
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 02 June 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.599552
This article is part of the Research Topic Anakoinosis: An Innovative Anticancer Therapy Targeting the Aberrant Cancer Tissue Homeostasis View all 9 articles
 Florian Lüke
Florian Lüke Dennis C. Harrer
Dennis C. Harrer Joachim Hahn
Joachim Hahn Matthias Grube
Matthias Grube Tobias Pukrop
Tobias Pukrop Wolfgang Herr
Wolfgang Herr Albrecht Reichle
Albrecht Reichle Daniel Heudobler*
Daniel Heudobler*Spontaneous remission in acute lymphoblastic leukemia (ALL) is a rare phenomenon, which typically involves a pattern of feverish or septic disease followed by quick but mostly transient remission. We report on two male patients (46-year-old (pt. 1) and 19-year-old (pt. 2)) with CD20 positive, BCR-ABL negative common B-ALL. Patient 1 had received dexamethasone and cyclophosphamide (1.2 g) as a prephase therapy, followed by rituximab and a cumulative dose of 200 mg daunorubicin combined with 2 mg vincristine as an induction therapy. Patient 2 was treated with a reduced therapy regimen (Vincristine 1 mg, dexamethasone and 80 mg daunorubicin, 12-month mercaptopurine maintenance) due to (alcohol-related) toxic liver failure and pontine myelinolysis. Both patients developed severe septic disease just few days into induction treatment. Patient 1 suffered from pulmonary mycosis, which had to be resected eventually. Histological work-up revealed invasive mucor mycosis. Patient 2 presented with elevated serum aspergillus antigen and radiographic pulmonary lesions, indicative of pulmonary mycosis. In both patients, chemotherapy had to be interrupted and could not be resumed. Both patients recovered under broad antimicrobial, antifungal and prophylactic antiviral therapy and achieved molecular complete remission. At data cut-off remissions had been on-going for 34 months (pt. 1) and 8 years (pt. 2). Short-term, reduced intensity induction chemotherapy accompanied by severe fungal infections was followed by long-lasting continuous complete remissions in ALL. Thus, we hypothesize that infection-associated immunogenic responses may not only prevent early relapse of ALL but could also eradicate minimal residual disease. The effects of combined cytotoxic therapy and severe infection may also be mimicked by biomodulatory treatment strategies aiming at reorganizing pathologically altered cellular signaling networks. This could reduce toxicity and comorbidity in adult patients requiring leukemia treatment. Therefore, these two cases should encourage systematic studies on how leukemia stroma interaction can be harnessed to achieve long lasting control of ALL.
Acute lymphoblastic leukemia (ALL) is an aggressive hematological neoplasia characterized by accumulation of aberrant, immature lymphatic blasts in bone marrow and peripheral blood. Its incidence is about 1.1/100,000 per year with an age peak in children below 5 years and a second smaller peak around 80 years of age. Due to improvements in therapy protocols 5-year overall survival in adults has risen from ∼30% in the years 1997–1999 to 41.1% in the years 2006–2008. Severe complications, such as infections and hemorrhage, are still a cause of significant morbidity and mortality (Sant et al., 2014).
Spontaneous remission (SR) in ALL is a rare phenomenon (Hirshberg and O’Regan; Höres et al., 2018), which typically involves a pattern of feverish or septic disease, followed by quick but mostly transient remission. Nevertheless, the majority of ALL patients do not show leukemia remission upon infections. Generally, “spontaneous” describes a partial or complete disappearance of a malignant disease without any treatment or a treatment, which is not considered adequate for inducing tumor or leukemia response (Everson 1964; Vernon 2018).
A rare, but continuously observed phenomenon, described since the 19th century is the occurrence of infections in the context of systemic tumor regression or remission. The very early pioneer of the theory that infections represent a kind of systemic therapy against cancer was William Bradley Coley, a surgeon who had foreseen the pivotal importance of tumor stroma interactions and “immune response” The surgeons Tilden Everson and Warren Cole reviewed 176 published cases of SR from 1900 to 1960. They also found co-occurring infection as a frequent theme in SR (O'Neill et al., 2009; Vernon 2018).
Clinical data clearly indicate that both inflammation and inflammation control have the capacity to control systemically spread cancer (Walter et al., 2012; Heudobler et al., 2018a). However, the molecular mechanisms, are not fully understood, yet (Cen et al., 2018; Heudobler et al., 2018a; Heudobler et al., 2018b). So far, clinical results indicate infection-associated inflammation is not sufficient for long-term leukemia control. Correspondingly, all spontaneous ALL remissions related to fever or sepsis showed leukemia relapse within a short period of time (Höres et al., 2018).
Here, we describe two cases receiving short-term induction therapy with reduced dose intensity followed by severe fungal infection necessitating discontinuation of any specific anti-leukemic therapy. Long-term continuous remissions could be observed in both cases.
ALL diagnosis was confirmed in both patients by cytomorphology and flow cytometry. Starting with diagnosis, antibiotic (Ciprofloxacin), anti-fungal (Fluconazol) and antiviral (Aciclovir) prophylaxis was administered according to institutional standards. Both patients were treated outside a clinical trial and gave their written, informed consent for publishing their cases. Data were analyzed retrospectively. Clinical outcomes were followed up until 08/2020. This study was approved by the ethics committee of the University of Regensburg (statement No.: 20-2012-104).
Patient 1 was a 46-year-old male with CD-20 positive common B-ALL (Figure 1A).Genetic and molecular-genetic analysis yielded a missing chromosome, 45, XY dic (9;12) (p13;p13) in ALL blasts, plus CDKN2A and 3’ETV6 deletion. He was allocated to the standard risk group of patients according to the GMALL guidelines. Yet, CDKN2 deletion might predict poor prognosis (Xu et al., 2015). Additionally, he suffered from leukemia-associated chronic subdural hematoma. Acute subdural bleeding, complicated by a still measurable activity of phenprocoumon taken because of atrial fibrillation and mechanic aortic valve replacement, was the reason for admission to our university hospital. By then, patient 1 had received dexamethasone and cyclophosphamide (cumulative 1.2 g) as prephase, followed by rituximab, a cumulative dose of 200 mg daunorubicin combined with one dose of 2 mg vincristine. He had also received two intrathecal therapies of 15 mg methotrexate each. Chemotherapy could not be resumed due to active intracranial bleeding. He developed a septic disease pattern eventually leading to intensive care unit (ICU) care because of cardiac decompensation 1 month after admission. A thoracic CT scan showed pronounced pulmonary infiltrates in the lower right lung (Figure 2A), suspicious for fungal infection. Antimicrobial therapy was adjusted empirically. This led to a significant improvement of the patient’s condition and he was transferred back to a normal ward. A dry cough and new pulmonary lesions in the left lung appeared, making further chemotherapy impossible (Figure 2 B). A bronchoalveolar lavage (BAL) revealed mucor mycosis. Since intensified conservative efforts to resolve this infection were unsuccessful, an atypical lung resection became necessary, which removed residual lesions (Figure 2C)
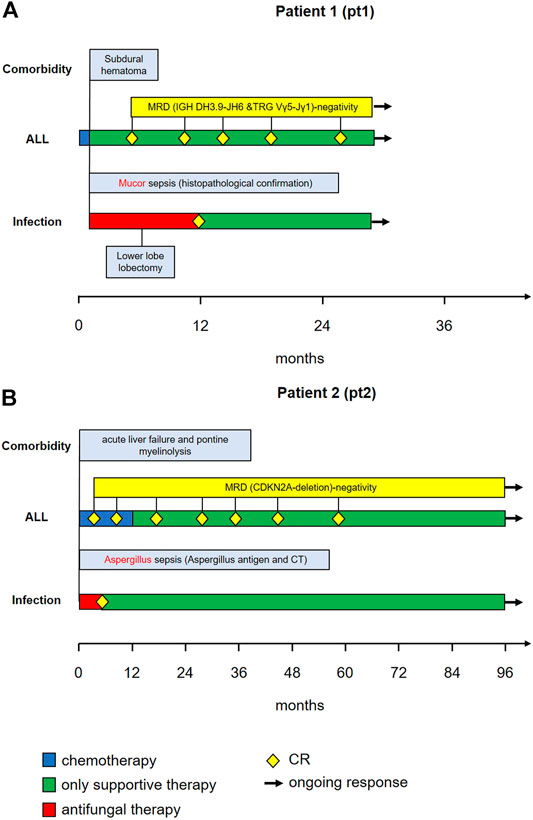
FIGURE 1. Course of relevant comorbidities (1st row), ALL treatment (2nd row), fungal infection (3rd row) and antimycotic treatment (4th row) in Patient 1 (panel A) and Patient 2 (panel B).
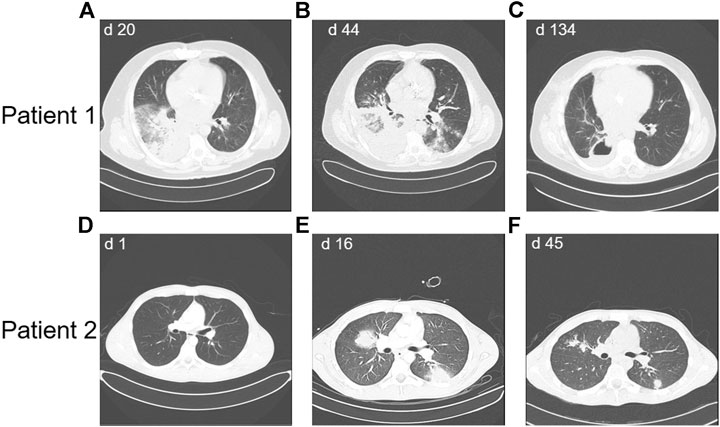
FIGURE 2. Thoracic CT scans showing the course of mycotic lesions in Patient 1 (panels A-C) and patient 2 (panels D-F), d = day after diagnosis of ALL.
Patient 2 (Figure 1B) was a 19-year-old male with CD-20 positive common B-ALL. Genetic and molecular-genetic analysis yielded a normal karyotype plus a CDKN2A deletion. He was allocated to the standard risk group according to GMALL guidelines. Similar to patient 1, a non-leukemia-associated severe comorbidity was present at admission. Due to chronic and acute alcohol abuse an acute liver failure with histologically confirmed liver necrosis and accompanying hemorrhagic-necrotizing hepatitis was diagnosed. An initial thoracic CT scan showed no intrapulmonal abnormalities (Figure 2D). Cranial magnetic resonance imaging revealed pontine myelinolysis manifesting with severe neurologic symptoms originating long-term mechanical ventilation starting 3 days after admission. On day 16 after admission, patient 2 developed, a fungal infection of the lung with typical infiltrates in a thoracic CT-scan (Figure 2D), corroborated by elevated aspergillus antigen (Galactomannan) in repeated serological samples. Galactomannan concentrations in serum decreased adequately during broad anti-microbial therapy from 1.6 ng/mL to < 0.5 ng/mL, accompanied by significant improvement of the pulmonary lesions (Figure 2E). In patient 2 the chemotherapy doses were considerably reduced due to the severe alcohol-associated, secondary diseases. Vincristine was administered with 1 mg weekly, thrice combined with dexamethasone and once daunorubicin 60 mg was added, followed by a one year 6-mercaptopurine (6-MP) maintenance. He also received intrathecal therapy with 15 mg methotrexate twice.
In both cases, the severity of comorbidities coupled with the early achievement of molecular complete remission (mCR) prompted, after discussion in an interdisciplinary leukemia board, the discontinuation of intensive leukemia therapy. Only patient 2 received a one-year maintenance therapy, which was stopped due to the slow recovery of the liver function and neurology.
Both patients recovered from fungal pneumonia under broad antimicrobial, while rapidly clearing peripheral leukemic blasts. Whereas patient 2 recovered with continuously size-decreasing residual lesions in both lungs, patient 1 developed one carnifying constant lesion (Figure 2B) of his mucor pneumonia necessitating an atypical resection of this lesion. The additional pulmonary lesions continuously decreased under on-going antimicrobial therapy.
Hematologic remission, paralleled by molecular complete remission (mCR) in the bone marrow samples, was diagnosed after 3 months in patient 1 and after 1 month in patient 2. This means that in both patients, remission occurred after the onset of severe pneumonia, during hematologic recovery phase and after discontinuation of dose-reduced induction treatment.
Molecular CR was measured by the clonal gene-rearrangement of immunoglobulin genes, which were below the detection level (patient 1) and a missing detection of the CDKN2A deletion in patient 2 at first control, respectively. mCR is now on-going for 34 months with 3-monthly controls in patient 1 and 8 years in patient 2 who was regularly followed-up with bone marrow punctures and minimal residual disease (MRD) diagnostics for 5 years.
Thus, short-term, reduced intensity induction chemotherapy paralleled by severe fungal infections was followed by a rapid on-set and long-lasting continuous complete remission of cALL.
Neither short-term, dose-reduced induction therapy nor feverish or septic events are commonly associated with long-term molecular complete remission in ALL.
There is evidence that the immune system can switch from an immunotolerant to an immune activated tumor microenvironment (TME) triggered by chemotherapy and severe fungal infection inducing SR (Diamond and Luhby 1951; Yoruk et al., 2008; Chen and Chuang 2009; Pluchart et al., 2015; Purohit et al., 2015). Possible mechanisms could include inhibition of leukemia re-growth (provoked by the phoenix rising pathway) and control of minimal residual disease (Hoelzer et al., 1996; Seif et al., 2009; Li et al., 2010; Paul et al., 2016). Moreover, pathogen-associated molecular patterns (PAMPs) recognized by toll-like receptor (TLR) 2, TLR3, TLR4, TLR9, and Dectin-1 can be found in aspergillus as well as mucor (McGettrick und O’Neill 2007; O’Neill et al., 2009). Fungal TLR ligands may target both, normal and malignant hematopoiesis (O’Neill et al., 2009; Mansour et al., 2012; Monlish et al., 2016). Since TLRs are widely distributed among different tissues throughout the body, their stimulation next to cytotoxic activity of chemotherapy might be responsible for augmenting antileukemic effects of the TME (Rolf et al., 2015; Heudobler et al., 2019). For example, exposure to beta-glucan released during fungal infections induces expansion of progenitors of the myeloid lineage, which is associated with the recruitment and activation of the innate immune system (Mitroulis et al., 2018). Also unmethylated CpG motifs derived from Aspergillus fumigatus DNA may activate the innate immune system by TLR-9 stimulation (Dalpke et al., 2002; Ramirez-Ortiz et al., 2008). One study using TLR agonists in B-cell precursor ALL cell lines revealed that treatment with TLR2 or TLR9 agonists stimulated allogeneic T cell responses demonstrating an important link between innate and adaptive immunity (Corthals et al., 2006). Moreover, TLR-9 activated plasmocytoid dendritic cells were shown to be a powerful tool for overcoming ALL resistance by NK cell-mediated killing and for reinforcing the graft versus leukemia (GvL) effect following allogeneic hematopoietic stem cell transplantation (HSCT) in an ALL mouse model of residual disease (Díaz-Rodríguez et al., 2017). Targeted stimulation of TLRs and involvement of the innate immune system is associated with enhanced activity of cytotoxic drugs. For instance, treatment of primary ALL samples with the specific TLR2/1 ligand, PAM3CSK4, triggers caspase-8-mediated apoptosis and sensitizes ALL cells to vincristine-mediated toxicity in vitro (Rolf et al., 2015). Immunization with the vaccine BCR-ABL/GM-CSF/IL-12 and the TLR-9 agonist dSLIM combined with 6-MP induces an innate and adaptive immune response and leads to a very high survival rate in an ALL mouse model (Köchling et al., 2012). Also, in a breast cancer model, intratumoral dendritic cells could enhance efficacy of anthracyclines highlighting the interaction of immune cells and cytotoxic therapy (Vacchelli et al., 2015). In animal models, TLR9 stimulation triggers the break of tumor tolerance and induces immunity against AML and ALL (Hossain et al., 2014; Jo et al., 2020). A phase I trial with the TLR9 agonist (GNKG168) supports the immunomodulatory capacity of a TLR9 agonists in vivo, showing that in children with acute leukemia and present minimal residual disease a unique immune activation pattern can be induced (Ronsley et al., 2019). Taken together, there is a substantial body of evidence, that modulation of the immune system by PAMPs can also induce antineoplastic effects in ALL.
While the two above presented cases show some interesting similarities, there are several limitations to our study. Firstly, we present a very small number of patients that were analyzed retrospectively. Secondly, both patients received different antineoplastic and antiinfectious treatment. Furthermore, we cannot show experimental data on the underlying mechanisms of our findings. Thus, it remains ultimately unclear as to why long-term leukemia control was achieved.
Thus, we need to learn how to create specific (tissue) conditions mimicking those of specific infections that support immune reactions against ALL. While TLR-agonists show poor monoactivity, combining them with e.g. cytotoxic drugs or cancer vaccines might prime the immune system beyond the establishment of an innate immunity to develop TH1 cytotoxic response against leukemia antigen-expressing cells (Ridnour et al., 2013). As our cases demonstrate, the measures needed to achieve a molecular complete response consisted of a much-shortened total treatment duration of only few weeks. This coordinated interlock of cytotoxic therapy and severe infection resembles biomodulatory therapy approaches. They aim at redirecting tumor-related pathologic homeostatic pathways (Heudobler et al., 2019). Since ALL-therapy regimens are currently being mixed up by the introduction of new drugs like monoclonal antibodies, bispecific antibodies and tyrosine kinase inhibitors (Raff et al., 2007; Paul et al., 2016), combined modality biomodulatory approaches should also be pursued. This might ultimately result in substantially shorter, less toxic therapy regimens by targeting leukemia and therapy-related stage dependent leukemia-stroma-based communicative interactions, as suggested by the two cases (Heudobler et al., 2019).
The data analyzed in this study is subject to the following licenses/restrictions: The datasets generated for this study are available on request to the corresponding author. Requests to access these datasets should be directed to ZGFuaWVsLmhldWRvYmxlckB1a3IuZGU=
This study was approved by the ethics committee of the University of Regensburg (statement No.: 20-208 2012-104). The patients/participants provided their written informed consent to participate in this study.
FL, DH, JH, MG, and AR treated the patients. FL, AR, DCH and DH wrote the manuscript. All authors revised the manuscript critically, approved the final manuscript, and agreed to be accountable for all aspects of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Cen, X., Liu, S., and Cheng, K. (2018). The Role of Toll-like Receptor in Inflammation and Tumor Immunity. Front. Pharmacol. 9, 878. doi:10.3389/fphar.2018.00878
Corthals, S. L., Wynne, K., She, K., Shimizu, H., Curman, D., Garbutt, K., et al. (2006). Differential Immune Effects Mediated by Toll-like Receptors Stimulation in Precursor B-Cell Acute Lymphoblastic Leukaemia. Br. J. Haematol. 132 (4), 051220022257006–051220022257458. doi:10.1111/j.1365-2141.2005.05893.x
Dalpke, A., Frey, M., Morath, S., Hartung, T., and Heeg, K. (2002). Interaction of Lipoteichoic Acid and CpG-DNA during Activation of Innate Immune Cells. Immunobiology 206 (4), 392–407. doi:10.1078/0171-2985-00189
Díaz-Rodríguez, Y., Cordeiro, P., Belounis, A., Herblot, S., and Duval, M. (2017). In Vitro differentiated Plasmacytoid Dendritic Cells as a Tool to Induce Anti-leukemia Activity of Natural Killer Cells. Cancer Immunol. Immunother. 66 (10), 13071307–13201320. doi:10.1007/s00262-017-2022-y
Everson, T. C. (1964). SPONTANEOUS REGRESSION OF CANCER. Ann. New York Acad. Sci. 114 (2), 721–735. doi:10.1111/j.1749-6632.1964.tb40991.x
Heudobler, D., Lüke, F., Vogelhuber, M., Klobuch, S., Pukrop, T., Herr, W., et al. (2019). Anakoinosis: Correcting Aberrant Homeostasis of Cancer Tissue-Going beyond Apoptosis Induction. Front. Oncol. 9, 1408. doi:10.3389/fonc.2019.01408
Heudobler, D., Rechenmacher, M., Lüke, F., Vogelhuber, M., Klobuch, S., Thomas, S., et al. (2018a). Clinical Efficacy of a Novel Therapeutic Principle, Anakoinosis. Front. Pharmacol. 9, 1357. doi:10.3389/fphar.2018.01357
Heudobler, D., Rechenmacher, M., Lüke, F., Vogelhuber, M., Pukrop, T., Herr, W., et al. (2018b). Peroxisome Proliferator-Activated Receptors (PPAR)γ Agonists as Master Modulators of Tumor Tissue. Ijms 19 (11), 3540. doi:10.3390/ijms19113540
Hirshberg, C., and O’Regan, B. (1993). Spontaneous Remission: An Annotated Bibliography. (Petaluma: Institute of Noetic Sciences), 713.
Hoelzer, D., Ludwig, W., Thiel, E., Gassmann, W., Löffler, H., Fonatsch, C., et al. (1996). Improved Outcome in Adult B-Cell Acute Lymphoblastic Leukemia. Blood 87 (2), 495–508. doi:10.1182/blood.v87.2.495.bloodjournal872495
Höres, T., Wendelin, K., and Kerstin, S.-E. (2018). Spontaneous Remission of Acute Lymphoblastic Leukemia: A Case Report. Oncol. Lett. 15 (1), 115–120. doi:10.3892/ol.2017.7288
Hossain, D. M. S., Dos Santos, C., Zhang, Q., Kozlowska, A., Liu, H., Gao, C., et al. (2014). Leukemia Cell-Targeted STAT3 Silencing and TLR9 Triggering Generate Systemic Antitumor Immunity. Blood 123 (1), 15–25. doi:10.1182/blood-2013-07-517987
Jo, S., Fotovati, A., Duque-Afonso, J., Cleary, M. L., van den Elzen, P., Seif, A. E., et al. (2020). Differential Depletion of Bone Marrow Resident B-ALL after Systemic Administration of Endosomal TLR Agonists. Cancers 12 (1), 169. doi:10.3390/cancers12010169
Köchling, J., Rott, Y., Arndt, S., Marschke, C., Schmidt, M., Wittig, B., et al. (2012). Prevention and Synergistic Control of Ph+ ALL by a DNA Vaccine and 6-mercaptopurine. Vaccine 30 (41), 5949–5955. doi:10.1016/j.vaccine.2012.07.037
Li, F., Huang, Q., Chen, J., Peng, Y., Roop, D. R., Bedford, J. S., et al. (2010). Apoptotic Cells Activate the “phoenix Rising” Pathway to Promote Wound Healing and Tissue Regeneration. Sci. signaling 3 (110), ra13. doi:10.1126/scisignal.2000634
Mansour, M. K., Tam, J. M., and Vyas, J. M. (2012). The Cell Biology of the Innate Immune Response toAspergillus Fumigatus. Ann. New York Acad. Sci. 1273, 78–84. doi:10.1111/j.1749-6632.2012.06837.x
McGettrick, A. F., and O'Neill, L. A. J. (2007). Toll-like Receptors: Key Activators of Leucocytes and Regulator of Haematopoiesis. Br. J. Haematol. 139 (2), 185–193. doi:10.1111/j.1365-2141.2007.06802.x
Mitroulis, I., Ruppova, K., Wang, B., Chen, L.-S., Grzybek, M., Grinenko, T., et al. (2018). Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 172 (1-2), 147–161.e12. doi:10.1016/j.cell.2017.11.034
Monlish, D. A., Bhatt, S. T., and Schuettpelz, L. G. (2016). The Role of Toll-like Receptors in Hematopoietic Malignancies. Front. Immunol. 7, 390. doi:10.3389/fimmu.2016.00390
O’Neill, Luke. A. J., Bryant, Clare. E., and Doyle, Sarah. L. (2009). Therapeutic Targeting of Toll-like Receptors for Infectious and Inflammatory Diseases and Cancer. Pharmacol. Rev. 61 (2), 177–197. doi:10.1124/pr.109.001073
Paul, S., Kantarjian, H., and Jabbour, E. J. (2016). Adult Acute Lymphoblastic Leukemia. Mayo Clinic Proc. 91 (11), 1645–1666. doi:10.1016/j.mayocp.2016.09.010
Raff, T., Gökbuget, N., Lüschen, S., Reutzel, R., Ritgen, M., Irmer, S., et al. (2007). Molecular Relapse in Adult Standard-Risk ALL Patients Detected by Prospective MRD Monitoring during and after Maintenance Treatment: Data from the GMALL 06/99 and 07/03 Trials. Blood 109 (3), 910–915. doi:10.1182/blood-2006-07-037093
Ramirez-Ortiz, Z. G., Specht, C. A., Wang, J. P., Lee, C. K., Bartholomeu, D. C., Gazzinelli, R. T., et al. (2008). Toll-like Receptor 9-dependent Immune Activation by Unmethylated CpG Motifs in Aspergillus fumigatus DNA. Iai, 76, 2123–2129. doi:10.1128/IAI.00047-08
Ridnour, L. A., Cheng, R. Y. S., Switzer, C. H., Heinecke, J. L., Ambs, S., Glynn, S., et al. (2013). Molecular Pathways: Toll-like Receptors in the Tumor Microenvironment-Poor Prognosis or New Therapeutic Opportunity. Clin. Cancer Res. 19 (6), 1340–1346. doi:10.1158/1078-0432.CCR-12-0408
Rolf, N., Kariminia, A., Ivison, S., Reid, G. S., and Schultz, K. R. (2015). Heterodimer-specific TLR2 Stimulation Results in Divergent Functional Outcomes in B-Cell Precursor Acute Lymphoblastic Leukemia. Eur. J. Immunol. 45 (7), 1980–1990. doi:10.1002/eji.201444874
Ronsley, R., Kariminia, A., Ng, B., Mostafavi, S., Reid, G., Subrt, P., et al. (2019). The TLR9 Agonist (GNKG168) Induces a Unique Immune Activation Pattern In Vivo in Children with Minimal Residual Disease Positive Acute Leukemia: Results of the TACL T2009-008 Phase I Study. Pediatr. Hematol. Oncol. 36 (8), 468–481. doi:10.1080/08880018.2019.1667461
Sant, M., Minicozzi, P., Mounier, M., Anderson, L. A., Brenner, H., Holleczek, B., et al. (2014). Survival for Haematological Malignancies in Europe between 1997 and 2008 by Region and Age: Results of EUROCARE-5, a Population-Based Study. Lancet Oncol. 15 (9), 931–942. doi:10.1016/S1470-2045(14)70282-7
Seif, A. E., Barrett, D. M., Milone, M., Brown, V. I., Grupp, S. A., and Reid, G. S. D. (2009). Long-term protection from Syngeneic Acute Lymphoblastic Leukemia by CpG ODN-Mediated Stimulation of Innate and Adaptive Immune Responses. Blood 114 (12), 2459–2466. doi:10.1182/blood-2009-02-203984
Vacchelli, E., Ma, Y., Baracco, E. E., Sistigu, A., Enot, D. P., Pietrocola, F., et al. (2015). Chemotherapy-induced Antitumor Immunity Requires Formyl Peptide Receptor 1. Science 350 (6263), 972–978. doi:10.1126/science.aad0779
Vernon, L. F. (2018). William Bradley Coley, MD, and the Phenomenon of Spontaneous Regression. ImmunoTargets Ther. 7, 29–34. doi:10.2147/ITT.S163924
Walter, B., Schrettenbrunner, I., Vogelhuber, M., Grassinger, J., Bross, K., Wilke, J., et al. (2012). Pioglitazone, Etoricoxib, Interferon-α, and Metronomic Capecitabine for Metastatic Renal Cell Carcinoma: Final Results of a Prospective Phase II Trial. Med. Oncol. 29 (2), 799–805. doi:10.1007/s12032-011-9982-0
Keywords: biomodulation, anakoinosis, pulmonary mycosis, inflammation, acute lymphoblastic leukaemia
Citation: Lüke F, Harrer DC, Hahn J, Grube M, Pukrop T, Herr W, Reichle A and Heudobler D (2021) Continuous Complete Remission in Two Patients with Acute Lymphoblastic Leukemia and Severe Fungal Infection Following Short-Term, Dose-Reduced Chemotherapy. Front. Pharmacol. 12:599552. doi: 10.3389/fphar.2021.599552
Received: 24 September 2020; Accepted: 18 May 2021;
Published: 02 June 2021.
Edited by:
Giovanni Martinelli, University of Bologna, ItalyReviewed by:
Michael James Burke, Medical College of Wisconsin, United StatesCopyright © 2021 Lüke, Harrer, Hahn, Grube, Pukrop, Herr, Reichle and Heudobler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Heudobler, ZGFuaWVsLmhldWRvYmxlckB1a3IuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.