
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 12 April 2021
Sec. Drugs Outcomes Research and Policies
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.576093
This article is part of the Research Topic Drug Repurposing for COVID-19 Therapy View all 50 articles
Chloroquine and its derivatives have been used since ages to treat malaria and have also been approved by the FDA to treat autoimmune diseases. The drug employs pH-dependent inhibition of functioning and signalling of the endosome, lysosome and trans-Golgi network, immunomodulatory actions, inhibition of autophagy and interference with receptor binding to treat cancer and many viral diseases. The ongoing pandemic of COVID-19 has brought the whole world on the knees, seeking an urgent hunt for an anti-SARS-CoV-2 drug. Chloroquine has shown to inhibit receptor binding of the viral particles, interferes with their replication and inhibits “cytokine storm”. Though multiple modes of actions have been employed by chloroquine against multiple diseases, viral diseases can provide an added advantage to establish the anti–SARS-CoV-2 mechanism, the in vitro and in vivo trials against SARS-CoV-2 have yielded mixed results. The toxicological effects and dosage optimization of chloroquine have been studied for many diseases, though it needs a proper evaluation again as chloroquine is also associated with several toxicities. Moreover, the drug is inexpensive and is readily available in many countries. Though much of the hope has been created by chloroquine and its derivatives against multiple diseases, repurposing it against SARS-CoV-2 requires large scale, collaborative, randomized and unbiased clinical trials to avoid false promises. This review summarizes the use and the mechanism of chloroquine against multiple diseases, its side-effects, mechanisms and the different clinical trials ongoing against “COVID-19”.
Chloroquine, commonly known for the anti-malarial applications has evolved gradually as a magic medicine, effective against many diseases including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple types of cancer and viruses. It has also been a molecule of choice among research community for studying the mechanism of autophagy, nanoparticles internalization, endocytosis and interlinked role of multiple signalling pathways in various diseases including cancer and autophagy (Pelt et al., 2018; Varisli et al., 2019).
Recent onset of the Coronavirus Disease-2019, a pandemic which has put the world on its knees, has again brought this “age-old drug” chloroquine and its derivatives into bright limelight. The disease has already spread worldwide and has killed more than 9,534,437 of the world population (Coronavirus Death Toll and Trends-Worldmeter, n.d.) and is still affecting millions. Multiple drugs are being tested, and the research community leaves no stone unturned to come up with an effective vaccine or drug to treat this wide-spread disease. Chloroquine and its derivatives have also emerged as a potential drug for effective treatment of this novel coronavirus (Smith et al., 2020; Touret and Lamballerie, 2020). Other potential drugs being tested for COVID-19 are remdesivir (GS-5734), lopinavir;ritonavir, Interferon alfacon-1 in conjunction with corticosteroids and Ribavirin in conjunction with corticosteroids (Smith et al., 2020; Wang M. et al., 2020). However, none of the drugs being researched has been approved by the World Health Organization (WHO) for the treatment of COVID-19 till now, keeping the room open for further research on chloroquine and the derivatives.
4-N-(7-chloroquinolin-4-yl)-1-N, 1-N-diethylpentane-1, 4-diamine, commonly known as chloroquine is a 4-aminoquinoline approved by FDA for treatment of malaria and inflammation–related diseases. It is a colorless and odourless crystal with a molecular mass of 319.9g/mol and available as a generic medicine (PubChem ID: 2719). Chloroquine is an inexpensive, water-soluble, weakly basic tertiary amine, which at physiological pH (7.2–7.4) is highly membrane permeable. However, inside the acidic organelles, it gets protonated and accumulates, raising the pH of the respective organelle. It can interfere with all the pH-dependent signalling and functioning of the endosome, lysosome, Golgi network, phagosome, and autophagosomes (Weyerhäuser et al., 2018). However, due to some side-effects of chloroquine, several derivatives, including hydroxychloroquine have been synthesized with similar efficacy but reduced toxicity.
Chloroquine and its derivatives (Figure 1), emerging as one of the most probable drugs alone or in combination against the battle of COVID-19, needs a detailed compilation and review so that the mechanisms elucidated by them against multiple diseases can be understood and co-related or used for the further vaccine and drug development for COVID-19. This review summarises chloroquine’s journey, from being an anti-malarial drug to a magic bullet against multiple diseases, its good and evil, results of clinical trials obtained so far and the future aspects, it holds along with its drawbacks as prophylaxis or drug to fight COVID-19.
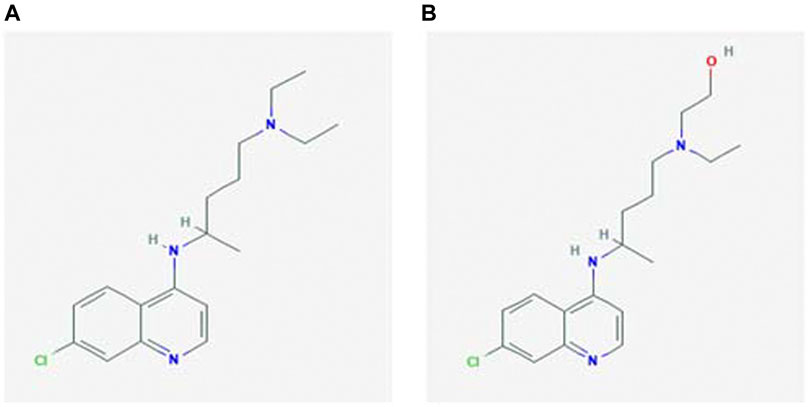
FIGURE 1. Chemical structure of (A) chloroquine and (B) hydroxychloroquine (National Center for Biotechnology Information, 2004).
To understand, how chloroquine inhibits malarial parasite, it is important to know the mechanism employed by Plasmodium sp. to hijack erythrocytes and use haemoglobin for their energy requirements. The Plasmodium sp. has a specialized acidic organelle known as digestive vacuole (DV) for degrading haemoglobin for its energy requirements following a cascade of protease activities (Pandey and Chauhan, 1998). The by-product of haemoglobin digestion is heme. Heme, when bound in haemoglobin is in the non-toxic ferrous form (Fe2+), but when free, it converts into very toxic ferric form (Fe3+) (Francis et al., 1994). To avoid toxicity, the parasite must evolve machinery to get rid of toxic heme, which is achieved by crystallization of heme called “hemozoin” or “malarial pigment” (Coronado et al., 2014). The formation of hemozoin takes place at considerably low pH where two heme units are linked together by iron carboxylate bonds. This unusual linkage is important for the synthesis and growth of an ordered insoluble crystal (Bohle et al., 1997; Coronado et al., 2014). Histidine rich protein (HRP) plays a vital role in the biocrystallization of hemozoin (Coronado et al., 2014). The hemozoin formed does not only detoxify the heme pigment for parasite but also adversely affects the human immune system, especially macrophages (Schwarzer et al., 2003).
There were several theories proposed regarding the mode of action of chloroquine to kill malaria pathogen as DNA binding agent (Parker and Irvin, 1952) protein synthesis inhibitor (Surolia and Padmanaban, 1991) polyamine metabolism inhibitor (Slater, 1993) and inhibitor of hemozoin crystallization (Orjih, 1997; Gorka et al., 2013). Most of the studies have shown chloroquine as a potent inhibitor of hemozoin crystallization. Sullivan. et al. (1996) postulated that chloroquine inhibits hemozoin formation by inhibiting HRP II. Again, Sullivan. et al. (1998) in their study concluded that chloroquine blocked the polymerization of free heme released during haemoglobin proteolysis in intraerythrocytic P. falciparum. Later in a review, Sullivan (2017) summarized that quinolones block every step of toxic heme crystal growth. DVs are acidic organelles with pH 5.0, where chloroquine can diffuse inside easily. However, the acidic pH yields diprotonation of the drug, inhibiting its movement out of the DV. The trapped diprotonic chloroquine inhibits the crystal growth of hemozoin, toxifying the malaria pathogen (Goldberg, 1993). Pandey and Tekwani et al. (1997) in their study established that chloroquine initiates a reverse reaction of conversion of hemozoin to monomeric heme (ferriprotoporphyrin IX) after interaction with malarial hemozoin, also termed as termed “hemozoin depolymerization”.
Developing resistance by Plasmodium sp. against chloroquine attributes to a point mutation in the genes coding for the chloroquine resistance transporter (PfCRT) present in DV (Martin et al., 2009; Chinappi et al., 2010). This protein avoids the accumulation of chloroquine by facilitating the efflux of the diprotonic chloroquine. However, the action of protein as a channel or a carrier is still debatable. Chinappi et al. (2010) in their study proposed that the protein acts as a carrier to exclude out both mono and diprotonic chloroquine. Reiling et al. (2018) proposed that pharmacological responses of sensitive and resistant malaria parasite towards chloroquine are also different.
Different strategies including alternative drugs, derivatives of chloroquine and combinational drug therapies have been used to combat the chloroquine-resistant malarial parasite. Clindamycin in combination with quinine was successfully used for the treatment of uncomplicated multidrug-resistant P. falciparum malaria in Thai patients (Pukrittayakamee et al., 2000). Artesunate-atovaquone-proguanil combination has proven successful for the treatment of the similar case of malaria (van Vugt et al., 2002). Primaquine, mefloquine, artesunate and artemisinins are some of the drugs used in the treatment of resistant malaria in India (Kalra et al., 2002). Treatment of chloroquine-resistant malaria using a combination of pyrimethamine, berberine, tetracycline or cotrimoxazole has been used successfully to treat chloroquine-resistant malaria in Africa (Sheng et al., 1997).
RA and LE are autoimmune diseases, where healthy tissues are attacked by the hyper-immune system causing inflammatory responses. RA is mainly characterized by pain, inflammation and stiffness around the joints, whereas LE is characterized in the early phase with arthritis, skin lesions, inflammation around the lungs and kidneys. Rhupus, is a syndrome which presents symptoms associated with both RA and LE (Macfarlane and Manzel, 1998; Thome et al., 2013).
Both chloroquine (CQ) and 4-hydroxychloroquine (HCQ) are extensively used as immune-modulators to treat RA and LE. There are evidence of both, pH-dependent and pH-independent role of chloroquine and its derivatives to inhibit the generation of autoantibodies and reducing the secretion of inflammatory cytokines (Macfarlane and Manzel, 1998; Thome et al., 2013).
CQ and HCQ both can enter acidic endosome and lysosome, remain there as CQ+ and CQ++, elevate their pH from 4.5 to 6.0, and interfere with their functions (Mindell, 2012). By interfering with endosome functions, it inhibits TLR7 and nine signalling and thus inhibits dendritic cell maturation. By changing the acidity of lysosome of antigen-presenting cells, CQ and HCQ, inhibits the presentation of the major histocompatibility (MHC) complex peptides to T cells, thus inhibiting the production of T helper cells and cytokines (Thome et al., 2013; Ponticelli and Moroni, 2017). It also inhibits calcium-dependent signalling, toll-like receptor signalling pathways, and iron metabolism in macrophages, thus suppressing production of IL-6, IL-1 and tumor necrosis factor-alpha (TNF-α) (Ponticelli and Moroni, 2017; Schrezenmeier and Dörner, 2020). Rand et al. (2008) in their experiment using atomic force microscopy (AFM) observed that hydroxychloroquine interferes with binding of antiphospholipid antibody–β2-glycoprotein I complexes to phospholipid bilayers, thus lowering down inflammation. Oh et al. (2016) concluded in their study that chloroquine reduces inflammation through p21-mediated suppression of T cell proliferation and Th1 cell differentiation.
Though the development of resistance against disease-modifying anti-rheumatic drugs (DMARDs) including chloroquine, has not been studied much, the role of ATP binding cassette (ABC) proteins responsible for drug efflux cannot be neglected (Jansen et al., 2003). A better understanding is needed in this field to establish alternative strategies and drug combination therapy for RA and LE.
Inhibition of cancer cell growth by chloroquine is a complex process. Table 1 summarizes the multi-ranged effects of chloroquine on multiple types of cancer cells. The primary mechanism employed by chloroquine and its derivative is inhibition of autophagy during cancer cell death. The pH-dependent accumulation of chloroquine inside lysosome leads to impairment of autophagosome degradation and thus inhibition of autophagy (Mauthe et al., 2018). It is also known to generate endoplasmic stress, lysosome and mitochondrial membrane depolarization in a reactive oxygen species (ROS) dependent manner, thus increasing apoptosis (Ganguli et al., 2014; Alam et al., 2016). Though chloroquine alone is not sufficient to depolarise membrane potential; it is generally used to sensitize chemo or radiotherapy, in an autophagy-dependent or independent manner (Maycotte et al., 2012; Makowska et al., 2016; Zhu et al., 2019). However, there are some severe kidney and organ injuries have also been reported after the use of chloroquine as the sensitizer to chemo and radiotherapy (Kimura et al., 2013).
Recent studies have revealed that chloroquine is also able to interfere with different metabolic pathways, including cholesterol, glucose, amino acids, and mitochondria metabolism (Weyerhäuser et al., 2018).
Chloroquine is also used to treat multidrug-resistant cancer by blocking drug extrusion by interfering with the ATP-binding cassette (ABC) transporter family and other transmembrane protein related to drug resistance (Szakács et al., 2006). A summary of mechanisms employed by chloroquine has been illustrated in Figure 2.
Generally, in response to intracellular bacterial or fungal pathogens, the first-line antimicrobial defence is initiated by the phagocytes. After being internalised by the phagocyte, a phagosome forms which further fuses with lysosomes. Through oxygen dependent and independent mechanisms, the bacteria are killed. This acidifies the phagolysosome to pH 4.5 and activates lysosomal enzymes. Several intracellular pathogenic bacteria and fungi evade this line of defence through different mechanisms such as, they lack the lysosomal pathway (ex. Bartonella sp.), escape before the fusion of phagosome and lysosome and survive in the cytosolic region (ex. Shigella sp, Rickettsia sp.), block lysosomal fusion and multiply in the phagosome (ex. Chlamydia sp, Salmonella sp, Mycobacterium sp, Yersinia sp), resistance to survival in phagolysosome (Coxiella burnetii, Tropheryma whipplei). Chloroquine treatment inhibits the growth of these intracellular pathogens by pH dependent iron deprivation and neutralising the phagolysosomal pH (Rolain et al., 2017). Lagier et al. (2014) reported the bactericidal combination treatment of doxycycline and hydroxychloroquine against the classic Tropheryma whipplei caused Whipple’s disease. The authors confirmed the effectivity of the combination treatment through in vitro studies and clinical trials (Lagier et al., 2014). Q fever, caused by Coxiella burnetii infection manifests into a severe complication of endocarditis. A combination of doxycycline and chloroquine derivates has been reported to reduce the mortality rate and is a prominent therapeutic intervention for Q fever. The mechanism of action is under investigation; however, it can be presumed that chloroquine increases the lysosomal pH and enhances the antibacterial activity of doxycycline (Alegre et al., 2012; Lagier et al., 2014).
The catastrophic impact of viral diseases on human has been observed since ages. From Spanish flu to COVID-19, humankind has always struggled to make a way out of socio-economic burden slapped by viral pathogens. The COVID-19 pandemic crisis has worsened the economic and health condition worldwide to such a level that had not been observed in the last 70years (https://www.un.org/development/desa/dspd/2020/04/social-impact-of-covid-19/). The COVID-19 outbreak is detrimental to old age, immuno-suppressive people, and a significant economic burden on indigenous and poor people.
Each virus has a different virulence factor, and the pathological consequences also differ from virus to virus. The knowledge of viral pathogenesis is neither accurate nor complete for most viral infections, especially for SARS-CoV-2. Novel SARS-CoV-2 is an enveloped single-stranded RNA virus belonging to the family Coronaviridae (Zheng, 2020) responsible for ongoing pandemic COVID-19. The main symptoms of this disease include fever, cough and fatigue, and it can lead to severe complications, having a mortality rate of 5.7% (Lechien et al., 2020) 50% of the COVID-19 positive patients are asymptomatic. The main symptoms in the early stages are headache (70%) loss of smell, and nasal obstruction. Cough, fever and dyspnoea are a sign of late infection (8–10days) (Lechien et al., 2020; Sajna and Kamat, 2020).
Discussing complete progress details about the viral pathogenesis will be beyond this review, however, in general, pathogenic virus and in particular, SARS-CoV-2 follows the following events to cause an infection.
a. Entry inside the cells in an endocytosis-dependent or independent manner.
Most of the human viruses follow an endocytosis-dependent entry inside the cells. The envelope spike glycoprotein of SARS-CoV and SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) receptor on target cells to facilitate entry (Li et al., 2020; Zhang et al., 2020). The spike “S” protein is responsible for the ACE2 receptor binding, whereas the cellular serine protease TMPRSS2 is required to prime the “S” protein (Hoffmann et al., 2020).
Xu et al. (2020) in their study found that the 3D structure of the receptor-binding domain in both the viruses is identical. SARS-CoV followed direct membrane fusion between the virus and plasma membrane as well as clathrin-dependent and -independent endocytosis mediated entry inside target cells (Wang et al., 2008; Kuba et al., 2010).
b. Viral replication inside target cells.
The replication mechanism of SARS-CoV and SARS-CoV2 is also found to be similar (Caly et al., 2020). After entry inside the target cells, the virus’s RNA genome is released in the cytoplasm, translated, and posttranslational modifications occur in endoplasmic-reticulum or Golgi apparatus. After the assembly of RNA and nucleocapsid proteins, the replicated virus particles are released by membrane fusion (Li et al., 2020).
c. Escaping immune surveillance
Most of the viral diseases survive inside human by escaping immune surveillance. Viruses of the family Coronaviridae are no exception. During the initial infection, the SARS-CoV-2 delays type 1 IFN production and avoids the recognition by pattern recognition receptors (PRRs), allowing uncontrolled viral replication, activating pro-inflammatory cytokines triggering “cytokine storm” (Huang et al., 2005; Li et al., 2020; Rothan and Byrareddy, 2020). Further, activation of specific Th1/Th17 enhances the inflammatory responses.
SARS-CoV-2 escapes activation of adaptive immunity by interfering differentiation and function of dendritic cells and defensins and a severe decrease in CD4+ and CD8+ T cells (Li X. et al., 2020; Li G. et al., 2020).
Chloroquine acts as a potent anti-viral agent by implying several mechanisms which have been listed in Table 2. The anti-viral mechanisms of chloroquine can further be exploited to develop it as a therapeutic agent against SARS-CoV-2.
Though studies are still ongoing on chloroquine as an inhibitor of SARS-CoV-2, the plausible mechanisms known from its use against various diseases can provide a substantial ground for further research and development of chloroquine as a potential drug against COVID-19. Multiple modes of actions of chloroquine against SARS-CoV-2 are as follows:
1. Inhibition of viral entry inside the target cells
Chloroquine can inhibit the binding of viral spike glycoprotein with ACE2 receptor on target cells to inhibit their entry. Chloroquine has shown potent inhibition of sugar modifying enzymes or glycosyltransferases and quinone reductase which have been involved in sialic acid biosynthesis of ACE2 receptor (Kwiek et al., 2004; Devaux et al., 2020). Wu et al. (2020) in their docking studies showed that chloroquine can potentially target Nsp3b or E-channel with the docking mfScores of–130.355 and–107.889, respectively, though experimental results are yet to be verified.
SARS-CoV-2 particles significantly resemble the nanoparticles with a size of 60–140nm and are spherical. Nanoparticles are known to exhibit their desired results by cell internalization (Kumari et al., 2017) which can effectively be inhibited by chloroquine. Chloroquine inhibits nanoparticles internalization by suppression of phosphatidylinositol binding clathrin assembly protein (PICALM), thus inhibiting clathrin-dependent endocytosis (Hu et al., 2020). The same principle can be applied for stopping the internalization of SARS-CoV-2 particles inside the target cells. Chloroquine can also play a vital role in interfering with the endocytosis of viral particles by increasing the pH of endosomes which has been explained earlier (Touret and Lamballerie, 2020). Interaction of TMPRSS2 with the ACE2 receptor is essential for facilitating SARS-CoV-2 entry (Matsuyama et al., 2020). Application of chloroquine with a known serine protease inhibitor can weaken the viral entry inside the cells (Markus et al., 2020). Serine protease inhibitor camostat mesylate has been observed to blocks TMPRSS2 activity in SARS-CoV-2 (Hoffmann et al., 2020).
2. Inhibition of viral replication and posttranslational modifications (PTM)
Chloroquine inhibits acidification of endosome and lysosome, stalling the virus inside endosomes and inhibiting the release of the viral RNA genome in the cytosol. Inhibition of lysosome acidification further hampers the fusion of endosome with the lysosome and upstream trafficking essential for viral replication (Devaux et al., 2020; Hu et al., 2020; Wang et al., 2020).
Inhibition of acidification further continues to work in favour of chloroquine against SARS-CoV-2 as it inhibits posttranslational modification in trans Golgi network (TGN). Lack of low pH in TGN interferes with functional proteases and glycosyl-transferases resulting in impaired PTM or non-infectious viral particles (Devaux et al., 2020; Touret and Lamballerie, 2020).
3. Inhibition of autophagy
Many of the human viruses employ autophagy for their replication inside the target cells (Table 2) (Yan et al., 2013; Calderon et al., 2019; Zhang et al., 2019). Though the role of autophagy in the proper functioning of SARS-CoV-2 is still under investigation, several results claim that autophagy is crucial for SARS-CoVs replication (Brest et al., 2020; Yang and Shen, 2020). Prentice et al. (2004) demonstrated the critical role of endogenous LC-3, a protein marker for autophagosomes in the replication of SARS-CoV. Chloroquine, being a well-established autophagy inhibitor can be a potential candidate for suppression of COVID-19.
4. Immuno-modulator and inhibition of “cytokine storm”
Chloroquine is widely used for the treatment of RA and SE based upon its immune-modulatory properties. As discussed in earlier sections, chloroquine inhibits pH-dependent toll-like signalling pathway in the endosome and inhibits the inflammatory response “cytokine storm”. The inhibition of toll-like signalling pathway prevents the recognition of viral antigen by dendritic cells (Devaux et al., 2020). It also enhances cytotoxic CD8+ T cell responses against viral antigens and exports soluble antibodies into the cytosol of the dendritic cell to fight the viral antigen.
Chloroquine interferes with viral antigen presentation via the lysosomal pathway and thus inhibits MHC II recognition of antigen, modulating the elevation of inflammatory responses (Kearney, 2020). Inhibition of TNFα, TNF α receptors and TNF α signalling by chloroquine plays a vital role in the suppression of “cytokine storm” (Touret and Lamballerie, 2020).
5. Interference with the metabolic pathways
As it is already known from the use of chloroquine against glioblastoma, this can regulate metabolic pathways especially lipid metabolisms in cells. Lipid metabolic pathways play an important role in viral entry and replication inside the target cells. SARS-CoV-2 infection interfered with the regulation of lipid metabolism with the higher concentration of free fatty acids, lysophosphatidylcholine, lysophosphatidylethanolamine, and phosphatidylglyceroland significant lower concentration of total cholesterol (TC), HDL-cholesterol and LDL-cholesterol levels in serum (Hu et al., 2020; Zheng et al., 2020). As observed during treatment of glioblastoma and SLE, chloroquine can also regulate metabolic pathways during SARS-CoV-2 infection as its therapeutic mode of action.
Apart from being an FDA approved anti-malaria, anti-RA, and anti-LE drug, chloroquine has been investigated against several other prevalent medical conditions. Table 3 summarises the use of chloroquine in the treatment of other diseases and its mode of action.
Chloroquine and the derivatives while using as an anti-malaria drugs in Mâncio Lima, Acre, Brazil, caused itching, stinging sensation, epigastric pain, and diarrhoea (Braga et al., 2015). It was explained by enhanced production of IgE, degranulation of mast cells and basophils creating allergy like reaction. However, severe side-effects including mental confusion, seizures, coma, and cardiovascular symptom, was not reported. Adedapo et al. (2009) observed that higher dosage of chlorpheniramine plus chloroquine (10 mg/kg daily for 3 days) in children below 5 years caused drowsiness and lower respiratory rates, though no additional benefits were obtained. Chloroquine is known to induce concentration-dependent cytotoxicity, which should always be optimized before finalizing the dosage.
Though optimized dosage and short-term treatment of RA with CQ and HCQ was considered safe, long-term use of CQ in a 64-year-old woman resulted in both restrictive and hypertrophic myocardiopathy auricular-ventricular blocks due to long term pH alteration in the lysosome (Cervera et al., 2001). Kelly et al. (1990) also focussed on the narrow margin between therapeutic uses of chloroquine against RA and the chloroquine poisoning. They reported the death of a 12-month-old infant after receiving 300 mg of chloroquine. They also highlighted the different dose optimization of chloroquine for adults and infants. Scherbel et al., (1965) in their clinical trials found that out of 741 patients treated with chloroquine derivative for SLE, 31-68% developed retinopathy and marked destruction of rod and con cells. However, no clear relationship between chloroquine dosage and retinal toxicity could be established. Lane et al., (2020) performed a multinational retrospective study to evaluate the risk of HCQ alone and in combination with azithromycin in 956,374 RA patients (18 years and above). It was observed that a 30-days dose of HCQ demonstrated no risk of adverse events. However, long term use of HCQ alone increased cardiovascular mortality. A combination of HCQ and azithromycin elevated the risk of heart failure even in the short term. Therefore, the authors suggest a careful consideration of benefit:risk ratio when starting HCQ treatment (Lane et al., 2020).
It is difficult to estimate the frequency of adverse events because many cases have been reported in more than one publication and lack a criterion for diagnosis (Scherbel et al., 1965). Hence, it is recommended to evaluate cardiac health with ECG and ophthalmological examinations for 6 months before prescribing a long-term treatment with chloroquine (Scherbel et al., 1965; Kelly et al., 1990; Cervera et al., 2001).
Chloroquine, as a chemotherapeutic agent against cancer, can act as a double-edged sword. It not only sensitizes the cancer cells but also the normal cells by blocking autophagy and impairing lysosome and endosomes' function (Kimura et al., 2013; Choi et al., 2018). Kidney is the most critically affected organ during chemotherapy with chloroquine with significant nephrotoxicity (Klionsky et al., 2016; Wang B. et al., 2020). Evangelisti et al. (2015) also reviewed the substantial side effects of chloroquine while treating acute leukaemia. Repurposing chloroquine against cancer was generally considered safe for short term treatment with optimized dosage. However, patients suffering from glucose-6-dehydrogenase deficiency, impaired hepatic and kidney diseases should always be cautious while practising chloroquine and derivatives as a chemotherapeutic agent (Verbaanderd et al., 2017). A clinical trial (ClinicalTrials.gov ID NCT04201457) by “Pediatric Brain Tumor Consortium” is ongoing to assess the safety and benefit of adding hydroxychloroquine to dabrafenib and/or trametinib in children with recurrent or progressive low grade or high-grade brain tumor with specific genetic mutations whose results are waited in February 2025 (https://clinicaltrials.gov/ct2/show/results/NCT04201457).
The standard and optimized dose of chloroquine as prophylaxis and during treatment of diseases do not bear significant toxicity, however, long term use of higher concentration of chloroquine can result in severe toxicity (Table 4). Use of less toxic derivatives such as hydroxychloroquine, optimized dosage, nanoencapsulation of the drug and combinational therapies have been used for reducing chloroquine toxicity and increasing efficacy (Amolegbe et al., 2018; Lima et al., 2018).
Hypokalemia toxicity is commonly observed in chloroquine and hydroxychloroquine overdose due to the intracellular shift of potassium. Clemessy et al. (1995), performed a retrospective study of 191 cases of chloroquine toxicity in which the initial clinical features included, gastro-intestinal disturbances, neurological impairment, and respiratory symptoms and eventual blockage of potassium channels contributed to hypokalaemia (Clemessy et al., 1995).
Neuropsychiatric manifestations including depression, psychosis, insomnia, agitation have also been reported due to acute or chronic use of chloroquine and hydroxychloroquine (Juurlink, 2020).
Hematologic toxicities are attributed to its long half-life in plasma which leads to accumulation in the blood cells. Lymphopenia, eosinophilia is typically observed immunologically mediated idiosyncratic drug reactions (Juurlink, 2020).
Prolongation of the QT interval due to both chloroquine and hydroxychloroquine has also been observed since the drugs interfere with vascular repolarization. It was observed that after a dose of 600 mg QTc increases 6.1 and 28 ms after a dose of 1,200 mg. However, this effect varied in younger age groups. In response to this treatment in COVID-19 patients, ventricular tachycardia and ventricular fibrillation and mortality were observed potentially due to the overdosage. Hence, in a COVID-19 setting FDA cautions the use of HCQ or CQ, but not in cases of malaria, lupus and RA (https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or). In severe COVID-19 cases where azithromycin was co-prescribed in combination with either chloroquine and hydroxychloroquine, Molina et al. (2020) reported no evidence of rapid anti-viral clearance or any associated clinical benefit in only 11 patients, possibly because they had significant comorbidities such as obesity, cancer, HIV infection (Molina et al., 2020). However, in a retrospective study 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Lagier et al. (2020) observed otherwise. Along with 3 days early treatment of HCQ-azithromycin resulting in faster viral load reduction, no cases of torsade de pointe or sudden death were observed. This could be because the patients belonged to mean age of 45 years, the treatment was initiated very early with a dosage of 200 mg of oral HCQ, three times daily for ten days and 500 mg of oral azithromycin on day 1 followed by 250 mg daily for the next four days, respectively.
Sacher et al., (2020) propose a pragmatic approach to mitigate the cardiac risk in the COVID-19 setting. The authors propose a cardiac algorithm for critically reviewing patient’s clinal history (use of other drugs that may extend QT interval, levels of serum K+, creatinine, and a recent 30 s ECG). In cases of QT intervals >500 ms, the authors recommend that a QT-prolonging drug should not be prescribed (Sacher et al., 2020).
Some rare immunologically mediated adverse reactions including Stevens-Johnson syndrome, toxic epidermal necrolysis, DRESS (drug reaction with eosinophilia and systemic symptoms), have been implicated in chloroquine and hydroxychloroquine treatment against viral diseases. Although rare, these conditions turn into serious entities when accompanied by liver or kidney injury (Juurlink, 2020).
Currently, there are multiple clinical trials underway to investigate the potential use of hydroxychloroquine and chloroquine alone or in combination against SARS-CoV-2 (Table 5). Some of the in vitro and in vivo results obtained with chloroquine and hydroxychloroquine supported their anti-viral role against SARS-CoV-2, (Andreani et al., 2020; Clementi et al., 2020; Fantini et al., 2020; Gao et al., 2020; Gautret et al., 2020; Liu et al., 2020; Weston et al., 2020; Yao et al., 2020), however, results of Gautret et al. (2020) faced severe criticism because of small sample size, overruling type I errors, inconsistency between study protocols and lack of blinding and a control arm even though the treatment resulted in viral load reduction. It is also very important to reproduce the in vitro results obtained with chloroquine in the in vivo studies and clinical trials to establish it as a safe and effective anti-SARS-CoV-2 drug.
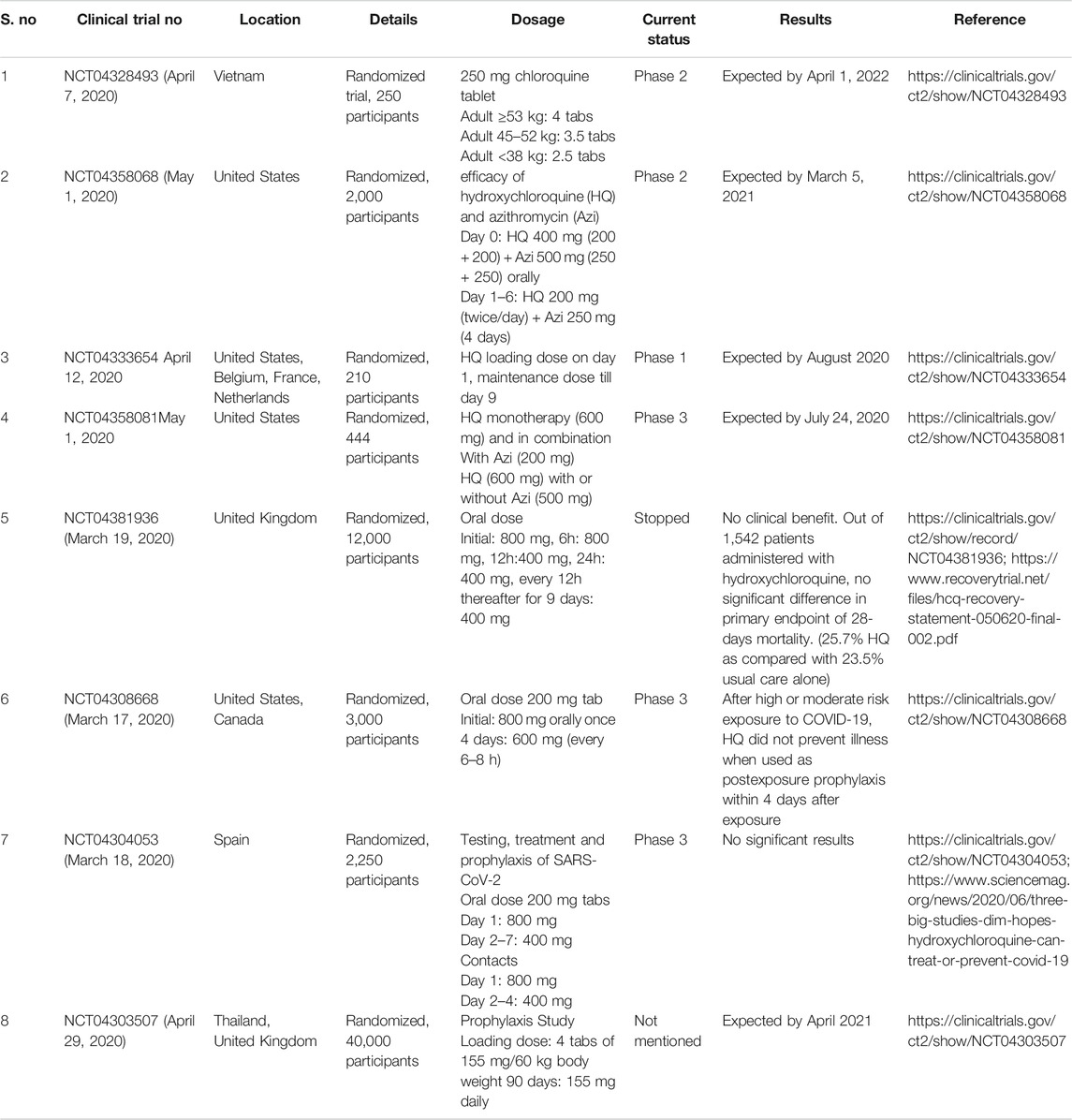
TABLE 5. Ongoing clinical trials to evaluate the potential of chloroquine and hydroxychloroquine against SARS-CoV-2.
The studies by Patel and coworkers (https://www.sciencemag.org/news/2020/06/mysterious-company-s-coronavirus-papers-top-medical-journals-may-be-unraveling; Mehra et al., 2020a; Mehra et al., 2020b), claimed to have performed a multinational registry analysis using a cloud-based health-care data analytics platform, Surgisphere Corporation, Chicago, IL, United States, on the usage of hydroxychloroquine or chloroquine with or without a macrolide for the treatment of COVID-19. They reported an increased risk of in-hospital mortality and de-novo ventricular arrhythmia in response to the treatment which led to the inference that hydroxychloroquine or chloroquine, when used alone or with a macrolide does not offer any benefit to the COVID-19 patients, which contributed to the halt in worldwide clinical trials by the WHO on May 25, 2020. The second study (Mehra et al., 2020b) claimed to negate the association of ACE inhibitors and angiotensin-receptor blockers (ARBs) with in-hospital COVID-19 deaths. Their analyses brought forth better survival rates due to the use of either ACE inhibitors or statins. However, the authors mentioned that since the study was not based on randomized trials, there could be a possibility of confounding and hence, concluded that an underlying cardiovascular disease is independently associated with an increased risk of in-hospital COVID-19 death.
Substantive red flags were raised by the rattled global scientific community because the doses in the reported cases were higher than those set by the United States FDA and discrepancies in the official COVID-19 mortality statistics, and sample size (https://www.sciencemag.org/news/2020/06/mysterious-company-s-coronavirus-papers-top-medical-journals-may-be-unraveling). Eventually, both the studies were retracted from the journals, The Lancet and The New England Journal of Medicine. Currently, clinal trials in various parts of the world have resumed to investigate the potential use of hydroxychloroquine in COVID-19 patients in response to WHO’s green signal (https://www.sciencemag.org/news/2020/06/mysterious-company-s-coronavirus-papers-top-medical-journals-may-be-unraveling). In another report by Geleris et al. (2020) reported no positive or negative observational effect of hydroxychloroquine on death or incubation risk on COVID-19 patients, however, this study did support the further randomized clinical trials of hydroxychloroquine testing its efficacy.
The United Kingdom’s mega RECOVERY trial (RECOVERY Collaborative Group, 2020) reported the ineffectiveness of hydroxychloroquine. The patients who received the treatment demonstrated a longer hospitalization duration, higher risk of mechanical ventilation or mortality than those who received the usual care. However, the study received sceptical reviews due to the high dosage issues: 800 mg at 0 and 6 h, followed by a 400 mg dose at 12 h and every 12 h thereafter for 9 days; which may have contributed to cardiovascular, neurological, and other toxicities. The authors chose this dosage based on extensive pharmacokinetic studies.
On December 2nd, 2020, the WHO Solidarity Trial Consortium published the findings of their trials on repurposed anti-viral drugs for COVID-19 (NCT04315948). The drugs included hydroxychloroquine, remdesivir, lopinavir, and interferon beta-1a in hospitalized COVID-19 patients. The randomized trials were evaluated for death rate according to age and requirement of mechanical ventilation. Like the RECOVERY trials, this one too reported negligible effect on mortality, ventilation, and hospitalization duration of COVID-19 patients (WHO Solidarity Trial Consortium, 2020).
Several ongoing clinical trials against COVID-19 with chloroquine/hydroxychloroquine alone or in a combination of drugs are the outcome of promising in vitro results and the hype created worldwide over the drug (Cortegiani et al., 2020). Giving too much of attention by the scientific community generates false promises and hampers the path of other potential drugs against COVID-19 in this pandemic era. Simultaneously, no negative feedback against chloroquine should be postulated without confirming the clinical trial results. Chloroquine being an age-old drug, has already been used against multiple diseases. If found effective, its inexpensive nature and already documented toxicity profile and dosage optimization can save time and a million lives. Though mixed results of chloroquine against COVID-19 have been obtained so far, there is an urgent need to test their effects and toxicity as a prophylactic drug, in mildly ill patients and severely ill patients of COVID-19. Moreover, one should never forget the thin line between chloroquine as a therapeutic agent and chloroquine poisoning (Kelly et al., 1990). An in-depth toxicity analysis of chloroquine and derivatives is required before confirming any comment for/against its use in time of COVID-19. The poor methods of clinal trials and its reporting has thus far been inadequate in proving the effective nature of hydroxychloroquine. Ferner and Aronson (2020) claim that the overuse of hydroxychloroquine will result in rare buy harmful cutaneous adverse reactions, hepatic problems and ventricular arrhythmias when prescribed in combination with azithromycin. In a recent study, Haque et al. (2021) reported the changes in purchasing patterns and pricing of hydroxychloroquine since March 2020 in India states. While no price and utilization changes were observed, hydroxychloroquine shortages were encountered due to the misinformation and management of COVID-19.
Among the rapidly changing guidelines in this pandemic era of COVID-19, WHO has revoked the ban on clinical trials with chloroquine against COVID-19 (https://www.sciencemag.org/news/2020/06/mysterious-company-s-coronavirus-papers-top-medical-journals-may-be-unraveling), however, FDA has cautioned its use outside of the hospital setting or a clinical trial due to risk of heart rhythm problems (https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or). Though the preventive or prophylactic potential of chloroquine and the derivatives are yet to be confirmed against COVID-19, extensive, collaborative, unbiased and random clinical trials are required instead of small and individual trials to conclude. Results of unprejudiced, statistically significant and ethical outcomes of clinical trials are eagerly awaited before sealing the fate of this age-old drug against COVID-19.
The “age-old” drug used to treat multiple diseases has generated mixed therapeutic responses against “COVID-19.” Chloroquine has been recognized as a miracle medicine to treat malaria, autoimmune diseases, cancer, viral, dermatological, and fungal infections. Different in vitro and in vivo studies have suggested the positive, neutral, and negative role of chloroquine and derivatives against SARS-CoV-2. Though some studies are still ongoing, different probable mechanisms have been reported in literature employed by chloroquine to inhibit SARS-CoV-2 infection or cause more harm than good. In this difficult situation where an effective anti-viral drug is urgently needed, a biased decision against or in favour of chloroquine can either generate a false sense of security or can add more anxiety in an already worse situation. However, the previous research done on chloroquine against multiple diseases can help establish its anti-SARS-CoV-2 mechanism, precautions to be taken to avoid chloroquine’s toxicity, and dosage–optimization to reach any conclusion.
MK conceptualized the idea. The manuscript was written, literature was collected and finalized by SK and MK.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MK is thankful to Council of Scientific and Industrial Research (CSIR), New Delhi and SK is grateful to University Grant Commission (UGC), New Delhi for providing the Senior Research Fellowship. Authors are also thankful to Anup Prasad, Scientist, Defence Research and Development Organization (DRDO) for critically reviewing the manuscript draft and providing his valuable inputs.
Adedapo, A. D. A., Ademowo, O. G., Adedapo, K. S., Demissie, K., and Osinubi, O. Y. O. (2009). Potential toxicity of chlorpheniramine plus chloroquine for the treatment of childhood malaria. Niger. J. Clin. Pract. 12 (3), 252–257.
Aghahowa, S. E., Obianwu, H. O., Isah, A. O., and Arhewoh, I. M. (2010). Chloroquine-induced pruritus. Indian J. Pharm. Sci. 72 (3), 283–289. doi:10.4103/0250-474X.70471
Alam, M. M., Kariya, R., Kawaguchi, A., Matsuda, K., Kudo, E., and Okada, S. (2016). Inhibition of autophagy by chloroquine induces apoptosis in primary effusion lymphoma in vitro and in vivo through induction of endoplasmic reticulum stress. Apoptosis 21, 1191–1201. doi:10.1007/s10495-016-1277-7
Al‐Bari, M. A. A. (2017). Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 5 (1), e00293. doi:10.1002/prp2.293
Alegre, P., da-Silva, A., Caminha, C., Lul, R. M., Kirschnick, L. S., and Galperim, B. (2012). Case report whipple’s disease: rare disorder and late diagnosis. Rev. Inst. Med. Trop. Sao Paulo 54 (5), 293–297. doi:10.1590/S0036-46652012000500010
Amolegbe, S. A., Hirano, Y., Adebayo, J. O., Ademowo, O. G., Balogun, E. A., Obaleye, J. A., et al. (2018). Mesoporous silica nanocarriers encapsulated anti-malarials with high therapeutic performance. Sci. Rep. 8, 3078. doi:10.1038/s41598-018-21351-8
Andreani, J., Le Bideau, M., Duflot, I., Jardot, P., Rolland, C., Boxberger, M., et al. (2020). In vitro testing of hydroxychloroquine and azithromycin on SARS-CoV-2 shows 2 synergistic effect. Microb. Pathog. 145, 104228. doi:10.1016/j.micpath.2020.104228
Arendt, P., and Gerding, H. (2017). Chloroquine retinopathy under inadequately weight adjusted dosage: early detection by multimodal imaging. Klin. Monbl. Augenheilkd. 234 (4), 543–545. doi:10.1055/s-0042-121573
Baruah, P., Bullenkamp, J., WilsonLee, P. O. G., Lee, M., Kaski, J. C., and Dumitriu, I. E. (2019). TLR9 mediated tumor-stroma interactions in human papilloma virus (HPV)-positive head and neck squamous cell carcinoma up-regulate PD-L1 and PD-L2. Front. Immunol. 10, 1644. doi:10.3389/fimmu.2019.01644
Beegle, S. H., Barba, K., Gobunsuy, R., and Judson, M. A. (2013). Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des. Devel. Ther. 7, 325–338. doi:10.2147/DDDT.S31064
Bhushan, P., Aggarwal, A., and Baliyan, V. (2014). Complete clearance of cutaneous warts with hydroxychloroquine: antiviral action? Indian J. Dermatol. 59 (2), 211. doi:10.4103/0019-5154.127694
Biot, C., Daher, W., Chavain, N., Fandeur, T., Khalife, J., Dive, D., et al. (2006). Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J. Med. Chem. 49, 2845–2849. doi:10.1021/jm0601856
Bohle, D. S., Dinnebier, R. E., Madsen, S. K., and Stephens, P. W. (1997). Characterization of the products of the heme detoxification pathway in malarial late trophozoites by X-ray diffraction. J. Biol. Chem. 272, 713–716. doi:10.1074/jbc.272.2.713
Boone, B. A., Murthy, P., Miller-Ocuin, J., Doerfler, W. R., Ellis, J. T., et al. (2018). Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer 18, 678. doi:10.1186/s12885-018-4584-2
Both, T., Zillikens, M. C., Schreuders-Koedam, M., Vis, M., Lam, W. K., Weel, A. E. A. M., et al. (2018). Hydroxychloroquine affects bone resorption both in vitro and in vivo. J. Cell. Physiol. 233 (2), 1424–1433. doi:10.1002/jcp.26028
Boya, P., Gonzalez-Polo, R. A., Poncet, D., Andreau, K., Vieira, H. L., et al. (2003). Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 22, 3927–3936. doi:10.1038/sj.onc.1206622
Braga, C. B. E., Martins, A. C., Cayotopa, A. D. E., Klein, W. W., Schlosser, A. R., da Silva, A. F., et al. (2015). Side effects of chloroquine and primaquine and symptom reduction in malaria endemic area (Mâncio Lima, Acre, Brazil). Interdiscip. Perspect. Infect. Dis. 2015, 346853. doi:10.1155/2015/346853
Brest, P., Benzaquen, J., Klionsky, D. J., Hofman, P., and Mograbi, B. (2020). Open questions for harnessing autophagy-modulating drugs in the SARS-CoV-2 War. Autophagy 16, 2267–2270. doi:10.20944/preprints202004.0022.v1
Bruinink, A., Zimmermann, G., and Riesen, F. (1991). Neurotoxic effects of chloroquine in vitro. Arch. Toxicol. 65, 480–484. doi:10.1007/bf01977360
Calderon, B. M., Danzy, S., Delima, G. K., Jacobs, N. T., Ganti, K., Hockman, M. R., et al. (2019). Dysregulation of M segment gene expression contributes to influenza A virus host restriction. PLoS Pathog. 15 (8), e1007892. doi:10.1371/journal.ppat.1007892
Caly, L., Druce, J. D., Catton, M. G., Jans, D. A., and Wagstaff, K. M. (2020). The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 178, 104787. doi:10.1016/j.antiviral.2020.104787
Calza, L., Attard, L., Manfredi, R., and Chiodo, F. (2002). Doxycycline and chloroquine as treatment for chronic Q fever endocarditis. J. Infect. 45 (2), 127–129. doi:10.1053/jinf.2002.0984
Casado, E., Gratacos, J., Tolosa, C., Martínez, J. M., Ojanguren, I., Ariza, A., et al. (2006). Antimalarial myopathy: an underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann. Rheum. Dis. 65 (3), 385–390. doi:10.1136/ard.2004.023200
Cervera, A., Espinosa, G., Font, J., and Ingelmo, M. (2001). Cardiac toxicity secondary to long term treatment with chloroquine. Ann. Rheum. Dis. 60 (3), 301. doi:10.1136/ard.60.3.301
Chinappi, M., Via, A., Marcatili, P., and Tramontano, A. (2010). On the mechanism of chloroquine resistance in Plasmodium falciparum. Plos One 5, e14064. doi:10.1371/journal.pone.0014064
Choi, Y., Bowman, J. W., and Jung, J. U. (2018). Autophagy during viral infection - a double-edged sword. Nat. Rev. Microbiol. 16, 341–354. doi:10.1038/s41579-018-0003-6
Chopra, A., Saluja, M., and Venugopalan, A. (2014). Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol. 66, 319–326. doi:10.1002/art.38221
Chou, H. L., Lin, Y. H., Liu, W., Wu, C. Y., Li, R. N., Huang, H. W., et al. (2019). Combination therapy of chloroquine and C₂-Ceramide enhances cytotoxicity in lung cancer H460 and H1299 Cells. Cancers (Basel) 11 (3), 370. doi:10.3390/cancers11030370
Chua, J., Senft, J. L., Lockett, S. J., Brett, P. J., Burtnick, M. N., DeShazer, D., et al. (2016). pH alkalinization by chloroquine suppresses pathogenic Burkholderia Type 6 secretion system 1 and multinucleated giant cells. Infect. Immun. 85 (1), e00586–16. doi:10.1128/IAI.00586-16
Clementi, N., Criscuolo, E., Diotti, R. A., Ferrarese, R., Castelli, M., Burioni, R., et al. (2020). Combined prophylactic and therapeutic use maximizes hydroxychloroquine Anti-SARS-CoV-2 effects in vitro. Front. Microbiol. 11, 1704. doi:10.1101/2020.03.29.014407
Clemessy, J.-L., Borron, S. W., Baud, F. J., Favier, C., Hantson, P. E., and Vicaut, E. (1995). Hypokalaemia related to acute chloroquine ingestion. Lancet 346 (8979), 877–880. doi:10.1016/s0140-6736(95)92711-5
Coronado, L. M., Nadovich, C. T., and Spadafora, C. (2014). Malarial hemozoin: from target to tool. Biochim. Biophys. Acta 1840, 2032–2041. doi:10.1016/j.bbagen.2014.02.009
Coronavirus Death Toll and Trends - Worldometer. (n.d.). Available at: https://www.worldometers.info/coronavirus/coronavirus-death-toll/ (Accessed January 30, 2021).
Cortegiani, A., Ingoglia, G., Ippolito, M., Giarratano, A., and Einav, S. (2020). A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 57, 279–283. doi:10.1016/j.jcrc.2020.03.005
Dai, W., Wu, Y., Bi, J., Wang, S., Li, F., Kong, W., et al. (2018). Anti-viral effects of ABMA against herpes simplex virus type 2 in vitro and in vivo. Viruses 10 (3), 119. doi:10.3390/v10030119
De Argila, D., Gonzalo, A., Pimentel, J., and Rovira, I. (1997). Isolated lichen planus of the lip successfully treated with chloroquine phosphate. Dermatol. 195 (3), 284–285. doi:10.1159/000245964
Devaux, D. A., Rolain, J., Colson, P., and Raoult, D. (2020). New insights on the anti-viral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents 55, 105938. doi:10.1016/j.ijantimicag.2020.105938
Dowall, S. D., Bosworth, A., Watson, R., Bewley, K., Taylor, I., Rayner, E., et al. (2015). Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo Guinea pig model. J. Gen. Virol. 96 (12), 3484–3492. doi:10.1099/jgv.0.000309
Elshazly, E. H., Zhang, S., Yu, L., Zhang, Y., Ke, L., and Gong, R. (2020). Hydroxychloroquine enhances anticancer effect of DOX/folate-phytosterol-carboxymethyl cellulose nanoparticles in A549 lung cancer cells. Trop. J. Pharma. Res. 19 (2), 219–225. doi:10.4314/tjpr.v19i2.1
Eng, C. H., Wang, Z., Tkach, D., Toral-Barza, L., Ugwonali, S., Liu, S., et al. (2016). Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc. Natl. Acad. Sci. U S A 113, 182–187. doi:10.1073/pnas.1515617113
Escobedo, A. A., Hanevik, K., Almirall, P., Cimerman, S., and Alfonso, M. (2014). Management of chronic .Giardia infection. Expert Rev. Anti Infect. Ther. 12, 1143–1157. doi:10.1586/14787210.2014.942283
Estes, M. L., Ewing-Wilson, D., Chou, S. M., Mitsumoto, H., Hanson, M., Shirey, E., et al. (1987). Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am. J. Med. 82, 447–455. doi:10.1016/0002-9343(87)90444-x
Evangelisti, C., Evangelisti, C., Chiarini, F., Lonetti, A., Buontempo, F., Neri, L. M., et al. (2015). Autophagy in acute leukemias: a double-edged sword with important therapeutic implications. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1853 (1), 14–26. doi:10.1016/j.bbamcr.2014.09.023
Fantini, J., Di Scala, C., Henri, S., and Yahi, N. (2020). Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents 55, 105960. doi:10.1016/j.ijantimicag.2020.105960
Fernandes, M. R., Soares, D. B. R., Thien, C. I., and Carneiro, S. (2018). Hydroxychloroquine ototoxicity in a patient with systemic lupus erythematosus. An. Bras. Dermatol. 93 (3), 469–470. doi:10.1590/abd1806-4841.20187615
Ferner, R. E., and Aronson, J. K. (2020). Chloroquine and hydroxychloroquine in covid-19. BMJ 369, m1432. doi:10.1136/bmj.m1432
Francis, S. E., Gluzman, I. Y., Oksman, A., Knickerbocker, A., Mueller, R., Bryant, M. L., et al. (1994). Molecular characterization and inhibition of a Plasmodium falciparum aspartic hemoglobinase. EMBO J. 13 (2), 306–317. doi:10.1002/j.1460-2075.1994.tb06263.x
Ganguli, A., Choudhury, D., Datta, S., Bhattacharya, S., and Chakrabarti, G. (2014). Inhibition of autophagy by chloroquine potentiates synergistically anti-cancer property of artemisinin by promoting ROS dependent apoptosis. Biochimie 107, 338–349. doi:10.1016/j.biochi.2014.10.001
Gao, J., Tian, Z., and Xu, Y. (2020). Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 14 (1), 72–73. doi:10.5582/bst.2020.01047
Gautret, P., Lagier, J. C., Parola, P., Meddeb, L., Mailhe, M., Doudier, B., et al. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 56, 105949. doi:10.1016/j.ijantimicag.2020.105949
Geleris, J., Sun, Y., Platt, J., Zucker, J., Baldwin, M., Hripcsak, G., et al. (2020). Observational Study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 382, 2411–2418. doi:10.1056/NEJMoa2012410
Gilman, A. L., Schultz, K. R., Goldman, F. D., Sale, G. E., Krailo, M. D., Chen, Z., et al. (2012). Randomized trial of hydroxychloroquine for newly diagnosed chronic graft-versus-host disease in children: a Children's Oncology Group study. Biol. Blood Marrow Transplant. 18 (1), 84–91. doi:10.1016/j.bbmt.2011.05.016
Goldberg, D. E. (1993). Hemoglobin degradation in Plasmodium-infected red blood cells. Semin. Cell Biol. 4, 355–361. doi:10.1006/scel.1993.1042
Gorka, A. P., Alumasa, J. N., Sherlach, K. S., Jacobs, L. M., Nickley, K. B., Brower, J. P., et al. (2013). Cytostatic versus cytocidal activities of chloroquine analogues and inhibition of hemozoin crystal growth. Antimicrob. Agents Chemother. 57, 356–364. doi:10.1128/AAC.01709-12
Hage, M. P., Al-Badri, M. R., and Azar, S. T. (2014). A favorable effect of hydroxychloroquine on glucose and lipid metabolism beyond its anti-inflammatory role. Ther. Adv. Endocrinol. Metab. 5 (4), 77–85. doi:10.1177/2042018814547204
Haque, M., Kumar, S., Charan, J., Bhatt, R., Islam, S., Dutta, S., et al. (2021). Utilisation, availability and price changes of medicines and protection equipment for COVID-19 among selected regions in India: findings and implications. Front. Pharmacol. 11, 582154. doi:10.3389/fphar.2020.582154
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280. doi:10.1016/j.cell.2020.02.052
Hu, T. Y., Frieman, M., and Wolfram, J. (2020). Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat. Nanotechnol. 15, 247–249. doi:10.1038/s41565-020-0674-9
Huang, K. J., Su, I. J., Theron, M., Wu, Y. C., Lai, S. K., Liu, C. C., et al. (2005). An interferon‐γ‐related cytokine storm in SARS patients. J. Med. Virol. 75 (2), 185–194. doi:10.1002/jmv.20255
Jancinová, V., Nosál, R., and Petriková, M. (1994). On the inhibitory effect of chloroquine on blood platelet aggregation. Thromb. Res. 74, 495–504. doi:10.1016/0049-3848(94)90270-4
Jansen, G., Scheper, R., and Dijkmans, B. (2003). Multidrug resistance proteins in rheumatoid arthritis, role in disease‐modifying anti-rheumatic drug efficacy and inflammatory processes: an overview. Scand. J. Rheumatol. 32 (6), 325–336. doi:10.1080/03009740310004333
Juurlink, D. N. (2020). Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ 192 (17), E450–E453. doi:10.1503/cmaj.200528
Kalra, S., Naithani, N., Mehta, S., and Kumar, R. (2002). Resistant Malaria: current concepts and therapeutic strategies. Med. J. Armed Forces India 58 (3), 228–233. doi:10.1016/S0377-1237(02)80136-8
Ke, X., Qin, Q., Deng, T., Liao, Y., and Gao, S. J. (2020). Heterogeneous responses of gastric cancer cell lines to tenovin-6 and synergistic effect with chloroquine. Cancers 12, 365. doi:10.3390/cancers12020365
Kearney, J. (2020). Chloroquine as a potential treatment and prevention measure for the 2019 novel coronavirus: a review. Preprint (Accessed March 17, 2020). doi:10.20944/preprints202003.0275.v1
Kelly, J. C., Wasserman, G. S., Bernard, W. D., Schultz, C., and Knapp, J. F. (1990). Chloroquine poisoning in a child. Ann. Emerg. Med. 19 (1), 47–50. doi:10.1016/s0196-0644(05)82141-9
Keshavarzi, F. (2016). Fungistatic effect of hydroxychloroquine, lessons from a case. Med. Mycol. Case Rep. 13, 17–18. doi:10.1016/j.mmcr.2016.09.003
Keyaerts, E., Li, S., Vijgen, L., Rysman, E., Verbeeck, J., Van Ranst, M., et al. (2009). Anti-viral activity of chloroquine against human coronavirus oc43 infection in newborn mice. Antimicrob. Agents Chemother. 53 (8), 3416–3421. doi:10.1128/AAC.01509-08
Kim, D., Ren, C., Ryou, C., and Li, J. (2019). Direct interaction of DNMT inhibitors to PrPC suppresses pathogenic process of prion. Acta Pharm. Sin. B 9, 952–959. doi:10.1016/j.apsb.2019.04.001
Kimura, T., Takabatake, Y., Takahashi, A., and Isaka, Y. (2013). Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res. 73 (1), 3–7. doi:10.1158/0008-5472.CAN-12-2464
King, M. A., Ganley, I. G., and Flemington, V. (2016). Inhibition of cholesterol metabolism underlies synergy between mTOR pathway inhibition and chloroquine in bladder cancer cells. Oncogene 35, 4518–4528. doi:10.1038/onc.2015.511
Klionsky, D. J., Abdelmohsen, K., Abe, A., Abedin, M. J., Abeliovich, H., Arozena, A. A., et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 12 (1), 1–222. doi:10.1080/15548627.2016.1139264
Kuba, K., Imai, Y., Ohto-Nakanishi, T., and Penninger, J. M. (2010). Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 128, 119–128. doi:10.1016/j.pharmthera.2010.06.003
Kumari, M., Shukla, S., Pandey, S., Giri, V. P., Bhatia, A., Tripathi, T., et al. (2017). Enhanced cellular internalization: a bactericidal mechanism more relative to biogenic nanoparticles than chemical counterparts. ACS Appl. Mater. Interfaces 9, 4519–4533. doi:10.1021/acsami.6b15473
Kwiek, J. J., Haystead, T. A., and Rudolph, J. (2004). Kinetic mechanism of quinone oxidoreductase 2 and its inhibition by the anti-malarial quinolines. Biochemistry 43, 4538–4547. doi:10.1021/bi035923w
Lagier, J. C., Fenollar, F., Lepidi, H., Giorgi, R., Million, M., and Raoult, D. (2014). Treatment of classic Whipple's disease: from in vitro results to clinical outcome. J. Antimicrob. Chemother. 69, 219–227. doi:10.1093/jac/dkt310
Lagier, J. C., Million, M., Gautret, P., Colson, P., Cortaredona, S., Giraud-Gatineau, A., et al. (2020). Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Travel Med. Infect. Dis. 36, 101791. doi:10.1016/j.tmaid.2020.101791
Lane, J. C. E., Weaver, J., Kostka, K., Duarte-Salles, T., Abrahao, M. T. F., Alghoul, H., et al. (2020). Risk of hydroxychloroquine alone and in combination with azithromycin in the treatment of rheumatoid arthritis: a multinational, retrospective study. Lancet Rheumatol. 2 (11), e698–e711. doi:10.1016/s2665-9913(20)30276-9
Lechien, J. R., Chiesa‐Estomba, C. M., Place, S., Laethem, Y. V., Cabaraux, P., Mat, Q., et al. (2020). Clinical and epidemiological characteristics of 1,420 European patients with mild‐to‐moderate coronavirus disease 2019. J. Intern. Med. 288 (3), 335–344. doi:10.1111/joim.13089
Leckie, M. J., and Rees, R. G. (2002). Stevens–Johnson syndrome in association with hydroxychloroquine treatment for rheumatoid arthritis. Rheumatology 41, 473–474. doi:10.1093/rheumatology/41.4.473
Lee, S., Kim, K. P., Yoo, C., Kim, D., Kim, C., Chae, H., et al. (2018). Autophagy inhibitor, chloroquine synergize oxaliplatin by modulating activity of cytosolic HMGB1 in pancreatic cancer. Cancer Res. 78 (13. Suppl), 1347. doi:10.1158/1538-7445.AM2018-1347
Li, C., Zhu, X., Ji, X., Quanquin, N., Deng, Y.Q., Tian, M., et al. (2017). Chloroquine, a FDA-approved drug, prevents zika virus infection and its associated congenital microcephaly in mice. EBioMedicine 24, 189–194. doi:10.1016/j.ebiom.2017.09.034
Li, G., Fan, Y., Lai, Y., Han, T., Li, Z., Zhou, P., et al. (2020). Coronavirus infections and immune responses. J. Med. Virol. 92, 424–432. doi:10.1002/jmv.25685
Li, X., Geng, M., Peng, Y., Meng, L., and Lu, S. (2020). Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharma. Anal. 10, 102–108. doi:10.1016/j.jpha.2020.03.001
Liang, R., Wang, L., Zhang, N., Deng, X., Su, M., Su, Y., et al. (2018). Development of small-molecule MERS-CoV inhibitors. Viruses 10 (12), 721. doi:10.3390/v10120721
Lima, T. L., Feitosa, R. D., Santos-Silva, D. E., Santos-Silva, D. M. A., Siqueira, E. M., et al. (2018). Improving encapsulation of hydrophilic chloroquine diphosphate into biodegradable nanoparticles: a promising approach against Herpes Virus Simplex-1 Infection. Pharmaceutics 10, 255. doi:10.3390/pharmaceutics10040255
Liu, D., Li, X., Zhang, Y., Kwong, J. S. W., Li, L., Zhang, Y., et al. (2018). Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: a systematic review and meta-analysis. Drug Des. Devel Ther. 12, 1685–1695. doi:10.2147/DDDT.S166893
Liu, J., Cao, R., Xu, M., Wang, X., Zhang, H., Hu, H., et al. (2020). Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6, 16. doi:10.1038/s41421-020-0156-0
Loricera, J., Calvo-Río, V., Mata, C., Ortiz-Sanjuán, F., González-López, M. A., Alvarez, L., et al. (2014). Urticarial vasculitis in northern Spain: clinical study of 21 cases. Med. (Baltimore) 93 (1), 53–60. doi:10.1097/MD.0000000000000013
Macfarlane, D. E., and Manzel, L. (1998). Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally relatedcompounds. J. Immunol. 160, 1122–1131.
Makowska, A., Eble, M., Prescher, K., Hoß, M., and Kontny, U. (2016). Chloroquine sensitizes nasopharyngeal carcinoma cells but not nasoepithelial cells to irradiation by blocking autophagy. PLoS One 11 (11), e0166766. doi:10.1371/journal.pone.0166766
Markus, D., Gottfried, L., Markus, M., Marina, R., Dario, B., and Danielle de, V. (2020). A SARS-CoV-2 prophylactic and treatment; a counter argument against the sole use of chloroquine. Am. J. Biomed. Sci. Res. 8 (4). doi:10.34297/AJBSR.2020.08.001283
Marois, I., Cloutier, A., Meunier, I., Weingartl, H. M., Cantin, A. M., and Richter, M. V. (2014). Inhibition of influenza virus replication by targeting broad host cell pathways. PLoS One 9 (10), e110631. doi:10.1371/journal.pone.0110631
Martin, R. E., Marchetti, R. V., Cowan, A. I., Howitt, S. M., Bröer, S., and Kirk, K. (2009). Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science 325, 1680–1682. doi:10.1126/science.1175667
Martín-García, R. F., Camacho, N. D., and Sánchez, J. L. (2003). Chloroquine-induced, vitiligo-like depigmentation. J. Am. Acad. Dermatol. 48, 981–983. doi:10.1067/mjd.2003.53
Martinson, J. A., Montoya, C. J., Usuga, X., Ronquillo, R., Landay, A. L., and Desai, S. N. (2010). Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: implication for T-cell activation. Antimicrob. Agents Chemother. 54 (2), 871–881. doi:10.1128/AAC.01246-09
Masmoudi, A., Abdelmaksoud, W., Turki, H., Hachicha, M., Marrekchi, S., Mseddi, M., et al. (2006). Beneficial effects of anti-malarials in the treatment of generalized granuloma annular in children. Tunis Med. 84 (2), 125–127.
Matsuyama, S., Nao, N., Shirato, K., Kawase, M., Saito, S., Takayama, I., et al. (2020). Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U S A 117 (13), 7001–7003. doi:10.1073/pnas.2002589117
Mauthe, M., Orhon, I., Rocchi, C., Zhou, X., Luhr, M., Hijlkema, K. J., et al. (2018). Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14 (8), 1435–1455. doi:10.1080/15548627.2018.1474314
Maycotte, P., Aryal, S., Cummings, C. T., Thorburn, J., Morgan, M. J., and Thorburn, A. (2012). Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy 8, 200–212. doi:10.4161/auto.8.2.18554
McGill, J. B., Johnson, M., Hurst, S., Cade, W. T., Yarasheski, K. E., Ostlund, R. E., et al. (2019). Low dose chloroquine decreases insulin resistance in human metabolic syndrome but does not reduce carotid intima-media thickness. Diabetol. Metab. Syndr. 11, 61. doi:10.1186/s13098-019-0456-4
Mehra, M. R., Ruschitzka, F., and Patel, A. N. (2020a). Retraction: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet 395, 1820. doi:10.1016/s0140-6736(20)31180-6
Mehra, M. R., Desai, S. S., Kuy, S., Henry, T. D., and Patel, A. N. (2020b). Retraction: cardiovascular disease, drug therapy, and mortality in COVID-19. N. Engl. J. Med. 382, 2582. doi:10.1056/NEJMc2021225
Mindell, J. A. (2012). Lysosomal acidification mechanisms. Annu. Rev. Physiol. 74, 69–86. doi:10.1146/annurev-physiol-012110-142317
Mitja, K., Izidor, K., and Mušič, E. (2000). Chloroquine-induced drug-toxic alveolitis. Pneumologie 54 (9), 395–397. doi:10.1055/s-2000-7180
Molina, J. M., Delaugerre, C., Le Goff, J., Mela-Lima, B., Ponscarme, D., Goldwirt, L., et al. (2020). No evidence of rapid anti-viral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 50, 384. doi:10.1016/j.medmal.2020.03.006
Nagaratnam, N., Chetiyawardana, A. D., and Rajiyah, S. (1978). Aplasia and leukaemia following chloroquine therapy. Postgrad. Med. J. 54, 108–112. doi:10.1136/pgmj.54.628.108
National Center for Biotechnology Information (2004). PubChem Compound Summary for CID 2719, Chloroquine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Chloroquine (Accessed April 17, 2020).
Nuri, E., Taraborelli, M., Andreoli, L., Tonello, M., Gerosa, M., Calligaro, A., et al. (2017). Long-term use of hydroxychloroquine reduces antiphospholipid antibodies levels in patients with primary antiphospholipid syndrome. Immunol. Res. 65 (1), 17–24. doi:10.1007/s12026-016-8812-z
Oh, S., Shin, J. H., Jang, E. J., Won, H. Y., Kim, H. K., Jeong, M. G., et al. (2016). Anti-inflammatory activity of chloroquine and amodiaquine through p21-mediated suppression of T cell proliferation and Th1 cell differentiation. Biochem. Biophys. Res. Commun. 474, 345–350. doi:10.1016/j.bbrc.2016.04.105
Orjih, A. U. (1997). Heme polymerase activity and the stage specificity of anti-malarial action of chloroquine. J. Pharmacol. Exp. Ther. 282, 108–112.
Pandey, A. V., and Chauhan, V. S. (1998). Heme polymerization by malarial parasite: a potential target for anti-malarial drug development. Curr. Sci. 75 (9), 911–918.
Pandey, A. V., and Tekwani, B. L. (1997). Depolymerization of malarial hemozoin: a novel reaction initiated by blood schizontocidal anti-malarials. FEBS Lett. 402, 236–240. doi:10.1016/s0014-5793(96)01536-0
Parhizgar, A. R., and Tahghighi, A. (2017). Introducing new anti-malarial analogues of chloroquine and amodiaquine: a narrative review. Iran J. Med. Sci. 42, 115–128.
Parker, F. S., and Irvin, J. L. (1952). The interaction of chloroquine with nucleic acids and nucleoproteins. J. Biol. Chem. 199, 897–909. doi:10.1016/s0021-9258(18)38529-6
Paton, N. I., Lee, L., Xu, Y., Ooi, E. E., Cheung, Y. B., Archuleta, S., et al. (2011). Chloroquine for influenza prevention: a randomized, double-blind, placebo-controlled trial. Lancet Infect. Dis. 11, 677–683. doi:10.1016/s1473-3099(11)70065-2
Pelt, J., Busatto, S., Ferrari, M., Thompson, E. A., Mody, K., and Wolfram, J. (2018). Chloroquine and nanoparticle drug delivery: a promising combination. Pharmacol. Ther. 191, 43–49. doi:10.1016/j.pharmthera.2018.06.007
Ponticelli, C., and Moroni, G. (2017). Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin. Drug Saf. 16, 411–419. doi:10.1080/14740338.2017.1269168
Prentice, E., McAuliffe, J., Lu, X., Subbarao, K., and Denison, M. R. (2004). Identification and characterization of severe acute respiratory syndrome coronavirus replicase proteins. J. Virol. 78, 9977–9986. doi:10.1128/JVI.78.18.9977-9986.2004
Puavilai, S., Kunavisarut, S., Vatanasuk, M., Timpatanapong, P., Sriwong, S. T., Janwitayanujit, S., et al. (1999). Ocular toxicity of chloroquine among Thai patients. Int. J. Dermatol. 38, 934–937. doi:10.1046/j.1365-4362.1999.00849.x
Pukrittayakamee, S., Chantra, A., Vanijanonta, S., Clemens, R., Looareesuwan, S., and White, N. J. (2000). Therapeutic responses to quinine and clindamycin in multidrug-resistant falciparum malaria. Antimicrob. Agents Chemother. 44 (9), 2395–2398. doi:10.1128/AAC.44.9.2395-2398.2000
Rand, J. H., Wu, X. X., Quinn, A. S., Chen, P. P., Hathcock, J. J., and Taatjes, D. J. (2008). Hydroxychloroquine directly reduces the binding of antiphospholipid antibodybeta2-glycoprotein I complexes to phospholipid bilayers. Blood 112 (5), 1687–1695. doi:10.1182/blood-2008-03-144204
Rangnekar, V. M. (2019). Chloroquine induction par-4 and treatment of cancer. U.S Patent No 10,512,641 10, 641.
RECOVERY Collaborative Group (2020). Effect of hydroxychloroquine in hospitalized patients with Covid-19. N. Eng. J. Med. 383, 2030–2040. doi:10.1056/NEJMoa2022926
Reiling, S. J., Krohne, G., Friedrich, O., Geary, T. G., and Rohrbach, P. (2018). Chloroquine exposure triggers distinct cellular responses in sensitive versus resistant Plasmodium falciparum parasites. Sci. Rep. 8, 11137. doi:10.1038/s41598-018-29422-6
Roberts, D., Jester, A., Southwood, T., Johnson, K., and Oestreich, K. (2018). Multifocal avascular necrosis of the lunate and triquetrum in a child with systemic lupus erythematosus. J. Rheum. Dis. Treat. 4, 63. doi:10.23937/2469-5726/1510063
Rodriguez-Caruncho, C., and Bielsa Marsol, I. (2014). Antimalarials in dermatology: mechanism of action, indications, and side effects. Actas Dermosifiliogr. 105 (3), 243–252. doi:10.1016/j.adengl.2012.10.021
Rolain, J. M., Colson, P., and Raoult, D. (2017). Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents 30, 297–308. doi:10.1016/j.ijantimicag.2007.05.015
Rothan, H. A., and Byrareddy, S. N. (2020). The epidemiology and pathogenesis of coronavirus disease (COVID-19) Outbreak. J. Autoimmun. 109, 102433. doi:10.1016/j.jaut.2020.102433
Sacher, F., Fauchier, L., Boveda, S., de Chillou, C., Defaye, P., Deharo, J. C., et al. (2020). Use of drugs with potential cardiac effect in the setting of SARS-CoV-2 infection. Arch. Cardiovasc. Dis. 113 (5), 293–296. doi:10.1016/j.acvd.2020.04.003
Sajna, K., and Kamat, S. (2020). Antibodies at work in the time of severe acute respiratory syndrome coronavirus 2. Cytotherapy 23, 101–110. doi:10.1016/j.jcyt.2020.08.009
Scherbel, A. L., Mackenzie, A. H., Nousek, J. E., and Atdjian, M. (1965). Ocular lesions in rheumatoid arthritis and related disorders with particular reference to retinopathy. N. Engl. J. Med. 273 (7), 360–366. doi:10.1056/nejm196508122730704
Scholnick, P. L., Epstein, J., and Marver, H. S. (1973). The molecular basis of the action of chloroquine in Porphyria Cutanea Tarda. J. Invest. Dermatol. 61, 226–232. doi:10.1111/1523-1747.ep12676478
Schrezenmeier, E., and Dörner, T. (2020). Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 16, 155–166. doi:10.1038/s41584-020-0372-x
Schultz, K. R., Su, W. N., Hsiao, C.-C., Doho, G., Jevon, G., Bader, S., et al. (2002). Chloroquine prevention of murine MHC-disparate acute graft-versus-host disease correlates with inhibition of splenic response to CpG oligodeoxynucleotides and alterations in T-cell cytokine production. Biol. Blood Marrow Transplant. 8 (12), 648–655. doi:10.1053/bbmt.2002.v8.abbmt080648
Schwarzer, E., Kühn, H., Valente, E., and Arese, P. (2003). Malaria-parasitized erythrocytes and hemozoin nonenzymatically generate large amounts of hydroxy fatty acids that inhibit monocyte functions. Blood 101, 722–728. doi:10.1182/blood-2002-03-0979
Seitz, C., Hugle, M., Cristofanon, S., Tchoghandjian, A., and Fulda, S. (2013). The dual PI3K/mTOR inhibitor NVP-BEZ235 and chloroquine synergize to trigger apoptosis via mitochondrial-lysosomal cross-talk. Int. J. Cancer 132, 2682–2693. doi:10.1002/ijc.27935
Sharma, O. P. (1998). Effectiveness of chloroquine and hydroxychloroquine in treating selected patients with sarcoidosis with neurological involvement. Arch. Neurol. 55 (9), 1248–1254. doi:10.1001/archneur.55.9.1248
Sheng, W. D., Jiddawi, M. S., Hong, X. Q., and Abdulla, S. M. (1997). Treatment of chloroquine-resistant malaria using pyrimethamine in combination with berberine, tetracycline or cotrimoxazole. East. Afr. Med. J. 74 (5), 283–284.
Shiryaev, S. A., Mesci, P., Pinto, A., Fernandes, I., Sheets, N., Shresta, S., et al. (2017). Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis. Sci. Rep. 7, 15771. doi:10.1038/s41598-017-15467-6
Singal, A. K., Kormos-Hallberg, C., Lee, C., Sadagoparamanujam, V. M., Grady, J. J., Freeman, D. H., et al. (2012). Low-dose hydroxychloroquine is as effective as phlebotomy in treatment of patients with porphyria cutanea tarda. Clin. Gastroenterol. Hepatol. 10 (12), 1402–1409. doi:10.1016/j.cgh.2012.08.038
Singal, A. K. (2019). Porphyria cutanea tarda: recent update. Mol. Genet. Metab. 128 (3), 271–281. doi:10.1016/j.ymgme.2019.01.004
Slater, A. F. G. (1993). Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol. Ther. 57 (2-3), 203–235. doi:10.1016/0163-7258(93)90056-j
Smith, T., Pharma, D., LeClaire, A., and Prosser, T. (2020). COVID-19 drug therapy – potential options. Amsterdam, Netherlands: Elsevier.
Stapley, L. (2001). Bone loss prevention by an anti-malarial drug. Trends Endocrinol. Metabol 12 (4), 146. doi:10.1016/s1043-2760(01)00396-4
Sullivan, D. J., Gluzman, I. Y., and Goldberg, D. E. (1996). Plasmodium hemozoin formation mediated by histidine-rich proteins. Sci 271, 219–222. doi:10.1126/science.271.5246.219
Sullivan, D. J., Matile, H., Ridley, R. G., and Goldberg, D. E. (1998). A common mechanism for blockade of heme polymerization by anti-malarial quinolones. J. Biol. Chem. 273, 31103–31107. doi:10.1074/jbc.273.47.31103
Sullivan, D. J. (2017). Quinolines block every step of malaria heme crystal growth. Proc. Natn Acad. Sci. U.S.A. 114, 7483–7485. doi:10.1073/pnas.1708153114
Supattapone, S., Nguyen, H. O. B., Cohen, F. E., Prusiner, S. B., and Scott, M. R. (1999). Elimination of prions by branched polyamines and implications for therapeutics. Proc. Natl. Acad. Sci. U.S.A 96 (25), 14529–14534. doi:10.1073/pnas.96.25.14529
Surolia, N., and Padmanaban, G. (1991). Chloroquine inhibits heme-dependent protein synthesis in Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 88, 4786–4790. doi:10.1073/pnas.88.11.4786
Szakács, G., Paterson, J. K., Ludwig, J. A., Booth-Genthe, C., and Gottesman, M. M. (2006). Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234. doi:10.1038/nrd1984
Teixeira, R. A., Martinelli Filho, M., Benvenuti, L. A., Costa, R., Pedrosa, A. A., and Nishióka, S. A. (2002). Cardiac damage from chronic use of chloroquine. A case report and review of the literature. Arq. Bras. Cardiol. 79, 85–88. doi:10.1590/s0066-782x2002001000009
Thome, R., Lopes, S. C. P., Costa, F. T. M., and Chen, T. C. (2013). Chloroquine: modes of action of an undervalued drug. Immunol. Lett. 153, 50–57. doi:10.1016/j.imlet.2013.07.004
Touret, F., and Lamballerie, X. (2020). Of chloroquine and COVID-19. Antivir. Res. 177, 104762. doi:10.1016/j.antiviral.2020.104762
Tsai, W.-P., Nara, P. L., Kung, H.-F., and Oroszlan, S. (1990). Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res. Hum. Retroviruses 6 (4), 481–489. doi:10.1089/aid.1990.6.481
van Loosdregt, J., Spreafico, R., Rossetti, M., Prakken, B. J., Lotz, M., and Albani, S. (2013). Hydroxychloroquine preferentially induces apoptosis of CD45RO+ effector T cells by inhibiting autophagy: a possible mechanism for therapeutic modulation of T cells. J. Allergy Clin. Immunol. 131 (5), 1443–1446. doi:10.1016/j.jaci.2013.02.026
van Vugt, M., Leonardi, E., Phaipun, L., Slight, T., Thway, K. L., McGready, R., et al. (2002). Treatment of uncomplicated multidrug-resistant falciparum malaria with artesunate-atovaquone-proguanil. Clin. Infect. Dis. 35, 1498–1504. doi:10.1086/344901
Varisli, L., Cen, O., and Vlahopoulos, S. (2019). Dissecting pharmacological effects of chloroquine in cancer treatment: interference with inflammatory signalling pathways. Immunol. 159, 257–278. doi:10.1111/imm.13160
Verbaanderd, C., Maes, H., Schaaf, M. B., Sukhatme, V. P., Pantziarka, P., Sukhatme, V., et al. (2017). Repurposing drugs in oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience 11, 781. doi:10.3332/ecancer.2017.781
Verschooten, L., Barrette, K., Van Kelst, S., Romero, N. R., Proby, C., De Vos, R., et al. (2012). Autophagy inhibitor chloroquine enhanced the cell death inducing effect of the flavonoid luteolin in metastatic squamous cell carcinoma cells. PLoS One 7 (10), e48264. doi:10.1371/journal.pone.0048264
Vincent, M. J., Bergeron, E., Benjannet, S., Erickson, B. R., Rollin, P. E., Ksiazek, T. G., et al. (2005). Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2, 69. doi:10.1186/1743-422x-2-69
Wang, T. F., and Lim, W. (2016). What is the role of hydroxychloroquine in reducing thrombotic risk in patients with antiphospholipid antibodies? Hematol. Am. Soc. Hematol. Educ. Program 2016 (1), 714–716. doi:10.1182/asheducation-2016.1.714
Wang, H., Yang, P., Liu, K., Guo, F., Zhang, Y., Zhang, G., et al. (2008). SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 18, 290–301. doi:10.1038/cr.2008.15
Wang, G., Chen, S., Edwards, H., Cui, X., Cui, L., and Ge, Y. (2014). Combination of chloroquine and GX15-070 (obatoclax) results in synergistic cytotoxicity against pancreatic cancer cells. Oncol. Rep. 32, 2789–2794. doi:10.3892/or.2014.3525
Wang, L. F., Lin, Y. S., Huang, N. C., Yu, C. Y., Tsai, W. L., Chen, J. J., et al. (2015). Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery. J. Interferon Cytokine Res. 35 (3), 143–156. doi:10.1089/jir.2014.0038
Wang, H., Liu, W., Yu, F., and Lu, L. (2016). Disruption of clathrin-dependent trafficking results in the failure of grass carp reovirus cellular entry. Virol. J. 13, 25. doi:10.1186/s12985-016-0485-7
Wang, F., Tang, J., Li, P., Si, S., Yu, H., Yang, X., et al. (2018). Chloroquine enhances the radiosensitivity of bladder cancer cells by inhibiting autophagy and activating apoptosis. Cell Physiol. Biochem. 45, 54–66. doi:10.1159/000486222
Wang, B., Guo, H., Ling, L., Ji, J., Niu, J., and Gu, Y. (2020). The chronic adverse effect of chloroquine on kidney in rats through an autophagy dependent and independent pathways. Nephron 144, 96–108. doi:10.1159/000503882
Wang, M., Cao, R., Zhang, L., Yang, X., Liu, J., Xu, M., et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30 (3), 269–271. doi:10.1038/s41422-020-0282-0
Weston, S., Coleman, C. M., Haupt, R., Logue, J., Matthews, K., and Frieman, M. (2020). FDA approved drugs with broad anti-coronaviral activity inhibit SARS-CoV-2 in vitro. bioRxiv. doi:10.1101/2020.03.25.008482
Weyerhäuser, P., Kantelhardt, S. R., and Kim, A. L. (2018). Re-purposing chloroquine for glioblastoma: potential merits and confounding variables. Front. Oncol. 8, 335. doi:10.3389/fonc.2018.00335
WHO Solidarity Trial Consortium (2020). Repurposed anti-viral drugs for COVID-19—interim WHO SOLIDARITY trial results. N. Eng. J. Med. 384, 497–511. doi:10.1056/NEJMoa2023184
Wielgo-Polanin, R., Lagarce, L., Gautron, E., Diquet, B., and Lainé-Cessac, P. (2005). Hepatotoxicity associated with the use of a fixed combination of chloroquine and proguanil. Int. J. Antimicrob. Agents 26, 176–178. doi:10.1016/j.ijantimicag.2005.04.019
Wondafrash, D. Z., Desalegn, T. Z., Yimer, E. M., Tsige, A. G., Adamu, B. A., and Zewdie, K. A. (2020). Potential effect of hydroxychloroquine in diabetes mellitus: a systematic review on preclinical and clinical trial studies. J. Diabetes Res. 2020, 5214751. doi:10.1155/2020/5214751
Wu, C., Liu, Y., Yang, Y., Zhang, P., Zhong, W., Wang, Y., et al. (2020). Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 10, 766–788. doi:10.1016/j.apsb.2020.02.008
Xu, X., Chen, P., Wang, J., Feng, J., Zhou, H., Li, X., et al. (2020). Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its Spike protein for risk of human transmission. Sci. China Life Sci. 63, 457–460. doi:10.1007/s11427-020-1637-5
Yan, Y., Zou, Z., Sun, Y., Li, X., Xu, K.-F., Wei, Y., et al. (2013). Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 23, 300–302. doi:10.1038/cr.2012.165
Yan, Y., Xu, Z., Dai, S., Qian, L., Sun, L., and Gong, Z. (2016). Targeting autophagy to sensitive glioma to temozolomide treatment. J. Exp. Clin. Cancer Res. 35, 23. doi:10.1186/s13046-016-0303-5
Yang, N., and Shen, H.-M. (2020). Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 16 (10), 1724–1731. doi:10.7150/ijbs.45498
Yao, X., Ye, F., Zhang, M., Cui, C., Huang, B., Niu, P., et al. (2020). In vitro anti-viral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 71, 732–739. doi:10.1093/cid/ciaa237
Zaidi, F. H., Rennie, C. A., Drinkwater, A. K., Sahu, D., Akyol, E., and Lotery, A. J. (2019). How to set up a hydroxychloroquine retinopathy screening service. Eye 33, 1679–1682. doi:10.1038/s41433-019-0418-y
Zhang, S., Yi, C., Li, C., Zhang, F., Peng, J., Wang, Q., et al. (2019). Chloroquine inhibits endosomal viral RNA release and autophagy-dependent viral replication and effectively prevents maternal to fetal transmission of Zika virus. Antiviral Res. 169, 104547. doi:10.1016/j.antiviral.2019.104547
Zhang, H., Penninger, J. M., Li, Y., Zhong, N., and Slutsky, A. S. (2020). Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 46, 586–590. doi:10.1007/s00134-020-05985-9
Zheng, Y., Ma, Y., Zhang, J., and Xie, X. (2020). COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 17, 259–260. doi:10.1038/s41569-020-0360-5
Zheng, J. (2020). SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 16 (10), 1678–1685. doi:10.7150/ijbs.45053
Zhu, Y., Li, J., Bai, Y., Wang, X., Duan, N., Jiang, H., et al. (2014). Hydroxychloroquine decreases the upregulated frequencies of Tregs in patients with oral lichen planus. Clin. Oral Investig. 18, 1903–1911. doi:10.1007/s00784-013-1176-z
Keywords: SARS-CoV-2, pH-dependent, autophagy, immunomodulatory, antiviral mechanism, toxicity
Citation: Kamat S and Kumari M (2021) Repurposing Chloroquine Against Multiple Diseases With Special Attention to SARS-CoV-2 and Associated Toxicity. Front. Pharmacol. 12:576093. doi: 10.3389/fphar.2021.576093
Received: 25 June 2020; Accepted: 08 February 2021;
Published: 12 April 2021.
Edited by:
Rafael Maldonado, Pompeu Fabra University, SpainReviewed by:
Brian Godman, University of Strathclyde, United KingdomCopyright © 2021 Kamat and Kumari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madhuree Kumari, bWFkaHVyZWVrQGlpc2MuYWMuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.