- 1School of Pharmaceutical Sciences, Jilin University, Changchun, China
- 2Institute of Special Animal and Plant Sciences, Chinese Academy of Agricultural Sciences, Changchun, China
Ginseng (Panax ginseng C. A. Mey.) is a traditional medicine that has been utilized for over 2000 years in Asia and shows varied pharmacological effects. Red ginseng (RG) is steamed and dried ginseng root and is considered to be more effective. Heating inactivates its catabolic enzymes and increases the activities of RG, which can improve the immune system, alleviate fatigue, and has anti-inflammatory effects and antioxidant activity. In addition, RG has a good anti-aging effect, but its mechanism is unclear. Senescence, a side-effect of normal developmental and metabolic processes, is a gradual decline in physiological integrity and function of the body. Senescence is usually associated with a variety of diseases, including neurodegenerative diseases and diabetes. Research on anti-aging and the prolongation of life span has always been a focus topic. In this study, we investigated the molecular mechanism of RG that results in prolonged the life span for male Drosophila melanogaster. Isobaric tag for relative and absolute quantitation (iTRAQ) was used to identify protein changes in an old male D. melanogaster treated with RG. The differential proteins were verified by qRT-PCR and western blotting. The results showed that 12.5 mg/ml RG prolonged its life span significantly. iTRAQ results showed that, compared to the control group, 32 upregulated proteins and 62 downregulated proteins displayed significantly differential expression in the RG group. In this study, we explored the pathways that RG may participate in that extend the life span of D. melanogaster, and the results showed that the PI3K/AKT/FoxO pathway was involved. In addition, 4E-BP increased and participated in the regulation of life span.
Introduction
Senescence is a gradual decline in the physiological integrity and function of the body, including molecules, cells, tissue structure, and function, as well as homeostasis (He and Jasper, 2014). Senescence is usually associated with various diseases, such as neurodegenerative diseases, diabetes, cardiovascular diseases, and cerebrovascular diseases. Research on anti-aging and the prolongation of life span has always been a point of focus.
Ginseng (Panax ginseng C. A. Mey.) is a traditional medicine that has been utilized for over 2000 years in Asia and has varied pharmacological effects (Wisniewski et al., 2009; Wu et al., 2017). The ancient Chinese Meteria Medica ShenNong BenCao Jing recorded that ginseng could be taken for a long time and prolong life. Modern pharmacological studies have shown that ginseng extract enhances the activity of superoxide dismutase in aged rats (Ramesh et al., 2012). In addition, ginseng has been shown to be cardioprotective because of its antioxidative, anti-arrhythmic, and calcium channel-antagonistic activities (Yuan 2015). Red ginseng (RG) is steamed and dried ginseng root, which is considered more effective. Heating inactivates its catabolic enzymes and increases the activities of RG, which can improve the immune system and alleviate fatigue and has anti-inflammatory effects and antioxidant activity; it allows effective coping with the metabolic dysfunction of senescent cells and functional decline (Wu et al., 2017; Ham et al., 2019). Furthermore, RG can protect the brain and spinal cord from neurodegeneration and extend the life span of Drosophila melanogaster (Wisniewski et al., 2009; Kim 2013; Liu et al., 2018).
D. melanogaster is an excellent model for research on senescence due to its short life span and easy maintenance (Allocca et al., 2018). In our previous experiments, we fed D. melanogaster RG, and the results showed that RG extended the life span of D. melanogaster in both males and females (Liu et al., 2018; Hou et al., 2020). However, the mechanism by which RG extends the life span of male D. melanogaster has not been elucidated. In this study, an isobaric tag for relative and absolute quantitation (iTRAQ) was used to identify protein changes in senile male D. melanogaster treated with RG and is the first study to reveal the changes in signaling pathways.
Materials and Methods
Materials
Red ginseng (6 years) was purchased from Changchun City (Jilin Province, China) and pulverized into a powder. The panaxoside content was determined by high-performance liquid chromatography method (Zhang et al., 2018), and the contents were (all in mg/g) Re 1.470, Rg1 1.836, Rf 1.01, Rb1 5.21, Rc 4.447, Rb2 3.211, Rb3 0.317, and Rd 4.453 (Figure 1).
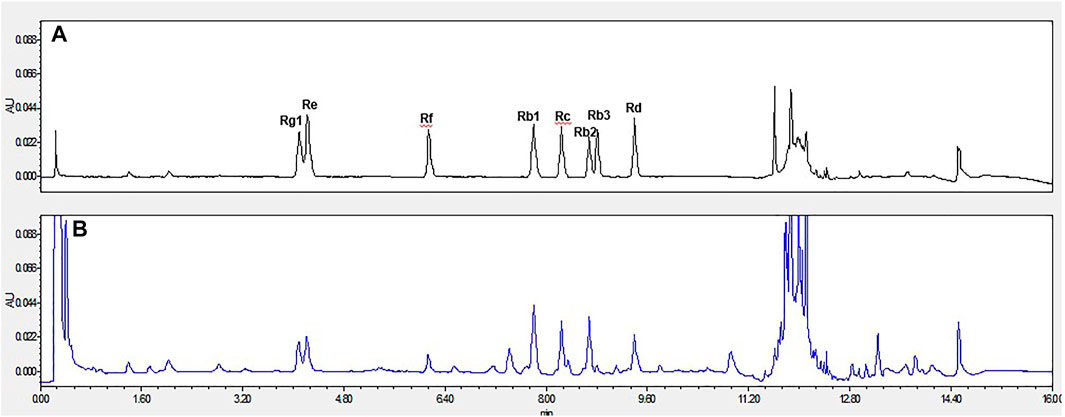
FIGURE 1. The representative UPLC chromatograms of ginsenosides. (A). The reference standards of ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, Rb3, Rd. (B). Red ginseng sample.; Abbreviation: UPLC, Ultra Performance Liquid Chromatography.
Red Ginseng Prolongs the Life Span of Male D. melanogaster
D. melanogaster (wild type) were a gift from Jilin Agricultural University (Changchun, China). All male D. melanogaster were housed in an artificial climate incubator at 25°C and 60% humidity with 12 h alternating dark and light phases. New eclosion D. melanogaster were separated by sex and males were subsequently divided into six groups (n = 60 each). D. melanogaster of the control group were fed a basic diet of water, agar, corn extract, sucrose, extra yeast powder, and propionic acid, and D. melanogaster of the RG group were fed basic diet supplemented with RG at a final concentration of 10.0, 12.5, 15.0, 17.5, or 20.0 mg/ml. The food was changed every 2–3 days. Survival was recorded at 8:00 AM for both the control and RG groups. D. melanogaster that died unnaturally were eliminated (accidental death), and the D. melanogaster that died naturally were removed from the cage. The test was repeated three times. All animals were handled in strict accordance with good animal practice according to the Animal Ethics Procedures and Guidelines of the People’s Republic of China, and the study was approved by The Animal Administration and Ethics Committee of Institute of Special Animal and Plant Sciences, Chinese Academy of Agricultural Sciences.
Climbing Ability Test
The male D. melanogaster of the control and RG groups were tested at the age of 36 days. The test was repeated three times. The locomotor activity of D. melanogaster was measured by climbing ability. D. melanogaster were placed into culture tubes (Diameter, 2.5 cm; Height, 12 cm). D. melanogaster was tapped down to the bottom of tube and then the maximum height that the D. melanogaster climbed up the tube was measured over 15 s (Iliadi and Boulianne 2010; Minois et al., 2001).
Protein Preparation
The male D. melanogaster of the control and RG group were anesthetized at the age of 36 days. Liquid nitrogen was added to the samples (The male D. melanogaster body) and ground into a fine powder. Then, the powder was dissolved in SDT buffer (4% sodium dodecyl sulfate, 0.1 M; dithiothreitol, 100 mM; Tris-HCl, pH 7.6). The protein content was tested using a BCA protein assay (Thermo Fisher Scientific, United States). Protein digestion was performed according to the filter-aided proteome preparation procedure (Wisniewski et al., 2009). The peptides were desalted on MILI-SPE Extraction disk cartridge (C18-SD), lyophilized, and 40 µL dissolution buffer was added.
Isobaric Tag for Relative and Absolute Quantitation Labeling
Each peptide mixture (100 μg) was labeled with the iTRAQ reagent-8 plex Multiplex Kit (AB SCIEX United Kingdom. Limited, United Kingdom). The peptide mixture of control-male-1 was labeled as a 113 tag. Control-male-2 was labeled as a 114 tag, and control-male-3 was labeled as a 115 tag. RG-male-1 was labeled as a 116 tag, RG-male-2 as a 117 tag, and RG-male-3 as a 118 tag.
LC-MS/MS Analysis
Each peptide mixture was analyzed by nano LC-MS/MS coupled to an EASY nLC (Thermo Fisher Scientific). The sample was loaded into the column (Thermo Scientific Acclaim PepMap100, 100 μm × 2 cm, nanoViper C18) by an automatic sampler and connected to an analytical column (Thermo Scientific EASY Column, 10 cm, ID75 μm, 3 μm, C18-A2) in buffer A (0.1% formic acid) and buffer B (84% acetonitrile and 0.1% formic acid) at a flow rate of 300 nL/min. LC-MS/MS analysis was performed on a Q Exactive mass spectrometer (Thermo Scientific). The mass spectrometer was detected in positive ion mode, and the precursor ions scanning range was 300–1,800 m/z. The automatic gain control (AGC) target was set to 1e6 and maximum inject time (IT) to 50 ms. Survey scans were acquired at a resolution of 70,000 at m/z 200 and the resolution for HCD spectra was set to 17,500 at m/z 200, and an isolation width of 2 m/z. Normalized collision energy was 30 eV, and the underfill ratio was defined as 0.1% (Liu et al., 2017; Yu et al., 2017). Raw mass spectrometry data were submitted to China National Genomics Data Center (CNCB-NGDC, https://bigd.big.ac.cn/omix/) BioProject accession number under PRJCA004725.
Proteomic Analysis
The mass spectrometry data were noted and quantified using the MASCOT engine (version 2.2; Matrix Science, London, United Kingdom) and Proteome Discoverer 1.4 (Thermo Fisher Scientific). The selected database was UniProt D. melanogaster 42524 20180327. fasta. The following options were used to identify proteins: peptide mass tolerance = ±20 ppm; fragment mass tolerance = 0.1 Da; enzyme = trypsin; max missed cleavages = 2; fixed modification: carbamidomethyl (C), iTRAQ4/8plex (N-term), iTRAQ4/8plex (K); and variable modification: xidation (M), iTRAQ4/8plex (Y); database pattern = decoy. The selection criteria for differential proteins were set as fold-change with a comparison >1.2 or <0.83, combined with an unadjusted p < 0.05. The Blast2GO program (https://www.blast2go.com/) was used to annotate the functions of the differentially expressed proteins. The Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used to analyze the pathway enrichment of significant proteins (Liu et al., 2017; Yu et al., 2017).
qRT-PCR
The identified differentially expressed proteins were examined at the transcriptional level by qRT-PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Proteintech, United States) was used as an internal reference. D. melanogaster from the control and RG group were euthanized at the age of 36 days. The test was repeated three times. Total RNA was extracted using the Total RNA Extraction Kit (UNIQ-10 Trizol, SK1321, Shanghai, China), and cDNA was synthesized using the Thermo Fisher Scientific cDNA Synthesis kit (EP0733). The 2−ΔΔCT method was used to analyze RNA expression. Primer sequences for qRT-PCR are shown in Table 1.
Western Blotting
The significant proteins were validated using western blotting. Male D. melanogaster from the control and RG group were euthanized at the age of 36 days. The test was repeated three times. Total protein was extracted from D. melanogaster with lysis buffer (Beyotime, Haimen, China) and Bullet Blender (NY, United States). Protein concentration was tested using the BCA protein assay reagent. GAPDH was used as the loading control. Samples were separated by 12% SDS-PAGE (Bio-Rad, Hercules, CA, United States) and transferred to polyvinylidene difluoride membranes (Millipore, United States). Membranes were blocked with 5% w/v nonfat dry milk (BD Biosciences, United States) and incubated with primary antibodies at 4°C overnight. The membranes were washed with Tris-buffered saline-Tween and incubated with horseradish peroxidase-labeled secondary antibodies (Proteintech, United States) for 1.5 h at 25°C. Finally, the enhanced chemiluminescence kit (GE Healthcare, United States) was used to visualize the immunobands. The protein bands were scanned using an imaging densitometer.
Statistical Analyses
SAS software (version 9.2) was used for statistical analysis. Fisher’s LSD (least significant difference) was used for the analysis of life span and climbing ability test data. The data of qRT-PCR and Western blotting were analyzed using t-test. A p value of less than 0.05 was considered statistically significant.
Result
Red Ginseng on Prolongs the Life Span of Male D. melanogaster
The male D. melanogaster in the RG group were fed RG at concentrations of 10.0, 12.5, 15.0, 17.5, or 20.0 mg/ml. The life spans of male D. melanogaster treated with 12.5 mg/ml RG and 15.0 mg/ml RG were significantly extended compared with the control group, while the life spans of male D. melanogaster treated with 20.0 mg/ml RG decreased (Figure 2). These results indicated that RG could prolong the life span of male D. melanogaster within the appropriate dose range, while an overdose of RG had a negative impact on the life span.
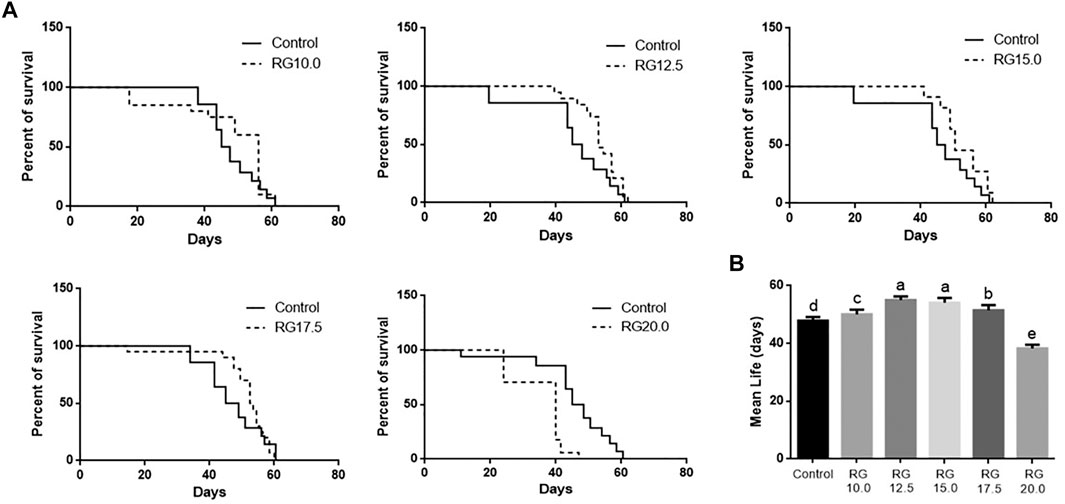
FIGURE 2. Red ginseng on prolonging life span of male D. melanogaster; B. Statistical analysis of data. The different lowercase letters mean significant difference at a 5% level with each treatment group.; Abbreviation: RG, red ginseng.
Climbing Ability Test
The male D. melanogaster of the control and RG groups were tested at the age of 36 days. Climbing activity had already been successfully used to evaluate the rate of aging in D. melanogaster. The male D. melanogaster treated with 12.5 mg/ml and 15.0 mg/ml RG had better climbing activity compared with the control group (Figure 3). These results indicated that RG could slow down the rate of aging.
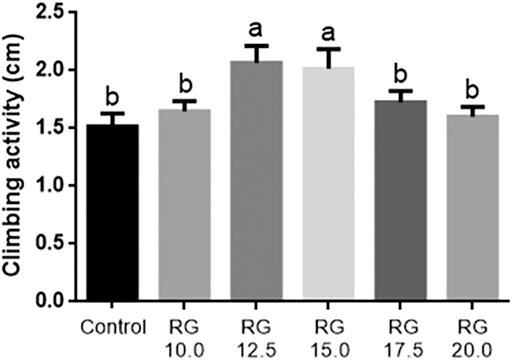
FIGURE 3. Climbing ability test.The different lowercase letters mean significant difference at a 5% level with each treatment group.; Abbreviation: RG, red ginseng.
Identification of Significantly Changed Proteins
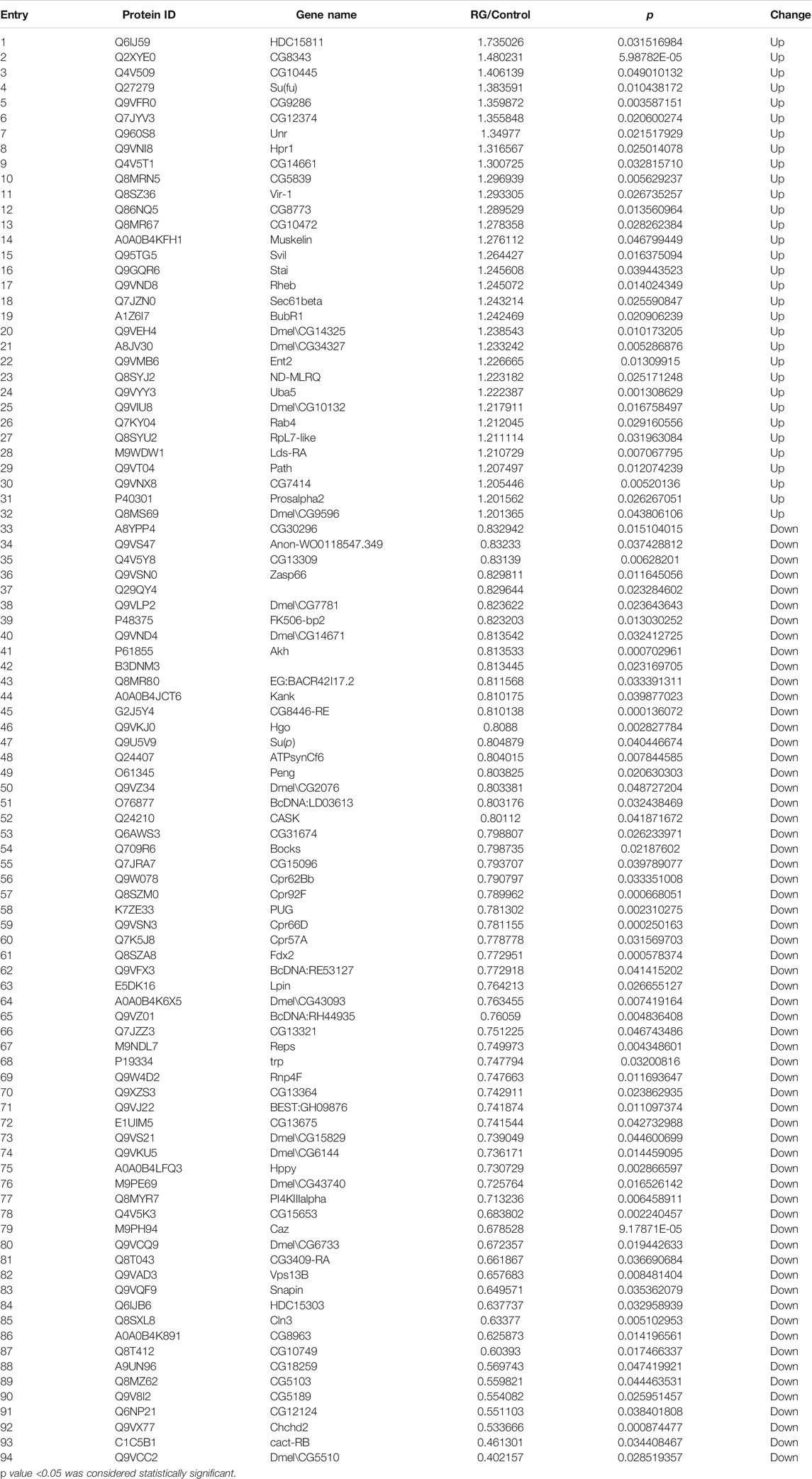
To investigate the effects of RG on male D. melanogaster life span, we treated male D. melanogaster with 12.5 mg/ml RG and used iTRAQ to identify protein changes. Using a threshold of >1.2 or <0.83 (p < 0.05), a total of 94 proteins were found to be differentially expressed between the RG and control groups. Of these, the expression of 32 proteins increased and the expression of 62 proteins decreased in the experimental group (Table 2). Clustering heatmaps and volcano plots showed significant changes in protein expression (Figures 4A,B).
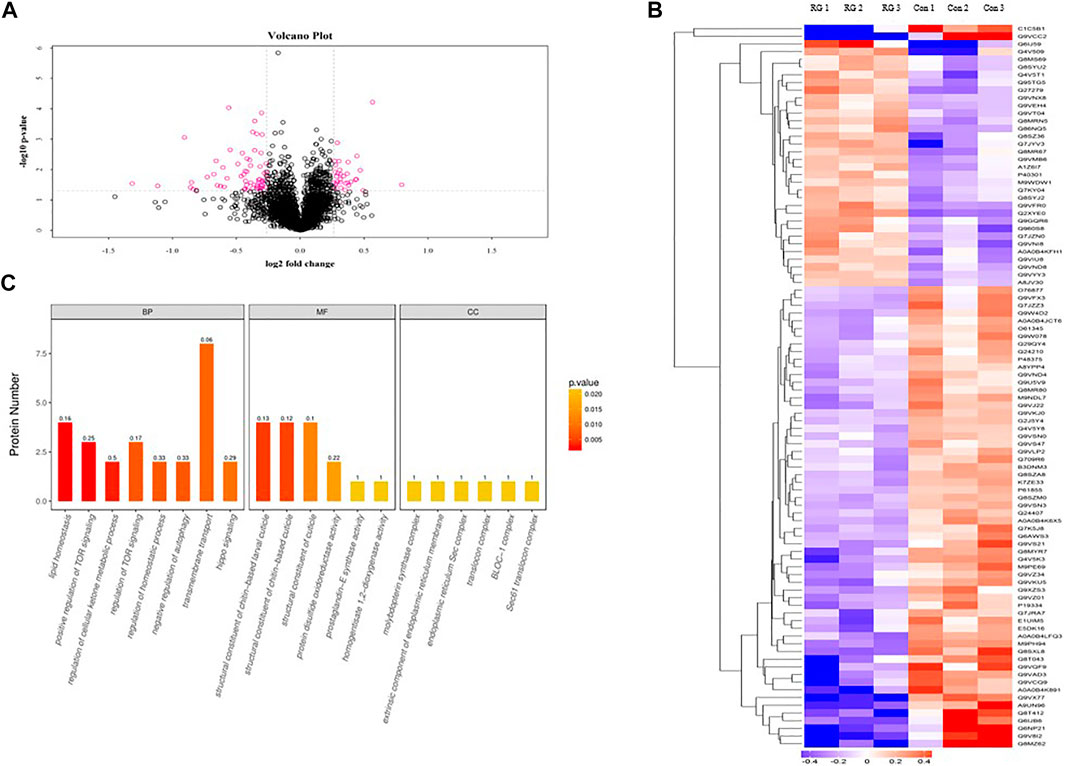
FIGURE 4. (A). Volcano plot showed the significant changes in proteins expression; The pink dots were the differentially expressed proteins in the figure (the multiple change was greater than 1.2 times and p value <0.05). The black dots were the proteins which had not changed. (B). Clustering heatmaps showed the significant changes in proteins expression; The red represented significantly up-regulated proteins. The blue represented significantly down-regulated proteins. Gray represented no protein quantitative information. (C). The significantly changed proteins were analyzed by the Blast2GO program. The abscissa represented the enriched GO terms in the figure. Biological Process, BP; Molecular Function, MF; Cellular Component, CC; The ordinate represented the number of differentially expressed proteins. The color gradient represented the size of the p value. The closer the color was to the red, the smaller the p value, and the higher the significance level of the enrichment degree of the corresponding GO functional category. The label above the bar chart showed the enrichment factor (Enrichment factor represented the ratio of the number of differentially expressed proteins annotated to a GO function to the number of all identified proteins annotated to that GO function).; Abbreviation: RG, red ginseng; Con, control.
Bioinformatics Analysis of Significantly Changed Proteins
The significantly changed proteins were analyzed by the Blast2GO program and Fisher’s exact test (p < 0.05). The enriched GO terms were from the following three categories: cellular component, molecular function, and biological process. The biological process category was significantly enriched in GO for proteins involved in lipid homeostasis, positive regulation of TOR signaling, and regulation of cellular ketone metabolic process. The molecular function category was significantly enriched in GO for structural constituents of the chitin-based larval cuticle, protein disulfide oxidoreductase activity, and prostaglandin-E synthase activity. The cellular component category was significantly enriched in GO for the extrinsic component of the endoplasmic reticulum membrane, molybdopterin synthase complex, and translocation complex (Figure 4C). Significantly changed proteins were analyzed by KEGG. The identified pathways were protein processing in the endoplasmic reticulum, oxidative phosphorylation, and the mTOR signaling pathway.
Validation of Significantly Changed Proteins by qRT-PCR
The mRNA levels of these differentially expressed proteins were tested by qRT-PCR. The mRNA level changes of CG9286, CG10472, FK506-bp2, Akh, hgo, CG31674, Lpin, BcDNA:RH44935, hppy, and Chchd2 were consistent with the changes analyzed by iTRAQ. No significant changes were evident in the levels of trp and Dmel\CG5510 between the control and RG groups (Figure 5).
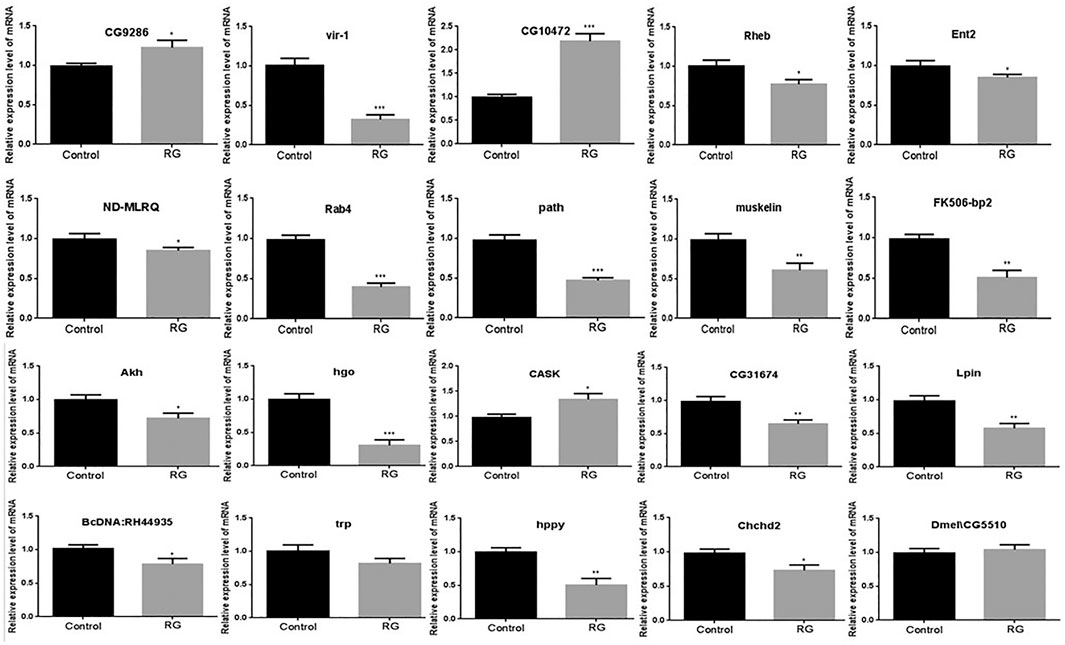
FIGURE 5. Validation of the significantly changed proteins by qRT-PCR. p value <0.05 was considered statistically significant.; Abbreviation: RG, red ginseng.
Validation of Proteins by Western Blotting
Expression of hppy, Lpin, Ent2, Rheb, and FK506-bp2 was tested by western blotting. The altered expressions of hppy, Lpin, Ent2, Rheb, and FK506-bp2 between the control and RG groups were consistent with the changes analyzed by iTRAQ. The expression of hppy, Lpin, and FK506-bp2 was downregulated and the expression of Ent2 and Rheb was upregulated (Figure 6).
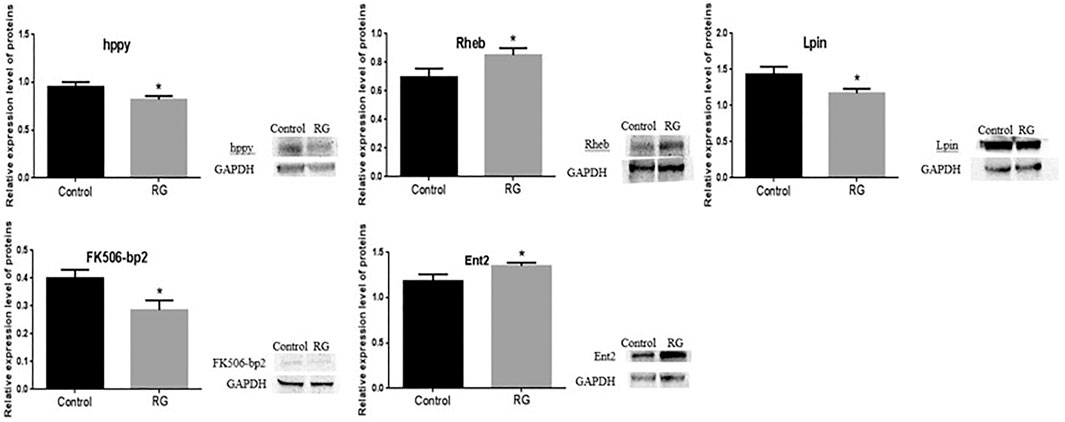
FIGURE 6. Expression of hppy, Rheb, Lpin, FK506-bp2 and Ent2 were confirmed using western blotting. P value < 0.05 was considered statistically significant.; Abbreviation: RG, red ginseng.
Effect of Hppy, Rheb, and Lpin on the PI3K/AKT/FoxO Pathway
The differentially expressed proteins hppy, Rheb, and Lpin participated in the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT)/forkhead box O (FoxO) signaling pathway and regulated the life span of D. melanogaster. We tested the changes of PI3K, AKT, p-AKT, mTOR, p-mTOR, S6K, 4E-BP, and FoxO proteins using western blotting. The expression of PI3K, AKT, and p-AKT was decreased in the RG group (p < 0.05), and the expression of mTOR and p-mTOR did not change significantly. The expression of 4E-BP and FoxO increased significantly (p < 0.05) and S6K did not change (Figures 7, 8).
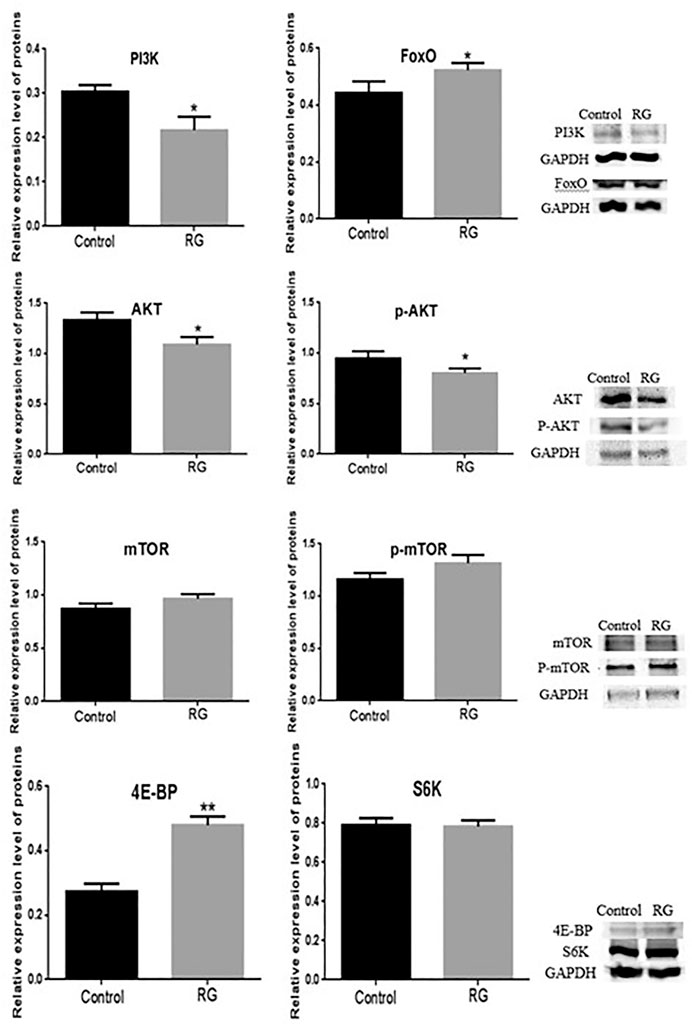
FIGURE 7. Expression of PI3K, AKT, p-AKT, mTOR, p-mTOR, 4E-BP, S6K and FoxO were confirmed using western blotting. P value < 0.05 was considered statistically significant.; Abbreviation: RG, red ginseng; Con, control; AKT, protein kinase B; mTOR, mechanistic target of rapamycin, PI3K, phosphatidylinositol 3 kinase; 4E-BP, eukaryotic translation initiation factor 4E binding protein; S6K, S6 protein kinase; FoxO, forkhead box O.
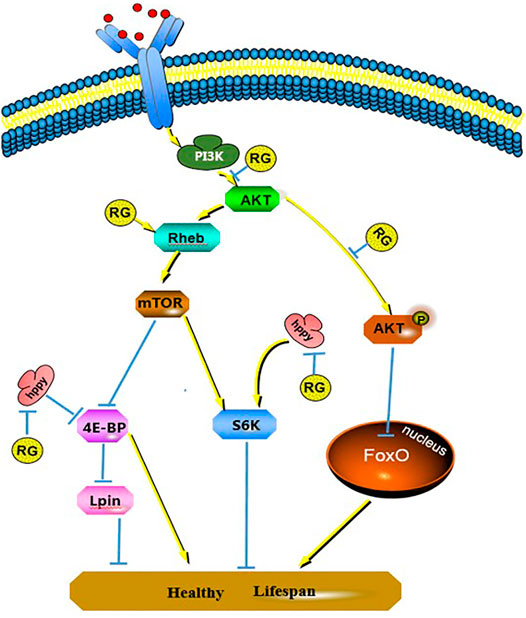
FIGURE 8. Changes of signal pathways induced by RG prolonging male D. melanogaster life span. P value < 0.05 was considered statistically significant.; Abbreviation: RG, red ginseng; Con, control; AKT, protein kinase B; mTOR, mechanistic target of rapamycin, PI3K, phosphatidylinositol 3 kinase; 4E-BP, eukaryotic translation initiation factor 4E binding protein; S6K, S6 protein kinase; FoxO, forkhead box O.
Discussion
Red ginseng prolongs the life span of D. melanogaster, but the mechanism has not been elucidated. In this study, iTRAQ was used to identify protein changes in senile (36 days old) male D. melanogaster. The iTRAQ examination revealed that a total of 94 proteins were differentially expressed in the RG group. Of these, the expression of 32 proteins increased and the expression of 62 proteins decreased. qRT-PCR revealed variations in the mRNA levels of CG9286, CG10472, FK506-bp2, Akh, hgo, CG31674, Lpin, BcDNA: RH44935, hppy, and Chchd2, which were consistent with the iTRAQ results. No significant changes were seen in the levels of trp and Dmel\CG5510 between the control and RG groups. The mRNA expression of vir-1, Rheb, Ent2, ND-MLRQ, Rab4, path, muskelin, and CASK after qRT-PCR detection were not consistent with iTRAQ results (Figure 4). This inconsistency in changes may be because mRNA is regulated by many regulatory factors during translation. The altered expression of hppy, Lpin, Ent2, Rheb, and FK506-bp2 found in western blotting were consistent with the results of iTRAQ.
The differentially expressed protein hppy, which is homologous to human MAP4K3, regulates various signaling pathways, including the mTOR pathway (Lam et al., 2010). The mTOR pathway plays an important role in the process of Drosophila senescence. A decrease in MAP4K3 level can reduce the activity of mTOR (Bryk et al., 2010). S6 protein kinase (S6K) and translation inhibitor (4E-BP) are targets of mTOR activity, and MAP4K3 also regulates the activity of S6K and 4E-BP (Weichhart 2018). The effect of 4E-BP on extending life span has been shown in Drosophila before (Hay 2011). In our study, western blotting results showed that the expression of hppy in male D. melanogaster was decreased after RG treatment. The expression of mTOR, p-mTOR, and S6K did not change. The expression of 4E-BP increased significantly (p < 0.05). The findings indicated that the decreased expression of hppy upregulated the expression of 4E-BP, directly inhibited protein synthesis, and participated in the regulation of life extension. In addition, MAP4K3 can also activate the JNK pathway and induce cell apoptosis. Based on these results, the decreased expression of hppy may inhibit the JNK pathway (Lam et al., 2010).
The differentially expressed protein Rheb, a homolog of Ras GTPase, participates in the PI3K/AKT/mTOR signaling pathway and upregulates Rheb activated mTOR activity (Karassek et al., 2010). Inhibiting mTOR activity and reducing protein synthesis can prolong the life span of organisms, including yeast, D. melanogaster, nematodes, and mice (Alic and Partridge 2011). However, long-term inhibition of mTOR activity can lead to inhibition of wound healing, anemia, proteinuria, pneumonia, and hypercholesterolemia (Lamming et al., 2012). The balance of the mTOR pathway is essential for cell health. In this study, the results showed that the expression of Rheb in male D. melanogaster was increased after RG treatment, and the expression of PI3K, AKT, and p-AKT was decreased in the RG group (p < 0.05). The expression of p-mTOR increased slightly in the RG group, but the difference was not significant (p > 0.05). Inactivation of AKT directly activates the FoxO family of proteins, which reduces oxidative stress, repairs DNA damage, and inhibits premature aging and cellular senescence (Zhang et al., 2011). Therefore, we tested the changes of FoxO protein, and the results showed that the expression of FoxO was increased (p < 0.05). The findings indicated that RG prolonged the life span of male D. melanogaster by regulating the PI3K/AKT/FoxO pathway. The mTOR pathway remained relatively balanced and was not overactivated. In addition, Rheb can reduce reactive oxygen species (ROS) and oxidative damage independently of the mTOR pathway (Ashraf et al., 2019). On feeding D. melanogaster RG, Rheb may reduce the oxidative damage and prolong the life span.
The differentially expressed protein Lpin, a homolog of human lipin, is a lipid protein regulated by the mTOR pathway. Aging is accompanied by the accumulation of Lpin (Romic et al., 2017). The expression of Lpin can be decreased by inhibiting mTOR and upregulating the expression of 4E-BP (Guo et al., 2019; Reue and Wang, 2019). The iTRAQ, qRT-PCR, and western blotting results showed that the expression of Lpin was decreased and 4E-BP was increased. The decreased expression of Lpin indicated that there was no excessive accumulation of lipids in the senile flies.
The differentially expressed protein Fk506-bp2 changed significantly, which is a binding protein. Fk506-bp2 is sensitive to oxidative stress and easily decomposes with the ryanodine receptor (Kreko-Pierce et al., 2016). The results showed that the expression of FK506-bp2 decreased, which may reduce the sensitivity to oxidative stress and oxidative damage.
The differentially expressed protein CG31674 is an oxidoreductase involved in oxidative stress (Yi et al., 2007; Fernandez-Ayala et al., 2010). Our results showed that the expression of CG31674 decreased. It was speculated that the activity of the redox reaction and oxidative damage may have decreased. Oxidative damage can induce aging. Significant prolongation of a healthy life span requires a reduction of all aging processes of an organism (Avril, et al., 1984).
Red ginseng prolonged the life span of male D. melanogaster through a complex biological process and may be used as a potential anti-aging drug. The iTRAQ examination revealed that a total of 94 proteins were differentially expressed in the RG group. Many proteins do not have primary antibodies and western blotting confirmatory experiments were not executed. In addition, D. melanogaster is a model organism and non-mammal. We will continue to study RG’s effect on prolonging life span and PI3K/AKT/FoxO pathway in mammals.
Conclusion
In this study, we explored the pathways that RG may participate in when extending the life span of D. melanogaster, and the results showed that the PI3K/AKT/FoxO pathway was involved. In addition, 4E-BP expression increased and participated in the regulation of life span.
Data Availability Statement
Raw mass spectrometry data were submitted to China National Genomics Data Center (CNCB-NGDC, https://bigd.big.ac.cn/omix/) under BioProject accession number PRJCA004725.
Author Contributions
JP conceived and designed the experiments. WH performed the experiments, analyzed the data, and contributed reagents/materials/analysis tools. WH and JP wrote the paper.
Funding
This work was supported by grants from the Jilin Science andTechnology Development Plan (No. 20180101261JC), the Central Public-Interest Scientific Institution Basal Research Fund (No. 1610342020008), the Jilin Provincial Department of Science and Technology China (No.20190201160JC), and the Jilin Province Development and Reform Commission (No. 2019C052-10).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alic, N., and Partridge, L. (2011). Death and Dessert: Nutrient Signalling Pathways and Ageing. Curr. Opin. Cel Biol. 23, 738–743. doi:10.1016/j.ceb.2011.07.006
Allocca, M., Zola, S., and Bellosta, P. (2018). The Fruit Fly, Drosophila melanogaster: Modeling of Human Diseases (Part II), Drosophila melanogaster - model for recent advances in genetics and therapeutics [M]. London: InTech Open, 131–156.
Ashraf, S., Kim, B. J., Park, S., Park, H., and Lee, S.-H. (2019). RHEB Gene Therapy Maintains the Chondrogenic Characteristics and Protects Cartilage Tissue from Degenerative Damage during Experimental Murine Osteoarthritis. Osteoarthritis and cartilage 27, 1508–1517. doi:10.1016/j.joca.2019.05.024
Avril, D. W., Anthony, D. B., and Alexander, H. (1984). Molecular biology of aging [M]. New York, Plenum Press, 16–17.
Bryk, B., Hahn, K., Cohen, S. M., and Teleman, A. A. (2010). MAP4K3 Regulates Body Size and Metabolism in Drosophila. Develop. Biol. 344, 150–157. doi:10.1016/j.ydbio.2010.04.027
Fernandez-Ayala, D. J., Chen, S., Kemppainen, E., O'Dell, K. M. C., and Jacobs, H. T. (2010). Gene Expression in a Drosophila Model of Mitochondrial Disease. PLoS One 5, e8549. doi:10.1371/journal.pone.0008549
Guo, Z., Cheng, X., Feng, X., Zhao, K., Zhang, M., Yao, R., et al. (2019). The mTORC1/4EBP1/PPARγ Axis Mediates Insulin-Induced Lipogenesis by Regulating Lipogenic Gene Expression in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 67, 6007–6018. doi:10.1021/acs.jafc.9b01411
Ham, S. W., Kim, J.-K., Jeon, H.-Y., Kim, E.-J., Jin, X., Eun, K., et al. (2019). Korean Red Ginseng Extract Inhibits Glioblastoma Propagation by Blocking the Wnt Signaling Pathway. J. Ethnopharmacology 236, 393–400. doi:10.1016/j.jep.2019.03.031
Hay, N. (2011). Interplay between FOXO, TOR, and Akt. Biochim. Biophys. Acta (Bba) - Mol. Cel Res. 1813, 1965–1970. doi:10.1016/j.bbamcr.2011.03.013
He, Y., and Jasper, H. (2014). Studying Aging in Drosophila. Methods 68, 129–133. doi:10.1016/j.ymeth.2014.04.008
Hou, W., Pei, J., Wang, Y. P., Zhang, J., Zheng, H. S., and Cui, R. (2020). Anti-ageing Effects of Red Ginseng on Female Drosophila melanogaster. J. Cel Mol Med. 24, 3751. doi:10.1111/jcmm.15029
Karassek, S., Berghaus, C., Schwarten, M., Goemans, C. G., Ohse, N., Kock, G., et al. (2010). Ras Homolog Enriched in Brain (Rheb) Enhances Apoptotic Signaling*. J. Biol. Chem. 285, 33979–33991. doi:10.1074/jbc.m109.095968
Kim, M. S. (2013). Korean Red Ginseng Tonic Extends Lifespan in D. melanogaster. Biomolecules Ther. 21, 241–245. doi:10.4062/biomolther.2013.024
Kreko-Pierce, T., Azpurua, J., Mahoney, R. E., and Eaton, B. A. (2016). Extension of Health Span and Life Span in Drosophila by S107 Requires the Calstabin Homologue FK506-BP2. J. Biol. Chem. 291, 26045–26055. doi:10.1074/jbc.m116.758839
Lam, D., Shah, S., de Castro, I. P., Loh, S. H. Y., and Martins, L. M. (2010). Drosophila Happyhour Modulates JNK-dependent Apoptosis. Cell Death Dis 1, e66. doi:10.1038/cddis.2010.44
Lamming, D. W., Ye, L., Katajisto, P., Goncalves, M. D., Saitoh, M., Stevens, D. M., et al. (2012). Rapamycin-induced Insulin Resistance Is Mediated by mTORC2 Loss and Uncoupled from Longevity. Science 335, 1638–1643. doi:10.1126/science.1215135
Liu, Q.-X., Zhang, W., Wang, J., Hou, W., and Wang, Y.-P. (2018). A Proteomic Approach Reveals the Differential Protein Expression in Drosophila melanogaster Treated with Red Ginseng Extract (Panax Ginseng). J. Ginseng Res. 42, 343–351. doi:10.1016/j.jgr.2017.04.006
Liu, X., Wang, J., Gao, L., Liu, H., and Liu, C. (2017). iTRAQ-Based Proteomic Analysis of Neonatal Kidney from Offspring of Protein Restricted Rats Reveals Abnormalities in Intraflagellar Transport Proteins. Cell Physiol Biochem 44, 185–199. doi:10.1159/000484626
Minois, N., Khazaeli, A. A., and J, W. (2001). Locomotor Activity as a Function of Age and Life Span in Drosophila melanogaster Overexpressing Hsp70. Exp. Gerontol. Curtsinger 36 (7), 1137–1153. doi:10.1016/s0531-5565(00)00263-1
Ramesh, T., Kim, S.-W., Sung, J.-H., Hwang, S.-Y., Sohn, S.-H., Yoo, S.-K., et al. (2012). Effect of Fermented Panax Ginseng Extract (GINST) on Oxidative Stress and Antioxidant Activities in Major Organs of Aged Rats. Exp. Gerontol. 47, 77–84. doi:10.1016/j.exger.2011.10.007
Reue, K., and Wang, H. (2019). Mammalian Lipin Phosphatidic Acid Phosphatases in Lipid Synthesis and beyond: Metabolic and Inflammatory Disorders. J. Lipid Res. 60, 728–733. doi:10.1194/jlr.s091769
Romic, S., Krskova, K., Olszanecki, R., Balazova, L., Lory, V., Koricanac, G., et al. (2017). Obesity- and Age-Related Alterations in FAT/CD36 Translocation and Lipin-1 Subcellular Localization in Skeletal Muscle of the Zucker Rats. Gen. Physiol. Biophys. 36, 399–406. doi:10.4149/gpb_2017010
Weichhart, T. (2018). mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 64, 127–134. doi:10.1159/000484629
Wisniewski, J. R., Zougman, A., Nagaraj, N., and Mann, M. (2009). Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 6, 359–362. doi:10.1038/nmeth.1322
Wu, X., Pei, J., Wang, Y., Zhang, J., Zheng, H., Cui, R., et al. (2017). Ginseng: An Nonnegligible Natural Remedy for Healthy Aging. Aging Dis. 8 (6) 708, doi:10.14336/AD.2017.0707
Yi, C. H., Sogah, D. K., Boyce, M., Degterev, A., Christofferson, D. E., and Yuan, J. (2007). A Genome-wide RNAi Screen Reveals Multiple Regulators of Caspase Activation. J. Cel Biol 179, 619–626. doi:10.1083/jcb.200708090
Yu, H., Wang, X., Xu, J., Ma, Y., Zhang, S., Yu, D., et al. (2017). iTRAQ-Based Quantitative Proteomics Analysis of Molecular Mechanisms Associated with Bombyx mori (Lepidoptera) Larval Midgut Response to BmNPV in Susceptible and Near-Isogenic Strains. J. Proteomics 165, 35–50. doi:10.1016/j.jprot.2017.06.007
Yuan, S. M. (2015). Potential Cardioprotective Effects of Ginseng Preparations. Pak J. Pharm. Sci. 28, 963–968 .
Zhang, H., Xu, S., Piao, C., Zhao, X., Tian, Y., Cui, D., et al. (2018). Post-planting Performance, Yield, and Ginsenoside Content of Panax Ginseng in Relation to Initial Seedling Size. Ind. Crops Prod. 125, 24–32. doi:10.1016/j.indcrop.2018.08.091
Keywords: red ginseng, anti-Aging, isobaric tag for relative and absolute quantitation, proteome, drosophila
Citation: Hou W and Pei J (2021) Proteomic Analysis of Red Ginseng on Prolonging the Life Span of Male Drosophila melanogaster. Front. Pharmacol. 12:618123. doi: 10.3389/fphar.2021.618123
Received: 16 October 2020; Accepted: 14 April 2021;
Published: 11 June 2021.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Paul Chazot, Durham University, United KingdomYu Chiang Hung, Kaohsiung Chang Gung Memorial Hospital, Taiwan
Copyright © 2021 Hou and Pei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Pei, cGVpamluQGpsdS5lZHUuY24=
 Wei Hou
Wei Hou Jin Pei1*
Jin Pei1*