
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 08 March 2021
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.586973
This article is part of the Research TopicOutcomes of Cardiovascular Drug Use in the Older PopulationView all 13 articles
 He-Ping Lei1,2,3†
He-Ping Lei1,2,3† Min Qin1,2,4†
Min Qin1,2,4† Li-Yun Cai1,2,5†
Li-Yun Cai1,2,5† Hong Wu6†
Hong Wu6† Lan Tang5
Lan Tang5 Ju-E Liu7
Ju-E Liu7 Chun-Yu Deng1,2
Chun-Yu Deng1,2 Yi-Bin Liu1,2
Yi-Bin Liu1,2 Qian Zhu1,2
Qian Zhu1,2 Han-Ping Li1,2
Han-Ping Li1,2 Wei Hu3
Wei Hu3 Min Yang1,2
Min Yang1,2 Yi-Zhun Zhu3*
Yi-Zhun Zhu3* Shi-Long Zhong1,2,4,7*
Shi-Long Zhong1,2,4,7*It is widely accepted that genetic polymorphisms impact atorvastatin (ATV) metabolism, clinical efficacy, and adverse events. The objectives of this study were to identify novel genetic variants influencing ATV metabolism and outcomes in Chinese patients with coronary artery disease (CAD). A total of 1079 CAD patients were enrolled and followed for 5 years. DNA from the blood and human liver tissue samples were genotyped using either Global Screening Array-24 v1.0 BeadChip or HumanOmniZhongHua-8 BeadChip. Concentrations of ATV and its metabolites in plasma and liver samples were determined using a verified ultra-performance liquid chromatography mass spectrometry (UPLC-MS/MS) method. The patients carrying A allele for the rs4148323 polymorphism (UGT1A1) showed an increase in 2-hydroxy ATV/ATV ratio (p = 1.69E−07, false discovery rate [FDR] = 8.66E−03) relative to the value in individuals without the variant allele. The result was further validated by an independent cohort comprising an additional 222 CAD patients (p = 1.08E−07). Moreover, the rs4148323 A allele was associated with an increased risk of death (hazard ratio [HR] 1.774; 95% confidence interval [CI], 1.031–3.052; p = 0.0198). In conclusion, our results suggested that the UGT1A1 rs4148323 A allele was associated with increased 2-hydroxy ATV formation and was a significant death risk factor in Chinese patients with CAD.
Atorvastatin (ATV), which reduces low-density lipoprotein cholesterol (LDL-C) by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, is among the most widely prescribed drugs for treating and preventing atherosclerotic disease events (Rosenson, 2006). The beneficial effects of ATV therapy in reducing the risk of cardiovascular morbidity and mortality have been well documented (Sever et al., 2003; Arca, 2007; Sillesen et al., 2008).
ATV is orally administered in the active acid form and is extensively metabolized by cytochrome P450 (CYP) 3A4 to form two major active metabolites, 2-hydroxy (2-OH) ATV and 4-hydroxy (4-OH) ATV (Park et al., 2008). Both metabolites are pharmacologically equivalent to parent ATV and significantly contribute to the circulating inhibitory activity for HMG-CoA reductase (Lennernas 2003). Glucuronidation, mediated via the enzymes UDP-glucuronosyltransferase (UGT) 1A1 and 1A3 (UGT1A1/3) in the liver, is the critical step in facilitating the conversion of the acid forms of ATV to the corresponding lactones (Prueksaritanont et al., 2002; Schirris et al., 2015). Thus, variations in the activities of drug metabolizing enzymes may result in lower or greater exposure to ATV.
Pharmacogenetic studies have shown that single-nucleotide polymorphisms (SNP) in genes related to absorption, distribution, metabolism and excretion (ADME) of drugs contribute to interindividual variability in drug efficacy and adverse effects (Lauschke et al., 2017; Guan et al., 2019). Failure to recognize these variants could lead to high systemic drug concentrations, which may increase rates of adverse events (Roden et al., 2019).
In this study, we focused particularly on the genes involved in ADME to identify novel genetic polymorphisms affecting plasma ATV and its metabolites concentrations and clinical outcomes of patients with coronary artery disease (CAD). Subsequently, we aimed to identify specific SNP associated with ATV metabolism in human liver microsomes (HLM).
This study was approved by the Medical Ethical Review Committee of Guangdong Provincial People's Hospital (Approval number GDREC2010137H) and Sun Yat-sen Memorial Hospital (Approval number CS07095) (Guangzhou, China), and conducted in accordance with the basic principles of the Declaration of Helsinki. All patients provided written informed consent.
A schematic diagram of this study was exposited in Figure 1. A total of 1079 CAD patients were categorized into two cohorts to discover and validate the effects of genetic variants on ATV metabolism and the risk of all-cause death. Thereafter, 55 HLM were enrolled to verify the effect of enzyme activity of UGT1A1 on ATV metabolism and the correlation between UGT1A1*6 and the formation rate of 2-OH ATV. All patients were sequentially enrolled in Guangdong Provincial People's Hospital between January 2010 and December 2013 according to the inclusion and exclusion criteria. Patients were followed up for all-cause death up to 5 years. CAD was defined as the presence of ≥50% stenosis in at least one major coronary artery based on coronary angiography. The inclusion criteria were patients with CAD aged 18–80 years who underwent percutaneous coronary intervention (PCI) and received ATV therapy. Exclusion criteria included renal impairment (serum creatinine >3 times the upper limit of normal (ULN), renal transplantation or dialysis); liver impairment (serum transaminase >3 times the ULN, or a diagnosis of cirrhosis); pregnancy or lactation; malignant disease; uncontrolled infection; worsening of any chronic disease; use of lipid-lowering drugs other than ATV.
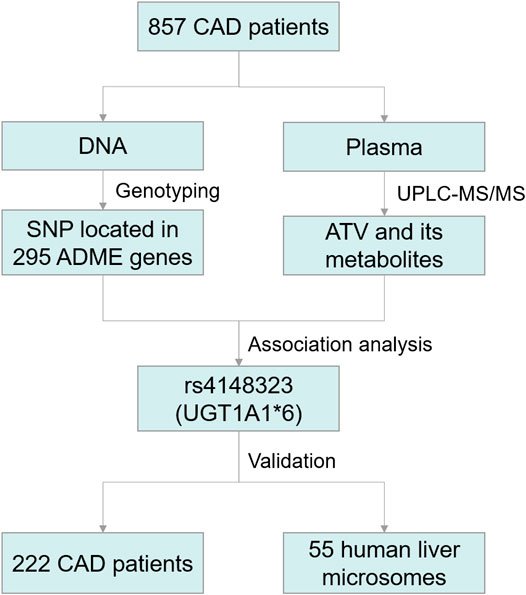
FIGURE 1. Schematic diagram of the Trial Protocol. CAD, coronary artery disease; ATV, atorvastatin; UGT1A1, UDP-glucuronosyltransferase 1A1; SNP, single-nucleotide polymorphisms; ADME, absorption, distribution, metabolism, and excretion.
All patients received ATV for at least seven consecutive days at a dose of 10–40 mg/day before blood samples were collected. The dose of ATV was chosen based on the discretion of the physician. Steady-state ATV concentrations could be reached after approximately 3 days (Cilla et al., 1996). Baseline medical information was collected from the hospital medical records, including demographics, medical history, biochemical measurements, and comedications. Drug compliance was monitored by contacting with the patients at hospitalization or hospital visit. Patients were contacted every 6 months via telephone for surveillance of all-cause death. Individuals who could not be contacted despite several attempts were considered as lost to follow-up.
Fasting venous blood (4 ml) was drawn into ethylenediaminetetraacetic acid (EDTA)-containing tubes 10–12 h after the last ATV dose. Samples were centrifuged 1900 g for 10 min at 4°C; plasma was collected and stored at −80°C until analysis.
The tumor resection margin of patients with liver cancer or the liver tissues of patients with benign liver diseases undergoing hepatectomy were collected at Sun Yat-Sen Memorial Hospital (Guangzhou, China) from September 2012 to May 2015 (n = 55). Specimens for microsome extraction were quickly prepared using the GENMED A Solution (GENMED Scientific Inc., Arlington, TX, United States) and stored in liquid nitrogen until use. HLM were prepared according to our previously published protocol (Liu et al., 2016). Protein concentration was determined by the Bradford protein assay kit (Bio-Rad, Hercules, CA, United States) with bovine serum albumin as standard.
Genomic DNA was extracted from blood samples using the TIANamp Genomic DNA Kit (Cat. no. DP304; TIANGEN Biotech, Beijing, China) per manufacturer's instructions. DNA quality and quantity were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States) and agarose gel electrophoresis, respectively.
In the discovery cohort, genotyping was performed for 857 DNA samples from patients with CAD on the Global Screening Array-24 v1.0 (GSA) BeadChip (Illumina Inc., San Diego, CA, United States) comprising 700,078 SNP. Genotyping procedures followed the Infinium HTS Assay protocol, and intensity data were normalized using Illumina's GenomeStudio software and calling algorithm. In the validation cohort comprising the other 222 patients with CAD, genotyping of UGT1A1 c.211G > A (rs4148323) was performed by TaqMan assay (Applied Biosystems, California, United States). DNA from the human liver samples (n = 55) were genotyped using the HumanOmniZhongHua-8 BeadChip (Illumina Inc., San Diego, CA, United States) comprising 900,015 SNP.
A standard quality control procedure was applied to the raw genotyping data to filter both unqualified SNP and samples prior to association analysis. Samples with call rates <95% were removed. SNP were excluded if they 1) did not map on autosomal chromosomes; 2) had a call rate <95%; 3) had a minor allele frequency (MAF) < 5%; and 4) were deviated from Hardy–Weinberg equilibrium (p-value < 1.0E−06). After quality control, 291194 SNP in the GSA BeadChip and 695778 SNP in the HumanOmniZhongHua-8 BeadChip were retained for analysis.
Concentrations of ATV and its acid (2-OH ATV and 4-OH ATV) and lactone metabolites (ATV lactone [ATV L], 2-OH ATV L and 4-OH ATV L) in plasma were measured by ultra-performance liquid chromatography mass spectrometry (UPLC-MS/MS). Our previous report has established the accuracy and reproducibility of this method (Cai et al., 2017).
The UGT1A1 activity in HLM was determined using the known substrate SN-38. The procedure was carried out based on our previously validated approach (Zhong et al., 2017).
A typical phase I and II enzymes mixing incubation system contains potassium phosphate buffer (50 mM, pH = 7.4), phase I Solution A and B, HLM (final concentration 0.35 mg/ml), ATV (final concentration 1.5 μg/ml), phase II Solution A and B in a total volume of 400 μL. Incubations were carried out for 60 min at 37°C in a shaking water bath. After the incubation, 60 µL ice-cold acetonitrile containing internal standard carbamazepine (100 ng/ml) were added to terminate the enzyme activity. All experiments were performed in triplicate. The samples were centrifuged at 15,000 g for 30 min at 4°C, and then ATV and its major metabolites in supernatant was analyzed by UPLC-MS/MS method as previously described (Cai et al., 2017).
Demographic and clinical characteristics were described as follows: continuous variables are presented as mean ± standard deviation (SD) and categorical variables are presented as counts (percentages). Normality was evaluated by the Shapiro–Wilk test. Natural-log transformation was performed prior to statistical analysis since the raw ATV analyte concentrations did not follow a normal distribution. Univariate linear regression analysis was used to assess the relationships between the baseline characteristics and plasma ATV concentration, and the significant characteristics (p-value < 0.05) were included into multivariate linear regression analysis.
In the discovery stage, SNP located in 295 candidate ADME genes from the PharmaADME website (http://www.pharmaadme.org/) were employed to association analysis. Chi-square test was used to estimate the Hardy-Weinberg equilibrium. Linear regression analysis under the additive mode was used to identify the associations between the candidate SNP and the concentrations of ATV, five metabolites (2-OH ATV, 4-OH ATV, ATV L, 2-OH ATV L and 4-OH ATV L) and five concentration ratios (2-OH ATV/ATV, 4-OH ATV/ATV, ATV L/ATV, 2-OH ATV L/ATV, 4-OH ATV L/ATV). In addition to sex, age and ATV dose, aspartate aminotransferase (AST) and creatinine (CREA) levels were also included for adjustment since they were significantly associated with plasma ATV concentration (Table 1). The linkage disequilibrium (LD) analyses were conducted to identify independent SNP between SNP pairs located in same chromosome and the r2 of two SNP exceeding 0.5 was considered in LD. The false discovery rate (FDR) was used to correct the number of SNP and association analyses for multiple hypothesis testing. The significant correlation (FDR < 0.05) between SNP and metabolite concentration ratio was repeatedly investigated in the validation cohort. For SNP pairs in LD, only the SNP with the most significant p value was selected.
Spearman correlation analysis was used to study the correlation between the UGT1A1 enzyme activity and the reduction of ATV as well as the formation rate of its five metabolites. To examine relations between the candidate SNP and the reduction of ATV as well as the formation of metabolites from ATV in 55 HLM, the independent sample t test or one-way ANOVA test was used for data conforming to normal distribution, whereas the nonparametric Mann–Whitney U or Kruskal–Wallis H test was used for data conforming to skewed distribution. Cox regression analysis was utilized to assess the association of SNP with all-cause death with results presented as hazard ratio (HR) and 95% confidence interval (CI). Cumulative event rates were estimated with the Kaplan–Meier method. A two-sided p-value < 0.05 was considered statistically significant.
All statistical analyses were carried out using PLINK (version 1.07, http://zzz.bwh.harvard.edu/plink/), R (version 3.4.3, https://www.r-project.org/) and GraphPad Prism 8 (GraphPad Software, San Diego, CA, United States).
Patients' demographic and clinical characteristics and their impact on the plasma ATV concentrations are presented in Table 1. In total, 1,079 Chinese patients with CAD who had received ATV therapy were sequentially recruited in the study and followed for 5 years. Univariate linear regression analysis indicated that patients with older age, higher ATV dose, higher levels of alanine aminotransferase (ALT), AST, CREA, CHOL and LDL-C tended to have a higher plasma ATV concentration, while patients with higher levels of high-density lipoprotein cholesterol (HDL-C) and apolipoprotein (a) [Apo (a)] tended to have a lower plasma ATV concentration. In the multivariate model, only ATV dose, AST and CREA levels remained independent predictors of plasma ATV concentrations (p = 2.69E−02, 1.80E−04 and 3.47E−04, respectively) in CAD patients (Table 1).
Ten SNP were found to have a significant effect on the concentration ratio of 2-ATV to ATV (FDR < 0.05, Table 2). Among these SNP, an exonic variant of rs4148323 in UGT1A1 was most strongly associated with an increase in the 2-OH ATV/ATV ratio. Five SNP (rs15524, rs4646457, rs4646450, rs776746 and rs4646458) in CYP3A5 also showed significant positive correlations with the formation of 2-OH ATV. Furthermore, an intergenic variant (rs10242455 between ZSCAN25 and CYP3A5) and three intronic variants (rs2687136 and rs2687134 in CYP3A7; rs3806598 in UGT1A10) were also significantly associated with 2-OH ATV/ATV ratio (Table 2). Further analysis indicated that rs4148323 was in strong LD with rs3806598, and rs15524 was in strong LD with the remaining seven loci located in chromosome 7 (r2 > 0.5). Finally, rs4148323 was further verified to be significantly correlated with 2-OH ATV/ATV ratio in an independent cohort comprising an additional 222 CAD patients (p = 1.08E−07, Figure 2A).

TABLE 2. Ten SNPs significantly associated with the concentration ratio of 2-OH ATV to ATV in 857 patients with CAD.
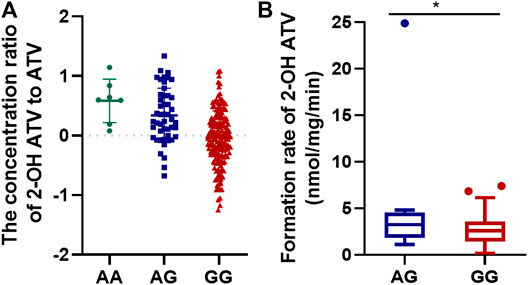
FIGURE 2. The effect of genotype of rs4148323 on the formation of 2-OH ATV in the 222 patients with CAD (A) and 55 HLM (B). ATV, atorvastatin; CAD, coronary artery disease; HLM, human liver microsomes.
To verify the effect of rs4148323 on the rates of formation of 2-OH ATV from ATV, the association between genotypes and 2-OH ATV formation rate was investigated in 55 HLM. The results showed that SNP rs4148323 in UGT1A1 was associated with changes in 2-OH ATV levels (5.30 ± 7.44 and 2.71 ± 1.68 nmol/mg/min for AG and GG, respectively; p = 0.026, Figure 2B).
Correlation between UGT1A1 activity and rates of microsomal metabolism of ATV and its metabolites are detailed in Figure 3. Higher UGT1A1 activity was associated with a markedly increased formation rates of 2-OH ATV, 4-OH ATV, 2-OH ATV L and 4-OH ATV L (r = 0.4208, p = 0.0026; r = 0.4285, p = 0.0021; r = 0.3476, p = 0.0144; r = 0.3512, p = 0.0133). In contrast, the activity of UGT1A1 was not correlated with the reduction rate of ATV and the formation rate of ATV L (p = 0.0805 and 0.8433, respectively) (Figure 3).
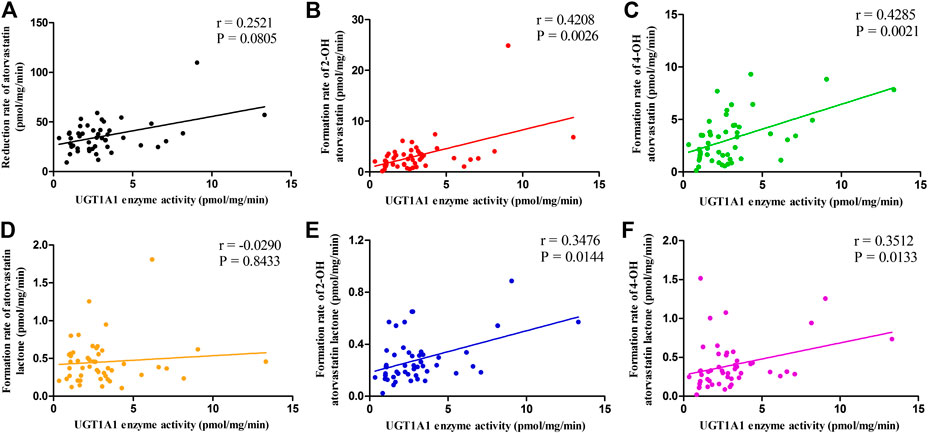
FIGURE 3. The correlations between UGT1A1 activity and the rates of microsomal metabolism of ATV (A), 2-OH ATV (B), 4-OH ATV (C), ATV L (D), 2-OH ATV L (E) and 4-OH ATV L (F). UGT1A1, UDP-glucuronosyltransferase 1A1; ATV, atorvastatin; ATV L, atorvastatin lactone.
In order to illustrate the genotype of rs4148323 whether has an effect on the poor prognosis of patients with CAD, we merged the discovery and validation cohorts to assess the association between genotypes and death risk. Due to the small number of patients with the AA genotype of rs4148323 (n = 16), the AG and AA individuals were grouped together into the AG + AA genotype group (the A allele carriers), for analysis. Kaplan-Meier survival analysis showed that the carriers of rs4148323 A allele have a higher risk of death than non-carriers (HR 1.774, 95% CI, 1.031–3.052; p = 0.0198) (Figure 4).
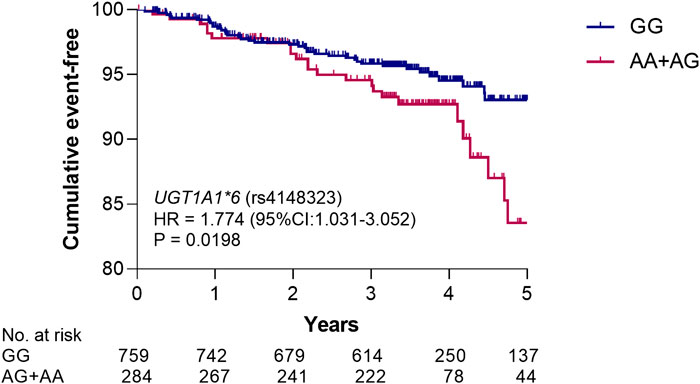
FIGURE 4. Kaplan–Meier analysis of rs4148323 for all-cause death risk. The p value was calculated by log-rank test. UGT1A1, UDP-glucuronosyltransferase 1A1; HR, hazard ratio; 95% CI, 95% confidence interval.
Our result showed that a variant of rs4148323, located in an UGT1A1 exon, increased the plasma ATV's active metabolite 2-OH ATV formation. This finding was further validated by an independent cohort comprising an additional 222 CAD patients and by the human liver microsome systems. Furthermore, the UGT1A1 rs4148323 A allele has a significantly higher risk of death in Chinese patients with CAD. Consequently, genotyping of rs4148323 might be useful for tailoring both the ATV dose and safety monitoring of CAD patients.
Despite tremendous progress due to lifestyle interventions and drug treatments, CAD remains one of the most significant cause of death worldwide (Georgia Karanasiou et al., 2018). ATV is a life-saving drug which leads to reduce cardiovascular events in patients with cardiovascular disease, providing substantial public health benefits (Crouch 2001). ATV exists in both the acid and lactone forms in vivo. The acid form is pharmacologically active, whereas the lactone form is inactive toward HMG-CoA reductase and has been associated with muscle-related adverse effects (Hermann et al., 2006; Skottheim et al., 2008). ATV-induced liver injury can be caused during ATV therapy. The higher hepatocellular concentration of ATV was found to increase the risk of hepatotoxicity since ATV induced cytotoxicity in HepaRG cells in a concentration-dependent manner (Fukunaga et al., 2016). We have previously shown that high plasma exposure of statins was associated with an increased risk of contrast-induced acute kidney injury in patients with CAD; therefore, statins should be used with caution in these patients (Cai et al., 2018). We also found that a higher plasma exposure of ATV and metabolites was linked to increased risk of death in CAD patients (Zhou et al., 2020).
Interindividual differences in efficacy of ATV may be caused not only by nongenetic factors, but also by genetic polymorphisms in drug metabolizing enzymes and transporters involved in ATV metabolism and elimination (Kivisto et al., 2004; Cho et al., 2012; Wei and Zhang 2015; Peng et al., 2018). UGT1A1 is an important member of the UGT1A family responsible for the conjugation and detoxification of numerous endogenous and exogenous compounds (Levesque et al., 2007). Defects in this enzyme result in unconjugated hyperbilirubinemia, such as Gilbert syndrome and Crigler–Najjar syndrome (Kadakol et al., 2000). The genetic polymorphism UGT1A1*6 (rs4148323, c.211G > A, Arg71Gly) is an exonic variant of the UGT1A1 gene on chromosome 2q37 and associated with reduced UGT1A1 activity (Bai et al., 2019). UGT1A1*6 is highly prevalent in East Asian populations but is absent in European and African populations (Dai et al., 2013). It has allele frequencies of 23%, 23%, 13%, and 0% among Chinese, Korean, Japanese, and German populations, respectively (Akaba et al., 1998). It was reported that one of the metabolic pathways of ATV is through UGT1A1-mediated glucuronidation (Schirris et al., 2015) and the A allele in UGT1A1 rs4148323 is associated with decreased UGT1A1 activity (Bai et al., 2019). Therefore, we speculated that the rs4148323 A allele might decrease glucuronidation activity for ATV and corresponding increase in 2-OH ATV production.
Many studies have reported genetic variants were associated with CAD pathogenesis (McPherson and Tybjaerg-Hansen 2016; Miao et al., 2018). Despite an enormous amount of research that has been done on the biological effect of UGT1A1 gene (Goon et al., 2016), few studies have assessed whether the rs4148323 SNP has a prognostic value on all-cause death among CAD patients. To our knowledge, we are the first to demonstrate that the rs4148323 A allele was associated with increased risk of death in CAD patients.
CYP3A5 is an important hepatic drug-metabolizing enzyme. Willrich et al. found that the CYP3A5*3A allele was associated with reduced cholesterol-lowering response to ATV in 139 non-African individuals with hypercholesterolemia (Willrich et al., 2008). In the present study, positive correlations were found between SNP (rs15524, rs4646457, rs4646450, rs776746 and rs4646458) in the CYP3A5 gene and the formation of 2-OH ATV. ATV and its active metabolites are subject to cellular membrane transport by organic anion-transporting polypeptide (OATP) transporters and P-glycoprotein (P-gp) (Bogman et al., 2001; Chen et al., 2005). Despite evidence that drug transporter polymorphisms could influence ATV metabolism (Lee et al., 2010; Wang et al., 2017), we did not observe such an association in vivo and the reason for this result is unclear.
Our study had two limitations. First, the study subjects were primarily Han ethnic Chinese, and that caution may be warranted in extrapolating our results to other populations. Second, the sample size was relatively small. In order to minimize the finding of false positive statistical associations, the p values were adjusted using the FDR.
In summary, the UGT1A1 rs4148323 A allele was found to be significantly associated with elevated 2-OH ATV formation, and might increase the risk of death in Chinese patients with CAD. The present study provides suggestive data, and genotyping large cohorts of CAD patients for rs4148323 in UGT1A1 gene will be required to unambiguously prove these findings.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: EMBL-EBI [Project: PRJEB42554; Analyses: ERZ1714343].
The studies involving human participants were reviewed and approved by the Medical Ethical Review Committee of Guangdong Provincial People’s Hospital and Sun Yat-sen Memorial Hospital (Guangzhou, China). The patients/participants provided their written informed consent to participate in this study.
H-PL, S-LZ, Y-ZZ, and HW wrote manuscript. S-LZ, Y-ZZ, H-PL, L-YC, LT, and MY designed research. MQ, L-YC, J-EL, H-PL, HW, C-YD, Y-BL, and QZ performed research. H-PL, MQ, QZ, L-YC, WH, and Y-BL analyzed data.
This study was funded by National Natural Science Foundation of China (No. 81872934, 81673514, 81202602, 81470440), the National key research and development program (No. 2017YFC0909301), and the Key research and development program of Guangdong Province, China (2019B020229003), Science and Technology Development Projects of Guangdong Province, China (No. 2017B0303314041), Science and Technology Program of Guangzhou, China (201510010282).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ATV atorvastatin
GLUC glucose
TRIG triglycerides
CHOL cholesterol
HDL-C high-density lipoprotein cholesterol
LDL-C low-density lipoprotein cholesterol
CREA creatinine
ALT alanine aminotransferase
AST aspartate aminotransferase
CK creatine kinase
CKMB creatine kinase MB
Lp (a) lipoprotein (a)
Apo (a) apolipoprotein (a)
CAD coronary artery disease
FDR false discovery rate
ADME absorption, distribution, metabolism, and excretion
CYP cytochrome P450
HLM human liver microsomes
HR hazard ratio
OR odds ratio
CI confidence interval
eGFR estimated glomerular filtration rate
MAF minor allele frequency
PCI percutaneous coronary intervention
CCB calcium channel blocker
ACEI angiotensin-converting enzyme inhibitor
PPI proton pump inhibitor
SNP single-nucleotide polymorphisms
LD linkage disequilibrium
UGT UDP-glucuronosyltransferase
ULN upper limit of normal
SD standard deviation
UPLC-MS/MS ultra-performance liquid chromatography mass spectrometry
HMG-CoA 3-hydroxy-3-methylglutaryl-coenzyme A
OATP organic anion transporting polypeptide
P-gp P-glycoprotein
Akaba, K., Kimura, T., Sasaki, A., Tanabe, S., Ikegami, T., Hashimoto, M., et al. (1998). Neonatal hyperbilirubinemia and mutation of the bilirubin uridine diphosphate-glucuronosyltransferase gene: a common missense mutation among Japanese, Koreans and Chinese. Biochem. Mol. Biol. Int. 46, 21–26. doi:10.1080/15216549800203512
Arca, M. (2007). Atorvastatin efficacy in the prevention of cardiovascular events in patients with diabetes mellitus and/or metabolic syndrome. Drugs 67 (Suppl. 1), 43–54. doi:10.2165/00003495-200767001-00005
Bai, J., Luo, L., Liu, S., Liang, C., Bai, L., Chen, Y., et al. (2019). Combined effects of UGT1A1 and SLCO1B1 variants on Chinese adult mild unconjugated hyperbilirubinemia. Front. Genet. 10, 1073. doi:10.3389/fgene.2019.01073
Bogman, K., Peyer, A. K., Torok, M., Kusters, E., and Drewe, J. (2001). HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br. J. Pharmacol. 132, 1183–1192. doi:10.1038/sj.bjp.0703920
Cai, L., Bai, X., Lei, H., Wu, H., Liu, Y., Zhu, Q., et al. (2018). High plasma exposure of statins associated with increased risk of contrast-induced acute kidney injury in Chinese patients with coronary artery disease. Front. Pharmacol. 9, 427. doi:10.3389/fphar.2018.00427
Cai, L., Zheng, Z., Wang, X., Tang, L., and Zhong, S. J. (2017). Simultaneous determination of atorvastatin and its metabolites in human plasma by UPLC-MS/MS. R. Soc. Chem., 9. 1038–1045. doi:10.1039/C6AY03113G
Chen, C., Mireles, R. J., Campbell, S. D., Lin, J., Mills, J. B., Xu, J. J., et al. (2005). Differential interaction of 3-hydroxy-3-methylglutaryl-coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab. Dispos 33, 537–546. doi:10.1124/dmd.104.002477
Cho, S. K., Oh, E. S., Park, K., Park, M. S., and Chung, J. Y. (2012). The UGT1A3*2 polymorphism affects atorvastatin lactonization and lipid-lowering effect in healthy volunteers. Pharmacogenet. Genomics. 22 (8), 598–605. doi:10.1097/FPC.0b013e3283544085
Cilla, D. D., Whitfield, L. R., Gibson, D. M., Sedman, A. J., and Posvar, E. L. (1996). Multiple-dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy subjects. Clin. Pharmacol. Ther. 60, 687–695. doi:10.1016/S0009-9236(96)90218-0
Crouch, M. A (2001). Effective use of statins to prevent coronary heart disease. Am. Fam. Physician 63, 323–324.
Dai, X., Wu, C., He, Y., Gui, L., Zhou, L., Guo, H., et al. (2013). A genome-wide association study for serum bilirubin levels and gene-environment interaction in a Chinese population. Genet. Epidemiol. 37, 293–300. doi:10.1002/gepi.21711
Fukunaga, K., Nakagawa, H., Ishikawa, T., Kubo, M., and Mushiroda, T. (2016). ABCB1 polymorphism is associated with atorvastatin-induced liver injury in Japanese population. BMC Genet. 17, 79. doi:10.1186/s12863-016-0390-5
Georgia Karanasiou, S., Nikolaos Tachos, S., Sakellarios, A., Conway, C., Pennati, G., Petrini, L., et al. (2018). In Silico analysis of stent deployment- effect of stent design. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 248, 4567–4570. doi:10.1109/EMBC.2018.8513205
Goon, C. P., Wang, L. Z., Wong, F. C., Thuya, W. L., Ho, P. C., and Goh, B. C. (2016). UGT1A1 mediated drug interactions and its clinical relevance. Curr. Drug Metab. 17, 100–106. doi:10.2174/1389200216666151103121253
Guan, Z. W., Wu, K. R., Li, R., Yin, Y., Li, X. L., Zhang, S. F., et al. (2019). Pharmacogenetics of statins treatment: efficacy and safety. J. Clin. Pharm. Ther. 44, 858–867. doi:10.1111/jcpt.13025
Hermann, M., Bogsrud, M. P., Molden, E., Asberg, A., Mohebi, B. U., Ose, L., et al. (2006). Exposure of atorvastatin is unchanged but lactone and acid metabolites are increased several-fold in patients with atorvastatin-induced myopathy. Clin. Pharmacol. Ther. 79, 532–539. doi:10.1016/j.clpt.2006.02.014
Kadakol, A., Ghosh, S. S., Sappal, B. S., Sharma, G., Chowdhury, J. R., and Chowdhury, N. R. (2000). Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum. Mutat. 16, 297–306. doi:10.1002/1098-1004(200010)16:4<297::Aid-humu2>3.0.Co;2-z
Kivisto, K. T., Niemi, M., Schaeffeler, E., Pitkala, K., Tilvis, R., Fromm, M. F., et al. (2004). Lipid-lowering response to statins is affected by CYP3A5 polymorphism. Pharmacogenetics 14, 523–525. doi:10.1097/01.fpc.0000114762.78957.a5
Lauschke, V. M., Milani, L., and Ingelman-Sundberg, M. (2017). Pharmacogenomic biomarkers for improved drug therapy-recent progress and future developments. AAPS J. 20, 4. doi:10.1208/s12248-017-0161-x
Lee, Y. J., Lee, M. G., Lim, L. A., Jang, S. B., and Chung, J. Y. (2010). Effects of SLCO1B1 and ABCB1 genotypes on the pharmacokinetics of atorvastatin and 2-hydroxyatorvastatin in healthy Korean subjects. Int. J. Clin. Pharmacol. Ther. 48, 36–45. doi:10.5414/cpp48036
Lennernas, H. (2003). Clinical pharmacokinetics of atorvastatin. Clin. Pharmacokinet. 42, 1141–1160. doi:10.2165/00003088-200342130-00005
Levesque, E., Girard, H., Journault, K., Lepine, J., and Guillemette, C. (2007). Regulation of the UGT1A1 bilirubin-conjugating pathway: role of a new splicing event at the UGT1A locus. Hepatology 45, 128–138. doi:10.1002/hep.21464
Liu, J. E., Ren, B., Tang, L., Tang, Q. J., Liu, X. Y., Li, X., et al. (2016). The independent contribution of miRNAs to the missing heritability in CYP3A4/5 functionality and the metabolism of atorvastatin. Sci. Rep. 6, 26544. doi:10.1038/srep26544
McPherson, R., and Tybjaerg-Hansen, A. (2016). Genetics of coronary artery disease. Circ. Res. 118, 564–578. doi:10.1161/CIRCRESAHA.115.306566
Miao, X., Chen, X., Xie, Z., and Lin, H. (2018). Tissue-specific network analysis of genetic variants associated with coronary artery disease. Sci. Rep. 8, 11492. doi:10.1038/s41598-018-29904-7
Park, J. E., Kim, K. B., Bae, S. K., Moon, B. S., Liu, K. H., and Shin, J. G. (2008). Contribution of cytochrome P450 3A4 and 3A5 to the metabolism of atorvastatin. Xenobiotica 38, 1240–1251. doi:10.1080/00498250802334391
Peng, C., Ding, Y., Yi, X., Shen, Y., Dong, Z., Cao, L., et al. (2018). Polymorphisms in CYP450 genes and the therapeutic effect of atorvastatin on ischemic stroke: a retrospective cohort study in Chinese population. Clin. Ther. 40, 469–477. doi:10.1016/j.clinthera.2018.02.002
Prueksaritanont, T., Subramanian, R., Fang, X., Ma, B., Qiu, Y., Lin, J. H., et al. (2002). Glucuronidation of statins in animals and humans: a novel mechanism of statin lactonization. Drug Metab. Dispos 30, 505–512. doi:10.1124/dmd.30.5.505
Roden, D. M., McLeod, H. L., Relling, M. V., Williams, M. S., Mensah, G. A., Peterson, J. F., et al. (2019). Pharmacogenomics Lancet 394, 521–532. doi:10.1016/S0140-6736(19)31276-0
Rosenson, R. S. (2006). Low high-density lipoprotein cholesterol and cardiovascular disease: risk reduction with statin therapy. Am. Heart J. 151, 556–563. doi:10.1016/j.ahj.2005.03.049
Schirris, T. J., Ritschel, T., Bilos, A., Smeitink, J. A., and Russel, F. G. (2015). Statin lactonization by uridine 5'-Diphospho-glucuronosyltransferases (UGTs). Mol. Pharm. 12, 4048–4055. doi:10.1021/acs.molpharmaceut.5b00474
Sever, P. S., Dahlöf, B., Poulter, N. R., Wedel, H., Beevers, G., Caulfield, M., et al. (2003). Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361, 1149–1158. doi:10.1016/s0140-6736(03)12948-0
Sillesen, H., Amarenco, P., Hennerici, M. G., Callahan, A., Goldstein, L. B., Zivin, J., et al. (2008). Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke 39, 3297–3302. doi:10.1161/STROKEAHA.108.516450
Skottheim, I. B., Gedde-Dahl, A., Hejazifar, S., Hoel, K., and Asberg, A. (2008). Statin induced myotoxicity: the lactone forms are more potent than the acid forms in human skeletal muscle cells in vitro. Eur. J. Pharm. Sci. 33, 317–325. doi:10.1016/j.ejps.2007.12.009
Wang, Y., Tian, Y., Lv, P., Chen, L., Luo, W., Jing, X., et al. (2017). The effect of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and 2-hydroxyatorvastatin in healthy Chinese people. Pharmazie 72, 365–368. doi:10.1691/ph.2017.6944
Wei, K. K., and Zhang, L. R. (2015). Interactions between CYP3A5*3 and POR*28 polymorphisms and lipid lowering response with atorvastatin. Clin. Drug Investig. 35, 583–591. doi:10.1007/s40261-015-0317-3
Willrich, M. A. V., Hirata, M. H., Genvigir, F. D. V., Arazi, S. S., Rebecchi, I. M. M., Rodrigues, A. C., et al. (2008). CYP3A5-3A allele is associated with reduced lowering-lipid response to atorvastatin in individuals with hypercholesterolemia. Clin. Chim. Acta 398, 15–20. doi:10.1016/j.cca.2008.07.032
Zhong, S., Han, W., Hou, C., Liu, J., Wu, L., Liu, M., et al. (2017). Relation of transcriptional factors to the expression and activity of cytochrome P450 and UDP-glucuronosyltransferases 1A in human liver: Co-expression network analysis. AAPS J. 19, 203–214. doi:10.1208/s12248-016-9990-2
Keywords: atorvastatin, coronary artery disease, UGT1A1∗6, polymorphisms, ADME genes, clinical outcomes
Citation: Lei H-P, Qin M, Cai L-Y, Wu H, Tang L, Liu J-E, Deng C-Y, Liu Y-B, Zhu Q, Li H-P, Hu W, Yang M, Zhu Y-Z and Zhong S-L (2021) UGT1A1 rs4148323 A Allele is Associated With Increased 2-Hydroxy Atorvastatin Formation and Higher Death Risk in Chinese Patients With Coronary Artery Disease. Front. Pharmacol. 12:586973. doi: 10.3389/fphar.2021.586973
Received: 24 July 2020; Accepted: 21 January 2021;
Published: 08 March 2021.
Edited by:
Loes Visser, Erasmus Medical Center, NetherlandsReviewed by:
Matthijs Becker, Spaarne Gasthuis, NetherlandsCopyright © 2021 Lei, Qin, Cai, Wu, Tang, Liu, Deng, Liu, Zhu, Li, Hu, Yang, Zhu and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Long Zhong, emhvbmdzbEBob3RtYWlsLmNvbQ==; Yi-Zhun Zhu, eXp6aHVAbXVzdC5lZHUubW8=
†These authors contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.