
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 15 February 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.617559
This article is part of the Research Topic Integrative Pharmacology-based Research on Traditional Medicine: Methodologies, Medical and Pharmacological Applications View all 73 articles
 Xiaomeng Zhang1
Xiaomeng Zhang1 Xiaoying Chen1
Xiaoying Chen1 Lei Wang1
Lei Wang1 Changhao He1
Changhao He1 Zhongyu Shi1
Zhongyu Shi1 Qian Fu1
Qian Fu1 Wenhui Xu2
Wenhui Xu2 Shujing Zhang1
Shujing Zhang1 Sumin Hu1*
Sumin Hu1*Ionizing radiation damage refers to acute, delayed, or chronic tissue damage associated with ionizing radiation. Specific or effective therapeutic options for systemic injuries induced by ionizing radiation have not been developed. Studies have shown that Chinese herbal Medicine or Chinese Herbal Prescription exhibit preventive properties against radiation damage. These medicines inhibit tissue injuries and promote repair with very minimal side effects. This study reviews traditional Chinese herbal medicines and prescriptions with radiation protective effects as well as their mechanisms of action. The information obtained will guide the development of alternative radioprotectants.
Ionizing radiation is essential in clinical diagnoses and treatment. It is an effective therapeutic strategy for cancer treatment. Approximately 50% of cancer patients are administered with radiotherapy to inhibit metastasis (Begg et al., 2011). Ionizing radiation causes clustered DNA damage and leads to persistent oxidative stress injuries to cellular macromolecules (Shuryak, 2019). Radiotherapy inhibits metastasis by inducing DNA damage. However, it causes unintended damage to normal cells by enhancing DNA double-strand breaks (DSBs). Double-strand breaks can be repaired through two major pathways: the non-homologous end-joining (NHEJ) and homologous recombination (HR). The NHEJ pathway occurs during the G0/G1 phase, while the HR repair pathway is only active in the late S and G2 phases (Shibata, 2017). Severe genetic changes such as chromosomal deletions and translocations in the repair process can stimulate tumorigenesis (Shrivastav et al., 2008; Gerelchuluun et al., 2015). According to the United Nations Scientific Committee, the effects of atomic radiation include mutations due to DNA deletions (Sankaranarayanan and Wassom, 2005), and epigenetic transmissions that affect generations (Horemans et al., 2019). Oxidative damage after exposure to ionizing radiation is a crucial reason for sustained injuries (Einor et al., 2016). Approximately 70% of cellular radiation damage is indirectly caused by water dissociation and reactive oxygen species (ROS). Free radicals are triggers for a state of constant oxidative stress (Anuranjani and Bala, 2014). Inflammation, immunity, and other associated signaling pathways are involved in the regulation of cellular damage by inducing cell senescence, apoptosis, and other cell fates (Santivasi and Xia, 2014). The pathological process is as shown in Figure 1. Ferroptosis is a novel form of cell death that is involved in pathological damage after irradiation (Lei et al., 2020). There is a need for the development of drugs that inhibit or repair pathological damages associated with radiation.
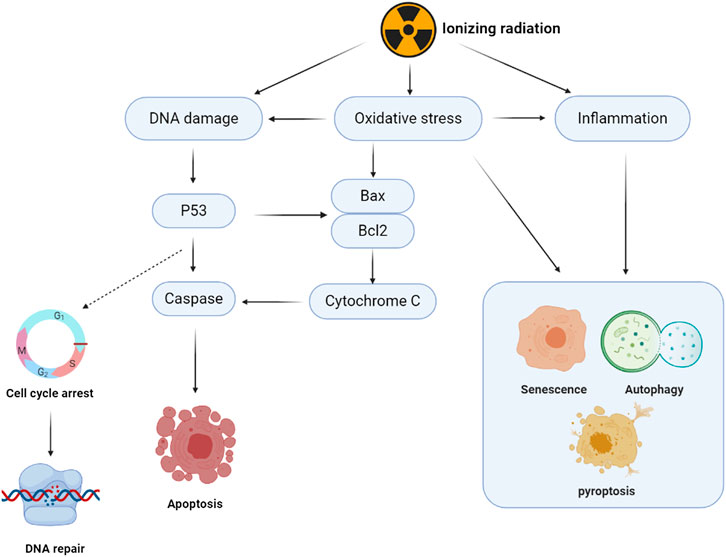
FIGURE 1. Schematic diagram of ionizing radiation damage mechanism. The damage of ionizing radiation to cell structure mainly has two ways: the direct damage to DNA and the indirect damage from accumulated ROS. DNA damage response and repair induced by ionizing radiation can lead to cell cycle arrest and activate DNA homologous recombination (HR) or non-homologous end joining (NHEJ) repair. DNA damage induces the phosphorylation of P53, promotes the expression of Bax and other apoptotic proteins, promotes the release of Cytochrome C from mitochondria, and activates Caspase from the intrinsic apoptosis pathway. P53 can induce the expression of downstream death receptors and death ligands, which triggers the extrinsic apoptosis pathway. Oxidative stress caused by ROS overproduction triggers DNA double-strand breaks that continue and exacerbate DNA damage. It also induces mitochondrial dysfunction and intrinsic apoptotic cascade reaction. Ionizing radiation increases the secretion of inflammatory factors and induces immune responses. Inflammatory factors and oxidative damage are involved in regulating cell fate, including autophagy, pyroptosis and senescence.
Current therapeutic strategies for radiation damage focus on minimizing DNA breaks, generation of antioxidants, scavenging free radicals, and inhibiting lipid peroxidation (Weiss and Landauer, 2003; Johnke et al., 2014; Smith et al., 2017). However, clinical applications of radioprotectants are limited because of its limited efficacies and severe side effects (Brizel, 2007; Citrin et al., 2010; Singh and Seed, 2019). Chinese herbal medicines have been used for thousands of years. They are widely used in clinical settings to treat various diseases, or are used in combination with western medicines to improve clinical efficacies (Li and Xu, 2011; Hao et al., 2017; Takayama and Iwasaki, 2017; Wang and Zhang, 2017; Yeh et al., 2017; Lei et al., 2019). As early as the 1960s, Chinese researchers had extended the research focused on the anti-radiation effects of Chinese herbal medicines. It has been found that Panax ginseng C.A.Mey., Ganoderma Lucidum Karst, Angelica sinensis (Oliv.) Diels, and other medicinal herbs exhibit varying degrees of anti-radiation effects. In addition, Chinese Herbal Prescriptions have been shown to facilitate physical recovery. Traditional Chinese medicinal herbs have complex chemical structures and biological activities. The Chinese Herbal Prescription, which is composed of more than one medicinal herb, is the embodiment of traditional Chinese medicine’s clinical applications. The Chinese Herbal Prescriptions can enhance the efficacy of a drug, reduce or neutralize the adverse effects of individual drugs and improve their therapeutic efficacies (Shen et al., 2017; Li et al., 2019b; Shi et al., 2019b). In recent years, researches on the treatment of ionizing radiation damage with Traditional Chinese Medicine are regularly emerging. The active compounds in Chinese medicinal herbs and Chinese Herbal Prescriptions exhibit significant effects on the reduction of oxidative stress and promote DNA repair. Their biological mechanisms involve the regulation of multiple signaling pathways.
In this review, we summarized recent advances that have been aimed at elucidating the functions and mechanisms of effective active ingredients of Chinese herbal medicines and prescriptions in preventing ionizing radiation associated damage. We aimed at establishing novel therapeutic avenues for the development and clinical applications of radiation protective drugs.
Active compounds in Chinese herbal medicines can scavenge for free radicals, reduce DNA damage, promote post-injury repair, and reduce cell apoptosis. Therefore, these herbs prevent radiation damage through different mechanisms. Below, we highlight a few representative active compounds and highlight their potential mechanisms and pharmacological activities in radioprotection (As shown in Table 1).
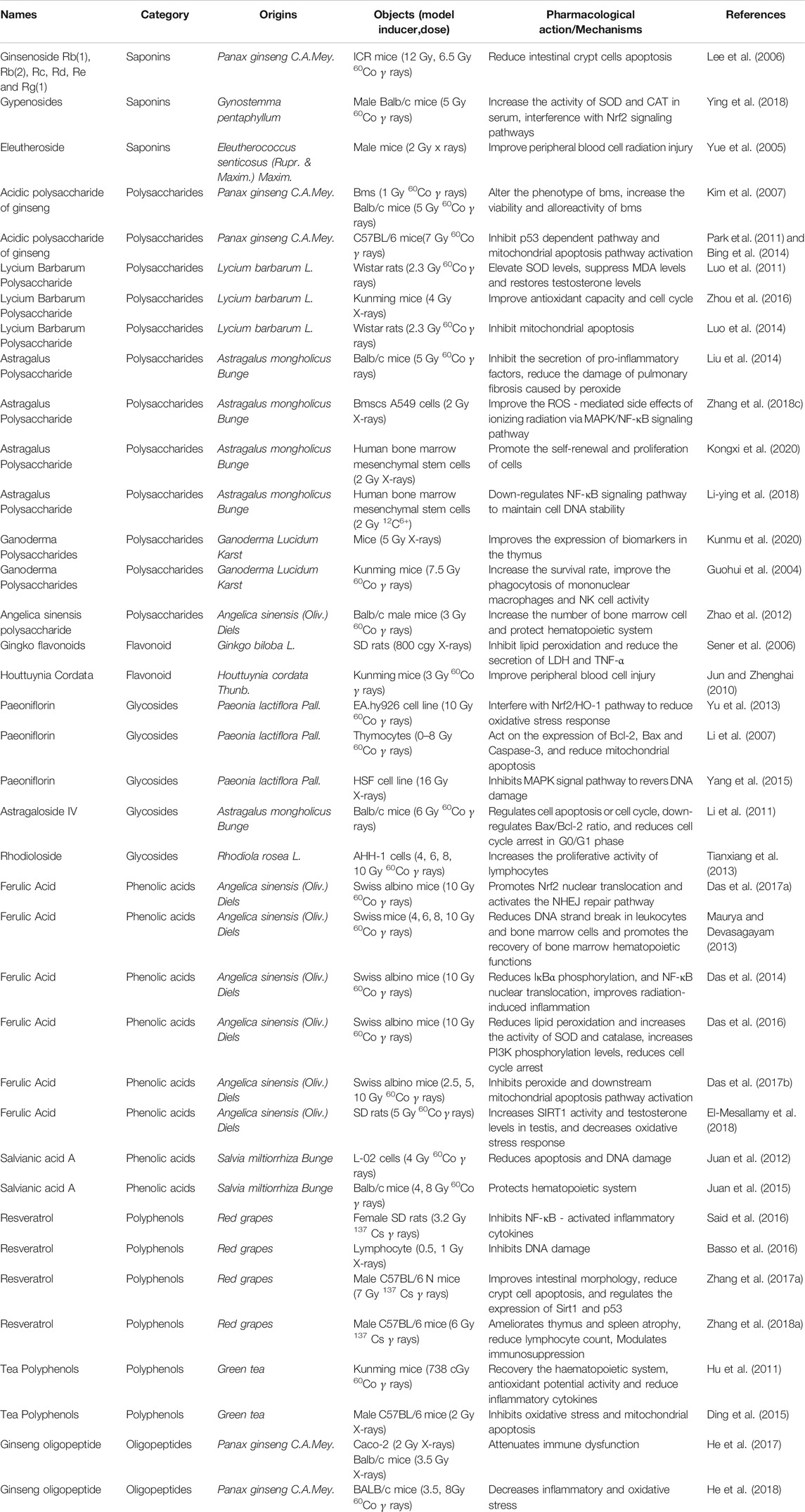
TABLE 1. Effects and Mechanisms of active compounds of Chinese Herbal Medicines in ionizing radiation damage.
Saponins are the main active compounds in many Chinese herbal medicines (Yang et al., 2014), especially in Panax ginseng C.A.Mey. Lee et al. established that the active ingredients in Panax ginseng C.A.Mey such as Ginsenoside Rc, Ginsenoside Rd, and Ginsenoside Re are radioprotective (Lee et al., 2006). The administration of Ginsenoside Rd and Ginsenoside Re in mice before irradiation enhanced the formation of endogenous splenic colonies and inhibited radiation-induced apoptosis of the intestinal crypt cells (Lee et al., 2006). Ginsenosides have a wide range of biological and pharmacological properties. They have been shown to be effective against neurological diseases, infectious diseases, and tumors (Rokot et al., 2016; Arring et al., 2018; Nguyen and Nguyen, 2019). Through intestinal biotransformation, Panax ginseng C.A.Mey. can be transformed into high pharmacological activity metabolites that act on multiple human tissues (Mancuso and Santangelo, 2017).
Other medicinal herbs have also been shown to contain saponins that play a role in radioprotection. Administration of Gypenoside before irradiation effectively increased serum superoxide dismutase (SOD) and CAT levels that inhibit the expression of Nrf2 and HO-1 (Ying et al., 2018). Saponins from Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. ameliorate peripheral blood cell damage associated with radiation (Yue et al., 2005).
Polysaccharides are widespread in animals, plants, and microorganisms. They from part of the primary substances that make up living things (Chen and Huang, 2018). Panax ginseng C.A.Mey. contains polysaccharides. The acidic polysaccharide of ginseng (APG) has been shown to increase IL-12 levels in bone marrow cells (BMs) of irradiated mice. Kim et al. speculated that APG contribute to the proliferation of CD4 (+) T lymphocytes and facilitate viability as well as alloreactivity by inducing phenotypic changes in BMs (Kim et al., 2007). APG inhibits the activation of the p53-dependent pathway and the mitochondrial apoptosis pathway. It down-regulates pro-apoptotic proteins (p53, BAX, cytochrome-c, and caspase-3), thereby, promoting the proliferation of crypt cells. These effects were shown to protect the small intestines of mice from radiation damage (Park et al., 2011; Bing et al., 2014).
Lycium barbarum L. is commonly used in traditional medicine to nourish the liver and kidney. Its active ingredient, the Lycium barbarum polysaccharide (LBP), exhibits significant antioxidant effects. Studies have reported that after multiple consecutive local 60Co γ-rays irradiation of rats’ testis, LBP enhanced testicular SOD activity, inhibited malondialdehyde (MDA) levels, promoted redox balance recovery, and restored the secretion of testosterone (Luo et al., 2011). In the hematopoietic system, LBP was shown to increase the antioxidant capacity of bone marrow mononuclear cells and mitigated cell cycle arrest by interfering with adhesion molecules CD44 and CD49d (Zhou et al., 2016). In addition, by acting on on Bcl-2 and Bax, LBP inhibits spermatogenic cell apoptosis by regulating mitochondrial membrane potential and inhibiting mitochondrial apoptosis (Luo et al., 2014).
Astragalus mongholicus Bunge is a qi-tonifying medicinal herb that is often used in qi deficiency syndromes (Li et al., 2014b). Radiation-induced lung injury is one of the most common and fatal complications of chest radiotherapy (Klein et al., 2016). After ionizing irradiation, alveolar epithelial cells in the lung exhibit a senescence phenotype and up-regulates the transcription of pro-inflammatory factors that induce pulmonary fibrosis (Beach et al., 2017). This condition manifests itself as breathlessness. The Astragalus polysaccharide is the active ingredient in Astragalus mongholicus Bunge. It inhibits thiobarbituric acid reactive substances and pro-inflammatory factors, but also activates SOD, catalase and glutathione. In addition, it reduces the damage of pulmonary fibrosis caused by peroxidation. Its mechanism of action is correlated with the expression of NF-κB, and this mechanism applys to radiation-induced liver injury (Liu et al., 2014).
Astragalus polysaccharide inhibits p38 phosphorylation, JNK, ERK1/2, NF-κB P65, and COX-2 protein expression levels. Evidence shows that it inhibits ionizing radiation-induced side effects through the ROS-mediated MAPK/NF-κB signaling pathway (Zhang et al., 2018c). Ionizing radiation decreases the capacity for cell proliferation. The Astragalus polysaccharide has been documented to promote self-renewal and proliferation of cells by elevating the expressions of peroxisome proliferator-activated receptor-γ (PPAR-γ), CCAAT/enhancer-binding protein α (C/EBPα) and protecting adipogenic differentiation functions (Kongxi et al., 2020). In vitro studies have also revealed that Astragalus polysaccharides enhance bone marrow mesenchymal stem cell proliferation by downregulating NF-κB signaling pathway-related proteins and maintaining DNA stability (Li-ying et al., 2018).
Ganoderma Lucidum Karst has qi replenishment and nerve soothing properties. It contains various biologically active components and pharmacological activities that are important in the control of multiple diseases (Jin et al., 2016; Ahmad, 2018). The Ganoderma lucidum polysaccharide is the main biologically active component of Ganoderma Lucidum Karst. Studies have shown that Ganoderma lucidum polysaccharide regulates the metabolism of endogenous substances (such as L-glutamic acid, taurine, and glycerophospholipid) that enhance the expression of relevant biomarkers in mice thymus and exert radioprotective effects (Kunmu et al., 2020). Guohui et al. reported that after exposing mice to radiation, Ganoderma Lucidum Karst increased mice survival rates and improved the phagocytic abilities of mononuclear macrophages and NK cells (Guohui et al., 2004).
The polysaccharides of Angelica sinensis (Oliv.) Diels have been documented to exhibit thymus and spleen protective indices, increase the number of red blood cells (RBC), white blood cells (WBC), and bone marrow cells of mice after irradiation. Therefore, they play a role in protecting the hematopoietic system (Zhao et al., 2012).
Flavonoids are widely distributed in plants and exhibit health promoting properties (Mukai, 2018). Ginkgo biloba L. contains flavonols and other active compounds (Mei et al., 2017) that are responsible for its free radical scavenging ability and antioxidant properties (Evans, 2013). Therefore, Ginkgo biloba L. prevents ionizing radiation mediated injuries by inhibiting oxidative stress. Sener et al. have detected rat lung, liver, kidney, and ileum. They reported that the administration of Ginkgo biloba L. before and after irradiation attenuated malondialdehyde (MDA) content and reduced DNA damage by inhibiting lipid peroxidation (Sener et al., 2006). Houttuynia cordata Thunb. has shown to clearing heat and removing toxicity, reducing swelling and draining pus (Ma et al., 2017). The flavonoid contents of Houttuynia cordata Thunb. have been shown to improve the state of peripheral blood cells after radiation, thereby reducing injury (Jun and Zhenghai, 2010).
Paeonia lactiflora Pall. is an herb that nourishes the blood, restrains yin, softens the liver, and relieves pain. Paeoniflorin is a component of Paeonia lactiflora Pall.. Paeoniflorin increased glutathione (GSH), SOD, and reduced MDA and lactate dehydrogenase (LDH) content in an endothelial cell model. In addition, it was shown to reduce oxidative stress responses by interfering with the Nrf2/HO-1 pathway (Yu et al., 2013). Another study showed that it inhibited ROS-mediated mitochondrial apoptosis and reduced ROS accumulation as well as intracellular cytosolic Ca2 + concentrations. Other than inhibiting the mitochondrial apoptotic pathway by acting on Bcl-2, Bax, and caspase-3 (Li et al., 2007), paeoniflorin also inhibited MAPK signaling pathway activation and reversed radiation-induced DNA damage (Yang et al., 2015).
Astragaloside IV is an active ingredient in Astragalus mongholicus Bunge that is involved in controlling apoptosis or cell cycle, down-regulation of the Bax/Bcl-2 ratio, inhibition of G0/G1 cell-cycle arrest, increasing the proliferative ability of bone marrow cells, and in protection against radiation induced damage to the hematopoietic system (Li et al., 2011).
Rhodiola rosea L. contains a variety of biologically active compounds with antioxidant, anti-inflammatory, and stress response properties (Amsterdam and Panossian, 2016; Nabavi et al., 2016). Glycosides in Rhodiola rosea L. were shown to stimulate lymphocytic cell proliferation after radiation (Tianxiang et al., 2013).
Angelica sinensis (Oliv.) Diels promotes blood nourishment and circulation and is often used to treat blood deficiency syndromes. Ferulic acid (FA) is a bioactive component of Angelica sinensis (Oliv.) Diels that reduces DNA damage. Studies have shown that administration of FA to mice 1 h before or after irradiation inhibited micronuclei formation in peripheral blood. FA promotes hematopoietic recovery by attenuating DSBs in white blood cells and bone marrow cells (Maurya and Devasagayam, 2013). After DNA damage, the PARPI repair mechanism is activated and regulates inflammatory mediators (such as cytokines, chemokines, and inducible Nitric Oxide synthase) (Bai and Virag, 2012), while SIRT1 negatively regulates PARP1 (Caito et al., 2010). After the exposure of mice to ionizing radiation, it was found that PARP1 activities and intracellular calcium concentrations increased in the testis, while SIRT1 activities and expression significantly decreased. FA reversed the expression of SIRT1, maintained testosterone levels, and reduced oxidative stress, while regulating PARP1 and cytosolic calcium concentrations to ameliorate spermatogenesis disorders (El-Mesallamy et al., 2018). Accumulated ROS enhances p53 nuclear transport, expands ataxia capillaries, and activates mutant protein (ATM). Using a radiation damage mice model, Das et al. showed that FA enhanced the nuclear translocation of nuclear factor Nrf2 and activated the NHEJ repair pathway in response to ROS-mediated oxidative stress and DNA damage (Das et al., 2017a). In addition to inhibiting DNA damage and promoting DNA repair, FA was also involved in the regulation of inflammatory pathways and related factors. It was shown to inhibit the expression of Cox-2 and inducible nitric oxide synthase 2 (iNOS-2) after irradiation, control the phosphorylation/activation of IKKα/β and IκBα pathways, and regulate the downstream NF-κB nuclear translocation, thus ameliorating radiation-induced inflammation (Das et al., 2014).
In the intestines, FA was shown to interfere with the ROS/NF-κB/Nrf2/p53-caspase 3-PARP axis. In this processes, it enhanced the expression of Mn-SOD and Heme oxygenase-1 (HO-1) that inhibited peroxidation, regulated phosphatidylserine and mitochondrial membrane potential, and suppressed the activation of downstream mitochondrial apoptotic pathways (Das et al., 2017b). Therefore, FA can regulate cell cycle while inhibiting lipid peroxidation and radiation-induced cell apoptosis. Ionizing radiation activates stress marker cyclin (Cdc42) and down-regulates the activation of survival pathways by inhibiting the phosphorylation of phosphatidylinositol 3-kinase (PI3K) and serine/threonine kinase (Akt). The phosphatase gene (PTEN) is a critical molecule that regulates the survival pathway of PI3K/Akt. Ionizing radiation significantly increases the expression of PTEN that promotes cell cycle arrest and inhibits survival-related pathways. It has been demonstrated that FA lowers the overexpression of Cdc42, apoptotic proteins (p53, p21, Bax, and PTEN), and increases PI3K phosphorylation. Moreover, reduced cell cycle arrest inhibits lipid peroxidation while increasing SOD and catalase activities (Das et al., 2016). After radiation, the senescence phenotype of normal cells can be observed with the cells undergoing inflammation and fibrosis (Li et al., 2018b). Due to its antioxidant and anti-inflammatory aspects, FA has been shown to ameliorate these conditions.
Salvia miltiorrhiza Bunge is an alternative therapeutic option for cardiovascular and cerebrovascular diseases. It has anti-inflammatory, antioxidant, and anti-cancer biologic properties (Shi et al., 2019a). Salvianic acid A is the active compound in Salvia miltiorrhiza Bunge that has been shown to inhibit apoptosis and reduce ionizing radiation associated DNA damage (Juan et al., 2012). An in vivo assay established that salvianic acid A protects the hematopoietic system and improves survival after radiation (Juan et al., 2015).
Polyphenols exhibit antioxidant properties that inhibit DNA damage caused by peroxide free radicals (Fraga et al., 2019). Resveratrol is a vital plant antitoxin that possesses antioxidant and anti-inflammatory effects. Resveratrol inhibits NF-κB - activated inflammatory cytokine secretion by up-regulating the expression of peroxisome proliferation-activated receptor (PPAR-4) and SIRT1. These effects prevent ionizing radiation-induced premature ovarian failure (Said et al., 2016). In vitro experiments, Basso et al. demonstrated that resveratrol is involved in inhibiting DNA damage after radiation through the assessment of human lymphocyte DNA damage, repair kinetics, and histone deacetylase activity (Basso et al., 2016). It also improves the morphology of the small intestine, reduces crypt cell apoptosis, regulates Sirt1 and acetylated p53, and has a radioprotective role (Zhang et al., 2017a). Resveratrol improves thymic and spleen atrophy, lymphocyte counts, and proliferation caused by ionizing radiation. Moreover, it inhibits serum levels of IL-2, IL-4, IL-7, and IFN-γ thereby regulating immune functions (Zhang et al., 2018a). Green tea is also rich in polyphenols. Tea polyphenols can improve hematopoietic functions after irradiation (Hu et al., 2011), inhibit oxidative stress and mitochondrial apoptosis as well as prevent radiation induced spermatogenic cell death (Ding et al., 2015).
The Ginseng oligopeptide (GOP) reduces the concentration of plasma diamine oxidase and LPS, and inhibits the secretion of IL-1 as well as TNF-α. It also protects the intestinal epithelial barrier by up-regulating the expression of tight junction proteins (ZO-1 and Occludin). It promotes intestinal repairs by suppressing the expression of apoptosis-related proteins (Bax and Caspase-3) and elevating lymphocyte (CD3 +, CD4 +, CD8 +) concentrations (He et al., 2017; He et al., 2018). He et al. reported that prophylactic administration of GOP exhibited radioprotective effects while post-treatment was beneficial for the quick repair of irradiation-induced injuries (He et al., 2017).
In summary, various radioprotective compounds occur in traditional Chinese medicines. This implies that the anti-radiation mechanisms of Chinese herbal medicines are multi-faceted. However, Chinese herbal contains sophisticated compounds. If only an active compound is used to explore the effect mechanism, there may be limitations.
Extracts from Chinese herbal medicines have been used to comprehensively elucidate on the anti-radiation mechanisms of Chinese Herbal Medicines (Table 2).
Studies have confirmed that Panax ginseng C.A.Mey. reduces the overall or local cancer rates induced by long-term exposures to radiation (Bespalov et al., 2014). Ionizing radiation causes acute myelosuppression and leads to the apoptosis of hematopoietic stem cells as well as hematopoietic progenitor cells. These pathological changes are the primary causes of death after exposure to moderate-to-high radiation doses (Shao et al., 2014). The extract of Panax ginseng C.A.Mey. was shown to increase bone marrow cells, spleen cells, and granulocyte-macrophage colony-forming units (CFU-GM) in mice while promoting the secretion of endogenous cytokines (IL-1, IL-6, and IL-12) to rejuvenate hematopoietic functions (Song et al., 2003). In addition, the extract inhibited the decrease in red blood cell counts, hemoglobin and hematocrit, as well as prevented radiation induced anemic symptoms (Verma et al., 2011). The extract of Panax ginseng C.A.Mey. has been reported to protect the hematopoietic system from ionizing radiation by inhibiting cyclooxygenase 2 (COX-2) and down-regulating activated p38 MAPK and PI3K/Akt pathways (Koo et al., 2013). Ionizing radiation-induced changes in the cellular microenvironment affect the immune system (Frey et al., 2017). Panax ginseng C.A.Mey. has immune regulation properties (Lee et al., 2005). Studies have shown that Panax ginseng C.A.Mey. elevates the mRNA expression of Th1 and Th2 cytokines and inhibits immunosuppression after radiation by stimulating normal spleen cells in mice (Han et al., 2005). Panax ginseng C.A.Mey. also exerts its radioprotective effects by inhibiting the expression of IL-1β in macrophages while simultaneously preventing the signal cascade of CHK2 and nuclear factor kappa B (NF-κB) (Lee et al., 2014). By destroying the intestinal epithelial barrier, radiation therapy enhances intestinal permeability and mucosal injury. Intestinal injuries lead to high plasma lipopolysaccharide (LPS) levels and elevated pro-inflammatory cytokine secretions that trigger a series of inflammatory reactions and bowel syndrome (Romesser et al., 2019). Moreover, Panax ginseng C.A.Mey. was shown to improve appetite in rats and reduced anorexic symptoms after radiation (Balaji Raghavendran et al., 2012). Ionizing radiation also causes damage to other tissues and organs. Oral administration of the extract of Panax ginseng C.A.Mey. before irradiation inhibited the suppression of serum creatine kinase and lactate dehydrogenase levels, suppressed urea and creatinine levels and further protected against irradiation-induced cardio-nephrotoxicity by enhancing antioxidant activities and inhibiting endothelial dysfunctions (Mansour, 2013). Through its antioxidant mechanisms, Panax ginseng C.A.Mey. can inhibit catalase activity, increase glutathione content, suppress the expression of IL-1β, TNF-α, and alleviates inflammation in radiation-induced lung injuries (Jang et al., 2015). Ionizing radiation leads acute skin damage (Park et al., 2018). The extract of Panax ginseng C.A.Mey. was shown to suppress the secretion of β-hexosaminidase, histamine, intracellular ROS, and internal Ca2+. It was also revealed that Black Panax ginseng C.A.Mey. inhibited mastocyte-mediated signaling activities, suppressed IL-4 serum levels, and ameliorated the symptoms and clinical signs of post-radiation allergic dermatitis (Kang et al., 2018). These studies suggest that Panax ginseng C.A.Mey. may be a useful herb against radiation associated damage.
Panax quinquefolius L. belongs to the Araliaceae family and has yin nourishment as well as heat clearing properties. From a clinical observation study, it was suggested that Panax quinquefolius L. ameliorated lymphocytic DNA damage after radiotherapy, suppressed micronucleus ratios in human lymphocytes, and enhanced total antioxidant capacities after exposure to radiation (Lee et al., 2010). In addition, this herb protects genes from acute damage in a short period. Studies have established that Panax quinquefolius L. tea protects cellular DNA from oxidative stress damage for at least 2 h (Szeto et al., 2015).
A fresh rhizome of Zingiber officinale Roscoe has been reported as being able to inhibit lipid peroxidation and excessive glutathione consumption (Jagetia et al., 2003), and improve mice survival after irradiation (Jagetia et al., 2004). Ji et al. demonstrated that the extract of Zingiber officinale Roscoe suppresses ionizing radiation-induced overproduction of ROS and DSBs in human mesenchymal stem cells. Its antioxidant mechanisms involve the induction of NRF2 nuclear translocation and activation of its downstream cell protection genes (HO- 1 and NQO-1) (Ji et al., 2017). Radiotherapy confers adverse side effects such as vomiting and nausea. These side effects have been attributed to ionizing radiation associated damage of the gastrointestinal, viscera and the vagus nerve to release serotonin that activates the brains vomiting center through serotonin receptors. Furthermore, radiation affects neural activity in the brain and activates specific sensory receptors. Zingiber officinale Roscoe can ameliorate nausea and vomiting. Zingiber officinale Roscoe inhibits the activation of related receptors, promotes neurobehavioral functions, and alleviates radiation-induced taste aversion and vomiting (Sharma et al., 2005). Besides, Zingiber officinale Roscoe plays a role in the regulation of inflammatory signaling pathways. Zingerone suppresses the MAPK signaling pathway, inhibits cytochrome P4502E1 as well as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and downregulates liver enzyme activities. Further, it has been shown to regulate the expression of inflammatory markers such as TLR4, iNOS, COX-2, and MPO (Mohamed and Badawy, 2019). Negative regulation of the TLR4 pathway by zingerone alleviates radiation-induced hepatic injury (Lee et al., 2018). Epidemiological studies have indicated that adults with congenital heart diseases are exposed to low-dose ionizing radiations during cardiac surgeries. These patients have an increased risk for cancer when compared to the general population (Cohen et al., 2018). Studies have shown that prophylactic administration of zingerone regulates serum lactate dehydrogenase, creatine kinase-MB activity and suppresses the expression of TNF-α as well as COX-2. It also inhibits DNA fragmentation, enhances mitochondrial complex activity, and interferes with the aggravation of ionizing radiation induced heart damage (Soliman et al., 2018). The antioxidant and anti-inflammatory effects of Zingiber officinale Roscoe in other tissue damage models have not been established.
Chest radiotherapy induces myocardial fibrosis (Curigliano et al., 2016) while whole-body radiation can lead to osteopontin (OPN) activation. OPN is a cytokine involved in myocardial fibrosis. The extract of Angelica sinensis (Oliv.) Diels inhibits radiation induced cardiac fibrosis by suppressing the expression of OPN, c-jun, and miRNA-21 as well as suppressing Troponin-I (Tn-I) levels (Ma et al., 2019). The main active components in Paeonia lactiflora Pall. have been shown to have radioprotective properties.
Different parts of Ginkgo biloba L. in the Ginkgo family can be used as alternative medicine. Ginkgo biloba L. leaves have been utilized in studies of radiation-associated injuries. Ionizing radiation induces permanent nervus cerebral defects, chronic microangiopathy, and blood-brain barrier dysfunctions. Radiation associated brain damage results in cerebrovascular abnormalities, demyelination, white matter necrosis, and cognitive impairment (Lumniczky et al., 2017). Abnormally elevated levels of catecholamine, epinephrine, norepinephrine, dopamine, and inflammatory factors are the major causes of radiation-induced brain injuries. Ismail et al. confirmed that the extract of Ginkgo biloba L. regulated the above mentioned indicators by suppressing lipid peroxidation (Ismail and El-Sonbaty, 2016). In addition, that the extract of Ginkgo biloba L. was shown to suppress the expressions of P53, Bcl-2 and inhibited apoptosis after radiation (Raafat et al., 2013).
Biologically active compounds in Portulaca oleracea L. include flavonoids, alkaloids, terpenoids, and sterols. These compounds have been shown to have antioxidant, anti-bacterial, and anti-inflammatory properties. Portulaca oleracea L. extracts alleviated lipid peroxidation in the liver and kidney of irradiated rats. It also suppressed MDA levels in tissues and inhibited total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-c), and maintained atherosclerosis indices (Abd El-Azime et al., 2014). From the mechanism perspective, Portulaca oleracea L. may also play an anti-inflammatory role by inhibiting TNF-α secretion, thereby preventing NF-κB nuclear translocation. This herb also plays an important role in regulating peroxidation (Zhou et al., 2015). Mentha canadensis L. from the Labiatae family inhibits radiation induced damage by scavenging for free radicals, its antioxidant, anti-inflammatory, anti-mutation activities, and enhancing DNA repair (Baliga and Rao, 2010).
The antioxidant properties of the Solanaceae Lycium barbarum L. are important in antagonizing mitochondrial apoptosis and inhibiting DNA damage. Duan et al. documented that Lycium barbarum L. increases the DNA content of red blood cells and hemoglobin, effectively inhibits P53, caspase-3, and caspase-6 while accelerating the recovery of splenic functions (Duan et al., 2015). Crataegus pinnatifida Bunge is rich in polyphenols and total flavonoids. An extract of Crataegus pinnatifida Bunge has been established to reduce lymphocytic micronucleus and lowers the effects of radiation (Hosseinimehr et al., 2009). While an extract of Hippophae rhamnoides L. scavenges for free radicals, prevents cell cycle arrest in the G2-M phase (Goel et al., 2003), and protects mitochondria and chromatin from radiation-induced damage (Shukla et al., 2006). Moreover, Hippophae rhamnoides L. protects against radiation-induced sperm injuries (Goel et al., 2006).
In conclusion, the above reviews show that a single Chinese herbal medicine can ameliorate radiation-induced damage in a variety of ways. These herbs have considerable potential as radioprotectants.
Traditional Chinese Medicine’s clinical efficacy exerted in the form of Chinese herbal prescriptions, is based on clinical symptoms and purposefully matched different Chinese medicines. Experimental and clinical studies have verified the therapeutic effect of Chinese Herbal Prescriptions (Zhang et al., 2016; Gao et al., 2017). Compared to a single herb or active compound, Chinese Herbal Prescription contains many active compounds with multiple therapeutic targets. These Prescriptions are suitable for the prevention and treatment of multiple ionizing radiation induced systemic damage. Synergism between the different herbs enhance therapeutic effects with reduced toxicity (Zhang et al., 2018b) (Table 3).
The hematopoietic system is highly sensitive to radiation. Hematopoietic cells exposed to ionizing radiation degenerate quickly, and undergo necrosis as well as apoptosis. In addition, ionizing radiation decreases the number of peripheral blood cells, especially neutrophils, lymphocytes, and platelets, leading to bleeding and anemia.
Siwu Tang is a classical Chinese herbal prescription that reinforces qi and nourishes the blood. It is comprised of four Chinese herbal medicines (Rehmannia glutinosa (Gaertn.) DC., Angelica sinensis (Oliv.) Diels, Conioselinum anthriscoides ‘Chuanxiong', and Paeonia lactiflora Pall.) (Sun et al., 2016). The active compounds in this prescription include fructose, paeoniflorin, and ferulic acid, among others. This Prescription promotes hematopoietic and immune system recovery after irradiation by increasing the number of peripheral white blood cells and bone marrow colony-forming units (Liang et al., 2006). Studies revealed that Siwu Tang induces the antioxidant Nrf2 pathway, partially inhibits DNA damage, prevents the activation of nuclear transcription factor activating protein-1 (AP-1) and NF-κB, thereby, inhibiting ionizing radiation-induced damage and oncogenesis (Liu et al., 2017).
Buzhong Yiqi Tang is a well-known Chinese herbal prescription with nearly 800 years of application. It is widely used a therapeutic option for spleen-qi deficiencies and post-illness symptoms (Hu et al., 2019; Liu et al., 2019). This prescription is composed of Astragalus mongholicus Bunge, Codonopsis pilosula (Franch.) Nannf., Atractylodes lancea (Thunb.) DC., Bupleurum chinense DC., Actaea cimicifuga L., Citrus reticulata Blanco, Angelica sinensis (Oliv.) Diels, and Glycyrrhiza glabra L.. This prescription has been shown to significantly elevate peripheral white blood cell counts in mice after irradiation and relieves platelet damage (Xiao-fang et al., 2013). Further, it suppresses lipid peroxides generated by the accumulation of free radicals and improves the hematopoietic microenvironment (Xiaoling et al., 2006).
Xuebijing (XBJ) is an injection of Chinese herbal prescription. It was approved by the National Medical Products Administration for the clinical management of septicemia. It contains Carthamus tinctorius L., Paeonia lactiflora Pall., Conioselinum anthriscoides 'Chuanxiong', Salvia miltiorrhiza Bunge, and Angelica sinensis (Oliv.) Diels. A previous study documented that XBJ improved the survival rate of irradiated mice by suppressing ROS production in bone marrow cells and alleviated radiation induced hematopoietic cell injury (Li et al., 2014a).
In the study of Yiqi Yangyin Fang (YYF: Astragalus mongholicus Bunge, Panax ginseng C.A.Mey., Ligustrum lucidum W.T.Aiton, Eclipta prostrata (L.) L., Angelica sinensis (Oliv.) Diels, Atractylodes macrocephala Koidz., Poria cocos (Schw.) Wolf, Glycyrrhiza glabra L.), Junling Zhang et al. indicated that this prescription increased the number of bone marrow cells, hematopoietic progenitor cells, and hematopoietic stem cells. It also improved bone marrow suppression after radiation by suppressing intracellular ROS levels (Zhang et al., 2017b).
Ionizing radiation damages the hematopoietic system, the thymus and the spleen. Radiation inhibits tissue and cell repair after injury and induces immunosuppression. Inhibition is proportional to the radiation dose. Hematopoietic and immune dysfunctions associated with ionizing radiation elevate tissue permeability, weaken body resistance, and predisposes the body to endogenous or exogenous infections.
HemoHIM is a prescription composed of three herbs with various biological and immunological activities. HemoHIM inhibits the continuous down-regulation of Th1 immune responses after radiation by regulating the IL-12 p70/pSTAT4 signaling pathway (Park et al., 2012). This prescription has also been shown to protect hematopoietic stem cells and speeds up the recovery of immune cells (Park et al., 2014).
Bushen Jiedu Recipe is an optimized combination of Liuwei Dihuang Pills. This prescription maintains kidney tone, nourishes yin, tonifies Qi and blood, clears heat and removes toxin. Yunjing et al. documented that Bushen Jiedu Recipe interfered with the expression of NF-κBp65 by regulating the TLR4 signaling pathway, suppressed white blood cell damage and protected the thymus and spleen (Yunjing et al., 2015; Lidan et al., 2016).
The Wumai Danghuang Oral Liquid is composed of Schisandra chinensis (Turcz.) Baill., Ophiopogon japonicus (Thunb.) Ker Gawl., Codonopsis pilosula (Franch.) Nannf., and Astragalus mongholicus Bunge. This prescription exhibits its anti-radiation effects by elevating SOD, degrading MDA and inhibiting the generation of free radicals (Chunhong et al., 2014). In addition, Wumai Danghuang Oral Liquid exhibits protective and repair effect on radiation induced immune injuries (Hang et al., 2013).
Houttuynia cordata Thunb. and its bioactive molecules have anti-inflammatory and antioxidant properties (Shingnaisui et al., 2018). Co-Herba Houttuyniae Oral Liquid contains Houttuynia cordata Thunb., Panax ginseng C.A.Mey., Lycium barbarum L.. This liquid suppresses the rate of radiation induced chromosomal aberrations (Lin et al., 2001). It also enhances the anti-stress ability of mice by improving immune functions. Moreover, Panax ginseng C.A.Mey. and Lycium barbarum L. improve immune functions and inhibit radiation effects by promoting the repair of damaged cells and tissues (Lin et al., 2002).
The radioprotective prescription (Astragalus mongholicus Bunge, Ganoderma Lucidum Karst, Lycium barbarum L., and Poria cocos (Schw.)Wolf) has been shown to improve the survival rate, white blood cell count, thymus index, spleen index, and the DNA content of bone marrow cells thereby reducing radiation induced immune damage in mice models (Liming et al., 2011).
Ionizing radiation severely damages the digestive system and causes pathological changes such as intestinal mucosal damage and liver fibrosis. These damages lead to digestive and absorptive dysfunctions, resulting in a series of clinical symptoms like diarrhea, nausea, and vomiting.
STW 5 is a herbal prescription with anti-inflammatory and antioxidant properties. This prescription inhibits oxidative stress responses, suppresses the levels of inflammation factors, and intestinal damage indices by regulating apoptosis-related factors to prevent intestinal mucosal damage after radiation (Khayyal et al., 2014). Prophylactic administration of STW 5 reduces the severity of radiation mucositis (El-Ghazaly et al., 2015).
Astragalus immortal prescription is composed of Rehmannia glutinosa (Gaertn.) DC., Ophiopogon japonicus (Thunb.) Ker Gawl. and Equus asinus L.. It is derived from Dunhuang medical papers (now stored in France, code: P.4038), and Astragalus mongholicus Bunge. This prescription has been shown to elevate the activities of GSH-Px and SOD while suppressing MDA levels in the liver. Furthermore, it protects the liver from radiation induced oxidative damage (Cai-qin et al., 2017).
The male reproductive system is very sensitive to ionizing radiation as it can damage the seminiferous epithelium and spermatogenic cells at all levels. These radiations can confer injuries to the reproductive system, and cause male infertility (Bates et al., 2016; Kesari et al., 2018). Radiation also causes DNA damage in spermatogenic cells, increases embryonic mortality, and offspring cancer susceptibility. It may induce hereditary changes.
Wuzi Yanzong Pill (WZYZ) is a Chinese herbal prescription that is used as a therapeutic option for male infertility. Clinically, it has significant therapeutic effects on oligospermia and asthenozoospermia. WZYZ improves sperm quality by suppressing DNA damage (Zhao et al., 2018). A double-blind randomized controlled trial confirmed that WZYZ is an excellent therapeutic option for men with low fertility who cannot be cured by conventional western medicines (Zhao et al., 2019). Pelvic exposure to radiation reduces testicular weight, sperm quality and leads to testicular oxidative stress and abnormal testicular structure. WZYZ protects against suppressed serum testosterone levels, reduces MDA levels and oxidative stress indices (OSI) in the testis. Its mechanism may be associated with up-regulation of PCNA (Ji et al., 2016).
The Yiqi Jiedu Decoction (YQJD) enhances testicular index, structural recovery of the testis, decreases the apoptotic rate of spermatogenic cells, and maintains spermatogenic functions after irradiation. These results suggest that YQJD plays a protective role by intervening in TLR5 downstream signaling pathways (Wang et al., 2020).
Oxidative stress leads to the pathogenesis of various human diseases and the aging process. Mitochondria is the energy center in cell metabolism, it regulates redox homeostasis, and plays a central role in diseases pathogenesis (Li et al., 2019a). Oxidative stress damages the mitochondria, accelerates excessive ROS production, activates the mitochondrial apoptotic pathway and induces apoptosis (Kim and Kim, 2018). Ionizing radiation mediated overproduction of ROS is associated with mitochondrial dysfunctions. ROS acts as a signaling molecule that initiates a series of cascade reactions (Wu et al., 2019). Changes in the mitochondrial membrane potential elevates the expressions of pro-apoptotic proteins and suppresses the expression of anti-apoptotic proteins (Sun et al., 2018). These events trigger the activation of caspase-3, and initiates the mitochondrial-dependent pathway (Li et al., 2018a). As previously stated, Chinese Herbal Medicines inhibit mitochondrial apoptosis by: i. inhibiting oxidative stress responses and suppressing the generation of ROS, and ii. Interfering with the expression of pathway associated factors, regulating the pro-apoptotic protein and anti-apoptotic protein ratios, as well as by suppressing Cytochrome C and caspase-3.
Mitogen-activated protein kinases (MAPK) family, including three significant members of extracellular signal-regulated kinases (ERK), p38 kinase, and c-Jun N-terminal kinase (JNK), participate in various physiological processes such as morphogenesis, cell growth, proliferation, apoptosis, and differentiation (Fang and Richardson, 2005; Lu et al., 2019). Ionizing radiation activates the classic MAPK signaling pathway, JNK, and P38 MAPK pathways. In addition, radiation-induced secreted cytokines enhance MAPK pathway responses in cells (Dent et al., 2003). Enhancement of the MAPK signal upregulates telomerase activity, initiates changes in chromatin distribution, and regulates the cell cycle (Shain et al., 2018). Activated p38 and JNK signaling pathways are involved in immune regulation (Wang et al., 2019a). After p38 activation, a mitochondrial apoptotic pathway is initiated (Choi et al., 2006; Niaudet et al., 2017). The PI3K/AKT signaling pathway is essential in the regulation of cell growth, migration, proliferation, and metabolism in mammals (Pompura and Dominguez-Villar, 2018). Activated PI3K/Akt pathway accelerates DSB repair. Ionizing radiation inhibits its activation (Toulany and Rodemann, 2015). This pathway is also involved in cell cycle and apoptosis regulation after exposure to ionizing radiation (Chen et al., 2018). Because of the wide reach of the MAPK and PI3K/AKT signaling pathways, Chinese Herbal Medicines regulate them through multiple targets.
The Nrf2/HO-1 signaling pathway antagonizes tissue and organ oxidative stress injuries by regulating antioxidant, anti-inflammatory, apoptosis, pyroptosis, ferroptosis, and autophagy processes. Nrf2 is a transcription factor and the core regulator of cellular redox. It stimulates gene expression through antioxidant response elements in gene promoters and protects cells against ROS induced DNA damage (Zimta et al., 2019). When exposure to ionizing radiation occurs, Nrf2 acts as a critical transcription factor that regulates antioxidant enzymes and protects tissues from oxidative stress damage. HO-1 regulates the expression of apoptotic and inflammatory factors. In addition, it also promotes angiogenesis by preventing oxidative damage (Loboda et al., 2016). Chinese Herbal Medicines trigger Nrf2 and enhances mRNA and protein expressions of HO-1. These events trigger the antioxidant pathway and inhibits ionizing radiation induced oxidative damage.
Ionizing radiation-induced DNA damage results in phosphorylation and activation of multiple transcription factors (such as NF-κB, p53, and MAPK) by stimulated ATM kinases. ROS is also involved in these processes (Purbey et al., 2017). After exposure to ionizing radiation, the secretion of various inflammatory cytokines (IL-1, IL-6, TNF-α, IFN-γ, COX-2) is elevated. Inflammatory cytokines recruit immune cells that regulate cell microenvironment with a crucial impact on local or systemic tissues (Harding et al., 2017). Chinese Herbal Medicines exhibit a two-way regulation effect on inflammatory factors. The first one is by inhibiting the secretion of inflammatory factors, preventing fibrosis and inflammatory lesions after irradiation. The second strategy is that it plays an immunomodulatory role by regulating signaling pathways such as TLRs and NF-κB to reduce apoptosis (Scholch et al., 2015; Liu et al., 2018).
Traditional Chinese Medicine exhibits its curative effects on multiple body systems (such as hematopoiesis, immunity, reproduction, respiration, and circulation) by inhibiting oxidative stress, reducing DNA damage, and regulating abnormally activated signaling pathways. The Mechanism is as shown in Figure 2. It is worth noting that Chinese Herbal Medicines are particularly useful in anti-lipid peroxidation. Recently, a new regulatory cell death method (ferroptosis) has attracted considerable attention. Excessive ROS results in membrane lipid peroxidation. The accumulation of iron-dependent lipid peroxides leads to ferroptosis. Radiation induced damage to the hematological system and the lungs can be relieved by intervening in ferroptosis-related pathways (Li et al., 2019c; Zhang et al., 2020). This is an avenue for Traditional Chinese Medicine research in future.
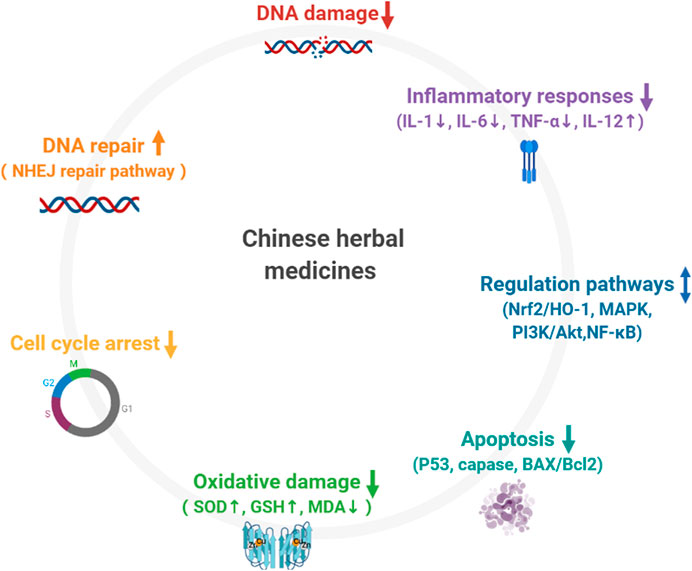
FIGURE 2. Mechanism of Chinese herbal medicines in preventing radiation injury. 1) Chinese herbal medicines reduce DNA damage; 2) Chinese herbal medicine promote DNA repair; 3) Chinese herbal medicines regulate cell cycle arrest; 4) Chinese herbal medicines prevent excessive accumulation of ROS and inhibits oxidative damage; 5) Chinese herbal medicine suppress extrinsic and intrinsic apoptotic pathways via reduction of P53, caspase, BAX/Bcl2 activation; 6) Chinese herbal medicines are involved in the regulation of inflammatory response; 7) Chinese herbal medicines participate in the regulation of multiple abnormally activated signaling pathways.
Ionizing radiation injuries are systemic damages that affect multiple organs and tissues. The Traditional Chinese Medicine characteristic theory lies in its holistic view: man and nature as a harmonious and unified whole, emphasizing the interactions between man and the environment, achieving a balance between the two. Simultaneously, various systems of the human body as a whole are connected physiologically and pathologically influence each other. Based on this holistic view, Chinese medicines and Chinese Herbal Prescriptions are suitable for use as therapeutic options for multi-system damages caused by ionizing radiation.
Compared to a single herb, the composition of Chinese Herbal Prescription is more complex and exhibits its therapeutic effects by having multiple targets (Yang et al., 2017; Wang et al., 2019b). The synergy between the Chinese medicines in the Chinese Herbal Prescriptions improves its efficacy while inhibiting toxic and side effects. A rationally designed Chinese Herbal Prescription will exhibit a better protective effect.
Chinese medicine has favorable economic benefits and is an economical option for the development of safe and effective radioprotectors. With positive effects, many Chinese Herbal Prescriptions have been used in clinical settings to reduce radiation induced damage. Studies on the anti-radiation activities and mechanisms of single Chinese medicines and their active compounds are limited. However, due to their sophisticated active compounds, it is difficult to elucidate on their potential radio-protective mechanisms. More studies are needed to evaluate the efficacy of these medicines. Systemic biology and network pharmacology applications may provide alternative methods and strategies for the applications of Chinese Herbal Prescriptions (Boezio et al., 2017; Tavassoly et al., 2018), and may, therefore, help in the development of innovative drugs for radiation protection.
XZ conceived the topic and wrote the manuscript. SH revised and modified the manuscript. XC, LW, CH, and ZS helped to revise the manuscript and draw the figures. QF, WX and SZ consulted the references.
This work was supported by the National Natural Science Foundation of China (No. 11675027, 12075035).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abd El-Azime, A. S., Hussein, E. M., and Ashry, O. M. (2014). Synergestic effect of aqueous purslane (Portulaca oleracea L.) extract and fish oil on radiation-induced damage in rats. Int. J. Radiat. Biol. 90 (12), 1184–1190. doi:10.3109/09553002.2014.926040
Ahmad, M. F. (2018). Ganoderma lucidum: persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 107, 507–519. doi:10.1016/j.biopha.2018.08.036
Amsterdam, J. D., and Panossian, A. G. (2016). Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 23 (7), 770–783. doi:10.1016/j.phymed.2016.02.009
Anuranjani, , and Bala, M. (2014). Concerted action of Nrf2-ARE pathway, MRN complex, HMGB1 and inflammatory cytokines - implication in modification of radiation damage. Redox. Biol. 2, 832–846. doi:10.1016/j.redox.2014.02.008
Arring, N. M., Millstine, D., Marks, L. A., and Nail, L. M. (2018). Ginseng as a treatment for fatigue: a systematic review. J. Alternative Compl. Med. 24 (7), 624–633. doi:10.1089/acm.2017.0361
Bai, P., and Virag, L. (2012). Role of poly(ADP-ribose) polymerases in the regulation of inflammatory processes. FEBS Lett. 586 (21), 3771–3777. doi:10.1016/j.febslet.2012.09.026
Balaji Raghavendran, H. R., Rekha, S., Cho, H. K., Jang, S. S., and Son, C. G. (2012). Ginsenoside rich fraction of Panax ginseng C.A. Meyer improve feeding behavior following radiation-induced pica in rats. Fitoterapia 83 (6), 1144–1150. doi:10.1016/j.fitote.2012.04.008
Baliga, M. S., and Rao, S. (2010). Radioprotective potential of mint: a brief review. J. Canc. Res. Therapeut. 6 (3), 255–262. doi:10.4103/0973-1482.73336
Basso, E., Regazzo, G., Fiore, M., Palma, V., Traversi, G., and Testa, A., et al. (2016). Resveratrol affects DNA damage induced by ionizing radiation in human lymphocytes in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen 806, 40–46. doi:10.1016/j.mrgentox.2016.07.005
Bates, G. E., Taub, R. N., and West, H. (2016). Fertility and cancer treatment. JAMA Oncol. 2 (2), 284. doi:10.1001/jamaoncol.2015.4143
Beach, T. A., Johnston, C. J., Groves, A. M., Williams, J. P., and Finkelstein, J. N. (2017). Radiation induced pulmonary fibrosis as a model of progressive fibrosis: contributions of DNA damage, inflammatory response and cellular senescence genes. Exp. Lung Res. 43 (3), 134–149. doi:10.1080/01902148.2017.1318975
Begg, A. C., Stewart, F. A., and Vens, C. (2011). Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Canc. 11 (4), 239–253. doi:10.1038/nrc3007
Bespalov, V. G., Alexandrov, V. A., Semenov, A. L., Kovan'Ko, E. G., and Ivanov, S. D. (2014). Anticarcinogenic activity of alpha-difluoromethylornithine, ginseng, eleutherococcus, and leuzea on radiation-induced carcinogenesis in female rats. Int. J. Radiat. Biol. 90 (12), 1191–1200. doi:10.3109/09553002.2014.932937
Bing, S. J., Kim, M. J., Ahn, G., Im, J., Kim, D. S., and Ha, D., et al. (2014). Acidic polysaccharide of Panax ginseng regulates the mitochondria/caspase-dependent apoptotic pathway in radiation-induced damage to the jejunum in mice. Acta Histochem. 116 (3), 514–521. doi:10.1016/j.acthis.2013.11.012
Boezio, B., Audouze, K., Ducrot, P., and Taboureau, O. (2017). Network-based approaches in pharmacology. Mol. Inform. 36 (10), 1–11. doi:10.1002/minf.201700048
Brizel, D. M. (2007). Pharmacologic approaches to radiation protection. J. Clin. Oncol. 25 (26), 4084–4089. doi:10.1200/JCO.2007.11.5816
Cai-qin, F., Yong-qi, L., Li-ying, Z., Xiao-ming, X., Tong-tong, Z., Juan, L., et al. (2017). Protective effects of Astragalus immortal prescription against liver oxidative stress in irradiated mice. Med. J. Chin. Peoples Lib. Army 42 (12), 1061–1065. doi:10.11855/j.issn.0577-7402.2017.12.07
Caito, S., Hwang, J. W., Chung, S., Yao, H., Sundar, I. K., and Rahman, I. (2010). PARP-1 inhibition does not restore oxidant-mediated reduction in SIRT1 activity. Biochem. Biophys. Res. Commun. 392 (3), 264–270. doi:10.1016/j.bbrc.2009.12.161
Chen, L., and Huang, G. (2018). Antitumor activity of polysaccharides: an overview. Curr. Drug Targets 19 (1), 89–96. doi:10.2174/1389450118666170704143018.
Chen, Y. A., Tzeng, D. T. W., Huang, Y. P., Lin, C. J., Lo, U. G., and Wu, C. L., et al. (2018). Antrocin sensitizes prostate cancer cells to radiotherapy through inhibiting PI3K/AKT and MAPK signaling pathways. Cancers 11 (1), 34. doi:10.3390/cancers11010034
Choi, S. Y., Kim, M. J., Kang, C. M., Bae, S., Cho, C. K., and Soh, J. W., et al. (2006). Activation of Bak and Bax through c-abl-protein kinase Cdelta-p38 MAPK signaling in response to ionizing radiation in human non-small cell lung cancer cells. J. Biol. Chem. 281 (11), 7049–7059. doi:10.1074/jbc.M512000200
Chunhong, X., Peng, L., Haibo, L., Mei, X., and Xia, Y. (2014). Influence of wumaidanghuang oral liquid on free radicals scavenging capacity in visceral organs of radiation injured mice. Chin. Pharm. 23 (7), 20–21.
Citrin, D., Cotrim, A. P., Hyodo, F., Baum, B. J., Krishna, M. C., and Mitchell, J. B. (2010). Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncol. 15 (4), 360–371. doi:10.1634/theoncologist.2009-S104
Cohen, S., Liu, A., Gurvitz, M., Guo, L., Therrien, J., Laprise, C., et al. (2018). Exposure to low-dose ionizing radiation from cardiac procedures and malignancy risk in adults with congenital heart disease. Circulation 137 (13), 1334–1345. doi:10.1161/CIRCULATIONAHA.117.029138
Curigliano, G., Cardinale, D., Dent, S., Criscitiello, C., Aseyev, O., and Lenihan, D., et al. (2016). Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA A Cancer J. Clin. 66 (4), 309–325. doi:10.3322/caac.21341
Das, U., Biswas, S., Sengupta, A., Manna, K., Chakraborty, A., and Dey, S. (2016). Ferulic acid (FA) abrogates ionizing radiation-induced oxidative damage in murine spleen. Int. J. Radiat. Biol. 92 (12), 806–818. doi:10.1080/09553002.2016.1230241
Das, U., Manna, K., Khan, A., Sinha, M., Biswas, S., and Sengupta, A., et al. (2017a). Ferulic acid (FA) abrogates γ-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radic. Res. 51 (1), 47–63. doi:10.1080/10715762.2016.1267345
Das, U., Manna, K., Sinha, M., Datta, S., Das, D. K., and Chakraborty, A., et al. (2014). Role of ferulic acid in the amelioration of ionizing radiation induced inflammation: a murine model. PloS One 9 (5), e97599. doi:10.1371/journal.pone.0097599
Das, U., Sengupta, A., Biswas, S., Adhikary, A., Dey Sharma, R., and Chakraborty, A., et al. (2017b). Alteration of murine duodenal morphology and redox signalling events by reactive oxygen species generated after whole body γ-irradiation and its prevention by ferulic acid. Free Radic. Res. 51 (11-12), 1–25. doi:10.1080/10715762.2017.1388916
Dent, P., Yacoub, A., Fisher, P. B., Hagan, M. P., and Grant, S. (2003). MAPK pathways in radiation responses. Oncogene 22 (37), 5885–5896. doi:10.1038/sj.onc.1206701
Ding, J., Wang, H., Wu, Z. B., Zhao, J., Zhang, S., and Li, W. (2015). Protection of murine spermatogenesis against ionizing radiation-induced testicular injury by a green tea polyphenol. Biol. Reprod. 92 (1), 6. doi:10.1095/biolreprod.114.122333
Duan, Y., Chen, F., Yao, X., Zhu, J., Wang, C., and Zhang, J., et al. (2015). Protective effect of Lycium ruthenicum murr. Against radiation injury in mice. Int. J. Environ. Res. Publ. Health 12 (7), 8332–8347. doi:10.3390/ijerph120708332
Einor, D., Bonisoli-Alquati, A., Costantini, D., Mousseau, T. A., and Moller, A. P. (2016). Ionizing radiation, antioxidant response and oxidative damage: a meta-analysis. Sci. Total Environ. 548-549, 463–471. doi:10.1016/j.scitotenv.2016.01.027
El-Ghazaly, M. A., El-Hazek, R. M., and Khayyal, M. T. (2015). Protective effect of the herbal preparation, STW 5, against intestinal damage induced by gamma radiation in rats. Int. J. Radiat. Biol. 91 (2), 150–156. doi:10.3109/09553002.2014.954059
El-Mesallamy, H. O., Gawish, R. A., Sallam, A. M., Fahmy, H. A., and Nada, A. S. (2018). Ferulic acid protects against radiation-induced testicular damage in male rats: impact on SIRT1 and PARP1. Environ. Sci. Pollut. Res. Int. 25 (7), 6218–6227. doi:10.1007/s11356-017-0873-6
Evans, J. R. (2013). Ginkgo biloba extract for age-related macular degeneration. Cochrane Database Syst. Rev. (1), CD001775. doi:10.1002/14651858.CD001775.pub2
Fang, J. Y., and Richardson, B. C. (2005). The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 6 (5), 322–327. doi:10.1016/S1470-2045(05)70168-6
Fraga, C. G., Croft, K. D., Kennedy, D. O., and Tomas-Barberan, F. A. (2019). The effects of polyphenols and other bioactives on human health. Food Funct. 10 (2), 514–528. doi:10.1039/c8fo01997e
Frey, B., Ruckert, M., Deloch, L., Ruhle, P. F., Derer, A., and Fietkau, R., et al. (2017). Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol. Rev. 280 (1), 231–248. doi:10.1111/imr.12572
Gao, L., Jia, C., Zhang, H., and Ma, C. (2017). Wenjing decoction (herbal medicine) for the treatment of primary dysmenorrhea: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 296 (4), 679–689. doi:10.1007/s00404-017-4485-7
Gerelchuluun, A., Manabe, E., Ishikawa, T., Sun, L., Itoh, K., and Sakae, T., et al. (2015). The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions. Radiat. Res. 183 (3), 345–356. doi:10.1667/RR13904.1
Goel, H. C., Kumar, I. P., Samanta, N., and Rana, S. V. (2003). Induction of DNA-protein cross-links by Hippophae rhamnoides: implications in radioprotection and cytotoxicity. Mol. Cell. Biochem. 245 (1-2), 57–67. doi:10.1023/a:1022809625826
Goel, H. C., Samanta, N., Kannan, K., Kumar, I. P., and Bala, M. (2006). Protection of spermatogenesis in mice against gamma ray induced damage by Hippophae rhamnoides. Andrologia 38 (6), 199–207. doi:10.1111/j.1439-0272.2006.00740.x
Guohui, T., Ling, M., and Hongmei, W. (2004). Studies of G .lucidum spores powcder on the immunoregulation and antiradiation. Chinese Journal of Food Hygiene 16 (2), 132–134. doi:10.13590/j.cjfh.2004.02.009
Han, S. K., Song, J. Y., Yun, Y. S., and Yi, S. Y. (2005). Ginsan improved Th1 immune response inhibited by gamma radiation. Arch Pharm. Res. (Seoul) 28 (3), 343–350. doi:10.1007/BF02977803
Hang, L., Peng, L., Mei, X., and Haibo, L. (2013). Effects of Wumai Danghuang oral liquid on immune function of radiation damage model mice. China. Pharm. 24 (47), 4442–4444. doi:10.6039/j.issn.1001-0408.2013.47.08
Hao, P., Jiang, F., Cheng, J., Ma, L., Zhang, Y., and Zhao, Y. (2017). Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J. Am. Coll. Cardiol. 69 (24), 2952–2966. doi:10.1016/j.jacc.2017.04.041
Harding, S. M., Benci, J. L., Irianto, J., Discher, D. E., Minn, A. J., and Greenberg, R. A. (2017). Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548 (7668), 466–470. doi:10.1038/nature23470
He, L. X., Wang, J. B., Sun, B., Zhao, J., Li, L., and Xu, T., et al. (2017). Suppression of TNF-α and free radicals reduces systematic inflammatory and metabolic disorders: radioprotective effects of ginseng oligopeptides on intestinal barrier function and antioxidant defense. J. Nutr. Biochem. 40, 53–61. doi:10.1016/j.jnutbio.2016.09.019
He, L. X., Zhang, Z. F., Zhao, J., Li, L., Xu, T., and Bin, S., et al. (2018). Ginseng oligopeptides protect against irradiation-induced immune dysfunction and intestinal injury. Sci. Rep. 8 (1), 13916. doi:10.1038/s41598-018-32188-6
Horemans, N., Spurgeon, D. J., Lecomte-Pradines, C., Saenen, E., Bradshaw, C., and Oughton, D., et al. (2019). Current evidence for a role of epigenetic mechanisms in response to ionizing radiation in an ecotoxicological context. Environ. Pollut. 251, 469–483. doi:10.1016/j.envpol.2019.04.125
Hosseinimehr, S. J., Mahmoudzadeh, A., Azadbakht, M., and Akhlaghpoor, S. (2009). Radioprotective effects of Hawthorn against genotoxicity induced by gamma irradiation in human blood lymphocytes. Radiat. Environ. Biophys. 48 (1), 95–98. doi:10.1007/s00411-008-0190-z
Hu, L., Yao, Z., Qin, Z., Liu, L., Song, X., and Dai, Y., et al. (2019). In vivo metabolic profiles of Bu-Zhong-Yi-Qi-Tang, a famous traditional Chinese medicine prescription, in rats by ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 171, 81–98. doi:10.1016/j.jpba.2019.04.001
Hu, Y., Cao, J. J., Liu, P., Guo, D. H., Wang, Y. P., and Yin, J., et al. (2011). Protective role of tea polyphenols in combination against radiation-induced haematopoietic and biochemical alterations in mice. Phytother Res. 25 (12), 1761–1769. doi:10.1002/ptr.3483
Ismail, A. F., and El-Sonbaty, S. M. (2016). Fermentation enhances Ginkgo biloba protective role on gamma-irradiation induced neuroinflammatory gene expression and stress hormones in rat brain. J. Photochem. Photobiol. B Biol. 158, 154–163. doi:10.1016/j.jphotobiol.2016.02.039
Jagetia, G., Baliga, M., and Venkatesh, P. (2004). Ginger (Zingiber officinale Rosc.), a dietary supplement, protects mice against radiation-induced lethality: mechanism of action. Cancer Biother. Radiopharm. 19 (4), 422–435. doi:10.1089/cbr.2004.19.422
Jagetia, G. C., Baliga, M. S., Venkatesh, P., and Ulloor, J. N. (2003). Influence of ginger rhizome (Zingiber officinale Rosc) on survival, glutathione and lipid peroxidation in mice after whole-body exposure to gamma radiation. Radiat. Res. 160 (5), 584–592. doi:10.1667/rr3057
Jang, S. S., Kim, H. G., Han, J. M., Lee, J. S., Choi, M. K., and Huh, G. J., et al. (2015). Modulation of radiation-induced alterations in oxidative stress and cytokine expression in lung tissue by Panax ginseng extract. Phytother Res. 29 (2), 201–209. doi:10.1002/ptr.5223
Ji, H. J., Wang, D. M., Wu, Y. P., Niu, Y. Y., Jia, L. L., and Liu, B. W., et al. (2016). Wuzi Yanzong pill, a Chinese polyherbal formula, alleviates testicular damage in mice induced by ionizing radiation. BMC Compl. Alternative Med. 16 (1), 509. doi:10.1186/s12906-016-1481-6
Ji, K., Fang, L., Zhao, H., Li, Q., Shi, Y., and Xu, C., et al. (2017). Ginger oleoresin alleviated γ-ray irradiation-induced reactive oxygen species via the Nrf2 protective response in human mesenchymal stem cells. Oxid. Med. Cell Longev. 2017, 1480294. doi:10.1155/2017/1480294
Jin, X., Ruiz Beguerie, J., Sze, D. M., and Chan, G. C. (2016). Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev. 4, CD007731. doi:10.1002/14651858.CD007731.pub2
Johnke, R. M., Sattler, J. A., and Allison, R. R. (2014). Radioprotective agents for radiation therapy: future trends. Future Oncol. 10 (15), 2345–2357. doi:10.2217/fon.14.175
Juan, G., Yanjun, Z., Guangzhou, A., Guozhen, G., and Junye, L. (2015). Protective-effects of salvianic acid A on ionizing irradiated mice. Lishizhen Medicine and Materia Medica Research 26 (8), 1811–1813. doi:10.3969/j.issn.1008-0805.2015.08.007
Juan, G., Yanjun, Z., Lihua, Z., and Guozhen, G. (2012). Protective effect of salvianic acid A on L-02DNA of human embryonic liver cells damaged by ionizing radiation. Lishizhen Medicine and Materia Medica Research 23 (2), 499–500.
Jun, B., and Zhenghai, L. (2010). The study on radioprotective effect of flavonoids from Houttuyniae cordata Chin. Arch. of Traditional Chinese Med. 28 (8), 1747–1748.
Kang, J. A., Song, H. Y., Byun, E. H., Ahn, N. G., Kim, H. M., and Nam, Y. R., et al. (2018). Gamma-irradiated black ginseng extract inhibits mast cell degranulation and suppresses atopic dermatitis-like skin lesions in mice. Food Chem. Toxicol. 111, 133–143. doi:10.1016/j.fct.2017.11.006
Kesari, K. K., Agarwal, A., and Henkel, R. (2018). Radiations and male fertility. Reprod. Biol. Endocrinol. 16 (1), 118. doi:10.1186/s12958-018-0431-1
Khayyal, M. T., Abdel-Naby, D. H., Abdel-Aziz, H., and El-Ghazaly, M. A. (2014). A multi-component herbal preparation, STW 5, shows anti-apoptotic effects in radiation induced intestinal mucositis in rats. Phytomedicine 21 (11), 1390–1399. doi:10.1016/j.phymed.2014.04.030
Kim, H. J., Kim, M. H., Byon, Y. Y., Park, J. W., Jee, Y., and Joo, H. G. (2007). Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J. Vet. Sci. 8 (1), 39–44. doi:10.4142/jvs.2007.8.1.39
Kim, S. H., and Kim, H. (2018). Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-A mini-review. Nutrients 10 (9). doi:10.3390/nu10091137
Klein, D., Schmetter, A., Imsak, R., Wirsdorfer, F., Unger, K., and Jastrow, H., et al. (2016). Therapy with multipotent mesenchymal stromal cells protects lungs from radiation-induced injury and reduces the risk of lung metastasis. Antioxid Redox Signal 24 (2), 53–69. doi:10.1089/ars.2014.6183
Kongxi, W., Yongqi, L., Yangyang, L., Liying, Z., Xiu, F., Wenjun, W., et al. (2020). Effects of astragalus polysaccharide on radiation-induced adipogenic differentiation of bonemarrow mesenchymal stem cells. J. Xi'an Jiaot. Univ. 41 (2), 304–308.
Koo, H. J., Jang, S. A., Yang, K. H., Kang, S. C., Namkoong, S., and Kim, T. H., et al. (2013). Effects of red ginseng on the regulation of cyclooxygenase-2 of spleen cells in whole-body gamma irradiated mice. Food Chem. Toxicol. 62, 839–846. doi:10.1016/j.fct.2013.10.009
Kunmu, D. L. Y., Jia-qi, F., Dong-hua, Y., and Chun-miao, Y. (2020). Thymic metabolomics for effect of Ganoderma polysaccharides on radiation-injured mice. Chinese Journal of Experimental Traditional Medical Formulae 26 (3), 102–109. doi:10.13422/j.cnki.syfjx.20191952
Lee, H. J., Kim, S. R., Kim, J. C., Kang, C. M., Lee, Y. S., and Jo, S. K., et al. (2006). In Vivo radioprotective effect of Panax ginseng C.A. Meyer and identification of active ginsenosides. Phytother Res. 20 (5), 392–395. doi:10.1002/ptr.1867
Lee, T. K., Johnke, R. M., Allison, R. R., O'Brien, K. F., and Dobbs, L. J. (2005). Radioprotective potential of ginseng. Mutagenesis 20 (4), 237–243. doi:10.1093/mutage/gei041
Lee, T. K., O'Brien, K. F., Wang, W., Johnke, R. M., Sheng, C., and Benhabib, S. M., et al. (2010). Radioprotective effect of American ginseng on human lymphocytes at 90 minutes postirradiation: a study of 40 cases. J. Alternative Compl. Med. 16 (5), 561–567. doi:10.1089/acm.2009.0590
Lee, W., Hwang, M. H., Lee, Y., and Bae, J. S. (2018). Protective effects of zingerone on lipopolysaccharide-induced hepatic failure through the modulation of inflammatory pathways. Chem. Biol. Interact. 281, 106–110. doi:10.1016/j.cbi.2017.12.031
Lee, Y. J., Han, J. Y., Lee, C. G., Heo, K., Park, S. I., and Park, Y. S., et al. (2014). Korean Red Ginseng saponin fraction modulates radiation effects on lipopolysaccharide-stimulated nitric oxide production in RAW264.7 macrophage cells. J. Ginseng. Res. 38 (3), 208–214. doi:10.1016/j.jgr.2014.02.001
Lei, G., Zhang, Y., Koppula, P., Liu, X., Zhang, J., and Lin, S. H., et al. (2020). The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 30 (2), 146–162. doi:10.1038/s41422-019-0263-3
Lei, Y., Yang, J., Li, Y., Yu, X., Deng, S., Xue, C., et al. (2019). Traditional Chinese medicine on treating epididymitis: a systematic review and meta-analysis protocol. Medicine (Baltim.) 98 (24), e15975. doi:10.1097/MD.0000000000015975
Li, C. R., Zhou, Z., Zhu, D., Sun, Y. N., Dai, J. M., and Wang, S. Q. (2007). Protective effect of paeoniflorin on irradiation-induced cell damage involved in modulation of reactive oxygen species and the mitogen-activated protein kinases. Int. J. Biochem. Cell Biol. 39 (2), 426–438. doi:10.1016/j.biocel.2006.09.011
Li, D. D., Luo, Z., Ling, S. C., Wu, K., Chen, G. H., and Cheng, J. (2018a). Mitochondrial apoptotic pathway mediated the Zn-induced lipolysis in yellow catfish Peteobagrus fulvidraco. Chemosphere 208, 907–915. doi:10.1016/j.chemosphere.2018.05.200
Li, D., Lu, L., Zhang, J., Wang, X., Xing, Y., and Wu, H., et al. (2014a). Mitigating the effects of Xuebijing injection on hematopoietic cell injury induced by total body irradiation with γ rays by decreasing reactive oxygen species levels. Int. J. Mol. Sci. 15 (6), 10541–10553. doi:10.3390/ijms150610541
Li, M., You, L., Xue, J., and Lu, Y. (2018b). Ionizing radiation-induced cellular senescence in normal, non-transformed cells and the involved DNA damage response: a mini review. Front. Pharmacol. 9, 522. doi:10.3389/fphar.2018.00522
Li, Q., Dong, Z., Lian, W., Cui, J., Wang, J., and Shen, H., et al. (2019a). Ochratoxin A causes mitochondrial dysfunction, apoptotic and autophagic cell death and also induces mitochondrial biogenesis in human gastric epithelium cells. Arch. Toxicol. 93 (4), 1141–1155. doi:10.1007/s00204-019-02433-6
Li, T. T., Wang, Z. B., Li, Y., Cao, F., Yang, B. Y., and Kuang, H. X. (2019b). The mechanisms of traditional Chinese medicine underlying the prevention and treatment of atherosclerosis. Chin. J. Nat. Med. 17 (6), 401–412. doi:10.1016/S1875-5364(19)30048-2
Li, X., Qu, L., Dong, Y., Han, L., Liu, E., and Fang, S., et al. (2014b). A review of recent research progress on the astragalus genus. Molecules 19 (11), 18850–18880. doi:10.3390/molecules191118850
Li, X., Zhuang, X., and Qiao, T. (2019c). Role of ferroptosis in the process of acute radiation-induced lung injury in mice. Biochem. Biophys. Res. Commun. 519 (2), 240–245. doi:10.1016/j.bbrc.2019.08.165
Li, Y. R., Cao, W., Guo, J., Miao, S., Ding, G. R., and Li, K. C., et al. (2011). Comparative investigations on the protective effects of rhodioside, ciwujianoside-B and astragaloside IV on radiation injuries of the hematopoietic system in mice. Phytother Res. 25 (5), 644–653. doi:10.1002/ptr.3313
Li, Z., and Xu, C. (2011). The fundamental theory of traditional Chinese medicine and the consideration in its research strategy. Front. Med. 5 (2), 208–211. doi:10.1007/s11684-011-0126-x
Li-ying, Z., Lei, W., Li-xin, Z., Yi-ming, Z., Xiao-min, X., Nan, D., et al. (2018). Protective effect of Astragalus Polysaccharide on heavy ionizing radiation on BMSCs and its mechanism related with NF-κB. Chin. J. Traditional Chin. Med. Pharm. 33 (12), 5576–5580.
Liang, Q. D., Gao, Y., Tan, H. L., Guo, P., Li, Y. F., and Zhou, Z., et al. (2006). Effects of four Si-Wu-Tang's constituents and their combination on irradiated mice. Biol. Pharm. Bull. 29 (7), 1378–1382. doi:10.1248/bpb.29.1378
Lidan, Y., Yunshuang, Y., Lei, G., Changpei, L., Xiaoyue, Z., Yunjing, Z., et al. (2016). The effect of bushen Jiedu fang on thymus and spleen of mice with radiation injury. J. Tradit. Chin. Med. 57 (1), 67–70. doi:10.13288/j.11-2166/r.2016.01.017
Liming, H., Peng, S., Linjing, Z., and Chengrong, S. (2011). Effects of radioprotection formula of TCM on acute injury induced by(60)Co γ-rays in mice. Chin. Pharm. Aff. 25 (2), 132–134.
Lin, H., Weiai, L., Xianjie, Z., and Rong, W. (2001). Effects of Co-herba Houttuyniae oral liquid on the immunological function and the cytogenetics of radiation injuries mice. Pharmaceutical J. of Chin. People’s Liberation Army 17 (3), 160–161. doi:10.3969/j.issn.1008-9926.2001.03.015
Lin, H., Zhengsheng, S., Romg, W., and Weiai, L. (2002). Effect of Co-herba Houttuyniae oral liquid to the stress function of R adiation injured mice. Pharmaceutical J. of Chin. People’s Liberation Army 18 (1), 44–45. doi:10.3969/j.issn.1008-9926.2002.01.016
Liu, L., Hu, L., Yao, Z., Qin, Z., Idehara, M., and Dai, Y., et al. (2019). Mucosal immunomodulatory evaluation and chemical profile elucidation of a classical traditional Chinese formula, Bu-Zhong-Yi-Qi-Tang. J. Ethnopharmacol. 228, 188–199. doi:10.1016/j.jep.2018.08.003
Liu, M. M., Huang, K. M., Yeung, S., Chang, A., Zhang, S., and Mei, N., et al. (2017). Inhibition of neoplastic transformation and chemically-induced skin hyperplasia in mice by traditional Chinese medicinal formula Si-Wu-Tang. Nutrients 9 (3). doi:10.3390/nu9030300
Liu, Y., Liu, F., Yang, Y., Li, D., Lv, J., and Ou, Y., et al. (2014). Astragalus polysaccharide ameliorates ionizing radiation-induced oxidative stress in mice. Int. J. Biol. Macromol. 68, 209–214. doi:10.1016/j.ijbiomac.2014.05.001
Liu, Z., Lei, X., Li, X., Cai, J. M., Gao, F., and Yang, Y. Y. (2018). Toll-like receptors and radiation protection. Eur. Rev. Med. Pharmacol. Sci. 22 (1), 31–39. doi:10.26355/eurrev_201801_14097
Loboda, A., Damulewicz, M., Pyza, E., Jozkowicz, A., and Dulak, J. (2016). Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 73 (17), 3221–3247. doi:10.1007/s00018-016-2223-0
Lu, M., Wang, Y., and Zhan, X. (2019). The MAPK pathway-based drug therapeutic targets in pituitary adenomas. Front. Endocrinol. 10, 330. doi:10.3389/fendo.2019.00330
Lumniczky, K., Szatmari, T., and Safrany, G. (2017). Ionizing radiation-induced immune and inflammatory reactions in the brain. Front. Immunol. 8, 517. doi:10.3389/fimmu.2017.00517
Luo, Q., Cui, X., Yan, J., Yang, M., Liu, J., and Jiang, Y., et al. (2011). Antagonistic effects of Lycium barbarum polysaccharides on the impaired reproductive system of male rats induced by local subchronic exposure to 60Co-γ irradiation. Phytother Res. 25 (5), 694–701. doi:10.1002/ptr.3314
Luo, Q., Li, J., Cui, X., Yan, J., Zhao, Q., and Xiang, C. (2014). The effect of Lycium barbarum polysaccharides on the male rats reproductive system and spermatogenic cell apoptosis exposed to low-dose ionizing irradiation. J. Ethnopharmacol. 154 (1), 249–258. doi:10.1016/j.jep.2014.04.013
Ma, C., Fu, Z., Guo, H., Wei, H., Zhao, X., and Li, Y. (2019). The effects of Radix Angelica Sinensis and Radix Hedysari ultrafiltration extract on X-irradiation-induced myocardial fibrosis in rats. Biomed. Pharmacother. 112, 108596. doi:10.1016/j.biopha.2019.01.057
Ma, Q., Wei, R., Wang, Z., Liu, W., Sang, Z., and Li, Y., et al. (2017). Bioactive alkaloids from the aerial parts of Houttuynia cordata. J. Ethnopharmacol. 195, 166–172. doi:10.1016/j.jep.2016.11.013
Mancuso, C., and Santangelo, R. (2017). Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food Chem. Toxicol. 107 (Pt A), 362–372. doi:10.1016/j.fct.2017.07.019
Mansour, H. H. (2013). Protective effect of ginseng against gamma-irradiation-induced oxidative stress and endothelial dysfunction in rats. EXCLI J. 12, 766–777.
Maurya, D. K., and Devasagayam, T. P. (2013). Ferulic acid inhibits gamma radiation-induced DNA strand breaks and enhances the survival of mice. Cancer Biother. Radiopharm. 28 (1), 51–57. doi:10.1089/cbr.2012.1263
Mei, N., Guo, X., Ren, Z., Kobayashi, D., Wada, K., and Guo, L. (2017). Review of Ginkgo biloba-induced toxicity, from experimental studies to human case reports. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 35 (1), 1–28. doi:10.1080/10590501.2016.1278298
Mohamed, H. E., and Badawy, M. M. M. (2019). Modulatory effect of zingerone against cisplatin or γ-irradiation induced hepatotoxicity by molecular targeting regulation. Appl. Radiat. Isot. 154, 108891. doi:10.1016/j.apradiso.2019.108891
Mukai, R. (2018). Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 82 (2), 207–215. doi:10.1080/09168451.2017.1415750
Nabavi, S. F., Braidy, N., Orhan, I. E., Badiee, A., Daglia, M., and Nabavi, S. M. (2016). Rhodiola rosea L. And alzheimer's disease: from farm to pharmacy. Phytother Res. 30 (4), 532–539. doi:10.1002/ptr.5569
Nguyen, N. H., and Nguyen, C. T. (2019). Pharmacological effects of ginseng on infectious diseases. Inflammopharmacology 27 (5), 871–883. doi:10.1007/s10787-019-00630-4
Niaudet, C., Bonnaud, S., Guillonneau, M., Gouard, S., Gaugler, M. H., and Dutoit, S.et al. (2017). Plasma membrane reorganization links acid sphingomyelinase/ceramide to p38 MAPK pathways in endothelial cells apoptosis. Cell. Signal. 33, 10–21. doi:10.1016/j.cellsig.2017.02.001
Park, E., Hwang, I., Song, J. Y., and Jee, Y. (2011). Acidic polysaccharide of Panax ginseng as a defense against small intestinal damage by whole-body gamma irradiation of mice. Acta Histochem. 113 (1), 19–23. doi:10.1016/j.acthis.2009.07.003
Park, H. R., Jo, S. K., Choi, N. H., and Jung, U. (2012). HemoHIM ameliorates the persistent down-regulation of Th1-like immune responses in fractionated γ-irradiated mice by modulating the IL-12p70-STAT4 signaling pathway. Radiat. Res. 177 (5), 676–684. doi:10.1667/rr2768.1
Park, H. R., Jo, S. K., Jung, U., Yee, S. T., and Kim, S. H. (2014). Protective effects of HemoHIM on immune and hematopoietic systems against γ-irradiation. Phytother Res. 28 (2), 245–251. doi:10.1002/ptr.4982
Park, S. J., Cho, W., Kim, M. S., Gu, B. K., Kang, C. M., and Khang, G., et al. (2018). Substance-P and transforming growth factor-β in chitosan microparticle-pluronic hydrogel accelerates regenerative wound repair of skin injury by local ionizing radiation. J. Tissue. Eng. Regen. Med. 12 (4), 890–896. doi:10.1002/term.2445
Pompura, S. L., and Dominguez-Villar, M. (2018). The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J. Leukoc. Biol. doi:10.1002/JLB.2MIR0817-349R
Purbey, P. K., Scumpia, P. O., Kim, P. J., Tong, A. J., Iwamoto, K. S., and McBride, W. H., et al. (2017). Defined sensing mechanisms and signaling pathways contribute to the global inflammatory gene expression output elicited by ionizing radiation. Immunity 47 (3), e421–e423. doi:10.1016/j.immuni.2017.08.017
Raafat, B. M., Saleh, A., Shafaa, M. W., Khedr, M., and Ghafaar, A. A. (2013). Ginkgo biloba and Angelica archangelica bring back an impartial hepatic apoptotic to anti-apoptotic protein ratio after exposure to technetium 99mTc. Toxicol. Ind. Health 29 (1), 14–22. doi:10.1177/0748233711433938
Rokot, N. T., Kairupan, T. S., Cheng, K. C., Runtuwene, J., Kapantow, N. H., and Amitani, M., et al. (2016). A role of ginseng and its constituents in the treatment of central nervous system disorders. Evid. Based Complement Alternat. Evid. Based Complement Alternat. Med. 2016, 2614742. doi:10.1155/2016/2614742
Romesser, P. B., Kim, A. S., Jeong, J., Mayle, A., Dow, L. E., and Lowe, S. W. (2019). Preclinical murine platform to evaluate therapeutic countermeasures against radiation-induced gastrointestinal syndrome. Proc. Natl. Acad. Sci. U. S. A. 116 (41), 20672–20678. doi:10.1073/pnas.1906611116
Said, R. S., El-Demerdash, E., Nada, A. S., and Kamal, M. M. (2016). Resveratrol inhibits inflammatory signaling implicated in ionizing radiation-induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly(ADP-ribose) polymerase 1 (PARP-1). Biochem. Pharmacol. 103, 140–150. doi:10.1016/j.bcp.2016.01.019
Sankaranarayanan, K., and Wassom, J. S. (2005). Ionizing radiation and genetic risks XIV. Potential research directions in the post-genome era based on knowledge of repair of radiation-induced DNA double-strand breaks in mammalian somatic cells and the origin of deletions associated with human genomic disorders. Mutat. Res. 578 (1-2), 333–370. doi:10.1016/j.mrfmmm.2005.06.020
Santivasi, W. L., and Xia, F. (2014). Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal 21 (2), 251–259. doi:10.1089/ars.2013.5668
Scholch, S., Rauber, C., Weitz, J., Koch, M., and Huber, P. E. (2015). TLR activation and ionizing radiation induce strong immune responses against multiple tumor entities. OncoImmunology 4 (11), e1042201. doi:10.1080/2162402X.2015.1042201
Sener, G., Kabasakal, L., Atasoy, B. M., Erzik, C., Velioglu-Ogunc, A., and Cetinel, S., et al. (2006). Ginkgo biloba extract protects against ionizing radiation-induced oxidative organ damage in rats. Pharmacol. Res. 53 (3), 241–252. doi:10.1016/j.phrs.2005.11.006
Shain, A. H., Joseph, N. M., Yu, R., Benhamida, J., Liu, S., and Prow, T., et al. (2018). Genomic and transcriptomic analysis reveals incremental disruption of key signaling pathways during melanoma evolution. Canc. Cell 34 (1), 45–55, e44. doi:10.1016/j.ccell.2018.06.005
Shao, L., Luo, Y., and Zhou, D. (2014). Hematopoietic stem cell injury induced by ionizing radiation. Antioxid Redox Signal 20 (9), 1447–1462. doi:10.1089/ars.2013.5635
Sharma, A., Haksar, A., Chawla, R., Kumar, R., Arora, R., and Singh, S., et al. (2005). Zingiber officinale Rosc. modulates gamma radiation-induced conditioned taste aversion. Pharmacol. Biochem. Behav. 81 (4), 864–870. doi:10.1016/j.pbb.2005.06.012
Shen, C. Y., Jiang, J. G., Yang, L., Wang, D. W., and Zhu, W. (2017). Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 174 (11), 1395–1425. doi:10.1111/bph.13631
Shi, M., Huang, F., Deng, C., Wang, Y., and Kai, G. (2019a). Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 59 (6), 953–964. doi:10.1080/10408398.2018.1474170
Shi, Q., Si, D., Bao, H., Yan, Y., Kong, Y., Li, C., et al. (2019b). Efficacy and safety of Chinese medicines for asthma: a systematic review protocol. Medicine (Baltim.) 98 (34), e16958. doi:10.1097/MD.0000000000016958
Shibata, A. (2017). Regulation of repair pathway choice at two-ended DNA double-strand breaks. Mutat. Res. 803-805, 51–55. doi:10.1016/j.mrfmmm.2017.07.011
Shingnaisui, K., Dey, T., Manna, P., and Kalita, J. (2018). Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: a review. J. Ethnopharmacol. 220, 35–43. doi:10.1016/j.jep.2018.03.038
Shrivastav, M., De Haro, L. P., and Nickoloff, J. A. (2008). Regulation of DNA double-strand break repair pathway choice. Cell Res. 18 (1), 134–147. doi:10.1038/cr.2007.111
Shukla, S. K., Chaudhary, P., Kumar, I. P., Samanta, N., Afrin, F., and Gupta, M. L., et al. (2006). Protection from radiation-induced mitochondrial and genomic DNA damage by an extract of Hippophae rhamnoides. Environ. Mol. Mutagen. 47 (9), 647–656. doi:10.1002/em.20251
Shuryak, I. (2019). Review of microbial resistance to chronic ionizing radiation exposure under environmental conditions. J. Environ. Radioact. 196, 50–63. doi:10.1016/j.jenvrad.2018.10.012
Singh, V. K., and Seed, T. M. (2019). The efficacy and safety of amifostine for the acute radiation syndrome. Expert Opin. Drug. Saf. 18 (11), 1077–1090. doi:10.1080/14740338.2019.1666104
Smith, T. A., Kirkpatrick, D. R., Smith, S., Smith, T. K., Pearson, T., and Kailasam, A., et al. (2017). Radioprotective agents to prevent cellular damage due to ionizing radiation. J. Transl. Med. 15 (1), 232. doi:10.1186/s12967-017-1338-x
Soliman, A. F., Anees, L. M., and Ibrahim, D. M. (2018). Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 391 (8), 819–832. doi:10.1007/s00210-018-1506-4
Song, J. Y., Han, S. K., Bae, K. G., Lim, D. S., Son, S. J., and Jung, I. S., et al. (2003). Radioprotective effects of ginsan, an immunomodulator. Radiat. Res. 159 (6), 768–774. doi:10.1667/0033-7587(2003)159[0768:reogai]2.0.co;2
Sun, J., Zhang, L., He, Y., Zhang, K., Wu, L., and Fan, Y., et al. (2016). To unveil the molecular mechanisms of qi and blood through systems biology-based investigation into Si-Jun-Zi-Tang and Si-Wu-Tang formulae. Sci. Rep. 6, 34328. doi:10.1038/srep34328
Sun, X., Zhang, H., Zhang, Y., Yang, Q., and Zhao, S. (2018). Caspase-dependent mitochondrial apoptotic pathway is involved in astilbin-mediated cytotoxicity in breast carcinoma cells. Oncol. Rep. 40 (4), 2278–2286. doi:10.3892/or.2018.6602
Szeto, Y. T., Sin, Y. S., Pak, S. C., and Kalle, W. (2015). American ginseng tea protects cellular DNA within 2 h from consumption: results of a pilot study in healthy human volunteers. Int. J. Food Sci. Nutr. 66 (7), 815–818. doi:10.3109/09637486.2015.1088937
Takayama, S., and Iwasaki, K. (2017). Systematic review of traditional Chinese medicine for geriatrics. Geriatr. Gerontol. Int. 17 (5), 679–688. doi:10.1111/ggi.12803
Tavassoly, I., Goldfarb, J., and Iyengar, R. (2018). Systems biology primer: the basic methods and approaches. Essays Biochem. 62 (4), 487–500. doi:10.1042/EBC20180003
Tianxiang, M., Jiuhong, W., Ning, S., and Hongzhu, G. (2013). Screening for radioprotective components of glycosides and alcohols from Rhodiola in vitro. Pharmaceutical Journal of Chinese People's Liberation Army 29 (3), 203–205.
Toulany, M., and Rodemann, H. P. (2015). Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Semin. Canc. Biol. 35, 180–190. doi:10.1016/j.semcancer.2015.07.003
Verma, P., Sharma, P., Parmar, J., Sharma, P., Agrawal, A., and Goyal, P. K. (2011). Amelioration of radiation-induced hematological and biochemical alterations in Swiss albino mice by Panax ginseng extract. Integr. Canc. Ther. 10 (1), 77–84. doi:10.1177/1534735410375098
Wang, A., Wang, L., Lu, X., Wang, Y., Chen, X., and Shi, Z., et al. (2020). A Chinese herbal prescription Yiqi Jiedu decoction attenuates irradiation induced testis injury in mice. Biomed. Pharmacother. 123, 109804. doi:10.1016/j.biopha.2019.109804
Wang, J., Shao, W., Niu, H., Yang, T., Wang, Y., and Cai, Y. (2019a). Immunomodulatory effects of colistin on macrophages in rats by activating the p38/MAPK pathway. Front. Pharmacol. 10, 729. doi:10.3389/fphar.2019.00729
Wang, W. J., and Zhang, T. (2017). Integration of traditional Chinese medicine and Western medicine in the era of precision medicine. J. Integr. Med. 15 (1), 1–7. doi:10.1016/S2095-4964(17)60314-5
Wang, Z., Lin, H. H., Linghu, K., Huang, R. Y., Li, G., and Zuo, H., et al. (2019b). Novel compound-target interactions prediction for the herbal formula hua-yu-qiang-shen-tong-Bi-fang. Chem. Pharm. Bull. (Tokyo) 67 (8), 778–785. doi:10.1248/cpb.c18-00808
Weiss, J. F., and Landauer, M. R. (2003). Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 189 (1-2), 1–20. doi:10.1016/s0300-483x(03)00149-5
Wu, Y., Chen, M., and Jiang, J. (2019). Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 49, 35–45. doi:10.1016/j.mito.2019.07.003
Xiao-fang, Y., Ya-nan, W., Zhe, Z., and Sheng-qi, W. (2013). Experimental research on the protection of buzhong Yiqi pills in low-dose radiation. World J. Integ. Traditional and Western Med. 8 (6), 560–562. doi:10.3969/j.issn.1673-6613.2013.06.007
Xiaoling, H., Qiujun, L., zhe, Z., Aidong, M., Xuejun, W., Mingsan, M., et al. (2006). Radiation protection effect of buzhong Yiqi pill and other proprietary Chinese medicines. Chin. J. Radio. Med. Protection 26 (4), 366–369. doi:10.3760/cma.j.issn.0254-5098.2006.04.017
Yang, J., Yan, Y., Liu, H., Wang, J., and Hu, J. (2015). Protective effects of acteoside against X-ray induced damage in human skin fibroblasts. Mol. Med. Rep. 12 (2), 2301–2306. doi:10.3892/mmr.2015.3630
Yang, Y., Laval, S., and Yu, B. (2014). Chemical synthesis of saponins. Adv. Carbohydr. Chem. Biochem. 71, 137–226. doi:10.1016/B978-0-12-800128-8.00002-9
Yang, Y., Zhang, G., Sun, Q., Liu, L., Peng, H., and Wang, J., et al. (2017). Simultaneous determination of 8 compounds in gancao-ganjiang-tang by HPLC-DAD and analysis of the relations between compatibility, dosage, and contents of medicines. Evid. Based Complement Alternat. Med. 2017, 4703632. doi:10.1155/2017/4703632
Yeh, M. L., Chiu, W. L., Wang, Y. J., and Lo, C. (2017). An investigation of the use of traditional Chinese medicine and complementary and alternative medicine in stroke patients. Holist. Nurs. Pract. 31 (6), 400–407. doi:10.1097/HNP.0000000000000238
Ying, N., Mei-na, Z., Jin-rui, C., Jian, C., and Wei, Z. (2018). Protection and mechanism of gypenosides on the oxidative injury induced by irradiation in mice. Central South Pharmacy 16 (7), 935–938. doi:10.7539/j.issn.1672-2981.2018.07.009
Yu, J., Zhu, X., Qi, X., Che, J., and Cao, B. (2013). Paeoniflorin protects human EA.hy926 endothelial cells against gamma-radiation induced oxidative injury by activating the NF-E2-related factor 2/heme oxygenase-1 pathway. Toxicol. Lett. 218 (3), 224–234. doi:10.1016/j.toxlet.2013.01.028
Yue, C., Bao-gui, W., Gui-ying, Z., and Wei-qun, Y. (2005). Protective effects of acanthopanax senticosus saponins on mice with irradiation damage induced by X-rays. J. Jilin Univ. (Med. Ed.) 31 (3), 423–425. doi:10.3969/j.issn.1671-587X.2005.03.031
Yunjing, Z., Yunshuang, Y., Lidan, Y., Xiaoyue, Z., Lei, D., and Rong, Z. (2015). Effect of BushenJiedu recipe on peripheral blood leukocytes and spleen NF-κBp65 of mice after radiation. J. Jinan Univ. (Nat. Sci. Med. Ed.) (6), 490–495. doi:10.11778/j.jdxb.2015.06.009
Zhang, H. P., Wang, L., Wang, Z., Xu, X. R., Zhou, X. M., and Liu, G., et al. (2018b). Chinese herbal medicine formula for acute asthma: a multi-center, randomized, double-blind, proof-of-concept trial. Respir. Med. 140, 42–49. doi:10.1016/j.rmed.2018.05.014
Zhang, H., Yan, H., Ying, J., Du, L., Zhang, C., and Yang, Y., et al. (2018a). Resveratrol ameliorates ionizing irradiation-induced long-term immunosuppression in mice. Int. J. Radiat. Biol. 94 (1), 28–36. doi:10.1080/09553002.2018.1408976
Zhang, H., Yan, H., Zhou, X., Wang, H., Yang, Y., and Zhang, J., et al. (2017a). The protective effects of Resveratrol against radiation-induced intestinal injury. BMC Compl. Alternative Med. 17 (1), 410. doi:10.1186/s12906-017-1915-9
Zhang, J., Li, H., Lu, L., Yan, L., Yang, X., and Shi, Z., et al. (2017b). The Yiqi and Yangyin Formula ameliorates injury to the hematopoietic system induced by total body irradiation. J. Radiat. Res. 58 (1), 1–7. doi:10.1093/jrr/rrw056
Zhang, L., Luo, Y., Lu, Z., He, J., Wang, L., and Zhang, L., et al. (2018c). Astragalus polysaccharide inhibits ionizing radiation-induced bystander effects by regulating MAPK/NF-kB signaling pathway in bone mesenchymal stem cells (BMSCs). Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 24, 4649–4658. doi:10.12659/MSM.909153
Zhang, W., Wang, S., Zhang, R., Zhang, Y., Li, X., and Lin, Y., et al. (2016). Evidence of Chinese herbal medicine Duhuo Jisheng decoction for knee osteoarthritis: a systematic review of randomised clinical trials. BMJ Open 6 (1), e008973. doi:10.1136/bmjopen-2015-008973
Zhang, X., Xing, X., Liu, H., Feng, J., Tian, M., and Chang, S., et al. (2020). Ionizing radiation induces ferroptosis in granulocyte-macrophage hematopoietic progenitor cells of murine bone marrow. Int. J. Radiat. Biol. 96 (5), 584–595. doi:10.1080/09553002.2020.1708993
Zhao, L., Wang, Y., Shen, H. L., Shen, X. D., Nie, Y., and Wang, Y., et al. (2012). Structural characterization and radioprotection of bone marrow hematopoiesis of two novel polysaccharides from the root of Angelica sinensis (Oliv.) Diels. Fitoterapia 83 (8), 1712–1720. doi:10.1016/j.fitote.2012.09.029
Zhao, M., Chan, C. P. S., Cheung, C. W. C., Alqawasmeh, O., Wang, R. C. C., and Wu, J. C. Y., et al. (2019). A double-blinded, randomized placebo-controlled trial on the effect of traditional Chinese medicine formula Wuzi Yanzong pill on improving semen qualities in men with suboptimal parameters. Trials 20 (1), 540. doi:10.1186/s13063-019-3647-2
Zhao, M. P., Shi, X., Kong, G. W. S., Wang, C. C., Wu, J. C. Y., Lin, Z. X., et al. (2018). The therapeutic effects of a traditional Chinese medicine formula wuzi Yanzong pill for the treatment of oligoasthenozoospermia: a meta-analysis of randomized controlled trials. Evid. Based Complement Alternat. Evid. Based Complement Alternat. Med. 2018, 2968025. doi:10.1155/2018/2968025
Zhou, J., Pang, H., Li, W., Liu, Q., Xu, L., and Liu, Q., et al. (2016). Effects of Lycium barbarum polysaccharides on apoptosis, cellular adhesion, and oxidative damage in bone marrow mononuclear cells of mice exposed to ionizing radiation injury. BioMed. Res. Int. 2016, 4147879. doi:10.1155/2016/4147879
Zhou, Y. X., Xin, H. L., Rahman, K., Wang, S. J., Peng, C., and Zhang, H. (2015). Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. BioMed. Res. Int. 2015, 925631. doi:10.1155/2015/925631
Keywords: ionizing radiation, oxidative damage, Chinese herbal medicines, Chinese herbal prescriptions, anti-radiation, radiation protective drugs
Citation: Zhang X, Chen X, Wang L, He C, Shi Z, Fu Q, Xu W, Zhang S and Hu S (2021) Review of the Efficacy and Mechanisms of Traditional Chinese Medicines as a Therapeutic Option for Ionizing Radiation Induced Damage. Front. Pharmacol. 12:617559. doi: 10.3389/fphar.2021.617559
Received: 15 October 2020; Accepted: 11 January 2021;
Published: 15 February 2021.
Edited by:
Hai Yu Xu, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Amit Krishna De, Indian Science Congress Association (ISCA), IndiaCopyright © 2021 Zhang, Chen, Wang, He, Shi, Fu, Xu, Zhang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sumin Hu, aHVzbUBidWNtLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.