
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 16 April 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.593434
A commentary has been posted on this article:
Commentary: Chinese Herbal Medicine Usage Reduces Overall Mortality in HIV-Infected Patients With Osteoporosis or Fractures
 Mao-Wang Ho1,2†
Mao-Wang Ho1,2† Te-Mao Li3†
Te-Mao Li3† Ju-Pi Li4,5†
Ju-Pi Li4,5† Jian-Shiun Chiou6
Jian-Shiun Chiou6 Mu-Lin Chiu3
Mu-Lin Chiu3 Chao-Jung Chen7,8
Chao-Jung Chen7,8 Chi-Fung Cheng6,8
Chi-Fung Cheng6,8 Fuu-Jen Tsai3,8,9
Fuu-Jen Tsai3,8,9 Yang-Chang Wu3
Yang-Chang Wu3 Ting-Hsu Lin8
Ting-Hsu Lin8 Chiu-Chu Liao8
Chiu-Chu Liao8 Shao-Mei Huang8
Shao-Mei Huang8 Yu-Ning Lin8
Yu-Ning Lin8 Chen-Hsing Chou6
Chen-Hsing Chou6 Wen-Miin Liang6*
Wen-Miin Liang6* Ying-Ju Lin3,8*
Ying-Ju Lin3,8*The survival of patients with HIV has greatly improved, due to Anti-Retroviral Therapy (ART). However, long-term HIV survivors often develop serious bone abnormalities, possibly due to the interplay of osteoblasts, osteoclasts, HIV ad ART. We evaluated in a nation-wide study in Taiwan the effect of Chinese herbal medicine (CHM) on overall mortality in HIV patients with osteoporosis or fractures. Enrollment period was between 1998 and 2011. Patients with osteoporosis or fractures before the HIV infection, and those with less than 14 days CHM use, were excluded. This left 498 patients, 160 CHM users, 338 without CHM. Univariate Kaplan-Meier and multivariate Cox regression analysis were used to compare the overall mortality in these 2 groups. Due to the nature of Chinese medicine, CHMs inevitably varied. We therefore also used rule mining and network analysis to determine which major CHM clusters were prescribed to the patients. CHM users had a much Lower mortality (hazard ratio (HR) = 0.43, 95% confidence interval (CI): 0.24–0.77, p < 0.005) and higher survival (p = 0.004, log-rank test). Although the CHMs greatly varied, network analysis identified one main cluster of strongly related CHM combinations (Chuan-Xiong-Cha-Tiao-San (CXCTS), Gan-Cao (GC; Glycyrrhiza uralensis Fisch.), Liu-He-Tang (LHT), Huang-Qin-Tang (HQT), Jia-Wei-Ping-Wei-San (JWPWS), and Dang-Gui-Long-Hui-Wan (DGLHuiW)). CHM as an additional treatment strongly improves overall survival in HIV-infected patients with osteoporosis and fractures.
With antiretroviral therapy (ART), HIV-positive and negative patients have similar lifespans (Pham and Mesplede, 2018; Zhang et al., 2019). Patients with HIV/AIDS who receive ART demonstrate delayed AIDS progression, improved quality of life, and lower all-cause mortality (Antiretroviral Therapy Cohort Collaboration, 2017; Lu et al., 2018). ART suppresses viral replication; it does not eliminate the virus. Discontinuation of ART results in drug resistance of HIV, viral reactivation, and disease progression (Meintjes et al., 2017; Dubrocq and Rakhmanina, 2018). Long-term living with HIV and ART use in HIV-infected patients are associated with adverse effects. These adverse effects include hyperlipidemia, cardiovascular disease, bone related abnormalities, diabetes, and renal disease (Kwong et al., 2006; De Wit et al., 2008; Capeau et al., 2012; Achhra et al., 2016; Grant et al., 2016; Hoy and Young, 2016; Ahmad et al., 2017; Dorjee et al., 2017; Hoy et al., 2017; Tsai et al., 2017; Nan et al., 2018).
Bone related abnormalities including low bone density, osteomalacia, osteonecrosis, osteopenia, osteoporosis, and fracture (Hoy and Young, 2016; Ahmad et al., 2017). Osteoporosis is a multifactorial systemic skeletal disease with low bone density, degeneration of bone architecture, bone fragility, and consequent increased risk of fracture (Ji and Yu, 2015; Sozen et al., 2017). A number of studies report that lower bone density was observed in HIV-infected patients when compared with non-infected individuals (Bruera et al., 2003; Amiel et al., 2004; Arnsten et al., 2007). The pathological mechanism between HIV and/or ART and bone related abnormalities remain to be elucidated, but are probably due to HIV and ART affecting the interactions between osteoclasts and osteoblasts. Furthermore, the loss of bone mineral density is frequently observed in HIV-infected patients with ART (Duvivier et al., 2009; van Vonderen et al., 2009; Grant et al., 2016; Hoy et al., 2017; Chisati et al., 2020b). HIV-infected patients placed on protease inhibitor (PI) regimens demonstrate bone loss in the spine, while the nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) regimen is associated with bone loss at the hip (Hoy et al., 2017).
Chinese herbal medicines (CHMs) are often used to treat bone related diseases, as they show anti-inflammatory, anti-osteopenia, anti-osteoporotic, and promote fracture healing activities (Chow et al., 1982; Chen et al., 2005; Li et al., 2011; Ma et al., 2011; Xiang et al., 2011; Shih et al., 2012; Wong et al., 2013; He and Shen, 2014; Mukwaya et al., 2014; Lin et al., 2015; Zhang et al., 2016; Hsiao et al., 2017; Wang et al., 2018b; Xi et al., 2018; Cheng et al., 2019a; Cheng et al., 2019b). However, none of these studies have been carried out in prospective randomized clinical trials in humans. These results encourage to analyze if CHM as additional therapy to improve osteoporosis and fractures management and survival among HIV-infected patients. We therefore analyzed in a population-based nationwide database from Taiwan, what the effect was of CHM treatment-or-not on the overall mortality in HIV-infected patients with osteoporosis or fractures.
This is a longitudinal study spanning 1995 through 2012 using the database of National Health Insurance Research Database in Taiwan (NHIRD; http://nhird.nhri.org.tw/). From the database, 3450 anonymized HIV-infected patients with osteoporosis or fractures were further identified during the period between 1998 and 2011 (Figures 1,2) (the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes for HIV infection: 042-044, V08; ICD-9-CM codes for osteoporosis: 7330; ICD-9-CM codes for fractures: 8050, 8052, 8054, 8056, 8058, 8060, 8062, 8064, 8068, 8070, 8072, 8074, 8075, 8080, 8082, 8084, 8088, 8090, 8100, 8110, 8120, 8122, 8124, 8130, 8132, 8134, 8138, 8140, 8200, 8202, 8208, 8210, 8212, 8220, 8230, 8232, 8238, 8240, 8242, 8244, 8246, and 8248). Of these, 2952 excluded for: 1) osteoporosis or fractures diagnosed before the diagnosis of HIV infection (n = 2,221); 2) less than 14 CHM cumulative prescription days within 1 year after osteoporosis or fractures (n = 731).
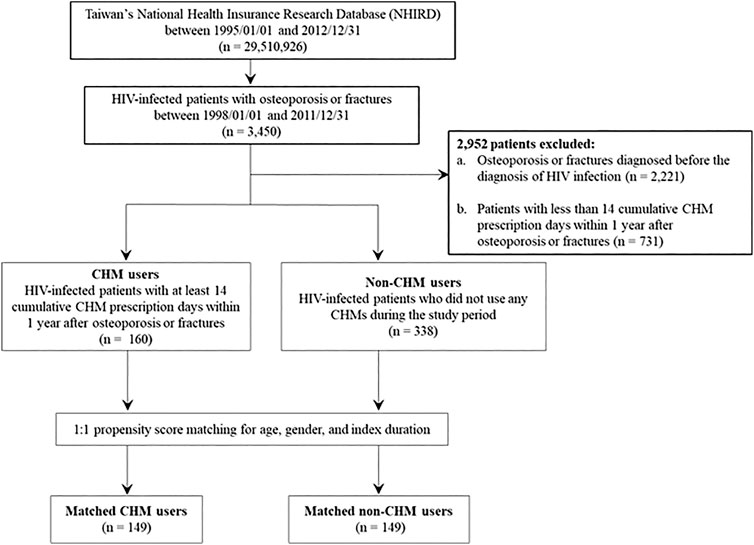
FIGURE 1. Flowchart for the enrollment of CHM and non-CHM users in HIV-infected patients with osteoporosis or fractures. CHM, Chinese herbal medicine.
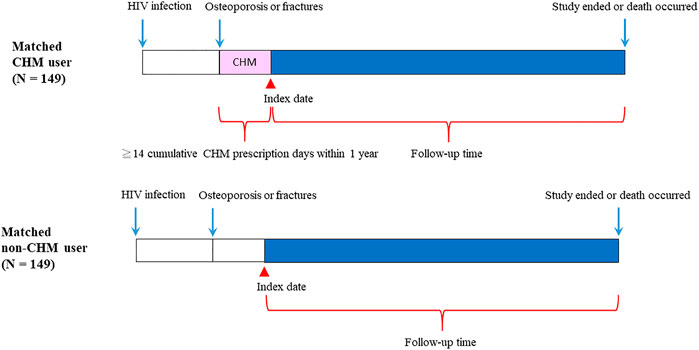
FIGURE 2. Follow-up times of CHM and non-CHM users in HIV-infected patients with osteoporosis or fractures. CHM, Chinese herbal medicine.
The flowchart for the selection for HIV-infected patients with osteoporosis or fractures is shown in Figures 1,2. Patients were initially diagnosed with HIV infection followed by osteoporosis or fractures (Figure 2). Between January 01, 1998 and December 31, 2011, 3450 HIV-infected patients with osteoporosis or fractures were identified (Figure 1). After exclusion, there were 498 patients with osteoporosis or fractures, including 160 CHM and 338 non-CHM users (Figure 1). The CHM users were defined as the patients who received CHMs for at least 14 days in the 12 months after osteoporosis or fractures (Figures 1,2).
Patients were classified as CHM users when they received more than 14 CHM cumulative prescription days among the first year after osteoporosis or fractures (n = 160, Figures 1,2). The index date started after the 14 cumulative CHM prescription days were accomplished (Figure 2). The CHM users received CHM therapies during the study period (Supplementary Table S2). On the other hand, the controls were classified as non-CHM users when they did not receive any CHMs for the study period (n = 338). To reduce potential confounding factors, these two groups were matched for age, gender, and index duration using the propensity score matching method (1:1 ratio) (Table 1). This resulted in 149 CHM and 149 non-CHM users (Figures 1,2; Table 1).
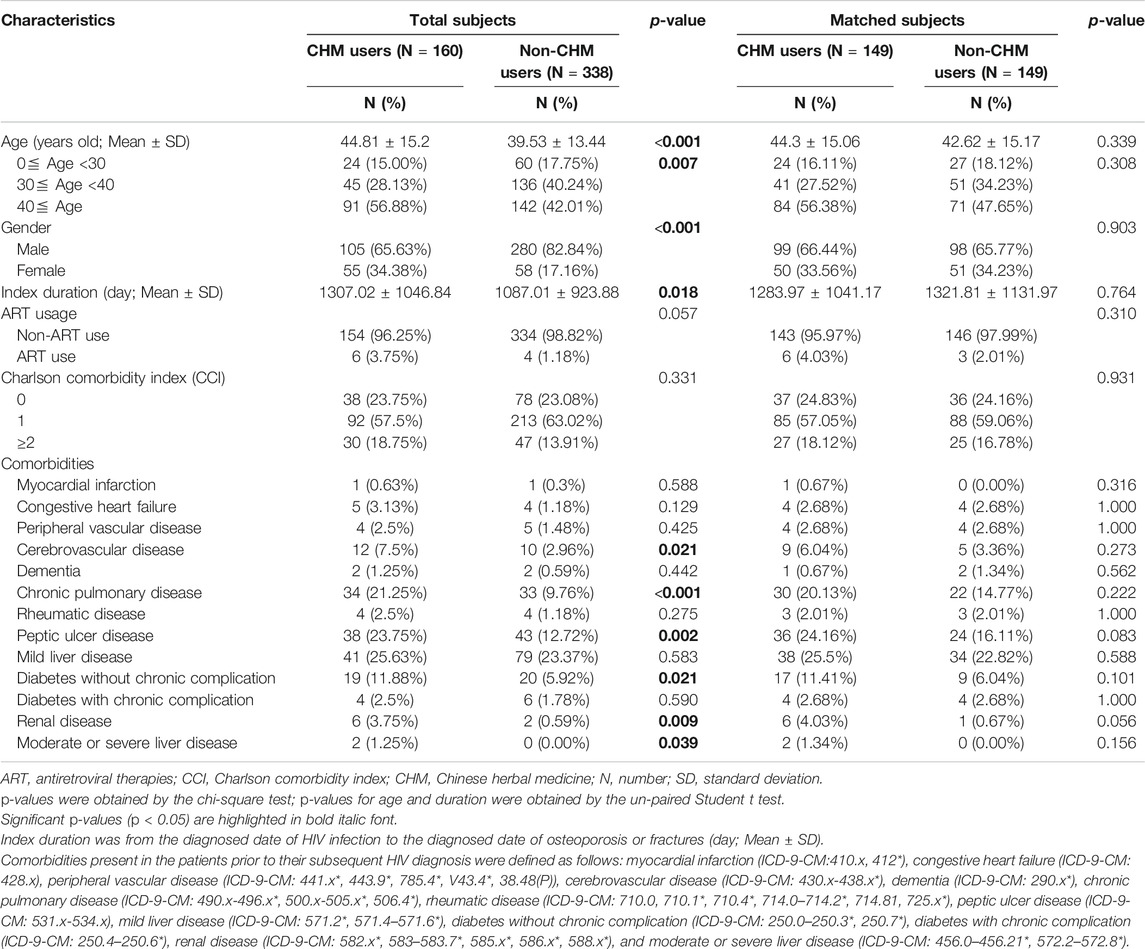
TABLE 1. Demographic characteristics of HIV-infected patients with osteoporosis or fractures according to Chinese herbal medicine usage in Taiwan.
Among these patients, the characteristics included age, gender, index duration (from the HIV infection diagnosed date to the diagnosed date of osteoporosis or fractures), Charlson comorbidity index (CCI), and comorbidities (Table 1). In this study, comorbidities were defined before HIV infection (Table 1). The ART usage was defined before the diagnosed date of osteoporosis or fractures (Table 1). The study was approved by the Institutional Review Board of the China Medical University Hospital (The ethics approval number: CMUH107-REC3-074(CR1)).
These CHMs are prescribed by licensed and experienced traditional Chinese medicine doctors in Taiwan, and they are served as traditional Chinese medicine in health care systems in Taiwan. CHMs include single herbs and herbal formulae. A single herb is made from the flower, root, stem, or leaf of a given plant. It is also made from an organ of an animal, insect, or mineral source. The herbal formulae are mixtures of a minimum of two single herbs. The CHM composition, frequency, and usage patterns are shown in Supplementary Table S1. CHMs are produced by pharmaceutical Good Manufacturing Practice companies with in Taiwan.
Association rule mining was performed, as previously described, using SAS software (version 9.4; SAS Institute, Cary, NC, United States). This association rule mining has been applied to discover studies in the relationships of these CHM prescriptions (Chen et al., 2014; Cheng et al., 2019b; Tsai et al., 2019). Chinese herbal medicine (CHM) product X (CHM_X) and CHM product Y (CHM_Y) were shown as the “items,” respectively. The CHM prescriptions were used as the “transactions,” with co-occurrences of CHM_X and CHM_Y (Table 3). This expression shows the relationship between the occurrences of CHM_X and CHM_Y. The strength of the association using this technique was expressed as support, confidence, and lift. Support is a measure of whether an association between CHM_X and CHM_Y happened by chance. The support (X) (%) value is the calculated joint probability of having both of CHM_X and CHM_Y, which is (the frequency of CHM_X and CHM_Y/total number of prescription) × 100%. Confidence is an indicator of how often CHM_Y appeared in transactions that contained CHM_X. The confidence value (CHM_X → CHM_Y; %) is the calculated conditional probability of having a prescription of CHM_Y among those who already have the prescription of CHM_X, which is given as (frequency of CHM_X and CHM_Y/frequency of CHM_X) x 100%. Lift is the ratio of observed support to expected support when X and Y are independent. The lift value is the confidence (CHM_X → CHM_Y) (%)/P (Y) (%) or confidence (CHM_Y → CHM_X) (%)/P (X) (%). A lift value greater than 1 indicates that the occurrences between the two CHM products are dependent and suggests a strong co-occurrence relationship between CHM_X and CHM_Y.
Network analysis was performed as previously described (Cheng et al., 2019a; Cheng et al., 2019b) (Figure 3). The single herb is expressed as a green circle, and the herbal formula is shown as a red circle. The prescription frequency of the single herb or herbal formula is shown (Supplementary Table S2) and is denoted as the circle size. The support value (%) (between CHM_X and CHM_Y) is shown in Table 3 and is expressed as the line size. The lift value is also shown in Table 3 and is represented as the line color. The connection strength between the paired CHM products is shown as the line size and line color. All data were employed using Cytoscape software (https://cytoscape.org/, version 3.7.0).
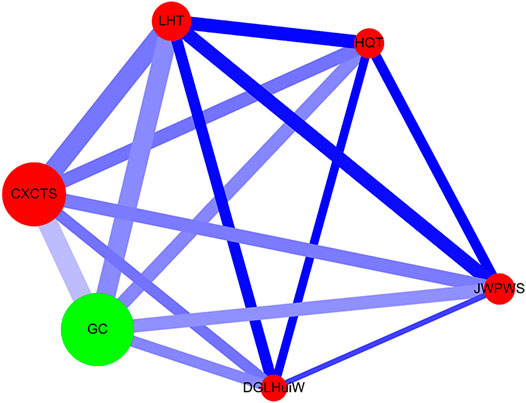
FIGURE 3. Network analysis for CHM prescription pattern in HIV-infected patients with osteoporosis or fractures. The lines connecting CHMs represent the support value: thicker lines represent higher support values (Support (X) (%)). The line color between CHMs shows the lift value: darker lines represent stronger connections with higher lift values. The red circle represents herbal formulas, while the green circle represents single herbs. The size of the circle for each CHM shows its prescription frequency: larger circles indicate higher prescription frequency. Support (X) (%) = Frequency of prescriptions of X and Y products/total prescriptions x 100%. Lift = Confidence (X → Y) (%)/P (Y) (%). Confidence (X → Y) (%) = Frequency of prescriptions of X and Y products/Frequency of prescriptions of X product x 100%. P (Y) (%) = Frequency of prescriptions of Y product/total prescriptions x 100%. CHM, Chinese herbal medicine; CXCTS, Chuan-Xiong-Cha-Tiao-San; DGLHuiW, Dang-Gui-Long-Hui-Wan; GC, Gan-Cao; HQT, Huang-Qin-Tang; JWPWS, Jia-Wei-Ping-Wei-San; LHT, Liu-He-Tang.
Age was expressed as continuous data (years, mean ± SD) and categorical data (numbers (percentages)) (Table 1). Index duration was expressed as continuous data (from the diagnosed date of HIV infection to the diagnosed date of osteoporosis or fractures) (day, Mean ± SD) (Table 1). Gender, antiretroviral therapies (ART) usage, Charlson comorbidity index (CCI) and comorbid conditions were expressed as categorical data (numbers (percentages)) (Table 1). The un-paired Student t-test was applied in continuous data (Supplementary Table S2). The Chi-squared test was used in categorical data. Univariate (crude) and multivariate (adjusted) Cox proportional hazard models were employed to evaluate the risk of overall mortality (Table 2). Multivariate-adjustments include age, gender, CHM use, ART use, and CCI (Table 2). For survival analysis, Kaplan-Meier method and the log-rank test were performed (Figure 4; Supplementary Table S4, S5). All data and statistical analyses were employed using SAS software (version 9.4; SAS Institute, Cary, NC, United States).
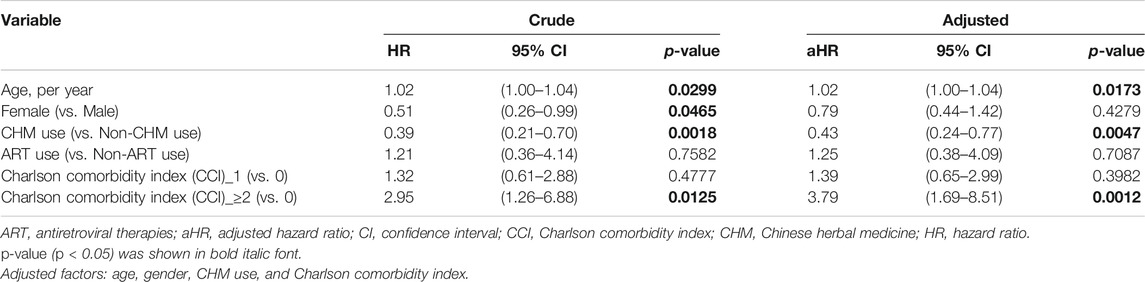
TABLE 2. Cox proportional hazard models for overall mortality in HIV-infected patients with osteoporosis or fractures.
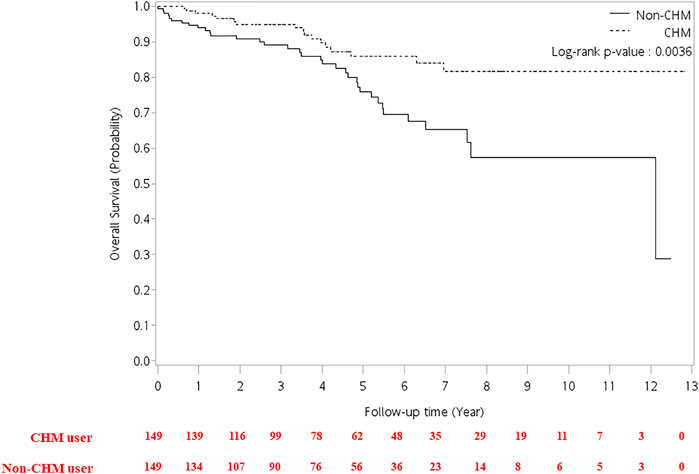
FIGURE 4. Cumulative incidence of overall survival between CHM and non-CHM users in HIV-infected patients with osteoporosis or fractures. CHM, Chinese herbal medicine.
The 160 CHM users received CHM therapies during the study period (Supplementary Table S3). The other 338 patients did not use any CHM at all. The demographic characteristics of total subjects are shown in Table 1. When compared with the 338 non-CHM users, the 160 CHM users were slightly older, more often females, had a longer index duration between HIV diagnosis date and the osteoporosis or fractures date, and had more often comorbidities (p < 0.05). To prevent the effects of these confounding factors, propensity score matching (1:1 ratio) was applied to match the two groups for age, gender, and index duration. After matching, each group had 149 HIV-infected patients with osteoporosis or fractures (Figures 1,2; Table 1).
The risk of overall mortality in patients with osteoporosis or fractures was evaluated by Cox proportional hazard models (Table 2). For univariate (crude) Cox proportional hazard model, there were differences in age, gender, CHM use, and comorbidities (p < 0.05). The univariate (crude) Cox proportional hazard model showed that patients showed a higher risk of overall mortality per year increase in age (Table 2; hazard ratio (HR): 1.021, 95% confidence interval (CI): 1.00–1.04, p = 0.0299). Female patients had a lower risk of overall mortality than male patients (HR: 0.51, 95% CI: 0.26–0.99, p = 0.0465). The CHM users had a lower risk of overall mortality than non-CHM users (HR: 0.39, 95% CI: 0.21–0.70, p = 0.0018). Patients with Charlson comorbidity index (CCI) ≥ 2 showed a higher risk of overall mortality than those who did not have any comorbidities (HR: 2.95, 95% CI: 1.26–6.88, p = 0.0125).
The multivariate Cox proportional hazard model showed that patients had a higher risk of overall mortality per year increase in age after adjusting for gender, CHM use, and Charlson comorbidity index (Table 2; adjusted hazard ratio (aHR): 1.02, 95% CI: 1.00–1.04, p = 0.0173). The CHM users had a lower risk of overall mortality than non-CHM users after adjusting for age, gender, and Charlson comorbidity index (aHR: 0.43, 95% CI: 0.24–0.77, p = 0.0047). Patients with Charlson comorbidity index (CCI) ≥ 2 showed a higher risk of overall mortality than those who did not have any comorbidities after adjusting for age, gender, and CHM use (aHR: 3.79, 95% CI: 1.69–8.51, p = 0.0012). Kaplan-Meier survival plots exhibited that there was a difference in the cumulative incidences of overall survival between the CHM and non-users (Figure 4; p = 0.0036, log-rank test). The cumulative incidence of overall survival was significantly higher in CHM users.
The commonly prescribed CHM products and compositions are listed for the HIV-infected patients with osteoporosis or fractures in Supplementary Table S1. LC-MS/MS analysis of active component standards and these 6 herbal extracts are also shown in Supplementary Figures S1–S6. According to the frequency of prescriptions (Supplementary Table S1), Chuan-Xiong-Cha-Tiao-San (CXCTS) was the most commonly herbal formula. The second and third formulas were Liu-He-Tang (LHT) and Jia-Wei-Ping-Wei-San (JWPWS), respectively. Gan-Cao (GC; Glycyrrhiza uralensis Fisch.) was the most commonly single herb.
Association rule analysis showed the 10 most commonly co-prescriptions of CHM products for HIV-infected patients with osteoporosis or fractures (Table 3). Higher levels of support, confidence, and lift values suggested stronger associations between the paired CHM products. According to the frequency of prescriptions, support, confidence, and lift values (Table 3), the most commonly used paired CHM products were Chuan-Xiong-Cha-Tiao-San (CXCTS) → Gan-Cao (GC; Glycyrrhiza uralensis Fisch.) (first co-prescription frequency: 172, support: 4.59%, confidence: 55.31%, lift: 5.75), followed by Liu-He-Tang (LHT) → CXCTS (second co-prescription frequency: 171, support: 4.57%, confidence: 97.71%, lift: 11.77), and LHT → GC (third co-prescription frequency: 169, support: 4.51%, confidence: 96.57%, lift: 10.05).
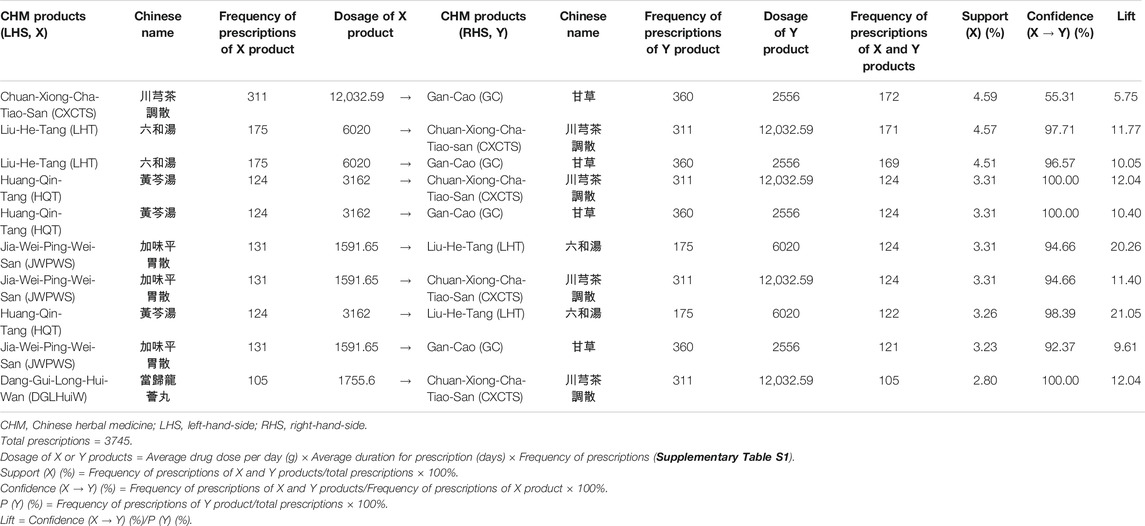
TABLE 3. Ten most commonly used co-prescriptions of CHM products for HIV-infected patients with osteoporosis or fractures in Taiwan.
Network analysis showed the CHM prescription network for patients with osteoporosis or fractures (Figure 3; Supplementary Figure S7). There were 149 patients who used 3,745 prescriptions by traditional Chinese medicine doctors (Table 3). Network analysis showed one main CHM cluster, including CXCTS, GC, LHT, HQT, JWPWS, and DGLHuiW. Our results show that these 6 CHMs are important for HIV-infected patients with osteoporosis or fractures.
Long-term living with HIV and ART use in HIV-infected patients are associated with adverse effects including bone related abnormalities. In this study, we investigated the effect of CHMs on the overall mortality in HIV-infected patients with osteoporosis or fractures in Taiwan. We found that CHM usage reduced the overall mortality for these patients. We also described their CHM prescription network; these included CXCTS, GC, LHT, HQT, JWPWS, and DGLHuiW. CHM treatment exhibited lower risks of overall mortality for HIV-infected patients with osteoporosis or fractures in Taiwan.
Reduced bone mineral density is observed in HIV-infected patients on ART therapy (Duvivier et al., 2009; van Vonderen et al., 2009; Grant et al., 2016; Hoy et al., 2017; Chisati et al., 2020b). Furthermore, Chisati et al., reported that low bone mineral density was also associated with low levels of physical activity among these patients (Chisati et al., 2020b). Maximal strength training for physical activity improves bone mineral density for people living with HIV and receiving ART (Chisati et al., 2020a). In this study, we observed that among HIV-infected patients with osteoporosis or fractures, CHM users showed a lower risk of overall mortality after adjusting for age, gender, ART use, and CCI. The cumulative incidence of overall survival was higher in CHM users, especially different between 4 and 8 years. These CHM users received CHM therapies during the study period (Supplementary Table S3); the non-CHM users did not receive any CHMs for the study period. CHMs may exhibit bone protection effect after long-term treatment. Studies have also suggested that CHM may be beneficial for bone metabolism through osteopenia prevention, anti-osteoporotic activities, promotion of fracture healing, and inhibition of inflammation (Chow et al., 1982; Chen et al., 2005; Li et al., 2011; Ma et al., 2011; Xiang et al., 2011; Wong et al., 2013; He and Shen, 2014; Zhang et al., 2016; Hsiao et al., 2017; Wang et al., 2018b; Xi et al., 2018; Lee et al., 2019). Among these studies, there were two review studies reported in human beings. Zhang et al., reported that there were 33 Traditional Chinese medicine (TCM) formulas commonly used to treat osteoporosis, exhibiting anti-osteoporotic effects in humans and animals (Zhang et al., 2016). Wang et al., reported that a natural compound from the traditional Chinese medicinal herb was effective in preventing postmenopausal osteoporosis observed from a 24-months randomized double-blind placebo-controlled clinical trial in humans (Wang et al., 2018b). Our study identified one main CHM cluster, which includes CXCTS, GC, LHT, HQT, JWPWS, and DGLHuiW.
Therapeutic approaches for treating osteoporosis or fracture inhibit further loss of bone density and strength (Kling et al., 2014). The bisphosphonates (Lewiecki, 2010), which are anti-bone resorption medications, are among the major clinical pharmacological treatments. Bisphosphonates, including alendronate, risedronate, ibandronate, and zoledronate have strong affinities for hydroxyapatite in bone and a long skeletal half-life; therefore, they inhibit bone resorption (Pazianas et al., 2014). Inhibition of osteoclast differentiation resulting in the suppression of bone resorption is one of the potential therapy targets for anti-osteoporotic and anti-fracture activities (Weivoda et al., 2020). Chinese herbs and their related natural compounds may prevent osteoporosis or fractures via inhibition of osteoclast activities. Ferulic acid is one of the natural compounds of Chuan-Xiong (CX; Rhizoma Chuanxiong; Ligusticum sinense Oliv.) (Lv et al., 2010; Li et al., 2012), and it is a component of CXCTS, Zhi-Ban-Xia (ZBX; Rhizoma Pinelliae Preparatum; Pinellia ternata (Thunb.) Makino) (Han et al., 2007), LHT, Huang-Qin (HQin; Radix Scutellariae; Scutellaria baicalensis Georgi) (Lu et al., 2011), HQT, Dang-Gui (DG; Radix Angelicae Sinensi; Angelica sinensis (Oliv.) Diels) (Giacomelli et al., 2017), and DGLHuiW. Ferulic inhibits osteoclast differentiation (Sagar et al., 2016; Doss et al., 2018) and the RANKL dependent NF-kB signaling pathway (Doss et al., 2018). Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is one of the natural compounds of CX (Li et al., 2012), and it is a component of CXCTS, Ren-Shen (RS; Radix Ginseng; Panax ginseng C. A. Mey.) (Chung et al., 2016), LHT, Hou-Po (HP; Cortex Magnoliae Officinalis; Magnolia officinalis Rehder and E. H. Wilson) (Shim et al., 2015), JWPWS, Bai-Shao (BS; Radix Paeoniae Alba; Paeonia lactiflora Pall.) (Liu et al., 2015), HQT, Zhi-Zi (ZZ; Fructus Gardeniae; Gardenia jasminoides J. Ellis) (Uddin et al., 2014), and DGLHuiW. Gallic acid also suppresses inflammatory and osteoclast activities (Karatas and Gevrek, 2020). Interestingly, herbal extracts of HP suppresses osteoclastogenesis and bone resorption (Shim et al., 2015). Apigenin (also known as 5,7,4′-Trihydroxyflavone) is one of the natural compounds of Bo-He (BH; Herba Menthae Haplocalycis; Mentha arvensis L.) (Xu et al., 2017), and it is a component of CXCTS, Chen-Pi (CP; Pericarpium Citri Reticulatae; Citrus reticulata Blanco) (Brito et al., 2014), JWPWS, HQin (Wang et al., 2018c), HQT, and DGLHuiW. It also inhibits osteoclastogenesis and prevents bone loss (Goto et al., 2015). Caffeic acid is one of the natural compounds of CX (Li et al., 2012), and it is a component of CXCTS, JWPWS, RS (Becker et al., 2015), LHT, DG (Li et al., 2009), DGLHuiW, and GC (Conidi et al., 2019). Caffeic acid shows suppresses bone destruction (Tang et al., 2006). Genistein is one of the natural compounds of ZZ (Wang et al., 2018a), and it is a component of DGLHuiW; it has anti-inflammatory and anti-osteoclastic properties (Lee et al., 2014; Bhattarai et al., 2017). Glycyrrhizic acid is one of the natural compounds of GC (Hennell et al., 2008), and it is a component of CXCTS, LHT, JWPWS, HQT; it suppresses osteoclast differentiation (Yin et al., 2019). Quercetin is one of the natural compounds of DG (Yue et al., 2017), and it is a component of DGLHuiW and GC (Jia et al., 1990). Quercetin induces the apoptosis of osteoclasts, inhibits bone loss, and attenuates cell signaling of tumor necrosis factor receptor family (Pang et al., 2006; Tsuji et al., 2009; Guo et al., 2012).
In this study, the limitations were the lacks of laboratory tests, education, occupation, and lifestyle in the database. However, we found that CHM may reduce risk of overall mortality in patients with osteoporosis or fractures, and may be useful for future investigations in randomized controlled trials (RCT) and functional studies in bone protection. Large-scale RCTs for these CHMs in HIV-infected patients should be performed to determine their relative effectiveness and safety, and to evaluate their potential interactions during regular treatments in these patients.
HIV-infected patients with osteoporosis or fractures who used CHMs as adjunctive therapy had a better survival rate. Based on association rules mining and network analysis, CXCTS, GC, LHT, HQT, JWPWS, and DGLHuiW are potential CHMs for these patients. Further investigations may be undertaken to validate the safety and efficacy of CHMs among these patients. An investigation into the mechanism of actions of the potential compounds of CHMs are required.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The study was approved by the Institutional Review Board of the China Medical University Hospital (The ethics approval number: CMUH107-REC3-074(CR1)). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Y-JL, M-WH, and J-PL wrote the manuscript and interpreted the data. C-JC, J-SC, M-LC, C-FC, T-ML, Y-CW, T-HL, C-CL, S-MH, Y-NL, and C-HC collected, assembled, and analyzed the data. F-JT and Y-JL provided study materials. W-ML and Y-JL designed, conceived the study, and amended the manuscript.
This study was supported by grants from China Medical University CMU109-S-18, CMU109-S-27, CMU109-MF-41, and CMU109-MF-126, China Medical University Hospital DMR-110-134 and DMR-110-152, and the Ministry of Science and Technology, Taiwan MOST 108-2314-B-039-044-MY3, MOST 109-2320-B-039-035-MY3, and MOST 109-2410-H-039-002.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the Health Data Science Center at the China Medical University Hospital for providing administrative, technical, and funding support. The funding entities for this study had no roles in the study design, data collection, data analysis, interpretation, or authorship of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.593434/full#supplementary-material.
Achhra, A. C., Nugent, M., Mocroft, A., Ryom, L., and Wyatt, C. M. (2016). Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr. Hiv/aids Rep. 13, 149–157. doi:10.1007/s11904-016-0315-y
Ahmad, A. N., Ahmad, S. N., and Ahmad, N. (2017). HIV infection and bone abnormalities. Toorthj. 11, 777–784. doi:10.2174/1874325001711010777
Amiel, C., Ostertag, A., Slama, L., Baudoin, C., N'guyen, T., Lajeunie, E., et al. (2004). BMD is reduced in HIV-infected men irrespective of treatment. J. Bone Miner Res. 19, 402–409. doi:10.1359/JBMR.0301246
Antiretroviral Therapy Cohort Collaboration (2017). Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 4, e349–e356. doi:10.1016/S2352-3018(17)30066-8
Arnsten, J. H., Freeman, R., Howard, A. A., Floris-Moore, M., Lo, Y., and Klein, R. S. (2007). Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 21, 617–623. doi:10.1097/qad.0b013e3280148c05
Becker, L. C., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Liebler, D. C., et al. (2015). Safety assessment of Panax spp root-derived ingredients as used in cosmetics. Int. J. Toxicol. 34, 5S–42S. doi:10.1177/1091581815610508
Bhattarai, G., Poudel, S. B., Kook, S.-H., and Lee, J.-C. (2017). Anti-inflammatory, anti-osteoclastic, and antioxidant activities of genistein protect against alveolar bone loss and periodontal tissue degradation in a mouse model of periodontitis. J. Biomed. Mater. Res. 105, 2510–2521. doi:10.1002/jbm.a.36109
Brito, A., Ramirez, J., Areche, C., Sepúlveda, B., and Simirgiotis, M. (2014). HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules. 19, 17400–17421. doi:10.3390/molecules191117400
Bruera, D., Luna, N., David, D. O., Bergoglio, L. M., and Zamudio, J. (2003). Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS. 17, 1917–1923. doi:10.1097/00002030-200309050-00010
Capeau, J., Bouteloup, V., Katlama, C., Bastard, J.-P., Guiyedi, V., Salmon-Ceron, D., et al. (2012). Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 26, 303–314. doi:10.1097/qad.0b013e32834e8776
Chen, H.-Y., Lin, Y.-H., Su, I. H., Chen, Y.-C., Yang, S.-h., and Chen, J.-l. (2014). Investigation on Chinese herbal medicine for primary dysmenorrhea: implication from a nationwide prescription database in Taiwan. Complement. Therapies Med. 22, 116–125. doi:10.1016/j.ctim.2013.11.012
Chen, K. M., Ge, B. F., Ma, H. P., Liu, X. Y., Bai, M. H., and Wang, Y. (2005). Icariin, a flavonoid from the herb Epimedium enhances the osteogenic differentiation of rat primary bone marrow stromal cells. Pharmazie. 60, 939–942.
Cheng, C.-F., Lin, J. C-F., Tsai, F.-J., Chen, C.-J., Chiou, J.-S., Chou, C.-H., et al. (2019a). Protective effects and network analysis of natural compounds obtained from Radix dipsaci, Eucommiae cortex, and Rhizoma drynariae against RANKL-induced osteoclastogenesis in vitro. J. Ethnopharmacology. 244, 112074. doi:10.1016/j.jep.2019.112074
Cheng, C. F., Lin, Y. J., Tsai, F. J., Li, T. M., Lin, T. H., Liao, C. C., et al. (2019b). Effects of Chinese herbal medicines on the risk of overall mortality, readmission, and reoperation in hip fracture patients. Front. Pharmacol. 10, 629. doi:10.3389/fphar.2019.00629
Chisati, E. M., Constantinou, D., and Lampiao, F. (2020a). Effects of maximal strength training on bone mineral density in people living with HIV and receiving anti-retroviral therapy: a pilot study. BMC Sports Sci. Med. Rehabil. 12, 67. doi:10.1186/s13102-020-00216-6
Chisati, E. M., Constantinou, D., and Lampiao, F. (2020b). Reduced bone mineral density among HIV infected patients on anti-retroviral therapy in Blantyre, Malawi: prevalence and associated factors. PLoS One. 15, e0227893. doi:10.1371/journal.pone.0227893
Chow, S. P., Yeung, H. W., Law, L. K., Chan, T. M., and Lau, C. (1982). The effect of davallina orientalis on bone healing—a preliminary report. Am. J. Chin. Med. 10, 101–106. doi:10.1142/s0192415x82000166
Chung, I.-M., Lim, J.-J., Ahn, M.-S., Jeong, H.-N., An, T.-J., and Kim, S.-H. (2016). Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 40, 68–75. doi:10.1016/j.jgr.2015.05.006
Conidi, C., Fuca, L., Drioli, E., and Cassano, A. (2019). A membrane-based process for the recovery of glycyrrhizin and phenolic compounds from licorice wastewaters. Molecules. 24, 2279. doi:10.3390/molecules24122279
De Wit, S., Sabin, C. A., Weber, R., Worm, S. W., Reiss, P., Cazanave, C., et al. (2008). Data collection on adverse events of AntiIncidence and risk factors for new-onset diabetes in HIV-infected patients: the data collection on adverse events of anti-HIV drugs (D:A:D) study. Diabetes Care. 31, 1224–1229. doi:10.2337/dc07-2013
Dorjee, K., Baxi, S. M., Reingold, A. L., and Hubbard, A. (2017). Risk of cardiovascular events from current, recent, and cumulative exposure to abacavir among persons living with HIV who were receiving antiretroviral therapy in the United States: a cohort study. BMC Infect. Dis. 17, 708. doi:10.1186/s12879-017-2808-8
Doss, H. M., Samarpita, S., Ganesan, R., and Rasool, M. (2018). Ferulic acid, a dietary polyphenol suppresses osteoclast differentiation and bone erosion via the inhibition of RANKL dependent NF-κB signalling pathway. Life Sci. 207, 284–295. doi:10.1016/j.lfs.2018.06.013
Dubrocq, G., and Rakhmanina, N. (2018). Antiretroviral therapy interruptions: impact on HIV treatment and transmission. HIV AIDS (Auckl). Vol. 10, 91–101. doi:10.2147/hiv.s141965
Duvivier, C., Kolta, S., Assoumou, L., Ghosn, J., Rozenberg, S., Murphy, R. L., et al. (2009). Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 23, 817–824. doi:10.1097/qad.0b013e328328f789
Giacomelli, N., Yongping, Y., Huber, F. K., Ankli, A., and Weckerle, C. S. (2017). Angelica sinensis (Oliv.) Diels: influence of value chain on quality criteria and marker compounds ferulic acid and Z-ligustilide. Medicines (basel). 4, 14. doi:10.3390/medicines4010014
Goto, T., Hagiwara, K., Shirai, N., Yoshida, K., and Hagiwara, H. (2015). Apigenin inhibits osteoblastogenesis and osteoclastogenesis and prevents bone loss in ovariectomized mice. Cytotechnology. 67, 357–365. doi:10.1007/s10616-014-9694-3
Grant, P. M., Kitch, D., Mccomsey, G. A., Collier, A. C., Koletar, S. L., Erlandson, K. M., et al. (2016). Long-term bone mineral density changes in antiretroviral-treated HIV-infected individuals. J. Infect. Dis. 214, 607–611. doi:10.1093/infdis/jiw204
Guo, C., Hou, G.-q., Li, X.-d., Xia, X., Liu, D.-x., Huang, D.-y., et al. (2012). Quercetin triggers apoptosis of lipopolysaccharide (LPS)-induced osteoclasts and inhibits bone resorption in RAW264.7 cells. Cell Physiol Biochem. 30, 123–136. doi:10.1159/000339052
Han, M. H., Yang, X. W., Zhong, G. Y., and Zhang, M. (2007). [Bioactive constituents inhibiting TNF-alpha production in fresh rhizome of Pinellia ternata]. Zhongguo Zhong Yao Za Zhi. 32, 1755–1759.
He, X., and Shen, Q. (2014). Salvianolic acid B promotes bone formation by increasing activity of alkaline phosphatase in a rat tibia fracture model: a pilot study. BMC Complement. Altern. Med. 14, 493. doi:10.1186/1472-6882-14-493
Hennell, J. R., Lee, S., Khoo, C. S., Gray, M. J., and Bensoussan, A. (2008). The determination of glycyrrhizic acid in Glycyrrhiza uralensis Fisch. ex DC. (Zhi Gan Cao) root and the dried aqueous extract by LC-DAD. J. Pharm. Biomed. Anal. 47, 494–500. doi:10.1016/j.jpba.2008.01.037
Hoy, J. F., Grund, B., Roediger, M., Schwartz, A. V., Shepherd, J., Avihingsanon, A., et al. (2017). Immediate initiation of antiretroviral therapy for HIV infection accelerates bone loss relative to deferring therapy: findings from the START bone mineral density substudy, a randomized trial. J. Bone Miner Res. 32, 1945–1955. doi:10.1002/jbmr.3183
Hoy, J., and Young, B. (2016). Do people with HIV infection have a higher risk of fracture compared with those without HIV infection?. Curr. Opin. HIV AIDS 11, 301–305. doi:10.1097/coh.0000000000000249
Hsiao, H. B., Wu, J. B., and Lin, W. C. (2017). (-)-Epicatechin 3-O-beta-D-allopyranoside prevent ovariectomy-induced bone loss in mice by suppressing RANKL-induced NF-kappaB and NFATc-1 signaling pathways. BMC Complement. Altern. Med. 17, 245. doi:10.1186/s12906-017-1737-9
Ji, M.-X., and Yu, Q. (2015). Primary osteoporosis in postmenopausal women. Chronic Dis. Translational Med. 1, 9–13. doi:10.1016/j.cdtm.2015.02.006
Jia, S. S., Ma, C. M., and Wang, J. M. (1990). Studies on flavonoid constituents isolated from the leaves of Glycyrrhiza uralensis Fisch. Yao Xue Xue Bao 25, 758–762.
Karatas, O., and Gevrek, F. (2020). 3,4,5-Trihydroxybenzoic acid attenuates ligature-induced periodontal disease in Wistar rats. Antiinflamm Antiallergy Agents Med. Chem. 20, 51-60. doi:10.2174/1871523019666200206094335
Kling, J. M., Clarke, B. L., and Sandhu, N. P. (2014). Osteoporosis prevention, screening, and treatment: a review. J. Women’s Health 23, 563–572. doi:10.1089/jwh.2013.4611
Kwong, G. P., Ghani, A. C., Rode, R. A., Bartley, L. M., Cowling, B. J., Da Silva, B., et al. (2006). Comparison of the risks of atherosclerotic events versus death from other causes associated with antiretroviral use. AIDS. 20, 1941–1950. doi:10.1097/01.aids.0000247115.81832.a1
Lee, H.-P., Chen, P.-C., Wang, S.-W., Fong, Y.-C., Tsai, C.-H., Tsai, F.-J., et al. (2019). Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. J. Funct. Foods. 52, 537–544. doi:10.1016/j.jff.2018.11.040
Lee, S.-H., Kim, J.-K., and Jang, H.-D. (2014). Genistein inhibits osteoclastic differentiation of RAW 264.7 cells via regulation of ROS production and scavenging. Int. J. Mol. Sci. 15, 10605–10621. doi:10.3390/ijms150610605
Lewiecki, E. M. (2010). Bisphosphonates for the treatment of osteoporosis: insights for clinicians. Ther. Adv. Chronic Dis. 1, 115–128. doi:10.1177/2040622310374783
Li, W., Tang, Y., Chen, Y., and Duan, J.-A. (2012). Advances in the chemical analysis and biological activities of chuanxiong. Molecules. 17, 10614–10651. doi:10.3390/molecules170910614
Li, X.-d., Liu, Z.-y., Chang, B., Liu, D.-x., Chen, B., Guo, C., et al. (2011). Panax notoginseng saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK and P38 MAPK signaling pathways. Cel Physiol Biochem. 28, 367–376. doi:10.1159/000331753
Li, X., Wu, X., and Huang, L. (2009). Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui). Molecules. 14, 5349–5361. doi:10.3390/molecules14125349
Lin, Y. J., Ho, T. J., Yeh, Y. C., Cheng, C. F., Shiao, Y. T., Wang, C. B., et al. (2015). Chinese herbal medicine treatment improves the overall survival rate of individuals with hypertension among type 2 diabetes patients and modulates in vitro smooth muscle cell contractility. PLoS One. 10, e0145109. doi:10.1371/journal.pone.0145109
Liu, J., Chen, L., Fan, C. R., Li, H., Huang, M. Q., Xiang, Q., et al. (2015). Qualitative and quantitative analysis of major constituents of Paeoniae radix Alba and Paeoniae radix rubra by HPLC-DAD-Q-TOF-MS/MS. Zhongguo Zhong Yao Za Zhi. 40, 1762–1770.
Lu, D.-Y., Wu, H.-Y., Yarla, N. S., Xu, B., Ding, J., and Lu, T.-R. (2018). HAART in HIV/AIDS treatments: future trends. Infect Disord Drug Targets. 18, 15–22. doi:10.2174/1871526517666170505122800
Lu, Y., Joerger, R., and Wu, C. (2011). Study of the chemical composition and antimicrobial activities of ethanolic extracts from roots of Scutellaria baicalensis Georgi. J. Agric. Food Chem. 59, 10934–10942. doi:10.1021/jf202741x
Lv, G., Cheng, S., Chan, K., Leung, K. S., and Zhao, Z. (2010). [Determination of free ferulic acid and total ferulic acid in Chuanxiong by high-performance liquid chromatography for quality assessment]. Zhongguo Zhong Yao Za Zhi. 35, 194–198. doi:10.4268/cjcmm20100217
Ma, C., Zhang, J., Fu, J., Cheng, L., Zhao, G., and Gu, Y. (2011). Up-regulation of VEGF by MC3T3-E1 cells treated with curculigoside. Phytother. Res. 25, 922–926. doi:10.1002/ptr.3449
Meintjes, G., Moorhouse, M. A., Carmona, S., Davies, N., Dlamini, S., Van Vuuren, C., et al. (2017). Adult antiretroviral therapy guidelines 2017. South. Afr. J. HIV Med. 18, 776. doi:10.4102/sajhivmed.v18i1.776
Mukwaya, E., Xu, F., Wong, M.-S., and Zhang, Y. (2014). Chinese herbal medicine for bone health. Pharm. Biol. 52, 1223–1228. doi:10.3109/13880209.2014.884606
Nan, C., Shaefer, M., Urbaityte, R., Oyee, J., Hopking, J., Ragone, L., et al. (2018). Abacavir use and risk for myocardial infarction and cardiovascular events: pooled analysis of data from clinical trials. Open Forum Infect. Dis. 5, ofy086. doi:10.1093/ofid/ofy086
Pang, J. L., Ricupero, D. A., Huang, S., Fatma, N., Singh, D. P., Romero, J. R., et al. (2006). Differential activity of kaempferol and quercetin in attenuating tumor necrosis factor receptor family signaling in bone cells. Biochem. Pharmacol. 71, 818–826. doi:10.1016/j.bcp.2005.12.023
Pazianas, M., Van Der Geest, S., and Miller, P. (2014). Bisphosphonates and bone quality. Bonekey Rep. 3, 529. doi:10.1038/bonekey.2014.24
Pham, H. T., and Mesplede, T. (2018). The latest evidence for possible HIV-1 curative strategies. Drugs Context. 7, 212522. doi:10.7573/dic.212522
Sagar, T., Rantlha, M., Kruger, M. C., Coetzee, M., and Deepak, V. (2016). Ferulic acid impairs osteoclast fusion and exacerbates survival of mature osteoclasts. Cytotechnology. 68, 1963–1972. doi:10.1007/s10616-016-0009-8
Shih, W. T., Yang, Y. H., and Chen, P. C. (2012). Prescription patterns of Chinese herbal products for osteoporosis in taiwan: a population-based study. Evid. Based Complement. Alternat Med. 2012, 752837. doi:10.1155/2012/752837
Shim, K.-S., Kim, T., Ha, H., Lee, C.-J., Lee, B., Kim, H. S., et al. (2015). Water extract of Magnolia officinalis cortex inhibits osteoclastogenesis and bone resorption by downregulation of nuclear factor of activated T cells cytoplasmic 1. Integr. Med. Res. 4, 102–111. doi:10.1016/j.imr.2015.02.002
Sozen, T., Ozisik, L., and Calik Basaran, N. (2017). An overview and management of osteoporosis. Eur. J. Rheumatol. 4, 46–56. doi:10.5152/eurjrheum.2016.048
Tang, Q. Y., Kukita, T., Ushijima, Y., Kukita, A., Nagata, K., Sandra, F., et al. (2006). Regulation of osteoclastogenesis by Simon extracts composed of caffeic acid and related compounds: successful suppression of bone destruction accompanied with adjuvant-induced arthritis in rats. Histochem. Cel Biol. 125, 215–225. doi:10.1007/s00418-005-0062-4
Tsai, F.-J., Cheng, C.-F., Chen, C.-J., Lin, C.-Y., Wu, Y.-F., Li, T.-M., et al. (2019). Effects of Chinese herbal medicine therapy on survival and hepatic outcomes in patients with hepatitis C virus infection in Taiwan. Phytomedicine. 57, 30–38. doi:10.1016/j.phymed.2018.09.237
Tsai, F.-J., Cheng, C.-F., Lai, C.-H., Wu, Y.-C., Ho, M.-W., Wang, J.-H., et al. (2017). Effect of antiretroviral therapy use and adherence on the risk of hyperlipidemia among HIV-infected patients, in the highly active antiretroviral therapy era. Oncotarget. 8, 106369–106381. doi:10.18632/oncotarget.22465
Tsuji, M., Yamamoto, H., Sato, T., Mizuha, Y., Kawai, Y., Taketani, Y., et al. (2009). Dietary quercetin inhibits bone loss without effect on the uterus in ovariectomized mice. J. Bone Miner Metab. 27, 673–681. doi:10.1007/s00774-009-0088-0
Uddin, R., Saha, M. R., Subhan, N., Hossain, H., Jahan, I. A., Akter, R., et al. (2014). HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv. Pharm. Bull. 4, 273–281. doi:10.5681/apb.2014.040
Van Vonderen, M. G., Lips, P., Van Agtmael, M. A., Hassink, E. A., Brinkman, K., Geerlings, S. E., et al. (2009). First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS. 23, 1367–1376. doi:10.1097/qad.0b013e32832c4947
Wang, X., Wang, G. C., Rong, J., Wang, S. W., Ng, T. B., Zhang, Y. B., et al. (2018a). Identification of steroidogenic components derived from Gardenia jasminoides ellis potentially useful for treating postmenopausal syndrome. Front. Pharmacol. 9, 390. doi:10.3389/fphar.2018.00390
Wang, Z.-L., Wang, S., Kuang, Y., Hu, Z.-M., Qiao, X., and Ye, M. (2018c). A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 56, 465–484. doi:10.1080/13880209.2018.1492620
Wang, Z., Wang, D., Yang, D., Zhen, W., Zhang, J., and Peng, S. (2018b). The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 29, 535–544. doi:10.1007/s00198-017-4255-1
Weivoda, M. M., Chew, C. K., Monroe, D. G., Farr, J. N., Atkinson, E. J., Geske, J. R., et al. (2020). Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nat. Commun. 11, 87. doi:10.1038/s41467-019-14003-6
Wong, K.-C., Pang, W.-Y., Wang, X.-L., Mok, S.-K., Lai, W.-P., Chow, H.-K., et al. (2013). Drynaria fortunei-derived total flavonoid fraction and isolated compounds exert oestrogen-like protective effects in bone. Br. J. Nutr. 110, 475–485. doi:10.1017/s0007114512005405
Xi, H.-R., Ma, H.-P., Yang, F.-F., Gao, Y.-H., Zhou, J., Wang, Y.-Y., et al. (2018). Total flavonoid extract of Epimedium herb increases the peak bone mass of young rats involving enhanced activation of the AC10/cAMP/PKA/CREB pathway. J. Ethnopharmacol. 223, 76–87. doi:10.1016/j.jep.2018.05.023
Xiang, M.-X., Su, H.-W., Hu, J., and Yan, Y.-J. (2011). Stimulative effects ofPolygonum amplexicaulevar.sinenseon osteoblastic MC3T3-E1 cells. Pharm. Biol. 49, 1091–1096. doi:10.3109/13880209.2011.568507
Xu, L. L., Xu, J. J., Zhong, K. R., Shang, Z. P., Wang, F., Wang, R. F., et al. (2017). Analysis of non-volatile chemical constituents of Menthae Haplocalycis Herba by ultra-high performance liquid chromatography-high resolution mass spectrometry. Molecules. 22, 1756. doi:10.3390/molecules22101756
Yin, Z., Zhu, W., Wu, Q., Zhang, Q., Guo, S., Liu, T., et al. (2019). Glycyrrhizic acid suppresses osteoclast differentiation and postmenopausal osteoporosis by modulating the NF-κB, ERK, and JNK signaling pathways. Eur. J. Pharmacol. 859, 172550. doi:10.1016/j.ejphar.2019.172550
Yue, S. J., Xin, L. T., Fan, Y. C., Li, S. J., Tang, Y. P., Duan, J. A., et al. (2017). Herb pair Danggui-Honghua: mechanisms underlying blood stasis syndrome by system pharmacology approach. Sci. Rep. 7, 40318. doi:10.1038/srep40318
Zhang, G., Luk, B. T., Wei, X., Campbell, G. R., Fang, R. H., Zhang, L., et al. (2019). Selective cell death of latently HIV-infected CD4(+) T cells mediated by autosis inducing nanopeptides. Cell Death Dis. 10, 419. doi:10.1038/s41419-019-1661-7
Keywords: HIV, osteoporosis, fracture, overall mortality, Chinese herbal medicine, network analysis
Citation: Ho M-W, Li T-M, Li J-P, Chiou J-S, Chiu M-L, Chen C-J, Cheng C-F, Tsai F-J, Wu Y-C, Lin T-H, Liao C-C, Huang S-M, Lin Y-N, Chou C-H, Liang W-M and Lin Y-J (2021) Chinese Herbal Medicine Usage Reduces Overall Mortality in HIV-Infected Patients With Osteoporosis or Fractures. Front. Pharmacol. 12:593434. doi: 10.3389/fphar.2021.593434
Received: 10 August 2020; Accepted: 29 March 2021;
Published: 16 April 2021.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Jan Baak, Stavanger Health Research Norway, NorwayCopyright © 2021 Ho, Li, Li, Chiou, Chiu, Chen, Cheng, Tsai, Wu, Lin, Liao, Huang, Lin, Chou, Liang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Miin Liang, d21saWFuZ0BtYWlsLmNtdS5lZHUudHc=; Ying-Ju Lin, eWpsaW4ua2F0aEBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.