiovascular effects of Helichrysum ceres S Moore [Asteraceae] ethanolic leaf extract in some experimental animal paradigms. Cardiovasc. J. Afr. 19, 246–253.
Naseri, M., Arabian, M., Badavi, M., and Ahangarpour, A. (2008). Vasorelaxant and hypotensive effects of Allium cepa peel hydroalcoholic extract in rat. Pak. J. Biol. Sci. 11, 1569–1575. doi:10.3923/pjbs.2008.1569.1575
Navedo, M. F., Nieves-Cintrón, M., Amberg, G. C., Yuan, C., Votaw, V. S., Lederer, W. J., et al. (2008). AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ. Res. 102, e1–e11. doi:10.1161/circresaha.107.167809
Nguelefack, T., Dimo, T., Mbuyo, E., Tan, P., Rakotonirina, S., and Kamanyi, A. (2005). Relaxant effects of the neutral extract of the leaves of Bidens pilosa Linn on isolated rat vascular smooth muscle. Phytother. Res. 19, 207–210. doi:10.1002/ptr.1646
Nguelefack, T., Fodem, C., Nguelefack-Mbuyo, E., Nyadjeu, P., Wansi, S., Watcho, P., et al. (2015). Endothelium nitric oxide-independent vasorelaxant effects of the aqueous extract from Stephania abyssinica on the isolated rat thoracic aorta. J. Comple. Integr. Med. 12, 15–21. doi:10.1515/jcim-2014-0022
Niazmand, S., Fereidouni, E., Mahmoudabady, M., and Mousavi, S. (2014). Endothelium-independent vasorelaxant effects of hydroalcoholic extract from Nigella sativa seed in rat aorta: the roles of Ca2+ and K+ channels. Biomed. Res. Int. 2014, 247054. doi:10.1155/2014/247054
Nie, H., Meng, L., Zhou, J., Fan, X., Luo-, Y., and Zhang, G. (2009). Imperatorin is responsible for the vasodilatation activity of Angelica dahurica var. Formosana regulated by nitric oxide in an endothelium-dependent manner. Chin. J. Integr. Med. 15, 442–447. doi:10.1007/s11655-009-0442-z
Nie, Q., Zhu, L., Zhang, L., Leng, B., and Wang, H. (2019). Astragaloside IV protects against hyperglycemia-induced vascular endothelial dysfunction by inhibiting oxidative stress and calpain-1 activation. Life. Sci. 232, 116662. doi:10.1016/j.lfs.2019.116662
Nishida, S., and Satoh, H. (2003). Mechanisms for the vasodilations induced by Ginkgo biloba extract and its main constituent, bilobalide, in rat aorta. Life Sci. 72, 2659–2667. doi:10.1016/s0024-3205(03)00177-2
Oh, K., Han, W., Wang, M., and Lee, B. (2007a). The effects of chronic treatment with Morus bombycis KOIDZUMI in spontaneously hypertensive rats. Biol. Pharm. Bull. 30, 1278–1283. doi:10.1248/bpb.30.1278
Oh, K., Ryu, S., Kim, Y., and Lee, B. (2007b). Large conductance Ca2+-activated K+ (BKCa) channels are involved in the vascular relaxations elicited by piceatannol isolated from Rheum undulatum rhizome. Planta Med. 73, 1441–1446. doi:10.1055/s-2007-990246
Oh, K. S., Choi, Y. H., Ryu, S. Y., Oh, B. K., Seo, H. W., Yon, G. H., et al. (2008). Cardiovascular effects of lignans isolated from Saururus chinensis. Planta Med. 74, 233–238. doi:10.1055/s-2008-1034310
Ojewole, J., Kamadyaapa, D., Gondwe, M., Moodley, K., and Musabayane, C. (2007). Cardiovascular effects of Persea americana Mill (Lauraceae) (avocado) aqueous leaf extract in experimental animals. Cardiovasc. J. Afr. 18, 69–76. doi:10.1111/j.1600-0765.1986.tb01485.x
Orallo, F., and Alzueta, A. (2001). Preliminary study of the vasorelaxant effects of (+)-nantenine, an alkaloid isolated from Platycapnos spicata, in rat aorta. Planta Med. 67, 800–806. doi:10.1055/s-2001-18848
Orallo, F., Alvarez, E., Basaran, H., and Lugnier, C. (2004). Comparative study of the vasorelaxant activity, superoxide-scavenging ability and cyclic nucleotide phosphodiesterase-inhibitory effects of hesperetin and hesperidin. Naunyn. Schmiedebergs Arch. Pharmacol. 370, 452–463. doi:10.1007/s00210-004-0994-6
Oskouei, D. S., Rikhtegar, R., Hashemilar, M., Sadeghi-Bazargani, H., Sharifi-Bonab, M., Sadeghi-Hokmabadi, E., et al. (2013). The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. J. Stroke Cerebrovasc. Dis. 22, e557–e563. doi:10.1016/j.jstrokecerebrovasdis.2013.06.010
Othman, R., Ibrahim, H., Mohd, M., Mustafa, M., and Awang, K. (2006). Bioassay-guided isolation of a vasorelaxant active compound from Kaempferia Galanga L. Phytomedicine 13, 61–66. doi:10.1016/j.phymed.2004.07.004
Ouedraogo, M., Ruiz, M., Vardelle, E., Carreyre, H., Coustard, J., Potreau, D., et al. (2011). From the vasodilator and hypotensive effects of an extract fraction from Agelanthus dodoneifolius (DC) Danser (Loranthaceae) to the active compound dodoneine. J. Ethnopharmacol. 133, 345–352. doi:10.1016/j.jep.2010.10.002
Pan, Z., Feng, T., Shan, L., Cai, B., Chu, W., Niu, H., et al. (2008). Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother Res. 22, 1428–1433. doi:10.1002/ptr.2364
Panahi, Y., Khalili, N., Hosseini, M. S., Abbasinazari, M., and Sahebkar, A. (2014). Lipid-modifying effects of adjunctive therapy with curcuminoids–piperine combination in patients with metabolic syndrome: results of a randomized controlled trial. Comple. Ther. Med. 22, 851–857. doi:10.1016/j.ctim.2014.07.006
Panahi, Y., Khalili, N., Sahebi, E., Namazi, S., Reiner, Ž., Majeed, M., et al. (2017). Curcuminoids modify lipid profile in type 2 diabetes mellitus: a randomized controlled trial. Comple. Ther. Med. 33, 1–5. doi:10.1016/j.ctim.2017.05.006
Paredes, A., Palacios, J., Quispe, C., Nwokocha, C., Morales, G., Kuzmicic, J., et al. (2016). Hydroalcoholic extract and pure compounds from Senecio nutans Sch. Bip (Compositae) induce vasodilation in rat aorta through endothelium-dependent and independent mechanisms. J. Ethnopharmacol. 192, 99–107. doi:10.1016/j.jep.2016.07.008
Peng, S., Li, Z., Zou, L., Liu, W., Liu, C., and McClements, D. J. (2018). Enhancement of curcumin bioavailability by encapsulation in sophorolipid-coated nanoparticles: an in vitro and in vivo study. J. Agric. Food Chem. 66, 1488–1497. doi:10.1021/acs.jafc.7b05478
Pfaltzgraff, E. R., and Bader, D. M. (2015). Heterogeneity in vascular smooth muscle cell embryonic origin in relation to adult structure, physiology, and disease. Dev. Dyn. 244, 410–416. doi:10.1002/dvdy.24247
Pinto, N. V., Assreuy, A. M. S., Coelho-de-Souza, A. N., Ceccatto, V. M., Magalhães, P. J. C., Lahlou, S., et al. (2009). Endothelium-dependent vasorelaxant effects of the essential oil from aerial parts of Alpinia zerumbet and its main constituent 1, 8-cineole in rats. Phytomedicine 16, 1151–1155. doi:10.1016/j.phymed.2009.04.007
Pires, S., de Assis, T., de Almeida, R., Filho, J., Julien, C., and de Medeiros, I. (2000). Endothelium-derived nitric oxide is involved in the hypotensive and vasorelaxant responses induced by the aqueous fraction of the ethanolic extract of the leaves of Albizia inopinata (Harms) G. P. Lewis in rats. Phytomedicine 7, 91–98. doi:10.1016/s0944-7113(00)80079-3
Poh, T., Ng, H., Hoe, S., and Lam, S. (2013). Gynura procumbens causes vasodilation by inhibiting angiotensin II and enhancing bradykinin actions. J. Cardiovasc. Pharmacol. 61, 378–384. doi:10.1097/fjc.0b013e31828685b3
Qiao, M.-M., Liu, F., Liu, Y., Guo, L., Zhou, Q.-M., Peng, C., et al. (2019). Curcumane C and (±)-curcumane D, an unusual seco-cadinane sesquiterpenoid and a pair of unusual nor-bisabolane enantiomers with significant vasorelaxant activity from Curcuma longa. Bioorg. Chem. 92, 103275. doi:10.1016/j.bioorg.2019.103275
Raimundo, J., Trindade, A., Velozo, L., Kaplan, M., Sudo, R., and Zapata-Sudo, G. (2009). The lignan eudesmin extracted from Piper truncatum induced vascular relaxation via activation of endothelial histamine H1 receptors. Eur. J. Pharmacol. 606, 150–154. doi:10.1016/j.ejphar.2009.01.038
Ramdé-Tiendrébéogo, A., Tibiri, A., Ouedraogo, M., Ouédraogo, S., Nacoulma, O., and Guissou, I. (2014). Study of antisickling and vasorelaxant activities and acute toxicity assessment of crude extracts of leaves of Ficus sycomorus L. (Moraceae). Pak. J. Biol. Sci. 17, 829–835. doi:10.3923/pjbs.2014.829.835
Ried, K., Frank, O., and Stocks, N. (2013). Aged garlic extract reduces blood pressure in hypertensives: a dose–response trial. Eur. J. Clin. Nutr. 67, 64–70. doi:10.1038/ejcn.2012.178
Roth, G. A., Johnson, C., Abajobir, A., Abd-Allah, F., Abera, S. F., Abyu, G., et al. (2017). Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 70, 1–25. doi:10.1016/j.jacc.2017.04.052
Sabzghabaee, A. M., Dianatkhah, M., Sarrafzadegan, N., Asgary, S., and Ghannadi, A. (2012). Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: a randomized, placebo controlled clinical trial. Med. Arch. 66, 198–200. doi:10.5455/medarh.2012.66.198-200
Salahdeen, H., Adebari, A., Murtala, B., and Alada, A. (2015). Potassium channels and prostacyclin contribute to vasorelaxant activities of Tridax procumbens crude aqueous leaf extract in rat superior mesenteric arteries. Afr. J. Med. Med. Sci. 44, 5–19.
Sasaki, Y., Goto, H., Tohda, C., Hatanaka, F., Shibahara, N., Shimada, Y., et al. (2003). Effects of curcuma drugs on vasomotion in isolated rat aorta. Biol. Pharm. Bull. 26, 1135–1143. doi:10.1248/bpb.26.1135
Senejoux, F., Girard, C., Kerram, P., Aisa, H., Berthelot, A., Bévalot, F., et al. (2010). Mechanisms of vasorelaxation induced by Ziziphora clinopodioides Lam. (Lamiaceae) extract in rat thoracic aorta. J. Ethnopharmacol. 132, 268–273. doi:10.1016/j.jep.2010.08.028
Senejoux, F., Girard, C., Aisa, H., Bakri, M., Kerram, P., Berthelot, A., et al. (2012). Vasorelaxant and hypotensive effects of a hydroalcoholic extract from the fruits of Nitraria sibirica Pall. (Nitrariaceae). J. Ethnopharmacol. 141, 629–634. doi:10.1016/j.jep.2011.08.012
Seiichiro, N., and Hiroyasu, S. (2004). Comparative vasodilating actions among terpenoids and flavonoids contained in Ginkgo biloba extract. Clin. Chim. Acta 339, 855. doi:10.1016/j.cccn.2003.10.004
Shah, A., and Gilani, A. (2011). Blood pressure lowering effect of the extract of aerial parts of Capparis aphylla is mediated through endothelium-dependent and independent mechanisms. Clin. Exp. Hypertens. 33, 470–477. doi:10.3109/10641963.2010.549273
Shaito, A., Thuan, D. T. B., Phu, H. T., Nguyen, T. H. D., Hasan, H., Halabi, S., et al. (2020). Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Front. Pharmacol. 11, 422. doi:10.3389/fphar.2020.00422
Sikora, J., Broncel, M., Markowicz, M., Chałubiński, M., Wojdan, K., and Mikiciuk-Olasik, E. (2012). Short-term supplementation with Aronia melanocarpa extract improves platelet aggregation, clotting, and fibrinolysis in patients with metabolic syndrome. Eur. J. Nutr. 51, 549–556. doi:10.1007/s00394-011-0238-8
Singh, A., Laribi, S., Teerlink, J. R., and Mebazaa, A. (2017). Agents with vasodilator properties in acute heart failure. Eur. Heart J. 38, 317–325. doi:10.1093/eurheartj/ehv755
Skiker, M., Mekhfi, H., Aziz, M., Haloui, B., Lahlou, S., Legssyer, A., et al. (2010). Artemisia herba-alba Asso relaxes the rat aorta through activation of NO/cGMP pathway and K(ATP) channels. J. Smooth Muscle Res. 46, 165–174. doi:10.1540/jsmr.46.165
Soares de Moura, R., Costa, S., Jansen, J., Silva, C., Lopes, C., Bernardo-Filho, M., et al. (2002). Bronchodilator activity of Mikania glomerata Sprengel on human bronchi and Guinea-pig trachea. J. Pharm. Pharmacol. 54, 249–256. doi:10.1211/0022357021778277
Sowers, J. R., Epstein, M., and Frohlich, E. D. (2001). Diabetes, hypertension, and cardiovascular disease: an update. Hypertension 37, 1053–1059. doi:10.1161/01.hyp.37.4.1053
Su, X., Duan, R., Sun, Y., Wen, J., Kang, D., Lee, H., et al. (2014). Cardiovascular effects of ethanol extract of Rubus chingii Hu (Rosaceae) in rats: an in vivo and in vitro approach. J. Physiol. Pharmacol. 65, 417–424.
Suárez, A., Ulate, G., and Ciccio, J. (2000). Hypotensive action of an aqueous extract of Pimenta dioica (Myrtaceae) in rats. Rev. Biol. Trop. 48, 53–58.
Sun, X., Ding, J., Li, H., Pan, N., Gan, L., Yang, X., et al. (2007). Activation of large-conductance calcium-activated potassium channels by puerarin: the underlying mechanism of Puerarin-mediated vasodilation. J. Pharmacol. Exp. Ther. 323, 391–397. doi:10.1124/jpet.107.125567
Sureda, A., Silva, A. S., Sánchez-Machado, D. I., López-Cervantes, J., Daglia, M., Nabavi, S. F., et al. (2017). Hypotensive effects of genistein: from chemistry to medicine. Chemi. Biol. Inter. 268, 37–46. doi:10.1016/j.cbi.2017.02.012
Suresh Kumar, P., Patel, J., and Saraf, M. (2008). Mechanism of vasorelaxant activity of a fraction of root extract of Sesamum indicum Linn. Indian. J. Exp. Biol. 46, 457–464. doi:10.1158/1535-7163.MCT-08-0363
Suzuki, A., Yamamoto, M., Jokura, H., Fujii, A., Tokimitsu, I., Hase, T., et al. (2007). Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. Am. J. Hypertens. 20, 508–513. doi:10.1016/j.amjhyper.2006.11.008
Tada, Y., Kagota, S., Kubota, Y., Nejime, N., Nakamura, K., Kunitomo, M., et al. (2008). Long-term feeding of Ginkgo biloba extract impairs peripheral circulation and hepatic function in aged spontaneously hypertensive rats. Biol. Pharm. Bull. 31, 68–72. doi:10.1248/bpb.31.68
Taiwo, I. A., Odeigah, P. G. C., Jaja, S., and Mojiminiyi, F. (2010). Cardiovascular effects of Vernonia amygdalina in rats and the implications for treatment of hypertension in diabetes. Researcher 2, 76–79.
Takagi, S., Goto, H., Shimada, Y., Nakagomi, K., Sadakane, Y., Hatanaka, Y., et al. (2005). Vasodilative effect of perillaldehyde on isolated rat aorta. Phytomedicine 12, 333–337. doi:10.1016/j.phymed.2003.08.004
Takashima, M., Kanamori, Y., Kodera, Y., Morihara, N., and Tamura, K. (2017). Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine 24, 56–61. doi:10.1016/j.phymed.2016.11.016
Tan, C., Ch’ng, Y., Loh, Y., Zaini Asmawi, M., Ahmad, M., and Yam, M. (2017). Vasorelaxation effect of Glycyrrhizae uralensis through the endothelium-dependent pathway. J. Ethnopharmacol. 199, 149–160. doi:10.1016/j.jep.2017.02.001
Tang, D., Chen, K., Huang, L., and Li, J. (2017). Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 13, 323–330. doi:10.1080/17425255.2017.1251903
Tang, F., Yan, Y. M., Yan, H. L., Wang, L. X., Hu, C. J., Wang, H. L., et al. (2020) Chuanxiongdiolides R4 and R5, phthalide dimers with a complex polycyclic skeleton from the aerial parts of Ligusticum chuanxiong and their vasodilator activity. Bioorg. Chem. 107, 104523. doi:10.1016/j.bioorg.2020.104523
Tep-Areenan, P., Sawasdee, P., and Randall, M. (2010). Possible mechanisms of vasorelaxation for 5,7-dimethoxyflavone from Kaempferia parviflora in the rat aorta. Phytother Res. 24, 1520–1525. doi:10.1002/ptr.3164
Testai, L., Silvio, C., Ammar, B., Luisa, P., Vincenzo, C., and Martinotti, E. (2005). Vasorelaxant effects of the chloroformic crude extract of Bupleurum fruticosum L. (Umbelliferae) roots on rat thoracic aorta. J. Ethnopharmacol. 96, 93–97. doi:10.1016/j.jep.2004.08.024
Tibiriçá, E., Almeida, A., Caillleaux, S., Pimenta, D., Kaplan, M., Lessa, M., et al. (2007). Pharmacological mechanisms involved in the vasodilator effects of extracts from Echinodorus Grandiflorus. J. Ethnopharmacol. 111, 50–55. doi:10.1016/j.jep.2006.10.030
Tokoudagba, J., Auger, C., Bréant, L., N'Gom, S., Chabert, P., Idris-Khodja, N., et al. (2010). Procyanidin-rich fractions from Parkia Biglobosa (Mimosaceae) leaves cause redox-sensitive endothelium-dependent relaxation involving NO and EDHF in porcine coronary artery. J. Ethnopharmacol. 132, 246–250. doi:10.1016/j.jep.2010.08.031
Tom, E., Girard, C., Dimo, T., Mbafor, J., Berthelot, A., and Demougeot, C. (2010). Vasorelaxant effects of extracts of the stem bark of Terminalia superba Engler and Diels (Combretaceae). J. Ethnopharmacol. 127, 335–340. doi:10.1016/j.jep.2009.10.036
Török, J. (2000). Histamine-induced relaxation in pulmonary artery of normotensive and hypertensive rats: relative contribution of prostanoids, nitric oxide and hyperpolarization. Physiol. Res. 49, 107–114. doi:10.1086/316715
Trebak, M. (2012). STIM/Orai signalling complexes in vascular smooth muscle. J. Physiol. 590, 4201–4208. doi:10.1113/jphysiol.2012.233353
Ushida, Y., Matsui, T., Tanaka, M., Matsumoto, K., Hosoyama, H., Mitomi, A., et al. (2008). Endothelium-dependent vasorelaxation effect of rutin-free tartary buckwheat extract in isolated rat thoracic aorta. J. Nutr. Biochem. 19, 700–707. doi:10.1016/j.jnutbio.2007.09.005
Waheed, A., Miana, G., Ahmad, S., and Khan, M. A. (2006). Clinical investigation of hypoglycemic effect of Coriandrum sativum in type-2 (NIDDM) diabetic patients. Pakistan J. Pharmacol. 23, 7–11.
Walch, L., Gascard, J. P., Dulmet, E., Brink, C., and Norel, X. (2000). Evidence for a M1 muscarinic receptor 1756 on the endothelium of human pulmonary veins. Br. J. Pharmacol. 130, 73–78. doi:10.1038/sj.bjp.0703301
Wang, Y., Shi, J., Wang, M., Che, C., and Yeung, J. (2009). Vasodilatory actions of xanthones isolated from a Tibetan herb, Halenia elliptica. Phytomedicine 16, 1144–1150. doi:10.1016/j.phymed.2009.03.015
Wansi, S., Nyadjeu, P., Nguelefack, T., Fodouop, S., Donatien, A., and Kamanyi, A. (2012) In vivo antioxidant and vasodilating activities of Gmelina arborea (Verberaceae) leaves hexane extract. J. Comple. Integr. Med 9, doi:10.1515/1553-3840.1623
Wicha, P., Tocharus, J., Nakaew, A., Pantan, R., Suksamrarn, A., and Tocharus, C. (2015). Ethyl rosmarinate relaxes rat aorta by an endothelium-independent pathway. Eur. J. Pharmacol. 766, 9–15. doi:10.1016/j.ejphar.2015.09.003
Wimmer, R. A., Leopoldi, A., Aichinger, M., Wick, N., Hantusch, B., Novatchkova, M., et al. (2019). Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510. doi:10.1038/s41586-018-0858-8
Wong, K., Chan, P., Yang, H., Hsu, F., Liu, I., Cheng, Y., et al. (2004). Isosteviol acts on potassium channels to relax isolated aortic strips of Wistar rat. Life Sci. 74, 2379–2387. doi:10.1016/j.lfs.2003.09.065
Xia, M., Gao, Q., Zhou, X., Qian, L., Shen, Z., Jiang, H., et al. (2007). Vascular effect of extract from mulberry leaves and underlying mechanism. Zhejiang Da Xue Xue Bao Yi Xue Ban 36, 48–53.
Xu, W.-Q., Xiong, Z. Z., Chen, T. T., Gao, X. Y., Yu, H., Zhang, S. Q., et al. (2012). Vasodilation effect of 2-benzyl-5-hydroxy-6-methoxy-3, 4-dihydroisoquinolin-1-one. Arch. Pharmacal Res. 35, 1471–1477. doi:10.1007/s12272-012-0818-z
Xu, Y. J., Elimban, V., and Dhalla, N. S. (2015). Reduction of blood pressure by store-operated calcium channel blockers. J. Cell Mol. Med. 19, 2763–2770. doi:10.1111/jcmm.12684
Yam, M., Tan, C., Ahmad, M., and Shibao, R. (2016). Mechanism of vasorelaxation induced by eupatorin in the rats aortic ring. Eur. J. Pharmacol. 789, 27–36. doi:10.1016/j.ejphar.2016.06.047
Yam, M. F., Tan, C. S., Ahmad, M., and Ruan, S. (2016). Vasorelaxant action of the chloroform fraction of Orthosiphon stamineus via NO/cGMP pathway, potassium and calcium channels. Am. J. Chin. Med. 44, 1413–1439. doi:10.1142/s0192415x16500798
Yamamoto, M., Suzuki, A., Jokura, H., Yamamoto, N., and Hase, T. (2008). Glucosyl hesperidin prevents endothelial dysfunction and oxidative stress in spontaneously hypertensive rats. Nutrition 24, 470–476. doi:10.1016/j.nut.2008.01.010
Yan, Y., Chen, Y., Lin, Y., Guo, J., Niu, Z., Li, L., et al. (2015). Brazilin isolated from the heartwood of Caesalpinia sappan L induces endothelium-dependent and -independent relaxation of rat aortic rings. Acta Pharmacol. Sin. 36, 1318–1326. doi:10.1038/aps.2015.113
Yanaga, A., Goto, H., Nakagawa, T., Hikiami, H., Shibahara, N., and Shimada, Y. (2006). Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol. Pharm. Bull. 29, 2415–2418. doi:10.1248/bpb.29.2415
Yılmaz, B., and Usta, C. (2013). Ellagic acid-induced endothelium-dependent and endothelium-independent vasorelaxation in rat thoracic aortic rings and the underlying mechanism. Phytother. Res. 27, 285–289. doi:10.1002/ptr.4716
Yin, Y., Ying, X., Luan, H., Zhao, Z., Lou, J., Wang, D., et al. (2014). UPLC-DAD/Q-TOF-MS based ingredients identification and vasorelaxant effect of ethanol extract of jasmine flower. Evid. Based Compl. Alternat. Med. 2014, 707908. doi:10.1155/2014/707908
Yoo, M., Lee, B., Choi, Y., Lee, J., Seo, J., Oh, K., et al. (2006). Vasorelaxant effect of the rootbark extract of Paeonia moutan on isolated rat thoracic aorta. Planta Med. 72, 1338–1341. doi:10.1055/s-2006-951678
Yu, L., and Liu, H. (2018). Perillaldehyde prevents the formations of atherosclerotic plaques through recoupling endothelial nitric oxide synthase. J. Cell Biochem. 119, 10204–10215. doi:10.1002/jcb.27362
Yun, W.J., Zhang, X. Y., Liu, T. T., Liang, J. H., Sun, C. P., Yan, J. K., et al. (2020) The inhibition effect of uncarialin A on voltage-dependent L-type calcium channel subunit alpha-1C: inhibition potential and molecular stimulation. Inter. J. Biol. Macromol. 159, 1022–1030. doi:10.1016/j.ijbiomac.2020.05.100
Yuzurihara, M., Ikarashi, Y., Goto, K., Sakakibara, I., Hayakawa, T., and Sasaki, H. (2002). Geissoschizine methyl ether, an indole alkaloid extracted from Uncariae ramulus et Uncus, is a potent vasorelaxant of isolated rat aorta. Eur. J. Pharmacol. 444, 183–189. doi:10.1016/s0014-2999(02)01623-0
Zhang, W., Zhang, C., Wang, X., Gao, P., Zhu, D., Chen, H., et al. (2006). Astragaloside IV dilates aortic vessels from normal and spontaneously hypertensive rats through endothelium-dependent and endothelium-independent ways. Planta Med. 72, 621–626. doi:10.1055/s-2006-931572
Zhang, C., Wang, X., Zhong, M., Liu, R., Li, H., Zhang, W., et al. (2007). Mechanisms underlying vasorelaxant action of astragaloside IV in isolated rat aortic rings. Clin. Exp. Pharmacol. Physiol. 34, 387–392. doi:10.1111/j.1440-1681.2007.04564.x
Zhang, T., Niu, C., Mai, W., Jing, H., and Liu, H. (2009). Vasorelaxational effects of total alkali Sophora Alopecuroids on rabbit aorta in vitro. Zhongguo Zhong Yao Za Zhi 34, 2379–2382.
Zhang, H. T., Wang, Y., Deng, X. L., Dong, M. Q., Zhao, L. M., and Wang, Y. W. (2010). Daidzein relaxes rat cerebral basilar artery via activation of large-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Eur. J. Pharmacol. 630, 100–106. doi:10.1016/j.ejphar.2009.12.032
Zhang, N., Zou, H., Jin, L., Wang, J., Zhong, M., Huang, P., et al. (2010). Biphasic effects of sodium danshensu on vessel function in isolated rat aorta. Acta Pharmacol. Sin. 31, 421–428. doi:10.1038/aps.2010.24
Zhao, Y., Zhang, X., Li, J., Bian, Y., Sheng, M., Liu, B., et al. (2016) Jujuboside B reduces vascular tension by increasing Ca2+ influx and activating endothelial nitric oxide synthase. PLoS One 11, e0149386. doi:10.1371/journal.pone.0149386
Keywords: traditional medicinal plants, natural products, vasodilation, mechanism, cardiovascular and cerebrovascular diseases
Citation: Tang F, Yan H-L, Wang L-X, Xu J-F, Peng C, Ao H and Tan Y-Z (2021) Review of Natural Resources With Vasodilation: Traditional Medicinal Plants, Natural Products, and Their Mechanism and Clinical Efficacy. Front. Pharmacol. 12:627458. doi: 10.3389/fphar.2021.627458
Received: 09 November 2020; Accepted: 29 January 2021;
Published: 01 April 2021.
Edited by:
Yu-Yo Sun, University of Virginia, United StatesReviewed by:
Simona Saponara, University of Siena, ItalyShaobo Ding, Southern Medical University, China
Copyright © 2021 Tang, Yan, Wang, Xu, Peng, Ao and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Peng, cGVuZ2NoZW5nY2hlbmdkdUAxMjYuY29t; Hui Ao, YW9odWkyMDA1QDEyNi5jb20=; Yu-Zhu Tan, dGFueXV6aHVAY2R1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Fei Tang1†
Fei Tang1† Cheng Peng
Cheng Peng Yu-Zhu Tan
Yu-Zhu Tan