- 1Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Sciences, Peking University, Beijing, China
- 2International Research Center for Medicinal Administration, Peking University Health Science Center, Beijing, China
Purpose: Cancer is a leading cause of morbidity and mortality worldwide. Off-label (OL) use of antineoplastic drugs to treat malignancies is prevalent. In this study, we quantified and characterized OL use of antineoplastic drugs to treat malignancies in China.
Methods: This was a retrospective study using nationwide data collected from 2008 to 2010. Use of antineoplastic drugs was considered OL if they were used for indications not reflected in the package insert published by the National Medical Products Administration at the time of prescription. Descriptive analysis and Spearman rank correlation were used to evaluate the frequency and pattern of OL drug use.
Results: In total, 51,382 patients with malignancies, 24 categories of antineoplastic drugs, and 77 types of malignancies treated with OL drugs were included in this study. Twenty commonly used antineoplastic drugs (ICD encoded as L01) were used OL in 10–61% of cases, and four commonly used endocrine therapy antineoplastic drugs (ICD encoded as L02) were used OL in 10–19% of cases. There was a significant negative correlation between the disease constituent ratio and the average OL use rate of antineoplastic drugs for various malignancies. In contrast, there was a significant positive correlation between the average OL use rate of antineoplastic drugs and the number of malignancies treated with OL drugs.
Conclusion: This study provided information regarding OL use of antineoplastic drugs for treatment of malignancies, and showed that OL use was prevalent. In addition, uncommon malignancies were more likely to be treated with OL antineoplastic drugs. Furthermore, more commonly used antineoplastic drugs were more likely to be used OL.
Introduction
Cancer is a leading cause of morbidity and mortality worldwide. Cancer is the second leading cause of death globally, and was responsible for an estimated 9.6 million deaths in 2018, and about 1 in 6 deaths are due to cancer. Approximately 70% of deaths from cancer occur in low- and middle-income countries (WHO, 2018). In the United States, 1.7 million new cancer cases and 0.6 million cancer deaths were projected to occur in 2019 (Siegel et al., 2019). In China, increased incidence of cancer is a major public health problem. Approximately 4.3 million new cancer cases and 2.9 million new cancer deaths occurred in China in 2018 (Chen et al., 2016; Feng et al., 2019). In addition, the survival rate of patients with malignancies has increased. Advances and improvements in diagnostic and therapeutic strategies have resulted in better control, and reduced death rates, related to cancer in countries such as the United States and Europe (De Angelis et al., 2014; Ferlay et al., 2015; WHO, 2018). However, low- and middle-income countries such as China have not experienced this reduction in death rates. Compared to the United States and United Kingdom, China has a lower cancer incidence, but a 30–40% higher cancer mortality rate (Feng et al., 2019). The higher mortality rate in China is a result of a lower diagnosis rate of early stage cancers and non-uniform clinical treatment strategies across regions.
Antineoplastic drugs are widely used to treat cancer (also known as malignancy). However, effective treatments for rare malignancies have not been developed (Saiyed et al., 2017b). Patients with advanced malignancies are more likely to accept the greater risk associated with new therapies. Off-label (OL) drug use (or unlabeled use) is use that is not included in the indications or dosing regimens listed in the approved labeling (Ling-li et al., 2012). Off-label drug use is controversial, but has shown some efficacy for treatment of malignancies. In clinical practice, OL use for treatment of advanced malignancies is common (Herrero Fernandez et al., 2019). Off-label drug use is particularly common for treatment of malignancies due to the existence of numerous cancer subtypes, difficulties involved in performing clinical trials, rapid diffusion of preliminary results, and delays in approval of new drugs by regulatory bodies (Lerose et al., 2011; Herbrand et al., 2019; Mascolo et al., 2020). Therefore, OL use of antineoplastic drugs to treat malignancies is prevalent.
The present study analyzed OL use of antineoplastic drugs for treatment of malignancies in China using data from the Nationwide Medical Insurance database from 2008 to 2010. This was the first study to evaluate OL use at the national level. In addition, this study contributed data from a low-to-middle-income economy (China). Several descriptive studies of OL use for treatment of malignancies have been performed in developed countries. Twenty percent of antineoplastic drugs prescribed to treat malignancies were OL in Italy (Lerose et al., 2011), 27.2% were OL in Switzerland (Joerger et al., 2014), and 35% were OL in Australia (Mellor et al., 2009). Our study characterized OL use of antineoplastic drugs for treatment of malignancies, and described the extent and pattern of OL use. Even though some of the OL indications in 2010 are now accepted practice–that is no longer OL use a few years later, but this study contributed to suggest future strategies that find new use of the old drugs through the OL use. Since the fact that expenditure in oncology medicines is increasingly rapidly, OL use is a more cost-effective choice. It is meaningful to undertake the study to assess the extent of OL use at the time to set a baseline and use the findings to suggest future strategies. We also documented the rationale for OL use. The goal of this study was to provide a basis for development of policies and treatment strategies for OL use of antineoplastic drugs.
Materials and Methods
Data Source
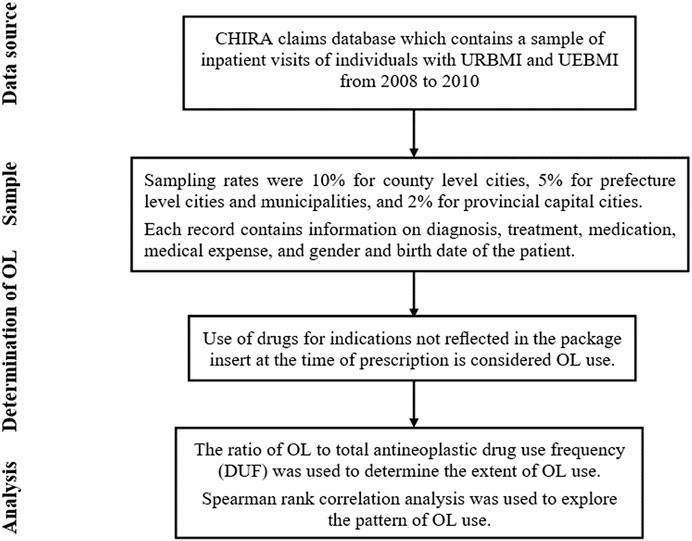
Data was acquired from the China Health Insurance Research Association (CHIRA) claims database, which contains a sample of inpatient visits of individuals with Urban Resident Basic Medical Insurance (URBMI) and Urban Employee Basic Medical Insurance (UEBMI) from 2008 to 2010. Urban Resident Basic Medical Insurance is public health insurance for urban non-working residents that covers children, adolescents, college students, elderly individuals, and individuals with disabilities. Urban Employee Basic Medical Insurance covers the working population (public and private sectors) in urban China. Therefore, URBMI and UEBMI are the two largest public insurance providers for urban residents, and cover nearly all urban populations in China. In 2010, the total number of enrollees in URBMI and UEBMI was 432.06 million1, which accounted for approximately 65% of the urban population in China2. It would be great to expand the data to longer years. However, such data is not available from our provider. A sample from 2008 to 2010 is the latest data available to us, and as far as we know, our data is the only source that covers a wide range of cities regarding inpatient visits and used to evaluate the OL use of antineoplastic drugs for treatment of malignancies. All data generated or analyzed during this study are included in this published article and its Supplementary Material information files. The studies involving human participants are reviewed and approved by Office of Biomedical Ethics Committee of Peking University (IRB00001052–19007).
Study Sample
Claims submitted to CHIRA were drawn from a few representative cities from the province level Healthcare Security Bureaus. Sampling rates were 10% for county level cities, 5% for prefecture level cities (non-provincial capital cities) and municipalities, and 2% for provincial capital cities. Each record contains information on diagnosis (based on the World Health Organization International Classification of Diseases and Related Health Problems (ICD-10), version 20103), treatment, medication, medical expense, and gender and birth date of the patient.
We used SQL Server 2005 and Network Analysis to select the discharge diagnostic codes (C00-C97) recognized as malignancies to investigate off-label use of antineoplastic drugs to treat malignancies.
Determination of OL use
Use of drugs for indications not reflected in the package insert published by the National Medical Products Administration at the time of prescription is considered OL use (Seetasith et al., 2017; Fernandez et al., 2019). A summary of results that matched ICD-10 diagnostic codes and the indications included in the package inserts are provided in Supplementary File S1. This standard is used worldwide for general epidemiology, health management, and clinical analysis (Deferio et al., 2018; Nittari et al., 2018).
Analysis
Descriptive analysis was used to determine the extent of OL use. The ratio of OL to total antineoplastic drug use frequency (DUF) was used to determine the extent of OL use (Prendergast et al., 2013). We also calculated the OL use rate of antineoplastic drugs for treatment of malignancies, and constructed an OL use spectrum for commonly used antineoplastic drugs.
Spearman rank correlation analysis was used to explore the pattern of OL use. First, we analyzed the correlation between the disease constituent ratio4 (DCR) and the average OL use rate of antineoplastic drugs for treatment of various malignancies. Then, we analyzed the correlation between the average OL use rate of antineoplastic drugs and the number of malignancies these drugs were used to treat. Please see Figure 1 for more details of the study design.
Results
Characteristics of the Sample
In this study, 51,382 patients with malignancies who were taking 36 commonly used antineoplastic drugs (frequency of usage above 200) were retrospectively studied. Among 36 commonly used antineoplastic drugs, only 24 antineoplastic drugs could be accurately identified as OL use (the indications of the remaining 12 drugs include a variety of uncertain tumors or the symptoms common to a variety of uncertain tumors). The 24 antineoplastic drugs were found to be off-label used in 77 categories of malignancies (Supplementary File S2).
Extent of OL Drug Use
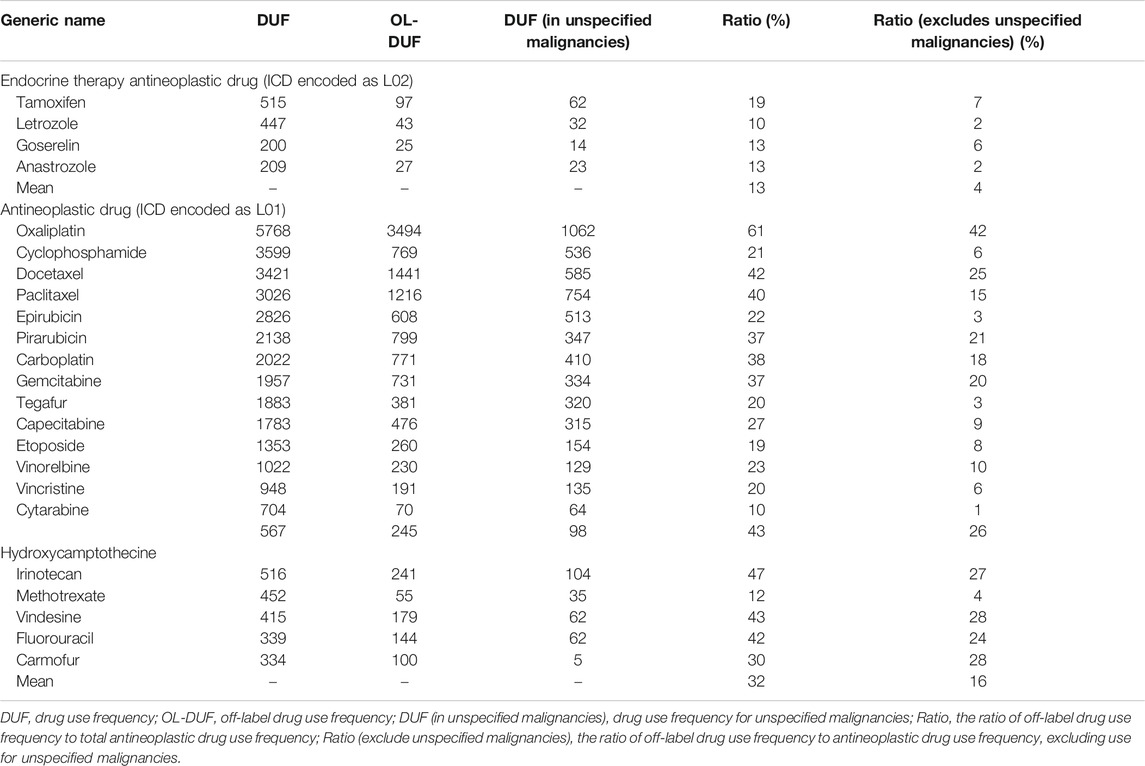
We calculated the ratio of OL drug use frequency (OL-DUF) to total antineoplastic DUF to determine the extent of OL use (Table 1). The results showed that OL use of antineoplastic drugs to treat malignancies was common. Twenty commonly used antineoplastic drugs (ICD encoded as L01) were used OL for 10–61% of cases, and four commonly used endocrine therapy antineoplastic drugs (ICD encoded as L02) were used OL for 10–19% of cases. The highest ratios of OL drug use were those for oxaliplatin (42%), docetaxel (25%), paclitaxel (15%), carboplatin (18%), gemcitabine (20%), vinorelbine (10%), hydroxycamptothecine (26%), irinotecan (27%), vindesine (28%), floxuridine (24%), and carmofur (28%). We constructed an OL use spectrum for 24 commonly used antineoplastic drugs used to treat 77 malignancies. (Figures 2A,B).
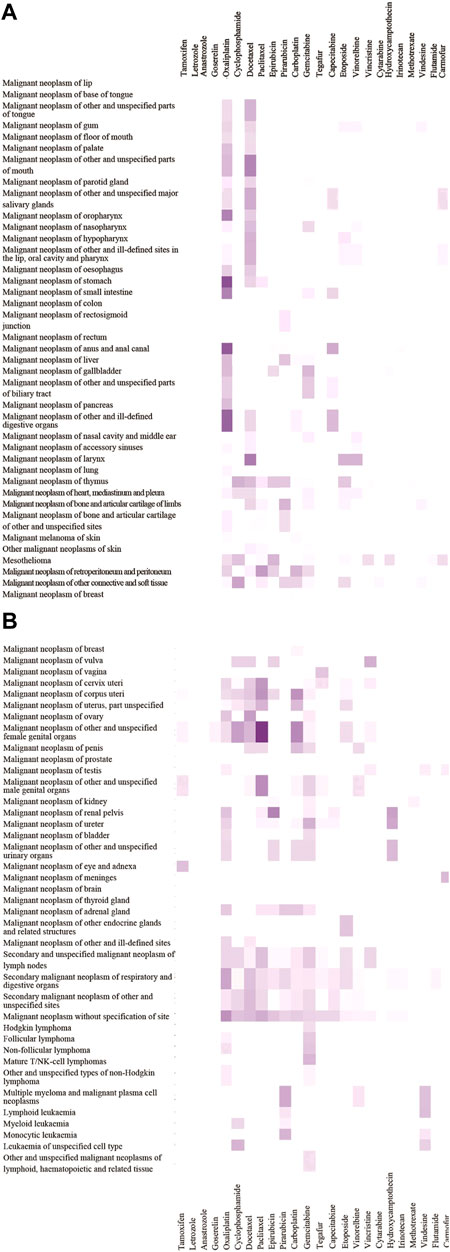
FIGURE 2. OL use spectrum of 24 antineoplastic drugs in 77 malignancies.The depth of color scale represents the extent of OL use rates (range 0–40%). The malignancies are arranged from top to bottom according to ICD codes (C00-C96). Antineoplastic drugs are sorted in descending order from left to right based on total drug use frequency.
Pattern of OL Drug Use
We used Spearman rank correlation analysis to evaluate patterns of OL use. The results showed a significantly negative correlation (correlation coefficient = −0.778, P = 0.000) between the disease constituent ratio (DCR) and the average OL use rate of antineoplastic drugs for treatment of various malignancies. (Figure 3). The results also showed a significant positive correlation (correlation coefficient = 0.797, P = 0.000) between the average OL use rate of antineoplastic drugs and the number of malignancies treated using OL drugs (Figure 4).

FIGURE 3. Scatter diagram of the average OL use rate of antineoplastic drugs used to treat malignancies.
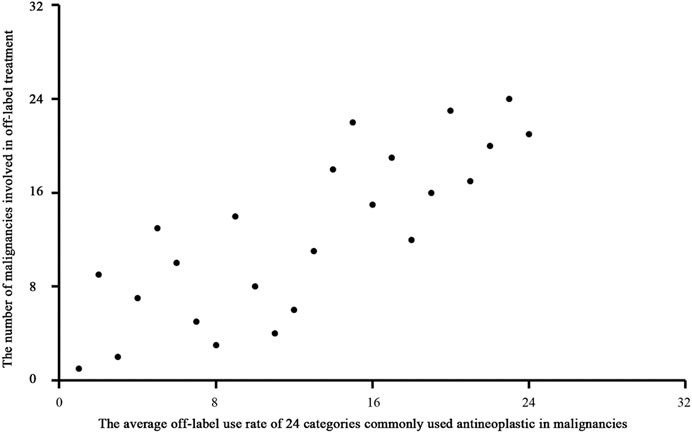
FIGURE 4. Scatter diagram of the average OL use rate of antineoplastic drugs and the number of malignancies treated using OL drugs.
Discussion
The results of this study indicated that OL use of antineoplastic drugs is common in clinical practice across China, which is consistent with the results of other studies (Saiyed et al., 2017a; De Giorgi et al., 2018; Smieliauskas et al., 2018). These results suggested that increased attention should be given to OL use of antineoplastic drugs for treatment of malignancies.
The OL use spectrum for commonly used antineoplastic drugs for treatment of malignancies constructed in this study showed a significant negative correlation between the disease constituent ratio and the average OL use rate of antineoplastic drugs for treatment of various malignancies. This result suggested that uncommon malignancies were more likely to be treated with OL antineoplastic drugs. Uncommon malignancies often do not have standardized treatment courses, and many clinicians may not have experience with treating these cancers. In addition, there was a significant positive correlation between the average OL use rate of antineoplastic drugs and the number of malignancies treated with OL drugs. These results suggested that commonly used antineoplastic drugs were more likely to be used OL to treat malignancies. This may have resulted from clinician familiarity with these drugs. Oxaliplatin is used to treat colorectal cancer, and it is commonly used to treat gastric cancer (42% OL use in this study). Oxaliplatin is the recommended first-line treatment for colorectal and gastric cancer according to the National Comprehensive Cancer Network (NCCN®) guidelines, which provides justification for OL use of oxaliplatin to treat gastric cancer. However, oxaliplatin was not recommended by the NCCN® for preoperative gastric cancer treatment until 2013. Our study showed that oxaliplatin was the most commonly used antineoplastic drug to treat gastric cancer in China from 2008 to 2010. Other studies also showed that oxaliplatin was commonly used to treat patients with gastric cancer during this period in China (Liu et al., 2007; Liu et al., 2008; Park et al., 2008; Jiang et al., 2011; Bang et al., 2012) and worldwide (Barone et al., 2007; Park et al., 2008; Koizumi et al., 2010; Lordick et al., 2010; Montagnani et al., 2011). These results indicated that commonly used antineoplastic drugs are often used OL to treat malignancies.
In conclusion, this study showed that OL use of antineoplastic drugs to treat malignancies was prevalent in China from 2008 to 2010. Furthermore, OL use of antineoplastic drugs was more common for treatment of uncommon malignancies. In addition, more commonly used antineoplastic drugs were more likely to be used OL.
It is meaningful to undertake the study to assess the extent of OL use at the time to suggest future traditional drug discovery strategies. Discover new use of the old drugs through the OL use is a more cost-effective future strategy This strategy is highly efficient, time saving, low-cost and minimum risk of failure. It maximizes the therapeutic value of a drug and consequently increases the success rate, which not only provides an effective alternative approach to traditional drug discovery process, but also offers a less expensive treatment plan for patients.
Our Study Suffered From Several Limitations
To measure OL use, we selected the first three digits of the ICD code for comparison with the labeled indications of each drug. Use was considered on-label for the disease subcategories under each three-digit ICD code. This may have resulted in an underrepresentation of OL use. The current empirical results cannot rule out more interpretations about the conditions of patients’ choice, since the characteristics of the patients submitted for chemotherapy were not available due to the limitation of data.
In addition, the data used in our study were collected from 2008 to 2010, which may not represent current OL use patterns for treatment of malignancies. However, no relevant studies have evaluated OL use of antineoplastic drugs for treatment of malignancies at the national level. Furthermore, the spectra of malignancies and treatment strategies have remained stable in China (Chen et al., 2016). Even though some treatments that were OL in 2010 are now no longer off-label 10°years later. For instance, capecitabine gets approved for gastric adenocarcinoma in 2018. But there are few such examples, which calls for speeding up the drug approval process in China. Propranolol, a non-selective beta-blocker, is an example of a well-known medicine that was reformulated for use in children with proliferating hemangiomas (Léauté-Labrèze et al., 2015). However, to this date (October 2020), it has not been granted in China. Therefore, our study may adequately describe OL use of antineoplastic drugs for treatment of malignancies, and may serve as a scaffold for future studies. Even though some of the OL indications in 2010 are now accepted practice. However, undertook the study to assess the extent of OL use at the time to set a baseline and use the findings to suggest future strategies.
Finally, our study did not evaluate the rationale for OL use of drugs. Further studies should evaluate the rationale for OL use of antineoplastic drugs to treat malignancies by referencing relevant standard treatment guidelines and clinical experience. OL use confronts some clinical and legal risks. OL prescribing is legal in the United States and in many other countries (Verbaanderd et al., 2020). However, there are no standardized regulations on OL use in China, and the law does not specify whether OL use is illegal. Therefore, Medical institutions take the responsibility for regulating and monitoring the OL use (Wu and Wu, 2014). describes a strategy to ensure oversight of patient safety, and prospectively assess efficacy for OL use, and provides a mechanism for the evolution and promotion of standards of medical care for this situation. Furthermore, not all countries support the reimbursement of OL use, so some OL treatments could be unaffordable to patients in China. There are some mature international experience waiting to be practiced to address inappropriate prescribing (Nayroles et al., 2017). This study provided the basis for further research to assess the impact of OL use, and may contribute to development of policies for OL use.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
All authors participated in study conception and design, data analysis, and data interpretation, are responsible for the integrity of the work, and approved the final version for publication. LS, JC, MW, and GXW designed the study. GXW, MW, and SH performed statistical analyses. GXW, HZ, and CZ wrote the manuscript, and all authors participated in manuscript revision. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The study was funded by the National Science and Technology Major Project of China (Project No: 2018ZX09721003–001–002) for financial support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank CHIRA for providing the data used in this study, and the National Major Science and Technology Projects of China (Project No: 2018ZX09721003–001–002) for financial support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.616453/full#supplementary-material.
Footnotes
1Please refer to page 74 of http://www.chinacdc.cn/tjsj/gjwstjsj/201205/W020120517331408782774.pdf.
2The total population living in urban area in China in 2010 was 665.58 million, according to population census in 2010.
3Please refer to World Health Organization. ICD-10 version 2010 website [Internet].
4Disease constituent ratio: Since the total number of samples from different regions is not proportional to the total residents, it is not meaningful to compare the number of cases of malignancies among different regions. In order to make the best use of the existing data and understand the geographical distribution characteristics of malignancies, we introduce the concept of disease composition ratio. The proportion of the number of patients with a certain malignancy in the total number of patients in a certain region is defined as the constituent ratio of this malignancies in this region.
References
Bang, Y. J., Kim, Y. W., Yang, H. K., Chung, H. C., Park, Y.-K., Lee, K. H., et al. (2012). Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy: a phase 3 open-label, randomised controlled trial. Lancet 379 (9813), 315–321. doi:10.1016/S0140-6736(11)61873-4
Barone, C., Basso, M., Schinzari, G., Pozzo, C., Trigila, N., D'Argento, E., et al. (2007). Docetaxel and oxaliplatin combination in second-line treatment of patients with advanced gastric cancer. Gastric Cancer 10 (2), 104–111. doi:10.1007/s10120-007-0415-x
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer statistics in China, 2015. CA Cancer J. Clin. 66 (2), 115–132. doi:10.3322/caac.21338
De Angelis, R., Sant, M., Coleman, M. P., Francisci, S., Baili, P., Pierannunzio, D., et al. (2014). Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5-a population-based study. Lancet Oncol. 15 (1), 23–34. doi:10.1016/s1470-2045(13)70546-1
De Giorgi, V., Grazzini, M., Benemei, S., Marchionni, N., Botteri, E., Pennacchioli, E., et al. (2018). Propranolol for off-label treatment of patients with melanoma. JAMA Oncol. 4 (2), e172908. doi:10.1001/jamaoncol.2017.2908
Deferio, J. J., Levin, T. T., Cukor, J., Banerjee, S., Abdulrahman, R., Sheth, A., et al. (2018). Using electronic health records to characterize prescription patterns: focus on antidepressants in nonpsychiatric outpatient settings. JAMIA Open 1 (2), 233–245. doi:10.1093/jamiaopen/ooy037
Feng, R. M., Zong, Y. N., Cao, S. M., and Xu, R. H. (2019). Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 39 (1), 22. doi:10.1186/s40880-019-0368-6
Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J., Rosso, S., Coebergh, J. W. W., Comber, H., et al. (2015). Reprint of: cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer 51 (9), 1201–1202. doi:10.1016/j.ejca.2015.05.004
Fernandez, M. H., Villaverde, R. M., Yustos, M. A., Exposito, F. N., Gonzalez, J. L. L., Infantes, M. R. L., et al. (2019). The off-label use of antineoplastics in oncology is limited but has notable scientific support in a university hospital setting. Front. Pharmacol. 10, 1210. doi:10.3389/fphar.2019.01210
Herbrand, A. K., Schmitt, A. M., Briel, M., Diem, S., Ewald, H., Hoogkamer, A., et al. (2019). Contrasting evidence to reimbursement reality for off-label use of drug treatments in cancer care: rationale and design of the CEIT-OLU project. ESMO Open 4 (6), e000596. doi:10.1136/esmoopen-2019-000596
Herrero Fernandez, M., Molina Villaverde, R., Arroyo Yustos, M., Navarro Expósito, F., Lopez Gonzalez, J. L., Luque Infantes, M. R., et al. (2019). The off-label use of antineoplastics in oncology is limited but has notable scientific support in a university hospital setting. Front. Pharmacol. 10, 1210. doi:10.3389/fphar.2019.01210
Jiang, J., Zheng, X., Xu, X., Zhou, Q., Yan, H., Zhang, X., et al. (2011). Prognostic significance of miR-181b and miR-21 in gastric cancer patients treated with S-1/Oxaliplatin or Doxifluridine/Oxaliplatin. PLoS One 6 (8), e23271. doi:10.1371/journal.pone.0023271
Joerger, M., Schaer-Thuer, C., Koeberle, D., Matter-Walstra, K., Gibbons-Marsico, J., Diem, S., et al. (2014). Off-label use of anticancer drugs in eastern Switzerland: a population-based prospective cohort study. Eur. J. Clin. Pharmacol. 70 (6), 719–725. doi:10.1007/s00228-014-1662-5
Koizumi, W., Takiuchi, H., Yamada, Y., Boku, N., Fuse, N., Muro, K., et al. (2010). Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study). Ann. Oncol. 21 (5), 1001–1005. doi:10.1093/annonc/mdp464
Léauté-Labrèze, C., Hoeger, P., Mazereeuw-Hautier, J., Guibaud, L., Baselga, E., Posiunas, G., et al. (2015). A randomized, controlled trial of oral propranolol in infantile hemangioma. N. Engl. J. Med. 372 (8), 735–746. doi:10.1056/NEJMoa1404710
Lerose, R., Musto, P., Aieta, M., Papa, C., and Tartarone, A. (2011). Off-label use of anti-cancer drugs between clinical practice and research: the Italian experience. Eur. J. Clin. Pharmacol. 68 (5), 505–512. doi:10.1007/s00228-011-1173-6
Ling-li, Z., You-ping, L., Li-nan, Z., Yi, L., Die, H., Yi, L., et al. (2012). Evidence-based evaluation on off-label drug use policies in 15 countries. Chin. J. Evid. Based Med. 12 (4), 426–435. doi:10.7507/1672-2531.20120069
Liu, B., Wei, J., Zou, Z., Qian, X., Nakamura, T., Zhang, W., et al. (2007). Polymorphism of XRCC1 predicts overall survival of gastric cancer patients receiving oxaliplatin-based chemotherapy in Chinese population. Eur. J. Hum. Genet. 15 (10), 1049. doi:10.1038/sj.ejhg.5201884
Liu, C., Sun, Q., Hang, X., Zhong, B., and Wang, D. (2008). Multicenter phase II study of capecitabine plus oxaliplatin as a first-line therapy in Chinese patients with advanced gastric cancer. Anticancer Drugs 19 (8), 825–831. doi:10.1097/cad.0b013e32830c457e
Lordick, F., Luber, B., Lorenzen, S., Hegewisch-Becker, S., Folprecht, G., Wöll, E., et al. (2010). Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br. J. Cancer 102 (3), 500–505. doi:10.1038/sj.bjc.6605521
Mascolo, A., Scavone, C., Bertini, M., Brusco, S., Punzo, F., Pota, E., et al. (2020). Safety of anticancer agents used in children: a focus on their off-label use through data from the spontaneous reporting system. Front. Pharmacol. 11, 621. doi:10.3389/fphar.2020.00621
Mellor, J. D., Bensted, K. E., and Chan, P. L. (2009). Off label and unlicensed prescribing in a specialist oncology center in Australia. Front. Pharmacol. 5(4), 242–246. doi:10.1111/j.1743-7563.2009.01239.x
Montagnani, F., Turrisi, G., Marinozzi, C., Aliberti, C., and Fiorentini, G. (2011). Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer 14 (1), 50–55. doi:10.1007/s10120-011-0007-7
Nayroles, G., Frybourg, S., Gabriel, S., Kornfeld, Å., Antoñanzas-Villar, F., Espín, J., et al. (2017). Unlocking the potential of established products: toward new incentives rewarding innovation in Europe. J. Market Access Health Pol. 5 (1), 1298190. doi:10.1080/20016689.2017.1298190
Nittari, G., Pallotta, G., Pirillo, I., Ricci, G., and Amenta, F. (2018). Evaluation of medical prescriptions and off-label use on board ships to improve healthcare quality. Eur. Rev. Med. Pharmacol. Sci. 22 (13), 4392–4400. doi:10.26355/eurrev_201807_15439
Park, Y. H., Lee, J.-L., Ryoo, B.-Y., Ryu, M.-H., Yang, S. H., Kim, B. S., et al. (2008). Capecitabine in combination with Oxaliplatin (XELOX) as a first-line therapy for advanced gastric cancer. Cancer Chemother. Pharmacol. 61 (4), 623–629. doi:10.1007/s00280-007-0515-7
Prendergast, M., Li, L., Evans, E., and Hall, E. (2013). Consistency in self-reports of drug use frequency by high-risk offenders over a 5-year interval. Prison J. 93 (4), 375–389. doi:10.1177/0032885513500616
Saiyed, M. M., Ong, P. S., and Chew, L. (2017a). Off-label drug use in oncology: a systematic review of literature. J. Clin. Pharm. Ther. 42 (3), 251–258. doi:10.1111/jcpt.12507
Saiyed, M. M., Ong, P. S., and Chew, L. (2017b). Perception of oncology practitioners towards off-label use of anticancer medicines in Singapore. J. Pharm. Health Serv. Res. 8 (2), 91–99. doi:10.1111/jphs.12175
Seetasith, A., Holdford, D., Shah, A., and Patterson, J. (2017). On-label and off-label prescribing patterns of erythropoiesis-stimulating agents in inpatient hospital settings in the US during the period of major regulatory changes. Res. Soc. Adm. Pharm. 13 (4), 778–788. doi:10.1016/j.sapharm.2016.07.005
Siegel, R. L., Miller, K. D., and Jemal, A. (2019). Cancer statistics, 2019. CA Cancer J. Clin. 69 (1), 7–34. doi:10.3322/caac.21551
Smieliauskas, F., Sharma, H., Hurley, C., de Souza, J. A., and Shih, Y.-C. T. (2018). State insurance mandates and off-label use of chemotherapy. Health Econ. 27 (1), e55–e70. doi:10.1002/hec.3537
Verbaanderd, C., Rooman, I., Meheus, L., and Huys, I. (2020). On-label or off-label? Overcoming regulatory and financial barriers to bring repurposed medicines to cancer patients. Front. Pharmacol. 10, 1664. doi:10.3389/fphar.2019.01664
WHO (2018). Cancer. Available at: http://www.who.int/es/news-room/fact-sheets/detail/cancer (Accessed December 31, 2019)..
Keywords: off-label use, antineoplastic drugs, malignancies, nationwide medical insurance data, retrospective study
Citation: Wei G, Wu M, Zhu H, Han S, Chen J, Zhai C and Shi L (2021) Off-Label Use of Antineoplastic Drugs to Treat Malignancies: Evidence From China Based on a Nationwide Medical Insurance Data Analysis. Front. Pharmacol. 12:616453. doi: 10.3389/fphar.2021.616453
Received: 12 October 2020; Accepted: 23 February 2021;
Published: 08 April 2021.
Edited by:
Ileana Mardare, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Brian Godman, University of Strathclyde, United KingdomDaniela Calina, University of Medicine and Pharmacy of Craiova, Romania
Copyright © 2021 Wei, Wu, Zhu, Han, Chen, Zhai and Shi This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luwen Shi, c2hpbHVAYmptdSxlZHUuY24=
 Guoxu Wei
Guoxu Wei Min Wu1
Min Wu1 He Zhu
He Zhu Sheng Han
Sheng Han Chenchen Zhai
Chenchen Zhai Luwen Shi
Luwen Shi