- 1Department of Breast Surgery, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
- 2Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Oncology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Background: The prevalence rate of hypertension and breast cancer increases with advancing age. Renin-angiotensin system inhibitors (RASIs), β-blockers (BBs), calcium channel blockers (CCBs), and diuretics are widely used to treat patients with hypertension. Although, the association between the use of antihypertensive medication and breast cancer has been highly debated, recent evidence supporting this association remains controversial.
Objective: To evaluate the association between the use of antihypertensive medication and the risk of breast cancer and its prognosis.
Methods: This study was conducted using data from the PubMed, Embase, and Cochrane Library databases retrieved for the period from January 2000 to April 2021. Articles and their references were checked and summary effects were calculated using random- and fixed-effects models. Heterogeneity test and sensitivity analysis were also performed.
Results: This meta-analysis included 57 articles, which were all related to breast cancer risk or prognosis. Assessment of breast cancer risk using the pooled data showed that the use of BBs or CCBs or diuretics was associated with increased cancer risk [BB: relative risk (RR) = 1.20, 95% confidence interval (CI) = 1.09–1.32; CCBs: RR = 1.06, 95% CI 1.03–1.08; diuretics: RR = 1.06, 95% CI 1.01–1.11]. Long-term use of diuretic increased the risk of breast cancer (RR = 1.10, 95% CI 1.01–1.20), whereas long-term RASIs treatment reduced the risk (RR = 0.78, 95% CI 0.68–0.91). In addition, we found that diuretic users may be related to elevated breast cancer-specific mortality [hazard ratio (HR) = 1.18, 95% CI 1.04–1.33], whereas using other antihypertensive medications was not associated with this prognosis in patients with breast cancer.
Conclusion: Using CCBs, BBs, and diuretics increased the risk of breast cancer. In addition, diuretics may elevate the risk of breast cancer-specific mortality. The long-term use of RASIs was associated with a significantly lower breast cancer risk, compared with non-users. Thus, this analysis provides evidence to support the benefits of the routine use of RASIs in patients with hypertension, which has important public health implications.
Introduction
In 2017, hypertension was identified as a major risk factor for cardiovascular disease globally, accounting for 10.4 (9.39–11.5) million deaths and 218 (198–237) million disability-adjusted life-years (Stanaway et al., 2018). Fortunately, antihypertensive therapy significantly reduces the risk of cardiovascular disease and death in various populations. A meta-analysis showed that reducing the systolic blood pressure by 10 mm Hg would reduce the risk of major cardiovascular disease, coronary heart disease, stroke, and heart failure by 20, 17, 27, and 28%, respectively, whereas all-cause mortality was reduced by 13% (Ettehad et al., 2016). As an effective measure to control blood pressure, antihypertensive medications are commonly prescribed worldwide, and many patients take these drugs for a long period.
Several studies have found that breast cancer is associated with a variety of antihypertensive medications, including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs), β-blockers (BBs), calcium channel blockers (CCBs), and diuretics. However, these results remain controversial. Raebel et al. (2017) concluded that long-term use of CCBs was not related to breast cancer risk and long-term use of ACEIs might protect against breast cancer. The Chang et al. (2016) conducted a nested case-control study of 794,533 women and did not find any relationship between antihypertensive medication use and breast cancer risk. In contrast, other studies found that long-term treatment with CCBs was associated with a higher risk of breast cancer; however, no association was observed with the use of diuretics, BB, and renin-angiotensin system inhibitors (RASIs) (Li et al., 2013; Gomez-Acebo et al., 2016). A population-based case-control study demonstrated that treatment with ACEIs for more than 5 years increased breast cancer risk by 14% [odds ratio (OR) = 1.14, 95% confidence interval (CI) = 1.06–1.22] (Hallas et al., 2012).
A recent systematic review of observational studies concluded that BB reduced the risk of breast cancer recurrence (Caparica et al., 2021), whereas ACEIs and CCBs were not associated with breast cancer development (Zhao et al., 2017a; Naghibzadeh et al., 2020). In addition, the result of a meta-analysis suggested an association between CCBs use and breast cancer risk (Wright et al., 2017). However, uncertainty still exists regarding the effect of long-term use of antihypertensive medications and a potential association between different populations, breast cancer sub-types, or both.
This question remains controversial and, therefore, in this updated meta-analysis, we aimed to assess the association between the use of various classes of antihypertensive medication and breast cancer risk, prognosis [breast cancer-specific mortality, recurrence, overall survival (OS), and disease specific survival (DSS)].
Materials and Methods
Data Sources and Search Strategy
Supplementary Table S1 shows the strategy we used to conduct a comprehensive literature search of the PubMed, Embase, and Cochrane Library electronic databases without any restriction regarding geographical parameters and publication type or language. We reviewed research articles published over a span of nearly 20 years rather than those published in the 1990s, because we considered that the technique and treatment strategies that affect patient prognosis and survival have improved with time. The search was based on the framework of adult populations exposed to antihypertensive medication compared with non-users and the articles were evaluated for diagnosis or progress of breast cancer. Based on a combination of MeSH terms, keywords, and substance names, we conducted the following string search: “antihypertensive medication,” “calcium channel blockers,” “beta blockers,” “angiotensin-converting enzyme inhibitors,” “angiotensin receptor blockers,” “renin-angiotensin system inhibitors,” or “diuretics” combined with “breast cancer” or “breast carcinoma.” In addition, the reference lists of other reviews or meta-analyses were manually searched to identify additional related articles. This meta-analysis is not registered in the PROSPERO database, but the process is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). The PRISMA 2020 checklist was shown in Supplementary Table S2.
Selection Criteria
Studies fulfilling the following inclusion criteria were included: 1) the exposed group included individuals who had received antihypertensive medication; 2) the study had a comparative design: antihypertensive medication users vs. non-users; 3) the outcome was breast cancer risk, breast cancer-specific mortality, recurrence, or survival (DSS or OS); 4) the study reported the relative risk (RR), OR, or hazard ratio (HR) with corresponding 95% CIs or sufficient data to calculate these parameters. When the results of multiple studies were from the same database, the study including the largest number of participants was included. The following types of studies were excluded from this meta-analysis: meta-analyses, systematic or narrative reviews, case studies, experimental laboratory articles, conference abstracts, commentaries, randomized controlled trials, repeated publications, or if the reference group was administered another class of antihypertensive medication.
Data Extraction and Quality Assessment
Two of the authors (Yuxiu Xie and Men Wang) extracted data from the included studies and any disagreements between them were resolved by discussions with between the two other relevant co-authors. We extracted the following information from the selected articles: first author and year of publication, source of data, country, study design, duration of follow-up, study outcome, sample size of participants, and covariates used for the adjustment of confounders. The risk estimate adjusted for the largest number of confounding factors was extracted.
The nine-star Newcastle–Ottawa Scale (NOS) was used to assess the methodological quality of cohort studies and case-control studies (Stang, 2010; Ma et al., 2020). A score of ≥7 was considered to indicate high quality.
Statistical Analysis
We conducted this meta-analysis to assess the association between the use of antihypertensive medication and breast cancer risk and prognosis. The summary risk estimates are presented as forest plots. The I2 test was used to assess potential heterogeneity between individual studies, and an I2 > 50% indicated significant heterogeneity (Higgins et al., 2003). A fixed-effects model was used when there was no significant heterogeneity among the studies; whereas, the random-effects models was used when there was. To explore the source of heterogeneity and evaluate the potential impact of variables, we conducted subgroup analyses based on study design, country, and duration of antihypertensive medication use in years (e.g., <5, 5–10, ≥10 years). Articles with results that showed separate risk estimates based on different classes of CCBs or a different pathological pattern of breast cancer without a summary result were treated as different studies according to the result. Funnel plots were generated and examined visually, and gauged Egger’s tests were performed to assess publication bias (Egger et al., 1997). Sensitivity analyses were conducted excluding one study at a time to test the robustness of this association.
Results
Characteristics and Quality of Studies
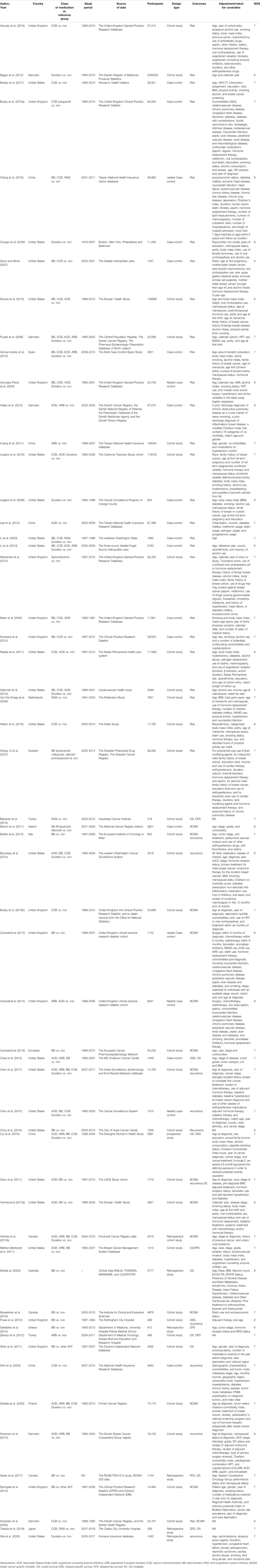
A total of 842 potentially eligible studies were retrieved from the selected databases during the initial search. After removing duplicates and further screening the titles and abstracts, 26 studies on breast cancer risk (Meier et al., 2000; Li et al., 2003; Gonzalez-Perez et al., 2004; Fryzek et al., 2006; Largent et al., 2006; Davis and Mirick, 2007; Van Der Knaap et al., 2008; Coogan et al., 2009; Largent et al., 2010; Huang et al., 2011; Hallas et al., 2012; Lee et al., 2012; Mackenzie et al., 2012; Biggar et al., 2013; Li et al., 2013; Saltzman et al., 2013; Devore et al., 2015; Numbere et al., 2015; Azoulay et al., 2016; Chang et al., 2016; Gomez-Acebo et al., 2016; Wilson et al., 2016; Brasky et al., 2017; Raebel et al., 2017; Busby et al., 2018b; Zheng et al., 2021), and 30 studies on breast cancer prognosis (Powe et al., 2010; Barron et al., 2011; Ganz et al., 2011; Melhem-Bertrandt et al., 2011; Shah et al., 2011; Şendur et al., 2012; Holmes et al., 2013a; Holmes et al., 2013b; Botteri et al., 2013; Cardwell et al., 2013; Chae et al., 2013; Sorensen et al., 2013; Boudreau et al., 2014; Cardwell et al., 2014; Sakellakis et al., 2014; Babacan et al., 2015; Chen et al., 2015; Springate et al., 2015; Cardwell et al., 2016; Choy et al., 2016; Chen et al., 2017; Spera et al., 2017; Busby et al., 2018a; Musselman et al., 2018; Cui et al., 2019; Takada et al., 2019; Modi et al., 2020; Santala et al., 2020; Shih et al., 2020; Wei et al., 2020) and one study on both risk and prognosis (Sorensen et al., 2000) finally met the inclusion criteria of this meta-analysis. The study selection process is depicted in a flow chart (Figure 1) and the characteristics of the included studies are summarized in Table 1. The sample sizes of the studies included for breast cancer risk ranged from 654 to 2,300,000 with a total of 3,726,281 participants, and that for breast cancer prognosis varied from 218 to 73,170 with a total of 270,745 participants. Although the adjusted covariates of individual studies differed, most risk estimates were adjusted for age, body mass index (BMI), alcohol intake, and hormone replacement therapy use. The quality scores in this analysis ranged from 6 to 8 stars (Supplementary Table S3).
Association of Antihypertensive Medication Use with Breast Cancer Risk
Pooled data from 18 studies related to breast cancer risk showed that the use of BBs was associated with increased cancer risk (RR = 1.20, 95% CI 1.09–1.32; Figure 2A and Table 2). A subgroup analysis showed a significant association with case-control (RR = 1.09, 95% CI 1.05–1.12), nested case-control (RR = 1.11, 95% CI 1.03–1.20) studies, and cohort (RR = 1.43, 95% CI 1.07–1.91) studies. In addition, a significant association was observed in Europe (RR = 1.41, 95% CI 1.16–1.70) and Asia (RR = 1.12, 95% CI 1.02–1.23), but not in North America (RR = 1.02, 95% CI 0.94–1.11). However, subgroup analysis of the duration of administration in years showed that the use of BBs even for ≥10 years was not associated with breast cancer risk.
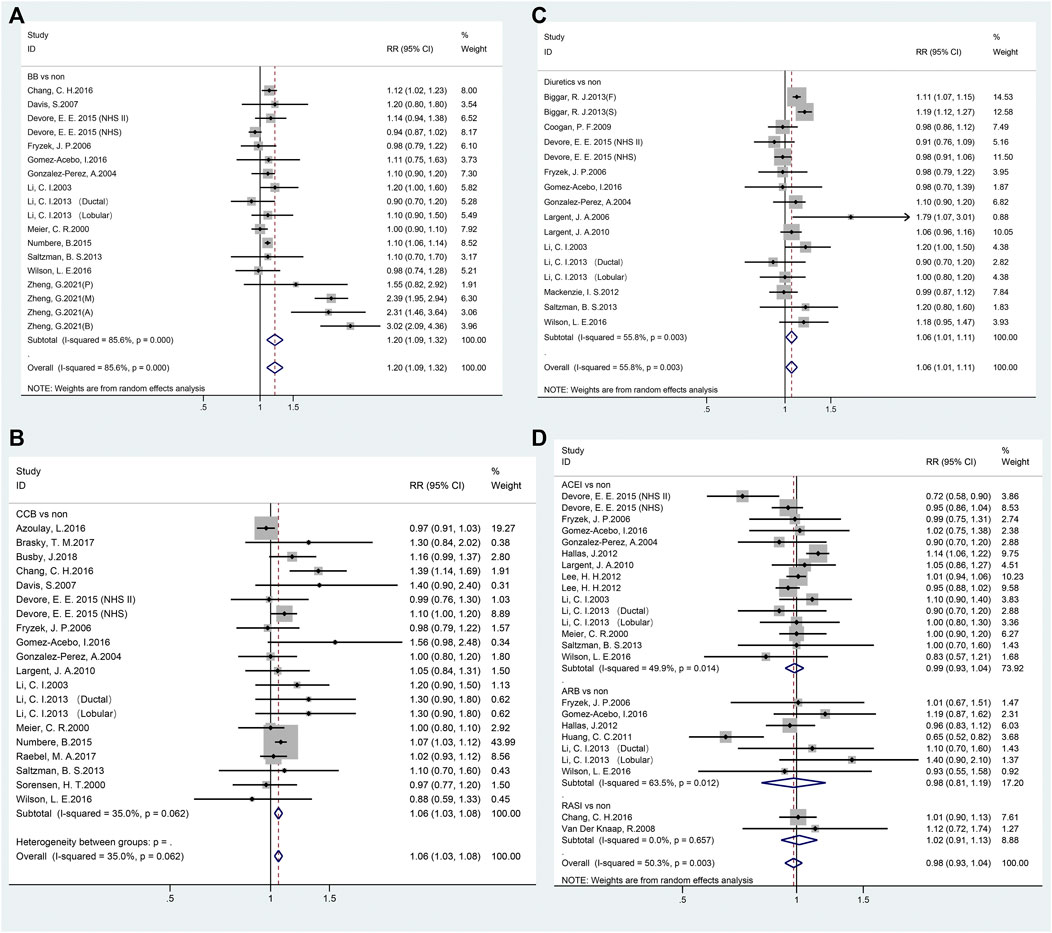
FIGURE 2. Forest plot of studies among the risk of breast cancer with antihypertensive medications use. Legends (A beta-blockers; B: calcium-channel blockers; C: diuretics; D: renin-angiotensin system inhibitors).
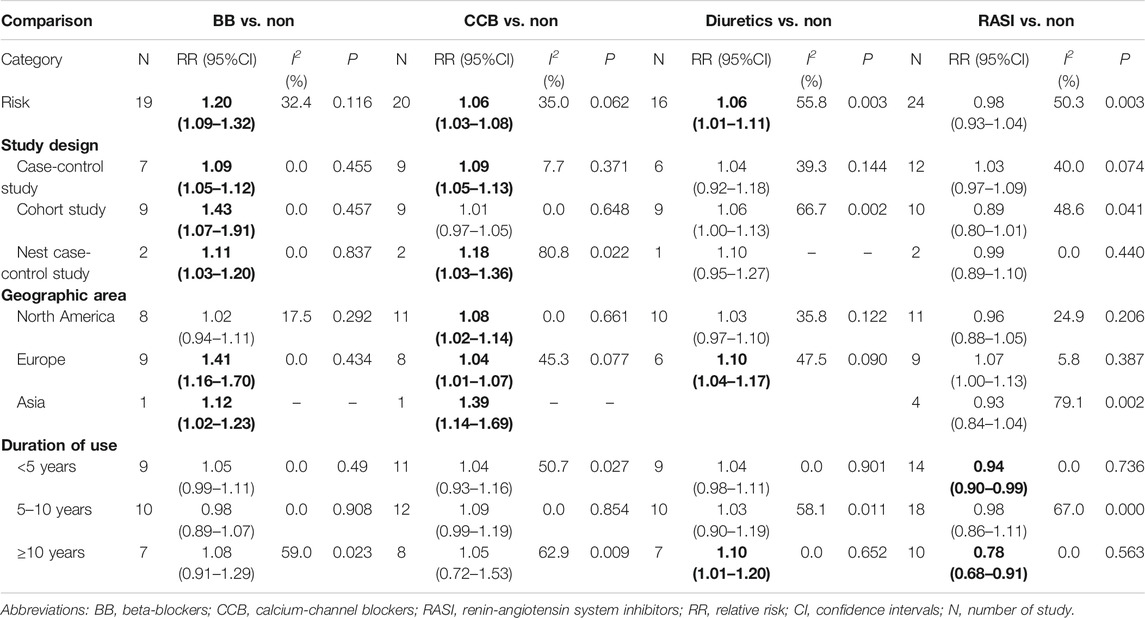
TABLE 2. The results of the association between antihypertensive medication use and breast cancer risk.
Twenty studies reported the relationship between CCBs and breast cancer risk (Figure 2B; Table 2). The pooled RR of breast cancer risk for CCBs use vs. non-use was 1.06 (95% CI 1.03–1.08), indicating a significant positive association. Case-control studies (RR = 1.09, 95% CI 1.05–1.13), nested case-control studies (RR = 1.18, 95% CI 1.03–1.36), and every area (North America: RR = 1.08, 95% CI 1.02–1.14; Europe: RR = 1.04, 95% CI 1.01–1.07; Asia: RR = 1.39, 95% CI 1.14–1.69) showed a positive association. There was no duration-response association between long-term use of CCB and breast cancer risk.
As shown in Figure 2C, exposure to diuretics (16 studies) showed a significant association where the pooled RR was 1.06 (95% CI 1.01–1.11). A significant relationship was noted in Europe (RR = 1.10, 95% CI 1.04–1.17) but not in North America (RR = 1.03, 95% CI 0.97–1.10). Notably, a significant association was observed with long-term use of diuretics (≥10 years; RR = 1.10, 95% CI 1.01–1.20, Table 2).
A total of 24 studies reported a connection between RASIs exposure of any duration and breast cancer risk. RASIs did not increase the risk of breast cancer with a pooled RR (0.98, 95% CI 0.93–1.04), as shown in Figure 2D. However, it is worth noting that RASIs played a protective role in breast cancer when studies were restricted to those where the duration of RASI use was <5 years (RR = 0.94, 95% CI 0.90–0.99) and ≥10 years (RR = 0.78, 95% CI 0.68–0.91). Fifteen and seven studies were related to ACEI and ARB use, respectively, and the results suggested that use of ACEI or ARB was not related to breast cancer risk (ACEI: RR = 0.99, 95% CI 0.93–1.04; ARB: RR = 0.98, 95% CI 0.81–1.19, Table 2).
Association of Antihypertensive Medication Use with Breast Cancer Prognosis Breast Cancer-Specific Mortality
Twenty-nine independent studies examined the association between antihypertensive medication use and breast cancer-specific mortality, with a pooled HR of 1.04 (95% CI 0.98–1.10). When HRs for the individual antihypertensive medication (BBs, CCBs, and RASIs) were calculated in subgroup analyses, no significant results were observed (Figure 3A). However, a significant association was observed in diuretic users (HR = 1.18, 95% CI 1.04–1.33).
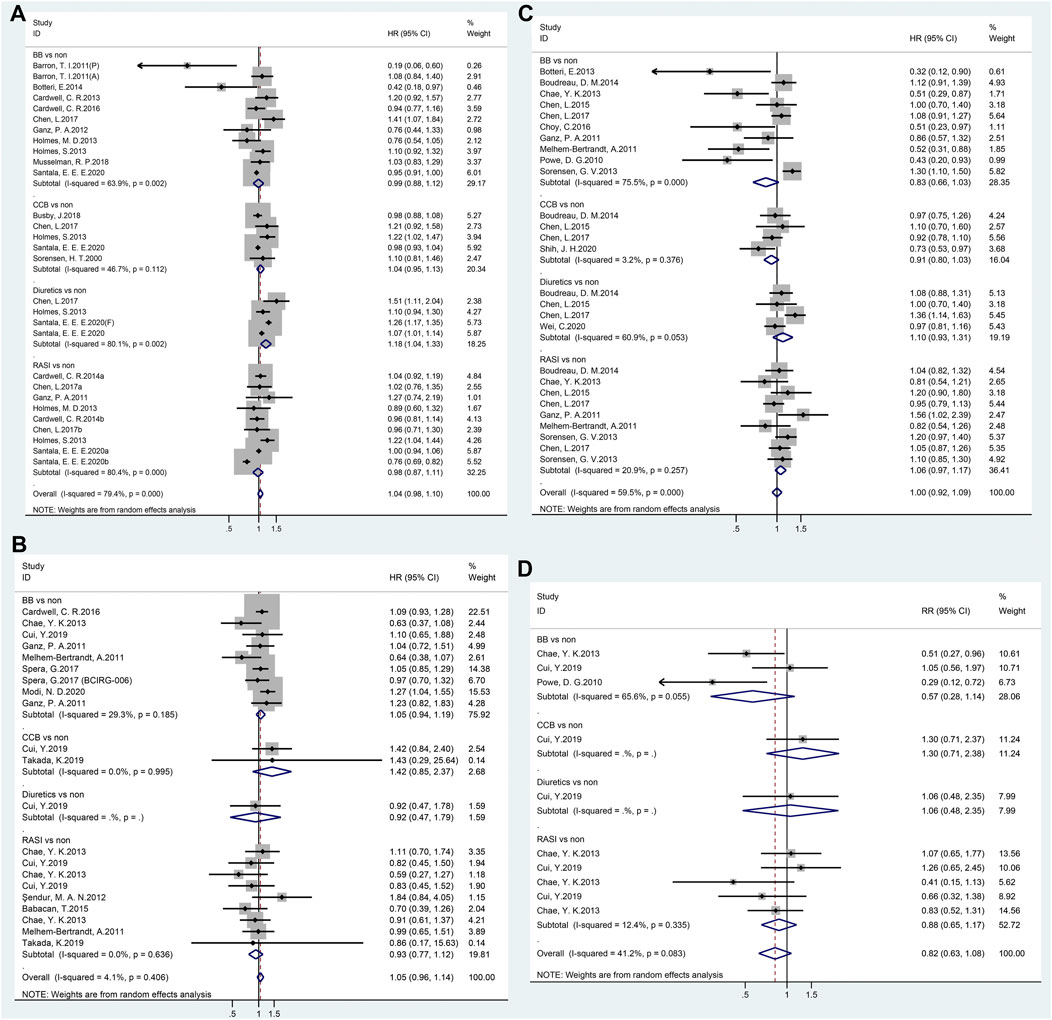
FIGURE 3. Forest plot of studies among the prognosis of breast cancer patients with antihypertensive medications use. Legends (A: breast cancer-specific mortality; B: overall survival; C: recurrence; D: disease-specific survival).
OS
Twenty-one studies revealed no significant link between antihypertensive medication use and breast cancer OS (HR = 1.05, 95% CI 0.96–1.14, Figure 3B). Furthermore, a subgroup analysis of the classes of antihypertensive medication showed no significant association was observed.
Recurrence
In total, 27 studies reported breast cancer recurrence with the use of antihypertensive medication. However, as shown in Figure 3C, no effect was observed for any type of antihypertensive medication (HR = 1.00, 95% CI 0.92–1.09).
DSS
The results of the meta-analyses on DSS are summarized in forest plots for all 10 studies; no association was found between antihypertensive medication use and breast cancer DSS (HR = 0.82, 95% CI 0.63–1.08, Figure 3D).
Risk of Publication Bias
The results of the Begg’s funnel plot and Egger’s regression test, showed no evidence of potential publication bias in articles reporting the use of BBs (P = 0.168), CCBs (P = 0.151), diuretics (P = 0.348), or RASIs (P = 0.397) in relation to the risk of breast cancer risk (Supplementary Figure S1). In addition, a potential publication bias was observed in studies on the use of antihypertensive medication with recurrence (P = 0.001), but not in other studies on prognosis (mortality: P = 0.717; OS: P = 0.096; DSS: P = 0.125) (Supplementary Figure S2).
Sensitivity Analysis
A sensitivity analysis was performed as shown in Supplementary Figures S3 and S4. There was no significant alteration in the pooled RRs or HRs after sequentially deleting a single study from the overall pooled analyses each time. In the sensitive analysis of diuretics users with risk of breast cancer, the pooled RR was not statistically significant after the deletion of an article (RR = 1.03, 95% CI 0.98–1.08) (Biggar et al., 2013).
Discussion
Our meta-analysis showed that the use of BBs, CCBs, or diuretics was associated with a moderate increase in the risk of breast cancer. Diuretic therapy for ≥10 years could increase breast cancer risk by 10%. Long-term use of RASIs exerted a protective effect against breast cancer, especially when used for ≥10 years. In addition, this meta-analysis indicated that diuretics are related to elevated breast cancer-specific mortality, but antihypertensive medication use did not affect recurrence, OS, or DSS.
Our conclusion that BBs were associated with increased breast cancer risk was consistent with this highly influential and large studies (Numbere et al., 2015; Chang et al., 2016; Zheng et al., 2021). The prospective study data minimizes the recall bias and in the study in question, some important confounders such as alcohol status, smoking status, BMI, medication and comorbidities, were adjusted. Similarly, the positive association between diuretics and breast cancer risk mainly depended on the study that evaluated cancer risk in 2.3 million Danish women who were followed-up for 28.8 million person-years (Biggar et al., 2013). The study reported that breast cancer risk increased in the first-year of diuretic use, but the association was attributed to reverse causality, such as where symptoms of cancer, including abdominal swelling or incidental findings such as hypertension, led to diuretic exposure. To exclude care practices that affect the risk from true etiologic impact, the study further examined risk only in women who were exposed to drugs for more than one year prior to diagnosis, and found a slightly increased risk of breast cancer with increasing duration of diuretic exposure. Consistent with its results, we observed a duration-response association between the duration of diuretic therapy ≥10 years and breast cancer risk. Studies found that thiazide diuretics, but not other diuretics, were linked to an increasing risk of breast cancer, and the authors considered the increase in insulin resistance due to thiazide diuretics as a possible explanation (Sarafidis et al., 2007; Ni et al., 2017). A recent study showed that the use of furosemide or other diuretics before breast cancer diagnosis increased the risk of breast cancer-related death (Santala et al., 2020). Our results showed that the breast cancer mortality increased with the use of diuretics; however, we failed to analyze the effect of diuretic subtypes on breast cancer prognosis because there were few relevant studies. Thus, we could not arrive at the conclusion that different subclass of diuretics had varying associations with breast cancer. Future studies are warranted to determine the potential difference among various diuretics.
Our meta-analysis showed a positive association between CCBs use and the risk of breast cancer, which was consistent with the results of previous studies (Wright et al., 2017; Busby et al., 2018a). The elevated levels of cytosolic calcium could affect the process of apoptosis through different signaling pathways such as caspase activation, induction of endonuclease activity, or the miRNA-524-5p-BRI3-extracellular signal-regulated kinase (Erk) pathway (Iwasawa et al., 2011; Guo et al., 2014; Zhao et al., 2017b). CCBs blocks calcium entry, inhibiting this vital process, which could destroy the body’s natural defense mechanism against cancer growth and promote cell survival by initiating autophagy (Sun et al., 2018). However, there is no conclusive evidence to accurately explain this observed link and the benefits of the long-term use of CCBs are controversial.
The association between RASIs and breast cancer remains controversial with some studies reporting that ARB/ACEI was not related to breast cancer risk (Teo, 2011; Datzmann et al., 2019), whereas another concluded that users of ACEIs had an increased risk (Hallas et al., 2012). However, our result demonstrated a significant (22%) reduction in breast cancer risk with ≥10 years, which is consistent with previously published observational studies (Huang et al., 2011; Li et al., 2013; Wang et al., 2013; Ni et al., 2017). In addition, in vivo and in vitro studies on RASIs have demonstrated a protective anti-inflammatory, anti-proliferative, and pro-apoptotic effect on breast cancer (Herr et al., 2008; George et al., 2010). Although the effect of RASIs on breast cancer is still contradictory, RASIs should be selected more often for patients undergoing antihypertensive therapy considering its availability, feasibility, and established safety with reduced breast cancer risk.
It is important to assess the possible source of heterogeneity. First, the significance of our results might have been affected by an indication bias, such as high blood pressure (Coogan, 2013), as both hypertension and cancer are associated with obesity, alcohol, smoking, cardiometabolic abnormalities, and other comorbidities. One study reported a relationship between hypertension and cancer (Han et al., 2017). In addition, in this meta-analysis, not all included studies adjusted for the confounding factor of hypertension which would cause a certain degree of heterogeneity. Second, in observational studies of the general population, it is important to determine a dose or duration-response relationship to properly interpret the potential link between drug exposure and cancer development. However, because of the differences in drug exposure time and dose between the included studies, the results will eventually be heterogeneous. Third, most of studies were from North America or Europe but a few studies are from Asia, which produced regional heterogeneity and limited the extent to which the results can be generalized. In the subgroup analysis according to geographical area, we found reduced between-study heterogeneity and observed different results, which indicated that the association varied among different areas. Finally, because the included studies were retrieved from some population registration database, the analysis results could reduce recall bias and misclassification bias to some extent. However, population-based registries also have some shortcomings, such as the limitation of potential confounders by stored data, and the fact that a prescription was collected does not necessarily indicate that the prescribed drugs were taken.
Our meta-analysis provides convincing and clear evidence of the relationship between antihypertensive drugs and the risk and prognosis of breast cancer. One strength of our study is that we conducted a stratified analysis according to the duration of antihypertensive drug use and found that using diuretics for ≥10 years could increase the risk of breast cancer, but using RASIs for ≥10 years would reduce the risk of breast cancer. This suggests that RASIs would be the best choice for patients with long-term antihypertensive drugs. Another strength of the study is that we focused on breast cancer risk and prognosis to obtain a more homogeneous group of studies. We studied the possible source of heterogeneity between studies, including study design, geographic area, and duration of antihypertensive medication use. It is noteworthy that there were also some limitations to this study. First, biases such as confounders, possible detection bias, and selection bias is inevitable in observational studies. Secondly, subgroup analyses based on age, sex, pathology of breast cancer, dose of antihypertensive medication, subclass of BB (selective BBs and non-selective BBs), CCBs (dihydropyridines and non-dihydropyridines), and diuretics (thiazide, loop, and potassium sparing diuretics) were not conducted because of the lack of relevant studies. Thirdly, our current summary evidence did not support a clear relation between long-term use of CCBs or BBs and breast cancer risk. Finally, significant heterogeneity was observed among studies focused on antihypertensive medication use and breast cancer risk and this persisted even when the data were stratified into subgroups.
The results of this meta-analysis demonstrated that BBs, CCBs, and diuretics were significantly associated with breast cancer risk. Unexpectedly, a beneficial effect of long-term use of RASI was observed against breast cancer risk. Additionally, according to the analysis of prognosis, no association was observed with any class of antihypertensive medication. Considering the limitations in this meta-analysis, further research is needed to fully clarify the association between antihypertensive medication use and breast cancer risk.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors read, critically reviewed and approved the final manuscript. YX and MW conducted the database searches, screened titles, abstracts and full-texts for eligibility, PX, YD, YZ, and SY performed study quality assessments. JC and ZD planed and designed the research; DZ, NL, and NW provided methodological support/advice; PX and YD tested the feasibility of the study; YX, MW, and DZ extract data; YX performed the statistical analysis; YX and MW wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all members of our study team for their wonderful cooperation and the original authors of the included studies for their results.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.609901/full#supplementary-material
References
Azoulay, L., Soldera, S., Yin, H., and Bouganim, N. (2016). Use of Calcium Channel Blockers and Risk of Breast Cancer. Epidemiology 27 (4), 594–601. doi:10.1097/ede.0000000000000483
Babacan, T., Balakan, O., Kuzan, T. Y., Sarici, F., Koca, E., Kertmen, N., et al. (2015). The Effect of Renin-Angiotensin-System Inhibition on Survival and Recurrence of N3+ Breast Cancer Patients. J. Buon 20 (1), 50–56. doi:10.1007/s10549-013-2654-3
Barron, T. I., Connolly, R. M., Sharp, L., Bennett, K., and Visvanathan, K. (2011). Beta Blockers and Breast Cancer Mortality: a Population—Based Study. Jco 29 (19), 2635–2644. doi:10.1200/jco.2010.33.5422
Biggar, R. J., Andersen, E. W., Wohlfahrt, J., and Melbye, M. (2013). Spironolactone Use and the Risk of Breast and Gynecologic Cancers. Cancer Epidemiol. 37 (6), 870–875. doi:10.1016/j.canep.2013.10.004
Botteri, E., Munzone, E., Rotmensz, N., Cipolla, C., De Giorgi, V., Santillo, B., et al. (2013). Therapeutic Effect of β-blockers in Triple-Negative Breast Cancer Postmenopausal Women. Breast Cancer Res. Treat. 140, 567–575. doi:10.1007/s10549-013-2654-3
Boudreau, D. M., Yu, O., Chubak, J., Wirtz, H. S., Bowles, E. J. A., Fujii, M., et al. (2014). Comparative Safety of Cardiovascular Medication Use and Breast Cancer Outcomes Among Women with Early Stage Breast Cancer. Breast Cancer Res. Treat. 144 (2), 405–416. doi:10.1007/s10549-014-2870-5
Brasky, T. M., Krok-Schoen, J. L., Liu, J., Chlebowski, R. T., Freudenheim, J. L., Lavasani, S., et al. (2017). Use of Calcium Channel Blockers and Breast Cancer Risk in the Women's Health Initiative. Cancer Epidemiol. Biomarkers Prev. 26 (8), 1345–1348. doi:10.1158/1055-9965.Epi-17-0096
Busby, J., Mills, K., Zhang, S.-D., Liberante, F. G., and Cardwell, C. R. (2018a). Postdiagnostic Calcium Channel Blocker Use and Breast Cancer Mortality. Epidemiology 29 (3), 407–413. doi:10.1097/ede.0000000000000814
Busby, J., Murray, L., Mills, K., Zhang, S.-D., Liberante, F., and Cardwell, C. R. (2018b). A Combined Connectivity Mapping and Pharmacoepidemiology Approach to Identify Existing Medications with Breast Cancer Causing or Preventing Properties. Pharmacoepidemiol. Drug Saf. 27 (1), 78–86. doi:10.1002/pds.4345
Caparica, R., Bruzzone, M., Agostinetto, E., De Angelis, C., Fêde, Â., Ceppi, M., et al. (2021). Beta-blockers in Early-Stage Breast Cancer: a Systematic Review and Meta-Analysis. ESMO Open 6 (2), 100066. doi:10.1016/j.esmoop.2021.100066
Cardwell, C. R., Coleman, H. G., Murray, L. J., Entschladen, F., and Powe, D. G. (2013). Beta-blocker Usage and Breast Cancer Survival: a Nested Case-Control Study within a UK Clinical Practice Research Datalink Cohort. Int. J. Epidemiol. 42 (6), 1852–1861. doi:10.1093/ije/dyt196
Cardwell, C. R., Mc Menamin, Ú. C., Hicks, B. M., Hughes, C., Cantwell, M. M., and Murray, L. J. (2014). Drugs Affecting the Renin-Angiotensin System and Survival from Cancer: a Population Based Study of Breast, Colorectal and Prostate Cancer Patient Cohorts. BMC Med. 12, 28. doi:10.1186/1741-7015-12-28
Cardwell, C. R., Pottegård, A., Vaes, E., Garmo, H., Murray, L. J., Brown, C., et al. (2016). Propranolol and Survival from Breast Cancer: A Pooled Analysis of European Breast Cancer Cohorts. Breast Cancer Res. 18 (1), 119. doi:10.1186/s13058-016-0782-5
Chae, Y. K., Brown, E. N., Lei, X., Melhem-Bertrandt, A., Giordano, S. H., Litton, J. K., et al. (2013). Use of ACE Inhibitors and Angiotensin Receptor Blockers and Primary Breast Cancer Outcomes. J. Cancer 4 (7), 549–556. doi:10.7150/jca.6888
Chang, C.-H., Chiang, C.-H., Yen, C.-J., Wu, L.-C., Lin, J.-W., and Lai, M.-S. (2016). Antihypertensive Agents and the Risk of Breast Cancer in Women Aged 55 Years and Older. J. Hypertens. 34(3), 558–566. doi:10.1097/hjh.0000000000000813
Chen, L., Chubak, J., Boudreau, D. M., Barlow, W. E., Weiss, N. S., and Li, C. I. (2017). Use of Antihypertensive Medications and Risk of Adverse Breast Cancer Outcomes in a SEER-Medicare Population. Cancer Epidemiol. Biomarkers Prev. 26 (11), 1603–1610. doi:10.1158/1055-9965.EPI-17-0346
Chen, L., Malone, K. E., and Li, C. I. (2015). Use of Antihypertensive Medications Not Associated with Risk of Contralateral Breast Cancer Among Women Diagnosed with Estrogen Receptor-Positive Invasive Breast Cancer. Cancer Epidemiol. Biomarkers Prev. 24 (9), 1423–1426. doi:10.1158/1055-9965.Epi-15-0547
Choy, C., Raytis, J. L., Smith, D. D., Duenas, M., Neman, J., Jandial, R., et al. (2016). Inhibition of β2-adrenergic Receptor Reduces Triple-Negative Breast Cancer Brain Metastases: The Potential Benefit of Perioperative β-blockade. Oncol. Rep. 35 (6), 3135–3142. doi:10.3892/or.2016.4710
Coogan, P. F. (2013). Calcium-Channel Blockers and Breast Cancer. JAMA Intern. Med. 173 (17), 1637–1638. doi:10.1001/jamainternmed.2013.9069
Coogan, P. F., Strom, B. L., and Rosenberg, L. (2009). Diuretic Use and the Risk of Breast Cancer. J. Hum. Hypertens. 23 (3), 216–218. doi:10.1038/jhh.2008.131
Cui, Y., Wen, W., Zheng, T., Li, H., Gao, Y.-T., Cai, H., et al. (2019). Use of Antihypertensive Medications and Survival Rates for Breast, Colorectal, Lung, or Stomach Cancer. Am. J. Epidemiol. 188 (8), 1512–1528. doi:10.1093/aje/kwz106
Datzmann, T., Fuchs, S., Andree, D., Hohenstein, B., Schmitt, J., and Schindler, C. (2019). Systematic Review and Meta-Analysis of Randomised Controlled Clinical Trial Evidence Refutes Relationship between Pharmacotherapy with Angiotensin-Receptor Blockers and an Increased Risk of Cancer. Eur. J. Intern. Med. 64, 1–9. doi:10.1016/j.ejim.2019.04.019
Davis, S., and Mirick, D. K. (2007). Medication Use and the Risk of Breast Cancer. Eur. J. Epidemiol. 22 (5), 319–325. doi:10.1007/s10654-007-9135-0
Devore, E. E., Kim, S., Ramin, C. A., Wegrzyn, L. R., Massa, J., Holmes, M. D., et al. (2015). Antihypertensive Medication Use and Incident Breast Cancer in Women. Breast Cancer Res. Treat. 150 (1), 219–229. doi:10.1007/s10549-015-3311-9
Egger, M., Smith, G. D., Schneider, M., and Minder, C. (1997). Bias in Meta-Analysis Detected by a Simple, Graphical Test. Bmj 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Ettehad, D., Emdin, C. A., Kiran, A., Anderson, S. G., Callender, T., Emberson, J., et al. (2016). Blood Pressure Lowering for Prevention of Cardiovascular Disease and Death: a Systematic Review and Meta-Analysis. The Lancet 387 (10022), 957–967. doi:10.1016/s0140-6736(15)01225-8
Fryzek, J. P., Poulsen, A. H., Lipworth, L., Pedersen, L., Nørgaard, M., McLaughlin, J. K., et al. (2006). A Cohort Study of Antihypertensive Medication Use and Breast Cancer Among Danish Women. Breast Cancer Res. Treat. 97 (3), 231–236. doi:10.1007/s10549-005-9091-x
Ganz, P. A., Habel, L. A., Weltzien, E. K., Caan, B. J., and Cole, S. W. (2011). Examining the Influence of Beta Blockers and ACE Inhibitors on the Risk for Breast Cancer Recurrence: Results from the LACE Cohort. Breast Cancer Res. Treat. 129 (2), 549–556. doi:10.1007/s10549-011-1505-3
George, A. J., Thomas, W. G., and Hannan, R. D. (2010). The Renin-Angiotensin System and Cancer: Old Dog, New Tricks. Nat. Rev. Cancer 10 (11), 745–759. doi:10.1038/nrc2945
Gómez-Acebo, I., Dierssen-Sotos, T., Palazuelos, C., Pérez-Gómez, B., Lope, V., Tusquets, I., et al. (2016). The Use of Antihypertensive Medication and the Risk of Breast Cancer in a Case-Control Study in a Spanish Population: The MCC-Spain Study. PLoS One 11 (8), e0159672. doi:10.1371/journal.pone.0159672
González-Pérez, A., Ronquist, G., and García Rodríguez, L. A. (2004). Breast Cancer Incidence and Use of Antihypertensive Medication in Women. Pharmacoepidem. Drug Safe. 13 (8), 581–585. doi:10.1002/pds.910
Guo, D.-Q., Zhang, H., Tan, S.-J., and Gu, Y.-C. (2014). Nifedipine Promotes the Proliferation and Migration of Breast Cancer Cells. PLoS One 9 (12), e113649. doi:10.1371/journal.pone.0113649
Hallas, J., Christensen, R., Andersen, M., Friis, S., and Bjerrum, L. (2012). Long Term Use of Drugs Affecting the Renin-Angiotensin System and the Risk of Cancer: a Population-Based Case-Control Study. Br. J. Clin. Pharmacol. 74 (1), 180–188. doi:10.1111/j.1365-2125.2012.04170.x
Han, H., Guo, W., Shi, W., Yu, Y., Zhang, Y., Ye, X., et al. (2017). Hypertension and Breast Cancer Risk: a Systematic Review and Meta-Analysis. Sci. Rep. 7, 44877. doi:10.1038/srep44877
Herr, D., Rodewald, M., Fraser, H. M., Hack, G., Konrad, R., Kreienberg, R., et al. (2008). Potential Role of Renin-Angiotensin-System for Tumor Angiogenesis in Receptor Negative Breast Cancer. Gynecol. Oncol. 109 (3), 418–425. doi:10.1016/j.ygyno.2008.02.019
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Holmes, M. D., Hankinson, S. E., Feskanich, D., and Chen, W. Y. (2013a). Beta Blockers and Angiotensin-Converting Enzyme Inhibitors' Purported Benefit on Breast Cancer Survival May Be Explained by Aspirin Use. Breast Cancer Res. Treat. 139 (2), 507–513. doi:10.1007/s10549-013-2553-7
Holmes, S., Griffith, E. J., Musto, G., and Minuk, G. Y. (2013b). Antihypertensive Medications and Survival in Patients with Cancer: a Population-Based Retrospective Cohort Study. Cancer Epidemiol. 37 (6), 881–885. doi:10.1016/j.canep.2013.09.001
Huang, C.-C., Chan, W.-L., Chen, Y.-C., Chen, T.-J., Lin, S.-J., Chen, J.-W., et al. (2011). Angiotensin II Receptor Blockers and Risk of Cancer in Patients with Systemic Hypertension. Am. J. Cardiol. 107 (7), 1028–1033. doi:10.1016/j.amjcard.2010.11.026
Iwasawa, R., Mahul-Mellier, A.-L., Datler, C., Pazarentzos, E., and Grimm, S. (2011). Fis1 and Bap31 Bridge the Mitochondria-ER Interface to Establish a Platform for Apoptosis Induction. Embo j 30 (3), 556–568. doi:10.1038/emboj.2010.346
Largent, J. A., Bernstein, L., Horn-Ross, P. L., Marshall, S. F., Neuhausen, S., Reynolds, P., et al. (2010). Hypertension, Antihypertensive Medication Use, and Breast Cancer Risk in the California Teachers Study Cohort. Cancer Causes Control 21 (10), 1615–1624. doi:10.1007/s10552-010-9590-x
Largent, J. A., McEligot, A. J., Ziogas, A., Reid, C., Hess, J., Leighton, N., et al. (2006). Hypertension, Diuretics and Breast Cancer Risk. J. Hum. Hypertens. 20 (10), 727–732. doi:10.1038/sj.jhh.1002075
Lee, H. H., Tsan, Y. T., Ho, W. C., Lin, M. H., Lee, C. H., Tseng, C. D., et al. (2012). PCN7 Angiotensin-Converting Enzyme Inhibitors Enhance the Effect of Cyclooxygenase Inhibitors on Breast Cancer: A Nationwide Case-Control Study. Value in Health 15 (7), A654. doi:10.1016/j.jval.2012.08.305
Li, C. I., Daling, J. R., Tang, M.-T. C., Haugen, K. L., Porter, P. L., and Malone, K. E. (2013). Use of Antihypertensive Medications and Breast Cancer Risk Among Women Aged 55–74 Years. JAMA Intern. Med. 173 (17), 1629–1637. doi:10.1001/jamainternmed.2013.9071
Li, C. I., Malone, K. E., Weiss, N. S., Boudreau, D. M., Cushing-Haugen, K. L., and Daling, J. R. (2003). Relation between Use of Antihypertensive Medications and Risk of Breast Carcinoma Among Women Ages 65–79 Years. Cancer 98 (7), 1504–1513. doi:10.1002/cncr.11663
Ma, L.-L., Wang, Y.-Y., Yang, Z.-H., Huang, D., Weng, H., and Zeng, X.-T. (2020). Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: what Are They and Which Is Better? Mil. Med Res 7 (1), 7. doi:10.1186/s40779-020-00238-8
Mackenzie, I. S., Macdonald, T. M., Thompson, A., Morant, S., and Wei, L. (2012). Spironolactone and Risk of Incident Breast Cancer in Women Older Than 55 years: Retrospective, Matched Cohort Study. BMJ 345, e4447. doi:10.1136/bmj.e4447
Meier, C. R., Derby, L. E., Jick, S. S., and Jick, H. (2000). Angiotensin-converting Enzyme Inhibitors, Calcium Channel Blockers, and Breast Cancer. Arch. Intern. Med. 160 (3), 349–353. doi:10.1001/archinte.160.3.349
Melhem-Bertrandt, A., Chavez-Macgregor, M., Lei, X., Brown, E. N., Lee, R. T., Meric-Bernstam, F., et al. (2011). Beta-blocker Use is Associated with Improved Relapse-free Survival in Patients with Triple-Negative Breast Cancer. J Clin Oncol. 29 (19), 2645–2652. doi:10.1200/jco.2010.33.4441
Modi, N. D., Tan, J. Q. E., Rowland, A., Koczwara, B., Kichenadasse, G., McKinnon, R. A., et al. (2020). The Influence of Pre-existing Beta-Blockers Use on Survival Outcomes in HER2 Positive Advanced Breast Cancer: Pooled Analysis of Clinical Trial Data. Front. Oncol. 10, 1130. doi:10.3389/fonc.2020.01130
Musselman, R. P., Bennett, S., Li, W., Mamdani, M., Gomes, T., van Walraven, C., et al. (2018). Association between Perioperative Beta Blocker Use and Cancer Survival Following Surgical Resection. Eur. J. Surg. Oncol. 44 (8), 1164–1169. doi:10.1016/j.ejso.2018.05.012
Naghibzadeh, N., Asgharzadeh, F., and Khazaei, M. (2020). Effect of Renin-Angiotensin System Inhibitors on Survival Rate of Patients with Breast Cancer: Systematic Review and Meta-Analysis. Iranian J. Obstet. Gynecol. Infertility 26 (6), 88–96. doi:10.22038/IJOGI.2020.16884
Ni, H., Rui, Q., Zhu, X., Yu, Z., Gao, R., and Liu, H. (2017). Antihypertensive Drug Use and Breast Cancer Risk: a Meta-Analysis of Observational Studies. Oncotarget 8 (37), 62545–62560. doi:10.18632/oncotarget.19117
Numbere, B., Fleming, K. M., Walker, A., and Card, T. R. (2017). Adrenergic Blockers and the Risk for Common Solid Cancers: a Case-Control Study. Eur. J. Cancer Prev. 26 (1), 86–93. doi:10.1097/cej.0000000000000218
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Plos Med. 18 (3), e1003583. doi:10.1371/journal.pmed.1003583
Powe, D. G., Voss, M. J., Zänker, K. S., Habashy, H. O., Green, A. R., Ellis, I. O., et al. (2010). Beta-blocker Drug Therapy Reduces Secondary Cancer Formation in Breast Cancer and Improves Cancer Specific Survival. Oncotarget 1 (7), 628–638. doi:10.18632/oncotarget.197
Raebel, M. A., Zeng, C., Cheetham, T. C., Smith, D. H., Feigelson, H. S., Carroll, N. M., et al. (2017). Risk of Breast Cancer with Long-Term Use of Calcium Channel Blockers or Angiotensin-Converting Enzyme Inhibitors Among Older Women. Am. J. Epidemiol. 185 (4), 264–273. doi:10.1093/aje/kww217
Sakellakis, M., Kostaki, A., Starakis, I., and Koutras, A. (2014). β-Blocker Use and Risk of Recurrence in Patients with Early Breast Cancer. Chemotherapy 60 (5–6), 288–289. doi:10.1159/000371871
Saltzman, B. S., Weiss, N. S., Sieh, W., Fitzpatrick, A. L., McTiernan, A., Daling, J. R., et al. (2013). Use of Antihypertensive Medications and Breast Cancer Risk. Cancer Causes Control 24 (2), 365–371. doi:10.1007/s10552-012-0122-8
Santala, E. E. E., Murto, M. O., Artama, M., Pukkala, E., Visvanathan, K., and Murtola, T. J. (2020). Angiotensin Receptor Blockers Associated with Improved Breast Cancer Survival-A Nationwide Cohort Study from Finland. Cancer Epidemiol. Biomarkers Prev. 29, 2376–2382. doi:10.1158/1055-9965.Epi-20-0711
Sarafidis, P. A., McFarlane, S. I., and Bakris, G. L. (2007). Antihypertensive Agents, Insulin Sensitivity, and New-Onset Diabetes. Curr. Diab Rep. 7 (3), 191–199. doi:10.1007/s11892-007-0031-5
Şendur, M. A. N., Aksoy, S., Yaman, S., Ozdemir, N. Y., Zengin, N., and Altundag, K. (2012). Efficacy of Angiotensin-Receptor Blockers on Demographic and Clinico-Pathological Characteristics of Breast Cancer. Breast 21 (3), 419–420. doi:10.1016/j.breast.2012.01.01010.1016/j.breast.2011.09.015
Shah, S. M., Carey, I. M., Owen, C. G., Harris, T., Dewilde, S., and Cook, D. G. (2011). Does β-adrenoceptor Blocker Therapy Improve Cancer Survival? Findings from a Population-Based Retrospective Cohort Study. Br. J. Clin. Pharmacol. 72 (1), 157–161. doi:10.1111/j.1365-2125.2011.03980.x
Shih, J. H., Kao, L. T., Chung, C. H., Liao, G. S., Fann, L. Y., Chien, W. C., et al. (2020). Protective Association between Calcium Channel Blocker Use and Breast Cancer Recurrence in Postsurgical Women: A Population‐Based Case‐Control Study in Taiwan. J. Clin. Pharmacol. 60, 785–792. doi:10.1002/jcph.1579
Sørensen, G. V., Ganz, P. A., Cole, S. W., Pedersen, L. A., Sørensen, H. T., Cronin-Fenton, D. P., et al. (2013). Use of β-Blockers, Angiotensin-Converting Enzyme Inhibitors, Angiotensin II Receptor Blockers, and Risk of Breast Cancer Recurrence: A Danish Nationwide Prospective Cohort Study. J Clin Oncol 31 (18), 2265–2272. doi:10.1200/jco.2012.43.9190
Sørensen, H. T., Olsen, J. H., Mellemkjaer, L., Marie, A., Steffensen, F. H., McLaughlin, J. K., et al. (2000). Cancer Risk and Mortality in Users of Calcium Channel Blockers. A Cohort Study. Cancer 89 (1), 165–170. doi:10.1002/1097-0142(20000701)89:1<165::aid-cncr21>3.0.co;2-g
Spera, G., Fresco, R., Fung, H., Dyck, J. R. B., Pituskin, E., Paterson, I., et al. (2017). Beta Blockers and Improved Progression-free Survival in Patients with Advanced HER2 Negative Breast Cancer: A Retrospective Analysis of the ROSE/TRIO-012 Study. Ann. Oncol. 28 (8), 1836–1841. doi:10.1093/annonc/mdx264
Springate, D. A., Ashcroft, D. M., Kontopantelis, E., Doran, T., Ryan, R., and Reeves, D. (2015). Can Analyses of Electronic Patient Records Be Independently and Externally Validated? Study 2—the Effect of—adrenoceptor Blocker Therapy on Cancer Survival: a Retrospective Cohort Study. BMJ Open 5 (4), e007299. doi:10.1136/bmjopen-2014-007299
Stanaway, J. D., Afshin, A., Gakidou, E., Lim, S. S., Abate, D., Abate, K. H., et al. (2018). Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks for 195 Countries and Territories, 1990–2017: a Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 392 (10159), 1923–1994. doi:10.1016/s0140-6736(18)32225-6
Stang, A. (2010). Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Sun, X., Yang, Y., Zhong, X. Z., Cao, Q., Zhu, X.-H., Zhu, X., et al. (2018). A Negative Feedback Regulation of MTORC1 Activity by the Lysosomal Ca2+ Channel MCOLN1 (Mucolipin 1) Using a CALM (Calmodulin)-dependent Mechanism. Autophagy 14 (1), 38–52. doi:10.1080/15548627.2017.1389822
Takada, K., Kashiwagi, S., Asano, Y., Goto, W., Takahashi, K., Fujita, H., et al. (2019). Verification of the Effects of Calcium Channel Blockers on the Immune Microenvironment of Breast Cancer. BMC Cancer 19 (1), 615. doi:10.1186/s12885-019-5828-5
Teo, K. K. (2011). Effects of Telmisartan, Irbesartan, Valsartan, Candesartan, and Losartan on Cancers in 15 Trials Enrolling 138,769 Individuals. J. Hypertens. 29 (4), 623–635. doi:10.1097/HJH.0b013e328344a7de
Van Der Knaap, R., Siemes, C., Coebergh, J.-W. W., Van Duijn, C. M., Hofman, A., and Stricker, B. H. C. (2008). Renin-angiotensin System Inhibitors, Angiotensin I-Converting Enzyme Gene Insertion/deletion Polymorphism, and Cancer. Cancer 112 (4), 748–757. doi:10.1002/cncr.23215
Wang, K.-L., Liu, C.-J., Chao, T.-F., Huang, C.-M., Wu, C.-H., Chen, T.-J., et al. (2013). Long-term Use of Angiotensin II Receptor Blockers and Risk of Cancer: a Population-Based Cohort Analysis. Int. J. Cardiol. 167 (5), 2162–2166. doi:10.1016/j.ijcard.2012.05.096
Wei, C., Bovonratwet, P., Gu, A., Moawad, G., Silverberg, J. I., Friedman, A. J., et al. (2020). Spironolactone Use Does Not Increase the Risk of Female Breast Cancer Recurrence: A Retrospective Analysis. J. Am. Acad. Dermatol. 83, 1021–1027. doi:10.1016/j.jaad.2020.05.081
Wilson, L. E., D’Aloisio, A. A., Sandler, D. P., and Taylor, J. A. (2016). Long-term Use of Calcium Channel Blocking Drugs and Breast Cancer Risk in a Prospective Cohort of US and Puerto Rican Women. Breast Cancer Res. 18 (1), 61. doi:10.1186/s13058-016-0720-6
Wright, C. M., Moorin, R. E., Chowdhury, E. K., Stricker, B. H., Reid, C. M., Saunders, C. M., et al. (2017). Calcium Channel Blockers and Breast Cancer Incidence: An Updated Systematic Review and Meta-Analysis of the Evidence. Cancer Epidemiol. 50 (Pt A), 113–124. doi:10.1016/j.canep.2017.08.012
Zhao, T., Guo, D., Gu, Y., and Ling, Y. (2017a). Nifedipine Stimulates Proliferation and Migration of Different Breast Cancer Cells by Distinct Pathways. Mol. Med. Rep. 16 (2), 2259–2263. doi:10.3892/mmr.2017.6818
Zhao, Y., Wang, Q., Zhao, X., Meng, H., and Yu, J. (2017b). Effect of Antihypertensive Drugs on Breast Cancer Risk in Female Hypertensive Patients: Evidence from Observational Studies. Clin. Exp. Hypertens. 40 (1), 22–27. doi:10.1080/10641963.2017.1288736
Keywords: antihypertensive drugs, breast cancer, meta-analysis, prognosis, risk
Citation: Xie Y, Wang M, Xu P, Deng Y, Zheng Y, Yang S, Wu Y, Zhai Z, Zhang D, Li N, Wang N, Cheng J and Dai Z (2021) Association Between Antihypertensive Medication Use and Breast Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:609901. doi: 10.3389/fphar.2021.609901
Received: 26 October 2020; Accepted: 29 April 2021;
Published: 13 May 2021.
Edited by:
Xian-Tao Zeng, Wuhan University, ChinaReviewed by:
Jinhong Zhu, Harbin Medical University Cancer Hospital, ChinaJiang-Bo Liu, The First Affiliated Hospital of Henan University of Science and Technology, China
Copyright © 2021 Xie, Wang, Xu, Deng, Zheng, Yang, Wu, Zhai, Zhang, Li, Wang, Cheng and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijun Dai, ZHpqMDkxMUAxMjYuY29t; Jing Cheng, Y2hlbmppbjExMThAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Yuxiu Xie
Yuxiu Xie Men Wang3†
Men Wang3† Yujiao Deng
Yujiao Deng Yi Zheng
Yi Zheng Si Yang
Si Yang Jing Cheng
Jing Cheng Zhijun Dai
Zhijun Dai