
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 05 March 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.593116
This article is part of the Research TopicTargeted Immunotherapy for CancerView all 23 articles
Though cancer therapeutics can successfully eradicate cancerous cells, the effectiveness of these medications is mostly restricted to several deleterious side effects. Therefore, to alleviate these side effects, antioxidant supplementation is often warranted, reducing reactive species levels and mitigating persistent oxidative damage. Thus, it can impede the growth of cancer cells while protecting the normal cells simultaneously. Moreover, antioxidant supplementation alone or in combination with chemotherapeutics hinders further tumor development, prevents chemoresistance by improving the response to chemotherapy drugs, and enhances cancer patients’ quality of life by alleviating side effects. Preclinical and clinical studies have been revealed the efficacy of using phytochemical and dietary antioxidants from different sources in treating chemo and radiation therapy-induced toxicities and enhancing treatment effectiveness. In this context, algae, both micro and macro, can be considered as alternative natural sources of antioxidants. Algae possess antioxidants from diverse groups, which can be exploited in the pharmaceutical industry. Despite having nutritional benefits, investigation and utilization of algal antioxidants are still in their infancy. This review article summarizes the prospective anticancer effect of twenty-three antioxidants from microalgae and their potential mechanism of action in cancer cells, as well as usage in cancer therapy. In addition, antioxidants from seaweeds, especially from edible species, are outlined, as well.
Oxygen is essential to aerobic life conditions and represents the main driving force for the maintenance of cell metabolism and viability. Simultaneously, oxygen also has a potential hazard due to its paramagnetic characteristics stimulating the formation of partially oxidized high reactive components, known as reactive oxygen species (ROS) (Francenia Santos-Sánchez et al., 2019). Though the metabolism of oxygen produces ROS in living organisms as by-products, they have a significant influence on cell signaling and redox homeostasis. Sometimes, ROS levels can be increased upon contacting with exogenous or endogenous sources, rendering a stress condition in the cell that is called oxidative stress. In such a state, the ROS level reaches a toxic threshold, and it manages to overcome the antioxidant system of the cell, thus escapes to elimination and remain in the cell. (Raza et al., 2017). These ROS give rise to negative oxidative stress that engenders some drastic changes in cellular function and metabolism through altering cellular signaling pathways, initiating genomic instability, or activating immunosuppression, which leads to carcinogenesis (Morry et al., 2017). Cancer cells are more sensitive to therapeutic drugs that produce excessive amounts of ROS or impair ROS scavenging capacity of cells, which provokes apoptosis (Mut-Salud et al., 2015).
Among a variety of treatments, chemotherapy remains the first choice of cancer treatment. Though drugs used in chemotherapy can successfully eliminate fast-growing cancerous tissues, these drugs can affect the mucous membranes of various organs. As a consequence, several side effects are noticed in cancer patients, such as anaphylaxis, a different type of cytopenia, toxicity to liver, heart, nephron, ear, and also nausea, vomiting, pain, diarrhea, alopecia, anorexia, cachexia, inflammation in mucous membranes, and asthenia (Oun et al.,2018). To compensate for these adverse effects, antioxidant supplements are often prescribed, which can help to ameliorate side effects while not affecting treatment efficacy (Ambrosone et al., 2019). Cancer survivors often consume vitamins or minerals supplements, natural plant-based products, or herbal medicines to alleviate the therapy-related side effects. The most common recommended antioxidants are vitamins, polyphenols, and carotenoids. Edible vegetables and fruits are an excellent reservoir of different antioxidant phytochemicals with varied antioxidant capacity and it has been recommended that intake of >400 g fruits and vegetables can prevent certain types of cancer (Miller and Snyder, 2012; Chester et al., 2019; Wall-Medrano and Olivas-Aguirre, 2020).
Besides these plant products, microalgae can be an excellent alternative producer of antioxidant compounds. Microalgae are often considered a mother lode of high value pharmaceutically important metabolites, like carotenoids, polyphenols, fatty acids, phycobiliproteins, vitamins, which are the outcomes of defense strategies of microalgae against stress factors (Chu, 2013). These bioactive compounds have proven antioxidant capability as well as in vitro and in vivo anticancer property as well. For example, microalgal tetraterpenoids are a good source of antioxidants and also have shown promising antitumor activity in different cell lines (Ferdous and Yusof, 2021). The activity of microalgal antioxidants is commensurate with or sometimes higher than that of plant or animal origin, which makes them a good supply of nutraceuticals for human health (Sansone and Brunet, 2019). Microalgae are getting more attention to exploit in pharmaceutical usage due to having a diverse and wide array of metabolites, accelerated growth rate, ability to grow to disregard the seasonal variation or extremity, not requiring cultivable land and supply of fresh water, and most importantly, not affect food crops (Khan et al., 2018). Microalgae and their metabolites, like astaxanthin, DHA are used popularly as a supplement. Chlorella and Spirulina are the two most commonly consumed healthy foods in the forms of powder, tablets, or capsules. Currently, Tetraselmis is joining the race, which is consumed as an antioxidant supplement. Microalgae-enriched food products are also a good source of nutraceuticals (Koyande et al., 2019). Additionally, seaweeds are also a good source of antioxidant molecules. Among these bioactive, fucoidans, phlorotannin, laminarin, and terpenoids are widely studied for their antioxidant activity (Gupta and Abu-Ghannam, 2011). Moreover, many Asian countries, like China, Indonesia, Japan, Korea, Malaysia, Thailand, and the Philippines, are the leading producers and consumers of edible seaweeds that contain these antioxidants in high amounts (Ferdouse et al., 2018).
However, antioxidant phytochemicals found in these algae have been claimed to exhibit chemo-preventive role in normal cells by suppressing radiation or chemotherapy-induced oxidative stress via activation of the antioxidant defense system in cells, prevention of ROS mediated genomic instability, and inhibition aberrant cell proliferation, metastasis, and angiogenesis. On top of these roles, in combination with chemotherapeutic agents, antioxidants can act as therapeutic agents. They can boost oxidative stress in tumor cells, disable transcription factors, switch on apoptosis-related signaling pathways, and impede signaling pathways involved in cell proliferation (Chikara et al., 2018). Nevertheless, there are still some controversies in the utilization of antioxidants in cancer therapy. This review clarifies reactive species as well as oxidative stress, and their roles in cancer development. Then, the classification and mode of action of antioxidants have been explained briefly. Finally, some well-known microalgal and seaweed antioxidants and their potential roles in cancer therapy are described.
Free radicals contain one or more unpaired electrons in their atoms’ outermost shell, which makes them strikingly reactive and more unstable. They are formed in our body naturally as byproducts during biological processes or from exogenous sources and can potentially harm cells. (Shrivastava et al., 2019). Free radicals are related to reactive oxygen species (ROS), reactive nitrogen species (RNS), reactive sulfur species (RSS), reactive carbonyl species (RCS), and reactive selenium species (RSeS) (Sies et al., 2017). These reactive species are continuously formed from endogenous and exogenous sources in our body. Endogenous sources comprise intracellular organelles, like peroxisomes, mitochondria, and extracellular components like inflammatory cells (macrophages, eosinophils, and neutrophils). On the other hand, exogenous sources include high ionizing radiation, environmental toxins (pollution, allergens, toxic metals like cadmium, lead, mercury, iron, arsenic, and pesticides, microorganisms, some drugs, cigarette smoke, alcohol, and dietary xenobiotics (Pizzino et al., 2017).
Among these reactive species, ROS are widely studied. ROS is generated in the cytosol by soluble cell components and cytosolic enzymes, on membranes of mitochondria, in the peroxisomes, in the endoplasmic reticulum, on the plasma membrane of the dysfunctional cells, and in the lysosomes (Di Meo et al., 2016). However, ROS is of two classes; one type consists of radicals with an unpaired electron in their outermost shell (superoxide anion, nitric oxide, hydroperoxyl, and peroxyl radicals, and hydroxyl radical); another class comprises non-radical ROS, and these ROS are without unpaired electron but still has the chemical reactivity, even can be changed to radical ROS, e.g., singlet oxygen, ozone, hydrogen peroxide, and hypochlorous acid (Chahal et al., 2018). In cell signaling, ROS can serve as secondary messengers, playing an essential role in a range of cellular processes by stimulating different signal transduction pathways that involve gene activation or cellular growth (Klaunig and Wang, 2018).
ROS reacting with nitric oxide gives rise to RNS and RSS, with thiols (Corpas and Barroso, 2015; Mut-Salud et al., 2015; Sies et al., 2017). RNS, nitrogen-containing oxidants, consist of nitric oxide (NO•) and nitrogen dioxide radical (NO2•), peroxynitrite (HNO3−), as well as other oxides of nitrogen. Similarly, reactive sulfur species (RSS) are sulfur-containing molecules, which include hydrogen sulfide (H2S), thiols (RSH), persulfides (RSSH), polysulfides, S-nitrosothiols (RSNO), hydrogen polysulfides, and sulfenic acids (RSOH), that have essential roles in the regulation of cellular systems (Xu et al., 2019).
As a notion in redox biology, the term oxidative stress has been mentioned for the first time in the book entitled “Oxidative Stress” in 1985. Oxidative stress (OS) occurs when there is a disproportion between generation and detoxification of RS by the biological system in cells (Di Meo et al., 2016). According to Helmut Sie, oxidative stress is “an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage.” Oxidative stress can exert two-sided actions, classified according to intensity, as oxidative eustress and oxidative distress. Low oxidant or reactive species exposure permits addressing particular targets for redox signaling, essential for maintaining normal physiology, which is called oxidative eustress. The basal level of OS augments the defense system through the expression of antioxidant compounds and proteins, yielding health benefits. Contrarily, excessive oxidant or RS challenge leads to disrupted redox signaling, causing deleterious effect, like macromolecular damage in intracellular organelles, inactivation of redox regulatory enzymes, or abnormal cellular proliferation and death, which is termed as oxidative distress (Niki, 2016; Go and Jones, 2017; Sies, 2020) (Figure 1). There are different types of oxidative stress which depend mainly on the generation source, such as nutritional, postprandial, photooxidative, radiation-induced, reductive, and nitroxidative, nitrosative, nitrative oxidative stress (Sies, 2019).
OS can play an important role in all phases of the oncogenic process (initiation, promotion, and progression), by activating different transcription factors, including nuclear factor (NF-κB), Nuclear factor erythroid 2-related factor 2 (Nrf2), hypoxia-inducible factor (HIF-1α), activator protein (AP), tumor protein (p53), β-catenin/Wnt signaling pathway, which helps in modulating the expression of immune and inflammatory-related genes and thus triggers carcinogenesis (Saed et al., 2017). Besides, ROS functions bidirectionally in cancer. It can be pro- and antitumorigenic. ROS can contribute to cancer development via a range of cancer signaling pathways, such as MAPK/AP-1/NF-κB, associated with cancer metastasis and angiogenesis. ROS can also trigger inflammation by activating NF-κB, AP-1, HIF-1a, growth factors, inflammatory cytokines, and chemokine. Conversely, elevated ROS level promotes oxidative stress-induced cancer cell death by triggering antitumorigenic signaling (Reczek and Chandel, 2017; Kashyap et al., 2019). Cancer cells always need to keep an elevated ROS level allowing the pro-tumorigenic cell signaling without inducing cell death. Moreover, the ROS scavenging mechanism is stimulated by tumor cells to maintain ROS levels below the cytotoxic level (Ilghami et al., 2020).
An increase in ROS has been implicated in enhanced cell growth, proliferation, survival and in the progression of carcinogenesis by regulating mitogen activated-protein kinase, protein kinase D (PKD) signaling pathways, transcription factors such as AP, NF-κB, HIF-1α and also through the negative regulation of phosphatases and protein tyrosine phosphatase 1B (PTP1B), epigenetic alterations in transcription factors and tumor suppressors, Nrf2 and p53, as well as by down-regulating the expression of E-cadherin tumor suppressor (Galadari et al., 2017; Moloney and Cotter, 2018).
ROS often act as mediators of DNA damage. When ROS accumulate cells through its overproduction, they are often associated with DNA interaction, producing ROS-interacting modification, such as inter-and intra-strand bindings or creating DNA-protein crosslinks, yielding altered gene expression. ROS cause DNA damage through oxidizing nucleoside bases and form DNA lesions, such as the formation of 8-oxo guanine, that generate DNA double-strand breaks (DSBs), if unrepaired. ROS accumulation creates mitochondrial DNA lesions, strand breaks, and finally, degradation. In addition, increased ROS through the activation of oncogenes influences the replication stress. ROS can oxidize dNTPs that can modify polymerase activity, breakdown of replication forks, and the formation of DSBs, which all together lead to genomic instability. Moreover, ROS induce activation of proteins associated with cell cycle checkpoint, leading to cell cycle arrest. Above all, these alterations of chromosomes give rise to genetic instability and ultimately lead to carcinogenesis (De Sá Junior et al., 2017; Srinivas et al., 2019).
Increased ROS engender cell cycle arrest, senescence, and apoptosis. Elevated intracellular ROS production promotes apoptosis via extrinsic or intrinsic pathways. Moreover, ROS trigger apoptosis by inactivating or enhancing the ubiquitination of anti-apoptotic protein, Bcl-2, and by reducing the levels of apoptosis regulator, Bax, and Bad. On the other hand, ROS can kill cancer cells through autophagy, an effective defense against OS damage. ROS cause inactivation of autophagy-related genes and can inhibit the negative regulator of autophagy (TORC1). ROS generated in the mitochondrial electron transport chain or by NADPH oxidases (NOXs), enhance necroptosis. Furthermore, tumor suppressor protein p53 causes cell death through ferroptosis (depends on intracellular iron) which is induced by increased ROS level (Perillo et al., 2020).
In metastasis, tumor cells are circulated from the primary site to other places in the body via blood and lymph. ROS can cause metastasis by inducing hypoxia-mediated MMPs (matrix metalloproteinases) and cathepsin expression. Increased ROS level may activate the MMP enzymes with the stimulation or modulation of a myriad number of tumor progression pathways or metastasis signaling pathways, respectively. Tumor migration can be caused by ROS providing that they are produced by activated growth factor receptors and integrin assembly and with the modulation of signaling kinases. ROS mediate FAK (cell motility controlling protein) activation, leading to cellular invasion. Moreover, ROS can activate the actin-binding protein, cofilin, and thus, help in cell migration.
However, metastasis can be induced by ROS by other mechanisms also, like proteolytic degradation of glycosaminoglycan (GAG) and other ECM components. An increased level of ROS can stabilize HIFα by impeding prolyl hydroxylases (PHDs) and, thus, VEGF (primary pro-angiogenic factor) activation, ultimately rendering angiogenesis and tumor progression (Galadari et al., 2017; Kashyap et al., 2019).
Chemoresistance is a primary cause of treatment ineffectiveness in cancer. P-glycoprotein (a transporter protein) is a multidrug resistance protein that involves the removal or efflux of several anticancer drugs from cancer cells. ROS can upregulate this protein, leading to chemoresistance and inhibiting cell death (Galadari et al., 2017).
Antioxidant was first defined by Halliwell et al., in 1989 as “any substance that, present in low concentrations compared to oxidizable substrates (carbohydrates, lipids, proteins or nucleic acids), significantly delays or inhibits the oxidation of the mentioned substrates” (Halliwell et al., 1992). The term ‘Antioxidant’ denotes that antioxidants are molecules that work against the activity of oxidants. Antioxidants can be defined as, chemicals that can inhibit or quench free radicals, that are formed as natural byproducts in the body during the biological process, and thus retarding oxidative damage (Chahal et al., 2018; Khurana et al., 2018).
Antioxidants, which are produced in our body through the metabolic process, are called endogenous antioxidants. Antioxidants can also be incorporated exogenously through foods and dietary supplements, which are called exogenous antioxidants. Besides, there is also another group of antioxidants that can be produced synthetically, which are widely used in the food industry (Mut-Salud et al., 2015).
Antioxidants can be classified based on their origin, activity, size, solubility, and mode of action (Figure 2).
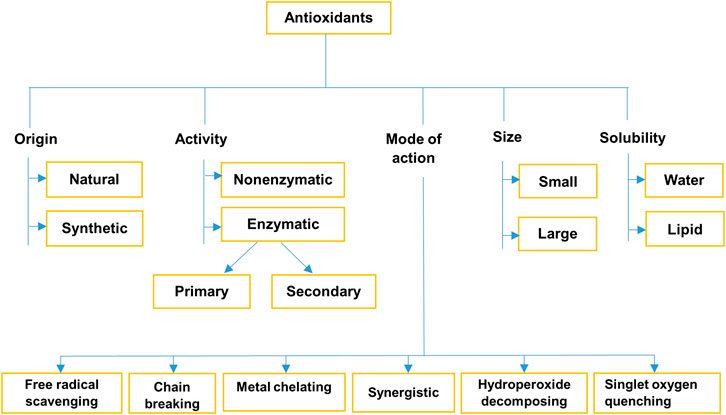
FIGURE 2. Classification of antioxidants (Nimse and Pal, 2015; Anwar et al., 2018; Chahal et al., 2018; Khurana et al., 2018; Azat Aziz et al., 2019).
Antioxidants give protection to the cells through three lines of defense. The first line of defense includes antioxidants hindering the formation of new free radicals. Enzymatic antioxidants such as SOD, CAT, GPx, and reduced glutathione; metal-binding proteins (ferritin and ceruloplasmin) and antioxidant minerals such as selenium, copper, and zinc. The second line comprises antioxidants, which are involved in scavenging free radicals, and thus preventing OS. Endogenous and exogenous antioxidants such as glutathione, albumin, CoQ, flavonoids, carotenoids, uric acid, and vitamins (A, C, and E) are involved in this group. Finally, different enzymatic antioxidants are the main player in the third line of defense, that repair the damaged DNA, intracellular protein, and other biomolecules. For example, DNA repair enzymes, proteases, peptidase, lipases, transferases, etc. (Surai et al., 2003; Mut-Salud et al., 2015).
Cancer is a term used for a cluster of analogous diseases that causes cells anywhere in the body commence to divide out of control and start proliferating in the surrounding or even distant tissues. It is the second-highest cause of mortality globally and accounts for approximately 9.6 million deaths in 2018 (WHO, 2020). Depending on the type of cancer and the malignancy, there is a range of cancer treatments, such as surgery, chemo-, radiation-, immuno-, targeted- and hormone therapy, stem cell transplant, or a combination of these therapies. Among them, chemotherapy remains the treatment of choice, integrated with surgery or other therapies. Commonly used chemotherapy drugs are the alkylating agents, anthracyclines (doxorubicin, daunorubicin, epirubicin, idarubicin, aclarubicin, and pirarubicin), epipodophyllotoxines, platinum-based drugs (cisplatin, carboplatin, and oxaliplatin), camptothecins, vinca alkaloids, taxanes, and antimetabolites, which are used for the treatment of a variety of cancers, such as breast, liver, ovarian, testicular, bladder, head and neck, lung cancer (He et al., 2018; Moiseeva, 2019; Ilghami et al., 2020). These drugs can cause more than 40 specific side effects and are broadly categorized into seven types, namely cardiotoxicity, hepatotoxicity, nephrotoxicity, ototoxicity, neurotoxicity, hematological toxicity, and gastrointestinal toxicity. (Oun et al., 2018). On the other hand, radiation therapy uses ionizing radiation to kill cells, by generating ROS, other organic radicals, and lipid peroxidation. Therefore, radiation induces an increase of free radicals which damage DNA and ultimately leads to cell death. This elevated ROS can affect the cellular antioxidant status as well (Mut-Salud et al., 2015; Ko and Formenti, 2019).
The goal of cancer treatment should be to kill cancer cells successfully and be attenuating therapy-induced genotoxicity in normal tissues and detoxifying harmful effects after treatment should be an additional goal of cancer treatment (Vilimanovich and Jevremovic, 2019). Therefore, antioxidant supplementation is often recommended to neutralize the effects of these chemotherapy drugs.
The usage of antioxidant supplements during cancer therapy can reduce oxidative damage in the surrounding healthy tissues, reduce side effects, and boost overall patient health and survival rate. (Calvani and Favre, 2019). These supplements can decrease cell growth, inhibit cell proliferation, and induce apoptosis in tumor cells. However, it has been estimated that 20–85% of cancer patients use antioxidant supplements, where the majority of consumers are breast cancer survivors. Also, patients with prostate, colorectal, and lung cancers prefer to take these supplements. When combined with certain types of chemotherapy, these nutraceuticals become more beneficial in treating cancer (Calvani et al., 2020).
Microalgal antioxidants are primarily composed of carotenoids, phenolics, flavonoids, polyunsaturated fatty acids, vitamins, sulfated polysaccharides, sterols, minerals, amino acids, phycobiliproteins as well as some other compounds like MAA, sulfolipids, Coenzyme Q, and peptides (Figure 3). From blue-green algae, antioxidant components like scytonemin, C-phycocyanin are also known as strong cytotoxic agents (Abd El-Hack et al., 2019). These phytochemicals have anti-cancerous properties as well (Table 1).
Vit A comprises retinol and its derivatives (retinoids). It is a collective term for many analogous compounds that can be classified into two groups based on the source. Vit A is derived from animal-based foods, such as beef liver, eggs, cod liver oil, butter, and yellow pigmented fruits, vegetables, and fortified grains, which are called preformed vitamin A. On the other hand, provitamin A (α- and β-carotene, β-cryptoxanthin) are available in colored fruits and vegetables, like in tomato, carrots, leafy greens, yams, and vegetable oils. This retinol is changed into retinoic acid and retinoids upon entering into the body (Fritz et al., 2011; Kim J. et al., 2019). Interestingly, some microalgae species contain Vit A which is much higher in amount compared to some fruits. For instance, Tetraselmis suecica contains Vit A (493,750 I.U./kg dry weight) in a higher amount than orange (14,728 I.U./kg dry weight). Isochrysis galbana, Dunaliella tertiolecta, Chlorella stigrnatophora, Chaetoceros calcitrans and Skeletonema costatum are also a rich source of Vit A and provitamin in comparison with other foods like cod liver oil, beef liver or parsley (Fabregas and Herrero, 1990; De Roeck-Holtzhauer et al., 1991). Cyanobacteria Aphanizomenon flos-aquae and Spirulina spp. are another reservoir of provitamin A (Kay and Barton, 1991). Chronopoulou et al., has reported that extraction of vit A from Tetradesmus Obliquus is in the highest amount through supercritical fluid extraction method (Chronopoulou et al., 2019).
Higher intake of dietary Vit A can remarkably decrease the ovarian, lung, gastric, pancreatic, and cervical carcinoma risk (Sanusi, 2019; Wang and He, 2020). Retinoic acid activates the extracellular-signal-regulated kinase (ERK) pathway and thus, promotes angiogenesis and metastasis in lung cancer. Retinoids in combination with chemotherapeutic drugs and other antioxidants inhibit cancer cell proliferation and thus increase the life span of cancer patients (Tripathi et al., 2019). Besides, natural and synthetic retinoids can prevent colorectal cancer progression (Abdel-Samad et al., 2019). Furthermore, retinol has a protective capacity against digestive cancers (Xie et al., 2019). Additionally, an increased dietary supplement of Vit A and β-carotene can improve hepatocellular carcinoma prognosis with an increased survival rate (Zhang et al., 2020).
Vit C can be obtained naturally in a variety of fruits, vegetables like green chili, thyme, parsley, guavas, black current, kiwis, lemon, and algae. It is commonly called ascorbic acid and is aqueous soluble (Padayatty and Levine, 2016). It is often considered a well-tolerated micronutrient. Vit C containing eleven microalgae species from different classes have been reported where Chaetoceros muelleri, Skeletonema costatum, Nannochloropsis oculata, and Nannochloris atomus showed higher amount of Vit C than others (Brown et al., 1997). Vitamin C is also commonly found in Spirulina spp., Chlorella spp., T. suecica, I. galbana, D. tertiolecta, Aph. flos-aquae, Pavlova lutheri, and Rhodomonas salina (Fabregas and Herrero, 1990; Kay and Barton, 1991; Brown et al., 1997).
However, Vit C can highly sensitize tumor cells compared to normal cells. Vit C acts as a prodrug by generating ascorbate radicals and H2O2, causing oxidative stress and ultimately kill cancer cells, which can be attained by the intravenous injection of Vit C. On the other hand, cancer cells can be damaged through epigenetic regulation, like DNA and histone demethylation, and reestablishing 5-hydroxymethylcytosine with oral administration of Vit C supplement. Moreover, Vit C supplementation can prevent tumor metastasis by collagen cross-linking, suppress cancer progression by HIF-1a degradation (Mustafi and Wang, 2019). It has been reported that higher doses of ascorbic acid alone or combinedly with conventional cancer drugs significantly impede cancer growth, but it should be administered intravenously. Oral administration of ascorbate causes only a moderate increase in its plasma concentration (Blaszczak et al., 2019).
In contrast, another study revealed that intravenous administration of Vit C of a lower dose with longer administration was better in treating cancer, though a high dose is still safe (Mikirova et al., 2019). Vit C can modulate infiltration of the tumor microenvironment by stimulating immune cells and delay cancer growth in breast, colorectal, melanoma, and pancreatic murine tumors (Magrì et al., 2020). Importantly, Vit C can kill cancer cells selectively and its activity depends on factors like the type of cancer and signaling pathways involved in the tumor development. In cancer stem cells, it can enter through sodium-dependent Vit C transporter 2 (SVCT2) and alter JHDM and TET protein. Besides it can enter via glucose transporters (GLUTs) and modify ROS, causing mitochondrial dysfunction and finally triggers Vit C-induced cell death (Satheesh et al., 2020). Vit C supplementation shows a protective effect in modulating inflammatory regulators in the case of esophageal adenocarcinoma (Abdel-Latif et al., 2019).
Vit D is also known as the “sunshine” vitamin since it can be gained through exposure to sunlight. Besides, this fat-soluble vitamin is available in foods like fishes rich in fat, egg yolk, dairy products like cheese, cod liver oil, beef liver, and mushrooms. Surprisingly, P. lutheri, T. suecica, S. costatum, and I. galbana can produce Vit D in a higher amount in comparison with cod liver oil, oyster, mushroom, egg, and liver (De Roeck-Holtzhauer et al., 1991). It has been reported that Vit D3 is found in the highest amount in UVB exposed Nannochloropsis oceanica (Ljubic et al., 2020).
In the liver, Vit D is metabolized into 25(OH)D (25-hydroxyvitamin D), which is a biomarker for Vit D status assessment (Marian, 2017). Studies showed that daily supplementation of Vit D is effective in improving relapse-free survival among digestive tract cancer patients and also in early-stage lung adenocarcinoma, with low bioavailable 25(OH)D levels (Akiba et al., 2018; Urashima et al., 2020). Besides, Vit D supplementation can reduce cancer-related mortality (Keum et al., 2019). Additionally, in the kidney, it is conceived that 25(OH)D can be converted to calcitriol by 1-alpha hydroxylase that can attach to Vit D specific receptors and has a significant effect on gene expression, and thus control cancer cell survival (Chatterjee et al., 2019).
Vit E, a lipid-soluble vitamin, is mainly found in nuts, seeds, vegetables, plant oils. Marine microalgae are an excellent source of Vit E and contain a larger amount of Vit D than other plant and animal sources. Studies showed that C. stigmatophora, C. calcitrans, P. lutheri, T. suecica, S. costatum, I. galbana, and D. tertiolecta possess ample amount of Vit D than olive oil, corn, bean, carrot, wheat or liver (Fabregas and Herrero, 1990; De Roeck-Holtzhauer et al., 1991). This Vit E can be classified into eight isoforms, namely α, β, δ, γ-tocopherol, and -tocotrienol (Peh et al., 2016). T. obliquus contains α and γ-tocopherol (Chronopoulou et al., 2019). Chlorella spp., Spirulina spp. and Aph. flos-aquae also have a significant level of Vit E (Kay and Barton, 1991; Kim et al., 2001).
Intake of vitamin E supplementation up to upper tolerable intake level (UL) of 300–1,000 mg/day is considered safe and effective in the reduction of mortality (Köpcke, 2019). Vitamin E supplementation has significant neuroprotective properties against cisplatin-induced ototoxicity (Villani et al., 2016) and also in cisplatin-induced nephrotoxicity, where a significant reduction in the serum levels of renal injury biomarker (NGAL) has been observed (Ashrafi et al., 2020). It has been reported that intake of high Vit E supplementation reduces total cancer and gastrointestinal cancer risk among patients with high selenium levels (Wang et al., 2019). Tocotrienols can selectively suppress cancer cells without harming the normal cells, where γ and δ tocotrienols have the highest anti-cancer activity. They can exert anti-cancer activity by inhibiting cell proliferation, arresting cell cycle, inhibition of angiogenesis by downregulation various growth factors, metastasis and inducing cell death (apoptosis, autophagy, and paraptosis) through different mechanisms that involve death receptor, caspase 9 activation, or Bax/Bcl ratio (Abraham et al., 2019; Constantinou et al., 2020). Besides, Vit E consumption reduces the risk of bladder cancer (Lin et al., 2019).
Vitamin K belongs to lipid-soluble vitamin, also known as ‘Koagulations vitamin,’ which is divided into two classes Vit K1 and K2, along with synthetic derivatives K3–K5. Vit K1 and K2 are also called phylloquinone and menaquinone, respectively, which are found in leafy vegetables, cheese, and curd (Kurosu and Begari, 2010). Vit K is also available in T. suecica, I. galbana, S. costatum, P. lutheri, Chlorella ellipsoidea, and T. obliquus where the level is significantly higher than milk, egg, or vegetables like spinach, cabbage (De Roeck-Holtzhauer et al., 1991; Kim et al., 2001; Chronopoulou et al., 2019).
Vit K and derivatives have been reported to exhibit anticancer property against cancer in the lung, liver, breast, prostate, blood, colon, and bladder. It can destroy cancer cells through several mechanisms, such as by increasing oxidative stress, by inducing apoptosis through the upregulation of Fas/FasL, NF-kB, p53, downregulating Bcl-2/Bcl-xl, Bax/Bak, and also through caspase-3 activation pathway, by inhibiting cell cycle through the inhibition of CDK-1 checkpoint and activation of CDK-1 inhibitors, p21. It can also induce autophagic death in different cancer cells (Dasari et al., 2017). Along with autophagy, Vit K2 can cause non-apoptotic cell death in breast cancer cell lines (Miyazawa et al., 2020). In combination with sorafenib, Vit K1 can cause apoptosis in hepatocellular carcinoma cells in vivo and in vitro through the activation of caspase pathways (Wei et al., 2010). In prostate cancer, Vit K2 has been reported to hinder metastasis and inducing apoptotic cell death (Vinjamuri et al., 2019).
Microalgae is a rich source of polyphenolic compounds that mainly consist of simple phenols, flavonoids, flavanones, isoflavone, flavonols, dihydroflavonols, flavones, flavan-3-ols, dihydrochalcones, proanthocyanidins. Among them, Flavones (Apigenin) and isoflavone (Genistein) have been reported to be found in P. tricornutum, Diacronema lutheri, P. purpureum, H. pluvialis, T. suecica, and C. vulgaris, while D. lutheri and H. pluvialis contain the most diverse classes of flavonoids (Goiris et al., 2014). In a study, Bulut et al., (2019), has assumed that flavonol (quercetin) from Scenedesmus sp., is one of the major contributors to its antioxidant property (Bulut et al., 2019). On the other hand, marine microalgae P. tricornutum, isolated from the Moroccan sea, produce protocatechuic acid which is considered to have antioxidant activity (Haoujar et al., 2019).
Euglena cantabrica having a high amount of phenolics (gallic acid and protocatechuic acid) shows the most effective radical scavenging activity which was even more than the conventional antioxidants (Jerez-Martel et al., 2017). Phenolic acids from Spirulina maxima displayed better radical scavenging activity and protection against microsomal lipid-peroxidation in the liver than commercial antioxidants (Abd El-Baky et al., 2009). Phenolic compounds are responsible for antioxidant activity tested for a myriad of microalgae, for instance, Nannochloropsis sp., Spirulina sp., D. salina, Navicula clavata, Chlorella sp., Tetraselmis sp., Porphyridium cruentum, P. tricornutum, Neochloris oleoabundans, C. calcitrans, Botryococcus braunii (Goiris et al., 2012; Hemalatha et al., 2013; Choochote et al., 2014; Zainoddin et al., 2018).
Along with antioxidant activity, these polyphenolic compounds exhibit anticarcinogenic activity as well. Jayshree et al., (2016), found that flavonoids, isolated from C. vulgaris as well as Chlamydomonas reinhardtii, were cytotoxic to breast cancer cells (Jayshree et al., 2016). Similarly, flavonoids in C. vulgaris extract can hinder proliferation in lung carcinoma (Wang et al., 2010). Spirulina maxima produce phenolic compounds that may stop proliferation and induce apoptosis in liver cancer cells (Wu et al., 2005). Likewise, phenolic compounds from C. vulgaris and I. galbana might be responsible for the anticancer activity against human liver cancer (Custódio et al., 2014; Raikar et al., 2018).
Polyphenols like quercetin, genistein, ellagic acid have significant anticancer properties. Genistein has displayed anticancer effects against breast, colon, lung, thyroid, gastric, and prostate cancers by modulating a variety of molecular targets, such as apoptotic markers caspases, Bcl and Bax, nuclear factor-κB, an inhibitor of NF-κB, mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase/Akt (PI3K/Akt), extracellular signal-regulated kinase 1/2 (ERK 1/2), and Wingless and integration 1/β-catenin (Wnt/ß-catenin) signaling pathway (Tuli et al., 2019). It can show pro-apoptotic, anti-proliferative, and anti-metastatic activities in vitro on PC3 prostate cancer cells through triggering apoptosis by activating caspase-3 related pathways, by reducing cell survival via inhibition of p38MAPK at both gene expression and protein levels, and by inhibiting metastasis through the blockage of MMP2 activity (Shafiee et al., 2020). Genistein is documented as clinically safe and effective when combined with standard fluoropyrimidine and platinum-based drug, oxaliplatin, with or without Bevacizumab, in the treatment of metastatic colon cancer (Pintova et al., 2019). Quercetin exerts its anti-cancer effects on different cancer cells through the regulation of PI3K/Akt/mTOR, Wnt-catenin, and MAPK/ERK1/2 pathways. It can induce tumor cell death by modulating the apoptotic pathway, enhancing the expression of pro-apoptotic proteins (Bax, Bad) as well as decreasing the expression level of anti-apoptotic proteins (Bcl, Mcl), and also affect the expression of TRAILR, FAS, TNFR1. Moreover, it hinders metastasis by reducing VEGF secretion, repressing the expression of the downstream regulatory factor AKT and MMP levels, and by inhibiting EMT progression. Furthermore, quercetin promotes protective autophagy in cancer cells by forming autophagic vacuoles and acidic vesicular organelles (AVOs), activating autophagic gens, and inhibiting Akt-mTOR signaling and stabilizing HIF-α expression (Reyes-Farias and Carrasco-Pozo, 2019; Tang et al., 2020). However, coadministration of sorafenib (0.1 µM) and quercetin 25 µM for 1 day has been exhibited a significant reduction in the cell proliferation rate and inhibition in cell adhesion and migration properties (Celano et al., 2020).
Another important phenolic compound in microalgae is ellagic acid (EA). EA can effectively reduce cisplatin (CP) induced nephrotoxicity and gonadotoxicity, by reducing peroxidative damage to tissue, when given together with CP to murine colon cancer model (Goyal et al., 2019). Moreover, EA in combination with doxorubicin and cisplatin can strongly hinder cell proliferation and engender mitochondria-mediated cell death in hepatocellular carcinoma cells in vitro and reduce side effects significantly (Zhong et al., 2019). In the multidrug-resistant glioma cells, EA combined with bevacizumab may show both inhibitory and suppressive role in bevacizumab-induced DNA repair, when treated for an extended period (Çetin et al., 2019).
β-Carotene (BC) is abundantly found in the human diet and popularly used as a food additive and coloring agent in the food industry (Bogacz-Radomska and Harasym, 2018). Microalgae Dunaliella salina possesses a copious amount of BC and is considered the richest source among other microalgae. BC from Dunaliella salina has been reported to kill human prostate cancer cells through apoptosis (Jayappriyan et al., 2013). Moreover, BC can be found readily in green microalgae Chlorella vulgaris, Asterarcys quadricellulare, and in cyanobacteria Spirulina sp. (Seshadri et al., 1991; Damergi et al., 2017; Singh et al., 2019).
BC suppresses the proliferation and self-renewal capacity of colon cancer stem cells (CSCs) through epigenetic modulation, involving expression of miRNAs and miRNA-mediated histone acetylation, and global DNA methylation (Kim D. et al., 2019). Though its negative relationship to lung cancer is widely studied, it can reduce lung cancer when combined with vitamin A (Yu et al., 2015). It has been documented that oral administration of beta-carotene-loaded solid lipid nanoparticles (BC-SLNs) enhances the bioavailability of BC and also the safety as well as the efficacy of BC. It sustains the release of BC from the lipid core and prolongs circulation time in the body (Jain et al., 2019). Besides, in methotrexate (MTX) therapy, BC loaded nanoparticles of zein (βC-NPs) significantly improve cellular uptake, reduces MTX-induced liver and kidney toxicity, and display elevated biopharmaceutical performance of BC orally (Jain et al., 2019).
Lutein is a carotenoid with a yellow-orange hue that is an important ingredient in the food, feed, and pharmaceutical industries. It is available in fruits, vegetables, and flowers, especially in marigold which is considered as a primary source (Becerra et al., 2020). Surprisingly, microalgae can produce up to six times higher lutein content compared to marigold and thus, is claimed to be a better alternative of lutein production (Lin et al., 2015). Lutein is produced at a higher amount in Chlorella protothecoides, C. sorokiniana, C. vulgaris, H. pluvialis, Parachlorella sp., Muriellopsis sp. and Scenedesmus obliquus (Li et al., 2001b; Shi et al., 2002; Blanco et al., 2007; Chan et al., 2013; Chen et al., 2016; Di Sanzo et al., 2018; Heo et al., 2018). Lutein from Botryococcus braunii has been reported to exhibit both in vitro and in vivo antioxidant activity (Rao et al., 2006).
Lutein augments the effect of the antiproliferation and apoptosis capacity of chemotherapy drugs and also can inhibit cell cycle progression, alone or combinedly with chemotherapy drugs, in the prostate cancer cell line. Moreover, lutein downregulates biomarker genes related to growth and survival in prostate cancer (Rafi et al., 2015). In another study, lutein has been shown anti-breast cancer activity by generating intracellular ROS level and also by inducing apoptotic cell death via downregulation of Bcl2 genes with the upregulation of pro-apoptotic genes and by enhancement of p53 signaling pathway. At the same time, lutein augments the anticancer activity of taxanes when administered combinedly in breast cancer cell lines (Gong et al., 2018). A similar result has been reported by Luan et al., (2018), where lutein plus doxorubicin hinders the growth of sarcoma cells, induces apoptosis, and also shows in vivo anti-tumor activity in a mouse model (Luan et al., 2018). Lutein also displays anti-proliferation activity in breast cancer cells by triggering the NrF2/ARE pathway and inactivating the NF-κB signaling pathway (Chang et al., 2018).
Astaxanthin (ATX), a red lipid-soluble xanthophyll carotenoid, is mostly available in microorganisms and has an important role in aquaculture, food, and pharmaceutical industries (Ambati et al., 2014). Haematococcus pluvialis is considered the finest production source of ATX industrially (Shah et al., 2016). This ATX from H. pluvialis hinders the oxidative stress inside the cells (Régnier et al., 2015). ATX is also obtained from other microalgae like C. sorokiniana, C. zofingiensi, Tetraselmis sp., Chlorococcum sp. and G. sulphuraria (Li et al., 2001a; Ip and Chen, 2005; Raman and Mohamad, 2012; Graziani et al., 2013).
ATX exhibits anti-proliferation activity against various cancer cells through blocking cell cycle at G0/G1 phase or G2-M phase, epigenetic alterations, or chromatin remodeling. It also induces apoptosis by downregulation of the antiapoptotic proteins while upregulation of the proapoptotic proteins. It also blocks angiogenesis and metastasis to distant tissues (Faraone et al., 2020). On combinatorial treatment with carbendazim, AXT potentiates the anti-proliferative effect of this drug by arresting MCF-7 cells at the G2/M phase (Atalay et al., 2019).
Fucoxanthin (FX) is an orange-hued marine carotenoid that is mainly obtained from algae. Fucoxanthin has many health benefits, especially antioxidative and antiproliferative capacity (Muthuirulappan and Francis, 2013). It has exhibited antitumor activity against a range of cancer types, namely osteosarcoma, leukemia, lymphoma, and also against colorectal, breast, prostate, hepatocellular, bladder cancer (Martin, 2015). Antioxidant activity of FX have been reported from Phaeodactylum tricornutum, Odontella aurita, I. galbana, C. calcitrans, D. salina, C. gracilis, Navicula sp., Thalassiosira sp., Pavlova lutheri, Cylindrotheca closterium (Rijstenbil, 2003; Xia et al., 2013; Neumann et al., 2019; Peraman and Nachimuthu, 2019). FX from P. tricornutum and C. calcitrans has been reported to show anticancer activity as well (Foo et al., 2018; Neumann et al., 2019). Furthermore, FX obtained from Conticribra weissflogii showed the anti-inflammatory property in the sepsis mouse model (Su et al., 2019). However, FX is also available in Nitzschia laevis, Chaetoceros muelleri, Amphora sp. and Tisochrysis lutea (Ishika et al., 2019; Sun et al., 2019; Mohamadnia et al., 2020).
The anticancer mechanism of FX is mainly directed by blocking the cell cycle at the G0/G1 phase with decreased cyclin D and also by apoptotic cell death with DNA degradation, chromatin condensation, or DNA laddering. FX also inhibits metastasis where a decreased level of MMPs has been observed. Besides, these mechanisms involved a myriad of pro-and anti-apoptotic proteins and many signaling pathways like caspase, PI3K/Akt/mTOR, JAK/STAT, MAPK, SAPK/JNK pathways (Kumar et al., 2013).
Zeaxanthin (ZX) is a yellow colored carotenoid and also found in orange or yellow colored fruits, vegetables, like corn, tangerine, squash, mango, honeydew, papaya, peach, yellow bell pepper, marigold, egg yolk, and in many microorganisms as well (Sajilata et al., 2008). On the other hand, ZX can be obtained from microalgae like in Synechocystis sp., Dunaliella salina, Chlorella saccharophila, C. ellipsoidea, C. pyrenoidosa, Scenedesmus almeriensis, S. obliquus, Porphyridium aerugineum, Microcystis aeruginosa, and Spirulina sp. (Lagarde et al., 2000; Chen et al., 2005; Inbaraj et al., 2006; Granado-Lorencio et al., 2009; Koo et al., 2012; Yu et al., 2012; Singh et al., 2013; El-Baz et al., 2019).
ZX from Nannochloropsis oculata, Scenedesmus obliquus, Porphyridium aerugineum has been reported to show the antioxidative property (Cho et al., 2011; Banskota et al., 2019). On the other hand, the anticancer activity of ZX has been reported in Porphyridium purpureum, where ZX induced apoptosis in cells of human melanoma through the augmentation of proapoptotic proteins (Bak, Bax) or pro-apoptotic factors (Bim, Bid) and the reduction of antiapoptotic proteins (Bcl-2), as well as through caspase 3 activation and DNA fragmentation. Moreover, ZX from this P. purpureum potentiates the efficacy of the chemotherapeutic drug, vemurafenib toward human melanoma (Juin et al., 2018). A similar apoptosis mechanism of ZX in melanoma cells was reported in another study as well (Bi et al., 2013).
Canthaxanthin (CTX), a ketocarotenoid, was found in Cantharellus cinnabarinus mushroom for the first time and now is gaining interest in the food and feed industry (De Miguel et al., 2001). This antioxidative and antitumorigenic CTX can be found in microalgae also. Microalgal species like Haematococcus pluvialis, Chlorella emersonii, C. zofingiensis. Coelastrella sp., Dactylococcus dissociates, Chlorococcum sp. and also in some cyanobacteria like Nodularia spumigena, Aphanizomenon flos-aqua, Trichormus variabilis, Anabaena sp. (Ben-Amotz, 1993; Malis et al., 1993; Li et al., 2006; Nobre et al., 2006; Hu et al., 2013; Grama et al., 2014; Janchot et al., 2019; Krajewska et al., 2019).
CTX showed anticancer activity by causing apoptosis in human colon adenocarcinoma as well as in melanoma cells (Palozza et al., 1998). Similarly, CTX from Aspergillus carbonarius has been reported to engender apoptosis in human prostate cancer cells (Kumaresan et al., 2008). Dietary intake of CTX has in vivo chemopreventive role in oral cancer (Tanaka et al., 1995).
Violaxanthin (VLX), an orange-hued carotenoid, is obtained mainly from fruits of similar color and also from leafy greens as well as microalgae. VLX has significant antioxidative activity. Yellow-green microalgae Eustigmatos cf. polyphem has been reported to produce VLX that has exhibited radical scavenging activity through DPPH and ABTS assays (Wang et al., 2018). Moreover, VLX has antiproliferative activity as well. VLX isolated from Dunaliella tertiolecta and Chlorella ellipsoidea has been revealed to inhibit breast cancer cells and colon cancer cells, respectively, and also induce apoptosis (Cha et al., 2008; Pasquet et al., 2011).
However, VLX from Chlorella vulgaris, N. oceanica, Dunaniella salina, Tetraselmis spp., Isochrysis galbana, Pavlova lutheri, P. salina, and Chaetoceros spp. has been reported to show antioxidative and anti-inflammatory activities (Soontornchaiboon et al., 2012; Ahmed et al., 2014; Kim H. et al., 2019; Kim et al., 2020).
Neoxanthin (NX), a pigment in spinach, is also available in microalgae. The antioxidative property of NX has been reported in Scenedesmus sp., Chlorella sp. and Tetraselmis suecica (Patias et al., 2017; Sansone et al., 2017). However, NX can also be isolated from Chlorella vulgaris, C. protothecoides, Ankistrodesmus gracilis, Scenedesmus quadricauda, Neochloris oleoabundans, Chlorella pyrenoidosa, Botryococcus braunii, Nephroselmis pyriformis (Tonegawa et al., 1998; Inbaraj et al., 2006; Magnusson et al., 2008; Chue et al., 2012).
NX has been reported to show anticancer activity against human prostate carcinoma and also responsible for the apoptosis in these cancer cells (Terasaki et al., 2007; Kotake-Nara et al., 2005; Kotake-Nara et al., 2001). In an animal model, NX exhibited anti-initiation activity and also hindered the promotion stage in tumor cells which was revealed through a two-step carcinogenesis study (Lin et al., 1995).
Ketocarotenoid siphonaxanthin (SPX) has been predominately found in microalgae and reported to show better anti-proliferative and anti-angiogenic activity than FX (Sugawara et al., 2014). For instance, SPX from green microalgae Codium fragile exhibited apoptosis in human leukemia cells through TRAIL induction with the augmentation of GADD45a and DR5 expression and reduced Bcl-2 and thus showed more effective anticancer property compared to FX (Ganesan et al., 2011). Similarly, this SPX displayed ex vivo antiangiogenic activity as well (Ganesan et al., 2010).
Cryptoxanthin is available in many microalgae like C. vulgaris, S. obliquus, Aphanothece microscopica Nageli, C. pyrenoidosa, C. zofingiensi, Chlamydomonas planctogloea, Selenastrum bibraianum, Coelastrum sphaericum, Parachlorella kessleri, Mougeotia sp., S. platensis, and P. cruentum (Jaime et al., 2005; Inbaraj et al., 2006; Patias et al., 2017; Di Lena et al., 2019; Soares et al., 2019). β-Cryptoxanthin obtained from Cyanophora paradoxa exerted cytotoxicity against human skin, breast, and lung cancer cells (Baudelet et al., 2013).
β-Cryptoxanthin blocks gastric cancer cells at the G0/G1 phase and induces apoptosis through caspase activation and Cyt C release (Gao et al., 2019). It also displayed anticancer property and apoptosis in HeLa cells (Gansukh et al., 2019). When combined with oxaliplatin, β-cryptoxanthin increased the potency of this chemotherapeutic drug and reduced its toxicity in colon carcinoma (Millán et al., 2015). Moreover, β-Cryptoxanthin hindered lung carcinoma both in vitro and in vivo experiments (Lian et al., 2006; Iskandar et al., 2016).
Omega-3 polyunsaturated fatty acids, mainly consisting of EPA, DHA as well as α-linolenic acid, is found predominately in fish oil, various plant sources (flaxseed, kiwifruit, chia), and in microalgae, which is effective in the treatment of a different form of cancers such as, breast, colorectal, prostate, ovarian, renal, liver, lung and some other types of cancer (Ashfaq et al., 2019). Microalgal fatty acids are frequently used as fish feed and also as a dietary supplement. EPA has been found in larger amounts in Chlorella minutissima, while α-linolenic acid in H. pluvialis and T. suecica (Rosa et al., 2005). In a study, DHA has been reported to be found in a high amount from Australian microalgae species Heterocapsa niei (Mansour et al., 2005). However, EPA and DHA are also obtained from Phaeodactylum sp., Thalassiosira sp., Skeletonema sp., Cryptomonas sp., Tetraselmis sp., Isochrysis sp., Nannochloropsis sp., Porphyridium sp., Chaetoceros sp. (Ryckebosch et al., 2012).
It has been reported that Omega-3 fatty acid supplementation with standard neoadjuvant cyclophosphamide, doxorubicin, and fluorouracil (CAF) chemotherapy and mastectomy improves overall survival and progression-free survival of locally advanced breast cancer patients, through decreasing expression levels of Ki-67 and VEGF leading to inhibition of proliferation and angiogenesis (Darwito et al., 2019). Higher intake of marine ω-3 polyunsaturated fatty acids (MO3PUFA) intake improves survival among stage III colon cancer patients with wild-type KRAS proto-oncogene and deficient DNA mismatch repair, which are responsible for tumor proliferation and survival (Song et al., 2019). Besides, co-supplementation of vitamin D and omega-3 fatty acids significantly reduces inflammatory biomarkers (TNF-a, IL-1b, IL-6, IL-8) and tumor marker, carcinoembryonic antigen in colorectal cancer patients (Haidari et al., 2020). It has been reported that omega-3 supplements can reduce cancer-related fatigue (CRF) in cancer patients under chemotherapy (Ansari et al., 2019). Though omega-3 polyunsaturated fatty acids (O3-PUFA) are widely known for reducing cancer-related fatigue, O6-PUFAs have been documented to significantly reduce CRF compared with O3-PUFA among breast cancer survivors (Peppone et al., 2019).
Microalgal is considered as an alternative source of producing some valuable commercial sterols like, β-sitosterol, stigmasterol, ergosterol, campesterol, and brassicasterol which have pharmaceuticals importance (Randhir et al., 2020). Sterols are found in Chlorella sp., Chlamydomonas sp., Scenedesmus sp., Ankistrodesmus sp., Nannochloropsis limnetica, Stephanodiscus hantzschii, Gomphonema parvulum, Cyclotella meneghiniana, Cryptomonas sp., Monoraphidium sp. (Martin-Creuzburg and Merkel, 2016). Along with antioxidative activity, microalgal sterols can show antitumor activity. A sterol-containing fraction of Nannochloropsis oculate exhibited anticancer property against human blood, lung, liver, and colon cancer cells (Sanjeewa et al., 2016). Similarly, fatty acid fractions of Nannochloropsis salina also showed cytotoxicity against breast cancer cells (Sayegh et al., 2016). Moreover, fatty acids from S. maxima have also been reported to show anticancer activity against breast cancer (Elkhateeb et al., 2020).
Sterols can stop tumor growth, metastasis, angiogenesis, and induce apoptosis through caspase-3 activation, Bax/Bcl2 enhancement, or blood cholesterol reduction (Ramprasath and Awad, 2015). Dietary intake of phytosterol can minimize the risk of cancer. For instance, β-sitosterol intake can hinder tumor growth in the human colon, lung, liver, prostate, and breast cancer cells (Jiang et al., 2019).
Microalgae is an excellent reservoir of polysaccharides that has different bioactivity, especially anti-inflammatory, antioxidant and anticancer. For instance, C. stigmatophora and P. tricornutum can produce polysaccharide extract with anti-inflammatory activity (Guzmán et al., 2003). Polysaccharides obtained from Tetraselmis spp., Pavlova viridis, Sarcinochrysis marina, Porphyridium sp. exhibited significant antioxidant activity revealed through antioxidant assays (Tannin-Spitz et al., 2005; Sun et al., 2014; Amna Kashif et al., 2018). In addition, polysaccharide extract of I. galbana and N. oculata has the antioxidant capacity and antiproliferative activity against HeLa cells (Hafsa et al., 2017). Nostoglycan, a polysaccharide isolated from Nostoc sphaeroides has been reported to give protection from oxidative stress, and also to stop the growth of lung cancer cells as well as to promote apoptosis through activation of the caspase-3 pathway (Li et al., 2018). Moreover, polysaccharide fraction of P. viridis displayed in vivo antitumor property (Sun et al., 2016).
An investigation on the exopolysaccharide-producing microalgae and cyanobacteria revealed that forty-five out of 166 strains were exopolysaccharide producers (Gaignard et al., 2019). Graesiella sp., isolated from Tunisian hot spring, possess EPS that have antioxidant activity and show cytotoxicity against human liver and colon cancer cells (Trabelsi et al., 2016). Similarly, C. pyrenoidosa, Chlorococcum sp., and Scenedesmus sp. produce EPS exhibiting antioxidative properties that also have the potential to kill human colon cancer cells (Zhang et al., 2019). On the other hand, sulfated polysaccharides (sPS) with antioxidant activity are extracted from Navicula sp. (Fimbres-Olivarria et al., 2018). sPS from Tribonema sp. showed antiproliferative and apoptosis in human hepatic carcinoma (Chen et al., 2019). P. cruentum having sPS showed in vitro and in vivo antitumor activity (Gardeva et al., 2009).
Phycobiliproteins, mainly composed of, phycocyanin, allophycocyanin, phycoerythrin phycoerythrocyanin, are light-harvesting colored protein found predominately in cyanobacteria and also in red algae. Phycobiliproteins have different bioactivities like, antioxidant, anti-inflammatory, anticancer, and others (Pagels et al., 2019). Phycocyanin (PC) plays a protective role against oxidative damage and exerts anticancer activity against different cancers. Arthrospira platensis produces PC which shows antioxidant activity revealed through DPPH assay (Pan-utai and Iamtham, 2019). PC isolated from Porphyra yezoensis exerted anticancer activity against human melanoma and laryngeal cancer cells in a dose-dependent way (Zhang et al., 2011). PC can block cell cycle at G0/G1 or G2/M phase and induce apoptosis through caspase 3 or 9 activations, reduction of Bcl-2/Bax, COX-2, p-ERK, PEG2, cyclin D1, and CDK4, DNA fragmentation, Cyt c release, ROS generation, reduction of NF-κB, Fas, p53, ICAM-1, CD44, Chromatin condensation. Moreover, PC also downregulates the genes involved in metastasis and angiogenesis. Besides, PC can promote autophagy through blocking Akt/mTOR/p70S6K pathways. Furthermore, PC can enhance the efficacy of chemotherapeutic drugs like doxorubicin, topotecan, betaine, when administered combinedly (Jiang et al., 2017).
Apart from these phycobiliproteins, microalgae also produce protein products, like whole-cell protein, protein hydrolysates, protein concentrates, and peptides which have different biological activities (Soto-Sierra et al., 2018). Microalgal peptides isolated from S. maxima, S. obliquus, and T. suecica have been reported to exert anti-inflammatory, antioxidant, and antimicrobial activity, respectively (Vo et al., 2013; Montone et al., 2018; Guzmán et al., 2019).
There is evidence that cancer is related to the interference in amino acid kinetics, which is indicated by an imbalance between plasma amino acids and a higher rate of whole-body turnover of protein and muscle protein breakdown, thus leads to muscle damage. Therefore, increased amino acid supplementation is recommended to promote the synthesis of muscle protein (van der Meij et al., 2019). Supplementation with branched-chain amino acids (BCAA) can control protein synthesis by triggering the mTORC1 pathway which promotes muscle protein balance. Amino acids like arginine and glutamine improve nutritional status in cancer patients undergoing surgery, chemotherapy, and radiotherapy by minimizing inflammation (Soares et al., 2020). In NSCLC, AAs suppress inflammation by increasing the number of CD4+ T cells and thus, improve immune status among patients receiving chemotherapy (Liu et al., 2018). However, Brown (1991) stated the presence of all 20 amino acids in 16 microalgae species, where aspartate and glutamate were the most abundant amino acids found in those microalgae. Lim et al., (2018) reported six dinoflagellates having 18 amino acids and glutamic acid was in the highest amount in all species. Additionally, leucine, alanine, valine, and glycine are found to be produced in higher amounts in C. sorokiniana and C. vulgaris (Ballesteros-Torres et al., 2019).
Mycosporine-like amino acids (MAAs) with the antioxidant property are also commonly found in microalgae. Xiong et al., (1999), reported the presence of five MAAs in Scenedesmus sp. and C. sorokiniana (Xiong et al., 1999). Llewellyn and Airs (2010) assessed 33 microalgae species and found six MAAs isolated from these microalgae. Among these microalgae, Glenodinuim foliaceum was the most prolific producer of MAAs, while shinorine was the most common MAA (Llewellyn and Airs, 2010).
Marine microalgae P. tricornutum, T. chuii, and N. granulate have macro minerals (Ca, P, Mg, K, Na, S) and microminerals (Cu, Fe, Mn, Se, Zn), while Botryococcus braunii and Porphyridium aerugineum possess all these minerals except Se (Fox and Zimba, 2018). Additionally, C. ellipsoidea contains major elements like Na, Mg, Al, K, Ca, Mn, Fe, Cu, and Zn (Kim et al., 2001). Moreover, cookies made from Spirulina and Chlorella are found high in Se content along with some other minerals Na, Mg, and P (Uribe-Wandurraga et al., 2020).
It has been reported that higher intake of calcium, magnesium, manganese, zinc, selenium, potassium, and iodine intakes, combined with lower intake of iron, copper, phosphorus, and sodium intake can reduce the risk of colorectal cancer incident in postmenopausal women (Swaminath et al., 2019). Supplementation of antioxidants multivitamin and mineral (AMM) protect cancer patients from radiotherapy or chemotherapy-induced oxidative stress, which is indicated by depletion of oxidative stress markers such as MDA and nitric oxide, and restores the endogenous and exogenous antioxidants (SOD, GPx, Vitamin C and Vitamin E) and essential trace element levels (zinc, copper, and selenium), as well (Patil and More, 2020). Moreover, a high daily intake of selenium is protective against cancer, though the effects vary with different cancers (Kuria et al., 2020).
Coenzyme Q (CoQ10), also known as ubiquinone, is a naturally occurring ubiquitous compound and also an important cofactor in oxidative phosphorylation in mitochondria and associated with cellular energy (ATP) production (Raizner, 2019). Microalgae Porphyridium purpureum has been claimed to produce CoQ10, as well as there is also evidence of the presence of CoQ10 in C. pyrenoidosa (Klein et al., 2011). Additionally, freeze-dried biomass of I. galbana showed a high amount of CoQ10 (Matos et al., 2019).
CoQ10 in combination with alpha-lipoic acid (ALA) prevent cisplatin-induced nephrotoxicity (Khalifa et al., 2020). It has been claimed that coenzyme Q10 inhibits human colon cancer (HCT116) cells through increased ROS and nitric oxide production, while regulating the increased expression of apoptosis-related genes and decreased expression of the anti-apoptotic gene, Bcl2 (Jang et al., 2017). A standard dose of 300 mg/day for 3 months of coenzyme Q10 supplementation has been proposed which can significantly increase antioxidant enzymes activities (SOD, CAT, and GPx) and decreases the levels of inflammatory markers in hepatocellular carcinoma patients after surgery (Liu et al., 2016). On the other hand, it has been observed that high proportion of patients with oral cancer has low ubiquinone and this deficiency is related to high risk of central obesity, hypertriglyceridemia, and metabolic syndrome (Chan et al., 2020). Similar deficiency is often observed in breast cancer also, where supplementation with CoQ10 has been suggested to reduce the adverse effects (Tafazoli, 2017).
Seaweeds are an important part of Asian cuisine and are rich in pharmaceutically important bioactive compounds. Seaweed antioxidants comprise mainly carotenoids, polyphenols, phycobilin (phycoerythrin and phycocyanin), sulfated polysaccharide, vitamin (A, C) (Cornish and Garbary, 2011). Sulfated polysaccharides and polyphenols from seaweed are not similar to microalgae. Carrageenans, fucoidans, ulvan, and porphyran are the most studied seaweed or macroalgal sulfated polysaccharides that have antioxidant and anticancer activity. Moreover, macroalgae also have non-sulfated polysaccharides like alginic acid, laminarin possessing antioxidative and antitumor properties (Venugopal, 2019). In the case of polyphenolic compounds, the presence of phlorotannins, tetraphloretol, fucophlorethol, eckol, difucol, fucodiphlorethol, phloroglucinol, diphlorethol have been reported from macroalgae (Mekinić et al., 2019). Among all the antioxidant-rich phenolic compounds, phlorotannins, are widely found in macroalgae, especially in brown algae (Montero et al., 2017). Fatty acids from Laurencia papillosa (red alga), sulfated polysaccharides from Pterocladia capillacea, meroterpenoids like sargachromanol, sargahydroquinoic and sargaquinoic acid from Sargassum serratifolium, sesquiterpenoids (isozonarol) from Dictyopteris undulata (brown alga) have been reported to exert high antioxidant property (Fleita et al., 2015; Kumagai et al., 2018; Omar et al., 2018; Lim et al., 2019). Besides these, a range of edible seaweeds with antioxidative properties is consumed globally (Table 2).
Dietary antioxidant supplements can act as a “double-edged sword” in cancer treatment due to their ability to kill cancer cells or to protect them (Favre, 2019). A high daily intake of nutraceutical supplementation may not be safe and may have toxic side effects. Therefore, it is necessary to differentiate the prophylactic dose from the therapeutic dose. A prophylactic dose protects healthy cells and tumor cells, while a therapeutic dose inhibits the growth of only cancer cells. (Calvani et al., 2020). In some cases, low concentrations of free radicals because of the high administration of antioxidant supplementation may promote the proliferation of neoplastic cells rather than interrupting it, thus causing cancer development (Valko et al., 2007). Similarly, herbal supplements are likely to carry a greater risk of pharmacokinetic (PK) interaction with chemotherapy agents compared with vitamin, mineral, and other supplements, which may decrease the efficacy of therapy or create an adverse effect (Luo and Asher, 2018).
The potential harmful or beneficial effect of an antioxidant often depends on its concentration, the presence of other antioxidants, and the concentration of endogenous antioxidants. Many antioxidants interact with the synergic effect with other antioxidants present in the formulation, which is known as “sparing effects.” Administration of a mixture of antioxidants exerts a higher biological effect due to their synergistic activity in various phases, which is more beneficial than a high amount of a single antioxidant (van Breda and de Kok, 2018).
Over the last few decades, there have been several in vitro and in vivo studies regarding the antioxidant therapies which have shown that daily intake of a specific dosage of antioxidant nutraceuticals is inversely related to cancer risk as well as enhances the treatment efficacy, nonetheless, randomized clinical trials have shown mixed results which are considered as a real conundrum for the extensive use of antioxidant supplements in cancer therapy. These inconsistent outcomes can be directed by several factors, such as dose, synergism, the bioavailability of antioxidants used, patients’ health status, type of cancer, lifestyle, tendency to supplement intake, and the duration of studies with other variables involved. Therefore, more controlled and well-defined clinical trials with newer approaches need to be conducted to accomplish a safe and effective antioxidant supplement system in cancer treatment. Likewise, there is a need for extensive research to explore novel antioxidant molecules from algae, and their purification strategies as well as in vivo investigations should be prioritized. More studies are needed to explore the actual antioxidant compounds present in several organic and aqueous extracts that have already shown in vitro antioxidant as well as anticancer activities, and to investigate their mechanism of action on the cellular system and their capability to potentiates chemotherapeutic drugs.
UF and ZB conceived the presented idea. UF wrote the manuscript in consultation with ZB. ZB reviewed and edited the manuscript.
This research is supported by Higher Institution Center of Excellence (HICOE) Research Grant (Innovative Vaccines and Therapeutics against Fish Diseases) (Project No. 6369100), and SATREPS (JICA-JST): COSMOS-MOHE G4-B Research Grant (Microalgae for Sustainable Aquaculture Health: Microalgae Vaccine Delivery System) (Project No. 6300866).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abd El-Baky, H. H., El Baz, F. K., and El-Baroty, G. S. (2009). Production of phenolic compounds from Spirulina maxima microalgae and its protective effects in Vitro toward hepatotoxicity model. Afr. J. Pharm. Pharmacol. 3 (4), 133–139. doi:10.5897/AJPP.9000240
Abd El-Hack, M. E., Abdelnour, S., Alagawany, M., Abdo, M., Sakr, M. A., Khafaga, A. F., et al. (2019). Microalgae in modern cancer therapy: current knowledge. Biomed. Pharmacother. 111, 42–50. doi:10.1016/j.biopha.2018.12.069
Abdel-Latif, M. M. M., Babar, M., Kelleher, D., and Reynolds, J. V. (2019). A pilot study of the impact of Vitamin C supplementation with neoadjuvant chemoradiation on regulators of inflammation and carcinogenesis in esophageal cancer patients. J. Cancer Res. Ther. 15, 185–191. doi:10.4103/jcrt.JCRT_763_16
Abdel-Samad, R., Aouad, P., and Darwiche, N. (2019). Natural and synthetic retinoids in preclinical colorectal cancer models. Anti-Cancer Drugs 30 (7), 655–669. doi:10.1097/CAD.0000000000000802
Abraham, A., Kattoor, A. J., Saldeen, T., and Mehta, J. L. (2019). Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 59 (17), 2831–2838. doi:10.1080/10408398.2018.1474169
Ahmed, F., Fanning, K., Netzel, M., Turner, W., Li, Y., and Schenk, P. M. (2014). Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 165, 300–306. doi:10.1016/j.foodchem.2014.05.107
Akiba, T., Morikawa, T., Odaka, M., Nakada, T., Kamiya, N., Yamashita, M., et al. (2018). Vitamin D supplementation and survival of patients with non-small cell lung cancer: a randomized, double-blind, placebo-controlled trial. Clin. Cancer Res. 24 (17), 4089–4097. doi:10.1158/1078-0432.CCR-18-0483
Ambati, R. R., Phang, S. M., Ravi, S., and Aswathanarayana, R. G. (2014). Astaxanthin: sources, extraction, stability, biological activities and its commercial applications–a review. Mar. Drugs 12 (1), 128–152. doi:10.3390/md12010128
Ambrosone, C. B., Zirpoli, G. R., Hutson, A. D., Mccann, W. E., Mccann, S. E., Barlow, W. E., et al. (2020). Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J. Clin. Oncol. 38 (8), 804–814. doi:10.1200/JCO.19.01203
Amna Kashif, S., Hwang, Y. J., and Park, J. K. (2018). Potent biomedical applications of isolated polysaccharides from marine microalgae Tetraselmis species. Bioproc. Biosyst. Eng. 41 (11), 1611–1620. doi:10.1007/s00449-018-1987-z
Ansari, H., Nouranian, M., Hajigholami, A., and Mahmudian, A. (2019). Effect of omega-3 fatty acid supplements on cancer-related fatigue in an outpatient setting: a randomized controlled trial. Middle East J. Cancer 10 (4), 333–340. doi:10.30476/mejc.2019.78605
Anwar, H., Hussain, G., and Mustafa, I. (2018). “Antioxidants from natural sources.” in Antioxidants in foods and its applications. Editors E. Shalaby, and G. M. Azzam (London, UK: IntechOpen), 3–28. doi:10.5772/intechopen.75961
Ashfaq, W., Rehman, K., Siddique, M. I., and Khan, Q. A. A. (2019). Eicosapentaenoic acid and docosahexaenoic acid from fish oil and their role in cancer research. Food Rev. Int. 36 (8), 795–814. doi:10.1080/87559129.2019.1686761
Ashrafi, F., Tabiei, M. N., Mousavi, S., Nematbakhsh, M., Sotoodehnasab, P., and Janbabaei, G. (2020). Does vitamin E mitigate cisplatin-induced nephrotoxicity in cancer patients: results from a randomized placebo-controlled clinical trial. Middle East J. Cancer 11 (2), 174–184. doi:10.30476/mejc.2019.78710.0
Atalay, P. B., Kuku, G., and Tuna, B. G. (2019). Effects of carbendazim and astaxanthin Co-treatment on the proliferation of MCF-7 breast cancer cells. In Vitro Cell Dev. Biol. Anim. 55 (2), 113–119. doi:10.1007/s11626-018-0312-0
Azat Aziz, M., Shehab Diab, A., and Mohammed, A. A. (2019). “Antioxidant categories and mode of action.” in Antioxidants, Editor E. Shalaby (London, UK: IntechOpen), 1–20. doi:10.5772/intechopen.83544
Ballesteros-Torres, J. M., Samaniego-Moreno, L., Gomez-Flores, R., Tamez-Guerra, R. S., Rodríguez-Padilla, C., and Tamez-Guerra, P. (2019). Amino acids and acylcarnitine production by Chlorella vulgaris and Chlorella sorokiniana microalgae from wastewater culture. PeerJ 7 (12), e7977. doi:10.7717/peerj.7977
Banskota, A. H., Sperker, S., Stefanova, R., McGinn, P. J., and O’Leary, S. J. B. (2019). Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycology 31 (1), 309–318. doi:10.1007/s10811-018-1523-1
Baudelet, P. H., Gagez, A. L., Bérard, J. B., Juin, C., Bridiau, N., Kaas, R., et al. (2013). Antiproliferative activity of Cyanophora paradoxa pigments in melanoma, breast and lung cancer cells, Mar. Drugs 11 (11), 4390–4406. doi:10.3390/md11114390
Becerra, M. O., Contreras, L. M., Lo, M. H., Díaz, J. M., and Herrera, G. C. (2020). Lutein as a functional food ingredient: stability and bioavailability. J Funct. Foods 66, 103771. doi:10.1016/j.jff.2019.103771
Ben‐Amotz, A. (1993). “Production of β-carotene and vitamins by the halotolerant Alga Dunaliella,”. in Pharmaceutical and bioactive natural products. Editor D. H. Attaway, and O. R. Zaborsky (Boston, MA:Springer), 1–20. doi:10.1007/978-1-4899-2391-2_11
Bergea, J. P., Debiton, E., Dumay, J., Durand, P., and Barthomeuf, C. (2002). In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J. Agric. Food Chem. 50, 6227–6232. doi:10.1021/jf020290y
Bi, M. C., Rosen, R., Zha, R. Y., McCormick, S. A., Song, E., and Hu, D. N. (2013). Zeaxanthin induces apoptosis in human uveal melanoma cells through bcl-2 family proteins and intrinsic apoptosis pathway. Evid. Based Complement. Altern. Med. 2013, 205082. doi:10.1155/2013/205082
Blanco, A. M., Moreno, J., Del Campooo, J. A., Rivas, J., and Guerrero, M. G. (2007). Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 73 (6), 1259–1266. doi:10.1007/s00253-006-0598-9
Blaszczak, W., Barczak, W., Masternak, J., Kopczynski, P., Zhitkovich, A., and Rubis, B. (2019). Vitamin C as a modulator of the response to cancer therapy. Molecules 24 (3), 453. doi:10.3390/molecules24030453
Bogacz-Radomska, Ludmila., and Harasym, J. (2018). β-Carotene-Properties and production methods. Food Qual. Saf. 2 (2), 69–74. doi:10.1093/fqsafe/fyy004
Brown, M. R. (1991). The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 145 (1), 79–99. doi:10.1016/0022-0981(91)90007-J
Brown, M. R., Jeffrey, S. W., Volkman, J. K., and Dunstan, G. A. (1997). Nutritional properties of microalgae for mariculture. Aquaculture 151 (1–4), 315–331. doi:10.1016/S0044-8486(96)01501-3
Bulut, O., Akın, D., Sönmez, Ç., Öktem, A., Yücel, M., and Öktem, H. A. (2019). Phenolic compounds, carotenoids, and antioxidant capacities of a thermo-tolerant Scenedesmus sp. (chlorophyta) extracted with different solvents. J. Appl. Phycology 31 (3), 1675–1683. doi:10.1007/s10811-018-1726-5
Calvani, M., and Favre, C. (2019). Antioxidant nutraceutical approach to ewing sarcoma: where is the trap? Biomed. J. Scientific Tech. Res. 17, 12805–12814. doi:10.26717/bjstr.2019.17.002999
Calvani, M., Pasha, A., and Favre, C. (2020). Nutraceutical boom in cancer : inside the labyrinth of reactive oxygen species. Int. J. Mol. Sci. 21 (6), 1936. doi:10.3390/ijms21061936
Celano, M., Maggisano, V., Bulotta, S., Allegri, L., Pecce, V., Abballe, L., et al. (2020). Quercetin improves the effects of sorafenib on growth and migration of thyroid cancer cells. Endocrine 67 (2), 496–498. doi:10.1007/s12020-019-02140-3
Çetin, A., Biltekin, B., and Degirmencioglu, S. (2019). Ellagic acid enhances the antitumor efficacy of bevacizumab in an in vitro glioblastoma model. World Neurosurg. 132, e59–65. doi:10.1016/j.wneu.2019.08.257
Cha, K. H., Koo, S. Y., and Lee, D. U. (2008). Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 56 (22), 10521–10526. doi:10.1021/jf802111x
Chahal, A., Saini, A. K., Chhillar, A. K., and Saini, R. V. (2018). Natural antioxidants as defense system against cancer. Asian J. Pharm. Clin. Res. 11 (5), 38–44. doi:10.22159/ajpcr.2018.v11i5.24119
Chan, M. C., Ho, S. H., Lee, D. J., Chen, C. Y., Huang, C. C., and Chang, J. S. (2013). Characterization, extraction and purification of lutein produced by an indigenous microalga Scenedesmus obliquus CNW-N. Biochem. Eng. J. 78, 24–31. doi:10.1016/j.bej.2012.11.017
Chan, M.-Y., Lee, B.-J., Chang, P.-S., Hsiao, H.-Y., Hsu, L.-P., Chang, C.-H., et al. (2020). The risks of ubiquinone and β -carotene deficiency and metabolic disorders in patients with oral cancer. BMC Cancer 20 (310), 1–9. doi:10.1186/s12885-020-06839-9
Chang, J., Zhang, Y., Li, Y., Lu, K., Shen, Y., Guo, Y., et al. (2018). NrF2/ARE and NF-ΚB pathway regulation may be the mechanism for lutein inhibition of human breast cancer cell. Future Oncol. 14 (8), 719–726. doi:10.2217/fon-2017-0584
Chatterjee, R., Erban, J. K., Fuss, P., Dolor, R., LeBlanc, E., Staten, M., et al. (2019). Vitamin D supplementation for prevention of cancer: the D2d cancer outcomes (D2dCA) study. Contemp. Clin. Trials 81, 62–70. doi:10.1016/j.cct.2019.04.015
Chen, C. Y., Jesisca, C. H., Hsieh, C., Lee, D. J., Chang, C. H., Chang, J. S., et al. (2016). Production, extraction and stabilization of lutein from microalga Chlorella sorokiniana MB-1. Bioresour. Technol. 200, 500–505. doi:10.1016/j.biortech.2015.10.071
Chen, F., Li, B. H., Wong, R. N. S., Ji, B., and Jiang, Y. (2005). Isolation and purification of the bioactive carotenoid zeaxanthin from the microalga Microcystis aeruginosa by high-speed counter-current chromatography. J. Chromatogr. A 1064 (2), 183–186. doi:10.1016/j.chroma.2004.12.065
Chen, X., Song, L., Wang, H., Liu, S., Yu, H., Wang, X., et al. (2019). Partial characterization, the immune modulation and anticancer activities of sulfated polysaccharides from filamentous microalgae Tribonema sp. Molecules 24 (2), 322. doi:10.3390/molecules24020322
Chester, K., Zahiruddin, S., Ahmad, A., Khan, W., Paliwal, S., and Ahmad, S. (2019). Bioautography-based identification of antioxidant metabolites of Solanum nigrum L. and exploration its hepatoprotective potential against D-galactosamine-induced hepatic fibrosis in rats. Pharmacognosy Mag. 15, 104–110. doi:10.4103/pm.pm_359_18
Chikara, S., Nagaprashantha, L. D., Singhal, J., Horne, D., Awasthi, S., and Singhal, S. S. (2018). Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett 413: 122–134. doi:10.1016/j.canlet.2017.11.002
Cho, Y. C., Cheng, J. H., Hsu, S. L., Hong, S. E., Lee, T. M., and Chang, C. M. J. (2011). Supercritical carbon dioxide anti-solvent precipitation of anti-oxidative zeaxanthin highly recovered by elution chromatography from Nannochloropsis oculata. Sep. Purif. Technol. 78 (3), 274–280. doi:10.1016/j.seppur.2011.02.017
Choochote, W., Suklampoo, L., and Ochaikul, D. (2014). Evaluation of antioxidant capacities of green microalgae. J. Appl. Phycology 26 (1), 43–48. doi:10.1007/s10811-013-0084-6
Chronopoulou, L., Dal Bosco, C., Di Caprio, F., Prosini, L., Gentili, A., Pagnanelli, F., et al. (2019). Extraction of carotenoids and fat-soluble vitamins from Tetradesmus obliquus microalgae: an optimized approach by using supercritical CO2. Molecules 24 (14). 2581. doi:10.3390/molecules24142581
Chu, W.-L. (2013). Biotechnological applications of microalgae. Biotechnological Appl. Microalgae 6 (126), 24–37. doi:10.1201/b14920
Chue, K. T., Ten, L. N., Oh, Y. K., Woo, S. G., Lee, M., and Yoo, S. A. (2012). Carotinoid compositions of five microalga species. Chem. Nat. Compd. 48 (1), 141–142. doi:10.1007/s10600-012-0183-7
Constantinou, C., Charalambous, C., and Kanakis, D. (2020). Vitamin E and cancer: an update on the emerging role of γ and δ tocotrienols. Eur. J. Nutr. 59 (3), 845–857. doi:10.1007/s00394-019-01962-1
Cornish, M. L., and Garbary, D. J. (2011). Antioxidants from Macroalgae : potential applications in human health and nutrition. Algae 25, 155–171. doi:10.4490/algae.2010.25.4.155
Corpas, F. J., and Barroso, J. B. (2015). Reactive sulfur species (RSS): possible new players in the oxidative metabolism of plant peroxisomes. Front. Plant Sci. 6, 116. doi:10.3389/fpls.2015.00116
Cui, C., Lu, J., Sun-Waterhouse, D., Mu, L., Sun, W., Zhao, M., et al. (2016). Polysaccharides from Laminaria japonica: structural characteristics and antioxidant activity. LWT-Food Sci. Technol. 73, 602–608. doi:10.1016/j.lwt.2016.07.005
Custódio, L., Soares, F., Pereira, H., Barreira, L., Vizetto-Duarte, C., João Rodrigues, M., et al. (2014). Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: possible application in the pharmaceutical and functional food industries. J. Appl. Phycology 26 (1), 151–161. doi:10.1007/s10811-013-0098-0
Damergi, E., Schwitzguébel, J. P., Refardt, D., Sharma, S., Holliger, C., and Ludwig, C. (2017). Extraction of carotenoids from Chlorella vulgaris using green solvents and syngas production from residual biomass. Algal Res. 25, 488–495. doi:10.1016/j.algal.2017.05.003
Darwito, D., Dharmana, E., Riwanto, I., Budijitno, S., Suwardjo, S., Purnomo, J., et al. (2019). Effects of omega-3 supplementation on ki-67 and VEGF expression levels and clinical outcomes of locally advanced breast cancer patients treated with neoadjuvant CAF chemotherapy: a randomized controlled trial report. Asian Pac. J. Cancer Prev. 20 (3), 911–916. doi:10.31557/APJCP.2019.20.3.911
Dasari, S., Ali, S. M., Zheng, G., Chen, A., Dontaraju, V. S., Bosland, M. C., et al. (2017). Vitamin K and its analogs: potential avenues for prostate cancer management. Oncotarget 8 (34), 57782–57799. doi:10.18632/oncotarget.17997
De Miguel, T., Sieiro, C., Poza, M., and Villa, T. G. (2001). Analysis of canthaxanthin and related pigments from Gordonia jacobaea mutants. J. Agric. Food Chem. 49 (3), 1200–1202. doi:10.1021/jf001169z
De Sá Junior, P. L., Dias Câmara, D. A., Porcacchia, A. S., Fonseca, P. M. M., Jorge, S. D., Araldi, R. P., et al. (2017). The roles of ROS in cancer heterogeneity and therapy. Oxidative Med. Cell Longevity 2017, 2467940. doi:10.1155/2017/2467940
Di Lena, G., Casini, I., Lucarini, M., and Lombardi-Boccia, G. (2019). Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 120, 810–818. doi:10.1016/j.foodres.2018.11.043
Di Meo, S., Reed, T. T., Venditti, P., and Victor, V. M. (2016). Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med. Cell Longevity 2016, 1245049. doi:10.1155/2016/1245049
Di Sanzo, G., Mehariya, S., Martino, M., Larocca, V., Casella, P., Chianese, S., et al. (2018). Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 16 (9), 334. doi:10.3390/md16090334
Dong, X., Bai, Y., Xu, Z., Shi, Y., Sun, Y., Janaswamy, S., et al. (2019). Phlorotannins from undaria pinnatifida sporophyll: extraction, antioxidant, and anti-inflammatory activities. Mar. Drugs 17 (8), 434. doi:10.3390/md17080434
El-Baz, F. K., Hussein, R. A., Saleh, D. O., and Abdel Jaleel, G. A. R. (2019). Zeaxanthin isolated from Dunaliella salina microalgae ameliorates age associated cardiac dysfunction in rats through stimulation of retinoid receptors. Mar. Drugs 17 (5), 1–15. doi:10.3390/md17050290
Elkhateeb, W., El-Sayed, H., Fayad, W., Galib Al Kolaibe, A., Emam, M., and Daba, G. (2020). In Vitro anti-breast cancer and antifungal bio-efficiency of some microalgal extracts. Egypt. J. Aquat. Biol. 24 (1), 263–279. doi:10.21608/ejabf.2020.70448
Fabregas, J., and Herrero, C. (1990). Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J. Ind. Microbiol. 5 (4), 259–263. doi:10.1007/BF01569683
Faraone, I., Sinisgalli, C., Ostuni, A., Armentano, M. F., Carmosino, M., Milella, L., et al. (2020). Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: a systematic review. Pharmacol. Res. 155, 104689. doi:10.1016/j.phrs.2020.104689
Favre, C. (2019). Antioxidant nutraceutical approach to ewing sarcoma: where is the trap? Biomed. J. Scientific Tech. Res. 17 (3), 12805–12814. doi:10.26717/bjstr.2019.17.002999
Ferdous, U. T., and Yusof, Z. N. B. (2021). “Algal terpenoids: a potential source of antioxidants for cancer therapy.” in Terpenes and Terpenoids. (London, UK: IntechOpen). doi:10.5772/intechopen.94122
Ferdouse, F., Løvstad Holdt, S., Smith, R., Murúa, P., and Yang, Z. (2018). The global status of seaweed production, trade and utilization (Rome, Italy: FAO Globefish Research Programme), Vol. 124.
Fimbres-Olivarria, D., Carvajal-Millan, E., Lopez-Elias, J. A., Martinez-Robinson, K. G., Miranda-Baeza, A., Martinez-Cordova, L. R., et al. (2018). Chemical characterization and antioxidant activity of sulfated polysaccharides from Navicula sp. Food Hydrocolloids 75, 229–236. doi:10.1016/j.foodhyd.2017.08.002
Fleita, D., El-Sayed, M., and Rifaat, D. (2015). Evaluation of the antioxidant activity of enzymatically-hydrolyzed sulfated polysaccharides extracted from red algae. Pterocladia capillacea. LWT-Food Sci. Technol. 63 (2), 1236–1244. doi:10.1016/j.lwt.2015.04.024
Foo, S. C., Yusoff, F. M., Imam, M. U., Foo, J. B., Ismail, N., Azmi, N. H., et al. (2018). Increased fucoxanthin in Chaetoceros calcitrans extract exacerbates apoptosis in liver cancer cells via multiple targeted cellular pathways. Biotechnol. Rep. 20, e00296. doi:10.1016/j.btre.2018.e00296
Fox, J. M., and Zimba, P. V. (2018). “Minerals and trace elements in microalgae”. in Microalgae in health and disease prevention (Academic Press). doi:10.1016/B978-0-12-811405-6.00008-6
Francenia Santos-Sánchez, N., Salas-Coronado, R., Villanueva-Cañongo, C., and Hernández-Carlos, B. (2019). “Antioxidant compounds and their antioxidant mechanism.” in Antioxidants (London, United Kingdom: IntechOpen), 1–28. doi:10.5772/intechopen.85270
Fritz, H., Kennedy, D., Fergusson, D., Fernandes, R., Doucette, S., Cooley, K., et al. (2011). Vitamin A and retinoid derivatives for lung cancer: a systematic review and meta analysis. PLoS One 6, e21107. doi:10.1371/journal.pone.0021107
Gaignard, C., Laroche, C., Pierre, G., Dubessay, P., Delattre, C., Gardarin, C., et al. (2019). Screening of marine microalgae: investigation of new exopolysaccharide producers. Algal Res. 44, 101711. doi:10.1016/j.algal.2019.101711
Galadari, S., Rahman, A., Pallichankandy, S., and Thayyullathil, F. (2017). Reactive oxygen species and cancer paradox: to promote or to suppress? Free Radic. Biol. Med. 104, 144–164. doi:10.1016/j.freeradbiomed.2017.01.004
Ganesan, P., Matsubara, K., Ohkubo, T., Tanaka, Y., Noda, K., Sugawara, T., et al. (2010). Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine 17 (14), 1140–1144. doi:10.1016/j.phymed.2010.05.005
Ganesan, P., Noda, K., Manabe, Y., Ohkubo, T., Tanaka, Y., Maoka, T., et al. (2011). Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta 1810 (5), 497–503. doi:10.1016/j.bbagen.2011.02.008
Gansukh, E., Nile, A., Sivanesan, I., Rengasamy, K. R. R., Kim, D.-H., Keum, Y.-S., et al. (2019). Chemopreventive E ff ect of β -cryptoxanthin on human cervical carcinoma (HeLa) cells is modulated through oxidative stress-induced apoptosis. Antioxidants 9 (28), 1–15.
Gao, M., Dang, F., and Deng, C. (2019). β-Cryptoxanthin induced anti-proliferation and apoptosis by G0/G1 arrest and AMPK signal inactivation in gastric cancer. Eur. J. Pharmacol. 859, 172528. doi:10.1016/j.ejphar.2019.172528
Gardeva, E., Toshkova, R., Minkova, K., and Gigova, L. (2009). Cancer protective action of polysaccharide, derived red microalga Porphyridium cruentum—a biological background. Biotechnol. Biotechnological Equipment 23 (Suppl. 1), 783–787. doi:10.1080/13102818.2009.10818540
Go, Y.-M., and Jones, D. P. (2017). Redox theory of aging : implications health disease. Clin. Sci. 131, 1669–1688. doi:10.1042/CS20160897
Goiris, K., Muylaert, K., Fraeye, I., Foubert, I., De Brabanter, J., and Cooman, L. D. (2012). Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycology 24 (6), 1477–1486. doi:10.1007/s10811-012-9804-6
Goiris, K., Muylaert, K., Voorspoels, S., Noten, B., De Paepe, D., Baart, G. J. E., et al. (2014). Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycology 50 (3), 483–492. doi:10.1111/jpy.12180
Gong, X., Smith, J. R., Swanson, H. M., and Rubin, L. P. (2018). Carotenoid lutein selectively inhibits breast cancer cell growth and potentiates the effect of chemotherapeutic agents through ROS-mediated mechanisms. Molecules 23 (4), 1–18. doi:10.3390/molecules23040905
Goyal, Y., Koul, A., and Ranawat, P. (2019). Ellagic acid ameliorates cisplatin toxicity in chemically induced colon carcinogenesis. Mol. Cell Biochem. 453 (1–2), 205–215. doi:10.1007/s11010-018-3446-1
Grama, B. S., Chader, S., Khelifi, D., Agathos, S. N., and Jeffryes, C. (2014). Induction of canthaxanthin production in a Dactylococcus microalga isolated from the Algerian sahara. Bioresour. Technol. 151, 297–305. doi:10.1016/j.biortech.2013.10.073
Granado-Lorencio, F., Herrero-Barbudo, C., Acién-Fernández, G., Molina-Grima, E., Fernández-Sevilla, J. M., Pérez-Sacristán, B., et al. (2009). In Vitro bioaccesibility of lutein and zeaxanthin from the microalgae Scenedesmus almeriensis. Food Chem. 114 (2), 747–752. doi:10.1016/j.foodchem.2008.10.058
Graziani, G., Schiavo, S., Nicolai, M. A., Buono, S., Fogliano, V., Pinto, G., et al. (2013). Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct. 4 (1), 144–152. doi:10.1039/c2fo30198a
Gupta, S., and Abu-Ghannam, N. (2011). Bioactive potential and possible health effects of edible Brown seaweeds. Trends Food Sci. Technol. 22 (6), 315–326. doi:10.1016/j.tifs.2011.03.011
Guzmán, F., Wong, G., Román, T., Cárdenas, C., Alvárez, C., Schmitt, P., et al. (2019). Identification of antimicrobial peptides from the microalgae Tetraselmis suecica (Kylin) butcher and bactericidal activity improvement. Mar. Drugs 17 (8), 453. doi:10.3390/md17080453
Guzmán, S., Gato, A., Lamela, M., Freire-Garabal, M., and Calleja, J. M. (2003). Anti-Inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytotherapy Res. 17 (6), 665–670. doi:10.1002/ptr.1227
Hafsa, M. B., Ismail, M. B., Garrab, M., Aly, R., Gagnon, J, and Naghmouchi, K. (2017). Antimicrobial, antioxidant, cytotoxic and anticholinesterase activities of water-soluble polysaccharides extracted from microalgae Isochrysis galbana and Nannochloropsis oculata. J. Serbian Chem. Soc. 82 (5), 509–522. doi:10.2298/JSC161016036B
Haidari, F., Abiri, B., Iravani, M., Ahmadi-Angali, K., and Vafa, M. (2020). Effects of vitamin D and omega-3 fatty acids Co-supplementation on inflammatory factors and tumor marker CEA in colorectal cancer patients undergoing chemotherapy: a randomized, double-blind, placebo-controlled clinical trial. Nutr. Cancer 72 (6), 948–958. doi:10.1080/01635581.2019.1659380
Halliwell, B., Gutteridge, J. M. C., and Cross, C. E. (1992). Free radicals, antioxidants, and human disease: where are we now. J. Lab. Clin. Med. 119 (6), 598–620. doi:10.5555/uri:pii:002221439290284R
Haoujar, I., Cacciola, F., Abrini, J., Mangraviti, D., Giuffrida, D., Oulad El Majdoub, Y., et al. (2019). The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from Mediterranean Morocco. Molecules 24 (22), 4037. doi:10.3390/molecules24224037
He, S., Li, C., Zhang, Q., Ding, J., Liang, X. J., Chen, X., et al. (2018). Tailoring platinum(IV) amphiphiles for self-targeting all-in-one assemblies as precise multimodal theranostic nanomedicine. ACS Nano 12 (7), 7272–7281. doi:10.1021/acsnano.8b03476
Hemalatha, A., Girija, K., Parthiban, C., Saranya, C., and Anantharaman, P. (2013). Antioxidant properties and total phenolic content of a marine diatom, Navicula clavata and green microalgae, Chlorella marina and Dunaliella salina. Pelagia Res. Libr. 4 (5), 151–157.
Heo, J., Shin, D. S., Cho, K., Cho, D. H., Lee, Y. J., and Kim, H. S. (2018). Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: optimization of lutein productivity via regulation of light intensity and carbon source. Algal Res. 33, 1–7. doi:10.1016/j.algal.2018.04.029
Hu, C. W., Chuang, L. T., Yu, P. C., and Nathan Chen, C. N. (2013). Pigment production by a new thermotolerant microalga coelastrella sp. F50. Food Chem. 138 (4), 2071–2078. doi:10.1016/j.foodchem.2012.11.133
Ilghami, R., Barzegari, A., Mashayekhi, M. R., Letourneur, D., Crepin, M., and Pavon-Djavid, G. (2020). The conundrum of dietary antioxidants in cancer chemotherapy. Nutr. Rev. 78 (1), 65–76. doi:10.1093/nutrit/nuz027
Inbaraj, B., Chien, J. T., and Chen, B. H. (2006). Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J. Chromatogr. A. 1102 (1–2), 193–199. doi:10.1016/j.chroma.2005.10.055
Ip, P. F., and Chen, F. (2005). Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem. 40 (2), 733–738. doi:10.1016/j.procbio.2004.01.039
Isaka, S., Cho, K., Nakazono, S., Abu, R., Ueno, M., Kim, D., et al. (2015). Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromolecules 74, 68–75. doi:10.1016/j.ijbiomac.2014.11.043
Ishika, T., Laird, D. W., Bahri, P. A., and Moheimani, N. R. (2019). Co-Cultivation and stepwise cultivation of Chaetoceros muelleri and Amphora sp. for fucoxanthin production under gradual salinity increase. J. Appl. Phycology 31 (3), 1535–1544. doi:10.1007/s10811-018-1718-5
Iskandar, A. R., Miao, B., Li, X., Hu, K. Q., Liu, C., and Wang, X. D. (2016). Β-Cryptoxanthin reduced lung tumor multiplicity and inhibited lung cancer cell motility by downregulating nicotinic acetylcholine receptor Α7 signaling. Cancer Prev. Res. 9 (11), 875–886. doi:10.1158/1940-6207.CAPR-16-0161
Jaime, L., Mendiola, J. A., Herrero, M., Soler-Rivas, C., Santoyo, S., Señorans, F. J., et al. (2005). Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD. J. Sep. Sci. 28 (16), 2111–2119. doi:10.1002/jssc.200500185
Jain, A., Sharma, G., Thakur, K., Raza, K., Shivhare, U. S., Ghoshal, G., et al. (2019). Beta-Carotene-Encapsulated solid lipid nanoparticles (BC-SLNs) as promising vehicle for cancer: an investigative assessment. AAPS PharmSciTech 20 (3), 100. doi:10.1208/s12249-019-1301-7
Janchot, ., Rauytanapanit, M., Honda, M., Hibino, T., Sirisattha, S., Praneenararat, T., et al. (2019). Effects of potassium chloride-induced stress on the carotenoids canthaxanthin, astaxanthin, and lipid accumulations in the green chlorococcal microalga strain TISTR 9500. J. Eukaryot. Microbiol. 66 (5), 778–787. doi:10.1111/jeu.12726
Jang, S., Lee, J., Ryu, S. M., Lee, H., Park, J.-R., Kim, H., et al. (2017). Effect of coenzyme Q10 via nitric oxide production and growth arrest of human colon cancer HCT116 cells. J. Prev. Vet. Med. 41 (2), 59–65. doi:10.13041/jpvm.2017.41.2.59
Jayappriyan, K. R., Rajkumar, R., Venkatakrishnan, V., Nagaraj, S., and Rengasamy, R. (2013). In Vitro anticancer activity of natural β-carotene from Dunaliella salina EU5891199 in PC-3 cells. Biomed. Prev. Nutr. 3 (2), 99–105. doi:10.1016/j.bionut.2012.08.003
Jayshree, A., Jayashree, S., and Thangaraju, N. (2016). Chlorella vulgaris and Chlamydomonas reinhardtii: effective antioxidant, antibacterial and anticancer mediators. Indian J. Pharm. Sci. 78 (5), 575–581. doi:10.4172/pharmaceutical-sciences.1000155
Jerez-Martel, I., García-Poza, S., Rodríguez-Martel, G., Rico, M., Afonso-Olivares, C., and Luis Gómez-Pinchetti, J. (2017). Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J. Food Qual. 2017 (4), 2924508. doi:10.1155/2017/2924508
Jiang, L., Wang, Y., Liu, G., Liu, H., Zhu, F., Ji, H., et al. (2018). C-Phycocyanin exerts anti-cancer effects via the MAPK signaling pathway in MDA-MB-231 cells. Cancer Cel Int. 18 (1), 12. doi:10.1186/s12935-018-0511-5
Jiang, L., Wang, Y., Yin, Q., Liu, G., Liu, H., Huang, Y., et al. (2017). Phycocyanin: a potential drug for cancer treatment. J. Cancer 8 (17), 3416–3429. doi:10.7150/jca.21058
Jiang, L., Zhao, X., Xu, J., Li, C., Yu, Y., Wang, W., et al. (2019). The protective effect of dietary phytosterols on cancer risk: a systematic meta-analysis. J. Oncol. 2019, 7479518. doi:10.1155/2019/7479518
Juin, C., de Oliveira Junior, R. G., Fleury, A., Oudinet, C., Pytowski, L., Bérard, J. B., et al. (2018). Zeaxanthin from Porphyridium purpureum induces apoptosis in human melanoma cells expressing the oncogenic BRAF V600E mutation and sensitizes them to the BRAF inhibitor vemurafenib. Braz. J. Pharmacognosy 28 (4), 457–467. doi:10.1016/j.bjp.2018.05.009
Kashyap, D., Tuli, H. S., Sak, K., Garg, V. K., Goel, N., Punia, ., et al. (2019). Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Curr. Pharmacol. Rep. 9 (11), 735. doi:10.3390/biom9110735
Kay, R. A., and Barton, Larry. L. (1991). Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 30 (6), 555–573. doi:10.1080/10408399109527556
Keum, N., Lee, D. H., Greenwood, D. C., Manson, J. E., and Giovannucci, E. (2019). Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann. Oncol. 30 (5), 733–743. doi:10.1093/annonc/mdz059
Khalifa, E. A., Ahmed, A. N., Hashem, K. S., and Allah, A. G. (2020). Therapeutic effects of the combination of alpha-lipoic acid ( ALA ) and coenzyme Q10 (CoQ10) on cisplatin-induced nephrotoxicity. Int. J. Inflamm. 2020, 1–11.
Khan, M. I., Shin, J. H., Kim, J. D., and Kim, J. D. (2018). The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cel Fact 17 (1), 36–21. doi:10.1186/s12934-018-0879-x
Khurana, R. K., Jain, A., Jain, A., Sharma, T., Singh, B., and Kesharwani, P. (2018). Administration of antioxidants in cancer: debate of the decade. Drug Discov. Today 23 (4), 763–770. doi:10.1016/j.drudis.2018.01.021
Kim, D., Kim, Y., and Kim, Y. (2019). Effects of β-carotene on expression of selected MicroRNAs, histone acetylation, and DNA methylation in colon cancer stem cells. J. Cancer Prev. 24 (4), 224–232. doi:10.15430/JCP.2019.24.4.224
Kim, H. M., Jung, J. H., Kim, J. Y., Heo, J., Cho, D. H., Kim, H. S., et al. (2019). The protective effect of violaxanthin from Nannochloropsis oceanica against ultraviolet B-induced damage in normal human dermal fibroblasts. Photochem. Photobiol. 95 (2), 595–604. doi:10.1111/php.13030
Kim, J., Kim, M., Lee, S., and Jin, E. S. (2020). Development of a Chlorella vulgaris mutant by chemical mutagenesis as a producer for natural violaxanthin. Algal Res. 46, 101790. doi:10.1016/j.algal.2020.101790
Kim, J., Park, M. K., Li, W. Q., Qureshi, A. A., and Cho, E. (2019). Association of vitamin A intake with cutaneous squamous cell carcinoma risk in the United States. JAMA Dermatol. 155 (11), 1260–1268. doi:10.1001/jamadermatol.2019.1937
Kim, S. K., Jeon, Y. J., Kim, W. S., Back, H. C., Park, P. J., Byun, H. G., et al. (2001). Biochemical composition of marine microalgae and their potential antimicrobial activity. J. Fish. Sci. Tech. 4 (2), 75–83.
Klaunig, J. E., and Wang, Z. (2018). Oxidative stress in carcinogenesis. Curr. Opin. Toxicol. 7, 116–121. doi:10.1016/j.cotox.2017.11.014
Klein, B. C., Bartel, S. J., Darsow, K. H., Naumann, I., Walter, C., Buchholz, R., et al. (2011). Identification OF coenzyme Q10 from porphyridium purpureum (rhodophyta) BY matrix-assisted laser desorption ionization curved field reflectron mass spectrometry1. J. Phycol 47 (3), 687–691. doi:10.1111/j.1529-8817.2011.00978.x
Ko, E. C., and Formenti, S. C. (2019). Radiation therapy to enhance tumor immunotherapy: a novel application for an established modality. Int. J. Radiat. Biol. 95 (7), 936–939. doi:10.1080/09553002.2019.1623429
Koo, S. Y., Cha, K. H., Song, D. G., Chung, D., and Pan, C. H. (2012). Optimization of pressurized liquid extraction of zeaxanthin from Chlorella ellipsoidea. J. Appl. Phycology 24 (4), 725–730. doi:10.1007/s10811-011-9691-2
Köpcke, W. (2019). Vitamin E in Human Health. Editors P. Weber, M. Birringer, J. B. Blumberg, M. Eggersdorfer, and J. Frank (Cham, Switzerland: Humana Press), 467. doi:10.1007/978-3-030-05315-4
Kotake-Nara, E., Asai, A., and Nagao, A. (2005). Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 220 (1), 75–84. doi:10.1016/j.canlet.2004.07.048
Kotake-Nara, E., Kushiro, M., Zhang, H., Sugawara, T., Miyashita, K., and Nagao, A. (2001). Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 131 (12), 3303–3306. doi:10.1093/jn/131.12.3303
Koyande, A. K., Chew, K. W., Rambabu, K., Tao, Y., Chu, D.-T., and Show, P.-L. (2019). Microalgae: a potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 8 (1), 16–24. doi:10.1016/j.fshw.2019.03.001
Krajewska, M., Szymczak-Żyła, M., Kobos, J., Witak, M., and Kowalewska, G. (2019). Canthaxanthin in recent sediments as an indicator of heterocystous cyanobacteria in coastal waters. Oceanologia 61 (1), 78–88. doi:10.1016/j.oceano.2018.07.002
Kumagai, M., Nishikawa, K., Matsuura, H., Umezawa, T., Matsuda, F., and Okino, T. (2018). Antioxidants from the Brown alga Dictyopteris undulata. Molecules 23 (5), 1214. doi:10.3390/molecules23051214
Kumar, S. R., Hosokawa, M., Miyashita, K., and Miyashita, K. (2013). Fucoxanthin: a marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 11 (12), 5130–5147. doi:10.3390/md11125130
Kumaresan, N., Sanjay, K. R., Venkatesh, K. S., Kadeppagari, R. K., Vijayalakshmi, G., and Umesh-Kumar, S. (2008). Partially saturated canthaxanthin purified from Aspergillus carbonarius induces apoptosis in prostrate cancer cell line. Appl. Microbiol. Biotechnol. 80 (3), 467–473. doi:10.1007/s00253-008-1538-7
Kuria, A., Fang, X., Li, M., Han, H., He, J., Aaseth, J. O., et al. (2020). Does dietary intake of selenium protect against cancer? A systematic review and meta-analysis of population-based prospective studies. Crit. Rev. Food Sci. Nutr. 60 (4), 684–694. doi:10.1080/10408398.2018.1548427
Kurosu, M., and Begari, E. (2010). Vitamin K2 in electron transport system: are enzymes involved in vitamin K2 biosynthesis promising drug targets? Molecules 15 (3), 1531–1553. doi:10.3390/molecules15031531
Lagarde, D., Beuf, L., and Vermaas, W. (2000). Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 66 (1), 64–72. doi:10.1128/aem.66.1.64-72.2000
Li, H. B., Chen, F., and Chen, F. (2001a). Preparative isolation and purification of astaxanthin from the microalga Chlorococcum sp. by high-speed counter-current chromatography. J. Chromatogr. A. 925 (1–2), 133–137. doi:10.1016/s0021-9673(01)01022-6
Li, H. B., Chen, F., Zhang, T. Y., Yang, F. Q., and Xu, G. Q. (2001b). Preparative isolation and purification of lutein from the microalga Chlorella vulgaris by high-speed counter-current chromatography. J. Chromatogr. A 905 (1–2), 151–155. doi:10.1016/S0021-9673(00)00987-0
Li, H. B., Fan, K. W., and Chen, F. (2006). Isolation and purification of canthaxanthin from the microalga Chlorella zofingiensis by high-speed counter-current chromatography. J. Sep. Sci. 29 (5), 699–703. doi:10.1002/jssc.200500365
Li, H., Su, L., Chen, S., Zhao, L., Wang, H., Ding, F., et al. (2018). Physicochemical characterization and functional analysis of the polysaccharide from the edible microalga Nostoc sphaeroides. Molecules 23 (2), 508. doi:10.3390/molecules23020508
Lian, F., Hu, K. Q., Russell, R. M., and Wang, X. D. (2006). Β-Cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor Β expression. Int. J. Cancer 119 (9), 2084–2089. doi:10.1002/ijc.22111
Lim, A. S., Jeong, H. J., Kim, S. J., and Ok, J. H. (2018). Amino acids profiles of six dinoflagellate species belonging to diverse families: possible use as animal feeds in aquaculture. Algae 33 (3), 279–290. doi:10.4490/algae.2018.33.9.10
Lim, S., Choi, A. H., Kwon, M., Joung, E. J., Shin, T., Lee, S. G., et al. (2019). Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 278, 178–184. doi:10.1016/j.foodchem.2018.11.058
Lin, J. H., Chen, S. J., Liu, H., Yan, Y., and Zheng, J. H. (2019). Vitamin E consumption and the risk of bladder cancer. Int. J. Vitam Nutr. Res. 89, 168–175. doi:10.1024/0300-9831/a000553
Lin, J. H., Lee, D. J., and Chang, J. S. (2015). Lutein production from biomass: marigold flowers versus microalgae. Bioresour. Technol. 184, 421–428. doi:10.1016/j.biortech.2014.09.099
Lin, J. K., Chen, W. C., and Hong, D. (1995). The inhibition of DMBA-induced carcinogenesis by neoxanthin in hamster buccal pouch. Nutr. Cancer 24 (3), 325–333. doi:10.1080/01635589509514421
Liu, H. T., Huang, Y. C., Cheng, S. B., Huang, Y. T., and Lin, P. T. (2016). Effects of coenzyme Q10 supplementation on antioxidant capacity and inflammation in hepatocellular carcinoma patients after surgery: a randomized, placebo-controlled trial. Nutr. J. 15 (1), 85. doi:10.1186/s12937-016-0205-6
Liu, L., Zhang, Y., Wei, J., Chen, Z., and Yu, J. (2019). A pilot study of amino acids in unresectable non-small-cell lung cancer patients during chemotherapy: a randomized serial N-of-1 trials design. Nutr. Cancer 71 (3), 399–408. doi:10.1080/01635581.2018.1515962
Liu, X., Yuan, W. Q., Sharma-Shivappa, R., and van Zanten, J. (2017). Antioxidant activity of phlorotannins from Brown algae. Int. J. Agric. Biol. Eng. 10 (6), 184–191. doi:10.25165/j.ijabe.20171006.2854
Ljubic, A., Jacobsen, C., Holdt, S. L., and Jakobsen, J. 2020. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3 Food Chem. 320), 126627. doi:10.1016/j.foodchem.2020.126627
Llewellyn, C. A., and Airs, R. L. (2010). Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar. Drugs 8 (4), 1273–1291. doi:10.3390/md8041273
Lopes, D., Melo, T., Meneses, J., Abreu, M. H., Pereira, R., Domingues, P., et al. (2019). A new look for the red macroalga palmaria palmata: a seafood with polar lipids rich in EPA and with antioxidant properties. Mar. Drugs 17 (9), 533. doi:10.3390/md17090533
Luan, R. L., Wang, P. C., Yan, M. X., and Chen, J. (2018). Effect of lutein and doxorubicin combinatorial therapy on S180 cell proliferation and tumor growth. Eur. Rev. Med. Pharmacol. Sci. 22 (5), 1514–1520. doi:10.26355/eurrev_201803_14501
Luo, Q., and Asher, G. N. (2018). Use of dietary supplements at a comprehensive cancer center. J. Altern. Complement. Med. 24 (9–10), 981–987. doi:10.1089/acm.2018.0183
Machu, L., Misurcova, L., Ambrozova, J. V., Orsavova, J., Mlcek, J., Sochor, J., et al. (2015). Phenolic content and antioxidant capacity in algal food products. Molecules 20 (1), 1118–1133. doi:10.3390/molecules20011118
Magnusson, M., Heimann, K., and Negri, A. P. (2008). Comparative effects of herbicides on photosynthesis and growth of tropical estuarine microalgae. Mar. Pollut. Bull. 56 (9), 1545–1552. doi:10.1016/j.marpolbul.2008.05.023
Magrì, A., Germano, G., Lorenzato, A., Lamba, S., Chilà, R., Montone, M., et al. (2020). High-Dose vitamin C enhances cancer immunotherapy. Sci. Translational Med. 12 (532), eaay8707. doi:10.1126/scitranslmed.aay8707
Malis, S. A., Cohen, E., and Ben Amotz, A. (1993). Accumulation of canthaxanthin in Chlorella emersonii. Physiologia Plantarum 87 (2), 232–236. doi:10.1111/j.1399-3054.1993.tb00148.x
Mansour, M. P., Frampton, D. M. F., Nichols, P. D., Volkman, J. K., and Blackburn, S. I. (2005). Lipid and fatty acid yield of nine stationary-phase microalgae: applications and unusual C24-C28 polyunsaturated fatty acids. J. Appl. Phycology 17 (4), 287–300. doi:10.1007/s10811-005-6625-x
Marian, M. J. (2017). Dietary supplements commonly used by cancer survivors: are there any benefits? Nutr. Clin. Pract. 32, 607–627. doi:10.1177/0884533617721687
Martin, L. J. (2015). Fucoxanthin and its metabolite fucoxanthinol in cancer prevention and treatment. Mar. Drugs 13 (8), 4784–4798. doi:10.3390/md13084784
Martin-Creuzburg, D., and Merkel, P. (2016). Sterols of freshwater microalgae: potential implications for zooplankton nutrition. J. Plankton Res. 38 (4), 865–877. doi:10.1093/plankt/fbw034
Matos, J., Cardoso, C., Gomes, A., Campos, A. M., Falé, P., Afonso, C., et al. (2019). Bioprospection of: Isochrysis galbana and its potential as a nutraceutical. Food Funct. 10 (11), 7333–7342. doi:10.1039/c9fo01364d
Mekinić, I. G., Skroza, D., Šimat, V., Hamed, I., Čagalj, M., and Perković, Z. P. (2019). Phenolic content of Brown algae (pheophyceae) species: extraction, identification, and quantification. Biomolecules 9 (6), 244. doi:10.3390/biom9060244
Mikirova, N., Casciari, J., and Hunninghake, R. (2019). Continuous intravenous vitamin C in the cancer treatment: Re-evaluation of a phase I clinical study. Funct. Foods Health Dis. 9 (3), 180–204. doi:10.31989/ffhd.v9i3.590
Millán, C. S., Soldevilla, B., Martín, P., Gil-Calderón, B., Compte, M., Pérez-Sacristán, B., et al. (2015). β-Cryptoxanthin synergistically enhances the antitumoral activity of oxaliplatin through ΔNP73 negative regulation in colon cancer. Clin. Cancer Res. 21 (19), 4398–4409. doi:10.1158/1078-0432.CCR-14-2027
Miller, P. E., and Snyder, D. C. (2012). Phytochemicals and cancer risk: a review of the epidemiological evidence. Nutr. Clin. Pract. 27 (5), 599–612. doi:10.1177/0884533612456043
Mise, T., Ueda, M., and Yasumoto, T. (2011). Production of fucoxanthin-rich powder from Cladosiphon okamuranus. Adv. J. Food Sci. Technol. 3 (1), 73–76.
Miyazawa, S., Moriya, S., Kokuba, H., Hino, H., Takano, N., and Miyazawa, K. (2020). Vitamin K2 induces non-apoptotic cell death along with autophagosome formation in breast cancer cell lines. Breast Cancer 27 (2), 225–235. doi:10.1007/s12282-019-01012-y
Mohamadnia, S., Tavakoli, O., Ali Faramarzi, M., and Shamsollahi, Z. (2020). Production of fucoxanthin by the microalga Tisochrysis lutea: a review of recent developments. Aquaculture 516, 734637. doi:10.1016/j.aquaculture.2019.734637
Moiseeva, A. A. (2019). Anthracycline derivatives and their anticancer activity. Ineos Open 2 (1), 9–18. doi:10.32931/io1902r
Moloney, J. N., and Cotter, T. G. (2018). ROS signalling in the biology of cancer. Semin. Cel Dev Biol 80, 50–64. doi:10.1016/j.semcdb.2017.05.023
Montero, L., Sánchez-Camargo, A. D. P., Ibáñez, E., and Gilbert-López, B. (2017). Phenolic compounds from edible algae: bioactivity and health benefits: bioactivity and Health Benefits. Curr. Med. Chem. 25 (37), 4808–4826. doi:10.2174/0929867324666170523120101
Montone, C. M., Capriotti, A. L., Cavaliere, C., La Barbera, G., Piovesana, S., Zenezini Chiozzi, R., et al. (2018). Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in Tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 410 (15), 3573–3586. doi:10.1007/s00216-018-0925-x
Morry, J., Ngamcherdtrakul, W., and Yantasee, W. (2017). Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 11, 240–253. doi:10.1016/j.redox.2016.12.011
Mustafi, S., and Wang, G. (2019). Vitamin C: epigenetic roles and cancer. Molecular nutrition: vitamins. Academic Press. doi:10.1016/B978-0-12-811907-5.00003-8
Mut-Salud, N., Álvarez, P. J., Aránega, A., Garrido, J. M., Carrasco, E., and Rodríguez-Serrano, F. (2015). Antioxidant intake and antitumor therapy: toward nutritional recommendations for Optimal results. Oxidative Med. Cell Longevity 2016, 6719534.
Muthuirulappan, S., and Francis, S. P. (2013). Anti-cancer mechanism and possibility of nano-suspension formulations for a marine algae product fucoxanthin. Asian Pac. J. Cancer Prev. 14 (4), 2213–2216. doi:10.7314/apjcp.2013.14.4.2213
Nagaraj, S., Rajaram, M. G., Arulmurugan, P., Baskaraboopathy, A., Karuppasamy, K., Jayappriyan, K. R., et al. (2012). Antiproliferative potential of astaxanthin-rich alga Haematococcus pluvialis flotow on human hepatic cancer (HepG2) cell line. Biomed. Prev. Nutr. 2 (3), 149–153. doi:10.1016/j.bionut.2012.03.009
Neumann, U., Derwenskus, F., Flister, V. F., Schmid-Staiger, U., Hirth, T., and Bischoff, S. C. (2019). Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in Vitro. Antioxidants 8 (6), 183. doi:10.3390/antiox8060183
Niki, E. (2016). Oxidative stress and antioxidants: distress or eustress? Arch. Biochem. Biophys. 595, 19–24. doi:10.1016/j.abb.2015.11.017
Nimse, S. B., and Pal, D. (2015). Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 5 (35), 27986–28006. doi:10.1039/c4ra13315c
Nobre, B., Marcelo, F., Passos, R., Beirão, L., Palavra, A., Gouveia, L., et al. (2006). Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 223 (6), 787–790. doi:10.1007/s00217-006-0270-8
Omar, H. H., Al-Judaibiand, A., and El-Gendy, A. (2018). Antimicrobial, antioxidant, anticancer activity and phytochemical analysis of the red alga, Laurencia papillosa. Int. J. Pharmacol. 14 (4), 572–583. doi:10.3923/ijp.2018.572.583
Oun, R., Moussa, Y., and Wheate, N. (2018). The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 47, 6645–6653. doi:10.1039/C8DT00838H
Padayatty, S. J., and Levine, M. (2016). Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 22 (6), 463–493. doi:10.1111/odi.12446
Pagels, F., Guedes, A. C., Amaro, H. M., Kijjoa, A., and Vasconcelos, V. (2019). Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications: chemistry and biotechnological applications Biotechnol. Adv. 37(3): 422–443. doi:10.1016/j.biotechadv.2019.02.010
Palozza, P., Maggiano, N., Calviello, G., Lanza, P., Piccioni, E., Ranelletti, F. O., et al. (1998). Canthaxanthin induces apoptosis in human cancer cell lines. Carcinogenesis 19 (2), 373–376. doi:10.1093/carcin/19.2.373
Pan-utai, W., and Iamtham, S. (2019). Extraction, purification and antioxidant activity of phycobiliprotein from arthrospira platensis. Process Biochem. 82, 189–198. doi:10.1016/j.procbio.2019.04.014
Pasquet, V., Morisset, P., Ihammouine, S., Chepied, A., Aumailley, L., Berard, J. B., et al. (2011). Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 9 (5), 819–831. doi:10.3390/md9050819
Patias, L. D., Fernandes, A. S., Petry, F. C., Mercadante, A. Z., Jacob-Lopes, E., and Zepka, L. Q. (2017). Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res. Int. 100, 260–266. doi:10.1016/j.foodres.2017.06.069
Patil, R. Y., and More, H. N. (2020). Antioxidants with multivitamin and mineral supplementation attenuates chemotherapy or radiotherapy-induced oxidative stress in cancer patients. Indian J. Pharm. Educ. Res. 54 (2), 484–490. doi:10.5530/ijper.54.2.55
Peh, H. Y., Daniel Tan, W. S., Liao, W., and Fred Wong, W. S. (2016). Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol. Ther. 162, 152–169. doi:10.1016/j.pharmthera.2015.12.003
Peppone, L. J., Inglis, J. E., Mustian, K. M., Heckler, C. E., A Padula, G. D., Mohile, S. G., et al. (2019). Multicenter randomized controlled trial of omega-3 fatty acids versus omega-6 fatty acids for the control of cancer-related fatigue among breast cancer survivors. JNCI Cancer Spectr. 3 (2), pkz005. doi:10.1093/jncics/pkz005
Peraman, M., and Nachimuthu, S. (2019). Identification and quantification of fucoxanthin in selected carotenoid-producing marine microalgae and evaluation for their chemotherapeutic potential. Pharmacognosy Mag. 15, S243–S249. doi:10.4103/pm.pm_64_19
Perillo, B., Di Donato, M., Pezone, A., Di Zazzo, E., Giovannelli, P., Galasso, G., et al. (2020). ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 52 (2), 192–203. doi:10.1038/s12276-020-0384-2
Pina, A. L., Costa, A. R., Lage-Yusty, M. A., and López-Hernández, J. (2014). An evaluation of edible red seaweed (chondrus crispus) components and their modification during the cooking process. LWT-Food Sci. Technol. 56 (1), 175–180. doi:10.1016/j.lwt.2013.08.006
Pintova, S., Dharmupari, S., Moshier, E., Zubizarreta, N., Ang, C., and Holcombe, R. F. (2019). Genistein combined with FOLFOX or FOLFOX–bevacizumab for the treatment of metastatic colorectal cancer: phase I/II pilot study. Cancer Chemother. Pharmacol. 84 (3), 591–598. doi:10.1007/s00280-019-03886-3
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., et al. (2017). Oxidative stress: harms and benefits for human health. Oxid Med. Cel Longev 2017, 8416763–8416813. doi:10.1155/2017/8416763
Rafi, M. M., Kanakasabai, S., Gokarn, S. V., Krueger, E. G., and Bright, J. J. (2015). Dietary lutein modulates growth and survival genes in prostate cancer cells. J. Med. Food 18 (2), 173–181. doi:10.1089/jmf.2014.0003
Raikar, S. M., Kalebar, V. U., and Adhoni, S. A. (2018). Screening of pharmacological and cytotoxic activities of fresh water lake isolated microalgae Chlorella vulgaris as-13 and Chlorella pyrenoidosa AS-6. Int. J. Bio-Technology Res. 8 (4), 1–8.
Raizner, A. E. (2019). Coenzyme Q10. Methodist Debakey Cardiovasc. J. 15 (3), 185–191. doi:10.1016/j.jchf.2014.12.006
Raman, R., and Mohamad, S. E. (2012). Astaxanthin production by freshwater microalgae Chlorella sorokiniana and marine microalgae Tetraselmis sp. Pak J. Biol. Sci. 15 (24), 1182–1186. doi:10.3923/pjbs.2012.1182.1186
Ramprasath, V. R., and Awad, A. B. (2015). Role of phytosterols in cancer prevention and treatment. J. AOAC Int. 98 (3), 735–738. doi:10.5740/jaoacint.SGERamprasath
Randhir, A., Laird, D. W., Maker, G., Trengove, R., and Moheimani, N. R. (2020). Microalgae: a potential sustainable commercial source of sterols. Algal Res. 46, 101772. doi:10.1016/j.algal.2019.101772
Rao, A. R., Sarada, R., Baskaran, V., and Ravishankar, G. A. (2006). Antioxidant activity of Botryococcus braunii extract elucidated in Vitro models. J. Agric. Food Chem. 54 (13), 4593–4599. doi:10.1021/jf060799j
Raza, M. H., Siraj, S., Arshad, A., Waheed, U., Aldakheel, F., Alduraywish, S., et al. (2017). ROS-Modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 143 (9), 1789–1809. doi:10.1007/s00432-017-2464-9
Reczek, C. R., and Chandel, N. S. (2017). The two faces of reactive oxygen species in cancer. Annu. Rev. Cancer Biol. 1 (1), 79–98. doi:10.1146/annurev-cancerbio-041916-065808
Régnier, P., Bastias, J., Rodriguez-Ruiz, V., Caballero-Casero, N., Caballo, C., Sicilia, D., et al. (2015). Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 13 (5), 2857–2874. doi:10.3390/md13052857
Reyes-Farias, M., and Carrasco-Pozo, C. (2019). The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int. J. Mol. Sci. 20 (13), 3177. doi:10.3390/ijms20123177
Rijstenbil, J. W. (2003). Effects of UVB radiation and salt stress on growth, pigments and antioxidative defence of the marine diatom Cylindrotheca closterium. Mar. Ecol. Prog. Ser. 254, 37–48. doi:10.3354/meps254037
De Roeck-Holtzhauer, Y., Quere, I., and Claire, C. (1991). Vitamin Analysis of five planktonic microalgae and one macroalga. J. Appl. Phycology 3 (3), 259–264. doi:10.1007/BF00003584
Rosa, A., Deidda, D., Serra, A., Deiana, M., A Dessì, M., and Pompei, R. (2005). Omega-3 fatty acid composition and biological activity of three microalgae species. J. Food Agric. Environ. 3 (2), 120–124.
Ryckebosch, E., Bruneel, C., Muylaert, K., and Foubert, I. (2012). Microalgae as an alternative source of omega-3 long chain polyunsaturated fatty acids. Lipid Technol. 24 (6), 128–130. doi:10.1002/lite.201200197
Saed, G. M., Diamond, M. P., and Fletcher, N. M. (2017). Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol. Oncol. 145 (3), 595–602. doi:10.1016/j.ygyno.2017.02.033
Sajilata, M. G., Singhal, R. S., and Kamat, M. Y. (2008). The carotenoid pigment zeaxanthin–a review. Compr. Rev. Food Sci. Food Saf. 7 (1), 29–49. doi:10.1111/j.1541-4337.2007.00028.x
Sanjeewa, K. K. A., Fernando, I. P. S., Samarakoon, K. W., Lakmal, H. H. C., Kim, E. A., Kwon, O. N., et al. (2016). Anti-Inflammatory and anti-cancer activities of sterol rich fraction of cultured marine microalga Nannochloropsis oculata. Algae 31 (3), 277–287. doi:10.4490/algae.2016.31.6.29
Sansone, C., and Brunet, C. (2019). Promises and challenges of microalgal antioxidant production. Antioxidants 8 (7), 199. doi:10.3390/antiox8070199
Sansone, C., Galasso, C., Orefice, I., Nuzzo, G., Luongo, E., Cutignano, A., et al. (2017). The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Scientific Rep. 7, 41215. doi:10.1038/srep41215
Sanusi, R. S. (2019). Outcome of combined neoadjuvant chemotherapy and vitamin A in advanced cervical carcinoma: a randomized double-blind clinical trial. Asian Pac. J. Cancer Prev. 20 (7), 2213–2218. doi:10.31557/APJCP.2019.20.7.2213
Satheesh, N. J., Samuel, S. M., and Büsselberg, D. (2020). Combination therapy with vitamin C could eradicate cancer stem cells. Biomolecules 10 (1), 79. doi:10.3390/biom10010079
Sayegh, F., Elazzazy, A., Bellou, S., Moustogianni, A., Elkady, A. I., Baeshen, M. N., et al. (2016). Production of polyunsaturated single cell oils possessing antimicrobial and anticancer properties. Ann. Microbiol. 66 (3), 937–948. doi:10.1007/s13213-015-1176-0
Seshadri, C. V., Umesh, B. V., and Manoharan, R. (1991). Beta-Carotene studies in Spirulina. Bioresour. Technol. 38 (2–3), 111–113. doi:10.1016/0960-8524(91)90140-F
Shafiee, G., Saidijam, M., Tayebinia, H., and Khodadadi, I. (2020). Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch. Physiol. Biochem., 1–9. doi:10.1080/13813455.2020.1717541
Shah, M. M., Liang, Y., Cheng, J. J., and Daroch, M. (2016). Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front. Plant Sci. 7, 531. doi:10.3389/fpls.2016.00531
Shi, X. M., Jiang, Y., and Chen, F. (2002). High-Yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol. Prog. 18 (4), 723–727. doi:10.1021/bp0101987
Shrivastava, A., Aggarwal, L. M., Mishra, S. P., and Khanna, H. D. (2019). Free radicals and antioxidants in normal versus cancerous cells—an overview. Indian J. Biochem. Biophys. 56, 7–19.
Sies, H. (2019). Oxidative stress: eustress and distress in redox homeostasis. Stress: physiology, biochemistry, and pathology. Academic Press. doi:10.1016/B978-0-12-813146-6.00013-8
Sies, H. (2020). Oxidative eustress and oxidative distress: introductory remarks. Oxidative stress. Academic Press. doi:10.1016/b978-0-12-818606-0.00001-8
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative stress. Annu. Rev. Biochem. 86, 715–748. doi:10.1146/annurev-biochem-061516-045037
Singh, D., Puri, M., Wilkens, S., Mathur, A. S., Tuli, D. K., and Barrow, C. J. (2013). Characterization of a new zeaxanthin producing strain of Chlorella saccharophila isolated from New Zealand marine waters. Bioresour. Technol. 143, 308–314. doi:10.1016/j.biortech.2013.06.006
Singh, D. P., Khattar, J. S., Rajput, A., Chaudhary, R., and Singh, R. (2019). High production of carotenoids by the green microalga Asterarcys quadricellulare PUMCC 5.1.1 under optimized culture conditions. Plos One 14 (9), e0221930. doi:10.1371/journal.pone.0221930
Soares, A. T., da Costa, D. C., Vieira, A. A. H., and Filho, N. R. A. (2019). Analysis of major carotenoids and fatty acid composition of freshwater microalgae. Heliyon 5 (4), e01529. doi:10.1016/j.heliyon.2019.e01529
Soares, J. D. P., Howell, S. L., Teixeira, F. J., and Pimentel, G. D. (2020). Dietary amino acids and immunonutrition supplementation in cancer-induced skeletal muscle mass depletion: a mini review. Curr. Pharm. Des. 26 (9), 970–978. doi:10.2174/1381612826666200218100420
Song, M., Ou, F. S., Zemla, T. J., Hull, M. A., Shi, Q., Limburg, P. J., et al. (2019). Marine omega-3 fatty acid intake and survival of stage III colon cancer according to tumor molecular markers in NCCTG phase III trial N0147 (alliance). Int. J. Cancer 145 (2), 380–389. doi:10.1002/ijc.32113
Soontornchaiboon, W., Joo, S. S., and Kim, S. M. (2012). Anti-Inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 35 (7), 1137–1144. doi:10.1248/bpb.b12-00187
Soto-Sierra, L., Stoykova, P., and Nikolov, Z. L. (2018). Extraction and fractionation of microalgae-based protein products. Algal Res. 36, 175–192. doi:10.1016/j.algal.2018.10.023
Srinivas, U. S., Tan, B. W. Q., Vellayappan, B. A., and Jeyasekharan, A. D. (2019). ROS and the DNA damage response in cancer. Redox Biol. 25, 101084. doi:10.1016/j.redox.2018.101084
Su, J., Guo, K., Huang, M., Liu, Y., Zhang, J., Sun, L., et al. (2019). Fucoxanthin, a marine xanthophyll isolated from Conticribra weissflogii ND-8: preventive anti-inflammatory effect in a mouse model of sepsis. Front. Pharmacol. 10, 906–917. doi:10.3389/fphar.2019.00906
Sugawara, T., Ganesan, P., Li, Z., Manabe, Y., and Hirata, T. (2014). Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs 12 (6), 3660–3668. doi:10.3390/md12063660
Sun, L., Chu, J., Sun, Z., and Chen, L. (2016). Physicochemical properties, immunomodulation and antitumor activities of polysaccharide from Pavlova viridis. Life Sci. 144, 156–161. doi:10.1016/j.lfs.2015.11.013
Sun, L., Wang, L., Li, J., and Liu, H. (2014). Characterization and antioxidant activities of degraded polysaccharides from two marine chrysophyta. Food Chem. 160, 1–7. doi:10.1016/j.foodchem.2014.03.067
Sun, P., Wong, C. C., Li, Y., He, Y., Mao, X., Wu, T., et al. (2019). A novel strategy for isolation and purification of fucoxanthinol and fucoxanthin from the diatom Nitzschia laevis. Food Chem. 277, 566–572. doi:10.1016/j.foodchem.2018.10.133
Surai, P. F., Karadas, F., and Sparks, N. H. (2003). “The importance of antioxidants in poultry.,” in 19th annual Carolina poultry conference, NC, USA, July 6–9, 2003, 38–56.
Swaminath, S., Um, C. Y., Prizment, A. E., Lazovich, D. A., and Bostick, R. M. (2019). Combined mineral intakes and risk of colorectal cancer in postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 28 (2), 392–399. doi:10.1158/1055-9965.EPI-18-0412
Tafazoli, A. (2017). Coenzyme Q10 in breast cancer care. Future Oncol. 13 (11), 1035–1041. doi:10.2217/fon-2016-0547
Tanaka, T., Makita, H., Ohnishi, M., Mori, H., Satoh, K., and Hara, A. (1995). Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Res. 55 (18), 4059–4064.
Tang, S. M., Deng, X. T., Zhou, J., Li, Q. P., Ge, X. X., and Miao, L. (2020). Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 121, 109604. doi:10.1016/j.biopha.2019.109604
Tannin-Spitz, T., Bergman, M., Van-Moppes, D., Grossman, S., and Arad, S. (2005). Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycology 17 (3), 215–222. doi:10.1007/s10811-005-0679-7
Terasaki, M., Asai, A., Zhang, H., and Nagao, A. (2007). A highly polar xanthophyll of 9′-cis -neoxanthin induces apoptosis in HCT116 human colon cancer cells through mitochondrial dysfunction. Mol. Cell Biochem. 300 (1–2), 227–237. doi:10.1007/s11010-006-9387-0
Tonegawa, I., Okada, S., Murakami, M., and Yamaguchi, K. (1998). Pigment composition of the green microalga Botryococcus braunii Kawaguchi-1. Fish. Sci. 64 (2), 305–308. doi:10.2331/fishsci.64.305
Torres, P., Nagai, A., Teixeira, D. I. A., Marinho-Soriano, E., Chow, F., and dos Santos, D. Y. A. C. (2019). Brazilian native species of gracilaria (gracilariales, rhodophyta) as a source of valuable compounds and as nutritional supplements. J. Appl. Phycology 31 (5), 3163–3173. doi:10.1007/s10811-019-01804-x
Trabelsi, L., Chaieb, O., Mnari, A., Abid-Essafi, S., and Aleya, L. (2016). Partial characterization and antioxidant and antiproliferative activities of the aqueous extracellular polysaccharides from the thermophilic microalgae graesiella sp. BMC Complement. Altern. Med. 16 (1), 210. doi:10.1186/s12906-016-1198-6
Tripathi, S. K., Pandey, K., Panda, M., Spinella, M. J., Rengasamy, K. R., and Biswal, B. K. (2019). The potential of retinoids for combination therapy of lung cancer: updates and future directions. Pharmacol. Res. 147, 104331. doi:10.1016/j.phrs.2019.104331
Tuli, H. S., Tuorkey, M. J., Thakral, F., Sak, K., Kumar, M., Sharma, A. K., et al. (2019). Molecular mechanisms of action of genistein in cancer: recent advances. Front. Pharmacol. 10, 1336. doi:10.3389/fphar.2019.01336
Urashima, M., Okuyama, M., Akutsu, T., Ohdaira, H., Kaji, M., and Suzuki, Y. (2020). Effect of vitamin D supplementation on survival of digestive tract cancer patients with low bioavailable 25-hydroxyvitamin d levels: a post hoc analysis of the AMATERASU randomized clinical trial. Cancers 12 (2), 347. doi:10.3390/cancers12020347
Uribe-Wandurraga, Z. N., Igual, M., García-Segovia, P., Martínez-Monzó, J., and Martínez-Monzó, J. (2020). In vitro bioaccessibility of minerals from microalgae-enriched cookies. Food Funct. 11 (3), 2186–2194. doi:10.1039/c9fo02603g
Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T. D., Mazur, M., and Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cel Biol. 39 (1), 44–84. doi:10.1016/j.biocel.2006.07.001
van Breda, S. G. J., and de Kok, T. M. C. M. (2018). Smart combinations of bioactive compounds in fruits and vegetables may guide new strategies for personalized prevention of chronic diseases. Mol. Nutr. Food Res. 62(1): 1700597. doi:10.1002/mnfr.201700597
van der Meij, B. S., Teleni, L., Engelen, M. P. K. J., and Deutz, N. E. P. (2019). Amino acid kinetics and the response to nutrition in patients with cancer. Int. J. Radiat. Biol. 95 (4), 480–492. doi:10.1080/09553002.2018.1466209
Venugopal, V. (2019). Sulfated and non-sulfated polysaccharides from seaweeds and their uses: an Overview. EC Nutr. 14 (2), 126–141.
Vilimanovich, U., and Jevremovic, S. A. (2019). Dihydroquercetin: a novel potent flavonoid antioxidant as an adjuvant for effective cancer treatment. EC Nutr. 14 (9), 660–674.
Villani, V., Zucchella, C., Cristalli, G., Galie, E., Bianco, F., Giannarelli, D., et al. (2016). Vitamin E neuroprotection against cisplatin ototoxicity: preliminary results from a randomized, placebo-controlled trial. Head Neck 38 (Suppl. 1), E2118–E2121. doi:10.1002/HED
Vinjamuri, S., Dontaraju, V. S., and Munirathinam, G. (2019). Prostate cancer and applications of vitamin K. Molecular nutrition: vitamins. Academic Press. doi:10.1016/B978-0-12-811907-5.00027-0
Vo, T. S., Ryu, B. M., and Kim, S. K. (2013). Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Foods 5 (3), 1336–1346. doi:10.1016/j.jff.2013.05.001
Wall-Medrano, A., and Olivas-Aguirre, F. J. (2020). Antioxidant phytochemicals in cancer prevention and therapy—an update. Functional foods in cancer prevention and therapy. Academic Press. doi:10.1016/b978-0-12-816151-7.00011-9
Wang, F., Huang, L., Gao, B., and Zhang, C. (2018). Optimum production conditions, purification, identification, and antioxidant activity of violaxanthin from microalga Eustigmatos Cf. Polyphem (eustigmatophyceae). Mar. Drugs 16 (6), 190. doi:10.3390/md16060190
Wang, H. M., Pan, J. L., Chen, C. Y., Chiu, C. C., Yang, M. H., Chang, H. W., et al. (2010). Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 45 (12), 1865–1872. doi:10.1016/j.procbio.2010.05.023
Wang, J., Guo, H., Lin, T., Song, Y., Zhang, H., Wang, B., et al. (2019). A nested case–control study on plasma vitamin E and risk of cancer: evidence of effect modification by selenium. J. Acad. Nutr. Diet. 119 (5), 769–781. doi:10.1016/j.jand.2018.11.017
Wang, L., Thilina, U. J., Yang, H.-W., Lee, H. G., Kang, M.-C., Sanjeewa, K. K. A., et al. (2020). Isolation, characterization, and antioxidant activity evaluation of a fucoidan from an enzymatic digest of the edible seaweed, hizikia fusiforme. Antioxidants 9, 363.
Wang, Q., and He, C. (2020). Dietary vitamin A intake and the risk of ovarian cancer: a meta-analysis. Biosci. Rep. 40, BSR20193979. doi:10.1042/BSR20193979
Wei, G., Wang, M., Hyslop, T., Wang, Z., and Carr, B. I. (2010). Vitamin K enhancement of sorafenib-mediated HCC cell growth inhibition in vitro and in vivo. Int. J. Cancer 127 (12), 2949–2958. doi:10.1002/ijc.25498
Wu, L. C., Annie Ho, J. A., Shieh, M. C., and Lu, I. W. (2005). Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J. Agric. Food Chem. 53 (10), 4207–4212. doi:10.1021/jf0479517
Xia, S., Wang, K., Wan, L., Li, A., Hu, Q., and Zhang, C. (2013). Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 11 (7), 2667–2681. doi:10.3390/md11072667
Xie, L., Song, Y., Lin, T., Guo, H., Wang, B., Tang, G., et al. (2019). Association of plasma retinol levels with incident cancer risk in Chinese hypertensive adults: a nested case-control study. Br. J. Nutr. 122 (3), 293–300. doi:10.1017/S000711451900120X
Xiong, F., Kopecky, J., and Nedbal, L. (1999). The occurrence of UV-B absorbing mycosporine-like amino acids in freshwater and terrestrial microalgae (chlorophyta). Aquat. Bot. 63 (1), 37–49. doi:10.1016/S0304-3770(98)00106-5
Xu, J., Xu, L. L., Zhou, Q. W., Hao, S. X., Zhou, T., and Xie, H. J. (2016). Enhanced in Vitro antioxidant activity of polysaccharides from enteromorpha prolifera by enzymatic degradation. J. Food Biochem. 40 (3), 275–283. doi:10.1111/jfbc.12218
Xu, S., Hamsath, A., Neill, D. L., Wang, Y., Yang, C. T., and Xian, M. (2019). Strategies for the design of donors and precursors of reactive sulfur species. Chem.A Eur. J. 25 (16), 4005–4016. doi:10.1002/chem.201804895
Yaich, H., Amira, A. B., Abbes, F., Bouaziz, M., Besbes, S., Richel, A., et al. (2017). Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol 105, 1430–1439. doi:10.1016/j.ijbiomac.2017.07.141
Yang, S., Wan, H., Wang, R., and Hao, D. (2019). Sulfated polysaccharides from Phaeodactylum tricornutum: isolation, structural characteristics, and inhibiting HepG2 growth activity in Vitro. PeerJ 7, e6409. doi:10.7717/peerj.6409
Yap, W. F., Tay, V., Tan, S. H., Yow, Y. Y., and Chew, J. (2019). Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 8 (3), 152. doi:10.3390/antibiotics8030152
Yu, B., Wang, J., Suter, P. M., Russell, R. M., Grusak, M. A., Wang, Y., et al. (2012). Spirulina is an effective dietary source of zeaxanthin to humans. Br. J. Nutr. 108 (4), 611–619. doi:10.1017/S0007114511005885
Yu, N., Su, X., Wang, Z., Dai, B., and Kang, J. (2015). Association of dietary vitamin A and β-carotene intake with the risk of lung cancer: a meta-analysis of 19 publications. Nutrients 7 (11), 9309–9324. doi:10.3390/nu7115463
Zainoddin, H. A. H., Hamzah, A., Jamari, Z., and Omar, W. A. W. (2018). Chemical profiles of methanolic extracts from two species of microalgae, Nannochloropsis sp. and Spirulina sp. Pertanika J. Trop. Agric. Sci. 41 (3), 1085–1096.
Zhang, D. M., Luo, Y., Yishake, D., Liu, Z. Y., He, T. T., Luo, Y., et al. (2020). Prediagnostic dietary intakes of vitamin A and β-carotene are associated with hepatocellular-carcinoma survival. Food Funct. 11 (1), 759–767. doi:10.1039/c9fo02468a
Zhang, J., Liu, L., Ren, Y., and Chen, F. (2019). Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int. J. Biol. Macromolecules 128, 761–767. doi:10.1016/j.ijbiomac.2019.02.009
Zhang, L. X., Cai, C. E., Guo, T. T., Gu, J. W., Xu, H. L., Zhou, Y., et al. (2011). Anti-Cancer effects of polysaccharide and phycocyanin from Porphyra yezoensis. J. Mar. Sci. Technol. 19 (4), 377–382.
Keywords: algae, antioxidant, cancer therapy, reactive species, dietary supplements, cancer
Citation: Ferdous UT and Yusof ZNB (2021) Medicinal Prospects of Antioxidants From Algal Sources in Cancer Therapy. Front. Pharmacol. 12:593116. doi: 10.3389/fphar.2021.593116
Received: 09 August 2020; Accepted: 19 January 2021;
Published: 05 March 2021.
Edited by:
Cara Haymaker, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Husain Yar Khan, Wayne State University, United StatesCopyright © 2021 Ferdous and Yusof. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zetty Norhana Balia Yusof, emV0dHlub3JoYW5hQHVwbS5lZHUubXk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.