- 1School of Nursing, Nanjing University of Chinese Medicine, Nanjing, China
- 2Public Teaching Department for Foreign Languages, Nanjing University of Chinese Medicine, Nanjing, China
Aim: We conducted a systematic review of high-quality randomized controlled trials (RCTs) to assess the efficacy and safety of Chinese herbal medicine (CHM) for the treatment of chemotherapy-induced leukopenia (CIL).
Methods: Eight electronic databases were searched from the date of inception to November 4, 2020 for high-quality RCTs that met the requirements of at least four key domains of the Cochrane risk of bias (RoB) tool. RevMan 5.3 was applied for the meta-analysis.
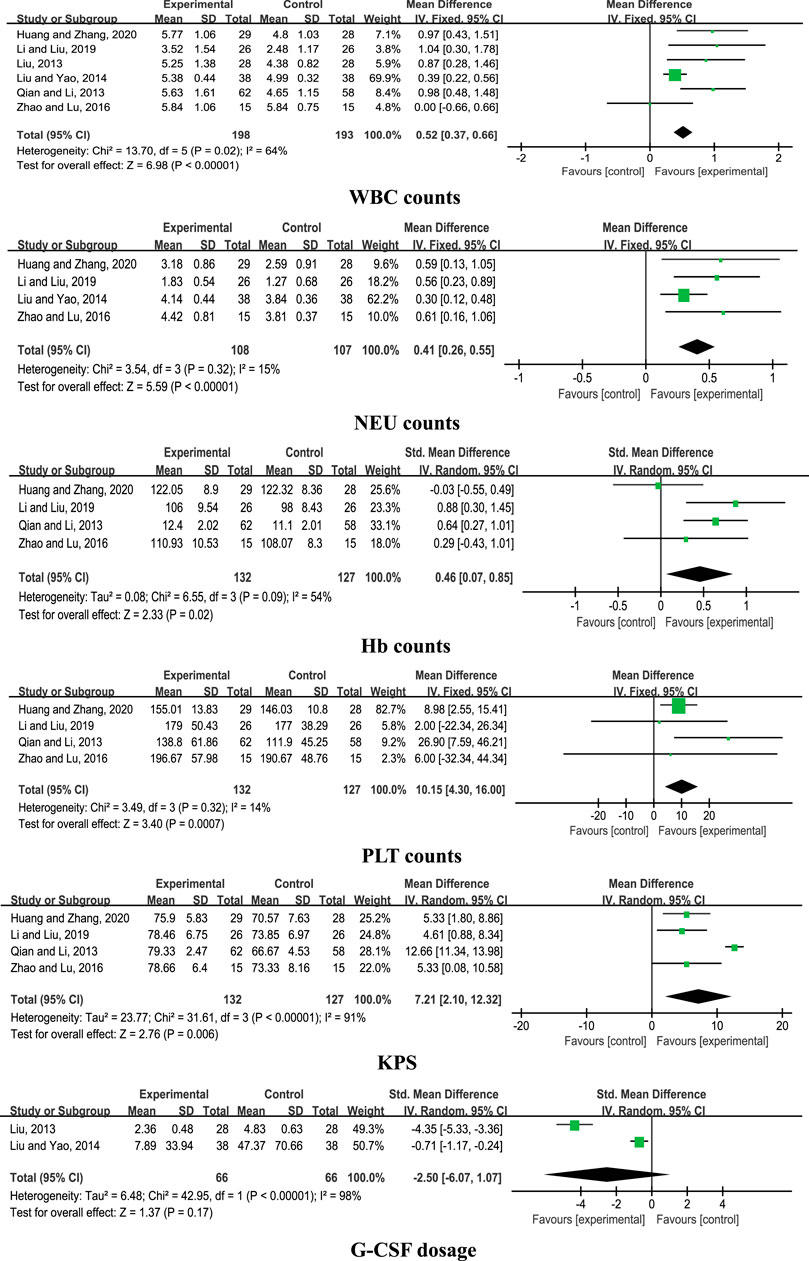
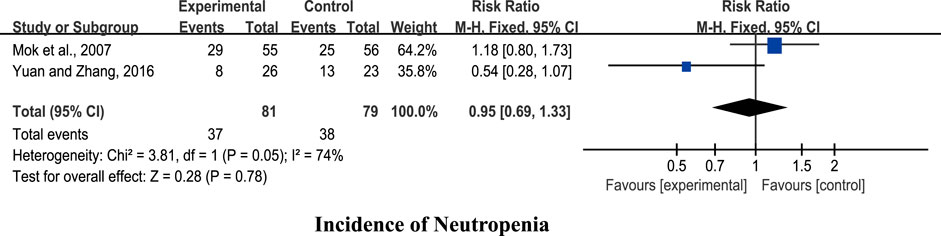
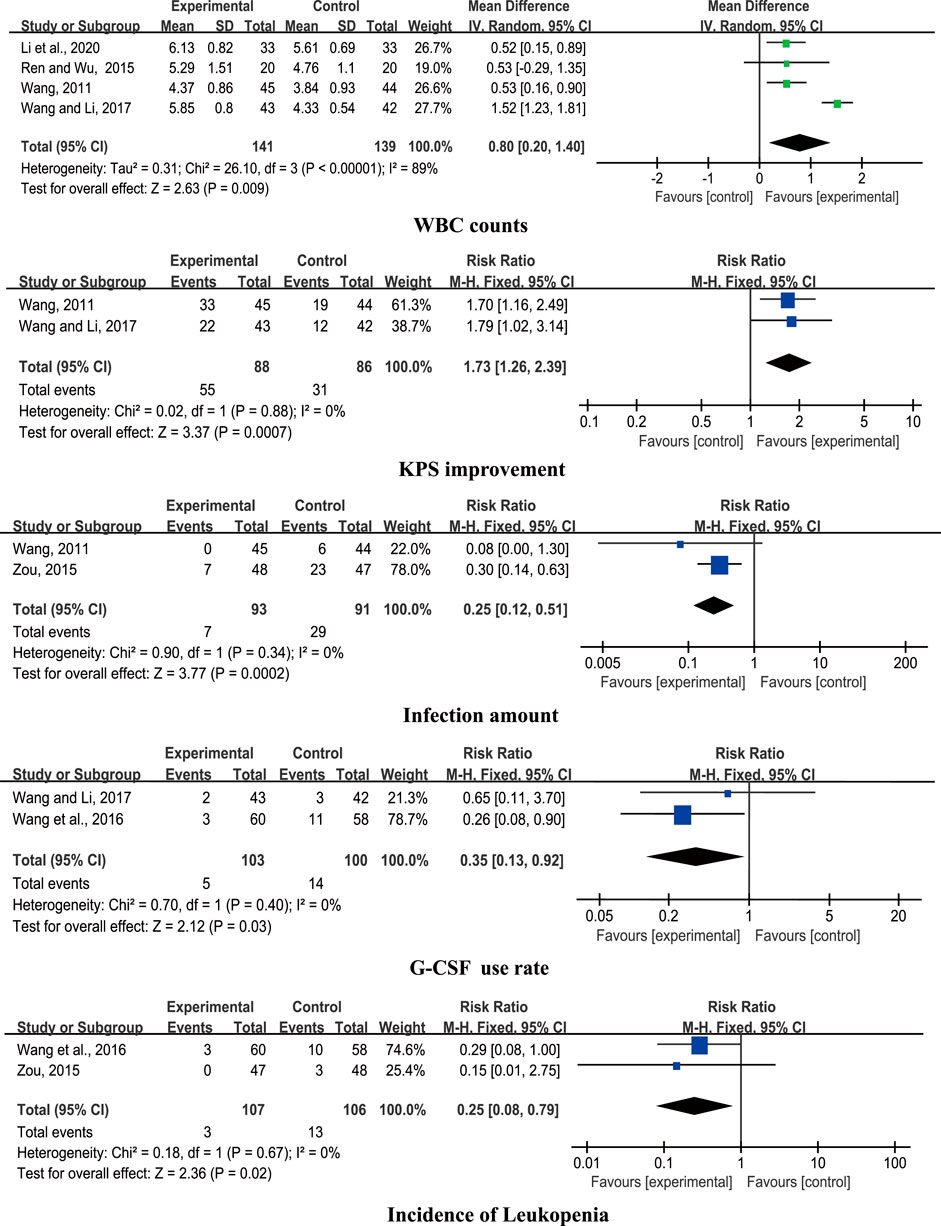
Results: Fourteen RCTs involving 1,053 patients were included. The pooled results showed that CHM + chemotherapy exerted greater beneficial effects on white blood cell (WBC), neutrophil (NEU), hemoglobin (Hb), and platelet (PLT) counts in addition to the Karnofsky performance scale (KPS) score, but showed no significant difference on granulocyte colony-stimulating factor (G-CSF) dosage compared with chemotherapy alone. Placebo (PBO) + chemotherapy and CHM + chemotherapy groups showed no significant differences in terms of reduction of the incidence of neutropenia. CHM + chemotherapy was superior to Western medicine (WM) + chemotherapy in improving the WBC count, KPS, infection amount, G-CSF use rate, and incidence of leukopenia. In addition, no severe adverse events were observed in the 14 RCTs.
Conclusion: CHM in combination with chemotherapy could effectively improve the clinical symptoms of CIL when compared with chemotherapy alone or Western medicine + chemotherapy, except when comparing with PBO + chemotherapy. While CHMs were generally safe for clinical use and exerted no severe side effects in the 14 RCTs, high-quality RCTs with larger sample sizes are essential to reduce study heterogeneity.
Introduction
Chemotherapy is widely applied for treatment of multiple cancer types, with one or more anticancer drugs generally used as part of a standardized chemotherapy regimen in the clinic (Johnstone et al., 2002). However, chemotherapy drugs often have poor targeting problems, and combined application of several drugs inevitably results in a series of adverse events (Sarah, 2013), such as bone marrow dysfunction, peripheral neuropathy, chronic pain, sleep disorders, nausea and vomiting, fatigue, and flushes, which not only negatively affect curative effects but also lead to severe patients’ discomfort and poor quality of life (QOL) posttreatment (Torre et al., 2015; Kato et al., 2019; Makary et al., 2019). Bone marrow suppression remains a major toxic effect (Yeshurun et al., 2014), which is characterized by a decrease in three critical cell types: leukocytes, erythrocytes, and platelets. Leukopenia is one of the most prominent effects of bone marrow suppression (Park et al., 2015) and often accompanied by severe infection and bleeding. Long-term usage of cytotoxic drugs is clearly associated with chemotherapy-induced leukopenia (CIL) (Merryman et al., 2012). To increase the white blood cell (WBC) counts within a short time frame for the maintenance of therapeutic efficacy and continue subsequent courses of treatment, granulocyte colony-stimulating factor (G-CSF) is commonly used to treat CIL (Winkler et al., 2016). However, while the effects of G-CSF are rapid, side effects, such as myalgia and fever, are also commonly reported (Yamauchi et al., 2014), making its usage less acceptable in the clinic. Moreover, for patients with severe myelosuppression, repeated treatment is required to maintain the curative effects of chemotherapy. Once medication is stopped, patients are prone to recurrent episodes of illness (Yang et al., 2015). Therefore, treatments that can facilitate effective and stable relief of CIL and promote patients’ QOL by consolidating the clinical value of previous chemotherapeutic regimens and ensuring continuation of therapy are currently a hot topic of research.
Recent studies have shown that traditional Chinese medicine (TCM) aiming to provide personalized treatment plans with multi-targeted and long-lasting effects together with fewer side effects (Zhang et al., 2015) can alleviate the adverse events of conventional chemotherapy (Li et al., 2019). Based on the key treatment concept of syndrome differentiation, TCM has been popularized and widely applied for patients on chemotherapy, including Chinese herbal medicine (CHM), Chinese patent drug, acupuncture, cupping, and other treatments (Li ta al., 2018). Accumulating reports have confirmed beneficial effects of TCM on adverse conditions resulting from bone marrow suppression after chemotherapy. In earlier pharmacological studies, administration of CHM as an adjuvant treatment significantly improved WBC counts in patients with CIL (Liu and Bi, 2015), relieved tumor-related fatigue and dizziness (Han and Jiang, 2018), elevated patients’ QOL (Li et al., 2019), and reduced toxic side effects (Jia et al., 2014). Data from previous meta-analyses clearly suggested that CHM is more effective than conventional oral Western medicine (WM) for the treatment of CIL induced by specific tumors (Li et al., 2015; Zhang et al., 2019), which we find enlightening, but limiting due to different CHM methods and tumor types. Two other systematic reviews and meta-analyses were not limited by the CHM method or tumor type (Li et al., 2016; Niu et al., 2018), but their clinical application and conclusive reliability were unfortunately affected by the low methodological quality of the included literature. Accordingly, we conducted a comprehensive advanced systematic review and meta-analysis of the effects of CHM on CIL, focusing on high-quality RCTs.
Materials and Methods
Our study was conducted according to the guidelines provided by the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009) and Cochrane Handbook (Higgins et al., 2011).
Search Strategy
We performed a comprehensive search of 4 English electronic databases (PubMed, Web of Science, Cochrane Library, and Elsevier) from the date of inception to November 4, 2020 and 4 Chinese electronic databases (China National Knowledge Infrastructure, Chinese Biomedical Database, Chinese VIP Information Database, and Wanfang Med Database). The following medical subject heading (MeSH) terms and free text words were used for the search: “Chinese Medicine,” “Chinese Herbal Medicine,” “Chinese patent medicine,” “leukopenia,” “hypoleucocytosis,” “hypolekocytosis,” “neutropenia,” and “bone marrow suppression.” In the Chinese electronic databases, keywords were searched in Chinese characters and Pinyin. There was no limitation on language used.
Inclusion and Exclusion Criteria
Inclusion Criteria
Inclusion criteria were based on the following:
(1) Type of participant: diagnosis of cancer with chemotherapy-induced leukopenia.
(2) Type of study: only high-quality randomized controlled trials (RCTs) related to Chinese herbal medicine in the treatment of CIL, which met the requirements of at least four key domains of the Cochrane risk of bias (RoB) tool, along with trials published in the form of dissertations were selected as eligible studies.
(3) Type of intervention: participants in the intervention groups were treated with CHM in combination with chemotherapeutic drugs. There was no limitation with regard to the form of CHM used (e.g., decoction, capsule, and granule), dosage, or treatment duration. The control groups used chemotherapy alone, chemotherapeutic drugs plus placebo, or chemotherapeutic drugs plus Western medicine, which used to raise leukocytes. All participants were treated via oral administration.
(4) Type of outcome measure: primary outcome measures were white blood cell (WBC), neutrophil (NEU), hemoglobin (Hb), and platelet (PLT) counts in addition to the incidence of leukopenia and neutropenia. Secondary outcome measures were the Karnofsky performance scale (KPS) score and improvement, infection amount, granulocyte colony-stimulating factor (G-CSF) dosage and use rate, and adverse events.
Exclusion Criteria
Exclusion criteria were as follows:
(1) Patients with leukopenia not caused by chemotherapy.
(2) Duplicate studies, review, animal experiments, and conference abstracts.
(3) Nonoral TCM methods, such as acupuncture, moxibustion, massage, and acupoint injection, in the intervention group or use of CHM drugs in the control group.
Data Extraction
Two reviewers (QW and HY) independently extracted the relevant data according to the predetermined inclusion and exclusion criteria. The following information was obtained using a standard data extraction form: 1) general information: publication year, language, and first author; 2) characteristics of participants: sample size, age, and gender; 3) intervention information: intervention method, medication dose, and course of treatment; and 4) outcome measures. To resolve any disagreements, the two reviewers discussed the issue or consulted the corresponding author (G-HX).
Risk of Bias Assessment
The RoB tool was used to assess the methodological quality of included studies (Higgins et al., 2011). Seven aspects were evaluated: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. RCTs that met the requirements of at least 4 of the above parameters were selected for the final analysis.
Statistical Analysis
RevMan 5.3 was used for statistical analysis. Chi-square test and I2 statistic were employed to assess the heterogeneity between intervention and control results. In cases where I2 was < 50% or chi-square p value was > 0.1, a fixed-effects model was used. Otherwise, a random-effects model was applied. p values <0.05 were considered statistically significant. The risk ratio (RR) with 95% confidence interval (CI) was used to calculate the dichotomous data, while the mean difference (MD) or standardized mean difference (SMD) was used to express the continuous data.
Assessment of Evidence Quality
The overall quality of the evidence for each outcome was assessed by two reviewers (QW and HY) in accordance with the methodology recommended by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) criteria (Schunemann et al., 2008). In the system, quality of the evidence varies in four levels: very low quality, low quality, moderate quality, and high quality. Risk of bias, inconsistency, indirectness, imprecision, and other factors (e.g., publication bias) are factors relating to lowering the level of evidence. According to specific criteria such as large I2 indicating inconsistency, the level of evidence from RCTs can be downgraded by one or two levels. Summary of findings table for outcomes was performed using GRADEpro GDT.
Results
Study Selection
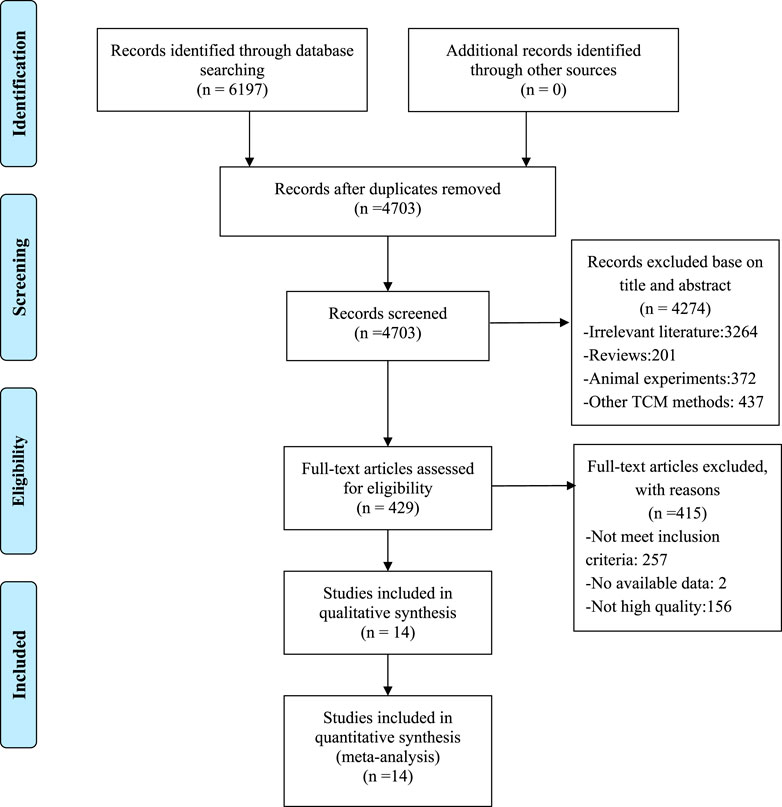
A total of 6,197 studies were identified after searching eight databases, from which 1,494 duplicates were removed. Among the remaining 4,703 records, 4,274 were excluded for various reasons after screening according to titles and abstracts. Specifically, 3,264 studies were irrelevant, 437 used other TCM methods, 372 were animal experiments, and 201 were reviews. Comprehensive reading of the full text of the remaining 429 articles resulted in exclusion of 415 studies due to the following reasons: 257 articles did not meet the inclusion criteria, two had no available data, and 156 had low methodological quality. Finally, 14 RCTs with RoB scores ≥4 were included (Mok et al., 2007; Wang, 2011; Liu, 2013; Qian and Li, 2013; Liu and Yao, 2014; Ren and Wu, 2015; Zou, 2015; Wang et al., 2016; Yuan and Zhang, 2016; Zhao and Lu, 2016; Wang and Li, 2017; Li and Liu, 2019; Huang and Zhang, 2020; Li et al., 2020). A flowchart of the screening process is presented in Figure 1.
Characteristics of Included Studies
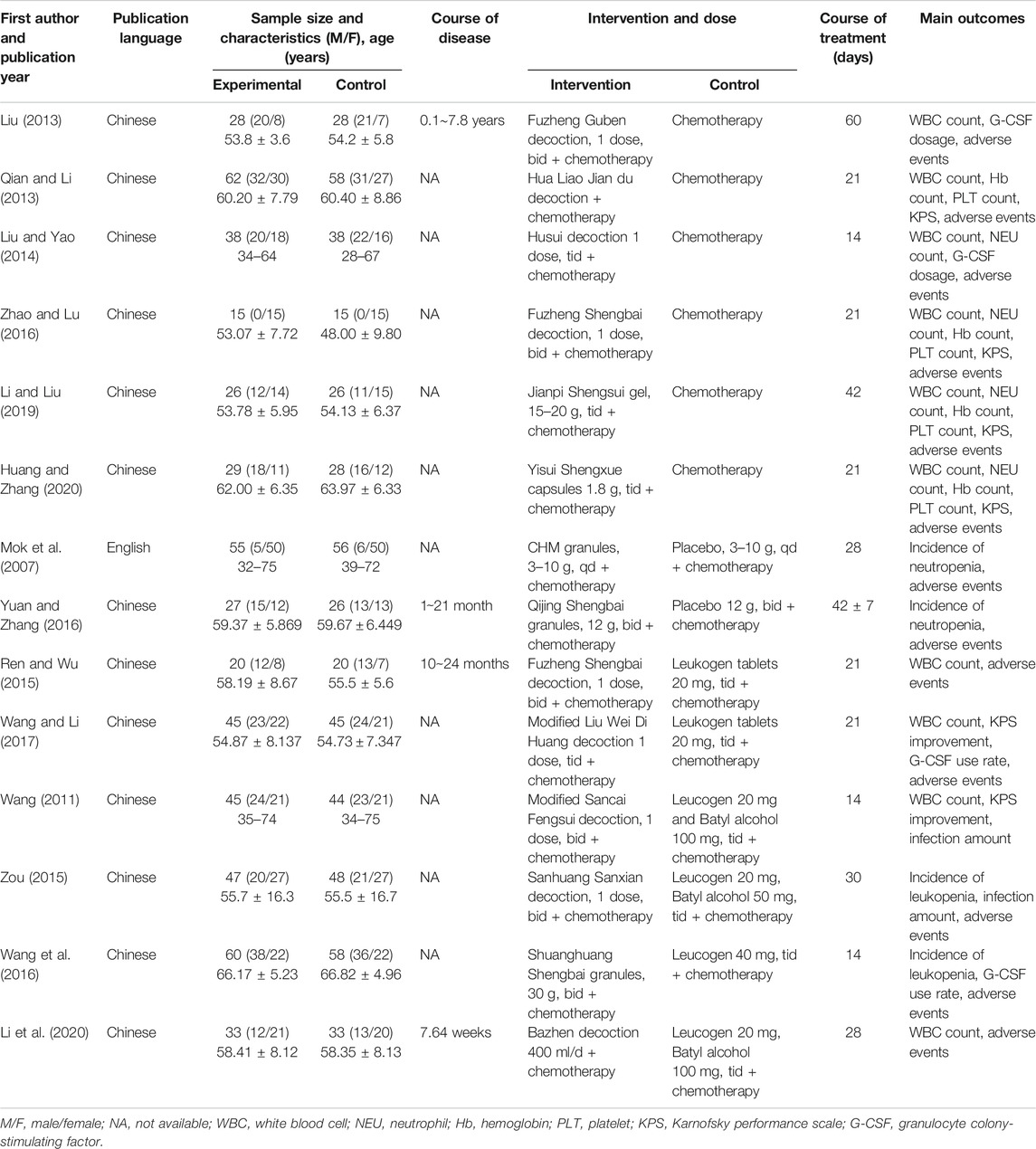
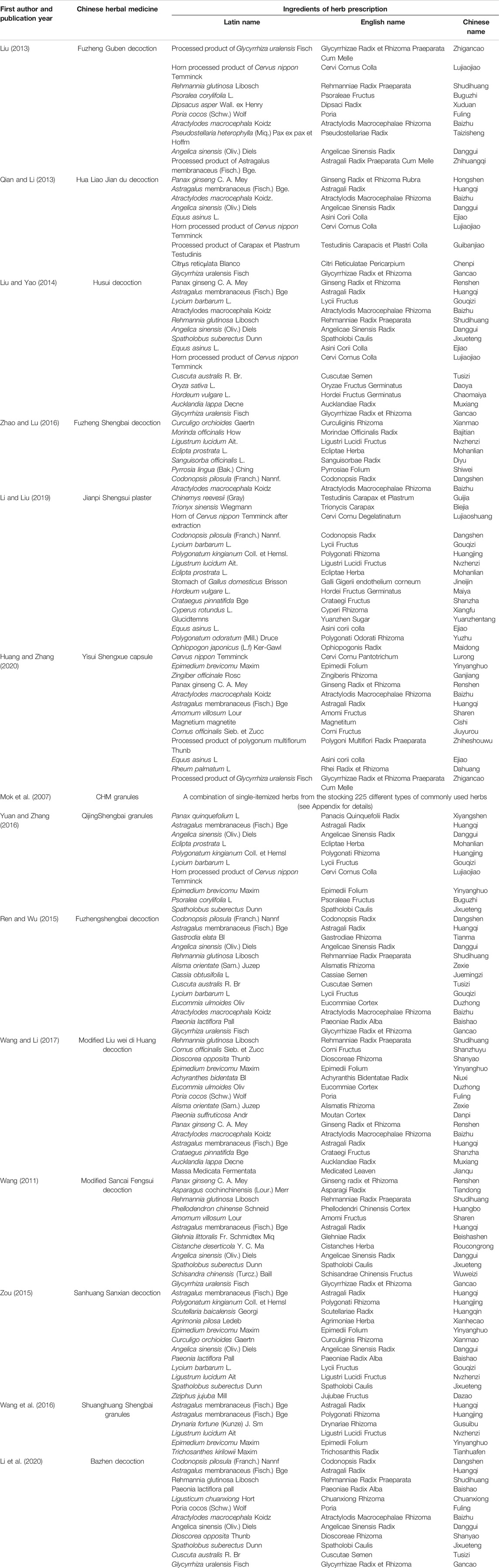
A total of 14 studies involving 1,053 patients were included. Only one study was published in English (Mok et al., 2007) and the remaining in Chinese (Wang, 2011; Liu, 2013; Qian and Li, 2013; Liu and Yao, 2014; Ren and Wu, 2015; Zou, 2015; Wang et al., 2016; Yuan and Zhang, 2016; Zhao and Lu, 2016; Wang and Li, 2017; Li and Liu, 2019; Huang and Zhang, 2020; Li et al., 2020). Sample sizes of studies published from 2007 to 2020 ranged from 30 to 120, which included a total of 530 patients in the treatment and 523 in the control groups. Six studies compared CHM + chemotherapy with chemotherapy alone (n = 391) (Liu, 2013; Qian and Li, 2013; Liu and Yao, 2014; Zhao and Lu, 2016; Li and Liu, 2019; Huang and Zhang, 2020), and two compared CHM + chemotherapy with placebo (PBO)+ chemotherapy (n = 164) (Mok et al., 2007; Yuan and Zhang, 2016). Six studies compared CHM + chemotherapy with WM + chemotherapy (n = 498) (Wang, 2011; Ren and Wu, 2015; Zou, 2015; Wang et al., 2016; Wang and Li, 2017; Li et al., 2020). The treatment courses lasted from 14 to 60 days. The preparations used for treatment in the intervention groups of the 14 RCTs were administered orally in the form of decoction (nine comparative analyses), granules (three comparative analyses), capsules, and gel (one comparative analysis separately). The characteristics of the 14 trials are presented in Table 1, and the components are described in Table 2.
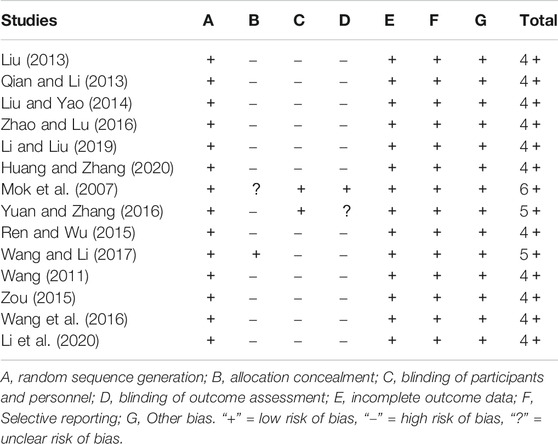
Risk of Bias Assessment
Risk of bias (ROB) data are shown in Table 3. All 14 RCTs specified the methods of random sequence generation. Only one study reported the allocation concealment method (Wang and Li, 2017). Two studies blinded both participants and personnel (Mok et al., 2007; Yuan and Zhang, 2016), and one blinded outcome assessment (Mok et al., 2007). As shown in the table, one article scored six points (Mok et al., 2007), two scored five points (Yuan and Zhang, 2016; Wang and Li, 2017), and eleven scored four points (Wang, 2011; Liu, 2013; Qian and Li, 2013; Liu and Yao, 2014; Ren and Wu, 2015; Zou, 2015; Wang et al., 2016; Zhao and Lu, 2016; Li and Liu, 2019; Huang and Zhang, 2020; Li et al., 2020).
Assessment of Efficacy
Chinese Herbal Medicine Plus Chemotherapy Versus Chemotherapy Alone
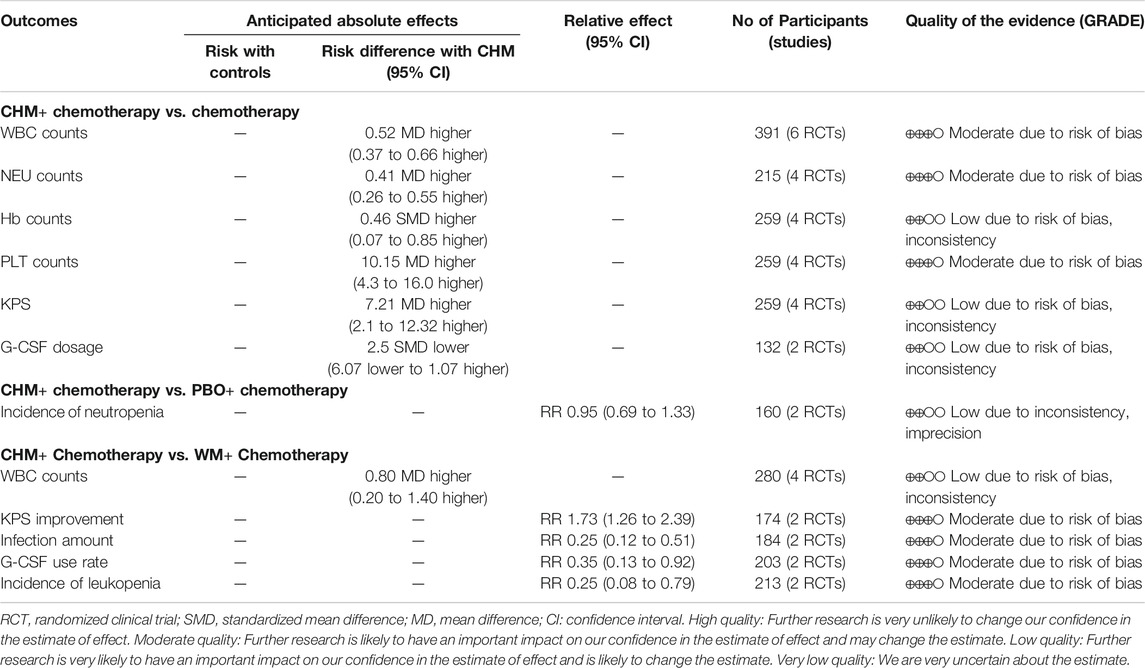
Six comparative studies (Liu, 2013; Qian and Li, 2013; Liu and Yao, 2014; Zhao and Lu, 2016; Li and Liu, 2019; Huang and Zhang, 2020) were included for analysis. The results of the meta-analysis disclosed a significant increase in WBC counts with CHM + chemotherapy, compared with chemotherapy alone (n = 391, MD = 0.52, 95% CI: 0.37 to 0.66, I2 = 64%, p < 0.00001). Data from pooled studies additionally showed that combined treatment with CHM and chemotherapy induced a significant increase in NEU, Hb, and PLT counts and KPS (p < 0.00001; p = 0.02; p = 0.0007; p = 0.006), aside from G-CSF dosage (p = 0.17; Figure 2).
Chinese Herbal Medicine Plus Chemotherapy Versus Placebo Plus Chemotherapy
Two studies (Mok et al., 2007; Yuan and Zhang, 2016) were included for analysis. The pooled results showed no significant differences in reducing the incidence of neutropenia (n = 164, RR 0.95, 95% CI: 0.69 to 1.33, I2 = 74%, p = 0.78; Figure 3).
Chinese Herbal Medicine Plus Chemotherapy Versus Western medicine Plus Chemotherapy
Six studies compared CHM + chemotherapy with WM + chemotherapy (Wang, 2011; Ren and Wu, 2015; Zou, 2015; Wang et al., 2016; Wang and Li, 2017; Li et al., 2020). The pooled results revealed significant differences in WBC counts, KPS improvement, infection amount, G-CSF use rate, and incidence of leukopenia (p < 0.05) between the groups (Figure 4).
Adverse Events
One study (Liu, 2013) reported vomiting and nausea in one case from the experimental group and nine cases from the control group. Both symptoms disappeared after reduction of the treatment dose. Another study (Mok et al., 2007) described occurrence of nausea, vomiting, and anorexia in both the experimental and control groups. In particular, CHM had a significant impact on control of nausea. Two studies (Wang and Li, 2017; Huang and Zhang, 2020) also reported nausea and vomiting in both experimental and control groups, with no significant differences in the two groups. Zou (2015) reported adverse events, such as slight muscle aches, fatigue, low-grade fever, nausea, and vomiting, which were relieved after symptomatic treatment, while Wang et al. (2016) noted one case of mild diarrhea in the intervention group and remission after dosage reduction. No severe adverse events were reported in three studies (Wang, 2011; Ren and Wu, 2015; Yuan and Zhang, 2016). Li et al. (2020) reported rash, dizziness, nausea, and vomiting in the experimental group, which indicated no significant difference compared with the control group. Five other studies (Qian and Li, 2013; Liu and Yao, 2014; Zhao and Lu, 2016; Li and Liu, 2019; Huang and Zhang, 2020) showed no significant differences in liver and kidney functions of patients from both experimental and control groups. The collective results from the 14 RCTs indicate that CHM exerts no severe side effects and is generally safe for human use.
Assessment of Evidence Quality
Comparing CHM + chemotherapy with chemotherapy alone or PBO + chemotherapy or WM + chemotherapy, the overall quality of evidence according to each outcome measures was moderate or low. The results of GRADE assessments are presented in Table 4.
Discussion
Cancer remains a severe public health problem with rapidly increasing incidence and mortality rates and is the leading cause of death worldwide (Bray et al., 2018; Siegel et al., 2020). Chemotherapy is currently the main treatment modality for cancer. However, CIL is the most common adverse effect of chemotherapy, which is directly associated with survival rates (Liu et al., 2013; Campos et al., 2015; Wyatt et al., 2015; Varughese et al., 2020). G-CSF is commonly used to relieve side effects and improve the QOL of patients with CIL (Disis, 2005; Ohnaka et al., 2013; Cornes et al., 2018). However, despite rapid effects, the drug is not suitable for long-term use in all patients owing to its high cost and secondary malignancy risk (Lyman et al., 2013; Lyman, et al., 2018). Thus, increasing number of patients have turned to complementary and alternative medicines to control symptoms and improve the QOL (Kuo et al., 2018; Knecht et al., 2020). CHM has received considerable research attention over the past few years and is widely applied following chemotherapy for various cancer types. Compared with G-CSF, the long-lasting effects and affordability of CHM have made it the preferred treatment of choice for many patients in China. Based on continued evaluation of clinical efficacy from meta-analyses together with accumulating experimental and pharmacological insights into their mechanisms of action, CHM drugs could increasingly benefit patients with chemotherapy-induced leukopenia (CIL) worldwide (Jia et al., 2015; Yang et al., 2017; Chen et al., 2018).
Trials included in previous meta-analyses (Li et al., 2016; Niu et al., 2018) had low methodological quality, with no information on blinding and allocation concealment. This updated systematic review included 14 high-quality studies that were divided into three subgroups to reduce heterogeneity, with a total of 1,053 selected patients. Earlier studies by Li et al. (2016) and Niu et al. (2018) had few evaluation indicators, and both showed superiority of CHM in improving the clinical efficacy rate. Niu and coworkers (2018) additionally demonstrated that CHM could enhance the KPS score.
In the current meta-analysis, the methodological quality was adjusted, the latest literature was included, and more specific indicators were evaluated, with the aim of providing a comprehensive and reliable reference for subsequent research. Pooled data on comparative analyses of CHM + chemotherapy and chemotherapy alone revealed that CHM had greater beneficial effects on several indicators, including WBC, NEU, Hb, and PLT counts, as well as KPS, but not G-CSF dosage. The CHM + chemotherapy group was non-inferior to the PBO + chemotherapy group for the incidence of neutropenia, supporting the findings of Liew et al. (2019) and Mok et al. (2007); CHM did not reduce hematologic toxicity associated with chemotherapy. The CHM + chemotherapy group was superior to the WM + chemotherapy group in terms of improvement of the WBC counts, KPS score, infection amount, G-CSF use rate, and incidence of leukopenia. In addition, CHM drugs were generally safe and induced no severe adverse events.
Our study has several limitations that should be taken into consideration. First, the methodological quality of the included studies was generally low. Although we searched both English and Chinese databases, potential selection bias may have been introduced since most of the included records retrieved were Chinese. Second, although all the trials met the requirements of at least four parameters of the Cochrane RoB tool, some methodological restrictions existed in primary studies. Only one study reported the method of allocation concealment that could lead to selection bias. Only two described blinding of participants and personnel, and one described blinding of outcome assessment. Missing blinding may cause detection bias. It is difficult to conduct adequate blinding in CHM RCTs due to different smells and tastes of CHM decoctions. Third, different components, doses, and duration of CHM interventions may have caused potential heterogeneity. Fourth, some indicators appeared less frequently, only in two studies. Finally, the small sample of subgroup and the quality of the evidence lead the conclusion to be more objective and explicit, giving us inspiration to design related clinical intervention.
Conclusion
CHM in combination with chemotherapy could improve clinical symptoms of CIL when compared with chemotherapy alone or Western medicine + chemotherapy, except when comparing with PBO + chemotherapy. While CHMs were generally safe and exerted no severe side effects in all 14 RCTs, larger sample sizes and high-quality RCTs are required to reduce study heterogeneity.
Author Contributions
QW designed the study and wrote the article. HY and QW performed the literature database search, data collection, extraction, and assessment of evidence quality. Q-qW and W-tL performed data analysis and rationalization of the results. B-bY helped with improving the writing of the manuscript. Y-mB and G-hX supervised all aspects of the study.
Funding
This study was supported by the Nursing Advantageous Discipline Construction Project in Nanjing University of Chinese medicine, Jiangsu Province (2019YSHL017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Appendix
Radix Scrophulariae, Polyporus, Coix Lacrymajobi, Semen Plantaginis, Herba Lysimachiae, Spora Lygodii, Herba Artemisiae Scopariae, Fructus Kochiae, Herba Dianthi, Herba Plantaginis, Pericarpium Arecae, Rhizoma Smilacis Glabrae, processed Radix Aconiti, Rhizoma Zingiberis, Cinnamomun cassia, Herba Asari, Fructus Evodiae, Radix Aconiti Kusnezoffii, Citrus Reticulata, Fructus Auranti Immaturus, Citrus medica, Cyperus Rotundus,Wuyao, Fructus Toosendan, Caulis Perillae, Massa Medicata Fermentata, Fructus Hordei Germinatus, Fructus Crataegi, Semen Raphani, Herba seu Radix Cirsii Japonici, Herba Cephalanoploris, Radix Sanguisorbae, Sophora Japonica, Thuja orientalis, Herba, Agrimoniae, Radizoma Bletillae, Pollen Typhae, Radix Notoginseng, Radix Sangyisorbae, Rhizoma Chuanxiong, Olibanum, Myrrha, Rhizoma Corydelis, Radix Curcumae, Rhizoma Curcumae, Rhizoma Sparganii, Radix Salviae Miltiorrhizae, Herba Leonuri, Semen Persicae, Flos CArthami, Faeces Trogopterori, Achyranthes Bidentata Blume, Cyathula Officinalis Kuan, Squama Manitia, Lignum Dalbergiae Odoriferae, Fructus Liquidambaris, processed Rhizoma Pinelliae, Rhizoma Pinelliae, Rhizoma Arisaematis, Rhizoma Typhonii, Radix Aconiti Coreani, Radix Platycodi, Flos Inulae, Bulbus Fritillariae Thunbergii, Rhizoma Cynanchi Stauntonii, Fructus Trichosanthis, Bulbus Fritillariae Cirrhosae, Caulis Bambusae in Taeniam, Bitter Apricot Kernel, Sargassum Fusiforme, Radix Stemonae, Radix Stemonae, Loquat leaf, Radix Scutellariae, Sangbaipi, Semen Lepidii, Stir-baked Flos Farfarae, Radix Peucedani, Ferrosoferric Oxide, Os Draconis Fossilia, Semen Ziziphi Spinosae, Semen Biotae, Radix Polygalae, Cortex Albiziae, Radix Codonopsis, Radix Pseudostellariae, Radix Astragali, Rhizoma Atractylodis Macrocephalae, Rhizoma Dioscoreae, Glycyrrhiza Uralensis, Radix Panacis Quinquefolii, Ziziphus Jujuba, Radix Morindae Officinalis, Herba Cistanches, Rhizoma Curculiginis, Herba Epimedii, Cortex Eucommiae, Radix Dipsaci, Rhizoma Cibotii, Rhizoma Drynariae, Fructus Psoraleae, Frucyus Alpiniae Oxyphyllae, Cuscuia Japonica, Herba Cynomorii, Radix Angelicae Sinensis, Rehmannia glutinosa, Radix Polygoni Multiflori, Radix Paeoniae Alba, Radix Ophiopogonis, Herba Dendrobii, Bulbus lilii, Fructus Lycii, Herba Ecliptae, Ligustrum Lucidum, Radix Glehniae, Rhizoma Atractylodis Macrocephalae, Fructus Schisandrae, Fructus Tritici Levis, Radix Oryzae Glutinosae, Radix Ephedrae, Fructus Corni, Fructus Rosae Laevigatae, Fructus Rubi, Concha Arcae, Folium Ginseng, Radix Adenophorae.
References
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clinicians 68, 394–424. doi:10.3322/caac.21492
Campos, M. I., Vieira, W. D. A., Campos, C. N., Aarestrup, F. M., and Aarestrup, B. J. V. (2015). Atorvastatin and trans-caryophyllene for the prevention of leukopenia in an experimental chemotherapy model in Wistar rats. Mol. Clin. Oncol. 3 (4), 825–828. doi:10.3892/mco.2015.544
Chen, D., Zhao, J., and Cong, W. (2018). Chinese herbal medicines facilitate the control of chemotherapy-induced side effects in colorectal cancer: progress and perspective. Front. Pharmacol. 9, 1442. doi:10.3389/fphar.2018.01442
Cornes, P., Gascon, P., Chan, S., Hameed, K., Mitchell, C. R., Field, P., Latymer, M., et al. (2018). Systematic review and meta-analysis of short- versus long-acting granulocyte colony-stimulating factors for reduction of chemotherapy-induced febrile neutropenia. Adv. Ther. 35 (11), 1816–1829. doi:10.1007/s12325-018-0798-6
Disis, M. L. (2005). Clinical use of subcutaneous G-CSF or GM-CSF in malignancy. Oncology (Williston Park, N.Y.) 19, 5–9.
Han, D. Y., and Jiang, M. (2018). Clinical efficacy of Qi Jiao Sheng Bai Capsule in preventing and treating leukopenia caused by chemotherapy in breast cancer. Beijing, China: Beijing University of Chinese Medicine.
Higgins, J. P. T., Altman, D. G., Gotzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Huang, D., and Zhang, Z. M. (2020). Clinical observation of the traditional Chinese medicine Yisui Shengxue capsule in the treatment of lung adenocarcinoma patients with myelosuppression after chemotherapy. MS thesis, Gansu, China: Gansu University of Chinese Medicine.
Jia, Y. J., Du, H. H., Yao, M., Cui, X. J., Shi, Q., Wang, Y. J., et al. (2015). Chinese herbal medicine for myelosuppression induced by chemotherapy or radiotherapy: a systematic review of randomized controlled trials. Evidence-Based Complement. Altern. Med. 2015, 690976. doi:10.1155/2015/690976.10.1155/2015/690976
Jia, Y. J., Yu, J. C., Yang, P. Y., Wang, X. Q., and Zhao, L. L. (2014). Discussion on the prevention and treatment of bone marrow suppression after chemotherapy by Fuzheng Jiedu Quyu. J. Traditional Chin. Med. 55 (3), 198–201. doi:10.13288/j.11-2166/r.2014.03.006
Johnstone, R. W., Ruefli, A. A., and Lowe, S. W. (2002). Apoptosis. Cell 108 (2), 153–164. doi:10.1016/S0092-8674(02)00625-6
Kato, Y., Okuma, Y., Watanabe, K., Yomota, M., Kawai, S., Hosomi, Y., et al. (2019). A single-arm phase II trial of weekly nanoparticle albumin-bound paclitaxel (nab-paclitaxel) monotherapy after standard of chemotherapy for previously treated advanced non-small cell lung cancer. Cancer Chemother. Pharmacol. 84 (2), 351–358. doi:10.1007/s00280-019-03843-0
Knecht, K., Kinder, D., and Stockert, A. (2020). Biologically-based complementary and alternative medicine use in cancer patients: the good, the bad, the misunderstood. Front. Nutr. 6, 196. doi:10.3389/fnut.2019.00196
Kuo, Y.-h., Tsay, S.-L., Chang, C.-C., Liao, Y.-c., and Tung, H.-H. (2018). Cancer impact, complementary/alternative medicine beliefs, and quality of life in cancer patients. J. Altern. Complement. Med. 24 (3), 276–281. doi:10.1089/acm.2016.0396
Li, C. Y., Yi, Z. B., Li, J. J., Zhao, J. L., and Wang, Q. Y. (2016). Meta analysis of spleen-invigorating and kidney-tonifying method in the treatment of leukopenia after tumor chemotherapy. J. Hunan Univ. CM 36 (10), 91–95. doi:10.3969/j.issn.1674070X.2016.10.0
Li, H., Ma, W., Ai, P., Zhang, H. M., and Ma, L. X. (2015). Treatment of chemotherapy-induced leucopenia in patients with malignant tumor by Chinese herbal medicine: a systematic review and meta-analysis of randomized clinical trials. Traditional West. Med. 35 (2), 157–166. doi:10.7661/CJIM.2015.02.015710.1016/s0254-6272(15)30002-9
Li, J., Niu, J., Yang, M., Ye, P., Zhai, J., Yuan, W., et al. (2019). Using single-patient (n-of-1) trials to determine effectiveness of traditional Chinese medicine on chemotherapy-induced leukopenia in gastric cancer: a feasibility study. Ann. Transl. Med. 7 (6), 124. doi:10.21037/atm.2019.02.03
Li, J., Tuo, L. Y., Zhang, C., Wang, J. F., and Xu, W. (2020). Clinical efficacy and mechanism of bazhen decoction on leukocyte reduction induced by chemotherapy in lung cancer. World Chin. Med. 15 (1), 94–98. doi:10.3969/j.issn.1673-7202.2020.01.018
Li, X. F., and Liu, Z. H. (2019). The study of effect of jianpi Shengsui plaster on prevention and treatment of myelosuppression after chemotherapy in non-small cell lung cancer. MS thesis, Guangzhou, China: Guangzhou University of Chinese Medicine.
Li, Y., Xiong, C., Qin, E., Yu, Z., Li, L., and Zhuang, G. (2018). Effectiveness of traditional Chinese medicine on chemo-radiotherapy induced leukaemia in patients with lung cancer: a meta-analysis. J. Tradit Chin. Med. 38 (5), 661–667. doi:10.1016/S0254-6272(18)30904-X
Liew, A. C. i., Peh, K.-K., Tan, B. S., Zhao, W., and Tangiisuran, B. (2019). Evaluation of chemotherapy-induced toxicity and health-related quality of life amongst early-stage breast cancer patients receiving Chinese herbal medicine in Malaysia. Support Care Cancer 27, 4515–4524. doi:10.1007/s00520-019-04724-1
Liu, A. Q., and Bi, H. X. (2015). Clinical reaserch on efficacy of Bazhen decoction on lung cancer patients with leucopenia after chemotherapy and the changes of serum IL-2. Nei Mongol J. Traditional Chin. Med. 5, 49–50. doi:10.16040/j.cnki.cn15-1101.2015.05.049
Liu, J., and Yao, D. J. (2014). The clinical observation of Husui prescription on treating leukopenia with chemotherapy. MS thesis, Chengdu, China: Chengdu University of Traditional Chinese Medicine.
Liu, W., Zhang, C. C., and Li, K. (2013). Prognostic value of chemotherapy-induced leukopenia in small-cell lung cancer. Cancer Biol. Med. 10, 92–98. doi:10.7497/j.issn.2095-3941.2013.02.005
Liu, Y. X. (2013). Fuzheng guben method combination chemotherapy bone metastases after chemotherapy leukopenia randomized controlled study. J. Pract. Traditional Chin. Intern. Med. 27 (5), 45–47. doi:10.3969/j.issn.1671-7813.2013.05(s).23
Lyman, G. H., Dale, D. C., Culakova, E., Poniewierski, M. S., Wolff, D. A., Kuderer, N. M., et al. (2013). The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann. Oncol. 24, 2475–2484. doi:10.1093/annonc/mdt226
Lyman, G. H., Yau, L., Nakov, R., and Krendyukov, A. (2018). Overall survival and risk of second malignancies with cancer chemotherapy and G-CSF support. Ann. Oncol. 29, 1903–1910. doi:10.1093/annonc/mdy311
Makary, P., Parmar, J. R., Mims, N., Khanfar, N. M., and Freeman, R. A. (2019). Patient counseling guidelines for the use of cannabis for the treatment of chemotherapy-induced nausea/vomiting and chronic pain. J. Pain Palliat. Care Pharmacother. 32 (4), 216–225. doi:10.1080/15360288.2019.1598531
Merryman, R., Stevenson, K. E., Gostic, W. J., Neuberg, D., O’Brien, J., Sallan, S. E., et al. (2012). Asparaginase-associated myelosuppression and effects on dosing of other chemotherapeutic agents in childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 59 (5), 925–927. doi:10.1002/pbc.24182
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 6, e1000097. doi:10.1371/journal.pmed.1000097
Mok, T. S. K., Yeo, W., Johnson, P. J., Hui, P., Ho, W. M., Lam, K. C., et al. (2007). A double-blind placebo-controlled randomized study of Chinese herbal medicine as complementary therapy for reduction of chemotherapy-induced toxicity. Ann. Oncol. 18 (4), 768–774. doi:10.1093/annonc/mdl465
Niu, X. F., Zhang, N. S., and Zhou, L. J. (2018). The treatment of Jianpi Yiqi Yangxue therapy meta analysis of clinical efficacy of chemotherapy for leukocyte reduction after malignant tumor chemotherapy. Nei Mongol J. Traditional Chin. Med. 37 (9), 92–95. doi:10.16040/j.cnki.cn15-1101.2018.09.062
Ohnaka, H., Tsukamoto, H., Nakamura, T., Yano, R., Watanabe, K., Igarashi, T., et al. (2013). Predictors of response of patients with solid tumors to granulocyte colony-stimulating factor. Int. J. Clin. Pharm. 35 (1), 45–50. doi:10.1007/s11096-012-9703-6
Park, M. S., Kim, D. H., Kim, D. H., Park, S. J., Hong, S. P., Kim, T. I., et al. (2015). Leukopenia predicts remission in patients with inflammatory bowel disease and Behcet’s disease on thiopurine maintenance. Dig. Dis. Sci. 60 (1), 195–204. doi:10.1007/s10620-014-3355-4
Qian, L., and Li, S. J. (2013). Clinical Research on curative effect of Hua Liao Jian Du Formula to bone marrow suppression caused by vinorelbine plus cisplatindissertation. Master’s Thesis. Chengdu (China): Chengdu University of Traditional Chinese Medicine.
Ren, X. Q., and Wu, X. Y. (2015). Fuzhengshengbai soup in the treatment of malignant tumor after chemotherapy leukopenia random parallel con trol study. J. Pract. Traditional Chin. Intern. Med. 29 (10), 31–32. doi:10.13729/j.issn.1671-7813.2015.10.14
Sarah, C. (2013). Is it time for a new paradigm for systemic cancer treatment? Lessons from a century of cancer chemotherapy. Front. Pharmacol. 4, 68. doi:10.3389/fphar.2013.00068
Schunemann, H. J., Oxman, A. D., Brozek, J., Glasziou, P., Bossuyt, P., Chang, S., et al. (2008). GRADE: assessing the quality of evidence for diagnostic recommendations. Evidence-Based Med. 13 (6), 162–163. doi:10.1136/ebm.13.6.162-a
Siegel, R. L., Miller, K. D., and Jemal, A. (2020). Cancer statistics, 2020. CA A. Cancer J. Clin. 70, 7–30. doi:10.3322/caac.21590
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., and Jemal, A. (2015). Global cancer statistics, 2012. CA: A Cancer J. Clinicians 65 (2), 87–108. doi:10.3322/caac.21262
Varughese, T., Joseph, J., and Menon, R. (2020). Efficacy of Jackfruit365 green jackfruit flour fortified diet on pegfilgrastim to prevent chemotherapy-induced leukopenia, irrespective of tumor type or drugs used-A retrospective study. Biomolecules 10 (2), 218. doi:10.3390/biom10020218
Wang, D., and Li, S. J. (2017). The clinical observation on treatment of bone marrow suppression after chemotherapy with modified Liu Wei Di Huang decoction. MS thesis, Chengdu, China: Chengdu University of Traditional Chinese Medicine.
Wang, J. L. (2011). Jiawei sancai fengsui tang treatment after chemotherapy leukopenia disease. Chin. J. Exp. Traditional Med. Formulae 17 (21), 248–250. doi:10.13422/j.cnki.syfjx.2011.21.007
Wang, L. F., Xiang, Y., Xu, Z. Y., Wang, Z. Q., Zhang, M., Deng, H. B., et al. (2016). Clinical observation of shuanghuang shengbai granules in preventing leukopenia after chemotherapy in lung adenocarcinoma. J. New Chin. Med. 48 (6), 194–196. doi:10.13457/jcnki.jncm.2016.06.086
Winkler, I. G., Wiercinska, E., Barbier, V., Nowlan, B., Bonig, H., and Levesque, J.-P. (2016). Mobilization of hematopoietic stem cells with highest self-renewal by G-CSF precedes clonogenic cell mobilization peak. Exp. Hematol. 44 (4), 303–314. doi:10.1016/j.exphem.2016.01.001
Wyatt, G., Sikorskii, A., Tesnjak, I., Victorson, D., and Srkalovic, G. (2015). Chemotherapy interruptions in relation to symptom severity in advanced breast cancer. Support Care Cancer 23, 3183–3191. doi:10.1007/s00520-015-2698-5
Yamauchi, T., Saito, H., Ito, M., Shichinohe, H., Houkin, K., and Kuroda, S. (2014). Platelet lysate and granulocyte-colony stimulating factor serve safe and accelerated expansion of human bone marrow stromal cells for stroke therapy. Transl. Stroke Res. 5 (6), 701–710. doi:10.1007/s12975-014-0360-z
Yang, S., He, X. H., Liu, P., Zhou, S. Y., Dong, M., Qin, Y., et al. (2015). Efficacy analysis of pegylated filgrastim as prophylaxis for chemotherapy-induced neutropenia. Chin. J. Clin. Oncol. 42 (12), 626–631. doi:10.3969/j.issn.1000-8179.20150577
Yang, Z. G., Liao, X., Lu, Y. F., Xu, Q. N., Tang, B. Z., Chen, X. R., et al. (2017). Add-on therapy with traditional Chinese medicine improves outcomes and reduces adverse events in hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Evidence-Based Complement. Altern. Med. 2017, 3428253. doi:10.1155/2017/3428253.10.1155/2017/3428253
Yeshurun, M., Labopin, M., Blaise, D., Cornelissen, J. J., Sengeloev, H., Vindelov, L., et al. (2014). Impact of postremission consolidation chemotherapy on outcome after reduced-intensity conditioning allogeneic stem cell transplantation for patients with acute myeloid leukemia in first complete remission: a report from the Acute Leukemia Working Party of. Cancer 120 (6), 855–863. doi:10.1002/cncr.28498
Yuan, X. M., and Zhang, H. L. (2016). Clinical observation of Qijingshengbai granules in preventing and treating neutropenia caused by chemotherapy on patients with NSCLC. MS thesis, Wulumuqi, China: Xinjiang Medical University.
Zhang, J. F., Liu, Y. X., Xu, Y. F., Li, S., and Yan, R. X. (2019). Traditional Chinese medicine combined with chemotherapy in treatment of postoperative gastric cancer: a meta-analysis. Chin. Arch. Traditional Chin. Med. 37 (8), 1819–1825. doi:10.13193/j.issn.1673-7717.2019.08.005
Zhang, K. L., Si, W. T., and Shao, H. M. (2015). Application of traditional Chinese medicine in the treatment of adverse drug reactions in cancer. J. Emerg. Traditional Chin. Med. 24 (7), 1212–1214. doi:10.3969/j.issn.1004-745X.2015.07.031
Zhao, M. M., and Lu, S. (2016). Clinical research of Fuzheng Shengbai Decoction in prevention and treatment of bone marrow suppression at the 1st chemotherapy after gynecological cancer surgerydissertation. Master’s Thesis. Nanjing (China): Nanjing University of Chinese Medicine.
Keywords: traditional Chinese medicine, systematic review, meta-analysis, Chinese herbal medicine, chemotherapy-induced leukopenia
Citation: Wang Q, Ye H, Wang Q-q, Li W-t, Yu B-b, Bai Y-m and Xu G-h (2021) Chinese Herbal Medicine for Chemotherapy-Induced Leukopenia: A Systematic Review and Meta-Analysis of High-Quality Randomized Controlled Trials. Front. Pharmacol. 12:573500. doi: 10.3389/fphar.2021.573500
Received: 05 August 2020; Accepted: 19 February 2021;
Published: 04 May 2021.
Edited by:
Bey Hing Goh, Monash University Malaysia, MalaysiaReviewed by:
Johanna Mahwahwatse Bapela, University of Pretoria, South AfricaAllah Bukhsh, Monash University, Australia
Copyright © 2021 Wang, Ye, Wang, Li, Yu, Bai and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ya-mei Bai, Y3pieW1AMTI2LmNvbQ==; Gui-hua Xu, Z3VpaHVhLnh1QG5qdWNtLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Qing Wang
Qing Wang Hui Ye1†
Hui Ye1†