
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol. , 10 March 2021
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.613837
This article is part of the Research Topic Recent Advances in Cardiotoxicity Testing, Volume I View all 13 articles
Evaluation of potential vascular injury is an essential part of the safety study during pharmaceutical development. Vascular liability issues are important causes of drug termination during preclinical investigations. Currently, preclinical assessment of vascular toxicity primarily relies on the use of animal models. However, accumulating evidence indicates a significant discrepancy between animal toxicity and human toxicity, casting doubt on the clinical relevance of animal models for such safety studies. While the causes of this discrepancy are expected to be multifactorial, species differences are likely a key factor. Consequently, a human-based model is a desirable solution to this problem, which has been made possible by the advent of human induced pluripotent stem cells (iPSCs). In particular, recent advances in the field now allow the efficient generation of a variety of vascular cells (e.g., endothelial cells, smooth muscle cells, and pericytes) from iPSCs. Using these cells, different vascular models have been established, ranging from simple 2D cultures to highly sophisticated vascular organoids and microfluidic devices. Toxicity testing using these models can recapitulate key aspects of vascular pathology on molecular (e.g., secretion of proinflammatory cytokines), cellular (e.g., cell apoptosis), and in some cases, tissue (e.g., endothelium barrier dysfunction) levels. These encouraging data provide the rationale for continuing efforts in the exploration, optimization, and validation of the iPSC technology in vascular toxicology.
Drug-induced vascular toxicity is a multifaceted problem besetting the pharmaceutical industry, the healthcare professionals, and most importantly the patients (Qureshi et al., 2011; Herrmann, 2020). Vascular toxicity that goes unidentified during preclinical and clinical drug studies can present a serious safety hazard to the patients, often leading to drug withdrawal from the market. In fact, multiple major drug withdrawals in the past two decades are attributed to increased vascular events such as strokes and heart attacks (Qureshi et al., 2011). In addition, numerous FDA-approved life-saving chemotherapies, while effective at combating tumor growth, can also result in a wide spectrum of vascular dysfunctions, including acute vasospasm, acute thrombosis, and acceleration of atherosclerosis (Herrmann, 2020). Prevention or mitigation of these debilitating effects in patients remains a challenge for scientists and clinicians.
There is a strong need for a model that can predict drug-induced vascular toxicity, provide insights into the underlying mechanisms, and test potential therapeutics. Currently, animal models such as mice and dogs are the standard preclinical models for toxicological evaluations. Despite their indispensable role in pharmaceutical development, there is a growing recognition that existing animal models alone are inadequate for the accurate prediction of drug toxicity in humans due to differences in physiology, metabolism, and molecular functions between species (Bailey et al., 2013; Chen et al., 2013). The advent and maturation of induced pluripotent stem cell (iPSC) technology presents a valuable opportunity to solve this problem by offering a human cell model. Genetically identical to the donors, iPSCs hold the promise to recapitulate individual predisposition to various risks (Kitani et al., 2019; Lam et al., 2019). Furthermore, recent advances in iPSC differentiation strategies now enable the efficient generation of all the major cell lineages of the human cardiovascular system, including cardiomyocytes (CMs) (Lian et al., 2012; Burridge et al., 2014), endothelial cells (ECs) (Lian et al., 2014; Olmer et al., 2018; Williams and Wu, 2019; Wang et al., 2020), smooth muscle cells (SMCs) (Cheung et al., 2012; Patsch et al., 2015), and cardiac fibroblasts (CFs) (Zhang et al., 2019a). These cells functionally and structurally resemble their counterpart primary cells. Among them, human iPSC-derived cardiomyocytes (iPSC-CMs) have already shown tremendous promise in predicting chemical-induced cardiac liabilities, as exemplified by the Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative led by the FDA (Colatsky et al., 2016; Fermini et al., 2016; Millard et al., 2018). Though similar large-scale studies have yet to be performed to validate the utility of iPSCs in vascular toxicology, emerging studies have shown encouraging results to justify further exploration of this technology as a candidate tool in preclinical and clinical investigations of drug-induced vascular toxicity. In this review, we will discuss the background of drug-induced vascular toxicity, and how iPSC technology may help us transform this field, with an emphasis on comparing the utility and drawbacks of different iPSC-based vascular models.
Unanticipated vascular toxicity is a significant cause for approved drugs to be withdrawn from the market (Qureshi et al., 2011). Using WITHDRAWN (Siramshetty et al., 2016), a publicly available database for discontinued and withdrawn drugs, we identified eight recent drug withdrawals caused by adverse vascular events. These drugs are diverse in applications, including nonsteroidal anti-inflammatory drugs (valdecoxib and rofecoxib), appetite suppressants (sibutramine, dexfenfluramine, and phenylpropanolamine), anti-diabetic drugs (benfluorex), constipation treatment (tegaserod) as well as treatment for Parkinson’s diseases (pergolide). The frequently observed risks of these drugs are strokes, myocardial infarctions (MIs), and valvular heart diseases. Among these drugs, the most recently withdrawn is sibutramine, which was found to increase the risk of non-fatal MIs by 28% and non-fatal strokes by 36% in a 2010 randomized study (James et al., 2010), and it was withdrawn from the United States market in the same year. Notably, the median time on the market of these medications, from their initial approvals to the withdrawals, is 16 years, with the longest for phenylpropanolamine (42 years) and the shortest for valdecoxib (3 years). Undoubtedly, the observed delay of these necessary withdrawals puts patients at risk and creates an extra burden to the healthcare system. Furthermore, these withdrawals highlight the inadequacy of the current system to accurately detect and predict drug-induced vascular dysfunctions.
Chemotherapy drugs are widely known for having cardiotoxic effects (Orphanos et al., 2009; McGowan et al., 2017). However, they also cause a wide spectrum of vascular dysfunctions such as hypertension, ischemic events, and thromboembolism (Cameron et al., 2016; Nebigil and Désaubry, 2018; Herrmann, 2020). In fact, the majority of chemotherapies, both conventional ones (e.g., alkylating agents, antimetabolites, and anthracyclines) and targeted ones (e.g., tyrosine kinase inhibitors, proteasome inhibitors, and monoclonal antibodies) have varying degrees of vascular toxicity (Herrmann et al., 2016; Luu et al., 2018; Sayed et al., 2019; Herrmann, 2020; Rhee et al., 2020). However, unlike those drugs withdrawn from the market, the life-saving nature of chemotherapeutic agents generally tips the scale in the benefit-risk assessment, allowing them to be continuously used to treat cancer patients despite the observed risk to the vasculature. Detection and management of chemotherapy—induced vascular injuries is an important yet highly challenging task, with several major difficulties. First, there is a lack of specific circulating biomarkers for early vascular injuries in humans (Louden et al., 2006; Mikaelian et al., 2014). Second, vascular susceptibility to drugs is patient-specific, depending on numerous pre-existing conditions of an individual (Oren and Herrmann, 2018). Lastly, the mode of injury varies significantly from drug to drug (Herrmann, 2020). Collectively, these challenges call for a new vascular model that allows us to perform patient-specific testing and discover specific biomarkers for vascular injuries.
The ideal platform to study drug toxicity should meet several key criteria. Primarily, the platform needs to faithfully recapitulate the pathological responses of the target organ on molecular, cellular, and tissue levels. From a translational standpoint, the source materials required for testing must be easily accessible and consistent in quality. Lastly, moving into the area of precision medicine, the testing platform should provide patient-specific risk prediction and facilitate the development of personalized prevention and treatment (Sayed et al., 2016; Wu et al., 2019). Conventional animal models and primary cell lines can at best partially fulfill the first two requirements, but are unlikely to meet the growing demand for personalized medicine.
The advent of iPSC technology offers us a valuable opportunity to transform the field of toxicology. Human iPSC-derived cells are genetically identical to the donor cells and hence are expected to respond to drugs in a patient-specific manner (Burridge et al., 2016; Kitani et al., 2019). In addition, the rapid progress in iPSC technology has enabled us to efficiently generate functional ECs (Cao et al., 2013; Olmer et al., 2018; Rosa et al., 2019) and SMCs (Biel et al., 2015; Patsch et al., 2015; Granata et al., 2017), two major cell types comprising the blood vessel. Furthermore, advances in tissue engineering (Chang and Niklason, 2017; Liu et al., 2018b; Ben-Shaul et al., 2019; Crosby et al., 2019) and organ-on-chip technologies (Mori et al., 2017; Zhang et al., 2018; Wnorowski et al., 2019) have enabled us to simulate the in vivo vascular structure and their biophysical environment at an unprecedented pace, making it possible to model more sophisticated pathological processes on multiple scales (Figure 1).
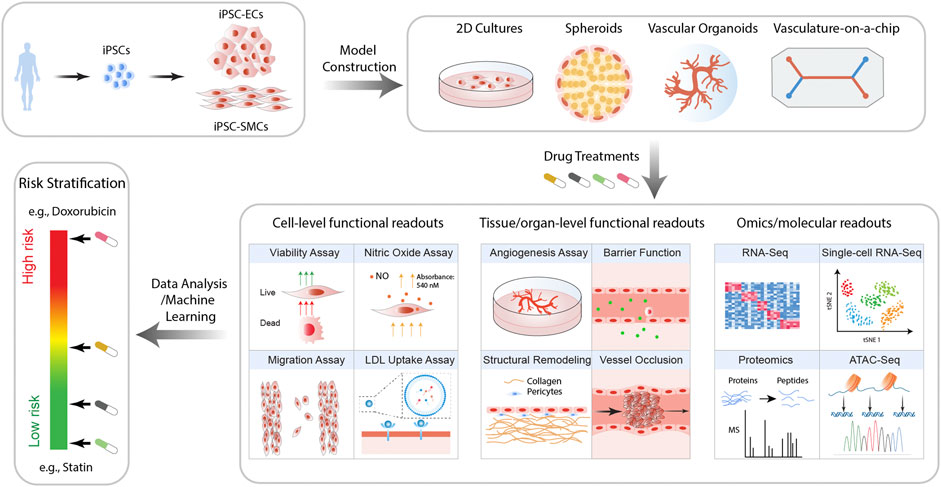
FIGURE 1. Proposed workflow for iPSC-based models for the prediction of drug-induced vascular injuries. Vascular cells such as ECs and SMCs are generated from patient iPSCs. These cells are then employed to construct in vitro models of varying complexity and physiological relevance, including 2D cultures, spheroids, organoids, and vasculature-on-chips. Upon treatment with drugs of interest, a wide range of functional and molecular readouts can be obtained, with which the risk of the drug for inducing vascular disorders is then calculated.
EC dysfunction plays a significant role in vascular diseases such as atherosclerosis (Davignon and Ganz, 2004), thrombosis (Yau et al., 2015), and hypertension (Brandes, 2014). The generation of iPSC-derived endothelial cells (iPSC-ECs) that resemble in vivo ECs is a critical step toward building an in vitro vascular model. In the past decade, numerous methods have been developed to produce functional ECs from iPSCs, which are well summarized in a recent review (Williams and Wu, 2019). The general principle is similar among different EC differentiation methods. Human iPSCs are usually first induced into the mesoderm by a cocktail of Activin A, BMP-4, and/or GSK3 inhibitors, and subsequently specified into ECs with VEGF, FGF2, and bFGF treatment (Lian et al., 2014; Palpant et al., 2017; Liu et al., 2018a). To further enrich ECs, the differentiated cells can be sorted for the CD31 positive population (Liu et al., 2018a). In a recent study from our lab, we demonstrated that iPSC-ECs exhibit a wide range of properties characteristic of primary ECs (Sayed et al., 2020). Specifically, iPSC-ECs showed morphological similarity to primary ECs with a cobblestone cell shape. Functionally, these cells increased nitric oxide (NO) production in response to shear stress or vasodilators such as acetylcholine and A23187. Metabolically, they can incorporate acetylated low—density lipoprotein (ac-LDL), another hallmark of primary ECs.
In addition to ECs, vascular SMCs are also essential for maintaining the homeostasis of a healthy vasculature. SMCs not only provide mechanical support for a vasculature, but also regulate blood vessel tone, inflammation, and vascular remodeling (Majesky et al., 2011; Lim and Park, 2014). SMC disorder is a major contributing factor to the development of common vascular diseases such as atherosclerosis (Bennett et al., 2016) and hypertension (Touyz et al., 2018). Early methods to generate SMCs from iPSCs relied on unguided differentiation to produce a highly heterogeneous population, followed by flow cytometry sorting for Flk1+/VE-cadherin- cells. These cells exhibited typical SMC morphology and expressed specific markers such as alpha-smooth muscle actin (αSMA) and calponin (CNN1) (Taura et al., 2009). Since then, SMC differentiation has evolved to be more efficient and targeted (Stephenson et al., 2020). For instance, Cheung et al. developed a set of protocols to produce lineage—specific vascular SMCs (Cheung et al., 2012; Cheung et al., 2014). These iPSC-SMCs not only express markers such as CNN1 and myosin heavy chain 11 (MYH11), but also differentially respond to cytokine stimulation depending on their lineages (Cheung et al., 2012). In addition to lineage specificity, Wanjare et al. reported that modulation of serum level and PDGF-BB could generate phenotype-specific (synthetic or contractile) SMCs from iPSCs (Wanjare et al., 2013). In this section, we will discuss the use of iPSC-ECs and iPSC-SMCs to model vascular injuries (Table 1).
To test the toxicity of a compound, the conventional approach is to apply it directly to a monolayer culture of target cells. Although they lack the sophisticated tissue architecture or biophysical stimulation (e.g., shear stress) present in vivo, numerous studies have demonstrated that iPSC-ECs or iPSC-SMCs cultured in monolayers respond to chemical or genetic stress in ways that are largely consistent with clinical observations or data from primary cells (Zhang et al., 2011; Cheung et al., 2012; Biel et al., 2015; Belair et al., 2016; Tang et al., 2017; Rieker et al., 2019; Chu et al., 2020). For instance, our lab recently found that e-cigarette liquid (e-liquid) triggered a broad array of molecular and functional disorders in iPSC-ECs, including activation of cell apoptosis, increased reactive oxygen species (ROS) production and LDL uptake, as well as reduced cell migration (Lee et al., 2019). Conditioned medium from e-liquid-treated iPSC-ECs induced macrophage polarization, suggesting a pro-inflammation effect of e-cigarettes on the cardiovascular system. Importantly, these data were corroborated by an analysis of the serum from e-cigarettes users, which revealed a similar upregulation of inflammatory cytokines (Lee et al., 2019).
A key advantage of a 2D model is the compatibility with high-throughput assays. Furthermore, the unlimited proliferative potential of iPSCs allows us to generate large quantities of cells required for large-scale screening experiments, which can be challenging when using primary cells. In fact, several studies have employed high-throughput platforms to investigate drug-induced vascular toxicity or to identify therapeutic agents using iPSC-ECs (Iwata et al., 2017; Sharma et al., 2017) or iPSC-SMCs (Zhang et al., 2019b). For instance, Iwata et al. used high-content imaging in a 384-well plate format to test a variety of compounds with or without vascular toxicity on iPSC-ECs (Iwata et al., 2017). Based on readouts including ATP content, nuclear content, cell viability, and tube formation, this platform successfully identified the toxic compounds with high reproducibility (Iwata et al., 2017). Moreover, the high-throughput format is also suitable for screening therapeutic compounds that could induce a desirable phenotype. Zhang et al. engineered a MYH11 reporter cell line in human embryonic stem cells (Zhang et al., 2019b). MYH11 expression was chosen as a surrogate for the desired contractile phenotype in SMCs. By doing so, they successfully identified RepSox, a small molecule inhibitor of TGF-β type 1 receptor, as a potent drug that could mitigate intimal hyperplasia, a condition characterized by the pathological switch of SMCs from the contractile to the synthetic phenotype.
Application of 3D culture is a common strategy to improve the physiological relevance of an in vitro model (Duval et al., 2017). Generally speaking, cells grown as 3D constructs experience more cell-cell interactions and cell-extracellular matrix (ECM) interactions than in 2D (Duval et al., 2017). In addition, a compact 3D environment creates diffusion gradients for biochemical signals, increasing the retention of secreted factors and allowing for a localized build-up of metabolic waste, all of which are typical features of the in vivo environment. Thanks to these advantages, 3D vascular models have been broadly exploited to investigate tumor angiogenesis and to test anti-angiogenic compounds (Taubenberger et al., 2016; Amann et al., 2017; Chiew et al., 2017; Klimkiewicz et al., 2017).
A simple method to generate vascular spheroids is to aggregate vascular cells, either stem cell-derived or primary, directly into 3D spheres. This can be done using the hanging-drop technique (Markou et al., 2020) or using an ultra—low attachment culture substrate (Moldovan et al., 2017). Interestingly, distinct cell types mixed together to form spheroids tend to spontaneously organize themselves into a structural hierarchy reminiscent of a native vasculature. For instance, Markou et al. generated small vascular spheroids (∼100 μm) from iPSC-ECs and iPSC-SMCs (Markou et al., 2020). The iPSC-ECs spontaneously formed the outer lining of these spheroids, allowing them to be in direct contact with the culture medium, whereas the iPSC-SMCs lined beneath the iPSC-ECs, forming the interior layer (Markou et al., 2020). Despite being a relatively crude model, these spheroids emulate some aspects of vascular anatomy that are not present in a 2D culture.
To create more sophisticated vascular organoids, biomaterials such as collagen, fibrin, and hyaluronic acid can be employed to initiate angiogenesis inside the organoids (Crosby et al., 2019; Crosby and Zoldan, 2019). Compared with cell—only spheroids, the introduction of biomaterials improves the control over the microenvironment, making it possible to develop high-fidelity vasculatures. This is well illustrated recently by Wimmer et al., who combined the use of fine-tuned iPSC vascular differentiation and a 3D matrix (collagen and Matrigel) to produce highly elaborate capillary vasculatures composed of ECs, pericytes, and basement membrane (Wimmer et al., 2019). These engineered microvessels have a hollow lumen and could integrate into the native vasculature upon transplantation in vivo, indicating the functionality of these capillaries. Upon exposure to diabetic stress consisting of hyperglycemia, tumor necrosis factor (TNF), and interleukin-6 (IL-6), these capillaries underwent massive thickening in the basement membrane as evidenced by collagen IV staining, a hallmark of diabetes-induced vasculopathy. Notably, identical treatment applied to iPSC-ECs or iPSC-SMCs in 2D cultures did not result in any increase in collagen IV accumulation, suggesting the necessity of the 3D structure to recapitulate this pathological change.
Microfluidic organ-on-a-chip technology is a fast-growing field arising from the convergence of engineering, material science, and human biology. Essentially, these microchips are miniaturized physiological systems engineered to emulate key features of native organ architecture and function (Atchison et al., 2017; Kurokawa et al., 2017; Gold et al., 2019). By modulating channel geometry, substrate stiffness, perfusion rate, and biochemical signals, microfluidic chips can model a wide range of vascular diseases on an organ scale. For instance, vascular chips can be engineered with inward protrusions in the middle of the channels to mimic the narrowing of arteries caused by atherosclerosis plaque (Westein et al., 2013). This special geometrical design resulted in platelet aggregation and increased vWF expression, in alignment with the increased thrombus events observed in animals and patients with atherosclerosis. Another example by Qiu et al. showed that the perfusion of microvascular chips in vitro using red blood cells (RBCs) harvested from sickle patients could cause vessel occlusions in the chip, similar to the vascular obstructions seen in sickle cell anemia patients (Qiu et al., 2018).
The fusion of iPSC technology and microfluidics is revealing even more exciting possibilities. Specifically, early vascular chips mainly relied on primary ECs like human umbilical vein endothelial cells (HUVECs) to generate a functional endothelium. While these HUVEC-based models are useful as a generic representation of vasculatures, they are not ideal models for organ-specific blood vessels. In fact, it is well-known that ECs in distinct organs differ in both molecular signature and function (Marcu et al., 2018; Paik et al., 2020b). By contrast, human iPSCs can be differentiated into organ-specific ECs, allowing us to manufacture more specialized and physiologically representative vasculatures. The blood-brain barrier (BBB), for instance, is one of the most specialized and clinically important vasculatures. Researchers have demonstrated that iPSC-derived brain microvascular ECs (iPSC-BMECs) can form a functional endothelium barrier in microfluidics and exhibit a large transendothelial electrical resistance (TEER) characteristic of the human BBB (Linville et al., 2019; Vatine et al., 2019). Furthermore, the engineered BBB exhibited selective permeability and remained functional while being perfused with human whole blood (Westein et al., 2013). In addition to modeling endothelium, microfluidic platforms can also be utilized to investigate vascular smooth muscle pathology in a patient-specific manner. Using microfluidics, iPSC-SMCs derived from progeroid patients were found to be more susceptible to detachment under flow-induced shear stress compared to the healthy control, and metalloprotease 13 (MMP13) was identified as a potential therapeutic target (Pitrez et al., 2020).
Generally speaking, there are two fundamental considerations in choosing in vitro models for vascular toxicity studies or toxicity studies in general. The first consideration is physiological relevance, which determines whether a model can precisely recapitulate the human response to a given drug. Overall, organoids and microfluidic vascular chips are expected to be more physiologically relevant than 2D models or simple vascular spheroids (Duval et al., 2017). The second factor to consider is experimental throughput. This factor is especially important during the early stage of drug research and development, when it is often necessary to screen tens of thousands of compounds. 2D models, despite their limited physiological relevance, are most commonly used for high-throughput screenings to identify toxic vs. therapeutic compounds. Therefore, a rational strategy would be to employ 2D models for early exploratory investigations that are focused on narrowing down the list of candidates or gaining preliminary insights. Afterward, 3D organoids or microfluidic platforms can be adopted to develop a more comprehensive and accurate understanding of the drug’s effects.
A universal concern regarding iPSC-derived cells is that they tend to be very heterogeneous, and this is no exception for iPSC-derived vascular cells (Karakikes et al., 2015; Paik et al., 2018; Kwong et al., 2019; Volpato and Webber, 2020). Drug testing using heterogeneous cell populations may lead to an inaccurate conclusion, especially if it is based on ensemble measurements (Altschuler and Wu, 2010). To tackle this issue, a variety of strategies can be employed, such as optimization of the differentiation protocol (Wang et al., 2020), purification using genetically engineered reporter cell lines (Hu et al., 2018), or magnetic-activated cell sorting (MACS) based on the desired surface marker (Li et al., 2017).
Future iPSC-based vascular models may benefit from using tissue-specific or vessel-specific cells to improve their physiological relevance. In recent years, it is increasingly appreciated that ECs or SMCs may vary considerably in both function and molecular signature depending on their subtype (Cheung et al., 2012; Bennett et al., 2016; Williams and Wu, 2019; Jambusaria et al., 2020). Some successful efforts have been made on this front, such as the development of brain microvascular chips (Linville et al., 2019; Vatine et al., 2019; Weaver and Valentin, 2019) and drug testing using lineage-specific SMCs (Cheung et al., 2012).
One representative iPSC technology at the forefront of transforming drug toxicology is the use of iPSC-derived cardiomyocytes (Gintant et al., 2019; Pang et al., 2019). In particular, large-scale efforts exemplified by the CiPA initiative have been coordinated by academia, industry, and regulatory bodies to explore and validate iPSC-CMs as a viable option for predicting cardiac liabilities in a translational setting (Colatsky et al., 2016; Blinova et al., 2017). Clearly, for iPSC-based vascular models to be accepted as a tool in preclinical or even clinical investigation, a similar pathway is inevitable. Particularly, iPSC vascular models should be rigorously tested with double-blinded experiments to quantify the specificity and sensitivity, and their performance needs to be compared with animal models.
The past decade has witnessed a bountiful rise of exciting technologies in the biomedical field. These technologies can be exploited to empower iPSC-based drug testing. 3D bioprinting technologies using iPSC-derived cells can help us build more realistic vasculatures on a chip (Kolesky et al., 2014; Maiullari et al., 2018). Single-cell technologies such as single-cell RNA sequencing can reveal hidden pathological changes at an unprecedented resolution (Chaudhry et al., 2019; Paik et al., 2020a). CRISPR-based gene editing tools together with patient-specific iPSCs may unveil the effects of single nucleotide polymorphism (SNP) on drug response and hence determine individual predisposition to toxicity (Pang et al., 2009; Burridge et al., 2016; Seeger et al., 2017; Garg et al., 2018; Ma et al., 2018). Machine learning supported with big data has also shown promise in improving the prediction of drug toxicity (Lau et al., 2019; Vo et al., 2020). Collectively, these technologies have led us to uncharted territory for toxicology. The utility of iPSC-based platforms in predicting vascular toxicity, or any drug-induced toxicity, is likely to be significantly expanded in combination with these state-of-the-art tools.
Prediction and understanding of drug-induced toxicity is a critical part of pharmaceutical development as well as patient care. The vascular system as the network for transporting nutrients and hormones is at a high risk of exposure to drug-induced off-target damages, as evidenced by notable major drug withdrawals caused by vascular events and frequent vascular complications observed in patients undergoing chemotherapy. However, the detection of drug-induced vascular toxicity in humans is difficult, partly because its manifestation may be slow and patient-specific (Cameron et al., 2016; Herrmann, 2020). In fact, it was suggested that surveillance of vascular health in certain cancer survivors should last several years after chemotherapy (Herrmann, 2020). Obviously, such a long time frame would be impractical in most translational and clinical settings. The use of animal models is intended to accelerate the discovery and understanding of drug toxicity. However, it is increasingly recognized that fundamental species differences on molecular, cellular, and tissue levels limit the predictive power of animal models for human toxicity (Bailey et al., 2013; Bailey et al., 2014). The invention of iPSC technology presents a paradigm shift improvement in our ability to predict and dissect drug-induced vascular injuries. For the first time, it is possible to establish a scalable and personalized drug testing platform. To be sure, iPSC models are still very immature for the time being, and issues such as consistency and physiological relevance are yet to be comprehensively examined. Nevertheless, with the rapid and continuous improvements made by researchers from different disciplines, we are optimistic that they will be an increasingly powerful tool in the future of vascular toxicology.
CT wrote the manuscript. CT, NC, MZ, and JW revised the manuscript and provided critical input.
This work was supported by the American Heart Association 20POST35080175 (CT) and 17MERIT33610009 (JW), the National Institutes of Health R01 HL141851, R01 HL141371, R01 HL146690, UH3 TR002588, and TRDRP 27IR-0012 (JW).
JW is a cofounder of Khloris Biosciences but has no competing interests, as the work presented here is completely independent.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Altschuler, S. J., and Wu, L. F. (2010). Cellular heterogeneity: do differences make a difference?. Cell 141, 559–563. doi:10.1016/j.cell.2010.04.033
Amann, A., Zwierzina, M., Koeck, S., Gamerith, G., Pechriggl, E., Huber, J. M., et al. (2017). Development of a 3D angiogenesis model to study tumour-endothelial cell interactions and the effects of anti-angiogenic drugs. Sci. Rep. 7, 2963. doi:10.1038/s41598-017-03010-6
Atchison, L., Zhang, H., Cao, K., and Truskey, G. A. (2017). A tissue engineered blood vessel model of hutchinson-gilford progeria syndrome using human iPSC-derived smooth muscle cells. Sci. Rep. 7, 8168. doi:10.1038/s41598-017-08632-4
Bailey, J., Thew, M., and Balls, M. (2014). An analysis of the use of animal models in predicting human toxicology and drug safety. Altern. Lab. Anim. 42, 181–199. doi:10.1177/026119291404200306
Bailey, J., Thew, M., and Balls, M. (2013). An analysis of the use of dogs in predicting human toxicology and drug safety. Altern. Lab. Anim. 41, 335–350. doi:10.1177/026119291304100504
Belair, D. G., Schwartz, M. P., Knudsen, T., and Murphy, W. L. (2016). Human iPSC-derived endothelial cell sprouting assay in synthetic hydrogel arrays. Acta Biomater. 39, 12–24. doi:10.1016/j.actbio.2016.05.020
Ben-Shaul, S., Landau, S., Merdler, U., and Levenberg, S. (2019). Mature vessel networks in engineered tissue promote graft-host anastomosis and prevent graft thrombosis. Proc. Natl. Acad. Sci. U. S. A. 116, 2955–2960. doi:10.1073/pnas.1814238116
Bennett, M. R., Sinha, S., and Owens, G. K. (2016). Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702. doi:10.1161/CIRCRESAHA.115.306361
Biel, N. M., Santostefano, K. E., DiVita, B. B., El Rouby, N., Carrasquilla, S. D., Simmons, C., et al. (2015). Vascular smooth muscle cells from hypertensive patient-derived induced pluripotent stem cells to advance hypertension pharmacogenomics. Stem Cell Transl. Med. 4, 1380–1390. doi:10.5966/sctm.2015-0126
Blinova, K., Stohlman, J., Vicente, J., Chan, D., Johannesen, L., Hortigon-Vinagre, M. P., et al. (2017). Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol. Sci. 155, 234–247. doi:10.1093/toxsci/kfw200
Brandes, R. P. (2014). Endothelial dysfunction and hypertension. Hypertension 64, 924–928. doi:10.1161/HYPERTENSIONAHA.114.03575
Burridge, P. W., Li, Y. F., Matsa, E., Wu, H., Ong, S. G., Sharma, A., et al. (2016). Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 22, 547–556. doi:10.1038/nm.4087
Burridge, P. W., Matsa, E., Shukla, P., Lin, Z. C., Churko, J. M., Ebert, A. D., et al. (2014). Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860. doi:10.1038/nMeth.2999
Cameron, A. C., Touyz, R. M., and Lang, N. N. (2016). Vascular complications of cancer chemotherapy. Can. J. Cardiol. 32, 852–862. doi:10.1016/j.cjca.2015.12.023
Cao, N., Liang, H., Huang, J., Wang, J., Chen, Y., Chen, Z., et al. (2013). Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell Res. 23, 1119–1132. doi:10.1038/cr.2013.102
Chang, W. G., and Niklason, L. E. (2017). A short discourse on vascular tissue engineering. Npj Regen. Med. 2, 1–8. doi:10.1038/s41536-017-0011-6
Chaudhry, F., Isherwood, J., Bawa, T., Patel, D., Gurdziel, K., Lanfear, D. E., et al. (2019). Single-cell RNA sequencing of the cardiovascular system: new looks for old diseases. Front. Cardiovasc. Med. 6, 173. doi:10.3389/fcvm.2019.00173
Chen, J., Kang, D., Xu, J., Lake, M., Hogan, J. O., Sun, C., et al. (2013). Species differences and molecular determinant of TRPA1 cold sensitivity. Nat. Commun. 4, 2501–2751. doi:10.1038/ncomms3501
Cheung, C., Bernardo, A. S., Pedersen, R. A., and Sinha, S. (2014). Directed differentiation of embryonic origin-specific vascular smooth muscle subtypes from human pluripotent stem cells. Nat. Protoc. 9, 929–938. doi:10.1038/nprot.2014.059
Cheung, C., Bernardo, A. S., Trotter, M. W., Pedersen, R. A., and Sinha, S. (2012). Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol. 30, 165–173. doi:10.1038/nbt.2107
Chiew, G. G. Y., Wei, N., Sultania, S., Lim, S., and Luo, K. Q. (2017). Bioengineered three-dimensional co-culture of cancer cells and endothelial cells: a model system for dual analysis of tumor growth and angiogenesis. Biotechnol. Bioeng. 114, 1865–1877. doi:10.1002/bit.26297
Chu, P. H., Chen, G., Kuo, D., Braisted, J., Huang, R., Wang, Y., et al. (2020). Stem cell-derived endothelial cell model that responds to tobacco smoke like primary endothelial cells. Chem. Res. Toxicol. 33, 751–763. doi:10.1021/acs.chemrestox.9b00363
Colatsky, T., Fermini, B., Gintant, G., Pierson, J. B., Sager, P., Sekino, Y., et al. (2016). The comprehensive in Vitro proarrhythmia assay (CiPA) initiative - update on progress. J. Pharmacol. Toxicol. Methods 81, 15–20. doi:10.1016/j.vascn.2016.06.002
Crosby, C. O., Valliappan, D., Shu, D., Kumar, S., Tu, C., Deng, W., et al. (2019). Quantifying the vasculogenic potential of induced pluripotent stem cell-derived endothelial progenitors in collagen hydrogels. Tissue Eng. Part. A. 25, 746–758. doi:10.1089/ten.tea.2018.0274
Crosby, C. O., and Zoldan, J. (2019). Mimicking the physical cues of the ECM in angiogenic biomaterials. Regen. Biomater. 6, 61–73. doi:10.1093/rb/rbz003
Davignon, J., and Ganz, P. (2004). Role of endothelial dysfunction in atherosclerosis. Circulation 109, III27. doi:10.1161/01.cir.0000131515.03336.f8
Duval, K., Grover, H., Han, L. H., Mou, Y., Pegoraro, A. F., Fredberg, J., et al. (2017). Modeling physiological events in 2D vs. 3D cell culture. Physiology 32, 266–277. doi:10.1152/physiol.00036.2016
Fermini, B., Hancox, J. C., Abi-Gerges, N., Bridgland-Taylor, M., Chaudhary, K. W., Colatsky, T., et al. (2016). A new perspective in the field of cardiac safety testing through the comprehensive in vitro proarrhythmia assay paradigm. J. Biomol. Screen. 21, 1–11. doi:10.1177/1087057115594589
Garg, P., Oikonomopoulos, A., Chen, H., Li, Y., Lam, C. K., Sallam, K., et al. (2018). Genome editing of induced pluripotent stem cells to decipher cardiac channelopathy variant. J. Am. Coll. Cardiol. 72, 62–75. doi:10.1016/j.jacc.2018.04.041
Gintant, G., Burridge, P., Gepstein, L., Harding, S., Herron, T., Hong, C., et al. (2019). Use of human induced pluripotent stem cell-derived cardiomyocytes in preclinical cancer drug cardiotoxicity testing: a scientific statement from the American Heart Association. Circ. Res. 125, e75–e92. doi:10.1161/RES.0000000000000291
Gold, K., Gaharwar, A. K., and Jain, A. (2019). Emerging trends in multiscale modeling of vascular pathophysiology: organ-on-a-chip and 3D printing. Biomaterials 196, 2–17. doi:10.1016/j.biomaterials.2018.07.029
Granata, A., Serrano, F., Bernard, W. G., McNamara, M., Low, L., Sastry, P., et al. (2017). An iPSC-derived vascular model of Marfan syndrome identifies key mediators of smooth muscle cell death. Nat. Genet. 49, 97–109. doi:10.1038/ng.3723
Herrmann, J. (2020). Vascular toxic effects of cancer therapies. Nat. Rev. Cardiol. 17, 503–522. doi:10.1038/s41569-020-0347-2
Herrmann, J., Yang, E. H., Iliescu, C. A., Cilingiroglu, M., Charitakis, K., Hakeem, A., et al. (2016). Vascular toxicities of cancer therapies: the old and the new--an evolving avenue. Circulation 133, 1272–1289. doi:10.1161/CIRCULATIONAHA.115.018347
Hu, Z., Wu, Y., Zhou, M., Wang, X., Pang, J., Li, Z., et al. (2018). Generation of reporter hESCs by targeting EGFP at the CD144 locus to facilitate the endothelial differentiation. Dev. Growth Differ. 60, 205–215. doi:10.1111/dgd.12433
Iwata, Y., Klaren, W. D., Lebakken, C. S., Grimm, F. A., and Rusyn, I. (2017). High-content assay multiplexing for vascular toxicity screening in induced pluripotent stem cell-derived endothelial cells and human umbilical vein endothelial cells. Assay Drug Dev. Technol. 15, 267–279. doi:10.1089/adt.2017.786
Jambusaria, A., Hong, Z., Zhang, L., Srivastava, S., Jana, A., Toth, P. T., et al. (2020). Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife 9, e51413. doi:10.7554/eLife.51413
James, W. P., Caterson, I. D., Coutinho, W., Finer, N., Van Gaal, L. F., Maggioni, A. P., et al. (2010). Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N. Engl. J. Med. 363, 905–917. doi:10.1056/NEJMoa1003114
Karakikes, I., Ameen, M., Termglinchan, V., and Wu, J. C. (2015). Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ. Res. 117, 80–88. doi:10.1161/CIRCRESAHA.117.305365
Kitani, T., Ong, S. G., Lam, C. K., Rhee, J. W., Zhang, J. Z., Oikonomopoulos, A., et al. (2019). Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation 139, 2451–2465. doi:10.1161/CIRCULATIONAHA.118.037357
Klimkiewicz, K., Weglarczyk, K., Collet, G., Paprocka, M., Guichard, A., Sarna, M., et al. (2017). A 3D model of tumour angiogenic microenvironment to monitor hypoxia effects on cell interactions and cancer stem cell selection. Cancer Lett. 396, 10–20. doi:10.1016/j.canlet.2017.03.006
Kolesky, D. B., Truby, R. L., Gladman, A. S., Busbee, T. A., Homan, K. A., and Lewis, J. A. (2014). 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. Weinheim 26, 3124–3130. doi:10.1002/adma.201305506
Kurokawa, Y. K., Yin, R. T., Shang, M. R., Shirure, V. S., Moya, M. L., and George, S. C. (2017). Human induced pluripotent stem cell-derived endothelial cells for three-dimensional microphysiological systems. Tissue Eng. Part. C Methods 23, 474–484. doi:10.1089/ten.tec.2017.0133
Kwong, G., Marquez, H. A., Yang, C., Wong, J. Y., and Kotton, D. N. (2019). Generation of a purified iPSC-derived smooth muscle-like population for cell sheet engineering. Stem Cell Rep. 13, 499–514. doi:10.1016/j.stemcr.2019.07.014
Lam, C. K., Tian, L., Belbachir, N., Wnorowski, A., Shrestha, R., Ma, N., et al. (2019). Identifying the transcriptome signatures of calcium channel blockers in human induced pluripotent stem cell-derived cardiomyocytes. Circ. Res. 125, 212–222. doi:10.1161/CIRCRESAHA.118.314202
Lau, E., Paik, D. T., and Wu, J. C. (2019). Systems-wide approaches in induced pluripotent stem cell models. Annu. Rev. Pathol. 14, 395–419. doi:10.1146/annurev-pathmechdis-012418-013046
Lee, W. H., Ong, S. G., Zhou, Y., Tian, L., Bae, H. R., Baker, N., et al. (2019). Modeling cardiovascular risks of E-cigarettes with human-induced pluripotent stem cell-derived endothelial cells. J. Am. Coll. Cardiol. 73, 2722–2737. doi:10.1016/j.jacc.2019.03.476
Li, Y., Green, M., Wen, Y., Wei, Y., Wani, P., Wang, Z., et al. (2017). Efficacy and safety of immuno-magnetically sorted smooth muscle progenitor cells derived from human-induced pluripotent stem cells for restoring urethral sphincter function. Stem Cell Transl. Med. 6, 1158–1167. doi:10.1002/sctm.16-0160
Lian, X., Bao, X., Al-Ahmad, A., Liu, J., Wu, Y., Dong, W., et al. (2014). Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep. 3, 804–816. doi:10.1016/j.stemcr.2014.09.005
Lian, X., Hsiao, C., Wilson, G., Zhu, K., Hazeltine, L. B., Azarin, S. M., et al. (2012). Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. U.S.A. 109, E1848–E1857. doi:10.1073/pnas.1200250109
Lim, S., and Park, S. (2014). Role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 47, 1–7. doi:10.5483/BMBRep.2014.47.1.285
Linville, R. M., DeStefano, J. G., Sklar, M. B., Xu, Z., Farrell, A. M., Bogorad, M. I., et al. (2019). Human iPSC-derived blood-brain barrier microvessels: validation of barrier function and endothelial cell behavior. Biomaterials 190-191, 24–37. doi:10.1016/j.biomaterials.2018.10.023
Liu, C., Cheng, L., Chen, C., and Sayed, N. (2018a). Generation of endothelial cells from human induced pluripotent stem cells. Bio-Protocol 8, 1–9. doi:10.21769/bioprotoc.3086
Liu, C., Oikonomopoulos, A., Sayed, N., and Wu, J. C. (2018b). Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development 145, dev156166. doi:10.1242/dev.156166
Louden, C., Brott, D., Katein, A., Kelly, T., Gould, S., Jones, H., et al. (2006). Biomarkers and mechanisms of drug-induced vascular injury in non-rodents. Toxicol. Pathol. 34, 19–26. doi:10.1080/01926230500512076
Luu, A. Z., Chowdhury, B., Al-Omran, M., Teoh, H., Hess, D. A., and Verma, S. (2018). Role of endothelium in doxorubicin-induced cardiomyopathy. JACC Basic Transl. Sci. 3, 861–870. doi:10.1016/j.jacbts.2018.06.005
Ma, N., Zhang, J. Z., Itzhaki, I., Zhang, S. L., Chen, H., Haddad, F., et al. (2018). Determining the pathogenicity of a genomic variant of uncertain significance using CRISPR/Cas9 and human-induced pluripotent stem cells. Circulation 138, 2666–2681. doi:10.1161/CIRCULATIONAHA.117.032273
Maiullari, F., Costantini, M., Milan, M., Pace, V., Chirivì, M., Maiullari, S., et al. (2018). A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 8, 13532–13615. doi:10.1038/s41598-018-31848-x
Majesky, M. W., Dong, X. R., Regan, J. N., and Hoglund, V. J. (2011). Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ. Res. 108, 365–377. doi:10.1161/CIRCRESAHA.110.223800
Marcu, R., Choi, Y. J., Xue, J., Fortin, C. L., Wang, Y., Nagao, R. J., et al. (2018). Human organ-specific endothelial cell heterogeneity. iScience 4, 20–35. doi:10.1016/j.isci.2018.05.003
Markou, M., Kouroupis, D., Badounas, F., Katsouras, A., Kyrkou, A., Fotsis, T., et al. (2020). Tissue engineering using vascular organoids from human pluripotent stem cell derived mural cell phenotypes. Front. Bioeng. Biotechnol. 8, 278. doi:10.3389/fbioe.2020.00278
McGowan, J. V., Chung, R., Maulik, A., Piotrowska, I., Walker, J. M., and Yellon, D. M. (2017). Anthracycline chemotherapy and cardiotoxicity. Cardiovasc. Drugs Ther. 31, 63–75. doi:10.1007/s10557-016-6711-0
Mikaelian, I., Cameron, M., Dalmas, D. A., Enerson, B. E., Gonzalez, R. J., Guionaud, S., et al. (2014). Nonclinical safety biomarkers of drug-induced vascular injury: current status and blueprint for the future. Toxicol. Pathol. 42, 635–657. doi:10.1177/0192623314525686
Millard, D., Dang, Q., Shi, H., Zhang, X., Strock, C., Kraushaar, U., et al. (2018). Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: results from a blinded CiPA pilot study. Toxicol. Sci. 164, 550–562. doi:10.1093/toxsci/kfy110
Moldovan, L., Barnard, A., Gil, C. H., Lin, Y., Grant, M. B., Yoder, M. C., et al. (2017). iPSC-derived vascular cell spheroids as building blocks for scaffold-free biofabrication. Biotechnol. J. 12. doi:10.1002/biot.201700444
Mori, N., Morimoto, Y., and Takeuchi, S. (2017). Skin integrated with perfusable vascular channels on a chip. Biomaterials 116, 48–56. doi:10.1016/j.biomaterials.2016.11.031
Nebigil, C. G., and Désaubry, L. (2018). Updates in anthracycline-mediated cardiotoxicity. Front. Pharmacol. 9, 1262. doi:10.3389/fphar.2018.01262
Olmer, R., Engels, L., Usman, A., Menke, S., Malik, M. N. H., Pessler, F., et al. (2018). Differentiation of human pluripotent stem cells into functional endothelial cells in scalable suspension culture. Stem Cell Rep. 10, 1657–1672. doi:10.1016/j.stemcr.2018.03.017
Oren, O., and Herrmann, J. (2018). Arterial events in cancer patients-the case of acute coronary thrombosis. J. Thorac. Dis. 10, S4367–S4385. doi:10.21037/jtd.2018.12.79
Orphanos, G. S., Ioannidis, G. N., and Ardavanis, A. G. (2009). Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 48, 964–970. doi:10.1080/02841860903229124
Paik, D. T., Cho, S., Tian, L., Chang, H. Y., and Wu, J. C. (2020a). Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat. Rev. Cardiol. 17, 457–473. doi:10.1038/s41569-020-0359-y
Paik, D. T., Tian, L., Williams, I. M., Rhee, S., Zhang, H., Liu, C., et al. (2020b). Single-cell RNA sequencing unveils unique transcriptomic signatures of organ-specific endothelial cells. Circulation 142, 1848. doi:10.1161/CIRCULATIONAHA.119.041433
Paik, D. T., Tian, L., Lee, J., Sayed, N., Chen, I. Y., Rhee, S., et al. (2018). Large-scale single-cell RNA-seq reveals molecular signatures of heterogeneous populations of human induced pluripotent stem cell-derived endothelial cells. Circ. Res. 123, 443–450. doi:10.1161/CIRCRESAHA.118.312913
Palpant, N. J., Pabon, L., Friedman, C. E., Roberts, M., Hadland, B., Zaunbrecher, R. J., et al. (2017). Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nat. Protoc. 12, 15–31. doi:10.1038/nprot.2016.153
Pang, G. S., Wang, J., Wang, Z., and Lee, C. G. (2009). Predicting potentially functional SNPs in drug-response genes. Pharmacogenomics 10, 639–653. doi:10.2217/pgs.09.12
Pang, L., Sager, P., Yang, X., Shi, H., Sannajust, F., Brock, M., et al. (2019). Workshop report: FDA workshop on improving cardiotoxicity assessment with human-relevant platforms. Circ. Res. 125, 855–867. doi:10.1161/CIRCRESAHA.119.315378
Patsch, C., Challet-Meylan, L., Thoma, E. C., Urich, E., Heckel, T., O’Sullivan, J. F., et al. (2015). Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 17, 994–1003. doi:10.1038/ncb3205
Pitrez, P. R., Estronca, L., Monteiro, L. M., Colell, G., Vazão, H., Santinha, D., et al. (2020). Vulnerability of progeroid smooth muscle cells to biomechanical forces is mediated by MMP13. Nat. Commun. 11, 4110–4116. doi:10.1038/s41467-020-17901-2
Qiu, Y., Ahn, B., Sakurai, Y., Hansen, C. E., Tran, R., Mimche, P. N., et al. (2018). Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat. Biomed. Eng. 2, 453–463. doi:10.1038/s41551-018-0224-z
Qureshi, Z. P., Seoane-Vazquez, E., Rodriguez-Monguio, R., Stevenson, K. B., and Szeinbach, S. L. (2011). Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiol. Drug Saf. 20, 772–777. doi:10.1002/pds.2155
Rhee, J. W., Ky, B., Armenian, S. H., Yancy, C. W., and Wu, J. C. (2020). Primer on biomarker discovery in cardio-oncology: application of omics technologies. JACC CardioOncol. 2, 379–384. doi:10.1016/j.jaccao.2020.07.006
Rieker, C., Migliavacca, E., Vaucher, A., Baud, G., Marquis, J., Charpagne, A., et al. (2019). Apolipoprotein E4 expression causes gain of toxic function in isogenic human induced pluripotent stem cell-derived endothelial cells. Arterioscler. Thromb. Vasc. Biol. 39, e195–e207. doi:10.1161/ATVBAHA.118.312261
Rosa, S., Praça, C., Pitrez, P. R., Gouveia, P. J., Aranguren, X. L., Ricotti, L., et al. (2019). Functional characterization of iPSC-derived arterial- and venous-like endothelial cells. Sci. Rep. 9, 3826–3915. doi:10.1038/s41598-019-40417-9
Sayed, N., Ameen, M., and Wu, J. C. (2019). Personalized medicine in cardio-oncology: the role of induced pluripotent stem cell. Cardiovasc. Res. 115, 949–959. doi:10.1093/cvr/cvz024
Sayed, N., Liu, C., and Wu, J. C. (2016). Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J. Am. Coll. Cardiol. 67, 2161–2176. doi:10.1016/j.jacc.2016.01.083
Sayed, N., Liu, C., Ameen, M., Himmati, F., Zhang, J. Z., Khanamiri, S., et al. (2020). Clinical trial in a dish using iPSCs shows lovastatin improves endothelial dysfunction and cellular cross-talk in LMNA cardiomyopathy. Sci. Transl. Med. 12, eaax9276. doi:10.1126/scitranslmed.aax9276
Seeger, T., Porteus, M., and Wu, J. C. (2017). Genome editing in cardiovascular biology. Circ. Res. 120, 778–780. doi:10.1161/CIRCRESAHA.116.310197
Sharma, A., Burridge, P. W., McKeithan, W. L., Serrano, R., Shukla, P., Sayed, N., et al. (2017). High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci. Transl. Med. 9, eaaf2584. doi:10.1126/scitranslmed.aaf2584
Siramshetty, V. B., Nickel, J., Omieczynski, C., Gohlke, B. O., Drwal, M. N., and Preissner, R. (2016). WITHDRAWN--a resource for withdrawn and discontinued drugs. Nucleic Acids Res. 44, D1080–D1086. doi:10.1093/nar/gkv1192
Stephenson, M., Reich, D. H., and Boheler, K. R. (2020). Induced pluripotent stem cell-derived vascular smooth muscle cells. Vasc. Biol. 2, R1–R15. doi:10.1530/vb-19-0028
Tang, L., Su, J., and Liang, P. (2017). Modeling cadmium-induced endothelial toxicity using human pluripotent stem cell-derived endothelial cells. Sci. Rep. 7, 14811–14812. doi:10.1038/s41598-017-13694-5
Taubenberger, A. V., Bray, L. J., Haller, B., Shaposhnykov, A., Binner, M., Freudenberg, U., et al. (2016). 3D extracellular matrix interactions modulate tumour cell growth, invasion and angiogenesis in engineered tumour microenvironments. Acta Biomater. 36, 73–85. doi:10.1016/j.actbio.2016.03.017
Taura, D., Sone, M., Homma, K., Oyamada, N., Takahashi, K., Tamura, N., et al. (2009). Induction and isolation of vascular cells from human induced pluripotent stem cells--brief report. Arterioscler. Thromb. Vasc. Biol. 29, 1100–1103. doi:10.1161/ATVBAHA.108.182162
Touyz, R. M., Alves-Lopes, R., Rios, F. J., Camargo, L. L., Anagnostopoulou, A., Arner, A., et al. (2018). Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 114, 529–539. doi:10.1093/cvr/cvy023
Vatine, G. D., Barrile, R., Workman, M. J., Sances, S., Barriga, B. K., Rahnama, M., et al. (2019). Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 24, 995. doi:10.1016/j.stem.2019.05.011
Vo, A. H., Van Vleet, T. R., Gupta, R. R., Liguori, M. J., and Rao, M. S. (2020). An overview of machine learning and big data for drug toxicity evaluation. Chem. Res. Toxicol. 33, 20–37. doi:10.1021/acs.chemrestox.9b00227
Volpato, V., and Webber, C. (2020). Addressing variability in iPSC-derived models of human disease: guidelines to promote reproducibility. Dis. Model. Mech. 13, dmm042317. doi:10.1242/dmm.042317
Wang, K., Lin, R.-Z., Hong, X., Ng, A. H., Lee, C. N., Neumeyer, J., et al. (2020). Robust differentiation of human pluripotent stem cells into endothelial cells via temporal modulation of ETV2 with modified mRNA. Sci. Adv. 6, eaba7606. doi:10.1126/sciadv.aba7606
Wanjare, M., Kuo, F., and Gerecht, S. (2013). Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc. Res. 97, 321–330. doi:10.1093/cvr/cvs315
Weaver, R. J., and Valentin, J. P. (2019). Today's challenges to de-risk and predict drug safety in human “Mind-the-Gap”. Toxicol. Sci. 167, 307–321. doi:10.1093/toxsci/kfy270
Westein, E., Van Der Meer, A. D., Kuijpers, M. J., Frimat, J. P., Van Den Berg, A., and Heemskerk, J. W. (2013). Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 110, 1357–1362. doi:10.1073/pnas.1209905110
Williams, I. M., and Wu, J. C. (2019). Generation of endothelial cells from human pluripotent stem cells. Atvb 39, 1317–1329. doi:10.1161/ATVBAHA.119.312265
Wimmer, R. A., Leopoldi, A., Aichinger, M., Wick, N., Hantusch, B., Novatchkova, M., et al. (2019). Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510. doi:10.1038/s41586-018-0858-8
Wnorowski, A., Yang, H., and Wu, J. C. (2019). Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models. Adv. Drug Deliv. Rev. 140, 3–11. doi:10.1016/j.addr.2018.06.001
Wu, J. C., Garg, P., Yoshida, Y., Yamanaka, S., Gepstein, L., Hulot, J. S., et al. (2019). Towards precision medicine with human iPSCs for cardiac channelopathies. Circ. Res. 125, 653–658. doi:10.1161/CIRCRESAHA.119.315209
Yau, J. W., Teoh, H., and Verma, S. (2015). Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 15, 130. doi:10.1186/s12872-015-0124-z
Zhang, B., Lai, B. F. L., Xie, R., Davenport Huyer, L., Montgomery, M., and Radisic, M. (2018). Microfabrication of angiochip, a biodegradable polymer scaffold with microfluidic vasculature. Nat. Protoc. 13, 1793–1813. doi:10.1038/s41596-018-0015-8
Zhang, H., Tian, L., Shen, M., Tu, C., Wu, H., Gu, M., et al. (2019a). Generation of quiescent cardiac fibroblasts from human induced pluripotent stem cells for in vitro modeling of cardiac fibrosis. Circ. Res. 125, 552–566. doi:10.1161/CIRCRESAHA.119.315491
Zhang, J., McIntosh, B. E., Wang, B., Brown, M. E., Probasco, M. D., Webster, S., et al. (2019b). A human pluripotent stem cell-based screen for smooth muscle cell differentiation and maturation identifies inhibitors of intimal hyperplasia. Stem Cell Rep. 12, 1269–1281. doi:10.1016/j.stemcr.2019.04.013
Keywords: vascular toxicity, IPSC disease modeling, drug testing, endothelial cells, smooth muscle cells, vascular organoids, vasculature-on-a-chip
Citation: Tu C, Cunningham NJ, Zhang M and Wu JC (2021) Human Induced Pluripotent Stem Cells as a Screening Platform for Drug-Induced Vascular Toxicity. Front. Pharmacol. 12:613837. doi: 10.3389/fphar.2021.613837
Received: 03 October 2020; Accepted: 22 January 2021;
Published: 10 March 2021.
Edited by:
Tamer M. A. Mohamed, University of Louisville, United StatesReviewed by:
Wolfgang F. Graier, Medical University of Graz, AustriaCopyright © 2021 Tu, Cunningham, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph C. Wu, am9ld3VAc3RhbmZvcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.