
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 29 April 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.563436
This study aimed to determine the composition and content of polyphenols in the dry extract obtained from the hydrodistilled residue by-product of the wild bergamot (Monarda fistulosa L., Lamiaceae Martinov family) herb (MFDE) and to evaluate its safety and pharmacological properties. The total phenolic content (TPC) in the MFDE was 120.64 mg GAE/g. The high-performance liquid chromatography (HPLC) analysis showed the presence of a plethora of phenolic compounds, including hydroxycinnamic acids and flavone derivatives in the MFDE, with rosmarinic acid and luteolin-7-O-glucoside being the main components. With an IC50 value of 0.285 mg/mL, it was found to be a strong DPPH radical scavenger. The acute toxicity study results indicate that the oral administration of MFDE to rats at the doses of 500–5,000 mg/kg did not produce any side effects or death in animals which indicates its safety. The results of the in vivo assay showed that the MFDE dose-dependently inhibited paw oedema and significantly reduced the number of writings in mice induced by the acetic acid injection suggesting its potent anti-inflammatory and analgesic activities, respectively. The conducted studies revealed that M. fistulosa hydrodistilled residue by-product could be regarded as a new natural source of polyphenols with valuable pharmacological properties.
Genus Monarda L. (Nepetoideae Burnett. of the Lamiaceae family) includes several species of herbaceous plants native to North America (Native plants, 2001). Nowadays, Monarda species are widely cultivated as ornamental and medicinal plants for local use, but they are not included in any Pharmacopoeia (American Herbal Pharmacopoeia, 2011; European Pharmacopoeia, 2016). The value of Monarda species is mainly based on their volatile constituents and ornamental features (Vysochina, 2020). It was found that only 49 original studies and reviews were retrieved from PubMed up to November 2020 about the chemical composition and biological activities of species from the Monarda genus (data obtained via searching the word “Monarda”). Scientifically confirmed pharmacological activities of Monarda plants were primarily revealed by the researchers due to the composition of essential oils isolated from the aerial parts of M. fistulosa L., M. didyma L., M. citriodoraCerv. ex Lag., and M. punctata L. (Fraternale et al., 2006; Zhilyakova et al., 2009; Geffre et al., 2011; Tabanca et al., 2013; Thompson et al., 2013; Li et al., 2014; Mattarelli et al., 2017; Shanaida et al., 2018b; Deepika et al., 2020; Ghosh et al., 2020; Marchioni et al., 2020).
The other groups of biologically active compounds of Monarda genus representatives such as flavonoids or hydroxycinnamic acids were studied less. Some of the scientific studies about the polyphenols composition of these species were published not only in the peer-reviewed journals (Banach and Olechnowicz-Stepien, 1986; Davies and Mazza, 1992; Savickiene et al., 2002; Krasyuk and Pupyrkina, 2016; Shanaida et al., 2018a; Shanaida et al., 2020; Vysochina, 2020). Flavonoids such as rutin, hyperoside, quercetin, quercitrin, and luteolin were found in the leaves and flowers of M. didyma grown in Lithuania by the HPLC method (Savickiene et al., 2002). Rosmarinic acid and flavonoids hyperoside, rutin, naringin, and naringenin were identified in the M. didyma herb by the high-performance thin layer chromatography (HPTLC) (HPTLC method for identification of medicinal plants (method collection), 2012). Davies and Mazza (1992) found that anthocyanin pelargonidin 3,5-diglucoside accounted for 17% of the total flavonoid content in the petals of M. fistulosa flowers. Among the other detected flavonoids were apigenin-7-O-glucosides, dihydroxyflavone 8-C-glucoside, and 5-hydroxyflavone (Davies and Mazza, 1992). Six flavonoid glucosides were isolated from the M. pectinata aerial part, i.e., acacetin-7-rutinoside, isosacuranetine-7-rutinoside, and luteolin-7-glucoside (Banach and Olechnowicz-Stepien, 1986). It was found that Monarda species introduced in the Republic of Bashkortostan (Russia) accumulated the total content of flavonoids calculated as luteolin equivalent at the levels of 1.57% in M. fistulosa, 1.63% in M. didyma, 1.61% in M. citriodora, 1.52% in M. hybrida Wender., and 0.91% in M. russelliana Nutt. (Krasyuk and Pupyrkina, 2016). Rosmarinic acid was found to be the main component of aqueous and methanol extracts obtained from the M. fistulosa herb grown in Ukraine (Shanaida et al., 2018a; Shanaida et al., 2020). The preventive and healing properties of polyphenols are mostly related to their antioxidant effects which could be useful in the treatment of inflammation, pain, cardiovascular diseases, neurodegenerative disorders, and cancer (Pudziuvelyte et al., 2020). Many chronic diseases of human and animal bodies lead to releasing reactive oxygen or nitrogen species, causing cell injury mainly through their oxidative degradation (Libby, 2007). As a result, inflammation is developing, nociceptors are sensitized, and it becomes painful (Luo et al., 2020).
Wild bergamot or bee balm (M. fistulosa) is a perennial herbaceous plant 60–90 cm tall, with erect branches and deltoid-lanceolate opposite leaves; its violet-pink corolla is strongly bilabiate; flowers are collected in the compact clusters 4-5 cm long at the tops of stems (Wagner, 2006; Thompson et al., 2013). This plant is easily cultivated, and as a consequence, it is widespread on different continents in a temperate climate as a garden ornamental, honey source, and medicinal plant (Krasyuk and Pupyrkina, 2016; Vysochina, 2020). M. fistulosa has been successfully introduced to the European countries. Research institutions are conducting the acclimatization and breeding of new cultivars of this species (Vysochina, 2020). Historically, among the Monarda representatives, M. fistulosa has been the most widely used effective local medicine across American Indian cultures in North America (Native plants, 2001). The aerial parts of this plant were used by many Native Americans to cure cold and flu, for the treatment of the oral cavity infections (gingivitis or dental caries), as a strong antiseptic for skin wounds, as an active diaphoretic, to relief headache and the flatulent colic, and in waterbaths for babies (Native plants, 2001; Vysochina, 2020; Wagner, 2006). The Cherokee used a warm poultice of bee balm to relieve headache (Wagner, 2006). Flambeau Ojibwe dried the whole aerial part of this plant and boiled it in a suitable vessel to obtain the essential oil for treatment of bronchial and catarrh conditions through inhalation (Wagner, 2006). Later, it was found that M. fistulosa essential oil is the natural source of thymol and p-cymene with strong antiseptic properties (Zhilyakova et al., 2009; Mattarelli et al., 2017).
Postdistillation wastes are obtained after the extraction of essential oils from the raw material of the plants belonging to the Lamiaceae, Asteraceae, Apiaceae, and Pinaceae families. It seems that using postdistillation wastes is quite a perspective in terms of the extraction of new biologically active substances possessing valuable pharmacological properties. As the amount of essential oil in the aerial parts of the Lamiaceae family representatives is not high (up to 3%), the majority of a plant raw material remains unused after the hydrodistillation (Gavarić et al., 2015). This scientific and practical field of using hydrodistilled residue by-products has been expanding rapidly for the species of this family for the last decade (Gavarić et al., 2015; Pogačar et al., 2016; Vovk et al., 2016; Baranauskienė et al., 2019; Mahmoudi et al., 2020; Shanaida, 2020). Nevertheless, HPLC assay revealed the high contents of such valuable polyphenols as rosmarinic acid (52.36–105.8 mg/g) and rutin (11.01–87.11 mg/g) in the dried extract developed from the postdistillation waste material of Thymus vulgaris L. (Gavarić et al., 2015). In another study (Pogačar et al., 2016), it was revealed that the extract obtained from the Thymus vulgaris post-hydrodistillation residue was rich in such bioactive phytochemicals as rosmarinic acid and flavone glucuronides. This extract effectively reduced the adhesion of Campylobacter jejuni to abiotic surfaces at low concentrations (0.2–12.5 μg/mL) (Pogačar et al., 2016). Hydrodistilled residue by-products from Ocimum basilicum L. leaves were also considered as a valuable source of rosmarinic acid and other polyphenols with noticeable antimicrobial, repellent, and antioxidant effects (Mahmoudi et al., 2020). Solid residues of Lavandula × intermedia and Thymus mastichina L. after the isolation of the essential oil were found as a source of polyphenols with antioxidant and metal chelating activities (Sánchez-Vioque et al., 2013). To the best of our knowledge, there are no studies related to hydrodistilled residue by-product of M. fistulosa aerial part.
The aim of this study was to characterize the phenolic profile in the dry extract developed from the hydrodistilled residue by-product of M. fistulosa herb (MFDE), to determine its safety, free radical scavenging, anti-inflammatory, and analgesic activities.
The herb of M. fistulosa was harvested during the flowering stage from the experimental plots in Ternopil Region (Ukraine, 49.5535°N, 25.5948°E), then dried in the shadow at a temperature of 25–35°C, and ground. The voucher specimens of the plant have been deposited in the herbarium of Pharmacognosy and Medical Botany Department of I. Horbachevsky Ternopil National Medical University.
The MFDE was obtained from the postdistillation waste of M. fistulosa herb in two stages (Shanaida, 2020): by aqueous extraction in the hydrodistillation process and by using 50% ethanol at the next stage as it was described for Salvia officinalis L. leaves by Vovk et al. (2016). 120.0 g of ground herb of M. fistulosa was used for obtaining the MFDE. The raw plant material was divided into four portions and subjected to hydrodistillation for 2 h (the ratio of the raw material to purified water was 1:15) (European Pharmacopoeia, 2016). After the separation of essential oil (1.87%), the aqueous extract was cooled, then filtered through a paper filter, and maintained for 24 h in a refrigerator (Shanaida, 2020). On the next stage, the decanted solution was evaporated in a vacuum rotary evaporator to 1/10 of an initial volume. Then, 1.2 L of 50% ethanol was added to the waste, and it was extracted using a boiling water heater for 40 min. After cooling, the filtrate was put in the refrigerator (for 24 h) to sediment ballast, then decanted, and evaporated to 1/10 of an initial volume. The obtained concentrated extracts were combined and dried in a vacuum spray dryer to obtain the MFDE.
Folin–Ciocalteu’s reagent, methanol, ethyl acetate, formic acid, acetic acid, gallic acid, Na2CO3, and AlCl3 were purchased from POCH S.A. (Gliwice, Poland). Standards of apigenin, luteolin, rutin, rosmarinic acid, caffeic acid, and chlorogenic acid for HPTLC, 2,2-diphenyl-1-picrylhydrazyl (DPPH), Trolox, carrageenan, and Tween-80 were purchased from Sigma-Aldrich (Poland). HPTLC silica gel 60 F254 plates, trifluoroacetic acid, acetonitrile, and standards for HPLC (chlorogenic acid, neochlorogenic acid, rosmarinic acid, caffeic acid, luteolin-7-O-glucoside, apigenin-7-O-glucoside, acacetin-7-O-glucoside, luteolin, and apigenin) were purchased from Merck (Germany). The tablets of “Diclophenac” (produced by chemical and pharmaceutical factory “Chervona zirka”, Kharkiv, Ukraine) and “Metamizole sodium (Analgin)” (produced by pharmaceutical factory “Darnitsa”, Kyiv, Ukraine) were purchased from the pharmacies in Ukraine. All the reagents and solvents were of analytical grade.
The TPC was measured according to the Folin–Ciocalteu method (Singleton and Rossi, 1965) with a Hitachi UV/VIS spectrophotometer and calculated as gallic acid equivalents (mg GAE/g extract). To plot the calibration curve, gallic acid was dissolved in purified water for obtaining the solutions in the range of 0.02–0.14 mg/mL (Shanaida et al., 2018a). Briefly, 0.1 mL of the aqueous solution of MFDE (concentration 0.5 mg/mL) was mixed with 1.5 mL of purified water, 0.1 mL of undiluted Folin-Ciocalteu reagent, and 0.3 mL of 20% Na2CO3. The obtained mixtures were incubated for 2 h in darkness at room temperature; the absorbance was measured with the spectrophotometer Hitachi U-2810 UV/VIS at 760 nm.
The HPTLC analysis of phenolic compounds was carried out using a CAMAG analytical system (Switzerland) (Stanek et al., 2019; Shanaida et al., 2020). The MFDE was dissolved in methanol (1.0 mg/mL) and filtered through a 0.45 μm Millipore filter to obtain a test solution. A standard solution consisted of the available reference standards dissolved in methanol (0.25 mg/mL). The test and standards solutions in a volume of 5 μL were applied to chromatographic plates (20 cm × 10 cm) using an automatic application device. The mobile phase consisted of ethyl acetate: formic acid: water (15:1:1). The detection of polyphenols spots was based on their natural fluorescence after the postchromatographic derivatization by 1% AlCl3 solution at 254 and 366 nm.
The HPLC analysis of polyphenols was performed using the Shimadzu HPLC-DAD system using the Phenomenex Luna C18 column (250 × 4.6 mm, 5 µm particle size) at 35°C. The UV absorption spectra of the available reference standards and the test samples were recorded in the range of 190–400 nm. Gradient elution conducted by mixing the mobile phases A (0.1% trifluoroacetic acid in water) and B (0.1% trifluoroacetic acid in acetonitrile) at a flow rate of 1.0 mL/min according to Shanaida et al. (2018a) (Table 1). The concentration ranges of reference standards for the calibration curve were 10.0–1000.0 μg/mL for rosmarinic acid and luteolin-7-O-glucoside and 1.0–100.0 μg/mL for other components; R2 was not less than 0.99 for all measurements.
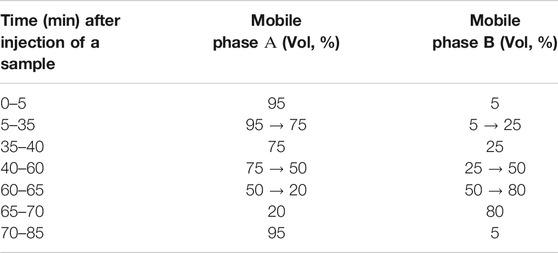
TABLE 1. Gradient of the mobile phases in HPLC analysis (Shanaida et al., 2018a).
The inhibition of DPPH radicals by the MFDE was studied using the Hitachi U-2810 UV/VIS spectrophotometer according to the method of Gougoulias and Mashev (2015). The MFDE and DPPH reagent were dissolved in methanol before the analysis. 0.1 mL of each test sample in various concentrations (0.1–1.0 mg/mL) was mixed in the flask with 1.9 mL of DPPH solution in methanol (25 μg/mL), and this mixture was incubated for 30 min in darkness at room temperature. The absorbance was measured at a wavelength of 517 nm. The concentration of the antioxidants presented in the test solution required to scavenge 50% of DPPH was expressed as an IC50 value; R2 = 0.9906. Trolox was used as a standard antioxidant.
All the in vivo experiments were conducted according to “European Convention for the Protection of Vertebrate Animals (1986)”.
Animals: mice (20–25 g) and albino rats (180–220 g) were used for the studies of acute toxicity and pharmacological activities of the MFDE. The animals were kept in plastic cages under the constant conditions (22 ± 2°C); they had free access to water and were fed ad libitum. After dividing into groups (n = 6–10), the animals fasted 12 h before the experiment.
Acute Toxicity Study: rats of both sexes in a ratio of 1:1 were orally given MFDE (500, 1,500, and 5,000 mg/kg bodyweight) once a day (Doklinichni doslidzhennia likarskykh zasobiv (metodychni rekomendacii), (2001). Different doses of the MFDE were dissolved in the vehicle (1% solution of Tween-80 in distilled water) and put into the stomach of rats through a tube. The animals were observed daily up to 14-days following the treatment. The fatality, general behavior, breath depth and rhythm, salivation, feed and water consumption, the character of excrements, condition of wool, mucous membranes, and dynamics of bodyweight before and during the experiment were analyzed daily.
Anti-Inflammatory Effect: the hind paw oedema of rats was produced by carrageenan (Doklinichni doslidzhennia likarskykh zasobiv (metodychni rekomendacii), (2001); Wang et al., 2012). Animals were divided into five groups (n = 6 animals/group). Each animal was injected with 0.1 mL of 1% carrageenan suspension into the right plantar aponeurosis for inducing the paw oedema. The animals were orally pretreated with the MFDE, diclofenac, or vehicle 60 min before the carrageenan injection. The rats in the control group were given the vehicle (1% solution of Tween-80 in distilled water), animals of 2–4 groups were pretreated with the MFDE in doses of 50, 100, or 200 mg/kg (experimental groups), and the 5th group was given the reference analgesic drug “Diclofenac” (8 mg/kg).
The paw volume oedema was measured plethysmometrically before and 1, 3, and 6 h after the injection of carrageenan. Anti-inflammatory activity (AIA) was expressed as the percentage reduction in oedema in the treated rats by comparison with the controls according to the following formula:
where dtreated is the difference in paw volume in the treated group, and dcontrol is the difference in paw volume in the control group.
Antinociceptive Activity: acetic acid induced stretching of the hind limbs and writhing of mice were provoked (Doklinichni doslidzhennia likarskykh zasobiv (metodychni rekomendacii), (2001); Magne et al., 2016). The mice were divided into five groups (n = 6 animals/group). The animals of the experimental groups were orally pretreated with the MFDE (50, 100, or 200 mg/kg), and the reference group was given “Metamizole sodium” (55 mg/kg) in 60 min before the intraperitoneal injection of 0.6% solution of acetic acid. The animals of the control group were pretreated before the intraperitoneal injection of acetic acid with the vehicle (1% solution of Tween-80 in distilled water). The mice were individually placed into the chambers. The number of stretching the hind limbs and writhing was counted for 20 min.
The percentage of analgesic activity (AA) was expressed as percentage decreasing in the number of “acetic acid cramps” in the treated animals concerning the controls as follows:
Statistical analyzes were performed using the Statistica software, version 13.1 (StatSoft). The HPLC analysis and the evaluation of TPC and DPPH free radical scavenging activity were carried out in triplicate. The acute toxicity study and anti-inflammatory and antinociceptive activities were carried out at a minimum of n = 6. The data were expressed as mean ± standard error of the mean (SEM). The results were analyzed using ANOVA one-way analysis of variance coupled with Dunnett’s test.
By using aqueous extraction in the hydrodistillation process and 50% ethanol at the next stage, a 23.17% MFDE yield was obtained. The TPC content was found to be 120.64 ± 2.65 mg GAE/g. This value was higher than those observed for other Lamiaceae species. Thus, Vlase et al. (2014) revealed that the TPC in the extracts of Ocimum basilicum and Hyssopus officinalis L. herbs cultivated in Romania (extraction with 70% ethanol on a waterbath) was 175.57 mg GAE/g and 77.72 mg GAE/g of raw material, respectively. The researchers revealed that TPC in the aerial parts of eight Lamiaceae species from Manipur (India) extracted with 80% ethanol varied in the range of 21.39–46.28 mg GAE/g of the herb (Khomdram and Potsangbam, 2011). The TPC in the Marrubium peregrinum L. herb collected in Serbia ranged from 27.26 to 89.78 mg GAE/g dependently of solvents (methanol, water, ethyl-acetate, acetone, or petroleum ether) used for maceration (Stanković, 2011). The TPC value of the 65–70% ethanol extracts of Salvia sclarea herb cultivated in Ukraine varied from 6.69 to 14.91 mg GAE per 1 gram of the herb (Hudz et al., 2019). The decoction of Origanum vulgare L. herb was characterized by the highest concentration of flavonoids and TPC than the infusion or hydroalcoholic extract (Martins et al., 2014). Thus, the mean TPC in the plant raw materials of the Lamiaceae representatives highly depends on the species of plant, its geographical origin, as well as solvents and extraction procedures chosen for analysis.
The chromatographic fingerprints of polyphenols in the MFDE were obtained using the HPTLC method. The zones sequence presented in the chromatograms of the reference standards and test solution scanned at 366 nm before and after derivatization with 1% AlCl3 solution are given in Figure 1. The chromatograms of the test solution demonstrated the most intense light blue zones at Rf = 0.75 corresponding to rosmarinic acid; weaker light blue zones of caffeic acid were presented just above the rosmarinic acid spots (Rf = 0.79). Furthermore, several unidentified fluorescent zones in different shades of blue and yellow colors were visible in the chromatograms of the test solution, especially after the derivatization. These spots could be considered as hydroxycinnamic acids or flavonoids, respectively (Stanek et al., 2019; Shanaida et al., 2020). Similar results were found for the methanol extract of the M. fistulosa herb (Shanaida et al., 2020).
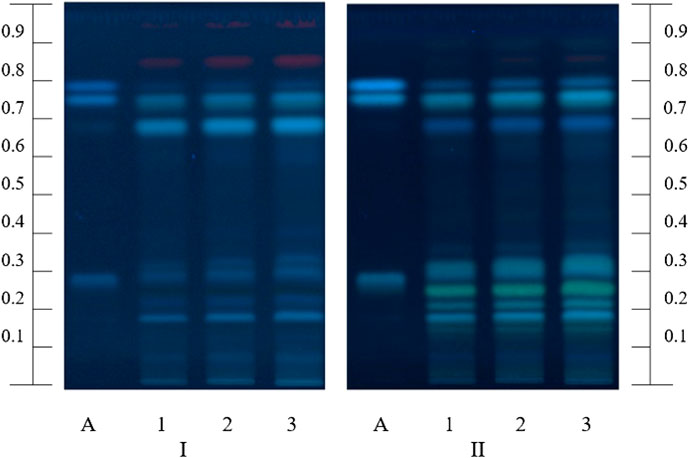
FIGURE 1. HPTLC fingerprints of the MFDE test solution (1–3) and polyphenol standards (A, chlorogenic, rosmarinic, and caffeic acids with increasing Rf) at λ = 366 nm before (I) and after (II) derivatization.
The quantification of phenolic compounds in the MFDE was conducted using the HPLC method. The amounts of several components including hydroxycinnamic acids (rosmarinic, caffeic, chlorogenic, and neochlorogenic) and flavone derivatives (luteolin-7-O-glucoside, apigenin-7-O-glucoside, acacetin-7-O-glucoside, luteolin, and apigenin) were higher than 5 mg/g (Table 2; Figure 2).
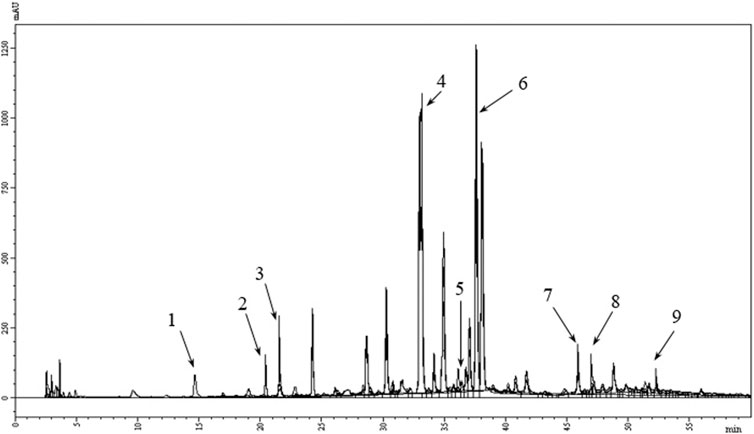
FIGURE 2. HPLC chromatogram of polyphenols in the MFDE. 1, neochlorogenic acid; 2, chlorogenic acid; 3, caffeic acid; 4, luteolin-7-O-glucoside; 5, apigenin-7-O-glucoside; 6, rosmarinic acid; 7, acacetin-7-O-glucoside; 8, luteolin; 9, apigenin.
Rosmarinic acid (Figure 3), a major component of the MFDE, possesses significant antioxidant, antinociceptive, anti-inflammatory, hepatoprotective, immunomodulatory, antidiabetic, antiviral, and antimicrobial properties revealed in both in vitro and in vivo studies (Petersen and Simmonds, 2003; Rocha et al., 2015; Alagawany et al., 2017; Luo et al., 2020). Numerous data about the chemical profiles of the representatives of Nepetoideae subfamily of the Lamiaceae family demonstrated that rosmarinic acid is their common predominant phenolic compound (Lamien-Meda et al., 2010; Zhu et al., 2014; Benedec et al., 2015; Shanaida et al., 2018a; Jakovljević et al., 2020). Among several Nepetoideae species investigated by Benedec et al. (2015), the Origanum vulgare herb contained the maximum amount of rosmarinic acid (12.40 mg/g). The aerial part of Satureja montana L. accumulated up to 7.84 μg/mg of rosmarinic acid, depending on the applied technological parameters and solvents (Jakovljević et al., 2020). Rosmarinic acid, luteolin, and apigenin were the major polyphenols of 80% methanol extract obtained from the Origanum vulgare herb (Martins et al., 2014). Caffeic acid, which was found by the HPLC as one of the major components of the MFDE, possesses the noticeable antioxidant, anti-inflammatory, antiviral, and anticancer activities (Touaibia et al., 2011; Spagnol et al., 2019). Findings of Spagnol et al. (2019) demonstrated that the antioxidant activity of caffeic acid was greater than that of ascorbic acid, and it showed a higher level of stability than the last one.
The antioxidant and anti-inflammatory effects of flavones found in the MFDE were proven by many researchers (Aziz et al., 2018; Park and Song, 2013; Seyoum et al., 2006; Song and Park, 2014; Žemlička et al., 2014). Luteolin-7-O-glucoside (Figure 3) and its aglycon luteolin potently strengthen the HO-1-mediated antioxidative effect (Park and Song, 2013; Song and Park, 2014). Luteolin is characterized by the most likely orthopositions of OH-groups to donate the hydrogen atom (4′-OH and 3′-OH) (Seyoum et al., 2006). The significant free radical scavenging activity of luteolin-7-O-glucoside was confirmed using the ABTS assay (Žemlička et al., 2014). Aziz et al. (2018) pointed out that plants with a prominent content of luteolin have been used in a folk medicine to treat inflammation-associated diseases. Numerous in silico, in vitro, and in vivo experiments confirmed the strong anti-inflammatory activity of this flavone derivative (Aziz et al., 2018). Antioxidant properties of apigenin are also well known, and it is considered to be an effective therapeutic agent in the treatment of inflammation, neurodegenerative disease, and even cancer (Salehi et al., 2019).
The in vitro DPPH radical scavenging assay showed that the MFDE possesses strong antiradical activity with an IC50 value of 0.285 mg/mL. It is possible to compare the obtained results of DPPH inhibition with data of the other researchers of Lamiaceae species. Several in vitro assays revealed a higher antioxidant potential of water extracts from the Nepeta spp. postdistillation residues comparatively to acetone extracts isolated from its dried waste (Baranauskienė et al., 2019). The extracts from the Ocimum basilicum and Hyssopus officinalis herbs prepared with 70% ethanol showed the prominent DPPH free radical inhibiting activity with IC50 values of 0.1249 and 0.1254 mg/mL, respectively (Vlase et al., 2014). The methanol extract of Marrubium peregrinum herb demonstrated stronger antioxidant activity against DPPH radical (IC50 = 0.187 mg/mL) than its water extract (IC50 = 0.481 mg/mL) which indicates the positive effect of lower alcohols on the extraction of polyphenols from the plant raw material (Stanković, 2011). As the free radical scavenging effect depends on the number and location of OH-groups, the major components of MFDE such as rosmarinic acid with 4 hydroxyl groups and luteolin-7-O-glucoside containing three of them in the aglycon part possess the significant antioxidant potential (Cao et al., 2005; Zhu et al., 2014; Benedec et al., 2015). The distinct antiradical activity of rosmarinic acid is amplified by the orthoposition of hydroxyl groups on the rings A and B (Cao et al., 2005).
Polyphenols are regarded as the main antioxidants of plant raw materials of many Lamiaceae species (Dai and Mumper, 2010; Khomdram and Potsangbam, 2011; Vlase et al., 2014; Zhu et al., 2014; Asha et al., 2015; Benedec et al., 2015; Gougoulias and Masev, 2015; Tungmunnithum et al., 2018; Jakovljević et al., 2020). The healing effect of Satureja montana extract rich in caffeic, rosmarinic, and syringic acids and flavonoid rutin against cyclophosphamide-induced testicular damage in rats was due to its antioxidative and antiapoptotic mechanisms (El Tawab et al., 2014). Origanum vulgare extracts demonstrated the highest antioxidant capacity among the other Lamiaceae species that is in line with the high contents of rosmarinic acid and other polyphenols. A lot of phenolic compounds demonstrated the free radicals scavenging and anti-inflammatory effects that could have preventive or therapeutic effects for neurodegenerative and cardiovascular diseases and cancer (Cory et al., 2018; Rytsyk et al., 2020). Some polyphenols exert even higher antioxidant activity than vitamins (Brglez Mojzer et al., 2016; Spagnol et al., 2019).
In the acute toxicity study, the general behavior of rats was recorded for 14 days after the MFDE administration. The results of this study indicate that the MFDE administered at doses of 500, 1,500, and 5,000 mg/kg did not induce any side effects or death of the experimental animals. No mortalities and other signs of adverse effects such as respiratory distress, convulsions, and changes of activity occurred in the animals tested during 14 days of behavioral observation. It suggests that up to 5,000 mg/kg the MFDE could be considered as safe.
Inflammation is a pathophysiological response of mammalian tissues to infections, injuries, allergies, or tumor growth. The administration of carrageenan as a high-molecular-weight polysaccharide into the animal paw leads to an inflammatory process as it is capable of releasing mediators associated with acute inflammation. It causes the time-dependent increase paw oedema (Luo et al., 2020). Nowadays, it is very important to develop effective herbal medicinal products with anti-inflammatory activity which possesses less side effects than synthetic drugs such as addiction and gastric ulcer.
The investigated MFDE significantly decreases in paw oedema of rats caused by the injection of carrageenan compared to the control group at 3 h of induced inflammation (Table 3). Anti-inflammatory activity was manifested by the MFDE in a dose-dependent manner; the highest tested dose (200 mg/kg) reduced paw oedema mostly (by 31.91% at the 3 h). The MFDE at a dose of 50 mg/kg did not demonstrate the noticeable anti-inflammatory effect when compared with the reference drug; this dose significantly inhibited paw oedema only after 3 and 6 h when compared with the control. The reference drug diclofenac caused a significant decrease paw oedema at all the hours of the experiment. According to different in vitro and in vivo studies (Petersen and Simmonds, 2003; Rocha et al., 2015; Luo et al., 2020), rosmarinic acid, which was found to be the predominant component of the MFDE, demonstrated the prominent anti-inflammatory effect due to the inhibition of lipoxygenase and cyclooxygenase pathways. Researchers revealed that administration of pure rosmarinic acid at a dose of 25 mg/kg reduced paw oedema in rats at 6 h by over 60% (Rocha et al., 2015). Such components of the MFDE as caffeic and chlorogenic acids (Touaibia et al., 2011; Naveed et al., 2018; Spagnol et al., 2019), as well as flavonoids such as apigenin, luteolin, and their glycosides (Xagorari et al., 2001; Aziz et al., 2018; Fan et al., 2018; Salehi et al., 2019), also possess the significant anti-inflammatory potential. Luteolin showed a significant anti-inflammatory activity in mice models induced by the carrageenan and reduced the number of abdominal constrictions caused by the acetic acid (Fan et al., 2018). Luteolin decreased the proinflammatory cytokine production in macrophages and endotoxin-stimulated phosphorylation cascade in murine (Xagorari et al., 2001). Thus, it can be speculated that the MFDE rich in rosmarinic acid and other polyphenols exhibits the anti-inflammatory activity via the inhibition of inflammatory cytokines production (Libby, 2007).
The writhing test induced by acetic acid is a widespread model to evaluate the peripheral antinociceptive effects of drugs (Karim et al., 2019). The pain provoked by the injection of acetic acid intraperitoneally is the consequence of irritation of chemosensitive nociceptors and causes releasing algogenic compounds such as prostaglandins, serotonin, histamine, and bradykinin (Magne et al., 2016). Table 4 shows that the analgesic effect the MFDE is manifested in a dose-dependent manner. The animals of the control group demonstrated 63.27 ± 1.49 writhing count, while the MFDE at a dose of 50 mg/kg reduced it to 49.8 ± 1.21. Higher doses of the extract decreased the writhing counts more significantly: 100 mg/kg to 36.24 ± 0.76 and 200 mg/kg to 30.85 ± 0.71. Thus, the MFDE in the highest dose (200 mg/kg) induced a noticeable reduction in the number of writhing in the mice provoked by the injection of acetic acid which was close to the reference drug. The revealed pharmacological effect of the MFDE could be attributed to a significant amount of polyphenols. A similar analgesic effect was found for the high dose of aqueous extract of Plectranthus glandulosus Hook. (Lamiaceae) leaves rich in phenolic compounds (Magne et al., 2016). The aqueous extract from the Rosmarinus officinalis L. leaves in a dose range of 100–400 mg/kg and rosmarinic acid at the doses of 10–40 mg/kg demonstrated their effectiveness in the management of pain and inflammation (Lucarini et al., 2013). The healing properties of rosmarinic acid were found in the experimental neuropathic pain in rats, and its anti-inflammatory and antiapoptotic activities have the key role in the observed antinociceptive effect (Rahbardar et al., 2018). In vitro and in vivo studies of the extracts prepared from the aerial parts of a lot of the Lamiaceae species from genera such as Mentha L., Ocimum L., Salvia L., Melissa L., Satureja L., and Rosmarinus L. showed their potent analgesic activity, and the revealed therapeutic effect could be attributed to the high polyphenol levels (Lucarini et al., 2013; Uritu et al., 2018).
This is the first research of the polyphenol profiles and pharmacological activities of the hydrodistilled residue by-product obtained from the M. fistulosa herb. The complex processing of the M. fistulosa raw material allows using rationally its post-hydrodistillation waste simultaneously with obtaining essential oil that enhances the overall profitability of this aromatic plant. The chromatographic analyses of polyphenols in the obtained MFDE revealed a high amount of rosmarinic acid, luteolin-7-O-glucoside, and other polyphenols with the valuable therapeutic properties. The high TPC in the MFDE correlated with its potent IC50 value. The administration of the obtained extract at the doses up to 5,000 mg/kg did not induce any toxic reactions. The in vivo assay revealed dose-dependent anti-inflammatory and analgesic activities of MFDE. Therefore, the results of this study indicate that hydrodistilled residue by-product from the M. fistulosa herb may represent a new natural source of polyphenols with significant pharmacological potential. The investigated extract could be also exploited as a health-promoting substance in food or cosmetic products.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The animal study was reviewed and approved by I. Horbachevsky Ternopil National Medical University.
MS conceived of the presented idea. NH and IJ-M verified the analytical methods. MS and IJ-M carried out the experiment. PW supervised the findings of this work. MS wrote the manuscript with support from NH, IJ-M, and PW. All the authors discussed the results and contributed to the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alagawany, M., Abd El-Hack, M. E., Farag, M. R., Gopi, M., Karthik, K., Malik, Y. S., et al. (2017). Rosmarinic acid: modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 18 (2), 1–10. doi:10.1017/S1466252317000081
American Herbal Pharmacopoeia (2011). Botanical pharmacognosy—microscopic characterization of botanical medicines. Boca Raton, FL: CRC Press, 735.
Asha, D., Mathew, L., and Kalappurakkal, R. (2015). Evaluation of HPTLC fingerprints of flavonoids and antioxidant activity of selected medicinal plants of Lamiaceae Family. IJPPR 7 (2), 240–245.
Aziz, N., Kim, M.-Y., and Cho, J. Y. (2018). Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J. Ethnopharmacology 225 (225), 342–358. doi:10.1016/j.jep.2018.05.019
Banach, R., and Olechnowicz-Stepien, W. (1986). The flavonoid fraction of some Monarda species. Herba Pol. 32 (3-4), 145–153.
Baranauskienė, R., Bendžiuvienė, V., Ragažinskienė, O., and Venskutonis, P. R. (2019). Essential oil composition of five Nepeta species cultivated in Lithuania and evaluation of their bioactivities, toxicity and antioxidant potential of hydrodistillation residues. Food Chem. Toxicol. 129, 269–280. doi:10.1016/j.fct.2019.04.039
Benedec, D., Hanganu, D., Oniga, I., Tiperciuc, B., Olah, N. K., Raita, O., et al. (2015). Assessment of rosmarinic acid content in six Lamiaceae species extracts and their antioxidant and antimicrobial potential. Pak J. Pharm. Sci. 28 (6), 2297–2303.
Brglez Mojzer, E., Knez Hrnčič, M., Škerget, M., Knez, Ž., and Bren, U. (2016). Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 21 (7), 901. doi:10.3390/molecules21070901
Cao, H., Cheng, W.-X., Li, C., Pan, X.-L., Xie, X.-G., and Li, T.-H. (2005). DFT study on the antioxidant activity of rosmarinic acid. J. Mol. Struct. THEOCHEM. 719 (1–3), 177–183. doi:10.1016/j.theochem.2005.01.029
Cory, H., Passarelli, S., Szeto, J., Tamez, M., and Mattei, J. (2018). The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 5, 87. doi:10.3389/fnut.2018.00087
Dai, J., and Mumper, R. J. (2010). Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15 (10), 7313–7352. doi:10.3390/molecules15107313
Davies, A. J., and Mazza, G. (1992). Separation and characterization of anthocyanins of Monarda fistulosa by high-performance liquid chromatography. J. Agric. Food Chem. 40 (8), 1341–1345. doi:10.1021/jf00020a009
Deepika, , , Singh, A., Chaudhari, A. K., Das, S., and Dubey, N. K. (2020). Nanoencapsulated Monarda citriodora Cerv. ex Lag. essential oil as potential antifungal and antiaflatoxigenic agent against deterioration of stored functional foods. J. Food Sci. Technol. 57 (8), 2863–2876. doi:10.1007/s13197-020-04318-4
Doklinichni doslidzhennia likarskykh zasobiv (metodychni rekomendacii) (2001). [Preclinical research of medicinal products]. Editor O. V. Stefanov Kyiv, Ukrainian: Avicenna, 73–209.
El Tawab, A. M. A., Shahin, N. N., and Abdel Mohsen, M. M. (2014). Protective effect of Satureja montana extract on cyclophosphamide-induced testicular injury in rats. Chem. Biol. Interact. 224, 196–205. doi:10.1016/j.cbi.2014.11.001
European Convention for the Protection of Vertebrate Animals (1986). European convention for the protection of vertebrate animals used for experimental and other scientific purposes. Strasbourg, France: Council of Europe, 53.
European Pharmacopoeia (2016). 9th Edn. Strasbourg, France. 9.0 , Available at: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition.
Fan, X., Du, K., Li, N., Zheng, Z., Qin, Y., Liu, J., et al. (2018). Evaluation of anti-nociceptive and anti-inflammatory effect of luteolin in mice. J. Environ. Pathol. Toxicol. Oncol. 37 (4), 351–364. doi:10.1615/JEnvironPatholToxicolOncol.2018027666
Fraternale, D., Giamperi, L., Bucchini, A., Ricci, D., Epifano, F., Burini, G., et al. (2006). Chemical composition, antifungal and in vitro antioxidant properties of Monarda didyma L. essential oil. J. Essent. Oil Res. 18, 581–585. doi:10.1080/10412905.2006.9699174
Gavarić, N., Kladar, N., Mišan, A., Nikolić, A., Samojlik, I., Mimica-Dukić, N., et al. (2015). Postdistillation waste material of thyme (Thymus vulgaris L., Lamiaceae) as a potential source of biologically active compounds. Ind. Crops Prod. 74 (15), 457–464. doi:10.1016/j.indcrop.2015.05.070
Geffre, C., Jacobs, J., Dixson, J., Bergmann, D., De Cory, J., and Gabel, M. (2011). Isolation and identification of anti-microbial compounds in Monarda fistulosa. Proc. South Dakota Acad. Sci. 90, 82.
Ghosh, M., Schepetkin, I. A., Özek, G., Özek, T., Khlebnikov, A. I., Damron, D. S., et al. (2020). Essential oils from Monarda fistulosa: chemical composition and activation of transient receptor potential A1 (TRPA1) Channels. Molecules 25 (21), E4873. doi:10.3390/molecules25214873
Gougoulias, N., and Mashev, N. (2015). Antioxidant activity and polyphenols content of some herbal teas of Lamiaceae family from Greece and Bulgaria. Oxid. Commun. 38 (1), 25–31.
HPTLC method for identification of medicinal plants (method collection) (2012). Monarda didyma. Available at: https://www.hptlc-association.org/methods/methods.cfm.
Hudz, N., Yezerska, O., Shanaida, M., Horčinová Sedláčková, V., and Wieczorek, P. P. (2019). Application of the Folin-Ciocalteu method to the evaluation of Salvia sclarea extracts. Phar. 66 (4), 209–215. doi:10.3897/pharmacia.66.e38976
Jakovljević, M., Vladić, J., Vidović, S., Pasto, K., Jokić, S., Molnar, M., et al. (2020). Application of deep eutectic solvents for the extraction of rutin and rosmarinic acid from Satureja montana L. and evaluation of the extracts antiradical activity. Plants 9 (2), 153. doi:10.3390/plants9020153
Karim, N., Khan, I., Khan, W., Khan, I., Khan, A., Halim, S. A., et al. (2019). Anti-nociceptive and anti-inflammatory activities of asparacosin A involve selective cyclooxygenase 2 and inflammatory cytokines inhibition: an in-vitro, in-vivo, and in-silico approach. Front. Immunol. 10, 581. doi:10.3389/fimmu.2019.00581
Khomdram, S. D., and Singh, P. K. (2011). Polyphenolic compounds and free radical scavenging activity in eight Lamiaceae herbs of Manipur. Not. Sci. Biol. 3, 108–113. doi:10.15835/nsb325638
Krasyuk, E. V., and Pupyrkina, K. A. (2016). Qualitative analysis and development of methods for quantitative determination of flavonoids in Monarda species introduced in the Republic of Bashkortostan. Med. Bull. Bashkortostan 11 (65), 73–77.
Lamien-Meda, A., Nell, M., Lohwasser, U., Börner, A., Franz, C., and Novak, J. (2010). Investigation of antioxidant and rosmarinic acid variation in the sage collection of the genebank in Gatersleben. J. Agric. Food Chem. 58, 3813–3819. doi:10.1021/jf903993f
Li, H., Yang, T., Li, F. Y., Yao, Y., and Sun, Z. M. (2014). Antibacterial activity and mechanism of action of Monarda punctata essential oil and its main components against common bacterial pathogens in respiratory tract. Int. J. Clin. Exp. Pathol. 7 (11), 7389–7398.
Libby, P. (2007). Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr. Rev. 65 (12 Pt 2), S140–S146. doi:10.1301/nr.2007.dec.s140-s146
Lucarini, R., Bernardes, W. A., Ferreira, D. S., Tozatti, M. G., Furtado, R., Bastos, J. K., et al. (2013). In vivo analgesic and anti-inflammatory activities of Rosmarinus officinalis aqueous extracts, rosmarinic acid and its acetyl ester derivative. Pharm. Biol. 51 (9), 1087–1090. doi:10.3109/13880209.2013.776613
Luo, C., Zou, L., Sun, H., Peng, J., Gao, C., Bao, L., et al. (2020). A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 28. doi:10.3389/fphar.2020.00153
Magne, A. L., Nguemfo, E. L., Zangueu, C. B., Ngando, H.-L., Belle, P. A., and Dongmo, A. B. (2016). Antinociceptive and anti-inflammatory effects of the aqueous leaves extract of Plectranthus glandulosus Hook. F. (Lamiaceae) in mice and rats. Pharmacologia 7 (1), 60–66. doi:10.5567/pharmacologia.2016.60.66
Mahmoudi, H., Marzouki, M., M'Rabet, Y., Mezni, M., Ait Ouazzou, A., and Hosni, K. (2020). Enzyme pretreatment improves the recovery of bioactive phytochemicals from sweet basil (Ocimum basilicum L.) leaves and their hydrodistilled residue by-products, and potentiates their biological activities. Arab. J. Chem. 13, 6451–6460. doi:10.1016/j.arabjc.2020.06.003
Marchioni, I., Najar, B., Ruffoni, B., Copetta, A., Pistelli, L., and Pistelli, L. (2020). Bioactive compounds and aroma profile of some Lamiaceae edible flowers. Plants 9 (6), 691. doi:10.3390/plants9060691
Martins, N., Barros, L., Santos-Buelga, C., Henriques, M., Silva, S., and Ferreira, I. C. (2014). Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: different performances regarding bioactivity and phenolic compounds. Food Chem. 158, 73–80. doi:10.1016/j.foodchem.2014.02.099
Mattarelli, P., Epifano, F., Minardi, P., Di Vito, M., Modesto, M., Barbanti, L., et al. (2017). Chemical composition and antimicrobial activity of essential oils from aerial parts of Monarda didyma and Monarda fistulosa cultivated in Italy. J. Essent. Oil Bearing Plants 20 (1), 76–86. doi:10.1080/0972060x.2016.1278184
Naveed, M., Hejazi, V., Abbas, M., Kamboh, A. A., Khan, G. J., Shumzaid, M., et al. (2018). Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed. Pharmacother. 97, 67–74. doi:10.1016/j.biopha.2017.10.064
Park, C. M., and Song, Y. S. (2013). Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr. Res. Pract. 7 (6), 423–429. doi:10.4162/nrp.2013.7.6.423
Petersen, M., and Simmonds, M. S. (2003). Rosmarinic acid. Phytochemistry 62 (2), 121–125. doi:10.1016/s0031-9422(02)00513-7
Pogačar, M. Š., Anja Klančnik, A., Bucar, F., Langerholc, T., and Možina, S. S. (2016). Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene and intestine epithelial cells. J. Sci. Food Agric. 96 (8), 2723–2730. doi:10.1002/jsfa.7391
Pudziuvelyte, L., Liaudanskas, M., Jekabsone, A., Sadauskiene, I., and Bernatoniene, J. (2020). Elsholtzia ciliata (Thunb.) Hyl. extracts from different plant parts: phenolic composition, antioxidant, and anti-inflammatory activities. Molecules 25 (5), 1153. doi:10.3390/molecules25051153
Rahbardar, M. G., Amin, B., Mehri, S., Mirnajafi-Zadeh, S. J., and Hosseinzadeh, H. (2018). Rosmarinic acid attenuates development and existing pain in a rat model of neuropathic pain: an evidence of anti-oxidative and anti-inflammatory effects. Phytomedicine 40, 59–67. doi:10.1016/j.phymed.2018.01.001
Rocha, J., Eduardo-Figueira, M., Barateiro, A., Fernandes, A., Brites, D., Bronze, R., et al. (2015). Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 116 (5), 398–413. doi:10.1111/bcpt.12335
Rytsyk, O., Soroka, Y., Shepet, I., Vivchar, Z., Andriichuk, I., Lykhatskyi, P., et al. (2020). Experimental evaluation of the effectiveness of resveratrol as an antioxidant in colon cancer prevention. Nat. Product. Commun. 15 (6), 1934578X2093274. doi:10.1177/1934578x20932742
Salehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A., et al. (2019). The therapeutic potential of apigenin. Int. J. Mol. Sci. 20 (6), 1305. doi:10.3390/ijms20061305
Sánchez-Vioque, R., Polissiou, M., Astraka, K., Mozos-Pascual, M. d. l., Tarantilis, P., Herraiz-Peñalver, D., et al. (2013). Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind. Crops Prod. 49, 150–159. doi:10.1016/j.indcrop.2013.04.053
Savickiene, N., Dagilyte, A., Barsteigiene, Z., Kazlauskas, S., and Vaiciūniene, J. (2002). [Analysis of flavonoids in the flowers and leaves of Monarda didyma L]. Medicina (Kaunas) 38 (11), 1119–1122.
Seyoum, A., Asres, K., and El-Fiky, F. K. (2006). Structure-radical scavenging activity relationships of flavonoids. Phytochemistry 67, 2058–2070. doi:10.1016/j.phytochem.2006.07.002
Shanaida, M., Golembiovska, O., Hudz, N., and Wieczorek, P. P. (2018a). Phenolic compounds of herbal infusions obtained from some species of the Lamiaceae family. Curr. Issues Pharm. Med. Sci. 31 (4), 194–199. doi:10.1515/cipms-2018-0036
Shanaida, M., Hudz, N., Korzeniowska, K., and Wieczorek, P. (2018b). Antioxidant activity of essential oils obtained from aerial part of some Lamiaceae species. Int. J. Green Pharm. 12 (3), 200–204. doi:10.22377/ijgp.v12i03.1952
Shanaida, M. I. (2020). Method of the development of herbal medicinal product with anti-inflammatory and analgesic activities from the Monarda fistulosa herb. Ukraine Patent No 120826.
Shanaida, M., Jasicka-Misiak, I., Makowicz, E., Stanek, N., Shanaida, V., and Wieczorek, P. (2020). Development of high-performance thin layer chromatography method for identification of phenolic compounds and quantification of rosmarinic acid content in some species of the Lamiaceae family. J. Pharm. Bioall. Sci. 12, 139–145. doi:10.4103/jpbs.jpbs_322_19
Singleton, W. S., and Rossi, J. A. (1965). Characterized oils for use in intravenous fat emulsions. Am. J. Clin. Nutr. 16, 16. doi:10.1093/ajcn/16.1.16
Song, Y. S., and Park, C. M. (2014). Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem. Toxicol. 65, 70–75. doi:10.1016/j.fct.2013.12.017
Spagnol, C. M., Assis, R. P., Brunetti, I. L., Isaac, V. L. B., Salgado, H. R. N., and Corrêa, M. A. (2019). In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 219, 358–366. doi:10.1016/j.saa.2019.04.025
Stanek, N., Kafarski, P., and Jasicka-Misiak, I. (2019). Development of a high performance thin layer chromatography method for the rapid qualification and quantification of phenolic compounds and abscisic acid in honeys. J. Chromatogr. A. 1598, 209–215. doi:10.1016/j.chroma.2019.04.052
Stanković, M. S. (2011). Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac J. Sci. 33, 63–72.
Tabanca, N., Bernier, U. R., Ali, A., Wang, M., Demirci, B., Blythe, E. K., et al. (2013). Bioassay-guided investigation of two Monarda essential oils as repellents of yellow fever Mosquito Aedes Aegypti. J. Agric. Food Chem. 61, 8573−8578. doi:10.1021/jf402182h
Thompson, T., Kiehne, P., Maroko, J., Kapsner, T. R., and Angerhofer, C. K. (2013). Seasonal variation in chemistry and biological activity of Monarda fistulosa. Planta Med. 79 (5), 11–17. doi:10.1055/s-0033-1336453
Touaibia, M., Jean-François, J., and Doiron, J. (2011). Caffeic acid, a versatile pharmacophore: an overview. Mini Rev. Med. Chem. 11 (8), 695–713. doi:10.2174/138955711796268750
Tungmunnithum, D., Thongboonyou, A., Pholboon, A., and Yangsabai, A. (2018). Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 5, 93–99. doi:10.3390/medicines5030093
Uritu, C. M., Mihai, C. T., Stanciu, G.-D., Dodi, G., Alexa-Stratulat, T., Luca, A., et al. (2018). Medicinal plants of the family Lamiaceae in pain therapy: a review. Pain Res. Manag. 2018, 1. doi:10.1155/2018/7801543
Vlase, L., Benedec, D., Hanganu, D., Damian, G., Csillag, I., Sevastre, B., et al. (2014). Evaluation of antioxidant and antimicrobial activities and phenolic profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 19 (19), 5490–5507. doi:10.3390/molecules19055490
Vovk, G. V., Koshovyi, O. M., Kovaliova, A. M., Rybak, V. A., Komisarenko, A. M., and Myha, M. M. (2016). Method of obtaining a dry extract from the Salvia officinalis leaves by the complex processing. Ukraine Patent No 110990.
Vysochina, G. I. (2020). Genus Monarda (Lamiaceae): chemical composition, biological activity and practical application (a review). Chem. Interest Sustain. Develop. 28 (2), 107–123. doi:10.15372/CSD2020209
Wang, M., Liu, J., Zhou, B., Xu, R., Tao, L., Ji, M., et al. (2012). Acute and sub-chronic toxicity studies of Danshen injection in Sprague-Dawley rats. J. Ethnopharmacol. 141, 96–103. doi:10.1016/j.jep.2012.02.005
Xagorari, A., Papapetropoulos, A., Mauromatis, A., Economou, M., Fotsis, T., and Roussos, C. (2001). Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 296 (1), 181–187.
Žemlička, L., Fodran, P., Lukeš, V., Vagánek, A., Slováková, M., Stasko, A., et al. (2014). Physicochemical and biological properties of luteolin-7-O-β-D-glucoside (cynaroside) isolated from Anthriscus sylvestris (L.) Hoffm. Monatsh. Chem. 145, 1307–1318. doi:10.1007/s00706-014-1228-3
Zhilyakova, E. T., Novikov, O. O., Naumenko, E. N., Krichkovskaya, L. V., Kiseleva, T. S., Timoshenko, E. Y., et al. (2009). Study of Monarda fistulosa essential oil as a prospective antiseborrheic agent. Bull. Exp. Biol. Med. 148 (4), 612–614. doi:10.1007/s10517-010-0777-7
Zhu, F., Asada, T., Sato, A., Koi, Y., Nishiwaki, H., and Tamura, H. (2014). Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 62, 885–892. doi:10.1021/jf404318j
Keywords: wild bergamot, herb, postdistillation waste, phenolic compounds, safety, anti-inflammatory activity, antiradical activity, analgesic activity
Citation: Shanaida M, Hudz N, Jasicka-Misiak I and Wieczorek PP (2021) Polyphenols and Pharmacological Screening of a Monarda fistulosa L. dry Extract Based on a Hydrodistilled Residue By-Product. Front. Pharmacol. 12:563436. doi: 10.3389/fphar.2021.563436
Received: 18 June 2020; Accepted: 15 February 2021;
Published: 29 April 2021.
Edited by:
Francesca Borrelli, University of Naples Federico II, ItalyReviewed by:
Giuseppe Annunziata, University of Naples Federico II, ItalyCopyright © 2021 Shanaida, Hudz, Jasicka-Misiak and Wieczorek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariia Shanaida, c2hhbmF5ZGFAdGRtdS5lZHUudWE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.